Abstract
Our objective was to explore the longitudinal trajectory of HbA1c in well-characterized youth (n=84) with normal weight and obesity during puberty. HbA1c rose from early puberty to Tanner 5, even in healthy, normal weight youth, revealing important implications for defining normal glycemia and prediabetes in adolescents.
With the rise in pediatric obesity, has come a parallel rise in youth-onset type 2 diabetes (T2D) incidence, with devistating consequences for those affected (1). However, even among those with risk factors, pediatric T2D incidence remains relatively low, hampering our ability to understand factors influencing progression to T2D. Large studies would be required to longitudinally define youth-specific predictors of T2D, including defining glycemic cut-points (ie, prediabetes) predicting progression. More fundamentally, little is known about the glycemic trajectory in adolescence and the impact of physiologic transient pubertal changes in insulin sensitivity and secretion. In a large cross-sectional study of 6th – 8th graders (HEALTHY Study), we demonstrated that the HbA1c normal distribution extends into the prediabetes range in healthy, normal weight adolescents (2). In fact, 2% of lean youth in HEALTHY had a prediabetes-range HbA1c; the highest among African American youth.
The Health Influences of Puberty (HIP) Study was designed to assess changes in insulin sensitivity and secretion in youth with normal weight and obesity as they progressed from early puberty to Tanner stage 5 (T5). HIP demonstrated that, though youth with obesity were substantially more insulin resistant during puberty, their compensatory insulin response, estimated by disposition index (DI), was similar to normal weight youth (3). However, in the HIP both insulin sensitivity and DI declined between early puberty and early T5, regardless of BMI. These data indicate that puberty affects both insulin sensitivity and secretion in normal-weight, healthy, youth.
A secondary objective was to assess the impact of puberty on glycemia over time and explore potential factors associated with HbA1c change during puberty. Our hypotheses were that HbA1c would rise during puberty and would be inversely associated with DI.
Methods:
The HIP study was approved by the Colorado Multiple Institutional Review Board and consent and assent were obtained from all participants. Detailed methods were previously published (3–5). Briefly, youth with normal weight (BMI 5th-85th%ile for age and sex) and obesity (BMI ≥ 95th%ile) recruited from general pediatrics and weight management clinics at Children’s Hospital Colorado and the surrounding community were enrolled in early puberty (T2–3). Exclusion criteria included: known history of diabetes, impaired glucose tolerance or impaired fasting glucose during an oral glucose tolerance test, dyslipidemia or hypertension requiring pharmacological intervention, genetic syndromes, other disorders or medications known to impact glucose metabolism or weight gain, current or recent use of an insulin sensitizer, proteinuria, or weight >300 pounds.
Tanner staging was performed by a pediatric endocrinologist every 6 months. Testicular volume was also assessed using a Prader orchidometer and assigned a Tanner stage equivalent4. Primary outcome visits occurred at 3 time points: Baseline (T2–3), T4, and T5, preceded by a 3-day standard macronutrient diet provided by the CU-Anschutz CTRC’s metabolic kitchen and 3 days of exercise restriction. Visits included frequently-sampled intravenous glucose tolerance testing (fsIVGTT), fasting laboratory studies, and dual x-ray absorptiometry (DXA) to measure percent fat mass. fsIVGTT and laboratory methodology were previously published3, 4. HbA1c was performed by high-performance liquid chromatography (Bio-Rad Variant TURBO, California, USA). Si, AIRg and DI were calculated using the Bergman minimal model5.
The analyses reported here are secondary and exploratory. Groups were compared using t-tests or the Mann-Whitney test for continuous variables, and chi-square or Fisher exact test for categorical variables. Linear models (unadjusted and adjusted for sex, race/ethnicity, baseline Tanner stage, and change in %fat over time) tested group differences in change in HbA1c from baseline (T2–3) to the end of study (T5). Mixed models assessed whether factors chosen based on their known contribution to puberty or to glycemia were associated with HbA1c at each time point. Factors included in the model were: leptin, adiponectin, highly sensitive c-reactive protein, dehydroepiandrosterone-sulfate, DI, and insulin-like growth factor-1.
Results:
Eight-four youth enrolled in HIP. Baseline demographic, anthropometric and laboratory characteristics are shown in the Table. As expected, youth with obesity had a higher BMI z-score, %fat and leptin, and lower insulin sensitivity and adiponectin. Fasting glucose was significantly higher in youth with obesity, but HbA1c was not statistically different (unadjusted).
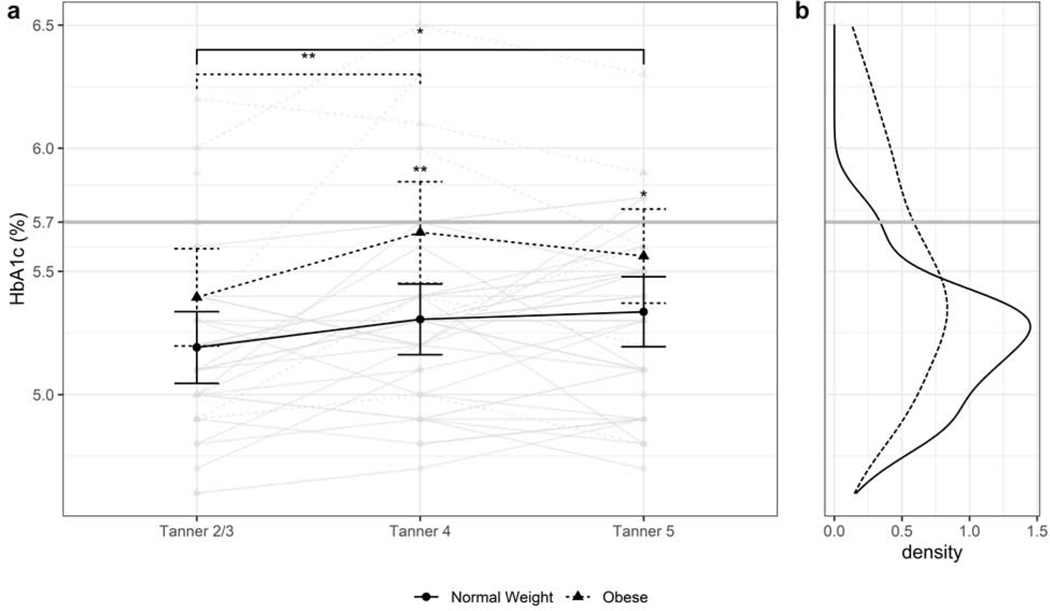
The Figure shows change in HbA1c over time (a), and distribution of HbA1c (b) in youth with normal weight and obesity. In the overall cohort, HbA1c rose between baseline (T2/3) and T4 (β=0.19±0.05, P < .001) and T5 (β=0.16±0.05, p=0.003), but did not change significantly between T4 and T5 (β=−0.03±0.04, p=0.79). HbA1c increased significantly in youth with normal weight at T4 (β=0.11±0.05, p=0.03) and T5 (β=0.14±0.05, p=0.006) compared with baseline, but not in youth with obesity. HbA1c was, however, higher in youth with obesity vs. normal weight at T4 (5.6±0.08% vs. 5.2±0.06%, p<0.001) and at T5 (5.5±0.08% vs. 5.2±0.06%, p=0.01). Importantly, although the overall HbA1c distribution was wider in youth with obesity, the tail crossed the cut-point for diagnosis of prediabetes (5.7%) in both cohorts. Time-dependent results were unchanged after adjusting for sex, race/ethnicity and after adding %fat to the model. However, group contrasts at each time point were no longer significant after adjusting for %fat.
DI was not significantly associated with HbA1c in either univariate regression (β=0.00006±0.00001, p=0.64) or in mixed models that included factors preselected to potentially contribute to changing HbA1c over time (β=0.00002±0.00001, p=0.23). Results were the same before and after adjusting for sex and race/ethnicity. In the mixed models, adjusted for sex and race/ethnicity, higher leptin (β=0.11±0.04, p=0.01) and lower adiponectin (β=−0.06±0.03, p=0.03) were significantly associated with higher HbA1c. After adding %fat to the model, the effect of adiponectin was similar (β=−0.07±0.03, p=0.01), but leptin was no longer significant (β=0.11±0.06, p=0.07).
Discussion:
This longitudinal analysis of a small, but deeply characterized, cohort extends our previous findings (3) that a subset of otherwise healthy youth experience mild elevation in HbA1c in adolescence. Moreover, our findings suggest that this HbA1c elevation may relate to pubertal changes in adipose tissue metabolism. These findings have important clinical implications. First, they raise a critical question about the definitions of normal and abnormal glycemia during adolescence. In particular, if HbA1c, and by extension glycemia, increases in all youth during adolescence, then the current criteria for prediabetes in adolescents based on adult norms may not be valid. Although HbA1c was, on average, higher in youth with obesity in HIP, the overall distribution overlapped between youth with normal weight and obesity. Thus, a “prediabetes-range” HbA1c may not mean the same thing in youth, even those with obesity, as it does in adults. Because only a small subset of youth with obesity progress to T2D and youth-onset T2D behaves more aggressively than adult-onset, it is critical to define risk for progression specific to adolescents. Better defining normal glycemia and prediabetes is a critical first step in developing more reliable criteria for T2D risk in youth.
Our finding that the rise in HbA1c did not relate to DI— an estimate of β-cell function relative to insulin sensitivity—was surprising, particularly given that we previously demonstrated that DI declines during puberty, both in youth with normal weight and with obesity (3). It is important to note that DI was based on IVGTT in HIP, whereas HbA1c represents glycemia in free-living conditions. Thus, post-prandial glucose, which contributes strongly to HbA1c, may play a large role in average glycemia during puberty, perhaps due to an altered incretin response or higher dietary glucose intake in youth vs. adults. HbA1c did correlate with adipokines. Leptin, which showed a positive association, is thought to help regulate glucose homeostasis (6), though it is typically thought to lower glucose in the healthy state. Leptin increases in all youth upon entry into puberty. In girls, leptin continues to rise throughout puberty, whereas in boys, leptin subsequently falls as puberty progresses (7). It is certainly possible that metabolic leptin resistance occurs during puberty, but further study is needed. Adiponectin is typically positively associated with insulin sensitivity, but a direct relationship with glycemia and adiponectin is less clear. Growth hormone is also elevated during puberty, which during fasting stimulates lipolysis, elevates free fatty acids and induces insulin resistance (8), which may impact different factors than assessed by IVGTT. Another potential explanation for changing glycemia during puberty is altered insulin clearance, which is affected by race (9, 10), is lower in obese youth than in obese adults (11), and correlates with declines in β-cell function in youth (12). However, little is currently known about how insulin clearance normally changes during childhood. Finally, changes in glucose mediated glucose uptake may play a role in average glycemia during puberty, another area in need of exploration.
Limitations of HIP include the relatively small sample size, the absence of continuous glucose monitoring to track free-living glycemic patterns, and lack of repeated oral glucose tolerance testing over time. It is also possible that IVGTT may not be sensitive enough to detect subtle changes in DI. Moreover, race and ethnicity may impact glycemia (13) as well as measurement of HbA1c (14), but the racial and ethnic differences between groups confound further analyses. Thus, more information is needed in future studies to determine the specific characteristics of the pubertal changes in average glycemia we have demonstrated.
In summary, in a longitudinal study of youth with normal weight and obesity, we found that average glycemia increases as puberty progresses, irrespective of BMI. This finding has important implications for defining prediabetes based on HbA1c in youth. Further studies are needed to better understand mediators of glucose homeostasis during puberty and define normal glycemia during adolescence.
Figure legend:
(a) Individual and mean changes in HbA1c in youth with normal weight or obesity. Mean HbA1c is significantly higher in youth with obesity at T4 and T5. In youth with normal weight, but not obesity, there is a significant rise in HbA1c from baseline to T4 and Tanner 5, suggesting an effect of puberty itself of normal glycemia. (b) Distribution of HbA1c in youth with normal weight and obesity. A proportion of youth in both groups experience an HbA1c above the American Diabetes Association-defined cut-point for prediabetes. *p<0.05, **p<0.01, ***p<0.001
Table 1:
Baseline study characteristics
| Variable* | Normal Weight | Obesity | p-value |
|---|---|---|---|
| N | 47 | 37 | |
| Race / Ethnicity n (%) | 0.001 | ||
| Hispanic | 12 (25.5) | 26 (70.3) | |
| White, Non-Hispanic | 23 (48.9) | 6 (16.2) | |
| Black | 8 (17.0) | 3 ( 8.1) | |
| Asian | 3 ( 6.4) | 0 ( 0.0) | |
| Other | 1 ( 2.1) | 2 ( 5.4) | |
| Age (years) | 12.0 (1.5) | 11.3 (1.2) | 0.02 |
| BMI Z-score | −0.01 (0.71) | 2.89 (1.08) | <0.001 |
| Tanner Stage n (%) | 0.94 | ||
| Tanner 2 | 29 (62) | 24 (65) | |
| Tanner 3 | 18 (38) | 13 (35) | |
| HbAlc (%) | 5.14 (0.25) | 5.34 (0.49) | 0.09 |
| Body fat (%) | 25.1 (6.4) | 41.31 (6.94) | <0.001 |
| Physical Activity (METS)^ | 65.66 (13.13) | 66.20 (14.26) | 0.86 |
| Insulin Sensitivity (Si, x10-4/min-1/mIU/mL ) | 8.49 (5.80) | 3.66 (4.36) | <0.001 |
| Insulin Secretion (AIRg, pIU/mL) | 591 (435) | 1831 (1072) | <0.001 |
| Disposition Index (DI, x10-4/min-1) | 3770(1639) | 4159 (1731) | 0.31 |
| Triglycerides (mg/dL) | 75.93 (38.28) | 106.30 (48.12) | 0.003 |
| HDL (mg/dL) | 50.98 (8.48) | 40.16 (8.27) | <0.001 |
| LDL (mg/dL) | 79.34 (22.94) | 86.51 (22.23) | 0.16 |
| Systolic Blood Pressure (mmHg) | 110.13 (9.68) | 118.03 (12.28) | 0.002 |
| Diastolic Blood Pressure (mmHg) | 67.02 (7.71) | 66.45 (7.89) | 0.75 |
| Fasting Glucose (mg/dL) | 83.09 (7.90) | 89.86 (16.63) | 0.02 |
| AST (IU/L) | 42.49 (14.69) | 40.89 (18.01) | 0.66 |
| ALT (IU/L) | 25.13 (9.63) | 34.78 (21.96) | 0.01 |
| IGF-1 (ng/mL) | 315.64 (103.38) | 260.95 (91.00) | 0.01 |
| Leptin (ng/mL) | 6.03 (5.66) | 31.30 (14.28) | <0.001 |
| Adiponectin (pg/mL) | 12.11 (4.40) | 9.57 (5.22) | 0.02 |
| CRP (mg/L) | 1.02 (2.63) | 6.28 (23.41) | 0.13 |
| DHEA-S (pg/dL) | 90.79 (61.01) | 95.76 (62.99) | 0.72 |
Data have been previously published1
All data are listed as mean ±SD unless otherwise specified;
estimated from the 3-day Physical Activity Recall Questionnaire2
Kelsey MM, Pyle L, Hilkin A, et al. The Impact of Obesity On Insulin Sensitivity and Secretion During Pubertal Progression: A Longitudinal Study. J Clin Endocrinol Metab. 2020;105(5).
Weston AT, Petosa RICH, Pate RR. Validation of an instrument for measurement of physical activity in youth. [Article]. Medicine & Science in Sports & Exercise. 1997;29(1):138–143.
Acknowledgments
The Health Influence of Puberty (HIP) Study was sponsored by the following grants: American Diabetes Association Junior Faculty Award (1–11-JF-23), Children’s Hospital Colorado Research Institute Research Scholar Award, Building Interdisciplinary Research Careers in Women’s Health NIH/NICHD BIRCWH K12 (HD057022–06), NIH/NCATS Colorado CTSA UL1 TR001082, Nutrition and Obesity Research Center Pilot Award NIH/NIDDK DK048520–13, Children’s Hospital Colorado Research Institute Bridge Award, University of Colorado School of Medicine Dean’s Bridge Award.
Portions of this study were presented at the virtual American Diabetes Association Meeting, June << >>, 2020.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. New England Journal of Medicine. 2017;376(15):1419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juras R, Tanner-Smith E, Kelsey M, Lipsey M, Layzer J. Adolescent Pregnancy Prevention: Meta-Analysis of Federally Funded Program Evaluations. American journal of public health. 2019;109(4):e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelsey MM, Pyle L, Hilkin A, Severn CD, Utzschneider K, Van Pelt RE, et al. The Impact of Obesity On Insulin Sensitivity and Secretion During Pubertal Progression: A Longitudinal Study. The Journal of clinical endocrinology and metabolism. 2020;105(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nokoff N, Thurston J, Hilkin A, Pyle L, Zeitler P, Nadeau KJ, et al. Sex Differences in Effects of Obesity on Reproductive Hormones and Glucose Metabolism in Early Puberty. The Journal of clinical endocrinology and metabolism. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelsey MM, Pyle L, Zeitler PS, Hilkin A, Utzschneider K, van Pelt RE, et al. The impact of obesity on insulin sensitivity and secretion during pubertal progression: a longitudinal study [Internet]. Figshare 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Souza A M, Neumann UH, Glavas MM, Kieffer TJ. The glucoregulatory actions of leptin. Mol Metab. 2017;6(9):1052–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blum WF, Englaro P, Hanitsch S, Juul A, Hertel NT, Muller J, et al. Plasma leptin levels in healthy children and adolescents: dependence on body mass index, body fat mass, gender, pubertal stage, and testosterone. The Journal of clinical endocrinology and metabolism. 1997;82(9):2904–10. [DOI] [PubMed] [Google Scholar]
- 8.Caprio S, Cline G, Boulware S, Permanente C, Shulman GI, Sherwin RS, et al. Effects of puberty and diabetes on metabolism of insulin-sensitive fuels. The American journal of physiology. 1994;266(6 Pt 1):E885–91. [DOI] [PubMed] [Google Scholar]
- 9.Gower BA, Granger WM, Franklin F, Shewchuk RM, Goran MI. Contribution of insulin secretion and clearance to glucose-induced insulin concentration in african-american and caucasian children. The Journal of clinical endocrinology and metabolism. 2002;87(5):2218–24. [DOI] [PubMed] [Google Scholar]
- 10.Uwaifo GI, Parikh SJ, Keil M, Elberg J, Chin J, Yanovski JA. Comparison of insulin sensitivity, clearance, and secretion estimates using euglycemic and hyperglycemic clamps in children. The Journal of clinical endocrinology and metabolism. 2002;87(6):2899–905. [DOI] [PubMed] [Google Scholar]
- 11.Metabolic Contrasts Between Youth and Adults With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes: I. Observations Using the Hyperglycemic Clamp. Diabetes Care. 2018;41(8):1696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galderisi A, Polidori D, Weiss R, Giannini C, Pierpont B, Trico D, et al. Lower Insulin Clearance Parallels a Reduced Insulin Sensitivity in Obese Youths and Is Associated With a Decline in beta-Cell Function Over Time. Diabetes. 2019;68(11):2074–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saaddine JB, Fagot-Campagna A, Rolka D, Narayan KM, Geiss L, Eberhardt M, et al. Distribution of HbA(1c) levels for children and young adults in the U.S.: Third National Health and Nutrition Examination Survey. Diabetes Care. 2002;25(8):1326–30. [DOI] [PubMed] [Google Scholar]
- 14.Bergenstal RM, Gal RL, Connor CG, Gubitosi-Klug R, Kruger D, Olson BA, et al. Racial Differences in the Relationship of Glucose Concentrations and Hemoglobin A1c Levels. Annals of internal medicine. 2017;167(2):95–102. [DOI] [PubMed] [Google Scholar]