Abstract
Background
Older adults with acute decompensated heart failure (ADHF) have persistently poor clinical outcomes. Cognitive impairment (CI) may be a contributing factor. However, the prevalence of CI and the relationship of cognition with other patient-centered factors such a physical function and quality-of-life (QOL) that also may contribute to poor outcomes are incompletely understood.
Methods
Older (≥60 years) hospitalized patients with ADHF were assessed for cognition [Montreal Cognitive Assessment (MoCA)], physical function [(short physical performance battery (SPPB), 6-minute walk distance (6MWD)], and QOL [Kansas City Cardiomyopathy Questionnaire (KCCQ), Short Form-12 (SF-12)].
Results
Among patients (N=198, 72.1±7.6 years), 78% screened positive for CI (MoCA <26) despite rare medical record documentation (2%). Participants also had severely diminished physical function (SPPB 6.0±2.5 units, 6MWD 186±100m) and QOL (scores <50). MoCA positively related to SPPB (ß=0.47, p<0.001), 6MWD ß=0.01, p=0.006) and inversely related to KCCQ Overall Score (ß=−0.05, p<0.002) and SF-12 Physical Component Score (ß=−0.09, p=0.006). MoCA was a small but significant predictor of SPPB, 6MWD, and KCCQ.
Conclusion
Among older hospitalized patients with ADHF, CI is highly prevalent, is underrecognized clinically, and is associated with severe physical dysfunction and poor QOL. Formal screening may reduce adverse events by identifying patients who may require more tailored care.
Keywords: acute decompensated heart failure, cognitive function, physical function, quality of life
INTRODUCTION
Older adults often experience progressive tandem decrements in cognitive and physical functioning that threaten their independence and quality-of-life (QOL).1,2 The severity of loss in QOL and overall functional ability varies, depending on interactions between aging and comorbid disease processes. Heart failure (HF) is among the most common systemic comorbidities of older adulthood. Given the highly vascularized nature of the brain and systemic effects of HF, particularly on cerebral perfusion, cognitive impairment (CI), may be a key, but clinically underrecognized, feature of HF.3–5 In addition, for older adults with HF, CI may be more complex and intensify decrements in physical function and QOL; the relationships among these different factors are not well understood.
CI may be particularly salient for older adults with acute decompensated heart failure (ADHF), the most frequent cause of hospitalization in patients ≥ 65 years of age and a leading cause of 30-day readmissions.6,7 Given their older age and advanced disease, CI may be most pronounced in ADHF as compared with chronic ambulatory patients, contributing to their persistently poor outcomes. CI could interfere with self-care, treatment regimens, and symptom recognition and management, and thereby, increase the risk of unplanned hospitalizations and even death.4,8,9 We recently reported that older patients with ADHF have marked and global deficits across cognitive, physical, and QOL domains.10–12 These deficits may interrelate to heighten the risk of functional disability and dependence. In order to develop tailored interventions to help prevent excess adverse outcomes, including re-hospitalizations, it is critical to understand the prevalence and nature of CI and its relationship with physical function and QOL in ADHF.
Accordingly, we performed systematic baseline assessments of cognition, physical function, and QOL in older adult hospitalized patients with ADHF who were consecutively enrolled in the National Institutes of Aging (NIA)-sponsored multicenter Rehabilitation Therapy in Older Acute Heart Failure Patients trial (REHAB-HF, clinicaltrials.gov #NCT02196038).13,14 We hypothesized that CI is highly prevalent in older adults with ADHF and that CI is associated with and is a predictor of physical dysfunction and reduced QOL. We examined: 1) the prevalence of CI; 2) the cognitive subdomains affected; and 3) the relationship of cognitive function with physical function and QOL.
METHODS
Study Design and Participants
This study is a cross-sectional analysis of the baseline assessment of the first n=202 consecutively enrolled participants in the ongoing, NIA-sponsored, multicenter physical intervention trial, REHAB-HF; 198 of whom had cognitive screening. The design of REHAB-HF, including eligibility criteria and ADHF diagnosis confirmation, have been previously described.13,14 Briefly, patients were ≥60 years old and hospitalized for ≥24 hours for ADHF [regardless of ejection fraction (EF)]. Patients were required to be independent with activities of daily living and with ambulation ≥4m (assistive devices permitted) and had a planned discharged to home. The study excluded patients with severe valvular disease and significant comorbidities such as advanced dementia, end-stage renal disease, or terminal illnesses. Written informed consent was obtained from all patients and the study was approved by the Institution Review Boards of all participating centers. The study was conducted at academic and community hospitals across northwestern, north central, and southeastern North Carolina and southeastern Pennsylvania.
Study Variables
Assessments were collected during hospitalization, after successful initial treatment for ADHF and achievement of clinical stability and just prior to discharge home. A trained assessor using standardized protocols assessed all functional measures, which included the Montreal Cognitive Assessment (MoCA), short physical performance battery (SPPB), and six-minute walk distance (6MWD).
Cognitive function was assessed using the MoCA.15 The MoCA assesses 8 different cognitive domains (visuospatial/executive, naming, memory, attention, language, abstraction, delayed recall, and orientation) and averages less than 10 minutes to administer, minimizing respondent burden and making it an ideal screening instrument for cardiovascular patients in the hospital setting.15,16 In the initial MoCA validation study by Nasreddine et al.,15 scores from patients with mild CI ranged from 19 to 25.2 out of total possible score of 30 and scores <19 were associated with dementia. A cut-off score of 26 had a 90% sensitivity and a 78% specificity for at least mild CI. Thus, a positive screen in this study was defined as a total score of <26.
The SPPB is an established, standardized, reproducible measure of physical function in older patients that strongly predicts clinical outcomes including hospitalization, death, and nursing facility placement.17,18 Each of the three components, balance, leg strength, and gait speed, is scored from 0–4, for a total score of 0–12; patients with a score of <10 are considered at high risk for mobility disability with lower scores identifying progressively higher risk. The 6MWD was assessed in an unobstructed hallway and patients were allowed to use an assistive device if needed. A distance of ≤300m identifies patients with severe functional impairment.19
Disease-specific QOL was assessed using the Kansas City Cardiomyopathy Questionnaire (KCCQ), a 23-item self-report questionnaire that addresses specific health domains pertaining to HF: physical limitation, symptoms, QOL, social limitation, symptom stability, and self-efficacy — all of which, except for self-efficacy, are aggregated into an Overall Summary Score (OSS).20 Values for the domains range from 0 to 100 with higher scores indicating better health status, lower symptom burden, and greater disease-specific QOL. General QOL was assessed using the common surveys, Short Form-12 (SF-12) physical and mental composite scores (PCS and MCS) and EuroQol-5D-5L (EQ-5D-5L).21,22
Statistical Analyses
Baseline sample characteristics are reported using frequencies (percentages) for categorical variables and means and standard deviations for continuous variables. Classification of cognitive impairment was based on a MoCA score of <26; a correction for education was used wherein one point was added for patients with ≤12 years of education or high school graduate equivalence.15 Relationships between MoCA score with physical function and QOL measures were analyzed using unadjusted and multivariate-adjusted regression. For adjusted analysis, raw MoCA score was the independent variable and was adjusted for sex, non-white race, and education, which have been shown a priori to affect cognition measures.23 Regression parameter estimates were reported. Potential predictors of physical function and QOL, including raw MoCA score, sex, non-white race, and education, were entered into a multiple linear regression model using stepwise selection with p<0.05 significance level. Additionally, the MoCA subdomain scores were entered into a multiple linear regression model with stepwise selection to explore potential predictors of physical function and QOL. All analyses were performed using SAS Enterprise Guide 7.1 (Cary, NC).
RESULTS
Participant Characteristics
A total of 198 consecutively enrolled patients with cognitive data were included in this analysis. Patients averaged 72.1±7.6 years of age, were mildly obese (BMI 33.2 kg/m2), and represented nearly equal proportions of males and females (46% vs. 54%) and whites and non-whites (52% vs. 48%). The majority (81%) had 12 years or more of formal education and 33% lived alone (Table 1).
Table 1:
Baseline Characteristics, Comorbidities, Medications of Older Hospitalized Patients with ADHF
| Characteristics | N=199 |
|---|---|
| Age, years | 72.1 ± 7.6 |
| Women | 108 (54%) |
| Non-white | 104 (52%) |
| Ejection Fraction <45% | 104 (52%) |
| BMI, kg/m2 | 33.2 ± 8.8 |
| NYHA Class | |
| II | 31 (15%) |
| III | 104 (52%) |
| IV | 44 (22%) |
| Patients with previous hospitalization within 6 months | 86 (43%) |
| Patients with previous heart failure hospitalizations within 6 months | 53 (32%) |
| All-cause hospitalizations within 6 months, total | 165 |
| Heart Failure hospitalizations in last 6 months, total | 82 (50%) |
| Less than high school education | 37 (19%) |
| Live alone | 66 (33%) |
| Comorbidities | |
| Hypertension | 184 (92%) |
| Diabetes mellitus | 110 (55%) |
| Atrial fibrillation | 92 (46%) |
| Chronic kidney disease | 65 (33%) |
| Depression | 33 (17%) |
| Stroke | 31 (16%) |
| Dementia/Cognitive impairment | 4 (2%) |
| Current medications | |
| Loop diuretic | 186 (94%) |
| Beta blocker | 166 (84%) |
| ACE or ARB | 125 (63%) |
| Aldosterone antagonist | 36 (18%) |
| Digoxin | 13 (13%) |
| Physical Function | |
| SPPB | 6.0 ± 2.5 |
| Balance score | 2.7 ± 1.3 |
| 4-meter walk score | 2.3 ± 1.0 |
| Chair stand score | 1.1 ± 1.1 |
| 6MWD, meters | 186 ± 100 |
| Quality of Life | |
| KCCQ OSS | 41 ± 21 |
| SF-12 PCS | 28 ± 9 |
| SF-12 MCS | 44 ± 14 |
| EQ5D-5L | |
| Mobility | 2.5 ± 1.0 |
| Self Care | 1.7 ± 0.9 |
| Usual Activities | 2.6 ± 1.2 |
| Pain/Discomfort | 2.4 ± 1.1 |
| Anxiety/Depression | 1.8 ± 1.0 |
| Thermometer/VAS (0–100) | 57 ± 22 |
Values presented as frequency (%) or mean ± standard deviation unless otherwise indicated.
Abbreviations: BMI, body mass index; ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; SPPB, Short Physical Performance Battery; 6MWD, six-minute walk distance; KCCQ, Kansas City Cardiomyopathy Questionnaire; OSS, Overall Summary Score; SF, Short Form; PCS, physical composite score; MCS, mental composite score; EQ-5D-5L, EuroQol-5D-5L; VAS, visual analog scale.
Comorbidity burden was high with the majority of patients having hypertension (92%) and diabetes mellitus (55%). Also, nearly half had atrial fibrillation (46%) and nearly a third had chronic kidney disease (33%). Those with reduced EF (HFrEF) or HF with preserved EF (HFpEF) were similarly distributed (52% vs. 48%). In the prior 6 months, the majority of patients (62%) had a HF-associated hospitalization and HF accounted for 50% of the total hospitalizations. Nearly 75% of patients were classified as either New York Heart Association Functional Class (NYHA) III (52%) or IV (22%).
Patients had severely reduced endurance (6MWD 186±100 m) and markedly impaired physical function (SPPB 6.0±2.5 units). HF-specific QOL (KCCQ) and general QOL (SF-12; EQ-5D-5L) were also severely diminished. Overall, QOL deficits were global, with self-reports of problems across mobility, self-care, usual activities, pain/discomfort, and anxiety/depression domains. Patients’ self-reported walking difficulty was consistent with the objective assessments demonstrating impaired physical function.
Cognitive Function Profile
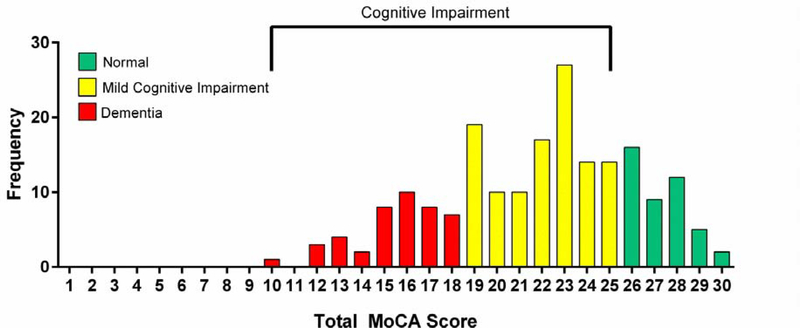
The total raw and education-corrected MoCA scores were 21.2±4.4 and 21.8±4.3, respectively (Table 2). The score distribution indicate that 155 of 199 (78%) of patients screened positive for CI (Figure 1). Of the 155 patients, 72% (N=111) of patients had a score of 19–25, an accepted range for mild CI, and 28% (N=43) of patients had a score less than 18, suggestive of dementia.15 However, only 2% of patients had dementia or CI documented in their medical record. Patients had global deficits across multiple cognitive subdomains; visuospatial/executive function and delayed recall were the most affected subdomains while orientation was the least affected subdomain (Table 2).
Table 2.
MoCA Total Score and Subdomain Scores of Older Hospitalized Patients with ADHF
| Cognitive Function Variable | Mean ± SD | % of Possible Score |
|---|---|---|
| MoCA score (30) | 21.2 ± 4.4 | 71% |
| MoCA score corrected for education* (30) | 21.8 ± 4.3 | 73% |
| MoCA Subdomains | ||
| Visuospatial/executive function score (5) | 2.9 ± 1.4 | 58% |
| Naming (3) | 2.6 ± 0.7 | 87% |
| Attention (6) | 4.5 ± 1.6 | 75% |
| Language (3) | 1.9 ± 0.9 | 63% |
| Abstraction (2) | 1.4 ± 0.8 | 70% |
| Delayed recall (5) | 2.2 ± 1.7 | 44% |
| Orientation (6) | 5.6 ± 0.8 | 93% |
1 point added for education ≤12 years or equivalence.
Abbreviations: MoCA, Montreal Cognitive Assessment
Figure 1. MoCA Score Distribution.
Distribution of education-corrected MoCA scores in patients ≥60 years of age hospitalized with acute decompensated heart failure with <26 cutoff marked. MoCA, Montreal Cognitive Assessment
Relationships of Cognitive Function with Physical Function and QOL
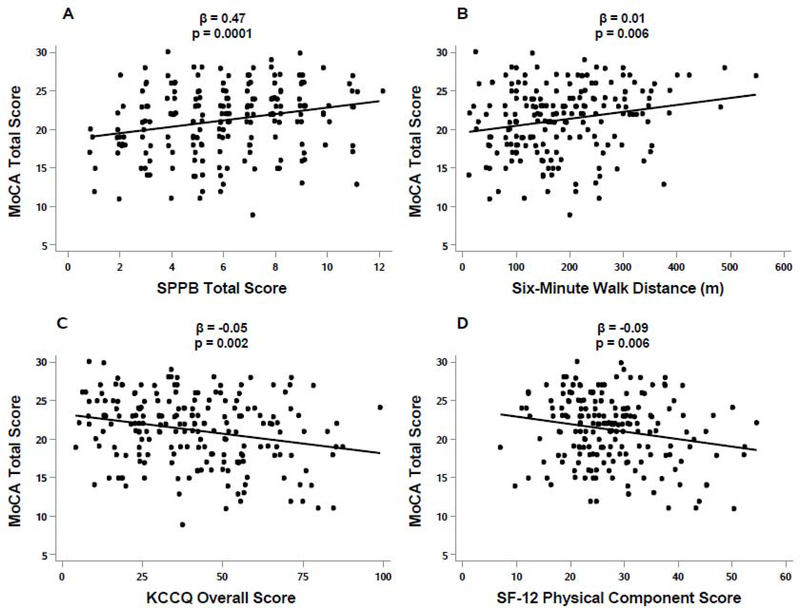
There were small but significant positive relationships between the education-corrected MoCA score and objective measures of physical function (SPPB ß=0.47, p=<0.001; 6MWD ß=0.01, p=0.006; Table 3, Figure 2A and 2B). There was a significant yet small inverse relationship between the education-corrected MoCA score and the HF-specific QOL KCCQ OSS (ß= –0.05, p<0.002; Table 3, Figure 2C) and the general QOL SF-12 PCS (ß= –0.09, p=0.006; Table 3, Figure 2D).
Table 3.
Unadjusted and Adjusted Relationships of MoCA Score with Participant Characteristics, Physical Function, and QOL
| Variable | Unadjusted* | Adjusted† | ||
|---|---|---|---|---|
| Estimate | p-value | Estimate | p-value | |
| Age | −0.06 ± 0.04 | 0.11 | −0.12 ± 0.04 | 0.005 |
| SPPB | 0.42 ± 0.12 | <0.001 | 0.47 ± 0.12 | <0.001 |
| Balance score | 0.77 ± 0.23 | 0.001 | 0.82 ± 0.23 | <0.001 |
| 4-meter walk score | 0.94 ± 0.30 | 0.002 | 0.95 ± 0.31 | 0.003 |
| Chair stand score | 0.45 ± 0.28 | 0.12 | 0.57 ± 0.28 | 0.046 |
| 6MWD | 0.01 ± 0.00 | 0.003 | 0.01 ± 0.00 | 0.006 |
| KCCQ OSS | −0.05 ± 0.01 | <0.001 | −0.05 ± 0.01 | 0.002 |
| SF-12 PCS | −0.09 ± 0.03 | 0.008 | −0.09 ± 0.03 | 0.006 |
| SF-12 MCS | −0.02 ± 0.02 | 0.34 | −0.02 ± 0.02 | 0.26 |
| EQ-5D-5L | ||||
| Mobility | 0.29 ± 0.31 | 0.34 | 0.14 ± 0.32 | 0.66 |
| Self Care | 0.01 ± 0.35 | 0.97 | −0.05 ± 0.35 | 0.89 |
| Usual Activities | 0.65 ± 0.25 | 0.011 | 0.55 ± 0.25 | 0.031 |
| Pain/Discomfort | 0.12 ± 0.28 | 0.67 | 0.25 ± 0.28 | 0.38 |
| Anxiety/Depression | 0.13 ± 0.31 | 0.67 | 0.03 ± 0.31 | 0.93 |
| Thermometer/VAS (0–100) | −0.02 ± 0.01 | 0.09 | −0.02 ± 0.01 | 0.16 |
Education-corrected MoCA total score.
Raw MoCA total score and adjusted for sex, race, and education.
Abbreviations: MoCA, Montreal Cognitive Assessment; SPPB, Short Physical Performance Battery; 6MWD, six-minute walk distance; KCCQ, Kansas City Cardiomyopathy Questionnaire; OSS, Overall Summary Score; SF, Short Form; PCS, physical composite score; MCS, mental composite score; EQ-5D-5L, EuroQol-5D-5L; VAS, visual analog scale.
Figure 2. Relationship Between Cognition and Physical Function and QOL.
Relationship between cognitive function and physical function and QOL in patients ≥60 years of age hospitalized with acute decompensated heart failure. There was a significant positive association between the total MoCA score and SPPB score (A) and 6MWD (B). There was a significant inverse association between the total MoCA score and KCCQ Overall Summary Score (C) and SF-12 Physical Composite Score. MoCA – Montreal Cognitive Assessment; SPPB, Short Physical Performance Battery; 6MWD, six-minute walk distance; KCCQ, Kansas City Cardiomyopathy Questionnaire; SF-12, Short Form.
In contrast, there was also a significant positive relationship between the education-corrected MoCA score and self-reported performance of Usual Activities (e.g., work, study, housework, family or leisure activities) in the EQ-5D-5L, (ß=0.55, p=0.031); Table 3). With the exception of age, adjusting for sociodemographic variables (sex, race, and education) on the raw MoCA score did not meaningfully change the aforementioned relationships (Table 3).
Predictors of Physical Function and Quality of Life
Raw MoCA score was a small yet significant independent predictor of the SPPB and 6MWD as well as QOL KCCQ (Supplemental Table 1). The predictive power of the MoCA score was similar in magnitude to that of age for KCCQ and SPPB, but less to that of sex for SPPB and 6MWD, and of EF for KCCQ, SPPB, and 6MWD. MoCA subdomains that were significant predictors included the following: visuospatial/executive function (ß= –2.70) and abstraction (ß= –0.48) for KCCQ, visuospatial/executive function (ß=0.48) for SPPB, and attention (ß=11.30) of 6MWD (Supplemental Table 2).
DISCUSSION
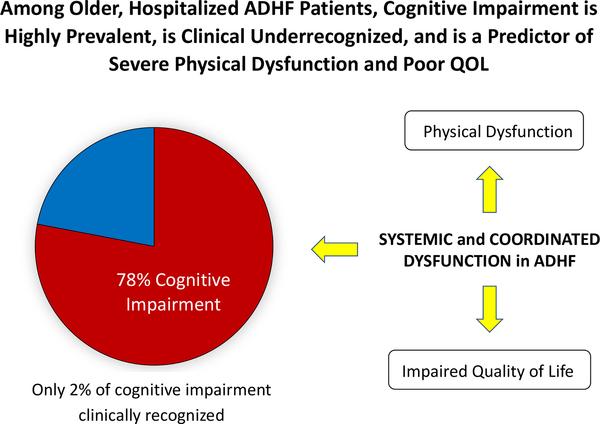
This study systematically assessed cognitive dysfunction and examined its relationships with multiple measures of other important patient outcomes, including physical function and QOL, across multiple academic and community sites in an older, diverse ADHF population. We found that 1) CI was highly prevalent; 2) CI involved predominantly visuospatial, executive function, and recall domains; and 3) CI was associated with severe physical dysfunction and poor QOL. Each of these impairments is clinically meaningful with prognostic implications despite not being typically addressed in current care models or disease management pathways. Concomitant cognitive and physical impairment implies coordinated, systemic dysfunction, which, subsequently, can jeopardize patient self-care, safety and independence (Figure 3).
Figure 3: Conceptual Model.
Among older patients hospitalized with acute decompensated heart failure, cognitive impairment is highly prevalent, is underrecognized clinically, and is a predictor of severe and widespread physical dysfunction and poor quality of life. Concomitant cognitive and physical impairment implies coordinated, systemic dysfunction, which has high potential to jeopardize adherence to heart failure management strategies, safety, and independent living. Screening for cognitive and physical dysfunction delivers crucial details regarding performance in daily life activities and health management, identifying those who may require more support and tailored care to reduce the risk of untoward adverse events and unnecessary hospitalizations.
CI Prevalence
Even though 78% of older patients with ADHF had MoCA scores suggesting the presence of mild CI or dementia, CI was only recognized clinically in a mere 2% of patients. Because patients with known severe CI or dementia were excluded from the present study, the actual prevalence of CI in the clinical setting is likely even greater. The high prevalence may also reflect the sensitivity of the MoCA, demonstrating its potential usefulness as screening tool for cognitive function in routine cardiovascular care. These results along with others indicate cognitive assessment using a standardized rapid screening instrument is feasible and can detect deficits not readily apparent through informal clinical assessment.4,9,16,24–26
CI and Self-Management
Successful HF management requires a high level of patient self-care management, including the ability to recognize symptoms and adhere to recommended self-monitoring and complex pharmaceutical and behavioral (diet, activity, mood, sleep) regimens. This in turn requires intact cognitive function.8,27 However, we found that older patients with ADHF performed poorly on items with executive, verbal recall, and visuospatial demands, extending emerging evidence in older patients with HF assessed with MoCA16,28 and in hospitalized patients with ADHF.24,25 Deficits in these domains suggest that older patients with ADHF are particularly challenged with reasoning, planning, problem-solving, short-term memory, and spatial relationships in the environment, thus jeopardizing their capacity for self-care management, instructional adherence, and safety upon hospital discharge to their home and community, respectively. Orientation was least affected, signifying that reliance on orientation alone to gauge cognitive capacity may be misplaced in this HF population.
Coordinated Dysfunction and Potential Mechanisms
Our results demonstrated that CI had a small yet significant and predictive relationship with physical impairments and poor QOL. Multiple cognitive subdomains were independent predictors including: visuospatial and executive function for SPPB, a multicomponent physical function test; attention for 6MWD, a physical endurance test; and visuospatial, executive function and abstraction for KCCQ, a multicomponent HF-specific QOL survey. These data imply that patients with ADHF may be especially challenged with multicomponent activities across both physical and psychological realms and with lengthy activities. Also, in this older, frail, population with multiple comorbidities, it is important to note that one of the domains (cognition, physical function, quality of life) does not fully capture the effects of HF disease burden and highlights the importance that all domains be assessed as potential targets for therapeutic intervention in patients with ADHF.
Interestingly, cognitive function was inversely correlated with HF-specific QOL KCCQ and general QOL SF-12 PCS. This suggests that patients with relatively preserved cognitive function may have either increased sensitivity to symptoms or a negative perception of their actual physical capability, while patients with lower cognitive function may lack insight into their actual physical deficits. Those who lack insight into physical deficits may be at more risk for untoward events such as falling or not recognizing warning signs associated with worsening HF, which is consistent with studies showing higher clinical events in patients with HF with CI.4,25 The potential applicability to clinical care could be that for those with relatively higher MOCA and lower QOL scores care could be directed toward improving QOL through emotional or psychological support strategies while for those with relatively lower MOCA and higher QOL scores care could be directed toward improving their physical function and safety through physical rehabilitation strategies.
Tandem declines in cognitive function and physical function imply that both may be connected by systemic signals. Potential pathophysiological links include HF-associated chronic hypoperfusion, systemic inflammation, and immune dysregulation.5,29–31 The inadequate delivery of nutrients and oxygen and the release of pro-inflammatory mediators elicit structural and functional changes in the brain and skeletal muscle to precipitate cognitive and physical decline. Moreover, as HF progresses and sedentary time increases, CI can worsen.32,33 This increased severity of CI can, in turn, affect motor pathways to accelerate physical decline, resulting in a vicious cycle of worsening.
Putative mechanisms for cerebral hypoperfusion include low cardiac output34 and cerebral microemboli35. It had been assumed that infarcts from conditions often accompanying HF such as atrial fibrillation and cardiomyopathy were the predominant causal factor of CI. Recently, however, the rigorously designed Cognition.Matters-HF trial demonstrated that older patients with HF had a greater risk for brain atrophy, especially in the medial temporal lobe that is critical for memory, compared with silent lacunae and brain infarctions, even after adjusting for comorbidities including atrial fibrillation.36 Also, greater atrophy associated with more severe CI, principally in memory and attention domains. Deficit in attention, in particular, was associated with higher NYHA classification and shorter 6MWD, substantiating our subdomain prediction results. Subsequently, identifying the specific pathways linking CI and physical dysfunction will inform the tailoring of interventions based on pathophysiology and functional capacity.
Given that successful pharmaceutical treatments for declines in cognitive and physical function are lacking, identifying alternative treatment strategies that mitigate concomitant cognitive and physical deficits in HF is central to comprehensive care. Physical rehabilitation holds promise as a widely accessible, low-cost intervention that may slow, reverse, or prevent cognitive decline in HF.37–39 Exercise is documented to increase cerebral perfusion and oxygenation by promoting neuroplasticity and neurogenesis and, in turn, cognitive functioning.40 This is particularly important given that neurons in the hippocampus of the medial temporal lobe, the main neural substrate for memory, have demonstrated neurogenesis and neuroplasticity.41,42 A recent study primarily in moderate to severe HF (NYHA II and III) found that baseline physical capacity (6MWD) was associated with lower MoCA scores.28 To date only one small study has examined the effects of aerobic exercise training on cognitive function specifically in severe HF (NYHA III) and found promising results.43 However, a much larger base of literature, including randomized controlled trials, suggests that exercise training improves cognitive function in other populations susceptible to cognitive decline, including older adults and those with stroke and dementia.44–46 The effects of exercise may also be examined in the REHAB-HF trial.
Strengths and Limitations
This study has several key strengths including a large, diverse representation of older patients hospitalized across academic and community settings with ADHF, use of multiple standardized instruments amenable to bedside administration, and acquisition of the patient-centered outcomes of cognition, physical function, and QOL within a multicenter trial. Assessment of cognitive subdomains and their relationships with physical function and QOL is also unique. The study also has some limitations. QOL is a multidimensional concept that requires the capacity to make complex judgements about life and involves several cognitive subdomains, such as attention, memory, language, and abstract thinking. Thus, the QOL surveys may not be as valid in patients with those subdomain deficits. Rigorous psychometric studies on QOL assessments in older patients with ADHF may be necessary. Although the MoCA was the single cognitive evaluation performed, the results are highly novel in this older hospitalized population and lays the groundwork for future studies of more detailed assessments. However, the recommended “normal” cutoff for the MoCA might be too high for the older hospitalized ADHF population; resolution would require comparison of the MoCA with neuropsychological testing and matched control data. In addition, although cognitive function in hospitalized patients with ADHF may improve after compensation,25 the accuracy of the MoCA could still be impacted by several factors, including acute illness recovery, reduced cerebral perfusion due to ADHF itself, poor sleep quality, medications, and delirium. Delirium was not assessed with a specific test (e.g., Confusion Assessment Method), however, patients were examined by the clinical team and study HF cardiologist. Patients deemed to have clinical delirium were not approached. Patients who were enrolled were deemed medically stable, cleared for discharge directly to their home, and capable of consent. Lastly, given the cross-sectional study design, we cannot assess the relative importance of the identified impairments to subsequent outcomes, although prior research indicates these impairments strongly predict subsequent clinical events.
Conclusion
Although the overwhelming majority of older hospitalized patients with ADHF had MoCA scores suggestive of CI, it was nearly always unrecognized clinically. Moreover, CI was global, affecting most domains of cognitive function, and was associated with severe impairments in physical function and QOL. Concomitant cognitive and physical impairment implies coordinated, systemic dysfunction, which has high potential to jeopardize patient HF self-management, safety, and functional independence. These findings suggest the potential utility of formal screening for CI and development of novel interventions to improve these important patient-centered outcomes (Figure 3).
Supplementary Material
HIGHLIGHTS.
In older hospitalized ADHF patients, cognitive dysfunction is prevalent.
Cognitive dysfunction is associated with severe physical function deficits.
Concomitant impairment can jeopardize HF management strategies and patient safety.
Screening may reduce adverse events by identifying those who need tailored care.
ACKNOWLEDGMENTS
Financial Disclosures: This study was supported in part by the following research grant awards from the National Institutes of Health: R01AG045551 and R01AG18915. Also supported in part by the Kermit Glenn Phillips II Chair in Cardiovascular Medicine at Wake Forest School of Medicine (DWK); the Claude D. Pepper Older Americans Independence Center (OAIC) NIH Grants P30AG021332 (DWK) and P30AG028716 (AMP); the Wake Forest Clinical and Translational Science Award, NIH Grant UL1TR001420, and the OAIC National Coordinating Center U24AG059624.
Conflict of Interest: Dr. Kitzman is a consultant for AstraZeneca, Abbvie, GlaxoSmithKline, Merck, Corvia Medical, Bayer, CinRx, Boehringer-Ingleheim, and St. Luke’s Medical Center in Kansas City, Kansas; received grant support from Novartis, AstraZeneca, Bayer, and St. Luke’s Medical Center in Kansas City, Kansas; and owns stock in Gilead Sciences. Dr. Mentz receives research support from the National Institutes of Health (U01HL125511–01A1 and R01AG045551–01A1), Akros, Amgen, AstraZeneca, Bayer, GlaxoSmithKline, Gilead, InnoLife, Luitpold/American Regent, Medtronic, Merck, Novartis and Sanofi; honoraria from Abbott, Amgen, AstraZeneca, Bayer, Boston Scientific, Janssen, Luitpold Pharmaceuticals, Medtronic, Merck, Novartis, and Sanofi; and has served on an advisory board for Amgen, AstraZeneca, Luitpold, Merck, Novartis and Boehringer Ingelheim. The other authors report no conflicts.
ABBREVIATIONS
- ADHF
acute decompensated heart failure
- CI
cognitive impairment
- MoCA
Montreal Cognitive Assessment
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- SPPB
short physical performance battery
- 6MWD
six-minute walk distance
- QOL
quality of life
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- SF-12
12-item short form health survey
- MCS
SF-12 mental composite score
- PCS
SF-12 physical composite score
- EQ-5D-5L
EuroQol-5D-5L questionnaire
- VAS
Visual Analog Scale
Footnotes
Sponsor’s Role: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Clouston SA, Brewster P, Kuh D, et al. The dynamic relationship between physical function and cognition in longitudinal aging cohorts. Epidemiol Rev. 2013;35:33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stijntjes M, Aartsen MJ, Taekema DG, et al. Temporal Relationship Between Cognitive and Physical Performance in Middle-Aged to Oldest Old People. J Gerontol A Biol Sci Med Sci. 2017;72(5):662–668. [DOI] [PubMed] [Google Scholar]
- 3.Cannon JA, Moffitt P, Perez-Moreno AC, et al. Cognitive Impairment and Heart Failure: Systematic Review and Meta-Analysis. J Card Fail. 2017;23(6):464–475. [DOI] [PubMed] [Google Scholar]
- 4.Dodson JA, Truong TT, Towle VR, Kerins G, Chaudhry SI. Cognitive impairment in older adults with heart failure: prevalence, documentation, and impact on outcomes. Am J Med. 2013;126(2):120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brassard P, Gustafsson F. Exercise Intolerance in Heart Failure: Did We Forget the Brain? Can J Cardiol. 2016;32(4):475–484. [DOI] [PubMed] [Google Scholar]
- 6.Krumholz HM, Nuti SV, Downing NS, Normand SL, Wang Y. Mortality, Hospitalizations, and Expenditures for the Medicare Population Aged 65 Years or Older, 1999–2013. JAMA. 2015;314(4):355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lovell J, Pham T, Noaman SQ, Davis MC, Johnson M, Ibrahim JE. Self-management of heart failure in dementia and cognitive impairment: a systematic review. BMC Cardiovasc Disord. 2019;19(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huynh QL, Negishi K, De Pasquale CG, et al. Cognitive Domains and Postdischarge Outcomes in Hospitalized Patients With Heart Failure. Circ Heart Fail. 2019;12(6):e006086. [DOI] [PubMed] [Google Scholar]
- 10.Reeves GR, Whellan DJ, Patel MJ, et al. Comparison of Frequency of Frailty and Severely Impaired Physical Function in Patients >/=60 Years Hospitalized With Acute Decompensated Heart Failure Versus Chronic Stable Heart Failure With Reduced and Preserved Left Ventricular Ejection Fraction. Am J Cardiol. 2016;117(12):1953–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reeves GRWD, O’Conner CM, Duncan P, Eggebeen JD, Morgan TM, Hewston LA, Pastva AM, Patel MJ, Kitzman D. A novel rehabilitation intervention for older patients with acute decompensated heart failure: The REHAB-HF Pilot Study. JACC Heart Fail. 2017, DOI: 10.1016/j.jchf.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warraich HJ, Kitzman DW, Whellan DJ, et al. Physical Function, Frailty, Cognition, Depression, and Quality of Life in Hospitalized Adults >/=60 Years With Acute Decompensated Heart Failure With Preserved Versus Reduced Ejection Fraction. Circ Heart Fail. 2018;11(11):e005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reeves GRWD, Duncan P, O’Conner CM, Pastva AM, Eggebeen JD, Hewston LA, Morgan TM, Reed SD, Mentz RJ, Rosenberg PB, Kitzman D. Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial: Design and rationale. Am Heart J. 2017;185(0):130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pastva AM, Duncan PW, Reeves GR, et al. Strategies for supporting intervention fidelity in the rehabilitation therapy in older acute heart failure patients (REHAB-HF) trial. Contemp Clin Trials. 2018;64:118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. [DOI] [PubMed] [Google Scholar]
- 16.Harkness K, Demers C, Heckman GA, McKelvie RS. Screening for cognitive deficits using the Montreal cognitive assessment tool in outpatients >/=65 years of age with heart failure. Am J Cardiol. 2011;107(8):1203–1207. [DOI] [PubMed] [Google Scholar]
- 17.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311(23):2387–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volpato S, Cavalieri M, Sioulis F, et al. Predictive value of the Short Physical Performance Battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci. 2011;66(1):89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Writing Committee M, Yancy CW, Jessup M, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):e240–327. [DOI] [PubMed] [Google Scholar]
- 20.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245–1255. [DOI] [PubMed] [Google Scholar]
- 21.EuroQol G EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. [DOI] [PubMed] [Google Scholar]
- 22.Ware J Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. [DOI] [PubMed] [Google Scholar]
- 23.O’Driscoll C, Shaikh M. Cross-Cultural Applicability of the Montreal Cognitive Assessment (MoCA): A Systematic Review. J Alzheimers Dis. 2017;58(3):789–801. [DOI] [PubMed] [Google Scholar]
- 24.Levin SN, Hajduk AM, McManus DD, et al. Cognitive status in patients hospitalized with acute decompensated heart failure. Am Heart J. 2014;168(6):917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kindermann I, Fischer D, Karbach J, et al. Cognitive function in patients with decompensated heart failure: the Cognitive Impairment in Heart Failure (CogImpair-HF) study. Eur J Heart Fail. 2012;14(4):404–413. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher R, Sullivan A, Burke R, et al. Mild cognitive impairment, screening, and patient perceptions in heart failure patients. J Card Fail. 2013;19(9):641–646. [DOI] [PubMed] [Google Scholar]
- 27.Dolansky MA, Hawkins MA, Schaefer JT, et al. Association Between Poorer Cognitive Function and Reduced Objectively Monitored Medication Adherence in Patients With Heart Failure. Circ Heart Fail. 2016;9(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vellone E, Chiala O, Boyne J, et al. Cognitive impairment in patients with heart failure: an international study. ESC Heart Fail. 2020;7(1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keller-Ross ML, Larson M, Johnson BD. Skeletal Muscle Fatigability in Heart Failure. Front Physiol. 2019;10:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ampadu J, Morley JE. Heart failure and cognitive dysfunction. Int J Cardiol. 2015;178:12–23. [DOI] [PubMed] [Google Scholar]
- 31.Shinmura K Frailty, heart failure, and cognitive impairment: a triangle in elderly people. Heart Metab. 2018;76:8–12. [Google Scholar]
- 32.Alosco ML, Spitznagel MB, Cohen R, et al. Decreased physical activity predicts cognitive dysfunction and reduced cerebral blood flow in heart failure. J Neurol Sci. 2014;339(1–2):169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alosco ML, Spitznagel MB, Sweet LH, Josephson R, Hughes J, Gunstad J. Cognitive dysfunction mediates the effects of poor physical fitness on decreased functional independence in heart failure. Geriatr Gerontol Int. 2015;15(2):174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woo MA, Ogren JA, Abouzeid CM, et al. Regional hippocampal damage in heart failure. Eur J Heart Fail. 2015;17(5):494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siachos T, Vanbakel A, Feldman DS, Uber W, Simpson KN, Pereira NL. Silent strokes in patients with heart failure. J Card Fail. 2005;11(7):485–489. [DOI] [PubMed] [Google Scholar]
- 36.Frey A, Sell R, Homola GA, et al. Cognitive Deficits and Related Brain Lesions in Patients With Chronic Heart Failure. JACC Heart Fail. 2018;6(7):583–592. [DOI] [PubMed] [Google Scholar]
- 37.Galioto R, Fedor AF, Gunstad J. Possible neurocognitive benefits of exercise in persons with heart failure. Eur Rev Aging Phys Act. 2015;12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gary RA, Brunn K. Aerobic exercise as an adjunct therapy for improving cognitive function in heart failure. Cardiol Res Pract. 2014;2014:157508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flint KM, Pastva AM, Reeves GR. Cardiac Rehabilitation in Older Adults with Heart Failure: Fitting a Square Peg in a Round Hole. Clin Geriatr Med. 2019;35(4):517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ang ET, Tai YK, Lo SQ, Seet R, Soong TW. Neurodegenerative diseases: exercising toward neurogenesis and neuroregeneration. Front Aging Neurosci. 2010;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merzenich MM, Van Vleet TM, Nahum M. Brain plasticity-based therapeutics. Front Hum Neurosci. 2014;8:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanne D, Freimark D, Poreh A, et al. Cognitive functions in severe congestive heart failure before and after an exercise training program. Int J Cardiol. 2005;103(2):145–149. [DOI] [PubMed] [Google Scholar]
- 44.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14(2):125–130. [DOI] [PubMed] [Google Scholar]
- 45.Oberlin LE, Waiwood AM, Cumming TB, Marsland AL, Bernhardt J, Erickson KI. Effects of Physical Activity on Poststroke Cognitive Function: A Meta-Analysis of Randomized Controlled Trials. Stroke. 2017;48(11):3093–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Groot C, Hooghiemstra AM, Raijmakers PG, et al. The effect of physical activity on cognitive function in patients with dementia: A meta-analysis of randomized control trials. Ageing Res Rev. 2016;25:13–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.