Abstract
DNA methylation provides a pivotal layer of epigenetic regulation in eukaryotes that has significant involvement for numerous biological processes in health and disease. The function of methylation of cytosine bases in DNA was originally proposed as a “silencing” epigenetic marker and focused on promoter regions of genes for decades. Improved technologies and accumulating studies have been extending our understanding of the roles of DNA methylation to various genomic contexts including gene bodies, repeat sequences and transcriptional start sites. The demand for comprehensively describing DNA methylation patterns spawns a diversity of DNA methylation profiling technologies that target its genomic distribution. These approaches have enabled the measurement of cytosine methylation from specific loci at restricted regions to single-base-pair resolution on a genome-scale level. In this review, we discuss the different DNA methylation analysis technologies primarily based on the initial treatments of DNA samples: bisulfite conversion, endonuclease digestion and affinity enrichment, involving methodology evolution, principles, applications, and their relative merits. This review may offer referable information for the selection of various platforms for genome-wide analysis of DNA methylation.
Keywords: DNA methylation, DNA hydroxymethylation, next-generation sequencing, bisulfite conversion, endonuclease digestion, affinity enrichment, microarray
1. DNA methylation and hydroxymethylation
1.1. The functions and patterns of DNA methylation
DNA methylation generally refers to the covalent addition of methyl groups to the 5-carbon of the cytosine rings occurring naturally in archaea, bacteria and eukaryotic species. It occurs primarily in the sequence context of CpG dinucleotides or CpHpG (H = A, T, C), which is in the linear 5’ to 3’ sequence of DNA where a cytosine and a guanine nucleotide are adjacent or one base apart[1–4]. The methylation of cytosine residues to create s-methylcytosine at CpG sites was initially reported independently by two research teams in 1975 that serve as a vital epigenetic modification in vertebrates[5, 6]. It was originally proposed as a “silencing” epigenetic mark[7]. As one of the most important epigenetic marks, DNA methylation has been widely appreciated as reversible and mitotically inheritable. DNA methylation plays key roles in local control of gene expression[8, 9], the establishment and maintenance of cellular identity[2, 10], the regulation of mammalian embryonic development[11–13] and other biological processes[14, 15].
CpG dinucleotides are randomly distributed throughout the genome and most of them are methylated, while a fraction of CpG dinucleotides are clustered with lower methylation levels, termed CpG island (CGI). Although there is still some controversy about the exact definition of a CGI[16], it is generally considered to be a region with over 200 bp and a CG dinucleotide percentage greater than 50%[2]. CpG sites in CGIs are mostly in a non-methylated state, which allows the region to avoid undergoing mutational 5-methycytosine (5mC) deamination to thymine. CGIs exist in more than half of the genes in a vertebrate genome. They are commonly enriched in gene promoter regions, often overlap transcription start sites (TSS), and their hypermethylation is frequently associated with transcriptional repression. There are several primary mechanisms by which DNA methylation of promoters and distal regulatory regions control gene expression[2]. Hypermethylation of CGIs in regulatory sequences and regions may destabilize nucleosomes and recruit related proteins, remodeling of chromatin structure, and subsequent inhibition of transcription[1]. Methyl-CpG-binding proteins (MBPs) can recognize and bind multiple methylated CpG sites in CGIs, which can manage site-specific chromatin reorganization through attracting epigenetic modifiers, resulting in transcriptional repression states[17]. In addition, methylated CpG sites can obstruct transcription initiation through blocking the binding of transcription factors, such as CREB, c-Myc and E2F1[18]. Moreover, DNA methylation can also remodel transcription complexes and interrupt gene transcription through repositioning nucleosomes[19].
Importantly, it has become increasing clear that DNA methylation in the bodies of genes, repetitive DNA sequences, and control regions outside of the TSS are involved in the regulation of gene expression[20, 21]. For example, heavily methylated CpG sites were found in the gene bodies of transcriptional activated genes in mammals[22–24] and plants[25–27]. DNA methylation distributed in the gene bodies might stimulate transcription elongation[7] and have an impact on splicing[28, 29]. Gene body methylation (mostly outside CGIs) can primarily silence repetitive elements of DNA, such as Alu, LINE1 and retroviruses[30]. On the other hand, methylation in DNA repeat sequences such as centromeres is likely to suppress transposable elements expression and thus have an important role in chromosomal and genome stability[31]. The hypermethylation in repetitive DNA sequences introduced from intragenomic parasites can prevent the transcription of the parasitic element and also may facilitate transcription initiation of the host genes[30]. Accumulating evidence also elucidates the important function of the methylation status in control regions such as insulators and enhancers outside TSS[32, 33].
DNA methylation is critical for embryonic development and the establishment and maintenance of cellular identity. Demethylation of DNA is accepted as being required for embryonic development and the pluripotency of embryonic stem cells (ESCs)[34, 35]. In the process of cell differentiation, ESCs gradually narrow their identities into different cell types along with the loss of pluripotency and plasticity. In addition, DNA methylation is attenuated during development in gene regulatory elements such as enhancers, accompanied by a global gain of DNA methylation in developmental-, gamete- and pluripotency-specific genes, resulting in differentiated cell types with different methylomic profiles[18]. Remarkably, disarranged reprogramming (elimination and reconstruction) of the methylome during early embryogenesis and gametogenesis can disrupt ontogenesis, leading to persistent physiological and metabolic changes in the fetus and altered susceptibility of the offspring to various diseases in later life[36–39].
1.2. The roles of DNA hydroxymethylation
5-Hydroxymethylcytosine (5hmC), a DNA pyrimidine nitrogen base derived from cytosine, was first discovered in bacteriophage DNA[40] and was detected in mammalian genomes for the first time in 1972[41]. In 2009, two independent research teams described the existence and function of 5hmC in mammalian embryonic stem cells[42] and neurons[43]. Since then, 5hmC and its natural creators, ten-eleven-translocation (TET) proteins, have received increasing interest concerning their roles in epigenetic modifications. 5hmC is now widely accepted as the sixth base after 5mC, the fifth base, in the mammalian genome[44]. In the process of an active DNA demethylation, TET proteins can oxidize 5mC to 5hmC, 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC)[42, 45–47], among which 5hmC is the most abundant form in almost all mammalian tissues and cells[43, 46, 48–50].
5hmC has been proven to be a fundamental and stable epigenetic modification in its own right[51–53]. In contrast to 5mC, the content of 5hmC is much lower in adult mammals. The distribution of 5hmC is variable, tissue-specific and non-random[54–56], which directly influences the equilibrium of DNA methylation in normal physiological activities[57]. 5hmC is abundant in embryonic stem cells and neurons[41–43, 58]. In the brain, 5hmC mainly exists at promoter regions, while 5mC is mostly found at intragenic regions[59]. In addition, the level of 5hmC is high in developed neuros and low in the stem cell areas[48, 60]. It is also noted that the content of 5hmC is dramatically decreased in various cancers, including brain, breast, liver, lung, skin, kidney, prostate and colon cancers[61–67]. These findings suggest the important roles of 5hmC in tumorigenesis and depleted 5hmC could be an important indicator in cancer diagnosis[67, 68]. Due to its enrichment in the central nervous system, 5hmC has been suggested to be pivotal in maintaining the structure and function of neurons[53, 69, 70], and its alterations were proven to be associated with nervous system diseases[71–75]. 5hmC also plays key roles in epigenome reprogramming[76, 77], DNA demethylation initiation[78, 79], cellular differentiation[80], aging[81, 82] and gene expression regulation[83, 84] in mammals. For example, contrary to 5mC, which is generally involved with the inhibition of gene expression, 5hmC has been shown to be associated with increased gene expression[59, 82].
Emerging evidences indicate that the roles of DNA methylation and hydroxymethylation vary with different genetic contexts and its function in the regulation of gene expression is more nuanced and complex than originally thought. A comprehensive understanding of DNA methylation and hydroxymethylaiton patterns and corresponding functions is necessary for interpreting changes of this biomarker observed in diseases. Much of the aforementioned information about DNA methylation and DNA hydroxymethylation status including genomic distribution and the function of different gene regions and elements are attributed, to a large extent, to the revolution in methylation analysis technologies. This especially holds true for improved genome-scale mapping of methylation over the past decades varying from restricted to a specific locus to the entire methylome at a single-base-pair resolution.
Here, we review the methodology of current and emerging DNA methylation and hydroxymethylation technologies, with an emphasis on the evolution, principles, and application of genome-wide DNA methylation profiling techniques. These approaches hold promise for facilitating our comprehensive understanding of DNA methylation patterns of any cells, tissues, or organisms in the context of growth, development, health, and diseases, as well as their interactions with environmental cues. This information may also offer referable suggestions for the selection of genome-wide analysis of DNA methylation from various platforms.
2. An overview of DNA hydroxymethylation detection methodology
After its rediscovery, 5hmC has attracted tremendous attention and accumulating evidence has revealed crucial biological roles of this base modification. Owing to the similar structures of 5mC and 5hmC, most prominent techniques, such as bisulfite sequencing[85, 86] and enzymatic approaches[86], for detection of 5mC cannot distinguish the rare 5hmC from 5mC. Therefore, the development of reliable and easy-to-use analytical technologies for large-scale mapping of 5hmC in the genome is critical and highly desirable. Here, we first provide an overview of DNA hydroxymethylation methodology.
In 2009, two independent research teams brought 5hmC back into the spotlight with 32P-radioactive labeling thin layer chromatography (TLC) technology[42, 43]. The TLC method is low cost, easy-to-use and has a detection limit of ~0.08% of total nucleotides, which allows detection of 5hmC from embryonic stem cells and brain tissues. However, TLC fails to yield a signal of 5hmC with genomic DNA extracted from cancer cells and other cultured cells[44]. In addition, the radioactive substrates required in TLC could be harmful to the operator and environment. Münzel et al. developed a more accurate liquid chromatography-mass spectroscopy (LC-MS) method with isotope-labeled 5hmC as an internal standard to investigate the distribution of 5hmC in mammal brains in 2010[87]. This method can quantify the 5hmC in all mouse tissues. To date, many improvements have been made based on LC-MS methods to detect 5hmC abundance in tissues and cells, such as LC-MS/MS[88], ultrapressure liquid-chromatography combined with tandem mass spectrometry (UPLC-MS)[89], UPLC-MS/MS[90] and liquid chromatography electrospray ionization tandem mass spectrometry with multiple reaction monitoring (LC-ESI-MS/MS-MRM)[91]. Those methods, however, are complex, exorbitantly priced and require skilled personnel[92].
Subsequently, antibody affinity pull-down techniques have become available to detect 5hmC[59, 77, 83, 93]. Hydroxymethylated DNA immunoprecipitation (hMeDIP) followed by high-throughput sequencing (hMeDIP-seq) provides a user-friendly and cost-effective strategy for genome-wide distribution of 5hmC[94–96]. Due to the density-dependent recognition of 5hmC, anti-5hmC antibody-based approaches are not very quantitative as expected. As an alternative, antibodies against 5-methylenesulonate, which is generated from 5hmC after a bisulfite treatment, have been shown to be more quantitative than 5hmC antibodies[97]. Anti-5hmC antibodies have also been used in immunohistochemistry (IHC) to investigate 5hmC distribution at CpG and non-CpG dinucleotides in specific cell types[54, 98–100].
In 2010, enzymatic labeling strategies began to be established by employing the T4 bacteriophage β-glucosyltransferase (β-GT) to selectively glycosylate 5hmC but not 5mC, providing a novel paradigm for distinguishing 5hmC from 5mC[55, 101]. Szwagierczak et al. used β-GT to transfer a 3H-labeled glucose to 5hmC and subsequently quantified 5hmC content by scintillation counting[55]. Similarly, Song et al. added an azide-labeled glucose to 5hmC, further chemically modified with biotin through copper-free click reaction[101]. 5hmC can then be detected and quantified in a simple dot-blot assay with avidin-horseradish peroxidase. Since the approach of β-GT labeling is able to modify every single 5hmC in the genomic DNA, this strategy improved the detection limit of 5hmC from 0.004%–0.006% of total nucleotides, allowing ultra-low levels of 5hmC detection in cancer cells and other cultured cells[102]. Besides radiolabeled and azide-labeled glucose, some other functional groups can also be introduced to 5hmC, such as aldehyde[97] and ketone[103]. As glycosylated 5hmC can make CCGG residues insensitive to Msp I, coupling β-GT-based glycosylation modification of 5hmC with restriction enzymes or endonuclease digestion, followed by massive parallel sequencing can reveal 5hmC distribution at single-base-pair resolution[70, 104]. However, this method can only target restriction enzyme cutting site and cannot cover 5hmC loci in noncleavable DNA regions[105–108]. β-GT-mediated glycosylation of 5hmC can also couple with recombinant TET enzyme to distinguish 5hmC at the genomic level, namely TET-assisted bisulfite sequencing (TAB-seq)[103]. Interestingly, glycosylated 5hmC can be recognized by J-binding protein 1 (JBP-1), which is another choice to isolate and immobilize 5hmC for quantification[109].
Another chemical transformation of 5hmC is oxidation. 5fC, a specific oxidative product of 5hmC treated with potassium perrhenate (KRuO4), behaves in a manner analogous to cytosine under bisulfite treatment, allowing only 5mC loci to be detected after oxidative conversion. Subtraction of oxidative bisulfite (oxBS)-generated methylation profile from standard bisulfite-converted methylation profiles can reveal the distribution of 5hmC within the genome. Thus, oxidative bisulfite followed by reduced representation bisulfite sequencing (RRBS) (oxRRBS)[110] and oxBS chemistry combined with Infinium 450K BeadChip (oxBS-450K)[111] have been developed for genomic 5hmC profiling. Recently, some electrogenerated chemiluminescence (ECL) biosensing platforms have been developing for quantitative detection of 5hmC based on the oxidation of 5hmC[112–115] as well.
In the demand of high sensitivity and resolution for 5hmC profiling, He and colleagues described a method for 5hmC detection with single-molecule, real-time (SMRT) DNA sequencing[47]. Modified nucleotides can slow down the incorporation of the DNA polymerase and this increased duration can be monitored in real time. He et al. introduced a chemical modification specific to 5hmC loci and the resulting HS-N3-5gmC induces prominent delays in incorporation kinetics, which can be identified algorithmically at single-base-pair resolution[47]. This method enables direct detection of 5hmC in original DNA and an unmodified template is no longer necessary, due to the markedly elevated interpulse duration[116, 117]. Another promising direction for single-base resolution profiling of 5hmC is nanopore sequencing[118]. However, further development is required before these third-generation sequencing technologies can be applied to widespread application. Perhaps a combination of the aforementioned approaches could provide an attractive solution.
3. Evolution of DNA methylation profiling methodology
In the eukaryotic genome, only a subset of target sequences is established with their unique methylation patterns by dedicated and highly conserved DNA methyltransferases (DNMT). These DNMTs include DNMT3A and DNMT3B, the so-called de novo DNA methyltransferase enzymes, and DNMT1, by which an established pattern of DNA methylation is maintained. The distribution of methylation, therefore, can demarcate regions of transcriptional potential and silence, effectively rendering genetic information to various genes and different cell types. The delineation of genomic distribution and regional patterns of DNA methylation has important implications for understanding its roles in cell identify, ontogenesis and diseases. This recognition has been largely responsible for prompting the massive effort into technological development for evaluating the distribution of methylation across the genome. There have been multiple approaches developed for DNA methylation analysis technologies, which fall broadly into two groups: typing and profiling. Both types of technologies are appropriate for high-throughput application[119–121]. Typing technologies, including restriction-, PCR- and mass spectrometry-based approaches, are typically used when a specific locus needs to be explored in multiple samples[119]. Examples of typing technologies are methylation-specific PCR (MSP) [122, 123], bisulfite sequencing PCR (BSP) [20], bisulfite PCR followed by restriction analysis (COBRA) [124] and bisulfite-PCR followed by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS)[124]. Herein, we place emphasis on reviewing the evolution of DNA methylome profiling methodology primarily based on different mediums and platforms (Figure 1).
Figure 1.
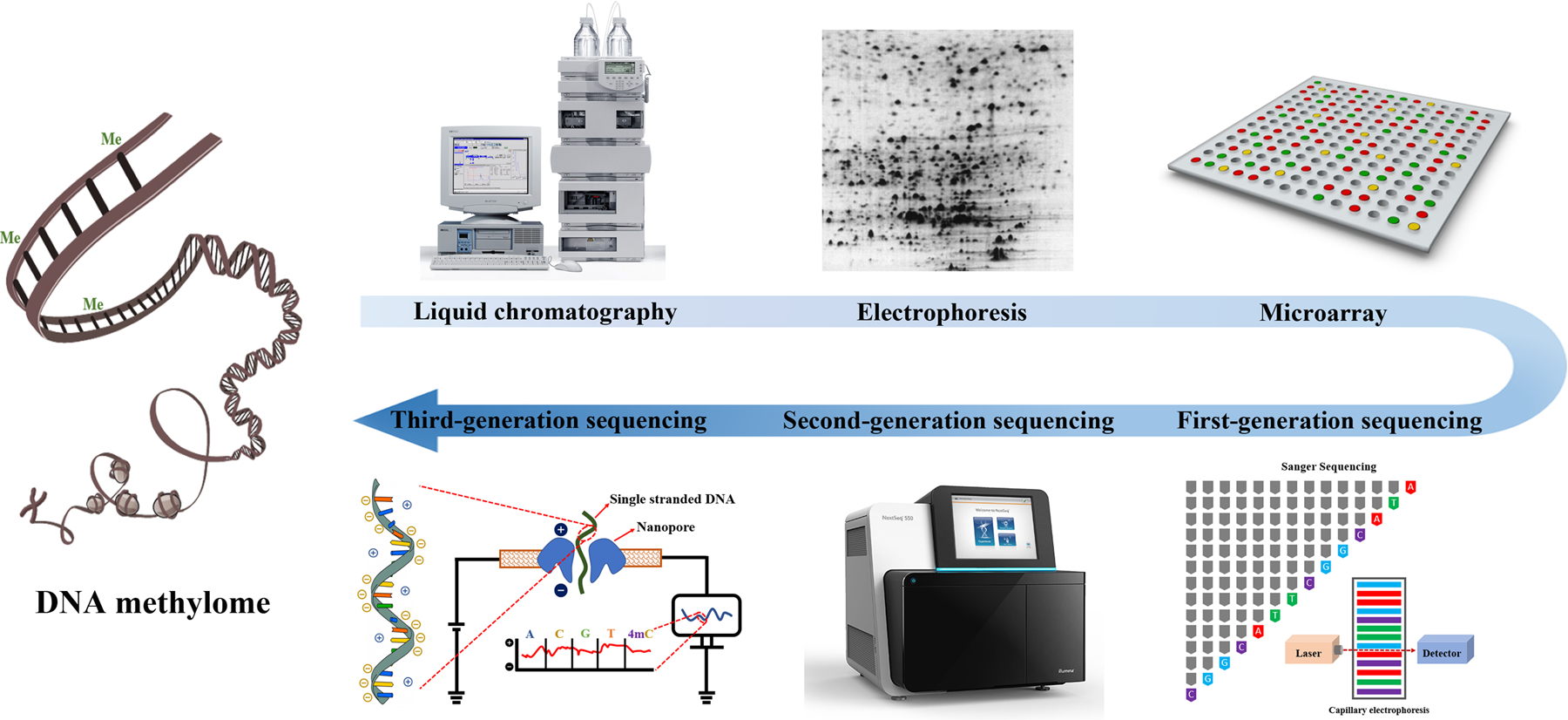
The evolution of DNA methylation profiling methodology.
It deserves a brief mention that liquid chromatography (LC) was the first platform used to quantify global DNA methylation represented by the total 5- methyldeoxycytidine (5mdC) content in DNA samples[125, 126], although this platform is not widely used anymore. Basically, DNA samples are hydrolyzed by DNase, alkaline phosphatase and exonuclease, successively or simultaneously[20]. After chromatographic separations, the genomic DNA methylation ratio (MR) is expressed as the percentage of 5mdC to the sum of 5mdC plus deoxycytidine (dC)[127]. LC-based profiling is fast and quantitative for experiments to gain an overall methylation level quickly. However, suitable internal standards for 5mdC and dC are not always readily available. A technical drawback of LC-based profiling is in obtaining the detailed information about specific positions with respect to where methylation occurs[128, 129]. This chromatographic technique still has strong vitality and may be improved with increased sensitivity when coupled with fluorescence[130], UV[131], and mass spectrometry (MS)[132]. Among these improvements, LC/MS provides an accurate and highly sensitive means for global DNA methylation quantification[133–135].
3.1. Electrophoresis-based techniques
The initial forays into genome-scale profiling technologies that allowed regional and positional information of DNA methylation across the genome to be derived were made with two-dimensional electrophoresis[136–140]. The restriction landmark genomic scanning (RLGS) technique used for these studies was first established and employed in 1991[141]. Since then, around 150 studies have used RLGS to profile DNA methylation[119]. As a typical example, RLGS was used to investigate genomic DNA methylation status of 1,184 unselected CpG-islands in 98 primary human tumors[142]. RLGS can target potential CpG sites relying on a methylation-sensitive restriction enzyme (MRE). This MRE can selectively cut certain genomic regions that are rich in CpG sites, or computational prediction of genome sequences where restriction fragments are potentially available[143]. Restriction fragments generated by enzyme digestion can then be separated by two-dimensional gel electrophoresis, which enables interrogation of CGIs with over 1,000 CpG sites in a single experiment. Thus, RLGS has been widely applied in site-, region- or organ-targeted studies, such as imprinted loci and tumors[136–140, 142]. In addition to RLGS, amplification of inter-methylated sites (AIMs)[144] and methylation-sensitive arbitrarily primed PCR (MS-AP-PCR)[145], have also been exploited to evaluate methylation status at the genomic level. Both AIMs and MS-AP-PCR rely on gel electrophoretic, differential patterns of PCR products and endonucleases such as methylation-sensitive or resistant restriction enzymes. Due to intensive labor and relatively low resolution, these gel-based profiling technologies are gradually being replaced.
3.2. Microarray hybridization techniques
The revolution of functional genomics truly benefited from the adaptation of microarray hybridization techniques[146–149]. The development of array hybridization began in the middle 1990s. Subsequently, advances and implementation of this technology greatly facilitated DNA methylomic analysis, which has a much higher resolution than gel-based profiling[150–152]. Based on different pretreatments of DNA samples, three major techniques have been adapted to array hybridization for methylome screening which are bisulfite conversion-, enzyme digestion-, and immunoprecipitation-based techniques.
In 2002, two research teams first described their application of microarrays on DNA methylation scanning[153, 154]. Essentially, they both employed a conventional sodium bisulfite (NaHSO3) treatment of genomic DNA[155]. Briefly, in the presence of NaHSO3, methylated cytosines are not affected while unmethylated cytosines are converted to uracil in denatured DNA[156]. The uracil is then converted into thymine during PCR amplification. The cytosine to thymine ratio in PCR amplification products (normally ~300–400 bp in size) can be used to compute methylation level at any given position. Hybridization of these PCR products onto a custom microarray containing probes that can discriminate unconverted and converted cytosines render a readout of any CpG site of interest. With respect to hybridization, a major drawback of bisulfite conversion-based array is bisulfite-treated DNA only has three different bases (A, T and G). The lack of cytosine bases reduces sequence complexity, resulting in greater redundancy and decreased hybridization specificity[119].
Tompa and her colleagues used a methylation-sensitive restriction endonuclease (an isoschizomer) to fractionate genomic DNA of Arabidopsis into segments with methylated and/or unmethylated CpG sites. These segments were then hybridized onto a custom microarray to obtain the first Arabidopsis methylome at a genome-wide level in 2002[157]. Since then, intense research efforts have been devoted to restriction-enzyme based methods[158]. These restriction enzymes include methylation-sensitive enzymes such as McrBC, and nonmethylated region-targeting endonucleases[159–161]. Such endonuclease digestion-based approaches have been aimed at sensitivity and quantification of methylation profiling and offer broader genomic coverage than bisulfite conversion-based microarrays[152, 162]. However, the resolution of enzyme digestion-based methods can never truly reach the whole genome level, as endonucleases only target those sequences that contain the restriction sites.
The existence of the high affinity of MBPs, such as methyl-CpG-binding domain (MBD) 2 and MBD3L1, contributes to the emergence of indirect approaches for DNA methylation profiling on microarray platforms. It is a milestone for methylation profiling that immunoprecipitation-based techniques were employed to get the first DNA methylome for Arabidopsis in 2006[25, 26]. In the same year, a methylated-CpG island recovery assay (MIRA) was also developed to determine DNA methylation patterns and identify frequent methylation of homeodomain-containing genes in lung cancer[163]. Briefly, fractions similar to that obtained in endonuclease digestion are achieved by immunoprecipitating the methylated fragments with a monoclonal antibody against methylated CpG sites from sheared genomic DNA samples. The immunoprecipitated methylated fractions are then hybridized onto a microarray against the input. The relative number of reads will be, therefore, used to calculate DNA methylation levels. Compared to the previously mentioned two types of techniques, immunoprecipitation-based array has less sequence bias, although its resolution is only hundreds of base pairs. It is not, however, always necessary to pursue single-base-pair resolution in many cases, as the range that contains correlated CpG sites adjacent to the targeted CpG position can be up to 1,000 bp[164].
The advent of microarray technologies has facilitated our understanding of DNA methylation patterns on a much broader level. Although continuous efforts have been made to achieve relatively more economical and comprehensive DNA profiles for an increasing sample size, it is a general trend that the array platforms will be gradually substituted by sequencing-based approaches, which is widely appreciated with their splendid accuracy and depth.
3.3. High-throughput sequencing techniques
The initiation of the first-generation sequencing-based methylation profiling was in 1984[165], when Church and Gilbert developed a genomic sequencing protocol that is applicable to the analysis of DNA methylation at single nucleotide resolution. In a Maxam and Gilbert sequencing system, they took the advantage of differential reactivities of hydrazine (N2H4) with unmethylated (high reactivity) and methylated (low reactivity) cytosines to generate discernible signals in cytosine bands. These signals can be further transformed into methylation levels. Bisulfite-converted genomic DNA is easy to use and particularly well suited for Sanger chemistry[166]. The development of bisulfite conversion-based sequencing, the “gold standard” for DNA methylation analysis, therefore, in the 1990s precipitated a breakthrough for sequencing-based methylation profiling. Related technologies have been commercially available since 2005. In 2007, pyrosequencing, a sequencing-by-synthesis method, was adapted to bisulfite sequencing[167]. Pyrosequencing can quantitatively monitor the real-time transformation of the incorporation of nucleotides into a proportional light signal through the conversion of released pyrophosphate. Importantly, numerous epigenome projects, such as the Human Epigenome Project (HEP, started in 2000) and the National Methylome 21 (NAME21, started in 2005), greatly improved sequencing-based techniques for methylome profiling, among which RRBS is a typical example[168].
Compared with the first generation sequencing (also known as Sanger sequencing or capillary sequencing), the exploitation of next-generation sequencing (NGS) (also called second-generation sequencing or massive parallel sequencing) platforms, such as Roche 454[169], Illumina[151], and Applied Biosystems[170], offer several orders of magnitude higher throughput per run. This generation of sequencing platforms enable fundamentally changed methylome profiling approaches in genetic medicine[171]. NGS platforms share the technical paradigm through clonally amplified and spatially separated DNA templates or flow cytometry (FCM)-separated single DNA molecules, rather than electrophoretic-separated chain-termination products employed in the Sanger sequencing[172]. NGS also allows for single-base-pair resolution of DNA methylation profiling. However, a major caveat of NGS is the reduced read length calls for massive bioinformatics computing.
To avoid progressively out-of-sync reads in the process of DNA synthesis between the amplified DNA stands and to preserve read quality, read length is much reduced in NGS technologies. The reduced length results in a critical limitation that requires approximative heuristics of bioinformatic computation, which in reverse increases the assembly errors[173]. By enabling reading the nucleotide sequences of single DNA molecules, a new class of DNA methylation profiling methods, termed third-generation sequencing (also known as long-read sequencing), is under active development[174]. In NGS techniques-based methylation profiling, the longer pattern of methylation information is lost due to the segmentation of long DNA molecules[175]. Third generation sequencing enables detection of DNA methylation of long-read single molecules in real-time, by virtue of the distinctive signal of 5mC from other nucleotide bases[117, 175].
In view of promising development of the emerging third-generation sequencing, a brief introduction of two platforms is worthwhile. Oxford Nanopore is a series of nanopore sequencing products that can analyze single molecules directly and electronically[176–178], and has been used for profiling the methylome[179]. Briefly, the methylation status in the nucleotides can produce distinctive electrical signals when each DNA strand passes through a nanopore. These signals can be transformed into 5mC levels via a hidden Markov model (HMM) [175]. SMRT, another sequencing platform developed by Pacific Biosciences (PacBio), can distinguish the distinctive fluorescent light pulse width of each methylation type[117], based on the properties of zero-mode waveguides[180]. Due to unresolved instability of involved molecular machinery, the third-generation sequencing platforms still generate higher error rates than second generation sequencing[181], yet targeted improvements are desirable.
4. Application of DNA methylation profiling techniques
As the third-generation sequencing is in the process of development, NGS platforms are still dominant in DNA methylation profiling approaches. DNA methylation information will be erased during PCR amplification due to the lack of endogenous and synthetic thermostable DNA methyltransferases in the PCR reaction. Almost all sequencing-based DNA methylation profiling approaches, therefore, rely on various pretreatments of genomic DNA[182–184]. As mentioned above, existing NGS-based DNA methylation analysis techniques fall into three main categories: bisulfite conversion, endonuclease digestion and affinity enrichment. Here, we review the principles, applications, and relative merits of some popular approaches for methylome analysis primarily based on different pretreatments of genomic DNA before amplification or hybridization. We also highlighted several microarray-based and commercially available capture-based platforms for DNA methylation profiling.
4.1. Sodium bisulfite conversion-based DNA methylation profiling techniques
As noted above, unmethylated cytosine residues in denatured genomic DNA can be deaminated and converted to thymine more rapidly than methylated cytosines once treated with sodium bisulfite[185]. This discovery turns an epigenetic difference into a genetic difference which has created a revolution of the technologies in DNA methylation analysis since the 1990s[166, 186]. As bisulfite-converted DNA is only comprised of three different bases, this limits its adaptation to array hybridization, especially for genome-scale DNA methylation analysis. Bisulfite-treated DNA, however, is particularly well suited for sequencing-based profiling. Due in large part to the exploitation of NGS platforms, bisulfite conversion-based approaches are enjoying a resurgence and are still playing leading roles in DNA methylome profiling currently.
Reduced representation bisulfite sequencing
The low sequence complexity of bisulfite-treated DNA increases the redundancy in the process of sequencing. RRBS was developed by Meissner et al. in 2005[168] to reduce sequencing redundancy via targeting only partial sequences of the genome (6%–12% CpGs). RRBS mainly covers promoter regions and repeated sequences that are relatively difficult to profile through conventional approaches[187, 188]. Two primary restriction enzymes, Msp I[189] (cuts at the recognition sequence CˇCGG) and Bgl II[168], are employed ahead of bisulfite conversion in RRBS to enrich CpG-containing fragments of genomic DNA.
Briefly, genomic DNA is first digested by a specific restriction enzyme to produce fragments (typically in the range of 50–300 bp) with CpG at the end[187]. Sticky double-stranded DNA ends resulting from enzyme digestion are repaired in the 3’ terminal, followed by adding an extra adenosine to both strands (referred to as A-Tailing). Then, DNA fragments are ligated with methylated sequence adapters which can prevent deamination of cytosines in the process of bisulfite conversion. The desired size of fragments is selected using gel electrophoresis for purification. Subsequently, those selected DNA fragments are treated with bisulfite to deaminate unmethylated cytosine into uracil. After PCR amplification and purification, DNA products are adapted onto sequencing platforms for sequencing (Figure 2). For instance, the Illumina Nextseq 550 platform is one of the options now used to sequence obtained DNA fragments.
Figure 2. Overview of WGBS and RRBS.
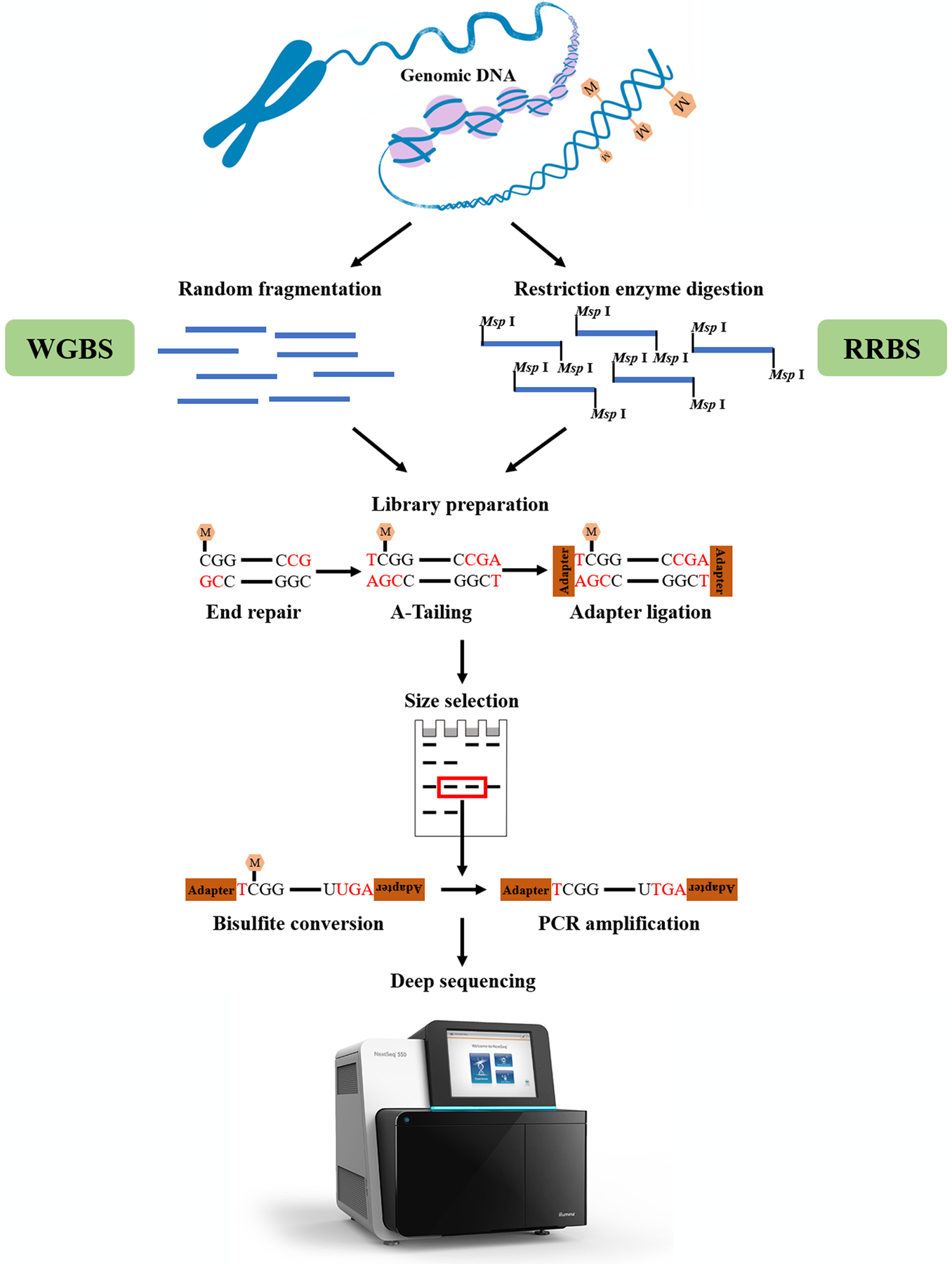
Msp I was used as an example for restriction enzymes employed in RRBS. “M” in the yellow hexagon indicates DNA methylation; RRBS, reduced representation bisulfite sequencing; WGBS, whole-genome bisulfite sequencing.
Owing to its high sensitivity, RRBS can be used to quickly analyze aberrant or overall methylation status in tumors[187, 190]. Especially, RRBS can targets those methylated areas that are difficult to be profiled with conventional bisulfite conversion-based sequencing approaches and repeated sequences observed in many tumor types[191, 192]. In addition, RRBS may be well suited for methylation states profiling in development biology. As RRBS uniquely interrogates a smaller portion of the genome that are CpG-rich through a specific restriction enzyme, it reduces the amount of sequencing required. RRBS is time and cost-effective and low samples input (10 – 300 ng) is required for effective and accurate analysis. Moreover, the quality of input DNA is less important for RRBS[187]. Notably, the restriction enzymes used in RRBS are not capable of covering all the CpG rich regions in the genome and some regions can only have a lower coverage. In addition, the non-proofreading polymerase used in the PCR portion can increase PCR sequencing errors in RRBS[168]. Incomplete denaturation of double-stranded DNA (bisulfite only converts single-stranded DNA) or reannealing in PCR amplification may also lead to incomplete bisulfite conversion. Several measures may be helpful for improving bisulfite conversion and reducing reannealing in PCR amplification. An extended proteinase K treatment in DNA extraction can help increase the quality and quantity of genomic DNA for bisulfite reaction[193]. Carriers, like glycogen and salmon sperm DNA, can be employed to promote bisulfite conversion and DNA increase precipitation[122]. Imbedding DNA in low-melting-point agarose block solution can reduce DNA losses and prevent DNA reannealing[155, 194]. Pre-denature of DNA prior to bisulfite conversion can improve the denaturation of double-stranded DNA[195]. Adding a high concentration (6 M) of urea into the bisulfite solution may also be helpful for destabilizing base-pairing in the DNA[196].
Whole genome bisulfite sequencing
Whole genome bisulfite sequencing (WGBS), also known as “Bisulfite sequencing”, “BS-seq”, “methyl-seq” or “methylC-seq”[197–200], is another technique that allows genome-wide DNA methylation analysis at a single CpG resolution. Compared with RRBS, the high-throughput data generated from WGBS enable absolute quantification of virtually all cytosines (excludes certain repetitive regions) in a genome under different biological contexts, via directly estimating the ratio of single-cytosine methylation. WGBS and RRBS are highly concordant with one another[201], and both need sodium bisulfite conversion of genomic DNA before sequencing on a NGS platform. In 2008, Cokus et al.[200] first implemented BS-seq to generate a map of DNA methylation patterns of A.thaliana on the Illumina 1G Genome Analyzer using Solexa sequencing technology. Since that time, WGBS has developed as a comprehensive and quantitative gold standard for DNA methylation profiling, especially focusing on differentially methylated CpGs (DMCs) and differentially methylated regions (DMRs), in many biological investigations[202].
Although intense research efforts have been devoted to the improvement of WGBS, the central and most widely used protocol for library preparation normally consists of genomic DNA fragmentation, (methylated) adapter ligation, bisulfite conversion and limited PCR amplification. Briefly, genomic DNA (50–100 ng) is first fragmented to ~200 bp. Fragmentation is then ligated with purified and methylated adapters which are compatible with the Illumina HiSeq platform. The DNA library is then treated with bisulfite followed by purification. The number of PCR amplification cycles is optimized using a qPCR assay, followed by library amplification and purification. Finally, a built library is sequenced on a sequencing platform[202] (Figure 2). Bismark[203] is used to control the quality of the raw data. Quality filtered data is then map to the genome to obtain DNA methylation information. As noted above, the detection of DMCs and DMRs is a key metric in WGBS data analysis. Numerous statistical methods or tools have been developed for optimal detection of DMCs and DMRs from WGBS data[204–212].
WGBS is still the gold standard for comprehensive and unbiased whole-genome DNA methylation profiling. It allows the observation of many types of methylation sites including CpG, CHG and CHH sequences that are presented in the samples of interest. The Roadmap Epigenomics Project supported by the US National Institutes of Health (NIH) recommends use of two replicates in WGBS experiments to reach a total coverage of 30 × (http://www.roadmapepigenomics.org/). However, WGBS is still very expensive which prohibits large-scale projects performed with biological replicates[213–216]. Thus, a statistical method, termed DSS-single, has been developed to detect DMRs from WGBS data that only have one replicate[217].
The standard protocol of WGBS library preparation requires a large amount of input DNA (minimum of 200–500 ng and up to 5 μg). Many methylome studies, however, only have a limited amount of samples[218]. Post-bisulfite adaptor tagging (PBAT)-based WGBS[219], and tagmentation-based WGBS (T-WGBS)[220–222] therefore, have been developed as commonly used alternative approaches. As an inherent step in conventional WGBS methods, bisulfite treatment follows the adaptor tagging of DNA templates, leading to substantial DNA fragmentation and increased requirement of input DNA. In PBAT-WGBS, bisulfite conversion precedes adaptor tagging via random primer extension, which enables the generation of abundant unamplified reads from only 100 ng of DNA[219]. T-WGBS employs a hyperactive Tn5 transposase to fragment the genomic DNA and appends sequencing adapters. This improvement allows the amount of input DNA required to be lower than 20 ng[222]. WGBS library preparation can also be affected by incomplete BS conversion, BS-induced DNA degradation, PCR amplification and DNA modifications, among which incomplete BS conversion is the main source of biases[223].
4.2. Endonuclease digestion-based DNA methylation profiling techniques
The restriction-modification system is a set of sequence-specific restriction endonucleases found generally in bacteria and archaea that can protect the host genome from genomic parasites[4]. Each restriction endonuclease has an associated DNA methyltransferase, which can protect the endogenous DNA from the action of the restriction enzyme via adding methyl groups onto the recognition site. As 5mC can inhibit some restriction endonucleases from cutting within their recognition sequences, this pattern of cutting provides a readout of DNA methylation status[224]. The tremendous potential of restriction endonucleases in molecular biology, therefore, is widely appreciated, especially in DNA methylation analysis of regions of interest. As examples, Hpa II/Msp I [23] (recognizes sequence CˇCGG) and Sma I/ Xma I (recognizes sequence CCCˇCGG) are two typical and widely used MRE pairs. MRE digestion followed by gel electrophoresis or PCR across the restriction site are sensitive techniques that were highly used in the past and are still applicable for some locus-specific studies.
Methylation-sensitive restriction enzyme sequencing
MRE enrichment approaches have been recently adapted to NGS platforms, namely MRE-seq. MRE-seq allows for allele-specific DNA methylation scanning and is capable of covering a broader region of the genome than conventional array hybridization[23, 225]. Typically, MREs, such as HpaII (CˇCGG), Hin6I (GˇCGC) and AciI (CˇCGC), are used parallelly in MRE-seq. These different MREs can be used to target non-overlapping sets of CpGs at a genomic scale, creating denser profiling. As MREs only recognize and cut DNA double-strands at unmethylated CpG sites (Figure 3), DNA methylation status is inferred by the inverse relationship between CpG methylation at MREs target sites and MRE-seq readout. Therefore, by generating a library of digested fragments via the digestion of a set of MREs, followed by massively parallel sequencing to converge millions of observations, we can infer the methylation status of a region of interest based on the number of times an enzyme cutting site is observed[23].
Figure 3. Key principle comparison of MRE-seq, MeDIP-seq and MBD-seq.
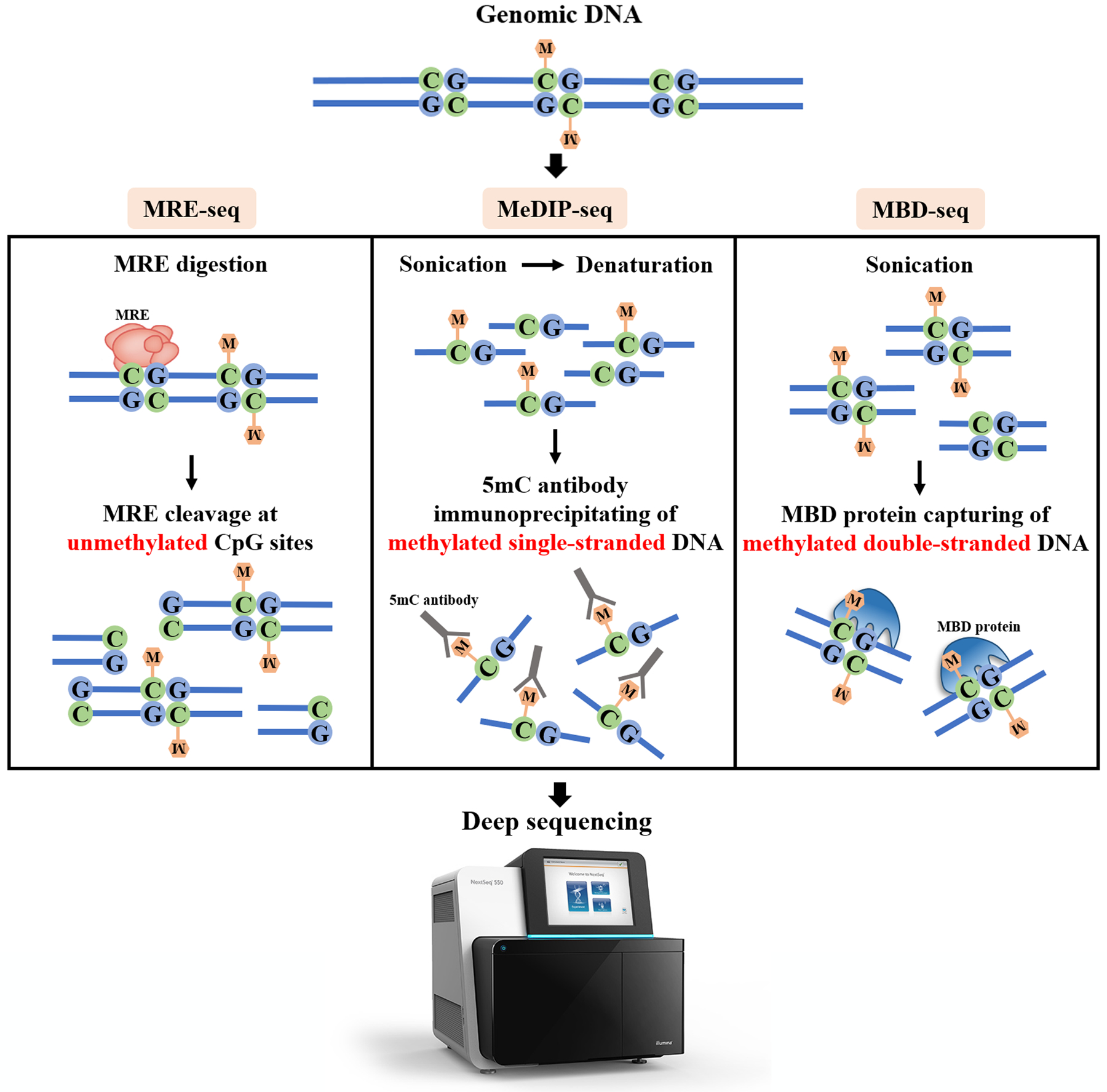
5mC, 5-methylcytosin; “M” in the yellow hexagon indicates DNA methylation; MBD, methyl-CpG-binding domain protein; MeDIP, methylated DNA immunoprecipitation; MRE, methylation sensitive restriction enzyme; -seq, sequencing.
In 2010, Maunakea et al. first used MRE-seq to investigate the role of intragenic methylation in regulating alternative promoters in gene bodies[225]. With multiple endonucleases, this approach can cover about 30% of a genome. In a typical MRE-seq protocol, genomic DNA is first digested in parallel by several (3~5) MREs (e.g., 37 °C for 3 h, two rounds). The same amount of each digest (e.g., 500 ng) is then combined into one tube, followed by size-selected electrophoresis on a 1% gel. Normally, a 100~300 bp gel slice is excised and purified. Library construction is then performed with single-end adapters, followed by end repair reaction and single-end oligo adaptor ligation. After PCR, amplification products are cleaned and examined for quality before sequencing. When mapped onto the reference genome, unmethylated CpG sites are revealed and can be further converted into methylation levels.
MRE-seq provides a user-friendly DNA methylation profiling approach at a single CpG resolution. However, as MRE-seq relies on the properties of different restriction enzymes employed, the availability of enzyme recognition sites in the genome limits its coverage. Incomplete digestion of MRE may tend to cause considerable false-positive results. Given that the fragments resulting from digestion may still contain important methylation information, an additional step of bisulfite conversion followed by MRE treatment, termed as MREBS, would target the methylation status of CpG sites within MRE fragments. Thus, MREBS allows the estimation of differential methylation across 60% of the genome[226]. In addition, methylated DNA immunoprecipitation (MeDIP)-seq (will be reviewed in detail below) is a good complementary technology for MRE-seq. MeDIP-seq and MRE-seq target either the methylated or unmethylated portion of the genome, and their combination has been proven to provide synergistic advantages[201, 225, 227, 228].
4.3. Affinity enrichment-based DNA methylation profiling techniques
As noted above, the primary limitation of MRE-based profiling technologies is that only specific restriction sites can be analyzed. For instance, the frequently used Hpa II only targets 3.9% of all CpGs in the human genome (nonrepetitive DNA)[229]. Bisulfite-conversion based approaches are always laborious and it is not easy to screen a large set of samples. To circumvent these constraints, strategies that target the isolation/fractionation of methylated sequence motifs based on the interaction of certain proteins with methylated DNA have been developed. These proteins include MBD family members, such as MBD2 and MBD3L1, that can selectively bind methylated DNA[230, 231]. Methyl-binding protein MECP2[232] is first demonstrated for the affinity purification of methylated DNA. Antibodies specific for 5mC (interaction with denatured DNA)[233] are also important choices. These affinity-enrichment methods have been largely adapted with array hybridization (reviewed above). Recently, most of them are coupled with sequencing platforms[234–236], providing genome-scale information of the immunoprecipitated DNA fragments. Here, we highlight two commonly used approaches: MeDIP-seq and MBD-seq (or MBDCap-seq). We also put them together for a brief comparison as these two strategies have shown good concordance[237].
Methylated DNA immunoprecipitation sequencing
In 2005, Weber and his colleagues first developed and employed MeDIP assay to acquire highly efficient enrichment of methylated DNA in normal and transformed human cells[233]. In this assay, a monoclonal antibody is raised against 5-methylcytidine (5mC, includes mC or mCG) which precipitates methylated DNA fragments. With the appropriate antibodies, hydroxymethylcytosine (hmC) (hMeDIP)[4, 77, 97] and possibly 5-formylcytosine and 5-carboxylcytosine can also be detected through MeDIP[235]. As antibodies employed in MeDIP were raised with equal specificity against particular methylated loci, MeDIP assay provides an unbiased and hypothesis-free approach. Upon combining MeDIP with NGS platform[234], termed MeDIP-seq, this approach can further offer a 100–300 bp resolution of the methylome at a genome-scale level. Notably, MeDIP-seq is cost-effectiveness compares to other affinity enrichment-based techniques[238].
In brief, genomic DNA is first sheared through conditionally optimized sonication to yield random fragments. These fragments range 100–500 bp in size, which is crucial for efficient immunoprecipitation and a reasonable resolution for further characterization. Sonicated DNA is then purified, end-repaired and adapter-ligated before proceeding further. As anti-5mC antibody has a higher affinity with single-stranded DNA, fragmentated DNA is therefore denatured at 95°C to destroy double-stranded DNA prior to primary antibody incubation to enrich DNA fragments. The next step is covering the immunoabsorbed DNA fragments with protein G coupled standard or magnetic beads and multiple washes follow. An adapter modified PCR is performed to prepare the library, followed by a regular gel extraction for final library size selection. After the library quality control with two independent PCR reactions, which include positive and negative controls, respectively. Finally, NGS platform is employed to obtain millions of raw reads.
Methyl-CpG-binding domain protein capture sequencing
MBD-seq is another affinity enrichment-based DNA methylome profiling strategy that only targets the methylated part of the genome. It is therefore more economically feasible than deep sequencing. MBD-seq is a powerful alternative to MeDIP-seq and has been demonstrated to be capable of identifying DMRs[201, 239–243]. MBD-seq has been shown to be able to replicate methylome-wide association studies (MWAS), leading to its consideration as a powerful technology[244].
To perform MBD-seq, 0.2–1 μg genomic DNA is first sonicated to random fragments. MBD protein coupled to beads is then used for capturing methylated DNA (double-stranded DNA). Following capture, a stepwise elution series is employed to enrich different CpG densities in the bound methylated DNA. After the library preparation, enriched DNA fragmentation is subjected to high-throughput sequencing on NGS platforms.
Comparison of MeDIP-seq and MBD-seq
Essentially, MeDIP-seq uses a 5mC-specific monoclonal antibody to precipitate single-stranded DNA fragments containing methylated CpG sites[233, 235, 245, 246], and MBD-seq captures methylated regions of double-stranded DNA with MBD proteins[230, 239, 247–250] (Figure 3). MeDIP tends to enrich for methylated fragments with a low CpG density, while the MBD-based strategies can capture different methylation densities by adjusting the salt fractionation employed[239]. Although both MeDIP-seq and MBD-seq are sensitive for detecting DMRs and their results are generally concordant[201, 251, 252], neither allows for single-base-pair resolution (~150 bp)[201, 228]. Both approaches are lack of coverage of unmethylated CpG sites as well. Hence, the exploration at CGIs of the genome may be limited as these areas are predominantly unmethylated[237]. Reports favor MBD-seq as more sensitive. However, MBD-seq is not capable of differentiating between 5mC and 5hmC[201]. Moreover, available MBD proteins for MBD-seq lack stable binding between MBD domain and hemimethylated DNA[253]. Importantly, affinity-based selection is generally biased towards hypermethylated regions. By contrast, MeDIP-seq demonstrates no bias for a specific DNA region and may provide a better genome-wide coverage[251, 252, 254].
4.4. Microarray-based DNA methylation profiling techniques
As reviewed above, primarily due to the high cost and significant technical expertise of data processing, WGBS is not always the most feasible approach for genome-scale mapping of methylated cytosines in large cohort studies[255]. The advance of DNA methylation arrays, Illumina BeadChip, provides a time- and cost-effective and user-friendly alternative. Illumina DNA BeadChip also shows good agreement with methylome profiling from other platforms[256]. The development of Illumina’s DNA methylation microarrays has been through four generations. The GoldenGate assay[257] is the first-generation Illumina DNA methylation BeadChip array system. It can analyze up to 1,536 promoter region CpG sites in 96 samples simultaneously, through incorporating multiplexed primer extension of bisulfite-converted genomic DNA (as low as 250 ng). Each CpG site is equipped with methylated and unmethylated primers, labelling with different dyes. The amplification products are hybridized onto Illumina universal bead arrays and each CpG site has up to 30 represented beads. This BeadArray platform has been successfully used to profile DNA methylation status of 371 genes in tumors, cancer cell lines and normal tissues, targeting adenocarcinoma-specific methylation markers[258, 259]. The Illumina Infinium HumanMethylation27 BeadChip (HM27)[256] is the second-generation platform. The coverage of HM27 has been scaled up to 27,578 CpG sites located in promoter regions. Of these, 27,324 CpG sites target ~1,000 cancer-related genes and a further 254 CpG sites are involved in ~100 microRNA genes. This protocol requires 0.5–1 μg of bisulfite-converted starting DNA and is particularly well suited for high-quality DNA extracted from blood samples and fresh frozen tissue. HM27 has also been adapted for formalin-fixed paraffin-embedded (FFPE)-extracted DNA[260]. To increase CpG coverage, two different assay chemistries, the Infinium Ⅰ and the Infinium Ⅱ assays, are employed in the third generation BeadChip platform, Infinium HumanMethylation450 (HM450)[261]. On this array, the Infinium Ⅰ assay is used for 136,476 CpGs, which has two bead types for each CpG site. The Infinium Ⅱ assay is adapted for 350,036 CpGs, each CpG site of which uses a single bead type. HM450 has provided a user-friendly platform to profile DNA methylome for many human genome-scale studies, including The Cancer Genome Atlas (TCGA) project[262]. This platform, however, presents a relatively low coverage of distal regulatory elements. In 2016, Illumina released the MethylationEPIC (EPIC) BeadChip[151] to specifically target these regions. EPIC interrogates a total of 863,904 CpG sites. These CpG sites include >90% of the CpGs inherited from HM450 and an additional 413,743 CpGs dedicated to FANTOM5 and ENCODE enhancers. This improvement of EPIC increases genome coverage of regulatory regions.
Infinium HumanMethylation450 platform
Basically, DNA methylation BeadChip arrays can convert the probe hybridization intensity values of bisulfite-treated DNA to a relative abundance of cytosines (methylated and unmethylated) at CpG sites of interest[263]. Here, we highlight HM450 as one of the most widely used platform. HM450 platform starts with the bisulfite conversion of 0.5–1 μg genomic DNA. Bisulfite-converted DNA is hybridized to arrays that contain two types of predesigned methylation-specific probes: methylated and unmethylated. Therefore, for each CpG site, a single-base extension of the probe incorporates a labeled and fluorescently stained nucleotide (ddNTP) at the 3’ CpG site. The BeadChip is then scanned on the Illumina iScan to measure the ratio of fluorescent signal. The proportion of DNA methylation at each CpG loci, so called beta-value (β), is calculated as M/(M+U+100). In this formula, M is the intensity of the methylated signal, U is the intensity of the unmethylated signal, and 100 is a constant offset used to regularize β values when intensities of both methylated and unmethylated cytosines are low. β is a continuous value, in which 0 indicates 0% methylation and 1 represents 100% methylation at a given CpG loci.
The HM450 BeadChip interrogates over 48,000 CpG sites across the human genome, which are enriched for 99.3% CpG residues. Almost half of the probes in the HM450 platform cover intergenic regions, including validated DMRs, DNase Ⅰ hypersensitive sites and bioinformatically predicted enhancers[261, 264]. Moreover, the HM450 platform has been adapted to FFPE-derived DNA, which is highly fragmented (150–300 bp). Thus, a lower amount (200 ng) of genomic DNA is now allowed to be profiled efficiently[260, 265]. The design of Illumina DNA methylation BeadChip arrays, however, is not hypothesis neutral. The inclusion and preselection of specific probes that interrogate only previously identified CpG sites will inevitably generate heavy biases. In addition, it is worthy of reconsideration that the “co-methylation assumption” assumes that CpG loci close to those probes-interrogated sites will be similarly methylated or unmethylated[164]. Further, the two types of probe on the array are behaviorally different. These BeadChip arrays are in essence single nucleotide polymorphisms (SNPs) microarrays, which means the SNPs may affect the filtering of probes and introduce further complexity into the data analysis pipelines[266]. These properties determine the sensitivity of the quantitative measurement of microarrays to the variation in experimental conditions and background[267]. Increasing data preprocessing methods, including background correction[263, 266, 268], inter-array normalization[266], probe-design bias adjustment[269–272] and dye bias correction[273], have been proposed to improve the quality of microarray data.
4.5. Other DNA methylome detection techniques
To date, the combination of different pretreatments of DNA samples followed by diverse bioinformatic analysis strategies has resulted in an array of emerging DNA methylation profiling techniques[119, 187, 199, 248, 274–276]. For example, an enhanced reduced representation bisulfite sequencing (ERRBS) integrated library preparation modification and downstream data processing has been developed. ERRBS has been shown to increase genome coverage of CpGs ( ~10% of genomic CpGs sites) [277, 278]. There are also some commercially available capture-based methods deserving a brief mention, although they are not widely used. In 2012, Agilent SureSelect Methyl-Seq (SSMethylSeq) was designed with biotinylated RNA oligos that can cover 3.7 M biologically relevant CpGs in human genome. Illumina TruSeq-Methyl capture EPIC (TruSeqEpic) was developed in 2016 as a complement and extension of EPIC BeadChip array. TruSeqEpic contains a DNA oligo pool that can covers 3.3 M biologically relevant CpGs. Roche NimbleGen SeqCap Epi CpGiant (CpGiant) capture platform was released in 2014 that is based on Roche’s SeqCap Epi enrichment system. This method can cover 2.8 M biologically relevant CpGs. These three capture-based platforms cover nearly all CpG sites of their target regions with low off-target ratio[279]. Importantly, capture libraries can be customized for areas of interest in the genome with relatively low sequencing costs[279]. However, these capture-based methods require a high amount (μg) of starting materials, which may limit their application in clinical trials[279].
5. Relative merits of different DNA methylation profiling approaches
The inherent property and complexity of different DNA methylation analysis methods derives from their competing strengths and weaknesses (Table 1). It is, therefore, challenging to have a straightforward comparison of various DNA methylome profiling techniques. First, the organism that is being studied with sequence-based platforms needs a reference genome. An appropriate selection from different DNA methylation profiling approaches is also influenced by the available quantity and quality of samples, the desired resolution and coverage, the needful accuracy and reproducibility as well as cost-restraints.
Table 1.
Potential sources of bias for different DNA profiling approaches.
| Source of bias | Bisulfite conversion-based |
Microarray-based |
Endonuclease digestion-based |
Affinity enrichment-based |
||
|---|---|---|---|---|---|---|
| WGBS | RRBS | Illumina DNA BeadChip | MRE-seq | MeDIP-seq | MBD-seq | |
| Incomplete bisulfite conversion | √ | √ | √ | |||
| Post-bisulfite conversion PCR | √ | √ | √ | |||
| Cross-hybridization | √ | |||||
| Fragment size variation | √ | |||||
| Copy number variation | √ | √ | ||||
| CpG density | √ | √ | ||||
indicates that the approach has this potential source of bias. MBD, methyl-CpG-binding domain protein; MeDIP, methylated DNA immunoprecipitation; MRE, methylation sensitive restriction enzyme; RRBS, reduced representation bisulfite sequencing; -seq, sequencing; WGBS, whole-genome bisulfite sequencing.
5.1. Sample quality and input
DNA methylation profiling platforms differ vastly in the requirement of sample quantity and quality (Figure 4). Generally, MRE-based methods tend to require high quantity, purity and integrity of genomic DNA although some targeted improvements have suitably broadened the acceptance of nucleic acid samples[280]. Affinity-base techniques, such as MeDIP- and MBD-based systems, are more tolerant of DNA purity and integrity. As the ratio of input DNA can affect the enrichment efficiencies of specific antibodies, it is worthy of careful consideration when determining the usage amount of input DNA in affinity-based approaches. Bisulfite-based profiling methods require DNA denaturation and purification before the adaptation onto sequencing platforms. Thus, the input DNA can be accepted with low quality. The application of MeDIP and bisulfite conversion-based methods is based on single-stranded DNA. These techniques are therefore compatible with previously denatured DNA samples. For example, FFPE-derived DNA, which is normally highly fragmented, has been successfully used to build libraries with the Illumina BeadChip array system[260, 281, 282].
Figure 4. The genome coverage and sample throughput of various DNA methylation profiling techniques.
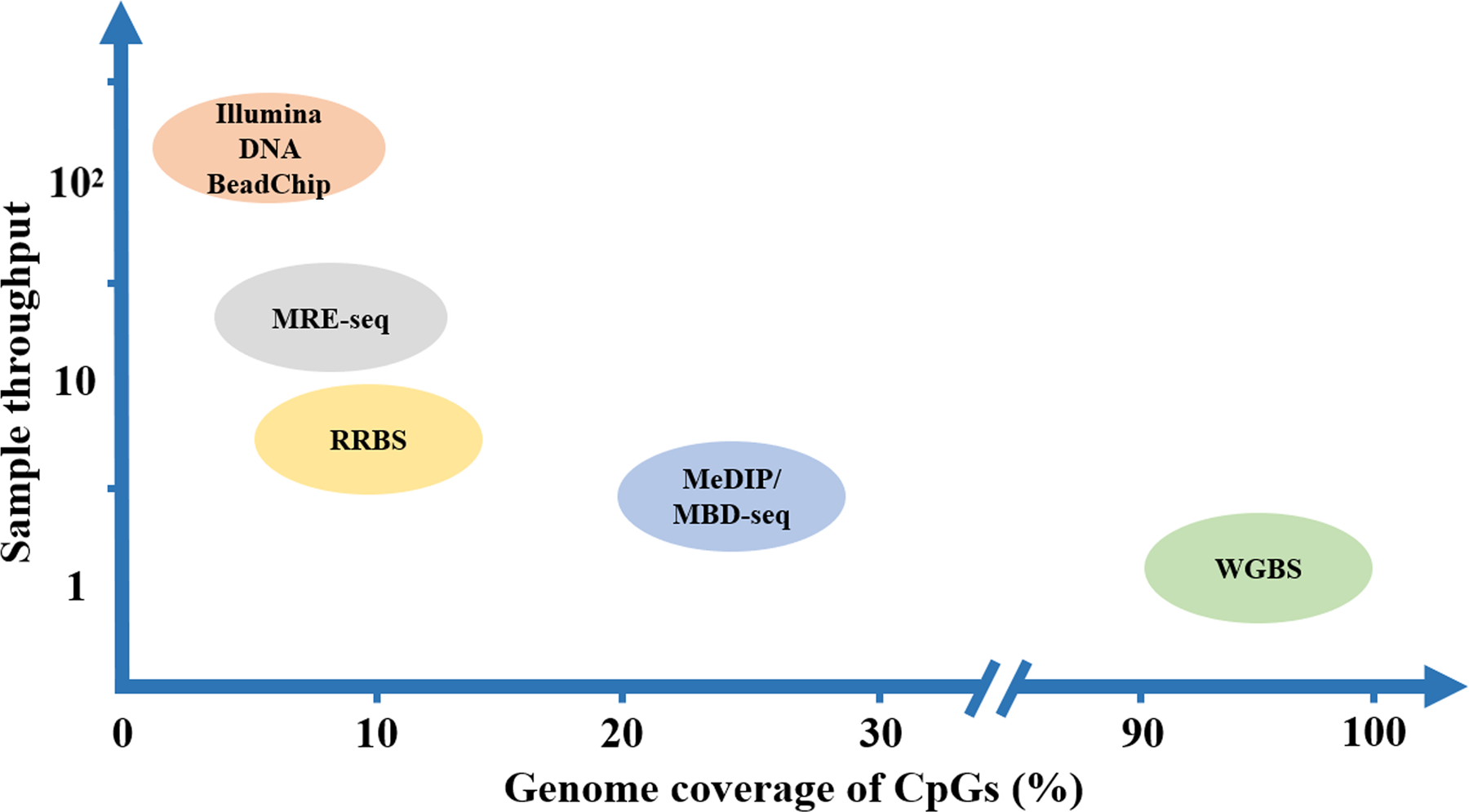
The genome coverage determined by the detectable percentage of CpG loci in the whole genome. The sample throughput represented by the number of samples that can be accepted for analysis per experiment. MBD, methyl-CpG-binding domain protein; MeDIP, methylated DNA immunoprecipitation; MRE, methylation sensitive restriction enzyme; RRBS, reduced representation bisulfite sequencing; -seq, sequencing; WGBS, whole-genome bisulfite sequencing.
5.2. Coverage and resolution
As noted, MREs can only target double-stranded DNA at recognizable unmethylated CpG loci. Enzyme-based methods commonly have their coverage and resolution close to the distribution of recognition sequences across the whole genome. For example, over 95% of CpG islands can be reached using techniques relying on Hpa II and Msp I[280]. Likewise, the genomic coverage and resolution of affinity-based profiling approaches, such as MBD-seq, rely on the affinity targets of methyl-binding proteins. However, affinity enrichment-based methods can only allow a low base-pair resolution (~150 bp) due to the antibody binding property. By contrast, bisulfite-based techniques are capable for a single CpG resolution throughout the genome (WGBS) and can reach to relatively difficult regions (RRBS) (Figure 4). Importantly, the fragment-size biases and base composition of NGS platforms may introduce new measuring errors and coverage limitations[283].
5.3. Sensitivity and accuracy
DNA methylation profiling techniques also vary in their sensitivity and accuracy. These techniques can be influenced by available sample size and quality, pretreatment of DNA sample, 5mC density in the target sequence as well as provided sequencing platforms. General genomic-level sequencing techniques are not very suitable for the detection of low-abundance DNA methylation states, which always need a deeper sequencing and will largely increase the cost. As aforementioned, MRE-based profiling methods are adept at low-CpG-density regions, whereas affinity-based approaches perform better for CpG-rich regions[284]. The length differences of DNA fragments are used to reveal DNA methylation information in many enzyme-based techniques. However, it is prone to sources of bias when this type of pretreatment of DNA is adapted to sequencing platform as library construction will be strongly influenced[283, 284]. In enzyme- and affinity-based techniques, the copy-number of short-reads that are uniquely aligned to particular regions of the genome is used to determine DNA methylation patterns. By contrast, bisulfite conversion-based sequencing extracts DNA methylation information from the sequence itself[4, 283]. Hence, bisulfite conversion-based sequencing tends to be sensitive, accurate and reproducible, and is well suited for single-base-level analysis[285, 286]. Incomplete bisulfite conversion induced by non-proofreading polymerase and re-annealing in PCR amplification is a major source of sequencing errors[168]. Spiked DNA controls, however, can be added to monitor the completion of sodium bisulfite conversion at various timepoints[287].
6. Concluding remarks and future perspective
Over the past decades, a veritable revolution in global DNA methylation profiling techniques has expanded our understanding of diverse aspects of genomic DNA methylation in health and disease. We are witnessing a deluge of sequence-based epigenetic data which encompasses not only the DNA methylome. As reviewed, nanopore-based third-generation sequencing platforms offer an exciting potential for direct sequencing of 5mC at the single molecule level with long reads and without sodium bisulfite conversion of DNA[286, 288–290]. Long-read sequencing has already stimulated the next revolution in high-throughput methylome analysis[291, 292]. Undoubtedly, the challenge in DNA methylation advances has been shifting from big data generation to data analysis. Bioinformatic information is already an important feature of epigenome databases[293]. In summary, when one wishes to make a choice from such a diversity of DNA methylome profiling platforms, biological purposes, available sample size and quality, desired resolution and accuracy, time and labor cost, as well as appropriation budget should be taken into a comprehensive consideration.
Highlights.
The function of DNA methylation varies by different gene regions.
An overview of DNA hydroxymethylation detection methodology.
Evolution of DNA methylation profiling methodology.
Long-read sequencing stimulates a new revolution in DNA methylome profiling.
Relative merits of different DNA methylome profiling approaches.
Acknowledgments
Funding
This work was supported by the National Institutes of Health (R01 CA178441 and R01 CA204346).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interests.
Shizhao Li: Conceptualization, Writing- Original draft preparation. Trygve O Tollefsbol: Conceptualization, Resources, Supervision, Writing- Reviewing and Editing, Project administration, Funding acquisition.
References
- [1].Bird A, DNA methylation patterns and epigenetic memory, Genes Dev 16(1) (2002) 6–21. [DOI] [PubMed] [Google Scholar]
- [2].Li S, Chen M, Li Y, Tollefsbol TO, Prenatal epigenetics diets play protective roles against environmental pollution, Clin. Epigenetics 11(1) (2019) 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jones PA, Takai D, The role of DNA methylation in mammalian epigenetics, Science 293(5532) (2001) 1068–1070. [DOI] [PubMed] [Google Scholar]
- [4].Laird PW, Principles and challenges of genome-wide DNA methylation analysis, Nature Reviews Genetics 11(3) (2010) 191–203. [DOI] [PubMed] [Google Scholar]
- [5].Holliday R, Pugh JE, DNA modification mechanisms and gene activity during development, Science 187(4173) (1975) 226–232. [PubMed] [Google Scholar]
- [6].Riggs AD, X inactivation, differentiation, and DNA methylation, Cytogenetic and Genome Research 14(1) (1975) 9–25. [DOI] [PubMed] [Google Scholar]
- [7].Jones PA, Functions of DNA methylation: islands, start sites, gene bodies and beyond, Nature Reviews Genetics 13(7) (2012) 484–492. [DOI] [PubMed] [Google Scholar]
- [8].Razin A, Cedar H, DNA methylation and gene expression, Microbiol. Mol. Biol. Rev 55(3) (1991) 451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schübeler D, Function and information content of DNA methylation, Nature 517(7534) (2015) 321–326. [DOI] [PubMed] [Google Scholar]
- [10].Jones PA, Taylor SM, Cellular differentiation, cytidine analogs and DNA methylation, Cell 20(1) (1980) 85–93. [DOI] [PubMed] [Google Scholar]
- [11].Feng S, Jacobsen SE, Reik W, Epigenetic reprogramming in plant and animal development, Science 330(6004) (2010) 622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fulka H, Mrazek M, Tepla O, Fulka J, DNA methylation pattern in human zygotes and developing embryos, Reproduction 128(6) (2004) 703–708. [DOI] [PubMed] [Google Scholar]
- [13].Li E, Beard C, Jaenisch R, Role for DNA methylation in genomic imprinting, Nature 366(6453) (1993) 362–365. [DOI] [PubMed] [Google Scholar]
- [14].Robertson KD, DNA methylation and human disease, Nature Reviews Genetics 6(8) (2005) 597–610. [DOI] [PubMed] [Google Scholar]
- [15].Das PM, Singal R, DNA methylation and cancer, J. Clin. Oncol 22(22) (2004) 4632–4642. [DOI] [PubMed] [Google Scholar]
- [16].Illingworth RS, Bird AP, CpG islands–’a rough guide’, FEBS Lett 583(11) (2009) 1713–1720. [DOI] [PubMed] [Google Scholar]
- [17].Fournier A, Sasai N, Nakao M, Defossez P-A, The role of methyl-binding proteins in chromatin organization and epigenome maintenance, Briefings in functional genomics 11(3) (2012) 251–264. [DOI] [PubMed] [Google Scholar]
- [18].Suelves M, Carrió E, Núñez-Álvarez Y, Peinado MA, DNA methylation dynamics in cellular commitment and differentiation, Briefings in functional genomics 15(6) (2016) 443–453. [DOI] [PubMed] [Google Scholar]
- [19].Teif VB, Beshnova DA, Vainshtein Y, Marth C, Mallm J-P, Höfer T, Rippe K, Nucleosome repositioning links DNA (de) methylation and differential CTCF binding during stem cell development, Genome Res 24(8) (2014) 1285–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li S, Zhu Y, Zhi L, Han X, Shen J, Liu Y, Yao J, Yang X, DNA Methylation Variation Trends during the Embryonic Development of Chicken, PLoS One 11(7) (2016) e0159230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yang X, Han H, De Carvalho DD, Lay FD, Jones PA, Liang G, Gene body methylation can alter gene expression and is a therapeutic target in cancer, Cancer Cell 26(4) (2014) 577–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jones PA, The DNA methylation paradox, Trends Genet 15(1) (1999) 34–37. [DOI] [PubMed] [Google Scholar]
- [23].Ball MP, Li JB, Gao Y, Lee J-H, LeProust EM, Park I-H, Xie B, Daley GQ, Church GM, Targeted and genome-scale strategies reveal gene-body methylation signatures in human cells, Nat. Biotechnol 27(4) (2009) 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hellman A, Chess A, Gene body-specific methylation on the active X chromosome, Science 315(5815) (2007) 1141–1143. [DOI] [PubMed] [Google Scholar]
- [25].Zilberman D, Gehring M, Tran RK, Ballinger T, Henikoff S, Genome-wide analysis of Arabidopsis thaliana DNA methylation uncovers an interdependence between methylation and transcription, Nat. Genet 39(1) (2007) 61–69. [DOI] [PubMed] [Google Scholar]
- [26].Zhang X, Yazaki J, Sundaresan A, Cokus S, Chan SW-L, Chen H, Henderson IR, Shinn P, Pellegrini M, Jacobsen SE, Genome-wide high-resolution mapping and functional analysis of DNA methylation in Arabidopsis, Cell 126(6) (2006) 1189–1201. [DOI] [PubMed] [Google Scholar]
- [27].Zhang X, Shiu S, Cal A, Borevitz JO, Global analysis of genetic, epigenetic and transcriptional polymorphisms in Arabidopsis thaliana using whole genome tiling arrays, PLoS genetics 4(3) (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shukla S, Kavak E, Gregory M, Imashimizu M, Shutinoski B, Kashlev M, Oberdoerffer P, Sandberg R, Oberdoerffer S, CTCF-promoted RNA polymerase II pausing links DNA methylation to splicing, Nature 479(7371) (2011) 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Laurent L, Wong E, Li G, Huynh T, Tsirigos A, Ong CT, Low HM, Sung KWK, Rigoutsos I, Loring J, Dynamic changes in the human methylome during differentiation, Genome Res 20(3) (2010) 320–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yoder JA, Walsh CP, Bestor TH, Cytosine methylation and the ecology of intragenomic parasites, Trends Genet 13(8) (1997) 335–340. [DOI] [PubMed] [Google Scholar]
- [31].Moarefi AH, Chédin F, ICF syndrome mutations cause a broad spectrum of biochemical defects in DNMT3B-mediated de novo DNA methylation, J. Mol. Biol 409(5) (2011) 758–772. [DOI] [PubMed] [Google Scholar]
- [32].Pikaart MJ, Recillas-Targa F, Felsenfeld G, Loss of transcriptional activity of a transgene is accompanied by DNA methylation and histone deacetylation and is prevented by insulators, Genes Dev 12(18) (1998) 2852–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hon GC, Rajagopal N, Shen Y, McCleary DF, Yue F, Dang MD, Ren B, Epigenetic memory at embryonic enhancers identified in DNA methylation maps from adult mouse tissues, Nat. Genet 45(10) (2013) 1198–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rai K, Huggins IJ, James SR, Karpf AR, Jones DA, Cairns BR, DNA demethylation in zebrafish involves the coupling of a deaminase, a glycosylase, and gadd45, Cell 135(7) (2008) 1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Costa Y, Ding J, Theunissen TW, Faiola F, Hore TA, Shliaha PV, Fidalgo M, Saunders A, Lawrence M, Dietmann S, NANOG-dependent function of TET1 and TET2 in establishment of pluripotency, Nature 495(7441) (2013) 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dolinoy DC, Huang D, Jirtle RL, Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development, Proceedings of the National Academy of Sciences 104(32) (2007) 13056–13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chang H-S, Anway MD, Rekow SS, Skinner MK, Transgenerational epigenetic imprinting of the male germline by endocrine disruptor exposure during gonadal sex determination, Endocrinology 147(12) (2006) 5524–5541. [DOI] [PubMed] [Google Scholar]
- [38].Milton AH, Hore SK, Hossain MZ, Rahman M, Bangladesh arsenic mitigation programs: lessons from the past, Emerging health threats journal 5(1) (2012) 7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Maloney B, Lahiri DK, Epigenetics of dementia: understanding the disease as a transformation rather than a state, The Lancet Neurology 15(7) (2016) 760–774. [DOI] [PubMed] [Google Scholar]
- [40].Wyatt G, Cohen S, A new pyrimidine base from bacteriophage nucleic acids, Nature 170(4338) (1952) 1072–1073. [DOI] [PubMed] [Google Scholar]
- [41].Penn N, Suwalski R, O’riley C, Bojanowski K, Yura R, The presence of 5-hydroxymethylcytosine in animal deoxyribonucleic acid, Biochem. J 126(4) (1972) 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1, Science 324(5929) (2009) 930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kriaucionis S, Heintz N, The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain, Science 324(5929) (2009) 929–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Song C-X, He C, The hunt for 5-hydroxymethylcytosine: the sixth base, Epigenomics 3(5) (2011) 521–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].He Y-F, Li B-Z, Li Z, Liu P, Wang Y, Tang Q, Ding J, Jia Y, Chen Z, Li L, Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA, Science 333(6047) (2011) 1303–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, He C, Zhang Y, Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine, Science 333(6047) (2011) 1300–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Song C-X, Clark TA, Lu X-Y, Kislyuk A, Dai Q, Turner SW, He C, Korlach J, Sensitive and specific single-molecule sequencing of 5-hydroxymethylcytosine, Nature methods 9(1) (2012) 75–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Globisch D, Münzel M, Müller M, Michalakis S, Wagner M, Koch S, Brückl T, Biel M, Carell T, Tissue distribution of 5-hydroxymethylcytosine and search for active demethylation intermediates, PLoS One 5(12) (2010) e15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ruzov A, Tsenkina Y, Serio A, Dudnakova T, Fletcher J, Bai Y, Chebotareva T, Pells S, Hannoun Z, Sullivan G, Lineage-specific distribution of high levels of genomic, Cell Res 21(9) (2011) 1332–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Pfaffeneder T, Hackner B, Truß M, Münzel M, Müller M, Deiml CA, Hagemeier C, Carell T, The discovery of 5-formylcytosine in embryonic stem cell DNA, Angewandte Chemie International Edition 50(31) (2011) 7008–7012. [DOI] [PubMed] [Google Scholar]
- [51].Münzel M, Globisch D, Carell T, 5-Hydroxymethylcytosine, the sixth base of the genome, Angewandte Chemie International Edition 50(29) (2011) 6460–6468. [DOI] [PubMed] [Google Scholar]
- [52].Branco MR, Ficz G, Reik W, Uncovering the role of 5-hydroxymethylcytosine in the epigenome, Nature Reviews Genetics 13(1) (2012) 7–13. [DOI] [PubMed] [Google Scholar]
- [53].Tan L, Shi YG, Tet family proteins and 5-hydroxymethylcytosine in development and disease, Development 139(11) (2012) 1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Coppieters N, Dieriks BV, Lill C, Faull RL, Curtis MA, Dragunow M, Global changes in DNA methylation and hydroxymethylation in Alzheimer’s disease human brain, Neurobiol. Aging 35(6) (2014) 1334–44. [DOI] [PubMed] [Google Scholar]
- [55].Szwagierczak A, Bultmann S, Schmidt CS, Spada F, Leonhardt H, Sensitive enzymatic quantification of 5-hydroxymethylcytosine in genomic DNA, Nucleic Acids Res 38(19) (2010) e181-e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Li W, Liu M, Distribution of 5-hydroxymethylcytosine in different human tissues, Journal of nucleic acids 2011 (2011). [DOI] [PMC free article] [PubMed]
- [57].Shi D-Q, Ali I, Tang J, Yang W-C, New insights into 5hmC DNA modification: generation, distribution and function, Frontiers in genetics 8 (2017) 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ito S, D’Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y, Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification, Nature 466(7310) (2010) 1129–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Jin S-G, Wu X, Li AX, Pfeifer GP, Genomic mapping of 5-hydroxymethylcytosine in the human brain, Nucleic Acids Res 39(12) (2011) 5015–5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Orr BA, Haffner MC, Nelson WG, Yegnasubramanian S, Eberhart CG, Decreased 5-hydroxymethylcytosine is associated with neural progenitor phenotype in normal brain and shorter survival in malignant glioma, PLoS One 7(7) (2012) e41036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Portela A, Esteller M, Epigenetic modifications and human disease, Nat. Biotechnol 28(10) (2010) 1057. [DOI] [PubMed] [Google Scholar]
- [62].Jones PA, Baylin SB, The epigenomics of cancer, Cell 128(4) (2007) 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ehrlich M, Cancer-linked DNA hypomethylation and its relationship to hypermethylation, DNA Methylation: Development, Genetic Disease and Cancer, Springer; 2006, pp. 251–274. [DOI] [PubMed] [Google Scholar]
- [64].Ehrlich M, Lacey M, DNA hypomethylation and hemimethylation in cancer, Epigenetic alterations in oncogenesis, Springer; 2013, pp. 31–56. [DOI] [PubMed] [Google Scholar]
- [65].Wilson AS, Power BE, Molloy PL, DNA hypomethylation and human diseases, Biochimica et Biophysica Acta (BBA)-Reviews on Cancer 1775(1) (2007) 138–162. [DOI] [PubMed] [Google Scholar]
- [66].Pfeifer GP, Kadam S, Jin S-G, 5-hydroxymethylcytosine and its potential roles in development and cancer, Epigenetics & chromatin 6(1) (2013) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Jin S-G, Jiang Y, Qiu R, Rauch TA, Wang Y, Schackert G, Krex D, Lu Q, Pfeifer GP, 5-Hydroxymethylcytosine is strongly depleted in human cancers but its levels do not correlate with IDH1 mutations, Cancer Res 71(24) (2011) 7360–7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Shukla A, Sehgal M, Singh TR, Hydroxymethylation and its potential implication in DNA repair system: a review and future perspectives, Gene 564(2) (2015) 109–118. [DOI] [PubMed] [Google Scholar]
- [69].Hahn MA, Qiu R, Wu X, Li AX, Zhang H, Wang J, Jui J, Jin S-G, Jiang Y, Pfeifer GP, Dynamics of 5-hydroxymethylcytosine and chromatin marks in Mammalian neurogenesis, Cell reports 3(2) (2013) 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Khare T, Pai S, Koncevicius K, Pal M, Kriukiene E, Liutkeviciute Z, Irimia M, Jia P, Ptak C, Xia M, 5-hmC in the brain is abundant in synaptic genes and shows differences at the exon-intron boundary, Nat. Struct. Mol. Biol 19(10) (2012) 1037–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Shu L, Sun W, Li L, Xu Z, Lin L, Xie P, Shen H, Huang L, Xu Q, Jin P, Genome-wide alteration of 5-hydroxymenthylcytosine in a mouse model of Alzheimer’s disease, BMC Genomics 17(1) (2016) 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Szulwach KE, Li X, Li Y, Song C-X, Wu H, Dai Q, Irier H, Upadhyay AK, Gearing M, Levey AI, 5-hmC–mediated epigenetic dynamics during postnatal neurodevelopment and aging, Nat. Neurosci 14(12) (2011) 1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Yao B, Lin L, Street RC, Zalewski ZA, Galloway JN, Wu H, Nelson DL, Jin P, Genome-wide alteration of 5-hydroxymethylcytosine in a mouse model of fragile X-associated tremor/ataxia syndrome, Hum. Mol. Genet 23(4) (2014) 1095–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wang F, Yang Y, Lin X, Wang J-Q, Wu Y-S, Xie W, Wang D, Zhu S, Liao Y-Q, Sun Q, Genome-wide loss of 5-hmC is a novel epigenetic feature of Huntington’s disease, Hum. Mol. Genet 22(18) (2013) 3641–3653. [DOI] [PubMed] [Google Scholar]
- [75].Papale LA, Zhang Q, Li S, Chen K, Keleş S, Alisch RS, Genome-wide disruption of 5-hydroxymethylcytosine in a mouse model of autism, Hum. Mol. Genet 24(24) (2015) 7121–7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Wossidlo M, Nakamura T, Lepikhov K, Marques CJ, Zakhartchenko V, Boiani M, Arand J, Nakano T, Reik W, Walter J, 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming, Nature communications 2(1) (2011) 1–8. [DOI] [PubMed] [Google Scholar]
- [77].Ficz G, Branco MR, Seisenberger S, Santos F, Krueger F, Hore TA, Marques CJ, Andrews S, Reik W, Dynamic regulation of 5-hydroxymethylcytosine in mouse ES cells and during differentiation, Nature 473(7347) (2011) 398–402. [DOI] [PubMed] [Google Scholar]
- [78].Hackett JA, Sengupta R, Zylicz JJ, Murakami K, Lee C, Down TA, Surani MA, Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine, Science 339(6118) (2013) 448–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Klug M, Schmidhofer S, Gebhard C, Andreesen R, Rehli M, 5-Hydroxymethylcytosine is an essential intermediate of active DNA demethylation processes in primary human monocytes, Genome Biol 14(5) (2013) R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Haffner MC, Chaux A, Meeker AK, Esopi DM, Gerber J, Pellakuru LG, Toubaji A, Argani P, Iacobuzio-Donahue C, Nelson WG, Global 5-hydroxymethylcytosine content is significantly reduced in tissue stem/progenitor cell compartments and in human cancers, Oncotarget 2(8) (2011) 627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Dzitoyeva S, Chen H, Manev H, Effect of aging on 5-hydroxymethylcytosine in brain mitochondria, Neurobiol. Aging 33(12) (2012) 2881–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Chen H, Dzitoyeva S, Manev H, Effect of aging on 5-hydroxymethylcytosine in the mouse hippocampus, Restor. Neurol. Neurosci 30(3) (2012) 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Wu H, D’Alessio AC, Ito S, Wang Z, Cui K, Zhao K, Sun YE, Zhang Y, Genome-wide analysis of 5-hydroxymethylcytosine distribution reveals its dual function in transcriptional regulation in mouse embryonic stem cells, Genes Dev 25(7) (2011) 679–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Colquitt BM, Allen WE, Barnea G, Lomvardas S, Alteration of genic 5-hydroxymethylcytosine patterning in olfactory neurons correlates with changes in gene expression and cell identity, Proceedings of the National Academy of Sciences 110(36) (2013) 14682–14687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Huang Y, Pastor WA, Shen Y, Tahiliani M, Liu DR, Rao A, The behaviour of 5-hydroxymethylcytosine in bisulfite sequencing, PLoS One 5(1) (2010) e8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Nestor C, Ruzov A, Meehan RR, Dunican DS, Enzymatic approaches and bisulfite sequencing cannot distinguish between 5-methylcytosine and 5-hydroxymethylcytosine in DNA, Biotechniques 48(4) (2010) 317–319. [DOI] [PubMed] [Google Scholar]
- [87].Münzel M, Globisch D, Brückl T, Wagner M, Welzmiller V, Michalakis S, Müller M, Biel M, Carell T, Quantification of the sixth DNA base hydroxymethylcytosine in the brain, Angewandte Chemie International Edition 49(31) (2010) 5375–5377. [DOI] [PubMed] [Google Scholar]
- [88].Godderis L, Schouteden C, Tabish A, Poels K, Hoet P, Baccarelli AA, Van Landuyt K, Global Methylation and Hydroxymethylation in DNA from Blood and Saliva in Healthy Volunteers, Biomed Res Int 2015 (2015) 845041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Cardenas A, Rifas-Shiman SL, Godderis L, Duca RC, Navas-Acien A, Litonjua AA, DeMeo DL, Brennan KJ, Amarasiriwardena CJ, Hivert MF, Gillman MW, Oken E, Baccarelli AA, Prenatal Exposure to Mercury: Associations with Global DNA Methylation and Hydroxymethylation in Cord Blood and in Childhood, Environ. Health Perspect 125(8) (2017) 087022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].De Nys S, Duca RC, Nawrot T, Hoet P, Van Meerbeek B, Van Landuyt KL, Godderis L, Temporal variability of global DNA methylation and hydroxymethylation in buccal cells of healthy adults: Association with air pollution, Environ. Int 111 (2018) 301–308. [DOI] [PubMed] [Google Scholar]
- [91].Le T, Kim KP, Fan G, Faull KF, A sensitive mass spectrometry method for simultaneous quantification of DNA methylation and hydroxymethylation levels in biological samples, Anal. Biochem 412(2) (2011) 203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Chen S, Dou Y, Zhao Z, Li F, Su J, Fan C, Song S, High-Sensitivity and High-Efficiency Detection of DNA Hydroxymethylation in Genomic DNA by Multiplexing Electrochemical Biosensing, Anal. Chem 88(7) (2016) 3476–80. [DOI] [PubMed] [Google Scholar]
- [93].Williams K, Christensen J, Pedersen MT, Johansen JV, Cloos PA, Rappsilber J, Helin K, TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity, Nature 473(7347) (2011) 343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Stroud H, Feng S, Kinney SM, Pradhan S, Jacobsen SE, 5-Hydroxymethylcytosine is associated with enhancers and gene bodies in human embryonic stem cells, Genome Biol 12(6) (2011) R54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Feldmann A, Ivanek R, Murr R, Gaidatzis D, Burger L, Schübeler D, Transcription factor occupancy can mediate active turnover of DNA methylation at regulatory regions, PLoS Genet 9(12) (2013) e1003994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Tan L, Xiong L, Xu W, Wu F, Huang N, Xu Y, Kong L, Zheng L, Schwartz L, Shi Y, Shi YG, Genome-wide comparison of DNA hydroxymethylation in mouse embryonic stem cells and neural progenitor cells by a new comparative hMeDIP-seq method, Nucleic Acids Res 41(7) (2013) e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Pastor WA, Pape UJ, Huang Y, Henderson HR, Lister R, Ko M, McLoughlin EM, Brudno Y, Mahapatra S, Kapranov P, Genome-wide mapping of 5-hydroxymethylcytosine in embryonic stem cells, Nature 473(7347) (2011) 394–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Chouliaras L, Mastroeni D, Delvaux E, Grover A, Kenis G, Hof PR, Steinbusch HW, Coleman PD, Rutten BP, van den Hove DL, Consistent decrease in global DNA methylation and hydroxymethylation in the hippocampus of Alzheimer’s disease patients, Neurobiol. Aging 34(9) (2013) 2091–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Lashley T, Gami P, Valizadeh N, Li A, Revesz T, Balazs R, Alterations in global DNA methylation and hydroxymethylation are not detected in Alzheimer’s disease, Neuropathol. Appl. Neurobiol 41(4) (2015) 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Takayama K, Misawa A, Suzuki T, Takagi K, Hayashizaki Y, Fujimura T, Homma Y, Takahashi S, Urano T, Inoue S, TET2 repression by androgen hormone regulates global hydroxymethylation status and prostate cancer progression, Nat Commun 6 (2015) 8219. [DOI] [PubMed] [Google Scholar]
- [101].Song CX, Szulwach KE, Fu Y, Dai Q, Yi C, Li X, Li Y, Chen CH, Zhang W, Jian X, Wang J, Zhang L, Looney TJ, Zhang B, Godley LA, Hicks LM, Lahn BT, Jin P, He C, Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine, Nat. Biotechnol 29(1) (2011) 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Song C-X, Szulwach KE, Fu Y, Dai Q, Yi C, Li X, Li Y, Chen C-H, Zhang W, Jian X, Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine, Nat. Biotechnol 29(1) (2011) 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Yu M, Hon GC, Szulwach KE, Song C-X, Jin P, Ren B, He C, Tet-assisted bisulfite sequencing of 5-hydroxymethylcytosine, Nat. Protoc 7(12) (2012) 2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Bhattacharyya S, Yu Y, Suzuki M, Campbell N, Mazdo J, Vasanthakumar A, Bhagat TD, Nischal S, Christopeit M, Parekh S, Steidl U, Godley L, Maitra A, Greally JM, Verma A, Genome-wide hydroxymethylation tested using the HELP-GT assay shows redistribution in cancer, Nucleic Acids Res 41(16) (2013) e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Zheng Y, Cohen-Karni D, Xu D, Chin HG, Wilson G, Pradhan S, Roberts RJ, A unique family of Mrr-like modification-dependent restriction endonucleases, Nucleic Acids Res 38(16) (2010) 5527–5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Song C-X, Yu M, Dai Q, He C, Detection of 5-hydroxymethylcytosine in a combined glycosylation restriction analysis (CGRA) using restriction enzyme TaqαI, Bioorg. Med. Chem. Lett 21(17) (2011) 5075–5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Wang H, Guan S, Quimby A, Cohen-Karni D, Pradhan S, Wilson G, Roberts RJ, Zhu Z, Zheng Y, Comparative characterization of the PvuRts1I family of restriction enzymes and their application in mapping genomic 5-hydroxymethylcytosine, Nucleic Acids Res 39(21) (2011) 9294–9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Szwagierczak A, Brachmann A, Schmidt CS, Bultmann S, Leonhardt H, Spada F, Characterization of PvuRts1I endonuclease as a tool to investigate genomic 5-hydroxymethylcytosine, Nucleic Acids Res 39(12) (2011) 5149–5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Robertson AB, Dahl JA, Vågbø CB, Tripathi P, Krokan HE, Klungland A, A novel method for the efficient and selective identification of 5-hydroxymethylcytosine in genomic DNA, Nucleic Acids Res 39(8) (2011) e55-e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Booth MJ, Branco MR, Ficz G, Oxley D, Krueger F, Reik W, Balasubramanian S, Quantitative sequencing of 5-methylcytosine and 5-hydroxymethylcytosine at single-base resolution, Science 336(6083) (2012) 934–937. [DOI] [PubMed] [Google Scholar]
- [111].Stewart SK, Morris TJ, Guilhamon P, Bulstrode H, Bachman M, Balasubramanian S, Beck S, oxBS-450K: a method for analysing hydroxymethylation using 450K BeadChips, Methods 72 (2015) 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Ma S, Sun H, Li Y, Qi H, Zheng J, Discrimination between 5-hydroxymethylcytosine and 5-methylcytosine in DNA via selective electrogenerated chemiluminescence (ECL) labeling, Anal. Chem 88(20) (2016) 9934–9940. [DOI] [PubMed] [Google Scholar]
- [113].Yin H, Yang Z, Wang H, Zhou Y, Ai S, Electrochemical biosensor for hydroxymethylated DNA detection and β-glucosyltransferase activity assay based on enzymatic catalysis triggering signal amplification, Sensors and Actuators B: Chemical 243 (2017) 602–608. [Google Scholar]
- [114].Zhang Y, Li Y, Wei Y, Sun H, Wang H, A sensitive signal-off electrogenerated chemiluminescence biosensing method for the discrimination of DNA hydroxymethylation based on glycosylation modification and signal quenching from ferroceneboronic acid, Talanta 170 (2017) 546–551. [DOI] [PubMed] [Google Scholar]
- [115].Wang H, Liu M, Bai W, Sun H, Li Y, Deng H, A convenient electrogenerated chemiluminescence biosensing method for selective detection of 5-hydroxymethylcytosine in genomic DNA, Sensors and Actuators B: Chemical 284 (2019) 236–242. [Google Scholar]
- [116].Salbert G, Weber M, Tracking genomic hydroxymethylation by the base, Nat Methods 9(1) (2011) 45–6. [DOI] [PubMed] [Google Scholar]
- [117].Flusberg BA, Webster DR, Lee JH, Travers KJ, Olivares EC, Clark TA, Korlach J, Turner SW, Direct detection of DNA methylation during single-molecule, real-time sequencing, Nature methods 7(6) (2010) 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Wanunu M, Cohen-Karni D, Johnson RR, Fields L, Benner J, Peterman N, Zheng Y, Klein ML, Drndic M, Discrimination of methylcytosine from hydroxymethylcytosine in DNA molecules, J. Am. Chem. Soc 133(3) (2011) 486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Beck S, Rakyan VK, The methylome: approaches for global DNA methylation profiling, Trends Genet 24(5) (2008) 231–237. [DOI] [PubMed] [Google Scholar]
- [120].Shen L, Waterland RA, Methods of DNA methylation analysis, Curr. Opin. Clin. Nutr. Metab. Care 10(5) (2007) 576–581. [DOI] [PubMed] [Google Scholar]
- [121].Huang T, Plass C, Liang G, Laird PW, Epi meets genomics: technologies for finding and reading the 5th base, The Epigenome: Molecular Hide and Seek, wiley; 2003, pp. 39–64. [Google Scholar]
- [122].Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB, Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands, Proceedings of the national academy of sciences 93(18) (1996) 9821–9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Sasaki M, Anast J, Bassett W, Kawakami T, Sakuragi N, Dahiya R, Bisulfite conversion-specific and methylation-specific PCR: a sensitive technique for accurate evaluation of CpG methylation, Biochem. Biophys. Res. Commun 309(2) (2003) 305–309. [DOI] [PubMed] [Google Scholar]
- [124].Xiong Z, Laird PW, COBRA: a sensitive and quantitative DNA methylation assay, Nucleic Acids Res 25(12) (1997) 2532–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Randt C, Linscheid M, Analysis of 5-methyl-deoxycytidine in DNA by micro-HPLC, Fresenius’ Zeitschrift für analytische Chemie 331(3–4) (1988) 459–463. [Google Scholar]
- [126].Wagner I, Capesius I, Determination of 5-methylcytosine from plant DNA by high-performance liquid chromatography, Biochimica et Biophysica Acta (BBA)-Nucleic Acids and Protein Synthesis 654(1) (1981) 52–56. [DOI] [PubMed] [Google Scholar]
- [127].Friso S, Choi S-W, Dolnikowski GG, Selhub J, A method to assess genomic DNA methylation using high-performance liquid chromatography/electrospray ionization mass spectrometry, Anal. Chem 74(17) (2002) 4526–4531. [DOI] [PubMed] [Google Scholar]
- [128].Ramsahoye BH, Nearest-neighbor analysis DNA Methylation Protocols, Springer; 2002, pp. 9–16. [Google Scholar]
- [129].Ramsahoye B, Measurement of genome wide DNA methylation by reversed-phase high-performance liquid chromatography, Methods 27(2) (2002) 156–161. [DOI] [PubMed] [Google Scholar]
- [130].Sonoki S, Lin J, Hisamatsu S, Liquid chromatographic determination of 5-methylcytosine in DNA with fluorescence detection, Anal. Chim. Acta 365(1–3) (1998) 213–217. [Google Scholar]
- [131].Magaña AA, Wrobel K, Caudillo YA, Zaina S, Lund G, Wrobel K, High-performance liquid chromatography determination of 5-methyl-2′-deoxycytidine, 2′-deoxycytidine, and other deoxynucleosides and nucleosides in DNA digests, Anal. Biochem 374(2) (2008) 378–385. [DOI] [PubMed] [Google Scholar]
- [132].Hu C-W, Lee H, Chen J-L, Li Y-J, Chao M-R, Optimization of global DNA methylation measurement by LC-MS/MS and its application in lung cancer patients, Anal. Bioanal. Chem 405(27) (2013) 8859–8869. [DOI] [PubMed] [Google Scholar]
- [133].Song L, James SR, Kazim L, Karpf AR, Specific method for the determination of genomic DNA methylation by liquid chromatography-electrospray ionization tandem mass spectrometry, Anal. Chem 77(2) (2005) 504–510. [DOI] [PubMed] [Google Scholar]
- [134].Humeny A, Beck C, Becker C-M, Jeltsch A, Detection and analysis of enzymatic DNA methylation of oligonucleotide substrates by matrix-assisted laser desorption ionization time-of-flight mass spectrometry, Anal. Biochem 313(1) (2003) 160–166. [DOI] [PubMed] [Google Scholar]
- [135].Liu Z, Liu S, Xie Z, Blum W, Perrotti D, Paschka P, Klisovic R, Byrd J, Chan KK, Marcucci G, Characterization of in vitro and in vivo hypomethylating effects of decitabine in acute myeloid leukemia by a rapid, specific and sensitive LC-MS/MS method, Nucleic Acids Res 35(5) (2007) e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Hayashizaki Y, Hirotsune S, Okazaki Y, Hatada I, Shibata H, Kawai J, Hirose K, Watanabe S, Fushiki S, Wada S, Restriction landmark genomic scanning method and its various applications, Electrophoresis 14(1) (1993) 251–258. [DOI] [PubMed] [Google Scholar]
- [137].Kawai J, Hirotsune S, Hirose K, Fushiki S, Watanabe S, Hayashizaki Y, Methylation profiles of genomic DNA of mouse developmental brain detected by restriction landmark genomic scanning (RLGS) method, Nucleic Acids Res 21(24) (1993) 5604–5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Plass C, Shibata H, Kalcheva I, Mullins L, Kotelevtseva N, Mullins J, Kato R, Sasaki H, Hirotsune S, Okazaki Y, Identification of Grf1 on mouse chromosome 9 as an imprinted gene by RLGS–M, Nat. Genet 14(1) (1996) 106–109. [DOI] [PubMed] [Google Scholar]
- [139].Song F, Smith JF, Kimura MT, Morrow AD, Matsuyama T, Nagase H, Held WA, Association of tissue-specific differentially methylated regions (TDMs) with differential gene expression, Proceedings of the National Academy of Sciences 102(9) (2005) 3336–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Allegrucci C, Wu Y-Z, Thurston A, Denning CN, Priddle H, Mummery CL, Ward-van Oostwaard D, Andrews PW, Stojkovic M, Smith N, Restriction landmark genome scanning identifies culture-induced DNA methylation instability in the human embryonic stem cell epigenome, Hum. Mol. Genet 16(10) (2007) 1253–1268. [DOI] [PubMed] [Google Scholar]
- [141].Hatada I, Hayashizaki Y, Hirotsune S, Komatsubara H, Mukai T, A genomic scanning method for higher organisms using restriction sites as landmarks, Proceedings of the National Academy of Sciences 88(21) (1991) 9523–9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Costello JF, Frühwald MC, Smiraglia DJ, Rush LJ, Robertson GP, Gao X, Wright FA, Feramisco JD, Peltomäki P, Lang JC, Aberrant CpG-island methylation has non-random and tumour-type–specific patterns, Nat. Genet 24(2) (2000) 132–138. [DOI] [PubMed] [Google Scholar]
- [143].Rouillard J-M, Erson AE, Kuick R, Asakawa J.-i., Wimmer K, Muleris M, Petty EM, Hanash S, Virtual genome scan: a tool for restriction landmark-based scanning of the human genome, Genome Res 11(8) (2001) 1453–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Frigola J, Ribas M, Risques R-A, Peinado MA, Methylome profiling of cancer cells by amplification of inter-methylated sites (AIMS), Nucleic Acids Res 30(7) (2002) e28-e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Liang G, Gonzalgo ML, Salem C, Jones PA, Identification of DNA methylation differences during tumorigenesis by methylation-sensitive arbitrarily primed polymerase chain reaction, Methods 27(2) (2002) 150–155. [DOI] [PubMed] [Google Scholar]
- [146].Yan PS, Perry MR, Laux DE, Asare AL, Caldwell CW, Huang TH-M, CpG island arrays: an application toward deciphering epigenetic signatures of breast cancer, Clin. Cancer Res 6(4) (2000) 1432–1438. [PubMed] [Google Scholar]
- [147].Yan PS, Chen C-M, Shi H, Rahmatpanah F, Wei SH, Caldwell CW, Huang TH-M, Dissecting complex epigenetic alterations in breast cancer using CpG island microarrays, Cancer Res 61(23) (2001) 8375–8380. [PubMed] [Google Scholar]
- [148].Balog RP, De Souza YEP, Tang HM, DeMasellis GM, Gao B, Avila A, Gaban DJ, Mittelman D, Minna JD, Luebke KJ, Parallel assessment of CpG methylation by two-color hybridization with oligonucleotide arrays, Anal. Biochem 309(2) (2002) 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Hatada I, Kato A, Morita S, Obata Y, Nagaoka K, Sakurada A, Sato M, Horii A, Tsujimoto A, Matsubara K, A microarray-based method for detecting methylated loci, J. Hum. Genet 47(8) (2002) 448–451. [DOI] [PubMed] [Google Scholar]
- [150].Moran S, Arribas C, Esteller M, Validation of a DNA methylation microarray for 850,000 CpG sites of the human genome enriched in enhancer sequences, Epigenomics 8(3) (2016) 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Pidsley R, Zotenko E, Peters TJ, Lawrence MG, Risbridger GP, Molloy P, Van Djik S, Muhlhausler B, Stirzaker C, Clark SJ, Critical evaluation of the Illumina MethylationEPIC BeadChip microarray for whole-genome DNA methylation profiling, Genome Biol 17(1) (2016) 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Schumacher A, Kapranov P, Kaminsky Z, Flanagan J, Assadzadeh A, Yau P, Virtanen C, Winegarden N, Cheng J, Gingeras T, Microarray-based DNA methylation profiling: technology and applications, Nucleic Acids Res 34(2) (2006) 528–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Gitan RS, Shi H, Chen C-M, Yan PS, Huang TH-M, Methylation-specific oligonucleotide microarray: a new potential for high-throughput methylation analysis, Genome Res 12(1) (2002) 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Adorján P, Distler J, Lipscher E, Model F, Müller J, Pelet C, Braun A, Florl AR, Gütig D, Grabs G, Tumour class prediction and discovery by microarray-based DNA methylation analysis, Nucleic Acids Res 30(5) (2002) e21-e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Olek A, Oswald J, Walter J, A modified and improved method for bisulphite based cytosine methylation analysis, Nucleic Acids Res 24(24) (1996) 5064–5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Hayatsu H, Wataya Y, Kai K, Iida S, Reaction of sodium bisulfite with uracil, cytosine, and their derivatives, Biochemistry 9(14) (1970) 2858–2865. [DOI] [PubMed] [Google Scholar]
- [157].Tompa R, McCallum CM, Delrow J, Henikoff JG, van Steensel B, Henikoff S, Genome-wide profiling of DNA methylation reveals transposon targets of CHROMOMETHYLASE3, Curr. Biol 12(1) (2002) 65–68. [DOI] [PubMed] [Google Scholar]
- [158].Murrell A, Rakyan VK, Beck S, From genome to epigenome, Hum. Mol. Genet 14(suppl_1) (2005) R3–R10. [DOI] [PubMed] [Google Scholar]
- [159].Bedell JA, Budiman MA, Nunberg A, Citek RW, Robbins D, Jones J, Flick E, Rohlfing T, Fries J, Bradford K, Sorghum genome sequencing by methylation filtration, PLoS Biol 3(1) (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Rabinowicz PD, Constructing gene-enriched plant genomic libraries using methylation filtration technology, Plant Functional Genomics, Springer; 2003, pp. 21–35. [DOI] [PubMed] [Google Scholar]
- [161].Yamada Y, Watanabe H, Miura F, Soejima H, Uchiyama M, Iwasaka T, Mukai T, Sakaki Y, Ito T, A comprehensive analysis of allelic methylation status of CpG islands on human chromosome 21q, Genome Res 14(2) (2004) 247–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Khulan B, Thompson RF, Ye K, Fazzari MJ, Suzuki M, Stasiek E, Figueroa ME, Glass JL, Chen Q, Montagna C, Comparative isoschizomer profiling of cytosine methylation: the HELP assay, Genome Res 16(8) (2006) 1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Rauch T, Li H, Wu X, Pfeifer GP, MIRA-assisted microarray analysis, a new technology for the determination of DNA methylation patterns, identifies frequent methylation of homeodomain-containing genes in lung cancer cells, Cancer Res 66(16) (2006) 7939–7947. [DOI] [PubMed] [Google Scholar]
- [164].Eckhardt F, Lewin J, Cortese R, Rakyan VK, Attwood J, Burger M, Burton J, Cox TV, Davies R, Down TA, DNA methylation profiling of human chromosomes 6, 20 and 22, Nat. Genet 38(12) (2006) 1378–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Church GM, Gilbert W, Genomic sequencing, Proceedings of the National Academy of Sciences 81(7) (1984) 1991–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Frommer M, McDonald LE, Millar DS, Collis CM, Watt F, Grigg GW, Molloy PL, Paul CL, A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands, Proceedings of the National Academy of Sciences 89(5) (1992) 1827–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Tost J, Gut IG, DNA methylation analysis by pyrosequencing, Nat. Protoc 2(9) (2007) 2265. [DOI] [PubMed] [Google Scholar]
- [168].Meissner A, Gnirke A, Bell GW, Ramsahoye B, Lander ES, Jaenisch R, Reduced representation bisulfite sequencing for comparative high-resolution DNA methylation analysis, Nucleic Acids Res 33(18) (2005) 5868–5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Mitchell SM, Ross JP, Drew HR, Ho T, Brown GS, Saunders NF, Duesing KR, Buckley MJ, Dunne R, Beetson I, A panel of genes methylated with high frequency in colorectal cancer, BMC Cancer 14(1) (2014) 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Lim J-Q, Tennakoon C, Li G, Wong E, Ruan Y, Wei C-L, Sung W-K, BatMeth: improved mapper for bisulfite sequencing reads on DNA methylation, Genome Biol 13(10) (2012) R82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Tucker T, Marra M, Friedman JM, Massively parallel sequencing: the next big thing in genetic medicine, The American Journal of Human Genetics 85(2) (2009) 142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Voelkerding KV, Dames SA, Durtschi JD, Next-generation sequencing: from basic research to diagnostics, Clin. Chem 55(4) (2009) 641–658. [DOI] [PubMed] [Google Scholar]
- [173].Treangen TJ, Salzberg SL, Repetitive DNA and next-generation sequencing: computational challenges and solutions, Nature Reviews Genetics 13(1) (2012) 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Bleidorn C, Third generation sequencing: technology and its potential impact on evolutionary biodiversity research, Systematics and biodiversity 14(1) (2016) 1–8. [Google Scholar]
- [175].Simpson JT, Workman R, Zuzarte PC, David M, Dursi LJ, Timp W, Detecting DNA methylation using the oxford nanopore technologies MinION sequencer, BioRxiv (2016) 047142.
- [176].Mikheyev AS, Tin MM, A first look at the Oxford Nanopore MinION sequencer, Mol. Ecol. Resour 14(6) (2014) 1097–1102. [DOI] [PubMed] [Google Scholar]
- [177].Loman NJ, Quinlan AR, Poretools: a toolkit for analyzing nanopore sequence data, Bioinformatics 30(23) (2014) 3399–3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Eisenstein M, Oxford Nanopore announcement sets sequencing sector abuzz, Nature Publishing Group, 2012. [DOI] [PubMed] [Google Scholar]
- [179].Simpson JT, Workman RE, Zuzarte P, David M, Dursi L, Timp W, Detecting DNA cytosine methylation using nanopore sequencing, Nature methods 14(4) (2017) 407. [DOI] [PubMed] [Google Scholar]
- [180].Levene MJ, Korlach J, Turner SW, Foquet M, Craighead HG, Webb WW, Zero-mode waveguides for single-molecule analysis at high concentrations, Science 299(5607) (2003) 682–686. [DOI] [PubMed] [Google Scholar]
- [181].Gupta PK, Single-molecule DNA sequencing technologies for future genomics research, Trends Biotechnol 26(11) (2008) 602–611. [DOI] [PubMed] [Google Scholar]
- [182].Callinan PA, Feinberg AP, The emerging science of epigenomics, Hum. Mol. Genet 15(suppl_1) (2006) R95–R101. [DOI] [PubMed] [Google Scholar]
- [183].Schones DE, Zhao K, Genome-wide approaches to studying chromatin modifications, Nature Reviews Genetics 9(3) (2008) 179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Pomraning KR, Smith KM, Freitag M, Genome-wide high throughput analysis of DNA methylation in eukaryotes, Methods 47(3) (2009) 142–150. [DOI] [PubMed] [Google Scholar]
- [185].Hayatsu H, Discovery of bisulfite-mediated cytosine conversion to uracil, the key reaction for DNA methylation analysis—a personal account, Proceedings of the Japan Academy, Series B 84(8) (2008) 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Susan JC, Harrison J, Paul CL, Frommer M, High sensitivity mapping of methylated cytosines, Nucleic Acids Res 22(15) (1994) 2990–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Gu H, Smith ZD, Bock C, Boyle P, Gnirke A, Meissner A, Preparation of reduced representation bisulfite sequencing libraries for genome-scale DNA methylation profiling, Nat. Protoc 6(4) (2011) 468. [DOI] [PubMed] [Google Scholar]
- [188].Smith ZD, Gu H, Bock C, Gnirke A, Meissner A, High-throughput bisulfite sequencing in mammalian genomes, Methods 48(3) (2009) 226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Meissner A, Mikkelsen TS, Gu H, Wernig M, Hanna J, Sivachenko A, Zhang X, Bernstein BE, Nusbaum C, Jaffe DB, Genome-scale DNA methylation maps of pluripotent and differentiated cells, Nature 454(7205) (2008) 766–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Versteeg R, Aberrant methylation in cancer, Am. J. Hum. Genet 60(4) (1997) 751. [PMC free article] [PubMed] [Google Scholar]
- [191].Bock C, Tomazou EM, Brinkman AB, Müller F, Simmer F, Gu H, Jäger N, Gnirke A, Stunnenberg HG, Meissner A, Quantitative comparison of genome-wide DNA methylation mapping technologies, Nat. Biotechnol 28(10) (2010) 1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Ehrlich M, DNA hypomethylation in cancer cells, Epigenomics 1(2) (2009) 239–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Warnecke PM, Stirzaker C, Song J, Grunau C, Melki JR, Clark SJ, Identification and resolution of artifacts in bisulfite sequencing, Methods 27(2) (2002) 101–107. [DOI] [PubMed] [Google Scholar]
- [194].Hajkova P, El-Maarri O, Engemann S, Oswald J, Olek A, Walter J, DNA-methylation analysis by the bisulfite-assisted genomic sequencing method, DNA methylation protocols, Springer; 2002, pp. 143–154. [DOI] [PubMed] [Google Scholar]
- [195].Li Y, Tollefsbol TO, DNA methylation detection: bisulfite genomic sequencing analysis, Epigenetics Protocols, Springer; 2011, pp. 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Paulin R, Davey MW, Piper AA, Grigg GW, Urea improves efficiency of bisulphite-mediated sequencing of 5′-methylcytosine in genomic DNA, Nucleic Acids Res 26(21) (1998) 5009–5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Urich MA, Nery JR, Lister R, Schmitz RJ, Ecker JR, MethylC-seq library preparation for base-resolution whole-genome bisulfite sequencing, Nat. Protoc 10(3) (2015) 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Feng S, Rubbi L, Jacobsen SE, Pellegrini M, Determining DNA methylation profiles using sequencing, High-Throughput Next Generation Sequencing, Springer; 2011, pp. 223–238. [DOI] [PubMed] [Google Scholar]
- [199].Lister R, Ecker JR, Finding the fifth base: genome-wide sequencing of cytosine methylation, Genome Res 19(6) (2009) 959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Cokus SJ, Feng S, Zhang X, Chen Z, Merriman B, Haudenschild CD, Pradhan S, Nelson SF, Pellegrini M, Jacobsen SE, Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning, Nature 452(7184) (2008) 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Harris RA, Wang T, Coarfa C, Nagarajan RP, Hong C, Downey SL, Johnson BE, Fouse SD, Delaney A, Zhao Y, Comparison of sequencing-based methods to profile DNA methylation and identification of monoallelic epigenetic modifications, Nat. Biotechnol 28(10) (2010) 1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Kernaleguen M, Daviaud C, Shen Y, Bonnet E, Renault V, Deleuze J-F, Mauger F, Tost J, Whole-genome bisulfite sequencing for the analysis of genome-wide DNA methylation and hydroxymethylation patterns at single-nucleotide resolution, Epigenome Editing, Springer; 2018, pp. 311–349. [DOI] [PubMed] [Google Scholar]
- [203].Krueger F, Andrews SR, Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications, Bioinformatics 27(11) (2011) 1571–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Akalin A, Kormaksson M, Li S, Garrett-Bakelman FE, Figueroa ME, Melnick A, Mason CE, methylKit: a comprehensive R package for the analysis of genome-wide DNA methylation profiles, Genome Biol 13(10) (2012) R87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Park Y, Figueroa ME, Rozek LS, Sartor MA, MethylSig: a whole genome DNA methylation analysis pipeline, Bioinformatics 30(17) (2014) 2414–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Hansen KD, Langmead B, Irizarry RA, BSmooth: from whole genome bisulfite sequencing reads to differentially methylated regions, Genome Biol 13(10) (2012) R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Benoukraf T, Wongphayak S, Hadi LHA, Wu M, Soong R, GBSA: a comprehensive software for analysing whole genome bisulfite sequencing data, Nucleic Acids Res 41(4) (2013) e55-e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Feng H, Conneely KN, Wu H, A Bayesian hierarchical model to detect differentially methylated loci from single nucleotide resolution sequencing data, Nucleic Acids Res 42(8) (2014) e69-e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Hebestreit K, Dugas M, Klein H-U, Detection of significantly differentially methylated regions in targeted bisulfite sequencing data, Bioinformatics 29(13) (2013) 1647–1653. [DOI] [PubMed] [Google Scholar]
- [210].Sun D, Xi Y, Rodriguez B, Park HJ, Tong P, Meong M, Goodell MA, Li W, MOABS: model based analysis of bisulfite sequencing data, Genome Biol 15(2) (2014) R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Saito Y, Tsuji J, Mituyama T, Bisulfighter: accurate detection of methylated cytosines and differentially methylated regions, Nucleic Acids Res 42(6) (2014) e45-e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Stockwell PA, Chatterjee A, Rodger EJ, Morison IM, DMAP: differential methylation analysis package for RRBS and WGBS data, Bioinformatics 30(13) (2014) 1814–1822. [DOI] [PubMed] [Google Scholar]
- [213].Zhao L, Sun M.-a., Li Z, Bai X, Yu M, Wang M, Liang L, Shao X, Arnovitz S, Wang Q, The dynamics of DNA methylation fidelity during mouse embryonic stem cell self-renewal and differentiation, Genome Res 24(8) (2014) 1296–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Lu F, Liu Y, Jiang L, Yamaguchi S, Zhang Y, Role of Tet proteins in enhancer activity and telomere elongation, Genes Dev 28(19) (2014) 2103–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Xie W, Barr CL, Kim A, Yue F, Lee AY, Eubanks J, Dempster EL, Ren B, Base-resolution analyses of sequence and parent-of-origin dependent DNA methylation in the mouse genome, Cell 148(4) (2012) 816–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Lee S-T, Muench MO, Fomin ME, Xiao J, Zhou M, de Smith A, Martín-Subero JI, Heath S, Houseman EA, Roy R, Epigenetic remodeling in B-cell acute lymphoblastic leukemia occurs in two tracks and employs embryonic stem cell-like signatures, Nucleic Acids Res 43(5) (2015) 2590–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Wu H, Xu T, Feng H, Chen L, Li B, Yao B, Qin Z, Jin P, Conneely KN, Detection of differentially methylated regions from whole-genome bisulfite sequencing data without replicates, Nucleic Acids Res 43(21) (2015) e141-e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, Nery JR, Lee L, Ye Z, Ngo Q-M, Human DNA methylomes at base resolution show widespread epigenomic differences, Nature 462(7271) (2009) 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Miura F, Enomoto Y, Dairiki R, Ito T, Amplification-free whole-genome bisulfite sequencing by post-bisulfite adaptor tagging, Nucleic Acids Res 40(17) (2012) e136-e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Adey A, Shendure J, Ultra-low-input, tagmentation-based whole-genome bisulfite sequencing, Genome Res 22(6) (2012) 1139–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Adey A, Morrison HG, Xun X, Kitzman JO, Turner EH, Stackhouse B, MacKenzie AP, Caruccio NC, Zhang X, Shendure J, Rapid, low-input, low-bias construction of shotgun fragment libraries by high-density in vitro transposition, Genome Biol 11(12) (2010) R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Wang Q, Gu L, Adey A, Radlwimmer B, Wang W, Hovestadt V, Bähr M, Wolf S, Shendure J, Eils R, Tagmentation-based whole-genome bisulfite sequencing, Nat. Protoc 8(10) (2013) 2022. [DOI] [PubMed] [Google Scholar]
- [223].Olova N, Krueger F, Andrews S, Oxley D, Berrens RV, Branco MR, Reik W, Comparison of whole-genome bisulfite sequencing library preparation strategies identifies sources of biases affecting DNA methylation data, Genome Biol 19(1) (2018) 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Bird AP, Southern EM, Use of restriction enzymes to study eukaryotic DNA methylation: I. The methylation pattern in ribosomal DNA from Xenopus laevis, J. Mol. Biol 118(1) (1978) 27–47. [DOI] [PubMed] [Google Scholar]
- [225].Maunakea AK, Nagarajan RP, Bilenky M, Ballinger TJ, D’Souza C, Fouse SD, Johnson BE, Hong C, Nielsen C, Zhao Y, Conserved role of intragenic DNA methylation in regulating alternative promoters, Nature 466(7303) (2010) 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Bonora G, Rubbi L, Morselli M, Ma F, Chronis C, Plath K, Pellegrini M, DNA methylation estimation using methylation-sensitive restriction enzyme bisulfite sequencing (MREBS), PLoS One 14(4) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Xing X, Zhang B, Li D, Wang T, Comprehensive whole DNA methylome analysis by integrating MeDIP-seq and MRE-seq, DNA Methylation Protocols, Springer; 2018, pp. 209–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Li D, Zhang B, Xing X, Wang T, Combining MeDIP-seq and MRE-seq to investigate genome-wide CpG methylation, Methods 72 (2015) 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Fazzari MJ, Greally JM, Epigenomics: beyond CpG islands, Nature Reviews Genetics 5(6) (2004) 446–455. [DOI] [PubMed] [Google Scholar]
- [230].Gebhard C, Schwarzfischer L, Pham T-H, Schilling E, Klug M, Andreesen R, Rehli M, Genome-wide profiling of CpG methylation identifies novel targets of aberrant hypermethylation in myeloid leukemia, Cancer Res 66(12) (2006) 6118–6128. [DOI] [PubMed] [Google Scholar]
- [231].Selker EU, Tountas NA, Cross SH, Margolin BS, Murphy JG, Bird AP, Freitag M, The methylated component of the Neurospora crassa genome, Nature 422(6934) (2003) 893–897. [DOI] [PubMed] [Google Scholar]
- [232].Cross SH, Charlton JA, Nan X, Bird AP, Purification of CpG islands using a methylated DNA binding column, Nat. Genet 6(3) (1994) 236–244. [DOI] [PubMed] [Google Scholar]
- [233].Weber M, Davies JJ, Wittig D, Oakeley EJ, Haase M, Lam WL, Schuebeler D, Chromosome-wide and promoter-specific analyses identify sites of differential DNA methylation in normal and transformed human cells, Nat. Genet 37(8) (2005) 853–862. [DOI] [PubMed] [Google Scholar]
- [234].Down TA, Rakyan VK, Turner DJ, Flicek P, Li H, Kulesha E, Graef S, Johnson N, Herrero J, Tomazou EM, A Bayesian deconvolution strategy for immunoprecipitation-based DNA methylome analysis, Nat. Biotechnol 26(7) (2008) 779–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Taiwo O, Wilson GA, Morris T, Seisenberger S, Reik W, Pearce D, Beck S, Butcher LM, Methylome analysis using MeDIP-seq with low DNA concentrations, Nat. Protoc 7(4) (2012) 617. [DOI] [PubMed] [Google Scholar]
- [236].Zhao M-T, Whyte JJ, Hopkins GM, Kirk MD, Prather RS, Methylated DNA immunoprecipitation and high-throughput sequencing (MeDIP-seq) using low amounts of genomic DNA, Cellular Reprogramming (Formerly Cloning and Stem Cells) 16(3) (2014) 175–184. [DOI] [PubMed] [Google Scholar]
- [237].Neary JL, Perez SM, Peterson K, Lodge DJ, Carless MA, Comparative analysis of MBD-seq and MeDIP-seq and estimation of gene expression changes in a rodent model of schizophrenia, Genomics 109(3–4) (2017) 204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Beck S, Taking the measure of the methylome, Nat. Biotechnol 28(10) (2010) 1026–1028. [DOI] [PubMed] [Google Scholar]
- [239].Serre D, Lee BH, Ting AH, MBD-isolated Genome Sequencing provides a high-throughput and comprehensive survey of DNA methylation in the human genome, Nucleic Acids Res 38(2) (2010) 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Li N, Ye M, Li Y, Yan Z, Butcher LM, Sun J, Han X, Chen Q, Wang J, Whole genome DNA methylation analysis based on high throughput sequencing technology, Methods 52(3) (2010) 203–212. [DOI] [PubMed] [Google Scholar]
- [241].Hogart A, Lichtenberg J, Ajay SS, Anderson S, Margulies EH, Bodine DM, Center NIS, Genome-wide DNA methylation profiles in hematopoietic stem and progenitor cells reveal overrepresentation of ETS transcription factor binding sites, Genome Res 22(8) (2012) 1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Lan X, Adams C, Landers M, Dudas M, Krissinger D, Marnellos G, Bonneville R, Xu M, Wang J, Huang TH-M, High resolution detection and analysis of CpG dinucleotides methylation using MBD-Seq technology, PLoS One 6(7) (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Nair SS, Coolen MW, Stirzaker C, Song JZ, Statham AL, Strbenac D, Robinson MD, Clark SJ, Comparison of methyl-DNA immunoprecipitation (MeDIP) and methyl-CpG binding domain (MBD) protein capture for genome-wide DNA methylation analysis reveal CpG sequence coverage bias, Epigenetics 6(1) (2011) 34–44. [DOI] [PubMed] [Google Scholar]
- [244].Aberg KA, McClay JL, Nerella S, Clark S, Kumar G, Chen W, Khachane AN, Xie L, Hudson A, Gao G, Methylome-wide association study of schizophrenia: identifying blood biomarker signatures of environmental insults, JAMA psychiatry 71(3) (2014) 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Mohn F, Weber M, Schübeler D, Roloff T-C, Methylated DNA immunoprecipitation (medip), DNA methylation, Springer; 2009, pp. 55–64. [DOI] [PubMed] [Google Scholar]
- [246].Jacinto FV, Ballestar E, Esteller M, Methyl-DNA immunoprecipitation (MeDIP): hunting down the DNA methylome, Biotechniques 44(1) (2008) 35–43. [DOI] [PubMed] [Google Scholar]
- [247].Klose RJ, Sarraf SA, Schmiedeberg L, McDermott SM, Stancheva I, Bird AP, DNA binding selectivity of MeCP2 due to a requirement for A/T sequences adjacent to methyl-CpG, Mol. Cell 19(5) (2005) 667–678. [DOI] [PubMed] [Google Scholar]
- [248].Brinkman AB, Simmer F, Ma K, Kaan A, Zhu J, Stunnenberg HG, Whole-genome DNA methylation profiling using MethylCap-seq, Methods 52(3) (2010) 232–236. [DOI] [PubMed] [Google Scholar]
- [249].Rauch T, Pfeifer GP, Methylated-CpG island recovery assay: a new technique for the rapid detection of methylated-CpG islands in cancer, Lab. Invest 85(9) (2005) 1172–1180. [DOI] [PubMed] [Google Scholar]
- [250].Rauch TA, Pfeifer GP, The MIRA method for DNA methylation analysis, DNA Methylation, Springer; 2009, pp. 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Stevens M, Cheng JB, Li D, Xie M, Hong C, Maire CL, Ligon KL, Hirst M, Marra MA, Costello JF, Estimating absolute methylation levels at single-CpG resolution from methylation enrichment and restriction enzyme sequencing methods, Genome Res 23(9) (2013) 1541–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Ellis RJ, Wang Y, Stevenson HS, Boufraqech M, Patel D, Nilubol N, Davis S, Edelman DC, Merino MJ, He M, Genome-wide methylation patterns in papillary thyroid cancer are distinct based on histological subtype and tumor genotype, The Journal of Clinical Endocrinology & Metabolism 99(2) (2014) E329–E337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Kangaspeska S, Stride B, Metivier R, Polycarpou-Schwarz M, Ibberson D, Carmouche RP, Benes V, Gannon F, Reid G, Transient cyclical methylation of promoter DNA, Nature 452(7183) (2008) 112–115. [DOI] [PubMed] [Google Scholar]
- [254].Walker DL, Bhagwate AV, Baheti S, Smalley RL, Hilker CA, Sun Z, Cunningham JM, DNA methylation profiling: comparison of genome-wide sequencing methods and the Infinium Human Methylation 450 Bead Chip, Epigenomics 7(8) (2015) 1287–1302. [DOI] [PubMed] [Google Scholar]
- [255].Stirzaker C, Taberlay PC, Statham AL, Clark SJ, Mining cancer methylomes: prospects and challenges, Trends Genet 30(2) (2014) 75–84. [DOI] [PubMed] [Google Scholar]
- [256].Bibikova M, Le J, Barnes B, Saedinia-Melnyk S, Zhou L, Shen R, Gunderson KL, Genome-wide DNA methylation profiling using Infinium® assay, Epigenomics 1(1) (2009) 177–200. [DOI] [PubMed] [Google Scholar]
- [257].Bibikova M, Fan J-B, GoldenGate® assay for DNA methylation profiling, DNA Methylation, Springer; 2009, pp. 149–163. [DOI] [PubMed] [Google Scholar]
- [258].Bibikova M, Lin Z, Zhou L, Chudin E, Garcia EW, Wu B, Doucet D, Thomas NJ, Wang Y, Vollmer E, High-throughput DNA methylation profiling using universal bead arrays, Genome Res 16(3) (2006) 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Lin Z, Thomas NJ, Bibikova M, Seifart C, Wang Y, Guo X, Wang G, Vollmer E, Goldmann T, Garcia EW, DNA methylation markers of surfactant proteins in lung cancer, Int. J. Oncol 31(1) (2007) 181–191. [PubMed] [Google Scholar]
- [260].Thirlwell C, Eymard M, Feber A, Teschendorff A, Pearce K, Lechner M, Widschwendter M, Beck S, Genome-wide DNA methylation analysis of archival formalin-fixed paraffin-embedded tissue using the Illumina Infinium HumanMethylation27 BeadChip, Methods 52(3) (2010) 248–254. [DOI] [PubMed] [Google Scholar]
- [261].Bibikova M, Barnes B, Tsan C, Ho V, Klotzle B, Le JM, Delano D, Zhang L, Schroth GP, Gunderson KL, High density DNA methylation array with single CpG site resolution, Genomics 98(4) (2011) 288–295. [DOI] [PubMed] [Google Scholar]
- [262].C.G.A.R. Network, Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia, N. Engl. J. Med 368(22) (2013) 2059–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Xu Z, Niu L, Li L, Taylor JA, ENmix: a novel background correction method for Illumina HumanMethylation450 BeadChip, Nucleic Acids Res 44(3) (2016) e20-e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Sandoval J, Heyn H, Moran S, Serra-Musach J, Pujana MA, Bibikova M, Esteller M, Validation of a DNA methylation microarray for 450,000 CpG sites in the human genome, Epigenetics 6(6) (2011) 692–702. [DOI] [PubMed] [Google Scholar]
- [265].Hosein A, Cocciardi S, Jayanthan J, Song S, Simpson P, G. Chenevix-Trench, The use of the Illumina FFPE Restoration Protocol to obtain suitable quality DNA for SNP-based CGH–a pilot study, Hered. Cancer Clin. Pract, Springer, 2012, p. A85. [Google Scholar]
- [266].Pidsley R, Wong CC, Volta M, Lunnon K, Mill J, Schalkwyk LC, A data-driven approach to preprocessing Illumina 450K methylation array data, BMC Genomics 14(1) (2013) 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Teschendorff AE, Menon U, Gentry-Maharaj A, Ramus SJ, Gayther SA, Apostolidou S, Jones A, Lechner M, Beck S, Jacobs IJ, An epigenetic signature in peripheral blood predicts active ovarian cancer, PLoS One 4(12) (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Triche TJ Jr, Weisenberger DJ, Van Den Berg D, Laird PW, Siegmund KD, Low-level processing of Illumina Infinium DNA methylation beadarrays, Nucleic Acids Res 41(7) (2013) e90-e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Dedeurwaerder S, Defrance M, Calonne E, Denis H, Sotiriou C, Fuks F, Evaluation of the Infinium Methylation 450K technology, Epigenomics 3(6) (2011) 771–784. [DOI] [PubMed] [Google Scholar]
- [270].Maksimovic J, Gordon L, Oshlack A, SWAN: Subset-quantile within array normalization for illumina infinium HumanMethylation450 BeadChips, Genome Biol 13(6) (2012) R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Touleimat N, Tost J, Complete pipeline for Infinium® Human Methylation 450K BeadChip data processing using subset quantile normalization for accurate DNA methylation estimation, Epigenomics 4(3) (2012) 325–341. [DOI] [PubMed] [Google Scholar]
- [272].Teschendorff AE, Marabita F, Lechner M, Bartlett T, Tegner J, Gomez-Cabrero D, Beck S, A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data, Bioinformatics 29(2) (2013) 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Davis S, Du P, Bilke S, Triche J, Bootwalla M, Methylumi: Handle Illumina Methylation Data. R package version 2.10.0, (2014).
- [274].Ushijima T, Detection and interpretation of altered methylation patterns in cancer cells, Nature Reviews Cancer 5(3) (2005) 223–231. [DOI] [PubMed] [Google Scholar]
- [275].Hatada I, Emerging technologies for genome-wide DNA methylation profiling in cancer, Critical Reviews™ in Oncogenesis 12(3–4) (2006). [DOI] [PubMed] [Google Scholar]
- [276].Heyn H, Esteller M, DNA methylation profiling in the clinic: applications and challenges, Nature Reviews Genetics 13(10) (2012) 679–692. [DOI] [PubMed] [Google Scholar]
- [277].Garrett-Bakelman FE, Sheridan CK, Kacmarczyk TJ, Ishii J, Betel D, Alonso A, Mason CE, Figueroa ME, Melnick AM, Enhanced reduced representation bisulfite sequencing for assessment of DNA methylation at base pair resolution, JoVE (Journal of Visualized Experiments) (96) (2015) e52246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Akalin A, Garrett-Bakelman FE, Kormaksson M, Busuttil J, Zhang L, Khrebtukova I, Milne TA, Huang Y, Biswas D, Hess JL, Base-pair resolution DNA methylation sequencing reveals profoundly divergent epigenetic landscapes in acute myeloid leukemia, PLoS Genet 8(6) (2012) e1002781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Kacmarczyk TJ, Fall MP, Zhang X, Xin Y, Li Y, Alonso A, Betel D, “Same difference”: comprehensive evaluation of four DNA methylation measurement platforms, Epigenetics & chromatin 11(1) (2018) 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Oda M, Glass JL, Thompson RF, Mo Y, Olivier EN, Figueroa ME, Selzer RR, Richmond TA, Zhang X, Dannenberg L, High-resolution genome-wide cytosine methylation profiling with simultaneous copy number analysis and optimization for limited cell numbers, Nucleic Acids Res 37(12) (2009) 3829–3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Killian JK, Bilke S, Davis S, Walker RL, Killian MS, Jaeger EB, Chen Y, Hipp J, Pittaluga S, Raffeld M, Large-scale profiling of archival lymph nodes reveals pervasive remodeling of the follicular lymphoma methylome, Cancer Res 69(3) (2009) 758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].De Ruijter TC, De Hoon JP, Slaats J, De Vries B, Janssen MJ, Van Wezel T, Aarts MJ, Van Engeland M, Tjan-Heijnen VC, Van Neste L, Formalin-fixed, paraffin-embedded (FFPE) tissue epigenomics using Infinium HumanMethylation450 BeadChip assays, Lab. Invest 95(7) (2015) 833–842. [DOI] [PubMed] [Google Scholar]
- [283].Dohm JC, Lottaz C, Borodina T, Himmelbauer H, Substantial biases in ultra-short read data sets from high-throughput DNA sequencing, Nucleic Acids Res 36(16) (2008) e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Irizarry RA, Ladd-Acosta C, Carvalho B, Wu H, Brandenburg SA, Jeddeloh JA, Wen B, Feinberg AP, Comprehensive high-throughput arrays for relative methylation (CHARM), Genome Res 18(5) (2008) 780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Pushkarev D, Neff NF, Quake SR, Single-molecule sequencing of an individual human genome, Nat. Biotechnol 27(9) (2009) 847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Eid J, Fehr A, Gray J, Luong K, Lyle J, Otto G, Peluso P, Rank D, Baybayan P, Bettman B, Real-time DNA sequencing from single polymerase molecules, Science 323(5910) (2009) 133–138. [DOI] [PubMed] [Google Scholar]
- [287].Campan M, Weisenberger DJ, Trinh B, Laird PW, MethyLight, DNA Methylation, Springer; 2009, pp. 325–337. [DOI] [PubMed] [Google Scholar]
- [288].Branton D, Deamer DW, Marziali A, Bayley H, Benner SA, Butler T, Di Ventra M, Garaj S, Hibbs A, Huang X, The potential and challenges of nanopore sequencing, Nanoscience and technology: A collection of reviews from Nature Journals, World Scientific 2010, pp. 261–268.
- [289].Clarke J, Wu H-C, Jayasinghe L, Patel A, Reid S, Bayley H, Continuous base identification for single-molecule nanopore DNA sequencing, Nature nanotechnology 4(4) (2009) 265. [DOI] [PubMed] [Google Scholar]
- [290].van Dijk EL, Jaszczyszyn Y, Naquin D, Thermes C, The third revolution in sequencing technology, Trends Genet 34(9) (2018) 666–681. [DOI] [PubMed] [Google Scholar]
- [291].Jain M, Fiddes IT, Miga KH, Olsen HE, Paten B, Akeson M, Improved data analysis for the MinION nanopore sequencer, Nature methods 12(4) (2015) 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Zheng GX, Lau BT, Schnall-Levin M, Jarosz M, Bell JM, Hindson CM, Kyriazopoulou-Panagiotopoulou S, Masquelier DA, Merrill L, Terry JM, Haplotyping germline and cancer genomes with high-throughput linked-read sequencing, Nat. Biotechnol 34(3) (2016) 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Zerbino DR, Achuthan P, Akanni W, Amode MR, Barrell D, Bhai J, Billis K, Cummins C, Gall A, Girón CG, Ensembl 2018, Nucleic Acids Res 46(D1) (2018) D754–D761. [DOI] [PMC free article] [PubMed] [Google Scholar]


