Abstract
Objective:
To compare aminotransferase with platelet ratio index (APRI), liver transplantation, and mortality rates between children with intestinal failure-associated liver disease (IFALD) who received fish oil lipid emulsion (FOLE) or soybean oil lipid emulsion (SOLE).
Study design:
In this multicenter integrated analysis, FOLE recipients (1 g/kg/d) (n=189) were compared with historical controls administered SOLE (up to 3 g/kg/d) (n=73).
Results:
Compared with SOLE, FOLE recipients had a higher direct bilirubin level at baseline (5.8 vs. 3.0 mg/dL, P < .0001). Among FOLE recipients, 65% experienced cholestasis resolution vs. 16% of SOLE (P<0.0001). APRI scores improved in FOLE (1.235 vs. 0.810 and 0.758, P<0.02) but worsened in SOLE recipients (0.540 vs. 2.564 and 2.098, P≤0.0003) when baseline scores were compared with cholestasis resolution and end of study, respectively. Liver transplantation was reduced in FOLE vs. SOLE (4% vs. 12%, P=0.0245). The probability of liver transplantation in relation to baseline DB was lower in FOLE vs SOLE recipients (1% vs. 9% at DB of 2 mg/dL; 8% vs 35% at DB of 12.87 mg/dL, P=0.0022 for both). Death rates were similar (FOLE vs. SOLE: 10% vs. 14% at DB of 2 mg/dL; 17% vs. 23% at a DB of 12.87 mg/dL, P=0.36 for both).
Conclusion
FOLE recipients experienced a higher rate of cholestasis resolution, lower APRI, and fewer liver transplants compared with SOLE. This demonstrates that FOLE may be the preferred parenteral lipid emulsion in children with IFALD when DB reaches 2 mg/dL.
Trial registration
Keywords: Soybean oil, intravenous lipid emulsion, Omegaven, APRI score, intestinal failure associated liver disease, liver transplant
Prolonged PN use can cause IF-associated liver disease (IFALD), defined as a serum direct bilirubin concentration (DB) ≥2 mg/dL.1 Unless PN is discontinued, cholestasis can progress from fibrosis to cirrhosis and death from end-stage liver disease.2 The Pediatric Intestinal Failure Consortium noted a 74.4% incidence of IFALD in children with IF, with a mortality rate of 27% and a liver transplant rate of 26%.3 Soybean oil intravenous (IV) lipid emulsions (SOLE) have been linked with IFALD, possibly due to their phytosterol, high omega-6 fatty acid, and low vitamin E content.4,5
In 2004, a fish oil IV lipid emulsion (FOLE) dosed at 1 g/kg/d was introduced in the US for compassionate use in patients with IFALD. Several single-site, observational studies have demonstrated that in comparison with SOLE, children who received FOLE achieved earlier resolution of cholestasis and had no evidence of essential fatty acid deficiency or growth failure.6–8 However, results for liver transplantation rates were inconsistent.9 Moreover, in some small case series reports, despite cholestasis reversal, hepatic fibrosis persisted in children who have received FOLE for IFALD.10,11
The objective of this report is to present data from a large, multicenter integrated analysis comparing FOLE vs. SOLE in infants with IFALD. We compared biochemical markers of liver injury including aspartate aminotransferase to platelet ratio index (APRI), a noninvasive marker for liver fibrosis, and rates of transplantation and mortality. These data were utilized for Food and Drug Administration (FDA) approval of FOLE as a sole source of intravenous lipid emulsion (ILE) in pediatric patients with IFALD.
Methods
Detailed methods have been previously described.12 Subjects from studies performed at Boston Children’s Hospital (BCH, Boston, MA) and Texas Children’s Hospital (TCH, Houston, TX) were included (clinicaltrials.gov study identifiers NCT00910104 and NCT00738101, respectively). Subjects with IFALD received open label treatment with a 10% FOLE (Omegaven, Fresenius Kabi, Bad Homburg, Germany) under compassionate use protocols and were prospectively followed. Enrollment began in September 2004 at BCH, and September 2007 at TCH and ended in July 2018. For comparison, data were retrieved from historical controls who had received 20% SOLE (Intralipid 20%, Baxter, Deerfield, IL) between February 1999 and September 2011 at BCH, TCH, and Mattel Children’s Hospital at the University of California Los Angeles (UCLA, Los Angeles, CA). Data collected until 30 June 2012 are included in this report.
The studies were conducted in accordance with the ethical principles stated in the Declaration of Helsinki, Good Clinical Practice, International Conference on Harmonisation Guidelines, and all applicable laws and regulations. Written informed consent was obtained from a parent or legal guardian of FOLE recipients prior to any study procedures. A waiver of consent was obtained for SOLE recipients. The study was approved by the local institutional review board at each site.
Inclusion and Exclusion Criteria
At BCH subjects were included if they were <2 years of age, DB ≥2mg/dL, anticipated PN need for ≥ 30 days, and failed to respond to standard therapies for IFALD. Criteria at TCH and UCLA were similar except the subjects were >14 days and <5 years of age and anticipated PN need ≥14 days. At TCH, subjects were required to receive >20% of their calories from PN. All FOLE recipients received SOLE prior to being switched to FOLE; no recipient received FOLE as their initial source of ILE.
Safety Evaluations
Study endpoints were the time to resolution of cholestasis (defined as achieving DB <2mg/dL), number of subjects achieving resolution of cholestasis, time to liver transplantation, number of subjects receiving liver transplantation, time to death, and number of subjects who died. Biochemical markers of liver injury included APRI score, DB, aspartate aminotransferase (AST), and alanine aminotransferase (ALT). Adverse events assessed included sepsis events and catheter-related bloodstream infections. A pediatric end-stage liver disease (PELD) score, used to estimate disease severity, was calculated post hoc for subjects who underwent liver transplantation at the time of listing.13
Statistical analyses
Statistical analyses were performed using the Statistical Analysis System (SAS) (version 9.3) (Cary, NC). The integrated analyses were performed based on the pooled safety data from the studies conducted at BCH and TCH. Results discussed in this report are for the Safety Population, which included all FOLE and SOLE subjects from BCH and TCH, and SOLE subjects from UCLA who could be matched to FOLE subjects at either BCH or TCH. Analyses were performed at baseline, the time of resolution of cholestasis, and at the end of study as defined in the previous report.12
Fisher’s exact test was used for categorical variables and Wilcoxon rank-sum test for continuous variables. The Chi-squared test or Fisher exact test was used to analyze difference in proportions between treatments. Changes from baseline to the time of resolution of cholestasis and end of the study were evaluated for DB, AST, and ALT using an analysis of covariance (ANCOVA) technique with baseline value and treatment as fixed effects. For missing values, the first value within 14 days after baseline or the last value within 14 days prior to resolution of cholestasis or end of study, as applicable, was used.
Time from baseline to resolution of cholestasis was assessed using Kaplan-Meier (KM) cumulative survival probabilities. If a subject discontinued the study without resolution of cholestasis (e.g., due to death or liver transplantation), then the subject’s time to resolution of cholestasis was censored using the last date with information. Estimates of time to resolution of cholestasis, a 95% confidence interval (CI) for median and quartiles, and number and percentage of subjects with resolution of cholestasis were analyzed by treatment. A log-rank test stratified by study was performed.
The time from baseline to death was analyzed using the KM method. If a subject discontinued the study, the subject’s time to death was censored using the last date with available information. KM estimates of time to death, a 95% CI for the median and quartiles, as well as the number and percentage of subjects who died and censored data were assessed by treatment. The time from baseline to liver transplant was similarly analyzed.
Results
FOLE recipients (n = 189) and 73 SOLE recipients were included in this study (Figure 1; available at www.jpeds.com). The proportion of subjects who completed the study was comparable for the FOLE and SOLE groups (64% vs. 63%). The most common reasons for study discontinuation in both groups were death and liver transplantation. Resumption of SOLE (after cholestasis resolution) was also a common reason for discontinuation in the FOLE group.
Figure 1 online.
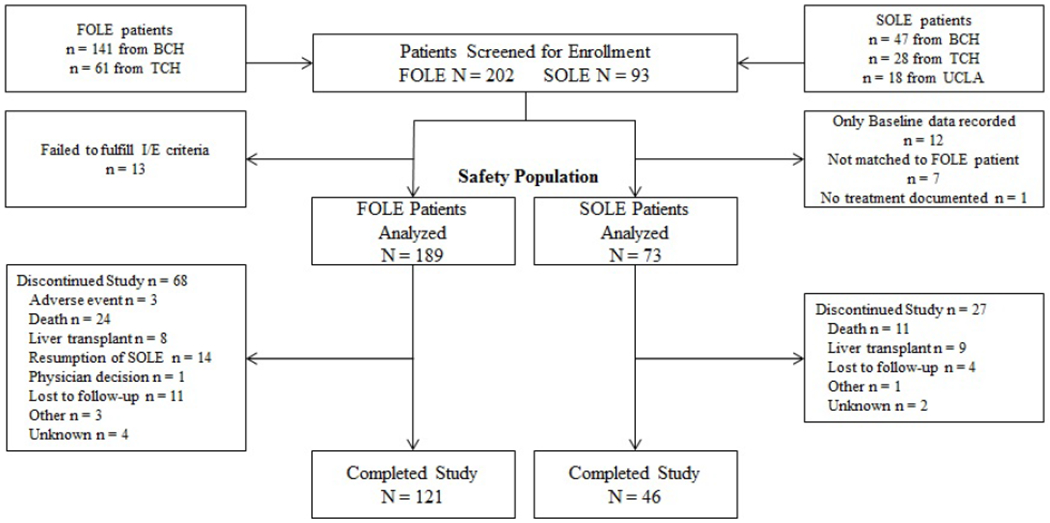
CONSORT Diagram. Disposition of FOLE and SOLE recipients.
A majority of subjects in both groups were male and White (Table I; available at www.jpeds.com). FOLE recipients had a lower median gestational age compared with SOLE recipients (P=0.0350). At baseline, median DB, ALT, and AST levels were higher in FOLE recipients compared with SOLE recipients (P<0.0001 for all). The median total PN (69.0 vs. 72.1 kcal/kg/d, P=0.8645) and enteral nutrition caloric intake (18.8 vs.19.9 kcal/kg/d, P=0.1886) were similar for FOLE and SOLE recipients through the time of resolution of cholestasis. The median ILE dose was lower in FOLE recipients vs. SOLE recipients (0.9 vs. 2.3 g/kg/d, P<0.0001). FOLE recipients received significantly higher dextrose doses compared with SOLE recipients (14.4 vs. 12.3 g/kg/d, P=0.0004).
Table I.
Demographic and baseline characteristics*
| Category Unit | FOLE N = 189 |
SOLE N = 73 |
P-value |
|---|---|---|---|
| Male sex | 109 (57.7) | 43 (58.9) | 0.8897 |
| White race | 119 (63.0) | 37 (50.7) | 0.2799 |
| Unknown race | 39 (20.6) | 23 (31.5) | |
| Gestational age (weeks) | 30.5 (26.0, 35.0) | 33.0 (28.0, 36.0) | 0.0350 |
| Postmenstrual age (weeks) | 41.0 (36.0, 52.0) | 38.0 (34.0, 43.0) | 0.0021 |
| n = 183 | n = 71 | ||
| Body weight, Z-score† | −1.48 (−2.55, −0.63) | −1.30 (−2.03, −0.56) | 0.1597 |
| n = 168 | n = 50 | ||
| Height/length , Z-score† | −1.86 (−3.04, −0.88) | −1.81 (−2.89, −1.16) | 0.4690 |
| n = 162 | n = 49 | ||
| Head circumference, Z-score† | −1.58 (−2.71, −0.62) | −1.19 (−1.62, −0.07) | 0.0156 |
| n = 189 | n = 73 | ||
| DB (mg/dL) | 5.80 (3.50, 9.00) | 3.00 (2.20, 4.40) | <0.0001 |
| n = 170 | n = 47 | ||
| Aspartate aminotransferase (U/L) | 133.53 (77.84, 209.58) | 70.66 (28.74, 133.53) | <0.0001 |
| n = 177 | n = 68 | ||
| Alanine aaminotransferase (U/L) | 88.02 (43.11, 185.63) | 35.33 (17.96, 82.63) | <0.0001 |
DB, conjugated or direct bilirubin, FOLE, fish oil intravenous lipid emulsion, SOLE, soybean oil intravenous lipid emulsion
Data are n (%) or median (interquartile range).
Z-scores represent age-adjusted values
APRI and Liver Indices
The median APRI score progressively improved in FOLE recipients from 1.235 at baseline to 0.810 at the time of resolution of cholestasis (P=0.0167), and to 0.758 at the end of study (P=0.0006). In contrast, the median APRI score worsened in SOLE recipients from 0.540 at baseline to 2.564 at the time of resolution of cholestasis (P<0.0001), and to 2.098 at the end of the study (P=0.0003) (Figure 2). Mean DB, AST, and ALT values showed a significant decrease in FOLE recipients compared with SOLE recipients (Table II; available at www.jpeds.com).
Figure 2.
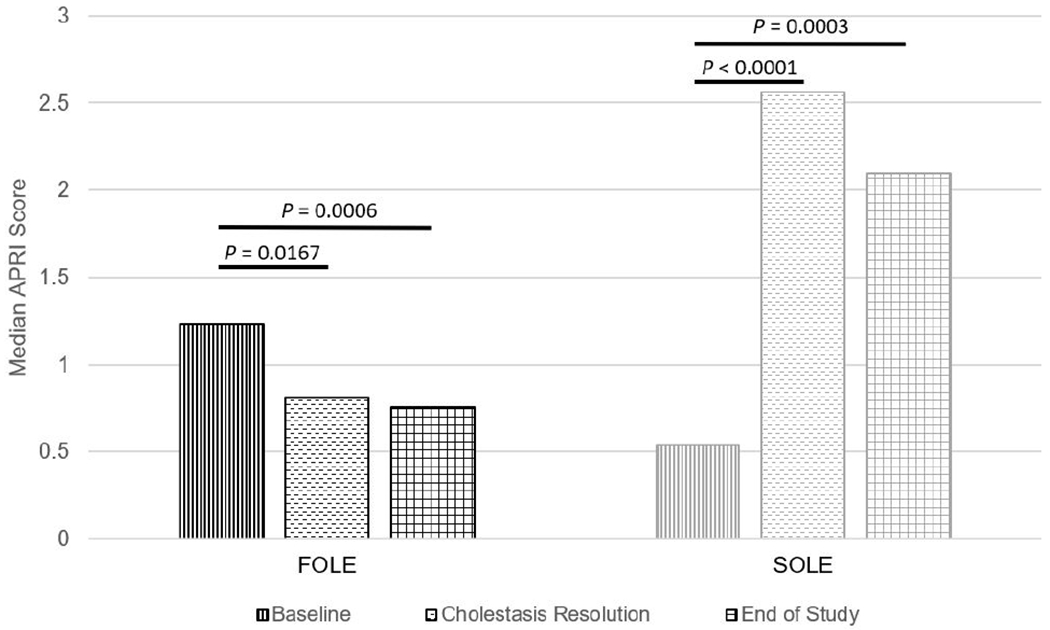
Comparison of median APRI score for FOLE recipients compared with SOLE recipients at baseline, resolution of cholestasis, and end of study. APRI, aspartate aminotransferase to platelet ratio index, FOLE, fish oil intravenous lipid emulsion, SOLE, soybean oil intravenous lipid emulsion.
Table II.
ANCOVA analysis of changes over time in liver function parameters*
| Parameter | Time Point (observations) |
|
|---|---|---|
| Group/Statistic | Resolution of cholestasis† | End of study‡ |
| DB (mg/dL), n | 247 | 236 |
| FOLE | −1.99 [−3.08, −0.91] | −2.55 [−3.72, −1.39] |
| SOLE | 3.95 [2.07, 5.84] | 4.34 [2.36, 6.33] |
| Difference | −5.95 [−8.16, −3.73] | −6.89 [−9.25, −4.54] |
| P-value§ | <0.0001 | <0.0001 |
| ALT (U/L), n | 214 | 207 |
| FOLE | 4.19 [−41.32, 49.70] | −13.77 [−61.68, 33.53] |
| SOLE | 125.15 [46.71, 204.19] | 128.74 [48.50, 208.98] |
| Difference | −121.56 [−213.17, −29.34] | −142.51 [−236.53, −48.50] |
| P-value§ | 0.0098 | 0.0031 |
| AST (U/L), n | 183 | 174 |
| FOLE | −13.17 [−76.65, 50.30] | −23.95 [−91.62, 43.11] |
| SOLE | 317.96 [173.65, 461.68] | 329.34 [180.84, 477.84] |
| Difference | −331.14 [−488.62, −173.05] | −353.29 [−517.37, −189.82] |
| P-value§ | <0.0001 | <0.0001 |
ALT, alanine aminotransferase, AST, aspartate aminotransferase, DB, direct or conjugated bilirubin, FOLE, fish oil intravenous lipid emulsion, SOLE, soybean oil intravenous lipid emulsion
Values represent least squares mean [95% confidence interval] using analysis of covariance (ANCOVA) model.
Resolution of cholestasis was defined as the time point at which DB was determined to be < 2.0 mg/dL.
End of study was defined as the time of study completion or discontinuation.
P-value for Class Effect/Covariate (Type III) by Treatment Group.
More FOLE recipients than SOLE recipients experienced resolution of cholestasis (65% vs. 16%, P<0.0001) (Figure 3). The estimated median time to resolution of cholestasis was 7.6 weeks [95% CI: 6.6, 8.6] for FOLE recipients, and could not be calculated due to a lack of events for SOLE recipients (P<0.0001). In the KM analysis, 100% of FOLE recipients experienced resolution of cholestasis by 52 weeks and this milestone was maintained through 104 weeks of treatment, while only 35% of SOLE recipients experienced resolution of cholestasis by 52 weeks (Figure 4). In a regression analysis, the probability of cholestasis resolution in relation to baseline DB was greater in the FOLE group vs. SOLE group (76% vs. 50% at a DB of 2 mg/dL and 50% vs. 19% at a DB of 12.87 mg/dL, all P-values <0.05) (Figure 5).
Figure 3.
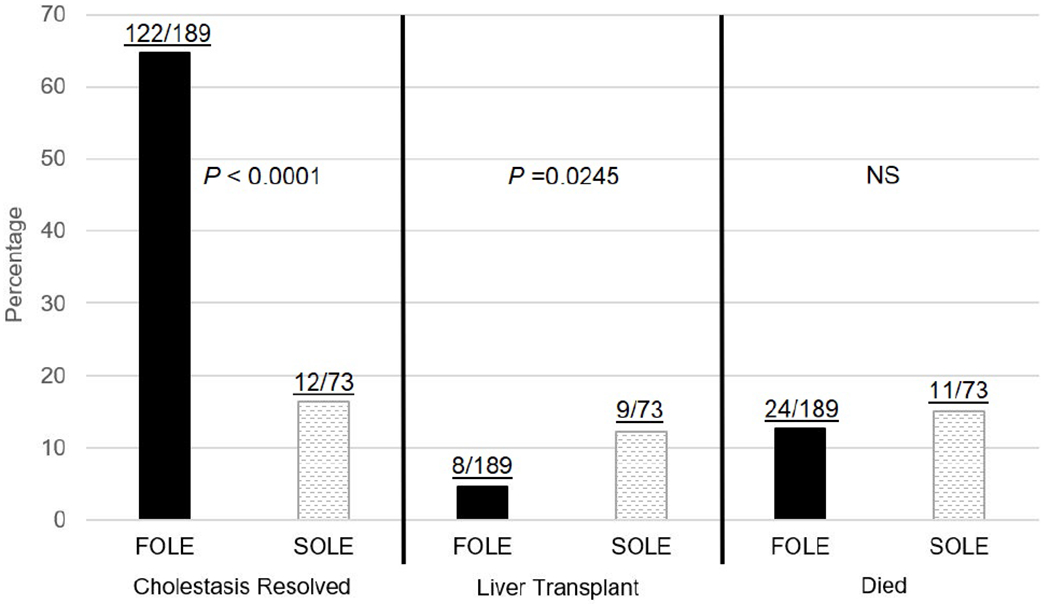
Percentage of subjects with resolution of cholestasis, liver transplantation, and who died by the end of the study. P-values are from Fisher’s exact test (2-sided). FOLE, fish oil intravenous lipid emulsion, NS, not significant, SOLE, soybean oil intravenous lipid emulsion.
Figure 4.
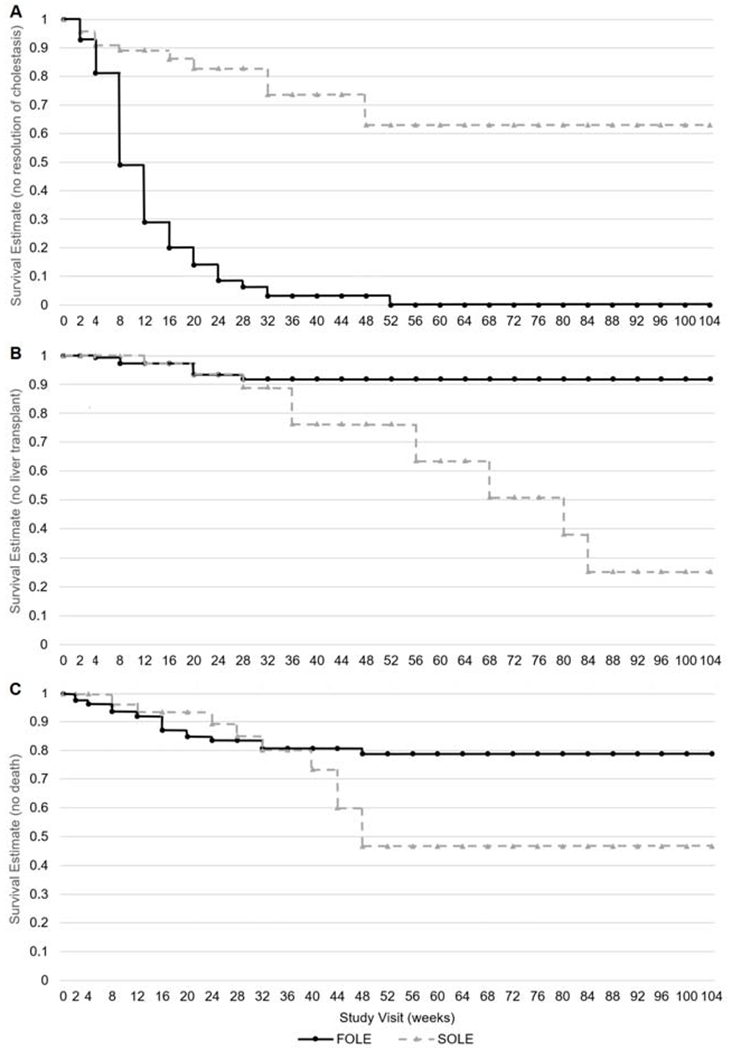
Kaplan-Meier survival estimates for the time to A, resolution of cholestasis, B, liver transplantation, and C, death for FOLE recipients compared with SOLE recipients. Resolution of cholestasis was defined as the time point when DB concentration returned to 2 mg/dL. Kaplan Meier curves were provided until last event or censoring, and were truncated after 2 years. Censoring data are not provided. FOLE, fish oil intravenous lipid emulsion, SOLE, soybean oil intravenous lipid emulsion.
Figure 5.
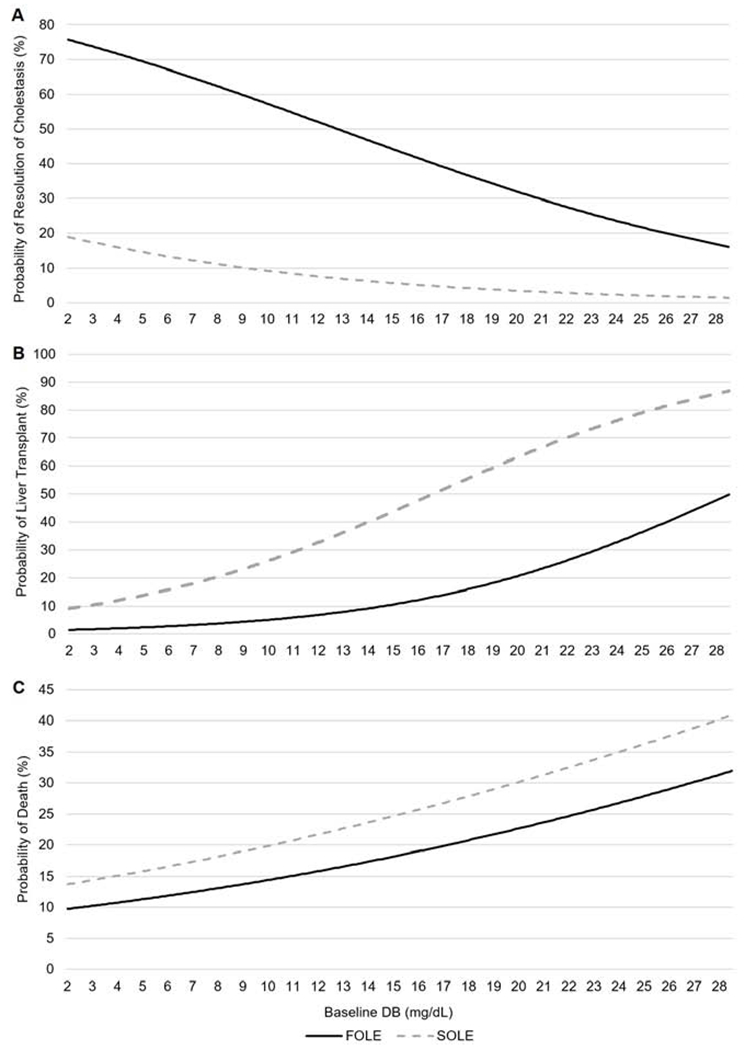
Regression analysis for the estimated probability of A, resolution of cholestasis, B, liver transplantation, and C, death by baseline DB for FOLE recipients compared with SOLE recipients. Resolution of cholestasis was defined as the time point when DB concentration returned to 2 mg/dL. For SOLE probability data, all values which were out of bound of what was observed in the data were truncated. FOLE, fish oil intravenous lipid emulsion, SOLE, soybean oil intravenous lipid emulsion.
Central Line Infections/Central Line Sepsis
In this current study, the number of patients having a central line infection/sepsis was greater in the FOLE group. Among FOLE recipients, 76/189 patients (40.2%) experienced a central line infection or central line sepsis in comparison with 25/73 SOLE recipients (34.2%) (P=0.3987). Nevertheless, when analyzed by frequency per subject-year of exposure, the number of central line infections/sepsis was significantly greater in the SOLE group (P=0.0007). SOLE recipients had approximately 2 central line infections or central line sepsis events per year (56 events per 27.9 subject-years) in comparison with only 1.14 events per year (168 events per 146.8 subject-years) in the FOLE group.
Liver Transplantation
Fewer FOLE recipients underwent liver transplantation compared with SOLE recipients (4% vs. 12%, P=0.0245) (Figure 3), even though the mean PELD score was marginally higher for FOLE recipients than for SOLE recipients (25.9 vs. 20.0, P=0.0612). The estimated median time to liver transplantation was 79.0 weeks [95% CI: 55.7, --] for SOLE recipients and could not be calculated due to lack of events for FOLE recipients (P=0.0310). In the KM analysis, 3% of subjects at 12 weeks in both groups had received a liver transplant. Among the subjects who continued to require ILE at 36 weeks, 8% of FOLE and 24% of SOLE recipients received a transplant. Among subjects who continued to require FOLE through 104 weeks, the need for liver transplantation remained stable at 8%. However, the need for liver transplantation among subjects who continued to receive SOLE at 56 weeks, 68 weeks, 80 weeks and 84 through 104 weeks was 36%, 49%, 62%, and 75%, respectively (Figure 4). In a regression analysis, the probability of liver transplantation in relation to baseline DB was lower in the FOLE group vs. SOLE group (1% vs. 9% at a DB of 2 mg/dL and 8% vs. 35% at a DB of 12.87 mg/dL, P=0.0022 for both) (Figure 5).
Mortality
The incidence of death was similar for FOLE and SOLE recipients (13% vs. 15%, P=0.6858) (Figure 3). Compared with SOLE recipients who died, FOLE recipients who died were more premature at birth (median gestational age of 26 weeks vs. 33 weeks) (P=0.0020) and had higher DB levels at baseline (6.2 mg/dL vs. 3.6 mg/dL, P=0.0505). The median time to death was estimated to be 45.4 weeks [95% CI: 36.9, –] for SOLE recipients, but could not be estimated for FOLE recipients (P=0.4986). In the KM analysis over time, among subjects who continued to require either FOLE or SOLE at 48 weeks, approximately 47% of SOLE were alive compared with 79% of FOLE at the same time point (Figure 4). In a regression analysis, the probability of death in relation to baseline DB was lower in the FOLE group vs. SOLE group (10% vs. 14% at a DB of 2 mg/dL and 17% vs. 23% at a DB of 12.87 mg/dL, P=0.36 for both) (Figure 5).
The events that resulted in death were similar for both groups and consisted primarily of respiratory and cardiac disorders. Four percent of FOLE recipients died because of general disorders, including multiple organ dysfunction syndrome, and 4% of SOLE recipients died because of their hepatobiliary disorders. None of the deaths in FOLE recipients were considered related to the study treatment. Events leading to death in 3 SOLE recipients were considered related to treatment: respiratory failure (1 subject) and hepatic failure-associated events (3 events in 1 subject and 1 event in 1 subject).
Discussion
In this large multicenter integrated safety analysis, children with IFALD who received FOLE demonstrated improved APRI scores and a decreased transplantation rate when compared with children who received SOLE. The probability of cholestasis resolution, transplantation, and death were dependent on baseline DB. These milestones were dependent on ILE type. Consistent with these results and previously published reports, when compared with SOLE recipients, FOLE recipients were more likely to experience cholestasis resolution and demonstrated improved liver indices over time.14,15
Concerns have been raised that, despite the normalization of biochemical and hematologic parameters with FOLE, liver fibrosis may progress.10,16 Although liver biopsy is the standard for fibrosis staging, it is an invasive procedure that requires anesthesia and is associated with bleeding, particularly in children with IFALD.17 APRI scores correlate with the progression of liver fibrosis in patients with cystic fibrosis, chronic hepatitis B infection, and biliary atresia.18–20 In a study of 15 infants with IF who underwent intestinal transplantation alone or in combination with liver transplant, APRI scores were associated with liver fibrosis progression.21 Similarly, in a group of pediatric IF patients, APRI scores correlated with bilirubin values and predicted cirrhosis.22 In pediatric patients with IFALD, an APRI score cut-off of 1.6 was associated with a sensitivity of 81% and specificity of 76% (area under the curve, 0.79 95% CI: 0.64-0.91) for advanced fibrosis.23
There are challenges when assessing APRI scores. Unlike other causes of cirrhosis or fibrosis that have a more predictable pattern, IFALD, similar to biliary atresia, is a cholestatic disorder, with an irregular pattern of fibrosis.24 Cirrhosis, however, is an irreversible condition and APRI has been considered more reliable in that situation. As reported by Mutanen et al, despite resolution of cholestasis and portal inflammation, significant liver fibrosis and steatosis persisted in patients even after weaning off PN.25 In that population-based, cross-sectional study on liver histology in pediatric IF, the authors reported that intracellular cholestasis was due to parenteral dextrose rather than ILE dose as it was their practice to avoid ILE among patients who develop signs of IFALD. This may have accounted for their observation that steatosis, more typically associated with adult PN patients26, was equally common during (50%) and after weaning off PN (45%) and correlated with duration of PN and absolute and age-adjusted small bowel length. Moreover, in the majority of cases, they reported that liver histology remained abnormal up to 9 years after weaning off PN.
Others, however, have reported that the type of ILE does influence fibrosis status. Pastor-Clerigues et al reported that in 10 adult patients with IFALD, inflammatory and profibrotic markers improved when ILE was switched from a soybean oil containing ILE to FOLE.27 In 2 patients administered FOLE for 4 months, the inflammatory, profibrotic, and clinical parameters of IFALD reversed within the first month of therapy. This response was maintained for the duration of FOLE therapy but decreased when soybean oil-containing ILE was reintroduced. The other patients receiving chronic soybean oil ILE showed elevated inflammatory and profibrotic parameters. As part of this same study, liver epithelial to mesenchymal transition (EMT) was induced by transforming growth factor beta 1 (TGFβ1) to evaluate in vitro liver fibrosis. FOLE suppressed the inflammatory response when in vitro human monocytes were stimulated with lipopolysaccharide but increased with soybean oil. In other experiments, TGFβ1 induced EMT that was suppressed by FOLE and enhanced by soybean oil.
The type of ILE may also influence the interpretation of APRI scores. The report by Diaz et al included only patients who received PN a minimum of three months and had a liver biopsy from January 2006 until November 2010.22 There was some selection bias as it was not customary to biopsy patients unless transplant was being considered. In that same study, patients ranging in age from 0.2 to 19 years (mean 2.03, SD 3.2 years) were included. Of those, 29 patients (60.4%) had been exposed to FOLE which may have also confounded the findings, as the use of FOLE may impact both AST and platelet values. [Unpublished data].28 Among those receiving FOLE, APRI scores improved as the AST values declined and platelet counts rose but similar to what was observed by Mutanen et al, histological changes lagged, making the APRI scores less useful in assessing fibrosis. In contrast, the APRI scores of cirrhotic patients did correlate with their histology.
Nandivada et al reviewed the natural history of cirrhosis in a cohort of children with cirrhosis due to IFALD whose biochemical cholestasis reversed after treatment with FOLE.14 In that study, the mean APRI decreased from 1.9 ± 1.8 at initiation of FOLE to 0.5 ± 0.3 at 12 months after cholestasis resolution (P<0.001). Transaminases also decreased after resolution of cholestasis and remained low: AST and ALT decreased from 162 IU/L and 126 IU/L at initiation of FOLE to 72 IU/L and 62 IU/L at 12 months after cholestasis resolution, respectively (P<0.001).
In our study, the median APRI scores, though high at baseline in the FOLE group, normalized and continued to progressively decline with FOLE, as demonstrated at the time of resolution of cholestasis and the end of study. These high APRI scores at baseline may have been the result of capillarization of the sinusoids as a result of hypersplenism or portal hypertension despite the fibrosis being mild and may be a limitation of using this noninvasive diagnostic tool.29 Moreover the etiology of thrombocytopenia seen in liver disease is multifactorial and may be due to lineage-specific thrombopoietin that is predominately produced in the liver and expressed by the hepatocytes, but is diminished when liver cell mass is severely damaged leading to reduced thrombopoiesis in the bone marrow with subsequent thrombocytopenia.30 Other factors such as sepsis, infection, and bacterial translocation, can also predispose IF patients to thrombocytopenia.31 Over time, infection rates and septic events improved in FOLE recipients which may have also contributed to the improved platelet counts. Thus, the decrease in APRI scores over time observed in the FOLE recipients suggests a gradual recovery of hepatic function as demonstrated by the improved platelet counts. Furthermore, despite the limitations associated with the use of the APRI score and although FOLE recipients did have some degree of fibrosis, our findings suggest fibrosis did not progress to cirrhosis and is consistent with the reduced incidence of liver transplantation seen in that population. In contrast, APRI scores increased in the SOLE group to values > 2.0, suggesting worsening fibrosis and possibly cirrhosis, which was reflected by a higher need for transplantation.
Similar to previous reports32, 33, the incidence of liver transplantation in our study was lower for FOLE recipients vs. SOLE recipients (4% vs. 12%, P=0.0245). The PELD score was designed for children under 12 years of age to estimate their liver disease progression and likely survival while awaiting liver transplantation and utilizes total serum bilirubin, international normalized ratio (INR), height, weight, and albumin.34 Despite having higher PELD scores at baseline, fewer FOLE recipients underwent liver transplantation compared with SOLE recipients, correlating with the FOLE recipients’ improved status. Further supporting these results, the incidence of transplantation was stable at 6.5% at 20 weeks and 8.0% at 84 weeks in FOLE recipients, and it gradually increased from 6.5% at 20 weeks to 74.6% at 84 weeks in SOLE recipients. In a case-cohort study of 91 infants with IFALD, when compared with a contemporary cohort of children who received SOLE, children who received FOLE demonstrated a significantly lower rate of death or liver transplantation (9.5% [n=1] vs. 34.7% [n=6], P=0.005).32 In this study, the probability of liver transplantation increased as baseline DB increased in both the FOLE group and SOLE group. However, this risk was approximately 4-fold higher (8% vs. 35% at DB of 12.87 mg/dL) in the SOLE group. In fact, some FOLE subjects with a PELD score ≥15 and baseline DB ≥15 mg/dL at the time of FOLE initiation still experienced biochemical resolution of their cholestasis.35 Nonetheless, like previous reports, this study suggests that early initiation of FOLE leads to better outcomes, particularly in preterm infants.9 To date, there is still no definitive test or factor to identify which patients have irreversible liver disease, suggesting that all PN-dependent patients with IFALD should be offered FOLE in a timely manner.
The use of FOLE may have contributed to our findings by lowering infection rates over time as catheter-related bloodstream infections and sepsis can also influence direct serum bilirubin concentrations. In this study, when analyzed by frequency per subject-year of exposure, the number of central line infections/sepsis events was significantly greater in SOLE recipients in comparison with those receiving FOLE.
With the improved care of children with IF, mortality from IFALD has decreased from approximately 27% prior to 2004 to > 10%.3,14 In this analysis, the incidence of death was similar for FOLE and SOLE recipients. However, no deaths were attributed to FOLE, whereas 3 deaths were considered related to SOLE. Despite a similar death incidence, at week 48 the likelihood of a SOLE recipient dying was more than double that for an FOLE recipient. This is notable because FOLE recipients were sicker at baseline and initiation with FOLE was reserved for subjects not expected to recover with conventional interventions. Moreover, deaths among FOLE recipients were more likely related to their prematurity at baseline and the severity of their underlying disease. In comparison, deaths among SOLE recipients were more likely to be related to liver disease.
Regarding study limitations, a randomized controlled trial (RCT) study design would have been preferable. However, ethical considerations, lack of equipoise, and recruitment challenges excluded the possibility of an RCT. Additional limitations include a relatively small sample size and changes in surgical, medical, and nutritional practice between the two eras that could not be controlled for in this study. Moreover, the use of APRI scores should be considered only as an ancillary tool to be used in conjunction with clinical status and biopsy/ultrasound findings when available.
The results from this integrated analysis demonstrate that FOLE monotherapy is a safe and well tolerated parenteral lipid for children with IFALD. Children who received FOLE experienced a higher rate of cholestasis resolution, had lower APRI scores, and had fewer liver transplants compared with those who received SOLE. Disease progression was improved when FOLE monotherapy was started as soon as DB reached 2 mg/dL. For these reasons, FOLE should be considered the preferred ILE source for children with IFALD who require parenteral lipids as soon as DB reaches 2 mg/dL.
Supplementary Material
Acknowledgements
We thank the following people and organizations for their invaluable assistance with the conduct of the studies: Alexis Potemkin, Alison O’Loughlin, The Center for Advanced Intestinal Rehabilitation Program, the Department of Pharmacy, Members of the Puder Lab, and the Vascular Biology Program at BCH; Keli Hawthorne, Steve Abrams, Laura Gollins, the Children’s Research Center, and the Department of Investigational Pharmacy at TCH; and Julie Calderon, Karrie V. Ly, Smurthi Murthy, Robert S. Venick, Stephen B. Shew, and James C. Y. Dunn at UCLA. We also acknowledge M.A.R.C.O. GmbH & Co, KG for assistance with the statistical analyses and TechMedWriting Services, LLC for medical writing assistance.
K.G. is a consultant for Pronova/BASF, Northsea Therapeutics, Xellia Pharmaceuticals, Pfizer Pediatric Center of Excellence, Baxter, and has received research support from Northsea Therapeutics, Otsuka Pharmaceutical Company, Alcresta, Fresenius Kabi, the FDA Orphan Drug Development Grant Program, and the March of Dimes. M.Pu. is a consultant for Pronova/BASF, Northsea Therapeutics, and has received research support from Northsea Therapeutics, Otsuka Pharmaceutical Company, Alcresta, Fresenius Kabi, the FDA Orphan Drug Development Grant Program, and the March of Dimes; Patent/Royalties for Omegaven are forthcoming. K.C. is a consultant for Fresenius Kabi, Mead Johnson, Prolacta, and Baxter and has received research support from Fresenius Kabi and NIH/NCATS KL2TR000122. M.Pr. is a consultant for Fresenius Kabi.
List of Abbreviations
- ALT
Alanine aminotransferase
- ANCOVA
Analysis of covariance
- APRI
Aspartate aminotransferase to platelet ratio index
- AST
Aspartate aminotransferase
- BCH
Boston Children’s Hospital
- DB
Direct or conjugated bilirubin
- FOLE
Fish oil intravenous lipid emulsion
- IFALD
Intestinal failure-associated liver disease
- IV
Intravenous
- PELD
Pediatric end-stage liver disease score
- SOLE
Soybean oil intravenous lipid emulsion
- TCH
Texas Children’s Hospital
- UCLA
University of California Los Angeles
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability The datasets generated and or analyzed during this study are available from the corresponding author on reasonable request.
References
- 1.Freund HR. Abnormalities of liver function and hepatic damage associated with total parenteral nutrition. Nutrition. 1991;7:1–5. [PubMed] [Google Scholar]
- 2.Chan S, McCowen KC, Bistrian BR, Thibault A, Keane-Ellison M, Forse RA, et al. Incidence, prognosis, and etiology of end-stage liver disease in patients receiving home total parenteral nutrition. Surgery. 1999;126:28–34. [DOI] [PubMed] [Google Scholar]
- 3.Squires RH, Duggan C, Teitelbaum DH, Wales PW, Balint J, Venick R, et al. Pediatric Intestinal Failure Consortium. Natural history of pediatric intestinal failure: initial report from the Pediatric Intestinal Failure Consortium. J Pediatr. 2012;161:723–8.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clayton PT, Whitfield P, Iyer K. The role of phytosterols in the pathogenesis of liver complications of pediatric parenteral nutrition. Nutrition. 1998;14:158–64. [DOI] [PubMed] [Google Scholar]
- 5.Ng K, Stoll B, Chacko S, Saenz de Pipaon M, Lauridsen C, Gray M, et al. Vitamin E in new-generation lipid emulsions protects against parenteral nutrition-associated liver disease in parenteral nutrition-fed preterm pigs. JPEN J Parenter Enteral Nutr. 2016;40:656–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Havranek T, Armbrecht E, Scavo LM. Compassionate use of omegaven in preterm neonates with parenteral nutrition associated direct hyperbilirubinemia. J Pediatr Neonatal Care. 2014;1:00004. [Google Scholar]
- 7.Strijbosch RA, Lee S, Arsenault DA, Andersson C, Gura KM, Bistrian BR, et al. Fish oil prevents essential fatty acid deficiency and enhances growth: clinical and biochemical implications. Metabolism. 2008;57:698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Meijer VE, Le HD, Meisel JA, Gura KM, Puder M. Parenteral fish oil as monotherapy prevents essential fatty acid deficiency in parenteral nutrition-dependent patients. J Pediatr Gastroenterol Nutr. 2010;50:212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calkins KL, Dunn JC, Shew SB, Reyen L, Farmer DG, Devaskar SU, et al. Pediatric intestinal failure-associated liver disease is reversed with 6 months of intravenous fish oil. JPEN J Parenter Enteral Nutr. 2014;38:682–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumoto CS, Kaufman SS, Island ER, Kallakury B, Yazigi NA, Khan KM, et al. Hepatic explant pathology of pediatric intestinal transplant recipients previously treated with omega-3 fatty acid lipid emulsion. J Pediatr. 2014;165:59–64. [DOI] [PubMed] [Google Scholar]
- 11.Mercer DF, Hobson BD, Fischer RT, Talmon GA, Perry DA, Gerhardt BK, et al. Hepatic fibrosis persists and progresses despite biochemical improvement in children treated with intravenous fish oil emulsion. J Pediatr Gastroenterol Nutr. 2013;56:364–9. [DOI] [PubMed] [Google Scholar]
- 12.Gura K, Premkumar MH, Calkins KL, Puder M. Intravenous Fish Oil Monotherapy as a Source of Calories and Fatty Acids Promotes Age-Appropriate Growth in Pediatric Patients with Intestinal Failure-Associated Liver Disease. J Pediatr. 2020. Ap;219:98–105. E4. doi: 10.1016/j.jpeds.2019.12.065. [DOI] [PubMed] [Google Scholar]
- 13.McDiarmid SV, Anand R, Lindblad AS; Principal investigators and institutions of the studies of pediatric liver transplant (SPLIT) research group. Development of a pediatric end-stage liver disease score to predict poor outcome in children awaiting liver transplantation. Transplantation. 2002;74:173–81. [DOI] [PubMed] [Google Scholar]
- 14.Nandivada P, Chang MI, Potemkin AK, Carlson SJ, Cowan E, O’Loughlin AA, et al. The natural history of cirrhosis from parenteral nutrition-associated liver disease after resolution of cholestasis with parenteral fish oil therapy. Ann Surg. 2015;261:172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nandivada P, Fell GL, Mitchell PD, Potemkin AK, OĽoughlin AA, Gura KM, et al. Long-term fish oil lipid emulsion use in children with intestinal failure-associated liver disease. JPEN J Parenter Enteral Nutr. 2017;41:930–7. [DOI] [PubMed] [Google Scholar]
- 16.Soden JS, Lovell MA, Brown K, Patrick DA, Sokol RJ. Failure of resolution of portal fibrosis during omega-3 fatty acid lipid emulsion therapy in two patients with irreversible intestinal failure. J Pediatr. 2010;156:327–331. [DOI] [PubMed] [Google Scholar]
- 17.Dezsőfi A, Baumann U, Dhawan A, Durmaz O, Fischler B, Hadzic N, et al. ; ESPGHAN Hepatology Committee. Liver biopsy in children: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr. 2015;60:408–20. [DOI] [PubMed] [Google Scholar]
- 18.Loaeza-del-Castillo A, Paz-Pineda F, Oviedo-Cárdenas E, Sánchez-Avila F, Vargas-Vorácková F. AST to platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosis. Ann Hepatol. 2008;7:350–7. [PubMed] [Google Scholar]
- 19.D’Souza RS, Neves Souza L, Isted A, Fitzpatrick E, Vimalesvaran S, Cotoi C, et al. AST-to-platelet ratio index in non-invasive assessment of long-term graft fibrosis following pediatric liver transplantation. Pediatr Transplant. 2016;20:222–6. [DOI] [PubMed] [Google Scholar]
- 20.Leung DH, Khan M, Minard CG, Guffey D, Ramm LE, Clouston AD, et al. Aspartate aminotransferase to platelet ratio and fibrosis-4 as biomarkers in biopsy-validated pediatric cystic fibrosis liver disease. Hepatology. 2015;62:1576–83. [DOI] [PubMed] [Google Scholar]
- 21.Mangus RS, O’Connor MG, Tector AJ, Lim JD, Vianna RM. Use of the aspartate aminotransferase to platelet ratio index to follow liver fibrosis progression in infants with short gut. J Pediatr Surg. 2010;45:1266–73. [DOI] [PubMed] [Google Scholar]
- 22.Díaz JJ, Gura KM, Roda J, Perez-Atayde AR, Duggan C, Jaksic T, et al. Aspartate aminotransferase to platelet ratio index correlates with hepatic cirrhosis but not with fibrosis in pediatric patients with intestinal failure. J Pediatr Gastroenterol Nutr. 2013;57:367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rumbo C, Martinez MI, Cabanne A, Trentadue J, Fernández A, Gondolesi G. Utility of aminotransferase/platelet ratio index to predict liver fibrosis in intestinal failure-associated liver disease in pediatric patients. JPEN J Parenter Enteral Nutr. 2017;41:884–89. [DOI] [PubMed] [Google Scholar]
- 24.Lind RC, Verkade HJ, Porte RJ, Hulscher JB. Aspartate transaminase-to-platelet ratio index is not correlated with severity of fibrosis or survival in children with biliary atresia. J Pediatr Gastroenterol Nutr. 2012;54:698. [DOI] [PubMed] [Google Scholar]
- 25.Mutanen A, Lohi J, Heikkilä P, Koivusalo AI, Rintala RJ, Pakarinen MP. Persistent abnormal liver fibrosis after weaning off parenteral nutrition in pediatric intestinal failure. Hepatology. 2013;58:729–38. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed S, Innes JK, Calder PC. Influence of different intravenous lipid emulsions on fatty acid status and laboratory and clinical outcomes in adult patients receiving home parenteral nutrition: A systematic review. Clin Nutr. 2020;S0261-5614(20)30379-4. [DOI] [PubMed] [Google Scholar]
- 27.Pastor-Clerigues A, Marti-Bonmati E, Milara J, Almudever , Cortijo J Anti-inflammatory and anti-fibrotic profile of fish oil emulsions used in parenteral nutrition-associated liver disease. PLoS One. 2014;9:e115404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gura KM, Diaz JJ, Roda J, Perez-Atayde AR, Duggan C, Jaksic T, et al. Aspartate aminotransferase to platelet index is predictive of cirrhosis but not of fibrosis in pediatric patients with intestinal failure. Presented at: 33th European Society for Parenteral and Parenteral Nutrition Congress on Clinical Nutrition & Metabolism; September 4, 2011; Gothenburg, Sweden. [Google Scholar]
- 29.Venturi C, Sempoux C, Bueno J, Ferreres Pinas JC, Bourdeaux C, Abarca-Quinones J, et al. Novel histologic scoring system for long-term allograft fibrosis after liver transplantation in children. Am J Transplant. 2012;12:2986–96. [DOI] [PubMed] [Google Scholar]
- 30.Peck-Radosavljevic M Thrombocytopenia in liver disease.Can J Gastroenterol. 2000. November;14 Suppl D:60D–66D. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell O, Feldman DM, Diakow M, Sigal SH. The pathophysiology of thrombocytopenia in chronic liver disease. Hepat Med. 2016;8:39–50. Published 2016 April 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puder M, Valim C, Meisel JA, Le HD, de Meijer VE, Robinson EM, et al. Parenteral fish oil improves outcomes in patients with parenteral nutrition-associated liver injury. Ann Surg. 2009;250:395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Premkumar MH, Carter BA, Hawthorne KM, King K, Abrams SA. High rates of resolution of cholestasis in parenteral nutrition-associated liver disease with fish oil-based lipid emulsion monotherapy. J Pediatr. 2013;162:793–98. [DOI] [PubMed] [Google Scholar]
- 34.Shneider BL, Neimark E, Frankenberg T, Arnott L, Suchy FJ, Emre S. Critical analysis of the pediatric end-stage liver disease scoring system: a single center experience. Liver Transpl. 2005;11:788–95. [DOI] [PubMed] [Google Scholar]
- 35.Nandivada P, Baker MA, Mitchell PD, O’loughlin AA, Potemkin AK, Anez-Bustillos L, et al. Predictors of Failure of Fish-Oil Therapy for Intestinal Failure-Associated Liver Disease in Children. Am J Clin Nutr. 2016;104:663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


