Abstract
Severe acute respiratory syndrome (SARS) coronavirus-2 (SARS-CoV-2), the causative viral agent for the ongoing COVID-19 pandemic, enters its host cells primarily via the binding of the SARS-CoV-2 spike (S) proteins to the angiotensin-converting enzyme 2 (ACE2). A number of other cell entry mediators have also been identified, including neuropilin-1 (NRP1) and transmembrane protease serine 2 (TMPRSS2). More recently, it has been demonstrated that transmembrane protease serine 4 (TMPRSS4) along with TMPRSS2 activate the SARS-CoV-2 S proteins, and enhance the viral infection of human small intestinal enterocytes. To date, a systematic analysis of TMPRSS4 in health and disease is lacking. In the present study, using in silico tools, the gene expression and genetic alteration of TMPRSS4 were analysed across numerous tumours and compared to controls. The observations were also expanded to the level of the central nervous system (CNS). The findings revealed that TMPRSS4 was overexpressed in 11 types of cancer, including lung adenocarcinoma, lung squamous cell carcinoma, cervical squamous cell carcinoma, thyroid carcinoma, ovarian cancer, cancer of the rectum, pancreatic cancer, colon and stomach adenocarcinoma, uterine carcinosarcoma and uterine corpus endometrial carcinoma, whilst it was significantly downregulated in kidney carcinomas, acute myeloid leukaemia, skin cutaneous melanoma and testicular germ cell tumours. Finally, a high TMPRSS4 expression was documented in the olfactory tubercle, paraolfactory gyrus and frontal operculum, all brain regions which are associated with the sense of smell and taste. Collectively, these data suggest that TMPRSS4 may play a role in COVID-19 symptomatology as another SARS-CoV-2 host cell entry mediator responsible for the tropism of this coronavirus both in the periphery and the CNS.
Keywords: transmembrane protease serine 4, pan-cancer, COVID-19, SARS-CoV-2, tropism
Introduction
Severe acute respiratory syndrome (SARS) coronavirus-2 (SARS-CoV-2) is the causative viral agent for the ongoing COVID-19 pandemic, with >75 million infected individuals and >2.38 million deaths worldwide, up to the writing of the present study (February 14, 2021) (1,2). During the writing of the present manuscript, a glimpse of hope appeared on the horizon with vaccinations being developed and administered globally (3-7).
It is now well-established that the entry of SARS-CoV-2 into cells is facilitated by its spike (S) proteins, mainly through binding to the angiotensin-converting enzyme 2 (ACE2) (8,9). Moreover, the SARS-CoV-2 S proteins are primed/activated by the transmembrane protease serine 2 (TMPRSS2), which appears to also play a key role in this viral infection (8,10,11). As such, there is increasing interest in identifying additional molecular mediators that may also facilitate the SARS-CoV-2 infection of host cells and promote further adverse COVID-19 symptoms. Accordingly, neuropilin-1 (NRP1) has been identified as a novel cellular mediator implicated in the SARS-CoV-2 infection (12-14), whilst similar evidence has also more recently emerged for TMPRSS4, another transmembrane protease. Indeed, TMPRSS4 - along with TMPRSS2 - appear to activate the SARS-CoV-2 S proteins, and enhance subsequent viral infection of human small intestinal enterocytes (15). These data from Zang et al, provide evidence that the intestine is an additional target organ for SARS-CoV-2 (15). Moreover, a recent study demonstrated that both ACE2 and TMPRSS2 genes were abundantly expressed in enterocytes of the lower gastrointestinal (GI) tract, whilst also exhibiting co-expression with TMPRSS4, particularly in the small intestine (16). Of note, whereas COVID-19 presentation usually includes fever, cough, pulmonary/respiratory symptoms, and loss of taste and smell (17,18), an increasing number of studies has also reported GI-related symptoms (e.g., diarrhoea, nausea and vomiting) in patients with COVID-19 (19-21). Indeed, a proportion of these patients with extra-pulmonary symptoms do not exhibit other respiratory symptoms (22).
Apart from respiratory and GI-related symptoms, it is now evident that patients with COVID-19 may experience a wide repertoire of neurological symptoms and complications (23-28), suggesting that SARS-CoV-2 can also attack the central nervous system (CNS), potentially via additional cell entry mediators, including NRP1 (14,29). Indeed, in silico analysis indicated that certain key mediators which facilitate the entry of SARS-CoV-2 into host cells, including ACE2, Cathepsin L (CTSL), TMPRSS2 and TMPRSS4, are expressed in the CNS, with the two transmembrane proteases being highly expressed in neurons (30).
Finally, increasing evidence suggests that cancer constitutes an additional risk factor for severe COVID-19 infection, with hospitalised patients with COVID-19 (particularly males and those receiving treatment with chemotherapy) exhibiting a poor prognosis and being associated with a high case-fatality rate (31). The authors of the present study, as well as other researchers have documented the differential expression of SARS-CoV-2 infection mediators in malignant states (11,32). TMPRSS2 in particular, was previously found to be significantly upregulated in rectum adenocarcinoma, as well as in prostate, breast and lung cancer (11). Notably, compared to never-smokers, a similar ACE2 and TMPRSS2 expression, but a higher TMPRSS4 expression, has been found in the human bronchial epithelial cells of current smokers when compared to never-smokers (33).
To date, there is a paucity of published data about the expression profile of TMPRSS4 in cancer. Therefore, present study curated pan-cancer data in terms of TMPRSS4 gene expression using in silico approaches. The TMPRSS4 expression profile is also further expanded by providing more evidence regarding the presence of this transmembrane protease in the CNS and GI track.
Data and methods
Bioinformatics analysis
The expression analysis of TMPRSS4 was validated through the Genotype-Tissue Expression (GTEx, www.gtexportal.org) and GEPIA (gepia.cancer-pku.cn). Information regarding the TCGA cohort pan-cancer data was acquired through cBioPortal (www.cbioportal.org/) and single cell analysis was done by using the Single Cell Portal (singlecell.broadinstitute.org/single_cell). Datasets accessed for pan-cancer analysis were the following: ACC, adrenocortical carcinoma; BLCA, bladder urothelial carcinoma; BRCA, breast invasive carcinoma; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; CHOL, cholangiocarcinoma; COAD, colon adenocarcinoma; DLBC, lymphoid neoplasm diffuse large B cell lymphoma; ESCA, oesophageal carcinoma; GBM, glioblastoma multiforme; HNSC, head and neck squamous cell carcinoma; KICH, kidney chromophobe; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LAML, acute myeloid leukaemia; LGG, brain lower grade glioma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; MESO, mesothelioma; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; PCPG, pheochromocytoma and paraganglioma; PRAD, prostate adenocarcinoma; READ, rectum adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma; STAD, stomach adenocarcinoma; TGCT, testicular germ cell tumours; THCA, thyroid carcinoma; THYM, thymoma; UCEC, uterine corpus endometrial carcinoma; UCS, uterine carcinosarcoma; and UVM, uveal melanoma. The visualisation of TMPRSS4 expression in the human brain was performed using the Allen brain atlas with 6 human donors, as assessed by microarray and presented as a heatmap (34). These data were acquired from a publicly available source which underwent all appropriate approvals by the Human Investigation Committees and Institutional Ethics Committees of each institute from which samples were obtained.
Statistical analysis
The method used for differential analysis in the present study was one-way ANOVA, using disease state (Tumour or Normal) as variables for calculating differential expression: Gene expression ~disease state. The expression data are first log2(TPM+1) transformed for differential analysis and the log2FC is defined as median (Tumor) – median (Normal). Genes with higher |log2FC| values and lower q values than pre-set thresholds are considered differentially expressed genes. For more information please visit: http://gepia.cancer-pku.cn/help.html.
Results
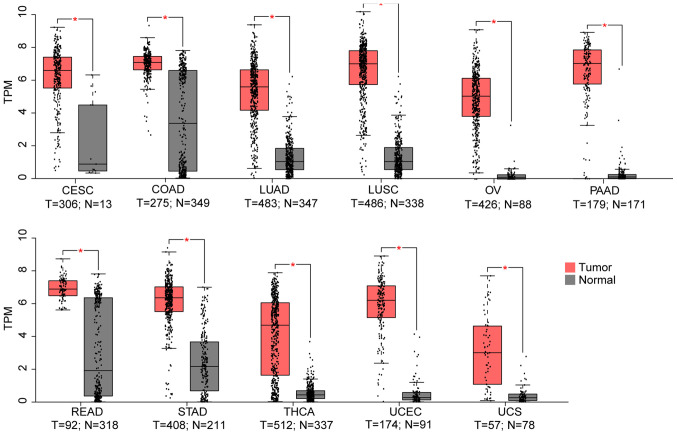
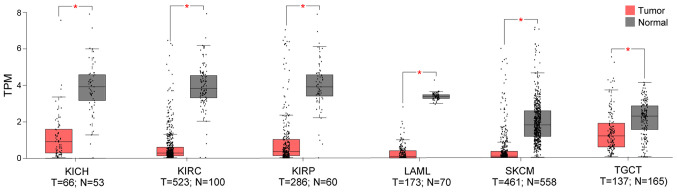
Using the TCGA and GTEX datasets, it was demonstrated that, compared to the normal control, TMPRSS4 was upregulated in 11 cancer datasets (i.e., CESC, COAD, LUAD, LUSC, OV, PAAD, READ, STAD, THCA, UCEC and UCS) (Fig. 1), while it was significantly downregulated in 6 datasets (i.e., KICH, KIRC, KIRP, LAML, SKCM and TGCT), with three of the latter consisting kidney tumours (i.e., KICK, KIRC and KIRP) (Fig. 2).
Figure 1.
From the 33 TCGA cancer datasets, 11 exhibited a higher TMPRSS4 expression in tumour samples. TMPRSS4 was highly expressed in lung cancers (LUAD and LUSC), gynecological cancers (CESC, OV, UCEC and UCS), pancreatic carcinoma (PAAD), colon adenocarcinoma (COAD), rectum carcinoma (READ), stomach adenocarcinoma (STAD) and thyroid cancer (THCA). T, tumour; N, normal; TPM, transcripts per million; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; OV, ovarian serous cystadenocarcinoma; UCEC, uterine corpus endometrial carcinoma; UCS, uterine carcinosarcoma.
Figure 2.
Out of the 33 TCGA cancer datasets, TMPRSS4 was significantly downregulated in 6 datasets (i.e., KICH, KIRC, KIRP, LAML, SKCM and TGCT), with three of these consisting kidney tumours (i.e., KICK, KIRC and KIRP) compared to normal tissues. KICH, kidney chromophobe; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LAML, acute myeloid leukaemia; SKCM, skin cutaneous melanoma; TGCT, testicular germ cell tumours; T, tumour; N, normal.
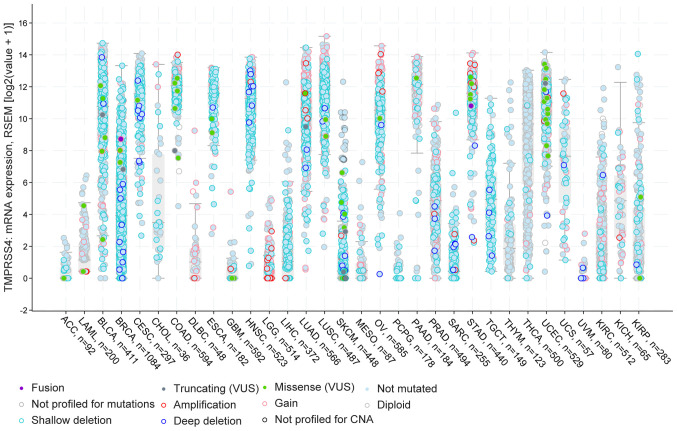
Furthermore, using the cBioportal pan-cancer panel, the present study identified specific cancer types with a number of TMPRSS4 amplifications in LGG, LUAD, STAD, deep deletions in BRCA, HNSC, and shallow deletions in BLCA, BRCA, CESC, ESCA, LUAD, LUSC, OV and TGCT. Furthermore, there were two datasets for thyroid cancers with a number of patients presenting diploid and not mutated versions of the gene, namely for THYM and THCA. Of note, in the majority of the studied cancers, the majority of the patients had deletions and partly some gains and amplifications (Fig. 3).
Figure 3.
cBioportal analysis of the 32 TCGA datasets with information about the gene dysrégulation. In BLCA, LUAD and LUSC there are a number of shallow deletions, while in BRCA and HNSC, high expression and more deep deletions are observed. In gynaecological cancers (CESC, OV, UCEC and UCS), there is high expression of the TMPRSS4 and a few patients present Gains and Missense 'variants of uncertain significant' (VUS). BLCA, bladder urothelial carcinoma; BRCA, breast invasive carcinoma; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; HNSC, head and neck squamous cell carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; OV, ovarian serous cystadenocarcinoma; UCEC, uterine corpus endometrial carcinoma; UCS, uterine carcinosarcoma.
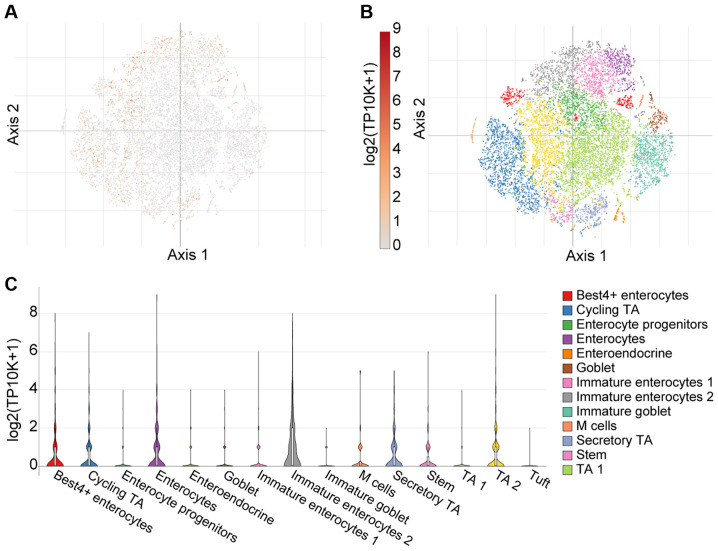
As aforementioned, there is emerging evidence suggesting abundant SARS-CoV-2 infection of the GI tract in severe COVID-19 cases (35). Herein, these observations were expanded upon using single cell analysis from 51 cell subsets (366,650 cells in total) in the colon mucosa of 18 ulcerative colitis and 12 healthy individuals using the Single Cell Portal (36). Using T-distributed Stochastic Neighbour Embedding (tSNE) - a machine learning algorithm for visualization - distinct cell sub-populations express TMPRSS4 (Fig. 4). Notably, a high TMPRSS4 expression was noted in immature enterocytes, BEST4-expressing enterocytes and enterocytes, as presented in Fig. 4C.
Figure 4.
Single cell analysis of colon mucosa, using the Single Cell Portal, revealed widespread expression of TMPRSS4. (A) Single-cell transcriptomics data are displayed as a spectral tSNE (T-distributed Stochastic Neighbour Embedding) plot of 366,650 cells, annotated according to known cell types; (B) sub-populations of cell types which are enriched for TMPRSS4 expression, with expression intensity demonstrated by a heat map; (C) single cell analysis using the Single Cell Portal, enriched for population subtypes, represented as violin plots. Notably, high TMPRSS4 expression was noted in immature enterocytes, BEST4-expressing enterocytes and enterocytes. TA1, TA2; transit-amplifying (TA) cells.
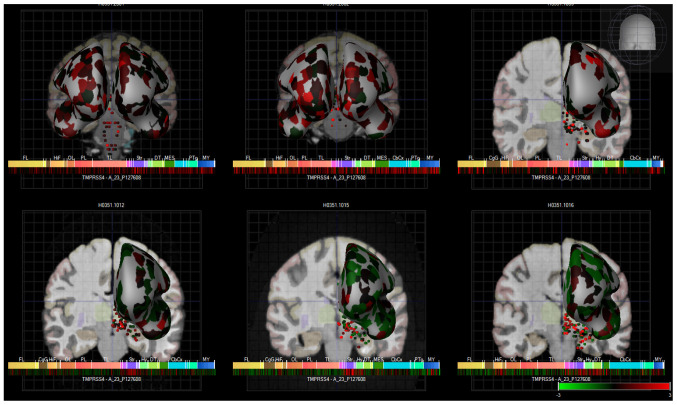
Finally, TMPRSS4 expression was also observed in various brain regions, based on a human microarray data set of 6 human brains (Fig. 5). These data were acquired from a publicly available source which underwent all appropriate approvals by the Human Investigation Committees and Institutional Ethics Committees of each institute from which samples were obtained.
Figure 5.
Heatmap expression of TMPRSS4 in the brains of 6 human donors, as assessed by microarray (Allen brain atlas). Global brain expression of TMPRSS4, as detected by microarray probes, with expression of cerebral areas indicated on the hemispheres and expression in brain nuclei indicated as dots. Heatmaps of expression are displayed below individual brains and organised by anterior to posterior regions with the frontal lobe (Fl), hippocampal formation (HiF), occipital lobe (Ol), parietal lobe (Pl), temporal lobe (Tl), striatum (Str), dorsal thalamus (dT), cerebral cortex (cbcx) and myelencephalon (MY) marked on the heatmap.
Fig. 5 presents these data as heatmaps of log2 expression values, showing that TMPRSS4 is expressed throughout the human brain. Of note, anosmia represents one of the common first symptoms of SARS-CoV-19 infection (37,38), and although there is global brain expression of TMPRSS4, a high TMPRSS4 expression is noted in brain regions important for the sense of smell and taste, namely in the olfactory tubercle (Log2 expression, 2.1±1), paraolfactory gyrus (Log2 expression, 1.3±0.3) and frontal operculum (Log2 expression, 1.5±0.5). These Log2 expression values are provided by the Allen Brain Atlas. Previous research has also demonstrated that TMPRSS4 exhibited the highest expression in neurons, with the neuronal rich cerebral cortex exhibiting a high TMPRSS4 expression (39). TMPRSS4 protein expression is 1.5 protein-transcripts per million (pTPM) in this region with the highest expression localised to the neuronal cells (ProteinAtlas_ENSG00000137648).
Discussion
In the present study, comprehensive evidence is presented regarding the peripheral tissue and CNS distribution of a new potential mediator of SARS-CoV-2 infection, namely TMPRSS4. Of note, it was demonstrated that TMPRSS4 is overexpressed in lung cancer; a condition that predisposes to severe COVID-19 (33,40,41). The current findings are in agreement with those of a previous study demonstrating the co-expression of TMPRSS4 with other key SARS-CoV-2 cell entry mediators (i.e., ACE2, ADAM17 and TMPRSS2) in bronchial epithelial cells from never smokers and current smokers (33). Notably, that study also demonstrated that the TMPRSS4 levels were elevated in smokers, suggesting that this may be an additional risk factor for SARS-CoV-2 infection (33). In the lung cancer cohorts investigated in the present study, a marginal difference was note in LUAD with a marked downregulation of TMPRSS4 in current smokers, whilst in patients with LUSC an increase in TMPRSS4 was noted in smokers compared to non-smokers (Fig. S1A and B). This is suggestive of potential tissue-specific effects, given that patients with LUAD have poorer prognosis than those with LUSC (42).
Another recent study also demonstrated a high expression of TMPRSS4 in the human endometrium, which increased with age, particularly in the early phases of the cycle (43). This is of increasing importance given that SARS-CoV-2 infectivity increases with age (44). The present study expanded on these observations and provided evidence of TMPRSS4 expression in other gynaecological tissues, demonstrating that TMPRSS4 is expressed in the cervix, vagina, fallopian tubes, ovaries, breast and uterus, with the first two being the primary organs in terms of a high expression (Fig. S1D). Moreover, it was demonstrated that TMPRSS4 was significantly upregulated in gynaecological malignancies, namely CESC, OV, UCEC and UCS. As age appears to play a role in the risk of COVID-19, the present study further investigated the expression of TMPRSS4 in the above-mentioned malignancies with age. In this respect, TMPRSS4 expression increased with age only in the case of UCEC (Fig. S1C). One of the major limitations of the present study was that in silico data were accessed, that did not allow us to perform statistical analysis with a post hoc test. Another limitation is the absence of survival analysis data. Future studies are required to determine whether TMPRSS4 can be of prognostic value in terms of overall- and/or progression free survival.
Recently, two different groups have described the involvement of TMPRSS4 as a SARS-CoV-2 entry mediator in the GI (15,16). In particular, ACE2, TMPRSS2 and TMPRSS4 have been shown to be co-expressed primarily in the small intestine (16). In another study, through a series of elegant experiments, it was shown that, apart from TMPRSS2, TMPRSS4 also enhanced SARS-CoV-2 infectivity in gut epithelial cells (15), suggesting that a leaky gut may allow SARS-CoV-2 to spread to other organs, including the liver. Future studies are required to focus on the role of these key cell entry mediators in GI tract organs, particularly in relation to extra-pulmonary manifestations of severe COVID-19.
Finally, it was demonstrated that TMPRSS4 is present in a number of anterior and posterior regions of the brain. This corroborates previous finding indicating expression in the cerebral cortex, hippocampus and caudate (45). Of note, other SARS-CoV-2 cell entry mediators (e.g., ACE2, TMPRSS2, NRP1 and CTSL) are also expressed in the CNS, raising the possibility of a synergy between multiple such proteins which may further drive the SARS-CoV-2 neurotropism (29,30,46).
In conclusion, the present study documents widespread TMPRSS4 protein expression in the CNS and GI tract, and provides further evidence supporting the potential involvement of TMPRSS4 in facilitating adverse COVID-19 outcomes in patients with certain cancers. Collectively, these data suggest that TMPRSS4 may be implicated in the symptomatology/complications of COVID-19, acting as another SARS-CoV-2 cell entry mediator responsible for the tropism of this novel coronavirus in the periphery and the CNS.
Supplementary Data
Acknowledgments
Not applicable.
Funding Statement
No funding was received.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Authors' contributions
PK, RK, JD, EK, JD, JLR, IK consulted the literature, and produced the figures and manuscript. MH, HSR, KC, JD, VA, DAS, JLR and AP contributed to the literature search, interpretation of the data and the critical revision of the manuscript. PK, JD, HSR, DAS, IK and EK contributed to the writing of the manuscript and final edits. IK and EK contributed equally to the conception of the study, data and literature analysis, as well as interpretation. All authors read and approved the final manuscript. JD is an alumnus of Brunel University London. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article. The other authors declare that they have no competing interests.
References
- 1.World Health Organization (WHO) Weekly Epidemiological Update on COVID-19. WHO; Geneva: 2021. [Google Scholar]
- 2.Docea AO, Tsatsakis A, Albulescu D, Cristea O, Zlatian O, Vinceti M, Moschos SA, Tsoukalas D, Goumenou M, Drakoulis N, et al. A new threat from an old enemy: Re-emergence of coronavirus (Review) Int J Mol Med. 2020;45:1631–1643. doi: 10.3892/ijmm.2020.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calina D, Hartung T, Docea AO, Spandidos DA, Egorov AM, Shtilman MI, Carvalho F, Tsatsakis A. COVID-19 vaccines: Ethical framework concerning human challenge studies. Daru. 2020;28:807–812. doi: 10.1007/s40199-020-00371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kostoff RN, Kanduc D, Porter AL, Shoenfeld Y, Calina D, Briggs MB, Spandidos DA, Tsatsakis A. Vaccine- and natural infection-induced mechanisms that could modulate vaccine safety. Toxicol Rep. 2020;7:1448–1458. doi: 10.1016/j.toxrep.2020.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calina D, Docea AO, Petrakis D, Egorov AM, Ishmukhametov AA, Gabibov AG, Shtilman MI, Kostoff R, Carvalho F, Vinceti M, et al. Towards effective COVID-19 vaccines: Updates, perspectives and challenges (Review) Int J Mol Med. 2020;46:3–16. doi: 10.3892/ijmm.2020.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calina D, Sarkar C, Arsene AL, Salehi B, Docea AO, Mondal M, Islam MT, Zali A, Sharifi-Rad J. Recent advances, approaches and challenges in targeting pathways for potential COVID-19 vaccines development. Immunol Res. 2020;68:315–324. doi: 10.1007/s12026-020-09154-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Oxford COVID Vaccine Trial Group Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, Geng Q, Auerbach A, Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwata-Yoshikawa N, Okamura T, Shimizu Y, Hasegawa H, Takeda M, Nagata N. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J Virol. 2019;93:e01815–e01818. doi: 10.1128/JVI.01815-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katopodis P, Anikin V, Randeva HS, Spandidos DA, Chatha K, Kyrou I, Karteris E. Pan-cancer analysis of transmembrane protease serine 2 and cathepsin L that mediate cellular SARS-CoV-2 infection leading to COVID-19. Int J Oncol. 2020;57:533–539. doi: 10.3892/ijo.2020.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bittmann S, Weissenstein A, Villalon G, Moschuring-Alieva E, Luchter E. Simultaneous treatment of COVID-19 with serine protease inhibitor camostat and/or cathepsin L inhibitor? J Clin Med Res. 2020;12:320–322. doi: 10.14740/jocmr4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, Franz J, Kuivanen S, Kallio K, Kaya T, Anastasina M, Smura T, et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and provides a possible pathway into the central nervous system. bioRxiv. doi: 10.1101/2020.06.07.137802. [DOI] [PMC free article] [PubMed]
- 14.Daly JL, Simonetti B, Klein K, Chen KE, Williamson MK, Anton-Plagaro C, Shoemark DK, Simon-Gracia L, Bauer M, Hollandi R, et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370:861–865. doi: 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zang R, Gomez Castro MF, McCune BT, Zeng Q, Rothlauf PW, Sonnek NM, Liu Z, Brulois KF, Wang X, Greenberg HB, et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol. 2020;5:1–15. doi: 10.1126/sciimmunol.abc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JJ, Kopetz S, Vilar E, Shen JP, Chen K, Maitra A. Relative abundance of SARS-CoV-2 entry genes in the enterocytes of the lower gastrointestinal tract. Genes (Basel) 2020;11:645. doi: 10.3390/genes11060645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, et al. China Medical Treatment Expert Group for Covid-19: Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerslake R, Hall M, Randeva HS, Spandidos DA, Chatha K, Kyrou I, Karteris E. Co-expression of peripheral olfactory receptors with SARS-CoV-2 infection mediators: Potential implications beyond loss of smell as a COVID-19 symptom. Int J Mol Med. 2020;46:949–956. doi: 10.3892/ijmm.2020.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng SC, Tilg H. COVID-19 and the gastrointestinal tract: More than meets the eye. Gut. 2020;69:973–974. doi: 10.1136/gutjnl-2020-321195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almeida JFM, Chehter EZ. COVID-19 and the gastrointestinal tract: What do we already know? Einstein (Sao Paulo) 2020;18:eRW5909. doi: 10.31744/einstein_journal/2020RW5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Syed A, Khan A, Gosai F, Asif A, Dhillon S. Gastrointestinal pathophysiology of SARS-CoV2 - a literature review. J Community Hosp Intern Med Perspect. 2020;10:523–528. doi: 10.1080/20009666.2020.1811556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, Hao SR, Jia HY, Cai H, Zhang XL, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Felice FG, Tovar-Moll F, Moll J, Munoz DP, Ferreira ST. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the central nervous system. Trends Neurosci. 2020;43:355–357. doi: 10.1016/j.tins.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Needham EJ, Chou SH-Y, Coles AJ, Menon DK. Neurological implications of COVID-19 infections. Neurocrit Care. 2020;32:667–671. doi: 10.1007/s12028-020-00978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mao XY, Jin WL. The COVID-19 pandemic: consideration for brain infection. Neuroscience. 2020;437:130–131. doi: 10.1016/j.neuroscience.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Q, Fan X, Hong H, Gu Y, Liu Z, Fang S, Wang Q, Cai C, Fang J. Comprehensive assessment of side effects in COVID-19 drug pipeline from a network perspective. Food Chem Toxicol. 2020;145:111767. doi: 10.1016/j.fct.2020.111767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sidiropoulou P, Docea AO, Nikolaou V, Katsarou MS, Spandidos DA, Tsatsakis A, Calina D, Drakoulis N. Unraveling the roles of vitamin D status and melanin during Covid-19 (Review) Int J Mol Med. 2021;47:92–100. doi: 10.3892/ijmm.2020.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies J, Randeva HS, Chatha K, Hall M, Spandidos DA, Karteris E, Kyrou I. Neuropilin-1 as a new potential SARS-CoV-2 infection mediator implicated in the neurologic features and central nervous system involvement of COVID-19. Mol Med Rep. 2020;22:4221–4226. doi: 10.3892/mmr.2020.11510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C, Mushumba H, Fitzek A, Allweiss L, Dandri M, et al. Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. Lancet Neurol. 2020;19:919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang K, Sheng Y, Huang C, Jin Y, Xiong N, Jiang K, Lu H, Liu J, Yang J, Dong Y, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: A multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:904–913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chai P, Yu J, Ge S, Jia R, Fan X. Genetic alteration, RNA expression, and DNA methylation profiling of coronavirus disease 2019 (COVID-19) receptor ACE2 in malignancies: A pan-cancer analysis. J Hematol Oncol. 2020;13:43. doi: 10.1186/s13045-020-00883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voinsky I, Gurwitz D. Smoking and COVID-19: Similar bronchial ACE2 and TMPRSS2 expression and higher TMPRSS4 expression in current versus never smokers. Drug Dev Res. 2020;81:1073–1080. doi: 10.1002/ddr.21729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, van de Lagemaat LN, Smith KA, Ebbert A, Riley ZL, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–399. doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma C, Cong Y, Zhang H. COVID-19 and the digestive system. Am J Gastroenterol. 2020;115:1003–1006. doi: 10.14309/ajg.0000000000000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smillie CS, Biton M, Ordovas-Montanes J, Sullivan KM, Burgin G, Graham DB, Herbst RH, Rogel N, Slyper M, Waldman J, et al. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell. 2019;178:714–730.e22. doi: 10.1016/j.cell.2019.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker A, Pottinger G, Scott A, Hopkins C. Anosmia and loss of smell in the era of covid-19. BMJ. 2020;370:m2808. doi: 10.1136/bmj.m2808. [DOI] [PubMed] [Google Scholar]
- 38.Veldhuizen MG, Small DM. Modality-specific neural effects of selective attention to taste and odor. Chem Senses. 2011;36:747–760. doi: 10.1093/chemse/bjr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darmanis S, Sloan SA, Zhang Y, Enge M, Caneda C, Shuer LM, Hayden Gephart MG, Barres BA, Quake SR. A survey of human brain transcriptome diversity at the single cell level. Proc Natl Acad Sci USA. 2015;112:7285–7290. doi: 10.1073/pnas.1507125112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Aberasturi AL, Redrado M, Villalba M, Larzabal L, Pajares MJ, Garcia J, Evans SR, Garcia-Ros D, Bodegas ME, Lopez L, et al. TMPRSS4 induces cancer stem cell-like properties in lung cancer cells and correlates with ALDH expression in NSCLC patients. Cancer Lett. 2016;370:165–176. doi: 10.1016/j.canlet.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 41.Larzabal L, Nguewa PA, Pio R, Blanco D, Sanchez B, Rodríguez MJ, Pajares MJ, Catena R, Montuenga LM, Calvo A. Overexpression of TMPRSS4 in non-small cell lung cancer is associated with poor prognosis in patients with squamous histology. Br J Cancer. 2011;105:1608–1614. doi: 10.1038/bjc.2011.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki K, Nagai K, Yoshida J, Nishimura M, Takahashi K, Yokose T, Nishiwaki Y. Conventional clinicopathologic prognostic factors in surgically resected nonsmall cell lung carcinoma. A comparison of prognostic factors for each pathologic TNM stage based on multivariate analyses. Cancer. 1999;86:1976–1984. doi: 10.1002/(SICI)1097-0142(19991115)86:10<1976::AID-CNCR14>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 43.Henarejos-Castillo I, Sebastian-Leon P, Devesa-Peiro A, Pellicer A, Diaz-Gimeno P. SARS-CoV-2 infection risk assessment in the endometrium: Viral infection-related gene expression across the menstrual cycle. Fertil Steril. 2020;114:223–232. doi: 10.1016/j.fertnstert.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nikolich-Zugich J, Knox KS, Rios CT, Natt B, Bhattacharya D, Fain MJ. SARS-CoV-2 and COVID-19 in older adults: What we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience. 2020;42:505–514. doi: 10.1007/s11357-020-00186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; Erratum. Geroscience. 2020;42:1013. doi: 10.1007/s11357-020-00193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guadarrama-Ortiz P, Choreño-Parra JA, Sánchez-Martínez CM, Pacheco-Sánchez FJ, Rodríguez-Nava AI, García-Quintero G. Neurological aspects of SARS-CoV-2 infection: mechanisms and manifestations. Front Neurol. 2020;11:1039. doi: 10.3389/fneur.2020.01039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frank S. Catch me if you can: SARS-CoV-2 detection in brains of deceased patients with COVID-19. Lancet Neurol. 2020;19:883–884. doi: 10.1016/S1474-4422(20)30371-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article.