Abstract
Background
COVID-19-related pleural effusions are frequently described during the ongoing pandemic.
Objectives
We described the incidence, characteristics, and outcomes of COVID-19-related pleural effusions based on the current evidence available in the literature.
Methods
We searched MEDLINE, Pubmed, and Google Scholar databases using keywords of “coronavirus disease 2019 (COVID-19),” “severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),” “pleural effusion,” “pleural fluid,” and “pleura” from January 1st, 2020 to January 31st, 2021.
Results
The incidence of pleural effusions was low at 7.3% among the 47 observational studies. Pleural effusions were commonly observed in critically ill patients and had Multisystem Inflammatory Syndrome (MIS). COVID-19-related pleural effusions were identified 5–7 days and 11 days, after hospital admission and onset of COVD-19 symptoms. The characteristic findings of pleural fluid were exudative, lymphocytic or neutrophilic-predominant pleural fluid with markedly elevated lactate dehydrogenase (LDH) levels and pleural fluid to serum LDH ratio.
Conclusion
A well-designed study is required to assess the significance of COVID-19-related pleural effusions during this current pandemic.
Keywords: Severe acute respiratory syndrome coronavirus 2, SARS-CoV-2, Coronavirus disease 2019, COVID-19, Pleural effusion, Pleura
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus is known to cause coronavirus disease 2019 (COVID-19), resulting in the ongoing pandemic.1 SARS-CoV-2 belongs to the same family of betacoronaviruses involving severe acute respiratory syndrome coronavirus (SARS-CoV-1) and middle east respiratory syndrome coronavirus (MERS-CoV) that are responsible for previous global outbreaks of respiratory illnesses such as severe acute respiratory syndrome (SARS) and middle east respiratory syndrome (MERS), respectively.1 COVID-19 has been well described to present with a wide variety of respiratory complications that range from self-limiting upper respiratory tract infection to severe acute respiratory failure in the form of acute respiratory distress syndrome (ARDS) from underlying pneumonia.2 , 3 The significance of pleural effusions in COVID-19 pneumonia has not been well assessed due to the rarity of the disease limited to case reports/series; no systematic review/meta-analysis has analyzed the importance of pleural effusions in the setting of the ongoing pandemic.4, 5, 6, 7, 8, 9, 10, 11, 12 The purpose of our review is to discuss the incidence, risk factors, outcomes, onset, and pleural fluid characteristic of pleural effusions in patients with COVID-19 pneumonia based on the current evidence available in the medical literature.
Methods
A literature search was performed through MEDLINE, Pubmed, and Google Scholar databases using keywords of “coronavirus disease 2019 (COVID-19),” “severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),” “pleural effusion,” “pleural fluid,” and “pleura” from January 1st, 2020 to January 31st, 2021. Articles published in the English language were selected, and any cited references were reviewed to identify relevant literature. All specified keywords were combined using the “OR” operator and “AND” operator for searching the literature. We selected articles that describe pleural effusions on chest imaging [using chest computed tomography (CT)] in adults and children due to COVID-19 infections from observational studies and case reports/series. We excluded articles that did not report pleural effusion in COVID-19 patients. All included studies in this review were analyzed for: study design (e.g., retrospective or prospective and cross-sectional, case-control, or cohort); study type (clinical or radiological characteristic); month/year; country; the number of patients; patient type (adults or adults and children); age of the patient (e.g., mean +/− standard deviation or median [interquartile range]); incidence of pleural effusions; location of pleural effusions (e.g., unilateral or bilateral); days to the diagnosis of first pleural effusion from admission; and other co-existing radiological features (e.g., frequency of pleural thickening, pleural retraction, pericardial effusion, and hilar/mediastinal lymphadenopathy) were described in Table 1 for COVID-19 observational studies and Table 2 for COVID-19 case reports/series. We similarly compared pleural effusions observed in other SARS and MERS viruses that were briefly summarized in Table 2. Lastly, we described the pleural effusions characteristics observed after pleural drainage was performed in four case reports/series as shown in Table 3 .
Table 1.
Summary of 47 observational studies for COVID-19 patients with pleural effusions.
| Author | Study Design | Study Type | Month, Year | Country | Patients (N) | Patient Type | Age (Y) Mean +/- SD, Median (IQR) | Pleural Effusion (%) | Pleural Effusion | Admission Days to 1st Pleural Effusion | Pleural Thickening (%) | Pleural Retraction (%) | Pericardial Effusion (%) | H/M Lymph-adenopathy (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | ||||||||||||||
| (%) | ||||||||||||||
| Arentz et al.40 | Cross-Sectional, Single-Center | Clinical | April 2020 | USA | 21 | Adults | 70.0 +/- NR | 23.8 | NR | NR | NR | NR | NR | NR |
| Bai et al.52 | Retrospective Cohort, Multi-Center | Clinical | August 2020 | China | 219 | Adults and Children | 45.0 +/- 15.0 | 4.0 | NR | NR | 15.0 | NR | NR | 3.0 |
| Blumfield et al.25 | Cross-Sectional, Single-Center | Clinical | July 2020 | USA | 16 | Children | 9.2 +/- 4.9 | 62.5 | NR | NR | NR | NR | 12.5 | NR |
| Bernheim et al.13 | Retrospective Cohort, Multi-Center | Clinical | June 2020 | China | 121 | Adults | 45.0 +/- 16.0 | 1.0 | Unilateral (100) | NR | NR | NR | NR | NR |
| Chen et al.22 | Retrospective Cohort, Multi-Center | Clinical | April 2020 | China | 61 | Adults and Children | 39.9 +/- 23.9 | 4.7 | NR | NR | NR | NR | NR | 2.3 |
| Cobb et al.58 | Retrospective Cohort, Multi-Center | Clinical | November 2020 | USA | 63 | Adults | 60.4 +/- 15.7 | 11.1 | NR | NR | NR | NR | NR | NR |
| Colombi et al.97 | Retrospective Cohort, Single-Center | Radiological | August 2020 | Italy | 236 | Adults | 68.0 +/- 2.0 | 20.0 | NR | NR | NR | NR | NR | 24 |
| Ding et al.76 | Retrospective Cohort, Single-Center | Radiological | April 2020 | China | 112 | Adults and Children | 55.8 +/- 16.1 | 17.0 | NR | NR | NR | NR | 4.5 | NR |
| Feng et al.20 | Retrospective Cohort, Multi-Center | Clinical | June 2020 | China | 442 | Adults and Children | 53.0 (40.0–64.0) | 5.7 | NR | NR | 53.8 | NR | NR | NR |
| Fu et al.14 | Retrospective Cohort, Single-Center | Clinical | July 2020 | China | 46 | Adults | NR | 8.7 | Bilateral (100) | NR | 54.3 | 37.0 | NR | NR |
| Gervaise et al.37 | Retrospective Cohort, Single-Center | Clinical | June 2020 | France | 72 | Adults | 62.3 +/- 17.8 | 22.0 | NR | NR | NR | NR | NR | NR |
| Guan et al.64 | Cross-Sectional, Single-Center | Radiological | March 2020 | China | 47 | Adults and Children | 42.0 +/- NR | 4.3 | NR | NR | NR | NR | NR | NR |
| Hameed et al. | Cross-Sectional, Single-Center | Radiological | January 2021 | UK | 35 | Children | 11.0 (6.0–14.0) | 11.4 | Bilateral (100) | NR | NR | NR | NR | 14.3 |
| Han et al.77 | Retrospective Cohort, Single-Center | Radiological | March 2020 | China | 17 | Adults | 40.0 +/- 10.0 | 23.5 | NR | 7 < | 76.5 | NR | NR | NR |
| Li et al.71 | Retrospective Cohort, Single-Center | Clinical | June 2020 | China | 83 | Adults | 45.5 +/- 12.3 | 8.4 | NR | NR | NR | NR | 4.8 | 8.4 |
| Li et al.19 | Retrospective Cohort, Single-Center | Clinical | March 2020 | China | 78 | Adults | 44.6 +/- 17.9 | 8.9 | Bilateral (83.3) | NR | NR | NR | NR | NR |
| Li et al.98 | Retrospective Cohort, Single-Center | Clinical | June 2020 | China | 51 | Adults | 58.0 +/- 17.0 | 2.0 | NR | NR | NR | NR | NR | NR |
| Liu et al.72 | Retrospective Cohort, Single-Center | Clinical | March 2020 | China | 73 | Adults and Children | 41.6 +/- 14.5 | 4.1 | NR | NR | NR | NR | NR | NR |
| Liu et al.23 | Cross-Sectional, Single-Center | Clinical | March 2020 | China | 55 | Adults and Children | NR | 23.6 | NR | NR | NR | NR | NR | NR |
| Long et al.99 | Retrospective Cohort, Single-Center | Clinical | March 2020 | China | 36 | Adults | 44.8 +/- 18.2 | 5.6 | NR | NR | NR | NR | NR | 2.8 |
| Martino et al.100 | Cross-Sectional, Single-Center | Radiological | June 2020 | Italy | 62 | Adults | 71.0 +/- 14.0 | 18.0 | NR | NR | 72.6 | NR | NR | NR |
| Majidi et al.21 | Cross-Sectional, Single-Center | Clinical | July 2020 | Iran | 552 | Adults | 51.2 +/- 14.8 | 7.6 | NR | NR | NR | NR | NR | 5.1 |
| Miro et al.101 | Retrospective Cohort, Multi-center | Clinical | January 2021 | Spain | 440 | Adults | 61.0 (46.0–77.0) | 2.5 | NR | NR | NR | NR | NR | NR |
| Mo et al.70 | Retrospective Cohort, Single-Center | Clinical | March 2020 | China | 155 | Adults | 54.0 (42.0–66.0) | 10.3 | NR | NR | NR | NR | NR | NR |
| Pakdemirli et al.102 | Cross-Sectional, Single-Center | Radiological | July 2020 | England | 18 | Adults | 57.0 (NR) | 39.0 | NR | NR | 50.0 | NR | NR | 17.0 |
| Shi et al.3 | Cross-Sectional, Single-Center | Clinical | April 2020 | China | 81 | Adults and Children | 49.5 +/- 11.0 | 5.0 | NR | NR | 32.0 | NR | NR | 6.0 |
| Song et al.15 | Retrospective Cohort, Single-Center | Clinical | April 2020 | China | 62 | Adults | 49.0 +/- 16.0 | 8.0 | Unilateral (75.0) | 5 < | NR | NR | 7.0 | 6.0 |
| Tabatabaei et al.39 | Retrospective Cohort, Single-Center | Radiological | April 2020 | Iran | 120 | Adults | 54.9 +/- 17.1 | 16.7 | NR | NR | NR | NR | NR | NR |
| Toubiana et al.30 | Prospective Cohort, Single-Center | Clinical | June 2020 | France | 21 | Children | 7.9 (3.7–16.6) | 14.3 | NR | NR | NR | NR | 47.6 | NR |
| Valverde et al.29 | Retrospective Cohort, Multi-Center | Clinical | January 2021 | Europe | 286 | Children | 8.4 (3.8 - 12.4) | 13.9 | NR | NR | NR | NR | 28 | NR |
| Vancheri et al.16 | Retrospective Cohort, Single-Center | Radiological | May 2020 | Italy | 180 | Adults | 65.0 +/- 16.0 | 6.6 | Unilateral (100) | NR | NR | NR | NR | NR |
| Wang et al.18 | Cross-Sectional, Single-Center | Radiological | May 2020 | China | 114 | Adults | 53.0 (NR) | 0.9 | NR | NR | NR | NR | NR | NR |
| Wang et al.75 | Cross-Sectional, Single-Center | Radiological | March 2020 | China | 90 | Adults | 45.0 +/−14.0 | 7.0 | Bilateral (100) | 11 < (Symptoms) | NR | NR | NR | NR |
| Wen et al.17 | Cross-Sectional, Multi-Center | Radiological | April 2020 | China | 82 | Adults | 46.0 +/- 15.0 | 1.0 | Unilateral (100) | NR | NR | NR | NR | NR |
| Wong et al.103 | Cross-Sectional, Single-Center | Radiological | March 2020 | China | 64 | Adults | 56.0 +/- 19.0 | 3.1 | NR | NR | NR | NR | NR | NR |
| Wu et al.104 | Cross-Sectional, Multi-Center | Clinical | February 2020 | China | 80 | Adults | 44.0 +/- 11.0 | 6.0 | NR | NR | NR | NR | 6.0 | 4.0 |
| Xiang et al.105 | Cross-Sectional, Multi-Center | Radiological | August 2020 | China | 53 | Adults | 53.0 +/- 16.0 | 1.9 | NR | NR | NR | NR | NR | NR |
| Xiong et al.74 | Cross-Sectional, Single-Center | Clinical | June 2020 | China | 42 | Adults | 49.5 +/- 14.1 | 11.9 | NR | NR | NR | NR | NR | 29.0 |
| Xu et al.106 | Cross-Sectional, Single-Center | Clinical | February 2020 | China | 90 | Adults | 50.0 (18.0–86.0) | 4.0 | NR | NR | 56.0 | NR | 1.0 | 1.0 |
| Xu et al.107 | Retrospective Cohort, Single-Center | Clinical | February 2020 | China | 41 | Adults and Children | 43.9 +/- 16.8 | 9.8 | NR | NR | NR | NR | NR | 2.4 |
| Yang et al.108 | Retrospective Cohort, Multi-Center | Clinical | February 2020 | China | 149 | Adults | 45.1 +/- 13.4 | 6.7 | NR | NR | NR | NR | NR | 4.7 |
| Yin et al.57 | Retrospective Cohort, Single-Center | Clinical | November 2020 | China | 30 | Adults | 52.7 +/- 15.1 | 13.3 | NR | NR | 90 | NR | 13.0 | 13.0 |
| Zhang et al.35 | Cross-Sectional, Single-Center | Clinical | July 2020 | China | 18 | Adults | 29.1 +/- 2.8 | 11.0 | NR | NR | NR | NR | NR | NR |
| Zhao et al.59 | Retrospective Cohort, Single-Center | Clinical | May 2020 | China | 101 | Adults and Children | 44.4 +/- 12.3 | 13.9 | NR | NR | NR | NR | NR | 1.0 |
| Zhou et al.60 | Retrospective Cohort, Single-Center | Clinical | June 2020 | China | 62 | Adults | 52.8 +/- 12.2 | 9.7 | NR | NR | 48.4 | 56.5 | NR | NR |
| Zhu et al.24 | Retrospective Cohort, Single-Center | Clinical | July 2020 | China | 72 | Adults | 55.6 +/- 12.8 | 6.9 | NR | NR | 52.8 | 31.9 | NR | NR |
| Zhu et al.109 | Retrospective Cohort, Single-Center | Clinical | March 2020 | China | 32 | Adults | 40.0 (27.0 – 53.0) | 6.0 | NR | NR | NR | NR | NR | 3.0 |
Abbreviations: IQR : interquartile range, NR : non-reported, N : number of patients, SD : standard deviation, Y : years.
Table 2.
Summary of 18 case reports/series for COVID-19 patients with pleural effusions and studies performed in SARS and MERS patients with pleural effusions.
| COVID-19 Case Reports/Series |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Study Design | Study Type | Month, Year | Country | Patients (N) | Patient Type | Age (Y) Mean +/- SD, Median (IQR) | Pleural Effusion (%) | Location of Pleural Effusion (%) | Admission Days to 1st Pleural Effusion | Pleural thickening (%) | Pleural Retraction (%) | Pericardial Effusion (%) | H/M Lymph-adenopathy (%) |
| Albarello et al.9 | Case Series | Radiological | February 2020 | Italy | 2 | Adults | 60s | 100 | Unilateral (50.0) | 7 < (Symptoms) | 100 | NR | 50.0 | 100 |
| Chiotos et al.110 | Case Series | Clinical | July 2020 | USA | 6 | Children | 8.5 +/- 3.5 | 16.7 | NR | NR | NR | NR | NR | NR |
| Chong et al.78 | Case Series | Clinical | September 2020 | USA | 4 | Adults | 56.5 +/- 8.9 | 100 | Unilateral (75.0) | 18 < | NR | NR | NR | NR |
| Fahad et al.45 | Case Report | Clinical | November 2020 | Iraq | 1 | Adult | 72.0 | 100 | Unilateral (100) | 10 < (Symptoms) | NR | NR | NR | NR |
| Gong et al.10 | Case Series | Clinical | July 2020 | China | 10 | Adults | 29.4 +/- 4.5 | 60 | Bilateral (100) | NR | NR | NR | NR | NR |
| Hussein et al.82 | Case Report | Clinical | October 2020 | Qatar | 1 | Adult | 52.0 | 100 | Unilateral (100) | 21 < (Symptoms) | 100 | NR | NR | NR |
| Lescure et al.111 | Case Series | Clinical | March 2020 | France | 5 | Adults | 47.0 +/- 18.1 | 20 | NR | NR | NR | NR | NR | NR |
| Lin et al.7 | Case Report | Clinical | February 2020 | China | 1 | Adult | 61.0 | 100 | Bilateral (100) | 9 < | NR | NR | NR | NR |
| Malik et al.6 | Case Report | Clinical | July 2020 | Pakistan | 1 | Adult | 38.0 | 100 | Bilateral (100) | NR | NR | NR | NR | NR |
| Matsuda et al.112 | Case Report | Clinical | October 2020 | Brazil | 1 | Child | 10.0 | 100 | Bilateral (100) | 7< (Symptoms) | NR | NR | 100 | NR |
| Mei et al.81 | Case Report | Clinical | July 2020 | Italy | 1 | Adult | 72.0 | 100 | Bilateral (100) | 6 < | NR | NR | NR | NR |
| Morris et al.31 | Case Series | Clinical | October 2020 | USA | 16 | Adults | 34.2 +/- 10.2 | 37.5 | NR | NR | NR | NR | 31.3 | 6.3 |
| Riphagen et al.27 | Case Series | Clinical | May 2020 | UK | 8 | Children | 8.9 +/- 3.4 | 37.5 | NR | NR | NR | NR | NR | NR |
| Rostad et al.34 | Case Series | Clinical | January 2021 | USA | 11 | Children | 9.0 +/- 3.5 | 81.8 | Bilateral (66.7) | NR | NR | NR | 81.8 | NR |
| Shi et al.11 | Case Report | Clinical | March 2020 | China | 1 | Adult | 57.0 | 100 | NR | 3 < | NR | NR | NR | NR |
| Sokolovsky et al.32 | Case Report | Clinical | June 2020 | USA | 1 | Adult | 36.0 | 100 | Right (100) | 7 < (Symptoms) | NR | NR | 100 | NR |
| Tham et al.46 | Case Series | Clinical | November 2020 | Singapore | 4 | Adults | 31.8 +/- 6.4 | 75.0 | Unilateral (100) | NR | NR | NR | NR | NR |
| Zhu et al.8 | Case Series | Clinical | February 2020 | China | 3 | Adults | 47.3 +/- 11.9 | 33.3 | Bilateral (100) | 11 < (Symptoms) | NR | NR | NR | NR |
| SARS Studies | ||||||||||||||
| Author | Study Design | Study Type | Month, Year | Country | Patients (N), | Patient Type | Age (Y) Mean +/- SD, Median (IQR) | Pleural Effusion (%) | Location of Pleural Effusion (%) | Admission Days to 1st Pleural Effusion | Pleural thickening (%) | Pleural Retraction (%) | Pericardial Effusion (%) | H/M Lymph-adenopathy (%) |
| Chan et al.113 | Cross-Sectional, Single-Center | Radiological | January 2004 | China | 27 | Adults | 45.0 (17.0–72.0) | 26.0 | NR | 18 < (Symptoms) | 59.0 | NR | NR | NR |
| Joynt et al.61 | Case Series | Radiological | February 2004 | China | 8 | Adults | 52.9 +/- 10.6 | 12.5 | Unilateral (100) | 14 < (Symptoms) | 12.5 | 12.5 | NR | NR |
| Muller et al.114 | Cross-Sectional, Multi-Center | Radiological | July 2003 | Canada and China | 25 | Adults | 44.0 (24.0 – 82.0) | 8.0 | Bilateral (100) | 9 < | NR | NR | NR | NR |
| Muller et al.115 | Cross-Sectional, Multi-Center | Radiological | January 2004 | Canada and China | 12 | Adults | 38.0 +/- NR | 8.3 | Bilateral (100) | NR | NR | NR | NR | NR |
| Ooi et al.116 | Cross-Sectional, Multi-Center | Radiological | March 2004 | Canada and China | 30 | Adults | 42.5 +/−12.2 | 7.1 | Bilateral (100) | 14 < (Symptoms) | NR | NR | NR | NR |
| Wong et al.67 | Cross-Sectional, Single-Center | Radiological | December 2004 | China | 70 | Adults | 39.4 +/- 12.8 | 2.9 | Bilateral (50.0) | NR | NR | NR | NR | NR |
| Wong et al.68 | Retrospective, Cohort, Single-Center | Radiological | August 2003 | China | 40 | Adults and Children | 37.2 +/- NR | 2.5 | Bilateral (100) | NR | NR | NR | NR | 2.5 |
| MERS Studies | ||||||||||||||
| Author | Study Design | Study Type | Month, Year | Country | Patients (N), | Patient Type | Age (Y) Mean +/- SD, Median (IQR) | Pleural Effusion (%) | Location of Pleural Effusion (%) | Admission Days to 1st Pleural Effusion | Pleural thickening (%) | Pleural Retraction (%) | Pericardial Effusion (%) | H/M Lymph-adenopathy (%) |
| Ajlan et al.47 | Case Series | Radiological | October 2014 | Saudi Arabia | 7 | Adults | 50.0 (19.0 – 83.0) | 42.8 | Bilateral (100) | 9 < (Symptoms) | NR | NR | NR | NR |
| Das et al.48 | Retrospective, Cohort, Single-Center | Radiological | September 2015 | Saudi Arabia | 55 | Adults and Children | 46.9 +/- NR | 30.9 | NR | 6 < | NR | NR | NR | NR |
| Das et al.49 | Case Series | Radiological | April 2015 | Saudi Arabia | 15 | Adults | 48.8 +/- 17.9 | 60.0 | NR | 3 < | NR | NR | NR | NR |
Abbreviations: IQR : interquartile range, NR : non-reported, N : number of patients, SD : standard deviation, Y : years.
Table 3.
Summary of pleural effusions characteristics in COVID-19 patients who received pleural drainage.
| Study | Chen et al.83 | Chong et al.81 | Chong et al.81 | Chong et al.81 | Chong et al.81 | Chong et al.81 | Hussein et al.85 | Mei et al.84 |
|---|---|---|---|---|---|---|---|---|
| Patients | Patient 1 | Patient 1 | Patient 2 | Patient 3 | Patient 3 | Patient 4 | Patient 1 | Patient 1 |
| Age, Gender | 12 M | 68 M | 62 F | 50 M | 50 M | 46 M | 52 M | 72 M |
| Comorbidities | None | HLD | HTN, HLD, CKD, Diabetes, CAD | None | None | HTN, HLD, CKD, Diabetes, CAD, Asthma | None | HTN |
| Systemic Anticoagulation | NR | Heparin | Heparin | Enoxaparin | Enoxaparin | Enoxaparin | NR | NR |
| Amount Pleural Fluid Removed | 300 ml | 3000 ml | 800 ml | 700 ml | 700 ml | 500 ml | NR | 600 ml |
| Laterality | Right | Left | Left | Left | Right | Right | Right | Bilateral |
| Pleural Fluid | ||||||||
| Color | Serous | Serosanguineous | Serous | Serous | Serosanguineous | Sanguineous | Serosanguineous | Serous |
| White Cell Count (per mm3) | 3060 | 1850 | 2719 | 476 | 600 | 7738 | 2450 | 120 |
| Neutrophil | 2% | 75% | 0% | 11% | 47% | 96% | 41% | NR |
| Lymphocyte | 98% | 9% | 75% | 50% | 30% | 1% | 45% | 92% |
| Eosinophil | NR | 0% | 0% | 0% | 0% | 1% | 9% | NR |
| Monocyte | NR | 5% | 10% | 39% | 22% | 2% | NR | NR |
| RBC (per mm3) | NR | 555,000 | 88,000 | 2000 | 133,000 | 1010,000 | NR | NR |
| Chemistry | ||||||||
| pH | NR | 7.45 | 7.43 | 7.72 | 7.8 | 7.57 | 7.5 | 7.35 |
| LDH (IU/L) | 291 | 2689 | 672 | 549 | 284 | 3651 | 1185 | 168 |
| Protein (gm/dL) | 4.6 | 4.5 | 3.6 | 2.6 | 2.2 | 3.1 | 6.0 | 2.3 |
| Glucose (mg/dL) | NR | 132 | 116 | 209 | 191 | 102 | 263 | 115 |
| Serum | ||||||||
| LDH (150–300 IU/L) | NR | 904 | 434 | 220 | 220 | 160 | 215 | 257 |
| Protein (6–8 gm/dL) | NR | 4.8 | 6.3 | 6.1 | 6.1 | 4.9 | 7.6 | 5.2 |
| Pleural Fluid/Serum LDH Ratio | NR | 3.0 | 1.5 | 2.5 | 1.3 | 22.8 | 5.5 | 0.7 |
| Pleural Fluid/Serum Protein Ratio | NR | 0.9 | 0.6 | 0.4 | 0.4 | 0.6 | 0.8 | 0.4 |
| Microbiology | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| SARS-CoV-2 PCR | NR | Positive | Positive | Positive | Positive | Positive | NR | Positive |
| Cytology | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
Abbreviations: F : female, M : male, CAD : coronary arterial disease, CKD : chronic kidney disease, HTN : hypertension, HLD : hyperlipidemia, NR : non-reported.
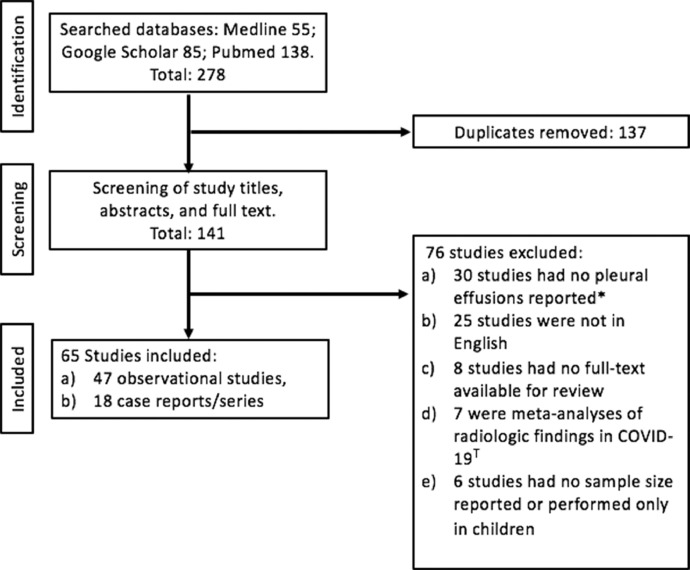
We screened a total of 141 studies (Fig. 1 ) and included 65 studies involving 47 observational studies and 18 case reports/series that described pleural effusion in either adults and/or children diagnosed with COVID-19 pneumonia (Fig. 1). Observational studies were divided into either clinical studies that described both the clinical and radiologic findings in COVID-19 patients or radiological studies that only reported the radiologic chest findings in COVID-19 patients. Among the 47 observational studies included in our review, 70.2% (33/47) were performed in China, (3/47) from the USA, and the remainder in the UK, France, Italy, Iran, and Spain (Table 1). We defined observational studies as studies that assessed a minimum of 15 patients and more. If there were less than 15 patients, it was categorized as either a case series (2–15 patients) or case reports (Table 2). Out of the 47 observational studies, 59.6% (28/47) were retrospective cohort studies, 38.3% (18/47) were cross-sectional studies, and the remainder 2.1% (1/47) was a prospective cohort study. The majority of observational studies, 70.2% (33/47), were clinical studies, and the remaining studies were radiological in COVID-19 patients. 27.3% (13/47) of observational studies had a large sample size (100 and more) of COVID-19 patients (Table 1). In all observational studies, pleural effusions were observed to be a bystander (minor secondary outcome) result or identified incidentally during subgroup analysis while assessing the many clinical and radiological characteristics, risk factors, and outcomes of hospitalized COVID-19 patients. No studies compared the baseline clinical characteristics, risk factors, and outcomes between COVID-19 patients who developed pleural effusions and those that did not develop pleural effusions. Therefore, it was not feasible to perform a statistical analysis due to the limited and heterogeneous data.
Fig. 1.
Flowchart for studies selected in review of COVID-19 patients with pleural effusions. *Observational studies that reported no incidence of pleural effusions in COVID-19 patients. T Meta-analyses assessing all the radiologic findings observed in COVID-19 patients.
Incidence of COVID-19-Related pleural effusion
A total of 4981 patients with COVID-19 patients at risk of developing pleural effusions were included in our review of 47 observational studies. Among the 47 observational studies included, no pleural fluid analysis was performed. In our review, the overall incidence of pleural effusions reported in COVID-19 pneumonia was 7.3% (365/4981) [Table 1]. In the 47 observational studies included, 70.2% (33/47) assessed adults, 21.3% (10/47) assessed both adults and children, and the remaining 8.5% (4/47) assessed only children. Among the 21.3% observational studies that assessed adults and children, the exact number of adults and children was not discussed further. The patient's age group ranged from 7.9 to 70.0 (Table 1). According to eight observational studies, the location of reported pleural effusions was unilateral in 66.8% (443/663) of cases, with the remainder bilateral in 33.2% (220/663) of patients.13, 14, 15, 16, 17, 18, 19 In the two largest observational studies included in our review by Majidi et al. and Feng et al., the incidence of pleural effusions were 7.6% and 5.7% among 552 and 442 COVID-19 patients, respectively.20 , 21 A high incidence of pleural effusions ranging from 13.3% to 62.5% was reported in 14 observational studies reviewed (Table 1). The possible explanation for the high incidence of pleural effusions were: a) Seven observational studies had a small sample size ranging between 16 and 55 COVID-19 patients,22, 23, 24, 25, 26, 27, 28 b) eight observational studies were single-center studies that focused on describing the radiological characteristics of COVID-19 patients,24 , 25 , 27 , 29, 30, 31, 32, 33 and c) five observational studies involved COVID-19-related pleural effusions among children.23 , 26 , 28 , 32 , 34
The similarities in the overall incidence of pleural effusions encountered in COVID-19 to SARS were observed in several radiological observational studies at 7.5% (16/212). However, in MERS, the incidence substantially increased to 37.7% (29/77) that was likely due to selection bias and small sample size [Table 2]. Pleural diseases such as pleural effusions and pneumothoraces may also suggest a more severe clinical course in critically ill MERS patients.35, 36, 37 The presence of pleural effusion in the setting of other infections causing pneumonia (parapneumonic effusions) has been well described among viral pathogens such as community-acquired influenza virus (A and B), adenovirus, measles, hantavirus, herpes simplex virus (HSV), Epstein-Barr virus (EBV), cytomegalovirus (CMV). Less frequently in varicella-zoster virus and human metapneumovirus.38, 39, 40 During zoonotic influenza outbreaks, the incidence of pleural effusions varies according to the influenza strain, such as swine influenza A H1N1 (7.7–8.3%), avian influenza A H5N1 (33.3%), and avian influenza A H7N9 (75–88.9%).38 , 39 , 41, 42, 43, 44 When comparing COVID-19- to Influenza-related pleural effusions, there exist conflicting evidence in the current literature. A single-center, retrospective cohort study by Yin et al. observed that pleural effusion occurs with higher frequency (16% versus 4%; p = 0.002) in those with influenza A H1N1 pneumonia compared to COVID-19 pneumonia.27 In contrast, a multi-center, retrospective, ICU study by Cobb et al. reported that the incidence of pleural effusions was no different (11.1% versus 13.7%; p = 0.65) when comparing COVID-19 patients, and influenza A and B patients admitted over a similar timeframe.45
Other additional chest CT findings related to pleural disease noted in COVID-19 patients were pleural thickening and pleural retraction. The incidence of pleural thickening was 46.9% (534/1139) in 11 observational studies (Table 1). Pleural retraction was only noted in three observational studies with a reported incidence of 41.7% (75/180).14 , 32 , 46 Although pleural thickening was commonly observed in elderly COVID-19 patients who were age 60 years and older (71.4% versus 40.9%; p = 0.011), which might indicate higher disease severity such as ARDS with greater radiologic lung involvement, the incidence of pleural traction and effusion remained similar, regardless of age group.47 In SARS patients, chest imaging during the late-stage of ARDS (two weeks and more) consisting of lung fibrosis and pleural thickening/retraction are similar to those seen in late-stage ARDS from alternative etiologies.48 It is also not unusual for patients with avian influenza A (H5N1) pneumonia to develop lung and pleural fibrosis (retraction) during the convalescent stage of ARDS.41 It has been suggested that to distinguish between patients with non-COVID-19 pneumonia and COVID-19 pneumonia, the radiologic finding of pleural effusion likely favors the diagnosis of former such as rhinovirus/enterovirus, human metapneumovirus, and influenza A H1N1 pneumonia; however, the significance of pleural thickening remains uncertain according to several observational studies by Bai et al. and Yin et al.27 , 40 , 49
Risk factors of COVID-19-related pleural effusions
The frequency of pleural effusions varies with age, according to a large observational study by Majidi et al. involving 552 COVID-19 patients.21 In this study, although the overall rate of pleural effusions detected was 7.3%, a higher amount of COVID-19 patients age 50 years and older (10% versus 5.2%; p = 0.037) developed pleural effusions than those 50 years and under. However, this result was not replicated in other smaller observational studies. Several observational studies comparing the clinical presentation between adults and children (18 years and younger) with COVID-19 pneumonia demonstrated that although adults tend to present with more severe clinical symptoms and chest CT involvement, the incidence of pleural effusion was not different.28 , 50 Similar findings were replicated in a separate observational study comparing elderly COVID-19 patients (age 60 years and older) versus younger COVID-19 patients where despite a greater chest CT involvement, the incidence of pleural effusion remained similar.47 A large, well-designed study controlling for confounding factors is required to assess whether age is a risk factor for developing COVID-19-related pleural effusions.
During the early course in the COVID-19 pandemic, data suggested that children appeared less likely to develop an infection or severe illness than adults.23 However, a new entity termed Multisystem Inflammatory Syndrome in Children (MIS-C) has been increasingly described in the current medical literature defined as a condition that primarily occurs in children that features both Kawasaki disease and toxic shock syndrome.51, 52, 53 MIS-C is often postulated to be an immune-mediated delayed host response triggered by a previous COVID-19 infection (post-infectious phenomenon) that occurs 2–4 weeks after the initial infection. Though, this hyperinflammatory disorder may even be a direct extension of active COVID-19 infections (cytokine storm).23 , 51 Several observational studies with various sample sizes demonstrated that the radiological findings of pleural effusions, pericardial effusions, and even ascites were highly variable at 13.9–62.5%, 12.5–47.6%, and 19.0–37.5%, respectively, in MIS-C patients.23 , 26 , 34 According to case reports/series, MIS was not just confined to children and has also been described in adults (aged 21 years and more) referred to as Multisystem Inflammatory Syndrome in Adults (MIS-A) who presented with pleural effusions.54 , 55 In the existing medical literature, the oldest COVID-19 patient ever diagnosed with pleural effusion secondary to MIS-A was a 50-year-old man who presented with three days of symptoms, tested positive for COVID-19 IgG antibodies with small pleural effusions noted on chest imaging, and improved clinically with immunosuppressive therapies of corticosteroids.54 It has even been suggested that radiologic findings that may help distinguish between MIS from COVID-19 infections are pleural effusions, pericardial effusions, ascites, and hilar/mediastinal lymphadenopathies that favor MIS, reflecting any underlying multisystemic inflammatory process.53 , 56 , 57 Case series by Rostad et al. described an incidence of pleural effusions in children was 81.8% for MIS-C compared to 6.3% (p < 0.01) during COVID-19 infections.57 In conclusion, as our knowledge continues to grow during this current pandemic, pleural effusions should be recognized as part of the constellation of imaging findings in MIS-C and even MIS-A to assist with early diagnosis and allow prompt treatment.
According to Liu et al., the incidence of pleural effusions was up to 29.3% (12/41) in pregnant COVID-19 patients versus 7.1% (1/14) in those who were not pregnant, but their study was underpowered to detect any significant difference.28 Pregnant COVID-19 patients who developed pleural effusions were in later stages of pregnancy at 35–41 weeks of gestation or during the postpartum period.10 , 28 , 58 In pregnant COVID-19 patients, the higher incidence of pleural effusion could be due to benign postpartum pleural effusion that occurs within 24 h of normal vaginal delivery due to transudation of fluid into the pleural space from a pregnancy-induced increase in systemic circulation hydrostatic pressure/blood volume and decreased oncotic pressure from hypoalbuminemia state.10 , 59 The repeated Valsalva maneuver during spontaneous delivery and elevated intraabdominal pressure from the gravid uterus will further increase intrathoracic pressure while impairing lymphatic drainage, promoting pleural effusion formation. Taking into consideration of other comorbidities, there was no association found between COVID-19 patients and the risk of developing pleural effusions in those with or without concurrent acute pulmonary embolism, according to a retrospective cohort study by Gervaise et al.60 When patients with active COVID-19 infections and those with MIS present with sepsis-like illness, aggressive IV fluid will be administered, leading to a clinical state of fluid overload that may precipitate the development of diffuse bilateral lung infiltrates with pleural effusions, attributable to depressed cardiac function, overwhelming systemic inflammatory response, and third spacing of fluids.6 , 17 , 22 , 29 , 56 , 57 , 61 At least 15% and more of COVID-19 patients developed either acute heart failure or kidney failure during their clinical course, increasing to more than 50% in critically ill patients.62 , 63 The clinical state of fluid overload has been described as a potential etiology in SARS-related pleural effusions.64 However, no study has been performed to determine the pre-existing comorbidities of congestive heart failure, hypoalbuminemia, chronic liver disease, and chronic renal failure with the risk of pleural effusions development in COVID-19 patients. The presence of SARS-CoV-2 was found in pleural effusions of 10 deceased COVID-19 patients during postmortem examination.65 However, the location of pleural effusions (unilateral/bilateral), time of development, and pleural fluid characteristics/cultures were not described. At the same time, many of the deceased COVID-19 patients had comorbidities of metastatic malignancies, cardiac, liver, and renal dysfunction, which are common etiologies of pleural effusions. The concomitant presence of co-infection such as active TB or stage IV lung malignancy has been described as predisposing factors for developing pleural effusions that are commonly lymphocytic-predominant with elevated LDH in COVID-19 patients, although limited to case reports/series.66 , 67 Therefore, a well-designed study is required to assess the relationship between pre-existing comorbidities against the risk of developing COVID-19-related pleural effusions.
Outcomes of COVID-19-related pleural effusion
The current overall mortality rate was around 3% for COVID-19 compared to other zoonotic outbreaks such as 2003 SARS outbreak and 2012 MERS outbreak, with an observed mortality rate of 6–11% and 43–60%, respectively.1 , 35, 36, 37 , 68, 69, 70, 71, 72, 73 Pleural effusions had been associated as an independent predictor for poor outcome in critically ill patients with ARDS from infectious and non-infectious etiologies.74 A large observational study of 476 COVID-19 patients by Feng et al. reported that although the overall incidence of pleural effusions was 5.7%, the incidence of pleural effusions was 18% in critically ill patients versus 3.1% (p < 0.001) in non-critically ill patients.32 Similar findings were noted collectively in several observational studies where critically ill COVID-19 patients had a greater likelihood of around 3.2-fold of developing pleural effusions at 33–43% versus 3–13% (p < 0.05) than non-critically ill COVID-19 patients.14 , 19 , 29 , 32 , 75 Moreover, studies by Li et al. and Liu et al. described that pleural effusions were solely seen on chest CT in critically ill COVID-19 patients.76 , 77 Although the inflammatory stress response in pregnant women towards viral pneumonia increased significantly during the middle and late stages of pregnancy, resulting in a rapid progression of the disease and severity of illness that may cause maternal-fetal death, the relationship between COVID-19-related pleural effusions and mortality in pregnancy were not assessed in observational studies and case series included in our review likely due to the small sample size.10 , 28
The association between pleural effusion and increased mortality has been described in MERS patients, where 63.2% of deceased than 13.9% of survivors (p = 0.001) developed pleural effusion.36 Influenza A H1N1 patients with ARDS and who suffered a poor clinical outcome have a greater likelihood of displaying pleural effusions.44 When comparing patients with avian influenza A, H7N9 infection lead to a higher incidence of pleural effusion (88.9%) versus H1N1 infection (7.7%) [p < 0.01] with an 8-fold increased risk of developing ARDS. However, this did not translate to significant mortality in that study.43 In patients with community-acquired pneumonia from both viral and bacterial microorganisms, bilateral pleural effusions and moderate to large pleural effusions are associated with a 7-fold and 3.4-fold increase in 30-day mortality.78 Therefore, more studies are required to confirm or refute the association between critically ill COVID-19 patients and the development of pleural effusions.
Onset of COVID-19-related pleural effusion
Pleural effusions likely represent a more severe inflammatory radiological evolution of COVID-19 pneumonia with increasing frequency during the hospital course and concomitant progression of opacities from ground-glass opacifications (GGOs) to consolidations on chest imaging.3 , 13 , 15 , 46 , 69 , 79 From the day of admission, the overall time to diagnosis of COVID-19-related pleural effusions using chest imaging was 5–7 days and more according to two observational studies.5 , 15 , 80 In contrast, when compared to the time of COVID-19 symptoms onset, it was 11 days and after (Table 1). A single-center, cross-sectional study utilizing serial chest CT scans demonstrated that pleural effusion incidence increased significantly from 12% to 38% (p = 0.01) when performed on day five and day 12 after the onset of COVID-19 symptoms.79 Ding et al. reported that the incidence of pleural effusions identified on chest imaging increased from 9.2% (5–9 days from onset of COVID-19 symptoms) to 27.9% (15–21 days from onset of COVID-19 symptoms) in their observational study.31 A single-center, retrospective study by Han et al. reported that the incidence of pleural effusions increases from 11.8% on week 2 to 23.5% on week 3 (p = 0.0071) of hospital admission.24 Lastly, a single-center, retrospective cohort study comparing chest CT features in early-phase and advanced-phase (eight days and more from symptoms onset) of COVID-19 pneumonia revealed that although there was no difference in the incidences of pleural thickening (42.5% versus 59.1%) and retraction (57.5% versus 54.5%), pleural effusions (2.5% versus 22.7%; p = 0.01) were predominantly seen in the advanced-phase.46 The delay in recognizing pleural effusions could be from the limited use of chest imaging (chest radiograph and CT) and pleural ultrasonography during the ongoing pandemic.81 The initial identification of pleural effusions on chest imaging is noted to be similar during the latter stages of SARS (14 days and more from the time of symptoms onset) and MERS (nine days and more from the time of symptoms onset) [Table 2]. In avian influenza A H5N1 patients, pleural effusions are frequently observed around a median of seven days after the onset of symptoms.41
Pleural fluid characteristics of COVID-19-related pleural effusion
Pleural effusions in many COVID-19 patients are under characterized due to concerns that pleural drainage is a potential aerosolized generating procedure leading to further transmission of the disease, although the evidence behind it remains poor.82 As many COVID-19 patients (Table 3) who received pleural drainage had positive RT-PCR for SARS-CoV-2, concerns exist for the risk of transmission during handling of the pleural fluid. To date, there has been no description of the pleural fluid characteristics of SARS-CoV-1 or MERS-CoV-associated pleural effusions, despite the variable incidence of pleural effusions reported in many observational studies (Table 2). As no pleural drainage was performed in the 47 observational studies included in our review, the pleural fluid characteristics in COVID-19 patients were limited to four case reports/series involving 7 COVID-19 patients (Table 3). In all four case reports/series, COVID-19-related pleural effusions were exudative in nature based on Light's criteria of pleural fluid to serum LDH ratio of 0.7 and more.81 , 83, 84, 85, 86 The white cell count differential in COVID-19-related pleural effusions was either lymphocytic or neutrophilic-predominant (Table 3). Pleural fluid LDH was markedly elevated compared to serum LDH among several COVID-19 patients that received pleural drainage. All COVID-19 patients had concomitant empyema excluded with negative pleural fluid cultures. The majority of COVID-19 patients included [ 5/7 (71.4%)], had positive RT-PCR results for SARS-CoV-2 in their pleural fluid, indicating an active viral infection of the pleural space. A case series by Chong et al. was the first to describe a series of critically ill COVID-19 patients with RT-PCR positive pleural effusions predominantly unilateral that required pleural drainage.81 Although no patients had congestive heart failure identified on echocardiogram, many patients had underlying comorbidities involving chronic kidney renal and lung disease. Pleural effusions were notably hemorrhagic [pleural fluid red blood cells (RBC) > 100,000/mm3] in this case series despite systemic anticoagulation being held during pleural drainage. Pleural fluid chemistry showed an overall marked elevation in LDH level of 1550 IU/L that persisted even after excluding sanguineous appearing pleural fluid.
Lymphocytic or neutrophilic-predominant white cell count differential has been noted in other viral-related pleural effusions such as adenovirus, influenza A H5N1, and human herpesvirus-6.87 A markedly elevated pleural fluid to serum LDH ratio of 1.3 had been suggested to support the diagnosis of COVID-19-related pleural effusions that were observed in many COVID-19 patients who received pleural drainage (Table 3).81 However, multiple confounding variables could explain the floridly elevated LDH level observed in several case reports/series of COVID-19 patients. High levels of LDH could be secondary to various etiologies such as complicated parapneumonic effusions, rheumatoid pleurisy, tuberculosis (TB), and lymphoma/lung malignancy.66 , 67 , 88 A few case reports/series reported of the concomitant findings of active TB and stage IV lung malignancy that were potential etiologies for pleural effusions in COVID-19 patients with pneumonia.66 , 67 A high pleural fluid to serum LDH ratio has also been observed in patients with other viral-related pleural effusions from influenza A H5N1, EBV, and adenovirus.87 , 89 Moreover, hemolysis of pleural fluid red blood cells (RBCs) might also contribute to elevation of pleural fluid LDH levels that were based on the serosanguineous and sanguineous pleural fluid appearance, commonly observed in the case series by Chong et al.81 However, a prospective study showed no increased risk in significant bleeding with drainage of pleural effusion in those receiving therapeutic anticoagulation.90 Therefore, it is possible that hemorrhagic pleural effusions encountered in a case series by Chong et al. were due to endothelial dysfunction-related microthrombi formation leading to infarct and hemorrhage of lung parenchyma extending into the pleura.81 These findings have been supported based on the autopsy results of COVID-19 patients.91 On autopsies performed in SARS patients, serosanguinous and bloody pleural fluid was commonly encountered with histopathological findings of focal hemorrhage in the pleura with adhesions regardless of the presence of vascular thrombosis.92 , 93 Nevertheless, due to the scarcity of pleural drainage being performed in COVID-19 patients, the typical characteristics findings in COVID-19-related pleural effusions remain elusive, despite the increasing incidence of pleural effusions reported in the literature.
Limitations
Our review had several limitations. First, the available data on pleural effusions in COVID-19 pneumonia was often limited due to the highly variable incidence reported across many observational studies compared to other common CT chest findings (Table 1). Furthermore, it remained challenging to assess if COVID-19-related pleural effusions were solely caused by SARS-CoV-2 infections because many COVID-19 patient's comorbidities were incompletely described, and iatrogenic etiologies were not adequately excluded. Second, the diagnosis of COVID-19 pneumonia was made by positive RT-PCR from the respiratory tract with the assistance of chest imaging in all of the studies included in our review. However, co-infection in COVID-19 patients by common non-coronavirus microorganisms such as adenovirus, influenza virus, Epstein-Barr virus (EBV), cytomegalovirus (CMV), and human metapneumovirus that can present as exudative pleural effusions with markedly elevated LDH were not entirely excluded in many observational studies included in our review.38 , 39 , 87 , 94 As all patients included in this review were hospitalized COVID-19 patients, secondary bacterial infection/pneumonia had not been adequately ruled out that might present with similar radiological features indistinguishable from COVID-19 pneumonia, considering pleural effusions occurred 5–7 days and more from time of admission.95, 96, 97, 98, 99 Third, 25 studies that we reviewed and excluded were published in non-English language (Fig. 1) that would have affected the generalizability of our study, in view of the ongoing worldwide pandemic since we (the authors) were not well versed in other languages. Fourth, in all observational studies, we observed pleural effusions to be a bystander (minor secondary outcome) result or identified incidentally during subgroup analysis while assessing the many characteristics, risk factors, and outcomes of hospitalized COVID-19 patients.7 , 14 , 20 , 75 , 77 Fifth, most diagnoses of COVID-19-related pleural effusions were made with the utilization of CT chest in observational studies included in our review, rendering the possible underdiagnosis of pleural effusions during this current pandemic. The use of routine chest imaging, including chest radiograph and CT, is understandably limited to reduce provider and radiology technician exposure to COVID-19 patients, unless a high clinical suspicion exists resulting from routine chest imaging may alter the overall decision-making and prognosis. Sixth, the high mortality rate associated with COVID-19 pneumonia may be an independent competing risk factor for the development of delayed pleural effusions (observed in many observational studies), leading to an unintended underestimation of the actual risk in non-deceased COVID-19 patients.16 , 24 , 31 , 46 , 79 Lastly, despite our exhaustive review of multiple studies available in the current medical literature (Table 4 ), there remains a lack of quality evidence to determine the association between COVID-19-related pleural effusions and: a) the length of hospitalization/ICU admission; b) duration of invasive mechanical ventilation requirement and the effects of pleural effusions on lung compliance; c) clinical and radiological characteristics predisposing to pleural effusions; d) management of acute respiratory failure in COVID-19 patients with pleural effusions; and e) significance and short/long term prognosis of COVID-19 patients with pleural effusions.
Table 4.
Summary of COVID-19-related pleural effusions.
| COVID-19-Related Pleural Effusions Summary Table: |
|---|
| What We know: |
| 1) Incidence: Low at 7.3%. Varies Across Numerous Observational Studies. |
| 2) Higher Incidence in Critically Ill And MIS COVID-19 Patients. |
| 3) Comorbidities: - Age = Likely Common in Elderly, - Pregnancy = Unclear Association. |
| 4) Similar Incidence With SARS-Related Pleural Effusions. |
| 5) Pleural Effusions : Distinguishing Radiological Features That Favor Non-COVID-19 And Non-SARS Viral Pneumonia. |
| 6) Time to Diagnosis: - 11 Days From Symptoms Onset And 5–7 Days From Admission, - Frequency Increases During The Course of COVID-19 Pneumonia (Advanced Pneumonia). |
| 7) Pleural Fluid Characteristics: - Exudative, Lymphocytic or Neutrophilic-Predominant, - Markedly Elevated LDH And Pleural Fluid To Serum (PF:S) LDH Ratio, - PF:S LDH Ratio of 1.3 < suggestive of COVID-19-Related Pleural Effusions. |
| Objectives For Future Studies: |
| 1) The Length of Hospitalization/ICU Admission. |
| 2) Duration of Invasive Mechanical Ventilation And Effects on Lung Compliance. |
| 3) Clinical Risk Factors And Radiological Prognosticators For Pleural Effusions. |
| 4) Management of Acute Respiratory Failure in The Setting of Pleural Effusions. |
| 5) Short And Long term Outcomes of Pleural Effusions. |
Conclusion
The reported incidence of pleural effusions in COVID-19 pneumonia is low at 7.3% among 47 observational studies included in our review. This finding is similar to the incidence of pleural effusions encountered in SARS patients (7.5%). The majority of COVID-19 related pleural effusions are unilateral 67.2%. It remains unclear if COVID-19-related pleural effusions are secondary to comorbid conditions rather than being directly related to SARS-CoV-2 infection. COVID-19 patients suffering from pneumonia who are critically ill or have MIS, shown in our review to have a greater tendency for developing pleural effusions. It is also uncertain if age is a possible risk factor for pleural effusions development in COVID-19 patients. COVID-19-related pleural effusions are commonly identified on 5–7 days after hospital admission and 11 days after symptoms onset. The frequency of pleural effusions identified on chest imaging may increase till day 21 from symptoms onset and resemble advanced stage of COVID-19 pneumonia. Pleural fluid characteristics in COVID-19 patients receiving pleural drainage will show an exudative, lymphocytic or neutrophilic-predominant cell differential with markedly elevated LDH and pleural fluid to serum LDH ratio. However, the evidence available in the current literature remained limited due to the variability in many observational studies reviewed on the actual incidence of pleural effusions and lack of pleural drainage. As our knowledge of COVID-19 disease continues to evolve during this current pandemic, we hope that more well-designed studies will be performed to describe the incidence, risk factors, outcomes, onset, and pleural fluid characteristics in COVID-19 patients with pleural effusions.
Declaration of Competing Interest
None
Acknowledgments
Funding
None
Author Contribution
All authors had access to the data and were involved in writing the manuscript.
References
- 1.Hui D.S., I Azhar E., Madani T.A., et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health — The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi H., Han X., Jiang N., et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung M., Bernheim A., Mei X., et al. CT Imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295(1):202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han R., Huang L., Jiang H., Dong J., Peng H., Zhang D. Early clinical and CT manifestations of coronavirus disease 2019 (COVID-19) Pneumonia. Published online 2020:6. [DOI] [PubMed]
- 6.Malik J., Javed N., Naeem H., Sattar R.A., Ikram U. COVID-19 associated pneumonia and pleural effusion masquerading as heart failure in rheumatic heart disease. Eur J Case Rep Intern Med. 2020;7(8) doi: 10.12890/2020_001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin C., Ding Y., Xie B., et al. Asymptomatic novel coronavirus pneumonia patient outside Wuhan: the value of CT images in the course of the disease. Clin Imaging. 2020;63:7–9. doi: 10.1016/j.clinimag.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albarello F., Pianura E., Di Stefano F., et al. 2019-novel Coronavirus severe adult respiratory distress syndrome in two cases in Italy: an uncommon radiological presentation. Int J Infect Dis. 2020;93:192–197. doi: 10.1016/j.ijid.2020.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong X., Song L., Li H., et al. CT characteristics and diagnostic value of COVID-19 in pregnancy. PLoS One. 2020;15(7) doi: 10.1371/journal.pone.0235134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi F., Yu Q., Huang W., Tan C. 2019 novel coronavirus (COVID-19) pneumonia with hemoptysis as the initial symptom: CT and clinical features. Korean J Radiol. 2020;21(5):537. doi: 10.3348/kjr.2020.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun D., Li H., Lu X.-.X., et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr. 2020;16(3):251–259. doi: 10.1007/s12519-020-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernheim A., Mei X., Huang M., et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. February 20, 2020 doi: 10.1148/radiol.2020200463. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Z., Tang N., Chen Y., et al. CT features of COVID-19 patients with two consecutive negative RT-PCR tests after treatment. Sci Rep. 2020;10(1):11548. doi: 10.1038/s41598-020-68509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song F., Shi N., Shan F., et al. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295(1):210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vancheri S.G., Savietto G., Ballati F., et al. Radiographic findings in 240 patients with COVID-19 pneumonia: time-dependence after the onset of symptoms. Eur Radiol. 2020;30(11):6161–6169. doi: 10.1007/s00330-020-06967-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wen Z., Chi Y., Zhang L., et al. Coronavirus disease 2019: initial detection on chest CT in a retrospective multicenter study of 103 chinese patients. Radiol: Cardiothorac Imaging. 2020;2(2) doi: 10.1148/ryct.2020200092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang K., Kang S., Tian R., Zhang X., Zhang X., Wang Y. Imaging manifestations and diagnostic value of chest CT of coronavirus disease 2019 (COVID-19) in the Xiaogan area. Clin Radiol. 2020;75(5):341–347. doi: 10.1016/j.crad.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li K., Fang Y., Li W., et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur Radiol. 2020;30(8):4407–4416. doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng Y., Ling Y., Bai T., et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201(11):1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majidi H., Bani-Mostafavi E.-.S., Mardanshahi Z., et al. High-resolution computed tomography finding in 552 patients with symptomatic COVID-19: first report from north of Iran. Emerg Radiol. 2020;27(6):633–639. doi: 10.1007/s10140-020-01819-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arentz M., Yim E., Klaff L., et al. Characteristics and outcomes of 21 critically Ill patients with COVID-19 in Washington state. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blumfield E., Levin T.L., Kurian J., Lee E.Y., Liszewski M.C. Imaging findings in multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease (COVID-19) Am J Roentgenol. 2021;216(2):507–517. doi: 10.2214/AJR.20.24032. [DOI] [PubMed] [Google Scholar]
- 24.Han X., Cao Y., Jiang N., et al. Novel coronavirus disease 2019 (COVID-19) pneumonia progression course in 17 discharged patients: comparison of clinical and thin-section computed tomography features during recovery. Clin Infect Dis. 2020;71(15):723–731. doi: 10.1093/cid/ciaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pakdemirli E., Mandalia U., Monib S. Characteristics of chest CT images in patients with COVID-19 pneumonia in London, UK. Cureus. September 7, 2020 doi: 10.7759/cureus.10289. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toubiana J., Poirault C., Corsia A., et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. June 3, 2020:m2094. doi: 10.1136/bmj.m2094. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin Z., Kang Z., Yang D., Ding S., Luo H., Xiao E. A comparison of clinical and chest CT findings in patients with Influenza A (H1N1) virus infection and coronavirus disease (COVID-19) Am J Roentgenol. 2020;215(5):1065–1071. doi: 10.2214/AJR.20.23214. [DOI] [PubMed] [Google Scholar]
- 28.Liu H., Liu F., Li J., Zhang T., Wang D., Lan W. Clinical and CT imaging features of the COVID-19 pneumonia: focus on pregnant women and children. J Infect. 2020;80(5):e7–e13. doi: 10.1016/j.jinf.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabatabaei S.M.H., Talari H., Moghaddas F., Rajebi H. CT features and short-term prognosis of COVID-19 pneumonia: a single-center study from Kashan, Iran. Radiol: Cardiothorac Imaging. 2020;2(2) doi: 10.1148/ryct.2020200130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martino A, Fiore E, Mazza EM, Minichiello S, Brogna B, Petronilla S, Megliola A,Musto L. CT features of coronavirus disease 2019 (COVID-19) pneumonia: experience of a single center in Southern Italy. Infez Med. 2020;28(suppl 1):104–110. [PubMed]
- 31.Ding X., Xu J., Zhou J., Long Q. Chest CT findings of COVID-19 pneumonia by duration of symptoms. Eur J Radiol. 2020;127 doi: 10.1016/j.ejrad.2020.109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao W., Zhong Z., Xie X., Yu Q., Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. Am J Roentgenol. 2020;214(5):1072–1077. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 33.Colombi D., Bodini F.C., Petrini M., et al. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology. 2020;296(2):E86–E96. doi: 10.1148/radiol.2020201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valverde I., Singh Y., Sanchez-de-Toledo J., et al. Acute cardiovascular manifestations in 286 children with multisystem inflammatory syndrome associated with COVID-19 infection in Europe. Circulation. 2021;143(1):21–32. doi: 10.1161/CIRCULATIONAHA.120.050065. [DOI] [PubMed] [Google Scholar]
- 35.Ajlan A.M., Ahyad R.A., Jamjoom L.G., Alharthy A., Madani T.A. Middle east respiratory syndrome coronavirus (MERS-CoV) infection: chest CT findings. Am J Roentgenol. 2014;203(4):782–787. doi: 10.2214/AJR.14.13021. [DOI] [PubMed] [Google Scholar]
- 36.Das K.M., Lee E.Y., Jawder S.E.A., et al. Acute middle east respiratory syndrome coronavirus: temporal lung changes observed on the chest radiographs of 55 patients. Am J Roentgenol. 2015;205(3):W267–S274. doi: 10.2214/AJR.15.14445. [DOI] [PubMed] [Google Scholar]
- 37.Das K.M., Lee E.Y., Enani M.A., et al. CT correlation with outcomes in 15 patients with acute middle east respiratory syndrome coronavirus. Am J Roentgenol. 2015;204(4):736–742. doi: 10.2214/AJR.14.13671. [DOI] [PubMed] [Google Scholar]
- 38.Kim E.A., Lee K.S., Primack S.L., et al. Viral pneumonias in adults: radiologic and pathologic findings. RadioGraphics. 2002;22(suppl_1):S137–S149. doi: 10.1148/radiographics.22.suppl_1.g02oc15s137. [DOI] [PubMed] [Google Scholar]
- 39.Koo H.J., Lim S., Choe J., Choi S.-.H., Sung H., Do K.-.H. Radiographic and CT features of viral pneumonia. RadioGraphics. 2018;38(3):719–739. doi: 10.1148/rg.2018170048. [DOI] [PubMed] [Google Scholar]
- 40.Bai H.X., Hsieh B., Xiong Z., et al. Performance of radiologists in differentiating COVID-19 from non-COVID-19 viral pneumonia at chest CT. Radiology. 2020;296(2):E46–E54. doi: 10.1148/radiol.2020200823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qureshi N.R., Hien T.T., Farrar J., Gleeson F.V. The radiologic manifestations of H5N1 avian influenza. J Thorac Imaging. 2006;21(4):259–264. doi: 10.1097/01.rti.0000213573.94032.53. [DOI] [PubMed] [Google Scholar]
- 42.Agarwal P.P., Cinti S., Kazerooni E.A. Chest radiographic and CT findings in novel swine-origin influenza A (H1N1) virus (S-OIV) infection. Published online 2009:6. [DOI] [PubMed]
- 43.Li H., Weng H., Lan C., et al. Comparison of patients with avian influenza A (H7N9) and influenza A (H1N1) complicated by acute respiratory distress syndrome. Medicine (Baltimore) 2018;97(12):e0194. doi: 10.1097/MD.0000000000010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoen K., Horvat N., Guerreiro N.F.C., de Castro I., de Giassi K.S. Spectrum of clinical and radiographic findings in patients with diagnosis of H1N1 and correlation with clinical severity. BMC Infect Dis. 2019;19(1):964. doi: 10.1186/s12879-019-4592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cobb N.L., Sathe N.A., Duan K.I., et al. Comparison of clinical features and outcomes in critically Ill patients hospitalized with COVID-19 versus influenza. Ann ATS. November 13, 2020 doi: 10.1513/AnnalsATS.202007-805OC. Published onlineAnnalsATS.202007-805OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou S., Wang Y., Zhu T., Xia L. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. Am J Roentgenol. March 5, 2020:1–8. doi: 10.2214/AJR.20.22975. Published online. [DOI] [PubMed] [Google Scholar]
- 47.Zhu T., Wang Y., Zhou S., Zhang N., Xia L. A comparative study of chest computed tomography features in young and older adults with corona virus disease (COVID-19) J Thorac Imaging. 2020;35(4):W97–W101. doi: 10.1097/RTI.0000000000000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joynt G.M., Antonio G.E., Lam P., et al. Late-stage adult respiratory distress syndrome caused by severe acute respiratory syndrome: abnormal findings at thin-section CT. Radiology. 2004;230(2):339–346. doi: 10.1148/radiol.2303030894. [DOI] [PubMed] [Google Scholar]
- 49.Liu X., Zhang H., Li Y., Liu J. Coronavirus disease 2019 (COVID-19) CT findings: a systematic review and meta-analysis. J Am Coll Radiol. 2020;17(6):9. doi: 10.1016/j.jacr.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen A., Huang J., Liao Y., et al. Differences in clinical and imaging presentation of pediatric patients with COVID-19 in comparison with adults. Radiol: Cardiothorac Imaging. 2020;2(2) doi: 10.1148/ryct.2020200117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaigany S., Gnirke M., Guttmann A., et al. An adult with Kawasaki-like multisystem inflammatory syndrome associated with COVID-19. Lancet. 2020;396(10246):e8–e10. doi: 10.1016/S0140-6736(20)31526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winant A.J., Blumfield E., Liszewski M.C., Kurian J., Foust A., Lee E.Y. Thoracic imaging findings of multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19: what radiologists need to know now. Radiol: Cardiothorac Imaging. 2020;2(4) doi: 10.1148/ryct.2020200346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morris S.B., Schwartz N.G., Patel P., et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection — United Kingdom and United States, March–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(40):1450–1456. doi: 10.15585/mmwr.mm6940e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sokolovsky S., Soni P., Hoffman T., Kahn P., Scheers-Masters J. COVID-19 associated Kawasaki-like multisystem inflammatory disease in an adult. Am J Emerg Med. 2021;39:253.e1–253.e2. doi: 10.1016/j.ajem.2020.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hameed S., Elbaaly H., Reid C.E.L., et al. Spectrum of imaging findings at chest radiography, US, CT, and MRI in multisystem inflammatory syndrome in children associated with COVID-19. Radiology. 2021;298(1):E1–E10. doi: 10.1148/radiol.2020202543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rostad B.S., Shah J.H., Rostad C.A., et al. Chest radiograph features of multisystem inflammatory syndrome in children (MIS-C) compared to pediatric COVID-19. Pediatr Radiol. 2021;51(2):231–238. doi: 10.1007/s00247-020-04921-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang L., Dong L., Ming L., et al. Severe acute respiratory syndrome coronavirus 2(SARS-CoV-2) infection during late pregnancy: a report of 18 patients from Wuhan, China. BMC Pregnancy Childbirth. 2020;20(1):394. doi: 10.1186/s12884-020-03026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gourgoulianis K.I., Karantanas A.H., Diminikou G., Molyvdas P.A. Benign postpartum pleural effusion. Eur Respir J. 1995;8(10):1748–1750. doi: 10.1183/09031936.95.08101748. [DOI] [PubMed] [Google Scholar]
- 60.Gervaise A., Bouzad C., Peroux E., Helissey C. Acute pulmonary embolism in non-hospitalized COVID-19 patients referred to CTPA by emergency department. Eur Radiol. 2020;30(11):6170–6177. doi: 10.1007/s00330-020-06977-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lal A., Mishra A.K., Sahu K.K. CT chest findings in coronavirus disease-19 (COVID-19) J Formos Med Assoc. 2020;119(5):1000–1001. doi: 10.1016/j.jfma.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chong W.H., Saha B. Relationship between severe acute respiratory syndrome coronavirus 2 and the etiology of acute kidney injury. Am J Med Sci. October 2020 doi: 10.1016/j.amjms.2020.10.025. Published onlineS000296292030478X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong K.T., Antonio G.E., Hui D.S.C., et al. Severe acute respiratory syndrome: radiographic appearances and pattern of progression in 138 patients. Radiology. 2003;228(2):401–406. doi: 10.1148/radiol.2282030593. [DOI] [PubMed] [Google Scholar]
- 65.Schaller T., Hirschbühl K., Burkhardt K., et al. Postmortem examination of patients with COVID-19. JAMA. 2020;323(24):2518–2520. doi: 10.1001/jama.2020.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fahad A.M., Al-Khalidi H.A., Abdulhameed Alhaideri Y.A., Majeed Altimimi Y.Q., Alshewered A.S. Pleural effusion in a patient with COVID-19 pneumonia and lung cancer: a case report. Respir Med Case Rep. 2020;31 doi: 10.1016/j.rmcr.2020.101302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tham S.M., Lim W.Y., Lee C.K., et al. Four patients with COVID-19 and tuberculosis, Singapore, April–May 2020. Emerg Infect Dis. 2020;26(11):2763–2765. doi: 10.3201/eid2611.202752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuiken T., Fouchier R.A., Schutten M., et al. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362(9380):263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guan C.S., Lv Z.B., Yan S., et al. Imaging features of coronavirus disease 2019 (COVID-19): evaluation on thin-section CT. Acad Radiol. 2020;27(5):609–613. doi: 10.1016/j.acra.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Groot R.J., Baker S.C., Baric R.S., et al. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the coronavirus study group. J Virol. 2013;87(14):7790–7792. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 72.Wong K., Antonio G.E., Hui D.S.C., et al. Severe acute respiratory syndrome. J Comput Assist Tomogr. 2004;28(6):6. doi: 10.1097/00004728-200411000-00010. [DOI] [PubMed] [Google Scholar]
- 73.Wong K.T., Antonio G.E., Hui D.S.C., et al. Thin-section CT of severe acute respiratory syndrome: evaluation of 73 patients exposed to or with the disease. Radiology. 2003;228(2):395–400. doi: 10.1148/radiol.2283030541. [DOI] [PubMed] [Google Scholar]
- 74.Lan C.-.C., Hsu H.-.H., Wu C.-.P., Lee S.-.C., Peng C.-.K., Chang H. Influences of pleural effusion on respiratory mechanics, gas exchange, hemodynamics, and recruitment effects in acute respiratory distress syndrome. J Surg Res. 2014;186(1):346–353. doi: 10.1016/j.jss.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 75.Mo P., Xing Y., Xiao Y., et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. March, 2020;16:ciaa270. doi: 10.1093/cid/ciaa270. Published online. [DOI] [Google Scholar]
- 76.Li K., Wu J., Wu F., et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Investig Radiol. 2020;55(6):327–331. doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu K.-.C., Xu P., Lv W.-.F., et al. CT manifestations of coronavirus disease-2019: a retrospective analysis of 73 cases by disease severity. Eur J Radiol. 2020;126 doi: 10.1016/j.ejrad.2020.108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Y-H, Marrie J. Do pulmonary radiographic findings at presentation predict mortality in patients with community-acquired pneumonia?:7. [PubMed]
- 79.Xiong Y., Sun D., Liu Y., et al. Clinical and high-resolution CT features of the COVID-19 infection: comparison of the initial and follow-up changes. Investig Radiol. March 2020:1. doi: 10.1097/RLI.0000000000000674. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y., Dong C., Hu Y., et al. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 2020;296(2):E55–E64. doi: 10.1148/radiol.2020200843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chong W.H., Huggins J.T., Chopra A. Characteristics of pleural effusion in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia. Am J Med Sci. September 2020 doi: 10.1016/j.amjms.2020.09.008. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aujayeb A. Clarification on pleural effusions in COVID-19. Radiol: Cardiothorac Imaging. 2020;2(3) doi: 10.1148/ryct.2020200330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen H.-.R., Zou H., Xue M., Chen Z.-.B., Chen W.-.X. A case of childhood COVID-19 infection with pleural effusion complicated by possible secondary mycoplasma pneumoniae infection. Pediatr Infect Dis J. 2020;39(7):e135–e137. doi: 10.1097/INF.0000000000002721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mei F. First detection of SARS-CoV-2 by real-time reverse transcriptase-polymerase chain reaction assay in pleural fluid. :4. [DOI] [PMC free article] [PubMed]
- 85.Hussein M., Haq I.U., Hameed M., et al. Pleural effusion as an isolated finding in COVID-19 infection. Respir Med Case Rep. 2020;31 doi: 10.1016/j.rmcr.2020.101269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Light R.W., Macgregor M.I., Luchsinger P.C., Ball W.C. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77(4):507–513. doi: 10.7326/0003-4819-77-4-507. [DOI] [PubMed] [Google Scholar]
- 87.Nestor J., Huggins T., Kummerfeldt C., DiVietro M., Walters K., Sahn S. Viral diseases affecting the pleura. J Clin Virol. 2013;58(2):367–373. doi: 10.1016/j.jcv.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 88.Sahn S.A. Getting the most from pleural fluid analysis: pleural fluid analysis. Respirology. 2012;17(2):270–277. doi: 10.1111/j.1440-1843.2011.02100.x. [DOI] [PubMed] [Google Scholar]
- 89.Soepandi P.Z., Burhan E., Mangunnegoro H., et al. Clinical course of avian influenza A(H5N1) in patients at the Persahabatan hospital, Jakarta, Indonesia, 2005–2008. Chest. 2010;138(3):665–673. doi: 10.1378/chest.09-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Puchalski J.T., Argento A.C., Murphy T.E., Araujo K.L.B., Pisani M.A. The safety of thoracentesis in patients with uncorrected bleeding risk. Ann ATS. 2013;10(4):336–341. doi: 10.1513/AnnalsATS.201210-088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8(7):681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ding Y., Wang H., Shen H., et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200(3):282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tse G.M.-.K. Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS) J Clin Pathol. 2004;57(3):260–265. doi: 10.1136/jcp.2003.013276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lansbury L., Lim B., Baskaran V., Lim W.S. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. May 27, 2020 doi: 10.1016/j.jinf.2020.05.046. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kanne J.P., Little B.P., Chung J.H., Elicker B.M., Ketai L.H. Essentials for radiologists on COVID-19: an update-radiology scientific expert panel. Radiology. 2020;296(2):E113–E114. doi: 10.1148/radiol.2020200527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Salehi S., Abedi A., Balakrishnan S., Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. Am J Roentgenol. 2020;14:1–7. doi: 10.2214/AJR.20.23034. Published online March. [DOI] [PubMed] [Google Scholar]
- 97.Zuo H. Contribution of CT features in the diagnosis of COVID-19. Vassilakopoulos TI, ed. Can Respir J. 2020;2020:1–16. doi: 10.1155/2020/1237418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ye Z., Zhang Y., Wang Y., Huang Z., Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. March 19, 2020 doi: 10.1007/s00330-020-06801-0. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reittner P., Ward S., Heyneman L., Johkoh T., Müller N.L. Pneumonia: high-resolution CT findings in 114 patients. Eur Radiol. 2003;13(3):515–521. doi: 10.1007/s00330-002-1490-3. [DOI] [PubMed] [Google Scholar]