Abstract
Voltage-dependent anion channel (VDAC), the most abundant mitochondrial outer membrane protein, is important for a variety of mitochondrial functions including metabolite exchange, calcium transport, and apoptosis. While VDAC’s role in shuttling metabolites between the cytosol and mitochondria is well established, there is a growing interest in understanding the mechanisms of its regulation of mitochondrial calcium transport. Here we review the current literature on VDAC’s role in calcium signaling, its biophysical properties, physiological function, and pathology focusing on its importance in cardiac diseases. We discuss the specific biophysical properties of the three VDAC isoforms in mammalian cells—VDAC 1, 2, and 3—in relationship to calcium transport and their distinct roles in cell physiology and disease. Highlighting the emerging evidence that cytosolic proteins interact with and regulate VDAC calcium permeability, we advocate for continued investigation into the VDAC interactome at the contact sites between mitochondria and organelles and its role in mitochondrial calcium transport.
Keywords: Voltage-dependent anion channel, Mitochondrial Outer Membrane, Mitochondrial Associated Membrane, α-Synuclein, Calcium signaling, Voltage gating, Ion selectivity, Protein-protein interaction
Graphical Abstract
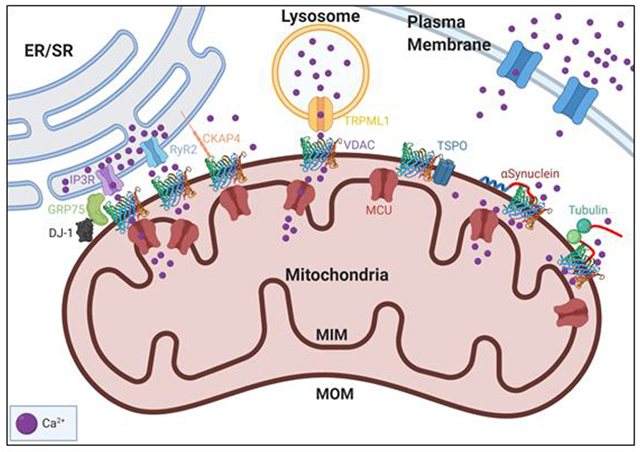
1. Introduction
Traditionally, the mitochondrial outer membrane (MOM) was regarded as just a barrier between the mitochondrial space and the cytosol. Emerging data has assigned more functions for the MOM, such as control over metabolite fluxes between the cytosol and mitochondria, tethering with the endoplasmic reticulum (ER), and lysosomes, and direct involvement in a cross-talk between mitochondria and proteins involved in metabolic and survival pathways. Some of these MOM functions are executed and regulated by the voltage-dependent anion channel (VDAC), the most abundant protein in the MOM. While VDAC’s role in conducting and regulating the MOM permeability to ions and small water-soluble metabolites is well-established [1–4], VDAC’s involvement in calcium (Ca2+) homeostasis is still unknown. In contrast to the mitochondria inner membrane (MIM), which has five known pathways for Ca2+ transport, the MOM does not have a specific Ca2+ transporter [5]. Tom40, the channel component of the translocase of the outer membrane (TOM complex) is a plausible candidate for a passive Ca2+ pathway [6]. The high cation selectivity of Tom40 [7] makes it an even more favorable candidate for Ca2+ transport than the weakly anion-selective VDAC. Indeed, Tom40 preference for cations matches positive charges of mitochondrial presequence matrix proteins [6, 8] and VDAC preference for anions matches a majority of negatively charged mitochondrial metabolites passing through it [4]. Such a straightforward comparison between two MOM major β-barrel channels raises the question of whether VDAC really plays an active role in Ca2+ regulation or it is merely an inert diffusion pore?
Initial studies on Ca2+ signaling using permeabilized cells relied on the assumption that the sheer number of highly conductive VDACs effectively constitutes no barrier to Ca2+, unlike the low copy number of Mitochondrial Calcium Uniporter (MCU) in the MIM, which regulates Ca2+ influx into the mitochondrial matrix (Figure 1) [9]. Though there are competing theories of the mechanism of Ca2+ flux through MCU (see discussion in [10]), it is well known that MCU shows positive cooperativity at elevated Ca2+ concentrations and therefore enhances Ca2+ flux at micromolar local Ca2+ concentrations [11]. In the cytosol, however, the global Ca2+ pool is generally around 100-500 nM [Ca2+] with peaks up to around 1-3 μM [12]. Therefore, rapid mitochondrial Ca2+ influx is achieved through tight contact sites between mitochondria and Ca2+ storage organelles such as ER, sarcoplasmic reticulum (SR), or lysosomes that are known as mitochondrial associated membranes (MAMs) [13]. MAMs have multi-protein complexes containing VDAC and Ca2+ channels that create highly localized Ca2+ microdomains for rapid Ca2+ transport across mitochondria [14] (Figure 1).
Figure 1. Ca2+ flux into the mitochondria through global cytoplasmic Ca2+ and Ca2+ microdomains.
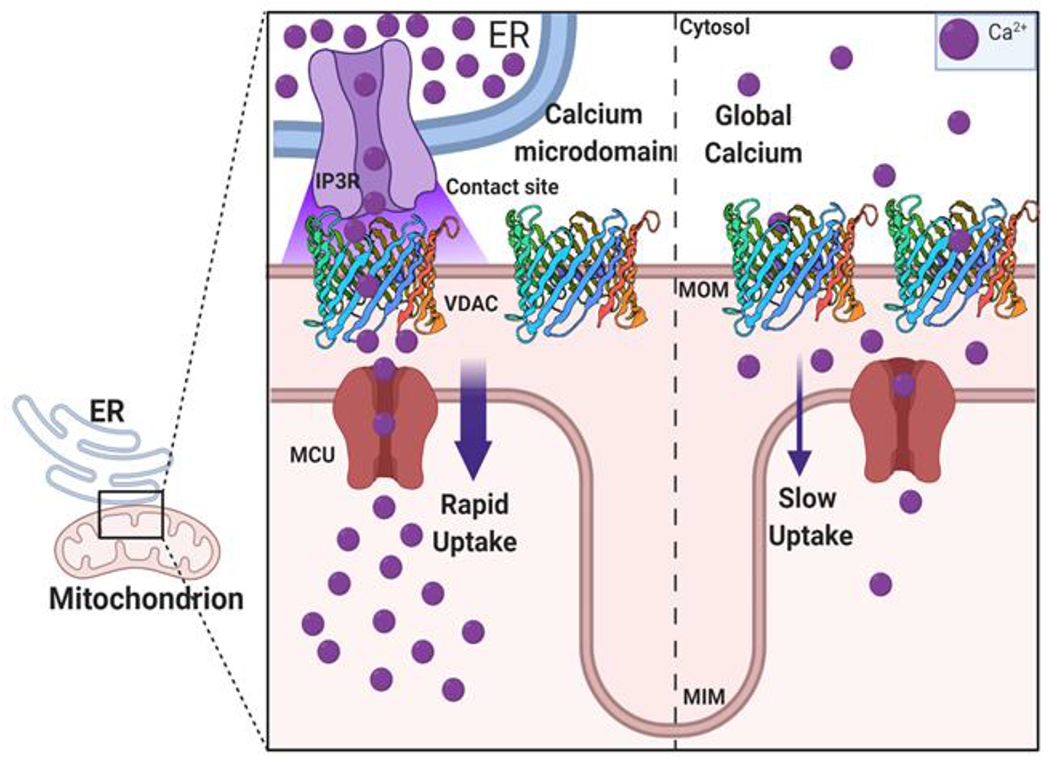
Mitochondria interact with organelles such as the endoplasmic reticulum (ER) through contact sites generating Ca2+ microdomains. These Ca2+ microdomains (left half), contain a multi-protein complex linking IP3R (an ER Ca2+ channel in purple) to VDAC (3D structure representation) in the mitochondrial outer member (MOM). The microdomains enable rapid transfer of Ca2+ (Ca2+ sparks) from the ER to mitochondria through VDAC and the MCU in the inner membrane (MIM). Global or capacitive uptake of Ca2+ from the cytosol (right half) is slower due to the lower local concentration of Ca2+compared to microdomains. Ca2+enters the cytosol from extracellular space or ER stores.
In early studies, VDAC was interpreted as an inert diffusive pore to solutes and metabolites. However, recent research has uncovered a wide variety of biological processes that alter the accessibility of VDAC, reigniting the debate on whether VDAC could be an active regulator of mitochondrial Ca2+ flux [15–20]. The proposed mechanisms of VDAC regulation of Ca2+ range from the inherent gating of VDAC, to VDAC’s interaction with other proteins and the surrounding lipid environment.
In this review, we revisit the role of VDAC in translocation and regulation of mitochondrial Ca2+ fluxes. We discuss the biophysical basis of VDAC Ca2+ transport, the impact of VDAC cytosolic regulators on its Ca2+ permeability, and VDAC’s role in mitochondria-organelle Ca2+ crosstalk with particular attention to the contribution of the individual VDAC isoforms in Ca2+ signaling. Finally, we discuss the possible physiological implications of VDAC Ca2+ transport in diseases. It is our hope that a deeper understanding of the mechanisms of VDAC regulation will help the researchers in interpreting mitochondrial Ca2+ experiments in the cells, recognizing new pathways that could regulate mitochondrial Ca2+ flux through VDAC, and developing novel therapies targeting VDAC Ca2+ transport.
2. VDAC’s role in Ca2+ exchange between mitochondria and organelles
Ca2+ is an important signaling molecule that regulates cell function through spatial and temporal Ca2+ release patterns [21]. Importantly, cytoplasmic Ca2+ concentration is maintained at low levels against its concentration in the extracellular space and intracellular stores. These levels are maintained through signaling mechanisms that either pump Ca2+ out into the extracellular space or the intracellular stores in organelles such as the ER, SR, and mitochondria [22]. Mitochondria exchange Ca2+ with the ER, SR, and lysosomes at close contact sites (MAMs). These contact sites have multi-protein complexes linking VDAC in the MOM to Ca2+ channels such as the ryanodine receptors (RyRs) at the SR, inositol 1,4,5-trisphosphate receptors (IP3Rs) at the ER, and TRPML1 at the lysosome [23, 24] (Figure 2). Such tight co-localization of multiple proteins involved in Ca2+ transduction is important for rapid Ca2+ transfer to mitochondria through a process known as Ca2+ “sparks” or “puffs”. Ca2+ sparks are localized microdomains of highly concentrated Ca2+ that result in transient micromolar concentration promoting rapid Ca2+ uptake across MCU [25]. Ca2+ sparks in the mitochondria mediate signaling pathways that control ATP supply, metabolism, and apoptosis in cell [26–28].
Figure 2. VDAC protein partners in Ca2+ regulation.
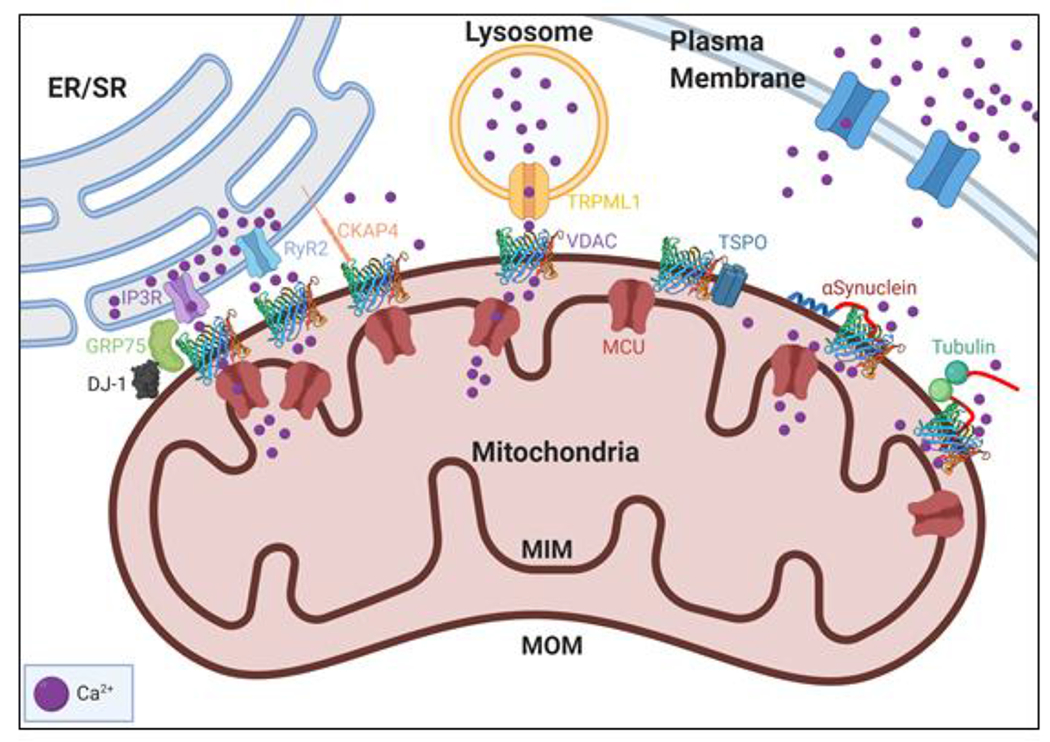
VDAC can regulate mitochondrial function through its interaction with multiple proteins such as various organelle Ca2+ channels and cytoplasmic and MOM proteins. VDAC facilitates efficient Ca2+ transfer to mitochondria by forming multi-protein complexes with Ca2+ channels in other organelles such as the IP3R-VDAC-GRP75-DJ-1 complex, the RyR2-VDAC, and TRPML1-VDAC on the ER, SR, and lysosome, respectively. ER-associated CKAP4 competes with the IP3R-GRP75 complex for interaction with VDAC. VDAC interacts with cytosolic free dimeric tubulin and PD-associated protein α-synuclein (αSyn). Tubulin and αSyn bind to the MOM and reversibly block VDAC by their disordered acidic C-terminal tails (shown in red). VDAC also interacts with TSPO, another MOM protein, which has been shown to affect Ca2+ uptake in cardiac disease.
Earlier studies on the role of MOM permeability in the uptake of Ca2+ focused on global or “capacitive” Ca2+ exposure whereupon an influx of Ca2+ into the cytoplasm saturates the mitochondria. For example, in HeLa cells, cytosolic to mitochondrial Ca2+ transfer, carried out by exposing permeabilized cells to extracellular Ca2+, was not altered by VDAC1 overexpression [17]. These experiments suggested that in basal conditions, flux across the VDAC is enough to saturate the MCU, and therefore overexpressing VDAC may not significantly alter flux. However, the same work showed that VDAC overexpression increases Ca2+ permeability at mitochondria-ER contact sites (MERCS). The authors further looked at the colocalization of VDAC-GFP at the MOM with the ER protein, calreticulin, and found that overexpression of VDAC did not alter the overlap between the ER and mitochondria. Nevertheless, using single-cell measurements of the ER to mitochondria Ca2+ transport, they found that overexpression of VDAC reduced the delay between ER Ca2+ release and subsequent mitochondrial uptake. These experiments suggest that VDAC overexpression does not increase the number of MERCS but does increase the permeability of MERCS to Ca2+. However, since colocalization methods have some obvious limitations, namely the diffraction limit of light precludes probing sub-250 nm structures such as the contact sites, novel methods that offer more accurate measurements of true MERCS such as split-GFP-based contact site sensor (SPLICS), FRET, and proximity labeling techniques should be used to further study these junctions [13, 29]. These data first indicated that the transfer of Ca2+ through the ER-MOM contacts critically depends on VDAC which forms a kind of “kinetic bottleneck” for the total mitochondrial Ca2+ homeostasis [17]. Altogether, these results indicate that VDAC is important for Ca2+ transfer from the ER and SR, where transient microdomains of Ca2+ flux propagate a wide variety of signaling responses.
Beyond mitochondria-ER contact sites, contacts between mitochondria and lysosomes were recently described (Figure 2). Wong et al. showed that the transient contacts between lysosomes and mitochondria induce mitochondrial fission [30]. Following up on these interesting results their group demonstrated that Ca2+ efflux from the lysosomes to the mitochondria is mediated by tethering of lysosomal-mitochondria contact sites through the transient receptor potential mucolipin 1 (TRPML1) [31]. Loss-of-function mutations in TRPML1 are known to cause mucolipidosis type IV (MLIV), a lysosomal storage disease that triggers severe neuronal impairment [32, 33]. Peng et al. showed that patient-derived fibroblasts harboring mutations in TRPML1 showed impaired mitochondrial Ca2+ uptake and this may be associated with mitochondrial damages found in MLIV [31]. In a coimmunoprecipitation pull-down for TRPML1, they found VDAC1, but not VDAC2 or VDAC3, in complex with TRPML1 [31]. They also found that mitochondrial Ca2+ uptake at mitochondria-lysosome contact sites is modulated by VDAC1 in the MOM and by MCU in the MIM. To test the role of VDAC1 in mitochondrial uptake of lysosomal Ca2+, the authors compared Ca2+ dynamics in cells expressing either wild-type human VDAC1 or its mutant E73Q with a single mutation in a putative Ca2+-binding site previously proposed by Israelson et al. [18]. The E73Q VDAC1 mutant showed a smaller increase in mitochondrial Ca2+ uptake than the wild-type. These results led authors to conclude that VDAC1 (but not VDAC2 or VDAC3) serves as a mediator of lysosomal Ca2+ uptake at mitochondria-lysosome contact sites, thus assigning a single residue, E73, a decisive role in this process. However, recent in vitro studies put in doubt the role of E73 as a unique VDAC1 Ca2+ binding site.
VDAC1 E73 residue was first suggested as a possible Ca2+ binding site [18, 19] (E72 according to the VDAC1 sequence used in these works) before the VDAC1 crystal structure was solved in 2008 [34–36]. which revealed that E73 is buried in the middle of the lipid membrane hydrocarbon core. Since then, this notorious residue has drawn attention from VDAC researchers [37] and especially in NMR spectroscopy and molecular dynamic (MD) simulation studies [36, 38–40]. Indeed, it is intuitively clear that the probability of a Ca2+ ion reaching the middle of the hydrocarbon core of the lipid bilayer is extremely low if not impossible. Interestingly, the same E73 residue was later identified as a specific binding site for cholesterol [35, 41, 42] and allopregnanolone [43, 44] by using NMR, photoaffinity labeling, and computational analysis. This raises a natural question of how the same residue could be a binding site for both a hydrophilic ion and a hydrophobic steroid. In addition, it should also be noted that E73 is not conserved among VDAC isoforms and species: mammalian VDAC3, VDAC from yeast [45], and fungi [37] have a non-charged glutamine Q73 (see also discussion in [46]). Recently, it was shown in experiments with wild-type mouse VDAC1 and mutants E73Q and E73A reconstituted into planar lipid membranes that E73 is not involved in VDAC1 gating [46], contrary to the early work prescribing a regulatory role for this residue [36, 38]. In the latest experiments with VDAC1 reconstituted into the planar membranes in pure CaCl2 gradient, our group showed that VDAC1 E73Q mutant has the same Ca2+ selectivity as the wild-type [47], at odds with the proposed role of E73 as a Ca2+ binding site. E73Q VDAC1 mutation may disrupt the Ca2+ signaling complex at the contact site or affect the VDAC-lipid interface. We have previously shown that a hydrophobic molecule, by binding to the external hydrophobic surface of VDAC β-barrel at the protein-lipid interface, could directly affect VDAC gating properties [48, 49]. This may hint at an explanation of why the E73Q VDAC1 mutant caused a reduction of lysosomal Ca2+ uptake shown by Peng, Wong et al. [31].
3. VDAC isoforms in Ca2+ transport
There are three mammalian isoforms of VDAC - VDAC1, VDAC2, and VDAC3 - encoded by individual genes for proteins of ~ 32 kDa. Each VDAC isoform has a tissue-specific expression pattern, hinting at unique roles for VDAC isoforms in organ physiology [50]. In general, VDAC1 and VDAC2 show higher levels of expression (~90%) than VDAC3 which is far less abundant (~10%), with an exception in the testis [45, 51]. Of the three isoforms, only embryonic knockout of VDAC2 is either lethal according to earlier studies [52] or resulted in developmentally impaired neonatal mice according to a recent study [53]. Embryonic knockout of VDAC1 and VDAC3 results in mice with physiologic impairments, such as bioenergetic defects in VDAC1 and VDAC3 knockout mice [54, 55] and infertility in VDAC3 knockout [56]. One of the outstanding questions in VDAC research is determining the relationship between specific physiological function and biophysical channel properties of each isoform. Recent data suggest that one of the distinguishing features of each isoform is their differential regulation and interaction with protein partners [52, 53, 57, 58].
Another imperative question in mitochondrial physiology is defining the role of each VDAC isoform in mediating Ca2+ signaling. Szabadai et al. first showed that VDAC1 forms a complex with IP3R mediated by the chaperone protein glucose-regulated protein 75 (grp75) [14]. In addition, the loss of grp75 dissociated the IP3R-VDAC1 interaction and impaired mitochondrial Ca2+ uptake after Ca2+ release from ER. Later it was shown that the IP3R-grp75 complex is unique to the VDAC1 isoform [16]. However, the uniqueness of VDAC1 to the complex has recently been called into question by Harada et al., who found that VDAC2 can also form a complex with IP3R [59]. Liu et al. has determined that another protein, DJ-1, mutated in familial forms of Parkinson’s disease (PD) is also a component of the IP3R-GRP75-VDAC1 complex [60]. Ablation of DJ-1 dissociated the IP3R-GRP75-VDAC1 complex and reduced mitochondrial Ca2+ uptake from the ER. Altogether, these findings indicate that a large VDAC-containing complex exists at the MERCS that can regulate Ca2+ flux (Figure 2). Given that the complex is regulated by its interaction with DJ-1 and the evidence that mitochondrial Ca2+ signaling is altered in PD, it is possible that the IP3R-GRP75-VDAC1 super-complex functions as a regulator of Ca2+ in neuronal cells where rapid Ca2+ signaling is imperative to meet high energy demands during neuronal activity.
Like VDAC1, VDAC2 has also been found in complexes with Ca2+ channels. Kee-Min and colleagues performed a screen to look for interacting partners of the Ryanodine Receptor 2 (RyR2), which mediates Ca2+ transport from the SR to the mitochondria in the heart [61]. They found VDAC2 as an interacting partner of RyR2 and showed that the two proteins colocalize using immunofluorescence and immuno-EM methods. As mentioned above, Harada et al. found that VDAC2 also colocalized with the IP3R-GRP75 complex in HeLa cells. In addition, they found that another ER-associated protein, cytoskeleton-associated protein 4 (CKAP4), bound to VDAC2 and detached it from the IP3R-GRP75 complex [59]. The ablation of CKAP4 led to enhanced MERCS formation and subsequent mitochondrial dysfunction caused by Ca2+ influx [59]. While most of the studies have been focused on Ca2+ transport involving the two major isoforms, VDAC1 and VDAC2, there has yet to be a concerted effort to establish a role of the minor isoform VDAC3 in Ca2+ transport.
Following their original work on VDAC1’s role in Ca2+ transport [17], Rizzuto’s group dissected the role of each VDAC isoform. De Stefani and colleagues found that knockout of individual VDAC isoform impaired Ca2+ transport to the mitochondria after IP3R agonist release [16]. Conversely, overexpression of an individual isoform enhanced the ER to mitochondrial Ca2+ transport. Interestingly, overexpression or silencing of VDAC2 or VDAC3 had a slightly higher effect on mitochondrial Ca2+ uptake than overexpression of VDAC1. The authors concluded that this difference may arise from the different Ca2+ transport properties of each isoform. However, this hypothesis has not been explored further.
Recently our group showed that all three VDAC isoforms have slight, but significantly different Ca2+ permeability with VDAC1 being more anionic and consequently less Ca2+ selective among three isoforms and VDAC3 more cationic and consequently more Ca2+ selective [47]. Interestingly, these data obtained on recombinant VDAC isoforms reconstituted into the planar lipid membranes, support an early hypothesis by De Stefani et al. [16], where the authors assigned the slight difference in mitochondrial Ca2+ uptake caused by individual VDAC isoform overexpression to the isoform-specific Ca2+ permeability of each isoform. We can speculate even further suggesting that the enhanced permeability to Ca2+ by VDAC3 found in our in vitro study, may be related to sperm defects and infertility in VDAC3 knockout mice due to the high expression levels of VDAC3 in the testes and sperm [56].
4. VDAC Permeability to Ca2+ - Biophysical Studies
Typically, ion channels are studied in situ using the patch-clamp method. However, the study of ion channels from cell organelles by this method is an experimentally daunting endeavor, because applying a patch-clamp to ~1 μm organelles, such as the mitochondrion, presents understandable experimental challenges. Therefore, the method of reconstituting organellular ion channel proteins into planar lipid membranes is, so far, the best available method to achieve quantitative characterization of ion channel properties. Mitochondrial VDAC is no exception. The experimental setup for VDAC reconstitution is schematically shown in Figure 3A. When reconstituted in physiologically relevant 150 mM KCl, VDAC forms large, ~0.7 nS [46] anion-selective channels (Cl−/K+ ~ 3) permeable to non-charged polymers up to 2-5 kDa [62, 63] and ATP and other negatively charged respiratory metabolites [2, 3]. The characteristic property of reconstituted VDACs, and its namesake, is gating. Gating refers to the ability of VDAC to move under the applied voltages of > 30 mV from a unique high conducting or “open” state to a variety of low conducting or so-called “closed” states [4, 64, 65] (Figure 3B). As most of the VDAC channel properties have been obtained and quantified in monovalent KCl and NaCl solutions, it was unclear whether VDAC would behave similarly in the presence of Ca2+ or in pure CaCl2 solutions. The answer to the former question can be found in the earlier works of Colombini’s group: most of the experiments with reconstituted VDAC were performed in the presence of non-physiological 2-5 mM Ca2+, which nonetheless did not measurably affect channel conductance, selectivity, or gating in 1 M KCl [15, 66, 67]. The answer to the latter question was obtained by Gincel et al. who were the first to demonstrate typical VDAC gating for channels reconstituted into pure 150/500 mM CaCl2 solutions [68]. The authors also found that the selectivity of the VDAC open state measured in pure CaCl2 gradient, is anionic with a permeability ratio between Cl− and Ca2+, PCl− /PCa2+, equal to ~3. Later, Tan and Colombini measured VDAC selectivity in a lower concentration of CaCl2 (in 80/20 mM CaCl2 gradient) and found that in this condition, Cl− permeability is ~25-fold higher than that of Ca2+ (Figure 4A) [15]. These results are not surprising considering the lower mobility of divalent Ca2+ than of monovalent Cl−, and that VDAC anionic selectivity increases with decreasing monovalent salt concentration [69]. Most importantly, Tan and Colombini showed that VDAC’s more cationic closed states have higher permeability for Ca2+ than the open state. They extrapolated their results to a physiologically low Ca2+ concentration of 1μM and found that a calculated Ca2+ flux through the closed states is about 4 to 10-fold higher than through the open state (Figure 4B). These results opened an important possibility that by gating, VDAC could regulate Ca2+ fluxes to and from mitochondria, thus providing evidence for an active role for VDAC in Ca2+ homeostasis rather than just being an inert pore. The immediate consequence of these findings is that VDAC in its open state facilitates fluxes of metabolites, but keeps Ca2+ flux low to maintain healthy mitochondrial respiration. In the closed states, VDAC diminishes metabolite fluxes but increases Ca2+ permeation. This state would attenuate respiration and accentuate apoptotic signals. The authors concluded that VDAC gating is not just a peculiar biophysical phenomenon but important physiological property to control MOM permeability [15].
Figure 3. Experimental assessment of VDAC properties.
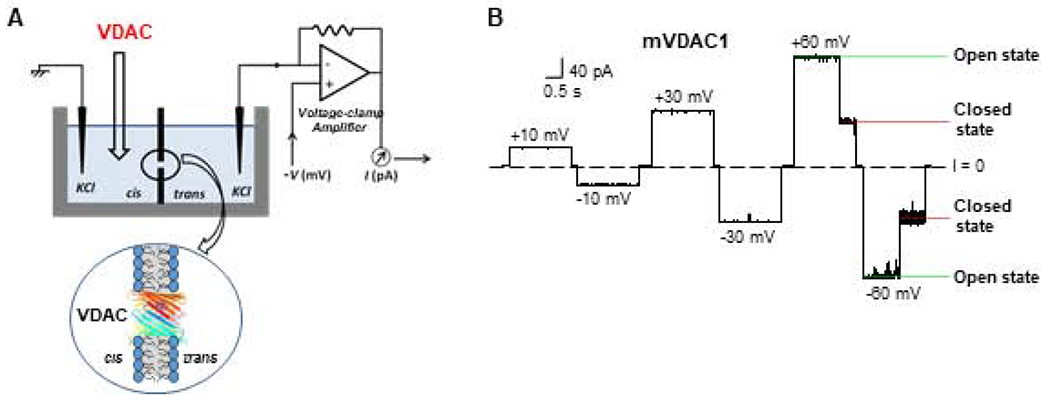
(A) A schematic experimental setup for VDAC reconstitution to the planar lipid membrane. The experimental chamber for planar lipid membrane formation consists of two compartments, cis and trans. Potential is defined as positive when it is greater at the side of VDAC addition (cis side). The current recording is performed in a voltage-clamp mode shown by the model circuit for current amplification and registration. Adapted by permission from Rostovtseva and Bezrukov (2015). Copyright © Springer International Publishing Switzerland 2015. (B) A representative single-channel current trace obtained with recombinant mouse VDAC1 reconstituted into the planar membrane at different applied voltages. Under high applied voltage (± 60 mV) channel conductance moves from the unique high conducting “open” state to a variety of low-conducting “closed” states. Relaxing voltage to 0 mV reopens the channel. Adapted from Queralt-Martin et al. J. Gen. Physiol. (2019). Copyright with permission © Rockefeller University Press 2019.
Figure 4. VDAC voltage gating enhances Ca2+ permeability.
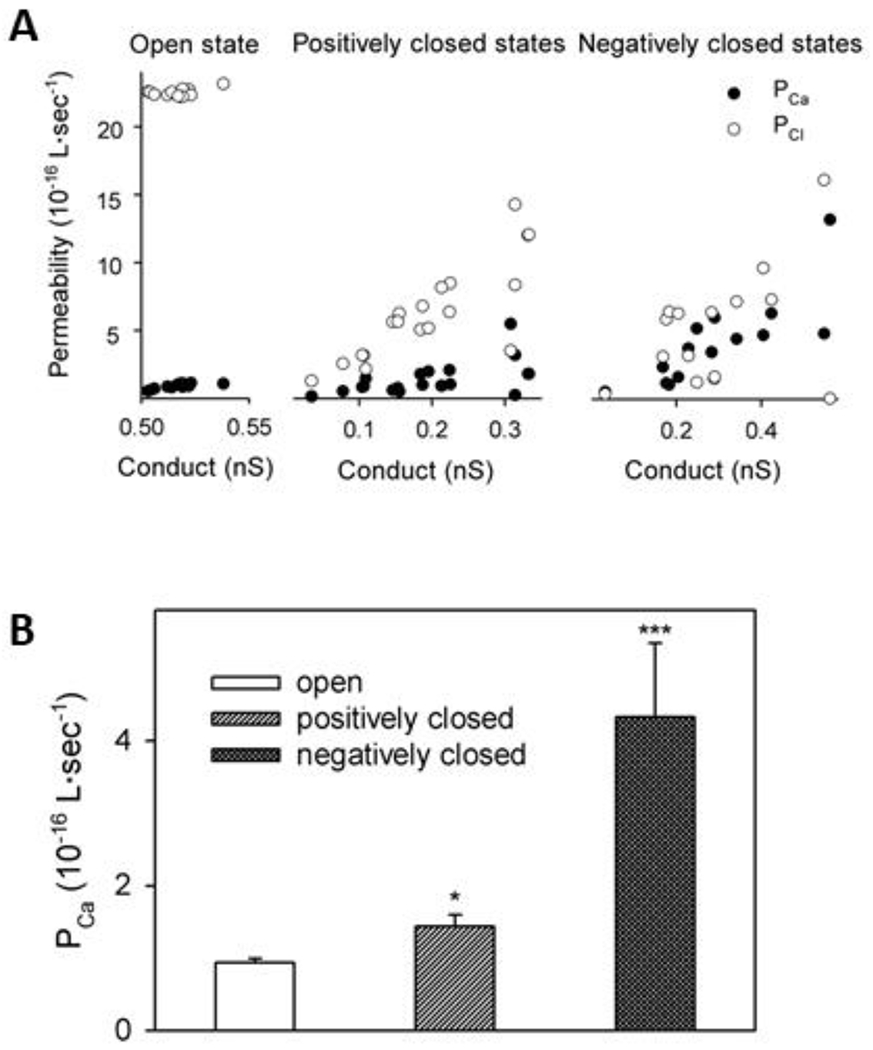
(A) Permeability of VDAC to Ca2+ and Cl− as a function of the total conductance of the channel. (B) Comparison of the permeability of Ca2+ for the VDAC open and closed states. Adapted from Tan and Colombini. Biochim. Biophys. Acta. (2007). Copyright with permission Copyright © 2007 Elsevier.
The discussions about the physiological relevance of VDAC gating, are confounded by open questions about a quantitative value for the transmembrane potential across the MOM in vivo. Common sense suggests that this potential should be negligible, due to the high abundance of the highly conductive VDACs in the MOM (see discussions, e.g. in [4, 15]). Colombini suggested that the main source of the potential across the MOM is the so-called Donnan potential which arises from a high concentration of VDAC-impermeable polyanions and polycations (e.g., cytochrome c of +9 charges) in the intermembrane space and the cytosol [4]. An intriguing theoretical model of the possible source of MOM potential developed by Lemeshko identifies the VDAC-hexokinase complex as a potential-generating “battery” [70, 71]. Lemeshko has given estimates of the MOM potential as high as 50 mV, negative at the cytoplasmic side of the MOM. In his model, the Gibbs free energy of kinase reactions is the driving force for the potential generation across the MOM. The model gives a new physiological relevance not only to the VDAC voltage-gating but also to the channel’s interaction with hexokinases and other cytosolic regulators such as tubulin. This leads us to another key question, namely: how does VDAC’s interaction with protein partners influence its permeability to Ca2+?
5. Regulation of VDAC Ca2+ transport
VDAC is known to interact with a quite impressive number of various genetically, structurally, and physiologically unrelated cytosolic and mitochondrial membrane proteins (see reviews by [58, 72, 73]). The former include glycolytic enzymes, hexokinase, dimeric tubulin, and neuronal proteins intimately involved in different neurological diseases such as α-synuclein (αSyn), Aβ peptide, tau, and SOD1 [73–79]. The latter include the cholesterol transporter, translocator protein 18 kDa (TSPO) [80], and Bcl-2 family proteins that ultimately link VDAC to apoptosis [52, 53, 81, 82]. Potentially, all these proteins can affect VDAC Ca2+ permeability, thus influencing mitochondrial Ca2+ homeostasis. Here we focus on two well-studied cases of VDAC cytosolic regulators αSyn and tubulin, as well as VDAC’s neighbor in the MOM, TSPO.
αSyn is a small, 140-amino acids, intrinsically disordered protein highly expressed in neurons. The abnormal accumulations of αSyn are considered to be the pathological hallmark of PD [83]. It is the major component of the Lewy bodies found in substantia nigra of the post-mortem brains of the PD patients where it exists in the fibrillar form [84]. Based on these observations most of the studies on the role of αSyn in PD and other neurodegeneration were focused on the pathological role of the αSyn aggregates. Under normal conditions, αSyn constitutes up to 1% of the total cytosolic protein in neurons [85] where it exists in predominantly monomeric form. Small amounts of αSyn are also found in peripheral tissues [86]. The point mutations, genomic duplications, and triplications found in the gene encoding αSyn provide further genetic evidence of αSyn connection with familial and sporadic forms of PD [86]. Specific relationships between αSyn toxicity and mitochondria dysfunction are well established [87, 88]. The latest work has found intriguing relationships between a newly discovered mutation in the ITPKB kinase that is protective against sporadic PD, αSyn aggregation, and mitochondrial Ca2+ uptake [89]. Inhibition of ITPKB was shown to enhance ER-to-mitochondria Ca2+ uptake, subsequently exacerbating αSyn aggregation and PD pathology via the impairment of autophagy. However, the precise role of αSyn, especially its monomeric form, in neurodegeneration remains enigmatic. In particular, monomeric and oligomeric αSyn are found associated with both mitochondrial membranes [90, 91], causing impairment of the mitochondria respiratory complexes [92], an increase of oxidative stress [93] and fission [94]. Recently our group using in situ and in vitro experiments showed that αSyn translocates into the mitochondria through VDAC [49, 76, 95]. Neuronally differentiated SHSY5Y cells with overexpressed wild type αSyn have been chosen as an appropriate cell model of PD [96]. The involvement of VDAC1 in αSyn translocation into mitochondria was confirmed by VDAC1 knockdown in these cells with siRNA [49]. The question of whether the other two VDAC isoforms also serve as pathways f6or the αSyn entry into mitochondria remains open until further studies. The experiments with recombinant mammalian VDAC1 and VDAC3 reconstituted into planar lipid membranes showed that αSyn effectively blocks and translocates through both VDACs [57, 76] suggesting that most likely αSyn enters mitochondria through all VDAC isoforms, but with different efficiency.
Figure 5A shows a typical electrophysiological experiment with reconstituted VDAC in the presence of αSyn. The ion current through the single VDAC channel is quiet, and the channel does not gate at the relatively low applied voltage (25 mV as in the experiment shown in Figure 5A). The channel can remain open at these experimental conditions for up a few hours with occasional transitions between the open and lower conducting states. Addition of nanomolar concentration of αSyn (50 nM as in Figure 5A) changes the situation dramatically by inducing fast fluctuations of current between the open and a well-defined blocked state with ~ 40% of the open state conductance [76]. The time-resolved blockage events in the milliseconds range are clearly seen in the inset of Figure 5A. It should be noted that αSyn-induced blockages are in striking contrast with the voltage-induced VDAC closure which is characterized by much longer-lasting closures (10-100 s) with a variety of conductances (Figure 3B) [97]. αSyn blocks VDAC from both sides of the channel (added to the both sides of a planar membrane, Figure 3A) when a negative potential is applied from the side of αSyn addition [76]. When the sign of the potential is reversed, no blockage events are detected. This observation and the fact that αSyn with truncated C-terminal induces orders of magnitude less blockages than the wild-type [76], suggest that the highly negatively charged 45-residue C-terminal domain of αSyn is responsible for the channel blockage. On the other hand, a synthetic peptide of the C-terminus of αSyn does not measurably block the channel up to 0.5 mM [76]. Altogether, these observations led us to propose a molecular mechanism where the acidic C-terminus of αSyn is captured inside the net positive VDAC pore while being anchored to the membrane surface by its N-terminus, preventing free translocation through the pore (Figure 5C). A single polypeptide strand of αSyn fits comfortably into the ~2.5-2.7 nm diameter VDAC pore. The blockage time increases exponentially with applied voltage up to a certain voltage – a “turnover” potential – which amplitude depends on experimental conditions such as lipid composition and salt concentration [76, 98]. At potentials higher than the turnover potential, the blockage time decreases with voltage signifying translocation of αSyn through the pore [76]. Thus, under certain conditions, αSyn can translocate through the VDAC pore into the mitochondria and target the electron transport complexes in the MIM leading to their impairment and mitochondria dysfunction, the conjecture that was supported by the loss of mitochondria potential in cells with overexpressed wild-type αSyn [49, 96].
Figure 5. Regulation of VDAC Ca2+ permeability by α-synuclein.
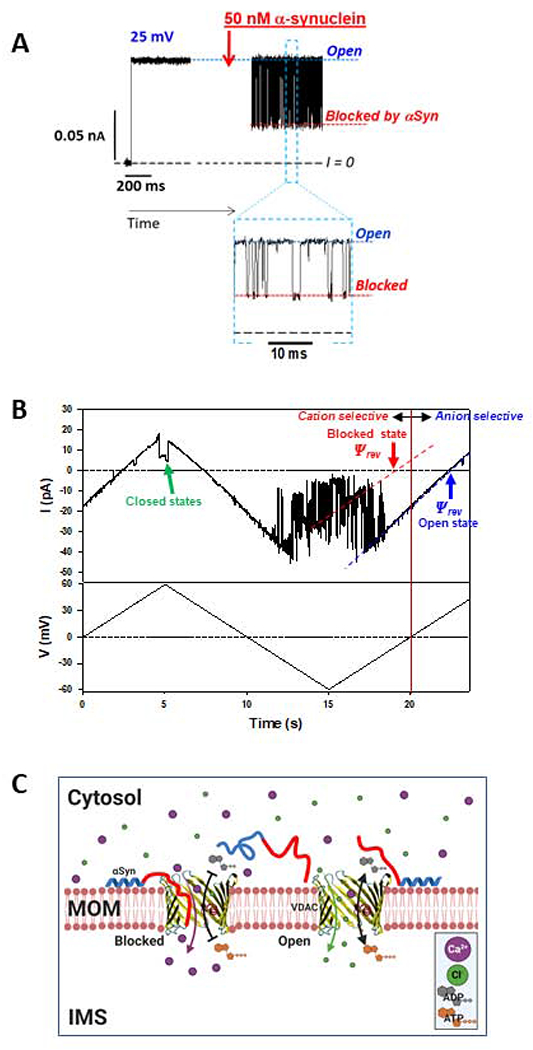
(A) A representative experiment showing a current record through a single VDAC in 1M KCl before and after the addition of 50 nM of α-synuclein (αSyn). αSyn induces fast characteristic blockage events best seen in a finer time scale in Inset. (B) Current through a single VDAC in response to applied triangular voltage wave (50 mHz, ±60 mV) obtained in 150mM/30mM CaCl2 gradient. Dashed blue and red lines indicate VDAC open and αSyn-blocked states, respectively. The intersection of these lines with the zero-current line (I = 0) corresponds to the reversal potential (Ψrev). Positive Ψrev corresponds to anionic selectivity of the open state and negative to cationic selectivity of the αSyn-blocked state. The voltage-induced closed states are indicated by the green arrow. Current records were digitally filtered using a 100 Hz filter. (C) A model of αSyn regulation of Ca2+ flux. Under applied negative voltage, acidic C-terminus of αSyn is captured inside the VDAC pore resulting in reversed VDAC selectivity and consequently in enhanced Ca2+ flux through the pore. When the channel is open, its slight anionic selectivity causes a reduced Ca2+ permeability and favors the fluxes of negatively charged metabolites such as ADP and ATP.
The fact most relevant for our current discussion is that when the highly negatively charged C-terminal domain of αSyn is transiently trapped inside the pore, the blocked state becomes cation selective [98, 99]. This leads to two important physiological consequences: impaired translocation of the negatively charged respiratory substrates such as ATP and ADP, which is due to the creation of electrostatic and steric barriers by the C-terminus inside the pore, and higher permeability for Ca2+ (Figure 5C). The increase in mitochondria uptake of Ca2+ released from the ER in response to αSyn overexpression in HeLa cells [100] supports the latter implication. The direct experimental evidence of the increased Ca2+ flux through the αSyn blocked state was obtained recently by our group in experiments with reconstituted VDAC [47]. The representative experiment in Figure 5B shows a single-channel trace obtained under the applied triangular voltage wave in a 150 mM /30 mM CaCl2 gradient in the presence of 50 nM αSyn in the cis compartment. Fast fluctuations of current between the open (indicated by the blue dashed line in Figure 5B) and blocked (indicated by the red dashed line) states correspond to time-resolved individual blockage events induced by aSyn. It can be seen that the reversal potential, Ψrev, (the voltage corresponding to zero current) has an opposite sign for the open and blocked states. A positive Ψrev corresponds to anion and negative to cation selectivity (Figure 5B). These results demonstrate that VDAC blocked by αSyn is significantly more permeable for Ca2+ than the open channel [47]. Indeed, the calculated permeability ratio between Cl− and Ca2+, PCl /PCa, reduces from 40.7 ± 9.8 for the open state to 0.58 ± 0.24 for the blocked state [47], which is a 70-fold change in favor of Ca2+ permeability in the blocked state. The cartoon in Figure 5C illustrates the proposed mechanism of αSyn regulation of Ca2+ transfer through VDAC. Therefore, αSyn interaction with VDAC might regulate mitochondrial Ca2+ uptake, but further genetic evidence is needed to conclusively surmise VDAC’s role in αSyn modulation of Ca2+ crosstalk between mitochondria and the ER [92, 100].
We speculate that another potent VDAC cytosolic regulator, dimeric tubulin [75, 95], can also regulate Ca2+ flux through VDAC by a similar mechanism as αSyn. It was shown that tubulin blocks the VDAC pore with its disordered negatively charged C-terminal tail and consequently reversed VDAC selectivity to cationic [62]. Therefore, we anticipate that the blockage of the VDAC pore by tubulin will increase Ca2+ permeability through the MOM.
VDAC is known to undergo post-translational modifications including phosphorylation [101, 102], ubiquitination [103, 104], and succination [105]. While none of these modifications have been shown to directly alter the Ca2+ transport properties of VDAC, they have been shown to affect VDAC interaction with other proteins that could in turn, indirectly affect Ca2+ signaling. For example, it was shown that phosphorylation of VDAC by glycogen synthase kinase-3β (GSK3β) in vitro, enhances VDAC blockage by tubulin [101]. Therefore, following the above speculations, VDAC phosphorylation could potentially affect its Ca2+ permeability via modulating VDAC interaction with tubulin. It was also suggested that depletion of the VDAC complex with GSK3ß and BCL-xL in response to reduced VDAC phosphorylation in steatosis may enhance mitochondrial permeability to Ca2+ and sensitize the cell to apoptosis[106]. Lastly, phosphorylation of VDAC was shown to displace the glycolytic enzyme Hexokinase2 (HK2) from VDAC1 [107, 108]. Concurrently the dissociation of HK2 from the MOM induces apoptosis via an influx of Ca2+ from the ER [109], thus letting us hypothesize that phosphorylation of VDAC may enhance mitochondrial Ca2+ uptake via HK2 displacement. Altogether, these observations open an intriguing possibility that other cytosolic proteins, yet to be discovered, could also regulate Ca2+ fluxes through VDAC. Future studies will reveal if this prediction is true.
Little is known about the mechanism of VDAC’s interaction with its neigboroughing membrane proteins in the crowded MOM environment. Apoptotic stimuli that evoke mitochondrial Ca2+ influx have been found to enhance VDAC expression and oligomer formation [110, 111]. VDAC’s natural potency to form ordered oligomeric arrays in MOM is well-known and was first demonstrated by electron micrographs by Mannella’s group [112] followed later by the work of Scheurung’s group [113] where supramolecular assembly of VDAC was demonstrated by atomic force microscopy in native MOM. Further, x-ray and NMR crystallography confirmed that VDAC could exist in dimeric conformation and form homo-oligomers of different sizes [114–116]. However, the important questions of how VDAC oligomerization affects Ca2+ homeostasis and whether oligomerization affects the Ca2+ permeability of the channel or regulates Ca2+ fluxes remain to be answered. Also, VDAC’s interaction and hetero-oligomerization with other MOM proteins and their potential role in Ca2+ regulation is also intriguing. For example, an increase in the expression of TSPO coincided with reduced mitochondrial Ca2+ uptake during heart failure, which was reversed in TSPO knockout mice [117]. The observed reduction in Ca2+ uptake could potentially be due to TSPO’s modulation of VDAC opening as hypothesized by Gatliff and Campanella [118]. Further studies on the interaction between TSPO and VDAC and its effect on VDAC Ca2+ transport are needed to understand their role in heart disease.
6. Role of VDAC in Ca2+ homeostasis in cardiac disease
Heart diseases are linked to mitochondrial dysfunction due to impaired Ca2+ uptake, bioenergetics, and mitophagy. For example, during ischemia-reperfusion injury, there is an increased translocation of GSK-3β to mitochondria which is known to modify VDAC by phosphorylation [119]. In addition, Gomez et al. showed that the inhibition of GSK-3β results in reduced Ca2+ overload and cell death [120]. Based on these two works we can hypothesize that VDAC phosphorylation may increase Ca2+ exchange through VDAC resulting in mitochondrial Ca2+ overload. Also, in a model of Duchenne muscular dystrophy, Viola et al. showed that the L-type Ca2+ channel regulates mitochondrial function by modulating VDAC function via cytoskeletal proteins [121]. Ca2+ influx through the L-type Ca2+ channel initiates contraction in the heart and increases mitochondrial membrane potential, ROS, NADH, and metabolic activity in a Ca2+-dependent manner [121]. The authors hypothesize that the absence of dystrophin results in the disarray of cytoskeletal proteins leading to the reduced metabolic activity due to the loss of communication between the L-type Ca2+ channel and VDAC through F-actin. However, perturbation of Ca2+-induced Ca2+ release (CICR) from the SR to mitochondria through the VDAC2-RyR2 channel complex due to cytoskeletal disarray may also affect energy production [122]
Given the lethality of VDAC2 knockout in mice models, it is of great interest to investigate the specific role of VDAC2 in cardiac function. During a heartbeat, the energy is required to translate the cardiac action potential to the physical contraction of cardiac myocytes by CICR to mitochondria through VDAC2-RyR2. Knockdown of VDAC2 increased intracellular Ca2+ levels upon electrical stimulation and decreased mitochondrial Ca2+ uptake after caffeine-induced activation of the RyR2 channels [61]. It was also found that VDAC2 knockdown modified mitochondrial Ca2+ uptake and the intensity and duration of SR Ca2+ sparks even though the autorhythmic and caffeine-induced Ca2+ transients were not affected [123]. Further evidence for VDAC2 as a modulator of rhythmic beating in the heart emerged when Shimizu and colleagues showed that overexpression of zebrafish VDAC2 (zfVDAC2) was antiarrhythmic in the zebrafish arrhythmia model [124]. They also found that a novel antiarrhythmic compound efsevin enhances mitochondrial uptake of Ca2+, which is mediated by its direct interaction with VDAC2, and the knockout of zfVDAC2 prevents efsevin’s protective effect. The authors proposed that efsevin enhanced zfVDAC2 permeability to Ca2+, thereby buffering aberrant Ca2+ signals in the heart. This was further confirmed by Wilting et al. who showed that efsevin binding to zfVDAC2 increases the channel’s cationic selectively, thus presumably increasing zfVDAC2 permeability to Ca2+ [125]. These studies show strong evidence for VDAC2’s specific role in Ca2+ homeostasis essential for proper cardiac function.
Taken together, the above studies tend to assign a specific role for VDAC, particularly VDAC2, in mitochondrial buffering of Ca2+ in cardiac physiology, suggesting that the lethality of VDAC2 knockout may be related to impaired cardiac function. The protective effect of efsevin in arrhythmia is promising, but further studies to discern the role of VDAC isoforms in ischemia/reperfusion and other heart diseases are needed to develop drugs to treat heart disease.
7. Conclusions and Perspectives
VDAC is well known for its role in shuttling metabolites between the cytosol and mitochondria. In this review, we have focused on its lesser-known function in Ca2+ transport and regulation of Ca2+ signaling at the mitochondria. VDAC is part of multi-protein complexes with specific organelle Ca2+ channels, which allows the generation of Ca2+ microdomains that facilitate rapid transfer of Ca2+ to mitochondria from the intracellular stores such as ER, SR, and lysosomes.
Biophysical studies of VDAC channel properties in the presence of Ca2+ suggest that VDAC could regulate Ca2+ fluxes to and from mitochondria by gating. Furthermore, these fluxes could be modulated by cytosolic VDAC regulators, such as neuronal protein αSyn. The distinct biophysical properties and physiological roles of VDAC isoforms in Ca2+ transport are especially interesting and warrant further study to understand the different effects of specific VDAC isoform knockouts, especially the lethality of VDAC2 knockout, in mice models. VDAC2 is also important in mitochondrial function in cardiac cells and could potentially be a target for drug development to treat heart diseases.
We hope that this review will help researchers to appreciate the contemporary state of research on VDAC’s role in Ca2+ homeostasis and promote further investigations into the isoform-specific role of VDACs and their regulation in Ca2+ signaling and mitochondrial function. As we move on from the traditional view of VDAC as an inert diffusion pore, we expect that future research will uncover the mechanisms by which VDAC controls mitochondrial Ca2+ fluxes.
Highlights.
VDAC plays an important role in mitochondrial calcium transport, regulation, and signaling.
VDAC is part of multi-protein complexes linking it to calcium channels in other organelles for efficient calcium exchange.
VDAC calcium transport can be regulated by voltage gating and by its interaction with proteins such as α-synuclein.
Calcium transport through VDAC is isoform specific.
Acknowledgments
This study was supported by the Intramural Research Program of the National Institutes of Health (NIH), Eunice Kennedy Shriver National Institute of Child Health Human Development (NICHD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interests
The authors declare that they have no competing interests with the contents of this article.
References
- 1.Rostovtseva TK and Bezrukov SM, VDAC regulation: role of cytosolic proteins and mitochondrial lipids. J Bioenerg Biomembr, 2008. 40(3): p. 163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodge T and Colombini M, Regulation of metabolite flux through voltage-gating of VDAC channels. J. Membr. Biol, 1997. 157(3): p. 271–279. [DOI] [PubMed] [Google Scholar]
- 3.Rostovtseva T and Colombini M, ATP flux is controlled by a voltage-gated channel from the mitochondrial outer membrane. J Biol Chem, 1996. 271(45): p. 28006–8. [DOI] [PubMed] [Google Scholar]
- 4.Colombini M, VDAC: The channel at the interface between mitochondria and the cytosol. Mol Cell Biochem, 2004. 256(1-2): p. 107–115. [DOI] [PubMed] [Google Scholar]
- 5.Hajnoczky G, Csordas G, and Yi M, Old players in a new role: mitochondria-associated membranes, VDAC, and ryanodine receptors as contributors to calcium signal propagation from endoplasmic reticulum to the mitochondria. Cell Calcium, 2002. 32(5-6): p. 363–77. [DOI] [PubMed] [Google Scholar]
- 6.Hill K, et al. Tom40 forms the hydrophilic channel of the mitochondrial import pore for preproteins [see comment]. Nature, 1998. 395(6701): p. 516–21. [DOI] [PubMed] [Google Scholar]
- 7.Kuszak AJ, et al. Evidence of Distinct Channel Conformations and Substrate Binding Affinities for the Mitochondrial Outer Membrane Protein Translocase Pore Tom40. J Biol Chem, 2015. 290(43): p. 26204–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Araiso Y, et al. Structure of the mitochondrial import gate reveals distinct preprotein paths.Nature, 2019. 575(7782): p. 395–401. [DOI] [PubMed] [Google Scholar]
- 9.Kirichok Y, Krapivinsky G, and Clapham DE, The mitochondrial calcium uniporter is a highly selective ion channel. Nature, 2004. 427(6972): p. 360–4. [DOI] [PubMed] [Google Scholar]
- 10.Boyman L and Lederer WJ, How the mitochondrial calcium uniporter complex (MCUcx) works. Proc Natl Acad Sci U S A, 2020. 117(37): p. 22634–22636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fieni F, et al. Activity of the mitochondrial calcium uniporter varies greatly between tissues. Nat Commun, 2012. 3: p. 1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Streb H, et al. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature, 1983. 306(5938): p. 67–9. [DOI] [PubMed] [Google Scholar]
- 13.Csordas G, et al. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell, 2010. 39(1): p. 121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szabadkai G, et al. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol, 2006. 175(6): p. 901–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan W and Colombini M, VDAC closure increases calcium ion flux. Biochim Biophys Acta, 2007. 1768(10): p. 2510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Stefani D, et al. VDAC1 selectively transfers apoptotic Ca2+ signals to mitochondria. Cell Death Differ, 2012. 19(2): p. 267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rapizzi E, et al. Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J Cell Biol, 2002. 159(4): p. 613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Israelson A, et al. Localization of the voltage-dependent anion channel-1 Ca2+-binding sites. Cell Calcium, 2007. 41(3): p. 235–244. [DOI] [PubMed] [Google Scholar]
- 19.Zaid H, et al. The voltage-dependent anion channel-1 modulates apoptotic cell death. Cell Death Differ, 2005. 12: p. 751–60. [DOI] [PubMed] [Google Scholar]
- 20.Bathori G, et al. Ca2+-dependent control of the permeability properties of the mitochondrial outer membrane and voltage-dependent anion-selective channel (VDAC). J Biol Chem, 2006. 281(25): p. 17347–58. [DOI] [PubMed] [Google Scholar]
- 21.Clapham DE, Calcium signaling. Cell, 2007. 131(6): p. 1047–58. [DOI] [PubMed] [Google Scholar]
- 22.Arruda AP and Hotamisligil GS, Calcium Homeostasis and Organelle Function in the Pathogenesis of Obesity and Diabetes. Cell Metab, 2015. 22(3): p. 381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pacher P, Thomas AP, and Hajnoczky G, Ca2+ marks: miniature calcium signals in single mitochondria driven by ryanodine receptors. Proc Natl Acad Sci U S A, 2002. 99(4): p. 2380–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lock JT and Parker I, IP3 mediated global Ca(2+) signals arise through two temporally and spatially distinct modes of Ca(2+) release. Elife, 2020. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng H and Lederer WJ, Calcium sparks. Physiol Rev, 2008. 88(4): p. 1491–545. [DOI] [PubMed] [Google Scholar]
- 26.Robb-Gaspers LD, et al. Coupling between cytosolic and mitochondrial calcium oscillations: role in the regulation of hepatic metabolism. Biochim Biophys Acta, 1998. 1366(1-2): p. 17–32. [DOI] [PubMed] [Google Scholar]
- 27.Szalai G, Krishnamurthy R, and Hajnoczky G, Apoptosis driven by IP(3)-linked mitochondrial calcium signals. EMBO J, 1999. 18(22): p. 6349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lederer JW, et al. Ca2+ sparks in heart muscle. J Muscle Res Cell Motil, 2004. 25(8): p. 602–3. [PubMed] [Google Scholar]
- 29.Hung V, et al. Proteomic mapping of cytosol-facing outer mitochondrial and ER membranes in living human cells by proximity biotinylation. Elife, 2017. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong YC, Ysselstein D, and Krainc D, Mitochondria-lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature, 2018. 554(7692): p. 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng W, Wong YC, and Krainc D, Mitochondria-lysosome contacts regulate mitochondrial Ca(2+) dynamics via lysosomal TRPML1. Proc Natl Acad Sci U S A, 2020. 117(32): p. 19266–19275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bassi MT, et al. Cloning of the gene encoding a novel integral membrane protein, mucolipidin- and identification of the two major founder mutations causing mucolipidosis type IV. Am J Hum Genet, 2000. 67(5): p. 1110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun M, et al. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum Mol Genet, 2000. 9(17): p. 2471–8. [DOI] [PubMed] [Google Scholar]
- 34.Ujwal R, et al. The crystal structure of mouse VDAC1 at 2.3 angstrom resolution reveals mechanistic insights into metabolite gating. Proc Natl Acad Sci U S A, 2008. 105(46): p. 17742–17747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hiller S, et al. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science, 2008. 321(5893): p. 1206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bayrhuber M, et al. Structure of the human voltage-dependent anion channel. Proc. Natl. Acad. Sci. U S A, 2008. 105(40): p. 15370–15375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colombini M, The published 3D structure of the VDAC channel: native or not? Trends Biochem. Sci, 2009. 34(8): p. 382–389. [DOI] [PubMed] [Google Scholar]
- 38.Villinger S, et al. Functional dynamics in the voltage-dependent anion channel. Proc Natl Acad Sci U S A, 2010. 107(52): p. 22546–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Briones R, et al. Voltage Dependence of Conformational Dynamics and Subconducting States of VDAC-1. Biophys J, 2016. 111(6): p. 1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaremko M, et al. High-Resolution NMR Determination of the Dynamic Structure of Membrane Proteins. Angew Chem Int Ed Engl, 2016. 55(35): p. 10518–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Budelier MM, et al. Photoaffinity labeling with cholesterol analogues precisely maps a cholesterol-binding site in voltage-dependent anion channel-1. J Biol Chem, 2017. 292(22): p. 9294–9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiser BP, et al. Computational investigation of cholesterol binding sites on mitochondrial VDAC. J Phys Chem B, 2014. 118: p. 9852–9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Budelier MM, et al. Click Chemistry Reagent for Identification of Sites of Covalent Ligand Incorporation in Integral Membrane Proteins. Anal Chem, 2017. 89(4): p. 2636–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng WWL, et al. Multiple neurosteroid and cholesterol binding sites in voltage-dependent anion channel-1 determined by photo-affinity labeling. Biochim Biophys Acta Mol Cell Biol Lipids, 2019. 1864(10): p. 1269–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Messina A, et al. VDAC isoforms in mammals. Biochim Biophys Acta, 2012. 1818: p. 1466–1476. [DOI] [PubMed] [Google Scholar]
- 46.Queralt-Martin M, et al. Assessing the role of residue E73 and lipid headgroup charge in VDAC1 voltage gating. Biochim Biophys Acta Bioenerg, 2019. 1860(1): p. 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosencrans WM, et al. α-Synuclein emerges as a potent regulator of VDAC-facilitated calcium transport. Cell Calcium, 2021. (in press)( 10.1101/2020.11.15.383729). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rostovtseva TK, et al. Targeting the Multiple Physiologic Roles of VDAC With Steroids and Hydrophobic Drugs. Front Physiol, 2020. 11: p. 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rovini A, et al. Molecular mechanism of olesoxime-mediated neuroprotection through targeting alpha-synuclein interaction with mitochondrial VDAC. Cell Mol Life Sci, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naghdi S and Hajnoczky G, VDAC2-specific cellular functions and the underlying structure. Biochim Biophys Acta, 2016. 1863(10): p. 2503–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rahmani Z, Maunoury C, and Siddiqui A, Isolation of a novel human voltage-dependent anion channel gene. Eur J Hum Genet, 1998. 6(4): p. 337–40. [DOI] [PubMed] [Google Scholar]
- 52.Cheng EH, et al. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science, 2003. 301(5632): p. 513–7. [DOI] [PubMed] [Google Scholar]
- 53.Chin HS, et al. VDAC2 enables BAX to mediate apoptosis and limit tumor development. Nat Commun, 2018. 9(1): p. 4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baines CP, et al. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol, 2007. 9(5): p. 550–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anflous-Pharayra K, et al. VDAC3 has differing mitochondrial functions in two types of striated muscles. Biochim Biophys Acta, 2011. 1807(1): p. 150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sampson MJ, et al. Immotile sperm and infertility in mice lacking mitochondrial voltage-dependent anion channel type 3. J Biol Chem, 2001. 276(42): p. 39206–12. [DOI] [PubMed] [Google Scholar]
- 57.Queralt-Martin M, et al. A lower affinity to cytosolic proteins reveals VDAC3 isoform-specific role in mitochondrial biology. J Gen Physiol, 2020. 152(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reina S and De Pinto V, Anti-Cancer Compounds Targeted to VDAC: Potential and Perspectives. Curr Med Chem, 2017. 24(40): p. 4447–4469. [DOI] [PubMed] [Google Scholar]
- 59.Harada T, et al. Palmitoylated CKAP4 regulates mitochondrial functions through an interaction with VDAC2 at ER-mitochondria contact sites. J Cell Sci, 2020. 133(21). [DOI] [PubMed] [Google Scholar]
- 60.Liu Y, et al. DJ-1 regulates the integrity and function of ER-mitochondria association through interaction with IP3R3-Grp75-VDAC1. Proc Natl Acad Sci U S A, 2019. 116(50): p. 25322–25328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Min CK, et al. Coupling of ryanodine receptor 2 and voltage-dependent anion channel 2 is essential for Ca(2)+ transfer from the sarcoplasmic reticulum to the mitochondria in the heart. Biochem J, 2012. 447(3): p. 371–9. [DOI] [PubMed] [Google Scholar]
- 62.Gurnev PA, Rostovtseva TK, and Bezrukov SM, Tubulin-blocked state of VDAC studied by polymer and ATP partitioning. FEBS Lett., 2011. 585(14): p. 2363–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blachly-Dyson E, et al. Probing the structure of the mitochondrial channel, VDAC, by site-directed mutagenesis: a progress report. J Bioenerg Biomembr, 1989. 21(4): p. 471–83. [DOI] [PubMed] [Google Scholar]
- 64.Colombini M, Blachly-Dyson E, and Forte M, VDAC, a channel in the outer mitochondrial membrane. Ion Channels, 1996. 4: p. 169–202. [DOI] [PubMed] [Google Scholar]
- 65.Rostovtseva TK, Tan W, and Colombini M, On the role of VDAC in apoptosis: fact and fiction. J Bioenerg Biomembr, 2005. 37(3): p. 129–42. [DOI] [PubMed] [Google Scholar]
- 66.Colombini M, Voltage gating in the mitochondrial channel, VDAC. J Membr Biol, 1989. 111(2): p. 103–111. [DOI] [PubMed] [Google Scholar]
- 67.Blachly-Dyson E, et al. Selectivity changes in site-directed mutants of the VDAC ion channel: structural implications. Science, 1990. 247(4947): p. 1233–6. [DOI] [PubMed] [Google Scholar]
- 68.Gincel D, Zaid H, and Shoshan-Barmatz V, Calcium binding and translocation by the voltage-dependent anion channel: a possible regulatory mechanism in mitochondrial function. Biochem J, 2001. 358(Pt 1): p. 147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zambrowicz EB and Colombini M, Zero-current potentials in a large membrane channel: a simple theory accounts for complex behavior. Biophys J, 1993. 65(3): p. 1093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lemeshko VV, VDAC electronics: 2. A new, anaerobic mechanism of generation of the membrane potentials in mitochondria. Biochim Biophys Acta, 2014. 1838(7): p. 1801–8. [DOI] [PubMed] [Google Scholar]
- 71.Lemeshko VV, VDAC electronics: 5. Mechanism and computational model of hexokinase-dependent generation of the outer membrane potential in brain mitochondria. Biochim Biophys Acta Biomembr, 2018. 1860(12): p. 2599–2607. [DOI] [PubMed] [Google Scholar]
- 72.Kanwar P, et al. VDAC and its interacting partners in plant and animal systems: an overview. Crit Rev Biotechnol, 2020. 40(5): p. 715–732. [DOI] [PubMed] [Google Scholar]
- 73.Caterino M, et al. Protein-protein interaction networks as a new perspective to evaluate distinct functional roles of voltage-dependent anion channel isoforms. Mol Biosyst, 2017. 13(12): p. 2466–2476. [DOI] [PubMed] [Google Scholar]
- 74.Al Jamal JA, Involvement of porin N, N-dicyclohexylcarbodiimide-reactive domain in hexokinase binding to the outer mitochondrial membrane. Protein J, 2005. 24: p. 1–8. [DOI] [PubMed] [Google Scholar]
- 75.Rostovtseva TK, et al. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proceedings of the National Academy of Sciences, 2008. 105(48): p. 18746–18751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rostovtseva TK, et al. alpha-Synuclein Shows High Affinity Interaction with Voltage-dependent Anion Channel, Suggesting Mechanisms of Mitochondrial Regulation and Toxicity in Parkinson Disease. J Biol Chem, 2015. 290(30): p. 18467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Magri A, et al. Hexokinase I N-terminal based peptide prevents the VDAC1-SOD1 G93A interaction and re-establishes ALS cell viability. Sci Rep, 2016. 6: p. 34802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Magri A and Messina A, Interactions of VDAC with Proteins Involved in Neurodegenerative Aggregation: An Opportunity for Advancement on Therapeutic Molecules. Curr Med Chem, 2017. 24(40): p. 4470–4487. [DOI] [PubMed] [Google Scholar]
- 79.Neumann D, et al. Two-color STED microscopy reveals different degrees of colocalization between hexokinase-I and the three human VDAC isoforms. PMC Biophys, 2010. 3(1): p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McEnery MW, et al. Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proceedings of the National Academy of Sciences, 1992. 89(8): p. 3170–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vander Heiden MG, et al. Bcl-xL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane. J Biol Chem, 2001. 276(22): p. 19414–9. [DOI] [PubMed] [Google Scholar]
- 82.Rostovtseva TK, et al. Bid, but not Bax, regulates VDAC channels. J Biol Chem, 2004. 279(14): p. 13575–83. [DOI] [PubMed] [Google Scholar]
- 83.Goedert M, Jakes R, and Spillantini MG, The Synucleinopathies: Twenty Years On. J Parkinsons Dis, 2017. 7(s1): p. S53–S71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Spillantini MG, et al. Alpha-synuclein in Lewy bodies. Nature, 1997. 388(6645): p. 839–40. [DOI] [PubMed] [Google Scholar]
- 85.Kruger R, Muller T, and Riess O, Involvement of alpha-synuclein in Parkinson’s disease and other neurodegenerative disorders. J Neural Transm (Vienna), 2000. 107(1): p. 31–40. [DOI] [PubMed] [Google Scholar]
- 86.Nussbaum RL, Genetics of Synucleinopathies. Cold Spring Harb Perspect Med, 2018. 8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reeve AK, et al. Aggregated alpha-synuclein and complex I deficiency: exploration of their relationship in differentiated neurons. Cell Death Dis, 2015. 6: p. e1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Elkon H, et al. Mutant and wild-type alpha-synuclein interact with mitochondrial cytochrome C oxidase. J Mol Neurosci, 2002. 18(3): p. 229–38. [DOI] [PubMed] [Google Scholar]
- 89.Apicco DJ, et al. The Parkinson’s disease-associated gene ITPKB protects against α-synuclein aggregation by regulating ER-to-mitochondria calcium release. Proc Natl Acad Sci U S A, 2021. 118(1): p. e2006476118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robotta M, et al. Alpha-synuclein binds to the inner membrane of mitochondria in an alpha-helical conformation. Chembiochem, 2014. 15(17): p. 2499–502. [DOI] [PubMed] [Google Scholar]
- 91.Li WW, et al. Localization of alpha-synuclein to mitochondria within midbrain of mice. Neuroreport, 2007. 18(15): p. 1543–6. [DOI] [PubMed] [Google Scholar]
- 92.Ludtmann MHR, et al. alpha-synuclein oligomers interact with ATP synthase and open the permeability transition pore in Parkinson’s disease. Nat Commun, 2018. 9(1): p. 2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Parihar MS, et al. Alpha-synuclein overexpression and aggregation exacerbates impairment of mitochondrial functions by augmenting oxidative stress in human neuroblastoma cells. Int J Biochem Cell Biol, 2009. 41(10): p. 2015–24. [DOI] [PubMed] [Google Scholar]
- 94.Nakamura K, et al. Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein. J Biol Chem, 2011. 286(23): p. 20710–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rostovtseva TK, et al. Lipids in Regulation of the Mitochondrial Outer Membrane Permeability, Bioenergetics, and Metabolism, in Molecular Basis for Mitochondrial Signaling. 2017. p. 185–215. [Google Scholar]
- 96.Gouarne C, et al. Olesoxime protects embryonic cortical neurons from camptothecin intoxication by a mechanism distinct from BDNF. Br J Pharmacol, 2013. 168(8): p. 1975–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rostovtseva TK and Bezrukov SM, Function and Regulation of Mitochondrial Voltage-Dependent Anion Channel, in Electrophysiology of Unconventional Channels and Pores,Delcour AH, Editor. 2015, Springer: Switzerland. p. 3–31. [Google Scholar]
- 98.Hoogerheide DP, et al. Real-Time Nanopore-Based Recognition of Protein Translocation Success. Biophys J, 2018. 114(4): p. 772–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoogerheide DP, et al. Mechanism of alpha-synuclein translocation through a VDAC nanopore revealed by energy landscape modeling of escape time distributions. Nanoscale, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Calí T, et al. α-Synuclein controls mitochondrial calcium homeostasis by enhancing endoplasmic reticulum-mitochondria interactions. Journal of Biological Chemistry, 2012. 287(22): p. 17914–17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Distler AM, Kerner J, and Hoppel CL, Post-translational modifications of rat liver mitochondrial outer membrane proteins identified by mass spectrometry. Biochim Biophys Acta, 2007. 1774(5): p. 628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen Y, Craigen WJ, and Riley DJ, Nek1 regulates cell death and mitochondrial membrane permeability through phosphorylation of VDAC1. Cell Cycle, 2009. 8(2): p. 257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ham SJ, et al. Decision between mitophagy and apoptosis by Parkin via VDAC1 ubiquitination. Proc Natl Acad Sci U S A, 2020. 117(8): p. 4281–4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang Y, et al. Nedd4 ubiquitylates VDAC2/3 to suppress erastin-induced ferroptosis in melanoma. Nat Commun, 2020. 11(1): p. 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Piroli GG, et al. Succination is Increased on Select Proteins in the Brainstem of the NADH dehydrogenase (ubiquinone) Fe-S protein 4 (Ndufs4) Knockout Mouse, a Model of Leigh Syndrome. Mol Cell Proteomics, 2016. 15(2): p. 445–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Martel C, et al. Glycogen synthase kinase 3-mediated voltage-dependent anion channel phosphorylation controls outer mitochondrial membrane permeability during lipid accumulation. Hepatology, 2013. 57(1): p. 93–102. [DOI] [PubMed] [Google Scholar]
- 107.Haloi N, et al. Structural Basis of Complex Formation Between Mitochondrial Anion Channel VDAC1 and Hexokinase-II. bioRxiv, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pastorino JG, Hoek JB, and Shulga N, Activation of glycogen synthase kinase 3ϐ disrupts the binding of hexokinase II to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Cancer research, 2005. 65(22): p. 10545–10554. [DOI] [PubMed] [Google Scholar]
- 109.Ciscato F, et al. Hexokinase 2 displacement from mitochondria-associated membranes prompts Ca2+-dependent death of cancer cells. EMBO reports, 2020: p. e49117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Keinan N, et al. The role of calcium in VDAC1 oligomerization and mitochondria-mediated apoptosis. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 2013. 1833(7): p. 1745–1754. [DOI] [PubMed] [Google Scholar]
- 111.Weisthal S, et al. Ca(2+)-mediated regulation of VDAC1 expression levels is associated with cell death induction. Biochim Biophys Acta, 2014. 1843(10): p. 2270–81. [DOI] [PubMed] [Google Scholar]
- 112.Mannella CA, Structure of the outer mitochondrial membrane: ordered arrays of porelike subunits in outer-membrane fractions from Neurospora crassa mitochondria. J Cell Biol, 1982. 94(3): p. 680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Goncalves RP, et al. Supramolecular assembly of VDAC in native mitochondrial outer membranes. J Mol Biol, 2007. 369(2): p. 413–8. [DOI] [PubMed] [Google Scholar]
- 114.Raschle T, et al. Structural and functional characterization of the integral membrane protein VDAC-1 in lipid bilayer nanodiscs. J Am Chem Soc, 2009. 131(49): p. 17777–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ujwal R, et al. Crystal packing analysis of murine VDAC1 crystals in a lipidic environment reveals novel insights on oligomerization and orientation. Channels (Austin), 2009. 3(3): p. 167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bergdoll LA, et al. Protonation state of glutamate 73 regulates the formation of a specific dimeric association of mVDAC1. Proc Natl Acad Sci U S A, 2018. 115(2): p. E172–E179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thai PN, et al. Cardiac-specific Conditional Knockout of the 18-kDa Mitochondrial Translocator Protein Protects from Pressure Overload Induced Heart Failure. Sci Rep, 2018. 8(1): p. 16213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gatliff J and Campanella M, The 18 kDa translocator protein (TSPO): a new perspective in mitochondrial biology. Curr Mol Med, 2012. 12(4): p. 356–68. [DOI] [PubMed] [Google Scholar]
- 119.Pastorino JG, Hoek JB, and Shulga N, Activation of glycogen synthase kinase 3beta disrupts the binding of hexokinase II to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Cancer Res, 2005. 65(22): p. 10545–54. [DOI] [PubMed] [Google Scholar]
- 120.Gomez L, et al. The SR/ER-mitochondria calcium crosstalk is regulated by GSK3beta during reperfusion injury. Cell Death Differ, 2016. 23(2): p. 313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Viola HM, et al. Impaired functional communication between the L-type calcium channel and mitochondria contributes to metabolic inhibition in the mdx heart. Proc Natl Acad Sci U S A, 2014. 111(28): p. E2905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu T and O’Rourke B, Enhancing mitochondrial Ca2+ uptake in myocytes from failing hearts restores energy supply and demand matching. Circ Res, 2008. 103(3): p. 279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Subedi KP, et al. Voltage-dependent anion channel 2 modulates resting Ca(2)+ sparks, but not action potential-induced Ca(2)+ signaling in cardiac myocytes. Cell Calcium, 2011. 49(2): p. 136–43. [DOI] [PubMed] [Google Scholar]
- 124.Shimizu H, et al. Mitochondrial Ca(2+) uptake by the voltage-dependent anion channel 2 regulates cardiac rhythmicity. Elife, 2015. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wilting F, et al. The antiarrhythmic compound efsevin directly modulates voltage-dependent anion channel 2 by binding to its inner wall and enhancing mitochondrial Ca(2+) uptake. Br J Pharmacol, 2020. 177(13): p. 2947–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]


