Abstract
Introduction
As the primary immune cells, macrophages play a key role in atherosclerotic progression. M2 macrophage polarization has been reported to promote tissue repair and attenuate plaque formation upon the expression of anti-inflammatory factors. Convallatoxin (CNT) is a natural cardiac glycoside with anti-inflammatory pharmacological properties. However, whether CNT protects against atherosclerosis (AS) and underlying mechanisms is unknown. This work was designed to explore the potential effects of CNT on atherosclerosis.
Methods
In this study, Apolipoprotein E deficiency (ApoE−/-) mice fed with high-fat diet were established, and CNT (50 or 100 μg/kg) were intragastrically administrated for 12 weeks every day. In vitro, RAW264.7 macrophages stimulated with ox-LDL were treated with CNT (50 or 100 nM) for 24 h. The specific PPARγ antagonist, GW9662, was used to block the PPARγ signaling pathway in vitro. Then, the atherosclerotic lesions, macrophage polarization markers, inflammatory cytokines and PPARγ signaling pathway were examined in further examinations.
Results
Our results showed that the atherosclerotic lesions were reduced by CNT, as demonstrated by the downregulation of serum lipid level and aortic plaque area in AS mice. Furthermore, we found that CNT treatment promoted the expression of M2 macrophage markers (Arg1, Mrc1, Retnla and Chi3l3), and decreased the levels of pro-inflammatory cytokines (IL-6 and TNF-α), accompanied by the increase of anti-inflammatory factor (IL-10) in aortic vessels of AS mice. In ox-LDL-induced RAW264.7 cells, CNT administration also facilitated macrophages polarizing towards M2 subtype and inhibited inflammatory responses. Furthermore, both the in vivo and in vitro experiments showed CNT could increase the expression of PPARγ, Integrin αv and Integrin β5, and GW9662 could block CNT-induced M2 macrophage polarization.
Conclusion
Taken together, these data suggest that CNT may promote M2 macrophage polarization to exert an anti-atherosclerotic effect, partially through activating PPARγ-Integrin αvβ5 signaling pathway.
Keywords: atherosclerosis, convallatoxin, macrophage polarization, ox-LDL, PPARγ-Integrin αvβ5 signaling pathway
Introduction
Atherosclerosis, the major type of cardiovascular diseases, is a chronic inflammatory disorder with significant incidence and mortality.1,2 It has been revealed to be characterized by the intimal lipid accumulation, arterial wall hyperplasia and vascular stenosis.3 Increasing evidences have shown that macrophages regulate the inflammatory mediator production, foam cell formation and plaque formation in the presence of various stimuli from diverse microenvironments.4,5 During this process, monocytes from circulatory system are recruited to endothelium, migrated into sub-intimal space and differentiated into M0 macrophages.6 Importantly, M0 macrophages have been demonstrated to extensively polarize towards M1 (a pro-inflammatory phenotype) or M2 (an anti-inflammatory phenotype) macrophages. In detail, M1 macrophages under the classical activation are shown to express significantly broad-spectrum pro-inflammatory cytokines and chemokines, ultimately aggravating the progression of atherosclerotic plaques;7 whereas the alternatively activated M2 macrophages may strengthen tissue repair and healing, and lessen plaque formation by inducing the expression of anti-inflammatory mediators.8,9 Thus, the polarized activation of macrophages may present a novel view for the prevention of atherosclerotic progression.
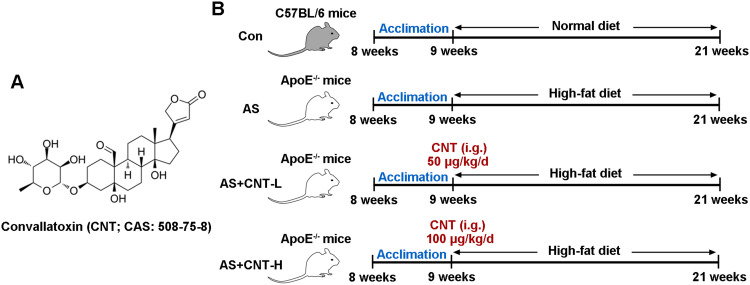
Convallatoxin (CNT) is one of the cardiac glycosides extracted from Adonis amurensis Regel et Radde, and its chemical structure has been shown in Figure 1A. It is generally available as an effective inhibitor of Na+/K+-ATPase to treat heart failure, which exerts a variety of pharmacological properties including anti-angiogenesis, anti-inflammation and anti-tumor.10,11 For example, CNT has been suggested to protect against skin inflammation in psoriatic-like mice.12 In colorectal cancer development, CNT is presented as a potential therapeutic candidate through regulating JAK2/STAT3 and mTOR/STAT3 signaling pathways.13 However, the role of CNT in atherosclerosis still needs further explanations.
Figure 1.
The chemical structure of Convallatoxin and flow diagram for experimental groups in vivo. (A) The chemical structure of Convallatoxin (CNT). (B) The flow diagram of animal experimental treatment.
Abbreviations: Con, control; AS, atherosclerosis; CNT-L, low dose of CNT; CNT-H, high dose of CNT; i.g., intragastrically.
Peroxisome proliferator-activated receptor gamma (PPARγ) is widely studied to play an important role in macrophages of atherosclerosis. It is found that the methylation of PPARγ promoter mediates the function of DNMT1 on increasing pro-inflammatory cytokine secretion and atherosclerotic plaque formation.14 PPARγ is able to promote macrophages polarizing towards M2-like phenotypes and inhibit M1 macrophage polarization.15–17 More importantly, a prior study shows that PPARγ activation is associated with the protective effects of CNT on experimental colitis.18 Therefore, the hypothesis of this study was that CNT might control the polarization of macrophage by activating PPARγ signaling pathway to prevent atherosclerosis.
In this work, the Apolipoprotein E deficiency (ApoE−/-) mice fed with high-fat diet and oxidized low-density lipoprotein (ox-LDL)-treated macrophages were prepared to examine the effect of CNT in vivo and in vitro.
Materials and Methods
Animal Studies
Procedures for animal experiments were conducted as the permission by the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University (2018077X), in accord with the Guide for the Care and Use of Laboratory Animals. Both male wild-type C57BL/6 mice and ApoE−/- mice (8-week old) were purchased from Beijing HFK bioscience (China), and acclimated in a 12-hour light/12-hour dark cycle for a week prior to the study.
The experimental groups are presented as Figure 1B. Briefly, all ApoE−/- mice were randomly divided into three groups: AS group; AS+CNT-L group; AS+CNT-H group. In AS group, ApoE−/- mice were fed with high-fat diet (42% kcal from fat and 0.2% cholesterol) for 12 weeks. In AS+CNT-L group, AS mice were injected with 50 μg/kg of CNT (abs47024074, Absin, China) intragastrically once a day from 9:00–10:00 for 12 weeks. In AS+CNT-H group, AS mice were injected with 100 μg/kg of CNT intragastrically every day from 9:00–10:00 for 12 weeks. Moreover, in Control group (Con), the wild-type C57BL/6 mice were fed with normal diet for 12 weeks. At the end of the experiments, mice were sacrificed, and aorta tissues and plasma were collected.
Immunofluorescence
The paraffin-embedded aorta tissue sections were deparaffinized and retrieved for antigen. Then, sections were stained with antibody against CD68 (sc-20060, Santa Cruz, USA) or CD163 (A8383, Abclonal, China) following fluorescence-labeled secondary antibodies. Sections were counterstained with DAPI (D106471, Aladdin, China) to characterize nuclei and visualized using a microscope (BX53, Olympus, Japan).
Cell Treatment
RAW264.7 macrophages (Zhong Qiao Xin Zhou Biotechnology, China) were cultured in DMEM medium (12100–046, Gibco, USA) containing 10% FBS (SH30084.03, Hyclone, USA). The cells were plated, and exposed to ox-LDL (100 μg/mL; UnionBiol, China) together with or without CNT (50 nM or 100 nM) for 24 h. The PPARγ inhibitor, GW9662 (1 μM; HY-16578, MCE, China) was used to treat cells for 2 h prior to ox-LDL treatment.
Flow Cytometry
RAW264.7 macrophages were collected and incubated with anti-CD163 antibody (11–1631-80, Thermo, USA) at 4°C in line with the manufacturer’s instructions in dark. Then, cells were analyzed by a flow cytometry system (NovoCyte, ACEA, USA).
Serum Lipid Measurement
At the end of animal model establishment, mice were sacrificed and blood serum was collected. Serum total cholesterol (TC; A111-1), triglycerides (TG; A110-1) and low-density lipoprotein cholesterol (LDL-C; A113-1) were determined using available commercial kits (Nanjing Jiancheng, China). The experimental protocols were performed in accordance with the description by the manufacturer.
Enzyme-Linked Immunosorbent Assay (ELISA)
Aortic tissues or RAW264.7 cell supernatants were harvested for ELISA. The ELISA kits of IL-6 (SEA079Mu), TNF-α (SEA133Mu), and IL-10 (SEA056Mu) were purchased from Uscn (China). The measurements for the concentrations were conducted following the manufacturer’s protocols.
Oil Red O Staining
The fixed aortic arteries were frozen in OCT compound and cut into 10-μm thickness of sections. Then, the tissue sections or fixed RAW264.7 cells were stained with Oil red O solution (O0625, Sigma, USA) to observe the plaque area in vivo and lipid droplet accumulation in vitro.
Quantitative Real-Time Polymerase Chain Reaction (qPCR)
Total RNAs were isolated from aortic tissues or RAW264.7 cells using a RNAsimple kit (DP419, Tiangen, China) and quantified with a spectrophotometer. The reverse-transcribed complementary DNA (cDNA) was used to gene amplification using a qPCR system (Exicycler 96, Bioneer, Korea) with SYBR Green PCR mix (SY1020, Solarbio, China). All specific primer sequences used in qPCR experiments are shown in Table 1. Expression level of GAPDH mRNA was used as a housekeeping control. The method of 2−ΔΔCT was carried out to analyze the results.
Table 1.
Primer Sequences Used in This Study
| Gene | Primer Sequences (5ʹ-3ʹ) |
|---|---|
| Arg1 | F: GGAAGACAGCAGAGGAGGTG |
| R: TCAGTCCCTGGCTTATGGTT | |
| Mrc1 | F: AGTGATGGTTCTCCCGTTTC |
| R: TGGGCTCAGGTAGTAGTGTTTT | |
| Retnla | F: CGTGGAGAATAAGGTCAAGGA |
| R: ACACCCAGTAGCAGTCATCCC | |
| Chi3l3 | F: ATGGCCTCAACCTGGACTG |
| R: TCCTGCTCCTGTGGAAGTG | |
| CD86 | F: ATGGGCTCGTATGATTGT |
| R: CTTCTTAGGTTTCGGGTG | |
| iNOS | F: CACCACCCTCCTCGTTC |
| R: CAATCCACAACTCGCTCC | |
| MARCO | F: AGGCGAATCTTTCCAACG |
| R: CCCAGAGCCACCTCCATA | |
| GAPDH | F: TGTTCCTACCCCCAATGTGTCCGTC |
| R: CTGGTCCTCAGTGTAGCCCAAGATG |
Abbreviations: F, forward primer; R, reverse primer.
Western Blot Analysis
Proteins from aortic tissues or macrophages were subjected to SDS-PAGE and transferred onto PVDF membrane (IPVH00010, Millipore, USA). Then, membranes were blocked in fresh non-fat milk, and incubated with primary antibodies against PPARγ (A0270, Abclonal), Integrin αv (AF5152, Affinity, China), Integrin β5 (AF0185, Affinity), Histone H3 (GTX122148, GeneTex, China) and GAPDH (60004-1-Ig, Proteintech, China). Afterwards, horseradish peroxidase-conjugated goat anti-rabbit antibody (SE134, Solarbio) or goat anti-mouse antibody (SE131, Solarbio) was prepared for an additional culture. The immunoblots were visualized using ECL reagent (PE0010, Solarbio).
Statistical Analysis
Data were analyzed using GraphPad Prism (GraphPad Software, USA). The Shapiro–Wilk normality test was used for determining data normal distribution. The data normally distributed were assessed using t-test between two groups, and one-way analysis of variance (ANOVA) and Bonferroni’s post hoc t-test among multiple groups. The statistics without normal distribution were analyzed by the non-parametric Kruskal–Wallis test. All experiments in vivo were repeated for six times, and in vitro were repeated for three times. A value of p < 0.05 indicated statistically significant.
Results
CNT Attenuated Atherosclerotic Lesions in vivo
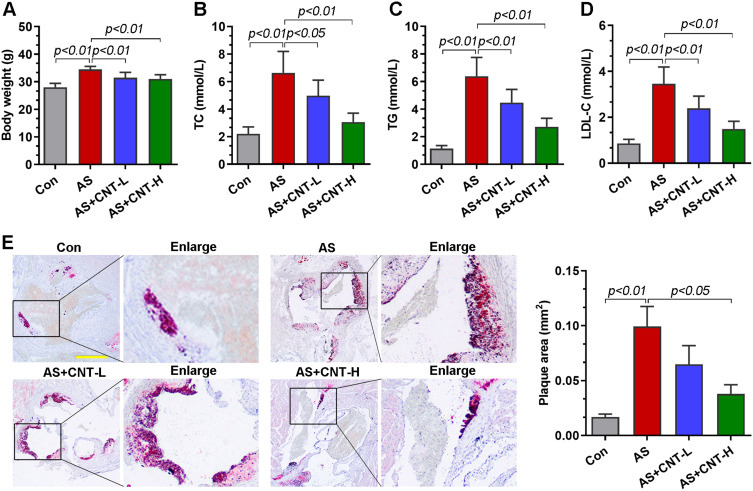
In vivo, the atherosclerotic models were established using the ApoE−/- mice fed with high-fat diet. We first determined the body weight and lipid alterations in mice. As shown in Figure 2A–D, the significantly increased body weight and serum levels of TC, TG and LDL-C in AS group were downregulated by CNT. Compared with that in AS mice, the area of atherosclerotic plaque was reduced in CNT-treated mice by Oil red O staining (Figure 2E). These results demonstrated the protective effects of CNT on atherosclerotic lesions.
Figure 2.
CNT attenuated atherosclerotic lesions in vivo. (A) Body weight. (B–D) Serum levels of TC, TG and LDL-C. (E) Oil red O staining and quantification of plaque areas in the aortic roots. The corresponding right panels showed the partially enlarged plaque areas labeled with black boxes. Bar=500 μm. Data were expressed as mean ± SD.
Abbreviations: Con, control; AS, atherosclerosis; CNT-L, low dose of CNT; CNT-H, high dose of CNT.
CNT Regulated Macrophage Polarization in vivo
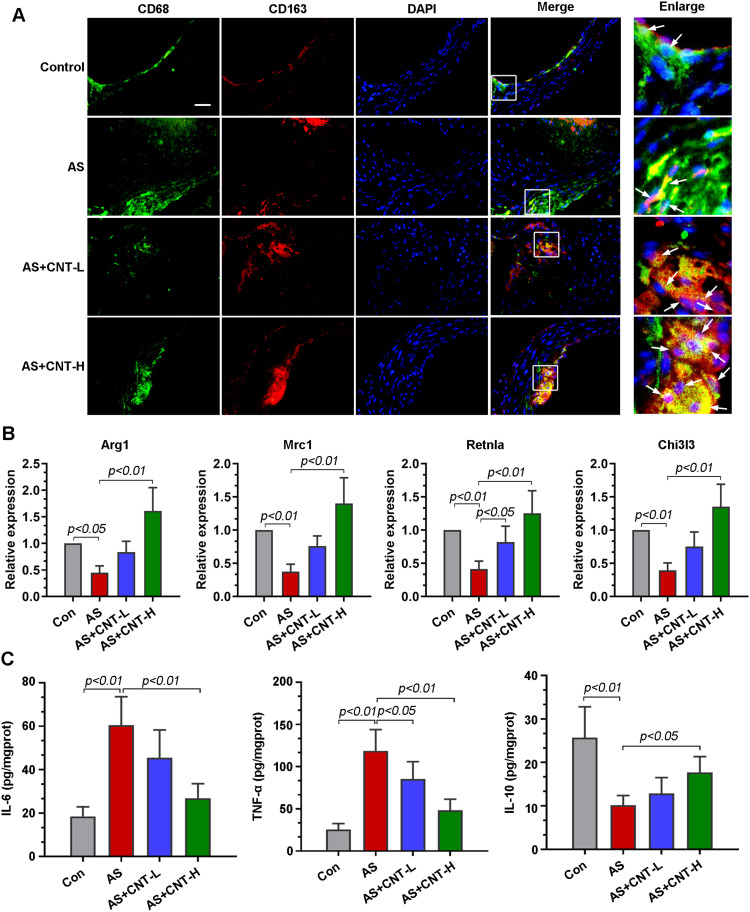
M1 and M2 macrophage polarization had been revealed to be involved in atherosclerotic progression. Double immunofluorescent colocalization results showed that, in comparison with that in AS mice, macrophages expressing CD68 had a high level of CD163 (an M2 marker) in aortic roots of mice treated with CNT (Figure 3A). CNT treatment significantly upregulated the expression of M2-associated genes, including Arg1, Mrc1, Rentla and Chi3l3 (Figure 3B). It also decreased pro-inflammatory cytokines (IL-6 and TNF-α), and increased anti-inflammatory factor (IL-10) in aortic tissues of AS mice (Figure 3C). The results also showed that the upregulated M1-associated genes (CD86, iNOS and MARCO) in aortas of AS mice were decreased by CNT treatment (Supplementary Figure 1). These data indicated that the role of CNT in atherosclerosis might be associated with M1/M2 macrophage polarization.
Figure 3.
CNT promoted M2 macrophage polarization in vivo. (A) Immunofluorescence staining of the co-localization of CD68 (green) and CD163 (red) in the aortic roots. The right panels were the corresponding partially enlarged images in white boxes. Bar=50 μm. (B) qPCR analysis of the relative mRNA levels of M2 markers in the aortic tissues, including Arg1, Mrc1, Retnla, and Chi3l3. (C) Contents of IL-6, TNF-α and IL-10 in the aortic tissues were determined by ELISA. Data were expressed as mean ± SD.
Abbreviations: Con, control; AS, atherosclerosis; CNT-L, low dose of CNT; CNT-H, high dose of CNT.
CNT Reduced ox-LDL-Induced Foam Cell Formation
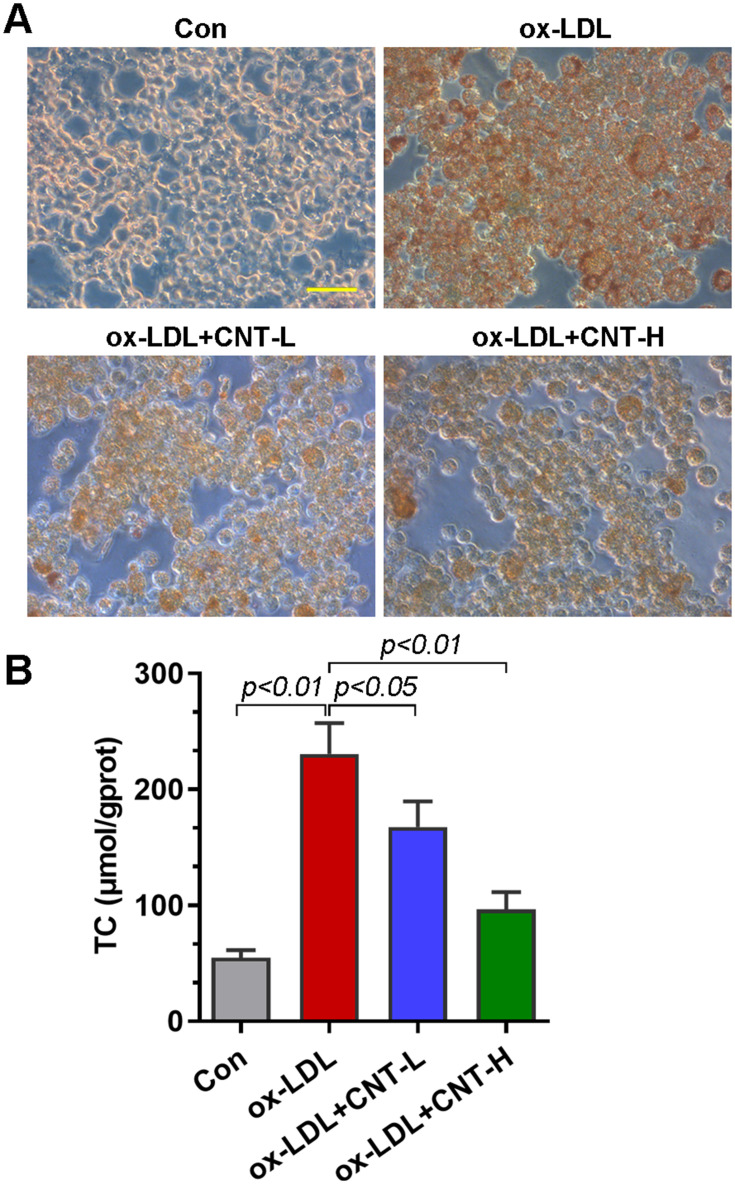
Given that foam cells derived from macrophages had a critical effect on atherosclerotic progression, we thus examined the role of CNT in ox-LDL-induced foam cells in vitro. As reflected by Oil red O staining, CNT reduced the lipid droplet accumulation in RAW264.7 cells induced by ox-LDL (Figure 4A). It also decreased ox-LDL-induced high contents of TC in macrophages (Figure 4B), suggesting the inhibitory role of CNT in foam cell formation.
Figure 4.
CNT reduced ox-LDL-induced foam cell formation. (A) Representative images of Oil red O staining in ox-LDL-treated RAW264.7 cells. Bar=50 μm. (B) TC contents in ox-LDL-treated cells. Data were expressed as mean ± SD.
Abbreviations: Con, control; CNT-L, low dose of CNT; CNT-H, high dose of CNT.
CNT Facilitated Macrophage Polarizing Towards M2 Phenotype in vitro
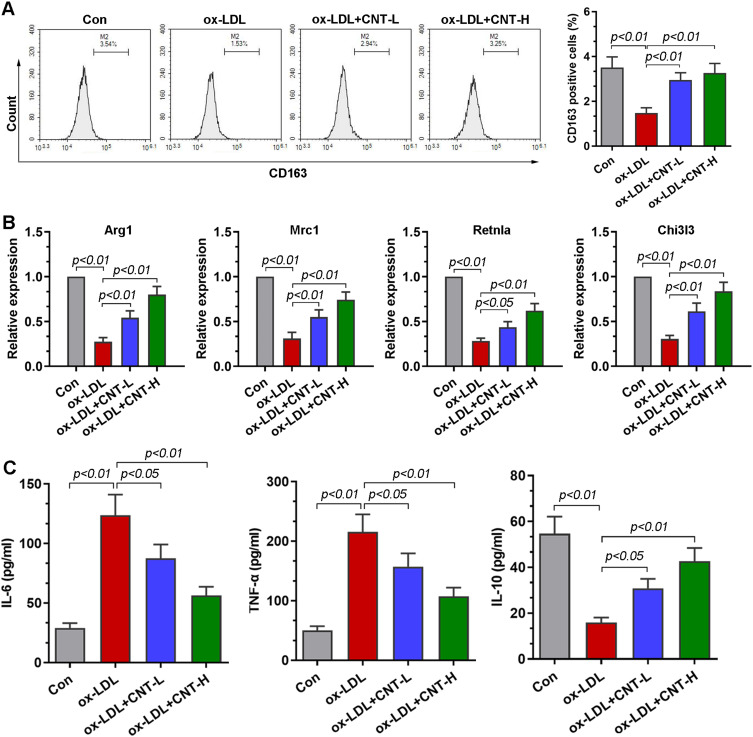
To further investigate the role of CNT in the polarization of macrophage, ox-LDL-induced RAW264.7 cells were used in vitro. Flow cytometry analysis showed that, in the presence of ox-LDL, the percentage of CD163+ cells was upregulated by CNT in RAW264.7 cells (Figure 5A). Similar to the results in vivo, in ox-LDL-treated RAW264.7 cells, the mRNA expression of M2 markers (Arg1, Mrc1, Retnla and Chi3l3) was also restored by CNT (Figure 5B). Furthermore, the decreased levels of IL-6 and TNF-α, accompanied by the increased expression of IL-10 were observed in ox-LDL-induced RAW264.7 cells after CNT treatment (Figure 5C). The in vitro results demonstrated that CNT was able to promote macrophages polarizing towards M2 subtypes.
Figure 5.
CNT facilitated macrophage polarizing towards M2 phenotype in vitro. (A) Flow cytometry analysis and quantification of the percentage of CD163 positive cells. (B) qPCR results of the relative expressions of M2 markers, including Arg1, Mrc1, Retnla, and Chi3l3. (C) ELISA assays of the levels of IL-6, TNF-α and IL-10 in cell supernatants. Data were expressed as mean ± SD.
Abbreviations: Con, control; CNT-L, low dose of CNT; CNT-H, high dose of CNT.
Involvement of PPARγ-Integrin αvβ5 Signaling Pathway in CNT-Enhanced M2 Macrophage Polarization
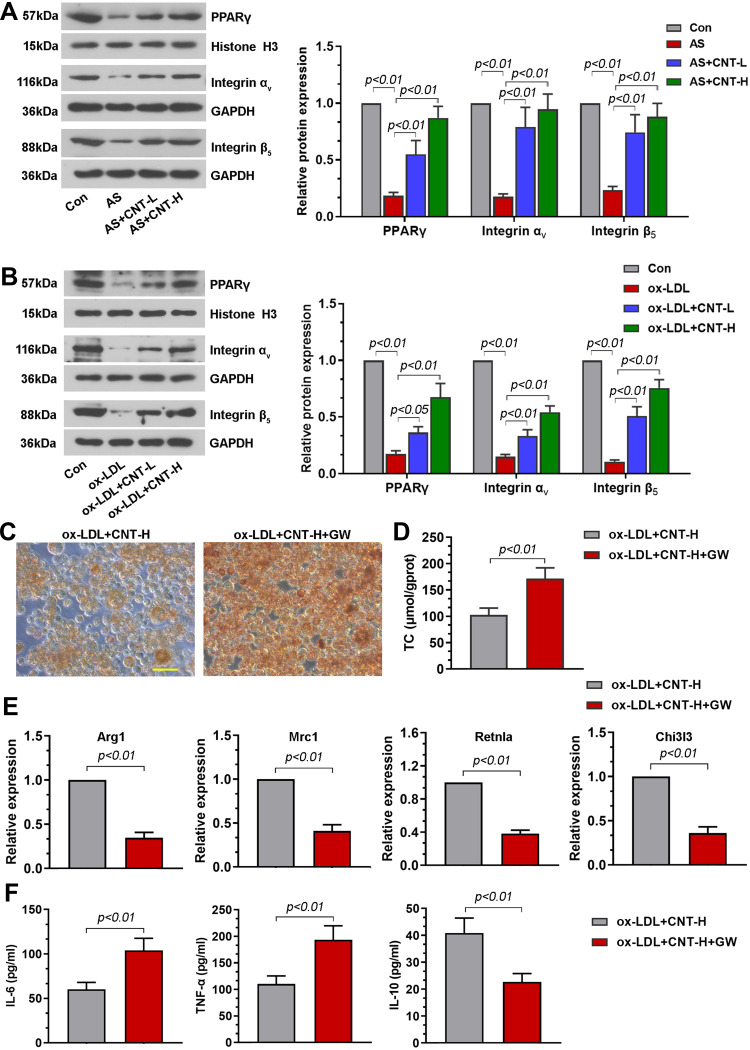
To further uncover the underlying mechanism of CNT on M2 macrophage, we paid attention to PPARγ-Integrin αvβ5 signaling pathway. Results in vivo demonstrated that the decreased PPARγ-related proteins (PPARγ, Integrin αV and Integrin β5) in atherosclerotic mice were dramatically elevated by CNT administration (Figure 6A). The similar tendency of PPARγ-Integrin αvβ5 pathway was also observed in vitro (Figure 6B). Then the specific PPARγ antagonist, GW9662 or T0070907 was employed to inhibit PPARγ expression. As shown in Figure 6C and D, GW9662 aggravated foam cell formation and upregulated TC contents in ox-LDL-treated RAW264.7 cells after CNT treatment. Inhibition of PPARγ using GW9662 also reduced M2-associated gene expressions and enhanced inflammatory responses (Figure 6E and F). Furthermore, the treatment of T0070907 significantly induced similar effects with GW9662 on CNT-induced M2 macrophage polarization, which are shown in Supplementary Figure 2. These results suggested that CNT activated PPARγ-Integrin αvβ5 signaling pathway to promote the polarization of macrophages towards M2-like phenotype.
Figure 6.
PPARγ antagonist, GW9662, blocked CNT-enhanced M2 macrophage polarization. (A and B) Western blot analysis and quantification of PPARγ, Integrin αv and Integrin β5 in the aortic tissues (A) and RAW264.7 cells (B). (C) Representative images of Oil red O staining in macrophages. Bar=50 μm. (D) Levels of TC in cells were measured by available kits. (E) Arg1, Mrc1, Retnla and Chi3l3 mRNA levels were assessed by qPCR. (F) IL-6, TNF-α and IL-10 contents in cell supernatants were tested using ELISA. Data were expressed as mean ± SD.
Abbreviations: Con, control; CNT-L, low dose of CNT; CNT-H, high dose of CNT.
Discussion
Atherosclerosis is an inflammatory cardiovascular disorder with complicated pathogenesis. The aim of this study was to investigate the effect of CNT on atherosclerotic progression. We demonstrated that CNT could reduce lipid accumulation and decrease plaque area in atherosclerotic mice. It also promoted macrophages polarizing towards M2 subtypes and activated PPARγ-Integrin αvβ5 pathway in vivo and in vitro. Inhibition of PPARγ with its antagonist blocked CNT-induced M2 macrophage polarization.
In recent years, growing researches were focused on determining the impacts of natural products on atherosclerosis. For instance, Zhong and the colleagues showed that halofuginone attenuated endothelial dysfunction by inhibiting oxidative stress and inflammation to prevent atherosclerosis.19 A flavonoid compound, corylin, was revealed to attenuate vascular inflammation, monocyte adhesion and vascular smooth muscle cell proliferation in ApoE−/- mice.20 Furthermore, the natural products, cardiac glycosides were found to be beneficial for the general hemodynamic parameters in chronic cerebral circulation insufficiency.21 However, whether CNT, one of cardiac glycosides, had potential functions in atherosclerosis are not well understood. Previous studies demonstrated that, in DSS-induced colitis, CNT ameliorated colitic inflammation through activating PPARγ and inhibiting NF-κB.18 Jiang and co-workers suggested an anti-psoriatic effect of CNT by suppressing inflammatory responses.12 Our results showed that CNT significantly lessened the lipid accumulation and plaque formation in AS mice, suggesting the possible anti-atherosclerotic role of CNT.
As we all know, macrophages had been currently identified as an important player in inflammatory responses during the process of atherosclerosis.22 Apart from lipid accumulation, endothelial dysfunction and inflammation, macrophage polarization towards M1 or M2 states also attracted a great deal of attention from scientists.23 Furthermore, the induction of ox-LDL could promote foam cell formation of macrophages, and modulate macrophage polarization.24–26 In this work, we demonstrated that CNT significantly enhanced macrophages polarizing towards M2 subtypes, inhibited pro-inflammatory cytokine expression and promoted anti-inflammatory mediator expression both in vivo and in vitro. The anti-inflammatory property of CNT in atherosclerosis was consistent with previous studies that reported in the experimental colitic or psoriatic models.12,18 Collectively, these findings indicated that CNT potentially induced macrophages polarizing towards M2 phenotypes to suppress inflammation in the progression of atherosclerosis.
Then, PPARγ-Integrin αvβ5 signaling pathway was focused to investigate the underlying mechanisms. Several studies had revealed the involvement of PPARγ in macrophage polarization. PPARγ was an important regulator to control lipid metabolism and inflammation in atherosclerosis,16,17,27 and its activation affected inflammatory states in macrophages.28 It had been well known that PPARγ played a key role in promoting M2 macrophage polarization and suppressing M1 macrophage polarization.15–17 Yao et al showed that PPARγ directly bound Integrin αvβ5 gene promoter, and it promoted macrophages polarized towards M2 subtypes.29 Our results suggested that CNT effectively activated PPARγ, which was consistent with the previous study.18 Inhibiting PPARγ with its antagonist, GW9662 or T0070907, greatly abrogated CNT-induced M2 macrophage polarization in vitro. Thus, these results prompted that the PPARγ-Integrin αvβ5 pathway participated in the regulation of CNT on M2 macrophage polarization. In the future, we will keep investigating the role of PPARγ using its antagonist in the in vivo experiments, which might be of significant importance. In addition, we demonstrated an inhibitory effect of CNT on M1 macrophage polarization. Consistent with these data, the previous study from DSS-induced colitic mice also demonstrated that the expression of iNOS protein could be inhibited by CNT.18 However, the underlying mechanism of CNT on M1 macrophage polarization remains to need further investigations. Although PPARγ had a function of protecting against atherosclerotic progression,14,30 the pro-atherogenic effect of PPARγ was also revealed to be mediated by NCOR1,31 suggesting the function of PPARγ might be controlled by different programs. The evidences by Yao et al also demonstrated that the inhibition of Integrin αv or/and Integrin β5 could not change M1-associated gene expression.29 Thus, another signaling pathway except PPARγ-Integrin αvβ5 pathway might also be participated in the regulation of CNT on M1 macrophage polarization. It still needs more investigations in our further study.
Conclusion
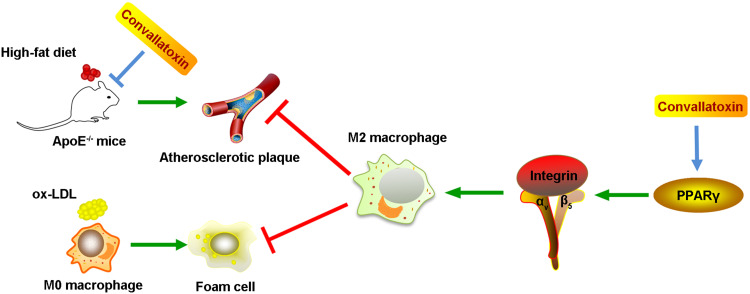
Taken together, our data highlight that CNT promotes the polarization of macrophages towards M2-like phenotypes through activating PPARγ-Integrin αvβ5 signaling pathway, thus reducing atherosclerotic plaque formation. The schematic diagram has been summarized in Figure 7, which presents a novel insight for the prevention of atherosclerosis.
Figure 7.
The schematic diagram of the potential molecular mechanism of the anti-atherosclerotic role of Convallatoxin.
Acknowledgments
This research was supported by grants from National Major Scientific and Technological Special Project for “Significant New Drugs Development” during the Thirteenth Five-year Plan Period (2017ZX09304017) and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding (Grant No. ZYLX201805).
Disclosure
The authors declare no conflicts of interest in this study.
References
- 1.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362(6423):801–809. doi: 10.1038/362801a0 [DOI] [PubMed] [Google Scholar]
- 2.Nigro J, Osman N, Dart AM, Little PJ. Insulin resistance and atherosclerosis. Endocr Rev. 2006;27(3):242–259. doi: 10.1210/er.2005-0007 [DOI] [PubMed] [Google Scholar]
- 3.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6(7):508–519. doi: 10.1038/nri1882 [DOI] [PubMed] [Google Scholar]
- 4.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17(11):1410–1422. doi: 10.1038/nm.2538 [DOI] [PubMed] [Google Scholar]
- 5.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7(2):77–86. doi: 10.1038/nrcardio.2009.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339(6116):161–166. doi: 10.1126/science.1230719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–737. doi: 10.1038/nri3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bi Y, Chen J, Hu F, Liu J, Li M, Zhao ZL. M2 macrophages as a potential target for antiatherosclerosis treatment. Neural Plast. 2019;2019:6724903. doi: 10.1155/2019/6724903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–6440. doi: 10.1002/jcp.26429 [DOI] [PubMed] [Google Scholar]
- 10.Ozaki H, Nagase H, Urakawa N. Involvement of the sugar moiety in the inhibitory action of the cardiac glycosides on the palytoxin-induced responses in vascular smooth muscles. J Pharmacol Exp Ther. 1984;231(1):153–158. [PubMed] [Google Scholar]
- 11.Shang X, Miao X, Yang F, et al. The genus as an important cardiac folk medicine: a review of the ethnobotany, phytochemistry and pharmacology. Front Pharmacol. 2019;10:25. doi: 10.3389/fphar.2019.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang B-W, Zhang W-J, Wang Y, et al. Convallatoxin induces HaCaT cell necroptosis and ameliorates skin lesions in psoriasis-like mouse models. Biomed Pharmacother. 2020;121:109615. doi: 10.1016/j.biopha.2019.109615 [DOI] [PubMed] [Google Scholar]
- 13.Zhang ZH, Li MY, Wang Z, et al. Convallatoxin promotes apoptosis and inhibits proliferation and angiogenesis through crosstalk between JAK2/STAT3 (T705) and mTOR/STAT3 (S727) signaling pathways in colorectal cancer. Phytomedicine. 2020;68:153172. doi: 10.1016/j.phymed.2020.153172 [DOI] [PubMed] [Google Scholar]
- 14.Yu J, Qiu Y, Yang J, et al. DNMT1-PPARgamma pathway in macrophages regulates chronic inflammation and atherosclerosis development in mice. Sci Rep. 2016;6:30053. doi: 10.1038/srep30053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Straus DS, Pascual G, Li M, et al. 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc Natl Acad Sci U S A. 2000;97(9):4844–4849. doi: 10.1073/pnas.97.9.4844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116–1120. doi: 10.1038/nature05894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82. doi: 10.1038/34178 [DOI] [PubMed] [Google Scholar]
- 18.Li MY, Zhang ZH, Wang Z, et al. Convallatoxin protects against dextran sulfate sodium-induced experimental colitis in mice by inhibiting NF-kappaB signaling through activation of PPARgamma. Pharmacol Res. 2019;147:104355. doi: 10.1016/j.phrs.2019.104355 [DOI] [PubMed] [Google Scholar]
- 19.Zhong M, Zhang X, Shi X, Zheng C. Halofuginone inhibits LPS-induced attachment of monocytes to HUVECs. Int Immunopharmacol. 2020;87:106753. doi: 10.1016/j.intimp.2020.106753 [DOI] [PubMed] [Google Scholar]
- 20.Chen -C-C, Li H-Y, Leu Y-L, Chen Y-J, Wang C-J, Wang S-H. Corylin inhibits vascular cell inflammation, proliferation and migration and reduces atherosclerosis in ApoE-deficient mice. Antioxidants (Basel). 2020;9(4). doi: 10.3390/antiox9040275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shkrobom SI. [Changes in the systemic hemodynamics of patients with chronic cerebrovascular insufficiency]. Zh Nevropatol Psikhiatr Im S S Korsakova. 1980;80(7):976–981. Russian. [PubMed] [Google Scholar]
- 22.Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists’ view. Eur Heart J. 2013;34(10):719–728. doi: 10.1093/eurheartj/ehs411 [DOI] [PubMed] [Google Scholar]
- 23.Chinetti-Gbaguidi G, Colin S, Staels B. Macrophage subsets in atherosclerosis. Nat Rev Cardiol. 2015;12(1):10–17. doi: 10.1038/nrcardio.2014.173 [DOI] [PubMed] [Google Scholar]
- 24.Yu X-H, Fu Y-C, Zhang D-W, Yin K, Tang C-K. Foam cells in atherosclerosis. Clin Chim Acta. 2013;424:245–252. doi: 10.1016/j.cca.2013.06.006 [DOI] [PubMed] [Google Scholar]
- 25.Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339 [DOI] [PubMed] [Google Scholar]
- 26.Seo JW, Yang EJ, Yoo KH, Choi IH. Macrophage differentiation from monocytes is influenced by the lipid oxidation degree of low density lipoprotein. Mediators Inflamm. 2015;2015:235797. doi: 10.1155/2015/235797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maréchal L, Laviolette M, Rodrigue-Way A, et al. The CD36-PPARγ pathway in metabolic disorders. Int J Mol Sci. 2018;19(5):1529. doi: 10.3390/ijms19051529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chawla A. Control of macrophage activation and function by PPARs. Circ Res. 2010;106(10):1559–1569. doi: 10.1161/CIRCRESAHA.110.216523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao Q, Liu J, Zhang Z, et al. Peroxisome proliferator-activated receptor gamma (PPARgamma) induces the gene expression of integrin alphaVbeta5 to promote macrophage M2 polarization. J Biol Chem. 2018;293(43):16572–16582. doi: 10.1074/jbc.RA118.003161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Q, Wei A, Chen F, et al. Enhancing PPARγ by HDAC inhibition reduces foam cell formation and atherosclerosis in ApoE deficient mice. Pharmacol Res. 2020;160:105059. doi: 10.1016/j.phrs.2020.105059 [DOI] [PubMed] [Google Scholar]
- 31.Oppi S, Nusser-Stein S, Blyszczuk P, et al. Macrophage NCOR1 protects from atherosclerosis by repressing a pro-atherogenic PPARgamma signature. Eur Heart J. 2020;41(9):995–1005. doi: 10.1093/eurheartj/ehz667 [DOI] [PubMed] [Google Scholar]