Abstract
Gonadal hormones influence neuronal organization and plasticity. Yet the consequences of altering their concentrations by administering contraceptive agents, which are used by most reproductive-age women in the United States, are unclear. Cross-sectional studies have found both larger and smaller cortical regions alongside a variety of mood alterations in women who use oral contraceptive pills (OCPs) compared to naturally-cycling women. The goal of this study, therefore, was to determine whether there is an effect of OCPs on MRI measures of prefrontal cortical brain structure that may influence regulation of mood. We performed a double-blind, placebo-controlled, randomized crossover study comparing effects of OCPs (0.15 mg levonorgestrel + 0.30 μg ethinyl estradiol) vs placebo (N = 26) on MRI measures of prefrontal cortical thickness and on mood, as indicated by self-report on the Daily Record of Severity of Problems, which also includes one item related to somatic symptoms. MRI measures that reflect cortical thickness were smaller bilaterally in the pars triangularis and in the pars opercularis and frontal pole of the right hemisphere during the OCP arm vs. placebo. Only the effect in the right pars triangularis survived multiple comparisons correction. Right pars triangularis MRI measures of cortical thickness were not related to mood symptoms, but negatively correlated across conditions with severity of somatic symptoms on the DSRP. The somatic symptoms and MRI measures may be independently related to the actions of steroid hormones in OCPs, with OCPs simultaneously inducing both more effects on MRI measures of cortical thickness and somatic symptoms.
1. Introduction
Investigating effects of hormonal contraceptives on the brain is important to understanding their role in public health because they may have influence that extends beyond their primary purpose of contraception. Oral contraceptive pills (OCPs) appear to influence mental health although the effect is neither uniformly positive or negative. There are reports that OCPs are associated with salutary effects on romantic relationships1 and mood2, specifically reduced risk of subthreshold panic disorder3 and depressive symptoms4. In contrast, two very large nationwide cohort studies (Ns > 475,000 and 1,000,000, respectively) found higher rates of depression diagnoses, antidepressant prescriptions,5 and suicidal behaviors (attempts and completions)6 in women who used hormonal contraceptives than those who did not. Adolescent girls using oral contraceptives had higher scores on inventories of depression symptoms compared to adolescent girls who did not use them7. A large proportion (27–51%) of women who used OCPs self-reported one or more mood symptoms while using them8–10, and those experiencing OCP-related mood problems were more likely to discontinue their use9. However, causal relationships between the use of OCPs and any of these outcomes cannot be established from observational studies, which cannot account for the many factors that vary between women who never use OCPs, those who start using OCPs and discontinue, and those who start OCPs and maintain their use long-term.
Hormonal contraceptives, including but not limited to OCPs, decrease endogenous hormone levels11 through substitution with higher-affinity, synthetic hormone analogs12, 13. Studies comparing women who take OCPs to naturally-cycling women have shown differences in brain structure: when measures were obtained using voxel-based morphometry, women using OCPs exhibited larger volumes of prefrontal cortices, pre- and postcentral gyri, parahippocampal and fusiform gyri, and temporal regions compared to women did not use contraceptives14. By contrast, smaller right putamen volume, measured with voxel-based morphometry15, and smaller thickness in the lateral orbitofrontal cortex and the posterior cingulate cortex, measured with cortical surface reconstruction and volumetric segmentation, have also been observed in women who use OCPs16. This disparity may partially reflect divergent actions of different types of OCs, as women who were using OCPs with androgenic progestins had smaller middle and superior frontal gyrus volumes compared to naturally cycling women, whereas those who used OCPs containing anti-androgenic progestins had larger volumes of the parahippocampal and fusiform gyri and cerebellum when measured using voxel-based morphometry17.
The ovarian hormones estrogen (17β-estradiol) and progesterone influence synaptic18–20 and neuronal plasticity21, with effects documented in the rodent cerebellum22, hippocampus23–25 and hypothalamus26, 27. Cortical excitability is also influenced by ovarian hormones28–30, which are distributed throughout the cerebral cortex31, 32 and are not limited to the reproductive neuroendocrine system per se. With the knowledge that brain circuitry and structure can change with experience and pharmacological interventions, including administration of metabolic hormones33–35, it stands to reason that if an intervention alters ovarian hormone levels, it may influence brain structure. Evidence increasingly points this way.
The lack of placebo-controlled, prospective studies in both the domains of brain imaging and mood preclude determination of which effects are causally linked to OC, and which are incidental findings linked to the populations who initiate, maintain, and/or discontinue OCP use. Therefore, to provide a single study with explanatory power to address this gap, we performed a prospective, double-blind, placebo-controlled, randomized crossover study in 26 women who self-reported daily measurements of mood and menstrual-related symptoms and underwent high-resolution structural MRI scans while they were using OCPs and placebo. Because prefrontal regions are important to cognitive control of emotion36, and have been previously observed to be smaller in women taking OCPs compared to naturally-cycling women16, prefrontal cortical thickness and mood were compared between the two intervention arms. We hypothesized that OCPs would increase negative mood and decrease prefrontal cortical thickness, and that the magnitudes of these two changes would correspond with one another.
2.0. Methods.
These methods were approved by the Institutional Review Board of UCLA before data collection began.
2.1. Participants.
Twenty-six healthy women, who had reported previous mood deterioration while using OCs, were recruited via Internet advertisements and completed the study. Participants were required to be 18–35 years of age, right-handed, and English speaking, and to endorse mood disturbances from previous use of birth control pills. Potential participants were excluded if they reported that they were current smokers, had a history of other drug abuse, had used hormonal contraceptives within the previous 3 months, were pregnant or nursing, were experiencing a current psychiatric disorder, had any major central nervous system damage or disease, had a history of claustrophobia, had any medical conditions or medications that would impact cerebral perfusion, or had non-removable metal that would interfere with MRI acquisition fidelity or safety.
Demographics for participants who completed both intervention arms are presented in Table 1. Participants who began in the OCP arm vs. those who began in the placebo arm were similar with respect to their ages and years of education. Ethnic backgrounds for each group were not compared due to small cell sizes, but are reported in Table 1.
Table 1:
Demographics of study completers.
| Starting arm: OCPs | Starting arm: Placebo | |
|---|---|---|
| Age (years, mean ± SD) | 28.8 ± 3.02 | 28.2 ± 4.43 |
| Years of education (mean ± SD) | 15.2 ± 1.56 | 15.7 ± 2.21 |
| Self-identified ethnic background | ||
| More than one ethnicity: | 2 | 4 |
| Asian: | 3 | 1 |
| Black: | 2 | 4 |
| Hispanic: | 4 | 1 |
| White: | 5 | 6 |
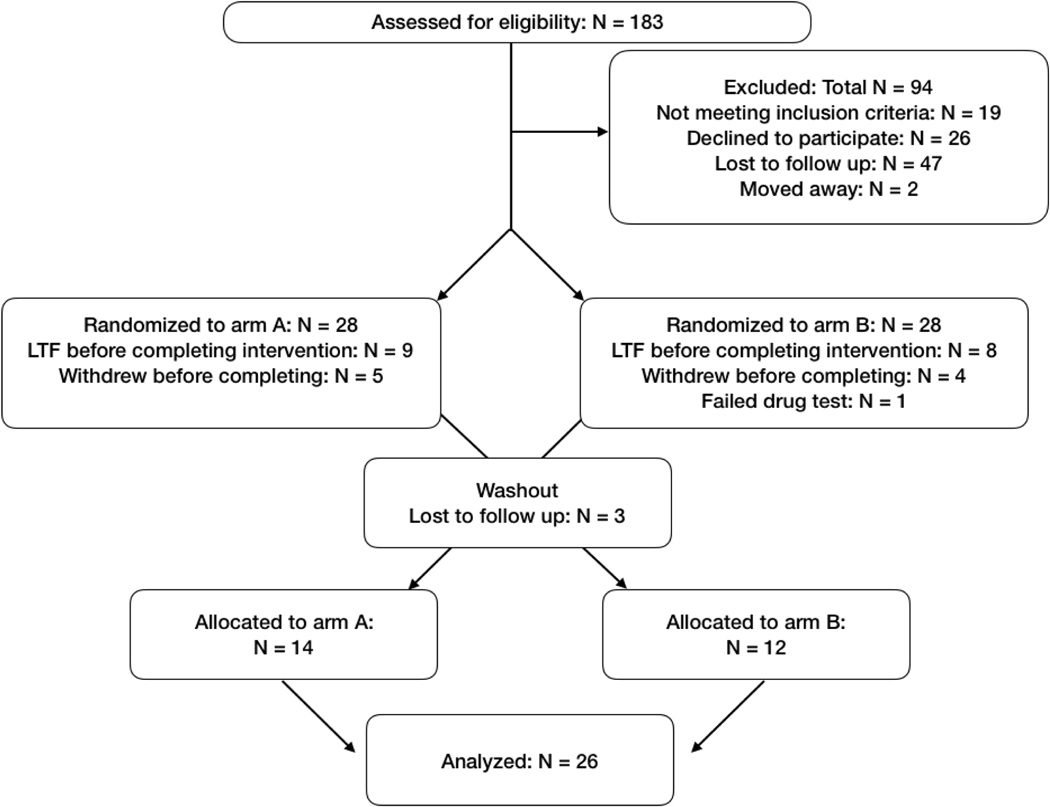
2.2.1. Study design (CONSORT diagram, Figure 1).
Figure 1:
CONSORT diagram describing the overall flow of the study, with the number of excluded participants and reasons for their exclusion at each phase
Those who successfully completed an initial phone screening were invited to an in-person screening, where written, informed consent was obtained after an in-depth description of study procedures. After the consenting process, eligibility was further determined by a comprehensive medical and psychiatric evaluation, including MRI safety and drug-use history questionnaires. Those who qualified were randomized into an intervention group and invited to complete two testing sessions spaced apart by a washout period lasting at least one menstrual cycle. Participants remained blind to study condition for the duration of the study. Investigators were unblinded after data analysis was complete.
Those who were deemed eligible and agreed to participate after the in-person screening were given a prescription for either 21 OC pills (0.30 μg ethinyl estradiol / 0.15 mg levonorgestrel) or placebo to be taken once daily, starting on the first day of menses, and they were scheduled for a testing session 18–21 days into each intervention arm, allowing sufficient time for OCPs to suppress ovulation37–40. Testing sessions included MRI, questionnaires, and cognitive testing, described in detail below. Participants were reminded 24 hours before each appointment to avoid caffeine, and when they arrived at the laboratory, were given tests to measure alcohol and carbon monoxide breath levels, in addition to urine toxicology and pregnancy tests to verify absence of recent drug use or ongoing pregnancy.
A washout period comprised of one complete menstrual cycle was included between intervention arms to allow for the effects of OCPs to dissipate and ovulation to return for the participants who began on the OCPs arm. After the washout period, an identical testing session was repeated for the other intervention arm. Counterbalancing was achieved by randomizing participants to one of the two starting arms using a random number generator, and then automatically assigning them to the other arm after the washout period. Thus, half of the participants were tested initially during the active pill phase, and the other half during the placebo pill phase.
2.2.2. Sample Size.
The sample size was determined prospectively based on effect sizes estimated from previous studies16. In total, 181 participants came to an in-person screening, and 26 eligible healthy women were identified to go on to complete both intervention arms. Data from two participants who completed arm A, but not arm B, and one participant who completed arm B, but not arm A, were included in subsequent analyses. These three participants were lost to follow-up (stopped responding to outreach from our staff). Brain imaging data from two participants was excluded due to motion (see 2.6. Methods: Structural MRI analysis for data cleaning procedures). Brain imaging data was not collected from one participant during arm A, and one participant during arm B, due to MRI time constraints. Therefore, total Ns for behavioral data (DRSP and BDI) were N = 28 for arm A and N = 27 for arm B. Total Ns for brain imaging data were N = 28 for arm A, and N = 25 for arm B. Crossover data were available for N = 26 (behavioral data) and N = 22 (brain imaging data).
2.3. Questionnaires.
The Daily Record of Severity of Problems (DRSP)41 was completed online each day that each participant was enrolled in the study. One participant’s scores were not included in analyses because she did not complete DRSPs for 6 days leading up to her MRI session. Only scores for the final 10 days leading up to each MRI were used for analyses to allow time for effects of OCPs to begin to appear; these scores were averaged together across the 10 days for each item. The DRSP includes one item measuring physical menstrual-related symptoms, and we did not remove this item from the inventory, although we had no a priori hypotheses regarding physical symptoms. On the day of scanning, participants also completed the Beck Depression Inventory (BDI)42.
2.4. Hormones.
Venipuncture was performed by a nurse or phlebotomist, and approximately 4 mL of whole blood was collected from each participant on each of the two testing days. 17β-estradiol and progesterone levels were measured by electrochemiluminescence with a detection threshold of 0.03 ng/mL (Roche Elecsys Immunoassay system, F. Hoffman-La Roche, Basel, Switzerland).
2.5. Scan Parameters.
Data were collected on a Siemens 3-Tesla Prisma Fit MRI Scanner (Siemens, Erlangen, Germany) equipped with a 32-channel head coil. A magnetized-prepared gradient echo (MPRAGE) sequence was used to collect the images, with slice thickness = 0.8 mm, TR = 2400 msec, TE = 2.24 msec, flip angle = 8o, acquisition time = 395 sec, and FOV = 240 × 256 mm2.
2.6. Structural MRI Analysis.
Anatomical MR images were processed using FreeSurfer 5.4.0 (http://surfer.nmr.mgh.harvard.edu) using the “recon-all” pipeline43, which generates a three-dimensional model of the cortical surface and provides local cortical thickness measurements, and then extracts mean thickness and volume within 34 automatically defined cortical parcellations for each hemisphere44, 45; the 9 parcellations in the prefrontal cortex were selected a priori for analysis, for a total of 18 comparisons (9 per hemisphere; see supplement for visualization). Detection of images with motion artifacts was performed automatically via the Qoala-T supervised learning quality control tool46, which flagged data from 2 participants for exclusion. Adding back these data did not change any results subsequently reported. Total cortical thickness in each hemisphere was also evaluated to test for a global vs. regional effect of OCPs.
2.7. Statistics.
Statistical analyses were performed in JMP Pro 14.0.0 (SAS Institute Inc., Cary, NC, USA). Effects of the intervention (OCPs vs. placebo) on cortical thickness and behavioral measurements were evaluated in a linear mixed model with participant as a random effect and intervention as a fixed effect. Because age is strongly related to cortical thickness in this age group47, it was included as a covariate in the model.
3. Results
3.1. Blinding success.
Participants were asked at the end of each intervention arm to report whether they thought they had just completed the OCP arm or placebo arm. Of 23 who provided usable responses (3 declined to answer or reported that they thought both arms were the same intervention), 14 (61%) correctly identified which intervention arm they had completed, and 9 did not (39%), an effect that did not differ significantly from chance [χ2 (1, N = 23) = 0.67, p = 0.41].
3.2. OCP compliance and washout.
Participants using OCPs had serum progesterone levels that ranged from 0.1 to 0.9 ng/mL, indicating that ovulation was successfully suppressed in all participants during the OCP arm. By comparison, serum progesterone levels during the placebo arm ranged from 0.1 to 25.9 ng/mL. These serum progesterone levels differed significantly between the two intervention arms, F(1,49) = 25.2, p < 0.0001. Estradiol levels ranged from 31 to 592 pg/mL during the placebo arm, and 0 (undetectable) to 296 pg/mL during the OCP arm; these values differed significantly, p = 0.0002.
Estradiol (t(21) = 2.91, p = 0.10) and progesterone (t(21) = 0.77, p = 0.73) levels did not differ during the placebo pill phase regardless of the order of the intervention arms (AB vs. BA) – i.e., estradiol levels were not significantly different in women who had not undergone the OCP arm yet compared to those who had. This suggests that the washout period was sufficient to allow hormone levels return to their baseline.
3.3. Effect of intervention on mood and menstrual-related symptoms.
OCs significantly increased total score of self-reported symptoms on the DRSP, F(1,49) = 4.268, p = 0.0498, Cohen’s d = 0.45. Post hoc tests showing effects of the intervention on each item on the DRSP are reported in Table 2. DRSP symptoms were higher during the OCP arm compared to placebo, and reached statistical significance on items 10 (felt overwhelmed), 11 (physical symptoms), 12 (reduced productivity), and 13 (social avoidance).
Table 2:
Effects of OCPs on menstrual-related mood and physical symptoms.
| DRSP Item | OCP arm: Mean ± Standard Deviation | Placebo arm: Mean ± Standard Deviation | p-value (OCP arm vs. placebo arm) | Cohen’s d |
|---|---|---|---|---|
| 1. Felt depressed | 1.940 ± 0.919 | 1.625 ± 0.600 | 0.115 | 0.41 |
| 2. Felt anxious | 2.007 ± 0.895 | 1.831 ± 0.600 | 0.350 | 0.23 |
| 3. Mood swings | 1.998 ± 0.945 | 1.607 ± 0.609 | 0.075 | 0.49 |
| 4. Felt angry | 1.892 ± 0.890 | 1.630 ± 0.606 | 0.192 | 0.34 |
| 5. Less interest | 1.981 ± 0.958 | 1.622 ± 0.609 | 0.056 | 0.45 |
| 6. Difficulty concentrating | 2.014 ± 1.081 | 1.708 ± 0.619 | 0.123 | 0.35 |
| 7. Fatigue | 2.421 ± 1.202 | 2.150 ± 2.150 | 0.323 | 0.16 |
| 8. Increased appetite | 2.089 ± 1.181 | 1.789 ± 0.715 | 0.209 | 0.31 |
| 9. Slept more | 2.168 ± 1.330 | 1.875 ± 0.870 | 0.302 | 0.26 |
| 10. Felt overwhelmed | 1.830 ± 0.861 | 1.501 ± 0.523 | 0.048* | 0.46 |
| 11. Physical symptoms | 2.166 ± 1.022 | 1.475 ± 0.443 | 0.0009*** | 0.88 |
| 12. Reduced productivity | 1.920 ± 0.921 | 1.462 ± 0.496 | 0.014* | 0.62 |
| 13. Social avoidance | 1.671 ± 0.745 | 1.347 ± 0.399 | 0.050 | 0.54 |
| 14. Relationship problems | 1.598 ± 0.672 | 1.364 ± 0.478 | 0.136 | 0.40 |
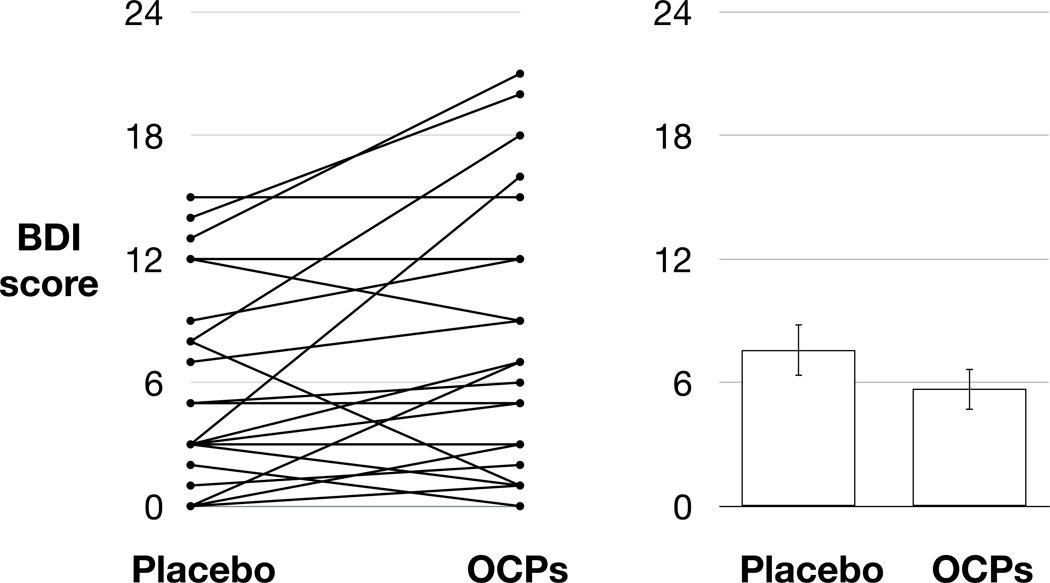
A linear mixed model including intervention arm (OC vs. placebo) as a fixed effect and participant as a random effect indicated that OCs also significantly increased symptoms of depression as assessed with the BDI, F(1,49)=6.12, p=0.0210, Cohen’s d = 0.33 (Figure 2).
Figure 2: OCPs significantly increased BDI scores.
The left panel plots individual scores during the placebo and OCP arms; the right panel depicts the mean (bars) and standard error of the mean (error bars) during each arm of the study.
3.4. Effects of OCPs on global brain structure.
A linear mixed model, entering intervention arm (OCP / placebo) as a fixed effect and participant as a random effect, with age as a covariate, showed no effect of OCPs on total intracranial volume (p = 0.19), mean cortical thickness (p = 0.15), or total cortical gray matter volume (p = 0.16).
3.5. Effects of OCPs on prefrontal cortical thickness.
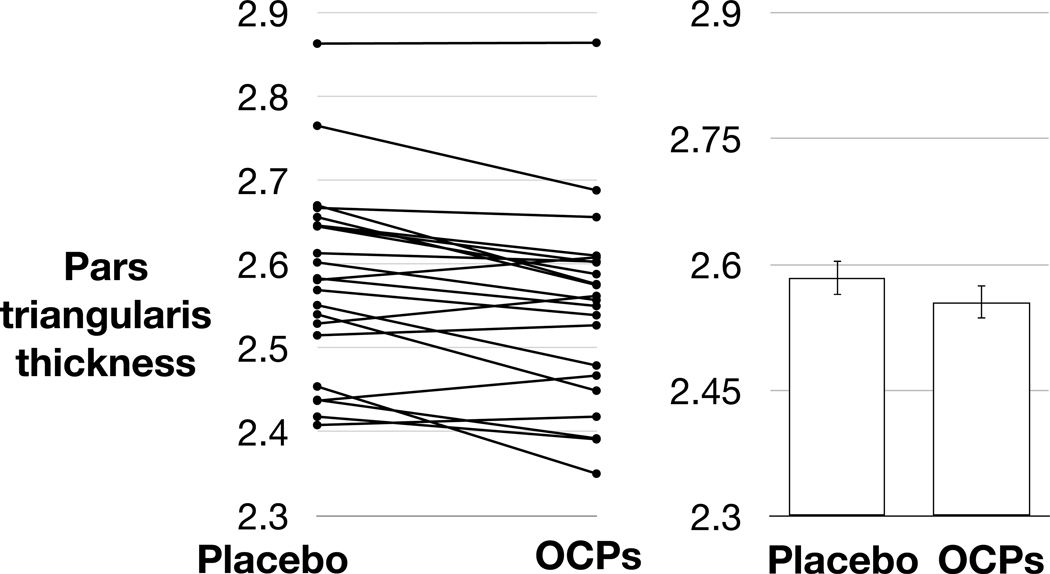
A linear mixed model, entering intervention arm (OCP / placebo) as a fixed effect and participant as a random effect, with age as a covariate, showed a significant reduction of cortical thickness in the right pars opercularis, right frontal pole, and bilateral pars triangularis when comparing the OCP arm to the placebo arm (p < 0.05, uncorrected) (see Table 3). Only the reduction in the right pars triangularis retained statistical significance after correcting for number of comparisons using the Benjamini-Hochberg procedure48 (Figure 3).
Table 3:
OCPs reduce prefrontal cortical thickness.In every prefrontal cortical subregion tested, in both hemispheres, thickness measurements were smaller during the OCP arm compared to placebo.
| Left hemisphere: OCPs vs placebo p-value (direction of effect); Cohen’s d | Right hemisphere: OCPs vs placebo p-value (direction of effect); Cohen’s d | |
|---|---|---|
| Prefrontal cortical subregion | ||
| caudal middle frontal | 0.52 (OCP < Placebo); 0.00 | 0.11 (OCP < Placebo); 0.08 |
| pars opercularis | 0.84 (Placebo < OCP); 0.07 | 0.008 (OCP < Placebo)‡; 0.30 |
| pars orbitalis | 0.13 (OCP < Placebo); 0.37 | 0.24 (OCP < Placebo); 0.15 |
| pars triangularis | 0.01 (OCP < Placebo) ‡; 0.37 | 0.001 (OCP < Placebo) Benjamini-Hochberg adjusted p-value = 0.018*; 0.26 |
| rostral middle frontal | 0.33 (OCP < Placebo); 0.16 | 0.15 (OCP < Placebo); 0.09 |
| superior frontal | 0.31 (OCP < Placebo); 0.03 | 0.26 (OCP < Placebo); 0.10 |
| frontal pole | 0.75 (OCP < Placebo); 0.04 | 0.03 (OCP < Placebo) ‡; 0.31 |
| lateral orbitofrontal | 0.09 (OCP < Placebo); 0.41 | 0.87 (OCP < Placebo); 0.11 |
| medial orbitofrontal | 0.80 (Placebo < OCP); 0.01 | 0.11 (OCP < Placebo); 0.30 |
This difference reached statistical significance, at α = 0.05, in the right pars opercularis, bilateral pars triangularis, and right frontal pole.
The effect in the right pars triangularis survived correction for multiple comparisons using the Benjamini-Hochberg false discovery rate adjustment.
Figure 3. OCPs reduce right pars triangularis thickness.
The left panel depicts pars triangularis thickness for each participant during the placebo and OCP arms. The right panel depicts mean (bars) pars triangularis thickness and standard error of the mean (error bars) during each intervention arm.
3.5. Exploratory tests of the relationship between cortical thickness and mood.
Tests were performed to evaluate possible relationships between mood symptoms and cortical thickness in the candidate regions identified by the structural analysis.
A linear mixed model entering right pars triangularis thickness as a fixed effect and participant as a random effect, with age entered as a covariate, indicated no significant linear relationship between pars triangularis thickness and BDI score (p = 0.59) or total DRSP score (p = 0.20). The relationship between right pars triangularis thickness and a DRSP item measuring physical menstrual-related symptoms (item 11) did reach significance only at the uncorrected level (F(1,49) = 8.23, p = 0.008; R2 = 0.15), with greater cortical thinning corresponding to more severe physical symptoms (breast tenderness, breast swelling, bloated sensation, weight gain, headache, joint or muscle pain, or other physical symptoms). No other symptoms correlated with right pars triangularis thickness (all ps > 0.05).
Entering the left pars triangularis into the model instead also showed no significant relationship between cortical thickness and BDI or total DRSP scores (ps > 0.05), and a significant negative relationship between cortical thickness and degree of social impairment (item 14; p = 0.04). This relationship did not survive correction for multiple comparisons.
Right pars opercularis and right frontal pole thickness did not correlate with BDI score or any DRSP symptoms (ps > 0.05).
4. Discussion
In this double-blind, placebo-controlled, randomized crossover trial, we tested the hypothesis that the use of OCPs is associated with a reduction in prefrontal cortical thickness, thereby adversely affecting cognitive control of emotion and producing the negative mood symptoms reported by some OCP users. In our sample of women with prior adverse mood effects, we found that 18–21 days of OCP use indeed reduced prefrontal cortical thickness, with the most pronounced effect in the right inferior frontal gyrus; OCPs also produced negative mood symptoms as self-reported on the BDI and DRSP. Contrary to our hypothesis, cortical thickness and mood symptoms were not related, suggesting that prefrontal cortical thinning is unlikely to be the mechanism by which OCPs affect mood, although this relationship may have been undetectable due to limitations outlined in more detail below. Exploratory tests suggested that OCP-induced changes in cortical thickness may instead be related to the severity of the experience of physical symptoms, such as breast tenderness and bloating, but this finding requires more study, as evaluating effects of OCPs on physical symptoms was not an a priori aim of this study. Inasmuch as the pars triangularis has an apparent role in pain processing (see meta-analysis 49), changes in pars triangularis thickness may reflect compensation for uncomfortable physical side effects produced by OCPs.
These findings are broadly consistent with one previous report of thinner prefrontal cortical regions in women using OCPs compared to those who are not16, and complement another hypothesis-driven study finding of smaller cortical regions in the right hippocampal / fusiform area in women who use OCs compared to naturally-cycling women50. Although other studies14, 17 have reported larger gray matter volumes in women who use OCs, it is likely that the discrepancy in findings is driven by the differences between the dozens of OCPs currently prescribed. Specifically, the progestins in OCPs are either androgenic or anti-androgenic, and at least one study has linked androgenic OCPs to smaller cortical regions, while use of anti-androgenic OCPs is linked to bigger cortical regions17. Consistent with these observations, the progestin used in this experiment (levonorgestrel) was androgenic, and cortical thinning resulted from using OCPs containing it.
Alternatively, discrepancies between studies may be related to differences in the samples selected, this study specifically having recruited women who reported adverse responses to OCPs. OCPs may produce different effects on brain structures in women who respond adversely to them and discontinue their use (as did the participants in this study) versus women who initiate and elect to continue OCP use (those tested in observational studies), producing a survivor bias in existing literature that would not appear here. This disparity between women who maintain or discontinue the use of OCPs may also be reflected in mood symptoms, as a recent study that evaluated BDI scores in women with ongoing OCP use found no significant differences between women who used OCPs and naturally-cycling controls15.
The inclusion criterion of having experienced worsening mood from use of OCPs was imposed to reduce variance in the group and to address the problem of adverse emotional responses to OCP use. The factors that predispose women to such responses are unknown, although research on other mental health conditions related to hormonal states may be informative. Women with premenstrual dysphoric disorder do not differ from healthy controls with respect to ovarian hormone levels, but growing evidence suggests that lower GABAA receptor plasticity in their brains may interact with ovarian hormone levels to produce affective symptoms (for review, see 51). Along these lines, brexanolone, a positive allosteric modulator of the GABAA receptor, has been used successfully to treat postpartum depression, again in the absence of any underlying difference in ovarian hormone levels in women with and without the condition52. No evidence indicates whether women with and without adverse emotional responses to OCPs share differences in GABA or other neurotransmitter signaling, but confirmatory evidence that OCPs lead to mood deterioration in some women emphasizes the need to identify factors that distinguish these women from those who respond positively or neutrally to OCPs.
This study detected relatively rapid changes in brain structure that occurred within 18–21 days of initiating OCP use, but provides no more granular understanding of the timeline of these effects than that they occur within 0–21 days. It is also unclear how long these changes persist, and whether they persist long-term. Long-term or permanent effects may be of a greater concern than transient ones. At least one investigation has demonstrated that longer durations of OCP use correspond to larger effects on gray matter volume17.
The biological plausibility of such rapid changes must be considered. Animal studies have revealed that exogenous estrogen administration can induce morphological changes in neural tissue, including dendritic growth, spinogenesis, and synaptogenesis, within 1–6 days53, and loss of synaptic spine density can be observed within 7 days following treatment with letrozole (an aromatase inhibitor that reduces estrogen production)54. In fact, the onset of these effects may be even more rapid, with some evidence showing that changes in dendritic spine density following progesterone treatment occurring within 2 h of administration25. Although it is not evident from structural MR images which, if any, of these phenomena produce the changes in cortical thickness measurements described here, such changes could occur on the timescale measured (18–21 days). Reverse translational studies in animal models could be helpful in clarifying the mechanism supporting OCP-induced changes in brain morphology observed here with structural MRI.
It is possible that the relatively rapid change in endogenous hormone levels produced by OCPs is responsible for the relatively rapid change in brain structure observed, and that a homeostatic process ultimately restores the brain to its pre-OCP state at some time >21 days after initiation of OCP use. However, this possibility has not been specifically investigated. Some efforts have been made to evaluate the duration of OCP effects after discontinuation, and one such investigation, which focused on the hippocampus and basal ganglia rather than prefrontal cortex, found a relationship between time since discontinuation and hippocampal volume but not basal ganglia volume55. This observation implies that discontinuation effects will differ by brain region, and that effects in some regions may be enduring. Rigorous investigations are needed to document the time course of OCP discontinuation effects.
The lack of information regarding timing (onset and termination of effects) represents one of the major limitations of this study. The relatively small sample (N = 26 completers) is another limitation, especially insofar as it constrained our analyses to evaluating regions where strong a priori hypotheses could be made. The population tested was also restricted to women with a history of adverse mood on OCPs, which restricts the generalizability of these findings. However, the use of a within-subject design increased our statistical power. The study also benefited from double-blinding, randomized order of treatment, and a placebo control. The use of a single oral contraceptive is both a strength and a weakness of the study -- dozens of formulations are prescribed clinically, and reducing the investigation to a single combination reduces the generalizability of the study. However, it also likely reduces the variance, increasing our ability to detect effects, and reduces some ambiguity in the interpretation of results compared to studies that combine many different formulations. In such studies, it is an open question as to whether effects are driven by subgroups of OC types. Finally, the lack of pre-registered hypotheses is another limitation of the study.
This investigation provides evidence that the use of OCPs is associated with changes in mood and brain structure, specifically reductions in cortical thickness in the right pars triangularis, in some women. Importantly, these are two distinct effects of OCPs and the effect on pars triangularis thickness does not explain the change in mood. This and the other limitations of the study highlight the need for more research in this area, especially focusing on the time-course of effects of OCPs and effects of different formulations.
Although tremendous strides have been made in developing safe, effective, and reversible contraceptives, unplanned pregnancies remain a major public health issue, as nearly half (45%)56 of pregnancies in the US are unintended. Knowledge of adverse unintended effects is critical to informed consent, and relevant to the 150 million women worldwide who currently use OCPs57. Identifying specific formulations that exacerbate these effects, and/or populations at risk for experiencing them, advances women’s health and thereby public health. We emphasize the need for screening to identify women at risk for adverse effects, and not a need to avoid or prematurely discontinue the use of OCPs out of concern that such effects may develop.
Supplementary Material
Acknowledgments
This work was supported by NIDA (R21DA040168 to EDL), the Marjorie Greene Family Trust, and the Thomas P and Katherine K Pike Chair in Addiction Studies (EDL).
Footnotes
conflicts of interest: We declare no conflicts of interest.
REFERENCES
- 1.Taggart TC, Eaton NR, Keyes KM, Hammett JF, Ulloa EC. Oral contraceptive use is associated with greater mood stability and higher relationship satisfaction. Neurology, Psychiatry and Brain Research 2018; 30: 154–162. [Google Scholar]
- 2.Hamstra DA, de Kloet ER, de Rover M, Van der Does W. Oral contraceptives positively affect mood in healthy PMS-free women: A longitudinal study. Journal of Psychosomatic Research 2017; 103: 119–126. [DOI] [PubMed] [Google Scholar]
- 3.Cheslack-Postava K, Keyes KM, Lowe SR, Koenen KC. Oral contraceptive use and psychiatric disorders in a nationally representative sample of women. Archives of Women’s Mental Health 2015; 18(1): 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keyes KM, Cheslack-Postava K, Westhoff C, Heim CM, Haloossim M, Walsh K et al. Association of Hormonal Contraceptive Use With Reduced Levels of Depressive Symptoms: A National Study of Sexually Active Women in the United States. American Journal of Epidemiology 2013; 178(9): 1378–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skovlund CW, Mørch LS, Kessing LV, Lidegaard Ø. Association of Hormonal Contraception With Depression. JAMA Psychiatry 2016; 73(11): 1154–1162. [DOI] [PubMed] [Google Scholar]
- 6.Skovlund CW, Mørch LS, Kessing LV, Lange T, Lidegaard Ø. Association of hormonal contraception with suicide attempts and suicides. American Journal of Psychiatry 2018; 175(4): 336–342. [DOI] [PubMed] [Google Scholar]
- 7.de Wit AE, Booij SH, Giltay EJ, Joffe H, Schoevers RA, Oldehinkel AJ. Association of Use of Oral Contraceptives With Depressive Symptoms Among Adolescents and Young Women. JAMA Psychiatry 2020; 77(1): 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shakerinejad G, Hidarnia A, Motlagh ME, Karami K, Niknami S, Montazeri A. Factors predicting mood changes in oral contraceptive pill users. Reproductive Health 2013; 10(1): 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westhoff CL, Heartwell S, Edwards S, Zieman M, Stuart G, Cwiak C et al. Oral contraceptive discontinuation: do side effects matter? American Journal of Obstetrics and Gynecology 2007; 196(4): 412. e411–412. e417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiebe ER, Brotto LA, MacKay J. Characteristics of women who experience mood and sexual side effects with use of hormonal contraception. Journal of Obstetrics and Gynaecology Canada 2011; 33(12): 1234–1240. [DOI] [PubMed] [Google Scholar]
- 11.Lobo RA, Stanczyk FZ. New knowledge in the physiology of hormonal contraceptives. Am J Obstet Gynecol 1994; 170(5 Pt 2): 1499–1507. [DOI] [PubMed] [Google Scholar]
- 12.Collins DC. Sex hormone receptor binding, progestin selectivity, and the new oral contraceptives. Am J Obstet Gynecol 1994; 170(5 Pt 2): 1508–1513. [DOI] [PubMed] [Google Scholar]
- 13.Fleischman DS, Navarrete CD, Fessler DMT. Oral Contraceptives Suppress Ovarian Hormone Production. Psychological Science 2010; 21(5): 750–752. [DOI] [PubMed] [Google Scholar]
- 14.Pletzer B, Kronbichler M, Aichhorn M, Bergmann J, Ladurner G, Kerschbaum HH. Menstrual cycle and hormonal contraceptive use modulate human brain structure. Brain Research 2010; 1348: 55–62. [DOI] [PubMed] [Google Scholar]
- 15.Sharma R, Smith SA, Boukina N, Dordari A, Mistry A, Taylor BC et al. Use of the birth control pill affects stress reactivity and brain structure and function. Hormones and Behavior 2020; 124: 104783. [DOI] [PubMed] [Google Scholar]
- 16.Petersen N, Touroutoglou A, Andreano JM, Cahill L. Oral contraceptive pill use is associated with localized decreases in cortical thickness. Human Brain Mapping 2015; 36(7): 2644–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pletzer B, Kronbichler M, Kerschbaum H. Differential effects of androgenic and anti-androgenic progestins on fusiform and frontal gray matter volume and face recognition performance. Brain Research 2015; 1596: 108–115. [DOI] [PubMed] [Google Scholar]
- 18.Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Natl Acad Sci U S A 2001; 98(23): 13391–13395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu F, Day M, Muñiz LC, Bitran D, Arias R, Revilla-Sanchez R et al. Activation of estrogen receptor-β regulates hippocampal synaptic plasticity and improves memory. Nature Neuroscience 2008; 11(3): 334–343. [DOI] [PubMed] [Google Scholar]
- 20.Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Hormones and Behavior 1998; 34(2): 140–148. [DOI] [PubMed] [Google Scholar]
- 21.Brinton RD. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends in Pharmacological Sciences 2009; 30(4): 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haraguchi S, Sasahara K, Shikimi H, Honda S-i, Harada N, Tsutsui K. Estradiol promotes purkinje dendritic growth, spinogenesis, and synaptogenesis during neonatal life by inducing the expression of BDNF. The Cerebellum 2012; 11(2): 416–417. [DOI] [PubMed] [Google Scholar]
- 23.Fester L, Rune GM. Sexual neurosteroids and synaptic plasticity in the hippocampus. Brain Research 2015; 1621: 162–169. [DOI] [PubMed] [Google Scholar]
- 24.Sato K, Akaishi T, Matsuki N, Ohno Y, Nakazawa K. β-Estradiol induces synaptogenesis in the hippocampus by enhancing brain-derived neurotrophic factor release from dentate gyrus granule cells. Brain research 2007; 1150: 108–120. [DOI] [PubMed] [Google Scholar]
- 25.Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. Journal of Comparative Neurology 1993; 336(2): 293–306. [DOI] [PubMed] [Google Scholar]
- 26.Naftolin F, Mor G, Horvath TL, Luquin S, Fajer AB, Kohen F et al. Synaptic remodeling in the arcuate nucleus during the estrous cycle is induced by estrogen and precedes the preovulatory gonadotropin surge. Endocrinology 1996; 137(12): 5576–5580. [DOI] [PubMed] [Google Scholar]
- 27.Parducz A, Perez J, Garcia-Segura L. Estradiol induces plasticity of GABAergic synapses in the hypothalamus. Neuroscience 1993; 53(2): 395–401. [DOI] [PubMed] [Google Scholar]
- 28.Inghilleri M, Conte A, Curra A, Frasca V, Lorenzano C, Berardelli A. Ovarian hormones and cortical excitability. An rTMS study in humans. Clinical neurophysiology 2004; 115(5): 1063–1068. [DOI] [PubMed] [Google Scholar]
- 29.Lee S, Chung SW, Rogasch NC, Thomson CJ, Worsley RN, Kulkarni J et al. The influence of endogenous estrogen on transcranial direct current stimulation: a preliminary study. European Journal of Neuroscience 2018; 48(4): 2001–2012. [DOI] [PubMed] [Google Scholar]
- 30.Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM. Effects of ovarian hormones on human cortical excitability. Annals of neurology 2002; 51(5): 599–603. [DOI] [PubMed] [Google Scholar]
- 31.Bixo M, Andersson A, Winblad B, Purdy RH, Bäckström T. Progesterone, 5alpha-pregnane-3,20-dione and 3alpha-hydroxy-5alpha-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain Res 1997; 764(1–2): 173–178. [DOI] [PubMed] [Google Scholar]
- 32.Bixo M, Bäckström T, Winblad B, Andersson A. Estradiol and testosterone in specific regions of the human female brain in different endocrine states. J Steroid Biochem Mol Biol 1995; 55(3–4): 297–303. [DOI] [PubMed] [Google Scholar]
- 33.Matochik JA, London ED, Yildiz BO, Ozata M, Caglayan S, DePaoli AM et al. Effect of leptin replacement on brain structure in genetically leptin-deficient adults. J Clin Endocrinol Metab 2005; 90(5): 2851–2854. [DOI] [PubMed] [Google Scholar]
- 34.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nature Reviews Neuroscience 2009; 10(9): 647–658. [DOI] [PubMed] [Google Scholar]
- 35.Videbech P, Yttri JE. [The effect of antidepressants on brain volume]. Ugeskr Laeger 2019; 181(38). [PubMed] [Google Scholar]
- 36.Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences 2012; 1251: E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz JL, Creinin MD, Pymar HC, Reid L. Predicting risk of ovulation in new start oral contraceptive users1 1The authors gratefully acknowledge Leslie A. Meyn, MS, for her statistical and editorial support. Obstetrics & Gynecology 2002; 99(2): 177–182. [DOI] [PubMed] [Google Scholar]
- 38.Danforth DR, Hodgen GD. “Sunday start” multiphasic oral contraception: ovulation prevention and delayed follicular atresia in primates. Contraception 1989; 39(3): 321–330. [DOI] [PubMed] [Google Scholar]
- 39.Killick S, Eyong E, Elstein M. Ovarian follicular development in oral contraceptive cycles**Supported by Wyeth International, Philadelphia, Pennsylvania. Fertility and Sterility 1987; 48(3): 409–413. [DOI] [PubMed] [Google Scholar]
- 40.Hoogland HJ, Skouby SO. Ultrasound evaluation of ovarian activity under oral contraceptives. Contraception 1993; 47(6): 583–590. [DOI] [PubMed] [Google Scholar]
- 41.Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Archives of Women’s Mental Health 2006; 9(1): 41–49. [DOI] [PubMed] [Google Scholar]
- 42.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review 1988; 8(1): 77–100. [Google Scholar]
- 43.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999; 9(2): 179–194. [DOI] [PubMed] [Google Scholar]
- 44.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; 31(3): 968–980. [DOI] [PubMed] [Google Scholar]
- 45.Fischl B, Van Der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH et al. Automatically parcellating the human cerebral cortex. Cerebral Cortex 2004; 14(1): 11–22. [DOI] [PubMed] [Google Scholar]
- 46.Klapwijk ET, Van De Kamp F, Van Der Meulen M, Peters S, Wierenga LM. Qoala-T: A supervised-learning tool for quality control of FreeSurfer segmented MRI data. Neuroimage 2019; 189: 116–129. [DOI] [PubMed] [Google Scholar]
- 47.Tamnes CK, Østby Y, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. Brain Maturation in Adolescence and Young Adulthood: Regional Age-Related Changes in Cortical Thickness and White Matter Volume and Microstructure. Cerebral Cortex 2009; 20(3): 534–548. [DOI] [PubMed] [Google Scholar]
- 48.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological) 1995; 57(1): 289–300. [Google Scholar]
- 49.Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage 2011; 54(3): 2492–2502. [DOI] [PubMed] [Google Scholar]
- 50.Pletzer B. Sex hormones and gender role relate to grey matter volumes in sexually dimorphic brain areas. Frontiers in neuroscience 2019; 13: 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hantsoo L, Epperson CN. Allopregnanolone in premenstrual dysphoric disorder (PMDD): Evidence for dysregulated sensitivity to GABA-A receptor modulating neuroactive steroids across the menstrual cycle. Neurobiology of Stress 2020; 12: 100213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walton N, Maguire J. Allopregnanolone-based treatments for postpartum depression: Why/how do they work? Neurobiology of Stress 2019; 11: 100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sasahara K, Shikimi H, Haraguchi S, Sakamoto H, Honda S-i, Harada N et al. Mode of action and functional significance of estrogen-inducing dendritic growth, spinogenesis, and synaptogenesis in the developing Purkinje cell. Journal of Neuroscience 2007; 27(28): 7408–7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fester L, Prange-Kiel J, Zhou L, Blittersdorf Bv, Böhm J, Jarry H et al. Estrogen-regulated synaptogenesis in the hippocampus: sexual dimorphism in vivo but not in vitro. The Journal of steroid biochemistry and molecular biology 2012; 131(1–2): 24–29. [DOI] [PubMed] [Google Scholar]
- 55.Pletzer B, Harris T, Hidalgo-Lopez E. Previous contraceptive treatment relates to grey matter volumes in the hippocampus and basal ganglia. Scientific Reports 2019; 9(1): 11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. New England Journal of Medicine 2016; 374(9): 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nations U. Contraceptive Use by Method 2019: Data Booklet (ST/ESA/SER.A/435). Department of Economic and Social Affairs, Population Division 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.