Abstract
Rationale
A subset of male rats that self-administer 3,4-methylenedioxypyrovalerone (MDPV) have unusually high levels of drug intake; however, factor(s) that influence this behavior (e.g., reinforcement history and sex) are unknown.
Objectives
Characterize the reinforcing potency and effectiveness of MDPV in female rats to determine whether: 1) a subset of females also develop high levels of MDPV self-administration (i.e., a high-responder phenotype); and 2) the degree to which the high-responder phenotype is influenced by various reinforcement histories (i.e., responding for cocaine or food).
Methods
Female Sprague Dawley rats initially responded for MDPV (0.032 mg/kg/infusion), cocaine (0.32 mg/kg/infusion), or food (45-mg grain pellet) under fixed ratio (FR) 1 and FR5 schedules of reinforcement. After 20 sessions, the cocaine- and food-history rats responded for MDPV for 20 additional sessions. Dose-response curves for MDPV were generated under FR5 and progressive ratio (PR) schedules of reinforcement.
Results
A subset of rats responding for MDPV developed high levels of MDPV intake. A history of responding for cocaine, but not food, inhibited the development of high levels of MDPV intake. Large individual differences were observed in the level of self-administration when MDPV was available under an FR5, but not PR, schedule of reinforcement.
Conclusions
MDPV functions as a powerful reinforcer in female rats, as has been previously reported in male rats. The substantial variability in MDPV self-administration between subjects may be related to individual differences in human drug-taking behavior.
Keywords: Cocaine, MDPV, synthetic cathinone, self-administration, females, individual differences, rat, reinforcement history, sex differences
Introduction
Women transition from initiating substance use to seeking treatment more rapidly than men (i.e., telescoping; Griffin et al. 1989; Hernandez-Avila et al. 2004; DeVito et al. 2014), suggesting a faster progression from initial use to substance use disorder (SUD). Women also use similar amounts of cocaine as men, despite initiating use later than men (Griffin et al. 1989; DeVito et al. 2014; Miguel et al. 2019). Similar sex-related differences have been reported in rats self-administering cocaine, including that female rats tend to acquire cocaine self-administration more rapidly and to a greater level than male rats (e.g., Lynch and Carroll 1999; Carroll et al. 2002; Hu et al. 2004; Lynch 2008; but see Caine et al. 2004). In addition, under progressive ratio (PR) schedules of reinforcement, females often reach greater breakpoints than males, suggesting that cocaine is a more effective reinforcer in female rats compared to male rats (Roberts et al. 1989; Lynch 2008; Cummings et al. 2011). After extinction of cocaine self-administration, females have also been reported to reinstate responding to a greater extent than male rats, which suggests potential sex differences in relapse-related behaviors (Lynch and Carroll 2000; Lynch and Taylor 2004). Despite well-established sex differences with cocaine, relatively few studies have examined the effects of another monoamine uptake inhibitor, 3,4-methylenedioxypyrovalerone (MDPV) in female subjects (King et al. 2015; Hambuchen et al. 2017; Javadi-Paydar et al. 2018; McClenahan et al. 2019), and only one of these evaluated MDPV self-administration (Javadi-Paydar et al. 2018).
MDPV is a synthetic cathinone, which are designer stimulants, often referred to as “Bath Salts”, and marketed as legal alternatives to illicit stimulants, including cocaine or methamphetamine. MDPV and other structurally-related cathinones have a high abuse liability and are more effective reinforcers in laboratory animals than cocaine and methamphetamine (Watterson et al. 2014; Gannon et al. 2017, 2018b; Collins et al. 2019). Additionally, we have previously reported that a subset of male rats that self-administer MDPV develop aberrant drug-taking behavior, including high levels of drug intake within a relatively short period of time, and high levels of responding during periods of signaled drug unavailability (i.e., post-infusion timeouts [TOs]; Gannon et al. 2017). Importantly, because this “high-responder” phenotype is associated with an upward shift in the dose-response curve for MDPV self-administration relative to the dose-response curve for “low-responder” rats, it is unlikely that individual differences in the reinforcing potency of MDPV contribute to these dysregulated patterns of drug taking (Gannon et al. 2017, 2018a). Because this phenotype has been reliably observed in rats responding for MDPV, and structurally-related cathinones, we have been interested in whether MDPV self-administration can be used in both male and female rats to model a core feature of SUDs that is largely absent from studies involving cocaine, the inability to control drug use. Interestingly, although rats that acquire cocaine self-administration do not appear to develop this high-responder phenotype, once the MDPV high-responder phenotype is established, high levels of drug intake are also observed when other stimulants (e.g., cocaine or methamphetamine) are made available for self-administration (Gannon et al. 2017), suggesting that it is an enduring SUD-like phenotype. Preliminary evidence suggests a history of cocaine self-administration may prevent or delay the development of this high-responder phenotype (Gannon et al. 2017), though the degree to which differences in operant or pharmacological history contribute to this prevention remains unclear.
Thus, the present study included two core experiments, the first of which tested hypotheses related to the reinforcing effects of MDPV in female rats, to determine whether: (1) high levels of MDPV self-administration and responding when drug is unavailable (i.e., high-responder phenotype) develops in female rats; (2) the high-responder phenotype develops in a similar proportion of female rats as male rats; and (3) there are differences in reinforcing potency (fixed ratio dose response curve) or reinforcing effectiveness (progressive ratio dose response curve) between high- and low-responder female rats. The second experiment tested hypotheses relating to interactions between reinforcement history and the development of the MDPV high-responder phenotype, to determine if: (1) a history of responding for cocaine would prevent/delay the development of the MDPV high-responder phenotype; and (2) any operant history (e.g., responding for food pellets) would be sufficient to prevent/delay the development of the MDPV high-responder phenotype.
Methods
Animals
Thirty, adult female Sprague-Dawley rats (200-225g) were obtained from Envigo (Indianapolis, IN) and singly housed in a temperature- and light-controlled (24°C; lights on 6am-8pm) environment. Rats had ad libitum access to water and rat chow, except for a brief period (3-5 days) where rats (n=3) that failed to meet acquisition criteria were mildly food-restricted (12-g/day; sufficient to maintain ≥95% of free-feeding bodyweight). All procedures were conducted during the light-cycle and in accordance with Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio and the Guide for Care and Use of Laboratory Animals (National Research Council 2011).
Surgical preparation
All rats were anesthetized with 2% isoflurane and prepared with an indwelling catheter in the left femoral vein and a vascular access button that was exteriorized in the mid-scapular region, as previously described (e.g., Doyle et al. 2020). Penicillin G (60,000 U/rat) was administered subcutaneously following surgery to prevent infection and 0.5 ml heparinized saline (100 U/ml) was flushed daily during the 5-7 days of recovery. Subsequent to the start of experimentation, rats were flushed daily with 0.2 ml of saline before and 0.5 ml of heparinized saline after each session.
Apparatus
Operant sessions were conducted in standard operant chambers (Med Associates Inc., St. Albans, VT), housed within ventilated, sound- and light-attenuated cubicles. Each chamber contained two levers with a set of red, yellow, and green LEDs above each lever. A white house light was located on the opposite wall. A variable speed syringe driver was used to deliver drug solutions through Tygon tubing connected to a fluid swivel and spring tether, held in place by a counterbalanced arm. In chambers where rats earned food reinforcers, a pellet dispenser delivered 45-mg grain-based pellets (Dustless Precision Pellets® Rodent, 45-mg; Bio-Serv, Flemington, NJ) to a lit food trough.
Self-administration
Acquisition
Rats initially responded for MDPV (0.032 mg/kg/infusion), cocaine (0.32 mg/kg/infusion), or food (1 grain-based pellet) under a fixed ratio (FR) 1 schedule of reinforcement during daily 90-min sessions. The doses of MDPV were selected due to the ~10-fold potency difference between MDPV and cocaine in behavioral assays (Baumann et al. 2013; Gatch et al. 2013; Collins et al. 2016; Schindler et al. 2016), and the relative position of both doses on the descending limb of the FR5 dose-response curve in male rats (Gannon et al. 2017). Illumination of a yellow LED above the active lever (counterbalanced across rats) signaled the start of the session; when a reinforcer was earned, all three LEDs above the active lever and the house light were illuminated for 5-sec, during which time no more reinforcers could be earned (i.e., a 5-sec timeout [TO]). Responses on the inactive lever, and on either lever during the 5-sec TO were recorded but had no scheduled consequence. After 10 sessions, rats that met acquisition criteria (i.e., ≥20 reinforcers and ≥80% responses on the active lever for 2 consecutive sessions) were advanced to an FR5:TO 5-sec schedule of reinforcement, whereas rats that failed to meet acquisition criteria within 10 sessions (cocaine-history: n=1; food-history: n=1) were mildly food restricted (12-g food/day) until acquisition criteria were met. One additional rat from the cocaine-history group never acquired responding and was excluded from all experiments and analyses beyond those related to the acquisition of responding.
Fixed Ratio 5 (FR5) Self-administration
Rats then responded for their assigned reinforcer under a FR5 schedule of reinforcement for 10 sessions and until meeting stability criteria (i.e., ± 20% of the mean for 3 consecutive sessions; no increasing or decreasing trend), or a maximum of 15 sessions. After this, the cocaine- and food-history groups self-administered 0.032 mg/kg/infusion MDPV for at least 20 sessions and until meeting stability criteria, or a maximum of 25 sessions. As we have done previously, rats were classified as either low- or high-responders based on the percentage of total active lever responses that occurred during the 5-sec TO periods during the final 3 sessions of this phase, where high-responder rats made ≥20% of their responses during TOs and low-responder rats made <20% of their responses during TOs (Gannon et al. 2017).
FR5 Dose-Response Curves
Dose substitution was used to generate full dose-response curves under the FR5:TO 5-sec schedule of reinforcement. The first dose evaluated was always 0.032 mg/kg/infusion, with the remaining doses of MDPV (0.001-0.1 mg/kg/infusion) and saline were evaluated in a pseudo-random order until stability criteria was reached (i.e., ± 20% of the mean for 3 consecutive sessions; no increasing or decreasing trend), or for a maximum of 10 sessions.
PR Dose-Response Curves
Upon completion of the FR5 dose-response curve, the schedule of reinforcement was changed to a progressive ratio (PR), where the response requirement was increased according to the following equation: ratio = (5e[infusion # * 0.2]) – 5 (Richardson and Roberts 1996). All rats first responded for 0.032 mg/kg/infusion MDPV, then the remaining doses of MDPV (0.0032-0.56 mg/kg/infusion) were evaluated in a pseudo-random order until stability criterion was reached (2 consecutive sessions within 2 infusions), or a maximum of 7 sessions. After completion of the MDPV dose-response curve, cocaine (0.32 mg/kg/infusion) was substituted, to allow for the cocaine dose-response to be established by dose substitution (0.032-1.78 mg/kg/infusion) under the PR schedule of reinforcement.
Statistical Analysis
Responding during the 10 sessions under FR1 and the 10 sessions under FR5 are shown as the mean ± SEM of the number of reinforcers earned and number of responses on the inactive lever. Mean number of reinforcers earned during the last 3 sessions of responding under FR1 and FR5 conditions for the original reinforcer, as well as mean number of sessions required to meet acquisition criteria were compared by a one-way ANOVA with Tukey’s post-hoc analyses, when appropriate. A two-way (dose and history/phenotype) repeated measures (dose) ANOVA compared both the FR5 and PR dose response curves, with Tukey’s post-hoc analyses, when appropriate.
To calculate the dose estimated to produce 50% of the maximum responding (ED50) under PR schedule of reinforcement, the dose-response curve for each rat was normalized by setting the number of infusions earned when saline was available as the 0% effect level, and setting the maximum number of infusions earned, regardless of dose, to the 100% effect level (i.e., Emax). These normalized dose response curves were fit using a linear regression of the data spanning the 20-80% effect levels; the dose that maintained 50% of the maximal responding was estimated for individual subjects, and the group mean and 95% confidence intervals (CI) were compared. To compare differences in sensitivity, the peak doses (i.e., the dose that maintained the greatest level of responding under FR5) were calculated for individual subjects; the group mean and 95% CI were compared between groups and were considered significantly different if the CI did not overlap. Area under the FR5 dose response curve (AUC) and the dose-independent Emax values (PR dose response curve) were compared between groups using one-way ANOVAs with Tukey’s post-hoc.
Drugs
Racemic MDPV HCl was synthesized by Agnieszka Sulima and Kenner Rice (Bethesda, MD). Cocaine HCl was provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). All drugs were dissolved in sterile 0.9% saline and administered intravenously in a volume of 0.1 ml/kg body weight.
Results
Most rats (MDPV-history: 100%; cocaine-history: 80%; food-history: 90%) met acquisition criteria (i.e., earning ≥20 reinforcers and making ≥80% of responses on the active lever) within the first 10 sessions of self-administration (Fig 1; left panels). The mean (± SEM) number of sessions to acquire responding for MDPV (group: 4.3 ± 0.7 sessions; high-responders: 5.0 ± 1.3; low-responders: 3.6 ± 0.5), cocaine (5.9 ± 0.8 sessions), or food (4.8 ± 0.8 sessions) did not differ (F=1.1, p=0.34). Though rats from the MDPV-history group appeared to earn more reinforcers (103.5 ± 29.8) than rats from the cocaine- (54.5 ± 2.5) or food-history (54.6 ± 5.6) groups at the end of the 10-day acquisition period, this difference was not significant (F[2,26]=2.3; p=0.12). However, after the response requirement was increased to FR5 for 10 sessions, there was a main effect of reinforcer (F[2,26]=3.7; p=0.04), with Tukey post-hoc analyses indicating that significantly more MDPV infusions (81.0 ± 17.3) were earned than food deliveries (37.3 ± 4.5; p=0.03), but there was no significant difference between the number of MDPV and cocaine infusions earned (57.8 ± 8.9; p=0.35). Throughout the course of the experiment, lever discrimination remained high (>90% of responses made on the active lever) and responses made during the TOs were almost exclusively on the active lever.
Fig 1.
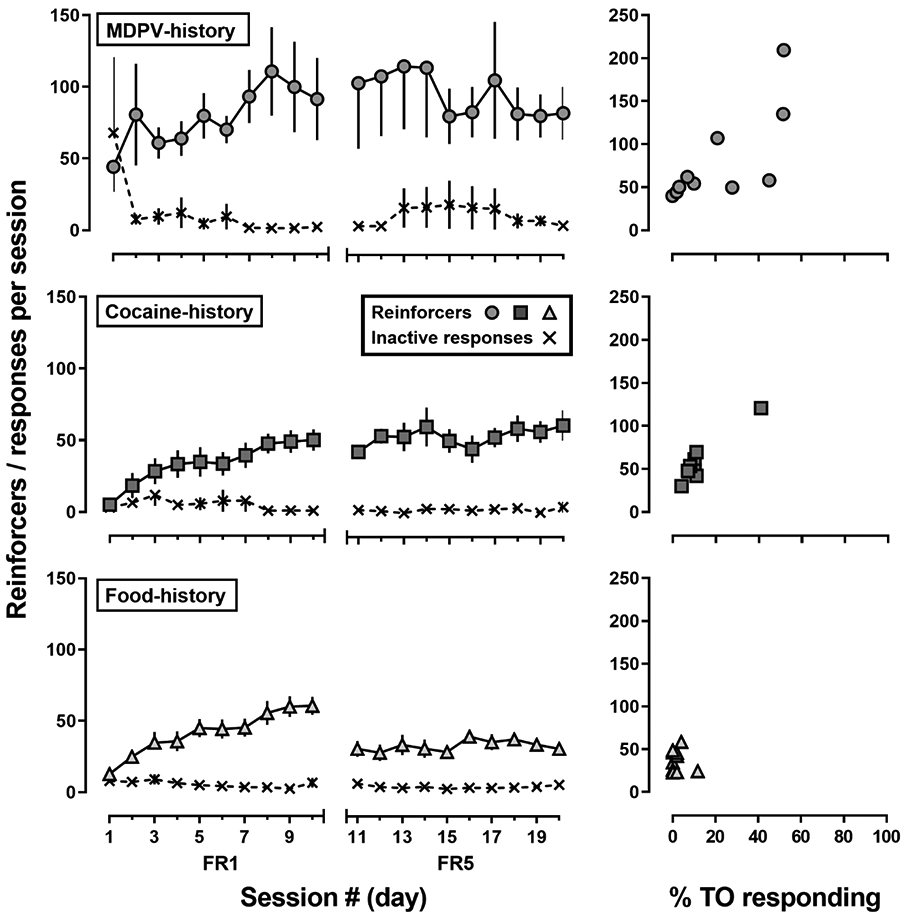
Reinforcers earned during 10 sessions of acquisition (fixed ratio [FR] 1) and 10 sessions of responding under a FR5 schedule of reinforcement in adult, female Sprague Dawley rats responding for 0.032 mg/kg/infusion MDPV (circles; n=10), 0.32 mg/kg/infusion cocaine (squares; n=9), or 1 grain-based pellet (triangles; n=10). Left panels represent mean (± 1 S.E.M.) number of reinforcers earned (circle, square or triangle) and responses on the inactive lever (x) across 20 sessions of self-administration. Abscissa: session number (day) where sessions 1-10 were responding under FR1 and sessions 11-20 were responding under FR5. Ordinates: mean number of reinforcers earned or responses per session. Right panels represent the correlation between mean percent timeout (TO) responding and the number of reinforcers earned during the final 3 sessions on FR5:TO 5-sec. Abscissa: percent responses made on the active lever during TOs versus total active lever responses. Ordinates: mean number of reinforcers earned
The percentage of active lever responses made during the TOs (%TO responding) compared to the mean number of infusions earned under an FR5 is also shown in Fig 1 (right panels). Five out of 10 rats from the MDPV-history group made more than 20% of total active lever responses during the TOs, compared to only 1 out of 9 from the cocaine-history and 0 out of 10 rats from the food-history groups. There was a significant correlation between %TO responding and infusions for rats in the MDPV-history (R2 = 0.54; p=0.016) and cocaine-history groups (R2 = 0.89; p<0.0001), however, the number of food deliveries and the %TO responding were not significantly correlated in the food-history group (p>0.05).
Figure 2 (left panels) depicts the mean number of reinforcers earned by individual subjects when the original reinforcer (i.e., 0.032 mg/kg/infusion MDPV, 0.32 mg/kg/infusion cocaine or 1 food pellet) was available, as well as when 0.032 mg/kg/infusion MDPV was substituted in the cocaine- and food-history groups. When rats originally responded for MDPV (MDPV-history group) there was a large amount of variability in the number of infusions earned (range: 169.3; SD: 54.4). In contrast, when rats originally responded for either cocaine infusions (range: 90.7; SD: 26.1), or food deliveries (range: 35.7; SD: 12.8) there was less inter-subject variability. When rats in the cocaine-history group responded for MDPV, the inter-subject variability remained small (range: 35.7; SD: 11.5); however, when rats from the food-history group were allowed to respond for MDPV, the inter-subject variability increased substantially (range: 148.0; SD: 55.0), and was comparable to that observed in the MDPV-history group. In addition, there was a significant correlation between %TO responding and number of MDPV infusions earned in all three groups (MDPV-history: R2 = 0.54, p=0.016; cocaine-history: R2 = 0.55, p=0.022; food-history: R2 = 0.65, p=0.005). Using the criterion established in male rats (i.e., high-responder rats make more than 20% of their active lever responses during TOs; Gannon et al. 2017, 2018a), 5 out of 10 rats from the MDPV-history group, and 6 out of 10 rats from the food-history group were classified as high-responders, whereas none of the rats from the cocaine-history group met the criterion (Fig 2).
Fig 2.
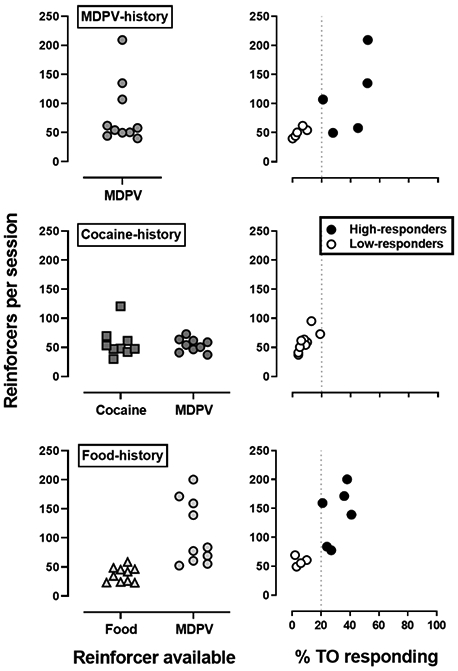
Mean number of reinforcers earned for individual subjects when responding for MDPV (circles), cocaine (squares) or food (triangles) in rats with a MDPV-history (top; n=10), cocaine-history (middle; n=9) or food-history (bottom; n=10). Left panels represent mean number of reinforcers earned during the final 3 sessions on FR5:TO 5-sec schedule of reinforcement for the original reinforcer and for MDPV. Abscissa: reinforcer available. Ordinates: mean number of reinforcers earned. Right panels represent the correlation between percent TO responding and mean number of 0.032 mg/kg/infusions of MDPV earned, where high-responders (filled symbols) made more than or equal to 20% of responses during the 5-sec TO and low-responders (open symbols) made less than 20% of responses during the TO. Abscissa: percent responses made on the active lever during TOs versus total active lever responses. Ordinates: mean number of MDPV infusions earned
Shown in Figure 3 are the dose-response curves for MDPV self-administration (FR5) generated in MDPV-history rats (top panel), cocaine-history rats (middle panel), and food-history rats (bottom panel). A two-way repeated measures ANOVA indicated a main effect of dose (F[1.6,39.4]=32.3, p<0.0001), a main effect of phenotype (F[4,24)=6.8, p=0.0008), and a dose x phenotype interaction (F[20,120]=3.4, p<0.0001). Tukey post-hoc analyses found that high-responders from the food-history group earned significantly more infusions of 0.0032 mg/kg/infusion MDPV than low-responder rats from the cocaine- or food-history groups (p<0.01 for both), and significantly more infusions of 0.032 mg/kg/infusion MDPV than low-responder rats from the MDPV-, cocaine-, or food-history groups (p<0.05 for both). The AUC for the MDPV dose-response curve for the high-responders from the food-history group was also significantly greater than the AUCs for low-responders from the cocaine- and food-history groups (Table 1). However, there were no significant differences in the reinforcing potency of MDPV, as measured by peak dose, among the three groups (Table 1).
Fig 3.
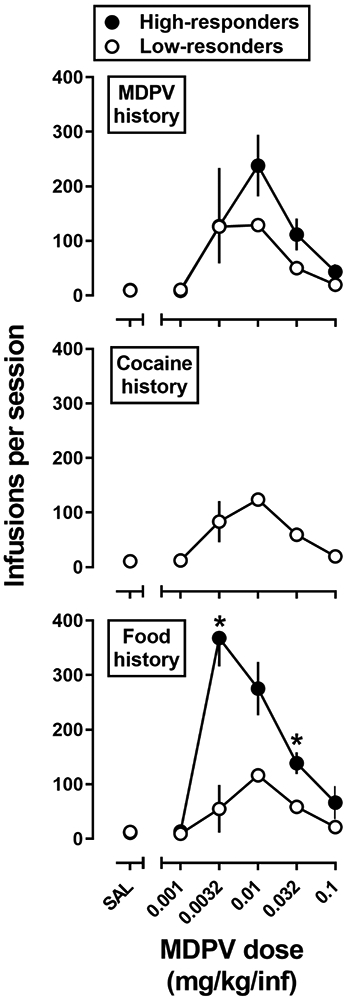
MDPV FR5 dose response curves in rats with a history of responding for MDPV (top; n=10), cocaine (middle; n=9), or food (bottom; n=10) in high- (filled symbols) and low-responder (open symbols) rats. Abscissa: SAL represents infusions of saline and the numbers refer to dose of MDPV available during each session, expressed as mg/kg/infusion on a log scale. Ordinate: total infusions obtained during the 90-minute session. Error bars represent ± 1 S.E.M.
Table 1.
Peak dose (measure of potency) area under the curve (AUC) calculated from the fixed ratio (FR5) dose response curve for MDPV, and ED50 (measure of potency) and Emax (measure of effectiveness) calculated from the progressive ratio dose response curves for MDPV and cocaine.
| Fixed ratio 5 dose response curve | Progressive ratio dose response curve | ||||||
|---|---|---|---|---|---|---|---|
| MDPV | MDPV | Cocaine | |||||
| Peak dose (95% CI) | AUC ± SEM | ED50 (95% CI) | Emax ± SEM | ED50 (95% CI) | Emax ± SEM | ||
| mg/kg | mg/kg | infusions | mg/kg | infusions | |||
| MDPV-history | Group (n=10) | 0.007 (0.005-0.010) | 209.3 ± 44.6 | 0.020 (0.016-0.027) | 28.0 ± 0.7 * | 0.16 (0.12-0.21) | 23.1 ± 0.8 |
| High-responders (n=5) | 0.008 (0.005-0.012) | 259.0 ± 80.6 | 0.019 (0.012-0.029) | 27.8 ± 1.3 * | 0.16 (0.11-0.23) | 24.1 ± 0.7 | |
| Low-responders (n=5) | 0.006 (0.004-0.011) | 159.5 ± 35.2 | 0.022 (0.016-0.030) | 28.1 ± 0.9 * | 0.16 (0.10-0.26) | 22.1 ± 1.4 | |
| Cocaine-history | Group/low-responders (n=8-9) | 0.007 (0.005-0.010) | 139.0 ± 20.7 | 0.021 (0.015-0.029) | 28.3 ± 1.5 * | 0.13 (0.10-0.17) | 24.1 ± 0.7 |
| Food-history | Group (n=8-10) | 0.005 (0.003-0.007) | 295.0 ± 58.0 $ | 0.020 (0.015-0.028) | 29.0 ± 0.6 * | 0.11 (0.08-0.12) | 22.9 ± 1.2 |
| High-responders (n=4-6) | 0.004 (0.003-0.006) | 411.1 ± 56.4 #$ | 0.022 (0.016-0.030) | 29.5 ± 1.0 * | 0.11 (0.06-0.18) | 22.5 ± 1.4 | |
| Low-responders (n=4) | 0.008 (0.004-0.013) | 121.0 ± 21.6 | 0.019 (0.009-0.037) | 28.4 ± 0.2 * | 0.12 (0.08-0.17) | 23.3 ± 2.3 | |
p<0.05 indicates a significant difference from the cocaine Emax for the same reinforcement history
p<0.05 indicates a significant difference from the low responders with the same reinforcement history
p<0.05 indicates a significant difference from rats with a cocaine history
Dose-response curves for MDPV and cocaine self-administration were also generated under a PR schedule of reinforcement (Fig 4). A repeated measures ANOVA to compare the MDPV dose-response curves across history and phenotype indicated a significant main effect of dose (F[2.7,61.3]=189.3, p<0.0001), but no effect of phenotype, and no significant dose x phenotype interaction. Similarly, for the cocaine dose-response curves, there was a significant main effect of dose (F[3.0,61.9]=213.1, p<0.0001), but no effect of phenotype, and no dose x phenotype interaction. MDPV was equi-potent and equi-effective in all groups, regardless of reinforcement history or phenotype (Table 1). Similarly, there were no significant differences in potency or effectiveness of cocaine to function as a reinforcer across groups. However, MDPV was a more effective reinforcer than cocaine and ~10 times more potent than cocaine in all groups (Table 1).
Fig 4.
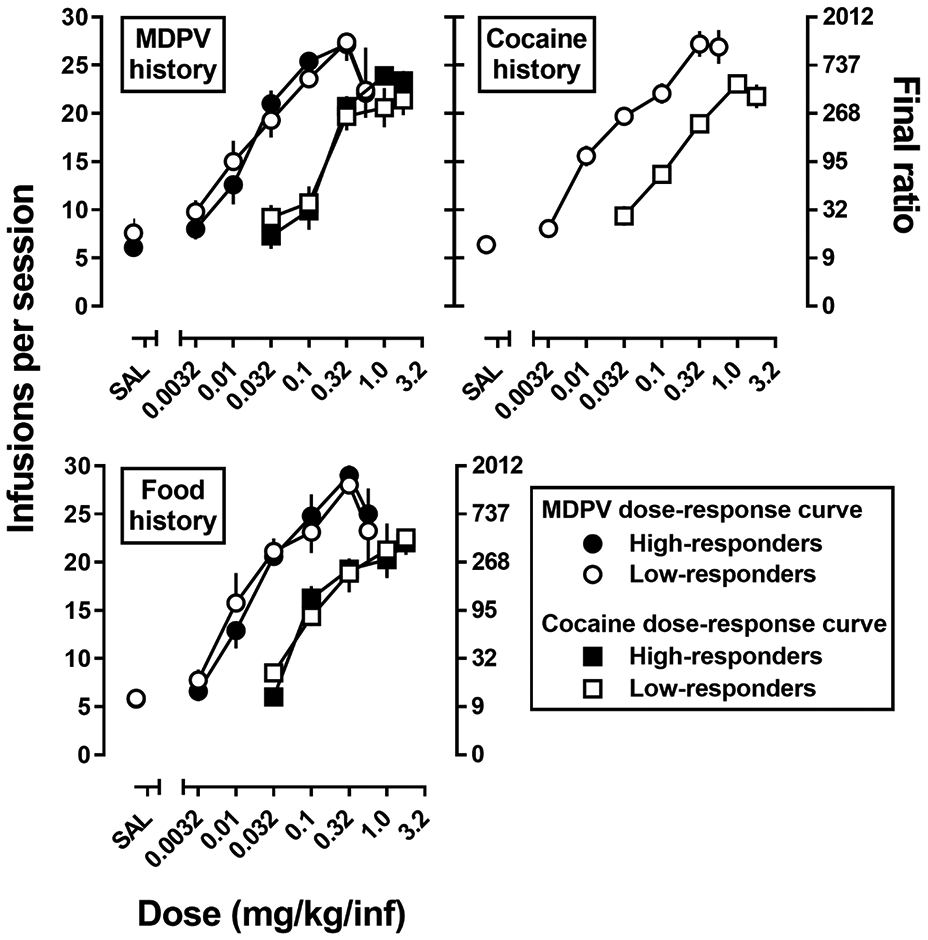
MDPV (circles) and cocaine (squares) progressive ratio dose response curves in rats with a history of responding for MDPV (top left panel; n=10), cocaine (top right panel; n=8-9), or food (bottom left panel; n=8-9) in high- (filled symbols) and low-responder (open symbols) rats. Abscissa: SAL represents infusions of saline and the numbers refer to dose of MDPV or cocaine available during each session, expressed as mg/kg/infusion on a log scale. Left ordinate: total infusions obtained during the session. Right ordinate: corresponding final ratio completed for each session. Error bars represent ± 1 S.E.M.
Discussion
Synthetic cathinones (bath salts) are used for their stimulant and euphoric properties, with users often reporting that these effects are greater for synthetic cathinones than for other stimulants, such as cocaine and methamphetamine (Winstock et al. 2011; Johnson and Johnson 2014). In rats and rhesus monkeys, MDPV and other pyrrolidine-containing cathinones have been reported to maintain high levels of responding under both fixed and progressive ratio schedules, as well as binge-like patterns of consumption (Watterson et al. 2014; Aarde et al. 2015b; Gannon et al. 2017, 2018a, 2018b; Javadi-Paydar et al. 2018; Collins et al. 2019); however, little is known about the reinforcing effects of MDPV in female subjects (Javadi-Paydar et al. 2018). Prior research using male rats suggested that a history of cocaine self-administration may prevent the development of high levels of MDPV intake; however, the broader impact of other reinforcement histories on the development of these high levels of MDPV intake are unknown. Thus, the present studies sought to characterize the reinforcing effects of MDPV, relative to cocaine, in female rats, and to investigate the role of reinforcement history on the development of high levels of MDPV self-administration. The first central finding was that MDPV was a significantly more effective reinforcer than cocaine in female rats, replicating a previous finding in male rats (Gannon et al. 2017, 2018b). The second major finding was that ~50% of rats self-administering MDPV developed the high-responder phenotype, and self-administered more MDPV across of range of doses under a FR schedule than low-responder rats, also replicating a previous finding in male rats (Gannon et al. 2017). However, there were no differences between high- and low-responder rats regarding the reinforcing effectiveness of either MDPV or cocaine. The third major finding was that a ~3-week history of responding for cocaine, but not food, was able to prevent or delay the development of high levels of MDPV intake. Taken together, these findings suggest that MDPV is a highly effective reinforcer in both male and female subjects, subsets of both male and female rats rapidly transition to high levels of MDPV intake, and this high-responder phenotype may be a model to study factors relating to individual differences in drug-taking in both sexes in order to better understand determinants contributing to vulnerability of humans to develop a SUD.
Women transition from first use to treatment-seeking more rapidly than men (Griffin et al. 1989; Hernandez-Avila et al. 2004; DeVito et al. 2014), suggesting they may progress from initial use to SUD in a shorter timeframe than men. We found that female rats rapidly acquired MDPV self-administration (~4 sessions), in a similar, but perhaps slightly faster time than males in a previous study (~5 sessions; Gannon et al. 2017). Importantly, even though the rate of acquisition was comparable between sexes, female rats earned three times as many infusions (~100 vs ~30) than male rats that were allowed to acquire MDPV self-administration under identical conditions in our laboratory (Gannon et al. 2017). These findings are somewhat consistent with literature on cocaine self-administration, which suggest that female rats acquire more quickly and to a higher level than male rats (Lynch and Carroll 1999; Carroll et al. 2002; Hu et al. 2004; Lynch 2008; but see Caine et al. 2004). Yet, it is interesting to note that in the present study, the rate and level of acquisition for cocaine self-administration in female rats was largely consistent with that previously reported for male rats self-administering cocaine under identical conditions in our laboratory (Gannon et al. 2017). Previous studies examining self-administration of other synthetic cathinones in female subjects also suggest that the reinforcing effects of α-pyrrolidinopentiophenone (α-PVP), methylone, mephedrone are not significantly different between male and female rats (Creehan et al. 2015; Aarde et al. 2015a; Vandewater et al. 2015; Javadi-Paydar et al. 2018; Marusich et al. 2019a, b); though, like the present study, these previous studies have all relied on historical comparisons rather than direct comparisons of male and female subjects in the same study.
Though there may be sex-related differences in MDPV acquisition, comparisons between the current study and a prior study in males (Gannon et al. 2017) suggest that any differences in the acquisition of responding are not due to sex-related differences in the reinforcing potency or effectiveness of MDPV. Both the dose that maintained peak levels of MDPV self-administration under an FR5 schedule of reinforcement (0.005 mg/kg/infusion), and the dose that maintained half maximal responding under the PR schedule of reinforcement (ED50 = 0.017 mg/kg/infusion) previously determined in male rats (Gannon et al. 2017) have overlapping confidence intervals with those determined in the current studies with female rats, suggesting that male and female rats are equally sensitive to the reinforcing effects MDPV. Similarly, the maximal reinforcing effectiveness of MDPV does not appear to differ as a function of sex, with both females and males earning a maximum of ~28 infusions of MDPV under a PR schedule of reinforcement (Gannon et al. 2017). Although numerous studies suggest that cocaine is a more effective reinforcer in female as compared to male rats (e.g., Roberts et al. 1989; Lynch 2008; Cummings et al. 2011), measures of reinforcing potency and effectiveness for cocaine in female rats were comparable to those we have previously reported using identical procedures in male rats (Gannon et al. 2017). Taken together, the results of the current studies in female rats and those of a previous study in male rats suggests that while sex-related differences might exist with regard to the rate and level of acquisition for stimulants, such as MDPV and cocaine, these differences were not apparent when full dose-response curves were evaluated under either FR5 or PR schedules of reinforcement, similar to previous reports with cocaine (Caine et al. 2004).
Like in male rats, a subset (~50%) of female rats developed high levels of MDPV intake and engaged in high levels of responding during the post-infusion TO (i.e., high-responder phenotype). Responding during post-infusion TOs is highly correlated with responding during periods of signaled drug unavailability (Gannon et al. 2017), which is thought to be an important aspect of SUD-related behavior (Deroche-Gamonet et al. 2004). Based on our previous study in male rats suggesting that a history of cocaine self-administration prevented or delayed the development of the MDPV high-responder phenotype, a second goal of these studies was to determine whether female rats that acquired responding for cocaine or a non-drug reinforcer (food pellets) would be similarly resistant to the development of the high-responder phenotype when MDPV was available. Similar to previous reports from our laboratory (Collins and France 2015; Gannon et al. 2017) that showed that male rats that acquired self-administration for 0.1, 0.32, or 1.0 mg/kg/infusion cocaine under limited access conditions all made less than 20% of their total responses during post-infusion TOs (i.e., none of them developed the high-responder phenotype), only one of ten female rats from the current study developed the high levels of TO responding when allowed to self-administer 0.32 mg/kg/infusion cocaine. Additionally, consistent with the findings reported in Gannon et al. 2017, the high-responder phenotype did not emerge after cocaine-trained female rats were transitioned to, and maintained on MDPV self-administration for a prolonged period of time (>60 sessions). Although female rats that acquired responding for food pellets also failed to exhibit patterns of responding consistent with the high-responder phenotype when responding was reinforced by food delivery, high rates of MDPV self-administration (and responding during post-infusion TOs) were observed in 6 of the 10 female rats once they were allowed to self-administer MDPV. Though the cocaine- and food-history groups had longer histories of reinforcement before classification as high- or low-responders (~40-45 sessions) compared to the MDPV-history rats (~20-25 sessions), the high-responder phenotype persisted throughout the generation of the MDPV dose-response curve (~55 sessions), suggesting the development of the phenotype, or lack thereof, is not due to differences in number of self-administration sessions. Together, these findings suggest a history of responding for cocaine, but not a more general history with operant contingencies, can prevent or delay the development of the high-responder phenotype; however, the mechanism(s) through which cocaine self-administration is capable of blocking/blunting the transition to high levels of MDPV self-administration is unclear. In order to better understand factors that contribute to differences in drug-taking behavior, future studies are necessary to determine whether a history of prior self-administration with drugs from other classes (e.g., opioids), or whether any cocaine exposure (e.g., non-contingent cocaine) may prevent the transition to high levels of MDPV self-administration.
Importantly, the high-responder rats generally self-administered more MDPV across a range of doses than the low-responder rats, resulting in an upward shift in the FR dose response curve and not a change in the peak dose, indicating that, like in male rats (Gannon et al. 2017), these differences in MDPV intake are not due to differences in sensitivity. MDPV was also a significantly more potent and effective reinforcer than cocaine (i.e., maintained higher breakpoints) in female rats, consistent with previous reports in male rats and rhesus monkeys (Gannon et al. 2017, 2018b; Collins et al. 2019). In contrast to what has been reported in male rats, there was no effect of the high-responder phenotype on the MDPV or cocaine PR dose response curves. This might be an important sex-related difference in aspects of the high-responder phenotype; however, it is also possible that it is due to the extended period of time (>60 sessions) before the PR dose response curves were generated.
MDPV self-administration reliably results in a subset of rats developing this high-responder phenotype, thus there are large individual differences in level of drug intake and responding when drug is not available, in both female and male subjects. Through historical comparisons, there do not appear to be large sex differences in MDPV self-administration between females and males; however, because of known sex/gender differences in cocaine use between women and men, it will be important to directly compare both sexes in future studies, especially when exploring factors that underlie the development of this novel, high-responder phenotype.
Acknowledgements:
The authors would also like to thank Kayla Galindo, Melson Mesmin, Karen Jimenez, and Raghad Akrouk for their technical assistance in the completion of these studies.
Funding: This research was supported by National Institutes of Health and National Institute on Drug Abuse (R01DA039146 [GTC] and R36DA050955 [MRD]), the jointly-sponsored National Institutes of Health Predoctoral Training Program in the Neurosciences (T32NS082145 [MRD]), and the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute of Alcohol Abuse and Alcoholism (KCR).
Footnotes
Conflict of interest: On behalf of all authors, the corresponding author states that there is no conflict of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Aarde SM, Creehan KM, Vandewater SA, Dickerson TJ, Taffe MA (2015a) In vivo potency and efficacy of the novel cathinone α-pyrrolidinopentiophenone and 3,4-methylenedioxypyrovalerone: Self-administration and locomotor stimulation in male rats. Psychopharmacology (Berl) 232:3045–3055. 10.1007/s00213-015-3944-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Dickerson TJ, Taffe MA (2015b) Binge-like acquisition of 3,4-methylenedioxypyrovalerone (MDPV) self-administration and wheel activity in rats. Psychopharmacology (Berl) 232:1867–1877. 10.1007/s00213-014-3819-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, et al. (2013) Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology 38:552–562. 10.1038/npp.2012.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, Mello NK (2004) Effect of gonadectomy and gonadal hormone replacement on cocaine self-administration in female and male rats. Neuropsychopharmacology 29:929–942. 10.1038/sj.npp.1300387 [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK (2002) Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: Phenotype and sex differences. Psychopharmacology (Berl) 161:304–313. 10.1007/s00213-002-1030-5 [DOI] [PubMed] [Google Scholar]
- Collins GT, Abbott M, Galindo K, Rush EL, Rice KC, and France CP (2016) Discriminative stimulus effects of binary drug mixtures: studies with cocaine, MDPV, and caffeine. J Pharmacol Exp Ther 359:1–10. 10.1124/jpet.116.234252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT and France CP (2015) Determinants of conditioned reinforcing effectiveness: dopamine D2-like receptor agonist-stimulated responding for cocaine-associated stimuli. Eur J Pharmacol 769:242–249. 10.1016/j.ejphar.2015.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Sulima A, Rice KC, France CP (2019) Self-administration of the synthetic cathinones 3,4-methylenedioxypyrovalerone (MDPV) and α-pyrrolidinopentiophenone (α-PVP) in rhesus monkeys. Psychopharmacology (Berl) 236:3677–3685. 10.1007/s00213-019-05339-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creehan KM, Vandewater SA, Taffe MA (2015) Intravenous self-administration of mephedrone, methylone and MDMA in female rats. Neuropharmacology 92:90–97. 10.1016/j.neuropharm.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JA, Gowl BA, Westenbroek C, Clinton SM, Akil H, Becker JB (2011) Effects of a selectively bred novelty-seeking phenotype on the motivation to take cocaine in male and female rats. Biol Sex Differ 2:. 10.1186/2042-6410-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV (2004) Evidence for addiction-like behavior in the rat. Science (80-) 305:1014–1017. 10.1126/science.1099020 [DOI] [PubMed] [Google Scholar]
- DeVito EE, Babuscio TA, Nich C, Ball SA, Carroll KM (2014) Gender differences in clinical outcomes for cocaine dependence: Randomized clinical trials of behavioral therapy and disulfiram. Drug Alcohol Depend 145:156–167. 10.1016/j.drugalcdep.2014.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle MR, Sulima A, Rice KC, Collins GT (2020) Interactions between reinforcement history and drug-primed reinstatement: Studies with MDPV and mixtures of MDPV and caffeine. Addict Biol e12904. 10.1111/adb.12904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Baumann MH, Walther D, et al. (2018a) The abuse-related effects of pyrrolidine-containing cathinones are related to their potency and selectivity to inhibit the dopamine transporter. Neuropsychopharmacology 43:2399–2407. 10.1038/s41386-018-0209-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Galindo KI, Mesmin MP, Sulima A, Rice KC, Collins GT (2018b) Relative reinforcing effects of second-generation synthetic cathinones: acquisition of self-administration and fixed ratio dose-response curves in rats. Neuropharmacology 134:28–35. 10.1016/j.neuropharm.2017.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Galindo KI, Rice KC, Collins GT (2017) Individual differences in the relative reinforcing effects of 3, 4-methylenedioxypyrovalerone under fixed and progressive ratio schedules of reinforcement in rats. J Pharmacol Exp Ther 361:181–189. 10.1124/jpet.116.239376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, and Forster MJ (2013) Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol 24:437–447. 10.1097/FBP.0b013e328364166d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin ML, Weiss RD, Mirin SM, Lange U (1989) A Comparison of Male and Female Cocaine Abusers. Arch Gen Psychiatry 46:122–126. 10.1001/archpsyc.1989.01810020024005 [DOI] [PubMed] [Google Scholar]
- Hambuchen MD, Hendrickson HP, Gunnell MG, McClenahan SJ, Ewing LE, Gibson DM, Berquist MD, Owens SM (2017) The pharmacokinetics of racemic MDPV and its (R) and (S) enantiomers in female and male rats. Drug Alcohol Depend 179:347–354. 10.1016/j.drugalcdep.2017.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR (2004) Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend 74:265–272. 10.1016/j.drugalcdep.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB (2004) Biological Basis of Sex Differences in the Propensity to Self-administer Cocaine. Neuropsychopharmacology 29:81–85. 10.1038/sj.npp.1300301 [DOI] [PubMed] [Google Scholar]
- Javadi-Paydar M, Harvey EL, Grant Y, Vandewater SA, Creehan KM, Nguyen JD, Dickerson TJ, Taffe MA (2018) Binge-like acquisition of α-pyrrolidinopentiophenone (α-PVP) self-administration in female rats. Psychopharmacology (Berl) 235:2447–2457. 10.1007/s00213-018-4943-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PS, Johnson MW (2014) Investigation of “bath salts” use patterns within an online sample of users in the United States. J Psychoactive Drugs 46:369–378. 10.1080/02791072.2014.962717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HE, Wakeford A, Taylor W, Wetzell B, Rice KC, Riley AL (2015) Sex differences in 3,4-methylenedioxypyrovalerone (MDPV)-induced taste avoidance and place preferences. Pharmacol Biochem Behav 137:16–22. 10.1016/j.pbb.2015.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ (2008) Acquisition and maintenance of cocaine self-administration in adolescent rats: Effects of sex and gonadal hormones. Psychopharmacology (Berl) 197:237–246. 10.1007/s00213-007-1028-0 [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME (1999) Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 144:77–82. 10.1007/s002130050979 [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME (2000) Reinstatement of cocaine self-administration in rats: Sex differences. Psychopharmacology (Berl) 148:196–200. 10.1007/s002130050042 [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR (2004) Sex differences in the behavioral effects of 24-h/day access to cocaine under a discrete trial procedure. Neuropsychopharmacology 29:943–951. 10.1038/sj.npp.1300389 [DOI] [PubMed] [Google Scholar]
- Marusich JA, Gay EA, Blough BE (2019a) Analysis of neurotransmitter levels in addiction-related brain regions during synthetic cathinone self-administration in male Sprague-Dawley rats. Psychopharmacology (Berl) 236:903–914. 10.1007/s00213-018-5011-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Gay EA, Watson SL, Blough BE (2019b) Synthetic cathinone self-administration in female rats modulates neurotransmitter levels in addiction-related brain regions. Behav Brain Res 376:112211. 10.1016/j.bbr.2019.112211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClenahan SJ, Hambuchen MD, Simecka CM, Gunnell MG, Berquist MD, Owens SM (2019) Cardiovascular effects of 3,4-methylenedioxypyrovalerone (MDPV) in male and female Sprague-Dawley rats. Drug Alcohol Depend 195:140–147. 10.1016/j.drugalcdep.2018.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel AQC, Jordan A, Kiluk BD, Nich C, Babuscio TA, Mari JJ, Carroll KM (2019) Sociodemographic and clinical outcome differences among individuals seeking treatment for cocaine use disorders. The intersection of gender and race. J Subst Abuse Treat 106:65–72. 10.1016/j.jsat.2019.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (2011) Guide for the care and use of laboratory animals, 8th ed. National Academy Press, Washington, DC [Google Scholar]
- Richardson NR, Roberts DCS (1996) Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy. J. Neurosci. Methods 66:1–11 10.1016/0165-0270(95)00153-0 [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Bennett SAL, Vickers GJ (1989) The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 98:408–411. 10.1007/BF00451696 [DOI] [PubMed] [Google Scholar]
- Schindler CW, Thorndike EB, Goldberg SR, Lehner KR, Cozzi NV, Brandt SD, and Baumann MH (2016) Reinforcing and neurochemical effects of the “bath salts” constituents 3,4-methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-Nmethylcathinone (methylone) in male rats. Psychopharmacology (Berl) 233:1981–1990. 10.1007/s00213-015-4057-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewater SA, Creehan KM, Taffe MA (2015) Intravenous self-administration of entactogen-class stimulants in male rats. Neuropharmacology 99:538–545. 10.1016/j.neuropharm.2015.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF (2014) Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV). Addict Biol 19:165–174. 10.1111/j.1369-1600.2012.00474.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstock A, Mitcheson L, Ramsey J, Davies S, Puchnarewicz M, Marsden J (2011) Mephedrone: Use, subjective effects and health risks. Addiction 106:1991–1996. 10.1111/j.1360-0443.2011.03502.x [DOI] [PubMed] [Google Scholar]


