Abstract
Metabolic disorders that include diabetes mellitus present significant challenges for maintaining the welfare of the global population. Metabolic diseases impact all systems of the body and despite current therapies that offer some protection through tight serum glucose control, ultimately such treatments cannot block the progression of disability and death realized with metabolic disorders. As a result, novel therapeutic avenues are critical for further development to address these concerns. An innovative strategy involves the vitamin nicotinamide and the pathways associated with the silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1), the mechanistic target of rapamycin (mTOR), mTOR Complex 1 (mTORC1), mTOR Complex 2 (mTORC2), AMP activated protein kinase (AMPK), and clock genes. Nicotinamide maintains an intimate relationship with these pathways to oversee metabolic disease and improve glucose utilization, limit mitochondrial dysfunction, block oxidative stress, potentially function as antiviral therapy, and foster cellular survival through mechanisms involving autophagy. However, the pathways of nicotinamide, SIRT1, mTOR, AMPK, and clock genes are complex and involve feedback pathways as well as trophic factors such as erythropoietin that require a careful balance to ensure metabolic homeostasis. Future work is warranted to gain additional insight into these vital pathways that can oversee both normal metabolic physiology and metabolic disease.
Keywords: Alzheimer’s disease, AMP activated protein kinase (AMPK), autophagy, apoptosis, circadian rhythm, clock genes, coronavirus disease 2019 (COVID-19), dementia, diabetes mellitus, erythropoietin, mechanistic target of rapamycin (mTOR), metformin, oxidative stress, poly-ADP-ribose polymerase (PARP), SARS-CoV-2, silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1), sirtuin, stem cells
1. The Global Impact of Metabolic Disease and Diabetes Mellitus
Diabetes mellitus (DM) is increasingly being targeted for the development of novel treatment strategies to limit death and disability for the world’s population (1–10). At least eighty percent of adults with DM live in low- and middle-income countries and almost five hundred million individuals suffer from DM (11–16). Interestingly, an additional four hundred million individuals are believed to either suffer from metabolic disease or be at risk for developing DM (16–19). The number of individuals with DM is expected to rise to seven hundred million individuals by the year 2045 (16). Approximately thirty-five million individuals, representing about ten percent of the population in the United States (US), are diagnosed with DM (4). Seven million individuals over the age of 18 remain undiagnosed with DM and almost thirty-five percent of adults in the US had prediabetes based on their fasting glucose and hemoglobin A1c (HbA1c) levels in the year 2018 (20). Prevalence of DM has also increased from nine and one-half percent during the period of 1999 to 2002 to twelve percent during the period of 2013 to 2016. Prevalence in the adult for DM can vary by factors that are influenced by socioeconomic status, such as education level. For example, thirteen percent of the adults with less than a high school education had DM compared to almost ten percent of individuals with a high school education and DM and seven and one-half percent of individuals with greater than a high school education and DM. Risk factors for the development of DM and its complications include tobacco consumption, physical inactivity, hypertension, and elevated serum cholesterol (6). Obesity is another risk factor for the development of DM and leads to impaired glucose tolerance (5, 21–27). Obesity and excess body fat can increase the risk of developing DM in young individuals (28) and can affect stem cell proliferation, aging, inflammation, oxidative stress injury, and mitochondrial function (23, 29–35).
In regards to the cost to care for DM, at least $20,000 United States Dollars (USD) are required to treat each individual with DM per year. The care for patients with DM equals approximately $760 billion USD (16). This care consumes more than seventeen percent of the Gross Domestic Product in the US (36). When considering the loss of function and disability that DM can cause in individuals, approximately sixty-nine billion USD are consumed from reduced productivity.
The toxic effects of DM involve all organs of the body and can affect all cellular systems (7). In the peripheral nervous system, at least seventy percent of individuals with DM can develop diabetic peripheral neuropathy. DM can result in autonomic neuropathy (37) and peripheral nerve disease (38–42). In the central nervous system, DM can cause insulin resistance and loss of cognition in patients with Alzheimer’s disease (AD) (3, 6, 7, 43–47). DM can affect several cellular pathways that lead to cognitive loss and dementia (13, 48–53). DM also has been tied to mental illness (54, 55), cerebral vascular injury (13, 18, 56–59), impairment of microglial activity (3, 43–45), and loss of stem cell development (13, 24, 48–52). DM leads to endothelial dysfunction (13, 47, 60–62), cardiovascular disease (19, 21, 61, 63–69), retinal disease (70–72), and immune and infectious disorders (73–79).
2. Novel Strategies for Metabolic Disease
With the growing prevalence of DM, the significant number of individuals that remain undiagnosed with DM, and the marked financial impact on all economies, innovative therapeutic strategies are critical for the treatment of metabolic disorders, such as DM. Although an early diagnosis of DM and quick treatment can offer limited protection and may block some progression of DM (14, 47, 80–84), tight serum glucose control does not ultimately prevent the complications that can arise during DM (6, 85). Careful nutritional and exercise management also can be important for DM care, but in some cases these strategies may be less than beneficial depending on the degree of reduced oral intake and a decrease in organ mass through processes that involve autophagy (86). DM also has additional risk factors when one considers the development of neurodegenerative disorders and cognitive loss that can be compounded by hypertension, low education in early life, and tobacco use (3, 6, 7, 87). For example, vascular disease as a result of DM may lead to loss of memory and dementia (6, 7, 46, 47, 88–91). One innovative strategy to meet the challenges for the treatment of metabolic disease involves the vitamin nicotinamide and the pathways associated with the silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1), the mechanistic target of rapamycin (mTOR), mTOR Complex 1 (mTORC1), mTOR Complex 2 (mTORC2), AMP activated protein kinase (AMPK), and clock genes.
3. Nicotinamide and Metabolic Disease
Nicotinamide, a vitamin, has an important role during metabolic dysfunction and DM (28, 81, 82, 92–95). Nicotinamide is the amide form of vitamin B3 (niacin) and it is obtained through synthesis in the body or as a dietary source and supplement, such as from animal sources or plants (95). Nicotinic acid is the alternative form of the water-soluble vitamin B3 (96). The primary form of niacin in dietary plant sources is nicotinic acid that is rapidly absorbed through the gastrointestinal epithelium (97). Nicotinamide is obtained through the conversion of nicotinic acid in the liver or through the hydrolysis of the coenzyme ß-nicotinamide adenine dinucleotide (NAD+). Once present, nicotinamide functions as the precursor for NAD+ (94, 98). In addition, nicotinamide is required for the synthesis of nicotinamide adenine dinucleotide phosphate (NADP+) (99). Nicotinamide is changed to its mononucleotide form (NMN) with the enzyme nicotinamide phosphoribosyl-transferase (NAMPT). NAMPT has two forms of intracellular and extracellular. NMN then is changed to the dinucleotides NAD+ and NAAD+ through nicotinamide/nicotinic acid mononucleotide adenylyltransferases (NMNATs). NAAD+ converts to NAD+ through NAD+ synthase (100) or NAD+ can be synthesized through nicotinamide riboside kinase that phosphorylates nicotinamide riboside (NR) to NMN (101, 102). Nicotinamide through NAD+ can be directly utilized by cells to synthesize NAD+ (92–95, 103). Nicotinamide participates in energy metabolism through the tricarboxylic acid cycle by utilizing NAD+ in the mitochondrial respiratory electron transport chain for the production of ATP, DNA synthesis, and DNA repair (104–106).
Specific concentrations of nicotinamide and NAD+ may be a critical factor for cell survival (103, 107, 108). Nicotinamide offers protection usually in a specific concentration range (98). Administration of nicotinamide in a range of 5.0 – 25.0 mmol/L can significantly protect neurons during oxidative stress injuries and apoptosis. This concentration range is similar to other injury paradigms in both animal models (109) and in cell culture models (94, 110, 111). Elevated concentrations of nicotinamide in some experimental models may not offer protection and can be detrimental (112, 113). Yet, increased administration of nicotinamide may be useful against tumorigenesis (114) and lead to apoptotic cell death in cancer cells (115, 116). Nicotinamide affects both phases of apoptotic cell death. Nicotinamide can prevent exposure of plasma membrane phosphatidylserine (PS) residues (117–123) to prevent inflammatory cell activation (98, 110, 111, 124). Nicotinamide can limit cardiovascular injury by blocking membrane PS exposure in vascular cells (94, 111), since membrane PS residue externalization in vascular cells can lead to hypercoagulation states (125) and cellular inflammation (126, 127). Nicotinamide can also reverse a previously sustained insult, since post-treatment studies with nicotinamide that can follow apoptotic injury in “real-time” show that early cellular apoptotic injury can be reversed (94, 110, 111, 124, 128, 129).
During cellular metabolism and DM, nicotinamide limits insulin resistance and glucose release with additional pathways to prevent the onset and progression of DM (130–132). Nicotinamide prevents skeletal muscle atrophy during DM (133), limits mitochondrial stress through AMP-activated protein kinase (AMPK) activation (134), and reduces inflammation of the brain during DM with niacin administration (135). In animal models, nicotinamide can maintain normal fasting blood glucose with streptozotocin-induced DM (136, 137) and block oxidative stress pathways that lead to cell death and apoptosis (111, 124, 138–140). In addition, nicotinamide can markedly improve glucose utilization, block excess lactate production, and improve electrophysiologic capacity in ischemic animal models (141). Oral nicotinamide administration at a dose of 1200mg/m2/day protects pancreatic β-cell function and prevents clinical disease in islet-cell antibody-positive first-degree relatives of type-1 DM (142). Patients with recent onset type-1 DM receiving nicotinamide (25mg/kg) in combination with intensive insulin therapy for up to two years experienced significantly reduce HbA1c levels (143). However, it is important to note that prolonged exposure of nicotinamide has been reported to result in impaired pancreatic β-cell function and cell growth (144, 145). Nicotinamide may block cytochromes P450 and hepatic metabolism (146). As a result, the duration of nicotinamide administration may influence the efficacy of this agent since long-term administration also has been reported to support glucose intolerance in some animal models (107).
4. Nicotinamide, SIRT1, and Autophagy
Nicotinamide is intimately tied to silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1) to oversee cellular function and survival during metabolic disease (13, 24, 76, 147–151). SIRT1 is a histone deacetylase that can transfer acetyl groups from ε-N-acetyl lysine amino acids to the histones of deoxyribonucleic acid (DNA) to control transcription (13, 47, 152–161). Nicotinamide and SIRT1 function through autophagic pathways that necessitate a tight oversight of SIRT1 activity (91, 162–165). During nicotinamide administration, mitochondrial autophagy (mitophagy) can lead to an increased NAD+/NADH ratio (107, 166, 167). In addition, chronic administration of nicotinamide can lead to skeletal muscle lipotoxicity and glucose intolerance during autophagy activation (107). As an inhibitor of SIRT1, nicotinamide through autophagy can limit cancer cell growth and in combination with chemotherapeutic agents lead to apoptotic cell death (168–171). Through SIRT1 inhibition, nicotinamide may exert anti-inflammatory properties and affect the transcriptional regulation of inflammatory genes (172). However, nicotinamide has been shown to be cytoprotective through SIRT1 to prevent palmitate-induced hepatotoxicity through SIRT1-dependent induction of autophagy (173).
Such observations for cellular protection with SIRT1 and nicotinamide are similar to work with the growth factor erythropoietin (EPO). EPO also protects against toxic metabolic environments through SIRT1 (76, 82, 174, 175). EPO increases metabolic activity and maintains adipose energy homeostasis in adipocytes to prevent metabolic dysfunction through the combined activation of PPAR-α and SIRT1 (176). EPO uses SIRT1 to modulate skeletal myogenic differentiation (177). In central nervous system endothelial cells, EPO promotes the subcellular trafficking of SIRT1 to the nucleus to promote vascular cell protection and to prevent mitochondrial depolarization, cytochrome c release, BCL2 associated agonist of cell death (Bad) activity, and caspase activation (178). EPO can increase survival of human cardiomyocytes that have mitochondrial dysfunction through the activation of SIRT1 during chemotherapy toxicity (154). EPO prevents the loss of neuronal cells in the brain through the up-regulation of SIRT1 (179). As a result, it is important to recognize the intimate relationship between nicotinamide and SIRT1. Dependent on the conditions, SIRT1 activity or inactivity with nicotinamide may promote cellular survival. In addition, through deacetylase reactions, SIRT1 can transfer the acetyl residue from the acetyllysine residue of histones to the ADP-ribose moiety of NAD+, resulting in the production of nicotinamide. Additional studies suggest that nicotinamide, although an initial inhibitor of SIRT1, may subsequently promote SIRT activity as well as a result of the cellular conversion of nicotinamide to NAD+ (93, 103).
5. Nicotinamide and the Mechanistic Target of Rapamycin (mTOR) Pathways
The mechanistic target of rapamycin (mTOR), a 289-kDa serine/threonine protein kinase, is increasingly being recognized as a critical pathway for nicotinamide to control cellular metabolism (6, 7, 24, 27, 79, 93, 180–182). mTOR also is termed the mammalian target of rapamycin and the FK506-binding protein 12-rapamycin complex-associated protein 1 (160, 161) and is encoded by a single gene FRAP1 (183–185). The target of rapamycin (TOR) was first discovered in Saccharomyces cerevisiae with the genes TOR1 and TOR2 (186). Employing rapamycin-resistant TOR mutants, TOR1 and TOR2 are now known to encode the Tor1 and Tor2 isoforms in yeast (187). Rapamycin is a macrolide antibiotic in Streptomyces hygroscopicus that blocks the activity of TOR and mTOR (18). mTOR is the primary component of the protein complexes mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2) (174, 188, 189). Rapamycin limits mTORC1 activity by binding to immunophilin FK-506-binding protein 12 (FKBP12) that attaches to the FKBP12 -rapamycin-binding domain (FRB) at the carboxy (C) -terminal of mTOR to interfere with the FRB domain of mTORC1 (190). Although not entirely clear on how rapamycin blocks mTORC1 activity, one pathway may involve allosteric changes on the catalytic domain as well as the inhibition of phosphorylation of protein kinase B (Akt) and p70 ribosomal S6 kinase (p70S6K) (191). mTORC1 is more sensitive to inhibition by rapamycin than mTORC2. Yet, chronic administration of rapamycin can inhibit mTORC2 activity as a result of the disruption of the assembly of mTORC2 (185, 192).
mTORC1 and mTORC2 are further divided into subcomponents. mTORC1 consists of Raptor, the proline rich Akt substrate 40 kDa (PRAS40), Deptor (DEP domain-containing mTOR interacting protein), and mammalian lethal with Sec13 protein 8, termed mLST8 (mLST8) (186). Binding of mTORC1 to its constituents occurs through the protein Ras homologue enriched in brain (Rheb) that phosphorylates the Raptor residue serine863 and other residues that include serine859, serine855, serine877, serine696, and threonine706 (193). The inability to phosphorylate serine863 reduces mTORC1 activity, as shown using a site-direct mutation of serine863 (194). mTOR can oversee Raptor activity which can be blocked by rapamycin (194). Deptor, an inhibitor of the mTOR pathway, blocks mTORC1 activity by binding to the FAT domain (FKBP12 -rapamycin-associated protein (FRAP), ataxia-telangiectasia (ATM), and the transactivation/transformation domain-associated protein) of mTOR. If the activity of Deptor is blocked, Akt, mTORC1, and mTORC2 activities are increased (195). PRAS40 decreases mTORC1 activity by preventing the association of p70 ribosomal S6 kinase (p70S6K) and the eukaryotic initiation factor 4E (eIF4E)-binding protein 1 (4EBP1) with Raptor (196, 197). mTORC1 is active after PRAS40 is phosphorylated by Akt. This releases PRAS40 from Raptor to sequester PRAS40 in the cell cytoplasm with the docking protein 14-3-3 (198–202). mLST8, in contrast, fosters mTOR kinase activity. This involves binding of p70S6K and 4EBP1 to Raptor (203). mLST8 also controls insulin signaling through the mammalian forkhead transcription factor FoxO3 (75, 204). mLST8 is also necessary for Akt and protein kinase C-α (PKCα) phosphorylation and is required for Rictor to associate with mTOR (204).
mTORC2 consists of Rictor, mLST8, Deptor, the mammalian stress-activated protein kinase interacting protein (mSIN1), and the protein observed with Rictor-1 (Protor-1) (183, 196). mTORC2 controls cytoskeleton remodeling through PKCα and cell migration through the Rac guanine nucleotide exchange factors P-Rex1 and P-Rex2 and through Rho signaling (205). mTORC2 increases the activity of protein kinases that includes glucocorticoid induced protein kinase 1 (SGK1), a member of the protein kinase A/protein kinase G/protein kinase C (AGC) family of protein kinases. Protor-1, a Rictor-binding subunit of mTORC2, activates SGK1 (206, 207). The kinase domain of mTOR phosphorylates mSIN1 and inhibits lysosomal degradation of this protein. Rictor and mSIN1 are also able to phosphorylate Akt at serine473 and foster threonine308 phosphorylation by phosphoinositide-dependent kinase 1 (PDK1) to enhance cell survival.
AMPK as part of the nicotinamide and mTOR pathways controls cellular metabolism as well (3, 64, 208, 209). AMPK prevents mTORC1 activity through the hamartin (tuberous sclerosis 1)/tuberin (tuberous sclerosis 2) (TSC1/TSC2) complex that blocks mTORC1 (159, 210). In addition, oversight of the TSC1/TSC2 complex is controlled though phosphoinositide 3-kinase (PI 3-K), Akt, and its phosphorylation of TSC2. Extracellular signal-regulated kinases (ERKs), protein p90 ribosomal S6 kinase 1 (RSK1), and glycogen synthase kinase −3β (GSK-3β) can also modulate the activity of the TSC1/TSC2 complex. TSC2 functions as a GTPase-activating protein (GAP) that converts G protein Rheb (Rheb-GTP) into the inactive GDP-bound form (Rheb-GDP). Once Rheb-GTP is active, Rheb-GTP then associates with Raptor to control the binding of 4EBP1 to mTORC1 and increase mTORC1 activity (211). AMPK phosphorylates TSC2 to increase GAP activity to change Rheb-GTP into the inactive Rheb-GDP and block mTORC1 activity (212).
The mTOR pathway is closely linked to cellular metabolic function and disease (6, 18, 40, 213–216). Activation of mTOR may decrease cognitive loss that can be a result of DM and other toxicities (2, 6, 160, 217–219). mTOR activation can prevent microglial injury during oxidative stress and block ß-amyloid (Aß) toxicity in neurons (200, 218, 220–224). mTOR activation also can limit diabetic neuropathy (41), alleviate pain sensitization in combination with SIRT1 activity (225), and reduce ischemic stroke injury in conjunction with circadian clock genes (6, 158, 226–228). Interestingly, decreased activity of mTOR has been shown to increase mortality in murine models of DM (229). During mTOR inhibition with rapamycin, reduced β-cell function, insulin resistance, and decreased insulin secretion can lead to the progression of DM (230). The correct translocation of glucose transporters to the plasma membrane in skeletal muscle are also affected during loss of mTOR activity (231). In patients with metabolic syndrome, mTOR activation is diminished. mTOR activity also appears necessary for proper metabolic function during inflammatory conditions with rheumatoid arthritis (232) and during diabetes-induced testicular dysfunction (182). This loss of mTOR activity may be responsible for insulin resistance and the increased risk of vascular thrombosis (233). Activation of mTOR pathways that oversee p70S6K and 4EBP1 can improve insulin secretion in pancreatic β-cells and increase resistance to β-cell streptozotocin toxicity and obesity in mice (234). Loss of p70S6K activity leads to hypo-insulinemia and glucose intolerance with diminished pancreatic β-cell size (235). mTOR activity has been shown to protect pancreatic β- cells against cholesterol-induced apoptosis (236), lead to enhanced neuronal cell survival in DM cellular models (237), reduce oxidative stress (24, 27, 42, 79, 238–242), and block glucolipotoxicity (243). mTOR activity can foster the differentiation of adipocytes (244), prevent endothelial cell dysfunction during hyperglycemia (62), and maintain glucose homeostasis (245). mTOR provides protection as an integral component of the Mediterranean diet to reduce obesity in the population. The diet may reduce Aβ toxicity in astrocytes through enhanced Akt activity by consumption of polyphenol of olives and olive oil that over time may prevent the onset or progression of AD (214).
Nicotinamide, and in a similar manner trophic factors, can provide protection against oxidative stress and DM through mTOR pathways. In relation to growth factors, such as insulin-like growth factor-1 (IGF-1) (246–249) and EPO (6, 22, 70, 83, 127, 250–254), these trophic factors rely upon mTOR pathways. EPO utilizes the mTOR pathway, such as PRAS40 and Akt, to increase cell survival (198, 255–257) and limit toxic cellular environments (221, 258–260). EPO also plays a significant role as a potential cellular protectant during aging (261) and DM with the modulation of mTOR pathways, in part, to preserve cell survival (18, 70, 82, 83, 127, 252, 262–266). In regards to nicotinamide, nicotinamide maintains a fine control over cellular metabolism through mTOR pathways such as p70S6K, 4EBP1, and AMPK. Both p70S6K and 4EBP1 in the mTOR pathway are required by nicotinamide to protect against radiation-induced apoptosis (267). p70S6K and 4EBP1 activation also can improve insulin secretion in pancreatic β-cells and increase resistance to β-cell streptozotocin toxicity and obesity in mice (234). Concerning AMPK, nicotinamide can reduce intracellular mitochondrial stress in hypoxic cardiomyocytes through the activation of AMPK (134). Similar to nicotinamide, biguanides and metformin also use AMPK to maintain cellular function. Metformin inhibits mTOR activity, promotes autophagy, and can function at times in an AMPK-independent manner (268). Through metformin, AMPK activation leads to autophagy induction and protects against diabetic apoptotic cardiac cell death (269). Metformin also has been shown to prevent lipid peroxidation in the brain and spinal cord and to reduce caspase activity during toxic insults (270). These observations of metformin to offer protection during DM may be associated with the ability of autophagic pathways to limit oxidative stress under some circumstances (22, 271). The protective role of metformin to regulate cellular metabolism through the inhibition of mTOR pathways and promotion of autophagy activation also has been recently highlighted as a novel means to lessen morbidity and mortality from the β-coronavirus family virion, SARS-CoV-2, and coronavirus disease 2019 (COVID-19) (79, 180, 272–279). Nicotinamide as well may function through such pathways and others, such as poly-ADP-ribose polymerase (PARP) (7, 81, 93, 94, 124, 148, 239, 280), to block illness from COVID-19 (281–283).
AMPK activation during metabolic disease can promote insulin sensitivity, fatty acid oxidation, and mitochondrial biogenesis. This leads to the generation of ATP and serves to limit oxidative stress (6, 93). AMPK activation can decrease disability and hyperalgesia from diabetic neuropathy in animal models (40). Diets associated with fish oil consumption can result in increased AMPK activity and block endothelial progenitor cell dysfunction and ischemic injuries (64). AMPK also can limit insulin resistance, since the loss of AMPK has been shown to lead to reduced tolerance for the development of insulin resistance (284). During periods of reduced dietary intake that may increase lifespan (285), AMPK activation also can shift to beneficial oxidative metabolism (286). This process has been shown to limit ischemic brain damage in diabetic animal models (287). In line with the vascular protective properties of nicotinamide (111, 128, 288), AMPK can limit insulin resistance (284) and protect endothelial progenitor cells during periods of hyperglycemia (64). AMPK activation also can strengthen memory retention in models of AD and DM (208), may assist with the elimination of Aß in the brain (289), facilitate tau clearance (290), and limit chronic inflammation in the nervous system (158, 164, 210).
AMPK oversees autophagy pathways and appears to require a fine control of activity similar to nicotinamide to achieve beneficial outcomes in metabolic disease. During metabolic disease, autophagy can remove misfolded proteins and eliminate non-functioning mitochondria to maintain β-cell function and prevent the onset of DM (291). Exercise in mice has been demonstrated to foster autophagy activation and regulate glucose homeostasis (292). Autophagy can improve insulin sensitivity during the administration of high fat diets in mice (284) and may protect microglia during acute glucose fluctuations (45). During periods of hyperglycemia, AMPK increases basal autophagy activity (156, 162) and prevents endothelial cell death (62, 293). AMPK can control autophagy during coronary artery disease (294), cholesterol efflux (295), endothelial dysfunction during hyperglycemia (62), and oxidative stress (296, 297). AMPK can promote anti-senescence activity and the increase of autophagic flux (298). Yet, as previously noted, activation of AMPK and autophagy pathways may require careful modulation during metabolic disease and DM (7, 9, 26, 43, 45, 75, 79). Enhanced activity of autophagy can lead to the loss of cardiac and liver tissue in diabetic rats during attempts to achieve glycemic control through diet modification (86). During elevated glucose exposure, toxic advanced glycation end products (AGEs) can yield autophagy activation and vascular smooth muscle proliferation that may lead to atherosclerosis (299) and cardiomyopathy (300). During elevated glucose exposure, autophagy can impair endothelial progenitor cells, lead to mitochondrial oxidative stress (301), and prevent angiogenesis (302). Chronic inflammatory conditions such as lichen planus also have been tied to mTOR inhibition and autophagy activation (303).
For cellular protection during metabolic disease, nicotinamide maintains a tight relationship with mTOR pathways and autophagy (24, 27, 79, 182, 238, 241, 304–308). Nicotinamide can reduce Aß toxicity and improve cognition (96, 309), limit metabolic dysfunction through the maintenance of mitochondria (81, 93, 134, 310), maintain metabolic homeostasis (282, 311, 312), block neural ischemic injury (313) and endothelial injury (314), and protect hypoxic myocardial cells through autophagy activation and the inhibition of mTOR (315). However, the loss of mTOR activity can be detrimental at times. During mTOR inhibition with rapamycin, reduced β-cell function, insulin resistance, and decreased insulin secretion can promote the DM progression (230). Decreased activity of mTOR can increase mortality in a mouse model of DM (229). Translocation of glucose transporters to the plasma membrane in skeletal muscle can be blocked in the absence of mTOR activity (231). Furthermore, limits in autophagy activation may be necessary. Interneuron progenitor growth in the brain requires mTOR activity with the inhibition of autophagy (316). Autophagy activation also can lead to injury of endothelial progenitor cells, result in mitochondrial oxidative stress, and prevent new blood vessel formation during elevated glucose exposure (302). Blockade of autophagy may also limit infarct size and rescue cerebral neurons during stroke and oxidative stress (307, 317). This work highlights a potential feedback mechanism with autophagy and mTOR, such as through AMPK, to block either excess AMPK or mTOR activity. As an example, if mTOR activity is unchecked during the inhibition of AMPK activity, mTOR and p70S6K can lead to glucose intolerance by inhibiting the insulin receptor substrate 1 (IRS-1) (318). In addition, mTOR inhibition may reduce stroke infarct size during models of DM (287), block cardiac hypertrophy (319), protect vascular cells from oxidative stress (241), prevent retinal degeneration (148), and also can be necessary for maintaining a balance between pancreatic β-cell proliferation and cell size (320). Therefore, feedback mechanisms involving nicotinamide, autophagy, mTOR, and AMPK, may be vital to achieve an appropriate balance of activity for cellular protection during metabolic disease.
6. Nicotinamide and Clock Genes
Circadian rhythm clock genes have a prominent role during cellular injury and metabolic disorders (6, 9, 158, 161, 321–327). Clock genes can impact endocrine metabolic disorders and cancer (228, 324, 325, 328), the development of DM (6, 322, 327, 329, 330), energy metabolism and aging (158, 321, 331–333), cellular metabolism and neurodegeneration (6, 332–337), mitochondrial energy maintenance (32, 325, 327, 338), diabetic retinal disease (9), and fasting plasma glucose release (329, 339). The mammalian circadian clock resides in the suprachiasmatic nucleus (SCN) located above the optic chiasm and receives light input from photosensitive ganglion cells in the retina. The SCN controls most overt circadian rhythms and relies upon the pineal gland, hypothalamic nuclei, and vasoactive intestinal peptide to oversee processes that involve the sleep wake cycle, release of hormones cortisol and melatonin, oxidative stress responses (340), and the regulation of body temperature (329). In regards to the clock gene family, members of the basic helix-loop-helix -PAS (Period-Arnt-Single-minded) transcription factor family, such as CLOCK and BMAL1 (341), control the expression of the genes Cryptochrome (Cry1 and Cry2) and Period (Per1, Per2, and Per3). Oversight and feedback are provided by PER:CRY heterodimers that translocate to the nucleus to block the transcription activated by CLOCK:BMAL1 complexes. Other regulatory loops consist of retinoic acid-related orphan nuclear receptors REV-ERBα, also known as NR1D1 (nuclear receptor subfamily 1, group D, member 1), and RORα that are activated by CLOCK:BMAL1 heterodimers. The REV-ERBα and RORα receptors bind retinoic acid-related orphan receptor response elements (ROREs) that exist in the BMAL1 promoter to repress and activate rhythmic transcription of BMAL1 by RORs and REV-ERBs, respectively. REV-ERBs can block transcription to result in circadian oscillation of BMAL1 (333, 342).
Clock gene pathways are associated with SIRT1, mTOR, and autophagy (6, 9, 158, 161, 226, 227, 325, 331, 338, 343–346). For example, melatonin, a pineal hormone that is involved in regulating circadian rhythm, relies upon autophagy pathways and mTOR to control processes of aging and neurodegeneration (331). Loss of mTOR activation has been shown to affect circadian rhythm and cognitive decline during prolonged space flight and microgravity (346). Cerebral ischemic infarction also may be influenced by an alteration in circadian rhythm genes and fluctuations in mTOR activity (226, 344). In relation to autophagy, circadian rhythm dysfunction that affects cognitive loss has been associated with autophagy induction (347). A basal circadian rhythm that oversees autophagy in animal models of AD may be required to limit cognitive decline and Aβ deposition (348). Changes in environmental homeostasis also can alter circadian rhythm that leads to depressed cognition function (158). As an example, chronic sleep fragmentation has been shown to alter autophagy proteins in the hippocampus and can impair memory and cognition (157, 190, 290, 343, 349, 350). In addition, autophagy activation with clock proteins may be required for cellular protection such as during cerebral ischemia, since loss of the PER1 circadian clock protein can worsen stroke pathology (344).
In light of the reliance of nicotinamide upon mTOR and autophagy pathways similar to clock genes, it may come as no surprise that nicotinamide can have an important role during circadian rhythm function. Nicotinamide and NAD+ play a critical role with circadian rhythm and clock genes that is tied to SIRT1, mTOR, and autophagy. Cellular NAD+ pools can fluctuate with circadian rhythmicity and with aging. SIRT1 can oversee clock gene expression through PER2 deacetylation (351). SIRT1 also in conjunction with CLOCK:BMAL1 can regulate the circadian expression of NAMPT that is necessary for the generation of NAD+. SIRT1 can be recruited to the NAMPT promoter to foster the circadian synthesis of its own coenzyme (352). In addition, the NAD+ pools can become more depressed with the loss of mitochondrial function that leads to cell injury as a result of the cellular NAD+ pools oscillating in tandem with free nicotinamide levels and affecting overall cell function and metabolism (353). Furthermore, it has been shown that the loss of the pathways of SIRT1 and mTOR during obesity can lead to the suppression of core circadian components CLOCK and BMAL1 and result in metabolic dysfunction. Studies show that the agent metformin can protect against such processes during obesity in murine models and can reverse impaired AMPK and SIRT1 function during the suppression of core circadian components CLOCK and BMAL1 (354). This work provides further evidence for circadian rhythm dysfunction linked to metabolic disease and the significant role of pathways associated with nicotinamide that include mTOR, AMPK, autophagy, and SIRT1. SIRT1 control of circadian rhythm and melatonin can affect glucose tolerance and DM (329) as well as inflammation during obesity (32). Through the oversight of circadian rhythms, SIRT1, an NAD+ dependent histone deacetylase, controls lipid metabolism, liver regeneration (355), and is involved with liver metabolism, aging, and clock genes (356). Nicotinamide, NAD+ pathways, SIRT1, mTOR can together influence circadian rhythm and clock genes to impact glucose tolerance, lipid metabolism, and cellular regeneration.
7. Future Perspectives
Metabolic disorders that include DM present significant challenges for maintaining the health of the world’s population. Approximately eighty percent of adults with DM are living in low- and middle-income countries and almost five hundred million individuals have DM. Furthermore, the number of individuals with DM is expected to rise to seven hundred million individuals by the year 2045. In addition, the care for patients with DM equals approximately $760 billion USD and consumes more than seventeen percent of the Gross Domestic Product in the US. Risk factors for the development of DM and its complications include tobacco consumption, physical inactivity, hypertension, elevated serum cholesterol, and obesity. Disorders such as DM can affect all organs and systems of the body. Although current therapies that offer tight serum glucose control may offer some protection, ultimately these strategies cannot block progression of metabolic disorders. For these reasons, novel therapeutic avenues are necessary to address metabolic disorders and DM. An exciting strategy involves the nicotinamide and the pathways associated with SIRT1, mTOR, mTORC1, mTORC2, AMPK, and clock genes.
Nicotinamide plays an important role during metabolic disease and DM (Figure 1). Nicotinamide can markedly improve glucose utilization, block excess lactate production, limit mitochondrial stress, reduce inflammation of the brain during DM, and reduce oxidative stress pathways. In addition, patients with recent onset type-1 DM receiving nicotinamide have experienced significantly reduce HbA1c levels and oral nicotinamide administration may protect pancreatic β-cell function to block clinical disease in islet-cell antibody-positive first-degree relatives of type-1 DM. However, it appears that specific concentrations of nicotinamide and NAD+ are critical factors for cell survival. Nicotinamide offers protection usually in a specific concentration range to protect against oxidative stress and cell death. Elevated concentrations of nicotinamide may not be protective and can be detrimental with the exception to promote apoptotic cell death in cancer cells. In addition, prolonged exposure of nicotinamide has been reported to result in impaired pancreatic β-cell function and glucose intolerance.
Figure 1: Downstream Nicotinamide Pathways during Metabolic Disease.
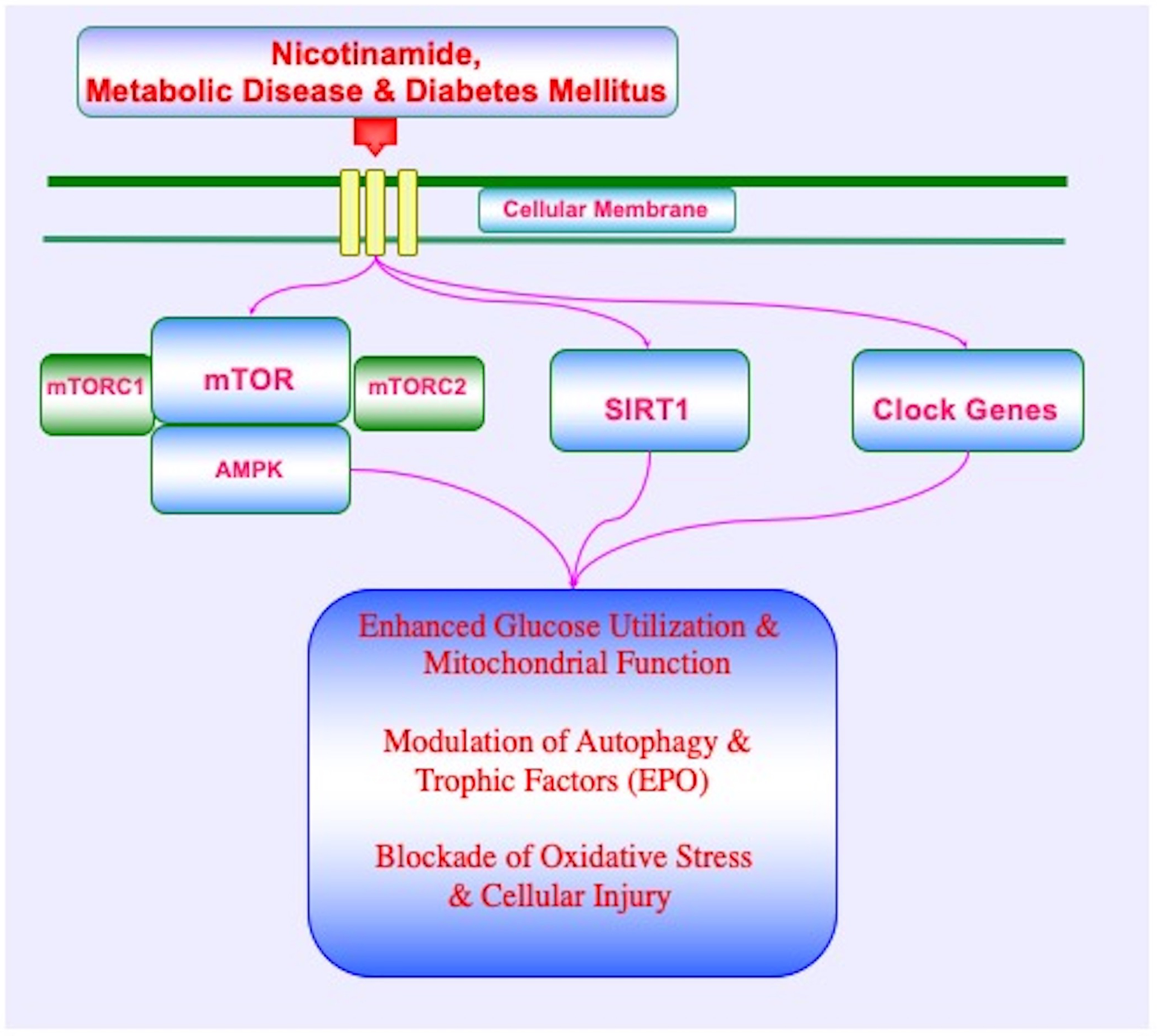
During metabolic disease and diabetes mellitus, nicotinamide employs the pathways of the silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1), the mechanistic target of rapamycin (mTOR), mTOR Complex 1 (mTORC1), mTOR Complex 2 (mTORC2), AMP activated protein kinase (AMPK), erythropoietin (EPO), and clock genes to maintain glucose homeostasis, enhance glucose utilization, oversee pathways of autophagy, and foster mitochondrial function. These pathways have complex interactions and involve feedback mechanisms that require a fine balance in activity to enhance cellular function and survival, block oxidative stress, and limit potential detrimental outcomes.
Interestingly, nicotinamide controls pathways of autophagy through SIRT1 that requires a close regulation of SIRT1 activity to oversee cellular survival. As an inhibitor of SIRT1, nicotinamide through autophagy can limit cancer cell growth and in combination with chemotherapeutic agents lead to apoptotic cell death. Through SIRT1 inhibition, nicotinamide may exert anti-inflammatory properties and affect the transcriptional regulation of inflammatory genes. Yet, nicotinamide at times may require SIRT1 since nicotinamide can prevent palmitate-induced hepatotoxicity through SIRT1-dependent induction of autophagy. Such observations for cellular protection with SIRT1 and nicotinamide are similar to studies with the growth factor EPO. For example, EPO increases metabolic activity and maintains adipose energy homeostasis through SIRT1. EPO also promotes the subcellular trafficking of SIRT1 to the nucleus to promote vascular cell protection and to prevent mitochondrial depolarization, cytochrome c release, BCL2 associated agonist of cell death (Bad) activity, and caspase activation. As a result, it is vital to appreciate the intimate relationship among nicotinamide, SIRT1, and cellular survival. Additionally, it is important to note that through deacetylase reactions, SIRT1 can transfer the acetyl residue from the acetyllysine residue of histones to the ADP-ribose moiety of NAD+ and lead to the production of nicotinamide. Furthermore, nicotinamide, although an initial inhibitor of SIRT1, may foster SIRT activity as a result of the cellular conversion of nicotinamide to NAD+.
Nicotinamide also is dependent upon the pathways of mTOR to influence cellular survival and metabolic disease. For example, p70S6K and 4EBP1 in the mTOR pathway are necessary for nicotinamide to protect against radiation-induced apoptosis and p70S6K and 4EBP1 activation also can improve insulin secretion in pancreatic β-cells and increase resistance to β-cell toxicity and obesity. In addition, nicotinamide can reduce intracellular mitochondrial stress through the activation of AMPK. As a downstream pathway of nicotinamide, AMPK oversees autophagy pathways and can promote insulin sensitivity, fatty acid oxidation, and mitochondrial biogenesis. Yet, the pathways of mTOR, AMPK, and autophagy require close oversight during metabolic disease and DM. During DM, toxic AGEs can promote autophagy activation and vascular smooth muscle proliferation that may lead to in atherosclerosis. Autophagy during periods of elevated glucose can impair endothelial progenitor cells, lead to mitochondrial oxidative stress, and block angiogenesis. In addition, during these periods of autophagy activation with the loss of mTOR activity, reduced β-cell function, insulin resistance, and decreased insulin secretion can promote DM progression. As a result, some biological conditions may require limits on autophagy activation since neuroprotection can be promoted at times during autophagy inhibition, such as during ischemic neuronal injury. Therefore, feedback mechanisms involving nicotinamide, autophagy, mTOR, and AMPK, may be required to achieve an appropriate balance of activity for cellular protection during metabolic disease.
Circadian rhythm clock genes also have a prominent role for nicotinamide during metabolic disorders. Clock genes can influence multiple processes for metabolism that include endocrine metabolic disorders and cancer, the development of DM, energy metabolism and aging, cellular metabolism and neurodegeneration, mitochondrial energy maintenance, diabetic retinal disease, and fasting plasma glucose release. Nicotinamide and NAD+ play a vital function with circadian rhythm and clock genes that are tied to SIRT1, mTOR, and autophagy. Cellular NAD+ pools can fluctuate with circadian rhythmicity. These NAD+ pools can become more depressed with the loss of mitochondrial function that leads to cell injury as a result of the cellular NAD+ pools oscillating in tandem with free nicotinamide levels and affecting overall cell function and metabolism. In addition, the loss of the pathways of SIRT1 and mTOR during obesity have been highlighted to result in the suppression of core circadian components CLOCK and BMAL1 and lead to metabolic dysfunction. Nicotinamide, through its oversight of NAD+ pathways, SIRT1, mTOR and clock genes can impact glucose tolerance, lipid metabolism, cellular regeneration, and cellular survival. Future work is warranted to gain further insight into these critical pathways that can oversee normal metabolic physiology as well as metabolic dysfunction.
Acknowledgments:
This research was supported by the following grants to Kenneth Maiese: American Diabetes Association, American Heart Association, NIH NIEHS, NIH NIA, NIH NINDS, and NIH ARRA.
Footnotes
Competing Interests: There are no conflicts of interest to declare.
References
- 1.Klimontov VV, Bulumbaeva DM, Fazullina ON, Lykov AP, Bgatova NP, Orlov NB, et al. Circulating Wnt1-inducible signaling pathway protein-1 (WISP-1/CCN4) is a novel biomarker of adiposity in subjects with type 2 diabetes. J Cell Commun Signal. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maiese K Novel nervous and multi-system regenerative therapeutic strategies for diabetes mellitus with mTOR. Neural regeneration research. 2016;11(3):372–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maiese K Impacting dementia and cognitive loss with innovative strategies: mechanistic target of rapamycin, clock genes, circular non-coding ribonucleic acids, and Rho/Rock. Neural regeneration research. 2019;14(5):773–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. 2020;CS 314227-A:1–30. [Google Scholar]
- 5.Liu L, Hu J, Yang L, Wang N, Liu Y, Wei X, et al. Association of WISP1/CCN4 with Risk of Overweight and Gestational Diabetes Mellitus in Chinese Pregnant Women. Dis Markers. 2020;2020:4934206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maiese K Cognitive impairment with diabetes mellitus and metabolic disease: innovative insights with the mechanistic target of rapamycin and circadian clock gene pathways. Expert Rev Clin Pharmacol. 2020;13(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maiese K Dysregulation of metabolic flexibility: The impact of mTOR on autophagy in neurodegenerative disease. Int Rev Neurobiol. 2020;155:1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng S, Li W, Hou N, Huang N. A Review of FoxO1-Regulated Metabolic Diseases and Related Drug Discoveries. Cells. 2020;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qi X, Mitter SK, Yan Y, Busik JV, Grant MB, Boulton ME. Diurnal Rhythmicity of Autophagy Is Impaired in the Diabetic Retina. Cells. 2020;9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaiou M circRNAs Signature as Potential Diagnostic and Prognostic Biomarker for Diabetes Mellitus and Related Cardiovascular Complications. Cells. 2020;9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haldar SR, Chakrabarty A, Chowdhury S, Haldar A, Sengupta S, Bhattacharyya M. Oxidative stress-related genes in type 2 diabetes: association analysis and their clinical impact. Biochemical genetics. 2015;53(4–6):93–119. [DOI] [PubMed] [Google Scholar]
- 12.Jia G, Aroor AR, Martinez-Lemus LA, Sowers JR. Invited Review: Over-nutrition, mTOR Signaling and Cardiovascular Diseases. Am J Physiol Regul Integr Comp Physiol. 2014;307(10):R1198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maiese K SIRT1 and stem cells: In the forefront with cardiovascular disease, neurodegeneration and cancer. World J Stem Cells. 2015;7(2):235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maiese K New Insights for Oxidative Stress and Diabetes Mellitus. Oxid Med Cell Longev. 2015;2015(2015:875961). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye Q, Fu JF. Paediatric type 2 diabetes in China-Pandemic, progression, and potential solutions. Pediatric diabetes. 2018;19(1):27–35. [DOI] [PubMed] [Google Scholar]
- 16.International Diabetes Federation. Diabetes. IDF Diabetes Atlas. 2019(9th Edition). [PubMed] [Google Scholar]
- 17.Harris MI, Eastman RC. Early detection of undiagnosed diabetes mellitus: a US perspective. Diabetes Metab Res Rev. 2000;16(4):230–6. [DOI] [PubMed] [Google Scholar]
- 18.Maiese K mTOR: Driving apoptosis and autophagy for neurocardiac complications of diabetes mellitus. World J Diabetes. 2015;6(2):217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maiese K, Chong ZZ, Shang YC. Mechanistic insights into diabetes mellitus and oxidative stress. Curr Med Chem. 2007;14(16):1729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maiese K Heightened Attention for Wnt Signaling in Diabetes Mellitus. Curr Neurovasc Res. 2020. [DOI] [PubMed] [Google Scholar]
- 21.Barchetta I, Cimini FA, Ciccarelli G, Baroni MG, Cavallo MG. Sick fat: the good and the bad of old and new circulating markers of adipose tissue inflammation. Journal of endocrinological investigation. 2019. [DOI] [PubMed] [Google Scholar]
- 22.Maiese K. Erythropoietin and diabetes mellitus. World J Diabetes. 2015;6(14):1259–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang AR, Yan XQ, Zhang C, Du CQ, Long WJ, Zhan D, et al. Characterization of Wnt1-inducible Signaling Pathway Protein-1 in Obese Children and Adolescents. Current medical science. 2018;38(5):868–74. [DOI] [PubMed] [Google Scholar]
- 24.Maiese K. Prospects and Perspectives for WISP1 (CCN4) in Diabetes Mellitus. Curr Neurovasc Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quesada I, de Paola M, Torres-Palazzolo C, Camargo A, Ferder L, Manucha W, et al. Effect of Garlic’s Active Constituents in Inflammation, Obesity and Cardiovascular Disease. Curr Hypertens Rep. 2020;22(1):6. [DOI] [PubMed] [Google Scholar]
- 26.Yamashima T, Ota T, Mizukoshi E, Nakamura H, Yamamoto Y, Kikuchi M, et al. Intake of ω−6 Polyunsaturated Fatty Acid-Rich Vegetable Oils and Risk of Lifestyle Diseases. Adv Nutr. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J, Suo H, Song J. Protective role of mitoquinone against impaired mitochondrial homeostasis in metabolic syndrome. Critical reviews in food science and nutrition. 2020:1–19. [DOI] [PubMed] [Google Scholar]
- 28.Maiese K, Chong ZZ, Shang YC, Hou J. Novel Avenues of Drug Discovery and Biomarkers for Diabetes Mellitus. Journal of clinical pharmacology. 2011;51(2):128–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cernea M, Tang W, Guan H, Yang K. Wisp1 mediates Bmp3-stimulated mesenchymal stem cell proliferation. Journal of molecular endocrinology. 2016;56(1):39–46. [DOI] [PubMed] [Google Scholar]
- 30.Curjuric I, Imboden M, Bridevaux PO, Gerbase MW, Haun M, Keidel D, et al. Common SIRT1 variants modify the effect of abdominal adipose tissue on aging-related lung function decline. Age (Dordr). 2016;38(3):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill JH, Solt C, Foster MT. Obesity associated disease risk: the role of inherent differences and location of adipose depots. Hormone molecular biology and clinical investigation. 2018;33(2). [DOI] [PubMed] [Google Scholar]
- 32.Liu Z, Gan L, Zhang T, Ren Q, Sun C. Melatonin alleviates adipose inflammation through elevating alpha-ketoglutarate and diverting adipose-derived exosomes to macrophages in mice. J Pineal Res. 2018;64(1):12455. [DOI] [PubMed] [Google Scholar]
- 33.Maiese K Picking a bone with WISP1 (CCN4): new strategies against degenerative joint disease. J Transl Sci. 2016;1(3):83–5. [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta J, Rayalam S, Wang X. Cytoprotective Effects of Natural Compounds against Oxidative Stress. Antioxidants (Basel, Switzerland). 2018;7(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75(3):207–46. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Medicare and Medicaid Services. National Health Expenditure Projections 2018–2027. wwwcmsgov. 2019. [Google Scholar]
- 37.Albiero M, Poncina N, Tjwa M, Ciciliot S, Menegazzo L, Ceolotto G, et al. Diabetes causes bone marrow autonomic neuropathy and impairs stem cell mobilization via dysregulated p66Shc and Sirt1. Diabetes. 2014;63(4):1353–65. [DOI] [PubMed] [Google Scholar]
- 38.Gomes MB, Negrato CA. Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetology & metabolic syndrome. 2014;6(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gomez-Brouchet A, Blaes N, Mouledous L, Fourcade O, Tack I, Frances B, et al. Beneficial effects of levobupivacaine regional anaesthesia on postoperative opioid induced hyperalgesia in diabetic mice. Journal of translational medicine. 2015;13(1):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atef MM, El-Sayed NM, Ahmed AAM, Mostafa YM. Donepezil improves neuropathy through activation of AMPK signalling pathway in streptozotocin-induced diabetic mice. Biochem Pharmacol. 2019;159:1–10. [DOI] [PubMed] [Google Scholar]
- 41.Dong J, Li H, Bai Y, Wu C. Muscone ameliorates diabetic peripheral neuropathy through activating AKT/mTOR signalling pathway. J Pharm Pharmacol. 2019;71(11):1706–13. [DOI] [PubMed] [Google Scholar]
- 42.Dai C, Xiao X, Zhang Y, Xiang B, Hoyer D, Shen J, et al. Curcumin attenuates colistin-induced peripheral neurotoxicity in mice. ACS Infect Dis. 2020. [DOI] [PubMed] [Google Scholar]
- 43.Caberlotto L, Nguyen TP, Lauria M, Priami C, Rimondini R, Maioli S, et al. Cross-disease analysis of Alzheimer’s disease and type-2 Diabetes highlights the role of autophagy in the pathophysiology of two highly comorbid diseases. Scientific reports. 2019;9(1):3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su M, Naderi K, Samson N, Youssef I, Fulop L, Bozso Z, et al. Mechanisms Associated with Type 2 Diabetes as a Risk Factor for Alzheimer-Related Pathology. Mol Neurobiol. 2019. [DOI] [PubMed] [Google Scholar]
- 45.Hsieh CF, Liu CK, Lee CT, Yu LE, Wang JY. Acute glucose fluctuation impacts microglial activity, leading to inflammatory activation or self-degradation. Scientific reports. 2019;9(1):840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu Z, Jiao R, Wang P, Zhu Y, Zhao J, De Jager P, et al. Shared Causal Paths underlying Alzheimer’s dementia and Type 2 Diabetes. Scientific reports. 2020;10(1):4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maiese K Sirtuins: Developing Innovative Treatments for Aged-Related Memory Loss and Alzheimer’s Disease. Curr Neurovasc Res. 2018;15(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maiese K, Chong ZZ, Shang YC, Hou J. FoxO proteins: cunning concepts and considerations for the cardiovascular system. Clin Sci (Lond). 2009;116(3):191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang PC, Ng YF, Ho S, Gyda M, Chan SW. Resveratrol and cardiovascular health--promising therapeutic or hopeless illusion? Pharmacol Res. 2014;90:88–115. [DOI] [PubMed] [Google Scholar]
- 50.Xiang L, Mittwede PN, Clemmer JS. Glucose Homeostasis and Cardiovascular Alterations in Diabetes. Comprehensive Physiology. 2015;5(4):1815–39. [DOI] [PubMed] [Google Scholar]
- 51.Xu YJ, Tappia PS, Neki NS, Dhalla NS. Prevention of diabetes-induced cardiovascular complications upon treatment with antioxidants. Heart failure reviews. 2014;19(1):113–21. [DOI] [PubMed] [Google Scholar]
- 52.Yao T, Fujimura T, Murayama K, Okumura K, Seko Y. Oxidative Stress-Responsive Apoptosis Inducing Protein (ORAIP) Plays a Critical Role in High Glucose-Induced Apoptosis in Rat Cardiac Myocytes and Murine Pancreatic beta-Cells. Cells. 2017;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fan X, Zhao Z, Wang D, Xiao J. Glycogen synthase kinase-3 as a key regulator of cognitive function. Acta biochimica et biophysica Sinica. 2020. [DOI] [PubMed] [Google Scholar]
- 54.Hadamitzky M, Herring A, Kirchhof J, Bendix I, Haight MJ, Keyvani K, et al. Repeated systemic treatment with rapamycin affects behavior and amygdala protein expression in rats. The international journal of neuropsychopharmacology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ignacio ZM, Reus GZ, Arent CO, Abelaira HM, Pitcher MR, Quevedo J. New perspectives on the involvement of mTOR in depression as well as in the action of antidepressant drugs. Br J Clin Pharmacol. 2016;82(5):1280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Rosa M, Malaguarnera L. Chitotriosidase: A New Inflammatory Marker in Diabetic Complications. Pathobiology. 2016;83(4):211–9. [DOI] [PubMed] [Google Scholar]
- 57.Maiese K, Chong ZZ, Hou J, Shang YC. Erythropoietin and oxidative stress. Curr Neurovasc Res. 2008;5(2):125–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tulsulkar J, Nada SE, Slotterbeck BD, McInerney MF, Shah ZA. Obesity and hyperglycemia lead to impaired post-ischemic recovery after permanent ischemia in mice. Obesity (Silver Spring, Md. 2015;24(2):417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiao FH, He YH, Li QG, Wu H, Luo LH, Kong QP. A genome-wide scan reveals important roles of DNA methylation in human longevity by regulating age-related disease genes. PLoS One. 2015;10(3):e0120388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arildsen L, Andersen JV, Waagepetersen HS, Nissen JBD, Sheykhzade M. Hypermetabolism and impaired endothelium-dependent vasodilation in mesenteric arteries of type 2 diabetes mellitus db/db mice. Diabetes & vascular disease research : official journal of the International Society of Diabetes and Vascular Disease. 2019;16(6):1479164119865885. [DOI] [PubMed] [Google Scholar]
- 61.Ding S, Zhu Y, Liang Y, Huang H, Xu Y, Zhong C. Circular RNAs in Vascular Functions and Diseases. Adv Exp Med Biol. 2018;1087:287–97. [DOI] [PubMed] [Google Scholar]
- 62.Pal PB, Sonowal H, Shukla K, Srivastava SK, Ramana KV. Aldose reductase regulates hyperglycemia-induced HUVEC death via SIRT1/AMPK-alpha1/mTOR pathway. Journal of molecular endocrinology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alexandru N, Popov D, Georgescu A. Platelet dysfunction in vascular pathologies and how can it be treated. Thromb Res. 2012;129(2):116–26. [DOI] [PubMed] [Google Scholar]
- 64.Chiu SC, Chao CY, Chiang EI, Syu JN, Rodriguez RL, Tang FY. N-3 polyunsaturated fatty acids alleviate high glucose-mediated dysfunction of endothelial progenitor cells and prevent ischemic injuries both in vitro and in vivo. The Journal of nutritional biochemistry. 2017;42:172–81. [DOI] [PubMed] [Google Scholar]
- 65.Maiese K Disease onset and aging in the world of circular RNAs. J Transl Sci. 2016;2(6):327–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maiese K, Chong ZZ, Shang YC, Hou J. Rogue proliferation versus restorative protection: where do we draw the line for Wnt and forkhead signaling? Expert opinion on therapeutic targets. 2008;12(7):905–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maiese K, Li F, Chong ZZ, Shang YC. The Wnt signaling pathway: Aging gracefully as a protectionist? Pharmacol Ther. 2008;118(1):58–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perez-Hernandez N, Vargas-Alarcon G, Posadas-Sanchez R, Martinez-Rodriguez N, Tovilla-Zarate CA, Rodriguez-Cortes AA, et al. PHACTR1 Gene Polymorphism Is Associated with Increased Risk of Developing Premature Coronary Artery Disease in Mexican Population. International journal of environmental research and public health. 2016;13(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thackeray JT, Radziuk J, Harper ME, Suuronen EJ, Ascah KJ, Beanlands RS, et al. Sympathetic nervous dysregulation in the absence of systolic left ventricular dysfunction in a rat model of insulin resistance with hyperglycemia. Cardiovasc Diabetol. 2011;10:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maiese K Novel applications of trophic factors, Wnt and WISP for neuronal repair and regeneration in metabolic disease. Neural regeneration research. 2015;10(4):518–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mishra M, Duraisamy AJ, Kowluru RA. Sirt1- A Guardian of the Development of Diabetic Retinopathy. Diabetes. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ponnalagu M, Subramani M, Jayadev C, Shetty R, Das D. Retinal pigment epithelium-secretome: A diabetic retinopathy perspective. Cytokine. 2017;95:126–35. [DOI] [PubMed] [Google Scholar]
- 73.Kell DB, Pretorius E. No effects without causes: the Iron Dysregulation and Dormant Microbes hypothesis for chronic, inflammatory diseases. Biological reviews of the Cambridge Philosophical Society. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin X, Zhang N. Berberine: Pathways to protect neurons. Phytotherapy research : PTR. 2018. [DOI] [PubMed] [Google Scholar]
- 75.Maiese K FoxO Transcription Factors and Regenerative Pathways in Diabetes Mellitus. Curr Neurovasc Res. 2015;12(4):404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maiese K, Chong ZZ, Shang YC, Wang S. Translating cell survival and cell longevity into treatment strategies with SIRT1. Rom J Morphol Embryol. 2011;52(4):1173–85. [PMC free article] [PubMed] [Google Scholar]
- 77.Woodhams L, Al-Salami H. The roles of bile acids and applications of microencapsulation technology in treating Type 1 diabetes mellitus. Therapeutic delivery. 2017;8(6):401–9. [DOI] [PubMed] [Google Scholar]
- 78.Zhao Y, Scott NA, Fynch S, Elkerbout L, Wong WW, Mason KD, et al. Autoreactive T cells induce necrosis and not BCL-2-regulated or death receptor-mediated apoptosis or RIPK3-dependent necroptosis of transplanted islets in a mouse model of type 1 diabetes. Diabetologia. 2015;58(1):140–8. [DOI] [PubMed] [Google Scholar]
- 79.Maiese K The Mechanistic Target of Rapamycin (mTOR): Novel Considerations as an Antiviral Treatment. Curr Neurovasc Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo T, Liu T, Sun Y, Liu X, Xiong R, Li H, et al. Sonodynamic therapy inhibits palmitate-induced beta cell dysfunction via PINK1/Parkin-dependent mitophagy. Cell death & disease. 2019;10(6):457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klimova N, Kristian T. Multi-targeted Effect of Nicotinamide Mononucleotide on Brain Bioenergetic Metabolism. Neurochem Res. 2019. [DOI] [PubMed] [Google Scholar]
- 82.Maiese K, Chong ZZ, Shang YC, Wang S. Novel directions for diabetes mellitus drug discovery. Expert opinion on drug discovery. 2013;8(1):35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Othman MAM, Rajab E, AlMubarak A, AlNaisar M, Bahzad N, Kamal A. Erythropoietin Protects Against Cognitive Impairment and Hippocampal Neurodegeneration in Diabetic Mice. Behavioral sciences (Basel, Switzerland). 2018;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yelumalai S, Giribabu N, Karim K, Omar SZ, Salleh NB. In vivo administration of quercetin ameliorates sperm oxidative stress, inflammation, preserves sperm morphology and functions in streptozotocin-nicotinamide induced adult male diabetic rats. Archives of medical science : AMS. 2019;15(1):240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Coca SG, Ismail-Beigi F, Haq N, Krumholz HM, Parikh CR. Role of intensive glucose control in development of renal end points in type 2 diabetes mellitus: systematic review and meta-analysis intensive glucose control in type 2 diabetes. Arch Intern Med. 2012;172(10):761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee JH, Lee JH, Jin M, Han SD, Chon GR, Kim IH, et al. Diet control to achieve euglycemia induces significant loss of heart and liver weight via increased autophagy compared with ad libitum diet in diabetic rats. Exp Mol Med. 2014;46:e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maiese K MicroRNAs for the Treatment of Dementia and Alzheimer’s Disease. Curr Neurovasc Res. 2019;16(1):1–2. [DOI] [PubMed] [Google Scholar]
- 88.Chen F, Liu Z, Peng W, Gao Z, Ouyang H, Yan T, et al. Activation of EphA4 induced by EphrinA1 exacerbates disruption of the blood-brain barrier following cerebral ischemia-reperfusion via the Rho/ROCK signaling pathway. Experimental and therapeutic medicine. 2018;16(3):2651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maiese K Dampening the Progression of Dementia. Curr Neurovasc Res. 2018;15(2). [DOI] [PubMed] [Google Scholar]
- 90.Ong WY, Wu YJ, Farooqui T, Farooqui AA. Qi Fu Yin-a Ming Dynasty Prescription for the Treatment of Dementia. Mol Neurobiol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maiese K Targeting the core of neurodegeneration: FoxO, mTOR, and SIRT1. Neural regeneration research. 2021;16(3):448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li F, Chong ZZ, Maiese K. Cell Life Versus Cell Longevity: The Mysteries Surrounding the NAD(+) Precursor Nicotinamide. Curr Med Chem. 2006;13(8):883–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maiese K New Insights for nicotinamide: Metabolic disease, autophagy, and mTOR. Frontiers in bioscience (Landmark edition). 2020;25:1925–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maiese K, Chong ZZ. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol Sci. 2003;24(5):228–32. [DOI] [PubMed] [Google Scholar]
- 95.Maiese K, Chong ZZ, Hou J, Shang YC. The vitamin nicotinamide: translating nutrition into clinical care. Molecules. 2009;14(9):3446–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Braidy N, Liu Y. NAD+ therapy in age-related degenerative disorders: A benefit/risk analysis. Exp Gerontol. 2020:110831. [DOI] [PubMed] [Google Scholar]
- 97.Rex A, Fink H. Pharmacokinetic aspects of reduced nicotinamide adenine dinucleotide (NADH) in rats. Front Biosci. 2008;13:3735–41. [DOI] [PubMed] [Google Scholar]
- 98.Li F, Chong ZZ, Maiese K. Navigating novel mechanisms of cellular plasticity with the NAD+ precursor and nutrient nicotinamide. Front Biosci. 2004;9:2500–20. [DOI] [PubMed] [Google Scholar]
- 99.Jackson TM, Rawling JM, Roebuck BD, Kirkland JB. Large supplements of nicotinic acid and nicotinamide increase tissue NAD+ and poly(ADP-ribose) levels but do not affect diethylnitrosamine-induced altered hepatic foci in Fischer-344 rats. J Nutr. 1995;125(6):1455–61. [DOI] [PubMed] [Google Scholar]
- 100.Wojcik M, Seidle HF, Bieganowski P, Brenner C. Glutamine-dependent NAD+ synthetase. How a two-domain, three-substrate enzyme avoids waste. J Biol Chem. 2006;281(44):33395–402. [DOI] [PubMed] [Google Scholar]
- 101.Khan JA, Forouhar F, Tao X, Tong L. Nicotinamide adenine dinucleotide metabolism as an attractive target for drug discovery. Expert opinion on therapeutic targets. 2007;11(5):695–705. [DOI] [PubMed] [Google Scholar]
- 102.Khan JA, Xiang S, Tong L. Crystal structure of human nicotinamide riboside kinase. Structure. 2007;15(8):1005–13. [DOI] [PubMed] [Google Scholar]
- 103.Maiese K Triple play: Promoting neurovascular longevity with nicotinamide, WNT, and erythropoietin in diabetes mellitus. Biomed Pharmacother. 2008;62(4):218–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Magni G, Amici A, Emanuelli M, Orsomando G, Raffaelli N, Ruggieri S. Enzymology of NAD+ homeostasis in man. Cell Mol Life Sci. 2004;61(1):19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lin SJ, Guarente L. Nicotinamide adenine dinucleotide, a metabolic regulator of transcription, longevity and disease. Curr Opin Cell Biol. 2003;15(2):241–6. [DOI] [PubMed] [Google Scholar]
- 106.Hageman GJ, Stierum RH. Niacin, poly(ADP-ribose) polymerase-1 and genomic stability. Mutat Res. 2001;475(1–2):45–56. [DOI] [PubMed] [Google Scholar]
- 107.Qi Z, Xia J, Xue X, He Q, Ji L, Ding S. Long-term treatment with nicotinamide induces glucose intolerance and skeletal muscle lipotoxicity in normal chow-fed mice: compared to diet-induced obesity. The Journal of nutritional biochemistry. 2016;36:31–41. [DOI] [PubMed] [Google Scholar]
- 108.Shear DA, Dixon CE, Bramlett HM, Mondello S, Dietrich WD, Deng-Bryant Y, et al. Nicotinamide Treatment in Traumatic Brain Injury: Operation Brain Trauma Therapy. J Neurotrauma. 2016;33(6):523–37. [DOI] [PubMed] [Google Scholar]
- 109.Kiuchi K, Yoshizawa K, Shikata N, Matsumura M, Tsubura A. Nicotinamide prevents N-methyl-N-nitrosourea-induced photoreceptor cell apoptosis in Sprague-Dawley rats and C57BL mice. Exp Eye Res. 2002;74(3):383–92. [DOI] [PubMed] [Google Scholar]
- 110.Lin SH, Vincent A, Shaw T, Maynard KI, Maiese K. Prevention of nitric oxide-induced neuronal injury through the modulation of independent pathways of programmed cell death. J Cereb Blood Flow Metab. 2000;20(9):1380–91. [DOI] [PubMed] [Google Scholar]
- 111.Chong ZZ, Lin SH, Maiese K. Nicotinamide Modulates Mitochondrial Membrane Potential and Cysteine Protease Activity during Cerebral Vascular Endothelial Cell Injury. J Vasc Res. 2002;39(2):131–47. [DOI] [PubMed] [Google Scholar]
- 112.Mahmoud YI, Mahmoud AA. Role of nicotinamide (vitamin B3) in acetaminophen-induced changes in rat liver: Nicotinamide effect in acetaminophen-damaged liver. Exp Toxicol Pathol. 2016. [DOI] [PubMed] [Google Scholar]
- 113.Naia L, Rosenstock TR, Oliveira AM, Oliveira-Sousa SI, Caldeira GL, Carmo C, et al. Comparative Mitochondrial-Based Protective Effects of Resveratrol and Nicotinamide in Huntington’s Disease Models. Mol Neurobiol. 2016. [DOI] [PubMed] [Google Scholar]
- 114.Jayaram HN, Kusumanchi P, Yalowitz JA. NMNAT Expression and its Relation to NAD Metabolism. Curr Med Chem. 2011;18(13):1962–72. [DOI] [PubMed] [Google Scholar]
- 115.Feng Y, Wang Y, Jiang C, Fang Z, Zhang Z, Lin X, et al. Nicotinamide induces mitochondrial-mediated apoptosis through oxidative stress in human cervical cancer HeLa cells. Life Sci. 2017. [DOI] [PubMed] [Google Scholar]
- 116.Kulkarni CA, Brookes P. Cellular Compartmentation and the Redox/Non-Redox Functions of NAD+. Antioxid Redox Signal. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hou J, Chong ZZ, Shang YC, Maiese K. Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation. Curr Neurovasc Res. 2010;7(2):95–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maiese K, Vincent AM. Membrane asymmetry and DNA degradation: functionally distinct determinants of neuronal programmed cell death. J Neurosci Res. 2000;59(4):568–80. [DOI] [PubMed] [Google Scholar]
- 119.Schutters K, Reutelingsperger C. Phosphatidylserine targeting for diagnosis and treatment of human diseases. Apoptosis. 2010;15(9):1072–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shang YC, Chong ZZ, Hou J, Maiese K. Wnt1, FoxO3a, and NF-kappaB oversee microglial integrity and activation during oxidant stress. Cell Signal. 2010;22(9):1317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Taveira GB, Mello EO, Souza SB, Monteiro RM, Ramos AC, Carvalho AO, et al. Programmed cell death in yeast by thionin-like peptide from Capsicum annuum fruits involving activation of capases and extracelullar H(+) flux. Bioscience reports. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wei L, Sun C, Lei M, Li G, Yi L, Luo F, et al. Activation of Wnt/beta-catenin Pathway by Exogenous Wnt1 Protects SH-SY5Y Cells Against 6-Hydroxydopamine Toxicity. J Mol Neurosci. 2013;49(1):105–15. [DOI] [PubMed] [Google Scholar]
- 123.Williams CJ, Dexter DT. Neuroprotective and symptomatic effects of targeting group III mGlu receptors in neurodegenerative disease. J Neurochem. 2014;129(1):4–20. [DOI] [PubMed] [Google Scholar]
- 124.Chong ZZ, Lin SH, Li F, Maiese K. The sirtuin inhibitor nicotinamide enhances neuronal cell survival during acute anoxic injury through Akt, Bad, PARP, and mitochondrial associated “anti-apoptotic” pathways. Curr Neurovasc Res. 2005;2(4):271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bombeli T, Karsan A, Tait JF, Harlan JM. Apoptotic vascular endothelial cells become procoagulant. Blood. 1997;89(7):2429–42. [PubMed] [Google Scholar]
- 126.Chong ZZ, Kang JQ, Maiese K. Angiogenesis and plasticity: role of erythropoietin in vascular systems. J Hematother Stem Cell Res. 2002;11(6):863–71. [DOI] [PubMed] [Google Scholar]
- 127.Maiese K, Chong ZZ, Shang YC. Raves and risks for erythropoietin. Cytokine Growth Factor Rev. 2008;19(2):145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chong ZZ, Lin SH, Maiese K. The NAD+ precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J Cereb Blood Flow Metab. 2004;24(7):728–43. [DOI] [PubMed] [Google Scholar]
- 129.Lin SH, Chong ZZ, Maiese K. Nicotinamide: A Nutritional Supplement that Provides Protection Against Neuronal and Vascular Injury. J Med Food. 2001;4(1):27–38. [DOI] [PubMed] [Google Scholar]
- 130.Ahangarpour A, Ramezani Ali Akbari F, Fathi Moghadam H. Effect of C-peptide Alone or in Combination with Nicotinamide on Glucose and Insulin Levels in Streptozotocin-Nicotinamide-Induced Type 2 Diabetic Mice. The Malaysian journal of medical sciences : MJMS. 2014;21(4):12–7. [PMC free article] [PubMed] [Google Scholar]
- 131.Folwarczna J, Janas A, Cegiela U, Pytlik M, Sliwinski L, Matejczyk M, et al. Caffeine at a Moderate Dose Did Not Affect the Skeletal System of Rats with Streptozotocin-Induced Diabetes. Nutrients. 2017;9(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ghasemi A, Khalifi S, Jedi S. Streptozotocin-nicotinamide-induced rat model of type 2 diabetes (review). Acta physiologica Hungarica. 2014;101(4):408–20. [DOI] [PubMed] [Google Scholar]
- 133.Guo S, Chen Q, Sun Y, Chen J. Nicotinamide protects against skeletal muscle atrophy in streptozotocin-induced diabetic mice. Archives of physiology and biochemistry. 2019;125(5):470–7. [DOI] [PubMed] [Google Scholar]
- 134.Lai YF, Wang L, Liu WY. Nicotinamide pretreatment alleviates mitochondrial stress and protects hypoxic myocardial cells via AMPK pathway. European review for medical and pharmacological sciences. 2019;23(4):1797–806. [DOI] [PubMed] [Google Scholar]
- 135.Lee HJ, Yang SJ. Supplementation with Nicotinamide Riboside Reduces Brain Inflammation and Improves Cognitive Function in Diabetic Mice. International journal of molecular sciences. 2019;20(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Reddy S, Bibby NJ, Wu D, Swinney C, Barrow G, Elliott RB. A combined casein-free-nicotinamide diet prevents diabetes in the NOD mouse with minimum insulitis. Diabetes Res Clin Pract. 1995;29(2):83–92. [DOI] [PubMed] [Google Scholar]
- 137.Hu Y, Wang Y, Wang L, Zhang H, Zhao B, Zhang A, et al. Effects of nicotinamide on prevention and treatment of streptozotocin-induced diabetes mellitus in rats. Chin Med J (Engl). 1996;109(11):819–22. [PubMed] [Google Scholar]
- 138.Chlopicki S, Swies J, Mogielnicki A, Buczko W, Bartus M, Lomnicka M, et al. 1-Methylnicotinamide (MNA), a primary metabolite of nicotinamide, exerts anti-thrombotic activity mediated by a cyclooxygenase-2/prostacyclin pathway. Br J Pharmacol. 2007;152(2):230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hara N, Yamada K, Shibata T, Osago H, Hashimoto T, Tsuchiya M. Elevation of cellular NAD levels by nicotinic acid and involvement of nicotinic acid phosphoribosyltransferase in human cells. J Biol Chem. 2007;282(34):24574–82. [DOI] [PubMed] [Google Scholar]
- 140.Ieraci A, Herrera DG. Nicotinamide Protects against Ethanol-Induced Apoptotic Neurodegeneration in the Developing Mouse Brain. PLoS Med. 2006;3(4):e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tam D, Tam M, Maynard KI. Nicotinamide modulates energy utilization and improves functional recovery from ischemia in the in vitro rabbit retina. Ann N Y Acad Sci. 2005;1053:258–68. [DOI] [PubMed] [Google Scholar]
- 142.Olmos PR, Hodgson MI, Maiz A, Manrique M, De Valdes MD, Foncea R, et al. Nicotinamide protected first-phase insulin response (FPIR) and prevented clinical disease in first-degree relatives of type-1 diabetics. Diabetes Res Clin Pract. 2006;71(3):320–33. [DOI] [PubMed] [Google Scholar]
- 143.Crino A, Schiaffini R, Ciampalini P, Suraci MC, Manfrini S, Visalli N, et al. A two year observational study of nicotinamide and intensive insulin therapy in patients with recent onset type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2005;18(8):749–54. [DOI] [PubMed] [Google Scholar]
- 144.Liu HK, Green BD, Flatt PR, McClenaghan NH, McCluskey JT. Effects of long-term exposure to nicotinamide and sodium butyrate on growth, viability, and the function of clonal insulin secreting cells. Endocr Res. 2004;30(1):61–8. [DOI] [PubMed] [Google Scholar]
- 145.Reddy S, Salari-Lak N, Sandler S. Long-term effects of nicotinamide-induced inhibition of poly(adenosine diphosphate-ribose) polymerase activity in rat pancreatic islets exposed to interleukin-1 beta. Endocrinology. 1995;136(5):1907–12. [DOI] [PubMed] [Google Scholar]
- 146.Gaudineau C, Auclair K. Inhibition of human P450 enzymes by nicotinic acid and nicotinamide. Biochem Biophys Res Commun. 2004;317(3):950–6. [DOI] [PubMed] [Google Scholar]
- 147.Maiese K Healing the Heart with Sirtuins and Mammalian Forkhead Transcription Factors. Curr Neurovasc Res. 2020;17(1):1–2. [DOI] [PubMed] [Google Scholar]
- 148.Pan YR, Song JY, Fan B, Wang Y, Che L, Zhang SM, et al. mTOR may interact with PARP-1 to regulate visible light-induced parthanatos in photoreceptors. Cell Commun Signal. 2020;18(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Potthast AB, Nebl J, Wasserfurth P, Haufe S, Eigendorf J, Hahn A, et al. Impact of Nutrition on Short-Term Exercise-Induced Sirtuin Regulation: Vegans Differ from Omnivores and Lacto-Ovo Vegetarians. Nutrients. 2020;12(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tang YL, Zhang CG, Liu H, Zhou Y, Wang YP, Li Y, et al. Ginsenoside Rg1 Inhibits Cell Proliferation and Induces Markers of Cell Senescence in CD34+CD38- Leukemia Stem Cells Derived from KG1α Acute Myeloid Leukemia Cells by Activating the Sirtuin 1 (SIRT1)/Tuberous Sclerosis Complex 2 (TSC2) Signaling Pathway. Med Sci Monit. 2020;26:e918207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang GZ, Deng YJ, Xie QQ, Ren EH, Ma ZJ, He XG, et al. Sirtuins and intervertebral disc degeneration: Roles in inflammation, oxidative stress, and mitochondrial function. Clin Chim Acta. 2020;508:33–42. [DOI] [PubMed] [Google Scholar]
- 152.Charles S, Raj V, Arokiaraj J, Mala K. Caveolin1/protein arginine methyltransferase1/sirtuin1 axis as a potential target against endothelial dysfunction. Pharmacol Res. 2017;119:1–11. [DOI] [PubMed] [Google Scholar]
- 153.Chong ZZ, Shang YC, Wang S, Maiese K. SIRT1: New avenues of discovery for disorders of oxidative stress. Expert opinion on therapeutic targets. 2012;16(2):167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cui L, Guo J, Zhang Q, Yin J, Li J, Zhou W, et al. Erythropoietin activates SIRT1 to protect human cardiomyocytes against doxorubicin-induced mitochondrial dysfunction and toxicity. Toxicol Lett. 2017;275:28–38. [DOI] [PubMed] [Google Scholar]
- 155.Geng C, Xu H, Zhang Y, Gao Y, Li M, Liu X, et al. Retinoic acid ameliorates high-fat diet-induced liver steatosis through sirt1. Science China Life sciences. 2017. [DOI] [PubMed] [Google Scholar]
- 156.Maiese K FoxO Proteins in the Nervous System. Anal Cell Pathol (Amst). 2015;2015:569392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Maiese K Forkhead transcription factors: new considerations for alzheimer’s disease and dementia. J Transl Sci. 2016;2(4):241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Maiese K Moving to the Rhythm with Clock (Circadian) Genes, Autophagy, mTOR, and SIRT1 in Degenerative Disease and Cancer. Curr Neurovasc Res. 2017;14(3):299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Maiese K Harnessing the Power of SIRT1 and Non-coding RNAs in Vascular Disease. Curr Neurovasc Res. 2017;14(1):82–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Maiese K The mechanistic target of rapamycin (mTOR) and the silent mating-type information regulation 2 homolog 1 (SIRT1): oversight for neurodegenerative disorders. Biochem Soc Trans. 2018;46(2):351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Maiese K Novel Treatment Strategies for the Nervous System: Circadian Clock Genes, Non-coding RNAs, and Forkhead Transcription Factors. Curr Neurovasc Res. 2018;15(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016;12(1):1–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Maiese K Programming apoptosis and autophagy with novel approaches for diabetes mellitus. Curr Neurovasc Res. 2015;12(2):173–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Maiese K Targeting molecules to medicine with mTOR, autophagy and neurodegenerative disorders. Br J Clin Pharmacol. 2016;82(5):1245–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Maiese K, Chong ZZ, Shang YC, Wang S. Targeting disease through novel pathways of apoptosis and autophagy. Expert opinion on therapeutic targets. 2012;16(12):1203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kim SW, Lee JH, Moon JH, Nazim UM, Lee YJ, Seol JW, et al. Niacin alleviates TRAIL-mediated colon cancer cell death via autophagy flux activation. Oncotarget. 2016;7(4):4356–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Maiese K The bright side of reactive oxygen species: lifespan extension without cellular demise. J Transl Sci. 2016;2(3):185–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Audrito V, Vaisitti T, Rossi D, Gottardi D, D’Arena G, Laurenti L, et al. Nicotinamide Blocks Proliferation and Induces Apoptosis of Chronic Lymphocytic Leukemia Cells through Activation of the p53/miR-34a/SIRT1 Tumor Suppressor Network. Cancer Res. 2011;71(13):4473–83. [DOI] [PubMed] [Google Scholar]
- 169.Wang T, Cui H, Ma N, Jiang Y. Nicotinamide-mediated inhibition of SIRT1 deacetylase is associated with the viability of cancer cells exposed to antitumor agents and apoptosis. Oncology letters. 2013;6(2):600–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang JG, Zhao G, Qin Q, Wang B, Liu L, Liu Y, et al. Nicotinamide prohibits proliferation and enhances chemosensitivity of pancreatic cancer cells through deregulating SIRT1 and Ras/Akt pathways. Pancreatology : official journal of the International Association of Pancreatology (IAP) [et al]. 2013;13(2):140–6. [DOI] [PubMed] [Google Scholar]
- 171.Han J, Shi S, Min L, Wu T, Xia W, Ying W. NAD(+) Treatment Induces Delayed Autophagy in Neuro2a Cells Partially by Increasing Oxidative Stress. Neurochem Res. 2011;36(12):2270–7. [DOI] [PubMed] [Google Scholar]
- 172.Zhang XM, Jing YP, Jia MY, Zhang L. Negative transcriptional regulation of inflammatory genes by group B3 vitamin nicotinamide. Mol Biol Rep. 2012;39(12):10367–71. [DOI] [PubMed] [Google Scholar]
- 173.Shen C, Dou X, Ma Y, Ma W, Li S, Song Z. Nicotinamide protects hepatocytes against palmitate-induced lipotoxicity via SIRT1-dependent autophagy induction. Nutr Res. 2017;40:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Maiese K Erythropoietin and mTOR: A “One-Two Punch” for Aging-Related Disorders Accompanied by Enhanced Life Expectancy. Curr Neurovasc Res. 2016;13(4):329–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Maiese K Regeneration in the nervous system with erythropoietin. Frontiers in bioscience (Landmark edition). 2016;21:561–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang L, Teng R, Di L, Rogers H, Wu H, Kopp JB, et al. PPARalpha and Sirt1 mediate erythropoietin action in increasing metabolic activity and browning of white adipocytes to protect against obesity and metabolic disorders. Diabetes. 2013;62(12):4122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang L, Jia Y, Rogers H, Wu YP, Huang S, Noguchi CT. GATA-binding protein 4 (GATA-4) and T-cell acute leukemia 1 (TAL1) regulate myogenic differentiation and erythropoietin response via cross-talk with Sirtuin1 (Sirt1). J Biol Chem. 2012;287(36):30157–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hou J, Wang S, Shang YC, Chong ZZ, Maiese K. Erythropoietin Employs Cell Longevity Pathways of SIRT1 to Foster Endothelial Vascular Integrity During Oxidant Stress. Curr Neurovasc Res. 2011;8(3):220–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wu H, Wang H, Zhang W, Wei X, Zhao J, Yan P, et al. rhEPO affects apoptosis in hippocampus of aging rats by upregulating SIRT1. Int J Clin Exp Pathol. 2015;8(6):6870–80. [PMC free article] [PubMed] [Google Scholar]
- 180.Blagosklonny MV. From causes of aging to death from COVID-19. Aging (Albany NY). 2020;12(11):10004–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Saenwongsa W, Nithichanon A, Chittaganpitch M, Buayai K, Kewcharoenwong C, Thumrongwilainet B, et al. Metformin-induced suppression of IFN-alpha via mTORC1 signalling following seasonal vaccination is associated with impaired antibody responses in type 2 diabetes. Scientific reports. 2020;10(1):3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tian Y, Xiao YH, Geng T, Sun C, Gu J, Tang KF, et al. Clusterin suppresses spermatogenic cell apoptosis to alleviate diabetes-induced testicular damage by inhibiting autophagy via the PI3K/AKT/mTOR axis. Biol Cell. 2020. [DOI] [PubMed] [Google Scholar]
- 183.Chong ZZ, Shang YC, Wang S, Maiese K. Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin. Prog Neurobiol. 2012;99(2):128–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jesko H, Stepien A, Lukiw WJ, Strosznajder RP. The Cross-Talk Between Sphingolipids and Insulin-Like Growth Factor Signaling: Significance for Aging and Neurodegeneration. Mol Neurobiol. 2019;56(5):3501–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Maiese K Molecules to Medicine with mTOR: Translating Critical Pathways into Novel Therapeutic Strategies. Academic Press, Elsevier. 2016;ISBN 9780128027332. [Google Scholar]
- 186.Maiese K, Chong ZZ, Shang YC, Wang S. mTOR: on target for novel therapeutic strategies in the nervous system. Trends Mol Med. 2013;19(1):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253(5022):905–9. [DOI] [PubMed] [Google Scholar]
- 188.Hwang SK, Kim HH. The functions of mTOR in ischemic diseases. BMB Rep. 2011;44(8):506–11. [DOI] [PubMed] [Google Scholar]
- 189.Martinez de Morentin PB, Martinez-Sanchez N, Roa J, Ferno J, Nogueiras R, Tena-Sempere M, et al. Hypothalamic mTOR: the rookie energy sensor. Curr Mol Med. 2014;14(1):3–21. [DOI] [PubMed] [Google Scholar]
- 190.Maiese K Taking aim at Alzheimer’s disease through the mammalian target of rapamycin. Ann Med. 2014;46(8):587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Xue Q, Nagy JA, Manseau EJ, Phung TL, Dvorak HF, Benjamin LE. Rapamycin inhibition of the Akt/mTOR pathway blocks select stages of VEGF-A164-driven angiogenesis, in part by blocking S6Kinase. Arterioscler Thromb Vasc Biol. 2009;29(8):1172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Maiese K Novel Stem Cell Strategies with mTOR. Molecules to Medicine with mTOR: Translating Critical Pathways into Novel Therapeutic Strategies, Academic Press, Elsevier. 2016:3–22. [Google Scholar]
- 193.Foster KG, Acosta-Jaquez HA, Romeo Y, Ekim B, Soliman GA, Carriere A, et al. Regulation of mTOR complex 1 (mTORC1) by raptor Ser863 and multisite phosphorylation. J Biol Chem. 2010;285(1):80–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang L, Lawrence JC Jr., Sturgill TW, Harris TE. Mammalian target of rapamycin complex 1 (mTORC1) activity is associated with phosphorylation of raptor by mTOR. J Biol Chem. 2009;284(22):14693–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, et al. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137(5):873–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Maiese K Driving neural regeneration through the mammalian target of rapamycin. Neural regeneration research. 2014;9(15):1413–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Malla R, Ashby CR Jr., Narayanan NK, Narayanan B, Faridi JS, Tiwari AK. Proline-rich AKT substrate of 40-kDa (PRAS40) in the pathophysiology of cancer. Biochem Biophys Res Commun. 2015;463(3):161–6. [DOI] [PubMed] [Google Scholar]
- 198.Chong ZZ, Shang YC, Wang S, Maiese K. PRAS40 Is an Integral Regulatory Component of Erythropoietin mTOR Signaling and Cytoprotection. PLoS ONE. 2012;7(9):e45456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Fonseca BD, Smith EM, Lee VH, MacKintosh C, Proud CG. PRAS40 is a target for mammalian target of rapamycin complex 1 and is required for signaling downstream of this complex. J Biol Chem. 2007;282(34):24514–24. [DOI] [PubMed] [Google Scholar]
- 200.Shang YC, Chong ZZ, Wang S, Maiese K. WNT1 Inducible Signaling Pathway Protein 1 (WISP1) Targets PRAS40 to Govern beta-Amyloid Apoptotic Injury of Microglia. Curr Neurovasc Res. 2012;9(4):239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wang H, Zhang Q, Wen Q, Zheng Y, Philip L, Jiang H, et al. Proline-rich Akt substrate of 40kDa (PRAS40): a novel downstream target of PI3k/Akt signaling pathway. Cell Signal. 2012;24(1):17–24. [DOI] [PubMed] [Google Scholar]
- 202.Xiong X, Xie R, Zhang H, Gu L, Xie W, Cheng M, et al. PRAS40 plays a pivotal role in protecting against stroke by linking the Akt and mTOR pathways. Neurobiol Dis. 2014;66:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kim DH, Sarbassov DD, Ali SM, Latek RR, Guntur KV, Erdjument-Bromage H, et al. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003;11(4):895–904. [DOI] [PubMed] [Google Scholar]
- 204.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11(6):859–71. [DOI] [PubMed] [Google Scholar]
- 205.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6(11):1122–8. [DOI] [PubMed] [Google Scholar]
- 206.Garcia-Martinez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem J. 2008;416(3):375–85. [DOI] [PubMed] [Google Scholar]
- 207.Pearce LR, Sommer EM, Sakamoto K, Wullschleger S, Alessi DR. Protor-1 is required for efficient mTORC2-mediated activation of SGK1 in the kidney. Biochem J. 2011;436(1):169–79. [DOI] [PubMed] [Google Scholar]
- 208.Du LL, Chai DM, Zhao LN, Li XH, Zhang FC, Zhang HB, et al. AMPK Activation Ameliorates Alzheimer’s Disease-Like Pathology and Spatial Memory Impairment in a Streptozotocin-Induced Alzheimer’s Disease Model in Rats. J Alzheimers Dis. 2015;43(3):775–84. [DOI] [PubMed] [Google Scholar]
- 209.Jiang T, Yu JT, Zhu XC, Wang HF, Tan MS, Cao L, et al. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol. 2014;171(13):3146–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Peixoto CA, de Oliveira WH, da Rocha Araujo SM, Nunes AKS. AMPK activation: Role in the signaling pathways of neuroinflammation and neurodegeneration. Exp Neurol. 2017;298(PtA):31–41. [DOI] [PubMed] [Google Scholar]
- 211.Sato T, Nakashima A, Guo L, Tamanoi F. Specific activation of mTORC1 by Rheb G-protein in vitro involves enhanced recruitment of its substrate protein. J Biol Chem. 2009;284(19):12783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577–90. [DOI] [PubMed] [Google Scholar]
- 213.Chong ZZ, Maiese K. Mammalian Target of Rapamycin Signaling in Diabetic Cardiovascular Disease. Cardiovasc Diabetol. 2012;11(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Crespo MC, Tome-Carneiro J, Pintado C, Davalos A, Visioli F, Burgos-Ramos E. Hydroxytyrosol restores proper insulin signaling in an astrocytic model of Alzheimer’s disease. BioFactors (Oxford, England). 2017;43(4). [DOI] [PubMed] [Google Scholar]
- 215.Gu HF, Li N, Tang YL, Yan CQ, Shi Z, Yi SN, et al. Nicotinate-curcumin ameliorates cognitive impairment in diabetic rats by rescuing autophagic flux in CA1 hippocampus. CNS Neurosci Ther. 2019;25(4):430–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Maiese K, Chong ZZ, Wang S, Shang YC. Oxidant Stress and Signal Transduction in the Nervous System with the PI 3-K, Akt, and mTOR Cascade. International journal of molecular sciences. 2013;13(11):13830–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Park JA, Lee CH. Temporal changes in mammalian target of rapamycin (mTOR) and phosphorylated-mTOR expressions in the hippocampal CA1 region of rat with vascular dementia. Journal of veterinary science. 2017;18(1):11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wang H, Li Q, Sun S, Chen S. Neuroprotective Effects of Salidroside in a Mouse Model of Alzheimer’s Disease. Cell Mol Neurobiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Shang N, Zhang P, Wang S, Chen J, Fan R, Chen J, et al. Aluminum-Induced Cognitive Impairment and PI3K/Akt/mTOR Signaling Pathway Involvement in Occupational Aluminum Workers. Neurotox Res. 2020. [DOI] [PubMed] [Google Scholar]
- 220.Cheng J, North BJ, Zhang T, Dai X, Tao K, Guo J, et al. The emerging roles of protein homeostasis-governing pathways in Alzheimer’s disease. Aging Cell. 2018;17(5):e12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Shang YC, Chong ZZ, Wang S, Maiese K. Prevention of beta-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging (Albany NY). 2012;4(3):187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Huang C, Wen C, Yang M, Li A, Fan C, Gan D, et al. Astaxanthin Improved the Cognitive Deficits in APP/PS1 Transgenic Mice Via Selective Activation of mTOR. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2020. [DOI] [PubMed] [Google Scholar]
- 223.Liu Y, Jin S, Yang YT, Bai Y, Yong H, Bao W. Cognitive dysfunction associated with activation of the mTOR signaling pathway after TSH suppression therapy in rats. Endocrine journal. 2020. [DOI] [PubMed] [Google Scholar]
- 224.Xu F, Na L, Li Y, Chen L. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell & bioscience. 2020;10:54. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 225.Yin Q, Wang JF, Xu XH, Xie H. Effect of lycopene on pain facilitation and the SIRT1/mTOR pathway in the dorsal horn of burn injury rats. Eur J Pharmacol. 2020:173365. [DOI] [PubMed] [Google Scholar]
- 226.Beker MC, Caglayan B, Yalcin E, Caglayan AB, Turkseven S, Gurel B, et al. Time-of-Day Dependent Neuronal Injury After Ischemic Stroke: Implication of Circadian Clock Transcriptional Factor Bmal1 and Survival Kinase AKT. Mol Neurobiol. 2018;55(3):2565–76. [DOI] [PubMed] [Google Scholar]
- 227.Ramanathan C, Kathale ND, Liu D, Lee C, Freeman DA, Hogenesch JB, et al. mTOR signaling regulates central and peripheral circadian clock function. PLoS Genet. 2018;14(5):e1007369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Angelousi A, Kassi E, Ansari-Nasiri N, Randeva H, Kaltsas G, Chrousos G. Clock genes and cancer development in particular in endocrine tissues. Endocrine-related cancer. 2019;26(6):R305–r17. [DOI] [PubMed] [Google Scholar]
- 229.Sataranatarajan K, Ikeno Y, Bokov A, Feliers D, Yalamanchili H, Lee HJ, et al. Rapamycin Increases Mortality in db/db Mice, a Mouse Model of Type 2 Diabetes. J Gerontol A Biol Sci Med Sci. 2016;71(7):850–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Fraenkel M, Ketzinel-Gilad M, Ariav Y, Pappo O, Karaca M, Castel J, et al. mTOR inhibition by rapamycin prevents beta-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes. 2008;57(4):945–57. [DOI] [PubMed] [Google Scholar]
- 231.Deblon N, Bourgoin L, Veyrat-Durebex C, Peyrou M, Vinciguerra M, Caillon A, et al. Chronic mTOR inhibition by rapamycin induces muscle insulin resistance despite weight loss in rats. Br J Pharmacol. 2012;165(7):2325–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Samimi Z, Izadpanah A, Feizollahi P, Roghani SA, Assar S, Zafari P, et al. The Association between the Plasma Sugar and Lipid Profile with the Gene Expression of the Regulatory Protein of mTOR (Raptor) in Patients with Rheumatoid Arthritis. Immunol Invest. 2020:1–12. [DOI] [PubMed] [Google Scholar]
- 233.Pasini E, Flati V, Paiardi S, Rizzoni D, Porteri E, Aquilani R, et al. Intracellular molecular effects of insulin resistance in patients with metabolic syndrome. Cardiovasc Diabetol. 2010;9:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hamada S, Hara K, Hamada T, Yasuda H, Moriyama H, Nakayama R, et al. Upregulation of the mammalian target of rapamycin complex 1 pathway by Ras homolog enriched in brain in pancreatic beta-cells leads to increased beta-cell mass and prevention of hyperglycemia. Diabetes. 2009;58(6):1321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Pende M, Kozma SC, Jaquet M, Oorschot V, Burcelin R, Le Marchand-Brustel Y, et al. Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature. 2000;408(6815):994–7. [DOI] [PubMed] [Google Scholar]
- 236.Zhou J, Wu J, Zheng F, Jin M, Li H. Glucagon-like peptide-1 analog-mediated protection against cholesterol-induced apoptosis via mammalian target of rapamycin activation in pancreatic betaTC-6 cells −1mTORbetaTC-6. Journal of diabetes. 2015;7(2):231–9. [DOI] [PubMed] [Google Scholar]
- 237.Liu YW, Zhang L, Li Y, Cheng YQ, Zhu X, Zhang F, et al. Activation of mTOR signaling mediates the increased expression of AChE in high glucose condition: in vitro and in vivo evidences. Mol Neurobiol. 2015. [DOI] [PubMed] [Google Scholar]
- 238.Deng D, Yan J, Wu Y, Wu K, Li W. Morroniside suppresses hydrogen peroxide-stimulated autophagy and apoptosis in rat ovarian granulosa cells through the PI3K/AKT/mTOR pathway. Human & experimental toxicology. 2020:960327120960768. [DOI] [PubMed] [Google Scholar]
- 239.Gallyas F Jr., Sumegi B, Szabo C. Role of Akt Activation in PARP Inhibitor Resistance in Cancer. Cancers. 2020;12(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Jayaraj RL, Beiram R, Azimullah S, Mf NM, Ojha SK, Adem A, et al. Valeric Acid Protects Dopaminergic Neurons by Suppressing Oxidative Stress, Neuroinflammation and Modulating Autophagy Pathways. International journal of molecular sciences. 2020;21(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Meng J, Chen Y, Wang J, Qiu J, Chang C, Bi F, et al. EGCG protects vascular endothelial cells from oxidative stress-induced damage by targeting the autophagy-dependent PI3K-AKT-mTOR pathway. Ann Transl Med. 2020;8(5):200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Dai C, Tang S, Biao X, Xiao X, Chen C, Li J. Colistin induced peripheral neurotoxicity involves mitochondrial dysfunction and oxidative stress in mice. Mol Biol Rep. 2019;46(2). [DOI] [PubMed] [Google Scholar]
- 243.Miao XY, Gu ZY, Liu P, Hu Y, Li L, Gong YP, et al. The human glucagon-like peptide-1 analogue liraglutide regulates pancreatic beta-cell proliferation and apoptosis via an AMPK/mTOR/P70S6K signaling pathway. Peptides. 2013;39:71–9. [DOI] [PubMed] [Google Scholar]
- 244.Jung CH, Lee DH, Ahn J, Lee H, Choi WH, Jang YJ, et al. gamma-Oryzanol Enhances Adipocyte Differentiation and Glucose Uptake. Nutrients. 2015;7(6):4851–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Malla R, Wang Y, Chan WK, Tiwari AK, Faridi JS. Genetic ablation of PRAS40 improves glucose homeostasis via linking the AKT and mTOR pathways. Biochem Pharmacol. 2015. [DOI] [PubMed] [Google Scholar]
- 246.Chen C, Xu Y, Song Y. IGF-1 gene-modified muscle-derived stem cells are resistant to oxidative stress via enhanced activation of IGF-1R/PI3K/AKT signaling and secretion of VEGF. Mol Cell Biochem. 2014;386(1–2):167–75. [DOI] [PubMed] [Google Scholar]
- 247.Nagel G, Peter RS, Rosenbohm A, Koenig W, Dupuis L, Rothenbacher D, et al. Association of Insulin-like Growth Factor 1 Concentrations with Risk for and Prognosis of Amyotrophic Lateral Sclerosis - Results from the ALS Registry Swabia. Scientific reports. 2020;10(1):736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Huang D, Shen S, Cai M, Jin L, Lu J, Xu K, et al. Role of mTOR complex in IGF-1 induced neural differentiation of DPSCs. Journal of molecular histology. 2019. [DOI] [PubMed] [Google Scholar]
- 249.Xie T, Ye W, Liu J, Zhou L, Song Y. The Emerging Key Role of Klotho in the Hypothalamus-Pituitary-Ovarian Axis. Reprod Sci. 2020. [DOI] [PubMed] [Google Scholar]
- 250.Maiese K, Hou J, Chong ZZ, Shang YC. A fork in the path: Developing therapeutic inroads with FoxO proteins. Oxid Med Cell Longev. 2009;2(3):119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Maiese K, Shang YC, Chong ZZ, Hou J. Diabetes mellitus: channeling care through cellular discovery. Curr Neurovasc Res. 2010;7(1):59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Montesano A, Bonfigli AR, De Luca M, Crocco P, Garagnani P, Marasco E, et al. Erythropoietin (EPO) haplotype associated with all-cause mortality in a cohort of Italian patients with Type-2 Diabetes. Scientific reports. 2019;9(1):10395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Maiese K, Chong ZZ, Hou J, Shang YC. Oxidative stress: Biomarkers and novel therapeutic pathways. Exp Gerontol. 2010;45(3):217–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. Jama. 2005;293(1):90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Lee HJ, Koh SH, Song KM, Seol IJ, Park HK. The Akt/mTOR/p70S6K Pathway Is Involved in the Neuroprotective Effect of Erythropoietin on Hypoxic/Ischemic Brain Injury in a Neonatal Rat Model. Neonatology. 2016;110(2):93–100. [DOI] [PubMed] [Google Scholar]
- 256.Maiese K, Chong ZZ, Shang YC, Wang S. Erythropoietin: new directions for the nervous system. International journal of molecular sciences. 2012;13(9):11102–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wang GB, Ni YL, Zhou XP, Zhang WF. The AKT/mTOR pathway mediates neuronal protective effects of erythropoietin in sepsis. Mol Cell Biochem. 2014;385(1–2):125–32. [DOI] [PubMed] [Google Scholar]
- 258.Esmaeili Tazangi P, Moosavi SM, Shabani M, Haghani M. Erythropoietin improves synaptic plasticity and memory deficits by decrease of the neurotransmitter release probability in the rat model of Alzheimer’s disease. Pharmacol Biochem Behav. 2015;130:15–21. [DOI] [PubMed] [Google Scholar]
- 259.Li YP, Yang GJ, Jin L, Yang HM, Chen J, Chai GS, et al. Erythropoietin attenuates Alzheimer-like memory impairments and pathological changes induced by amyloid beta42 in mice. Brain Res. 2015;1618:159–67. [DOI] [PubMed] [Google Scholar]
- 260.Sun J, Martin JM, Vanderpoel V, Sumbria RK. The Promises and Challenges of Erythropoietin for Treatment of Alzheimer’s Disease. Neuromolecular Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Maiese K Preserving Brain Function During Development and Aging with Erythropoietin. Curr Neurovasc Res. 2019. [DOI] [PubMed] [Google Scholar]
- 262.Chong ZZ, Hou J, Shang YC, Wang S, Maiese K. EPO Relies upon Novel Signaling of Wnt1 that Requires Akt1, FoxO3a, GSK-3beta, and beta-Catenin to Foster Vascular Integrity During Experimental Diabetes. Curr Neurovasc Res. 2011;8(2):103–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Chong ZZ, Shang YC, Maiese K. Vascular injury during elevated glucose can be mitigated by erythropoietin and Wnt signaling. Curr Neurovasc Res. 2007;4(3):194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Gradinaru D, Margina D, Ilie M, Borsa C, Ionescu C, Prada GI. Correlation between erythropoietin serum levels and erythrocyte susceptibility to lipid peroxidation in elderly with type 2 diabetes. Acta physiologica Hungarica. 2015;102(4):400–8. [DOI] [PubMed] [Google Scholar]
- 265.Hamed S, Bennett CL, Demiot C, Ullmann Y, Teot L, Desmouliere A. Erythropoietin, a novel repurposed drug: an innovative treatment for wound healing in patients with diabetes mellitus. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2014;22(1):23–33. [DOI] [PubMed] [Google Scholar]
- 266.Niu HS, Chang CH, Niu CS, Cheng JT, Lee KS. Erythropoietin ameliorates hyperglycemia in type 1-like diabetic rats. Drug design, development and therapy. 2016;10:1877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Lin F, Xu W, Guan C, Zhou M, Hong W, Fu L, et al. Niacin protects against UVB radiation-induced apoptosis in cultured human skin keratinocytes. Int J Mol Med. 2012;29(4):593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kalender A, Selvaraj A, Kim SY, Gulati P, Brule S, Viollet B, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11(5):390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.He C, Zhu H, Li H, Zou MH, Xie Z. Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes. 2013;62(4):1270–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Oda SS. Metformin Protects against Experimental Acrylamide Neuropathy in Rats. Drug development research. 2017;78(7). [DOI] [PubMed] [Google Scholar]
- 271.Zimmerman MA, Biggers CD, Li PA. Rapamycin treatment increases hippocampal cell viability in an mTOR-independent manner during exposure to hypoxia mimetic, cobalt chloride. BMC Neurosci. 2018;19(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lally M, Tsoukas P, Halladay C, O’Neil E, Gravenstein S, Rudolph JL. Metformin is associated with Decreased 30-day Mortality among Nursing Home Residents Infected with SARS-CoV2. Journal of the American Medical Directors Association. 2020;October:S1525-8610(20)30924–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Teixeira LB, Santos WC. The mTOR pathway as a target for SARS-COV-2: Rapamycin as a possible alternative pharmacological therapeutic for COVID-19. Act Farma Terap. 2020;18(2):102–8. [Google Scholar]
- 274.Bramante C, Ingraham N, Murray T, Marmor S, Hoversten S, Gronski J, et al. Observational Study of Metformin and Risk of Mortality in Patients Hospitalized with Covid-19. medRxiv. 2020. [Google Scholar]
- 275.Cavalli E, Bramanti A, Ciurleo R, Tchorbanov AI, Giordano A, Fagone P, et al. Entangling COVID-19 associated thrombosis into a secondary antiphospholipid antibody syndrome: Diagnostic and therapeutic perspectives (Review). Int J Mol Med. 2020;46(3):903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Cicco S, Cicco G, Racanelli V, Vacca A. Neutrophil Extracellular Traps (NETs) and Damage-Associated Molecular Patterns (DAMPs): Two Potential Targets for COVID-19 Treatment. Mediators Inflamm. 2020;2020:7527953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Coto-Montes A, Boga JA. ER stress and autophagy induced by SARS-CoV-2: The targets for melatonin treatment. Melatonin Research. 2020;3(3):346–61. [Google Scholar]
- 278.Zheng, L’i LEFF-M, Concetta Maria. Potential Therapeutic Options for COVID-19. Infectious Microbes and Diseases. 2020;2(3):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Cava C, Bertoli G, Castiglioni I. A protein interaction map identifies existing drugs targeting SARS-CoV-2. BMC Pharmacol Toxicol. 2020;21(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Yanez M, Jhanji M, Murphy K, Gower RM, Sajish M, Jabbarzadeh E. Nicotinamide Augments the Anti-Inflammatory Properties of Resveratrol through PARP1 Activation. Scientific reports. 2019;9(1):10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Heer CD, Sanderson DJ, Voth LS, Alhammad YMO, Schmidt MS, Trammell SAJ, et al. Coronavirus infection and PARP expression dysregulate the NAD Metabolome: an actionable component of innate immunity. J Biol Chem. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Doroftei B, Ilie OD, Cojocariu RO, Ciobica A, Maftei R, Grab D, et al. Minireview Exploring the Biological Cycle of Vitamin B3 and Its Influence on Oxidative Stress: Further Molecular and Clinical Aspects. Molecules. 2020;25(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Miller R, Wentzel AR, Richards GA. COVID-19: NAD(+) deficiency may predispose the aged, obese and type2 diabetics to mortality through its effect on SIRT1 activity. Med Hypotheses. 2020;144:110044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Liu Y, Palanivel R, Rai E, Park M, Gabor TV, Scheid MP, et al. Adiponectin stimulates autophagy and reduces oxidative stress to enhance insulin sensitivity during high fat diet feeding in mice. Diabetes. 2014;64(1):36–48. [DOI] [PubMed] [Google Scholar]
- 285.Balan V, Miller GS, Kaplun L, Balan K, Chong ZZ, Li F, et al. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem. 2008;283(41):27810–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Moroz N, Carmona JJ, Anderson E, Hart AC, Sinclair DA, Blackwell TK. Dietary restriction involves NAD -dependent mechanisms and a shift toward oxidative metabolism. Aging Cell. 2014;13(6):1075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Liu P, Yang X, Hei C, Meli Y, Niu J, Sun T, et al. Rapamycin Reduced Ischemic Brain Damage in Diabetic Animals Is Associated with Suppressions of mTOR and ERK1/2 Signaling. Int J Biol Sci. 2016;12(8):1032–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Stevens MJ, Li F, Drel VR, Abatan OI, Kim H, Burnett D, et al. Nicotinamide reverses neurological and neurovascular deficits in streptozotocin diabetic rats. J Pharmacol Exp Ther. 2007;320(1):458–64. [DOI] [PubMed] [Google Scholar]
- 289.Zhao H, Wang ZC, Wang KF, Chen XY. Abeta peptide secretion is reduced by Radix Polygalaeinduced autophagy via activation of the AMPK/mTOR pathway. Molecular medicine reports. 2015;12(2):2771–6. [DOI] [PubMed] [Google Scholar]
- 290.Zhang ZH, Wu QY, Zheng R, Chen C, Chen Y, Liu Q, et al. Selenomethionine mitigates cognitive decline by targeting both tau hyperphosphorylation and autophagic clearance in an Alzheimer’s disease mouse model. J Neurosci. 2017;37(9):2449–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Liu Z, Stanojevic V, Brindamour LJ, Habener JF. GLP1-derived nonapeptide GLP1(28–36)amide protects pancreatic beta-cells from glucolipotoxicity. J Endocrinol. 2012;213(2):143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481(7382):511–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Weikel KA, Cacicedo JM, Ruderman NB, Ido Y. Knockdown of GSK3beta Increases Basal Autophagy and AMPK Signaling in Nutrient-laden Human Aortic Endothelial Cells. Bioscience reports. 2016;36(5):pii e00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Dong Y, Chen H, Gao J, Liu Y, Li J, Wang J. Molecular machinery and interplay of apoptosis and autophagy in coronary heart disease. J Mol Cell Cardiol. 2019;136:27–41. [DOI] [PubMed] [Google Scholar]
- 295.An T, Zhang X, Li H, Dou L, Huang X, Man Y, et al. GPR120 facilitates cholesterol efflux in macrophages through activation of AMPK signaling pathway. The FEBS journal. 2020(April 3). [DOI] [PubMed] [Google Scholar]
- 296.Shokri Afra H, Zangooei M, Meshkani R, Ghahremani MH, Ilbeigi D, Khedri A, et al. Hesperetin is a potent bioactivator that activates SIRT1-AMPK signaling pathway in HepG2 cells. Journal of physiology and biochemistry. 2019. [DOI] [PubMed] [Google Scholar]
- 297.Zhao D, Sun X, Lv S, Sun M, Guo H, Zhai Y, et al. Salidroside attenuates oxidized lowdensity lipoproteininduced endothelial cell injury via promotion of the AMPK/SIRT1 pathway. Int J Mol Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Zhang H, Yang X, Pang X, Zhao Z, Yu H, Zhou H. Genistein protects against ox-LDL-induced senescence through enhancing SIRT1/LKB1/AMPK-mediated autophagy flux in HUVECs. Mol Cell Biochem. 2019;455(1). [DOI] [PubMed] [Google Scholar]
- 299.Hu P, Lai D, Lu P, Gao J, He H. ERK and Akt signaling pathways are involved in advanced glycation end product-induced autophagy in rat vascular smooth muscle cells. Int J Mol Med. 2012;29(4):613–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Lee Y, Hong Y, Lee SR, Chang KT. Autophagy contributes to retardation of cardiac growth in diabetic rats. Lab Anim Res. 2012;28(2):99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Martino L, Masini M, Novelli M, Beffy P, Bugliani M, Marselli L, et al. Palmitate activates autophagy in INS-1E beta-cells and in isolated rat and human pancreatic islets. PLoS ONE. 2012;7(5):e36188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Kim KA, Shin YJ, Akram M, Kim ES, Choi KW, Suh H, et al. High glucose condition induces autophagy in endothelial progenitor cells contributing to angiogenic impairment. Biol Pharm Bull. 2014;37(7):1248–52. [DOI] [PubMed] [Google Scholar]
- 303.Wang L, Wu W, Chen J, Li Y, Xu M, Cai Y. miR122 and miR199 synergistically promote autophagy in oral lichen planus by targeting the Akt/mTOR pathway. Int J Mol Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Dorvash M, Farahmandnia M, Tavassoly I. A Systems Biology Roadmap to Decode mTOR Control System in Cancer. Interdiscip Sci. 2020;12(1):1–11. [DOI] [PubMed] [Google Scholar]
- 305.Johri MK, Lashkari HV, Gupta D, Vedagiri D, Harshan KH. mTORC1 restricts hepatitis C virus RNA replication through ULK1-mediated suppression of miR-122 and facilitates post-replication events. J Gen Virol. 2020;101(1):86–95. [DOI] [PubMed] [Google Scholar]
- 306.Maiese K New Challenges and Strategies for Cardiac Disease: Autophagy, mTOR, and AMP-Activated Protein Kinase. Curr Neurovasc Res. 2020. [DOI] [PubMed] [Google Scholar]
- 307.Yu S, Xin W, Jiang Q, Li A. Propofol exerts neuroprotective functions by down-regulating microRNA-19a in glutamic acid-induced PC12 cells. BioFactors (Oxford, England: ). 2020. [DOI] [PubMed] [Google Scholar]
- 308.Zhou Q, Zhou S, Wang H, Li Y, Xiao X, Yang J. Stable silencing of ROR1 regulates cell cycle, apoptosis, and autophagy in a lung adenocarcinoma cell line. Int J Clin Exp Pathol. 2020;13(5):1108–20. [PMC free article] [PubMed] [Google Scholar]
- 309.Fu L, Liu C, Chen L, Lv Y, Meng G, Hu M, et al. Protective Effects of 1-Methylnicotinamide on Abeta1–42-Induced Cognitive Deficits, Neuroinflammation and Apoptosis in Mice. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2019. [DOI] [PubMed] [Google Scholar]
- 310.Castro-Portuguez R, Sutphin GL. Kynurenine pathway, NAD(+) synthesis, and mitochondrial function: Targeting tryptophan metabolism to promote longevity and healthspan. Exp Gerontol. 2020:110841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Li J, Lu Y, Li N, Li P, Su J, Wang Z, et al. Muscle metabolomics analysis reveals potential biomarkers of exercisedependent improvement of the diaphragm function in chronic obstructive pulmonary disease. Int J Mol Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Osorio Alves J, Matta Pereira L, Cabral Coutinho do Rego Monteiro I, Pontes Dos Santos LH, Soares Marreiros Ferraz A, Carneiro Loureiro AC, et al. Strenuous Acute Exercise Induces Slow and Fast Twitch-Dependent NADPH Oxidase Expression in Rat Skeletal Muscle. Antioxidants (Basel, Switzerland). 2020;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Tong Y, Elkin KB, Peng C, Shen J, Li F, Guan L, et al. Reduced Apoptotic Injury by Phenothiazine in Ischemic Stroke through the NOX-Akt/PKC Pathway. Brain sciences. 2019;9(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Zhao C, Li W, Duan H, Li Z, Jia Y, Zhang S, et al. NAD(+) precursors protect corneal endothelial cells from UVB-induced apoptosis. Am J Physiol Cell Physiol. 2020. [DOI] [PubMed] [Google Scholar]
- 315.Li W, Zhu L, Ruan ZB, Wang MX, Ren Y, Lu W. Nicotinamide protects chronic hypoxic myocardial cells through regulating mTOR pathway and inducing autophagy. European review for medical and pharmacological sciences. 2019;23(12):5503–11. [DOI] [PubMed] [Google Scholar]
- 316.Ka M, Smith AL, Kim WY. MTOR controls genesis and autophagy of GABAergic interneurons during brain development. Autophagy. 2017:0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Yin B, Liang H, Chen Y, Chu K, Huang L, Fang L, et al. EGB1212 post-treatment ameliorates hippocampal CA1 neuronal death and memory impairment induced by transient global cerebral ischemia/reperfusion. Am J Chin Med. 2013;41(6):1329–41. [DOI] [PubMed] [Google Scholar]
- 318.Kang S, Chemaly ER, Hajjar RJ, Lebeche D. Resistin promotes cardiac hypertrophy via the AMP-activated protein kinase/mammalian target of rapamycin (AMPK/mTOR) and c-Jun N-terminal kinase/insulin receptor substrate 1 (JNK/IRS1) pathways. J Biol Chem. 2011;286(21):18465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Zhao Z, Liu H, Guo D. Aliskiren attenuates cardiac dysfunction by modulation of the mTOR and apoptosis pathways. Braz J Med Biol Res. 2020;53(2):e8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Gu Y, Lindner J, Kumar A, Yuan W, Magnuson MA. Rictor/mTORC2 is essential for maintaining a balance between beta-cell proliferation and cell size. Diabetes. 2011;60(3):827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.De Nobrega AK, Luz KV, Lyons LC. Resetting the Aging Clock: Implications for Managing Age-Related Diseases. Adv Exp Med Biol. 2020;1260:193–265. [DOI] [PubMed] [Google Scholar]
- 322.Egstrand S, Olgaard K, Lewin E. Circadian rhythms of mineral metabolism in chronic kidney disease-mineral bone disorder. Curr Opin Nephrol Hypertens. 2020;29(4):367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Finger AM, Kramer A. Mammalian circadian systems: Organization and modern life challenges. Acta Physiol (Oxf). 2020:e13548. [DOI] [PubMed] [Google Scholar]
- 324.Ma D, Hou L, Xia H, Li H, Fan H, Jia X, et al. PER2 inhibits proliferation and stemness of glioma stem cells via the Wnt/β‑catenin signaling pathway. Oncology reports. 2020;44(2):533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Mocayar Marón FJ, Ferder L, Reiter RJ, Manucha W. Daily and seasonal mitochondrial protection: Unraveling common possible mechanisms involving vitamin D and melatonin. J Steroid Biochem Mol Biol. 2020;199:105595. [DOI] [PubMed] [Google Scholar]
- 326.Tatullo M, Marrelli B, Zullo MJ, Codispoti B, Paduano F, Benincasa C, et al. Exosomes from Human Periapical Cyst-MSCs: Theranostic Application in Parkinson’s Disease. Int J Med Sci. 2020;17(5):657–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Zhang H, Liang J, Chen N. Do not neglect the role of circadian rhythm in muscle atrophy. Ageing research reviews. 2020;63:101155. [DOI] [PubMed] [Google Scholar]
- 328.Zhang Y, Peng X, Yang H, Zhao H, Xia B, You Y. The expression of the circadian gene TIMELESS in non-small-cell lung cancer and its clinical significance. Int J Clin Exp Pathol. 2020;13(9):2297–304. [PMC free article] [PubMed] [Google Scholar]
- 329.Hardeland R Melatonin and the pathologies of weakened or dysregulated circadian oscillators. J Pineal Res. 2017;62(1):12377. [DOI] [PubMed] [Google Scholar]
- 330.Liu H, Chen A. Roles of sleep deprivation in cardiovascular dysfunctions. Life Sci. 2019;219:231–7. [DOI] [PubMed] [Google Scholar]
- 331.Jenwitheesuk A, Nopparat C, Mukda S, Wongchitrat P, Govitrapong P. Melatonin regulates aging and neurodegeneration through energy metabolism, epigenetics, autophagy and circadian rhythm pathways. International journal of molecular sciences. 2014;15(9):16848–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Cronin P, McCarthy MJ, Lim ASP, Salmon DP, Galasko D, Masliah E, et al. Circadian alterations during early stages of Alzheimer’s disease are associated with aberrant cycles of DNA methylation in BMAL1. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2017;13(6):689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Hood S, Amir S. Neurodegeneration and the Circadian Clock. Frontiers in aging neuroscience. 2017;9:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Maiese K Novel Pathways of Autophagy for the Treatment of Nervous System Disorders. Autophagy and Cardiometabolic Diseases: From Molecular Mechanisms to Translational Medicine, Academic Press, Elsevier. 2018:187–97. [Google Scholar]
- 335.Frankel S, Ziafazeli T, Rogina B. dSir2 and longevity in Drosophila. Exp Gerontol. 2011;46(5):391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Hardeland R Neuroprotection by radical avoidance: search for suitable agents. Molecules. 2009;14(12):5054–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Vaccaro A, Issa AR, Seugnet L, Birman S, Klarsfeld A. Drosophila Clock Is Required in Brain Pacemaker Neurons to Prevent Premature Locomotor Aging Independently of Its Circadian Function. PLoS Genet. 2017;13(1):e1006507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Rossetti ML, Esser KA, Lee C, Tomko RJ Jr., Eroshkin AM, Gordon BS. Disruptions to the Limb Muscle Core Molecular Clock Coincide with Changes in Mitochondrial Quality Control following Androgen Depletion. Am J Physiol Endocrinol Metab. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Troisi RJ, Cowie CC, Harris MI. Diurnal variation in fasting plasma glucose: implications for diagnosis of diabetes in patients examined in the afternoon. Jama. 2000;284(24):3157–9. [DOI] [PubMed] [Google Scholar]
- 340.Patel SA, Velingkaar NS, Kondratov RV. Transcriptional Control of Antioxidant Defense by the Circadian Clock. Antioxid Redox Signal. 2014;20(18):2997–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Lin F, Chen Y, Li X, Zhao Q, Tan Z. Over-expression of circadian clock gene Bmal1 affects proliferation and the canonical Wnt pathway in NIH-3T3 cells. Cell Biochem Funct. 2013;31(2):166–72. [DOI] [PubMed] [Google Scholar]
- 342.Bunney BG, Li JZ, Walsh DM, Stein R, Vawter MP, Cartagena P, et al. Circadian dysregulation of clock genes: clues to rapid treatments in major depressive disorder. Mol Psychiatry. 2015;20(1):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.He Y, Cornelissen-Guillaume GG, He J, Kastin AJ, Harrison LM, Pan W. Circadian rhythm of autophagy proteins in hippocampus is blunted by sleep fragmentation. Chronobiology international. 2016;33(5):553–60. [DOI] [PubMed] [Google Scholar]
- 344.Rami A, Rawashdeh O. The hippocampal autophagic machinery is depressed in the absence of the circadian clock protein PER1 that may lead to vulnerability during cerebral ischemia. Curr Neurovasc Res. 2017;14(3):207–14. [DOI] [PubMed] [Google Scholar]
- 345.Chen B, Tan Y, Liang Y, Li Y, Chen L, Wu S, et al. Per2 participates in AKT-mediated drug resistance in A549/DDP lung adenocarcinoma cells. Oncology letters. 2017;13(1):423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Wu X, Li D, Liu J, Diao L, Ling S, Li Y, et al. Dammarane Sapogenins Ameliorates Neurocognitive Functional Impairment Induced by Simulated Long-Duration Spaceflight. Frontiers in pharmacology. 2017;8:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Bondy S, Maiese K. Aging and Age-Related Disorders. Springer Science. 2010;ISBN: 9781607616016. [Google Scholar]
- 348.Chen X, Kondo K, Motoki K, Homma H, Okazawa H. Fasting activates macroautophagy in neurons of Alzheimer’s disease mouse model but is insufficient to degrade amyloid-beta. Scientific reports. 2015;5:12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Dong W, Wang R, Ma LN, Xu BL, Zhang JS, Zhao ZW, et al. Influence of age-related learning and memory capacity of mice: different effects of a high and low caloric diet. Aging Clin Exp Res. 2016;28(2):303–11. [DOI] [PubMed] [Google Scholar]
- 350.Min JJ, Huo XL, Xiang LY, Qin YQ, Chai KQ, Wu B, et al. Protective effect of Dl-3n-butylphthalide on learning and memory impairment induced by chronic intermittent hypoxia-hypercapnia exposure. Scientific reports. 2014;4:5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134(2):317–28. [DOI] [PubMed] [Google Scholar]
- 352.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324(5927):654–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Oblong JE. The evolving role of the NAD+/nicotinamide metabolome in skin homeostasis, cellular bioenergetics, and aging. DNA Repair (Amst). 2014;23:59–63. [DOI] [PubMed] [Google Scholar]
- 354.Caton PW, Kieswich J, Yaqoob MM, Holness MJ, Sugden MC. Metformin opposes impaired AMPK and SIRT1 function and deleterious changes in core clock protein expression in white adipose tissue of genetically-obese db/db mice. Diabetes Obes Metab. 2011;13(12):1097–104. [DOI] [PubMed] [Google Scholar]
- 355.Bellet MM, Masri S, Astarita G, Sassone-Corsi P, Della Fazia MA, Servillo G. Histone Deacetylase SIRT1 Controls Proliferation, Circadian Rhythm, and Lipid Metabolism during Liver Regeneration in Mice. J Biol Chem. 2016;291(44):23318–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Sato S, Solanas G, Peixoto FO, Bee L, Symeonidi A, Schmidt MS, et al. Circadian Reprogramming in the Liver Identifies Metabolic Pathways of Aging. Cell. 2017;170(4):664–77 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]


