Abstract
Introduction
Cancer-related fatigue (CRF) is one of the most distressing and persistent symptoms reported during pediatric acute lymphoblastic leukemia (ALL) therapy; however, information on the pathways underlying CRF severity is limited. Therefore, we conducted global metabolomics profiling of cerebrospinal fluid (CSF) samples to provide insight into the underlying mechanisms of CRF.
Methods
Fatigue in pediatric ALL patients (2012–2017) was assessed during post-induction therapy approximately 6-months post-diagnosis. Post-induction CSF was collected on 171 participants, comprising discovery (n=86) and replication (n=85) cohorts. We also conducted secondary validation using diagnostic CSF from 48 replication cohort participants. CSF metabolomic profiling was performed using gas chromatography-mass spectrometry (MS) and liquid chromatography-MS/MS. Kendall’s rank correlation was used to evaluate associations between metabolite abundance and CRF. False discovery rate (FDR) was used to account for multiple comparisons.
Results
Participants were 56% male and 59% Hispanic with a mean age at diagnosis of 8.5 years. A total of 274 CSF-derived metabolites were common to the discovery and replication cohorts. Eight metabolites were significantly associated with fatigue in the discovery cohort (p<0.05), of which three were significant in the replication cohort, including FDR-corrected associations with gamma-glutamylglutamine (pcombined = 6.2E-6) and asparagine (pcombined = 3.5E-4). Notably, the abundance of gamma-glutamylglutamine in diagnostic CSF samples was also significantly associated with fatigue (p=0.0062).
Conclusion
The metabolites identified in our assessment have been implicated in neurotransmitter transportation and glutathione recycling, suggesting glutamatergic pathways or oxidative stress may contribute to ALL-associated CRF. This information could inform targeted therapies for reducing CRF in at-risk individuals.
Keywords: Patient-reported Symptoms, Cancer-related Fatigue, Pediatric Acute Lymphoblastic Leukemia, Metabolomics, Cerebrospinal fluid, Biomarkers
INTRODUCTION
Children and adolescents with cancer report significantly more fatigue than their counterparts without cancer [1, 2]. In fact, cancer-related fatigue (CRF) is one of the most prevalent and distressing symptoms reported during childhood cancer therapy [3–7]. While acute symptoms of CRF typically improve over time [8, 9], symptoms may persist across the cancer care continuum for a subset of patients, with chronic fatigue present in 20–30% of long-term survivors [10, 11]. Given CRF is a pervasive symptom and source of significant morbidity in pediatric oncology populations, it is an important target for research and intervention.
CRF is frequently conceptualized as subjective physical or mental exhaustion which is not explained by recent activity and is not fully alleviated by rest [12, 13]. The etiology of CRF is likely multifaceted, with host-, disease-, and treatment-related components. Among pediatric patients with acute lymphoblastic leukemia (ALL), the most common malignancy diagnosed in individuals less than 15 years of age [14], there is considerable variability in the incidence and intensity of CRF. Of the clinical, demographic, and treatment intensity factors commonly evaluated [1, 15, 16], only exposure to corticosteroids during pediatric ALL treatment has been consistently associated with fatigue severity [16, 17]. Given that children receiving treatment for ALL are at risk of fatigue and its related complications, there is a growing need to better understand the mechanisms and pathophysiology of CRF in pediatric patients with ALL. Identifying the biological underpinnings of CRF may result in refined phenotyping and risk stratification of CRF to inform treatment and prevention efforts. To date, most studies exploring the biological mechanisms of CRF have targeted inflammatory pathways [18]. Because targeted studies are often informed by our limited understanding of disease etiology, they may not be robust to provide novel insight into the pathophysiology of CRF. Metabolomics technology, which systematically quantifies hundreds to thousands of metabolites, has been used to characterize the response to leukemia therapy and identify biomarkers of outcomes [19, 20], but has not been applied to study CRF. Specifically, metabolomics profiling of cerebrospinal fluid (CSF), a readily available and biologically relevant resource for CRF biomarker discovery, may yield insight into the physiological response to leukemia therapy in the central nervous system. Therefore, the objective of this study was to discover and replicate fatigue-associated metabolites using untargeted metabolomics profiling of CSF samples systematically collected on a prospective cohort of pediatric patients with ALL.
METHODS
The current study analyzed a subset of participants enrolled in a multi-site prospective study of symptoms. The design and methods of the parent study have been published [9]. Briefly, eligible participants for the current study included pediatric patients with newly-diagnosed ALL or lymphoblastic lymphoma, aged 2–18 years at diagnosis, and treated on or according to a Children’s Oncology Group ALL protocol (AALL0434, AALL0932, AALL1122, AALL1131, AALL1231). Treatment details for each protocol are available at www.clinicaltrials.gov. Treatment generally included one month of remission induction therapy consisting of corticosteroids, intrathecal methotrexate, and pegaspargase. Patients with high and very-high risk disease also typically received vincristine and daunorubicin. Post-induction therapy lasting six to eight months consisted of courses of, asparagine, corticosteroids, cytarabine, doxorubicin, mercaptopurine, intrathecal and intravenous methotrexate, and vincristine. Finally, two to three years of maintenance therapy typically included oral corticosteroids, mercaptopurine, and methotrexate in combinations with intravenous vincristine and intrathecal methotrexate. Symptom assessments were designed to evaluate symptoms (e.g., fatigue, sleep disturbance, nausea, pain) reported at the following phases of therapy: end of induction and approximately six months post-diagnosis, nine months post-diagnosis, and 12 months post-diagnosis. This analysis was restricted to fatigue reported by participants treated at Texas Children’s Hospital (Houston, Texas, USA) during post-induction chemotherapy (~6-months post-diagnosis), aligning with the start of delayed intensification therapy for patients treated on standard, high-, or very high-risk post-induction protocols or on day 113 of consolidation therapy of patients treated on low-risk protocols. This time point was selected to capture fatigue during one of the most intensive phases of pediatric ALL therapy when the level of symptom distress is typically high [4]. Eligible participants included patients free of developmental or neurologic disorders. Given the potential influence of less common treatment exposures on fatigue, patients with a history of relapse, bone marrow transplant, or radiation therapy prior to the delayed intensification fatigue assessment were excluded. The research procedures were reviewed and approved by a Baylor College of Medicine institutional review board. As appropriate, informed consent was obtained from the legal guardian and assent or consent obtained from research participants.
Sample Collection and Metabolomic Profiling
The study population was divided into two batches, comprising a discovery (diagnosed 2012–2015) and a replication (diagnosed 2015–2017) cohort, based on time of study enrollment. Aliquots (150 microliter) of CSF were obtained during scheduled therapeutic lumbar punctures at the time of the fatigue assessment (i.e., start of delayed intensification therapy) to facilitate cross-sectional associations. Additional scheduled diagnostic CSF samples were available on a subset of the participants enrolled in the replication population (n=48), providing the ability to explore potential temporal associations between fatigue during therapy and biomarkers present in treatment naïve samples. CSF samples were centrifuged and stored at −80°C. Metabolomic profiling were completed for the discovery cohort in 2016 and the replication cohort in 2019 by Metabolon (Metabolon, Inc., Morrisville, NC, USA). Untargeted metabolomic profiling was performed following previously described methods [21]. Briefly, methanol was added to each sample and vigorously shaken for two minutes to remove protein and dissociate protein-bound metabolites. Following centrifugation, the extract was divided into fractions, including fractions for reverse phase ultrahigh performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) with positive ion conditions for hydrophilic compounds, positive ion conditions for hydrophobic compounds, basic negative ion conditions, and a negative ionization after elution from a hydrophilic interaction chromatography column. Raw data files from the MS analysis were extracted and quality controlled. Compounds identities were determined based on the similarity of retention time/index, mass to charge ratio, and MS/MS spectral data to Metabolon’s library of known compounds. Each metabolite level was quantified based on the area-under-the-curve for each detected peak. The abundance of identified metabolites were scaled to a median of one, with missing values imputed to the minimum observed value for a given metabolite. Metabolites which were only identified in a single sample were excluded from statistical analyses.
Fatigue Assessment
To the extent possible, fatigue questionnaires were obtained at the time of the lumbar puncture procedure and CSF collection. If patients were not able to complete questionnaires at the time of the LP then questionnaires were typically obtained within one week of the lumbar puncture. Self- or parent proxy reported measures of fatigue were systematically collected using electronic data capture or paper questionnaires of validated instruments administered on computerized tablets [22–24]. Briefly, participants were asked to report symptoms over the previous two weeks, and surveys were available in both Spanish and English. Children and adolescents age ≥7 years self-reported symptoms using the 10-item Child Fatigue Scale (7–12 years) or 13-item Adolescent Fatigue Scale (≥13 years). Caregiver proxy reports of fatigue were collected on individuals <7 years of age using the 17-item Parent Fatigue Scale. As described previously [9], responses were summed across each item on the questionnaires and standardized scores (mean = 50, standard deviation [SD] = 10) were calculated separately for each scale to facilitate comparisons across the three scales. Higher scores were indicative of higher levels of fatigue.
Clinical Information
Information including age at diagnosis, treatment protocol, cancer diagnosis (B-lineage ALL, T-lineage ALL, lymphoblastic lymphoma), leukemia central nervous system (CNS) involvement at diagnosis, and post-induction treatment risk group (low/standard, high/very high) was abstracted from electronic medical records. Because excess adiposity may exacerbate CRF [25], heights in meters and weights in kilograms were abstracted at diagnosis and the time of the fatigue assessment and compared to the Centers for Disease Control and Prevention age- and sex-specific growth charts to calculate age- and sex-adjusted body mass index (BMI) z-scores [26]. Participants completed demographic questionnaires to collect information on race, ethnicity, and gender.
Statistical analysis
Descriptive statistics were calculated for clinical and demographic characteristics of the discovery and replication populations, including median and range of continuous measures and counts and proportions for categorical variables. The association between each clinical and demographic factor and fatigue assessed at the beginning of delayed intensification therapy was evaluated in unadjusted and adjusted linear regression models, accounting for other potentially relevant clinical variables, including age at diagnosis, BMI z-score, gender, race/ethnicity, diagnosis (B-lineage ALL, T-lineage ALL, or lymphoblastic lymphoma), and post-induction treatment risk group.
Kendall’s tau, a non-parametric bivariate rank correlation coefficient, was calculated to measure the strength of the cross-sectional association between standardized fatigue and the scaled abundance of each detected metabolite. Non-parametric rank-order correlation was the primary statistical comparison due to concerns over the non-normal distribution of many metabolites and the potential for influential outliers. However, to account for potential confounding, we compared the distribution of clinical and demographic factors for the top candidate metabolites using the Kruskal-Wallis test. Additionally, we generated linear regression models for the association between top candidate metabolites and fatigue with and without adjusting for possible confounding factors, including gender, post-induction treatment risk group, age at diagnosis, BMI z-score, and metabolite batch (for combined analysis). Metabolites with a p-value<0.05 in the discovery population were evaluated in the replication cohort. Metabolites with a p-value<0.05 in the replication cohort, consistent directions of effect in both cohorts, and a false discovery rate (FDR) corrected p-value<0.05 in the combined population were considered replicated. Using the discovery threshold significance level of p<0.05, the study was adequately powered (>80%) to identify correlation coefficients between metabolites and fatigue exceeding 0.30 in the discovery and replication populations. Finally, to investigate temporal relationships between fatigue and metabolites identified in the cross-sectional analyses, Kendall’s rank correlation was calculated for replicated metabolites using diagnostic CSF samples available on a subset of the replication cohort, with a p-value<0.05 used to define statistical significance. All analyses were conducted in R version 3.6.3 statistical software.
RESULTS
A description of the clinical and demographic characteristics of the discovery (n=86) and replication (n=85) cohorts is provided in Table 1. The distribution of most participant characteristics was similar for the discovery and replication populations (p>0.05), with the exception of a slightly higher proportion of Hispanics (p=0.02) and patients treated on AALL1231 (p=0.007) in the replication population. Overall, the study population had a mean age at diagnosis of 8.48 years (SD = 4.39) and a mean BMI z-score at diagnosis of 0.44 (SD = 1.18). The population was 56.1% male, 85.4% B-lineage ALL, with 63.7% receiving high- or very high-risk treatment. Fatigue assessments were completed a median of 184 days after diagnosis (range = 147–261), with little difference between discovery (median = 180; range: 147261) and replication (median = 184; range: 149 – 240) cohorts. Clinical and demographic factors were not significantly associated with fatigue (Table 2), with the exception of high- and very high-risk patients reporting about half a standard deviation less fatigue than low and average risk patients (p=0.004).
Table 1.
Clinical and demographic characteristics of the discovery and replication populations
| Discovery Population (N=86) |
Replication Population (N=85) |
|
|---|---|---|
| Median age at diagnosis, year (range) | 8.39 (2.61 – 17.48) | 8.57 (2.67 – 17.63) |
| Median age at survey, year (range) | 8.90 (3.16 – 17.93) | 9.09 (3.22 – 18.07) |
| Median BMI at survey, z-score (range) | 0.30 (−3.64 – 2.39) | 0.58 (−2.35 – 2.61) |
| Gender, n(%) | ||
| Male | 48 (55.8) | 48 (56.5) |
| Female | 38 (44.2) | 37 (43.5) |
| Race/Ethnicity, n(%) | ||
| Non-Hispanic White | 30 (34.9) | 13 (15.3) |
| Hispanic | 43 (50.0) | 59 (69.4) |
| Non-Hispanic Black | 8 (9.3) | 6 (7.1) |
| Non-Hispanic Other | 5 (5.8) | 7 (8.2) |
| Diagnosis, n(%) | ||
| B-lineage ALL | 76 (88.4) | 70 (82.4) |
| T-lineage ALL | 9 (10.5) | 11 (12.9) |
| Lymphoblastic lymphoma | 1 (1.1) | 4 (4.7) |
| Post-induction protocol, n(%) | ||
| AALL0434 | 7 (8.1) | 1 (1.2) |
| AALL0932 | 31 (36.1) | 22 (25.9) |
| AALL1122 | 2 (2.3) | 2 (2.4) |
| AALL1131 | 44 (51.2) | 47 (55.3) |
| AALL1231 | 2 (2.3) | 13 (15.3) |
| CNS involvement at diagnosis, n(%)1 | ||
| No CNS involvement | 59 (71.1) | 55 (68.8) |
| CNS involvement | 24 (28.9) | 25 (31.2) |
| End induction risk group, n(%) | ||
| Low/Standard | 36 (41.9) | 26 (30.6) |
| High/Very High | 50 (58.1) | 59 (69.4) |
| Fatigue scale, n(%) | ||
| Parent | 41 (47.7) | 36 (42.4) |
| Child | 28 (32.6) | 29 (34.1) |
| Adolescent | 17 (19.8) | 20 (23.5) |
| Median standardize fatigue score (range) | 49.86 (31.28 – 81.17) | 48.79 (30.32 – 73.89) |
CNS involvement not available on lymphoblastic lymphoma patients
Body mass index, BMI; acute lymphoblastic leukemia, ALL; central nervous system, CNS
Table 2.
Observed associations between clinical and demographic factors and post-induction fatigue during pediatric ALL therapy (n=171)
| Unadjusted Model |
Adjusted1 Model |
|||
|---|---|---|---|---|
| Beta (SE) | P-val | Beta (SE) | P-val | |
| Age at diagnosis, year | −0.08 (0.17) | 0.63 | 0.21 (0.20) | 0.31 |
| BMI z-score at survey | −0.35 (0.65) | 0.58 | −0.57 (0.66) | 0.39 |
| Gender | 0.12 | 0.17 | ||
| Male | Ref. | Ref. | ||
| Female | −2.35 (1.51) | −2.18 (1.50) | ||
| Race/Ethnicity | 0.90 | 0.96 | ||
| Non-Hispanic White | Ref. | Ref. | ||
| Hispanic | −1.13 (1.80) | −0.60 (1.88) | ||
| Non-Hispanic Black | −1.28 (3.04) | −1.54 (3.14) | ||
| Non-Hispanic Other | −2.01 (3.23) | −0.13 (3.63) | ||
| Diagnosis | 0.07 | 0.18 | ||
| B-lineage ALL | Ref. | Ref. | ||
| T-lineage ALL | −2.51 (2.32) | −3.17 (2.36) | ||
| Lymphoblastic lymphoma2 | −9.25 (4.42) | -- | ||
| CNS involvement at diagnosis, n(%) | 0.15 | 0.61 | ||
| No CNS involvement | Ref. | Ref. | ||
| CNS involvement | −2.42 (0.92) | −0.93 (1.80) | ||
| End induction risk group | 0.005 | 0.010 | ||
| Low/Standard | Ref. | Ref. | ||
| High/Very High | −4.36 (1.53) | −4.86 (1.87) | ||
Model adjusted for variables listed in table
Lymphoblastic lymphoma not included in adjusted models which include CNS involvement at diagnosis as a covariate
Body mass index, BMI; acute lymphoblastic leukemia, ALL; central nervous system, CNS
A total of 313 metabolites were detected and evaluated in the CSF of the discovery cohort (Supplemental Table S1). We identified eight metabolites significantly associated (p<0.05) with post-induction fatigue in the discovery cohort, including positive correlations observed for dimethylglycine, allantoin, ribitol, and dimethylmalonic acid and inverse correlations for gamma-glutamylglutamine, 3-methoxytyrosine, asparagine, and myo-inositol (Table 3). Overall, 409 metabolites were detected in the CSF of the replication cohort (Supplemental Table S2), including 274 (87.0%) of the named metabolites also identified in the discovery cohort. We observed consistent directions of effect for each of the candidate metabolites in the discovery and replication cohorts, including statistically significant (p<0.05) correlations between fatigue in the replication cohort and the abundance of gamma-glutamylglutamine, dimethylglycine, and asparagine (Table 3). In the combined population, statistically significant associations were observed between fatigue and the post-induction CSF abundance of gamma-glutamylglutamine (tau = −0.23, p = 6.2×10−6) and asparagine (tau = −0.18, p = 3.5×10−4) after correcting for multiple comparisons (FDR <0.05). Additionally, the candidate metabolites were not strongly associated with most clinical and demographic variables (Supplemental Table S3) and we observed similar associations between the top candidate metabolites and fatigue in both the unadjusted and multivariable linear regression models (Supplemental Table S4), suggesting the observed associations for the top metabolites were not likely explained by confounding.
Table 3.
CSF metabolites associated with post-induction fatigue in the discovery cohort (p <0.05) and observed associations in replication and combined populations
| Discovery Cohort (n=86) | Replication Cohort (n=85) | Combined Population (n=171) | ||||||
|---|---|---|---|---|---|---|---|---|
| Biochemical Name | Pathway | Tau | P-val | Tau | P-val | Tau | P-val | FDR |
| gamma-Glutamylglutamine | Peptide | −0.25 | 0.00070 | −0.23 | 0.0019 | −0.23 | 0.0000062 | 0.0017 |
| 3-methoxytyrosine | Amino Acid | −0.22 | 0.0028 | −0.08 | 0.30 | −0.13 | 0.0089 | 0.31 |
| Dimethylglycine | Amino Acid | 0.18 | 0.013 | 0.16 | 0.035 | 0.17 | 0.0013 | 0.12 |
| Asparagine | Amino Acid | −0.17 | 0.020 | −0.20 | 0.0060 | −0.18 | 0.00035 | 0.047 |
| Allantoin | Nucleotide | 0.17 | 0.023 | 0.08 | 0.27 | 0.11 | 0.032 | 0.58 |
| Myo-inositol | Lipid | −0.16 | 0.032 | −0.10 | 0.18 | −0.12 | 0.017 | 0.40 |
| Ribitol | Carbohydrate | 0.15 | 0.035 | 0.04 | 0.58 | 0.10 | 0.064 | 0.61 |
| Dimethylmalonic acid | Lipid | 0.15 | 0.035 | 0.14 | 0.063 | 0.14 | 0.0062 | 0.24 |
Cerebrospinal fluid, CSF
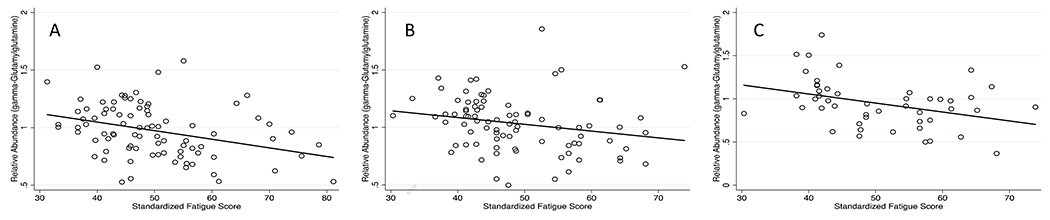
We attempted to further replicate the observed association between candidate biomarkers and post-induction fatigue using diagnostic CSF samples collected on 48 treatment-naïve individuals included in the replication cohort. The clinical and demographic characteristics of individuals with a diagnostic sample were similar to that of the underlying source population (Table 4). The abundance of asparagine in diagnostic CSF samples was not significantly correlated with post-induction fatigue (tau = −0.11, p = 0.27, results not shown). However, the abundance of gamma-glutamylglutamine at diagnosis was significantly associated with post-induction fatigue severity (tau = −0.27, p = 0.0062), demonstrating an inverse correlation similar to that observed cross-sectionally in the discovery and replication cohorts (Figure 1).
Table 4.
Clinical characteristics of discovery cohort with CSF samples evaluated at diagnosis
| Pediatric ALL Patients (n=48) |
|
|---|---|
| Median age at diagnosis, year (range) | 8.43 (3.0–17.4) |
| Median age at survey, year (range) | 8.90 (3.46–17.95) |
| Median BMI at diagnosis, z-score (range) | 0.42 (−3.09–2.43) |
| Gender, n(%) | |
| Male | 27 (56.2) |
| Female | 21 (43.8) |
| Race/Ethnicity, n(%) | |
| Non-Hispanic White | 9 (18.8) |
| Hispanic | 35 (72.9) |
| Non-Hispanic Black | 4 (8.3) |
| Non-Hispanic Other | 0 (0.0) |
| Diagnosis, n(%) | |
| B-lineage ALL | 43 (89.6) |
| T-lineage ALL | 5 (10.4) |
| Lymphoblastic lymphoma | 0 (0.0) |
| CNS involvement at diagnosis, n(%) | |
| No CNS involvement | 40 (83.3) |
| CNS involvement | 8 (16.7) |
| Treatment risk group, n(%) | |
| Low/Standard | 17 (35.4) |
| High/Very High | 31 (64.6) |
| Fatigue scale, n(%) | |
| Parent | 17 (35.4) |
| Child | 19 (39.6) |
| Adolescent | 12 (25.0) |
| Median standardize fatigue score (range) | 48.56 (30.32–73.89) |
Body mass index, BMI; acute lymphoblastic leukemia, ALL
Figure 1.
Observed correlation between standardize fatigue scores assessed at start of delayed intensification therapy and the abundance of gamma-glutamylglutamine in the cerebrospinal fluid of participants included in the discovery cohort (A), replication cohort (B), and diagnostic samples collected on the replication cohort (C)
DISCUSSION
This prospective evaluation of fatigue during pediatric ALL chemotherapy is the first study to perform an untargeted screening of the metabolic state of the central nervous system to discover biochemical correlates of CRF. Utilizing CSF samples collected prior to and during pediatric ALL therapy, we identify and replicate novel metabolic profiles associated with CRF reported during one of the most intense phases of treatment. Specifically, we found that the abundance of asparagine and gamma-glutamylglutamine was cross-sectionally associated with fatigue at the start of delayed intensification therapy in children with ALL. Importantly, the association between CRF and gamma-glutamylglutamine was further validated using CSF samples obtained at diagnosis. Given that therapeutic improvement has led to long-term survival rates approaching 90% in children with ALL [27], there is a growing need to better characterize the factors which contribute to reduced quality of life in patients and survivors. This work partly addresses this need by advancing our limited understanding of the mechanisms of CRF in pediatric ALL.
Asparagine has previously been identified as a marker of fatigue in various population. For example, urinary levels of asparagine have been linked to fatigue, with higher levels reported among fatigued patients with breast cancer post-radiation therapy and lower levels identified in individuals with chronic fatigue [28, 29]. The association observed between fatigue and asparagine in the current study may reflect variable patient response to asparaginase chemotherapy. Asparaginase hydrolyzes asparagine, forming ammonia and aspartic acid. Leukemic cells are particularly vulnerable to asparagine depletion, because they lack sufficient asparagine synthetase activity to replenish intracellular asparagine [30]. Asparagine synthetase catalyzes the conversion of aspartate and glutamine to form glutamate and asparagine. Although asparagine is typically considered a nonessential amino acid, deficiency in the expression of asparagine synthetase results in a dependence on extracellular asparagine pools. Because the blood-brain barrier transport system regulates amino acid concentrations in the central nervous system, CSF concentrations of asparagine and other key amino acids are approximately one-tenth of the levels detected in plasma [31]. Importantly, genetic variants in the gene encoding asparagine synthetase lead to disruptions in neurological development and function in children without any observed abnormalities in other organ systems [32], supporting a central role for asparagine homeostasis in the maintenance of neurologic processes. Further work is needed to elucidate the potential mechanisms linking CSF asparagine to cancer-related fatigue in children with ALL.
gamma-Glutamylglutamine demonstrated the strongest statistical correlation with fatigue of all evaluable metabolites in cross-sectional analyses of both the discovery and replication cohorts (Supplemental Tables S1 & S2). Notably, the concentrations of the dipeptide at diagnosis were also correlated with the severity of fatigue during treatment, indicating baseline metabolomic profiles in chemotherapy-naïve patients might be predictive of fatigue later during treatment. gamma-Glutamylglutamine was first detected as a widely distributed acidic dipeptide in the human brain [33], and differential CSF concentrations of gamma-glutamylglutamine have been implicated in unmedicated patients with schizophrenia disorders [34] and infants with urea cycle disorders [35]. gamma-Glutamyl amino acids are intermediates in the gamma-glutamyl cycle. Some evidence suggests the gamma-glutamyl cycle may be involved in regulating amino acid homeostasis across the blood-brain barrier [36]. The initial step of the cycle is catalyzed by gamma-glutamyl transferase (GGT), a plasma membrane bound enzyme involved in transporting gamma-glutamyl dipeptides and glutathione across the luminal membrane of endothelial cells lining the blood-brain barrier. GGT catalyzes the transfer of the glutamyl moiety of glutathione (GSH) to an amino acid acceptor, of which L-glutamine a good substrate in humans [37]. The gamma-glutamyl amino acid is imported across the luminal membrane by transport systems independent of free amino acids [36]. Intracellular gamma-glutamyl cyclotransferase catalyzes the conversion of the gamma-glutamyl amino acids to the free amino acid and 5-oxoproline, which is then hydrolyzed to form glutamate. Emerging evidence suggests that elevated concentrations of 5-oxoproline in endothelial cells activates Na+-dependent abluminal transport systems [38], which are oriented such that they aid in the active removal of amino acids from the extracellular fluid of the central nervous system. Thus, upregulation of this system may play an important role in protecting against excessive levels of amino acids in the central nervous system, including neurotransmitters and neurotransmitter precursors [39]. In particular, GGT may be involved in glutamate and glutamine transport in the brain [40, 41]. GGT has been linked to glutamatergic structures in the specific regions of the brain [42], and GGT inhibitors have been shown to alter glutamate uptake in brain cell cultures [43].
The inverse relationship observed between gamma-glutamylglutamine and fatigue in the current study may reflect a deficiency in the gamma-glutamyl transferase system or available glutamine pools. Although not among top metabolites identified in the discovery cohort, glutamine levels were consistently lower (Supplemental Tables S1 & S2) in the CSF of fatigued patients (overall tau = −0.14, p = 0.005). These observations are consistent with a glutamatergic hypothesis of CRF. Alternatively, because GGT is involved in the breakdown, synthesis, and transport of glutathione [44], disruptions in the metabolite intermediates of the gamma-glutamyl cycle may compromise the cellular response to oxidative stress. In its reduced form (GSH), glutathione is a critical scavenger of reactive oxygen species and is involved in xenobiotic detoxification [45]. The ratio of reduced to oxidized glutathione (GSH/GSSG) is often considered a marker of oxidative stress. Notably, Rodgers et al. prospectively monitored GSH/GSSG ratio in the CSF of 38 children with ALL and found lower ratios significantly correlated with higher levels of fatigue during post-induction therapy [46].
Unlike other commonly reported symptoms during childhood ALL therapy, such as nausea and pain, well-established pharmacologic interventions to mitigate CRF are lacking. A recent meta-analysis suggests that pharmacologic interventions with erythropoietins or stimulants may result in statistically significant reductions in CRF [47]. However, the use of pharmacologic agents is limited by the absence of clinically relevant effects, possible adverse consequences, and a lack of studies in pediatric populations. Non-pharmacologic interventions may improve fatigue symptoms while minimizing the potential for adverse responses [48]. In particular, physical activity interventions have demonstrated efficacy for improving CRF in pediatric populations [49]; however, adherence to exercise programs among the most fatigued patients remains a concern. Given the paucity of feasible and effective treatment strategies, it is not surprising that CRF is untreated in the vast majority of pediatric cancer patients [50]. Efforts to manage and mitigate CRF are likely to benefit from an improved understanding of the factors contributing to fatigue severity during childhood cancer treatment. The results of this study suggest that oxidative stress and glutamatergic pathways are promising candidates for future intervention research.
Strengths of the current study include the prospective collection of standardized measures of fatigue using validated questionnaires. Questionnaires were available in both English and Spanish languages, ensuring representation from an ethnically diverse research population. Importantly, we conducted untargeted metabolomic profiling of CSF samples, which were systematically collected during scheduled diagnostic and clinical lumbar punctures. These samples are a biologically relevant biofluid for the detection of biomarkers of CRF and provide a window into the metabolic state of the central nervous system at the time of sample collection. Still, our findings should be considered in light of several limitations. First, given our sample size, the current study was likely underpowered to identify modest to weak biochemical profiles associated with CRF. However, our sample of more than 170 comprehensively-phenotyped patients is notable given how infrequent pediatric ALL is in the general population. Furthermore, restricting our analysis to individuals treated with contemporary ALL therapy reduces the potential for confounding due to heterogenous diagnoses or treatment exposures. We did not observe differences in the associations between CSF metabolites and fatigue across racial and ethnic groups; however, our study sample did include a high proportion of individuals of Hispanic ethnicity compared to the broader U.S. population of patients with pediatric ALL. As a consequence, our findings may not be generalizable to other cancers, therapeutic regimens, or populations.
CRF is a distressing and highly pervasive symptom during childhood cancer therapy [3–7] and remains an unremitting complaint in up to one-third of long-term survivors [10, 11]. Even modest declines in quality of life, if persistent, can result in a considerable loss of quality of life years, highlighting the need for novel approaches to address CRF in this vulnerable population. Corticosteroid treatment and sleep-wake disruptions during pediatric ALL therapy likely contribute to fatigue distress [2, 16, 17]. However, because pediatric patients with ALL do not typically receive corticosteroids during delayed intensification therapy, exposure to corticosteroids is unlikely to explain fatigue reported in the current study. Consistent with the existing literature [1, 15, 16], we did not observe strong associations between fatigue and most clinical and demographic factors in the current study. The lack of our understanding of the underpinnings of CRF in susceptible populations underscores the need for new approaches in understanding this important outcome. The current study adds to our limited understanding of fatigue in children with ALL by identifying differences in the abundance of asparagine and gamma-glutamylglutamine metabolites among fatigued individuals. Differences in the relative abundance of these metabolites appear to occur in the absence of significant alterations in the broader constellation of CSF biochemicals. Whether the CSF abundance of gamma-glutamylglutamine and other candidate metabolites play a functional role in etiology of fatigue should be the subject of future research. Furthermore, additional work is needed to determine whether altered metabolomic profiles can aid in the identification of pediatric cancer survivors at increased risk of persistent fatigue. Ultimately, this line of investigation may aid in the development of new prevention and treatment approaches informed by an improved understanding of the etiology and risk factors for cancer related fatigue.
Supplementary Material
DISCLOSURES AND ACKNOWLEDGEMENTS
This work was supported in part by the National Institutes of Health National Cancer Institute (R01CA1693398, K07CA218362), Leukemia Research Foundation Hollis Brownstein Research Grants Program (to A.L.B), the Cancer Prevention & Research Institute of Texas (CPRIT RP190132), and the St. Baldrick’s Foundation Consortium Research Grant (522277) Reducing Ethnic Disparities in Acute Leukemia (REDIAL) Consortium. The funding sources had no involvement in the study design; collection, analysis, or interpretation of data; report writing; or decision to submit the manuscript for publication.
Footnotes
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Daniel LC, Brumley LD, and Schwartz LA, Fatigue in adolescents with cancer compared to healthy adolescents. Pediatr Blood Cancer, 2013. 60(11): p. 1902–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steur LMH, et al. , Sleep-wake rhythm disruption is associated with cancer-related fatigue in pediatric acute lymphoblastic leukemia. Sleep, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baggott CR, et al. , An evaluation of the factors that affect the health-related quality of life of children following myelosuppressive chemotherapy. Support Care Cancer, 2011. 19(3): p. 353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hockenberry MJ, et al. , The influence of oxidative stress on symptom occurrence, severity, and distress during childhood leukemia treatment. Oncol Nurs Forum, 2014. 41(4): p. E238–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erickson JM, Fatigue in adolescents with cancer: a review of the literature. Clin J Oncol Nurs, 2004. 8(2): p. 139–45. [DOI] [PubMed] [Google Scholar]
- 6.Dupuis LL, et al. , Validation of the Symptom Screening in Pediatrics Tool in Children Receiving Cancer Treatments. J Natl Cancer Inst, 2018. 110(6): p. 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibson F, et al. , Heavy to carry: a survey of parents’ and healthcare professionals’ perceptions of cancer-related fatigue in children and young people. Cancer Nurs, 2005. 28(1): p. 27–35. [DOI] [PubMed] [Google Scholar]
- 8.Baggott C, et al. , Changes in children’s reports of symptom occurrence and severity during a course of myelosuppressive chemotherapy. J Pediatr Oncol Nurs, 2010. 27(6): p. 307–15. [DOI] [PubMed] [Google Scholar]
- 9.Hockenberry MJ, et al. , Symptom Trajectories in Children Receiving Treatment for Leukemia: A Latent Class Growth Analysis With Multitrajectory Modeling. J Pain Symptom Manage, 2017. 54(1): p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeller B, et al. , Chronic fatigue in adult survivors of childhood cancer: associated symptoms, neuroendocrine markers, and autonomic cardiovascular responses. Psychosomatics, 2014. 55(6): p. 621–9. [DOI] [PubMed] [Google Scholar]
- 11.Zeller B, et al. , Chronic fatigue in long-term survivors of childhood lymphomas and leukemia: persistence and associated clinical factors. J Pediatr Hematol Oncol, 2014. 36(6): p. 438–44. [DOI] [PubMed] [Google Scholar]
- 12.Hinds PS, et al. , Comparing patient, parent, and staff descriptions of fatigue in pediatric oncology patients. Cancer Nurs, 1999. 22(4): p. 277–88; quiz 288–9. [DOI] [PubMed] [Google Scholar]
- 13.Berger AM, et al. , NCCN Clinical Practice Guidelines Cancer-related fatigue. J Natl Compr Canc Netw, 2010. 8(8): p. 904–31. [DOI] [PubMed] [Google Scholar]
- 14.Ward E, et al. , Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin, 2014. 64(2): p. 83–103. [DOI] [PubMed] [Google Scholar]
- 15.Crabtree VM, et al. , Changes in sleep and fatigue in newly treated pediatric oncology patients. Support Care Cancer, 2015. 23(2): p. 393–401. [DOI] [PubMed] [Google Scholar]
- 16.Hinds PS, et al. , Dexamethasone alters sleep and fatigue in pediatric patients with acute lymphoblastic leukemia. Cancer, 2007. 110(10): p. 2321–30. [DOI] [PubMed] [Google Scholar]
- 17.Yeh CH, et al. , Clinical factors associated with fatigue over time in paediatric oncology patients receiving chemotherapy. Br J Cancer, 2008. 99(1): p. 23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bower JE, Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat Rev Clin Oncol, 2014. 11(10): p. 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bannur Z, et al. , The differential metabolite profiles of acute lymphoblastic leukaemic patients treated with 6-mercaptopurine using untargeted metabolomics approach. Clin Biochem, 2014. 47(6): p. 427–31. [DOI] [PubMed] [Google Scholar]
- 20.Schraw JM, et al. , Metabolomic profiling identifies pathways associated with minimal residual disease in childhood acute lymphoblastic leukaemia. EBioMedicine, 2019. 48: p. 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans AM, et al. , High resolution mass spectrometry improves data quanitity and quality as compared to unit mass resolution mass spectrometry in high-throghput profiling metabolomics. Metabolomics, 2014. 4(2): p. 1–7. [Google Scholar]
- 22.Hinds PS, et al. , Psychometric and clinical assessment of the 10-item reduced version of the Fatigue Scale-Child instrument. J Pain Symptom Manage, 2010. 39(3): p. 572–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandrell BN, et al. , Psychometric and clinical assessment of the 13-item reduced version of the fatigue scale-adolescent instrument. J Pediatr Oncol Nurs, 2011. 28(5): p. 287–94. [DOI] [PubMed] [Google Scholar]
- 24.Hockenberry MJ, et al. , Three instruments to assess fatigue in children with cancer: the child, parent and staff perspectives. J Pain Symptom Manage, 2003. 25(4): p. 319–28. [DOI] [PubMed] [Google Scholar]
- 25.Fosså A, et al. , Metabolic analysis of amino acids and vitamin B6 pathways in lymphoma survivors with cancer related chronic fatigue. PLoS One, 2020. 15(1): p. e0227384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuczmarski RJ, et al. , 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11, 2002(246): p. 1–190. [PubMed] [Google Scholar]
- 27.Pui CH, et al. , Childhood Acute Lymphoblastic Leukemia: Progress Through Collaboration . J Clin Oncol, 2015. 33(27): p. 2938–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hugh Dunstan R, et al. , Altered amino acid homeostasis and the development of fatigue by breast cancer radiotherapy patients: A pilot study. Clin Biochem, 2011. 44(2–3): p. 208–15. [DOI] [PubMed] [Google Scholar]
- 29.Niblett SH, et al. , Hematologic and urinary excretion anomalies in patients with chronic fatigue syndrome. Exp Biol Med (Maywood), 2007. 232(8): p. 1041–9. [DOI] [PubMed] [Google Scholar]
- 30.Su N, et al. , Correlation between asparaginase sensitivity and asparagine synthetase protein content, but not mRNA, in acute lymphoblastic leukemia cell lines. Pediatr Blood Cancer, 2008. 50(2): p. 274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scholl-Burgi S, et al. , Amino acid cerebrospinal fluid/plasma ratios in children: influence of age, gender, and antiepileptic medication. Pediatrics, 2008. 121(4): p. e920–6. [DOI] [PubMed] [Google Scholar]
- 32.Ruzzo EK, et al. , Deficiency of asparagine synthetase causes congenital microcephaly and a progressive form of encephalopathy. Neuron, 2013. 80(2): p. 429–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanazawa A and Sano I, The distribution of gamma-L-glutamyl-L-glutamine in mammalian tissues. J Neurochem, 1967. 14(5): p. 596–8. [DOI] [PubMed] [Google Scholar]
- 34.Do KQ, et al. , gamma-Glutamylglutamine and taurine concentrations are decreased in the cerebrospinal fluid of drug-naive patients with schizophrenic disorders. J Neurochem, 1995. 65(6): p. 2652–62. [DOI] [PubMed] [Google Scholar]
- 35.Hammond JW, et al. , gamma-Glutamylglutamine identified in plasma and cerebrospinal fluid from hyperammonaemic patients. Clin Chim Acta, 1990. 194(2–3): p. 173–83. [DOI] [PubMed] [Google Scholar]
- 36.Hawkins RA, et al. , Structure of the blood-brain barrier and its role in the transport of amino acids. J Nutr, 2006. 136(1 Suppl): p. 218s–26s. [DOI] [PubMed] [Google Scholar]
- 37.Szewczuk A and Connell GE, Specificity of gamma-glutamyl cyclotransferase. Can J Biochem, 1975. 53(6): p. 706–12. [DOI] [PubMed] [Google Scholar]
- 38.Lee WJ, et al. , Role of oxoproline in the regulation of neutral amino acid transport across the blood-brain barrier. J Biol Chem, 1996. 271(32): p. 19129–33. [DOI] [PubMed] [Google Scholar]
- 39.Samuels S, Fish I, and Freedman LS, Effect of gamma-glutamyl cycle inhibitors on brain amino acid transport and utilization. Neurochem Res, 1978. 3(5): p. 619–31. [DOI] [PubMed] [Google Scholar]
- 40.Lisy V, et al. , Glutamate uptake into cerebral cortex slices is reduced in the presence of a gamma-glutamyl transpeptidase inhibitor. Experientia, 1983. 39(1): p. 111. [DOI] [PubMed] [Google Scholar]
- 41.Kvamme E, et al. , Developmental change of endogenous glutamate and gamma-glutamyl transferase in cultured cerebral cortical interneurons and cerebellar granule cells, and in mouse cerebral cortex and cerebellum in vivo. Neurochem Res, 1985. 10(7): p. 993–1008. [DOI] [PubMed] [Google Scholar]
- 42.Stastny F, et al. , Changes in the activity of gamma-glutamyl transpeptidase induced by kainic acid and surgical lesions of the hippocampal formation in young rats. Brain Res, 1988. 462(1): p. 56–61. [DOI] [PubMed] [Google Scholar]
- 43.Jankaskova B, Lisy V, and Stastny F, Effect of gamma-glutamyl transpeptidase inhibitors on the transport of glutamate into neuronal and glial primary cultures. Int J Dev Neurosci, 1992. 10(3): p. 225–30. [DOI] [PubMed] [Google Scholar]
- 44.Orlowski M and Meister A, The gamma-glutamyl cycle: a possible transport system for amino acids. Proc Natl Acad Sci U S A, 1970. 67(3): p. 1248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rae CD and Williams SR, Glutathione in the human brain: Review of its roles and measurement by magnetic resonance spectroscopy. Anal Biochem, 2017. 529: p. 127–143. [DOI] [PubMed] [Google Scholar]
- 46.Rodgers C, et al. , Fatigue and Oxidative Stress in Children Undergoing Leukemia Treatment . Biol Res Nurs, 2016. 18(5): p. 515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomlinson D, et al. , Pharmacologic interventions for fatigue in cancer and transplantation: a meta-analysis. Curr Oncol, 2018. 25(2): p. e152–e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruddy KJ, Barton D, and Loprinzi CL, Laying to rest psychostimulants for cancer-related fatigue? J Clin Oncol, 2014. 32(18): p. 1865–7. [DOI] [PubMed] [Google Scholar]
- 49.Chang CW, et al. , Systematic review and meta-analysis of nonpharmacological interventions for fatigue in children and adolescents with cancer. Worldviews Evid Based Nurs, 2013. 10(4): p. 208–17. [DOI] [PubMed] [Google Scholar]
- 50.Hyslop S, et al. , Symptom documentation and intervention provision for symptom control in children receiving cancer treatments. Eur J Cancer, 2019. 109: p. 120–128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.