Abstract
Objective:
To investigate if magnetic resonance imaging (MRI) is an accurate predictor for death or moderate-severe disability at 18–22 months of age among infants with neonatal encephalopathy in a trial of cooling initiated at 6–24 hours.
Study design:
Sub-group analysis of infants ≥ 36 weeks of gestation with moderate-severe neonatal encephalopathy randomized at 6–24 postnatal hours to hypothermia or usual care in a multicenter trial of late hypothermia. MRI scans were performed per each center’s practice and interpreted by two central readers using the NICHD injury score (six levels, normal to hemispheric devastation). Neurodevelopmental outcomes were assessed at 18–22 months of age.
Results:
Of 168 enrollees, 128 had an interpretable MRI and were seen in follow-up (n=119) or died (n=9). MRI findings were predominantly acute injury and did not differ by cooling treatment. At 18–22 months, death or severe disability occurred in 20.3%. No infant had moderate disability. Agreement between central readers was moderate (weighted Kappa 0.56, 95% confidence interval 0.45–0.67). The adjusted odds of death or severe disability increased 3.7-fold (95% confidence interval 1.8–7.9) for each increment of injury score. The area under the curve for severe MRI patterns to predict death or severe disability was 0.77 and the positive and negative predictive values were 36% and 100%, respectively.
Conclusion:
MRI injury scores were associated with neurodevelopmental outcome at 18–22 months among infants in the Late Hypothermia Trial. However, the results suggest caution when using qualitative interpretations of MRI images to provide prognostic information to families following perinatal hypoxia-ischemia.
Trial registration
Keywords: imaging, hypoxic-ischemic encephalopathy, brain cooling
Magnetic resonance imaging (MRI) is the modality of choice to image the newborn brain following hypoxic-ischemic encephalopathy (HIE).1 The patterns of brain injury among newborn infants with HIE include white matter injury reflecting a watershed pattern or a predominant basal ganglia nuclei and thalamic pattern.2, 3 These patterns parallel those of primates after partial, prolonged asphyxia or a shorter complete asphyxia event, respectively.4 Severity and duration of hypoxia-ischemia, and associated conditions (eg, inflammation, pre-conditioning events) modify the pattern and degree of brain injury. Multiple randomized controlled trials (RCT) of hypothermia initiated at <6 hours of age for HIE reported that brain MRI performed in the days to weeks following birth helps predict neurodevelopmental outcome at 18 months.5–7
The Late Hypothermia Trial was an RCT of initiating hypothermia at 6–24 hours after birth for moderate or severe encephalopathy (NCT 00614744)8. The trial was designed for infants for whom hypothermia could not be initiated within 6 hours due to late transport, or who had evolution of encephalopathy including occurrence of seizures beyond 6 hours. After an in-utero presumed hypoxic-ischemic event, infants may have similar phenotypes (e.g., encephalopathy, biochemical evidence of impaired placental gas exchange) that may represent a single sentinel event, multiple repetitive events, an event before birth superimposed on an intrinsically vulnerable brain (two hit hypothesis),9 a pre-existing injury,1 or a diagnosis other than global hypoxia-ischemia. Evidence of brain injury remote from birth or a focal injury (e.g. stroke) may be unrecognized at birth. The latter may be more common in infants who present with encephalopathy after 6 hours, and may affect the treatment response and prognosis.
The objectives of this planned secondary analysis were to determine if MRI serves as an accurate predictor of death or disability at 18–22 months among infants in the Late Hypothermia Trial, to determine if MRI abnormalities were consistent with an acute perinatal hypoxic-ischemic event and not injury remote from birth (non-acute), and to compare the agreement among central and local readers for the extent and distribution of injury.
Methods:
This was a predefined sub-group analysis of the Late Hypothermia RCT, performed among 21 centers of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN). Criteria for eligibility and trial details have been published.8 This analysis included all enrolled infants who had an MRI performed during their neonatal hospitalization. We excluded infants with no MRI, a non-interpretable MRI, or no follow-up. This study was approved by each participating center’s institutional review board and was performed under a waiver of consent or was covered by the trial consent.
MRIs obtained for clinical assessment were used and were acquired at 1.5 or 3.0 Tesla. Imaging sequences followed usual center practice; the most common sequences (in decreasing frequency) were T1 and T2 weighted images, diffusion weighted images, gradient echo/susceptibility weighted images and T2 weighted fluid attenuated inversion recovery. MRIs were de-identified and sent to the data coordinating center (RTI International, Research Triangle Park, North Carolina). If an infant had multiple MRIs, imaging obtained after the intervention (cooling or usual care) was prioritized.
MRIs were interpreted by two central readers, both pediatric neuroradiologists experienced in interpretation of neonatal brain MRI.10, 11 The central readers were masked to patient data except the gestational age and postnatal age at MRI acquisition. They classified injury using a version of the NICHD injury score used in the first NRN hypothermia trial.6 Injury was characterized qualitatively using 6 levels of increasing severity (0, 1A, 1B, 2A, 2B and 3), (Figure 1; available at www.jpeds.com). Injury scores were clarified from the original description6 to affirm that an infarction in a vascular distribution would be scored as 2B (not 1B).
Figure 1: MRI Levels of Injury after Hypoxic-Ischemic Encephalopathy and 18–22 Month Outcome.
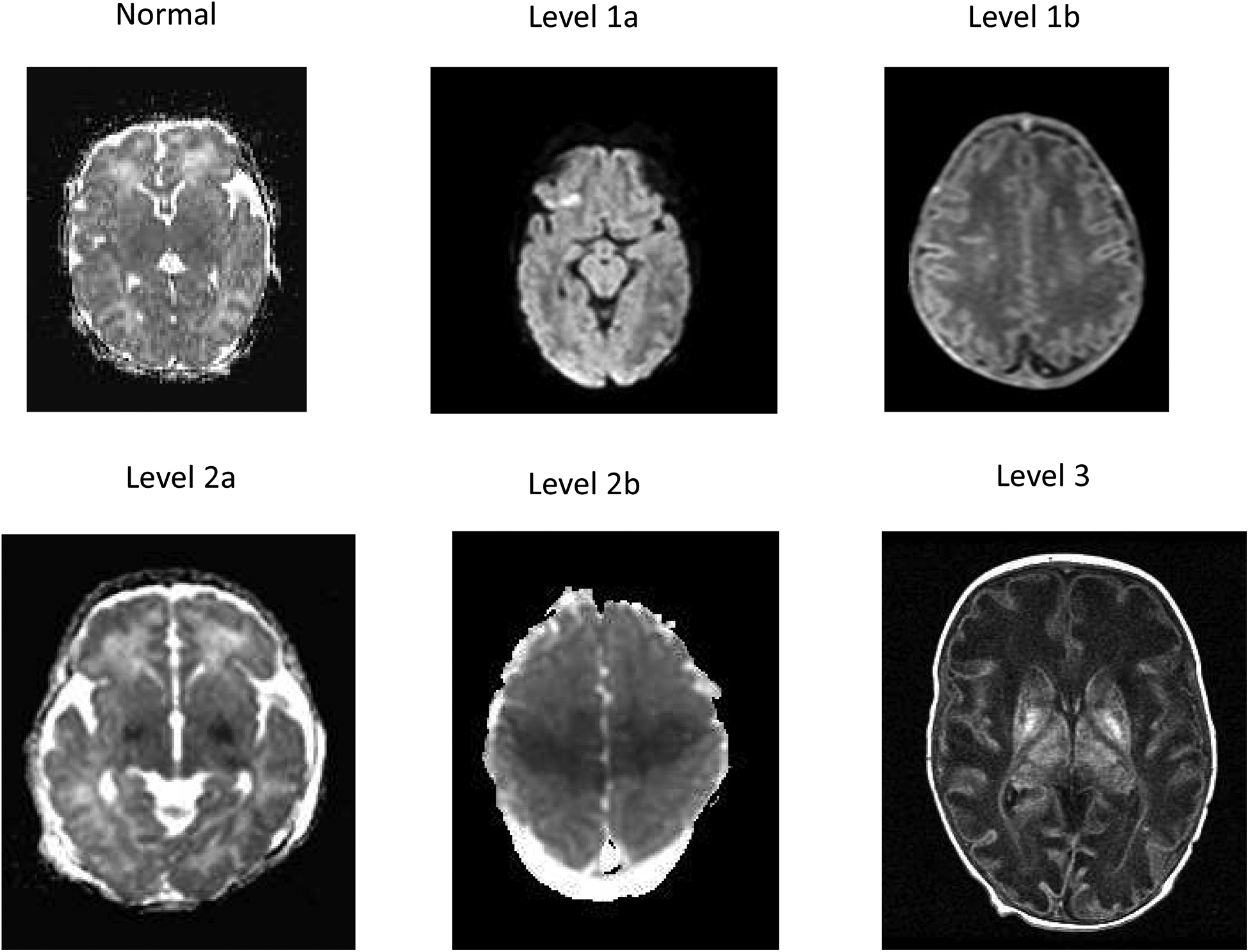
Representative images of the MRI levels of injury based on the NICHD pattern of signal abnormalities6 are presented and defined as follows:
Level 0: Normal signal throughout the brain on a diffusion image
Level 1a: Minimal cerebral lesions only without involvement of basal ganglia (BG), or thalamus (T), or anterior/posterior limb of the internal capsule (ALIC/PLIC respectively) and no areas of watershed infarction. A diffusion weighted image reveals a punctate lesion in the frontal region.
Level 1b: More extensive cerebral lesions not corresponding to a watershed or vascular distribution without BGT, PLIC, ALIC, involvement. A T1 weighted image indicates multiple high intensity punctate lesions in the white matter bilaterally.
Level 2a: Any BGT, ALIC, PLIC involvement or watershed infarction noted without any other cerebral lesions. A diffusion weighted image indicates abnormal signal intensity in the medial BGT and decreased signal intensity in the posterior limb of the internal capsule bilaterally.
Level 2b: Involvement of either BGT, ALIC, PLIC or areas of watershed/vascular distribution and additional cerebral lesions. A diffusion weighted image indicates restricted diffusion in the BGT and in the peri-rolandic and posterior parasagittal regions bilaterally.
Level 3: Cerebral hemispheric devastation. A T1 weighted image indicates global involvement of the white matter with attenuated signal intensity, a simplified cortical gray matter pattern and increased signal intensity in the BGT with loss of signal intensity of the PLIC.
Ten MRIs encompassing normal and a spectrum of injury were used to train central readers on the NICHD injury score, and results were discussed on conference calls. The extent of agreement on the training MRIs was not quantified. Each central reader was randomly assigned half of the MRIs for interpretation. MRIs considered normal did not undergo further readings. MRIs considered abnormal (abnormal tissue signal abnormality, NICHD score ≥ 1A, past injury, hemorrhage) were reviewed by the second central reader. Differences in assignment of the injury score were adjudicated by the central readers and the Late Hypothermia Trial MRI sub-committee. MRIs were also interpreted by local readers (primarily neuroradiologists), one for each participating NRN center. Local readers reviewed MRIs performed at their site unaware of treatment group and assigned an injury score after review of the publication describing the scoring.6
Outcomes:
Acute brain injury was assessed by signal intensity in basal ganglia/thalamic (BGT), anterior and posterior limb of the internal capsule (ALIC, PLIC), watershed infarct, cortical and hemispheric involvement. Non-acute changes included global cerebral atrophy, thinning of the corpus callosum, ventricular dilatation, cystic lesions, cortical dysplasia, cerebellar hypoplasia, midline structural abnormality, or longstanding hemorrhage. Signal abnormality was qualitatively graded for extent in the BGT (normal, minimal, moderate or severe) and in the PLIC (normal, equivocal or abnormal) consistent with prior descriptions.5 Agreement between central readers was determined for the NICHD injury score and for the BGT and PLIC injury. Agreement between local and central readers was determined for the NICHD injury score. Death or disability (moderate or severe) at 18–22 months of age was assessed by certified examiners as described previously.8
Sample size and statistical analysis plan:
The sample size was the number of infants enrolled in the Late Hypothermia Trial who had an interpretable MRI with an outcome at 18–22 months. Infants’ characteristics were compared with those not included for potential bias. Continuous variables were described using mean and standard deviation (SD) or median and interquartile range (IQR), and categorical variables were described using frequency and percentage. Comparisons (Wilcoxon and t-tests, and two-sided chi-square or Fisher exact tests) were considered significant with a P value of < 0.05.
Central reader interpretations including adjudicated readings were used to analyze associations between MRI findings and infant outcome. The unadjusted association between the NICHD injury score and death or disability was assessed using a Cochran Armitage linear trend test with the 6 injury levels as an ordinal variable. Predictive values were derived for the unadjusted prediction of death or disability by severe MRI abnormalities (injury score 2A, 2B and 3) versus normal or lesser abnormalities (injury score 0, 1A, and 1B).
Multivariable logistic regression assessed whether the NICHD injury score was an independent predictor of death or disability. Variables considered included baseline characteristics (gestational age, birth weight, sex, Apgar score at 5 minutes, umbilical cord pH and base deficit), delivery room interventions (intubation, chest compressions, emergency medication), characteristics at randomization (seizures, level of encephalopathy), treatment (hypothermia/control), and MRI (NICHD level of injury and age at MRI acquisition). Injury score was entered as a dichotomous variable (2A, 2B or 3 versus 0, 1A or 1B); center was not included given the small number of patients in multiple centers. Backward elimination was implemented until all remaining variables were statistically significant at p-value of 0.05. Treatment, although not significant, was included in the final model as a control variable. Results were expressed as adjusted odds ratios (OR) and 95% confidence intervals (95% CI).
Agreement for the assignment of the NICHD injury score was assessed using a weighted kappa.12 The injury score assigned by local readers represented a composite of multiple local readers because each local reader only interpreted MRIs performed in their center. RTI conducted the statistical analyses using SAS software (version 9.4).
Results:
The Late Hypothermia trial enrolled 168 infants. The consort diagram (Figure 2; available at www.jpeds.com) depicts the exclusions leading to 128 infants with an MRI and a known outcome (follow-up, n=119 or died, n=9) for this analysis. Infants with an MRI did not differ from infants without an MRI or an uninterpretable MRI except for use of inotropic support at randomization (Table I; available at www.jpeds.com).
Figure 2:
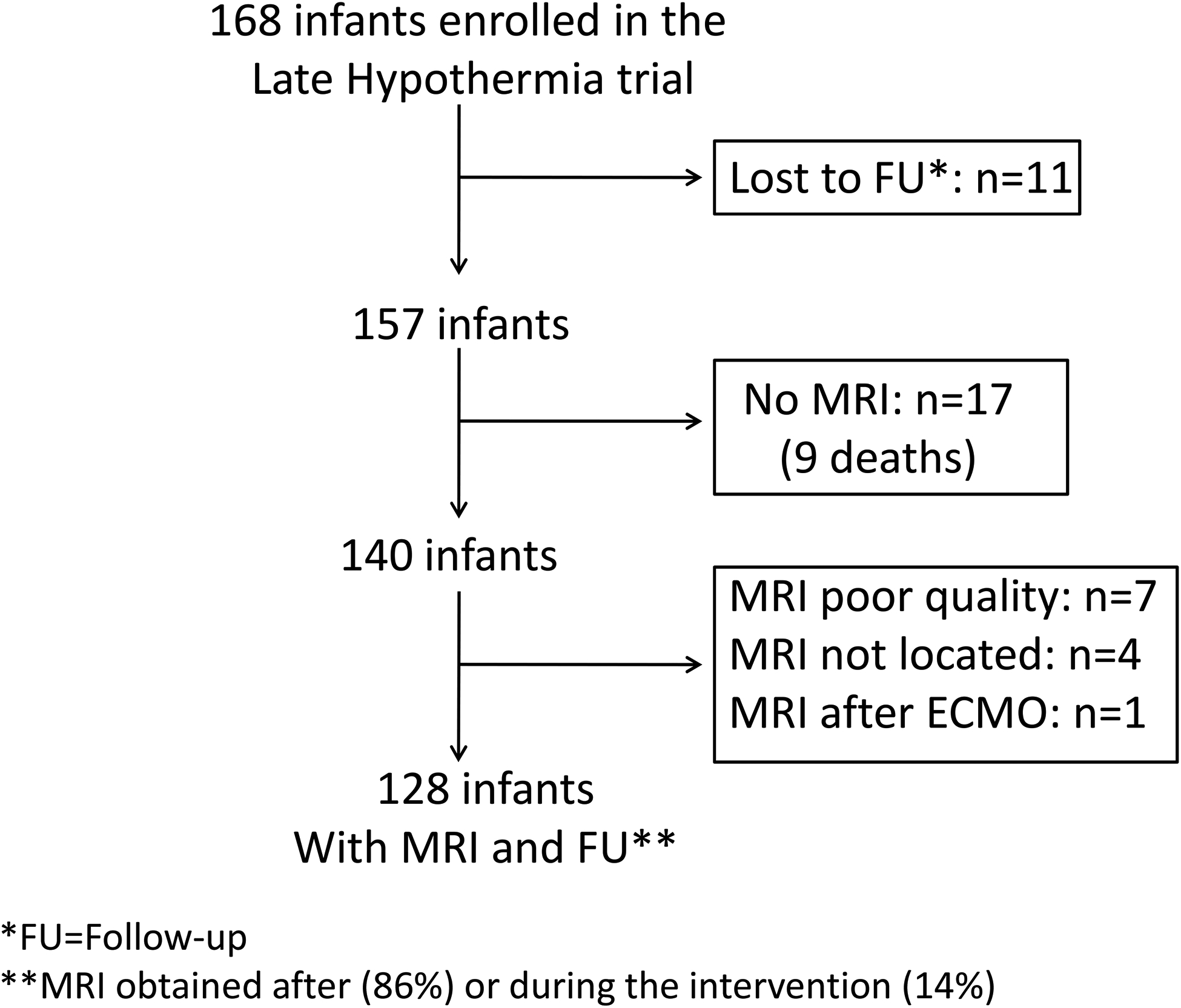
Flow diagram of infants enrolled in the Late Hypothermia trial who were analyzed for the secondary study.
Table 1 (online):
Maternal and Neonatal Characteristics of Infants with an MRI vs Without an MRI or an Uninterpretable MRI
| Characteristics | Infants with MRI (n=128) | Infants without MRI1 (n=29) | p-value2,3,4 |
|---|---|---|---|
| Maternal | |||
| Intrapartum Complications-No. (%) | |||
| Fetal decelerations | 90/128 (70.3%) | 20/28 (71.4%) | >0.99 |
| Cord mishap (prolapse, rupture, compression) | 20/128 (15.6%) | 1/29 (3.5%) | 0.13 |
| Uterine rupture | 3/128 (2.3%) | 1/29 (3.5%) | 0.56 |
| Maternal pyrexia (≥ 37.6°C) | 14/126 (11.1%) | 3/29 (10.3%) | >0.99 |
| Placental problems (abruption, previa) | 13/128 (10.2%) | 3/29 (10.3%) | >0.99 |
| Chorioamnionitis, clinical | 10/124 (8.1%) | 2/29 (6.9%) | >0.99 |
| Emergency cesarean delivery | 73/128 (57.0%) | 20/29 (69.0%) | 0.24 |
| Infant | |||
| Gestational age, wks, mean±SD | 39±1 (N=128) | 39±1 (N=29) | 0.95 |
| Birth weight, g, mean±SD | 3362±522 (N=128) | 3240±565 (N=29) | 0.26 |
| Delivery Room Intubation | 70/126 (55.6%) | 17/29 (58.6%) | 0.84 |
| Delivery Room Chest Compressions | 33/126 (26.2%) | 9/29 (31.0%) | 0.65 |
| Outborn | 108/128 (84.4%) | 27/29 (93.1%) | 0.37 |
| Apgar score, 5 min, median (IQR)5 | 4 (3–6) (N=128) | 4 (3–5) (N=29) | 0.61 |
| Apgar score, 10 min, median (IQR)5 | 6 (4–7) (N=104) | 6 (4–7) (N=27) | 0.94 |
| Cord Blood, mean±SD | |||
| pH | 6.98±0.16 (N=97) | 6.93±0.1 (N=19) | 0.22 |
| Base deficit (mEq/L) | 14.27±5.69 (N=81) | 14.25±3.53 (N=16) | 0.98 |
| Age at randomization, hrs, mean±SD | 15±5 (N=128) | 16±5 (N=29) | 0.73 |
| Level of Encephalopathy, No. (%) | |||
| Moderate encephalopathy | 118/128 (92.2%) | 24/29 (82.8%) | 0.16 |
| Severe encephalopathy | 10/128 (7.8%) | 5/29 (17.2%) | 0.16 |
| Inotropic support at randomization, No. (%) | 19/128 (14.8%) | 12/29 (41.48%) | <0.01 |
| Infants randomized to cooling, No. (%) | 66/128 (51.6%) | 12/29 (41.38%) | 0.32 |
Includes infants with outcome and without MRI or with unreadable MRI
Two-sample t-test for difference between characteristic mean (percentage) between infants with MRI and No-MRI
Two-sample Fisher Exact test (for small samples) for difference between characteristic percentage between infants with MRI and No-MRI
Wilcoxon Rank Test for medians
IQR: interquartile range
At 18–22 months, no disability was observed in 76 children (59.4%), and death or survival with any level of disability was observed in 52 children (40.6%). Among participants with death or disability (n=52), 26 had mild disability (50.0%), none had moderate disability, 17 had severe disability (32.7%), and 9 died (17.3%). Infants with death or severe disability were clustered among injury scores of 2A, 2B, and 3 for both central and local readers (Figure 3). In contrast, infants with mild or no disability were distributed across all injury scores, although the largest percentage of infants had a normal MRI. An increasing MRI injury score was associated with an increasing probability of death or severe disability (unadjusted p<0.0001, both central and local readers). Unadjusted prediction of death or severe disability at 18–22 months by severe MRI abnormalities (injury score 2A, 2B, and 3, Table 2) showed a low positive predictive value and likelihood ratio, a high negative predictive value and a low negative likelihood ratio, and an area under the curve of 0.77 and 0.80 for central and local readers, respectively. The age at MRI acquisition (median, IQR) among cooled and non-cooled infants was 7 days (6–11) and 6 days (5–7), respectively (p=0.001). The distribution of MRI injury score did not differ between treatment groups (Table 3) and was bilateral in 91% of infants.
Figure 3: MRI Injury Scores after Hypoxic-Ischemic Encephalopathy and 18–22 Month Outcome.
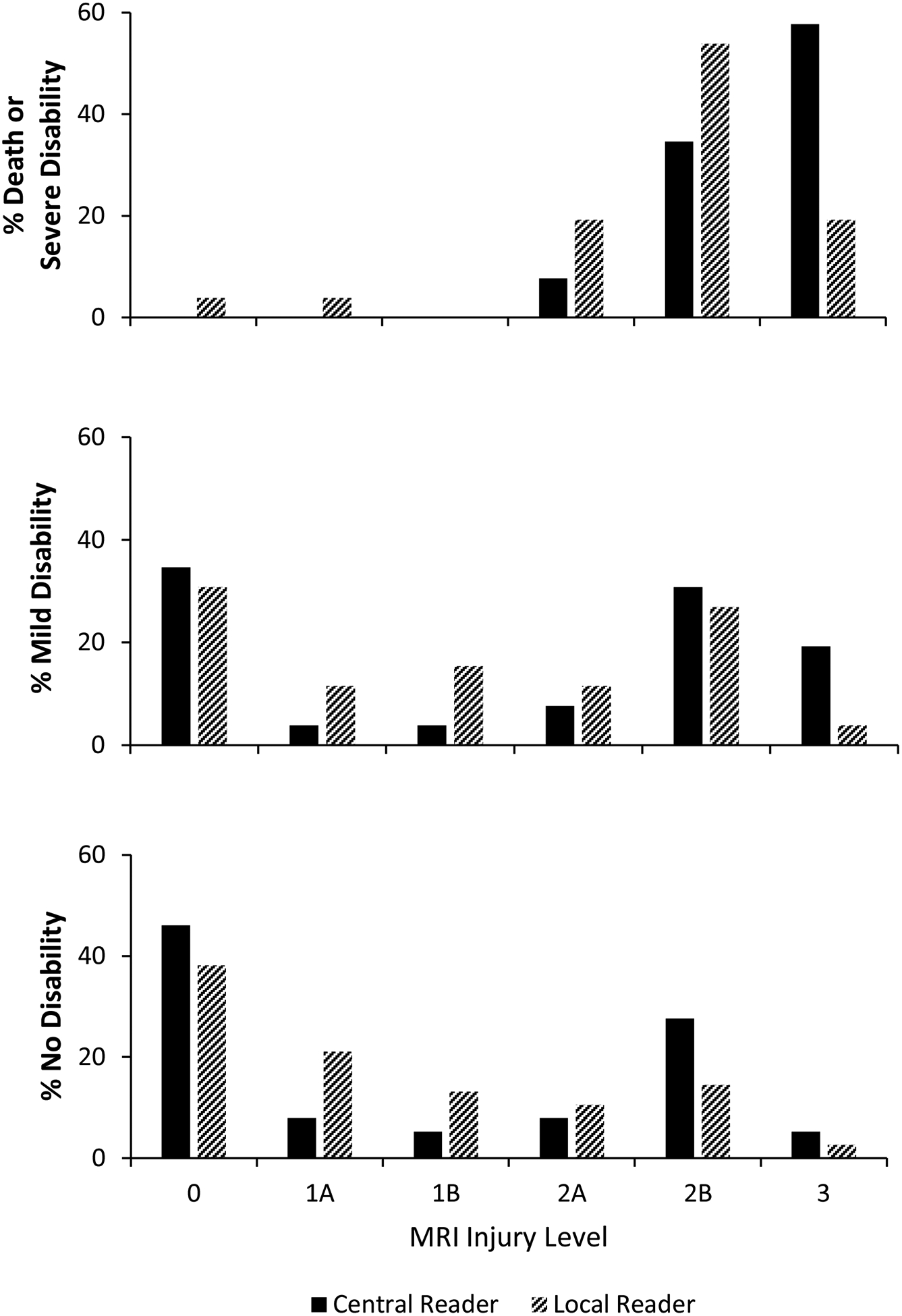
The 18–22 month outcome is plotted as function of the NICHD injury score for infants with death or severe disability (top panel), and for infants with mild disability (middle panel) or without disability (bottom panel). The injury score was determined by the central readers and included adjudicated interpretations. Black columns represent central readers and hashed columns represent local readers.
Table 2:
| Central Readers | Local Readers | |
|---|---|---|
| Sensitivity | 100% (87,100%)3 | 92% (75%,99%) |
| Specificity | 55% (45%,65%) | 69% (60%,78%) |
| Positive Predictive Value | 36% (25%,47%) | 43% (30%,56%) |
| Negative Predictive Value | 100% (94%,100%) | 97% (90%,100%) |
| Positive Likelihood Ratio | 2.2 | 3.0 |
| Negative Likelihood Ratio | 0.0 | 0.04 |
| Area under the curve | 0.77 (0.73,0.82) | 0.80 (0.74,0.87) |
Severe MRI abnormalities included NICHD levels 2A, 2B and 3
Unadjusted analyses
95% confidence intervals in parentheses
Table 3:
Distribution of the MRI Level of Injury by Treatment Group
| MRI Level of Injury* | Hypothermia (n=66) | Control (n=62) | ||
|---|---|---|---|---|
| Age of MRI (days) | N (%) | Age of MRI (days) | N (%) | |
| 0 | 8.6±3.4 | 24 (36.4) | 7.9±6.1 | 20 (32.3) |
| 1a | 8.0±4.1 | 4 (6.1) | 7.7±3.8 | 3 (4.8) |
| 1b | 8.5±2.1 | 2 (3.0) | 7.0±1.0 | 3 (4.8) |
| 2a | 5.8±0.4 | 5 (7.6) | 6.0±2.8 | 5 (8.1) |
| 2b | 9.2±3.4 | 18 (27.3) | 5.6±1.8 | 20 (32.3) |
| 3 | 8.0±3.6 | 13 (19.7) | 6.1±3.1 | 11 (17.7) |
Data are presented as the mean ± standard deviation. There was no difference in the distribution of injury among infants treated with hypothermia compared to non-cooled control infants (p=0.97)
Assignment of level of injury was per the Central reader or adjudicated reading if the central readers differed in their interpretations.
In the logistic regression analysis, the NICHD injury score was associated with death or severe disability after adjustment for level of encephalopathy, age at MRI and treatment (Table 4). There were no interactions with treatment group. The odds of death or severe disability increased by a factor of 3.75 with each increment of injury score (95% CI, 1.77–7. 94) for central readers and by a factor of 2.3 (95% CI, 1.6–3.5) for local readers.
Table 4:
Variables Associated with Death or Severe Disability after Hypoxic-Ischemic Encephalopathy
| Characteristics | Adjusted Odds Ratio (95% CI)1 |
|---|---|
| NICHD Injury score | 3.75 (1.77, 7.94) |
| Level of encephalopathy (Severe vs Moderate) | 8.84 (1.01, 76.92) |
| Age at MRI (≥7 days vs <7 days) | 0.28 (0.08, 1.00) |
| Treatment (Hypothermia vs Control) | 0.94 (0.28, 3.18) |
Adjusted odds ratios and 95% confidence intervals for variables used in a logistic regression to predict death or severe disability assessed at 18–22 months. Increasing MRI injury scores and severe encephalopathy compared to moderate encephalopathy was associated with increased odds of death or severe disability after adjustment for age at MRI acquisition and treatment. MRI acquired at ≥ 7 days compared to < 7 days were associated with reduced odds of death or severe disability (the upper confidence interval without rounding was 0.996).
Non-acute injury was present in 14 infants (10.9%) and included cerebral atrophy (4.8%), thinning of the corpus callosum (2.4%), and ventricular dilatation (10.9%). Ventricular dilatation was mild in 13 infants and moderate in 1 infant. There were no infants with cystic lesions, cortical dysplasia, cerebellar hypoplasia or long-standing hemorrhage. The age of MRI acquisition (median, IQR) was older (11, 6–14 days) among infants with non-acute injury compared with infants without (6, 5–8 days, p<0.0001). Infarction in a watershed distribution (between vascular territories) occurred in 37 infants (28.9%). Infarction in an arterial vascular distribution was noted in 17 infants (13.3%): 2 with right sided lesions, 6 with left sided lesions, and 9 with bilateral lesions. Because infarction in an arterial vascular distribution was higher than expected, analyses were repeated post-hoc after removal of these 17 infants. The unadjusted prediction of death or disability by severe MRI injury scores was unchanged (Table 5; available at www.jpeds.com) as was the adjusted odds of death or disability (OR 3.6, 95% CI, 1.7 – 7.7). Ten of 17 infants (59%) with infarction in an arterial vascular distribution had disability (mild-3, severe-7).
Table 5 (on-line):
Prediction of Death or Severe Disability by Severe MRI Abnormalities among Infants without Infarction in an Arterial Distribution1,2
| Central Readers | Local Readers | |
|---|---|---|
| Sensitivity | 100% (87,100%)3 | 92% (75%,99%) |
| Specificity | 55% (45%,65%) | 69% (60%,78%) |
| Positive Predictive Value | 36% (25%,47%) | 43% (30%,56%) |
| Negative Predictive Value | 100% (94%,100%) | 97% (90%,100%) |
| Positive Likelihood Ratio | 2.2 | 3.0 |
| Negative Likelihood Ratio | 0.0 | 0.04 |
| Area under the curve | 0.80 (0.75,0.85) | 0.80 (0.71,0.88) |
Severe MRI abnormalities included NICHD levels 2A, 2B and 3
Unadjusted analyses
95% confidence intervals in parentheses
Moderate agreement was observed between central readers for the NICHD injury score and signal abnormality classifications for the BGT and PLIC (NICHD injury score kappa: 0.56, 95% CI 0.45–0.67; BGT kappa: 0.55, 95% CI 0.43–0.67; PLIC kappa: 0.55, 95% CI 0.42–0.69). Agreement between local readers as a group and central reader 1 was substantial (kappa 0.73, 95% CI, 0.63–0.83), but moderate for central reader 2 (kappa 0.53, 95% CI, 0.40–0.67).
Discussion:
Among infants enrolled in the Late Hypothermia Trial, most MRI signal abnormalities were consistent with acute injury. Lower MRI injury scores were predictive of no disability or mild disability. However, higher injury scores did not accurately predict death or severe disability at 18–22 months and could be observed among infants considered normal or with mild disability. Of concern, the agreement for MRI classification between central readers, and between local and central readers was suboptimal. In addition, a surprisingly high percentage of infants had infarction in an arterial vascular distribution.
MRI is an integral part of the evaluation of infants with HIE and is used either alone or in conjunction with other assessments to counsel families regarding prognosis1 and in decisions regarding withdrawal of life support.13 This study used the NICHD injury score which is based primarily on the location of injury, and confirms prior observations that increasing extent of MRI abnormalities is predictive of death or disability at 18–22 months and during early childhood.6, 14 These observations add to cohort studies2, 3, 15–17 and clinical trials5–7 indicating that conventional MRI is a biomarker for neurodevelopmental outcome after HIE. However, the positive predictive value of severe MRI abnormalities (injury pattern 2A, 2B, and 3) to predict death or severe disability was poor compared with the NRN Hypothermia Trial initiated at <6 hours using one central reader.6 Elements of the injury score that may contribute to the positive predictive value are the use of location without qualification of injury extent, the degree of confluence of signal abnormality to define infarction, and the absence of an injury hierarchy involving basal ganglia/thalamic and white matter injury. The definitions of the injury score were clarified among central readers and would not change the classification as evidenced by the post-hoc analysis without infants with vascular infarction. MRIs were acquired at an earlier age in the Late Hypothermia Trial compared with the NRN trial at <6 hours (median 10 days, IQR 7–21), and later imaging may have a more evolved injury pattern. In contrast, Rutherford et al reported no difference in identified abnormalities before and after 8 days.5
The NICHD injury score was conceptualized to capture the extent of tissue damage with a single score and avoid assessing multiple brain regions to derive injury thresholds. This would simplify categorization of MRIs by neuroradiologists and provide a score with prognostic information. The moderate agreement between central readers for the injury score was disappointing, and is similar to a prior report for T1 and T2 weighted images being less concordant than diffusion images.18 Elements of the injury score that may contribute to poor agreement may reflect the same variables as listed for the positive predictive value of the injury score. In addition, the kappa values reflected agreement for MRIs interpreted as abnormal, and the absence of MRIs interpreted as normal may bias the agreement to lower values. Disagreement for “normal” MRIs maybe possible. The NICHD injury score was independently associated with death or disability but the confidence intervals were wide. In this multi-center trial, MRI sequences, post-acquisition processing and field strength were not harmonized across centers to enhance generalizability. These variables should not affect the extent of agreement between central readers. It may be of interest to compare our data with other published scoring systems that have included enhanced semi-quantitative scoring of recognized patterns of injury, and in some reports, weighting of specific brain regions to derive regional and total brain summary scores highly predictive of outcome.19, 20 In contrast, there are reports of the lack of reliability for MRI assessment of brain injury among preterm infants21–23 and term infants with encephalopathy.18 The results of the current study highlight the need for more objective MRI measures such as brain magnetic resonance spectroscopy biomarkers,24–26 diffusion tensor imaging to assess brain microstructure,27 and machine learning approaches to evaluate MRI data.28, 29
Enrollment of infants at 6–24 hours after birth could lead to inclusion of infants with a pre-existing injury or other diagnoses contributing to encephalopathy such as perinatal stroke, malformations, metabolic defects and congenital infections. MRI findings among infants in the Late Hypothermia Trial were predominantly acute injury, similar to a prior report30. When non-acute injury was observed, it was associated with a later age of MRI acquisition, and could reflect postnatal evolution of an acute injury, rather than injury remote from birth. An unanticipated finding was that 13% of infants had infarction in an arterial vascular distribution which is higher than the 3% noted in a prior report.30 There is overlap in the presentation of infants with strokes and HIE; seizures are common in neonatal arterial ischemic stroke (NAIS), occur most frequently between 12 and 72 hours after birth, and may be accompanied by encephalopathy.31 A potential role for hypoxia-ischemia in the development of NAIS has been raised,32 and the coexistence of NAIS and HIE has been reported.33, 34 All infants in the Late Hypothermia Trial with apparent NAIS met inclusion criteria of impaired placental gas exchange (some combination of a sentinel event, fetal acidemia, and need for resuscitation) accompanied by encephalopathy. These observations further support a biologic link between HIE and NAIS among infants with encephalopathy beyond 6 hours of age.
Strengths of this secondary analysis were its prospective design, a representative sub-group of the Late Hypothermia Trial, two central readers for MRI interpretation, and inclusion of local readers for insight into clinical MRI interpretation. Weaknesses that may impact maximizing the predictive value of an MRI under ideal circumstances include MRI acquisition over a range of post-natal ages which differed between treatment groups,35 lack of harmonization of MRI systems and sequences, and not including normal images in the adjudication process. The current 2A injury score does not separate watershed infarction from BGT and/or PLIC injury which likely have different outcomes. Other weaknesses include the inability to adjust for center, a relatively small sample of infants with death or severe disability, which limited the prediction of outcome for a specific injury score, and the early age of follow-up which may limit recognition of mild and moderate disability.
The moderate agreement between central readers suggest caution when using qualitative or even semi-quantitative interpretations of MRI images to provide prognostic information to families following HIE. The absence of severe injury on MRI, whether interpreted by central or local readers, can be used to reassure families. This study identified limitations in the predictive value of severe MRI abnormalities (injury scores 2A, 2B or 3) for neurodevelopmental outcome. Clinicians should be aware of limitations of structural MRI and qualitative scoring systems when counseling families.
Acknowledgments
We are indebted to our medical and nursing colleagues (Appendix) and the infants and their parents who agreed to take part in this study. We acknowledge Breda Munoz, PhD, for statistical analysis.
Additional members and participating hospitals of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network
NRN Steering Committee Chairs - Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine (2006–2011), Chicago, Illinois; Richard A. Polin, MD, Division of Neonatology, College of Physicians and Surgeons, Columbia University, New York, New York (2011-present).
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island, Providence, Rhode Island (U10 HD27904) – Martin Keszler, MD; William Oh, MD; Betty R. Vohr, MD; Elizabeth C. McGowan, MD; Barbara Alksninis, RNC PNP; Kristin Basso, MaT RN; Joseph Bliss, MD PhD; Carmena Bishop; Robert T. Burke, MD MPH; William Cashore, MD; Melinda Caskey, MD; Dan Gingras, RRT; Nicholas Guerina, MD PhD; Katharine Johnson, MD; Mary Lenore Keszler, MD Andrea M. Knoll; Theresa M. Leach, MEd CAES; Martha R. Leonard, BA BS; Emilee Little, RN BSN; Bonnie E. Stephens, MD; Elisa Vieira, RN BSN; Victoria E. Watson, MS CAS.
Case Western Reserve University, Rainbow Babies & Children’s Hospital, Cleveland, Ohio (U10 HD21364) – Anna Maria Hibbs, MD; Deanne E. Wilson-Costello, MD; Nancy S. Newman, RN; Beau Batton, MD; Monika Bhola, MD; Juliann M. Di Fiore, BSEE; Harriet G. Friedman, MA; Bonnie S. Siner, RN; Eileen K. Stork, MD; Gulgun Yalcinkaya, MD; Arlene Zadell, RN.
Children’s Mercy Hospital, University of Missouri Kansas City School of Medicine, Kansas City Missouri (U10 HD68284) – Eugenia K. Pallotto, MD MSCE; Howard W. Kilbride MD; Cheri Gauldin, RN BS CCRC; Anne Holmes RN MSN MBA-HCM CCRC; Kathy Johnson RN, CCRC; Allison Knutson, BSN RNC-NIC.
Cincinnati Children’s Hospital Medical Center, University of Cincinnati Medical Center, and Good Samaritan Hospital, Cincinnati, Ohio (U10 HD27853, UL1 TR77) – Kurt Schibler, MD; Kimberly Yolton, PhD; Cathy Grisby, BSN CCRC; Teresa L. Gratton, PA; Stephanie Merhar, MD MS; Sandra Wuertz, RN BSN CLC.
Duke University School of Medicine, University Hospital, University of North Carolina, and Duke Regional Hospital, Durham, North Carolina (U10 HD40492, UL1 TR1117) – C. Michael Cotten, MD MHS; Kimberley A. Fisher, PhD FNP-BC IBCLC; Sandra Grimes, RN BSN; Joanne Finkle, RN JD; Ricki F. Goldstein, MD; Kathryn E. Gustafson, PhD; William F. Malcolm, MD; Patricia L. Ashley, MD PhD; Kathy J. Auten, MSHS; Melody B. Lohmeyer, RN MSN; Matthew M. Laughon, MD MPH; Carl L. Bose, MD; Janice Bernhardt, MS RN; Cindy Clark, RN; Diane D. Warner, MD MPH; Janice Wereszcsak, CPNP; Sofia Aliaga, MD, MPH.
Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown, Atlanta, Georgia (U10 HD27851, UL1 TR454) – David P. Carlton, MD; Barbara J. Stoll, MD; Ellen C. Hale, RN BS CCRC; Yvonne Loggins, RN; Diane I. Bottcher, MSN RN; Colleen Mackie, BS RT; Maureen Mulligan LaRossa, RN; Ira Adams-Chapman, MD; Lynn C. Wineski, RN MS; Sheena L. Carter, PhD.
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Pregnancy and Perinatology Branch, Bethesda, Maryland –Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children at Indiana University Health, and Eskenazi Health, Indianapolis, Indiana (U10 HD27856, UL1 TR6) – Heidi M. Harmon, MD MS; Lu-Ann Papile, MD; Anna M. Dusick, MD (deceased); Susan Gunn, NNP-BC CCRC; Dianne E. Herron, RN CCRC; Abbey C. Hines, PsyD; Darlene Kardatzke, MD (deceased); Carolyn Lytle, MD MPH; Heike M. Minnich, PsyD HSPP; Leslie Richard, RN; Lucy C. Smiley, CCRC; Leslie Dawn Wilson, BSN CCRC.
McGovern Medical School at The University of Texas Health Science Center at Houston, Children’s Memorial Hermann Hospital, Houston, Texas (U10 HD21373) – Kathleen A. Kennedy, MD MPH; Elizabeth Allain, MS; Carrie M. Mason, MA LPA; Julie Arldt-McAlister, MSN APRN; Katrina Burson, RN BSN; Allison G. Dempsey, PhD; Andrea F. Duncan, MD MSClinRes; Patricia W. Evans, MD; Carmen Garcia, RN BSN; Charles E. Green, PhD; Margarita Jimenez, MD MPH; Janice John, CPNP; Patrick M. Jones, MD MA; M. Layne Lillie, RN BSN; Karen Martin, RN; Sara C. Martin, RN BSN; Georgia E. McDavid, RN; Shannon McKee EdS; Patti L. Pierce Tate, RCP; Shawna Rodgers, RN, BSN; Saba Khan Siddiki, MD; Daniel K. Sperry, RN; Sharon L. Wright, MT (ASCP).
Nationwide Children’s Hospital and The Ohio State University Wexner Medical Center, Columbus, Ohio (U10 HD68278) – Pablo J. Sánchez, MD; Leif D. Nelin, MD; Sudarshan R. Jadcherla, MD; Patricia Luzader, RN; Christine A. Fortney, PhD RN; Jennifer LGrothause, BA RN BSN.
RTI International (U10 HD36790) – Dennis Wallace, PhD; Marie G. Gantz, PhD; Kristin M. Zaterka-Baxter, RN BSN CCRP; Margaret M. Crawford, BS CCRP; Scott A. McDonald, BS; Jamie E. Newman, PhD MPH; Jeanette O’Donnell Auman, BS; James W. Pickett II, BS; Patricia Yost, BS.
Stanford University and Lucile Packard Children’s Hospital, Palo Alto, California (U10 HD27880, UL1 TR93) – Krisa P. Van Meurs, MD; David K. Stevenson, MD; M. Bethany Ball, BSc CCRC; Barbara Bentley, PsychD MS Ed; Valerie Y. Chock, MD MS Epi; Elizabeth F. Bruno, PhD; Alexis S. Davis, MD MS EPI; Maria Elena DeAnda, PhD; Anne M. DeBattista, RN PNP PhD; Beth Earhart, PhD; Lynne C. Huffman, MD; Jean G. Kohn, MD MPH; Casey E. Krueger, PhD; Melinda S. Proud, RCP; William D. Rhine, MD; Nicholas H. St. John, PhD; Heather Taylor, PhD; Hali E. Weiss, MD.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama, Birmingham, Alabama (U10 HD34216, M01 RR32) – Waldemar A. Carlo, MD; Myriam Peralta-Carcelen, MD MPH; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN; Vivien A. Phillips, RN BSN; Richard V. Rector, PhD; Sally Whitley, MA OTR-L FAOTA.
University of Iowa and Mercy Medical Center, Iowa City, Iowa (U10 HD53109, UL1 TR442) – Tarah T. Colaizy, MD MPH; Jane E. Brumbaugh, MD; Karen J. Johnson, RN BSN; Diane L. Eastman, RN CPNP MA; Michael J. Acarregui, MD MBA; Jacky R. Walker, RN; Claire A. Goeke, RN; Jonathan M. Klein, MD; Nancy J. Krutzfield, RN MA; Jeffrey L. Segar, MD; John M. Dagle, MD PhD; Julie B. Lindower, MD MPH; Steven J. McElroy, MD; Glenda K. Rabe, MD MME; Robert D. Roghair, MD; Lauritz R. Meyer, MD; Dan L. Ellsbury, MD; Donia B. Campbell, RNC-NIC; Cary R. Murphy, MD; Vipinchandra Bhavsar, MB BS.
University of New Mexico Health Sciences Center, Alburquerque, New Mexico (U10 HD53089, UL1 TR41) – Robin K. Ohls, MD; Conra Backstrom Lacy, RN; Sandra Sundquist Beauman, MSN RNC; Sandra Brown, BSN; Erika Fernandez, MD; Andrea Freeman Duncan, MD; Janell Fuller, MD; Elizabeth Kuan, RN, BSN; Jean R. Lowe, PhD.
University of Pennsylvania, Hospital of the University of Pennsylvania, Pennsylvania Hospital, and Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania (U10 HD68244) – Barbara Schmidt, MD MSc; Haresh Kirpalani, MB MSc; Sara B. DeMauro, MD MSCE; Kevin C. Dysart, MD; Soraya Abbasi, MD; Toni Mancini, RN BSN CCRC; Dara M. Cucinotta, RN; Judy C. Bernbaum, MD; Marsha Gerdes, PhD; Hallam Hurt, MD.
University of Rochester Medical Center, Golisano Children’s Hospital, and the University of Buffalo Women’s and Children’s Hospital of Buffalo, Rochester, New York (U10 HD68263, UL1 TR42) – Carl D’Angio, MD; Satyan Lakshminrusimha, MD; Nirupama Laroia, MD; Gary J. Myers, MD; Kelley Yost, PhD; Stephanie Guilford, BS; Rosemary L. Jensen; Karen Wynn, NNP RN; Osman Farooq, MD; Anne Marie Reynolds, MD MPH; Holly I.M. Wadkins, MA; Ashley Williams, MS Ed; Joan Merzbach, LMSW; Patrick Conway, MS; Melissa Bowman, MSN; Michele Hartley-McAndrew, MD; William Zorn, PhD; Cait Fallone, MA; Kyle Binion, BS; Constance Orme; Ann Marie Scorsone, MS, CCRC; Ashley Williams.
University of Texas Southwestern Medical Center, Parkland Health & Hospital System, and Children’s Medical Center Dallas, Dallas, Texas (U10 HD40689) – Luc P. Brion, MD; Lina F. Chalak, MD MSCS; Roy J. Heyne, MD; Lijun Chen, PhD RN; Diana M. Vasil, MSN BSN RNC-NIC; Sally S. Adams, MS RN CPNP; Catherine Twell Boatman, MS CIMI; Alicia Guzman; Elizabeth T. Heyne, MS MA PA-C PsyD; Lizette E. Lee, RN; Melissa H. Leps, RN; Linda A. Madden, BSN RN CPNP; Nancy A. Miller, RN; Emma Ramon, RNC-NIC RN BSN.
University of Utah Medical Center, Intermountain Medical Center, LDS Hospital, and Primary Children’s Medical Center, Salt Lake City, Utah (U10 HD53124) – Bradley A. Yoder, MD; Karen A. Osborne, RN BSN CCRC; Cynthia Spencer, RNC; R. Edison Steele, RN; Mike Steffen, PhD; Karena Strong, RN BSN; Kimberlee Weaver-Lewis, RN BSN; Shawna Baker, RN; Sarah Winter, MD; Karie Bird, RN BSN; Jill Burnett, RNC BSN.
Wayne State University, University of Michigan, Hutzel Women’s Hospital and Children’s Hospital of Michigan, Detroit, Michigan (U10 HD21385) – Beena G. Sood, MD MS; Rebecca Bara, RN BSN; Kirsten Childs, RN BSN; Lilia C. De Jesus, MD; Bogdan Panaitescu, MD; Sanjay Chawla, MD; Jeannette E. Prentice, MD; Laura A. Goldston, MA; Eunice Hinz Woldt, RN MSN; Girija Natarajan, MD; Monika Bajaj, MD; John Barks, MD; Mary Christensen, RT; Stephanie A. Wiggins, MS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
List of additional investigators and participating hospitals of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network is available in the Appendix (www.jpeds.com).
Data Sharing:
Data in this paper may be requested through a data use agreement. Further details are available at https://neonatal.rti.org/index.cfm?fuseaction=DataRequest.Home.
The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Center for Research Resources, and the National Center for Advancing Translational Sciences provided grant support for the Neonatal Research Network’s Late Hypothermia Trial (recruitment 4/17/2008 – 7/13/2014 and follow-up from 10/17/2009 – 9/12/2016) through cooperative agreements. Participating NRN sites collected data and transmitted it to RTI International, the data coordinating center (DCC) for the network, which stored, managed, and analyzed the data for this study. While NICHD staff did have input into the study design, conduct, analysis, and manuscript drafting, the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. On behalf of the NRN, A.D. (DCC Principal Investigator), Breda Munoz, PhD, and B.D. (DCC Statistician) had full access to all of the data in the study, and with the NRN Center Principal Investigators, take responsibility for the integrity of the data and accuracy of the data analysis. The authors declare no conflicts of interest.
Portions of this study were presented at the Pediatric Academic Societies annual meeting, May 6–9, 2017, San Francisco, CA
References
- 1.American College of Obstetricians and Gynecologists, American Academy of Pediatrics. Neonatal Encephalopathy and Neurologic Outcome, 2nd Ed. Washington, DC: American College of Obstetricians and Gynecologists; 2014. [Google Scholar]
- 2.Barkovich AJ, Hajnal BL, Vigneron D, Sola A, Partridge JC, Allen F, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol. 1998;19:143–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Miller SP, Ramaswamy V, Michelson D, Barkovich AJ, Holshouser B, Wycliffe N, et al. Patterns of brain injury in term neonatal encephalopathy. J Pediatr. 2005;146:453–60. [DOI] [PubMed] [Google Scholar]
- 4.Myers RE. Two patterns of perinatal brain damage and their conditions of occurrence. Am J Obstet Gynecol. 1972;112:246–76. [DOI] [PubMed] [Google Scholar]
- 5.Rutherford M, Ramenghi LA, Edwards AD, Brocklehurst P, Halliday H, Levene M, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 2010;9:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shankaran S, Barnes PD, Hintz SR, Laptook AR, Zaterka-Baxter KM, McDonald SA, et al. Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2012;97:F398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheong JL, Coleman L, Hunt RW, Lee KJ, Doyle LW, Inder TE, et al. Prognostic utility of magnetic resonance imaging in neonatal hypoxic-ischemic encephalopathy: substudy of a randomized trial. Arch Pediatr Adolesc Med. 2012;166:634–40. [DOI] [PubMed] [Google Scholar]
- 8.Laptook AR, Shankaran S, Tyson JE, Munoz B, Bell EF, Goldberg RN, et al. Effect of Therapeutic Hypothermia Initiated After 6 Hours of Age on Death or Disability Among Newborns With Hypoxic-Ischemic Encephalopathy: A Randomized Clinical Trial. JAMA. 2017;318:1550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badawi N, Kurinczuk JJ, Keogh JM, Alessandri LM, O’Sullivan F, Burton PR, et al. Intrapartum risk factors for newborn encephalopathy: the Western Australian case-control study. BMJ. 1998;317:1554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rollins N, Booth T, Morriss MC, Sanchez P, Heyne R, Chalak L. Predictive value of neonatal MRI showing no or minor degrees of brain injury after hypothermia. Pediatr Neurol. 2014;50:447–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shankaran S, Laptook AR, McDonald SA, Hintz SR, Barnes PD, Das A, et al. Acute Perinatal Sentinel Events, Neonatal Brain Injury Pattern, and Outcome of Infants Undergoing a Trial of Hypothermia for Neonatal Hypoxic-Ischemic Encephalopathy. J Pediatr. 2017;180:275–8 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furukawa TA, Straus SE, Bucher HC, Agoritsas T, Guyatt G. Diagnostic Tests. In: Guyatt G, Rennie D, Meade MO, Cook DJ, editors. Users’ Guides to the Medical Literature: A Manual for Evidence-Based Clinical Practices. 3rd ed. New York: McGraw Hill; 2015. p. 345–35. [Google Scholar]
- 13.Wilkinson D. MRI and withdrawal of life support from newborn infants with hypoxic-ischemic encephalopathy. Pediatrics. 2010;126:e451–8. [DOI] [PubMed] [Google Scholar]
- 14.Shankaran S, McDonald SA, Laptook AR, Hintz SR, Barnes PD, Das A, et al. Neonatal Magnetic Resonance Imaging Pattern of Brain Injury as a Biomarker of Childhood Outcomes following a Trial of Hypothermia for Neonatal Hypoxic-Ischemic Encephalopathy. J Pediatr. 2015;167:987–93 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okereafor A, Allsop J, Counsell SJ, Fitzpatrick J, Azzopardi D, Rutherford MA, et al. Patterns of brain injury in neonates exposed to perinatal sentinel events. Pediatrics. 2008;121:906–14. [DOI] [PubMed] [Google Scholar]
- 16.Steinman KJ, Gorno-Tempini ML, Glidden DV, Kramer JH, Miller SP, Barkovich AJ, et al. Neonatal watershed brain injury on magnetic resonance imaging correlates with verbal IQ at 4 years. Pediatrics. 2009;123:1025–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez-Biarge M, Diez-Sebastian J, Kapellou O, Gindner D, Allsop JM, Rutherford MA, et al. Predicting motor outcome and death in term hypoxic-ischemic encephalopathy. Neurology. 2011;76:2055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goergen SK, Ang H, Wong F, Carse EA, Charlton M, Evans R, et al. Early MRI in term infants with perinatal hypoxic-ischaemic brain injury: interobserver agreement and MRI predictors of outcome at 2 years. Clin Radiol. 2014;69:72–81. [DOI] [PubMed] [Google Scholar]
- 19.Trivedi SB, Vesoulis ZA, Rao R, Liao SM, Shimony JS, McKinstry RC, et al. A validated clinical MRI injury scoring system in neonatal hypoxic-ischemic encephalopathy. Pediatr Radiol. 2017;47:1491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weeke LC, Groenendaal F, Mudigonda K, Blennow M, Lequin MH, Meiners LC, et al. A Novel Magnetic Resonance Imaging Score Predicts Neurodevelopmental Outcome After Perinatal Asphyxia and Therapeutic Hypothermia. J Pediatr. 2018;192:33–40 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart AR, Smith MF, Rigby AS, Wallis LI, Whitby EH. Appearances of diffuse excessive high signal intensity (DEHSI) on MR imaging following preterm birth. Pediatr Radiol. 2010;40:1390–6. [DOI] [PubMed] [Google Scholar]
- 22.Calloni SF, Cinnante CM, Bassi L, Avignone S, Fumagalli M, Bonello L, et al. Neurodevelopmental outcome at 36 months in very low birth weight premature infants with MR diffuse excessive high signal intensity (DEHSI) of cerebral white matter. Radiol Med. 2015;120:1056–63. [DOI] [PubMed] [Google Scholar]
- 23.Morel B, Antoni G, Teglas JP, Bloch I, Adamsbaum C. Neonatal brain MRI: how reliable is the radiologist’s eye? Neuroradiology. 2016;58:189–93. [DOI] [PubMed] [Google Scholar]
- 24.Barta H, Jermendy A, Kolossvary M, Kozak LR, Lakatos A, Meder U, et al. Prognostic value of early, conventional proton magnetic resonance spectroscopy in cooled asphyxiated infants. BMC Pediatr. 2018;18:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lally PJ, Montaldo P, Oliveira V, Soe A, Swamy R, Bassett P, et al. Magnetic resonance spectroscopy assessment of brain injury after moderate hypothermia in neonatal encephalopathy: a prospective multicentre cohort study. Lancet Neurol. 2019;18:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitra S, Kendall GS, Bainbridge A, Sokolska M, Dinan M, Uria-Avellanal C, et al. Proton magnetic resonance spectroscopy lactate/N-acetylaspartate within 2 weeks of birth accurately predicts 2-year motor, cognitive and language outcomes in neonatal encephalopathy after therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed. 2019;104:F424–F32. [DOI] [PubMed] [Google Scholar]
- 27.Azzopardi D, Edwards AD. Magnetic resonance biomarkers of neuroprotective effects in infants with hypoxic ischemic encephalopathy. Semin Fetal Neonatal Med. 2010;15:261–9. [DOI] [PubMed] [Google Scholar]
- 28.Rajkomar A, Dean J, Kohane I. Machine Learning in Medicine. N Engl J Med. 2019;380:1347–58. [DOI] [PubMed] [Google Scholar]
- 29.Siddiqui MF, Reza AW, Kanesan J. An Automated and Intelligent Medical Decision Support System for Brain MRI Scans Classification. PLoS One. 2015;10:e0135875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cowan F, Rutherford M, Groenendaal F, Eken P, Mercuri E, Bydder GM, et al. Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet. 2003;361:736–42. [DOI] [PubMed] [Google Scholar]
- 31.Ferriero DM, Fullerton HJ, Bernard TJ, Billinghurst L, Daniels SR, DeBaun MR, et al. Management of stroke in neonates and children: A scientific statement from the American Heart Association/American Stroke Association. Stroke. 2019;50:e51–e96. [DOI] [PubMed] [Google Scholar]
- 32.Michoulas A, Basheer SN, Roland EH, Poskitt K, Miller S, Hill A. The role of hypoxia-ischemia in term newborns with arterial stroke. Pediatr Neurol. 2011;44:254–8. [DOI] [PubMed] [Google Scholar]
- 33.deVeber GA, Kirton A, Booth FA, Yager JY, Wirrell EC, Wood E, et al. Epidemiology and Outcomes of Arterial Ischemic Stroke in Children: The Canadian Pediatric Ischemic Stroke Registry. Pediatr Neurol. 2017;69:58–70. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Biarge M, Cheong JL, Diez-Sebastian J, Mercuri E, Dubowitz LM, Cowan FM. Risk factors for neonatal arterial ischemic stroke: The importance of the intrapartum period. J Pediatr. 2016;173:62–8e1. [DOI] [PubMed] [Google Scholar]
- 35.Bednarek N, Mathur A, Inder T, Wilkinson J, Neil J, Shimony J. Impact of therapeutic hypothermia on MRI diffusion changes in neonatal encephalopathy. Neurology. 2012;78:1420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


