Abstract
Glial subtype diversity is an emerging topic in neurobiology and immune-mediated neurological diseases such as multiple sclerosis (MS). We discuss recent conceptual and technological advances that allow a better understanding of the transcriptomic and functional heterogeneity of oligodendrocytes (OLs), astrocytes, and microglial cells under inflammatory–demyelinating conditions. Recent single cell transcriptomic studies suggest the occurrence of novel homeostatic and reactive glial subtypes and provide insight into the molecular events during disease progression. Multiplexed RNA in situ hybridization has enabled ‘mapping back’ dysregulated gene expression to glial subtypes within the MS lesion microenvironment. These findings suggest novel homeostatic and reactive glial cell type function both in immune-related processes and neuroprotection relevant to understanding the pathology of MS.
Tools and Concepts for Glial Subtype-Specific Transcriptomic Profiling
Multiplex RNA transcriptomic technologies, introduced over two decades ago, have revolutionized the investigation of biology and human disease. Recently, techniques such as single-cell and single-nuclei RNA-sequencing (scRNA-seq and snRNA-seq; see Glossary) and spatial transcriptomics have been applied to study human brain gene regulation with high resolution (Figure 1). This has been a major advantage over previous ex vivo approaches that do not reflect the transcriptomic state of tissue resident cell types, considering the tissue microenvironment and their interaction with surrounding cells [1,2]. Classical ex vivo approaches have also favored studying myeloid cell types including monocytes and brain macrophages, which are easier to isolate and examine in culture than neurons or macroglial cells. It is now feasible to disentangle complex molecular and cell-type-specific diversity of neuroglial, innate, and adaptive immune cells under neuroinflammatory conditions, such as multiple sclerosis (MS) in humans. MS pathology is highly heterogeneous [3] and affects both gray matter (GM) and white matter (WM) areas of the central nervous system (CNS) [4].
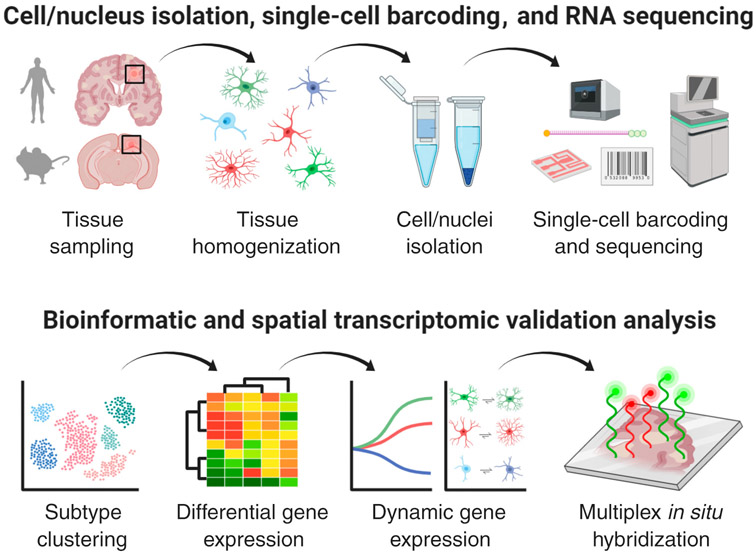
Figure 1. Tools to Perform Glial Cell Type-Specific Transcriptomic Profiling.
Upper panel illustrates high-throughput single-cell/nucleus RNA-sequencing (sc/snRNA-seq) discovery platform starting from glial cells/nuclei isolated from human multiple sclerosis (MS) or mouse experimental autoimmune encephalomyelitis (EAE) brain tissues with the presence of focal inflammatory–demyelinating lesions. Lower panel depicts bioinformatics analysis platform to analyze sc/snRNA-seq data featuring glial subtype clustering, differential (e.g., MS/EAE reactive versus healthy homeostatic subtypes) and dynamic (pseudotime/progressive) trajectory gene expression analysis followed by multiplex in situ RNA hybridization to visualize cell-type-specific transcriptomic changes in the inflamed lesion microenvironment. This figure was created using BioRender (https://biorender.com/).
Until recently, it has not been possible to dissect the cell-type-specific molecular changes within lesion and non-lesion areas in an unbiased way. With respect to MS and experimental autoimmune encephalomyelitis (EAE), a widely used MS model in rodents, snRNA-seq has proven a good method to isolate individual nuclei from frozen tissues for neuron and glial-cell-type-specific transcriptomic profiling [5,6]. scRNA-seq has proven to be a robust technique to isolate and sequence cells lacking long processes from fresh tissues using flow cytometric cell sorting, such as myeloid cells including microglia [1,7,8], astrocytes [9], and oligodendrocyte progenitor cells (OPCs) [10]. In combination with transgenic mouse models, cell-type-specific CNS gene expression studies have enabled research into early and late phases of neuroinflammation and make it possible to address certain questions related to subtype-specific reactive glial cell states along this temporal trajectory. Additionally, key transcriptomic features have been mapped back to the inflamed human MS tissue by spatial profiling using multiplex RNA in situ hybridization techniques [5,6,8] and imaging mass cytometry [11,12]. This unprecedented, detailed view of the cell-type-specific transcriptomic landscape has resulted in a new understanding of reactive glial subtypes at various inflammatory lesion stages in time and space. However, deciphering such complexity has challenges.
Several issues arise when conceptualizing strategies to deconvolute the cellular and molecular landscape of inflammatory demyelination in both human MS and respective animal models. A major consideration is the relationship between shared and divergent glial-cell-type-specific pathologies across different anatomical locations and inflammatory lesion stages. For example, glial subtype diversity becomes crucial during an inflammatory insult with a shift from a homeostatic/resting to a reactive/disease state, which then may have dichotomous fates, with either reparation or continued effector pathology that ultimately can contribute to dictating the tissue response and fate during lesion progression (Figure 2, Key Figure). It is likely that these cell-type-specific trajectories during lesion progression are inextricably linked to the spatial position of the cell type within the surrounding microenvironment. In particular, both homeostatic and reactive glial subtypes can occur side by side in normal-appearing (homeostatic) and demyelinating lesion (reactive) areas with respect to MS pathology.
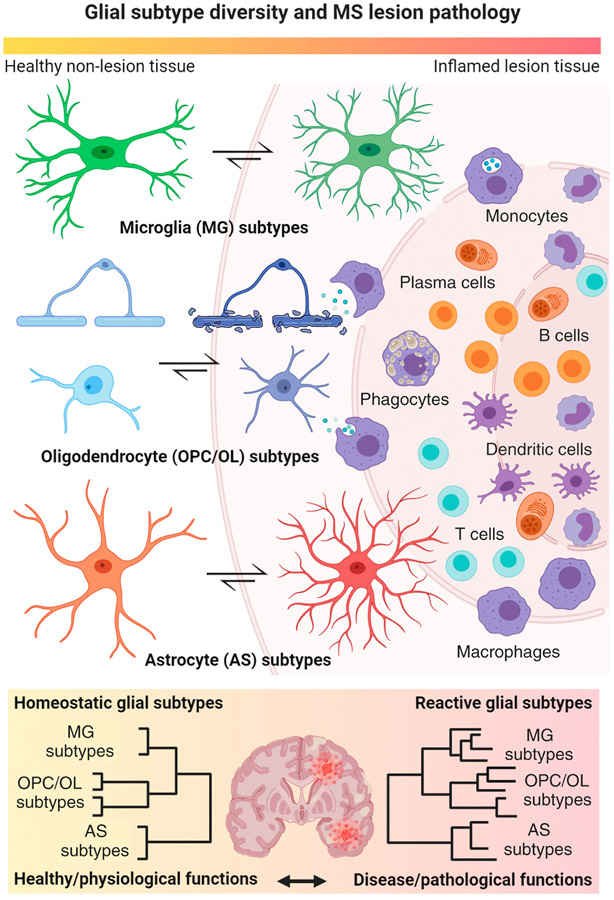
Figure 2. Key Figure. Homeostatic and Reactive Glial Cell Type Diversity in Inflammatory Demyelination.
Main cartoon highlights dynamic transformative states of glial cell types (left center) in response to a focal inflammatory–demyelinating lesion as regularly seen in multiple sclerosis (MS) with blood–brain barrier breakdown and immune cell infiltration into the surrounding parenchyma (right). Note a gradual morphological transformation of glial subtypes from a homeostatic (left) physiological to a reactive pathological state (center) relative to the inflamed lesion area. Under inflammatory–demyelinating conditions, astrocytes, and myeloid cells such as microglia regularly transform into reactive subtypes with enlarged cell bodies and retracted processes. Conversely, oligodendrocyte (OL) lineage cells including OL precursor cells (OPCs) and myelinating cells (OLs) become reactive and exhibit high metabolic stress. Note that most of them undergo cell death close to the demyelinating lesion rim. Lower panel illustrates increased subtype diversity in reactive glial cell populations isolated from inflamed MS brains compared with homeostatic subtype diversity in healthy brains by means of hierarchical clustering with respect to physiological versus pathological subtype functions. This figure was created using BioRender (https://biorender.com/).
The purpose of this review is to provide a comprehensive overview of glial subtype diversity (Box 1) in inflammatory demyelination with an emphasis on transcriptomic profiling in human MS and its animal models (Figure 3). We summarize the present knowledge of the spatial and temporal trajectories of homeostatic and reactive glial cell types under inflammatory–demyelinating conditions and how they can contribute to tissue damage and remodeling in MS (Figure 4). We review recent findings that provide novel perspectives on cell-type-specific pathological mechanisms that might be targeted by rational therapeutics in MS, as well as in other chronic inflammatory–demyelinating diseases.
Box 1. CNS Glial Cell Types of Relevance in MS Pathology.
OLs are the myelinating cells of the CNS and originate from OPCs. Together with astrocytes, these macroglial cells comprise neuroepithelial derivatives as compared with microglial cells. As demyelination is the pathological hallmark in MS, OLs have long been recognized as natural targets in MS pathobiology. Under physiological conditions, myelin functions in axonal ensheathment and supports saltatory conduction and axonal integrity under developmental and homeostatic conditions [13]. Recent cell-type-specific gene expression studies have revealed a high level of diversity during development [26,157] and disease conditions such as in human MS [5,6] and mouse EAE [10,16]. In particular, the finding that reactive OLs express immune-associated markers related to antigen presentation and immune cell migration, suggests the possibility that OL lineage cells are not just targets but also active players in disease progression and tissue inflammation in MS [5,6,10,16].
Astrocytes are the most abundant glial cell population in the mammalian CNS with crucial and highly specialized roles to support neurons, prune excitatory synapses [105], establish and maintain network circuitry, as well as saltatory conduction and ion and water homeostasis along fiber tracts [158]. These functions are compromised under inflammatory conditions, in which astrocytes need to compensate for extracellular disturbances in the concentration of various soluble factors such as neurotransmitters, as well as water and ions [77]. In this sense, astrocytes can also be direct targets of the immune response; for example, as in the context of NMO; a human disease characterized by antibodies against the water channel protein AQP4 [99]. Astrocytes can be activated through microglia or other immune cell subtypes that eventually result in transcriptomic and morphological changes, such as swelling and secretion of inflammatory agents including complement factors in both rodents and humans [68,159,160].
Microglia are yolk-sac-derived myeloid cells of the mammalian CNS with an array of homeostatic functions during development and normal brain function, with essential roles in regulating synapse development, pruning, and excitability, and serving as one of the primary phagocytes and regulators of inflammation [107,108]. They have emerged as key players in the initiation and spread of inflammation across multiple neurological diseases. This is reflected in myriad new studies pointing to microglia as key cellular determinants of demyelinating disease progression, neurodegeneration, and remyelination potential [107,108,117]. Besides being possible drivers of disease progression, subtypes of microglia are now being considered as part of the underlying etiology of several neurological diseases including MS [161-163].
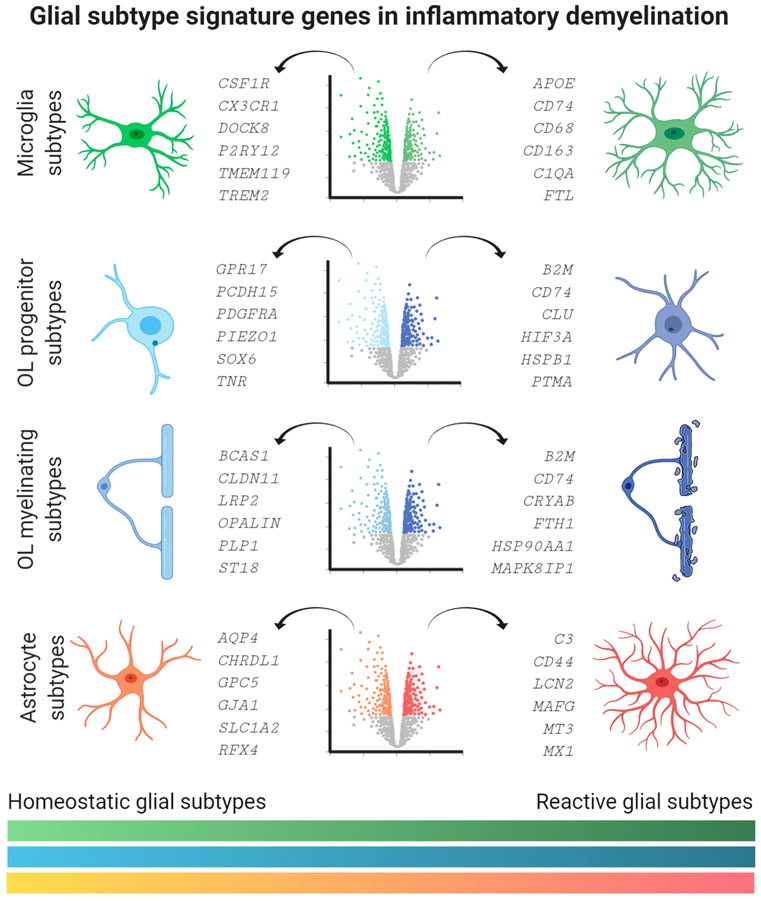
Figure 3. Human Transcriptomic Marker Genes Characterizing Homeostatic and Reactive Glial Subtypes.
Cartoon lists homeostatic (left) versus reactive (right) glial subtype signature genes by means of volcano plot visualization. Marker genes were selected by their specific enrichment in microglia, oligodendrocyte (OL) precursor, and myelinating cells, as well as astrocytes based on recent single-cell/nucleus RNA-sequencing (sc/snRNA-seq) studies from human control and multiple sclerosis (MS) tissues. See Boxes 2-4 in the main text for details about signature genes and their functional relevance. Note color spectrum on the bottom illustrates dynamic changes in gene expression between homeostatic and reactive glial subtypes according to cell type color code. This figure was created using BioRender (https://biorender.com/).
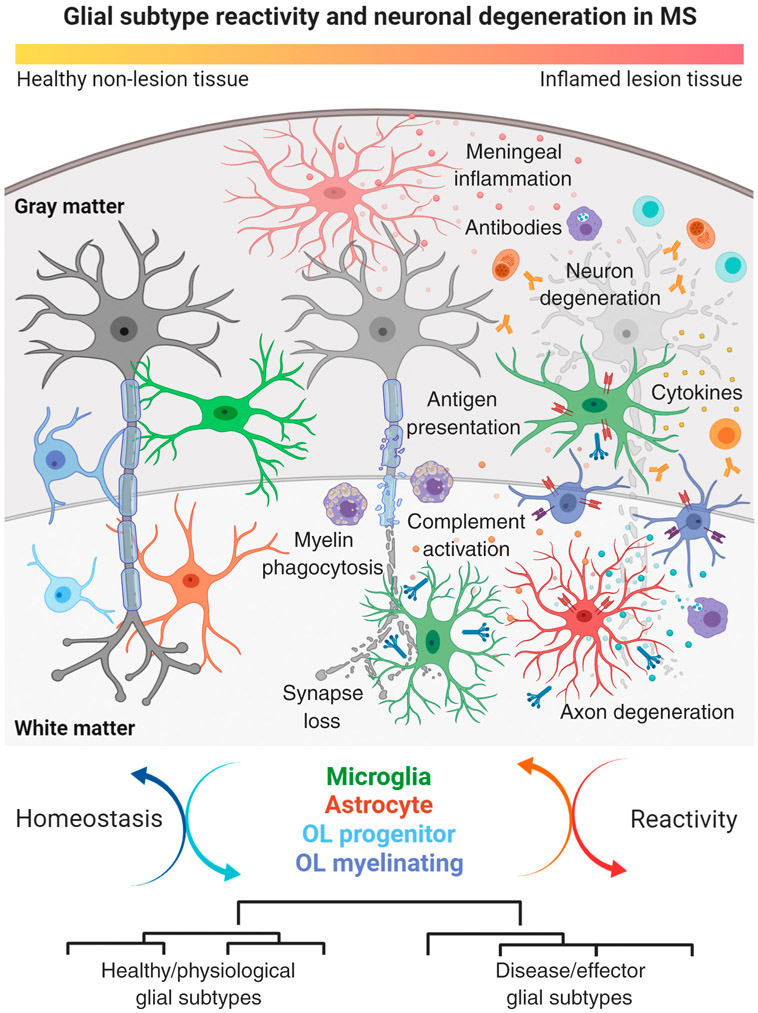
Figure 4. Morphological and Functional Changes in Homeostatic and Reactive Glial Subtypes Relative to Neuron Damage in Multiple Sclerosis (MS) Lesions.
Main cartoon illustrates the sequence of tissue damage in leukocortical (affecting both cortical gray matter and subcortical white matter) MS lesion with presence of infiltrating immune cell types. Note gradual neuronal degeneration from left (healthy state) to right (degenerated state) with axonal degeneration and synapse loss followed by retrograde injury and damage to neuronal cell bodies. Neuron degeneration is paralleled by morphological and functional changes in glial cell types including complement secretion and activation, antigen presentation via MHC class I and II, cytokine production, and complement factor release. Other factors are meningeal inflammation and tissue infiltration of monocyte and lymphocyte subtypes producing antibodies and cytokines, thus further corroborating tissue damage in MS lesion areas. Lower panel demonstrates dynamic cycling of glial subtype states between homeostatic and reactive conditions relative to the inflammatory stage of MS lesions. Note that different glial cells can transform from healthy/physiological subtypes into disease/effector subtypes. This figure was created using BioRender (https://biorender.com/). Abbreviation: OL, oligodendrocyte.
Subtype Diversity of Precursor and Myelinating OLs: Implications for MS and Experimental Inflammatory Demyelination
Isolation of intact OL lineage cells, including precursor and myelinating cells, from brain tissues is challenging and possibly the reason why less information about molecular subtype-specific markers is available, compared with cells of the myeloid cell lineage. However, recent studies have shown diverse functions for OPCs in health and demyelination [13]. In response to an inflammatory insult, OPCs, such as NG2 progenitor cells, with a core set of physiological properties including expression of voltage-gated Na+ channels and ionotropic glutamate receptors [13], have the potential to become reactive and ‘inflammatory’ cells (Figure 2). These cells are characterized by their capacity to release chemokines, as well as execute phagocytosis and antigen cross-presentation to cytotoxic CD8+ T cells, thus firmly establishing them as a major components of the innate immune response in the CNS of both mice and humans [10, 14-17]. In addition, OPCs have diverse homeostatic functions, including angiogenesis [18], the formation of synaptic connections with neurons, the production of the neurotransmitter glutamate, and the capacity to provide energy in the form of lactate for neurons via monocarboxylate transporters [19-21] – a scenario that becomes important under chronic hypoxic tissue damage as observed in MS lesions [22]. Another key function of homeostatic OLs is ion buffering and maintenance of axon integrity through potassium homeostasis by channels such as KIR4.1 [23,24]; this is relevant as the expression of this channel has been reported to be downregulated at the protein and RNA levels in chronic MS lesion areas in postmortem brain tissues relative to healthy controls [6,25].
Initial RNA-seq studies of postnatal OPCs taken from the brain and spinal cord of mice suggested a remarkable degree of similarity in transcriptomic profiles [26]. However, recent work using sc/snRNA-seq and proteomic studies has highlighted a high level of transcriptomic heterogeneity comprising several immature OPC and myelinating OL subtypes (Box 2, Figure 3), especially under chronic inflammatory–demyelinating conditions such as mouse EAE and human MS [5,10,27]. Although subtype-specific transcriptomic profiling of OL lineage cells helps understand the diversity across anatomical regions and lesion versus non-lesion areas, future studies need to assess if the diversity in gene expression translates into functional heterogeneity of subtypes in health and disease.
Box 2. OL Transcriptomic Diversity in Inflammatory Demyelination.
Utilizing scRNA-seq in human tissues, it became feasible to precisely dissect heterogeneity of the OL lineage with the identification of several progenitor (BCAN, PCDH15, PDGFRA, PIEZO1, SOX6, and TNR) [5,26,44,157], transitional (BCAS1, ENPP6, and GPR17) [26], and mature (CLDN11, LRP2, OPALIN, PLP1, and ST18) [6,91] transcriptomic markers along developmental trajectories (see Figure 3 in the main text). For example, lineage-specific marker genes encode transcription factors (SOX6 and ST18) and proteins involved in cell–cell contact (CLDN11 and GPR17), sensing of environmental cues (BCAN, PIEZO1, and TNR) as well as lipid and myelin formation (LRP2, OPALIN, and PLP1). Key marker genes have been confirmed under functional [164] and inflammatory–demyelinating conditions [5,27,165,166] and by proteomic technologies [167]. Human studies utilizing snRNA-seq with tissues from deceased individuals diagnosed with MS [5,6], Alzheimer’s disease [168], or major depression [169], have implicated a high number of dysregulated genes in the OL lineage with disease. In MS and EAE, several disease-specific enriched genes and pathways are related to antigen presentation (B2M and CD74) [5,6,10,16], iron metabolism (FTH1 and FTL) [5,170], and severe cell stress (CRYAB, HSP90AA1, and MAPK8IP1) [5,6].
From another angle, under inflammatory–demyelinating conditions (lesions) in both mice and human MS, the exposure of OPCs to cytokines such as interferon (IFN)-γ can suppress differentiation into mature OLs; it can also activate canonical cytokine transcriptional pathways in mice [28,29]. Of note, OPCs can also phagocytose antigens, which then undergo endolysosomal processing resulting in the functional presentation on MHC class I and II molecules and subsequent activation of CD8+ and CD4+ T cells (Figure 3) [10,16,30]. Moreover, the specific activation of immunoproteasome subunits in OPCs by IFN-γ mediates crosspresentation of exogenous antigens to cytotoxic CD8+ T cells, which in turn can kill OPCs as target cells, as shown in an adoptive transfer T cell mouse model [16]. While the capacity of antigen presentation is well described in dendritic cells (DCs), it appears that OPCs retain this capacity perhaps as an intrinsic mechanism to facilitate clearance of virus during CNS infections [15,31,32]. Crosspresentation is advantageous in the short term for clearing pathogens but in the setting of chronic inflammation, as it occurs in autoimmune diseases such as MS, it could be deleterious and might potentially explain the paucity of OPCs and failed remyelination in some cases of MS (Figure 4).
In some pathological descriptions of MS postmortem tissues there are dysfunctional OPCs or premyelinating OLs, which fail to differentiate into mature myelin-producing OLs [33]. These may be transcriptionally reprogrammed to perpetuate inflammation, as demonstrated by the presence of OL lineage cells that are immunoreactive for the markers SOX10 and PSMB8, only in areas of demyelination in MS brain tissues [16]. The proliferative and repair capacity of OPCs can be further suppressed by contact-dependent inhibition with glycoprotein-expressing cells, such as neighboring NG2+ OPCs or CD44+ reactive astrocytes, even in areas without myelin; and also, by interfering with their beneficial role in tissue repair after experimental demyelination in mice, as shown in demyelinated MS lesions [34,35]. In addition, OPC differentiation and remyelination can be suppressed by glial released products such as chondroitin sulfate proteoglycans and hyaluronans, as shown in lysolecithin-induced demyelination [36,37]. Therefore, developing treatments that specifically target proteoglycans or interfere with proteoglycan signaling might provide an interesting option to ideally promote repair and OPC differentiation.
Recent work has highlighted the role of aging and cellular senescence in limiting remyelination by OPCs. Specifically, aged mice (10–12 months old) fail to remyelinate after focal lysolecithin-induced glial toxic injury due to impaired OPCs and can be rescued by heterochronic parabiosis with circulating factors from young mice (5–7 weeks old) including CCR2+ monocytes [38]. Indeed, the beneficial roles of transforming growth factor (TGF)-β, activin, insulin-like growth factor (IGF)-1, and interleukin (IL)-4I1, highlight the inducible nature of OPCs in response to alternatively activated macrophages [39-41]. Also, old mouse OPCs are less capable to remyelinate due to epigenetic restraints driven via inefficient histone deacetylase (HDAC) recruitment to relevant genomic regions [42]. Nonetheless, the net balance in disease, especially over time, is for remyelination to fail, and recent efforts suggest that dysfunctional metabolic pathways associated with aging may be an explanation, at least in part [43]. Indeed, OPC senescence in mice, which can be part of the normal aging process and might be related to increased tissue stiffness during aging via PIEZO1 signaling [44], could be rescued through induction of AMP kinase by metformin; a drug used to treat type 2 diabetes mellitus [45]. Ablating senescent OPCs with the senolytic compounds dasatinib and quercetin attenuates deficits in an Alzheimer's disease model (APP/PS1 mutant mice), relative to untreated mice [46]. On the one hand, whether intrinsic or acquired deficits in early precursor lineages also contribute to disease remains unclear; however, recent work with human induced pluripotent stem cells (iPSC) lines from individuals with MS raises the possibility that these cells may also be susceptible to becoming dysfunctional and have senescent or even toxic profiles [47-49]. On the other hand, human postmortem tissue studies have demonstrated that remyelination can be extensive in aged MS brains, albeit, without knowing when the remyelination had happened during their lifetime [35,50]. These age-related features are therefore important when considering the development of future candidate cell-based treatments using human iPSC cells to promote CNS repair in diseases such as MS.
In addition, these studies have raised the question of whether new myelin formation in MS lesions can originate from pre-existing old OLs or can be the consequence of enhanced OPC proliferation and differentiation of new OPCs into myelinating OLs [51]. This is of particular interest, as old OLs might exhibit a reduced repair capacity versus younger counterparts. To address this issue, individual nuclei from OL cells were isolated; genomic integration of 14C (radiocarbon dating) was measured in samples derived from human postmortem brain tissues, including different types of MS lesions [52]. As the presence of 14C indicated that these cells would have been present for decades, it has been concluded that the majority of OLs in MS lesions might originate from pre-existing, rather than dividing and newly generated cells. Such studies and MS lesion snRNA-seq data [6] support the conclusion that OLs may have a limited regenerative potential in chronic demyelinated MS lesions, perhaps due to OL differentiation block, or age-related limitations due to remyelination failure in MS [53], warranting further investigation.
In addition, genetic outside variants, SNPs with only weak linkage disequilibrium to risk genes identified in genome-wide association studies [54], may interact with risk SNPs to influence target gene expression mediating CNS-specific cell-intrinsic risk that might account for impaired OPC maturation and incomplete remyelination [55]. This highlights recent developments that aim to link genomic variants – potentially indicating a specific disease risk – to cell-type-specific functions and properties utilizing novel single-cell omics approaches [56,57]. Further, there is growing evidence that subsets of mature OLs and neurons exhibit overlapping features of immune activation and oxidative stress in response to the perpetual inflammation and demyelination occurring in lesion and non-lesion areas [6]. Stressed and degenerating OLs at the lesion rim express MHC class I and express genes associated with iron overload similar to myeloid cell subtypes [6] (Figure 2), and it is likely that they contribute to the persistent ‘phase rim’ seen on susceptibility-weighted magnetic resonance imaging (MRI) in chronic-active MS lesions [58]. Humoral factors secreted by lymphoid aggregates in the meninges are another pathological feature that can further contribute to OL pathology and subpial demyelination, a hallmark of cortical lesion pathology in MS [59]. Finally, there is evidence that motor learning can stimulate and enhance the remyelinating capacity during development experimental demyelination in mice, providing additional insight into the putative beneficial properties of OLs during inflammatory demyelination [60,61].
In summary, OL lineage cells seem to be comprised of two distinct populations: an abundant proliferative OPC pool with diverse functions, and mature myelin producing OLs with large metabolic demands. It is reasonable to hypothesize that OPCs likely play an important supportive role in homeostasis but can also respond to tissue injury either by differentiating into new OLs or by facilitating immune responses, depending on microenvironmental cues. While it is well established that immune OPCs fail to make myelin and can promote cytotoxicity resulting in their depletion, it is unknown whether their transcriptional profile is associated with a terminally differentiated phenotype or is reversible upon resolution of inflammation. As a successful remyelination would not only afford recovery of rapid axonal conduction but also be neuroprotective, it therefore represents a pressing therapeutic goal in MS to ideally help improve clinical outcomes.
Astrocyte Subtype Diversity: Relevance for MS and Experimental Inflammatory Demyelination
Owing to their prominent histopathological contribution to MS, reactive or gliotic astrocytes [62] have become eponymous to the disease: the demyelinated core of the multiple focal lesions consists of a dense network of astrocytic processes, which build the sclerotic scar [3,63].
While traditionally thought to be a homogeneous population, transgenic mouse studies have shown diversified developmental programs, as well as diversified expression and functions of resting astrocytes [64-66]. Recent work in mice has demonstrated that reactive astrocytes can adopt at least two different polarization states based on their transcriptomic signature and functional properties, also depending on the injury modality [67,68] (Figure 2). Reactive astrocytes expressing complement factors such as C3 and Serping1, a regulator of the complement system [67], have been described as an ‘A1’ subtype that can be rapidly induced after systemic administration of lipopolysaccharide (LPS) in mice [67] and have been found in a subset of inflammatory MS lesions [68]. By contrast, other reactive astrocytes are linked to brain ischemia and were previously called A2 [67,68]. Other panreactive astrocyte markers such as Lcn2 and Cxcl10 are strongly induced in both A1 and A2 subtypes [67,68] and can facilitate the recruitment of T cells, as shown in loss-of-function studies in mouse EAE [69,70], providing further evidence that reactive astrocytes can actively modulate adaptive immune cell function [71,72]. In fact, inflammatory astrocytes rely on an intimate crosstalk with microglia and, with respect to the A1 subtype, can become polarized by microglia-mediated secretion of the proinflammatory cytokines IL-1α, tumor necrosis factor (TNF), and by C1q [68]. Notably, activation of nuclear factor (NF)-κB signaling in astrocytes can induce C3 production [73], and inhibition of the pathway has decreased cytokine and chemokine release [e.g., TNF-α, IFN-γ, and chemokine CC ligand (CCL)5] and has been shown to be neuroprotective, as evidenced from ameliorated pathology and neurological symptoms in mouse EAE [74].
Traditionally, the astroglial scar was thought to be an inhibitory barrier to repair and axonal regrowth in particular owing to the abundance of chondroitin sulfate proteoglycans [75]. However, recent work using transgenic mouse models to prevent the formation of astrocytic scars in spinal cord injury has revealed that reactive scar astrocytes can also promote neuronal plasticity and actively contribute to axonal regeneration in the presence of neurotrophic factors such as NT-3 and brain-derived neurotrophic factor (BDNF) [76]. Scar-forming astrocytes not only express axon-growth-supporting molecules but are also crucial in maintaining tissue integrity [76] and in shielding the spread of immune cell infiltration [77]. These features illustrate that reactive astrocytes can have beneficial functions in addition to their detrimental effects on enhancing tissue inflammation and neuronal damage, which might also be the case in MS (Figure 4).
With respect to cytokines and soluble factors, reactive astrocytes can respond to environmental and microglial stimuli through type I IFN and aryl hydrocarbon receptor (AHR) signaling as shown in EAE mice and MS lesions [78] or, as shown in a follow-up study in EAE mice, through ErbB1 and vascular endothelial growth factor receptor (VEGFR)1 signaling in microglia [79]. For example, microglia-driven Ahr loss-of-function strategies in EAE mice helped in the understanding of the crosstalk between astrocytes and microglia and how this essential interplay can be regulated by microbial metabolites in the context of inflammatory demyelination [79]. This sheds light on other recent studies in mice and humans suggesting that the microbiome can directly stimulate regulatory T and B cells, which can then migrate to the brain to regulate pathology in EAE and MS [80-82]. In another set of scRNA-seq and astrocyte-specific chromatin sequencing experiments in both mouse EAE and human MS, decreased Nrf2 expression and conversely increased Mafg expression were reported in EAE astrocytes relative to controls [9]. This suggested that certain reactive astrocytes might have a reduced capacity to protect against oxidative stress – an important pathological feature in MS/EAE pathology [83], as recently shown in a set of scRNA-seq studies in mouse EAE focusing on myeloid cell subtypes [84]. This proinflammatory astrocyte subtype can be expanded though granulocyte–macrophage colony-stimulating factor (GM-CSF), produced by T cells [9]. Therefore, astrocytes can be polarized in various ways, which can result in reciprocal responses that either promote (e.g., through VEGFR1 and MAFG) or control (e.g., through type I IFN and ErbB1) inflammation and scar formation.
Morphologically, astrocytes divide into two broad regionally restricted categories: protoplasmic astrocytes of GM and fibrous astrocytes of WM areas (Figure 4) [85]. Transcriptomic profiling in mice has suggested substantial molecular heterogeneity between astrocytes from different anatomical locations, including the cerebral cortex, olfactory bulb, hippocampus, striatum, thalamus, hypothalamus, midbrain, cerebellum, and spinal cord [72,86-92]. Therefore, it is likely that astrocyte diversity might be a key determinant of the fate of MS lesions that can occur throughout the entire CNS. However, it is unclear to what extent the discovery of astrocyte subtypes under homeostatic conditions translates into discrete region-specific reactive astrocyte signatures during inflammatory demyelination and whether there are actually disease and species-specific differences between rodents and humans with respect to EAE and MS. Recently, based on snRNA-seq and transcriptomic MS lesion mapping, dysregulated genes could be mapped back to either GM or WM astrocytes [6]. Accordingly, striking differences between cortical and subcortical astrocyte reactivity in MS lesions have been previously reported [35,72]. Specifically, GM astrocytes in cortical human MS lesions appeared to harbor decreased expression of genes involved in glutamate and potassium homeostasis (SLC1A2, GLUL, and KCNJ10), whereas WM astrocytes in subcortical lesion areas are characterized by upregulated marker genes GFAP and CD44, transcription factors BCL6 and FOS as well as EDNRB [6] (Box 3, Figure 3). Notably, endothelin receptor B (EDNRB) signaling has been reported to negatively regulate remyelination in focal lysolecithin-induced demyelination in mice [93], and CD44 is a receptor for hyaluronan that blocks OPC differentiation in mice [37].
Box 3. Astrocyte Transcriptomic Diversity in Inflammatory Demyelination.
Astrocyte-specific sorting and sequencing strategies have significantly helped establish a clearer picture of the transcriptomic signatures of both homeostatic and reactive cells in human MS and mouse EAE. By unsupervised snRNA-seq and multiplex RNA in situ hybridization profiling of human MS lesion and non-lesion tissue areas, subcortical WM astrocytes (CD44 and LINC01088) have been distinguished from cortical GM counterparts (GPC5 and SLC1A2), and pan-astrocyte gene signatures have been identified (ALDH1L1, AQP4, and RFX4) [6,95] with partial interspecies overlap. Under inflammatory–demyelinating disease states, the reactive single-cell astrocyte transcriptome undergoes dramatic changes. These changes are driven by a number of marker genes enriched in astrocyte subtypes as shown for MS and EAE by snRNA-seq [6] and scRNA-seq [9] workflows. Such marker genes encode transcription factors related to cell plasticity and immune function (BCL6, FOS, MAFG, and XBP1) [6,9,171] and genes related to interferon (AHR and MX1) [68,78], metal homeostasis (CP, LCN2, and MT3) [6,67,172], and growth factor signaling (ERBB1), as well as complement production (C3 and SERPING1) and extracellular matrix reorganization (CD44) [6,67,68]. At the same time, genes encoding homeostatic proteins are downregulated (GLUL and KCNJ10) [6].
These findings suggest that differences in WM and GM characteristics might contribute to different outcomes and severity of inflammatory-demyelinating lesions in MS, with or without underlying meningeal inflammation. Several reports in mice and humans indicate that marginal astrocytes in subpial areas of the cerebral cortex have unique properties distinct from protoplasmic astrocytes of other cortical layers based on gene expression [6,88,92,94,95], morphology [96], and electrophysiological [94] properties, and share characteristics of fibrous astrocytes, for example, high expression of Id3 and Gfap [86,92,95]. It is possible that the pathological involvement of marginal astrocytes in subpial MS lesions is crucial for meningeal disintegration resulting in immune cell infiltration and influx of inflammatory cytokines into cortical areas, but this remains to be tested [97]. In addition, astrocytes play important roles in blood–brain barrier integrity, establishing ion and water homeostasis, and this can be compromised under inflammatory–demyelinating conditions as observed in neuromyelitis optica (NMO), a condition similar to MS with the presence of antibodies and T cells targeting the astrocyte-specific water channel, AQP4 [98,99]. Further, dysregulation of AQP4 and other astrocyte-specific ion channels such as KIR4.1, have been described in MS lesions [25,100]. Recent data also emphasize the possibility that anti-AQP4 antibodies might promote leukocyte transmigration across the blood–brain barrier as suggested from in vitro astrocyte coculture assays, although this remains to be further tested [101]. It is reasonable to speculate that these findings might be directly linked to maladaptive events driven by a subset of reactive astrocytes that eventually worsen pathology in MS and related diseases – a possibility that merits further attention.
Of note, subcortical WM lesions are known to be more aggressive and expansive compared with GM lesions in MS [102,103]. For example, the gene encoding for the immune-attractant lipocalin-2 (Lcn2) has been reported to be upregulated in reactive EAE astrocytes from WM versus GM areas and spinal cord [72]. Astrocytes in MS WM lesions have also been characterized by the intracellular presence of myelin in combination with upregulation of the lysosomal marker LAMP1 and the cell surface scavenger receptor LRP1 – important for uptake of lipoproteins [104]. This suggests a capacity to digest myelin debris and a function for reactive astrocytes in MS. Although phagocytosis and synaptic stripping has been classically linked to microglia and myeloid cell function, a recent report found that astrocytes can ‘prune’ excitatory synapses [105]. Reactive mouse astrocytes can lose their synapse-pruning role under inflammatory conditions in vitro [68]. Notably, clearance of myelin is a key mechanism enabling the proper repair of damaged WM [106]. During development, the complement cascade and MHC molecules are involved in the elimination of neurological synapses [89], and overactivation of these pathways might result in degradation of synapses and concomitant neurodegeneration, representing an important feature of MS pathology (Figure 4) [72]. Indeed, in EAE, astrocyte-derived transcripts of the complement pathway have been reported to be enriched in the optic nerve suggesting further region-constrained heterogeneity in response to inflammatory demyelination [72]. Thus, together with their role as antigen-presenting and potentially phagocytosing cells, reactive astrocytes can adopt functions of classical innate immune cell types in a possibly region-restricted manner.
In recent years, it has become clear that homeostatic astrocytes exhibit a high level of inter-regional diversity throughout the CNS, as observed by transcriptomic, functional, and morphological studies. More recent studies also suggest that there is a similar if not higher degree of subtype diversity across different reactive astrocyte subtypes. These subtypes might have detrimental and beneficial functions, which might be modulated by the regional microenvironment. It will be important to fully characterize the breadth of astrocytes subtypes so that these might be potentially targeted in a stratified manner in putative future MS therapies.
Microglia and Myeloid Subtype Diversity: Impact in MS and during Experimental Inflammatory Demyelination
Besides their homeostatic functions, including roles in cleaning debris, regulating synapse development/plasticity and myelination, reactive microglia are found in a number of CNS diseases across species, which includes inflammatory–demyelinating conditions such as MS and its related animal models [107,108]. However, until recently, determining the presence and functions of microglia versus infiltrating monocytes has been challenging due to the lack of cell-specific markers and methodological tools. Dissecting the different myeloid cell populations including microglia subtypes is therefore vital for improving our understanding and treatment of MS, which is characterized by blood–brain barrier dysfunction and transmigration of immune cells into the brain (Figure 2).
Microglia are key drivers of the inflammatory process and in propagating degeneration across multiple diseases. This includes microglial function in driving reactive astrocyte subtypes, for example, via the production of inflammatory molecules C1q, TNF, and IL-1α [68]. Another key function of microglia in the diseased CNS is the dismantling of neuronal synapses. During normal development of the healthy CNS, microglia engulf and eliminate synaptic connections that initially form in excess (i.e., synaptic pruning) – an essential mechanism during development, and for fine-tuning neuronal circuits [109]. Recent work has shown a similar function leading to synapse loss in inflammatory demyelinating disease; several studies have demonstrated synapse loss in MS [110,111] and mouse EAE [112-114]. Profound synapse loss was demonstrated in the thalamus of human MS by immunoreactivity for glutamatergic synapses, as well as in marmoset and mouse EAE brain tissue samples postmortem [115] relative to corresponding controls. Moreover, synapse loss in EAE was attributed to a C3-dependent mechanism and attenuated by viral expression of a C3 inhibitor at glutamatergic synapses in the thalamus [115]. In these models, C3-dependent synapse loss always occurred in the presence of T lymphocytes and reactive astrocytes and, notably, could occur in the absence of significant demyelination or axonal pathology [115] (Figure 4). This is relevant as it raises the possibility that synapse loss and GM atrophy in this model might occur independently from other MS-related pathological events along WM fiber tracts. Indeed, these new data are intriguing in light of recent work in humans demonstrating that visual system pathology in MS – measured by optical coherence tomography – is highly correlated with the expression of genetic variants in complement genes, including C3 [116]. These studies open a new avenue to explore complement- and microglia-mediated synapse loss in MS pathology and raise the question of whether microglia that engulf and eliminate synapses can represent a specialized subset of microglia that might be therapeutically targeted.
Besides roles in driving the degenerative process in the CNS, microglia can play regenerative roles in myelin repair [117]. For instance, microglia-derived endothelin-2 and microglia necroptosis can promote OPC differentiation and remyelination [41,118,119], which might change during aging when microglia can inhibit OPC differentiation through TGFβ-dependent signaling [120]. In the young adult CNS, studies using a focal lysolecithin-driven model in mice have shown that many microglia in the diseased condition first assume a more reactive, inflammatory profile including expressing the markers inducible nitric oxide synthase (iNOS), TNF, and CCL2, and then undergo necroptotic cell death [41,119]. These proinflammatory cells undergo necrotic cell death followed by a second wave of proregenerative myeloid cells characterized by the markers Arg-1, CD206, and IGF, that promote remyelination, likely through type I IFN signaling, although this remains to be further tested [117]. This is interesting in the context of recent work in mice suggesting that CD11c+ microglia within the developing brain promote myelination, and a population of CD11c+ cells expands in both focal and multifocal inflammatory–demyelinating mouse models [121,122]. scRNA-seq and proteomic studies in mouse EAE have shown that this CD11c+ population steadily increases from disease onset to peak disease, and then decreases in chronic disease phases, suggesting that this cell subtype might be associated with the early inflammatory phase of demyelination [123,124]. Thus, it is intriguing to consider that these CD11c+ microglia might be proregenerative, myelinogenic cells, in the context of demyelinating diseases such as MS, and perhaps a potential therapeutic target for promoting regeneration.
Recent cell type-specific RNA-seq [7,8] and myeloid-cell-specific proteomic studies [123,124] resulted in a clearer picture of the diverse peripheral and CNS-derived myeloid cell types, including microglia, under inflammatory–demyelinating conditions (Box 4). These studies have demonstrated that CNS-derived myeloid cells can be further subdivided into diverse homeostatic and reactive subtypes [1,8]. Through this work, Hexb was identified as a novel panmicroglia core gene in mice [125], and genes such as TMEM119 and P2RY12 were identified to distinguish resident, homeostatic microglia from reactive, disease-associated microglial subtypes expressing markers such as CLEC7A, SPP1, APOE, GPNMB, CD163, and CD74, which overlap in humans [6,8,126] and mice [1,7,8] (Figure 3). Another single-cell study in EAE further subdivided disease-associated microglia into four distinct subclusters; all of which are associated with demyelinated lesions and express LY86 [7]. Ccl4 expression also identifies a major population of activated myeloid cells under inflammatory–demyelinating conditions, including MS lesions [1,8,127]. Previous studies in mice have also suggested marker genes Mrc1, Lyve1, and Siglec1 as being associated with CNS-derived myeloid cells, versus Ly6c2 and Ccr2 being associated with monocyte-derived myeloid cells [7], but the relevance of these markers in human MS pathology requires further investigation. Beyond identifying cell-type-specific markers, scRNA-seq has furthered our understanding of cell-type-specific functions of microglia and other macrophage populations. For example, microglia were considered the antigen-producing cells of the CNS. However, scRNA-seq in mouse EAE has revealed that the most prominent antigen-presenting cell gene signature in the CNS is from peripheral hematopoietic stem-cell-derived cells, versus resident CNS microglia [7]. Live imaging has further revealed that Ccr2+ peripheral-derived myeloid lineage cells have longer contacts with encephalitogenic T cells in EAE mice [7]. This is coupled with work showing that the most efficient antigen presentation during EAE is via DCs [32,128-130].
Box 4. Microglia Transcriptomic Diversity in Inflammatory Demyelination.
Transcriptomic analysis of peripheral and CNS-resident myeloid cells obtained from human biopsies based on population [173] or scRNA-seq [8,174] and snRNA-seq [6,175] workflows, revealed several homeostatic and activated cellular states. Of note, while there are significant differences during aging [176], a large number of microglia-enriched genes (C1QA, CSF1R, CX3CR1, DOCK8, P2RY12, TMEM119, and TREM2) showed overlap between humans and mice [1,6,8,126,163,173], suggesting similar homeostatic and reactive transcriptomic patterns during inflammatory demyelination. Cell-type-specific transcriptomics have demonstrated the enrichment of several key inflammatory pathways characteristic for different myeloid cell populations in the human brain, such as antigen presentation (CD74 and HLA-DRB1) [6-8], complement activation (C1QA, C1QB, and C1QC) [1,6,174], iron uptake and metabolism (CD163, LCN2, and FTL) [6,7,174], cell–cell interaction (CCL4, CXCL10, GPNMB, and SPP1) [1,6-8,177], lipid binding (APOE) [6,8,84], phagocytosis (CD68, S100A9, and TREM2) [6,7,145,175], and oxidative stress (GGT1 and GGTLC1) [84] in MS and EAE. Note that several marker genes such as C1QA, CD74, SPP1, and TREM2 that are upregulated in reactive subtypes are also expressed at baseline level in homeostatic cells.
Further studies in subcortical human MS lesions (postmortem) [6,131,132] and EAE marmosets [133] have demonstrated that the majority of iron-laden myeloid cells present in chronic-active lesions surrounding blood vessels and along the lesion rims (sometimes referred to as slowly expanding or ‘smoldering’ lesions) could be identified by a mix of reactive markers such as the hemoglobin–haptoglobin receptor CD163 and iron storage markers hepcidin and ferritin light and heavy chains, linking myeloid cell activation to dysregulated iron homeostasis [6,131-133]. These chronic-active MS lesions are associated with long-term disability and have been shown by 7-tesla MRI to grow in space and time [134,135]. The findings thus beg a further understanding of how iron accumulates, and to what extent it can impact microglial and other glial cell functions, and under which circumstances it might contribute to chronic tissue damage and disease progression.
With new Cre recombinase mouse lines and pharmacological strategies to ablate microglia with Csf1r kinase inhibitors [125,136-140], the ability to more specifically manipulate and dissect functions of myeloid cells under inflammatory demyelinating conditions is being realized. Recent studies have revealed a complex function for microglia with both detrimental and beneficial roles in the disease process. For example, when Tak1 – a key transcriptional regulator of inflammation – is deleted in microglia under the transcriptional control of Cx3cr1, EAE is attenuated [46]. Using a Csf1r kinase inhibitor to ablate microglia in cuprizone-induced demyelination in mice, prophylactic treatment blocked excessive demyelination; and, treatment after demyelination onset enhanced GM but not WM remyelination [47,48]. Similarly, administration of a Csf1r kinase inhibitor after EAE induction attenuated disease severity and promoted recovery [49]. This is, however, in contrast to earlier work that used either minocycline to dampen inflammation or clodronate-liposome-mediated macrophage depletion in spinal cord demyelination to show impaired remyelination [50,51]. Of note, Csf1r inhibition affects other myeloid-lineage populations and, possibly binds platelet-derived growth factor receptor (PDGFR)α on OPCs at high enough doses; therefore, conclusions regarding microglial specificity should be coupled with other more microglia-specific gene-targeting strategies [141,142]. In summary, these studies provide initial first evidence for a direct functional, albeit multifaceted role, for myeloid cells under inflammatory demyelination.
Another important aspect of microglial biology is some of the putative and beneficial roles that have been ascribed to reactive, proinflammatory, microglia subtypes. For instance, it is well established that myelin debris is inhibitory to remyelination, and early clearance by reactive microglia appears to be an important event in the regenerative process [106,143]. Moreover, using Myd88−/− mice and zebrafish, a recent study has found that proinflammatory MyD88-dependent responses were essential for microglial degradation of myelin and for promoting remyelination [144]. Similar results have been identified in mice deficient for triggering receptor expressed on myeloid cells 2 (Trem2−/− mice) – an immunoglobin superfamily receptor necessary for transition from a homeostatic to a disease-associated reactive phenotype [145-147]. Of note, cuprizone-induced demyelination in Trem2−/− mice resulted in failure of microglia to fully transition to a reactive phenotype and demonstrated defects in microglial clearance of myelin debris, leading to impaired remyelination [148,149]. Similar impairments in myelin clearance and impaired remyelination have also been observed in fractalkine-receptor-deficient Cx3cr1−/− mice, which are highly enriched in microglia relative to controls [150], emphasizing the important role of myeloid cells in tissue repair during inflammatory demyelination. In cuprizone and Pelizaeus–Merzbacher disease mouse models, myelin fragments that fail to degrade were found and can associate with lipofuscin granules within microglia [151] – a process also observed during normal aging. In this study, accumulation and failure of myelin degradation was exacerbated upon microglia-specific ablation of Rab7, which interfered with the lysosomal pathway of protein degradation, including myelin glycoproteins [151]. Consistent with sustained undigested myelin material, a recent snRNA-seq study has found a myeloid cell subtype in postmortem MS brain tissue that is enriched for myelin transcripts [6]. As further validation in vitro, upon myelin phagocytosis, microglial cells can retain myelin RNAs for several days. However, the consequences of undigested myelin RNA, lipids, and protein, to reactive microgliosis and inflammation in MS remain open questions, but certainly merit further attention.
In summary, microglia and other myeloid lineage cells play diverse roles in the MS brain. These roles can be detrimental and lead to propagation of inflammation, tissue destruction, and CNS atrophy. However, both pro- and anti-inflammatory microglia can play beneficial roles in clearing debris and promoting regeneration. With the same cell type performing seemingly opposing roles, targeting these cells to potentially slow neurodegeneration and promote regeneration remains challenging. Technological tools, the identification of new molecular mechanisms by which microglia contribute to multiple components of the disease process, as well as achieving increased ability to map these cells spatially and temporally in the MS brain can facilitate these pursuits.
Concluding Remarks
Recent technological advances in multiplex and single-cell transcriptomics have enabled deeper insight into glial cell type ontogeny, heterogeneity, and function under inflammatory conditions, in which concomitant demyelination is present. Recent single-cell gene expression studies and transcriptomic tissue mapping (Figure 1), which included postmortem MS tissues, have further advanced our understanding of glial subtypes across various CNS regions at different stages of inflammation (Figure 2); however, they have also left us with remaining unsolved problems (see Outstanding Questions).
Outstanding Questions.
To what extent is glial cell type heterogeneity reflected in regionally diversified responses under inflammatory–demyelinating conditions. To what extent do glial subtypes differ across species during homeostasis and reactivity; a concept that becomes essential when conducting preclinical studies in animal models.
Do reactive glial subtype polarization states exist and spatially change during inflammatory lesion progression? This is a key question that needs to be addressed in future studies applying high-resolution spatial omics approaches, including epigenomics, transcriptomics, proteomics, and metabolomics.
Can reactive glial cells be manipulated in a regionally stratified manner to support beneficial states and/or inhibit detrimental states? Understanding the precise molecular state of a particular glial subtype within its anatomical surrounding is key when developing interventional strategies to limit the expansion of lesions and prevent further damage to neurons.
While the glial response was initially interpreted as solely reactive, mounting evidence now implicates reactive glial cells as effector cells that may mediate and perpetuate the immune response and tissue injury. For example, antigen presentation via MHC class I and II proteins is a characteristic feature of reactive glial subtypes, which highlights important immune-related functions of both reactive OL [5,6,10,16,32] and astrocyte lineage [152-154] cells (Figure 3). Similar to professiona antigen-presenting cells such as DCs, glial cells have the capacity to phagocytose antigens and present them on MHC class II molecules promoting a specific immune response [104,105]. However, microglia might be not as competent at antigen presentation via MHC class I compared with DCs [32], and MHC-class-I-expressing OPCs represent a particularly vulnerable cell population to immune cell cytotoxicity during inflammation compared with microglia and DCs [16]. Another example of aberrant immune signaling is the release of neurotoxic factors such as complement cascade components that can mediate synaptic pathology and facilitate neuronal degeneration [68,115] (Figure 3). Also, many of the MS risk gene variants in humans originally implicated in peripheral immune responses, such as those that regulate NF-κB in astrocytes [155,156], now appear to be expressed in a wide range of CNS glial subtypes.
Glial cell types play diverse and sometimes opposing roles in MS pathobiology. These roles can be detrimental and, in some instances, lead to the propagation of inflammation, tissue destruction, and CNS atrophy, as shown for aberrant immune signaling that can result in neuronal damage via complement factors [115] and self-destruction via MHC class I antigen presentation [16]. However, pro- and anti-inflammatory glial subtypes can also play beneficial roles in clearing debris and promoting repair, as shown for astrocyte-mediated myelin phagocytosis [68], or proregenerative myeloid cells [117]. With the same cell type performing seemingly multifaceted roles, targeting these subtypes in an attempt to slow neurodegeneration and conversely promote regeneration will prove challenging (Figure 4).
Understanding the different molecular states of glial subtypes in MS lesion pathology will therefore be crucial to identify subtype-specific therapeutic targets. Of note, in that sense, MS pathobiology is unique and different compared with other primarily degenerative (e.g., Alzheimer’s disease) or inflammatory (e.g., viral encephalitis) diseases, which lack recurrent phases of inflammatory–demyelinating lesions that can occur throughout the entire CNS. Thus, strategies to decode diversity and function of glial subtypes in MS need to take into account intrinsic transcriptomic and functional differences of glial subtypes between anatomical compartments and CNS subregions. Furthermore, due to the relapsing-remitting nature of MS, it could be possible that glial cells cycle between different subtypes according to the specific inflammatory stage (Figures 2 and 4). Hence, a task for future studies includes identifying these subtype-specific key regulators of disease processes and develop interventional strategies to modulate glial subtype-specific pathways along spatial and temporal disease trajectories in MS. This might also help us to better understand and eventually promote anti-inflammatory and proregenerative cell subtypes to facilitate repair in MS.
Highlights.
Single-cell transcriptomic technologies have revolutionized our understanding of homeostatic and reactive glial cell types during disease progression in experimental models and in human multiple sclerosis (MS) tissue samples, which can reflect different stages of tissue damage.
Homeostatic and reactive glial cell types show intra- and inter-regional heterogeneity in different central nervous system (CNS) areas and within inflammatory–demyelinating lesions.
Reactive glial subtypes are highly polarized based on morphological, transcriptomic, and functional criteria, having seemingly opposing roles in either promoting or suppressing inflammation and further tissue damage.
Glial subtype-specific transcriptomic profiling has helped identify novel homeostatic and reactive subtype markers that will be useful for the development of novel biomarkers and therapeutic targets for future studies in MS.
Acknowledgments
This work was supported by intramural funding provided by the Medical Faculty Mannheim of Heidelberg University (to L.S.), research grants from the Hertie Foundation (medMS MyLab, P1180016 to L.S.), the Wellcome Trust (D.H.R.), a Wellcome Trust PhD studentship (PSAG/097 to T.B.), the European Research Council (‘DecOmPress’ ERC StG to L.S., and ‘Myel-IN-Crisis’ ERC AdG to D.H.R.), the Adelson Medical Research Foundation (D.P.S., D.H.R.), the National Multiple Sclerosis Society (FG-1902-33617 to L.S., D.H.R. and RG-1907-34756 to P.A.C.), the German Research Foundation (SCHI 1330/2-1, to L.S.) and grants from the National Institutes of Health (NS083513 to D.H.R., R37NS041435 to P.A.C., and R01MH113743 to D.P.S.), and the Department of Defense (W81XWH191062 to P.A.C.).
Glossary
- Antigen presentation
display of antigen molecules on the surface of antigen-presenting cells, such as DCs, in association with MHC class II molecules when presented to CD4+ helper T cells, or with MHC class I when presented to CD8+ cytotoxic T cells.
- Blood–brain barrier
highly selective and tightly regulated microvasculature interface comprised by different cell types, including endothelial and specialized stromal cells such as pericytes, as well as glial subtypes; astrocytes extend end feet to blood vessels and enwrap them to form the glial limitans.
- Central nervous system (CNS)
nervous system including gray and white matter areas of the brain, the cerebellum and spinal cord, and adjacent fiber tracts such as the olfactory tract and the optic nerve.
- Complement factors
proteins of the innate immune system; help clearance of microbes and damaged cells and promote inflammation through opsonization, anaphylatoxins, and formation of a cell-killing membrane attack complex.
- Cuprizone-induced demyelination
toxic demyelinating lesion model, for example, to study kinetics of oligodendrocyte precursor cell differentiation and remyelination; rodents fed the copper chelator cuprizone develop demyelination, typically of the corpus callosum of the brain, due to OL death.
- Demyelination
breakdown of normal myelin, typical of MS and related diseases; myelin sheaths and myelin-producing cells are damaged and eventually destroyed, resulting in permanent scar formation and damage to nerve fibers.
- Experimental autoimmune encephalomyelitis (EAE)
classic and usually monophasic MS animal model, which is primarily T cell-mediated and in which demyelination is induced by an autoimmune response against myelin proteins such as MOG35–55 peptide; usually results in multifocal demyelinating lesions along WM tracts.
- Fibrous astrocytes
subtype of homeostatic astrocytes based on morphological features comprising small cells with elongated fibers associated with WM areas along the CNS.
- Gray matter (GM)
essential compartment of various anatomical areas of the CNS such as the neocortex or the hippocampus; enriched in protoplasmic astrocytes, neurons, and synapses.
- Heterochronic parabiosis
shared blood circulation resulting in cross-circulation of humoral factors and cells in multiorgan systems.
- Homeostatic glia
glial cells under healthy conditions with a physiological phenotype characterized by their diverse functions, for example, sensing of and responding to environmental stimuli, supporting synapse and axonal firing, and regulating other surrounding neuronal and glial cell types
- Lesion stages
MS lesions can occur in both WM and GM; acute lesions are characterized by their inflammatory activity with active phagocytosis of myelin components; chronic-active lesions show enhanced, and chronic-inactive lesions show low-level inflammatory activity at lesion rims.
- Lipofuscin granules
lipid-containing residues derived from incomplete lysosomal digestion; considered to be a classic pathological aging pigment, typically found in aged neurons and less in glial cells.
- Lysolecithin-induced demyelination
toxic demyelinating lesion model to study the kinetics of OPC differentiation and remyelination by focal injection of lysolecithin, (lysophosphatidylcholine), typically into WM tracts.
- Marginal astrocytes
subtype of homeostatic astrocytes found close to the meninges, (subpial astrocytes), and blood vessels, (perivascular astrocytes).
- Meningeal disintegration
process in which the physiological barrier function of the meninges is impaired and associated with accumulation of immune cells in meningeal niches, as well as parenchymal immune cell infiltration
- Multiple sclerosis (MS)
most common inflammatory–demyelinating disease of both GM and WM of the CNS; mainly presents in young adults with a typical early relapse–remitting stage followed by chronic progressive disease resulting in accrual of permanent disability; pathological key features of MS are demyelination, astrogliosis, and a variable degree of inflammation and neuron degeneration.
- Pelizaeus–Merzbacher disease
X-linked recessive leukodystrophy, a genetic neurological disease that primarily affects oligodendrocytes in the CNS; caused by mutations in the PLP1 gene, encoding a major myelin protein.
- Protoplasmic astrocytes
subtype of homeostatic astrocytes based on morphological features comprising large cell bodies consisting of highly branched bushy processes associated with GM (e.g., the cerebral cortex).
- Reactive astrogliosis
general response of astrocytes to CNS injury; astrocytes drastically change their gene expression profile and morphology, become hypertrophic and retract their processes; demyelinated MS lesions are composed of reactive astrocytes (astroglial scar) forming a dense meshwork, characteristic of intermediate filaments, for example, glial fibrillary acidic protein.
- Reactive glia
general term for glial cells that respond to tissue injury and infiltrating immune cells, playing important roles as both effector cells actively participating in tissue remodeling, and as responsive cells reacting to the disease microenvironment.
- Reactive microgliosis
general response of microglia to CNS injury with morphological features, for example, enlarged cell bodies and contraction of processes, as well as dramatic changes in gene expression.
- Single-cell/nucleus RNA-sequencing
microfluidic technology that allows capturing and sequencing thousands of individual cells or nuclei for subsequent cell-type-specific cluster and differential gene expression analysis.
- Spatial transcriptomics
methods to perform (multiplex) in situ RNA hybridization and spatial visualization based on genes of interest (supervised) versus in situ transcriptomics (unsupervised) based on barcoded oligonucleotides that capture RNA to analyze transcripts in tissue sections.
- White matter (WM)
essential compartment of various anatomical areas of the CNS; enriched in fibrous astrocytes, oligodendrocytes, and myelinated axon fiber tracts, including short- and long-range axons from projection neurons.
Footnotes
Declaration of Interests
The authors have no interests to declare.
References
- 1.Hammond TR et al. (2019) Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 50, 253–271 e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cahoy JD et al. (2008) A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci 28, 264–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reich DS et al. (2018) Multiple sclerosis. N. Engl. J. Med 378, 169–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haider L et al. (2016) The topograpy of demyelination and neurodegeneration in the multiple sclerosis brain. Brain J. Neurol 139, 807–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jakel S et al. (2019) Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 566, 543–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schirmer L et al. (2019) Neuronal vulnerability and multilineage diversity in multiple sclerosis. Nature 573, 75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jordao MJC et al. (2019) Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science 363 [DOI] [PubMed] [Google Scholar]
- 8.Masuda T et al. (2019) Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388–392 [DOI] [PubMed] [Google Scholar]
- 9.Wheeler MA et al. (2020) MAFG-driven astrocytes promote CNS inflammation. Nature 578, 593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falcão AM et al. (2018) Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat. Med 24, 1837–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramaglia V et al. (2019) Multiplexed imaging of immune cells in staged multiple sclerosis lesions by mass cytometry. Elife 8, e48051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park C et al. (2019) The landscape of myeloid and astrocyte phenotypes in acute multiple sclerosis lesions. Acta Neuropathol. Commun 7, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergles DE and Richardson WD (2015) Oligodendrocyte development and plasticity. Cold Spring Harb. Perspect Biol 8, a020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrington EP et al. (2020) Immune cell modulation of oligodendrocyte lineage cells. Neurosci. Lett 715, 134601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez-Castaneda A et al. (2020) The active contribution of OPCs to neuroinflammation is mediated by LRP1. Acta Neuropathol. 139, 365–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirby L et al. (2019) Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nat Commun. 10, 3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiow LR et al. (2017) Reactive astrocyte COX2-PGE2 production inhibits oligodendrocyte maturation in neonatal white matter injury. Glia 75 469–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuen TJ et al. (2014) Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell 158, 383–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee Y et al. (2012) Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergles DE et al. (2000) Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405, 187–191 [DOI] [PubMed] [Google Scholar]
- 21.Xin W et al. (2019) Oligodendrocytes support neuronal glutamatergic transmission via expression of glutamine synthetase. Cell Rep. 27, 2262–2271 e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trapp BD and Stys PK (2009) Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 8, 280–291 [DOI] [PubMed] [Google Scholar]
- 23.Larson VA et al. (2018) Oligodendrocytes control potassium accumulation in white matter and seizure susceptibility. Elife 7, e34829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schirmer L et al. (2018) Oligodendrocyte-encoded Kir4.1 function is required for axonal integrity. eLife 7, 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schirmer L et al. (2014) Differential loss of KIR4.1 immunoreactivity in multiple sclerosis lesions. Ann. Neurol 75, 810–828 [DOI] [PubMed] [Google Scholar]
- 26.Marques S et al. (2016) Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science (New York, N.Y.) 352, 1326–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fard MK et al. (2017) BCAS1 expression defines a population of early myelinating oligodendrocytes in multiple sclerosis lesions. Sci. Transl. Med 9, eaam7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chew LJ et al. (2005) Interferon-gamma inhibits cell cycle exit in differentiating oligodendrocyte progenitor cells. Glia 52, 127–143 [DOI] [PubMed] [Google Scholar]
- 29.Lin W et al. (2006) Interferon-gamma inhibits central nervous system remyelination through a process modulated by endoplasmic reticulum stress. Brain 129, 1306–1318 [DOI] [PubMed] [Google Scholar]
- 30.Piatek P et al. (2019) Multiple sclerosis CD49d(+)CD154(+) as myelin-specific lymphocytes induced during remyelination. Cells 9, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joffre OP et al. (2012) Cross-presentation by dendritic cells. Nat. Rev. Immunol 12, 557–569 [DOI] [PubMed] [Google Scholar]
- 32.Ji Q et al. (2013) MHC class I-restricted myelin epitopes are cross-presented by Tip-DCs that promote determinant spreading to CD8(+) T cells. Nat. Immunol 14, 254–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang A et al. (2000) NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J. Neurosci 20, 6404–6412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes EG et al. (2013) Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat. Neurosci 16, 668–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang A et al. (2012) Cortical remyelination: a new target for repair therapies in multiple sclerosis. Ann. Neurol 72, 918–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lau LW et al. (2012) Chondroitin sulfate proteoglycans in demyelinated lesions impair remyelination. Ann. Neurol 72, 419–432 [DOI] [PubMed] [Google Scholar]
- 37.Sloane JA et al. (2010) Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc. Natl. Acad. Sci. U. S. A 107, 11555–11560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruckh JM et al. (2012) Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 10, 96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dillenburg A et al. (2018) Activin receptors regulate the oligodendrocyte lineage in health and disease. Acta Neuropatho. 135, 887–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Psachoulia K et al. (2016) IL4I1 augments CNS remyelination and axonal protection by modulating T cell driven inflammation. Brain 139, 3121–3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miron VE et al. (2013) M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci 16, 1211–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen S et al. (2008) Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat. Neurosci 11, 1024–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosko L et al. (2019) Oligodendrocyte bioenergetics in health and disease. Neuroscientist 25, 334–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Segel M et al. (2019) Niche stiffness underlies the ageing of central nervous system progenitor cells. Nature 573, 130–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neumann B et al. (2019) Metformin restores CNS remyelination capacity by rejuvenating aged stem cells. Cell Stem Cell 25, 473–485 e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang P et al. (2019) Senolytic therapy alleviates Abeta-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer's disease model. Nat. Neurosci 22, 719–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicaise AM et al. (2019) Cellular senescence in progenitor cells contributes to diminished remyelination potential in progressive multiple sclerosis. Proc. Natl. Acad. Sci. U. S. A 116, 9030–9039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicaise AM et al. (2017) iPS-derived neural progenitor cells from PPMS patients reveal defect in myelin injury response. Exp. Neurol 288, 114–121 [DOI] [PubMed] [Google Scholar]
- 49.Morales Pantoja IE et al. (2020) iPSCs from people with MS can differentiate into oligodendrocytes in a homeostatic but not an inflammatory milieu. PLoS One 15, e0233980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patrikios P et al. (2006) Remyelination is extensive in a subset of multiple sclerosis patients. Brain 129, 3165–3172 [DOI] [PubMed] [Google Scholar]
- 51.Neumann B et al. (2020) Problems and pitfalls of identifying remyelination in multiple sclerosis. Cell Stem Cell 26, 617–619 [DOI] [PubMed] [Google Scholar]
- 52.Yeung MSY et al. (2019) Dynamics of oligodendrocyte generation in multiple sclerosis. Nature 566, 538–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuhlmann T et al. (2008) Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain J. Neurol 131, 1749–1758 [DOI] [PubMed] [Google Scholar]
- 54.Corradin O et al. (2016) Modeling disease risk through analysis of physical interactions between genetic variants within chromatin regulatory circuitry. Nat. Genet 48, 1313–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Factor DC et al. (2020) Cell type-specific intralocus interactions reveal oligodendrocyte mechanisms in MS. Cell 181, 382–395 e21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watanabe K et al. (2019) Genetic mapping of cell type specificity for complex traits. Nat. Commun 10, 3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corces MR et al. (2020) Single-cell epigenomic analyses implicate candidate causal variants at inherited risk loci for Alzheimer's and Parkinson's diseases. Nat. Genet 52, 1158–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Absinta M et al. (2019) Association of chronic active multiple sclerosis lesions with disability in vivo. JAMA Neurol. 76, 1474–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bevan RJ et al. (2018) Meningeal inflammation and cortical demyelination in acute multiple sclerosis. Ann. Neurol 84, 829–842 [DOI] [PubMed] [Google Scholar]
- 60.Bacmeister CM et al. (2020) Motor learning promotes remyelination via new and surviving oligodendrocytes. Nat. Neurosci 23, 819–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McKenzie IA et al. (2014) Motor skill learning requires active central myelination. Science (New York, N.Y.) 346, 318–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liddelow SA and Barres BA (2017) Reactive astrocytes: production, function, and therapeutic potential. Immunity 46, 957–967 [DOI] [PubMed] [Google Scholar]
- 63.Charcot JM (1868) Histologie de la Sclérose en Plaques, Gazette Des Hopitaux [Google Scholar]
- 64.Hochstim C et al. (2008) Identification of positionally distinct astrocyte subtypes whose identities are specified by a homeodomain code. Cell 133, 510–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Molofsky AV et al. (2014) Astrocyte-encoded positional cues maintain sensorimotor circuit integrity. Nature 509, 189–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Farmer WT et al. (2016) Neurons diversify astrocytes in the adult brain through sonic hedgehog signaling. Science 351, 849–854 [DOI] [PubMed] [Google Scholar]
- 67.Zamanian JL et al. (2012) Genomic analysis of reactive astrogliosis. J. Neurosci 32, 6391–6410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liddelow SA et al. (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nam Y et al. (2014) Lipocalin-2 protein deficiency ameliorates experimental autoimmune encephalomyelitis: the pathogenic role of lipocalin-2 in the central nervous system and peripheral lymphoid tissues. J. Biol. Chem 289, 16773–16789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mills Ko E et al. (2014) Deletion of astroglial CXCL10 delays clinical onset but does not affect progressive axon loss in a murine autoimmune multiple sclerosis model. J. Neuroinflammation 11, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clarke LE et al. (2018) Normal aging induces A1-like astrocyte reactivity. Proc. Natl. Acad. Sci. U. S. A 115, E1896–E1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Itoh N et al. (2018) Cell-specific and region-specific transcriptomics in the multiple sclerosis model: Focus on astrocytes. Proc. Natl. Acad. Sci. U. S. A 115, E302–E309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lian H et al. (2015) NFkappaB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer's disease. Neuron 85, 101–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brambilla R et al. (2009) Transgenic inhibition of astroglial NF-kappa B improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation. J. Immunol. 182, 2628–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Silver J and Miller JH (2004) Regeneration beyond the glial scar. Nat. Rev. Neurosci 5, 146–156 [DOI] [PubMed] [Google Scholar]
- 76.Anderson MA et al. (2016) Astrocyte scar formation aids central nervous system axon regeneration. Nature 532, 195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sofroniew MV (2015) Astrocyte barriers to neurotoxic inflammation. Nature reviews. Neuroscience 16, 249–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rothhammer V et al. (2016) Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med 22, 586–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rothhammer V et al. (2018) Microglial control of astrocytes in response to microbial metabolites. Nature 557, 724–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Probstel AK et al. (2020) Gut microbiota-specific IgA(+) B cells traffic to the CNS in active multiple sclerosis. Sci. Immunol 5, eabc7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rojas OL et al. (2019) Recirculating intestinal IgA-producing cells regulate neuroinflammation via IL-10. Cell 177, 492–493 [DOI] [PubMed] [Google Scholar]
- 82.Benakis C et al. (2016) Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat. Med 22, 516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mahad DH et al. (2015) Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 14, 183–193 [DOI] [PubMed] [Google Scholar]
- 84.Mendiola AS et al. (2020) Transcriptional profiling and therapeutic targeting of oxidative stress in neuroinflammation. Nat. Immunol 21, 513–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Y and Barres BA (2010) Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr. Opin. Neurobiol 20, 588–594 [DOI] [PubMed] [Google Scholar]
- 86.Zeisel A et al. (2015) Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347, 1138–1142 [DOI] [PubMed] [Google Scholar]
- 87.Chai H et al. (2017) Neural circuit-specialized astrocytes: transcriptomic, proteomic, morphological, and functional evidence. Neuron 95, 531–549 e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morel L et al. (2017) Molecular and functional properties of regional astrocytes in the adult brain. J. Neurosci 37, 8706–8717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boisvert MM et al. (2018) The aging astrocyte transcriptome from multiple regions of the mouse brain. Cell Rep. 22, 269–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saunders A et al. (2018) Molecular diversity and specializations among the cells of the adult mouse brain. Cell 174, 1015–1030 e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zeisel A et al. (2018) molecular architecture of the mouse nervous system. Cell 174, 999–1014 e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Batiuk MY et al. (2020) Identification of region-specific astrocyte subtypes at single cell resolution. Nat. Commun 11, 1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hammond TR et al. (2014) Astrocyte-derived endothelin-1 inhibits remyelination through notch activation. Neuron 81, 588–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morel L et al. (2019) Intracortical astrocyte subpopulations defined by astrocyte reporter Mice in the adult brain. Glia 67, 171–181 [DOI] [PubMed] [Google Scholar]
- 95.Bayraktar OA et al. (2020) Astrocyte layers in the mammalian cerebral cortex revealed by a single-cell in situ transcriptomic map. Nat. Neurosci 23, 500–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lanjakornsiripan D et al. (2018) Layer-specific morphological and molecular differences in neocortical astrocytes and their dependence on neuronal layers. Nat. Commun 9, 1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gardner C et al. (2013) Cortical grey matter demyelination can be induced by elevated pro-inflammatory cytokines in the subarachnoid space of MOG-immunized rats. Brain J. Neurol 136, 3596–3608 [DOI] [PubMed] [Google Scholar]
- 98.Sagan SA et al. (2016) Tolerance checkpoint bypass permits emergence of pathogenic T cells to neuromyelitis optica autoantigen aquaporin-4. Proc. Natl. Acad. Sci. U. S. A 113, 14781–14786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lucchinetti CF et al. (2014) The pathology of an autoimmune astrocytopathy: lessons learned from neuromyelitis optica. Brain Pathol. 24, 83–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Prineas JW and Lee S (2019) Multiple sclerosis: destruction and regeneration of astrocytes in acute lesions. J. Neuropathol. Exp. Neurol 78, 140–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Takeshita Y et al. (2017) Effects of neuromyelitis optica-IgG at the blood-brain barrier in vitro. Neurol. Neuroimmunol. Neuroinflamm 4, e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Prins M et al. (2015) Pathological differences between white and grey matter multiple sclerosis lesions. Ann. N. Y. Acad. Sci 1351, 99–113 [DOI] [PubMed] [Google Scholar]
- 103.Albert M et al. (2007) Extensive cortical remyelination in patients with chronic multiple sclerosis. Brain Pathol. 17, 129–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ponath G et al. (2017) Myelin phagocytosis by astrocytes after myelin damage promotes lesion pathology. Brain 140, 399–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee JH et al. (2020) Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis. Nature Published online December 23, 2020. 10.1038/s41586-020-03060-3 [DOI] [PubMed] [Google Scholar]
- 106.Kotter MR et al. (2006) Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J. Neurosci 26, 328–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Voet S et al. (2019) Microglia in central nervous system inflammation and multiple sclerosis pathology. Trends Mol. Med 25, 112–123 [DOI] [PubMed] [Google Scholar]
- 108.O'Loughlin E et al. (2018) Microglial phenotypes and functions in multiple sclerosis. Cold Spring Harb. Perspect. Med 8, a028993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wilton DK et al. (2019) Neuron-glia signaling in synapse elimination. Annu. Rev. Neurosci 42, 107–127 [DOI] [PubMed] [Google Scholar]
- 110.Dutta R et al. (2011) Demyelination causes synaptic alterations in hippocampi from multiple sclerosis patients. Ann. Neurol 69, 445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Albert M et al. (2017) Synaptic pathology in the cerebellar dentate nucleus in chronic multiple sclerosis. Brain Pathol. 27, 737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Araujo SES et al. (2017) Inflammatory demyelination alters subcortical visual circuits. J. Neuroinflammation 14, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jurgens T et al. (2016) Reconstruction of single cortical projection neurons reveals primary spine loss in multiple sclerosis. Brain 139, 39–46 [DOI] [PubMed] [Google Scholar]
- 114.Bellizzi MJ et al. (2016) Platelet-activating factor receptors mediate excitatory postsynaptic hippocampal injury in experimental autoimmune encephalomyelitis. J. Neurosci 36, 1336–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Werneburg S et al. (2020) Targeted complement inhibition at synapses prevents microglial synaptic engulfment and synapse loss in demyelinating disease. Immunity 52, 167–182 e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fitzgerald KC et al. (2019) Early complement genes are associated with visual system degeneration in multiple sclerosis. Brain 142, 2722–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lloyd AF and Miron VE (2019) The pro-remyelination properties of microglia in the central nervous system. Nat. Rev. Neurol 15, 447–458 [DOI] [PubMed] [Google Scholar]
- 118.Yuen TJ et al. (2013) Identification of endothelin 2 as an inflammatory factor that promotes central nervous system remyelination. Brain 136, 1035–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lloyd AF et al. (2019) Central nervous system regeneration is driven by microglia necroptosis and repopulation. Nat. Neurosci 22, 1046–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Baror R et al. (2019) Transforming growth factor-beta renders ageing microglia inhibitory to oligodendrocyte generation by CNS progenitors. Glia 67, 1374–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wlodarczyk A et al. (2014) Comparison of microglia and infiltrating CD11c(+) cells as antigen presenting cells for T cell proliferation and cytokine response. J. Neuroinflammation 11, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Remington LT et al. (2007) Microglial recruitment, activation, and proliferation in response to primary demyelination. Am. J. Pathol 170, 1713–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mrdjen D et al. (2018) High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48, 380–395.e6 [DOI] [PubMed] [Google Scholar]
- 124.Ajami B et al. (2018) Single-cell mass cytometry reveals distinct populations of brain myeloid cells in mouse neuroinflammation and neurodegeneration models. Nat. Neurosci 21, 541–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Masuda T et al. (2020) Novel Hexb-based tools for studying microglia in the CNS. Nat. Immunol 21, 802–815 [DOI] [PubMed] [Google Scholar]
- 126.Olah M et al. (2020) Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer's disease. Nat. Commun 11, 6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kang SS et al. (2018) Microglial translational profiling reveals a convergent APOE pathway from aging, amyloid, and tau. J. Exp. Med 215, 2235–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bailey SL et al. (2007) CNS myeloid DCs presenting endogenous myelin peptides 'preferentially' polarize CD4+ T(H)-17 cells in relapsing EAE. Nat. Immunol 8, 172–180 [DOI] [PubMed] [Google Scholar]
- 129.Greter M et al. (2005) Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat. Med 11, 328–334 [DOI] [PubMed] [Google Scholar]
- 130.Giles DA et al. (2018) CNS-resident classical DCs play a critical role in CNS autoimmune disease. J. Clin. Invest 128, 5322–5334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zrzavy T et al. (2017) Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain J. Neurol 140, 1900–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hametner S et al. (2013) Iron and neurodegeneration in the multiple sclerosis brain. Ann. Neurol 74, 848–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee NJ et al. (2019) Potential role of iron in repair of inflammatory demyelinating lesions. J. Clin. Invest 129, 4365–4376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Absinta M et al. (2016) Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J. Clin. Invest 126, 2597–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dal-Bianco A et al. (2017) Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 133, 25–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yona S et al. (2013) Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Parkhurst CN et al. (2013) Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155, 1596–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kaiser T and Feng G (2019) Tmem119-EGFP and Tmem119-CreERT2 transgenic mice for labeling and manipulating microglia. eNeuro 6 ENEURO.0448–18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McKinsey GL et al. (2020) A new genetic strategy for targeting microglia in development and disease. Elife 9, e54590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Green KN et al. (2020) To kill a microglia: a case for CSF1R inhibitors. Trends Immunol. 41, 771–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu Y et al. (2019) Concentration-dependent effects of CSF1R inhibitors on oligodendrocyte progenitor cells ex vivo and in vivo. Exp. Neurol 318, 32–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lei F et al. (2020) CSF1R inhibition by a small-molecule inhibitor is not microglia specific; affecting hematopoiesis and the function of macrophages. Proc. Natl. Acad. Sci. U. S. A 117, 23336–23338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Neumann H et al. (2009) Debris clearance by microglia: an essential link between degeneration and regeneration. Brain 132, 288–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cunha MI et al. (2020) Pro-inflammatory activation following demyelination is required for myelin clearance and oligodendrogenesis. J. Exp. Med 217, e20191390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Krasemann S et al. (2017) The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47, 566–581 e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ulland TK and Colonna M (2018) TREM2 - a key player in microglial biology and Alzheimer disease. Nat. Rev. Neurol 14, 667–675 [DOI] [PubMed] [Google Scholar]
- 147.Keren-Shaul H et al. (2017) A unique microglia type associated with restricting development of Alzheimer's disease. Cell 169, 1276–1290 e17 [DOI] [PubMed] [Google Scholar]
- 148.Poliani PL et al. (2015) TREM2 sustains microglial expansion during aging and response to demyelination. J. Clin. Invest 125, 2161–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cantoni C et al. (2015) TREM2 regulates microglial cell activation in response to demyelination in vivo. Acta Neuropathol. 129, 429–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lampron A et al. (2015) Inefficient clearance of myelin debris by microglia impairs remyelinating processes. J. Exp. Med 212, 481–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Safaiyan S et al. (2016) Age-related myelin degradation burdens the clearance function of microglia during aging. Nat. Neurosci 19, 995–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Soos JM et al. (1998) Astrocytes express elements of the class II endocytic pathway and process central nervous system autoantigen for presentation to encephalitogenic T cells. J. Immunol 161, 5959–5966 [PubMed] [Google Scholar]
- 153.Fontana A et al. (1984) Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature 307, 273–276 [DOI] [PubMed] [Google Scholar]
- 154.Lee SC et al. (1990) Multiple sclerosis: a role for astroglia in active demyelination suggested by class II MHC expression and ultrastructural study. J. Neuropathol. Exp. Neurol 49, 122–136 [DOI] [PubMed] [Google Scholar]
- 155.Ponath G et al. (2018) Enhanced astrocyte responses are driven by a genetic risk allele associated with multiple sclerosis. Nat. Commun 9, 5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.International Multiple Sclerosis Genetics, C (2019) Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science 365, eaav7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Huang W et al. (2020) Origins and proliferative states of human oligodendrocyte precursor cells. Cell 182, 594–608.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ben Haim L and Rowitch DH (2017) Functional diversity of astrocytes in neural circuit regulation. Nat. Rev. Neurosci 18, 31–41 [DOI] [PubMed] [Google Scholar]
- 159.Michailidou I et al. (2015) Complement C1q-C3-associated synaptic changes in multiple sclerosis hippocampus. Ann. Neurol 77, 1007–1026 [DOI] [PubMed] [Google Scholar]
- 160.Ramaglia V et al. (2012) C3-dependent mechanism of microglial priming relevant to multiple sclerosis. Proc. Natl. Acad. Sci. U. S. A 109, 965–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Prinz M et al. (2011) Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat. Neurosci 14, 1227–1235 [DOI] [PubMed] [Google Scholar]
- 162.Hammond TR et al. (2019) Immune signaling in neurodegeneration. Immunity 50, 955–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Colonna M and Butovsky O (2017) Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol 35, 441–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xiao L et al. (2016) Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat. Neurosci 19, 1210–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Baxi EG et al. (2017) Lineage tracing reveals dynamic changes in oligodendrocyte precursor cells following cuprizone-induced demyelination. Glia 65, 2087–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen Y et al. (2009) The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat. Neurosci 12, 1398–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sharma K et al. (2015) Cell type- and brain region-resolved mouse brain proteome. Nat. Neurosci 18, 1819–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mathys H et al. (2019) Single-cell transcriptomic analysis of Alzheimer's disease. Nature 570, 332–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nagy C et al. (2020) Single-nucleus transcriptomics of the prefrontal cortex in major depressive disorder implicates oligodendrocyte precursor cells and excitatory neurons. Nat. Neurosci 23, 771–781 [DOI] [PubMed] [Google Scholar]
- 170.Velmeshev D et al. (2019) Single-cell genomics identifies cell type-specific molecular changes in autism. Science 364, 685–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wheeler MA et al. (2019) Environmental control of astrocyte pathogenic activities in CNS inflammation. Cell 176, 581–596 e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chen Z et al. (2019) Multi-copper ferroxidase deficiency leads to iron accumulation and oxidative damage in astrocytes and oligodendrocytes. Sci. Rep 9, 9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gosselin D et al. (2017) An environment-dependent transcriptional network specifies human microglia identity. Science 356, eaal3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sankowski R et al. (2019) Mapping microglia states in the human brain through the integration of high-dimensional techniques. Nat. Neurosci 22, 2098–2110 [DOI] [PubMed] [Google Scholar]
- 175.Zhou Y et al. (2020) Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer's disease. Nat. Med 26, 131–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Galatro TF et al. (2017) Transcriptomic analysis of purified human cortical microglia reveals age-associated changes. Nat. Neurosci 20, 1162–1171 [DOI] [PubMed] [Google Scholar]
- 177.Giladi A et al. (2020) Cxcl10+monocytes define a pathogenic subset in the central nervous system during autoimmune neuroinflammation. Nat. Immunol 21, 525–534 [DOI] [PubMed] [Google Scholar]