Abstract
Background
The survival rates in population-based series of glioblastoma (GBM) differ substantially from those reported in clinical trials. This discrepancy may be attributed to that patients recruited to trials tend to be younger with better performance status. However, the proportion and characteristics of the patients in a population considered either eligible or ineligible for trials is unknown. The generalizability of trial results is therefore also uncertain.
Methods
Using the Cancer Registry of Norway and the Brain Tumor Database at Oslo University Hospital, we tracked all patients within a well-defined geographical area with newly diagnosed GBM during the years 2012–2017. Based on data from these registries and the medical records, the patients were evaluated for trial eligibility according to criteria employed in recent phase III trials for GBM.
Results
We identified 512 patients. The median survival was 11.7 months. When we selected a potential trial population at the start of concurrent chemoradiotherapy (radiotherapy [RT]/ temozolomide [TMZ]) by the parameters age (18–70 y), passed surgery for a supratentorial GBM, Eastern Cooperative Oncology Group (ECOG) ≤2, normal hematologic, hepatic and renal function, and lack of severe comorbidity, 57% of the patients were excluded. Further filtering the patients who progressed during RT/TMZ and never completed RT/TMZ resulted in exclusion of 59% and 63% of the patients, respectively. The survival of patients potentially eligible for trials was significantly higher than of the patients not fulfilling trial eligibility criteria (P < .0001).
Conclusions
Patients considered eligible for phase III clinical trials represent a highly selected minority of patients in a real-world GBM population.
Keywords: clinical trials, eligibility, glioblastoma, selection bias, survival
Key Points.
A minority of GBM patients are eligible for clinical trials.
Patients in trials are not representative for a real-world GBM population.
The extrapolation of trial results to the real-world population is uncertain.
Importance of the Study.
Clinical trials are fundamental for therapeutic advances in GBM and according to the National Comprehensive Cancer Network, the best management of a cancer patient is in a trial. However, by current trial inclusion and exclusion criteria, only a selected group of patients can be considered eligible for trials. This bias not only restricts patients from receiving the best care, but also makes it difficult to extrapolate trial results to a real-world population. Here, we estimated the proportion of GBM patients who did not fulfill eligibility criteria for trial participation and compared the characteristics of patients considered trial participants to those excluded. We found that approximately 60% of patients were ineligible for trials. These patients were older, had worse performance status, received less treatment and had worse survival. This implies that the current trial landscape inadequately reflects the population, and that generalizability of trials results into clinical practice carries considerable uncertainty.
The median survival of patients with newly diagnosed glioblastoma (GBM), the most frequent and most malignant primary brain tumor, is commonly claimed to be about 15 months.1 Although valid for the subgroup of patients who are entered into randomized clinical trials (RCT) and typically undergo multimodal treatment with surgery, radiotherapy (RT) and chemotherapy with temozolomide (TMZ), this survival rate contrasts observations made in unselected populations of real-world GBM patients, where the survival is reported to be considerably shorter.2–5
The better outcome of patients in clinical trials is attributed to the selected population of patients being studied. From a heterogeneous patient population, trials infer a selection bias and recruit a more homogenous group of a well-defined population of patients. This selection aims to minimize confounders that might affect trial outcome.6 Compared to real-world patients, phase III trials in GBM usually enroll patients who are younger, have a more favorable performance status, and are more likely to have undergone tumor resection surgery,7–16 all of which are established as strong prognostic factors that influence survival.17–19 As a consequence, patients in trials have better outcomes than can be expected in a population setting, where inclusion and exclusion criteria do not exist and follow-up might be less rigorous.20
There is, however, a lack of data describing the proportion and characteristics of the patients who do not fulfill standard eligibility criteria for phase III clinical trials in GBM. The generalizability of trial results is also therefore uncertain. Due to several prospectively maintained national and regional databases that can be linked, combined with a single-payer universal health care system, Norway has a unique opportunity for population-based studies. Here, we utilized data from the Cancer Registry of Norway and the Brain Tumor Database at the Oslo University Hospital to evaluate the proportion and characteristics of real-world patients with newly diagnosed GBM that fulfill or violate eligibility criteria in phase III trials.
Materials and Methods
Population Base
Each person in Norway is registered in The National Population Register with a unique ID number and contact information that facilitates contact between health care officials and individual patients. In this study, we collected data from the counties Akershus, Buskerud, Oslo, Telemark, Vestfold, and Østfold since all patients from these counties receive their oncological treatment at Oslo University Hospital (OUH). This defined geographical area comprised 2.2 million people (43.9% of the Norwegian population) during the study period.
Cancer Registries
To identify the patients in this study, we used the Cancer Registry of Norway (CRN) and the Brain Tumor Database (BTD) at Oslo University Hospital.
The CRN was founded in 1951, and it maintains a prospective database of all tumors diagnosed in Norway, including both malignant and benign tumors of the central nervous system (CNS). Reporting to the registry is compulsory by law, and the registry is based on clinicians’ reports, pathology reports, and information from death certificates reporting neoplastic disease. The quality of the registry is maintained by ensuring that missing reports from attending clinicians or pathologists are requested by direct contact. Data from the CRN have undergone quality control and are valid for population studies.21 The tumors from the time period in this study were coded according to the third revision of the International Classification of Diseases for Oncology (ICD-O-3, primary GBM, site codes 710–719, and histology codes 9440–9442). The registry also retrieves data electronically, ensuring information on oncological treatment (RT and TMZ) in all patients. The unique identification number of each individual is maintained by the government and change to death status is conveyed to the CRN. This study has used data from the CRN. The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by the CRN is intended nor should be inferred.
The BTD is a prospective database containing details of all tumor resections and biopsies carried out in the Department of Neurosurgery, OUH. The registry retrieves data electronically from every patient, including name, unique ID number, sex, age at diagnosis, tumor localization, type of surgical treatment, pathology, and survival.
Patient Data
Using the BTD we identified all patients diagnosed with GBM (n = 512) between 2012 and 2017 from the defined counties. By using the CRN, we could also identify the patients who were diagnosed by MRI only. Two of the authors independently undertook a systematic review of the medical records of the individual patients in order to obtain the required data which included the following; sex, age at diagnosis, tumor localization (dichotomized into supra- or infratentorial), performance status by the Eastern Cooperative Oncology Group (ECOG) evaluated preoperatively, as well as before the start, at midway and at the end of concurrent RT/TMZ, type of primary surgery (no surgery, stereotactic biopsy, open biopsy or resection), primary oncological treatment (RT and TMZ), other comorbidities (heart, hepatic, immunologic, lung, psychiatric, or renal disease, coagulation insufficiency, previous malignancy, previous oncological treatment, and other disorders of the CNS, eg, dementia), medication use before the diagnosis of GBM (number of drugs taken daily that required prescription from a physician), the use and doses of steroids before, midway and at the end of concurrent RT/TMZ, disease status (progressive or not progressive disease) until finished radiochemotherapy phase, and blood levels of white blood cells (WBC), thrombocytes (Tbc), creatinine, bilirubin, aspartate amino transferase (AST), and alanine amino transferase (ALT). The time of death is recorded in both the CRN and BTD as obtained from the Norwegian Population Register. The last date of follow-up was July 6, 2020.
Definition of Variables
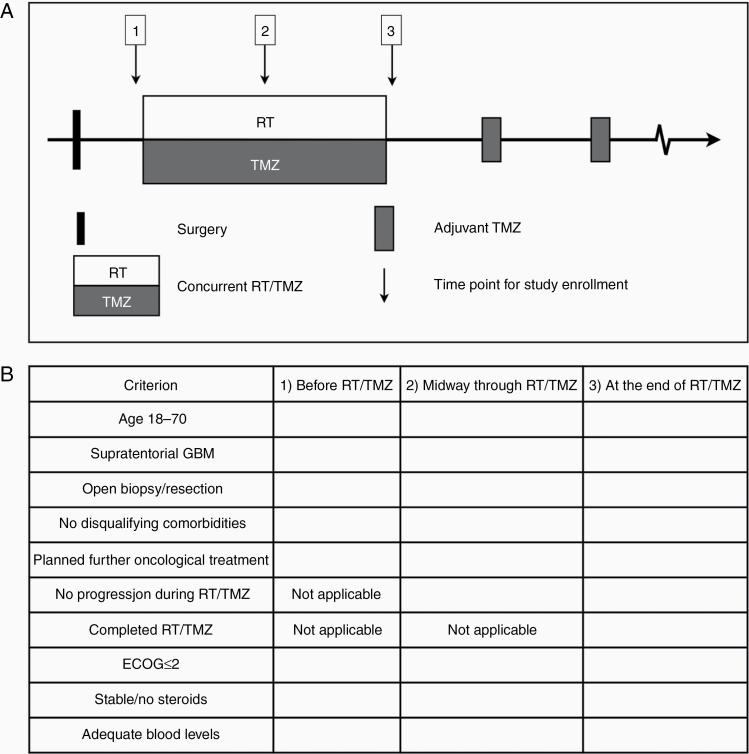
We used the phase III trials in newly diagnosed GBM that were published over the last 10 years as reference trials.7–16 We assessed the parameters used for inclusion and exclusion of patients in the respective trials (reviewed in Supplementary Table S1) and derived a list of the most commonly and uniformly used criteria that a patient must fulfill to be enrolled in a phase III trial for newly diagnosed GBM. These variables concern patient age, comorbidities, performance status, physiologic parameters (hematologic, hepatic, renal function), tumor localization, pathology, oncological treatment, and glucocorticoid use, as elaborated below. Based on the time of randomization used in different trials (Supplementary Table S1), we defined 3 time points for potential study recruitment: (1) before concurrent RT/TMZ, (2) midway through concurrent RT/TMZ, and (3) at the end of the concurrent RT/TMZ.
We used the ECOG scale to classify patient performance status as this is the scale used prospectively by physicians at OUH. In cases where the ECOG status was not specified in the medical records, it was assessed retrospectively by 2 independent researchers. Tumors that extended both supra- and infratentorial were categorized as infratentorial. The surgical procedure (stereotactic biopsy, open biopsy, and resection) was categorized according to the procedural description by the neurosurgeon. We did not distinguish between subtotal and total resections. Adjuvant treatment was considered completed in patients who received a radiation dose of ≥54 Gy and ≥5 weeks of concurrent TMZ (75 mg/m2). Patients were categorized as having received a suboptimal dose of RT if they received <54 Gy. This group included older patients who received hypofractionated treatment (3 Gy × 10 or 2.67 Gy × 15) and patients where RT was stopped because of side effects, tumor progression, or death. Patients were categorized as having discontinued TMZ treatment if TMZ was withdrawn before the completion of the fifth week (out of 6 weeks) of the concurrent phase due to side effects, toxicity, progression, or death.
We defined threshold levels of hematologic, hepatic, and renal function according to levels commonly used in published phase III trials in GBM.7–16 Patients were excluded if they had WBC < 1.5 ×109/L, tbc < 100 × 109/L, creatinine >150 µmol/L, bilirubin >34 µmol/L or AST/ALT >3 times above the upper reference limit of the hospital laboratory at the defined time points. For disqualifying comorbidities, we used the diseases commonly listed as reasons for trial exclusion according to published phase III trials.7–16 This included patients with active heart disease (NYHA 3–4, unstable angina pectoris, myocardial infarction within last 6 months), active hepatic disease (hepatic insufficiency), or active lung disease (COPD requiring hospitalization within the last 6 months), patients with bleeding disorders, immunologic disorders with inherited or acquired immunodeficiency or iatrogenic immunosuppression other than glucocorticoid use due to GBM, recent (within the last 6 months) intracranial bleeding, recent (within the last 6 months) abscess or infection of the CNS, previous oncological disease other than nonmelanoma skin cancer and carcinoma in situ of the cervix, patients with severe psychiatric disease (eg, schizophrenia requiring recent hospitalization) and patients with developmental disorders (eg, infantile autism) or dementia. Pregnancy was also categorized as an exclusion criterion. Patients taking ≥48 mg methylprednisolone (≥16 mg TID) or ≥12 mg dexamethasone (≥4 mg TID) daily, were classified as receiving high-doses of steroids. Patients who received increasing doses of steroids up to the predefined time points for potential enrollment in a trial were also classified into a separate group. Polypharmacy was considered present if a patient was taking ≥5 drugs requiring a prescription from a physician (except for newly started drugs related to GBM).
Ethics
The study was approved by The Norwegian Regional Committee for Medical Research Ethics (REK 2018/2295) and the data protection officers at the CRN and OUH (PVO 2017/7084).
Statistical Considerations
We constructed a custom-made database using FileMaker Pro 16.0.5. Data analysis and graphic presentation were performed using GraphPad Prism 8.0, Microsoft Excel 16.3, and Keynote 10.2. Population characteristics are presented as observed counts, weighted percentages, and medians. Overall survival was calculated from the time of surgery to the time of death by the Kaplan–Meier method. In patients lacking tissue-based diagnosis survival was calculated from the time of diagnosis as defined by the CRN to the time of death. Differences between groups were compared by unpaired nonparametric Mann–Whitney U test. Survival between different groups and associations with overall survival were compared by the log-rank test. A P-value <.05 was considered significant.
Results
Population Characteristics
A total of 512 patients were diagnosed with GBM between 2012 and 2017, representing an incidence of 3.89 per 100 000/y. The diagnosis was made via tissue examination by a pathologist in 484 cases (94.5%), while 28 (5.5%) had their diagnosis derived from radiologic imaging only, as they were not considered candidates to undergo an invasive procedure. The median age of the entire population at diagnosis was 64 years, 133 patients (26%) were >70 years, and the median age of the patients with and without a tissue-based diagnosis was 63 and 81 years, respectively. There was a slight male predominance (1.53:1). Ten patients (2%) had disease involving both supra- and infratentorial regions, while 10 (2%) had solely infratentorial disease localized in the cerebellum (n = 5), brain stem (n = 3), or spinal cord (n = 2). A good performance status (ECOG ≤2) at the time of diagnosis was observed in 469 patients (91.6%). Another previous or concurrent oncological disease was the most common comorbidity (n = 75, 14.6%). Further population characteristics are outlined in Table 1 and Supplementary Figure S1.
Table 1.
Population Characteristics
| Number (%) | Median age (range) in years | Median survival (95% CI), months | HR (95% CI) | P-value | |
|---|---|---|---|---|---|
| All patients | 512 (100) | 64 (10–97) | 11.7 (10.8–12.8) | - | - |
| Basis of diagnosis | |||||
| Pathology | 485 (94.5) | 63 (10–89) | 12.3 (11.4–13.2) | Reference | - |
| Radiology | 28 (5.5) | 81 (68–97) | 2.4 (1.5–3.7) | 6.54 (2.59–16.53) | <.0001 |
| Gender | |||||
| Male | 310 (60.5) | 64 (10–97) | 11.6 (10.8–12.8) | Reference | - |
| Female | 202 (39.5) | 64 (27–87) | 11.9 (9.8–13.7) | 0.87 (0.72–1.04) | .13 |
| Localization | |||||
| Supratentorial | 492 (96.0) | 64 (11–97) | 11.8 (11.0–12.9) | Reference | - |
| Supra- and infratentorial | 10 (2.0) | 49 (10–73) | 7.4 (0.5–31.2) | 1.13 (0.56–2.26) | .73 |
| Infratentorial only | 10 (2.0) | 62 (17–69) | 8.3 (1.3–40.2) | 1.12 (0.56–2.24) | .74 |
| Performance status, preoperatively | |||||
| ECOG 0 | 341 (66.6) | 62 (10–83) | 13.7 (12.4–14.8) | Reference | - |
| ECOG 1 | 99 (19.3) | 66 (34–94) | 9.3 (7.9–10.8) | 1.79 (1.37–2.35) | <.0001 |
| ECOG 2 | 29 (5.7) | 67 (35–86) | 7.0 (4.0–12.9) | 2.02 (1.18–3.45) | .0003 |
| ECOG 3 | 16 (3.1) | 68 (45–84) | 3.6 (1.5–12.4) | 2.92 (1.27–6.69) | <.0001 |
| ECOG 4 | 24 (4.7) | 73 (11–97) | 2.8 (1.1–6.0) | 2.78 (1.39–5.54) | <.0001 |
| N/A | 3 (0.6) | 85 (83–87) | 2.0 (2.0–2.3) | - | - |
| Surgical procedure | |||||
| Resection | 413 (80.7) | 63 (10–84) | 13.2 (12.4–14.1) | Reference | - |
| Open biopsy | 33 (6.4) | 69 (36–80) | 8.4 (4.2–10.7) | 2.33 (1.39–3.91) | <.0001 |
| Stereotactic biopsy | 38 (7.4) | 65 (22–89) | 3.7 (3.1–7.8) | 3.23 (1.84–5.67) | <.0001 |
| No surgery | 28 (5.5) | 81 (68–97) | 2.4 (1.5–3.7) | 8.03 (2.89–22.32) | <.0001 |
| Age at diagnosis | |||||
| <40 y | 31 (6.0) | 32 (10–39) | 31.2 (11.8–45.0) | Reference | - |
| 40–49 y | 51 (10) | 46 (40–49) | 15.4 (11.6–21.8) | 1.45 (0.88–2.37) | .15 |
| 50–59 y | 110 (21.5) | 54 (50–59) | 14.3 (12.9–17.0) | 1.76 (1.19–2.61) | .012 |
| 60–69 y | 172 (33.6) | 65 (60–69) | 12.5 (11.2–13.9) | 2.34 (1.68–3.26) | <.0001 |
| ≥70 y | 148 (28.9) | 74 (70–97) | 6.6 (4.8–8.4) | 3.47 (2.53–4.75) | <.0001 |
Baseline characteristics of the patient population in the present study reported as observed counts and percentages, median age with range, median survival with 95% CI and hazard ratios of death.
ECOG, Eastern Cooperative Oncology Group.
Patterns of Care and Survival in a GBM Population
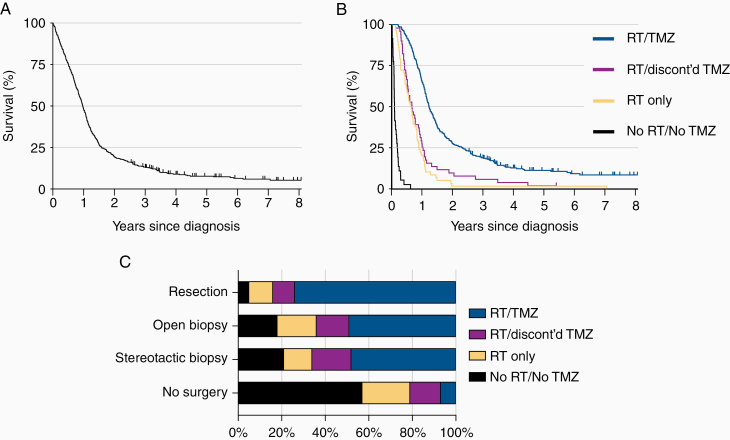
The median survival in the total population (n = 512) was 11.7 months, with 2- and 5-year survival rates of 19.3% and 7.4%, respectively (Figure 1A). The median survival times for patients with and without a tissue diagnosis were 12.3 and 2.4 months, respectively.
Figure 1.
Patterns of care and survival in a GBM population. (A) Overall survival in the whole population. Vertical line represents censored events. (B) Overall survival of pathologically confirmed GBM stratified according to postoperative oncological treatment. (C) Receipt of RT and TMZ among the patients by type of surgery. GBM, glioblastoma; RT, radiotherapy; TMZ, temozolomide.
Among patients with a histological diagnosis (n = 484), 336 patients (69.4%) completed concurrent RT/TMZ, whereas 51 (10.5%) received RT (any dose) and started, but discontinued TMZ, 58 (12.0%) received RT only (any dose), and 36 (7.5%) received no RT or TMZ. In the group where GBM was confirmed by tissue analysis, the median survival was 14.8 months (95% CI 13.8–16.4) for patients who completed concurrent RT/TMZ, 8.0 months (6.2–10.9) for patients who received RT (any dose) and started, but discontinued TMZ, 7.6 months (5.7–9.7) for patients who received RT only (any dose), and 1.1 months (0.9–1.9) for patients who did not receive any additional oncological therapy (Figure 1B, P < .0001).
Of the patients who received TMZ in the concurrent phase, 1.5% (n = 6) developed leucopenia below the predefined threshold level of 1.5 × 109/L corresponding to a grade 3 toxicity or higher, 8.3% (n = 33) developed thrombocytopenia below the predefined threshold level of 100 × 109/L corresponding to a grade 2 toxicity or higher, and 2.3% (n = 9) elevated ALT, AST or bilirubin levels above the predefined levels corresponding to a grade 2 toxicity or higher, that required discontinuation of TMZ. Further treatment and survival characteristics are outlined in Figure 1C and Supplementary Table S2.
Proportion of Patients Found Ineligible for a Phase III Trial
Phase III trials conducted in patients with newly diagnosed GBM over the last 10 years have enrolled patients at 3 different time points; (1) before the concurrent RT/TMZ (n = 5),8,10,11,14,16 (2) midway through RT/TMZ (n = 1),9 and (3) at the end/after completion of concurrent RT/TMZ (n = 4, Figure 2A).7,12,13,15 Although inclusion and exclusion criteria varied between individual trials, they broadly encompassed the same set of criteria (Supplementary Table S1). Commonly, these trials recruited patients within defined age limits (18–70 y), with a supratentorial located tumor, who underwent an open biopsy or surgical resection for tissue-based diagnosis and had a good performance status (ECOG ≤2). On the other hand, they typically excluded patients with biochemical signs of hematologic, renal, or hepatic insufficiency, other predefined disqualifying comorbidities (eg, recent intracranial bleeding or infection, decompensated heart or lung disease, history of previous malignancy), disease progression before the time of randomization and patients taking increasing or high doses of steroids (Supplementary Table S1). Based on the inclusion and exclusion criteria that were used in the majority of the reference trials,7–16 we established a list of criteria a patient had to fulfill to be considered eligible for trial recruitment at 3 different time points (Figure 2B).
Figure 2.
Time points and criteria for trial recruitment. (A) Potential time points for study enrollment during the concurrent RT/TMZ-phase (inset 1 to 3). (B) The inclusion and exclusion criteria a patient must fulfill to be considered trial participation in this study.
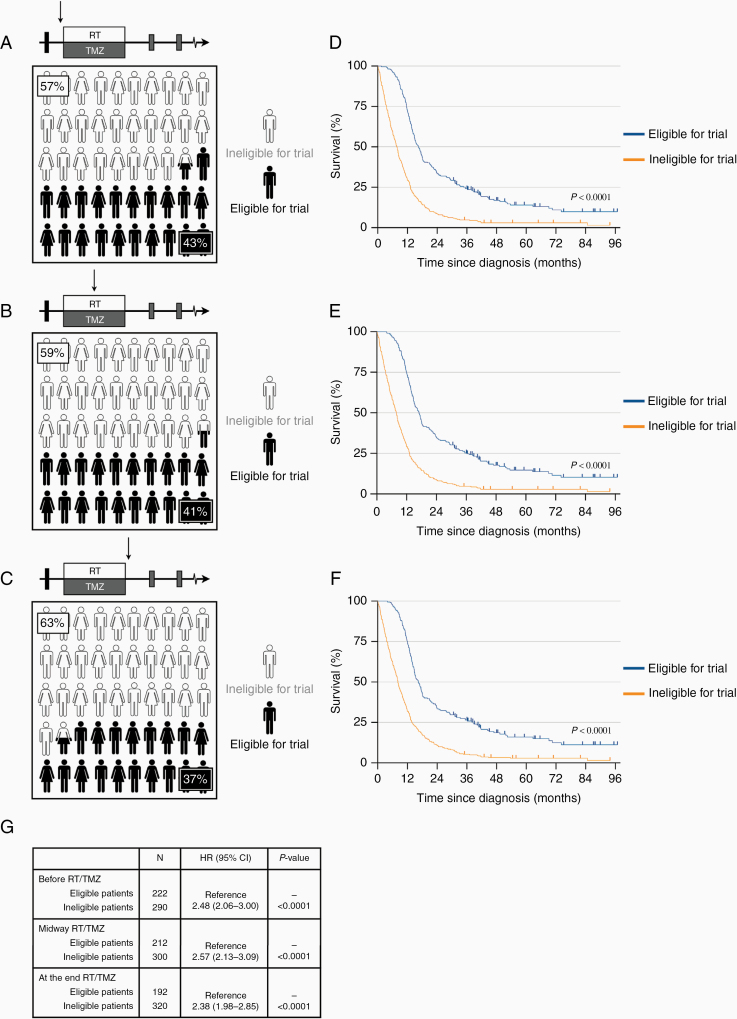
Using these criteria, we simulated enrollment before planned concurrent RT/TMZ by filtering by age (18–70), localization (supratentorial), undergone open biopsy or surgical resection, no disqualifying comorbidity, good performance status (ECOG ≤2), stable doses, or no glucocorticoids, and adequate hematologic, hepatic and renal function. Patients were considered ineligible if they failed one or more of these criteria. This selection excluded 290 (57%) of the patients from a potential clinical trial (Figure 3A, Supplementary Table S3). We next simulated potential enrollment midway through concurrent RT/TMZ treatment by adding the criterion that patients had to have started concurrent RT/TMZ treatment without signs of disease progression. With this scenario, 300 (59%) patients were ineligible for trial participation (Figure 3B). When we finally filtered by the criterion that patients had to have completed concurrent RT/TMZ, that is, simulating inclusion following completion of the concurrent radiochemotherapy phase, 320 (63%) of the patients were excluded (Figure 3C).
Figure 3.
Simulated study group derived from a GBM population. (A) When filtering patients according to the selected study criteria, 57% of patients were considered ineligible for a clinical trial before the concurrent RT/TMZ. (B) Midway through RT/TMZ, the filtering excluded 59% of the patients, and (C) 63% of the patients were considered ineligible at the end of concurrent RT/TMZ. (D–F) Survival curves of patients found eligible compared to those found ineligible. There was a significant survival advantage (P < .0001) with over 2 times increased median, 2-y and 5-y survival at all time points for the patients found eligible for trials. (G) Hazard ratios of death of the patients found ineligible compared to those found eligible. RT, radiotherapy; TMZ, temozolomide.
Survival Characteristics of the Potential Study Groups
We next compared the survival characteristics for eligible versus ineligible patients at different time points. Patients who potentially could have been included in a trial before the start of concurrent RT/TMZ had a median survival of 16.4 months, and the 2- and 5-year survival rates were 33.3% and 8.6%, respectively. Comparable data for the ineligible patients were 7.7 months, 14.0% and 3.0% (Figure 3D). Similar differences in survival were found at the two other time points for study inclusion (Figure 3E, F). The survival advantage for patients who fulfilled the eligibility criteria for trials at each time point was statistically significant (P < .0001, log-rank test). Compared to the group of patients who were considered ineligible for trials, the median, 2-year and 5-year survival rates were increased >2 times in the group of patients considered trial candidates (Figure 3D–F, Table 2).
Table 2.
Characteristics of Patients Considered Trial Eligible
| Before RT/TMZ | Midway RT/TMZ | At the end of RT/TMZ | ||||
|---|---|---|---|---|---|---|
| Eligible | Ineligible | Eligible | Ineligible | Eligible | Ineligible | |
| Median age, years | 58.0 | 69.0 | 58.0 | 68.5 | 58.0 | 68.0 |
| Male:female ratio | 1.7:1 | 1.4:1 | 1.6:1 | 1.5:1 | 1.9:1 | 1.4:1 |
| ECOG, median | 0 | 1 | 0 | 1 | 0 | 1 |
| Surgical resection, % | 98 | 67 | 98 | 68 | 98 | 70 |
| Median survival, months | 16.4 | 7.7 | 17.0 | 7.9 | 16.4 | 8.2 |
| 2-y survival, % | 33.3 | 14.0 | 34.4 | 14.7 | 33.3 | 16.0 |
| 5-y survival, % | 8.6 | 3.0 | 8.7 | 2.9 | 10.9 | 2.9 |
Characteristics of GBM patients considered to be trial eligible compared to those found ineligible for trials at different time points.
ECOG, Eastern Cooperative Oncology Group; GBM, glioblastoma.
Patient Characteristics of the Potential Study Groups
We further compared the characteristics of patients who were considered eligible for a trial to those considered ineligible. Before the start of concurrent RT/TMZ, compared to median age of 69.0 years for those found ineligible, the median age of patients who fulfilled the criteria for study enrollment was 58.0 years (P < .0001, Mann–Whitney test). Similar differences were found at both of the other time points for study enrollment (both P < .0001). In the group of patients considered eligible for trial recruitment, compared to a median ECOG of 1 among the ineligible patients, the median ECOG performance status was 0. Compared to 67% among ineligible patients, a total of 98% of patients considered trial candidates had undergone tumor resection surgery. The characteristics between the groups are outlined in Table 2.
We used data from the reference trials for external validation.7–16 Except for the survival rate, which was extracted from the control group of each trial, we utilized data from both randomized cohorts in the trials. We left out survival data from 2 trials8,16 that exclusively recruited MGMT-methylated tumors (as they have significantly better survival), and from one trial with no control group.15 We found that our data on patients (age, male to female ratio, ECOG status, a proportion that underwent surgical resection, median- and 2-year survival) considered to be candidates for trial recruitment corresponded well with the patients that de facto had been participants in clinical trials for GBM (Table 3).
Table 3.
Patient Characteristics of Trial Participants
| Reference phase 3 trials in GBM (n = 10) | |||
|---|---|---|---|
| Trial Participants | No. of Trials | References | |
| Median age of randomized cohorts, years (range) | 56.5 (54.0–59.0) | 9a | 8–16 |
| Male:female ratio (range) | 1.5:1 (1.2–2.1:1) | 10 | 7–16 |
| ECOG, median | 0-1b | 10 | 7–16 |
| Surgical resection, median % (range) | 97 (87–100) | 10 | 7–16 |
| Median survival, months (range) | 16.8 (16.0–20.0) | 7c | 7–9,11–14 |
| 2-year survival, median % (range) | 33.9 (30.1–40.0) | 5d | 7,9,11–13 |
Characteristics of trial participants extracted from the phase 3 trials used as reference trials.
ECOG, Eastern Cooperative Oncology Group.
aOne study did not report median age of the patients.
bPerformance status is reported over an interval as it was converted from KPS in 8 studies that could not discern a true median value.
cThree studies were excluded from survival calculations as 2 recruited MGMT-methylated tumors and one did not have an adequate control group.
dOnly 5 studies specifically reported 2-year survival characteristics.
Discussion
Our study provides a quantitative portrait of the patients who can be considered either eligible or ineligible for enrollment into phase III trials for newly diagnosed GBM. Our main result is that only a minority of the patients are eligible for inclusion into a typical RCT. Compared to a real-world GBM population, this minority formed a more homogeneous group consisting of younger patients with a better performance status that were more likely to have undergone tumor resection surgery. As these factors are associated with improved survival,17–19 it was not surprising that compared to those considered ineligible for trial participation, survival rates were markedly improved.
Our conclusion depends on; (1) that the study population represents a true real-world GBM population with patterns of care corresponding to that seen in recent population-based series, and (2) that our eligibility criteria result in the selection of a group of patients who has the characteristics of patients included in recent RCTs.
All patients within our defined geographical area with an intracranial lesion suspicious of GBM were referred to one hospital (OUH). Irrespective of how the diagnosis of GBM is made and the treatment modalities are applied, all patients are by law reported to the national CRN, where the diagnosis and further details of the disease are stored with the patient’s home address and unique national ID. The risk for missing individual patients in the current study was therefore small. We found an annual incidence of 3.89/100 000. The median age at diagnosis was 64 years, 26% of patients were over 70 years old and there was a slight male predominance. In addition, 2% presented with the infratentorial disease. These data are in accordance with several epidemiological series over time and across different GBM populations,22–29 indicating that our population corresponds well to established nonadjustable epidemiologic features in GBM.
We further found that the diagnosis was made without tissue examination (ie, by MRI only) in 5.5% of the cases, compared to around 5–20% in previous reports.2–4,30–32 Among the patients with a tissue diagnosis, resection surgery was performed in 80%, and 2/3 completed concurrent RT/TMZ. These levels correspond to patterns of care in other population-based series (70–90% of patients undergoing resection surgery,18,33,34 and approximately 60% receiving first-line treatment of concurrent RT/TMZ2,3,34,35), as do our data on overall, 2- and 5-year survival rates.4,5,26,35 We, thus, conclude that also the patterns of care and survival characteristics in this study are comparable to those in other recent population-based series.
When comparing the characteristics of the cohorts of patients who were included in the reference trials to the group found eligible for a typical RCT in this study, we found the median age to be 56.5 years (range 54.0–59.0) in the reference trials, and 58.0 years in the patients we found trial eligible. Compared to a median for 97% (range: 87–100%) in the reference trials, resection surgery was performed in 98% of patients considered trial candidates. Before comparing performance status before treatment, we converted performance data from all studies to the same scale (ECOG).36 Compared to a median of 0–1 in the reference trials (as several trials reported their baseline data over a range, we could not discern the true median other than over a range), the median ECOG among the patients we found eligible was 0. Lastly, depending on the different time points the median overall survival of patients we found eligible for a potential RCT ranged from 16.4–17.0 months versus 16.8 (range: 16.0–20.0) months in the reference trials. Correspondingly, the median 2-year survival ranged from 33.3–34.4% in our data versus 33.9% (30.1–40.0%) in the reference trials. Collectively, our data, therefore, indicate that the selection criteria we applied in the current study resulted in a group representative for patients typically included in a phase III trial in GBM. This substantiates our main conclusion that only a minority of patients with a newly diagnosed GBM is eligible for inclusion into a typical RCT.
The eligibility criteria applied in the reference trials were largely equivalent with respect to performance status, mode of diagnosis, physiological parameters, and disqualifying comorbidities.7–16 Although only 4 studies used 70 years as an upper age limit, enrolled patients tended to be younger also in the investigation where no such restriction was imposed.9,10,13 In line with the Stupp trial,37 we chose to use 70 years as an upper limit for potential trial inclusion. Still, we ended up with 58.0 years as the median age for trial eligible patients, compared to median age of the randomized patients ranging from 54.0 to 59.0 in the reference trials.7–16 As the median age at diagnosis in our unselected population was 64 years, we suspect that there has been a tendency to exclude older patients even in the studies where the age limit was not applied.
Phase III clinical trials are fundamental for establishing standards of care. Our data, where approximately 60% of patients are excluded from trials, suggest that current trial protocols only provide treatment guidance to a minority of the patients. As the generalizability of trial results is restricted to the population that has been studied, it remains uncertain how the majority should be treated. Unfortunately, the patients with the worst prognosis, where new treatment options are needed the most, are represented in this group.
It is well known that patients in clinical trials may be selected to such an extent that study results do not directly apply to the real-world situation.6,38 In brain tumors, skewed inclusion was described for anaplastic glioma by Macdonald and Cairncross’ group over 3 decades ago.39 Later studies evaluating survival of glioma patients described significant survival advantage of those considered eligible for experimental trials.40,41 In accordance with our data, these studies described the systematic skewness of younger patients with better performance status undergoing more extensive surgical treatment in the population considered trial eligible. Despite standards developed for brain tumor therapy trials,42,43 it remains a problem that only a minority of patients are included in such trials.
The major strengths of this study lie in the unique surveillance of GBM within a well-defined geographical area enabled by the CRN and BTD, as well as in the number of patients, the individual-level data, and that the results were derived from a real-world population setting. Moreover, the data were restricted to one health center only (OUH), thereby reducing the possible confounding effect of differences in access to health care services between health centers.
The major limitation of the study is the retrospective sampling of some of the data from individual patient records. As this introduces a degree of subjectivity primarily affecting scoring of ECOG status and patient comorbidity, 2 independent researchers went through the data of each patient. The retrospective design, moreover, cannot discern certain variables that might affect trial eligibility, such as logistical issues as well as attitudes and willingness to participate in a trial, that further exclude patients from RCTs.7–16,38 A further limitation is the lack of molecular data, especially IDH- and MGMT methylation status. This aspect is important as the stratification of patients according to molecular data will be important in future trials.
Concerns have been raised with regard to the low accrual rate of patients into neuro-oncological trials, which is estimated to be only approximately 10%.44 Barriers for trial accrual are related both to physicians (eg, awareness), and organizations/institutions (eg, infrastructure, economy).45 In addition, adjustments of eligibility criteria for trials to increase enrollment have gained increasing attention in the academic community in recent years.46,47 In this study, most patients were excluded based on age and comorbidities. With an increasingly aging population, it is expected that the number of patients with GBM will increase.5 To enroll more patients in trial accepting a wider age span, as well as allowing patients with a history of the malignant disease whose natural history or treatment does not have the potential to interfere with trial endpoints, may therefore be the two most useful criteria to increase the fraction of eligible patients.
In summary, we have provided a quantitative portrait of the selection bias of GBM patients in phase III clinical trials. We have shown that only a minority of patients in a real-world GBM population can be expected to fulfill standard eligibility in such trials and that these patients are younger, have better performance status and fewer comorbidities, and are more likely to have undergone tumor resection. Consequently, cohorts included in RCTs have improved survival compared to an unselected real-world population. This selection bias should be taken into consideration when trial results are used to guide treatment.
Supplementary Material
Acknowledgments
The authors would like to thank Sissel Reinlie and Frode Kolstad, former and present Head of the Department of Neurosurgery, and Håvard Attramadal, Director of Institute for Surgical Research, Oslo University Hospital, for supporting a great research environment. Preliminary results of this study were presented at the 24th annual meeting of The Society of Neuro-Oncology in Arizona (US) in 2019.
Funding
This study was supported by The Norwegian Cancer Society (GRANT# 144402).
Conflict of interest statement. The authors declare no potential conflicts of interest.
Authorship Statement: Conceived the study and study design: E.S., P.B., E.O.V.M., E.H., and I.A.L. Collected data: E.S., M.A.S., T.B.J., E.H., and I.A.L. Assisted in analyses: E.S., M.A.S, and I.A.L. Interpreted the data: E.S., T.B.J., P.B., E.O.V.M., E.H., and I.A.L. Wrote the manuscript: E.S., E.O.V.M., and I.A.L. All authors have seen and approved the final manuscript and submission.
References
- 1. Stupp R, Hegi ME, Mason WP, et al. . Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 2. Rønning PA, Helseth E, Meling TR, Johannesen TB. A population-based study on the effect of temozolomide in the treatment of glioblastoma multiforme. Neuro Oncol. 2012;14(9):1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yabroff KR, Harlan L, Zeruto C, Abrams J, Mann B. Patterns of care and survival for patients with glioblastoma multiforme diagnosed during 2006. Neuro Oncol. 2012;14(3):351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu P, Du XL, Lu G, Zhu J-J. Survival benefit of glioblastoma patients after FDA approval of temozolomide concomitant with radiation and bevacizumab: A population-based study. Oncotarget. 2014;5(0):44015–44031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Korja M, Raj R, Seppä K, et al. . Glioblastoma survival is improving despite increasing incidence rates: a nationwide study between 2000 and 2013 in Finland. Neuro Oncol. 2018;21(3):370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rothwell PM. External validity of randomised controlled trials: “To whom do the results of this trial apply?” The Lancet. 2005;365(9453):82–93. [DOI] [PubMed] [Google Scholar]
- 7. Gilbert MR, Wang M, Aldape KD, et al. . Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stupp R, Hegi ME, Gorlia T, et al. . Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. The Lancet Oncology. 2014;15(10):1100–1108. [DOI] [PubMed] [Google Scholar]
- 9. Gilbert MR, Dignam JJ, Armstrong TS, et al. . A randomized trial of bevacizumab for newly diagnosed glioblastoma. New Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chinot OL, Wick W, Mason W, et al. . Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 11. Westphal M, Heese O, Steinbach JP, et al. . A randomised, open label phase III trial with nimotuzumab, an anti-epidermal growth factor receptor monoclonal antibody in the treatment of newly diagnosed adult glioblastoma. Eur J Cancer. 2015;51(4):522–532. [DOI] [PubMed] [Google Scholar]
- 12. Weller M, Butowski N, Tran DD, et al. ; ACT IV trial investigators . Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18(10):1373–1385. [DOI] [PubMed] [Google Scholar]
- 13. Stupp R, Taillibert S, Kanner A, et al. . Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma. JAMA. 2017;318(23):2306–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kong DS, Nam DH, Kang SH, et al. . Phase III randomized trial of autologous cytokine-induced killer cell immunotherapy for newly diagnosed glioblastoma in Korea. Oncotarget. 2017;8(4):7003–7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liau LM, Ashkan K, Tran DD, et al. . First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med. 2018:16(142). doi: 10.1186/s12967-018-1507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herrlinger U, Tzaridis T, Mack F, et al. ; Neurooncology Working Group of the German Cancer Society . Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Lancet. 2019;393(10172):678–688. [DOI] [PubMed] [Google Scholar]
- 17. Laws ER, Parney IF, Huang W, et al. ; Glioma Outcomes Investigators . Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99(3):467–473. [DOI] [PubMed] [Google Scholar]
- 18. Helseth R, Helseth E, Johannesen TB, et al. . Overall survival, prognostic factors, and repeated surgery in a consecutive series of 516 patients with glioblastoma multiforme. Acta Neurol Scand. 2010;122(3):159–167. [DOI] [PubMed] [Google Scholar]
- 19. Brown TJ, Brennan MC, Li M, et al. . Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncology. 2016;2(11):1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shahar T, Nossek E, Steinberg DM, et al. . The impact of enrollment in clinical trials on survival of patients with glioblastoma. J Clin Neurosci. 2012;19(11):1530–1534. [DOI] [PubMed] [Google Scholar]
- 21. Larsen IK, Småstuen M, Johannesen TB, et al. . Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer. 2009;45(7):1218–1231. [DOI] [PubMed] [Google Scholar]
- 22. Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109(1):93–108. [DOI] [PubMed] [Google Scholar]
- 23. Bauchet L, Mathieu-Daudé H, Fabbro-Peray P, et al. . Oncological patterns of care and outcome for 952 patients with newly diagnosed glioblastoma in 2004. Neuro Oncol. 2010;12(7):725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gramatzki D, Dehler S, Rushing EJ, et al. . Glioblastoma in the Canton of Zurich, Switzerland revisited: 2005 to 2009. Cancer. 2016;122(14):2206–2215. [DOI] [PubMed] [Google Scholar]
- 25. Ostrom QT, Bauchet L, Davis FG, et al. . The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 2014;16(7):896–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ostrom QT, Cioffi G, Gittleman H, et al. . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019;21(Supplement_5):v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stark AM, van de Bergh J, Hedderich J, Mehdorn HM, Nabavi A. Glioblastoma: clinical characteristics, prognostic factors and survival in 492 patients. Clin Neurol Neurosurg. 2012;114(7):840–845. [DOI] [PubMed] [Google Scholar]
- 28. Adams H, Chaichana KL, Avendaño J, Liu B, Raza SM, Quiñones-Hinojosa A. Adult cerebellar glioblastoma: understanding survival and prognostic factors using a population-based database from 1973 to 2009. World Neurosurg. 2013;80(6):e237–e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cho HJ, Zhao J, Jung SW, et al. . Distinct genomic profile and specific targeted drug responses in adult cerebellar glioblastoma. Neuro Oncol. 2019;21(1):47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Climans SA, Ramos RC, Laperriere N, Bernstein M, Mason WP. Outcomes of presumed malignant glioma treated without pathological confirmation: a retrospective, single-center analysis. Neurooncol Pract. 2020;7(4):446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dressler EV, Liu M, Garcia CR, et al. . Patterns and disparities of care in glioblastoma. Neurooncol Pract. 2019;6(1):37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gulati S, Jakola AS, Johannesen TB, Solheim O. Survival and treatment patterns of glioblastoma in the elderly: a population-based study. World Neurosurg. 2012;78(5):518–526. [DOI] [PubMed] [Google Scholar]
- 33. Scoccianti S, Magrini SM, Ricardi U, et al. . Patterns of care and survival in a retrospective analysis of 1059 patients with glioblastoma multiforme treated between 2002 and 2007: a multicenter study by the Central Nervous System Study Group of Airo (Italian Association of Radiation Oncology). Neurosurgery. 2010;67(2):446–458. [DOI] [PubMed] [Google Scholar]
- 34. Hansen S, Rasmussen BK, Laursen RJ, et al. . Treatment and survival of glioblastoma patients in Denmark: The Danish Neuro-Oncology Registry 2009–2014. J Neuro-Oncol. 2018;139(2):479–489. [DOI] [PubMed] [Google Scholar]
- 35. Fabbro-Peray P, Zouaoui S, Darlix A, et al. . Association of patterns of care, prognostic factors, and use of radiotherapy–temozolomide therapy with survival in patients with newly diagnosed glioblastoma: a French national population-based study. J Neuro-Oncol. 2019;142(1):91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ma C, Bandukwala S, Burman D, et al. . Interconversion of three measures of performance status: an empirical analysis. Eur J Cancer. 2010;46(18):3175–3183. [DOI] [PubMed] [Google Scholar]
- 37. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 38. Kennedy-Martin T, Curtis S, Faries D, Robinson S, Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials. 2015;16:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Winger MJ, Macdonald DR, Schold SC Jr, Cairncross JG. Selection bias in clinical trials of anaplastic glioma. Ann Neurol. 1989;26(4):531–534. [DOI] [PubMed] [Google Scholar]
- 40. Florell RC, Macdonald DR, Irish WD, et al. . Selection bias, survival, and brachytherapy for glioma. J Neurosurg. 1992;76(2):179–183. [DOI] [PubMed] [Google Scholar]
- 41. Kirby S, Brothers M, Irish W, et al. . Evaluating glioma therapies: modeling treatments and predicting outcomes. J Natl Cancer Inst. 1995;87(24):1884–1888. [DOI] [PubMed] [Google Scholar]
- 42. Perry JR, DeAngelis LM, Schold SC Jr, et al. . Challenges in the design and conduct of phase III brain tumor therapy trials. Neurology. 1997;49(4):912–917. [DOI] [PubMed] [Google Scholar]
- 43. Chang SM, Reynolds SL, Butowski N, et al. . GNOSIS: guidelines for neuro-oncology: standards for investigational studies-reporting of phase 1 and phase 2 clinical trials. Neuro Oncol. 2005;7(4):425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vanderbeek AM, Rahman R, Fell G, et al. . The clinical trials landscape for glioblastoma: is it adequate to develop new treatments? Neuro Oncol. 2018;20(8):1034–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee EQ, Chukwueke UN, Hervey-Jumper SL, et al. . Barriers to accrual and enrollment in brain tumor trials. Neuro Oncol. 2019;31(5):536–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim ES, Bruinooge SS, Roberts S, et al. . Broadening eligibility criteria to make clinical trials more representative: american society of clinical oncology and friends of cancer research joint research statement. J Clin Oncol. 2017;35(33):3737–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee EQ, Weller M, Sul J, et al. . Optimizing eligibility criteria and clinical trial conduct to enhance clinical trial participation for primary brain tumor patients. Neuro Oncol. 2020;22(5):601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.