Abstract
Syzygium aromaticum has a diversity of biological activities due to the chemical compounds found in its plant products such as total phenolic compounds and flavonoids. The present work describes the chemical analysis and antimicrobial, antioxidant, and antitrypanosomal activity of the essential oil of S. aromaticum. Eugenol (53.23%) as the major compound was verified by gas chromatography-mass spectrometry. S. aromaticum essential oil was more effective against S. aureus (MIC 50 μg/mL) than eugenol (MIC 250 μg/mL). Eugenol presented higher antioxidant activity than S. aromaticum essential oil, with an EC50 of 12.66 and 78.98 µg/mL, respectively. S. aromaticum essential oil and eugenol exhibited Trypanosoma cruzi inhibitory activity, with IC50 of 28.68 ± 1.073 and 31.97 ± 1.061 μg/mL against epimastigotes and IC50 of 64.51 ± 1.658 and 45.73 ± 1.252 μg/mL against intracellular amastigotes, respectively. Both compounds presented low cytotoxicity, with S. aromaticum essential oil displaying 15.5-fold greater selectivity for the parasite than the cells. Nitrite levels in T. cruzi-stimulated cells were reduced by essential oil (47.01%; p = 0.002) and eugenol (48.05%; p = 0.003) treatment. The trypanocidal activity of S. aromaticum essential oil showed that it is reasonable to use it in future research in the search for new therapeutic alternatives for trypanosomiasis.
1. Introduction
The clove (Syzygium aromaticum) belongs to the family Myrtaceae originating from the Maluku Islands, in eastern Indonesia. It is a tree up to 12 meters high that can be grown in coastal areas at high altitudes, and its best known part is the flower buds that are produced after four years of planting and collected before flowering [1, 2]. The main chemotype of flower buds essential oil is its major compound, eugenol, a volatile phenylpropanoid widely used in the pharmaceutical industry. It also contains eugenol acetate, β-caryophyllene, and humulene [3]. S. aromaticum has demonstrated antiviral activity against food-borne viruses [4], antimicrobial activity, with low concentrations able to inhibit bacteria growth [5], and effectiveness in the treatment of bacterial infections [6].
Infectious diseases can be caused by several species, with the main pathogens related to bacteria. These infections are a serious problem in public health, due to their direct impact on society. They are caused by pathogenic microorganisms that invade the host organism, overcome its defenses, and cause tissue damage [7]. A further concern is the high rates of resistance of microorganisms, which occur in diseases such as food poisoning, one of the most common causes of death in developing countries. Most reports are associated with bacterial contamination, which requires the development of new antimicrobial agents with the ability to interfere in various activities of the bacterial cell [8].
Other diseases that have attracted attention due to their impact on public health are those caused by protozoa, such as Chagas disease, caused by the protozoan Trypanosoma cruzi. T. cruzi is a parasite with continental dispersion present in 21 countries in Latin America. There is a worldwide estimate of around 6 to 7 million infections and approximately 75 million living in endemic areas at risk of infection [9]. Nifurtimox and benznidazole are the only treatments used in patients with Chagas disease, causing about 40% of side effects in adult patients, making treatment adherence difficult [9–11]. There are still cases of strains resistant to these treatments [12].
Natural products have a chemical composition rich in secondary metabolisms, which play an important role in plant physiology. They have a variety of potential biological benefits, such as antioxidant, anti-inflammatory, anticancer, antibacterial, and antifungal activities, which act as its defense mechanism against predatory microorganisms. In addition, there are currently hundreds of drugs based on active compounds isolated from plants [13]. Products of plant origin have been described throughout history as therapeutic resources and used in several nations, with ancient civilizations using plant food and other substances as medicine. Their experiments with herbs resulted in both successes and failures, with the latter being associated with serious adverse effects. The discovery of the useful and harmful properties of plants helps in their use [14]. Plants are no longer merely a therapeutic option for needy populations; they have become a promising source of new molecules of therapeutic interest. Therefore, many species still need to be investigated.
In view of the aforementioned research, the use of natural origin products as antimicrobials was evident. The exploration of the biological activity of chemical substances in medicinal plants constitutes a potential form of alternative disease control, generating the development of models that make the sustainable production systems and the conservation of biodiversity and natural resources feasible. Thus, this research aimed to study the chemical properties of the essential oil of S. aromaticum, as well as verifying its possible antimicrobial, antioxidant, and antitrypanosomal properties.
2. Materials and Methods
2.1. Reagents
Anhydrous sodium sulfate, ethanol, ethyl acetate, dimethyl sulfoxide (DMSO), eugenol, 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), penicillin, streptomycin, N-benzyl-2-nitro-1H-imidazole-1-acetamide (benznidazole), Brewer thioglycollate medium, RPMI 1640 medium, 3-(4,5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT), sulfanilamide, H3PO4, N-(1-naphthyl)ethylenediamine, and sodium nitrite were purchased from Sigma, St Louis, MO, USA. Giemsa's azur-eosin-methylene blue, Brain Heart Infusion broth, Mueller–Hinton agar, and Mueller–Hinton broth were purchased from MERK, Darmstadt, Germany. Fetal bovine serum (FBS) was purchased from Gibco, Gaithersburg, MD, USA.
2.2. Plant Material
The floral buds of S. aromaticum were purchased from the central market of the city of São Luís, Maranhão, Brazil (2° 31′ 48″ S, 44° 18′ 10″ W). For the extraction of oil, dry flower buds were selected in an oven with air circulation at 37 °C/48 h, and soon afterwards they were sprayed in a knife mill in the Physics-Chemistry Laboratory of the Food and Water Quality Control Program (PCQA), Federal University of Maranhão.
2.3. Essential Oil Extraction
The extraction of the essential oil of S. aromaticum was carried out with 300 g of the ground vegetable product, and it was diluted in water in the proportion of 1 : 10 by hydrodistillation using the Clevenger system for 3h at 100 °C. The essential oil collected was dried with anhydrous sodium sulfate (Na2SO4), and the final volume found was used to determine the yield through the mass/volume ratio by measuring the density. Mass/volume ratios were calculated from the mass (g) of the initial vegetal material and the volume (mL) of essential oil obtained after extraction. The essential oil samples were kept at 25°C and then weighed. For the verification of biological activity in vitro, the essential oil and the reference drugs were diluted in DMSO, and subsequently serial dilutions were made in an appropriate culture medium until reaching a final concentration below 1% DMSO.
2.4. Physical-Chemical Analysis of Essential Oil
Some physical-chemical analyses were performed on the essential oil of S. aromaticum for density measured with a glass pycnometer, refractive index calculated with ABBE 2WAJ refractometer (PCE Instruments, Southampton, United Kingdom), color and appearance that were visually verified by three different people, and determination of solubility that is carried out through the ratio of 1: 1 of oil and ethanol 80% until its complete solubilization.
2.5. Gas Chromatography-Mass Spectrometry (GC-MS)
The chemical characterization of the essential oil of the floral buds of S. aromaticum was determined by gas chromatograph coupled to Shimadzu QP5000 mass spectrometer (Shimadzu, Kyoto, Japan), equipped with a capillary column ZB-5ms (5% phenyl arylene) 95% dimethylpolysiloxane, with HP 5MS electronic impact detector of 70 eV (40-500 Da) and transfer temperature of 280°C. Aliquots were injected in splitless mode with a volume of 0.3 µL in ethyl acetate (automatic injector CP-8410), fixing the following conditions: high purity helium as carrier gas; injector temperature maintained at 280°C, split mode (1 : 10); followed by an initial temperature of 40°C and a final temperature of 300°C, an initial time of 5 min and a final time of 7.5 min at 8/min.
2.6. Antioxidant Assay
The determination of antioxidant activity was carried out according to the methodology suggested by Re et al., with modifications [15]. The test was carried out with a radical of ABTS which was prepared by the reaction of 5.0 mL of a 3.840 μg/mL solution of ABTS with 88 µL of the 37.840 μg/mL potassium persulfate solution. The mixture was left in the dark at room temperature for 16 hours. Immediately after mixing, it was diluted in ethanol until an absorbance of 0.7 at 734 nm was obtained. In a dark environment, an aliquot of 30 µL of each concentration of essential oil (200 to 15 μg/mL) and eugenol (90 to 5 μg/mL) was transferred in test tubes containing 3.0 mL of the radical cation ABTS and homogenized in a tube shaker, and after 6 minutes the absorbance of the reaction mixture was read on a spectrophotometer at a length of 734 nm. The tests were performed in triplicate, and the capture of the free radical was expressed as a percentage of inhibition (% I) of the ABTS radical cation according to the following equation: % inhibition = (absorbance of the solution of the ABTS radical - absorbance of the sample)/(ABTS absorbance radical solution) × 100 [16]. It was also determined that the efficient concentration or EC50% represents the concentration of the sample necessary to sequester 50% of the ABTS root, in which the essential oil will be considered active when it has EC50% < 500 μg/mL [17].
2.7. Bacterial Strains and Culture Conditions
The tests were carried out at the Microbiology Laboratory of Food and Water Quality Control at the Federal University of Maranhão (PCQA-UFMA). Microbial strains from the American Type Culture Collection (ATCC), Escherichia coli (ATCC 25922), Staphylococcus aureus (ATCC 12600), and Pseudomonas aeruginosa (ATCC 27853), were tested at a cellular concentration of 108 UFC/mL following the McFarland scale, recommended by the Clinical and Laboratory Standards Institute [18].
2.8. Antimicrobial Assays
The disk diffusion test was performed using the 75 μL inoculum of the microbial suspension (1.5 × 108 CFU/mL) seeded on Mueller–Hinton agar. Soon after, the disks containing 50 µL of essential oil and eugenol were fixed on the agar surface. The plates were incubated in a bacteriological oven at 35°C for 24 hours. The diameters of the inhibition halos were measured, including the disk diameter, in triplicate [19]. After checking the antimicrobial potential on the strains tested by the disk diffusion method, the oil was subjected to the determination of the minimum inhibitory concentration (MIC), in which an aliquot of the essential oil of S. aromaticum and eugenol was transferred to a test tube containing Mueller–Hinton broth. Then, serial dilutions were performed, resulting in concentrations of 1000, 500, 250, 200, 100, 75, 50, 25, 15, and 5 μg/mL. Microbial suspensions containing 1.5 × 108 CFU/mL were added at each concentration and incubated at 35°C for 24 h. After the incubation period, the MIC of the oil was determined, being defined as the lowest concentration that visibly inhibited bacterial growth (absence of visible turbidity) [18].
2.9. Parasites
Trypomastigote forms of Trypanosoma cruzi (SC2005 strain) were obtained from Vero cells infected and used to infect the macrophages. Epimastigote forms originated from the suspension of cell culture trypomastigotes in 3 mL of LIT medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL of penicillin, and 100 μg/mL of streptomycin and were incubated in an oven at 28°C until complete differentiation of parasites.
2.10. Antiepimastigote Assay
Epimastigote forms of T. cruzi (106 parasites/mL) from a 2–4-day-old culture were added to 96-well plates, followed by the addition of different concentrations of either S. aromaticum essential oil or eugenol (1000–7.81 µg/mL), obtained by serial dilutions (1 : 2), at a final volume of 100 µL per well, for 72 hours. The controls were identified as follows: blank (wells without parasites), untreated control (parasites and DMSO 1%), and reference drug (benznidazole). With the aid of the hemocytometer and light microscopy, viability was evaluated by counting parasites, and the results were used to calculate the IC50 (50% inhibition of parasite growth) following the formula: IC50 = (sample counting)/(control counting) × 100 [20].
2.11. Animals and Ethical Statements
Animals were purchased from the Institute of Science and Technology in Biomodels of the Oswald Cruz Foundation. All procedures involving female 4–6-week-old BALB/c mice were performed in accordance with the National Council for the Control of Animal Experimentation (CONCEA) and approved by the Ethics Committee on Animal Care and Utilization (CEUA/IOC-L018/2018).
2.12. Obtaining Peritoneal Macrophages and Cell Culture
Peritoneal macrophages were obtained from BALB/c mice, elicited for 72 hours with 3 mL 3% Brewer thioglycollate medium, and maintained in RPMI 1640 medium supplemented with 10% FBS, 100 U/mL of penicillin, and 100 µg/mL of streptomycin, overnight at 37°C and 5% CO2.
2.13. Cytotoxicity Assay
Different concentrations, obtained by serial dilutions (1 : 2), of essential oils (1000–7.8 μg/mL) or benznidazole (200–0.78 μg/mL) up to a final volume of 100 μL per well were placed in 96-well plates with peritoneal macrophages (5 × 105 cells/mL). The controls were categorized as blank (wells with culture medium without cells), untreated control (cells and DMSO 1%), and reference drug (benznidazole). After 24 h, the cell viability was analyzed by the MTT colorimetric method [21]. Absorbance was measured in a spectrophotometer at 570 nm wavelength. The concentration inhibiting 50% of cell growth (CC50) was calculated following the formula: CC50 = (sample absorbance-blank absorbance)/(control absorbance-blank absorbance) × 100 [22].
2.14. Activity against Intracellular Amastigotes and Selectivity Index (SI)
Initially, trypomastigote forms of T. cruzi from cultured Vero cells were obtained in vitro. BALB/c peritoneal macrophages, cultured in 24-well plates (5 × 105 cells/well) with coverslips, were infected with trypomastigotes using the ratio of parasite/cell 10 : 1, at 37°C and 5% CO2 for 6 h. Afterwards, well plates were washed with phosphate-buffered saline (PBS, pH 7.2) to remove the noninternalized parasites. The infected cells were treated with different concentrations of S. aromaticum essential oil or eugenol (200–12.5 µg/mL) for 24 h. For light microscopy analysis, the coverslips with the infected and treated cells were fixed with Bouin and stained with Giemsa's azur-eosin-methylene blue. The IC50 was calculated from the total of intracellular amastigotes from 100 cells. The mean number of amastigotes per cell was obtained from the number of intracellular amastigotes in 100 cells divided by the number of infected cells. Benznidazole was used as the reference drug. The selectivity index (SI) was obtained from the ratio between cytotoxicity of BALB/c peritoneal macrophages and activity against intracellular amastigote (SI = CC50/IC50) [23].
2.15. Nitrite Quantification
BALB/c peritoneal macrophages (5 × 106 cells/mL) were treated with S. aromaticum essential oil (200 µg/mL) or eugenol (200 µg/mL) and either stimulated or not stimulated with T. cruzi trypomastigotes (5 × 107 parasites/mL). After 48 hours of incubation, the supernatant of cultures was collected and the analysis of nitrite quantification was carried out with Griess reagent. Briefly, in 96-well plates, 50 µL of culture supernatant and 50 µL of Griess reagent were added (25 µL of sulfanilamide 1% in 2.5% H3PO4 solution and 25 µL of N-(1-naphthyl)ethylenediamine 0.1% solution), followed by incubation in a dark environment for 10 minutes, and read at 570 nm on the spectrophotometer. The nitrite values were obtained from the standard curve of sodium nitrite (100–1.5 µM) [24].
2.16. Statistical Analysis
The numerical results from at least two independent assays were expressed as mean ± standard deviation, and the IC50 and CC50 determination were performed with the GraphPad Prism 7.00 software package (GraphPad Software, San Diego, CA, USA). The Mann–Whitney test was used to analyze the results, and the difference at p < 0.05 was considered as significant.
3. Results
3.1. Chemical Composition of S. aromaticum Essential Oil
The essential oil presented a yield of 3.8%, a density of 0.989 g/mL at 25°C, and a refractive index (ND 25) of 1.595. It was soluble in 90% (v/v) ethanol at a ratio of 1 : 2 and exhibited a transparent yellow color with a clear appearance in all samples. Chemical compounds in S. aromaticum essential oil evaluated by GC-MS are shown in Figure 1. Five major compounds were determined in the S. aromaticum essential oil and enumerated in order of elution and retention time. The major constituent was eugenol, representing 52.53% (Table 1).
Figure 1.
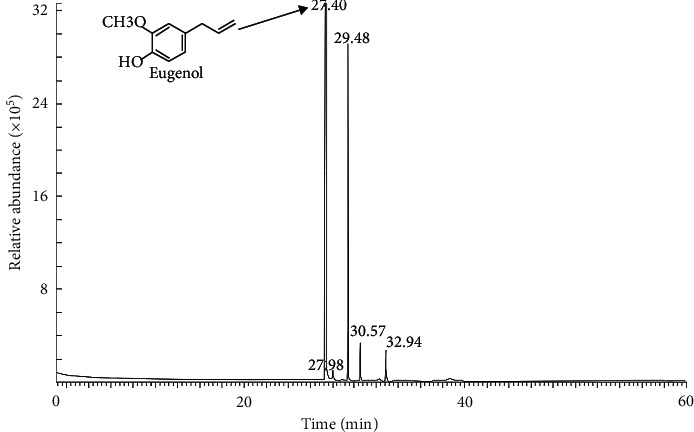
Chromatogram of Syzygium aromaticum essential oil.
Table 1.
Chemical composition of Syzygium aromaticum essential oil.
| Peak | Compounds | RT (min)1 | Kovats index | PA (%)2 |
|---|---|---|---|---|
| 1 | Eugenol | 27.40 | 1389 | 52.53 |
| 2 | Copaene | 27.98 | 1368 | 2.05 |
| 3 | Caryophyllene | 29.48 | 1538 | 37.25 |
| 4 | Humulene | 30.57 | 1498 | 4.11 |
| 5 | Eugenyl acetate | 32.94 | 1515 | 4.05 |
1Retention time. 2Peak area percentage in relation to peak total area.
3.2. Antioxidant Activity of S. aromaticum Essential Oil
Syzygium aromaticum essential oil and eugenol presented concentration-dependent antioxidant activity, as observed in the graph that relates S. aromaticum essential oil and eugenol concentration to the percentage of inhibition of the ABTS radical (Figure 2). The calculated EC50 was 78.98 µg/mL for S. aromaticum essential oil and 12.66 µg/mL for eugenol.
Figure 2.
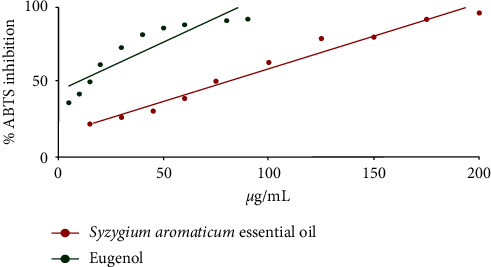
Inhibition of the ABTS radical by Syzygium aromaticum essential oil and eugenol.
3.3. Antimicrobial Activity of S. aromaticum Essential Oil
To assess antimicrobial activity, the disk diffusion test was performed. When checking the diameter of inhibition halo against Gram-positive (S. aureus) and Gram-negative (E. coli and P. aeruginosa) bacteria, the essential oil and eugenol showed a greater halo against S. aureus (Figure 3). The MIC assay revealed the significant antimicrobial activity of S. aromaticum essential oil, showing a similar effect against E. coli and P. aeruginosa and being more effective against S. aureus than eugenol (Table 2).
Figure 3.
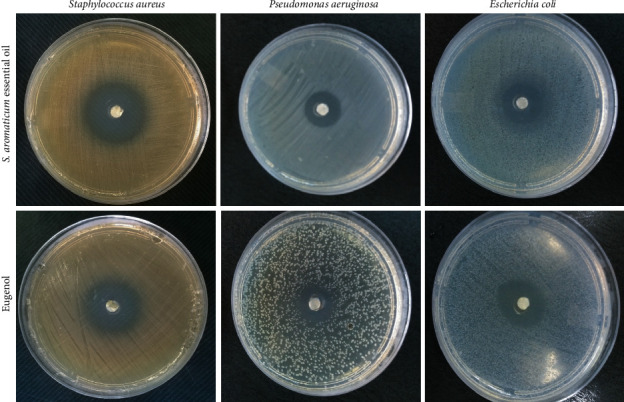
Inhibition zones of Syzygium aromaticum essential oil and eugenol on different bacterial cultures after 24 hours of treatment.
Table 2.
Inhibitory zone diameters and minimum inhibitory concentration of Syzygium aromaticum essential oil and eugenol on different bacterial cultures after 24 hours of treatment.
| Antimicrobial assay | Compounds | Bacteria strain | ||
|---|---|---|---|---|
| E. coli | S. aureus | P. aeruginosa | ||
| Inhibition zones (mm) | S. aromaticum | 22.67 ± 0.577a | 25.00 ± 1.000a | 14.00 ± 1.000a |
| Eugenol | 20.33 ± 0.577a | 22.33 ± 0.577b | 11.00 ± 1.000b | |
| Gentamycin | 13.33 ± 1.155b | 20.33 ± 0.577b | 16.67 ± 0.577c | |
| Ampicillin | 13.67 ± 0.577b | — | 8.33 ± 1.155d | |
|
| ||||
| MIC (μg/mL) | S. aromaticum | 100.0 ± 0.00a | 50.0 ± 0.00a | 200.0 ± 0.00a |
| Eugenol | 100.0 ± 0.00a | 250.0 ± 0.00b | 200.0 ± 0.00a | |
| Gentamycin | 16.00 ± 0.00b | 8.00 ± 0.00c | – | |
| Polymyxin B | — | — | 16.00 ± 0.00b | |
MIC: minimum inhibitory concentration. Data represent mean ± standard deviation of experiment carried out in triplicate. Different letters in the same column in each antimicrobial assay mean statistical difference between groups.
3.4. Antitrypanosomal Activity, Cytotoxicity, and Selective Index of S. aromaticum Essential Oil
The inhibitory concentrations of S. aromaticum essential oil and eugenol against epimastigotes and intracellular amastigotes of T. cruzi are presented in Table 3. Both compounds presented concentration-dependent inhibitory activity, presenting differences in growth inhibition on epimastigote forms only at 7.8 μg/mL (p = 0.0321) (Figure 4(a)). The similar inhibition activity of S. aromaticum essential oil and eugenol was evidenced by the absence of statistical differences between their IC50 values. In contrast, activity against intracellular amastigotes showed slightly larger differences in S. aromaticum essential oil and eugenol, with eugenol exhibiting greater activity than essential oil at the highest analyzed concentration (p = 0.0286) (Figure 4(b)). Compared to epimastigote activity, both compounds resulted in a reduction in intracellular amastigote inhibition, with S. aromaticum essential oil displaying a 2.2-fold increase in IC50 value against intracellular amastigotes compared to the IC50 against epimastigote forms.
Table 3.
Antitrypanosomal activity, BALB/c peritoneal macrophage cytotoxicity, and selectivity index of Syzygium aromaticum essential oil.
| Compound | Trypanosoma cruzi IC50 (μg/mL) | Peritoneal macrophage CC50 (μg/mL) | SI | |
|---|---|---|---|---|
| Epimastigote | Intracellular amastigote | |||
| S. aromaticum essential oil | 28.68 ± 1.073a | 64.51 ± 1.658a | >1000a | >15.5a |
| Eugenol | 31.97 ± 1.061a | 45.73 ± 1.252b | 292.7 ± 1.229b | 6.4b |
| Benznidazole | 1.950 ± 1.066b | 0.4726 ± 1.163c | 187.2 ± 1.125c | 396.1c |
IC50: inhibitory concentration for 50% of parasites; CC50: cytotoxic concentration for 50% of cells; SI: selectivity index, obtained from ratio CC50/IC50 intracellular amastigote. Data represent mean ± standard deviation of at least two independent experiments carried out in triplicate. Different letters in the same column mean statistical difference between groups.
Figure 4.

Dose-response curve of antitrypanosomal activity of Syzygium aromaticum essential oil and eugenol. Activity against epimastigotes (a) and intracellular amastigote of Trypanosoma cruzi (b) after 72 and 24 hours of treatment, respectively. Data represent mean ± standard deviation of the experiment realized in triplicate; ∗p < 0.05 when compared by Mann–Whitney test.
Cytotoxicity assay revealed that S. aromaticum essential oil did not exhibit toxicity for BALB/c peritoneal macrophages even at the highest concentration analyzed (2,000 μg/mL). Eugenol displayed greater cytotoxicity than S. aromaticum essential oil, directly influencing its selectivity index. Thus, although eugenol exhibited better activity against intracellular amastigotes, S. aromaticum essential oil exhibited a higher SI value (Table 3).
The parameters of infection analysis (Figure 5) showed that S. aromaticum essential oil treatment significantly reduced the number of amastigotes per 100 cells at 200 μg/mL (p = 0.0068) and 100 μg/mL (p = 0.0460) (Figure 5(a)), while eugenol reduced the number of amastigotes per 100 cells (p = 0.0095, Figure 5(b)) and the mean number of amastigotes per infected cells (p = 0.0112, Figure 5(d)) at 200 μg/mL. The reductions in intracellular amastigotes of T. cruzi after treatment with S. aromaticum essential oil and eugenol can be seen in Figure 5(e).
Figure 5.

BALB/c peritoneal macrophages infected with Trypanosoma cruzi and treated with Syzygium aromaticum essential oil or eugenol for 24 hours. Parameters of infection (a-d) and light microscopy (e). Intracellular amastigotes (black arrows) and remains of amastigotes (red arrows) inside macrophages. The images and data (mean ± standard deviation) represent two independent experiments performed in quadruplicate. ∗p < 0.05 and ∗∗p < 0.01 when compared with the untreated control group by the Mann–Whitney test. Giemsa, 40x objective.
3.5. S. aromaticum Essential Oil Induces Nitrite Levels Reduction in T. cruzi-Infected Peritoneal Macrophages
Nitrite levels were measured in the supernatant of BALB/c peritoneal macrophages and showed a decrease in cells treated with S. aromaticum essential oil (0.22 ± 0.067 μM NaNO2) and eugenol (0.32 ± 0.155 μM NaNO2), with reductions of 57.69% and 38.46%, respectively, when compared to the untreated cells (0.52 ± 0.224 μM NaNO2), although these reductions were not statistically significant. However, a significant reduction in the nitrite levels of cells stimulated with T. cruzi and treated with S. aromaticum essential oil (0.71 ± 0.123 μM NaNO2, p = 0.002) and eugenol (0.69 ± 0.126 μM NaNO2, p = 0.003) was observed, with reductions of 47.01% and 48.05% respectively, when compared to stimulated and untreated cells (1.34 ± 0.152 μM NaNO2) (Figure 6).
Figure 6.

Nitrite quantification in the supernatant of the BALB/c peritoneal macrophage treated with Syzygium aromaticum essential oil (200 g/mL) or eugenol (200 g/mL) and stimulated or not with Trypanosoma cruzi. Data represent mean ± standard deviation of experiment realized in sextuplicate; ∗∗p < 0.01, ∗∗∗p < 0.001 when compared with untreated and unstimulated macrophages or, between brackets, with the Mann–Whitney test.
4. Discussion
This research studied the chemical composition and antimicrobial, antioxidant, and antitrypanosomal activity of S. aromaticum essential oil, describing for the first time its activity against intracellular amastigotes of T. cruzi and its inhibitory effect on T. cruzi-stimulated peritoneal macrophages. The physical-chemical parameters (color, appearance, solubility, density, and refractive index) were evaluated to ensure the integrity and quality of the essential oil, as environmental factors (light, heat, air, and humidity) can influence the change in the chemotype of plant species. The physical characteristics of S. aromaticum essential oil were similar to the patterns described in previous studies [25].
Numerous studies have identified and quantified similar chemical compounds in the essential oils of S. aromaticum, revealing that this species has a similar chemotype to eugenol-rich chemotype. Eugenol was identified as a major compound (90.3%) in an essential oil extracted from the south of Brazil, in addition to β-caryophyllene (4.83%) and eugenol acetate (1.87%) [26], while another study, also on essential oil of cloves from the south of Brazil, observed the presence of eugenol (56.06%) and caryophyllene (39.63%) in greater quantities [27], very similar to the amount found in the present study. S. aromaticum essential oils obtained in China [28] and Italy [29] also had eugenol as their major compound, with 90.84% and 77.9%, respectively. The amount of eugenol contained in the essential oil and the difference between the compounds can be directly related to the different geographic areas where the plant has grown up, which can be influenced or changed by biotic and abiotic factors such as seasonality, stage of development, age of the plant, and climatic conditions [30]. In addition, the extraction method used to obtain the oil can also affect its chemical composition, as distillation and storage conditions are capable of influencing the content of its volatile metabolites [31]. Differences in chemical composition can be directly related to pharmacological properties, as noted in the antimicrobial activity tests in the present study.
S. aromaticum essential oil was active against standard strains of Gram-negative and Gram-positive bacteria, showing inhibition halo ranges between 14 and 25 mm by the disk diffusion test. These results agree with data from literature, in which a clove essential oil of more than 90% eugenol was more efficient against Gram-negative bacteria but showed smaller halos than those in our research for Gram-positive bacteria [26]. Another eugenol-rich clove essential oil presented inhibition zones of 28.3 and 28.1 mm for S. aureus and E. coli, respectively, indicating the susceptibility of the bacteria to the essential oil [27]. The study by Cimanga et al. [32] used inhibition halo size (IHS) to classify antimicrobial activity as follows: IHS≥15 mm strong inhibition; 10≤IHS <15 moderate inhibition; and IHS <10 inactive. When the results of the present study are compared with literature, a pattern can be observed, with S. aromaticum essential oil being considered as strong inhibitor for S. aureus and E. coli and a moderate inhibitor for P. aeruginosa.
The inhibition halos induced by S. aromaticum essential oil were higher than eugenol standard. It has been reported that essential oil possesses antimicrobial activity due to the presence of eugenol, which is described as the most important component of cloves, but such activity has also been found to be due to other phenolic compounds [33, 34]. Our results make it evident that the biocomplex is responsible for such activity, reinforcing the results of MIC determination.
The MIC of S. aromaticum essential oil displayed values between 50 and 200 μg/mL. Holetz et al. [35] classified samples with MIC values below 100 μg/mL as presenting good antibacterial activity, from 100 to 500 μg/mL as moderate activity, and from 500 to 1000 μg/mL as weak activity and those above 1000 μg/mL as inactive. Based on this, the essential oil analyzed in this study exhibited good inhibitory activity against the S. aureus strain and moderate activity against E. coli and P. aeruginosa. Eugenol demonstrated moderate activity against all the strains in the present study. When our data was compared with previous work, it was observed that a Brazilian eugenol-rich clove essential oil had MIC value of 60 μg/mL for the E.coli and S. aureus strain [36], a result close to that found in our study for the Gram-positive strain, while a clove essential oil with more than 90% eugenol resulted in MIC of 1,318 μg/mL for E.coli [29]. Eugenol was analyzed against an E.coli strain and exhibited 3,284 μg/mL [37] and 1,600 μg/mL MIC values [38]. Different results from those found in the present study may be related to the type of bacterial culture used. Eugenol was evaluated against standard strains and clinical isolates of S. aureus presenting MIC value of 256 μg/mL for the standard strain, while the other 25 strains had MIC values ranging from 128 to 512 μg/mL [39].
Selles et al. verified eugenol as the major compound of clove and found that EC50 value of 4.82 ± 0.06×10−2 µg/mL for S. aromaticum essential oil eliminated the DPPH radical, less than the value found in our research, while the MIC against bacteria obtained in this study ranged from 1.36 to 2.72 mg/mL, values lower than those found in our study [40]. Kaur et al. observed the presence of twenty-one compounds with eugenol as the main compound, also verifying the antioxidant activity of clove essential oil by DPPH with EC50 in the range of 10.87 to 31.63 μg/mL [41].
Eugenol is a chemotype found in several plant products and is one of the main constituents of clove essential oil. It is used in both food and cosmetics industries as a flavoring agent, and it is an excellent antimicrobial, having activity against fungi and bacteria [42]. Eugenol has free hydroxyls in its structure that may be responsible for the antimicrobial activity verified in this research, since the antimicrobial activity of nitric oxide (NO) is conferred by its free hydroxyl groups. It was deduced that the hydroxyl group in eugenol links to proteins, preventing enzymatic action. The cell membrane suffered ruptures in the presence of the essential oil because it is rich in lipophilic compounds. This damage directly affects the maintenance of cellular pH and the balance of the inorganic ions. The main factors responsible for this damage are monoterpenes and sesquiterpenes, which produce different effects in different microorganisms [43].
Eugenol and S. aromaticum essential oil demonstrated great capacity for the elimination of ABTS radical, agreeing with the data reported on the antioxidant activity of clove essential oils with a chemotype rich in eugenol [44]. The same result was observed in a study of the antioxidant activity of the essential oil from various parts of the plant S. aromaticum, which demonstrated its ability to reduce the ABTS radical [45].
Medicinal plants have shown therapeutic components in their composition with potential candidates for new drugs [46]. Among these components, essential oils and secondary plant metabolites are considered as alternatives or additions to antiparasitic therapies [47]. The trypanocidal activity of several essential oils is described in literature [48, 49]. Due to the challenges in the development of new therapeutic agents for Chagas disease, we reported the effect of S. aromaticum essential oil and its major component eugenol on the growth of T. cruzi epimastigotes and intracellular amastigotes.
In the present study, the effects obtained when T. cruzi was treated with clove essential oil showed that epimastigotes are more susceptible to treatment than intracellular amastigotes. The activity of S. aromaticum essential oil may be in part attributed to the presence of eugenol, which makes up over half its composition. Thus, in an attempt to identify the compound responsible for the activity observed in the essential oil, the eugenol was analyzed. Eugenol demonstrated antitrypanosomal activity when incubated with T. cruzi; however, due to its cytotoxicity on macrophages, eugenol becomes less selective than S. aromaticum essential oil.
There are reports that S. aromaticum exhibited activity against trypomastigote forms of Trypanosoma brucei brucei, with IC50 values of 10.4 μg/mL. However, it also exhibited cytotoxicity on macrophages (CC50 22.4 μg/mL) and low SI (2.2) [50], unlike what was observed in the present study. A study analyzed S. aromaticum essential oil against T. cruzi (Y strain), obtaining IC50/24 h values of 99.5 μg/mL for epimastigotes and 57.5 μg/mL for bloodstream trypomastigotes. Eugenol was also evaluated, presenting IC50/24h values of 246 μg/mL for epimastigotes and 76 μg/mL for trypomastigotes [51].
A similarity was observed between the inhibitory activities of S. aromaticum essential oil and eugenol against T. cruzi epimastigote, and so activity against intracellular amastigotes was also analyzed. To our knowledge, this is the first description of S. aromaticum essential oil activity against T. cruzi intracellular amastigote forms. As there is a lack of data about S. aromaticum essential oil activity against T. cruzi intracellular amastigotes in literature, we found this activity similar to that observed against intracellular amastigotes of Leishmania donovani (15.24 μg/mL) [52], a protozoa that belongs to the same Trypanosomatidae family of T. cruzi. Eugenol displayed better activity against the intracellular amastigotes than S. aromaticum essential oil. Parameters of infection analysis corroborated the intracellular amastigote results, showing that eugenol was able to decrease the number of amastigotes per 100 cells and the mean amastigotes per infected cells, while S. aromaticum essential oil only decreased amastigote numbers per 100 cells, although in a concentration inferior to that presented by eugenol.
The cell death mechanism of intracellular amastigotes may be an event associated with the activation of macrophage microbicidal mechanisms, particularly the increase in the production of NO levels [53], which can be indirectly measured by the quantification of nitrite in the supernatant of BALB/c peritoneal macrophages. Therefore, in an attempt to better understand activity against intracellular amastigote forms, we carried out an analysis of the nitrite quantification of T. cruzi-stimulated peritoneal macrophages treated with S. aromaticum essential oil or eugenol. A significant decrease in nitrite levels was observed in cells stimulated with T. cruzi and treated with essential oil or eugenol. Eugenol notably modulates NO production, inducing suppression of NO production and iNOS protein expression in carbon tetrachloride-induced liver injury in rats [54] and in nicotine-induced murine peritoneal macrophages [55]. Eugenolol and glyceryl-isoeugenol, two derivatives of eugenol, suppressed LPS-induced iNOS expression by downregulating NF-kB and AP-1 through the inhibition of the MAPKs and Akt/IkB-alpha signaling pathways [56]. The inhibition of NO production observed in macrophages treated with S. aromaticum essential oil is probably due to the known NO suppression by eugenol. Therefore, there are probably other possible mechanisms involved in the trypanocidal activity of S. aromaticum essential oil against intracellular amastigotes, which are not involved in nitric oxide. Further studies should elucidate these mechanisms.
5. Conclusions
Analysis of the chemical composition of S. aromaticum essential oil identified eugenol as its major compound. S. aromaticum essential oil and eugenol inhibit the growth of Gram-positive bacteria S. aureus and have antioxidant potential. Inhibitory activity against the epimastigotes and intracellular amastigotes of T. cruzi, associated with low cytotoxicity, demonstrated the selectivity of S. aromaticum essential oil against the parasite. In addition, the NO inhibition observed in T. cruzi-stimulated macrophages treated with S. aromaticum essential oil showed that there are probably other possible mechanisms involved in their trypanocidal activity, which are not related to NO. S. aromaticum essential oil trypanocidal activity provides optimism about its use in further research seeking new therapeutic alternatives to trypanosomiasis.
Acknowledgments
This work was supported by the Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil) (grant number: Finance Code 001 88887.363006/2019-00) and the Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ)) (grant number: E-26/010.001759/2019). The APC was funded by Oswaldo Cruz Institute (Instituto Oswaldo Cruz (IOC)). Dr. Fernando Almeida-Souza is postdoctoral researcher fellow of CAPES (grant no. 88887.363006/2019-00). Dr. Ana Lucia Abreu-Silva is research productivity fellow of National Scientific and Technological Development Council (Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)) (grant no. 309885/2017-5).
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Authors' Contributions
Amanda Mara Teles, João Victor Silva-Silva, Adenilde Nascimento Mouchrek, and Fernando Almeida-Souza contributed equally to this work.
References
- 1.Cortés-Rojas D. F., de Souza C. R. F., Oliveira W. P., Salvador M. J. Clove (Syzygium aromaticum): a precious spice. Asian Pacific Journal of Tropical Biomedicine. 2014;4(2):90–96. doi: 10.1016/s2221-1691(14)60215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamatou G. P., Vermaak I., Viljoen A. M. Eugenol--from the remote Maluku Islands to the international market place: a review of a remarkable and versatile molecule. Molecules (Basel, Switzerland) 2012;17(6) doi: 10.3390/molecules17066953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Mello V., Prata M. C., da Silva M. R., et al. Acaricidal properties of the formulations based on essential oils from Cymbopogon winterianus and Syzygium aromaticum plants. Parasitology Research. 2014;113(12):4431–4437. doi: 10.1007/s00436-014-4121-4. [DOI] [PubMed] [Google Scholar]
- 4.Aboubakr H. A., Nauertz A., Luong N. T., et al. In Vitro antiviral activity of clove and ginger aqueous extracts against feline calicivirus, a surrogate for human norovirus. Journal of Food Protection. 2016;79(6) doi: 10.4315/0362-028X.JFP-15-593. [DOI] [PubMed] [Google Scholar]
- 5.de Oliveira T. L., das Graças Cardoso M., de Araújo Soares R., Ramos E. M., Piccoli R. H., Tebaldi V. M. Inhibitory activity of Syzygium aromaticum and cymbopogon citratus (DC.) stapf. Essential oils against Listeria monocytogenes inoculated in bovine ground meat. Brazilian Journal of Microbiology. 2013;44(2) doi: 10.1590/S1517-83822013005000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faujdar S. S., Bisht D., Sharma A. Antibacterial activity of Syzygium aromaticum (clove) against uropathogens producing ESBL, MBL, and AmpC beta-lactamase: are we close to getting a new antibacterial agent? Journal of Family Medicine and Primary Care. 2020;9(1):180–186. doi: 10.4103/jfmpc.jfmpc_908_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bechtle M., Chen S., Efferth T. Neglected diseases caused by bacterial infections. Current Medicinal Chemistry. 2010;17(1) doi: 10.2174/092986710789957814. [DOI] [PubMed] [Google Scholar]
- 8.Pisoschi A. M., Pop A., Georgescu C., Turcuş V., Olah N. K., Mathe E. An overview of natural antimicrobials role in food. European Journal of Medicinal Chemistry. 2018;143 doi: 10.1016/j.ejmech.2017.11.095. [DOI] [PubMed] [Google Scholar]
- 9.WHO. Chagas disease. Geneva, Switzerland: 2020. https://www.who.int/news-room/facts-in-pictures/detail/chagas-disease. [Google Scholar]
- 10.Salomon C. J. First century of Chagas’ disease: an overview on novel approaches to nifurtimox and benzonidazole delivery systems. Journal of Pharmaceutical Sciences. 2020;101(3) doi: 10.1002/jps.23010. [DOI] [PubMed] [Google Scholar]
- 11.Jackson Y., Wyssa B., Chappuis F. Tolerance to nifurtimox and benznidazole in adult patients with chronic Chagas’disease. The Journal of Antimicrobial Chemotherapy. 2019;75(3) doi: 10.1093/jac/dkz473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apt W. Current and developing therapeutic agents in the treatment of Chagas disease. Drug Design, Development and Therapy. 2020;4:243–253. doi: 10.2147/dddt.s8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zakaryan H., Arabyan E., Oo A., Zandi K. Flavonoids: promising natural compounds against viral infections. Archives of Virology. 2017;162(9):2539–2551. doi: 10.1007/s00705-017-3417-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cragg G. M., Newman D. J. Natural products: a continuing source of novel drug leads. Biochimica et biophysica acta. 2013;1830(6) doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine. 1999;26(9):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 16.El Babili F., Bouajila J., Souchard J. P., et al. Oregano: chemical analysis and evaluation of its antimalarial, antioxidant, and cytotoxic activities. Journal of Food Science. 2020;76(3) doi: 10.1111/j.1750-3841.2011.02109.x. [DOI] [PubMed] [Google Scholar]
- 17.Campos M. G., Webby R. F., Markham K. R., Mitchell K. A., Da Cunha A. P. Age-induced diminution of free radical scavenging capacity in bee pollens and the contribution of constituent flavonoids. Journal of Agricultural and Food Chemistry. 2003;51(3) doi: 10.1021/jf0206466. [DOI] [PubMed] [Google Scholar]
- 18.CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard. 4th. WAYNE, PA, USA: CLSI; 2009. [Google Scholar]
- 19.Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. American Journal of Clinical Pathology. 1966;45(4):493–496. [PubMed] [Google Scholar]
- 20.da Silva V. D., Almeida-Souza F., Teles A. M., et al. Chemical composition of Ocimum canum Sims. essential oil and the antimicrobial, antiprotozoal and ultrastructural alterations it induces in Leishmania amazonensis promastigotes. Industrial Crops and Products. 2018;119:201–208. [Google Scholar]
- 21.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 2011;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira I. S. S., Colares A. V., Cardoso F. O., et al. Vernonia polysphaera baker: anti-inflammatory activity in vivo and inhibitory effect in LPS-stimulated RAW 264.7 cells. PLoS One. 2011;14:12. doi: 10.1371/journal.pone.0225275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teles A. M., Rosa T., Mouchrek A. N., Abreu-Silva A. L., Calabrese K. D. S., Almeida-Souza F. Cinnamomum zeylanicum, origanum vulgare, and curcuma longa essential oils: chemical composition, antimicrobial and antileishmanial activity. Evidence-Based Complementary and Alternative Medicine. 2019;2019:12. doi: 10.1155/2019/2421695.2421695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almeida-Souza F., de Souza C.. a.S., Taniwaki N. N., et al. Morinda citrifolia Linn. fruit (Noni) juice induces an increase in NO production and death of Leishmania amazonensis amastigotes in peritoneal macrophages from BALB/c. Nitric Oxide. 2016;58:51–58. doi: 10.1016/j.niox.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Barros Gomes P. R., Mouchrek Filho V. E., Ferreira Rabêlo W., et al. Caracterização química e citotoxicidade do óleo essencial do cravo-da-índia (Syzygium aromaticum) Revista Colombiana de Ciencias Químico - Farmacéuticas. 2018;47:37–52. [Google Scholar]
- 26.Silvestri J. D. F., Paroul N., Czyewski E., et al. Perfil da composição química e atividades antibacteriana e antioxidante do óleo essencial do cravo-da-índia (Eugenia caryophyllata Thunb.) Revista Ceres. 2018;57:589–594. [Google Scholar]
- 27.Radünz M., da Trindade M. L. M., Camargo T. M., et al. Antimicrobial and antioxidant activity of unencapsulated and encapsulated clove (Syzygium aromaticum, L.) essential oil. Food Chemistry. 2019;276 doi: 10.1016/j.foodchem.2018.09.173. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y., Wang Y., Zhu X., Cao P., Wei S., Lu Y. Antibacterial and antibiofilm activities of eugenol from essential oil of Syzygium aromaticum (L.) merr. & L. M. Perry (clove) leaf against periodontal pathogen porphyromonas gingivalis. Microbial Pathogenesis. 2017;113 doi: 10.1016/j.micpath.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 29.Ebani V. V., Najar B., Bertelloni F., Pistelli L., Mancianti F., Nardoni S. Chemical composition and in vitro antimicrobial efficacy of sixteen essential oils against Escherichia coli and Aspergillus fumigatus isolated from poultry. Journal of Veterinary Science. 2018;5(3) doi: 10.3390/vetsci5030062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrade B. F. M. T., Nunes Barbosa L., Probst I. P., Fernandes Júnior A. Antimicrobial activity of essential oils. Journal of Essential Oil Research. 2017;26(1):34–40. [Google Scholar]
- 31.Besten M. A., Jasinski V. C. G., Costa Â.d.G. L. C., et al. Chemical composition similarity between the essential oils isolated from male and female specimens of each five Baccharis species. Journal of the Brazilian Chemical Society. 2020;23:1041–1047. [Google Scholar]
- 32.Cimanga K., Kambu K., Tona L., et al. Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the democratic republic of Congo. Journal of Ethnopharmacology. 2020;79(2) doi: 10.1016/s0378-8741(01)00384-1. [DOI] [PubMed] [Google Scholar]
- 33.Chaieb K., Hajlaoui H., Zmantar T., et al. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): a short review. Phytotherapy Research. 2007;21(6):501–506. doi: 10.1002/ptr.2124. [DOI] [PubMed] [Google Scholar]
- 34.Hu Q., Zhou M., wei S. Progress on the antimicrobial activity research of clove oil and eugenol in the food antisepsis field. Journal of Food Science. 2018;83(6):1476–1483. doi: 10.1111/1750-3841.14180. [DOI] [PubMed] [Google Scholar]
- 35.Holetz F. B., Pessini G. L., Sanches N. R., Cortez D. A., Nakamura C. V., Filho B. P. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Memórias do Instituto Oswaldo Cruz. 2002;97(7):1027–1031. doi: 10.1590/s0074-02762002000700017. [DOI] [PubMed] [Google Scholar]
- 36.Beraldo C., Daneluzzi N. S., Scanavacca J., Doyama J. T., Fernandes Júnior A., Moritz C. M. F. Eficiência de óleos essenciais de canela e cravo-da-índia como sanitizantes na indústria de alimentos. Pesquisa Agropecuária Tropical. 2019;43:436–440. [Google Scholar]
- 37.Hemaiswarya S., Doble M. Synergistic interaction of eugenol with antibiotics against Gram negative bacteria. Phytomedicine. 2020;16(11):997–1005. doi: 10.1016/j.phymed.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Pei R. S., Zhou F., Ji B. P., Xu J. Evaluation of combined antibacterial effects of eugenol, cinnamaldehyde, thymol, and carvacrol against E. Coli with an improved method. Journal of Food Science. 2009;74(7) doi: 10.1111/j.1750-3841.2009.01287.x. [DOI] [PubMed] [Google Scholar]
- 39.Qiu J., Feng H., Lu J., et al. Eugenol reduces the expression of virulence-related exoproteins in <em>Staphylococcus aureus</em>. Applied and Environmental Microbiology. 2010;76(17):5846–5851. doi: 10.1128/AEM.00704-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selles S. M. A., Kouidri M., Belhamiti B. T., Ait Amrane A. Chemical composition, in-vitro antibacterial and antioxidant activities of Syzygium aromaticum essential oil. Food & Function Journal. 2020;14(4):2352–2358. [Google Scholar]
- 41.Kaur K., Kaushal S., Rani R. Chemical composition, antioxidant and antifungal potential of clove (Syzygium aromaticum) essential oil, its major compound and its derivatives. Journal of Essential Oil Bearing Plants. 2013;22(5):1195–1217. [Google Scholar]
- 42.Marchese A., Barbieri R., Coppo E., et al. Antimicrobial activity of eugenol and essential oils containing eugenol: a mechanistic viewpoint. Critical Reviews in Microbiology. 2017;43(6) doi: 10.1080/1040841X.2017.1295225. [DOI] [PubMed] [Google Scholar]
- 43.Rahman A., Kang S. C. Inhibition of foodborne pathogens and spoiling bacteria by essential oil and extracts of Erigeron ramosus (Walt.) B.S.P, Journal of Food Safety. 2009;29(2):176–189. [Google Scholar]
- 44.Hamed S. F., Sadek Z., Edris A. Antioxidant and antimicrobial activities of clove bud essential oil and eugenol nanoparticles in alcohol-free microemulsion. Journal of Oleo Science. 2020;61(11) doi: 10.5650/jos.61.641. [DOI] [PubMed] [Google Scholar]
- 45.Alfikri F. N., Pujiarti R., Wibisono M. G., Hardiyanto E. B. Yield, quality, and antioxidant activity of clove (Syzygium aromaticum L.) bud oil at the different phenological stages in young and mature trees. Scientifica (Cairo) 2020;2020 doi: 10.1155/2020/9701701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boy H. I. A., Rutilla A. J. H., Santos K. A., et al. Recommended medicinal plants as source of natural products: a review. Digital Chinese Medicine. 2018;1(2):131–142. [Google Scholar]
- 47.Pérez G. S., Ramos-Lopez M. A., Sanchez-Miranda E., Fresán-Orozco M. C., Pérez-Ramos J. Antiprotozoa activity of some essential oils. Journal of Medicinal Plants Research. 2012;6(15):p. 8. [Google Scholar]
- 48.Habila N., Agbaji A. S., Ladan Z., et al. Evaluation of in vitro activity of essential oils against trypanosoma brucei brucei and trypanosoma evansi. Journal of Parasitology Research. 2010;2010 doi: 10.1155/2010/534601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le T. B., Beaufay C., Nghiem D. T., Pham T. A., Mingeot-Leclercq M. P., Quetin-Leclercq J. Evaluation of the anti-trypanosomal activity of Vietnamese essential oils, with emphasis on curcuma longa L. And its components. Molecules (Basel, Switzerland) 2019;24(6) doi: 10.3390/molecules24061158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Costa S., Cavadas C., Cavaleiro C., Salgueiro L., do Céu Sousa M. In Vitro susceptibility of trypanosoma brucei brucei to selected essential oils and their major components. Experimental Parasitology. 2018;190 doi: 10.1016/j.exppara.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 51.Santoro G. F., Cardoso M. G., Guimarães L. G., Mendonça L. Z., Soares M. J. Trypanosoma cruzi: activity of essential oils from Achillea millefolium L., Syzygium aromaticum L. and Ocimum basilicum L. on epimastigotes and trypomastigotes. Experimental Parasitology. 2020;116(3):283–290. doi: 10.1016/j.exppara.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 52.Islamuddin M., Sahal D., Afrin F. Apoptosis-like death in leishmania donovani promastigotes induced by eugenol-rich oil of Syzygium aromaticum. Journal of Medical Microbiology. 2013;63 doi: 10.1099/jmm.0.064709-0. [DOI] [PubMed] [Google Scholar]
- 53.Müller I., Pedrazzini T., Farrell J. P., Louis J. T-cell responses and immunity to experimental infection with leishmania major. Annual Review of Immunology. 1989;7 doi: 10.1146/annurev.iy.07.040189.003021. [DOI] [PubMed] [Google Scholar]
- 54.Fathy M., Khalifa E. M. M. A., Fawzy M. A. Modulation of inducible nitric oxide synthase pathway by eugenol and telmisartan in carbon tetrachloride-induced liver injury in rats. Life Sciences. 2019;216 doi: 10.1016/j.lfs.2018.11.031. [DOI] [PubMed] [Google Scholar]
- 55.Kar Mahapatra S., Bhattacharjee S., Chakraborty S. P., Majumdar S., Roy S. Alteration of immune functions and Th1/Th2 cytokine balance in nicotine-induced murine macrophages: immunomodulatory role of eugenol and N-acetylcysteine. International Immunopharmacology. 2020;11(4) doi: 10.1016/j.intimp.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 56.Yeh J. L., Hsu J. H., Hong Y. S., et al. Eugenolol and glyceryl-isoeugenol suppress LPS-induced iNOS expression by down-regulating NF-kappaB and AP-1 through inhibition of MAPKS and AKT/IkappaBalpha signaling pathways in macrophages. International Journal of Immunopathology and Pharmacology. 2011;24(2) doi: 10.1177/039463201102400208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


