Abstract
Objectives:
We sought to determine the frequency of unexpectedly low natriuretic peptide (NP) levels in a clinical population.
Background:
Higher NP concentrations are typically observed as a compensatory response to elevated cardiac wall stress. Under these conditions, low NP levels may be indicative of a “NP deficiency.”
Methods:
We identified 3 clinical scenarios in which high B-type natriuretic peptide (BNP) levels would be expected: (A) hospitalization for heart failure (HF), (B) abnormal cardiac structure or function, or (C) abnormal hemodynamics. In Vanderbilt’s electronic health record, 47,970 adult patients had BNP measurements. A total of 13,613 patients had at least one of the 3 conditions (hospitalized HF, n=9,153; abnormal cardiac structure/function, n=7,041; abnormal hemodynamics, n=363). We quantified the frequency of low BNP levels. We performed whole exome sequencing of the NPPB gene in a subset of 9 patients.
Results:
Very low BNP levels (<50 pg/ml) were observed in 4.9%, 14.0%, and 16.3% of patients with hospitalized HF, abnormal cardiac structure/function, or abnormal hemodynamics, respectively. A small proportion (0.1–1.1%) in each group had BNP levels below detection limits. Higher body mass index was the strongest predictor of unexpectedly low BNP. Exome sequencing did not reveal coding variations predicted to alter detection of BNP by clinical assays.
Conclusions:
A subset of patients with confirmed HF or cardiac dysfunction have unexpectedly low BNP levels. Obesity is the strongest correlate of unexpectedly low BNP levels. Our findings support the existence of NP deficiency, which may render some individuals more susceptible to volume or pressure overload.
Keywords: natriuretic peptide, b-type natriuretic peptide, BNP, natriuretic peptide deficiency, electronic health record
Introduction
Natriuretic peptides (NP) are cardiac hormones with beneficial effects on renal, vascular, and myocardial function. (1–3) NPs serve a key role as counter-regulatory hormones against sodium overload and excessive neurohormonal activation. Animals with NP deficiency have salt-sensitive hypertension, cardiac hypertrophy, and glucose intolerance. (4) It has not been established whether humans can have NP deficiency, analogous to other endocrine deficiencies.
Due to an endocrine feedback loop, deficient NP production cannot be reliably determined by assessing NP levels alone. Circulating NP concentrations are determined not only by the ability to produce, secrete, and clear the peptides, but also by underlying cardiac wall stress, the principal trigger for NP release. One strategy for determining whether NP deficiency exists is to identify individuals with unexpectedly low NP levels in the context of a large, obvious stimulus for NP release. This is analogous to the assessment of other hormonal deficiencies. For instance, adrenal insufficiency can be diagnosed by documenting inappropriately low cortisol levels in the setting of a large stimulus, e.g. critical illness.
Hypothesis and Purpose
The most potent drivers of NP release are volume and pressure overload. Thus, we examined a large database of patients admitted to an academic medical center over 2 decades, to identify patients with conditions characterized by marked volume or pressure overload. We assessed for the presence of unexpectedly low B-type natriuretic peptide (BNP) levels using stringent thresholds. Further, we performed whole exome sequencing on DNA from a small subset of patients with particularly low BNP measurements.
Methods
Study sample
The Vanderbilt University Medical Center Institutional Review Board approved this study. We queried the de-identified version of the Vanderbilt University Medical Center electronic health record, known as the “Synthetic Derivative,” which contains approximately 3 million records and spans approximately 20 years. (5) The Synthetic Derivative is linked to BioVU, the Vanderbilt DNA biobank that currently contains ~250,000 DNA samples. (5) Among patients at least 18 years of age who ever had a circulating BNP level measured (n= 47,970), we identified 3 groups of patients with conditions associated with obvious cardiac pressure or volume overload: (1) patients hospitalized with an acute HF exacerbation, (2) patients with markedly abnormal cardiac structure or function by echocardiography, and (3) patients with abnormal invasive hemodynamics. Patient records were extracted through June 2017. The groups were not mutually exclusive; an individual patient could belong to one or more groups. For individuals who had more than one BNP value measured during an episode of significant cardiac dysfunction, the lowest value (and the characteristics associated with that value) was used.
The hospitalized HF group included patients with a BNP measurement during or within 24 hours prior to a hospitalization for HF. A hospitalization for HF was defined by the presence of an ICD-9 diagnosis code of HF (425.* or 428.*) plus the administration of at least one dose of intravenous diuretics during that hospitalization. The abnormal echocardiogram group included patients who had a BNP measurement within 90 days of an echocardiogram demonstrating severely reduced left ventricular ejection fraction (LVEF ≤ 35%) or left ventricular hypertrophy (LVH), defined as left ventricular mass >162 g in females or >224 g in males. (6) LVEF was measured using Simpson’s biplane method, or visually-estimated if the biplane value was not reported. Left ventricular mass (grams) was calculated using the formula 0.8 × {1.04 × [([LVEDd + IVSd + PWTd]3 − LVEDd3)]} + 0.6, in accordance with American Society of Echocardiography recommendations. (6) The abnormal hemodynamics group included individuals who had a BNP measurement within 1 day of a cardiac catheterization showing a left ventricular end-diastolic pressure (LVEDP) ≥ 20 mmHg, pulmonary capillary wedge pressure ≥ 20 mmHg, right atrial pressure ≥ 20 mmHg, or cardiac index < 2 L/min/m2 measured by thermodilution or Fick. Details regarding the validation of the hemodynamic information in the clinical database have been provided previously. (7,8)
BNP measurements
Plasma BNP concentrations have been measured at Vanderbilt University Medical Center since 2002, using one of two immunoassays. From 2002 until September 2013, the Biosite BNP assay was used (Biosite Diagnostics, San Diego, CA). Between 2002 and 2007, the Biosite assay was implemented using the Biosite Triage platform, and from 2007 to 2013, it was run on the Beckman Coulter DXI platform. Since October 2013, the Abbott Architect assay has been used (Abbott Laboratories, Abbott Park, IL). Both assays have coefficients of variation of <12% and lower limits of detection of 10 pg/ml.
Covariates
Age was determined at the date of BNP measurement. Body mass index (BMI) was calculated using the closest weight value within 1 day before or after the BNP measurement, and the closest adult height value without time restriction before or after the BNP measurement. The closest heart rate, systolic and diastolic blood pressure, creatinine, and glucose values on or within 1 day before the date of BNP measurement were extracted. Values outside the biologically plausible ranges for covariates were censored. The presence or absence of medical comorbidities was determined based on ICD-9 codes and other data from the electronic medical record, as described previously. (9,10)
Exome sequencing
Amino acid alterations from coding variation in the gene encoding proBNP (NPPB) could contribute to apparently low BNP concentrations by interfering with the immunoassays. Thus, we performed whole exome sequencing (WES) in 9 patients with unexpectedly low BNP levels. WES was performed in the Vanderbilt Technologies for Advanced Genomics (VANTAGE) core facility. Targeted capture was performed using the Illumina Truseq Exome Kit v1.2. Short sequence reads generated on the Illumina HiSEQ 2500 instrument were aligned to the human reference assembly GRCh37 using the Burrows-Wheeler Aligner’s (BWA) ‘mem’ function. (11) Only high-quality base-calls (PHRED-like score >30) were included, and low-quality reads were soft trimmed. PICARD/SamTools(12) were used to mark duplicate reads, sort and index the alignments which then underwent quality score recalibration, variant calling, and filtering using the Genome Alignment Toolkit v3(13) and their “whole-exome variant calling best practices” workflow (14) (https://software.broadinstitute.org/gatk/best-practices/workflow). Analysis ready variants were annotated and extracted using SnpEFF/SnpSIFT. (15) Variants likely to result in loss of function or nonsense-mediated decay were identified using SnpEFF prediction algorithms and include single nucleotide variants introducing a stop codon, altering the normal reading frame, disrupting an essential splice site, or resulting in deletion of critical or large portions of protein coding sequence. (15,16)
Statistical Analysis
As the three groups were not mutually exclusive, all analyses were performed within group and no between group comparisons were made. We utilized several thresholds to define “low” BNP levels, ranging from 100 pg/ml (the Food and Drug Administration cutoff for the diagnosis of HF among dyspneic patients) to 10 pg/ml (the assays’ lower limit of detection). Correlates of low BNP levels were identified using multivariable-adjusted logistic regression models with the outcome of BNP < 50 pg/ml. Covariates were selected based on identifying the strongest predictors of continuous BNP levels in proportional odds models, and included BMI, LVEF, age, creatinine, sex, race, and systolic blood pressure. Highly skewed data underwent log transformation prior to inclusion in regression models.
Numbers of missing variables are reported in Supplemental Table 1. Missing data were addressed using multiple imputation and bootstrapping. Multiple imputation was repeated 10 times. All 10 imputed datasets were used in the model fitting, and coefficient estimates were averaged for 10 imputations. The variance-covariance matrix for parameter estimates was also adjusted for the variability introduced by multiple imputation. Partial effects for parameters strongly associated with BNP levels were plotted. Two-tailed p-values <0.05 were considered statistically significant. R version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria) was used for the statistical analyses.
Results
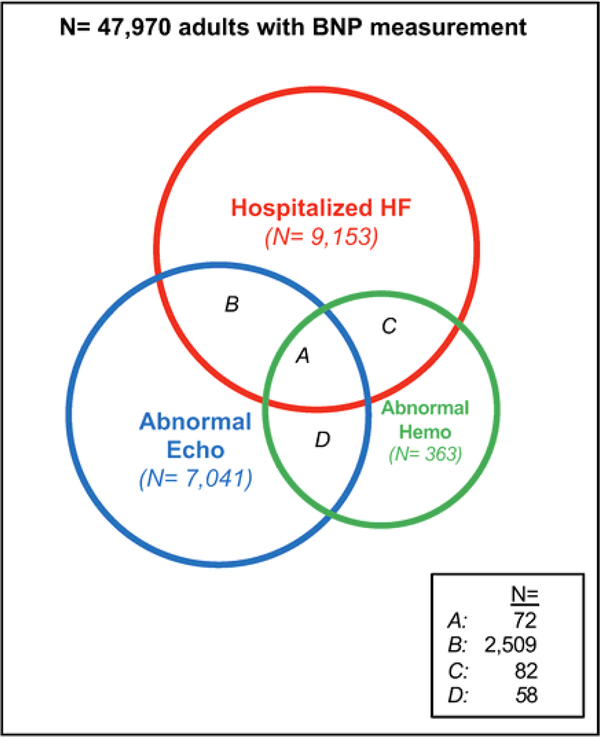
Among 47,970 patients with at least one BNP measurement, 13,613 (28.4%) met criteria for at least one of the clinical groups of HF or cardiac dysfunction (Figure 1). A total of 9,153 patients had a BNP measured during a hospitalization for acute HF, 7,041 patients had a BNP measured within 90 days of a markedly abnormal echocardiogram, and 363 individuals had a BNP measured within 1 day of abnormal hemodynamics (Figure 1). Characteristics of patients in these 3 groups are shown in Table 1. A total of 72 patients met criteria for all 3 categories of cardiac dysfunction.
Figure 1. Individuals who met criteria for groups of cardiac dysfunction.
Among 47,970 individuals with at least one BNP measurement, 9,153 patients had a BNP measured in the setting of a hospitalization for HF (“Hospitalized HF”), 7,041 individuals had a BNP measured in the setting of an echocardiogram showing abnormal cardiac structure or function (“Abnormal Echo”), and 363 individuals had a BNP measured in the setting of abnormal invasive hemodynamics (“Abnormal Hemo”). Sections A, B, C, and D represent individuals who met criteria for more than 1 group of cardiac dysfunction.
Table 1:
Characteristics of groups with significant cardiac dysfunction
| Hospitalized HF (N = 9,153) | Abnormal Echo (N = 7,041) | Abnormal Hemodynamics (N = 363) | |
|---|---|---|---|
| BNP, pg/ml | 530 (227, 1164) | 274 (100, 744) | 277 (87, 932) |
| Age, years | 66 (56, 76) | 64 (54, 74) | 63 (53, 72) |
| Male | 56% | 53% | 62% |
| Race, white | 81% | 76% | 83% |
| BMI, kg/m2 | 29.6 (25.0, 35.9) | 30.3 (25.7, 36.6) | 29.7 (24.9, 34.9) |
| BMI Category: | |||
| lean (BMI<25 kg/ m2) | 25% | 21% | 26% |
| overweight (25≤BMI<30 kg/m2) | 27% | 27% | 26% |
| obese (BMI≥30 kg/m2) | 48% | 52% | 48% |
| CAD | 67% | 58% | 87% |
| hypertension | 85% | 82% | 76% |
| diabetes mellitus | 21% | 46% | 39% |
| CKD | 37% | 25% | 20% |
| atrial fibrillation | 42% | 27% | 17% |
| Heart rate, bpm | 88 (75, 104) | 82 (71, 96) | 84 (75, 95) |
| Systolic BP, mm Hg | 121 (106, 140) | 124 (108, 142) | 119 (103, 133) |
| Diastolic BP, mm Hg | 67 (58, 79) | 68 (58, 80) | 64 (55, 78) |
| Creatinine, mg/dL | 1.28 (0.96, 1.86) | 1.20 (0.91,1.77) | 1.10 (0.88, 1.42) |
| Glucose, mg/dL | 119 (98, 157) | 112 (94,151) | 125 (102,180) |
| Septal Wall thickness, mm | 12.0 (11.0, 14.0) | 12.3 (11.0, 14.0) | 12.0 (11.0, 13.4) |
| Posterior Wall thickness, mm | 11.0 (9.6, 12.5) | 12.0 (10.0, 13.0) | 11.0 (9.0, 12.0) |
| LV internal diameter in diastole, mm | 49.4 (43.0, 57.0) | 50.8 (45.3, 57.0) | 48.0 (42.0, 55.0) |
| LV mass, g | 227 (176, 291) | 245 (202, 296) | 206 (166, 284) |
| Prevalence of LVH, % | 83% | 99% | 79% |
| LVEF, % | 50 (28, 55) | 52 (30, 55) | 48 (30, 55) |
| Preserved LVEF | 50% | 53% | 48% |
| LVEDP, mm Hg | 21 (15, 28) | 23 (15, 29) | 26 (22, 30) |
| PCWP, mm Hg | 20 (14, 27) | 21 (15, 27) | 22 (17, 27) |
| Right atrial pressure, mmHg | 12 (6, 16) | 12 (8, 16) | 13 (8, 17) |
| Cardiac Index, L/min/m2 | 2.4 (1.9, 3.0) | 2.4 (1.9, 3.0) | 2.3 (1.8, 2.9) |
Continuous variables are reported as median (25th percentile, 75th percentile). BNP, B-type natriuretic peptide; HF, heart failure; Echo, echocardiogram; CAD, coronary artery disease; CKD, chronic kidney disease; bpm, beats per minute; BP, blood pressure; LV, left ventricular; LVH, left ventricular hypertrophy; LVEF, left ventricular ejection fraction; LVEDP, left ventricular end-diastolic pressure; PCWP, pulmonary capillary wedge pressure
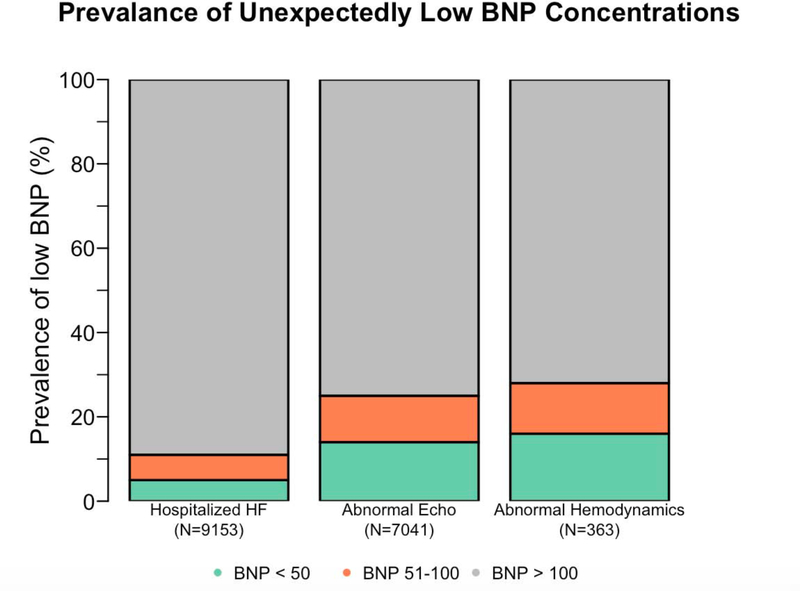
The frequencies of BNP levels below pre-specified thresholds are shown in Figure 2. BNP levels below 100 pg/ml were present in 10.7% (95% CI, 10.1% to 11.4%), 24.9% (95% CI, 23.9% to 25.9%), and 28.1% (95% CI, 23.6% to 33.1%) of patients with hospitalized HF, abnormal echocardiograms, and abnormal hemodynamics, respectively. BNP levels below 50 pg/ml were present in 4.9% (95% CI, 4.4% to 5.3%), 14.0% (95% CI, 13.2% to 14.8%), and 16.3% (95% CI, 12.6% to 20.5%) of patients with hospitalized HF, abnormal echocardiograms, and abnormal hemodynamics, respectively. Of the 72 patients who met all 3 definitions of cardiac dysfunction, 4 (6%) had BNP levels < 50 pg/ml. A small percentage of individuals even had BNP levels below 10 pg/ml. BNP levels below 10 pg/ml were present in 0.4% (95% CI, 0.3% to 0.6%), 1.1% (95% CI, 0.9% to 1.4%), and 0.2% (95% CI, 0.003% to 1.7%) of patients with hospitalized HF, abnormal echocardiograms, and abnormal hemodynamics, respectively.
Figure 2. Prevalence of unexpectedly low BNP concentrations.
BNP levels below 50 pg/ml (represented by green shading) and BNP levels below 100 pg/ml (represented by combination of green and orange shading) are presented for patients with hospitalized HF, abnormal echocardiograms, and abnormal hemodynamics, respectively. BNP, B-type natriuretic peptide; HF, heart failure; Echo, echocardiogram.
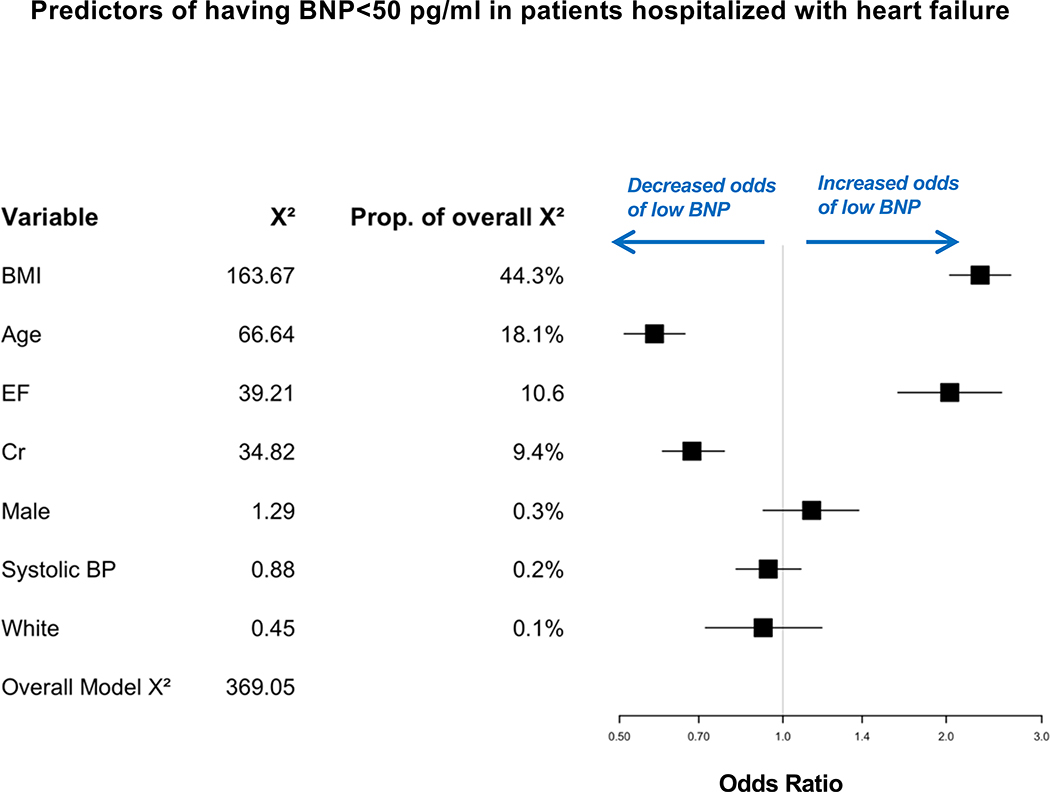
Characteristics of individuals with BNP concentrations below 50 pg/ml are shown in Table 2. Among patients with a BNP below 50 pg/ml in the context of a hospitalization for HF, the median age was 58 years, and 77% were obese. Next, we determined predictors of BNP levels below 50 pg/ml in the hospitalized HF group, the largest group. In multivariable analyses, the strongest predictors of having a BNP level below 50 pg/ml were higher BMI (which explained 44% of the overall Chi-square), followed by younger age, higher LVEF, and lower creatinine (Central Illustration, p<0.001 for all). In the hospitalized HF group, a BMI of 36.6 k/m2 (75th percentile), compared with a BMI of 25 kg/m2 (25th percentile), was associated with an adjusted odds of 2.31 (95% CI, 2.03 to 2.62) for low BNP levels. Characteristics of patients hospitalized with HF who had low BNP (< 50 pg/ml) stratified according to preserved vs. reduced LVEF are shown in Supplemental Table 2. Differences between patients with preserved and reduced LVEF in the subset with low BNP levels mirror those present in the broader population, namely HF with preserved ejection fraction patients tended to be older, with more obesity and diabetes, higher blood pressure, and less coronary artery disease.
Table 2.
Characteristics of patients with BNP<50 pg/ml and BNP≥50 pg/ml in patients hospitalized with heart failure
| Hospitalized HF |
||
|---|---|---|
| BNP < 50 pg/ml |
BNP ≥ 50 pg/ml |
|
| N (%) | 444 (4.9%) | 8709 (95.1%) |
| BNP, pg/ml | 28 (18, 37) | 575 (264, 1212) |
| Age, years | 58 (49, 66) | 67 (57, 76) |
| Male | 51% | 56% |
| Race, white | 77% | 81% |
| BMI, kg/m2 | 37.1 (31.2, 45.1) | 29.3 (24.8, 35.5) |
| BMI Category: | ||
| lean (BMI<25 kg/ m2) | 9% | 26% |
| overweight (25≤BMI<30 kg/m2) | 14% | 28% |
| obese (BMI≥30 kg/ m2) | 77% | 46% |
| CAD | 55% | 67% |
| Hypertension | 86% | 85% |
| Diabetes mellitus | 27% | 21% |
| CKD | 25% | 38% |
| Atrial fibrillation | 20% | 43% |
| Heart rate, bpm | 94 (81, 106) | 88 (74, 104) |
| Systolic BP, mmHg | 123 (110, 138) | 121 (105, 140) |
| Diastolic BP, mmHg | 69 (61,80) | 67 (58, 78) |
| Creatinine, mg/dL | 1.07 (0.83, 1.44) | 1.29 (0.97, 1.89) |
| Glucose, mg/dL | 121 (101, 166) | 119 (98, 156) |
| Septal Wall thickness, mm | 12 (11, 14) | 12 (11, 14) |
| Posterior Wall thickness, mm | 11 (10, 12) | 11 (10, 13) |
| LV internal diameter in diastole, mm | 46 (40, 50) | 50 (43, 57) |
| LV mass, g | 200 (158, 246) | 229 (177, 294) |
| LVH | 75% | 83% |
| LVEF, % | 55 (50, 55) | 48 (28, 55) |
| Prevalence of Preserved LVEF, % | 79% | 49% |
| LVEDP, mmHg | 24 (18, 28) | 21 (15, 28) |
| PCWP, mmHg | 10 (5, 22) | 20 (14, 27) |
| Right atrial pressure, mmHg | 6 (3, 17) | 12 (6, 16) |
| Cardiac Index, L/min/m2 | 2.2 (2.1,3.0) | 2.4 (1.9, 3.0) |
Continuous variables are reported as median (25th percentile, 75th percentile). BNP, B-type natriuretic peptide; HF, heart failure; Echo, echocardiogram; CAD, coronary artery disease; CKD, chronic kidney disease; bpm, beats per minute; BP, blood pressure; LV, left ventricular; LVH, left ventricular hypertrophy; LVEF, left ventricular ejection fraction; LVEDP, left ventricular end-diastolic pressure; PCWP, pulmonary capillary wedge pressure
Central Illustration. Predictors of having BNP levels <50 pg/ml in patients hospitalized with heart failure.
In multivariable analyses, the strongest predictors of having BNP levels below 50 pg/ml in the setting of hospitalization with HF were higher BMI, followed by younger age, higher EF, and lower creatinine (p<0.001 for all).
We performed whole exome sequencing in 9 patients with very low BNP levels (including 2 patients with BNP at or below the detection threshold) in the setting of HF and/or severe cardiac dysfunction. Patient characteristics and pertinent clinical histories are reported in Table 3. Whole exome sequencing generated a mean of 13.9 million reads per individual, with an average of 99.9% mapping to the reference genome and 80.5% mapping to the target sequence (Supplemental Table 3). Coverage of genes in the NP pathway (i.e., NPPB, CORIN, FURIN) was good, with 35 of 41 exons sequenced having 100% of base positions covered with a depth >5x. Coverage was poor for only 3 exons (all terminal exons for respective genes): CORIN exon 1 (bases covered at 5× 12%), FURIN exon 1 (bases covered at 5× 0%), and FURIN exon 16 (bases covered at 5× 31%) (Supplemental Table 4). We did not identify synthetic pathway loss of function variants or variants that would affect epitopes for either the Biosite or Abbott BNP assays. (17) Loss of function variants in the NP clearance receptor, NPR3, were found in two individuals.
Table 3.
Clinical characteristics of patients with inappropriately low BNP who underwent whole exome sequencing
| BNP value, pg/ml | Age (years) | Sex | Race | BMI, kg/m2 | Ejection fraction, % | Creatinine, mg/dl | LVEDP, mm Hg | Clinical features |
|---|---|---|---|---|---|---|---|---|
| <10 | 65 | F | W | 36.8 | 0.65 | 25 | High LVEDP; Older age | |
| <10 | 66 | M | W | 31.8 | 55 | 1.03 | 20 | High LVEDP; Older age |
| 11 | 61 | M | B | 27.0 | 55 | 0.88 | LVH; Hx of HF | |
| 13 | 55 | M | W | 31.2 | 45 | 1.00 | LVH; Hx of HF | |
| 17 | 62 | M | W | 23.4 | 62 | 2.29 | 20 | High LVEDP; High creatinine |
| 18 | 43 | F | W | 33.9 | 55 | 1.34 | LVH; Hx of HF | |
| 30 | 55 | F | 24.6 | 11 | 0.8 | Hospitalized for HF, Low EF | ||
| 31 | 69 | M | W | 28.0 | 50 | 1.45 | Hospitalized for HF; Older age | |
| 37 | 71 | F | W | 22.5 | 34 | High LVEDP; Older age |
Hx, history; HF, heart failure; LVH, left ventricular hypertrophy; LVEDP, left ventricular end diastolic pressure; EF, ejection fraction; M, male; F, female; W, white; B, black
Discussion
We used a large database containing approximately 48,000 patients with BNP measurements to identify individuals with unexpectedly low BNP levels in the setting of established HF, cardiac dysfunction, or hemodynamic overload. Our data support the hypothesis that a relative NP deficiency may exist in some individuals. Understanding the causes of deficiency may have important clinical implications, given the pivotal role of the NP system in the endogenous response to salt retention, vasoconstriction, and adverse cardiac remodeling.
Epidemiologic studies have demonstrated that among healthy populations, circulating NP levels are lower in certain individuals, such as obese compared with lean individuals (18), men compared with women (19), and blacks compared with whites. (9) Studies in healthy individuals may not establish whether a true “deficiency” exists, however, because there is little stimulus for NP release in the absence of cardiac wall stress. Large clinical databases are better suited to examining scenarios under which low hormone levels might be inappropriate. For example, just as a “normal” cortisol level of 8 mcg/dl is inappropriate for critically ill patients in an intensive care unit, a BNP level less than 50 pg/ml is probably inappropriate in a patient with severe cardiac dysfunction or HF.
In the present study, we examined 3 groups of patients in which a large stimulus to BNP release should exist. Though discordance between clinical status and measured BNP levels was present in the minority of patients, it was not rare. Between 5% and 16% of patients within each group of severe cardiac dysfunction had BNP levels less than 50 pg/ml, a threshold that is 50% lower than the upper end of the “normal range” for conventional BNP assays. Among patients in whom the highest likelihood of elevated BNP levels would be expected, namely, those hospitalized with HF, with abnormal hemodynamics at cardiac catheterization, and reduced LVEF or LVH on echocardiography, we found the proportion of NP deficient individuals was approximately 6%. Remarkably, 38 patients with hospitalized HF (0.4%) had barely detectable BNP levels (detection threshold, 10 pg/ml). As a comparison, the prevalence of inappropriately low cortisol levels during critical illness has been estimated as 13–30%. (20,21) In our study, the percentage of patients with BNP levels below 50 pg/ml would likely be even higher if BNP(1–32) (the biologically active segment of BNP) were measured by mass spectrometry. Prior studies have found that BNP(1–32) measured by mass spectrometry is low in many patients with HF, and that the commonly used BNP clinical immunoassays measure inactive BNP fragments in addition to the active BNP(1–32) segment. (22)
Our study was not specifically designed to assess the precise prevalence of potential NP deficiency, because the decision to obtain a BNP measurement was non-random, particularly in earlier time periods, when BNP testing was less common, and we used relatively stringent hemodynamic and cardiac structure and function thresholds to ensure specificity for a heart failure phenotype. Nonetheless, our data may be a better representation of what is found in actual clinical practice than results obtained from clinical trials or smaller observational studies, the sources of most of our information regarding BNP in HF. In the Breathing Not Properly trial, Maisel and colleagues found that among individuals with HF as the cause of acute dyspnea, 3% had a BNP level below 50 pg/ml, (23) lower than the prevalence found in our clinical cohort. In 318 patients with HF, Mehra et al. demonstrated that circulating BNP levels were lower in obese compared with non-obese patients (mean BNP levels 205 and 335 pg/ml, respectively). (24) Neither these studies nor others focused specifically on characterizing patients with very low levels.
The cause of unexpectedly low BNP levels is uncertain, and different explanations may account for the finding in different subsets of the population. For some individuals, low BNP levels may reflect a “relative” deficiency from the cumulative effect of non-cardiac factors known to affect NP production or clearance. For instance, obesity was the most prominent clinical correlate of low levels, consistent with studies in healthy populations and animal models. (18,25–27) The mechanisms of low NP levels in obesity are not fully established. Proposed mechanisms include impaired NP production, possibly related to insulin resistance, (27,28) and/or increased NP clearance due to higher expression levels of the NP clearance receptor. (29,30) Other correlates of low BNP levels in the multivariable analyses (younger age, preserved LVEF) might be evidence of lesser degrees of stimulus for BNP secretion and a normally responsive NP system.
On the other hand, a subset of individuals had no identifiable factor associated with low BNP levels, raising the possibility that some individuals have an intrinsic endocrine deficiency. Among the 9 patients selected for exome sequencing, 5 had BMI in the non-obese range and 7 were white (African-American individuals are known to have lower BNP levels). (9,31) None of these individuals had coding sequence variation that would be expected to interfere with the epitopes for the BNP assays, reducing the likelihood of measurement artifact. Further, 6 of 9 patients had multiple BNP measurements in the clinical system, all of which were less than 110 pg/ml. In public databases, natural coding variation in the mature BNP transcript has been described at position 25, (32) which is adjacent to the sequence bound by the Abbott assay detection antibody (positions 26–32). (17) None of the individuals sequenced in our sample had a variant at this position.
Limitations of our study should be noted. Misclassification bias is possible due to our definitions for the 3 groups with a HF phenotype. The combination of ICD-9 codes for heart failure/cardiomyopathy plus intravenous diuretics has previously been demonstrated to have high positive and negative predictive values (~95%) compared with a manually-adjudicated clinical diagnosis. (33) In the abnormal cardiac structure and function group, BNP measures were taken as those closest to and within 90 days of the echocardiogram. Clinical management decisions that may have resulted from the BNP and/or echocardiogram results that could have impacted the other could not be ascertained from the EHR. That said, the results in the echocardiography group are largely consistent with the other 2 groups, for which there is greater specificity, i.e. acute HF and objectively measured hemodynamics. The hemodynamic criteria for defining the abnormal hemodynamics cohort were substantially beyond clinically abnormal values. These stringent criteria were applied to enhance specificity for a large stimulus for which high BNP values would be expected. The net effect could be an underestimation of the prevalence of unexpectedly low BNP levels. In addition, 3 different BNP assay platforms were used in the clinical lab over the course of this study. All 3 platforms have a lower limit of detection of 10 pg/ml, and a BNP level below 50 pg/ml would be considered unexpectedly low in the setting of significant cardiac dysfunction for all assay platforms. In a sensitivity analyses, results were similar when assay type was included as covariate in the multivariable-adjusted analyses. Finally, whether prognosis varies according to the presence of NP insufficiency could not be accurately assessed using the current data, as patients could obtain care outside of that captured by our electronic medical record system. Addressing prognosis in relation to NP insufficiency is a future direction.
In conclusion, unexpectedly low BNP levels are present in a subset of patients with overt HF, cardiac dysfunction, or hemodynamic overload. These findings suggest that a relative or absolute NP deficiency can exist in certain individuals. Further investigation is needed to define the clinical and physiologic consequences of NP deficiency in humans.
Supplementary Material
Clinical Perspectives:
Natriuretic peptides are cardiac-derived hormones that counteract increased cardiac stress. Patients with low natriuretic peptide levels in the setting of elevated cardiac stress, e.g. heart failure, may have a “natriuretic peptide deficiency.”
Translational Outlook:
Future studies are needed to define the clinical and physiologic consequences of natriuretic peptide deficiency in humans, and whether correcting natriuretic peptide deficiency can improve outcomes.
Acknowledgments
Sources of Funding: This research was supported by Career Development Award #IK2 CX001678 from the U.S. Department of Veterans Affairs Clinical Sciences Research and Development (CSR&D) Program, Vanderbilt University Medical Center Faculty Research Scholars award, and National Institute of Health Awards: National Heart, Lung, and Blood Institute grants K12 HL109019, 1K23HL128928-01A1, 1K23HL146887-01, R01 HL131532, 1 R01 HL146588, 3 R01 HL146588-01S1, 1 U01 HL125212-01, and R01HL140074; National Institute of Diabetes and Digestive and Kidney Diseases T32DK007061, and the National Center for Advancing Translational Sciences of the National Institutes of Health UL1TR000445 (Vanderbilt).
Abbreviations List:
- NP
natriuretic peptide
- BNP
B-type natriuretic peptide
- BMI
body mass index
- HF
heart failure
- LVEF
left ventricular ejection fraction
- LVH
left ventricular hypertrophy
- LVEDd
left ventricular end-diastolic diameter
- IVSd
interventricular septal thickness end-diastole
- PWTd
posterior wall thickness end-diastole
- LVEDP
left ventricular end-diastolic pressure
- WES
whole exome sequencing
- BWA
Burrows-Wheeler Aligner’s
Footnotes
Relationship with Industry: The authors report no relationships with industry.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Newton-Cheh C, Larson MG, Vasan RS et al. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet 2009;41:348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vasan RS, Glazer NL, Felix JF et al. Genetic variants associated with cardiac structure and function: a meta-analysis and replication of genome-wide association data. JAMA 2009;302:168–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. [Review] [67 refs]. New England Journal of Medicine 1998;339:321–328. [DOI] [PubMed] [Google Scholar]
- 4.Tamura N, Ogawa Y, Chusho H et al. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci U S A 2000;97:4239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roden DM, Pulley JM, Basford MA et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther 2008;84:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang RM, Badano LP, Mor-Avi V et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39 e14. [DOI] [PubMed] [Google Scholar]
- 7.Assad TR, Hemnes AR, Larkin EK et al. Clinical and Biological Insights Into Combined Post- and Pre-Capillary Pulmonary Hypertension. J Am Coll Cardiol 2016;68:2525–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assad TR, Maron BA, Robbins IM et al. Prognostic Effect and Longitudinal Hemodynamic Assessment of Borderline Pulmonary Hypertension. JAMA Cardiol 2017;2:1361–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta DK, de Lemos JA, Ayers CR, Berry JD, Wang TJ. Racial Differences in Natriuretic Peptide Levels: The Dallas Heart Study. JACC Heart Fail 2015;3:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel DK, Green KD, Fudim M, Harrell FE, Wang TJ, Robbins MA. Racial differences in the prevalence of severe aortic stenosis. J Am Heart Assoc 2014;3:e000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009;25:1754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Handsaker B, Wysoker A et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009;25:2078–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKenna A, Hanna M, Banks E et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 2010;20:1297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DePristo MA, Banks E, Poplin R et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet 2011;43:491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cingolani P, Platts A, Wang le L et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 2012;6:80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacArthur DG, Balasubramanian S, Frankish A et al. A systematic survey of loss-offunction variants in human protein-coding genes. Science 2012;335:823–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu S, Ping P, Zhu Q, Ye P, Luo L. Brain Natriuretic Peptide and Its Biochemical, Analytical, and Clinical Issues in Heart Failure: A Narrative Review. Front Physiol 2018;9:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang TJ, Larson MG, Levy D et al. Impact of obesity on plasma natriuretic peptide levels. Circulation 2004;109:594–600. [DOI] [PubMed] [Google Scholar]
- 19.Lam CS, Cheng S, Choong K et al. Influence of sex and hormone status on circulating natriuretic peptides. J Am Coll Cardiol 2011;58:618–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sibbald WJ, Short A, Cohen MP, Wilson RF. Variations in adrenocortical responsiveness during severe bacterial infections. Unrecognized adrenocortical insufficiency in severe bacterial infections. Ann Surg 1977;186:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peeters B, Meersseman P, Vander Perre S et al. Adrenocortical function during prolonged critical illness and beyond: a prospective observational study. Intensive Care Med 2018;44:1720–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller WL, Phelps MA, Wood CM et al. Comparison of mass spectrometry and clinical assay measurements of circulating fragments of B-type natriuretic peptide in patients with chronic heart failure. Circ Heart Fail 2011;4:355–60. [DOI] [PubMed] [Google Scholar]
- 23.Maisel AS, Krishnaswamy P, Nowak RM et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 2002;347:161–7. [DOI] [PubMed] [Google Scholar]
- 24.Mehra MR, Uber PA, Park MH et al. Obesity and suppressed B-type natriuretic peptide levels in heart failure. JAmCollCardiol 2004;43:1590–1595. [DOI] [PubMed] [Google Scholar]
- 25.Miyashita K, Itoh H, Tsujimoto H et al. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes 2009;58:2880–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cannone V, Boerrigter G, Cataliotti A et al. A genetic variant of the atrial natriuretic peptide gene is associated with cardiometabolic protection in the general community. J Am Coll Cardiol 2011;58:629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan AM, Cheng S, Magnusson M et al. Cardiac natriuretic peptides, obesity, and insulin resistance: evidence from two community-based studies. J Clin Endocrinol Metab 2011;96:3242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H, Thoonen R, Yao V et al. Regulation of B-type natriuretic peptide synthesis by insulin in obesity in male mice. Experimental physiology 2015. [DOI] [PubMed] [Google Scholar]
- 29.Pivovarova O, Gogebakan O, Kloting N et al. Insulin up-regulates natriuretic peptide clearance receptor expression in the subcutaneous fat depot in obese subjects: a missing link between CVD risk and obesity? J Clin Endocrinol Metab 2012;97:E731–9. [DOI] [PubMed] [Google Scholar]
- 30.Clerico A, Giannoni A, Vittorini S, Emdin M. The paradox of low BNP levels in obesity. Heart Fail Rev 2012;17:81–96. [DOI] [PubMed] [Google Scholar]
- 31.Gupta DK, Claggett B, Wells Q et al. Racial differences in circulating natriuretic peptide levels: the atherosclerosis risk in communities study. J Am Heart Assoc 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karczewski KJ, Francioli LC, Tiao G et al. Variation across 141,456 human exomes and genomes reveals the spectrum of loss-of-function intolerance across human protein-coding genes. bioRxiv 2019:531210. [Google Scholar]
- 33.Ahlers MJ, Lowery BD, Farber-Eger E et al. Heart Failure Risk Associated With Rheumatoid Arthritis-Related Chronic Inflammation. J Am Heart Assoc 2020;9:e014661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.