Abstract
Objectives:
To describe the academic concerns and risk strata of children with sickle cell disease (SCD) as identified through a parent-directed screening tool and to compare the rates of these concerns with actual school service utilization in the clinic population.
Study design:
We completed a retrospective review of patients with SCD referred to the school intervention program during the 2017–2018 and 2018–2019 school years due to a school-related concern raised by parents or noted by the clinical team. All parents completed the Brief School Needs Inventory (BSNI), a validated parent-response tool used to stratify academic risk. Rates of special education services, grade retention, and results from neuropsychological testing were captured. Clinical history, the use of disease-modifying therapy, and results from laboratory and neuroimaging studies were also obtained. Descriptive statistics were performed to examine demographic information, clinical history, and BSNI results.
Results:
137 unique patients (age range – 14 months to 19 years) completed the BSNI during the study period, for a total of 181 events. According to BSNI risk-stratification, 45% of patients were deemed low, 36% moderate, and 19% high academic risk. Over half of parents were concerned about their ability to advocate for their child’s needs. Despite legal qualification for a Section 504 accommodation plan, only 20% had established plans. Academic concerns were common with 31% of children reporting an Individualized Education Program and 20% with grade retention/remediation.
Conclusions:
Concerns for academic challenges remain high amongst parents of children with SCD; however, school service utilization remains disproportionately low due to numerous reasons.
Keywords: sickle cell anemia, neurocognition, individualized education program, stroke, silent infarction
Sickle cell disease (SCD) is a common and life-threatening inherited disorder of hemoglobin, affecting over 100,000 persons in the US and millions worldwide.1,2 The acute and chronic complications of SCD, affecting nearly every organ system, begin as early as the first year of life and without adequate treatment, result in significant morbidity and early mortality.3 Neurologic complications are among the most common and devastating effects of untreated SCD.4 The clinical severity of these complications is wide, ranging from overt stroke to more subtle neurocognitive deficits.5–8 Although the risk of overt stroke (11% before the age of 20 years),5 silent cerebral infarction (33% of children by age 10 years)9 and cerebral vasculopathy among children with the more common and severe sickle cell genotypes (sickle cell anemia or SCA) is well-recognized, the subtle SCA-related damage to brain tissue is often overlooked. Many children with normal brain MRI/MRA studies and transcranial Doppler (TCD) velocities who are not receiving disease-modifying therapy with either hydroxyurea or chronic blood transfusion therapy have significant neurocognitive deficits primarily affecting executive functioning and attention.8,10 Measures of full-scale intelligence for children with SCA are significantly lower than non-affected sibling and community controls.6,8,10
Although many of these neurologic insults are often referred to as silent, they negatively impacting not only academic achievement,11 but also subsequent job attainment and financial stability later in life, with estimated unemployment rates as high as 44% for adults with SCD.12 Rates of specialized school service utilization in children with SCD remain underreported, but with data suggesting approximately 37%, whereas grade retention rates range from 28–40% in adolescents with SCD.13,14 Although neurocognition and general intelligence are significant contributors to academic achievement, there are a number of additional factors that influence school performance, including socioeconomic status, parental education, and lack of family cohesion.16–19 In addition, disease severity influences academic achievement,11 primarily due to school absences for acute SCD complications requiring frequent clinic visits and prolonged hospitalizations. Finally, patients with SCD are disproportionately of minority populations1 and are affected by social determinants of health,20 which are associated with worse health outcomes in general.21
Many educators are unaware of the cognitive deficits and increased need for support services for children with SCD;22 further, the recommendation for school assessments in this population has not yet been incorporated into national SCD care guidelines.23 Our objectives with this study were to first describe the academic concerns and risk strata of our pediatric population with SCD as identified through a parent-directed screening tool and to compare the rates of these concerns/risks with actual school service utilization in our clinic population.
Methods
Brief School Needs Inventory
The Cincinnati Children’s Hospital Medical Center (CCHMC) employs a full-time licensed educator who acts a liaison between the family, medical team, and school to provide education to school staff regarding a child’s diagnosis and to support planning for needed school services to minimize educational problems related to diagnosis and treatment. Children with SCD (all genotypes) can be referred to the school liaison by any member of the clinical team, including physicians, nurse practitioners, nurse care managers, social workers, or psychologists for any school-related concern, real or anticipated. Upon receiving a referral, the school liaison interviews the family to complete the Brief School Needs Inventory (BSNI), a tool designed and validated at CCHMC to determine a child’s educational risk based on academic and psychosocial history and parental responses; a full description of the development and validation of the BSNI is outside the scope of this paper and has been published elsewhere.24
In completing the BSNI, parents are asked to indicate whether their child has had, currently has, or if they anticipate concerns in the following areas: grade retention, school attendance, academic performance, school supports/accommodations, peer relationships, emotions or behavior at school, and the parent’s ability to explain his/her child’s medical needs to the school; current or unresolved concerns receive more weight, as do academic over social concerns (Figure, A). Item responses generate a preliminary numeric score from 0–20. This numeric score, representing parental concerns, contributes approximately a third to the overall composite educational risk score (low, moderate, or high). The other two thirds are determined by the school liaison’s assessment of the family’s preparedness to advocate to the school on the child’s behalf and the anticipated impact of the child’s current health status on his/her school participation; the sum of the contribution of all three creates the final qualitative educational risk of low, moderate, or high (Figure, B; for the full BSNI, see Appendix 1 [available at www.jpeds.com]). Although for completeness the school liaison asks about current/past school service utilization, the latter does not factor into the final BSNI score. Rather, the score helps facilitate a tiered service model for increasing levels of intervention based on the patient’s unique level of need. Tier 1 services include supportive documentation (see form diagnosis letter, Appendix 2 [available at www.jpeds.com) and consultation with the family, Tier 2 expands to include phone/virtual contact with the child’s school team, and Tier 3 services include the school liaison’s in person participation in school team meetings and the sharing of neuropsychological evaluation reports with the school team to inform educational planning. Although in our clinic the school liaison provides the most support for patients in regard to advocating to school personnel, practitioners provide clinical evidence for school services as needed.
FIGURE 1. The Brief School Needs Inventory.
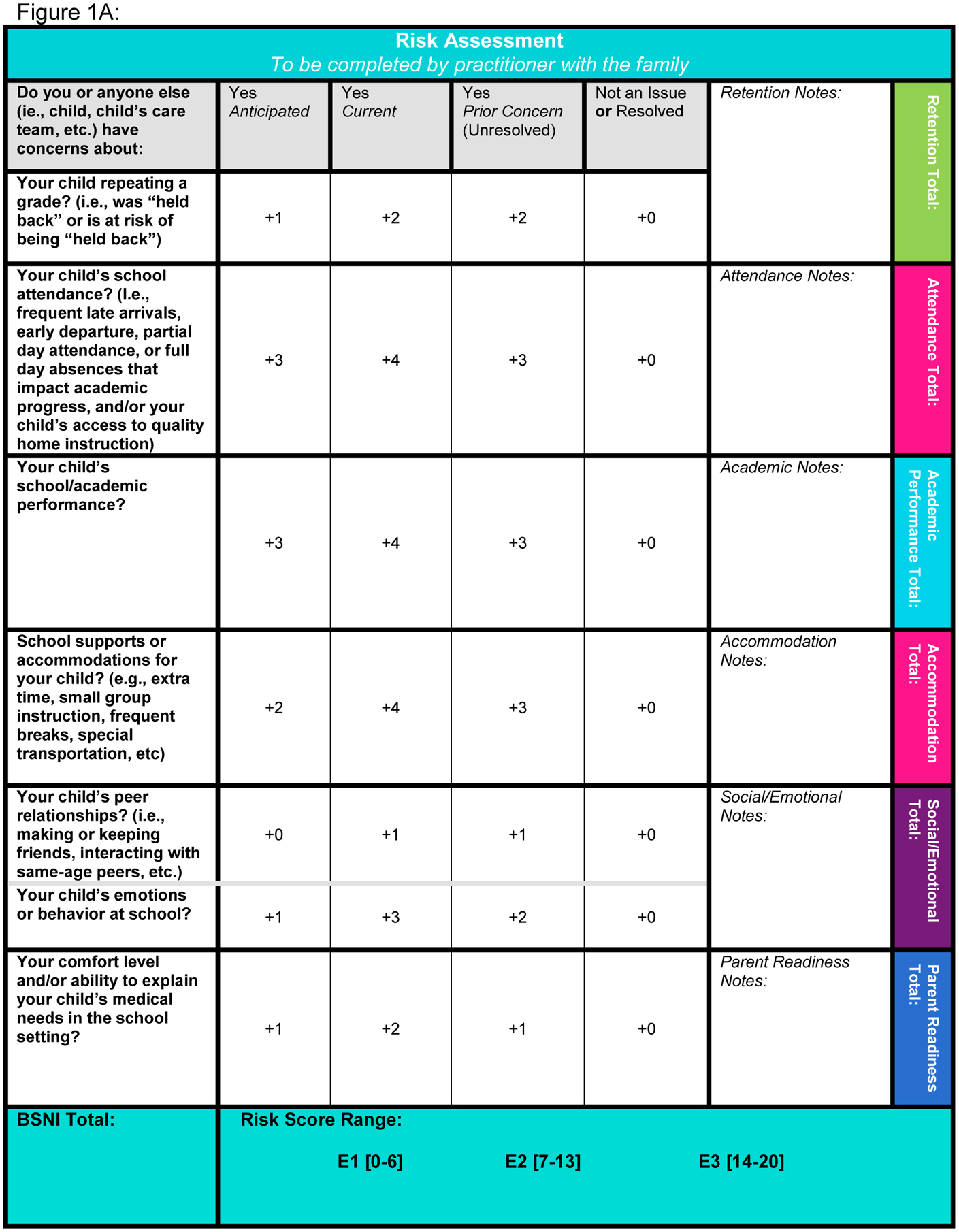
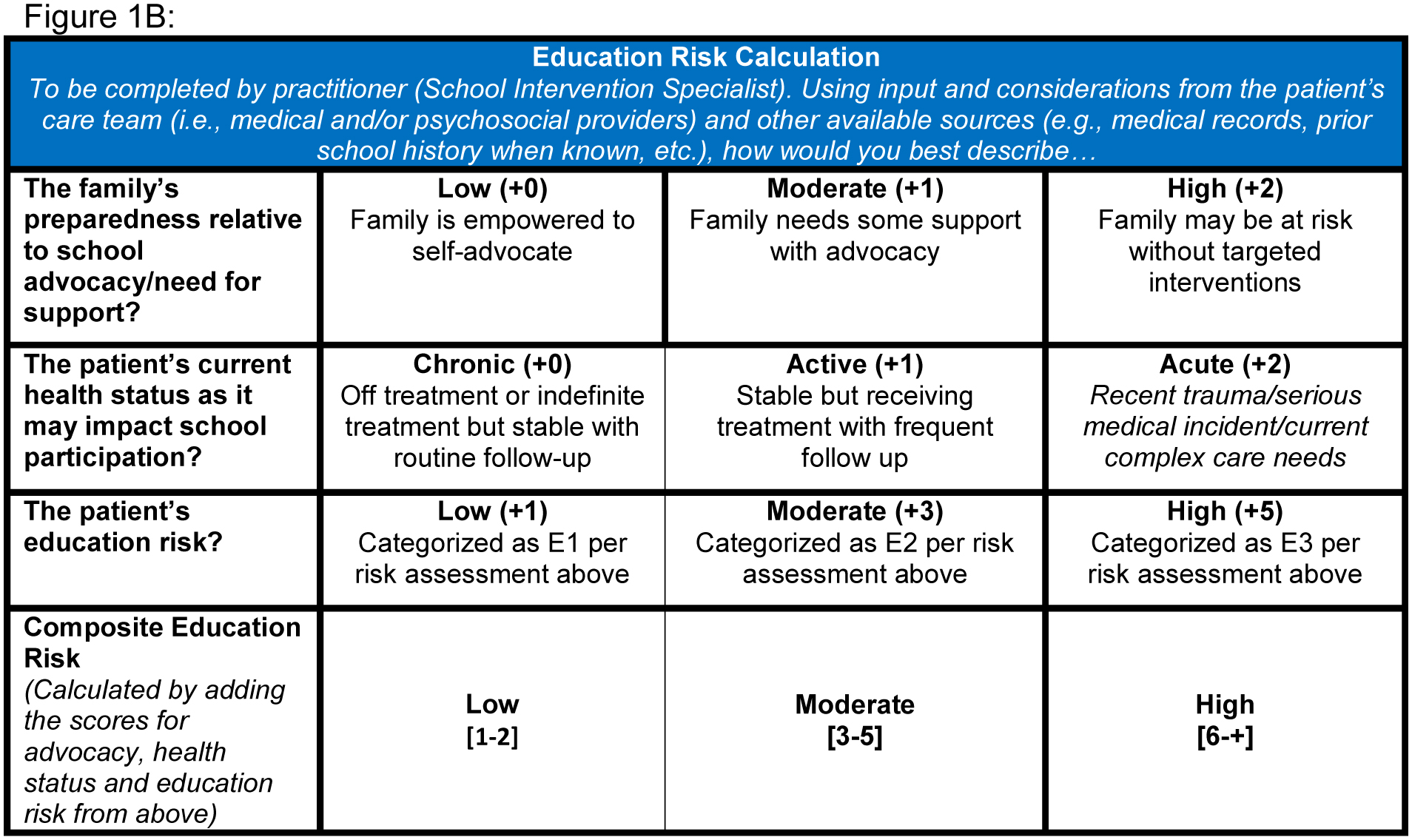
The Brief School Needs Inventory (BSNI) is composed of two primary parts, including A) seven parent-directed response items that determine the “educational risk score” and B) the school liaison’s assessment of the parent’s comfort level with working with the school, the child’s current health needs, and the risk assessment score from above, which combine for the overall composite numerical score (0–20), E1 = low education risk, E2 = moderate education risk, E3 = high education risk.
Routine Neuroanatomical and Neurocognitive Screening Guidelines
The CCHMC SCD Clinical Practice Guidelines (Appendix 3; available at www.jpeds.com) include routine and comprehensive evaluation of neuroanatomical and neurocognitive status. Specifically, for children with the more severe genotypes (HbSS and HbS-β0thalassemia) both formal neuropsychological testing and brain MRI/MRA are recommended beginning at age five years and every five years until transition to an adult hematology provider, typically at age 21 years. Also in the high risk genotype group, transcranial Doppler studies are performed beginning at age two years and at least every year thereafter depending upon results. Children with the generally less severe genotypes (HbSC and HbS-β+thalassemia) are referred for brain imaging or neuropsychological testing only as clinically indicated. Assessment of academic status is recommended at least annually for all children, regardless of genotype.
Retrospective Review
The BSNI data were reviewed for all patients with SCD completed during the 2017–2018 and 2018–2019 school years; patients were identified using the school liaison’s records. The results of the BSNI are then uploaded to a flowsheet within the electronic medical record (EMR); all information was validated by also reviewing the school liaison’s electronic progress notes detailing interactions with each family and school personnel. The frequency of grade retention and the receipt of special education services, including an individualized education program (IEP) or 504 accommodation plan were captured from the school liaison’s electronic records. To distinguish between the two formalized service plan documents, 504 accommodation plans solely allow accommodations for the student to access general education, as well as for allowances related to his/her diagnosis, such as extra water breaks and nurse access, while IEPs additionally provide direct instruction for identified educational needs, including related services. IEPs are only developed after a student has been found eligible through the evaluation process;25 any prior 504 accommodation plan will be incorporated into the IEP. In accordance with Section 504 of the Rehabilitation Act of 1973, children typically qualify for a 504 accommodation plan by meeting the legal requirement of having a disability that substantially limits one or more major life activities, though individually must still be determined to have such needs. Under the Individuals with Disabilities Education Act (IDEA), SCD is listed as a qualifying diagnosis under the category of Other Health Impaired – Minor provided there is evidence of an adverse effect on the child’s educational performance.26 Of note, private schools are not required to offer the specialized school service plans.
Basic demographic and disease-related data, including zip code, patients’ disease history, use of disease-modifying therapy, and whether the patient had had formal neuropsychological testing and brain imaging were also recorded from the EMR. Using patients’ nine digit zip codes, we determined their Area Deprivation Index (ADI) using the publicly available Neighborhood Atlas through the University of Wisconsin School of Medicine and Public Health.27 The ADI calculates the degree of neighborhood or census group-level disadvantage using 17 indicators of poverty, employment status, housing quality, and education;28,29 neighborhood disadvantage has been linked with poorer health outcomes across various chronic diseases.29–32 We divided our population into those with national standardized scores from the 0–50th percentiles (least disadvantaged) and then into deciles to the most disadvantaged (100th percentile).
Statistical Analysis
Descriptive statistics were performed to examine demographic information, clinical history, and BSNI results. Statistical analysis was completed using R.33 The study was approved by the CCHMC Institutional Review Board with a waiver of informed consent; all personal health information was de-identified following extraction from the electronic medical record.
Results
Demographic and Clinical Factors
For the combined school years of 2017–2018 and 2018–2019, 179 patients were referred to the school liaison. Of these, parents of 137 patients (49% female, 99% Black and non-Hispanic) completed the BSNI for a total of 181 events. Of the other 42 patients, nine families did not respond to efforts to make contact, and another thirty-three did not undergo the full BSNI due to grade level (young daycare or post-secondary) or having moved away.
Of the 137 patients who completed the BSNI during the study period, all sickle cell genotypes were represented with 70% of patients having the HbSS genotype (Table I). The mean age was 10.5±4.5 years (range 14 months to 19 years). The entire grade spectrum was represented, ranging from daycare/preschool to post-secondary education. All school types were represented with the majority of patients attending public school (75%). A majority of patients (76%) lived in neighborhoods with national ADI scores of at least the 50th percentile (more disadvantaged) and many (29%) lived in the highest decile of disadvantaged neighborhoods (ADI scores 91–100th percentiles).
TABLE 1.
Demographic & Socioeconomic Characteristics
| Total N = 137* (%) | ||
|---|---|---|
| Genotype | HbSS | 96 (70) |
| HbSC | 35 (26) | |
| HbS- β0thalassemia | 1 (0.7) | |
| HbS-β+thalassemia | 5 (3.6) | |
| Grade | preschool/pre-K/kindergarten | 26 (19) |
| elementary | 55 (40) | |
| middle | 20 (15) | |
| high school | 34 (25) | |
| college | 2 (1.2) | |
| School Type** | public | 103 (95) |
| private | 10 (7) | |
| charter | 13 (10) | |
| home school/online | 4 (3) | |
| Area Deprivation Index (0–100)# | Least disadvantaged (<50) | 32 (24) |
| 50–60 | 9 (7) | |
| 61–70 | 9 (7) | |
| 71–80 | 15 (11) | |
| 81–90 | 28 (21) | |
| 91–100 | 38 (29) | |
unless otherwise specified
excluding daycare & college level, out of 130
Out of 131 due to six patients having moved, inability to confirm prior address
Of the 97 patients with the high-risk genotypes HbSS and HbS-β0thalassemia, 95 (98%) were receiving disease-modifying therapy. Specifically, 76 (78%) were prescribed hydroxyurea and 19 (20%) received chronic monthly transfusions or erythrocytapheresis. Of the 40 patients with non-SCA genotypes (HbSC or HbS-β+thalassemia), five (13%) were prescribed hydroxyurea and one received monthly erythrocytapheresis due to frequent acute and chronic pain.
Ninety-four patients (HbSS and HbS-β0thalassemia genotypes only) underwent routine TCD studies for stroke risk; 90% were normal without evidence of increased risk (Table 2). Five patients included in the study had a history of overt stroke. Approximately half (53%) of patients had undergone a brain MRI/MRA, mainly those with either HbSS or HbS-β0thalassemia. Although the majority of MRI/MRAs were normal, 27% showed evidence of silent cerebral infarcts, 5% showed evidence of past overt stroke, and 12% had cerebral vasculopathy, including Moyamoya syndrome. Despite clinical guidelines recommending neuropsychological testing for all children with the higher risk genotypes after five years of age, only 33% of those eligible had completed formal neuropsychological testing at least once at any time in the past. An additional 20% of these children with the high risk genotypes began the process of obtaining neuropsychological testing but did not complete the three required visits for the entire evaluation; 17/19 (89%) were from the 50–100th most disadvantaged neighborhoods with 8/19 (42%) from the highest decile of neighborhood disadvantage.
TABLE 2.
Clinical & Laboratory Data
| Genotype (137) | Baseline laboratory values | Disease-modifying therapy | TCD* | MRI/A** | Neuropsychological Testing | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Hb±95%CI (g/dL) | Mean abs retic±95% CI (109/mcL) | Mean %HbF±95% CI | None | Hydroxyurea | Chronic transfusions | normal | conditional | abnormal | Normal | Infarction (CVA/SCI)# | none | Yes | No | incomplete | |
| HbSS + Hb −β0 thalassemia (97) | 9.5 (1.2) | 230 (120) | 20 (12) | 2 | 76 | 19 | 74 | 6 | 4 | 33 | 23 | 33 | 27 | 52 | 18 |
| HbSC (35) | 11 (1.0) | 140 (50) | N/A | 24 | 10 | 1 | N/A | 6 | 1 | 26 | 9 | 26 | 0 | ||
| HbS-β+ thalassemia (5) | 12 (1.1) | 150 (50) | N/A | 5 | 0 | 0 | N/A | 0 | 0 | 5 | 1 | 3 | 1 | ||
three excluded due to age; also 5 patients with prior CVA & 5 with closed bone windows
one patient with HbSS unknown; total greater than 137 due to patients with multiple MRI findings; not listed are other findings: aneurysm, Moyamoya syndrome, non-SCD-related findings
CVA = clinical cerebrovascular accident; SCI = silent cerebral infarct; CVA refers to evidence of past stroke
BSNI Results & Evidence of Academic Challenges
The mean score on the BSNI was 7.2 (±5.6) (0–20 scale) with 45% of patients in the low, 36% moderate, and 19% high-risk categories (Table 3). There were no significant differences in the overall risk distribution across the two years when comparing unique patients with total events (P = .57–0.83). During the first year but not the second, 58 patients had an evaluation; 11 of these patients had graduated high school, two had moved, and 22 were deemed low-risk during the 2017–2018 school year, suggesting that further evaluation by the school liaison was not needed. Another 23 were categorized as moderate or high-risk, but were not followed during the 2018–2019 school year. Of the 44 patients who underwent the BSNI both years, almost 65% had changes in their risk stratification with nine moving to a lower risk level and 19 moving to a higher risk level, including seven increasing from “low-risk” to “high-risk”. We attempted to determine the reasons for this abrupt increase; we identified that two had moved from kindergarten to first grade, one changed schools and was concerned about losing prior services, three were at risk for failing subjects, and one was unclear, but may have been due to a language barrier.
TABLE 3.
BSNI Risk Stratification & Academic Challenges
| School Interventionist Assessment | Unique Patients (N = 137) | ||
|---|---|---|---|
| Low (%) | Moderate (%) | High (%) | |
| Composite education risk | 62 (45) | 49 (36) | 26 (19) |
| Parental Concern | Yes (%) | No (%) |
|---|---|---|
| Grade retention | 22 (16) | 115 (84) |
| School attendance | 53 (39) | 84 (61) |
| School/academic performance | 38 (28) | 99 (72) |
| Supports or accommodations | 78 (57) | 59 (43) |
| Peer relationships | 16 (12) | 121 (88) |
| Emotions/behavior at school | 19 (14) | 118 (86) |
| Comfort with explaining medical/educational needs | 69 (50) | 68 (50) |
| School Service Use | Yes (%) | No (%) | In process/almost/incomplete (%) |
|---|---|---|---|
| 504 medical plan* | 27 (20) | 101 (76) | 5 (4) |
| Individualized education plan (IEP)** | 41 (31) | 92 (69) | 1 (1) |
| Grade retention*** | 15 (13) | 92 (80) | 8 (7) |
4 patients unclear
3 patients unclear
22 excluded due to age
The most commonly reported concerns by parents were challenges in obtaining supports/accommodations for their child’s needs (reported by 57%), explaining their child’s medical needs to school personnel (reported by 50%), and concerns about their child’s school attendance and academic performance (reported by 39% and 28%, respectively). Parents were less concerned about grade retention, peer relationships, and their child’s behavior. The rate of academic challenges was consistent with parental concerns; 28% of respondents reported having a 504 accommodation plan in place, and 31% reported having an IEP, representing almost 60% of this high-risk population being supported on a formal school plan during the two years under study. Thirteen percent of patients had been retained at least one year during their academic career, and another seven percent of parents reported that their child had almost been retained; these children instead had either completed summer remediation or school personnel had requested retention. Importantly, only 9/23 (39%) of children who were retained or almost retained had an established IEP.
School Service Utilization & Imaging Results Stratified by Educational Risk
When evaluated more closely across educational risk status as determined by the BSNI, there was a nonsignificant increase in the rate of IEPs across educational risk from 28% in the low risk group to 42% in the high-risk group. The rate of 504 accommodation plans followed a similar trajectory from 19% in the low risk to 29% in the high risk groups. When combined as school service utilization, 48% of the low risk group, 53% of the moderate risk group, and 71% of the high risk group had either an IEP or 504 accommodation plan. There was a significant difference between the overall rate of school service utilization between the low and high risk groups (p = 0.024). Concerning grade retention, there was a trend towards significance with an increase in grade retention/almost retention with increasing educational risk (14% low risk, 24% moderate risk, 32% high risk; difference between low vs high risk, p = 0.066).
We also evaluated imaging findings by risk stratification; unfortunately, the comparison across MRI/MRA status was limited as 64/137 patients had not undergone an MRI/MRA. Rates of normal brain MRI/MRA were the same across all three risk levels (57% low risk, 51% moderate risk, and 56% high risk). Similarly, the rates of stroke (cerebrovascular accident + silent infarctions) were also the same across risk levels (32% low risk, 31% moderate risk, and 30% high risk). For those children who underwent routine TCD screening (HbSS and HbS-β0thalassemia genotypes), the high educational risk group had a lower rate of normal TCD velocities (low risk 76%, moderate risk 84%, and high risk 59%); the difference between the moderate and high risk groups reached statistical significance (p = 0.011) but not between the low and high risk groups. There were no significant differences in conditional or abnormal TCD velocities across the three groups, likely due to small numbers of children with either of the former.
Discussion
This review allowed for a broad cross-sectional approximation of the rates and types of academic challenges in the pediatric and adolescent population with SCD. The majority of parents in our referred cohort reported having challenges in obtaining appropriate accommodations and explaining their child’s needs to school personnel. In addition, almost 60% of patients were deemed at elevated risk for academic challenges via the BSNI screening tool. Although a true prevalence rate could not be calculated, the dichotomy between the elevated percentage of patients identified by the clinical team as having academic concerns or challenges and the actual rates of school service utilization were concerning. Congruous with studies in adolescents, in which the prevalence of grade retention is between 28–40%,13,14 we found that over 20% of our referred population had been or had almost been retained. This proportion held when looking solely at elementary school-aged children, as compared with a national average of 2–6%.34 Our data are consistent with published studies demonstrating that children with SCD have grade retention rates higher than national, state, and local norms.35
Most American patients with SCD are Black and due to the historical effects of systemic racism, are disproportionately affected by health-related disparities and social determinants of health.20 These racial disparities affect Black individuals in all aspects of life, including income, access to health care, health care outcomes, and educational achievement. when subdivided by race, Black students have higher high school dropout rates than their White peers.36 Our studied population demonstrated similar sociodemographic challenges, as a disproportionate number of patients live in the most disadvantaged neighborhoods in the country, including one quarter living in the tenth most disadvantaged neighborhoods. However, the academic challenges for children with SCD extend beyond racial and socioeconomic disparities, as children with SCD disproportionately have higher rates of school retention and special education services compared with non-affected children in local school districts.35 The rate of academic challenges in a cohort of children with SCD was double that of demographically and socioeconomically-matched peers.37 Compared with sibling and unaffected matched community controls, individuals with SCD score lower on measures of full scale intelligence, which decline proportionately with the presence of silent cerebral infarcts and a history of overt stroke;8 thus, their academic and life-related challenges are compounded by the socioeconomic and racial barriers that unequally affect this population.38
Published data suggests that the rate of specialized education services (either an IEP or 504 accommodation plan) for children with SCD is 34–37%.13,35 One-half of our patients receive services which may be due to this population being self-identified as high-risk, but also the efforts of our school liaison to work directly with schools to advocate for patients’ needs. Our school liaison completed 102 in-service sessions with school personnel during the two years under study. Our center published the results of a randomized pilot trial of school intervention for children with SCD in 2004; children who were randomized to the intervention arm, which included in-service sessions with the child’s teacher and peers, had significantly lower school absences compared with those randomized to a more passive approach.39 Implementation of another dedicated school intervention program increased the number of patients with SCD with known overt and silent cerebral infarctions who received IEPs.40
We recognize that many programs may not have the resources to support an extensive hospital-based school intervention program. In an SCD program in Minnesota, which includes a clinic-embedded neuropsychologist/school liaison, a limiting factor was a reliance on philanthropic support for these additional services, given that none were reimbursable.41 However, even with limited resources, efforts by the healthcare provider to screen and include discussions about school with parents are free and feasible to add to the clinic visit. In a systematic review of school experiences of children with chronic illness returning to school after a prolonged absence, the smoothest returns involved structured communication between health care personnel, the school, and family.42 The American Academy of Pediatrics (AAP) outlined the pediatrician’s central role in development and implementation of individual family service plans, as well as care coordination for children with chronic illness.43 Further, school personnel often are unaware of the neurocognitive deficits children with SCD face,22 which the pediatrician can help mitigate by his/her involvement.
The BSNI is a useful and easily administered tool for identifying those children most in need of intervention, which may allow for appropriate allocation of clinic or hospital resources. Other studies have also shown the utility of similarly brief screening tools at identifying high-risk patients in the clinic setting.44–46 At a minimum, most patients with SCD should qualify for a 504 accommodation plan, or similar health/medical plan; the treating provider can provide support through documentation of medical necessity and recommendations. Form letters (Appendix 2) describing the challenges and special medical and academic needs for children with SCD should be provided to all families and schools annually. An AAP policy statement emphasizes the pediatrician’s role in assisting with documentation for a 504 accommodation plan and for advocating for specialized school services.47
Our study has several limitations. The fact that those referred to the school liaison were identified by the clinical team as being at higher risk for academic challenges suggests that our cohort may not be representative of our entire population with SCD. Due to the retrospective nature of this study, we were unable to determine the rates of school service utilization in those clinic patients not referred to the school intervention program. Due to a lack of longitudinal data, we were unable to evaluate associations between neuroanatomical changes, treatment history, and academic challenges. Our goal in the future is for all patients to undergo the BSNI yearly regardless of referral to the school interventionist. Due to the retrospective design of this study and its reliance on parent-reported information, some of the data were missing and could not be verified; all school service utilization data were self-reported and not verified with the school districts. In addition, the missing data could have unintentionally created bias, either by under- or over-estimating school service utilization and grade retention rates. Finally, as not all eligible patients underwent MRI/MRA or formal neuropsychological testing, the rate of abnormal findings may be falsely elevated as children with more severe clinical concerns were referred.
In conclusion, this study confirms the high rates of and more clearly details the academic challenges for children and adolescents with SCD. These challenges are found in many patients with SCD who do not have overt neuroanatomical changes and are likely multi-factorial in nature. Academic challenges begin early in elementary school and become more prevalent and pronounced with advancing grade level. A lack of appropriate assistance through specialized school services only compounds this problem. Thus, even if a formal clinic or hospital-based school intervention program is not feasible, all healthcare providers of children with SCD should implement universal screening for academic risk given the importance of education to ensure later success in adulthood.
Supplementary Material
Acknowledgments
Funded by NHLBI 1K23HL128885.
Abbreviations:
- ADI
area deprivation index
- BSNI
brief school needs inventory
- IEP
individualized education program
- MRI/A
magnetic resonance imaging/angiography
- SCA
sickle cell anemia
- SCD
sickle cell disease
- TCD
transcranial Doppler
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this study were presented at the American Society of Hematology annual meeting, December 7-10, 2019, Orlando, Florida.
References
- 1.Hassell K Population estimates of sickle cell disease in the U.S. Am J Prev Med 2010; 38:S512–21. [DOI] [PubMed] [Google Scholar]
- 2.Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN. Global burden of sickle cell anaemia in children under five, 2010–2050: modelling based on demographics, excess mortality, and interventions. PLoS Med 2013; 10:e1001484. doi: 10.1371/journal.pmed.1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leikin SL, Gallagher D, Kinney TR, Sloane D, Klug P, Rida W. Mortality in children and adolescents with sickle cell disease: Cooperative Study of Sickle Cell Disease. Pediatrics 1989; 84:500–8. [PubMed] [Google Scholar]
- 4.DeBaun MR, Kirkham FJ. Central nervous system complications and management in sickle cell disease. Blood 2016; 127:829–38. [DOI] [PubMed] [Google Scholar]
- 5.Ohene-Frempong K, Weiner SJ, Sleeper LA, Miller ST, Embury S, Moohr JW, et al. Cerebrovascular accidents in sickle disease: rate and risk factors. Blood 1998; 91:288–94. [PubMed] [Google Scholar]
- 6.Armstrong FD, Thompson RJ Jr, Wang W, Zimmerman R, Pegelow CH, Miller S, et al. Cognitive functioning and brain magnetic resonance imaging in children with sickle Cell disease. Neuropsychology Committee of the Cooperative Study of Sickle Cell Disease. Pediatrics 1996; 97:864–70. [PubMed] [Google Scholar]
- 7.Vichinsky EP, Neumayr LD, Gold JI, Weiner MW, Rule RR, Truran D, et al. ; Neuropsychological Dysfunction and Neuroimaging Adult Sickle Cell Anemia Study Group. Neuropsychological dysfunction and neuroimaging abnormalities in neurologically intact adults with sickle cell anemia. JAMA 2010; 303:1823–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prussien KV, Jordan LC, DeBaun MR, Compas BE. Cognitive function in sickle cell disease across domains, cerebral infarct status, and the lifespan: a meta-analysis. J Pediatr Psychol 2019; 44:948–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pegelow CH, Macklin EA, Moser FG, Wang WC, Bello JA, Miller ST, et al. Longitudinal changes in brain magnetic resonance imaging findings in children with sickle cell disease. Blood 2002; 99:3014–8. [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Enos L, Gallagher D, Thompson R, Guarini L, Vichinsky E, et al. Neuropsychologic performance in school-aged children with sickle cell disease: a report from the Cooperative Study of Sickle Cell Disease. J Pediatr 2001; 139:391–7. [DOI] [PubMed] [Google Scholar]
- 11.Smith KE, Patterson CA, Szabo MM, Tarazi RA, Barakat LP. Predictors of academic achievement for school age children with sickle cell disease. Adv Sch Ment Health Promot 2013; 6:5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanger M, Jordan L, Pruthi S, Day M, Covert B, Merriweather B, et al. Cognitive deficits are associated with unemployment in adults with sickle cell anemia. J Clin Exp Neuropsychol 2016; 38:661–71. [DOI] [PubMed] [Google Scholar]
- 13.Crosby LE, Joffe NE, Irwin MK, Strong H, Peugh J, Shook L, et al. School performance and disease interference in adolescents with sickle cell disease. Phys Disabil 2015; 34:14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herron S, Bacak SJ, King A, DeBaun MR. Inadequate recognition of education resources required for high-risk students with sickle cell disease. Arch Pediatr Adolesc Med 2003; 157:104. [DOI] [PubMed] [Google Scholar]
- 15.King A, Rodeghier MJ, Panepinto JA, Strouse JJ, Casella JF, Quinn CT, et al. Silent cerebral infarction, income, and grade retention among students with sickle cell anemia. Am J Hematol 2014; 89:E188–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King AA, Strouse JJ, Rodeghier MJ, Compas BE, Casella JF, McKinstry RC, et al. Parent education and biologic factors influence on cognition in sickle cell anemia. Am J Hematol 2014; 89:162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ladd RJ, Valrie CR, Walcott CM. Risk and resilience factors for grade retention in youth with sickle cell disease. Pediatr Blood Cancer 2014; 61:1252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sirin SR. Socioeconomic status and academic achievement: a meta-analytic review of research. Review of Educational Research 2005; 75:417–53. [Google Scholar]
- 19.Davis-Kean PE. The influence of parent education and family income on child achievement: the indirect role of parental expectations and the home environment. J Fam Psychol 2005; 19:294–304. [DOI] [PubMed] [Google Scholar]
- 20.Power-Hays A, Li S, Mensah A, Sobota A. Universal screening for social determinants of health in pediatric sickle cell disease: A quality-improvement initiative. Pediatr Blood Cancer (2020). doi: 10.1002/pbc.28006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Commission on Social Determinants of Health [homepage on the internet]. Geneva: World Health Organization; [updated 2008; cited 2020 Apr 2]. Closing the gap in a generation: health equity through action on the social determinants of health. Final Report of the Commission on Social Determinants of Health. Available from: https://apps.who.int/iris/bitstream/handle/10665/43943/9789241563703_eng.pdf;jsess [Google Scholar]
- 22.King AA, Herron S, McKinstry R, Bacak S, Armstrong M, White D, et al. A multidisciplinary health care team’s efforts to improve educational attainment in children with sickle-cell anemia and cerebral infarcts. J Sch Health 2006; 76:33–7. [DOI] [PubMed] [Google Scholar]
- 23.National Heart, Lung and Blood institute [homepage on the internet]. Bethesda, MD: NHLBI; [updated 2014 Sept; cited 2020 Apr 2]. Evidence-based management of sickle cell disease: Expert panel report 2014. Available from: https://www.nhlbi.nih.gov/sites/default/files/media/docs/sickle-cell-disease-report%20020816_0.pdf [Google Scholar]
- 24.Elam M, Murphy C, Irwin MK. Validity, reliability, and feasibility of the Brief School Needs Inventory: Evaluating educational risk for students with chronic health conditions. Psychooncology 2019; 28:1483–9. [DOI] [PubMed] [Google Scholar]
- 25.US Department of Education [homepage on the internet]. Washington, D.C.: USDE; [updated 2019 Aug 30; cited 2020 Feb 19]. Laws & Guidance: Special Education & Rehabilitative Services: A guide to the individualized education program. Available from: https://www2.ed.gov/parents/needs/speced/iepguide/index.html#introduction [Google Scholar]
- 26.Center for Parent Information & Resources [homepage on the internet]. Newark, NJ: US Dept of Edu: Office of Special Education Programs; [cited 2020 Mar 22]. Available from: https://www.parentcenterhub.org/section504/ [Google Scholar]
- 27.2015 Area Deprivation Index v.2.0 [Internet]. Madison, WI: University of Wisconsin School of Medicine and Public Health; [updated 2015; cited 2020 Mar 8]. Available from: https://www.neighborhoodatlas.medicine.wisc.edu/ [Google Scholar]
- 28.Singh GK. Area deprivation and widening inequalities in US mortality, 1969–1998. Am J Public Health 2003; 93:1137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kind AJ, Jencks S, Brock J, Yu M, Bartels C, Ehlenbach W, et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med 2014; 161:765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu J, Kind AJH, Nerenz D. Area deprivation index predicts readmission risk at an urban teaching hospital. Am J Med Qual 2018; 33:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durfey SNM, Kind AJH, Buckingham WR, DuGoff EH, Trivedi AN. Neighborhood disadvantage and chronic disease management. Health Serv Res 2019; 54:206–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ludwig J, Sanbonmatsu L, Gennetian L, Adam E, Duncan GJ, Katz LF, et al. Neighborhoods, obesity, and diabetes- a randomized social experiment. N Engl J Med 2011; 365:1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.R Core Team. R: A language and environment for statistical computing [internet]. Vienna, Austria: R Foundation for Statistical Computing; c2017; [updated 2019 Jul 5; cited 2020 Mar 8]. Available from: http://www.R-project.org/ [Google Scholar]
- 34.Warren JR, Hoffman E, Andrew M. Patterns and trends in grade retention rates in the United States, 1995–2010. Educ Res 2014; 43:433–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Epping AS, Myrvik MP, Newby RF, Panepinto JA, Brandow AM, Scott JP. Academic attainment findings in children with sickle cell disease. J Sch Health 2013; 83:548–53. [DOI] [PubMed] [Google Scholar]
- 36.McFarland J, Cui J, Holmes J, Wang X. US Department of Education [Internet]. Washington, DC: National Center for Education Statistics. [cited 2020 Nov 8]. Trends in high school dropout and completion rates in the United States: 2019 Compendium Report. Available from: https://nces.ed.gov/pubs2020/2020117.pdf [Google Scholar]
- 37.Schatz J Brief report: Academic attainment in children with sickle cell disease. J Pediatr Psychol 2004; 29:627–33. [DOI] [PubMed] [Google Scholar]
- 38.Power-Hays A, McGann PT. When actions speak louder than words – racism and sickle cell disease. N Engl J Med (2020). doi: 10.1056/NEJMp2022125. [DOI] [PubMed] [Google Scholar]
- 39.Koontz K, Short AD, Kalinyak K, Noll RB. A randomized, controlled pilot trial of a school intervention for children with sickle cell anemia. Journal of Pediatric Psychology 2004; 29:7–17. [DOI] [PubMed] [Google Scholar]
- 40.King AA, Herron S, McKinstry R, Bacak S, Armstrong M, White D, et al. A multidisciplinary health care team’s efforts to improve educational attainment in children with sickle-cell anemia and cerebral infarcts. J Sch Health 2006; 76:33–37. [DOI] [PubMed] [Google Scholar]
- 41.Lum A, Wakefield CE, Donnan B, Burns MA, Fardell JE, Marshall GM. Understanding the school experiences of children and adolescents with serious chronic illness: a systematic meta-review. Child Care Health Dev 2017; 43:645–62. [DOI] [PubMed] [Google Scholar]
- 42.American Academy of Pediatrics Council on Children With Disabilities, Cartwright JD. Provision of educationally related services for children and adolescents with chronic diseases and disabling conditions. Pediatrics 2007; 119:1218–23. [DOI] [PubMed] [Google Scholar]
- 43.Wills KE, Nelson SC, Hennessy J, Nwaneri MO, Miskowiec J, McDonough E, et al. Transition planning for youth with sickle cell disease: embedding neuropsychological assessment into comprehensive care. Pediatrics 2010; 126:S151–59. [DOI] [PubMed] [Google Scholar]
- 44.Schatz J, Schlenz A, Reinman L, Smith K, Roberts CW. Developmental screening in pediatric sickle cell disease: disease-related risk and screening outcomes in 4 year olds. J Dev Behav Pediatr 2017; 38:654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hood AM, Reife I, King AA, White DA. Brief screening measures identify risk for psychological difficulties among children with sickle cell disease. J Clin Psychol Med Settings 2020; 27:651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karkoska K, Zaheer S, Chen V, Fishbein J, Appiah-Kubi A, Aygun B. A pilot study to screen for poor academic performance in children with sickle cell disease in the outpatient setting. Pediatr Blood Cancer (2020). doi: 10.1002/pbc.28196. [DOI] [PubMed] [Google Scholar]
- 47.Allison MA, Attisha E, Council on School Health. The link between school attendance and good health. Pediatrics (2019). doi: 10.1542/peds.2018-3648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


