Abstract
We measured percent body fat by air-displacement plethysmography in 86 infants born at <32 weeks of gestation randomized to receive either high-volume (180–200 ml/kg/day) or usual-volume feeding (140–160 ml/kg/day). High-volume feeding increased percent body fat by ≤2% at 36 weeks of postmenstrual age (within a predefined range of equivalence).
Trial registration
Keywords: body composition, neonatal adiposity, body fat, clinical trial, postnatal growth, anthropometric measurements, premature infants
Clinicians often prescribe human milk fortifiers and infant formulas to increase energy and protein intake and prevent postnatal growth failure in infants born preterm1. When this practice of high-energy feeding is insufficient to avoid postnatal growth failure2, clinicians can combine high-energy feeding with high-volume feeding3.
In a clinical trial of high-volume feeding, 224 infants born at <32 weeks of gestation were randomized to receive either 180–200 ml/kg/day or 140–160 ml/kg/day. The trial showed that high-volume feeding increased anthropometric measurements at study completion, but the effects on body composition were not reported4. Promoting rapid growth with high-volume feeding could alter fat mass (FM) accretion and increase the risk of obesity and metabolic syndrome in adulthood5, 6.
This ancillary study hypothesized that adiposity estimated by air-displacement plethysmography at 36 weeks of postmenstrual age (PMA) is equivalent in infants born preterm receiving high and usual-volume feeding.
METHODS
A detailed description of the high-volume feeding trial is published elsewhere4. Briefly, infants born at <32 weeks of gestation were included. Infants with patent ductus arteriosus, necrotizing enterocolitis, gastrointestinal or neurologic malformations, and terminal illness were excluded. Patient recruitment began in January 2015. In 2016, the outcome of body composition was added, the clinicaltrials.gov registration was updated, and the consent form was modified. On the modified consent form, parents could opt-in for infant body composition measurements at 36 weeks PMA. Written consent was obtained before randomization. A computer-generated, random-block sequence was used to randomize infants. Numbered, opaque, sealed envelopes were opened in sequential order to allocate the intervention. The intervention was not masked. The study protocol was approved by the UAB Institutional Review Board.
Infants were randomized to receive either high-volume feeding (180–200 ml/kg/d) or usual-volume feeding (140–160 ml/kg/d). The primary outcome was percent body fat (%BF) estimated by air-displacement plethysmography at 36 weeks PMA or hospital discharge (whichever occurred first). Other outcomes included FM z-scores7, fat-free mass (FFM) z-scores, postnatal growth failure (weight <10th percentile), growth rate from birth to 36 weeks PMA, and anthropometric measurements at 36 weeks PMA.
A 24-hour enteral intake was recorded at the time of enrollment and weekly after that. The weekly caloric intake was calculated after study completion. These calculations were made assuming that unfortified human milk had 1.5 g of protein per 100 kcal, that 24 kcal/oz fortified human milk had 3.3 g of protein per 100 kcal, and that 24 kcal/oz formula had 3.6 g of protein per 100 kcal. Length was measured from stretched heel to top of head using a flexible tape measure. Growth rate was calculated using the exponential method8. Infant body composition was measured with the PeaPod®(Life Measurement Instruments, Concord, CA).
During the trial, enteral nutrition was administered as an intermittent bolus gavage every 3 hours. Feeding volumes were started at 20–30 ml/kg/day and then increased by 20–30 ml/kg/day. When full enteral feeding was established, bovine-based fortifiers were added to human milk. If the supply of human milk was insufficient, preterm formula was prescribed. Donor milk was not offered.
We estimated that the mean ± SD %BF was 15 ± 39. To exclude clinically meaningful differences between groups, we predefined the range of equivalence to be −2 to 2% and calculated a sample size of 86 infants anticipating that 10% of them would exit the study after randomization (α=0.05; power: 80%).
For the intention-to-treat analysis of the primary outcome, the mean %BF values in both groups were compared with an equivalence test. Secondary outcomes were assessed with superiority analyses (t test for continuous variables and chi-square test for categorical variables). Longitudinal differences in human milk feeding rates and differences in volume, caloric, protein, and fat intake between groups were analyzed with a repeated-measures mixed model. All statistical analyses were performed using JMP Pro (SAS Institute Inc, Cary, NC).
RESULTS
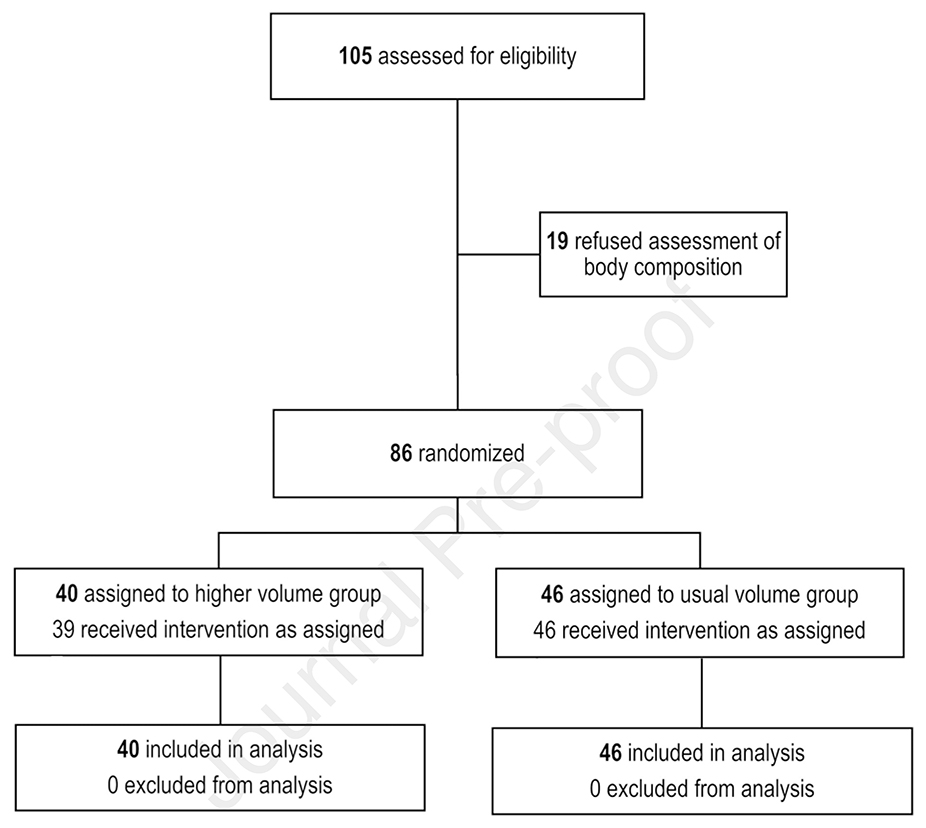
From 8/2016–11/2017, 105 infants met the eligibility criteria for inclusion in the trial; 86 infants randomized to either high or usual-volume feeding had body composition measurements at 36 weeks PMA (Figure 1; available at www.jpeds.com). Baseline characteristics did not differ between groups (Table 1).
Figure 1.
Flow diagram
Table 1.
Baseline characteristics
| High Volume group (n = 40) | Usual Volume group (n = 46) | |
|---|---|---|
| Demographic characteristics | ||
| Birth weight in grams, mean ± SD | 1458 ± 236 | 1483 ± 276 |
| Gestational age in weeks, median (IQR) | 31 (30–31) | 30 (30–31) |
| Weight-for-age percentile at birth*, mean ± SD | 47 ± 24 | 53 ± 23 |
| Male, n (%) | 18 (45) | 26 (57) |
| Black, n (%) | 21 (53) | 27 (59) |
| Apgar score at 5 minutes, median (IQR) | 8 (7–9) | 8 (7–9) |
| Feeding volume at study entry in ml/kg/day, median (IQR) | 120 (120–140) | 120 (120–147) |
| Any human milk feeding at study entry, n (%) | 32 (80) | 43 (93) |
| Postnatal age at study entry in days, median (IQR) | 7 (5–10) | 7 (6–9) |
Z-scores were estimated using the Fenton 2013 growth curves.
The mean ± SD %BF was 15.1 ± 3.7 in the high-volume feeding group and 14.1 ± 3.5 in the usual-volume feeding group (within the predefined range for equivalence). Other body composition measurements did not differ between groups (Table 2). The differences in baseline characteristics and growth outcomes between infants with body composition measurements and those without body composition measurements included in the high-volume feeding trial were not significant (Table 3; available at www.jpeds.com).
Table 2.
Nutritional intake and growth outcomes
| High volume group (n=40) | Usual volume group (n=46) | P | |
|---|---|---|---|
| Length of study participation in days, median (IQR) | 27 (22 – 31) | 28 (23 – 32) | 0.72 |
| Growth rate in g/kg/day, mean ± SD | |||
| From study entry to study completion | 20.3 ± 5.0 | 18.4 ± 4.0 | 0.05 |
| From birth to 36 weeks PMA | 13.5 ± 3.7 | 11.8 ± 3.1 | 0.03 |
| Postnatal age at assessment in days, mean ± SD | 35 ± 9 | 36 ± 10 | 0.47 |
| Body composition z-scores at 36 weeks PMA, mean ± SD | |||
| Fat mass | 1.1 ± 0.9 | 0.9 ± 1.0 | 0.36 |
| Fat-free mass | −1.5 ± 1.1 | −1.2 ± 1.6 | 0.40 |
| % Body fat | 1.7 ± 0.9 | 1.4 ± 0.9 | 0.16 |
| Anthropometrics at 36 weeks PMA*, mean ± SD | |||
| Weight in grams | 2308 ± 325 | 2256 ± 305 | 0.44 |
| Weight z-score | −0.8 ± 0.7 | −0.7 ± 0.8 | 0.42 |
| Length z-score | −0.6 ± 0.7 | −0.8 ± 0.8 | 0.44 |
| Head circumference z score | −0.2 ± 0.7 | −0.2 ± 0.7 | 0.64 |
| Differences between weight z-score at birth and weight z-score at 36 weeks PMA, mean ± SD | −0.8 ± 1.0 | −0.8 ± 1.1 | 0.88 |
| Postnatal growth failure defined as weight at 36 wk postmenstrual age <10th percentile, n (%) | 10 (25) | 10 (22) | 0.72 |
| Any human milk feeding at study completion, n (%) | 24 (60) | 31 (67) | 0.42 |
| Exclusive human milk feeding at study completion, n (%) | 14 (35) | 18 (39) | 0.69 |
| Length of stay after randomization in days, mean ± SD | 39 ± 14 | 40 ± 15 | 0.62 |
Z-scores were estimated using the Fenton 2013 growth curves.
Table 3.
Baseline characteristics and growth outcomes for infants with and without body composition measurements
| Body Composition Cohort (n=86) | Main Cohort (n=131) | P | |
|---|---|---|---|
| Demographic characteristics | |||
| Birth weight in grams, mean ± SD | 1471 ± 257 | 1435 ± 253 | 0.31 |
| Gestational age in weeks, median (IQR) | 31 (30–31) | 31 (29–31) | 0.18 |
| Male, n (%) | 44 (51) | 60 (46) | 0.47 |
| Black, n (%) | 48 (56) | 67 (52) | 0.39 |
| Apgar score at 5 minutes, median (IQR) | 8 (7–9) | 8 (7–8) | 0.10 |
| Feeding volume at study entry in ml/kg/day, median (IQR) | 120 (120–145) | 120 (120–147) | 0.49 |
| Growth rate in g/kg/day, mean ± SD | |||
| From study entry to study completion | 19.3 ± 5 | 19.3 ± 5 | 0.98 |
| Anthropometrics at 36 weeks PMA, mean ± SD | |||
| Weight in grams | 2280 ± 314 | 2277 ± 333 | 0.95 |
| Length in centimeters | 45 ± 2 | 45 ± 2 | 0.69 |
| Head circumference in centimeters | 32 ± 1 | 31 ± 1 | 0.01 |
| Differences between weight at entry and weight at completion in grams, mean ± SD | 892 ± 324 | 913 ± 344 | 0.66 |
| Length of stay in days, mean ± SD | 47 ± 16 | 48 ± 20 | 0.59 |
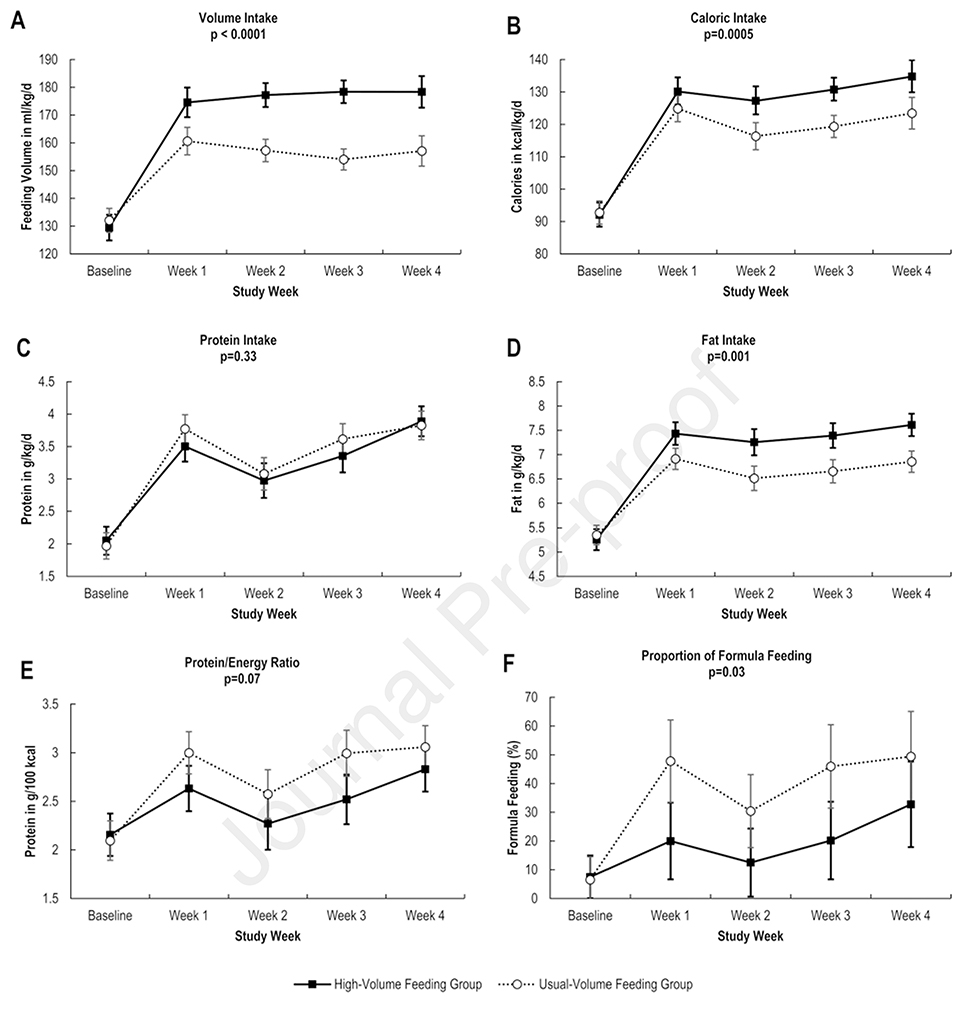
The weekly caloric intake increased by approximately 10 kcal/kg/day with highvolume feeding. Protein intake did not differ between groups, but fat intake was significantly higher in the high-volume feeding group (Figure 2; available at www.jpeds.com). In a posthoc analysis, 215 weeks of enteral intake data in the high-volume feeding group were compared with 249 weeks of enteral intake data in the usual-volume group. For this analysis, each infant contributed with an average of 5 weeks of enteral intake data. The analysis showed that infants in the high-volume feeding group had more weeks of unfortified human milk intake (38% vs 25%; p=0.001) and fewer weeks of 27 kcal/oz formula intake (2% vs. 9%; p=0.001).
Figure 2.
Longitudinal changes in volume intake (A), caloric intake (B), protein intake (C), fat intake (D), protein to energy intake (E), and formula feeding (F). Mean values and 95% confidence intervals were adjusted with repeated measures analyses that assumed an unstructured variance and accounted for subject-to-subject variability (random effects) and a fixed interaction term between the study groups and time (fixed effects).
The median length of hospital stay was 35 days (IQR: 29–40). There were no serious events related to body composition measurements.
DISCUSSION
This randomized trial revealed that the effect of high-volume feeding on %BF of infants born at <32 weeks of gestation was within a clinically meaningful range of equivalence when compared with the effect of usual-volume feeding. At 36 weeks PMA, high-volume feeding with either fortified human milk or preterm formula increased %BF by no more than 2%. In underpowered, superiority analyses, no differences in FM or FFM measurements were found. High-volume feeding increased the growth rate from birth to 36 weeks PMA.
Our study results suggest that the higher growth rate, weight, and z-scores of critical anthropometric measurements found at study completion in infants randomized to high-volume feeding4 were not the result of substantial increases in %BF. They also indicate that among infants in the high-volume feeding group, the protein intake, along with the increased energy and fat intakes, was sufficient to support appropriate protein gain. A comprehensive systematic review recently underlined the benefits of the early enteral intake of energy, fat, and protein on growth rates, brain structure, and neurodevelopment10. Our findings provide moderate to high-quality evidence that increases in %BF due to high-volume feeding do not offset these potential benefits.
It is not clear if the previously described rapid increase in %BF from birth to 36 weeks PMA is an adaptive response to the extrauterine environment independent of high-energy feeding11 or an early sign of increased susceptibility to adverse metabolic outcomes9, 12, including the disproportionate increase in %BF observed in adults born preterm6, 13. FFM accretion is a more unequivocal outcome of high-energy, high-protein feeding. Higher energy and protein intake are consistently associated with higher FFM gains14. Because FFM at 36 weeks PMA is associated with a lower risk of adverse neurodevelopmental outcomes in childhood2, 15, FFM is a compelling outcome to show the potential benefits of higher energy and higher protein intake, particularly in infants without a history of intrauterine growth restriction. We did not observe significant FFM gains in the high-volume feeding group, but our study was underpowered for this outcome and other anthropometric outcomes that often correlate with higher FFM gains. If we had measured body composition in all the infants included in the high-volume feeding trial, we would have achieved 80% power to detect a true difference of 0.5 in FFM z-scores between groups.
Randomization and the use of air-displacement plethysmography, a non-invasive and accurate method, were the main strengths of the study. Other strengths were the strict control of the study intervention for several weeks, the successful assessment of body composition in all the study participants, and the sufficient sample size to test our hypothesis. We considered our range of equivalence clinically meaningful (i.e., an absolute increase of 2% in %BF). However, because this arbitrary range includes relative increases in %BF of 10% or more, we acknowledge that this range may be considered unacceptable.
The main limitations of the study were the lack of masking, the methods used to measure length, and the single-center study design. Lack of masking could have resulted in reduced compliance, delayed fortification, and limited use of 27 kcal/oz formula in the high-volume feeding group. If the lack of masking had not affected fortification practices and protein/energy ratios, protein intake would have increased as much as fat or energy intake in the high-volume feeding group and supported not only growth but also FFM gains. Length measured with an inaccurate method could have affected the body composition calculations. The single-center study design is also a limitation because it reduces the external validity of our results. Other limitations were the indirect method used to calculate caloric intake and the lack of longitudinal data on FM accretion from birth to 36 weeks PMA.
In conclusion, this trial suggests that high-volume feeding with a relatively “low” protein/energy ratio supports weight gain and body fat accretion within a clinically meaningful range of equivalence when compared with usual-volume feeding with a relatively “high” protein/energy ratio.
Supplementary Material
Acknowledgments
A.S. is supported by a research grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K23HD102554). M.J. is supported by the UAB NORC T32 program. The funder/sponsor did not participate in the work. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. A.S. received honoraria from the Lockwood Group for participation in Mead Johnson advisory board meetings. A.S. and P.L. filed a patent for an instrumented feeding bottle. W.C. serves on the board of directors of MEDNAX. The other authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].American Academy of Pediatrics Committee on Nutrition: Nutritional needs of low-birthweight infants. Pediatrics. 1985;75:976–86. [PubMed] [Google Scholar]
- [2].Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117:1253–61. [DOI] [PubMed] [Google Scholar]
- [3].Abiramalatha T, Thomas N, Gupta V, Viswanathan A, McGuire W. High versus standard volume enteral feeds to promote growth in preterm or low birth weight infants. Cochrane Database Syst Rev. 2017;9:CD012413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Travers CP, Wang T, Salas AA, Schofield E, Dills M, Laney D, et al. Higher or Usual Volume Feedings in Very Preterm Infants: A Randomized Clinical Trial. J Pediatr. 2020. [DOI] [PubMed] [Google Scholar]
- [5].Euser AM, Finken MJ, Keijzer-Veen MG, Hille ET, Wit JM, Dekker FW, et al. Associations between prenatal and infancy weight gain and BMI, fat mass, and fat distribution in young adulthood: a prospective cohort study in males and females born very preterm. Am J Clin Nutr. 2005;81:480–7. [DOI] [PubMed] [Google Scholar]
- [6].Morrison KM, Ramsingh L, Gunn E, Streiner D, Van Lieshout R, Boyle M, et al. Cardiometabolic Health in Adults Born Premature With Extremely Low Birth Weight. Pediatrics. 2016;138. [DOI] [PubMed] [Google Scholar]
- [7].Norris T, Ramel SE, Catalano P, Caoimh CN, Roggero P, Murray D, et al. New charts for the assessment of body composition, according to air-displacement plethysmography, at birth and across the first 6 mo of life. Am J Clin Nutr. 2019;109:1353–60. [DOI] [PubMed] [Google Scholar]
- [8].Cormack BE, Embleton ND, van Goudoever JB, Hay WW Jr., Bloomfield FH. Comparing apples with apples: it is time for standardized reporting of neonatal nutrition and growth studies. Pediatr Res. 2016;79:810–20. [DOI] [PubMed] [Google Scholar]
- [9].Johnson MJ, Wootton SA, Leaf AA, Jackson AA. Preterm birth and body composition at term equivalent age: a systematic review and meta-analysis. Pediatrics. 2012;130:e640–9. [DOI] [PubMed] [Google Scholar]
- [10].Ottolini KM, Andescavage N, Keller S, Limperopoulos C. Nutrition and the developing brain: the road to optimizing early neurodevelopment: a systematic review. Pediatr Res. 2020;87:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Simon L, Frondas-Chauty A, Senterre T, Flamant C, Darmaun D, Roze JC. Determinants of body composition in preterm infants at the time of hospital discharge. Am J Clin Nutr. 2014;100:98–104. [DOI] [PubMed] [Google Scholar]
- [12].Wiedmeier JE, Joss-Moore LA, Lane RH, Neu J. Early postnatal nutrition and programming of the preterm neonate. Nutr Rev. 2011;69:76–82. [DOI] [PubMed] [Google Scholar]
- [13].Raju TNK, Buist AS, Blaisdell CJ, Moxey-Mims M, Saigal S. Adults born preterm: a review of general health and system-specific outcomes. Acta Paediatr. 2017;106:1409–37. [DOI] [PubMed] [Google Scholar]
- [14].Lingwood BE, Al-Theyab N, Eiby YA, Colditz PB, Donovan TJ. Body composition in very preterm infants before discharge is associated with macronutrient intake. Br J Nutr. 2020;123:800–6. [DOI] [PubMed] [Google Scholar]
- [15].Ramel SE, Gray HL, Christiansen E, Boys C, Georgieff MK, Demerath EW. Greater Early Gains in Fat-Free Mass, but Not Fat Mass, Are Associated with Improved Neurodevelopment at 1 Year Corrected Age for Prematurity in Very Low Birth Weight Preterm Infants. J Pediatr. 2016;173:108–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.