Abstract
Banked allogeneic or ‘off-the-shelf’ (OTS) T cells from healthy human donors are being developed to address the limitations of autologous cell therapies. Potential challenges of OTS T cell therapies are associated with their allogeneic origin and the possibility of graft-versus-host disease (GvHD) and host-versus-graft immune reactions. While the risk of GvHD from OTS T cells has been proved to be manageable in clinical studies, approaches to prevent immune rejection of OTS cells are at an earlier stage of development. We provide an overview of strategies to generate OTS cell therapies and mitigate alloreactivity-associated adverse events, with a focus on recent advances for preventing immune rejection.
‘Off-the-shelf’ cell therapies
Autologous cell therapies utilize a patients’ own immune cells, including T cells, NK cells, innate-like T cells (see Glossary), to treat human cancers and have achieved remarkable clinical success against advanced malignancies[1–3]. Currently, T cells expressing chimeric antigen receptors (CAR-T), specifically, autologous CD19-CAR-T cell products axicabtagene ciloleucel, brexucabtagene autoleucel and tisagenlecleucel, are the only US FDA-approved cell products[4–6]. Despite clinical success and commercialization, autologous cell therapies in general, and CAR-T cells in particular, require complex patient-specific manufacturing that limits their scalability and often result in therapeutic products with unpredictable potency. In addition, the expense and potential for manufacturing failure or disease progression during cell product generation further restrict accessibility to autologous cell therapies[7,8].
To address these key hurdles, recent efforts have focused on developing well characterized allogeneic or ‘off-the-shelf’ (OTS) cell therapies manufactured from healthy donors and then banked to offer immediate availability and high potency at a reduced cost[9]. Potential limitations of OTS cell therapies are associated with their allogeneic origin and the possibility of graft-versus-host disease (GvHD) and host-versus-graft immune reactions. Both reactions are common adverse events associated with all types of allogeneic cell/tissue/organ transplantation and are mainly mediated by alloreactive CD4+ and CD8+ T and NK cells[10]. Alloreactive T cells, through their native T cell receptors (TCRs), recognize mismatches of cell surface markers between the donor and recipient, including the major histocompatibility complex (MHC) and minor histocompatibility antigens (MiHAs)[11–15]. According to the ‘missing self’ hypothesis alloreactive NK cells may be activated through NK cell receptors (NKRs) that sense the lack of certain MHC molecules on the allogenic target [16–18]. Due to the polymorphic nature of MHCs and MiHAs in humans, each individual has a unique combination of MHC/MiHA, and mismatches between the transplant donor and recipient can trigger an immune response.
Whether donor-recipient mismatch results in GvHD or rejection is dictated by the donor-recipient balance between the number and frequency of alloreactive cells[19]. Specifically, GvHD is caused when the donor alloreactive cells dominate and recognize healthy patient tissues as foreign and destroy them. It is commonly observed after allogenic hematopoietic stem cell transplantation (HSCT), since the patients’ immune and hematopoietic systems are ablated by cytotoxic drugs and/or radiation and a large number of donor cells are often transferred to ensure successful engraftment. Conversely, host-versus-graft activity or immune rejection occurs when host alloreactive lymphocytes (including T, B and NK cells) target the mismatched transplant. This complication is more frequently seen after solid organ transplantation, since patients are less immunocompromised than after HSCT and the transplanted organs contain fewer alloreactive lymphocytes than the HSCT graft[19].
In principle, either GvHD or rejection may occur after OTS cell therapy, although clinical data remain too scarce to know which will dominate. As discussed above, immunocompromised patients should have a higher risk of GvHD, while immunocompetent recipients should more likely reject the infused OTS product, prematurely terminating the desired anti-tumor activity[20]. Rejection not only causes unpredictable pharmacokinetics and suboptimal clinical outcomes, but also may lead to the formation of memory immune-cell populations that will speedily reject subsequent infusions of OTS products from the same donor. As current trials of T cell therapies include lymphodepleting regimens prior to T cell infusion, GvHD has been observed as an adverse event on rare occasions[21,22], whereas the incidence of immune rejection remains to be determined in the clinic.
In this review, we first briefly summarize strategies to generate OTS cell therapies and mitigate GvHD following infusion of allogeneic CAR-T cells, many of which have shown clinical benefit. Then, we focus on recent approaches to address the complex clinical problem of preventing immune rejection of OTS cells.
Sources of OTS T cells
Currently, most therapeutic polyclonal T cells are sourced from peripheral blood mononuclear cells (PBMCs), which are easily accessible and have demonstrated clinical activity[1,4–6]. As discussed below, emerging evidence suggests that alternative sources of OTS T cells, such as T cells derived from human umbilical cord blood (UCB) and induced pluripotent stem cells (iPSCs), may possess certain intrinsic advantages over PBMC-derived T-cells, including lower immunogenicity and greater proliferative capacity.
UCB-derived cells
Investigators have reported certain promising clinical results using UCB-derived CD19-CAR-NK cells for various lymphoid tumors[23], while the efficacy and safety of UCB-derived CAR-T cells remains under preclinical evaluation. UCB grafts have been used as a source of allogeneic stem cells with relatively low incidence and severity of GvHD[25] in transplant recipients. However, we do not yet know whether UBC-derived CAR-T cells also possess lower alloreactivity than PBMC-derived ones and if this property also reduces their anti-tumor efficacy in vivo.
iPSC-derived cells
Technological advances have enabled us to genetically engineer and robustly differentiate ex vivo iPSCs into immune cell populations that include T[27–29] and NK cells[30,31]. iPSC-derived immune effectors should establish a well-characterized cell bank with the potential for extensive expansion, allowing repeat dosing of multiple recipients, and minimal batch-to-batch functional variation[32]. These properties make iPSCs an attractive source of OTS cell products. Ongoing phase I clinical studies are evaluating iPSC-derived CAR-NK cell therapies in various hematological malignancies (NCT04245722i, NCT04555811ii, NCT04614636iii)[33,34], while OTS iPSC-derived CAR-T cells are still under development and are expected to be evaluated soon in patients with B-cell malignancies (NCT04629729)iv[35].
Approaches to preventing GvHD
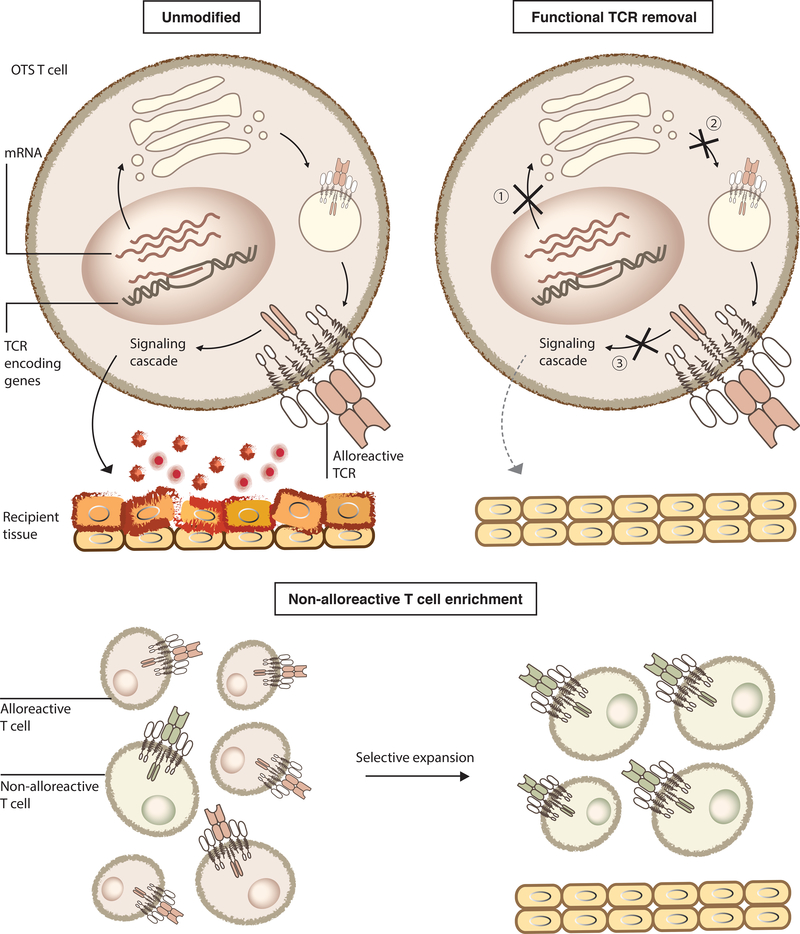
Several manufacturing strategies have been used to mitigate the risk of GvHD from OTS T cells (Figure 1). The common goal of these approaches is to exclude T cells expressing the functional alloreactive TCR from the final infusion product. One approach is to remove the endogenous TCR by knocking out one or two genes encoding components of the TCR complex, such as TRAC (T Cell Receptor Alpha Constant), TRBC (T Cell Receptor Beta Constant), and CD3ε[21,36–39]. Since only the fully assembled TCR complex can be transported to the cell membrane, removing any of its components will prevent surface expression of functional TCRs. This strategy is being evaluated in several Phase I clinical trials and has shown promising preliminary results, as recently reviewed[9].
Figure 1. Approaches to preventing GvHD mediated by OTS T cells.
Top: TCRs of unmodified OTS alloreactive T cells are translated, assembled, transported to cell surface, recognize mismatched recipients’ healthy tissues, initiate downstream signaling and release effector molecules, such as cytolytic enzymes and inflammatory cytokines, leading to tissue damage. This can be prevented by removing functional TCRs from cell surface through ① knocking out one or two genes encoding components of the TCR complex, ② trapping TCR in the endoplasmic reticulum (ER) through anti-CD3ε PEBLs, or ③ preventing alloreactive TCR signaling by expressing TIM, a dominant-negative form of CD3ζ. Bottom: An alternative direction is to selectively expand non-alloreactive immune effectors to include in the final product.
Preclinical studies have also demonstrated that knocking-in the CAR construct at the TRAC locus may simultaneously abrogate endogenous TCR expression and enhance CAR T cell function. In this system, the CD19 CAR transgene replaces the endogenous TRAC gene, thus preventing assembly of the full TCR complex and allowing more physiologically regulated CAR expression[40]. A third approach is to express artificial proteins that retain one or several TCR components intracellularly. Examples include a CD3ε protein expression blocker (PEBL) that traps CD3ε subunits intracellularly by using an anti-CD3ε single-chain binder anchored in the endoplasmic reticulum via the KDEL sequence[41].
In an alternative approach to reduce functional alloreactivity, researchers attenuated the strength of TCR signaling in OTS cells by enforced expression of a small peptide called TCR inhibitory molecule (TIM): a competitive antagonist to wildtype CD3ζ for TCR complex binding[42]. The resulting TCR with TIM cannot induce downstream signaling after stimulation, thereby inhibiting the effector function of alloreactive T cells. This approach is currently under clinical evaluation (NCT03692429)v in combination with an NKG2D-based CAR for the treatment of metastatic colorectal cancer: preliminary Phase I results (primary outcome: occurrence of dose-limiting toxicities) reported 6/12 patients with tumor reduction without GvHD following 35 injections of the OTS CAR-T product, but still requires extensive validation[43].
As an alternative to TCR removal or functional blockade, investigators have used T cells with defined TCR specificity or enriched for non-alloreactive cells as OTS products; these have included a variety of virus-specific T cells[44–46] and innate-like T cells[47–51]. Moreover, in general, several clinical studies have shown the safety and lack of severe GvHD in patients treated with these allogenic cell products[44–46,50,51]. Although these products do not require TCR modification, in general, manufacturing these products appears to be lengthier compared to polyclonal αβT cell products owing to their lower frequencies in peripheral blood, necessitating longer ex vivo expansion [24].
Regardless of the approach, ensuring the purity of the final product is essential: one clinical study showed that even a CD19 CAR-T product that was 99.3% TCR-negative could induce GvHD[21]. Nonetheless, these approaches are starting to prove effective in clinical trials, suggesting that the risk of GvHD from OTS CAR T cells might become a manageable concern.
Preventing immune rejection: passive approaches
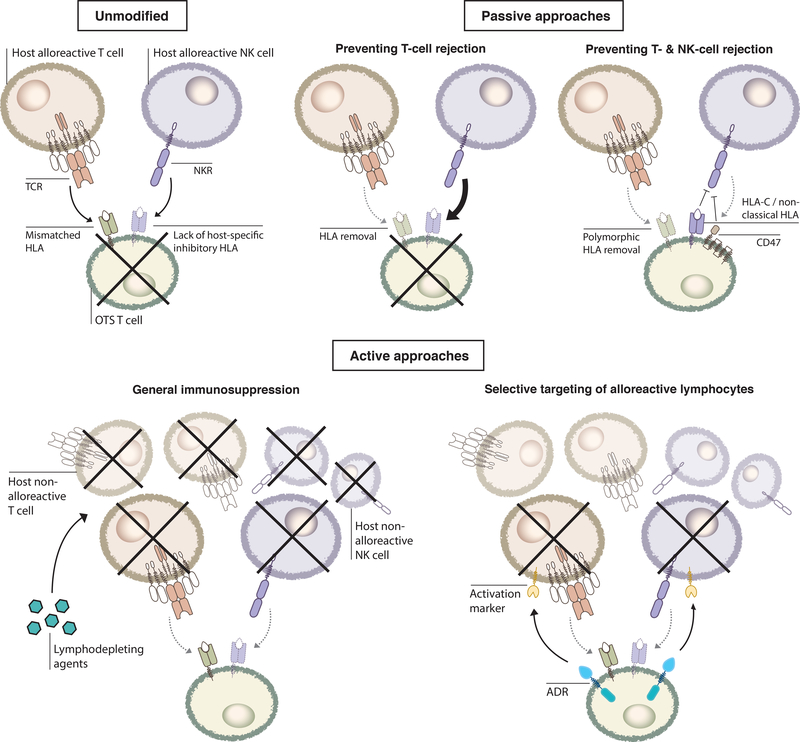
While controlling GvHD only deals with alloreactive T cells in the OTS product, suppressing immune rejection is a more complex problem that will need to tackle multiple arms of the host immune system. The earliest phylogeny of multicellular animals, such as sea anemones, already have mechanisms to distinguish ‘self’ from ‘non-self,’ and the human innate and adaptive immune systems possess multilayered defenses against foreign antigens. A successful strategy to prevent rejection therefore needs to address all layers of defense simultaneously, since defeat of any one simply exposes a subsequent protective mechanism (Figure 2).
Figure 2. Approaches to preventing immune rejection of OTS T cells.
Top: Host alloreactive T cells recognize mismatched HLAs on unmodified OTS T cells and cause rejection. Some host NK cells may also sense the lack of certain host HLA class I molecules on the mismatched OTS cells and facilitate rejection. HLA knockout protects against alloreactive T-cell recognition but results in stronger NK-mediated rejection. Additional strategies to suppress NK activation include overexpression of HLA-E/G, CD47, or retaining HLA-C and non-classical HLA class I molecules on the OTS T cells. Bottom: Alternatively, active approaches can be used, through either non-selectively suppressing host immunity (lymphodepleting agents including CD52/CD38 mAbs and FOLFOX) or selectively eliminating activated alloreactive lymphocytes (ADR targeting activation marker 4-1BB).
Removing MHCs to prevent T cell rejection
One of the earliest attempts to avoid allogeneic rejection by removing MHC molecules on OTS cells offers an example of this multilayered defense. As the recognition of surface MHC class I on OTS cells by host cytotoxic CD8+ T cells is considered one major mechanism of immune rejection, researchers developed OTS cells without surface MHC class I expression, commonly achieved by gene knockout of β2-microglobulin (B2m) [37,52]. To avoid potential recognition by CD4+ alloreactive T cells, a number of studies showed the benefit of further disrupting MHC class II expression on OTS T cells by deleting regulatory elements that control the transcription of MHC class II genes, such as MHC class II transactivator (CIITA) and RFXANK[53–57]. While these approaches are still undergoing clinical evaluation, additional engineering strategies are likely needed to minimize the risk of rejection by NK cells, as discussed below.
Strategies to prevent NK cell-mediated rejection
Although removal of MHC class I and II, singly or together, mitigates activation of alloreactive T cells, pre-clinical evidence suggests that MHCnull OTS T cells may become more susceptible to NK cell-mediated host immunosurveillance in vitro and in vivo in mouse models [39,55–58]. Although the exact mechanisms underlying NK cell-mediated rejection remain to be elucidated, using various models, several laboratory groups have shown that complete removal of MHC molecules -- to reduce the extent of inhibitory signaling produced by engaging inhibitory NK receptors with MHCs-- inevitably unleashed NK cytotoxicity against MHCnull OTS T cells [59–61].
Although the clinical relevance of NK cell-mediated rejection has yet to be investigated in patients receiving OTS CAR-T cells, NK cell recognition of MHCnull OTS cells may pose a greater threat than alloreactive T cells. First, in lymphodepleted patients, NK-cell recovery is generally faster than pan-T-cell recovery[62,63]. Secondly, in contrast to T cells, whose number and target repertoire gradually decrease after puberty due to thymic involution and immunosenescence[64], NK cell numbers increase with age[65]. Finally, NK cells eliminate targets more rapidly than CD8+ T cells, as no prior priming or clonal expansion is needed. Alloreactive CD8+ T cells, by contrast, might likely predominantly be in their naïve state and require lengthy antigen presentation, clonal expansion, and differentiation to gain cytotoxic potency[66].
Additional countermeasures are thus required to prevent NK cell-mediated rejection. Outside the cell therapy field, regenerative medicine has developed several cloaking strategies in the iPSC platform, focusing on extending the persistence of iPSC-derived tissues or organs in transplant recipients. Cancer immunotherapy is now exploring the feasibility of adopting these anti-rejection approaches for use in primary T cells.
One cloaking approach is the enforced expression of non-classical MHC class I molecules such as HLA-E and HLA-G[52,67]. These antigens have limited polymorphism and therefore host T cells do not recognize them as ‘non-self’. Instead, they inhibit NK cell activity by binding to inhibitory NKRs, such as CD94/NKG2A/B binding to HLA-E[68–70]. However, this approach may only be effective against the subset of NK-cells expressing cognate inhibitory NKRs. For example, while HLA-E inhibits NK cell function through CD94/NKG2A/B receptors it stimulates other NK cells via the activating CD94/NKG2C receptor[68].
In a second approach, one study showed that over-expression of CD47, a ‘don’t eat me’ signal for macrophages[71], could also prevent NK cell recognition of MHC Class I/II-deleted iPSCs[56]. Specifically, murine iPSCs with MHC class I and II knockout plus transgenic CD47 expression (B2m−/−Ciita−/−Cd47 tg) readily engrafted in both syngeneic and allogenic mouse strains, while wildtype (WT) iPSCs or iPSCs with only MHC class I and II deletion (B2m−/−Ciita−/−) failed to persist in allogenic hosts[56]. The investigators then showed in two different humanized mouse models (NSG-SGM3 and BLT) that human MHC-ablated iPSCs with CD47 overexpression evaded allogeneic immune rejection in vivo, with reduced production of the inflammatory IFNgamma cytokine and IgM antibodies relative to WT iPSCs, suggesting less activation of both cellular and humoral immune compartments. Through in vitro coculture with isolated murine or human NK cells and CD47 blockade using blocking antibodies, the authors further showed that CD47 overexpression enabled MHC-deficient mouse (B2m−/−Ciita−/−Cd47 tg) and human (B2M−/−CIITA−/−CD47 tg) iPSCs to prevent NK activation in a CD47-dependent manner, as the addition of a blocking antibody completely abolished the protective effect. Given the known interactions between CD47 and macrophages, the authors also repeated the murine iPSC engraftment experiment in a macrophage-depleted allogeneic host (through clodronate pretreatment) and observed similar immune-evading properties in B2m−/−Ciita−/−Cd47 tg iPSCs. These results suggested that CD47 might likely directly interact with NKRs to prevent NK cell activation, but the underlying mechanisms of this phenomenon remain unclear[56]. However, given the heterogeneity of NK cells, it will also be important to investigate whether this method might be widely applicable to all NK subsets, and certainly merits further attention.
For the third approach, investigators selectively deleted HLA-A and HLA-B from iPSCs but the cells retained expression of HLA-C and other non-classical HLA-E/F/G expression[55]. The authors showed that these HLA-A−/−HLA-B−/− cells escaped allo-recognition in vitro by both cytotoxic CD8+ T cells and NK cells, while β2m-knockout iPSCs that lacked all HLA class I alleles did not. Similar protective effects against HLA-A−/−HLA-B−/− iPSCs were replicated in immunodeficient NRG mouse models co-transplanted with either alloreactive human T cells or NK cells[55]. Of note, in their short-term co-culture assay with NK cells, only iPSCs retaining HLA-C, but not HLA-A/B/C triple knockout cells, evaded NK recognition. These studies indicated that expression of non-classical HLA alleles alone is insufficient to suppress NK cell activation. Subsequent coculture studies demonstrated that this approach can be further combined with MHC class II knockout through CIITA disruption to abrogate the activity of alloreactive CD4+ T cells[55]. An additional benefit is that unlike β2m deleted cells, these cells may be able to present a few antigens through residual HLAs, which may allow their own clearance by the immune system in situations where the transplanted iPSCs themselves are mutated or infected with a pathogen, thereby representing a putative safety mechanism[55]. Regarding feasibility and scalability, since HLA-C has limited polymorphism, the authors predicted that 12 alleles of HLA-C might be immunologically compatible with more than 90% of the world’s population[55]. Although the authors did not delve into the molecular mechanism underlying rejection prevention, previous studies suggested that HLA-C molecules could inhibit NK cell activation through binding of KIR2DL1/2/3[49]. Similar to the above strategy overexpressing HLA-E, this method might only suppress rejection mediated by NK cell subsets expressing these specific inhibitory NKRs.
Overall, these data suggest that immune rejection by both T and NK cells may be managed by ‘hiding’ OTS T cells using MHC modifications and additional engineering strategies. Nevertheless, preclinical evaluation of these former iPSC technologies in the CAR-T platform is still underway.
Preventing immune rejection: active approaches
General immunosuppression
While passive strategies conceal OTS cells from host immune surveillance, investigators are also exploring active approaches to counter host immunity. For example, lymphodepleting agents, such as the monoclonal antibody (mAb) alemtuzumab targeting CD52, can non-selectively deplete host lymphocytes prior to CAR-T cell infusion[22,38]. Disruption of the CD52 gene in OTS CAR T cells allows them to avoid binding circulating CD52 mAb, thus enabling them to engraft and expand in the host[22,38]. Preclinical proof-of-concept studies suggest that CD52-deficient CD19 CAR T cells engrafted in a xenograft mouse model of B cell lymphoma in the presence of alemtuzumab, can mediate uncompromised anti-tumor effects, i.e. comparable survival as standard CD19 CAR-T cells (orthotopic CD19 lymphoma murine model) [38]. Results from subsequent phase I clinical studies (NCT02746952vi, NCT02808442vii) indicated that B-cell leukemia patients receiving alemtuzumab presented increased expansion of allogeneic CD52−/− CD19 CAR T cells compared to patients who did not undergo this lymphodepletion regimen; indeed, complete responses (CR) were observed in 14 out of 17 evaluable patients treated with alemtuzumab combined with cyclophosphamide and fludarabine[22]. However, infections and prolonged cytopenia -- known complications associated with alemtuzumab treatment-- were observed in most patients. No discernable CAR-T expansion and early recovery of endogenous T and NK cells were noted in four patients who received only cyclophosphamide/fludarabine conditioning without alemtuzumab, which suggested the possibility of allogeneic rejection[22]. A similar strategy is currently under clinical translation and uses CD38-deficient allogeneic NK cells in conjunction with a CD38 mAb daratumumab to treat multiple myeloma, and awaits further investigation [34,72].
As an alternative to mAb-mediated lymphodepletion, the aforementioned open-label, Phase I study in patients with unresectable metastatic colorectal cancer testing OTS NKG2D-based CAR-T (NCT03692429)v used FOLFOX chemotherapy (a combination of folinic acid, 5-fluorouracil, and oxaliplatin) [43]. CAR-T expansion was observed following all injections, and preliminary observations suggested tumor regression in 50% of patients, which might have indicated a certain degree of rejection control via FOLFOX. Peak engraftment of circulating CAR-T cells, however, was reduced after the second and third cell infusions compared to the initial dose, suggesting the putative persistence or recovery of allogeneic rejection mechanisms, although this remains to be rigorously validated [43].
Indeed, larger sample sizes and longer follow-up studies are required to assess the impact of pan-lymphodepleting agents on anti-tumor responses and on putative opportunistic infections; in addition, it will be important to characterize the kinetics of hematopoietic system recovery and OTS cell persistence to evaluate the potential effect of anti-rejection mechanisms on host immunity. The effects of CD52 and CD38 knockout on therapeutic cells must also be better characterized as it is still unclear whether removing those genes leads to functional changes in T cells.
Selective elimination of alloreactive T and NK cells
As prolonged pan-immunosuppression may increase the risk of opportunistic infections, approaches specifically targeting alloimmune components may be preferable. Recently, our group developed a novel anti-rejection strategy by actively and selectively targeting alloreactive T and NK cells[39]. As activated T and NK cells transiently upregulate the costimulatory receptor 4–1BB (CD137) on their surface, this marker allowed us to specifically target alloreactive lymphocytes in their activated and cytotoxic state while sparing resting and non-alloreactive immune cells. We developed a chimeric receptor, termed an alloimmune defense receptor (ADR), the expression of which enabled therapeutic OTS T cells to recognize and eliminate 4–1BB+ T and NK cells. ADR-expressing T cells exerted potent cytotoxicity against activated T and NK cells but not resting lymphocytes in vitro. ADR T cells were also protected against allogeneic rejection mediated by allogeneic T and NK cells in mixed lymphocyte reaction (MLR) assays in vitro, as well as in mouse models of allogeneic T-cell therapy of hematopoietic and solid cancers (e.g. NSG (MHCnull), engrafted with human PBMC [39].
To evaluate the feasibility of using ADR to generate OTS T cells that can resist host rejection and eradicate tumor, we co-expressed both ADR and a CD19 CAR in the same T cell. We demonstrated that CAR.ADR T cells were cytotoxic against both activated lymphocytes and CD19+ NALM6 tumor cells in vitro[39]. Finally, we established a mouse model of allogeneic cell therapy in which immunodeficient NSG mice were simultaneously engrafted with human CD19+ leukemia and healthy donor pan-T cells. In this model, adoptive transfer of unmodified allogeneic CD19 CAR T cells produced only transient anti-tumor activity (leukemia) due to rejection by pre-engrafted allogeneic T cells within two weeks post-infusion. In contrast, CAR.ADR T cells were protected from immune rejection, resulting in long-term persistence (> eight weeks) and durable remission in 17/19 mice[39]. In subsequent studies using this model, ADR and the CD19 CAR were co-expressed in T cells that had been TCR-edited to ablate their capacity to induce GvHD (TCR KO). These TCR-negative CAR.ADR T cells were equally well protected from immune rejection, enabling long-term anti-tumor activity and mouse survival[39]. Similar results were observed in a neuroblastoma xenograft model using allogeneic GD2 CAR.ADR T cells[39]. Therefore, ADR-armed OTS CAR T cell products can retain anti-tumor activity and resist allogeneic rejection from the host immune system in vivo in certain mouse models. In principle, the ADR strategy could be combined with different CAR constructs to treat a variety of tumors, which certainly merits future attention.
Preliminary data from these ADR studies also suggested that protective immunity might be conserved. Not all activated T cells upregulate 4–1BB, and thus, the in vitro co-culture results described in these studies suggested that in the presence of ADR T cells, virus-specific T cells could largely retain their ability to clear virus-infected cells in vitro [39]. Moreover, the restricted expression pattern of 4–1BB expression in healthy human tissues, as determined by immunohistochemistry (only in some activated lymphocytes) suggested that the ADR approach might be more selective than other pan-lymphodepletion methods and might cause less toxicity, although this remains to be rigorously tested. Presumably, ‘safety switches’ (reviewed in [73]) might be added to the CAR.ADR cell products to effectively terminate activity on demand. However, this also remains to be further tested.
Other lymphocyte activation markers might also be explored as potential ADR targets. For instance, several other co-stimulatory molecules in the tumor necrosis factor receptor superfamily (TNFRSF), of which 4–1BB is a member, share a similarly restricted expression pattern on activated human lymphocytes[74]. All these receptors provide additional co-stimulation during lymphocyte activation but different cell subsets (e.g., CD4+ versus CD8+ T cells, virus-specific versus tumor-specific T cells) may express different combinations of co-stimulatory molecules[74–76]. Aside from T and NK cells, B cells and antigen presenting cells can also transiently upregulate TNFRs upon activation and be targeted, which may prevent the initial priming of adaptive alloimmunity and production of allo-antibodies mediating rejection[77]. Since cell surface expression of these activation-induced TNFRs is usually transient and depends on the specific activation signal, our knowledge of their expression pattern remains limited. Thus, a comprehensive screening to identify activation molecules expressed highly and frequently in alloreactive immune cells (T/B/NK and antigen presenting cells) – relative to other non-alloreactive cells-- might improve the specificity of ADRs, and awaits further testing.
It is also worth noting that potential applications of ADR strategy can extend beyond suppressing rejection. Alloreactive lymphocytes also play an important role in GvHD pathogenesis and ADR T cells might be potentially used for this indication as well. Future studies will need to determine the optimal ADR target(s) for each individual application.
Concluding remarks
Steps toward making OTS therapeutics a standard of care
Recent reports suggest that banked immune effectors can represent a clinically safe and feasible therapeutic platform, with low incidence of GvHD. However, approaches to prevent immune rejection and ensure OTS therapies retain their antitumor activity are at an earlier stage of development. To date, available methods to prevent allogeneic rejection utilize either a passive strategy to hide OTS cells from the host immune system or an active approach to completely or selectively abrogate host alloreactivity. While passive strategies may be insufficient to prevent rejection mediated through certain NK subsets as well as alloreactive T cells recognizing MiHAs, active methods may compromise patient long-term immunity, increasing the risk and severity of opportunistic infections. By offering selective targeting of alloimmunity, ADRs may represent a balance between these two approaches, although their safety and efficacy awaits clinical evaluation. Clinical data and improved understanding of the immunological mechanisms underlying allogeneic rejection (see Outstanding Questions) can benefit anti-rejection strategies and facilitate more widespread implementation of OTS immune effector therapies. Combined with existing strategies to prevent GvHD, we are headed towards a new era of affordable and accessible candidate cell therapies for cancer.
Outstanding questions.
Host defensive mechanisms against mismatched OTS cells warrant further studies. What is the role of mismatched MiHA recognition in alloimmune rejection? Which NKRs are involved in rejection and what are their roles?
Can we predict a patient’s susceptibility to GvHD or rejection following OTS T cell treatment clinically?
Would reducing (instead of removing) surface MHC expression on OTS T cells help mitigate both T and NK cell responses?
What are the consequences of extended persistence if OTS T cells are modified to resist rejection?
As prolonged persistence may not be necessary after tumor clearance, what is the length of time that we ought to expect anti-rejection mechanisms to function in patients?
Can immune tolerance to OTS cells be induced in patients without additional anti-rejection measures? Recent results from a phase I study (NCT03056339)viii using allogeneic CAR-NK cells for the treatment of B-cell lymphoid tumors (36 participants; primary outcomes: dose, toxicity, and efficacy) reported long-term persistence of the infused allogeneic, partially HLA-matched UCB-derived CAR-NK cells for up to one-year post infusion[23]. A closer look at the recovery of patient hematopoiesis, particularly T and NK cell counts, may give us a clearer mechanistic insight of response to OTS treatment: were the cells not rejected because of the permissive environment created by lymphodepletion prior to CAR-NK cell treatment or the development of immune tolerance?
Highlights.
There are recent efforts to develop ‘off-the-shelf’ (OTS) cell therapies manufactured from healthy donors and then banked to offer immediate availability and high potency at a reduced cost.
Potential limitations of OTS cell therapies are linked to their allogeneic origin and the possibility of graft-versus-host disease (GvHD) and host-versus-graft immune reactions.
Manufacturing strategies mitigating the risk of GvHD from OTS T cells aim to exclude T cells expressing the alloreactive TCR from the final infusion product. These approaches are proving highly effective in clinical trials.
Approaches to prevent immune rejection of OTS cells are at an earlier stage of development. Current methods can be categorized into passive strategies that hide OTS cells from the host immune system and active strategies that completely or selectively abrogate host alloreactivity.
Acknowledgements
The authors thank Catherine Gillespie for editing the manuscript. This project was supported by the National Cancer Institute (F99CA253757, P50CA126752 and P01CA094237) SU2C/AACR 604817 Meg Vosburg T cell Lymphoma Dream Team, and the Leukemia and Lymphoma Society. Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research.
Disclosures
HEH is a co-founder with equity in Allovir and Marker Therapeutics, serves on Advisory Boards for Gilead, Tessa Therapeutics, Novartis, PACT Pharma and Kiadis and has research funding from Tessa Therapeutics and Kuur Therapeutics
MKB is a co-founder with equity in Allovir, Marker Therapeutics and Tessa Therapeutics, serves on Advisory Boards for Tessa Therapeutics, Allogene, Memgen, Kuur Therapeutics. Walking Fish Therapeutics, Tscan, Abintus and Turnstone Biologics
M.M., F.M. and M.K.B. are co-inventors on a patent related to ADRs and methods of their use, licensed to Fate Therapeutics and Allogene Therapeutics
Glossary
- innate-like T cells
T cell subsets with innate-like properties such as limited and highly conserved TCR repertoires, and MHC-independent target recognition (e.g. natural killer T (NKT) cells, mucosal associated invariant T (MAIT) cells, and gamma-delta T (gdT) T cells)
- allogeneic / ‘off-the-shelf’ (OTS) cell therapies
therapeutic cells derived from a donor rather than the patient
- graft-versus-host disease
potentially adverse event post allogeneic transplant where the donor graft recognizes the recipient’s tissues as foreign and causes damage
- host-versus-graft immune reactions
potentially adverse event post allogeneic transplant where the recipient’s immune system recognizes the donor graft as foreign and causes rejection
- ‘missing self’ hypothesis
NK cells recognize and eliminate cells that do not express self MHC class I molecules
- chimeric antigen receptors (CARs)
artificial receptor proteins consisting of antigen-binding and T-cell signaling domains that render T cells the new ability to target a specific protein in an MHC-independent manner
- major histocompatibility complex (MHC)
large locus on vertebrate DNA containing a set of closely linked genes that code for cell surface proteins essential for antigen presentation. CD8+ T cells specifically recognize peptides presented by MHC class I; CD4+ T cells bind to peptides presented by MHC class II. Human MHCs are human leukocyte antigens (HLAs)
- minor histocompatibility antigens (MiHAs)
amino acid polymorphisms in cellular proteins originating from genetic disparities between individuals; can lead to differential presentation of antigenic peptides by MHC molecules and cause recognition by alloreactive T cells, e.g. during transplantation between HLA-matched siblings
- polymorphic
A gene is considered polymorphic if it has more than one allele and each of its alleles occurs in the population at a rate of at least 1%, which suggests that the allele combination each individual possesses will be highly diverse
- lymphodepleting regimens
chemotherapy regimens that destroy patient lymphocytes
- umbilical cord blood
blood in the placenta and umbilical cord after childbirth; good source for stem cells that can be used to treat hematopoietic and genetic disorders
- induced pluripotent stem cells
somatic cells reprogrammed to possess pluripotency, via introduction of four genes encoding transcription factors
- TCR complex
a protein complex on T cell surface consisting of TCRa, TCRb, CD3e, CD3g, CD3d, and CD3z
- KDEL sequence
target peptide sequence located on the C-terminal end of a protein, which allows it to be retrieved from the Golgi apparatus by retrograde transport to the endoplasmic reticulum (ER) lumen if accidentally exported out of ER
- NKG2D
cytotoxic surface receptor expressed by NK cells and some T cell subsets
- β2-microglobulin
universal protein component of the fully assembled MHC class I complex. Only fully assembled class I complexes can be transported to cell membrane
- MHC class II transactivator (CIITA)
regulatory protein activating transcription from MHC class II promoters
- RFXANK
regulatory protein activating transcription from MHC class II promoters
- non-classical MHC class I
in humans, classical MHC class I alleles are HLA-A/B/C, and non-classical ones are HLA-E/F/G/H. Non-classical class I molecules are less polymorphic
- ‘don’t eat me’ signal
CD47 can bind to SIRPα on macrophages and inhibit phagocytosis
- CD38
glycoprotein on the surface of many lymphocytes including T, B and NK cells
- alloimmune defense receptor (ADR)
chimeric receptor that binds activation markers on lymphocytes and elicits T-cell activation and degranulation via the intracellular CD3ζ chain
- GD2
disialoganglioside overexpressed on tumors of neuroectodermal origin
Footnotes
Resources
This trial is listed at https://clinicaltrials.gov/ct2/show/NCT04245722
This trial is listed at https://clinicaltrials.gov/ct2/show/NCT04555811
This trial is listed at https://clinicaltrials.gov/ct2/show/NCT04614636
This trial is listed at https://clinicaltrials.gov/ct2/show/NCT04629729
This trial is listed at https://clinicaltrials.gov/ct2/show/NCT03692429
This trial is listed at https://clinicaltrials.gov/ct2/show/NCT02746952
This trial is listed at https://clinicaltrials.gov/ct2/show/NCT02808442
This trial is listed at https://clinicaltrials.gov/ct2/show/NCT03056339
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.June CH and Sadelain M (2018) Chimeric Antigen Receptor Therapy. N. Engl. J. Med. 379, 64–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minetto P et al. Harnessing NK Cells for Cancer Treatment. Frontiers in Immunology, 10. 06-December-(2019), Frontiers Media S.A., 2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sebestyen Z et al. Translating gammadelta (γδ) T cells and their receptors into cancer cell therapies. Nature Reviews Drug Discovery, 19. 01-March-(2020), Nature Research, 169–184 [DOI] [PubMed] [Google Scholar]
- 4.Wang M et al. (2020) KTE-X19 CAR T-Cell therapy in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 382, 1331–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neelapu SS et al. (2017) Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-Cell lymphoma. N. Engl. J. Med. 377, 2531–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maude SL et al. (2018) Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Köhl U et al. (2018) CAR T Cells in Trials: Recent Achievements and Challenges that Remain in the Production of Modified T Cells for Clinical Applications. Hum. Gene Ther. 29, 559–568 [DOI] [PubMed] [Google Scholar]
- 8.Lin JK et al. (2019) Cost effectiveness of chimeric antigen receptor T-cell therapy in multiply relapsed or refractory adult large B-cell lymphoma. J. Clin. Oncol. 37, 2105–2119 [DOI] [PubMed] [Google Scholar]
- 9.Depil S et al. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nature Reviews Drug Discovery, 19. 01-March-(2020), Nature Research, 185–199 [DOI] [PubMed] [Google Scholar]
- 10.Perkey E and Maillard I (2018) New Insights into Graft-Versus-Host Disease and Graft Rejection. Annu. Rev. Pathol. Mech. Dis. 13, 219–245 [DOI] [PubMed] [Google Scholar]
- 11.Lakkis FG and Lechler RI (2013) Origin and biology of the allogeneic response. Cold Spring Harb. Perspect. Med. 3, (8):a014993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marino J et al. Allorecognition by T lymphocytes and allograft rejection. Frontiers in Immunology, 7. 14-December-(2016), Frontiers Media S.A., 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felix NJ and Allen PM Specificity of T-cell alloreactivity. Nature Reviews Immunology, 7. 21-November-(2007), Nature Publishing Group, 942–953 [DOI] [PubMed] [Google Scholar]
- 14.Falkenburg JHF et al. (2003) Minor histocompatibility antigens in human stem cell transplantation. Exp. Hematol. 31, 743–751 [DOI] [PubMed] [Google Scholar]
- 15.Chao NJ (2004) Minors come of age: Minor histocompatibility antigens and graft-versus-host disease. Biol. Blood Marrow Transplant. 10, 215–223 [DOI] [PubMed] [Google Scholar]
- 16.Velardi A et al. Natural killer cell allorecognition of missing self in allogeneic hematopoietic transplantation: a tool for immunotherapy of leukemia. Current Opinion in Immunology, 21. October-(2009), Curr Opin Immunol, 525–530 [DOI] [PubMed] [Google Scholar]
- 17.Colonna M et al. (1993) Generation of allospecific natural killer cells by stimulation across a polymorphism of HLA-C. Science (80-. ). 260, 1121–1124 [DOI] [PubMed] [Google Scholar]
- 18.Ruggeri L et al. (2002) Effectiveness of donor natural killer cell aloreactivity in mismatched hematopoietic transplants. Science (80-. ). 295, 2097–2100 [DOI] [PubMed] [Google Scholar]
- 19.Peter Gale R and Reisner Y (1986) GRAFT REJECTION AND GRAFT-VERSUS-HOST DISEASE: MIRROR IMAGES. Lancet 327, 1468–1470 [DOI] [PubMed] [Google Scholar]
- 20.Torikai H and Cooper LJN Translational implications for off-the-shelf immune cells expressing chimeric antigen receptors. Molecular Therapy, 24. 01-August-(2016), Nature Publishing Group, 1178–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qasim W et al. (2017) Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci. Transl. Med. 9, eaaj2013. [DOI] [PubMed] [Google Scholar]
- 22.Benjamin R et al. (2020) Genome-edited, donor-derived allogeneic anti-CD19 chimeric antigen receptor T cells in paediatric and adult B-cell acute lymphoblastic leukaemia: results of two phase 1 studies. Lancet 396, 1885–1894 [DOI] [PubMed] [Google Scholar]
- 23.Liu E et al. (2020) Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 382, 545–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heczey A, Courtney AN, Montalbano A et al. (2020) Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: an interim analysis. Nat Med 26, 1686–1690 [DOI] [PubMed] [Google Scholar]
- 25.Juric MK et al. Milestones of hematopoietic stem cell transplantation - From first human studies to current developments. Frontiers in Immunology, 7. 09-November-(2016), Frontiers Media S.A., 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwoczek J et al. (2018) Cord blood-derived T cells allow the generation of a more naïve tumor-reactive cytotoxic T-cell phenotype. Transfusion 58, 88–99 [DOI] [PubMed] [Google Scholar]
- 27.Themeli M et al. (2013) Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat. Biotechnol. 31, 928–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ando M and Nakauchi H (2017) Òff-the-shelf’ immunotherapy with iPSC-derived rejuvenated cytotoxic T lymphocytes. Exp. Hematol. 47, 2–12 [DOI] [PubMed] [Google Scholar]
- 29.Nagano S et al. (2020) High Frequency Production of T Cell-Derived iPSC Clones Capable of Generating Potent Cytotoxic T Cells. Mol. Ther. - Methods Clin. Dev. 16, 126–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu H et al. (2020) Pluripotent stem cell-derived NK cells with high-affinity noncleavable CD16a mediate improved antitumor activity. Blood 135, 399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y et al. (2018) Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell 23, 181–192.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nianias A and Themeli M Induced Pluripotent Stem Cell (iPSC)–Derived Lymphocytes for Adoptive Cell Immunotherapy: Recent Advances and Challenges. Current Hematologic Malignancy Reports, 14. 15-August-(2019), Current Science Inc., 261–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodridge JP et al. (2019) FT596: Translation of First-of-Kind Multi-Antigen Targeted Off-the-Shelf CAR-NK Cell with Engineered Persistence for the Treatment of B Cell Malignancies. Presented at: 61st ASH Annual Meeting & Exposition. Blood 134, 301 [Google Scholar]
- 34.Bjordahl R et al. (2019) FT538: Preclinical Development of an Off-the-Shelf Adoptive NK Cell Immunotherapy with Targeted Disruption of CD38 to Prevent Anti-CD38 Antibody-Mediated Fratricide and Enhance ADCC in Multiple Myeloma When Combined with Daratumumab. Presented at: 61st AS. Blood 134, 133–133 [Google Scholar]
- 35.Chang C et al. (2019) FT819: Translation of Off-the-Shelf TCR-Less Trac-1XX CAR-T Cells in Support of First-of-Kind Phase I Clinical Trial. Presented at: 61st ASH Annual Meeting & Exposition. Blood 134, 4434–4434 [Google Scholar]
- 36.Torikai H et al. (2012) A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood 119, 5697–5705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren J et al. (2017) Multiplex Genome Editing to Generate Universal CAR T Cells Resistant to PD1 Inhibition. Clin. Cancer Res. 23, 2255–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poirot L et al. (2015) Multiplex Genome-Edited T-cell Manufacturing Platform for ‘Off-the-Shelf’ Adoptive T-cell Immunotherapies. Cancer Res. 75, 3853–3864 [DOI] [PubMed] [Google Scholar]
- 39.Mo F et al. (2020) Engineered off-the-shelf therapeutic T cells resist host immune rejection. Nat. Biotechnol. DOI: 10.1038/s41587-020-0601-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eyquem J et al. (2017) Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamiya T et al. (2018) A novel method to generate T-cell receptor-deficient chimeric antigen receptor T cells. Blood Adv. 2, 517–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michaux A et al. (2018) Expression of a TIM8 Peptide Reduces Alloreactivity of T Cells Facilitating an Allogeneic NKG2D Chimeric Antigen Receptor T Cell Therapy Approach. Presented at: 21st ASGCT Annual Meeting. Mol. Ther. 26, 214 [Google Scholar]
- 43.Prenen H et al. (2019) Results from the completed dose-escalation of the alloSHRINK phase I study evaluating the allogeneic NKG2D-based CAR T-cell therapy CYAD-101 in metastatic colorectal cancer patients. Presented at: SITC 2019 Annual Meeting. J. Immunother. Cancer 7(Suppl 1), P330 [Google Scholar]
- 44.Tzannou I et al. (2017) Off-the-Shelf Virus-Specific T Cells to Treat BK Virus, Human Herpesvirus 6, Cytomegalovirus, Epstein-Barr Virus, and Adenovirus Infections After Allogeneic Hematopoietic Stem-Cell Transplantation. J. Clin. Oncol. 35, 3547–3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Melenhorst JJ et al. (2010) Allogeneic virus-specific T cells with HLA alloreactivity do not produce GVHD in human subjects. Blood 116, 4700–4702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leen AM et al. (2013) Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood 121, 5113–5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heczey A et al. (2014) Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy. Blood 124, 2824–2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin J et al. (2019) Development of an Allogeneic Universally Tolerated NKT Cell Platform for Off-the-Shelf Cancer Immunotherapy. Presented at: 22nd ASGCT Annual Meeting. Mol. Ther. 27, 321 [Google Scholar]
- 49.Lowdell MW et al. (2001) Non-MHC-restricted cytotoxic cells: their roles in the control and treatment of leukaemias. Br. J. Haematol. 114, 11–24 [DOI] [PubMed] [Google Scholar]
- 50.Wilhelm M et al. (2014) Successful adoptive transfer and in vivo expansion of haploidentical γδ T cells. J. Transl. Med. 12, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alnaggar M et al. (2019) Allogenic Vγ9Vδ2 T cell as new potential immunotherapy drug for solid tumor: a case study for cholangiocarcinoma. J. Immunother. Cancer 7, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torikai H et al. (2013) Toward eliminating HLA class I expression to generate universal cells from allogeneic donors. Blood 122, 1341–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holling TM et al. (2002) Activated Human T Cells Accomplish MHC Class II Expression Through T Cell-Specific Occupation of Class II Transactivator Promoter III. J. Immunol. 168, 763–770 [DOI] [PubMed] [Google Scholar]
- 54.Krawczyk M et al. (2004) Long Distance Control of MHC Class II Expression by Multiple Distal Enhancers Regulated by Regulatory Factor X Complex and CIITA. J. Immunol. 173, 6200–6210 [DOI] [PubMed] [Google Scholar]
- 55.Xu H et al. (2019) Targeted Disruption of HLA Genes via CRISPR-Cas9 Generates iPSCs with Enhanced Immune Compatibility. Cell Stem Cell 24(4):566–578.e7 [DOI] [PubMed] [Google Scholar]
- 56.Deuse T et al. (2019) Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 37, 252–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kagoya Y et al. (2020) Genetic Ablation of HLA Class I, Class II, and the T-cell Receptor Enables Allogeneic T Cells to Be Used for Adoptive T-cell Therapy. Cancer Immunol. Res. 8, 926–936 [DOI] [PubMed] [Google Scholar]
- 58.Storkus WJ et al. (1987) NK susceptibility varies inversely with target cell class I HLA antigen expression. J. Immunol. 138, 1657–9 [PubMed] [Google Scholar]
- 59.Raulet DH (2006) Missing self recognition and self tolerance of natural killer (NK) cells. Semin. Immunol. 18, 145–150 [DOI] [PubMed] [Google Scholar]
- 60.Kärre K (2008) Natural killer cell recognition of missing self. Nat. Immunol. 9, 477–480 [DOI] [PubMed] [Google Scholar]
- 61.Shifrin N et al. (2014) NK cell self tolerance, responsiveness and missing self recognition. Semin. Immunol. 26, 138–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Den Brink MRM et al. (2015) Immune reconstitution following stem cell transplantation. Hematology 2015, 215–219 [DOI] [PubMed] [Google Scholar]
- 63.Ogonek J et al. Immune reconstitution after allogeneic hematopoietic stem cell transplantation. Frontiers in Immunology, 7. 17-November-(2016), Frontiers Media S.A., 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pangrazzi L and Weinberger B T cells, aging and senescence. Experimental Gerontology, 134. 01-June-(2020), Elsevier Inc., 110887. [DOI] [PubMed] [Google Scholar]
- 65.Hazeldine J and Lord JM The impact of ageing on natural killer cell function and potential consequences for health in older adults. Ageing Research Reviews, 12. September-(2013), Elsevier, 1069–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosenberg J and Huang J CD8+ T cells and NK cells: parallel and complementary soldiers of immunotherapy. Current Opinion in Chemical Engineering, 19. 01-March-(2018), Elsevier Ltd, 9–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gornalusse GG et al. (2017) HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat. Biotechnol. 35, 765–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Braud VM et al. (1998) HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 391, 795–799 [DOI] [PubMed] [Google Scholar]
- 69.Lee N et al. (1998) HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc. Natl. Acad. Sci. 95, 5199–5204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lieto LD et al. (2006) The human CD94 gene encodes multiple, expressible transcripts including a new partner of NKG2A/B. Genes Immun. 7, 36–43 [DOI] [PubMed] [Google Scholar]
- 71.Brown EJ and Frazier WA Integrin-associated protein (CD47) and its ligands. Trends in Cell Biology, 11. 01-March-(2001), Elsevier Current Trends, 130–135 [DOI] [PubMed] [Google Scholar]
- 72.Bjordahl R et al. (2019) FT576: A Novel Multiplexed Engineered Off-the-Shelf Natural Killer Cell Immunotherapy for the Dual-Targeting of CD38 and Bcma for the Treatment of Multiple Myeloma. Presented at: 61st ASH Annual Meeting & Exposition. Blood 134, 3214–3214 [Google Scholar]
- 73.Jones BS et al. (2014) Improving the safety of cell therapy products by suicide gene transfer. Front. Pharmacol. 5, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Watts TH (2005) TNF/TNFR FAMILY MEMBERS IN COSTIMULATION OF T CELL RESPONSES. Annu. Rev. Immunol. 23, 23–68 [DOI] [PubMed] [Google Scholar]
- 75.Croft M (2003) Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat. Rev. Immunol. 3, 609–620 [DOI] [PubMed] [Google Scholar]
- 76.Croft M (2014) The TNF family in T cell differentiation and function – Unanswered questions and future directions. Semin. Immunol. 26, 183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Firl DJ et al. A paradigm shift on the question of b cells in transplantation? Recent insights on regulating the alloresponse. Frontiers in Immunology, 8. 02-February-(2017), Frontiers Research Foundation, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]