Abstract
Family members – mainly spouses and partners – are the primary caregivers for individuals with Alzheimer’s disease and related dementias (ADRD), chronic progressive illnesses requiring increasing levels of care. We performed a retrospective observational analysis comparing depressive symptoms of 16,650 older individuals with partners without ADRD, and those recently (within 2 years) or less recently diagnosed (≥2 years prior), controlling for lagged sociodemographic and health characteristics. The mean number of reported depressive symptoms was 1.2 (SD=1.8). Compared to respondents with partners with no ADRD, having a partner with any ADRD was associated with a 0.35 increase (95% CI: 0.30, 0.41), or 30% increase, in depressive symptoms. A less recent partner diagnosis was associated with a 33% increase while a recent diagnosis was associated with a 27% increase. Clinically meaningful and longitudinally worsening depressive symptoms amplify the need to prioritize partner health and family-centered care following an ADRD diagnosis.
Introduction
Of an estimated $200 billion in annual U.S. care costs for Alzheimer’s disease and related dementias (ADRD), three-quarters is attributed to long-term care and home-based care provided by unpaid caregivers (Hurd, Martorell, Delavande, Mullen, & Langa, 2013; Mehta & Yeo, 2017). More than 16 million family caregivers provide an average of nearly 22 hours of informal, unpaid care per caregiver per week (Alzheimer’s Association, 2019). This substantial provision of care reflects the extensive physical, functional and behavioral issues associated with the disease that increase over time. Furthermore, informal caregiving time for care recipients with ADRD may significantly increase with worsening ADRD severity and for individuals that are partnered or married (Hajek et al., 2016).
It is widely known that family caregiving acts a chronic stressor and places considerable burden on caregivers of care recipients with debilitating medical conditions such as cancer and stroke (Bevans & Sternberg, 2012; Schulz, Beach, Czaja, Martire, & Monin, 2020). Factors associated with burden including neuropsychiatric symptoms, loss of relationship closeness and limited caregiver sense of competence are understood to negatively affect the emotional health of family members who provide supportive care (Fauth et al., 2012; van der Lee, Bakker, Duivenvoorden, & Dröes, 2017). Particularly for spousal and partner caregivers (Givens, Mezzacappa, Heeren, Yaffe, & Fredman, 2014), who comprise over 60% of ADRD caregivers (Alzheimer’s Association, 2019).
While it is believed that providing informal care for a partner with ADRD leads to poorer emotional health, the exact nature of the relationship over time is unclear. It is likely that the emotional health of the partner or spousal caregiver worsens generally, but varies over time. For instance, after an ADRD diagnosis, a partner caregiver’s emotional health status may decline just after a diagnosis but later return to its pre-diagnosis level. In support of this hypothesis, the mental health of older individuals is often unchanged following major life events (Atchley, 1989). Partner caregivers may be resilient to events, stabilizing or even improving in health even while care recipient health deteriorates (Hoffman, Burgard, Mendez-Luck, & Gaugler, 2019).
Alternatively, a partner caregiver’s emotional health may continuously decline over time due to the progressive deteriorating nature of ADRD, resulting in increased care demands of the care recipient. Because the condition of a care recipient with ADRD can critically influence health, behavioral changes related to ADRD could have cumulative effects on a partner caregiver’s emotional health over time
Prior research has observed high rates of emotional health issues (anxiety and depression incident rates as high as 60%) among spousal caregivers of individuals previously diagnosed with ADRD (Joling, 2015). Additional studies that use cross-sectional designs have reported relationships between ADRD caregiving and poor caregiver emotional health (Adams, 2008). These studies did not account for time-varying effects of caregiving and did not control for baseline depression, and thus were unable to isolate the marginal impact of an ADRD diagnosis on the emotional health of partner caregivers or identify whether the length of exposure influenced caregiver outcomes.
This study aimed to assess the temporal effects of an ADRD diagnosis on the emotional health of partner caregivers, examining both short- and lengthier associations. We used a nationally representative dataset to evaluate depressive symptoms of older adult partner caregivers of individuals who were diagnosed with ADRD less recently (≥2 years prior) and more recently (within 2 years). This study focused on older adult caregivers as they may be most subject to emotional strains of caregiving due to longer-term relationships with their partner and because of instrumental challenges (e.g. aging related health changes, limited social connections, limited access to quality and consistent healthcare) that could affect their ability to care for their partner with ADRD over time.
Methods
Data
This retrospective, observational analysis used pooled data from the 8 waves of the 2000 to 2014 Health and Retirement Study (HRS). The HRS is a longitudinal household study that surveys approximately 20,000 Americans ages 51 and older approximately every two years, with each year-specific survey defined as a wave (Health and Retirement Study, 2018). It captures sociodemographic, health and functioning information, including for both spouses/partners in households with married or partnered individuals. These data were chosen as they allow for the creation of a robust, longitudinal dataset containing linked data for each member of a spousal/partner dyad over time plus validated measures of cognitive functioning and depressive symptoms (Gianattasio, Wu, Glymour, & Power, 2019; Langa et al., 2009).
Study Population
Older respondents (ages 51 years and older) who met the following inclusion criteria were included in the study sample: completed at least two adjacent waves of the HRS from 2000-2014 (e.g., the 2002 and 2004 waves); had a spouse or partner (of any age) who also responded to the two or more adjacent surveys; and had complete data for model covariates detailed below. These criteria resulted in a final study sample of 62,457 observations for 16,650 unique older respondents. The number of observations per respondent ranged from 1-7, with an average of 3.8 (SD=2.2). Given repeated observations (HRS waves) among the respondents, the unit of analysis was a “person-wave,” or respondent-specific answers to HRS surveys collected every two years. Given inclusion in our sample of both members of spousal/partner dyads, we refer to one spouse as the “respondent” and the partner/spouse of that individual as the “partner;” in cases where the respondent’s partner was diagnosed with ADRD, we refer to the respondent as the “respondent caregiver” and the partner with ADRD as the “care recipient.”
Outcome
The primary outcome of emotional health was operationalized in this study as the number of self-reported depressive symptoms of the respondent, based upon the Center for Epidemiologic Studies Depression (CES-D) scale (Radloff, 1977), a valid and reliable indicator with a range of 0-8 (Karim, Weisz, Bibi, & ur Rehman, 2015). Respondents were asked to indicate (yes/no) whether, over the past week (prior to the survey administration), they felt the following much of the time: depressed, everything is an effort, sleep is restless, felt alone, felt sad, could not get going, felt happy, and enjoyed life. The latter two items were reverse coded and one point was then given for each yes response. A higher score indicates the presence of more depressive symptoms.
Main Predictors of Interest
The ADRD status of the respondent’s partner was the main predictor of interest, defined categorically as: no ADRD diagnosis ever, a less recent diagnosis (≥2 years) or a recent ADRD diagnosis (less than 2 years). ADRD status of the partner was identified using questions from HRS survey about whether their spouse or partner ever received a diagnosis of dementia, Alzheimer’s disease, or other memory-related disorder or had received such a diagnosis since the prior survey wave (where the average time between survey waves was approximately two years) (Gaugler, Jutkowitz, Peterson, & Zmora, 2018). ADRD categories for the respondent’s partner were then created by first coding those partners never having received an ADRD diagnosis and those having received any ADRD diagnosis. Those partners who had received an ADRD diagnosis were further categorized into recent diagnosis of < 2 years and those with a longer diagnosis of ≥2 years resulting in 3 categories of partner ADRD status: never diagnosed with ADRD; ADRD diagnosis within 2 years; ADRD diagnosis received 2 or more years earlier.
Therefore, respondents with partners reporting no ADRD initially but later diagnosed with ADRD would be categorized initially as having a partner with no ADRD but in subsequent waves as having a partner with ADRD. If the respondent’s depressive symptoms were measured in the same wave in which the partner developed ADRD, then the respondent would be considered to have “recent” exposure (<2 years); if the respondent’s depressive symptoms were measured in waves following the partner’s ADRD diagnosis, then the respondent was considered to have “longer” exposure (≥2 years). In the analysis, the never ADRD diagnosis of the respondent’s partner was the reference group.
Covariates
Depression and ADRD diagnoses are associated with a number of sociodemographic, health and functioning characteristics among older adults (Bauer, Schwarzkopf, Graessel, & Holle, 2014; Fiest, Currie, Williams, & Wang, 2011; Hybels et al., 2006). Potential confounders of the relationship between respondent depressive symptoms and the ADRD status of the partner were included as covariates in this study. Demographic variables of the respondent included age (continuous variable, ≥51), sex (dummy variable: 0=male, l=female), race/ethnicity (dummy variables for non-Hispanic white, African American, Hispanic, and other races), level of education (dummy variables for less than high school, GED or high school graduate, some college, college degree and above), and total household annual income (continuous variable). Variables relating to the health of the respondent were having ever smoked (dummy variable: 0=no, 1=yes); 7 separate dummies indicating ever having been diagnosed with the following chronic conditions (high blood pressure, diabetes, lung disease, heart disease, stroke, cancer, or arthritis); and variables reflecting current health status: number of days of drinking alcoholic beverages per week (discrete variable: 0-7); body mass index (BMI) (continuous variable), number of limitations with activities of daily living (ADLs) (discrete variable: 0-5); number of limitations with instrumental activities of daily living (IADLs) (discrete variable: 0-5); and baseline number of prior depressive symptoms (i.e., symptoms measured in the “baseline” wave, which was prior to that in which the outcome was measured). Each of these variables was measured for the older respondent (and not the partner); the only information recorded for the partner was ADRD status in the “baseline” wave.
To ensure temporality in the analysis (e.g. covariates of the respondent preceding report of her or his depressive symptoms), all time-varying covariates were lagged by one survey wave (Hoffman, Hays, Wallace, Shapiro, & Ettner, 2017). Covariates were measured in wave w-1 (or “baseline”) and the outcome and predictor of interest were measured in wave w (or “follow-up,” approximately 2 years later) (Figure 1).
Figure 1.
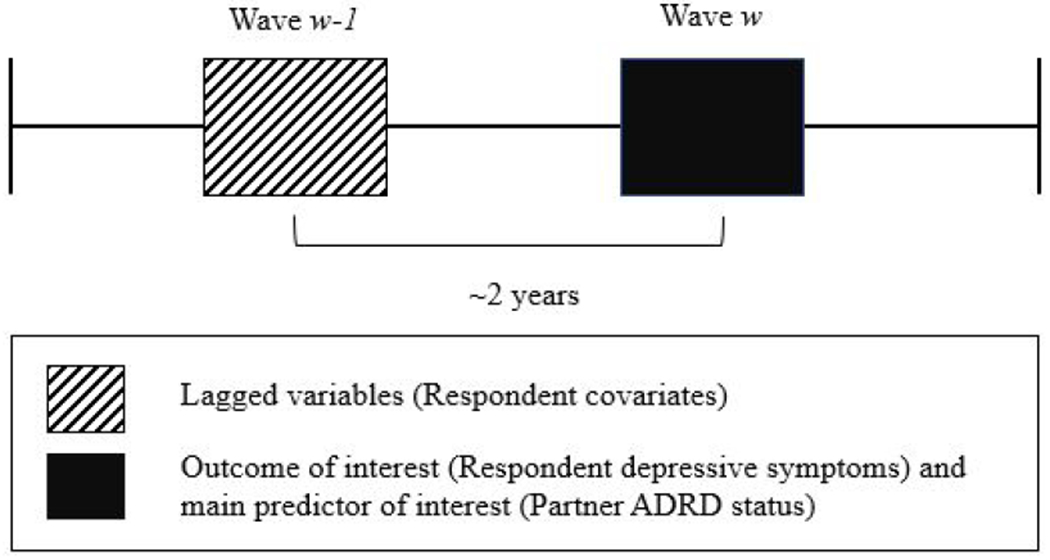
Illustration of Lagged Baseline Covariates and Follow-up Depressive Symptoms
Analysis
Descriptive statistics were calculated, overall and according to ADRD diagnosis status. Appropriate tests (chi-square, t-tests and Kruskal Wallis tests) were used to examine differences in respondent characteristics across partner ADRD diagnosis status.
Because the outcome variable – number of depressive symptoms of the respondent - had a skewed distribution, lacked equidispersion, and contained a large number of zeroes (32,188 of 62,457, or 51.5% of observations), zero-inflated negative binomial regression models were used (Hidayat & Pokhrel, 2010). Coefficients from the negative binomial regression models are incident rate ratios (IRRs), which indicate (among respondents with any probability of having depressive symptoms) the change in the respondent’s expected count of depressive symptoms according to ADRD status of the partner. Marginal effects representing incremental change in the probability of respondent depressive symptoms, after covariate adjustment, were computed using Stata’s margins command. A marginal effect of 0.30 for recent partner ADRD diagnosis would indicate that, all else equal, having a partner recently diagnosed is associated with a 0.30 increase in depressive symptoms of the respondent.
A first model regressed respondent depressive symptoms at follow-up on whether or not a partner was ever diagnosed with ADRD (regardless of diagnosis recency). A second model regressed the same respondent outcome on dummy variables indicating less recent (≥2 years) and recent (<2 years) partner ADRD diagnoses. Both models adjusted for baseline (lagged) covariates of the respondent, including depressive symptoms. Cluster-robust standard errors were used to account for the inclusion of repeated observations (person-wave) from some of the respondents. The coefficients on the predictors of interest represent the incremental change (from baseline to follow-up) in depressive symptoms for respondents associated with more recent and longer exposures, compared to no exposure, to a partner with ADRD.
In sensitivity analyses, we included two additional covariates unavailable for all years of the study period. These were respondent use of prescription medications for anxiety and/or depression (a variable available in the HRS beginning in 2006) and frequency of light physical activity (≥3 times per week, 1-2 times/week, 1-3 times per month, less than 1 time per month, and never; available beginning in 2004). Examples of light physical activity included walking, gardening, golfing, dancing and bowling. The sensitivity models used the same methods as in the primary analyses but were restricted to years 2006-2014 (controlling for prescription medications for anxiety and/or depression) and 2004-2014 (controlling for frequency of light physical activity). These findings were compared to the main models that were re-estimated using the same respective years of data as the two sensitivity models (because the years of data available were more limited for these analyses, the sample sizes were comparatively smaller than the main analyses). The same analytic sample was used in each of these sensitivity analyses to ensure appropriateness of the comparisons using the different model risk-adjustors; absent use of the same samples, differences in model coefficients could have been due to differing samples rather than differences reflecting changes in the risk-adjustment strategies.
We also re-estimated models using fixed effects specifications in order to control for within-person unobservable factors that may have biased our main results. With fixed effects, respondents serve as their own controls over time, which allows for adjustment for time-invariant factors that may confound the relationship of interest. All data were analyzed using Stata’s version IC 15.1 (StataCorp, 2017).
This study was determined to be not regulated by the affiliated university’s institutional review board.
Results
Descriptive Statistics
As shown in Table 1, across the overall sample of person-waves, respondents’ mean age was 65.6 years (SD: 8.9), the majority were female (50.5%), and non-Hispanic white (77.4%). Mean annual household income was 78,034 (SD: 126,599). The mean depressive symptoms of partner caregivers at “follow up” (wave w) and at “baseline” (wave w-1) was 1.2 (SD=1.8).
Table 1.
Sociodemographic and Health Characteristics of Respondents Overall and by Partner ADRD Status (2000-2014)
| Partner ADRD Status |
|||||
|---|---|---|---|---|---|
| Overall (n=62,457) |
No partner ADRD (n=59,805) |
Any diagnosis (n=2,652) |
Less recently diagnosed (n=1,677) |
Recently diagnosed (n=975) |
|
| Mean (SD) follow-up depressive symptoms* | 1.2 (1.8) | 1.2 (1.8) | 2.1 (2.2) | 2.2 (2.3) | 1.8 (2.1) |
| Mean (SD) baseline depressive symptoms* | 1.2 (1.8) | 1.1 (1.7) | 1.9 (2.2) | 2.1 (2.2) | 1.6 (2.1) |
| Mean (SD) age* | 65.6 (8.9) | 65.4 (8.8) | 71.5 (9.5) | 71.2 (9.8) | 72.2 (9.0) |
| Male (%)* | 49.5 | 50.1 | 37.4 | 37.5 | 37.4 |
| Race/Ethnicity (%)* | |||||
| Non-Hispanic White | 77.4 | 77.5 | 75.2 | 75.8 | 77.6 |
| African-American | 10.6 | 10.6 | 11.2 | 12.3 | 9.4 |
| Hispanic | 9.6 | 9.6 | 11.3 | 11.5 | 11.1 |
| Other races | 2.4 | 2.4 | 2.2 | 2.4 | 1.9 |
| Education (%)* | |||||
| < High school | 16.8 | 16.4 | 25.1 | 26.7 | 22.4 |
| High school | 35.2 | 35.2 | 35.5 | 34.9 | 36.7 |
| Some college | 23.0 | 23.0 | 23.0 | 22.5 | 15.9 |
| College and above | 25.0 | 25.4 | 16.4 | 15.9 | 17.2 |
| Mean (SD) household income* | 78,034 (126,599) | 79,306 (128,182) | 49,358 (77,024) | 47,829 (75,002) | 51,985 (82,680) |
| Ever smoked (% yes) | 57.0 | 57.1 | 55.1 | 54.1 | 56.8 |
| Chronic medical conditions (%) | |||||
| High blood pressure* | 57.0 | 53.2 | 65.0 | 60.9 | 60.7 |
| Diabetes* | 18.8 | 18.6 | 21.8 | 23.1 | 19.6 |
| Lung Disease* | 8.2 | 8.0 | 13.3 | 13.5 | 12.9 |
| Heart disease* | 22.2 | 21.8 | 30.1 | 31.5 | 27.6 |
| Stroke* | 4.9 | 4.8 | 7.1 | 7.7 | 6.2 |
| Cancer* | 13.1 | 12.9 | 18.0 | 18.6 | 16.8 |
| Arthritis* | 56.4 | 55.9 | 68.1 | 68.0 | 68.3 |
| Mean weekly alcohol consumption (number days) (SD)* | 1.3 (2.2) | 1.3 (2.2) | 0.9 (2.0) | 0.9 (1.9) | 1.0 (2.0) |
| Mean BMI (SD)* | 28.2 (5.5) | 28.2 (5.5) | 27.6 (5.8) | 27.7 (5.9) | 27.4 (5.5) |
| Mean (SD) ADL limitations* | 0.2 (0.6) | 0.2 (0.6) | 0.4 (0.9) | 0.4 (0.9) | 0.3 (0.8) |
| Mean (SD) IADL limitations* | 0.1 (0.5) | 0.1 (0.5) | 0.2 (0.7) | 0.3 (0.7) | 0.2 (0.6) |
p <0.05
Note: Characteristics are measured for older respondents. Respondent characteristics included in the column indicating partner with any ADRD diagnosis (‘Any diagnosis’) includes respondents who had a partner with either a recent or less recent ADRD diagnosis. Partner less recently diagnosed indicates a respondent who had a partner that received any ADRD diagnosis 2 or more years earlier. Partner recently diagnosed indicates a respondent who had a partner that received any ADRD diagnosis in the last 2 years. Follow-up depressive symptoms for respondents were those reported at wave w, while baseline depressive symptoms were those reported at wave w-1. For instance, wave w could indicate the 2004 HRS wave while wave w-1 could indicate the 2002 HRS wave. ADL = activities of daily living, ADRD = Alzheimer’s Disease or Related Dementias, BMI = body mass index, IADLs = instrumental activities of daily living, SD = standard deviation.
Compared to the 59,805 observations of respondents with partners without ADRD, those with a partner with any ADRD (recent or less recent) were older (71.5, SD: 9.5 vs. 65.4 , SD: 8.8) (p<0.001), were more often women (62.6% vs. 49.1%, p<0.001), more often of minority racial/ethnic background (24.8% vs. 22.5%, p=0.01) and were less educated (22.4% vs. 16.4%, p<0.001). Mean annual household income was also lower for respondents with partners who had any ADRD (whether diagnosed more or less recently) ($49,358, SD=$77,024) compared to those with partners with no ADRD ($79,306, SD=$128,182) (p<0.001). More respondents who had partners with any ADRD diagnoses (recent or less recent) compared to those with partners with no ADRD diagnosis reported having chronic medical conditions. They also reported more difficulties with ADLs (0.4, SD: 0.9 vs. 0.2, SD: 0.6) and IADLs (0.2, SD: 0.7 vs. 0.1, SD: 0.5).
Adjusted Results
A first model compared depressive symptoms at “follow-up” for respondents whose partner had any diagnosis (regardless of recency) versus no ADRD diagnosis, controlling for “baseline” covariates. Being a respondent with a partner having versus not having any ADRD diagnosis increased the number of respondent depressive symptoms by a factor of 1.3 (IRR:1.30, p<0.001) (Table 2). This was equivalent to respondents with partners with any ADRD having 0.35 (p<0.001) more depressive symptoms (marginal difference) than those with partners with no ADRD diagnosis. Given a mean of 1.15 symptoms among respondents in the no ADRD diagnosis group, this amounted to a [0.35/1.15 =] 30% increase in respondent depressive symptoms.
Table 2.
Incident Rate Ratios, Marginal Differences, and Percentage Change in Respondent Depressive Symptoms according to Partner ADRD Status, 2000-2014 (n=62,457)
| Partner ADRD Status |
Incident Rate Ratio [95% CI] |
Marginal Difference [95% CI] |
p |
Percentage Change (%) |
|---|---|---|---|---|
| 1.30 | 0.35 | |||
| Any Diagnosis | [1.25, 1.35] | [0.30, 0.41] | <0.001 | 30 |
| Less Recent | 1.32 | 0.38 | ||
| Diagnosis | [1.26, 1.38] | [0.31, 0.44] | <0.001 | 33 |
| 1.27 | 0.31 | |||
| Recent Diagnosis | [1.19, 1.35] | [0.22, 0.41] | <0.001 | 27 |
Note: Partner with any diagnosis includes those with a recent or less recent diagnosis. A less recent diagnosis indicates a respondent who had a partner that received any ADRD diagnosis 2 or more years earlier. A recent diagnosis indicates a respondent who had a partner that received any ADRD diagnosis in the last 2 years. Two separate zero-inflated negative binomial models were estimated. The first model’s results are presented in the first row. Results from the second model, which compared recent and less recent partner ADRD diagnosis with no partner ADRD diagnosis, are presented in the second and third rows. Percentage changes are based on marginal effects given a mean of 1.15 depressive symptoms among caregivers of partners without ADRD (e.g., [0.35/1.15]=30%).
A second model compared the outcome of respondent depressive symptoms at follow-up by recency of partner ADRD diagnosis, controlling for baseline covariates. Compared to respondents whose partners did not have any ADRD diagnosis, having a partner less recently diagnosed increased the number of respondent depressive symptoms (IRR 1.32; marginal increase of 0.38 depressive symptoms,p<0.001), as did having a partner more recently diagnosed (IRR: 1.27; marginal increase of 0.31 depressive symptoms, p<0.001) (Table 2). Based on these marginal effects, percentage increases in respondents’ depressive symptoms when having a partner with less and more recent ADRD diagnoses represented [0.38/1.15 =] 33% and [0.31/1.15 =] 27% average increases in respondents’ depressive symptoms, respectively.
Sensitivity Models
Results were robust to sensitivity analyses, with model coefficients nearly the same in models controlling for additional covariates or using fixed effects specifications when compared to the main models (see Appendix A).
Discussion
In this study of the effect of length of exposure following a partner’s ADRD diagnosis on older American’s depressive symptoms, we report two main findings. First, having a partner with any ADRD diagnosis compared to no ADRD diagnosis was associated with a 30% overall increase in depressive symptoms of caregivers. Second, the increases in depressive symptoms of caregivers were greater when they had longer exposure to a partner’s ADRD diagnosis, with 33% versus 27% increases in depressive symptoms for caregivers with partners with less recent (≥ 2 years) and more recent (<2 years) ADRD diagnoses, respectively. To our knowledge, these are the first estimates of the incremental effects of diagnosis of ADRD on a partner caregiver’s emotional health in a large, nationally representative sample. Collectively, these findings highlight the cumulative, clinically meaningful impact of a partner’s ADRD diagnosis on the emotional health of older partner caregivers.
Prior research has observed a substantially increased incidence (37%−48%) of diagnosed depressive disorders associated with older adults having a spouse with ADRD (Ballard, Eastwood, Gahir, & Wilcock, 1996; Joling et al., 2015). However, these studies recruited many spousal caregivers that already had elevated depressive symptoms at the time of study recruitment. Because individuals with high baseline depressive symptoms are more likely to reach a clinical threshold for depression, these studies’ estimates of clinical depression associated with ADRD care were unlikely to have captured the incremental effects of a partner’s ADRD diagnosis on spousal health.
However, the 30% incremental increases in depressive symptoms we observed in this study are clinically meaningful (Jensen et al., 2017) and concerning; for instance, the 0.38 increase for longer-exposed spouses represents ~30% of the non-exposed sample’s standard deviation, where ≥30% suggests clinical significance (McLeod, Cappelleri, & Hays, 2016). A 0.5-standard deviation increase in depressive symptoms of older adults has previously been linked to a 30% increase in falls (Hoffman, Hays, Wallace, Shapiro, & Ettner, 2017), while poorer health (Han, 2002) and cognitive decline (Köhler et al., 2010) can also result from such increased depressive symptoms among older adults. In all, the effects of an ADRD diagnosis are sizeable and grow over time, with implications for partner welfare as well partners’ ability to provide quality care to individuals with ADRD; this may be particularly important given the growing size of the older adult population who will be living longer with chronic conditions including ADRD.
Earlier work identifies as important the closeness of family relationships for health outcomes including attenuated declines for individuals with dementia in emotionally close relationships, particularly among spouses (Norton et al., 2009). A substantial literature has also identified interdependence in the health outcomes, or shared trajectories of health, among older adult dyads including dementia caregivers and their care recipients (Kershaw et al., 2015). Our findings reinforce the idea of these dynamic influences on the health of individuals within family dyads (Elder, 1995; Kim & Moen, 2002) and may additionally illustrate the persistent investments individuals make in their partners’ welfare (Rusbult & Buunk, 1993). The intimacy of the spousal relationship may explain partners’ sustained commitments of support and caregiving despite the growing emotional costs of helping a partner with a progressive disease, in turn heightening the need to “care for the caregiver” as behavioral and care needs exacerbate over time.
Limitations
This study had several limitations. First, we pooled data from multiple survey waves to expand the study sample and thereby increase statistical power. This use of repeated observations could reduce standard errors, resulting in falsely positive associations between partner ADRD status and depressive symptoms of caregivers. However, we clustered standard errors to address this concern, as is often done; moreover, sensitivity analyses using fixed effects models that explicitly modeled the hierarchical data were performed, with similar results to the main models. Therefore, the large sample size should be seen as a strength, particularly in light of prior work in this area that has used convenience samples with fewer than several hundred study participants (Ballard, Eastwood, Gahir, & Wilcock, 1996; Joling et al., 2015).
Second, for our primary predictor of interest, we identified ADRD by combining responses for dementia, Alzheimer’s disease and memory-related diseases. Different dementia types are associated with varying degrees of decline and behavioral disturbances (Gill et al.,2013; Steinberg et al., 2006), which can differentially affect caregiver burden and emotional health (Campbell et al., 2008). Delineating different ADRD types in clinical practice is often difficult and can result in multiple dementia types being comprised under a single dementia label. For clinical purposes, then, understanding the incremental impact of any of these dementia-related diagnoses for a partner caregiver’s health is of importance; however, this study was unable to evaluate the impacts of specific types of ADRD diagnoses on partner caregiver emotional health
Third, this analysis did not explore the age of dementia onset as a covariate. This may be an important factor to explore in future research to further understand how early versus later onset diagnoses may differentially affect spousal caregivers, as younger onset dementia may be associated with greater caregiver depressive symptoms compared to diagnoses confirmed later in life (Millenaar et al., 2016).
Fourth, we used self- and proxy-reported diagnoses of ADRD, which may not reflect when the disease first presented. Cognitive declines also likely preceded ADRD diagnoses, meaning caregivers’ burdens may have already increased prior to any diagnosis. If anything, this would result in an underestimation of the impact of a diagnosis on spousal mental health, given high levels of burden experienced by caregivers of individuals with mild cognitive impairment (Connors et al., 2019).
These limitations notwithstanding, the large and growing cumulative impact of an individual’s ADRD diagnosis on a partner’s emotional health is suggestive of critical, potentially unmet emotional health needs for older Americans caring for partners with ADRD. The interdependent nature of health between both members of the dyad observed in this study and earlier research further support the increasingly recognized need for clinical, family-centered care planning throughout the disease process in consideration of needs of both clinically treated individuals and their partner caregivers (Griffin et al., 2019; Lyons & Lee, 2018; Noel, Kaluzynski, & Templeton, 2015). Absent such efforts, patients with ADRD may have less than optimal support from family members.
This research magnifies the need for including family caregivers into care plans involving progressive chronic illness by exemplifying the interdependent, dynamic relationship between caregiver emotional health and care recipient cognitive health. Programs designed for couples affected by ADRD should be prioritized that encompass the needs of both partners independently, as well as the well-being of the couple as a dyad (Hill, Yeates, & Donovan, 2018). Interventions designed for dyads in early stages of ADRD are important and can be effective (Whitlatch, Judge, Zarit, & Femia, 2006), but longer-term initiatives to support the dyad throughout the lifetime of the disease should be further substantiated. Absent recognition and ongoing support of caregivers’ investments and potential emotional declines during care recipients’ disease progression, the quality of care support may suffer, along with the well-being of the caregiver; providers should not expect, then, that the level of informal support will necessarily be consistent over time during the course of the disease.
From a policy perspective, the results portend more challenges for family caregivers given the growth of the older adult ADRD population (Matthews et al., 2019), even as programs to support caregivers remain poorly funded. Limited or conditional coverage for resources such as respite care for family caregivers through Medicare and state-level programs including the Program of All-inclusive Care for the Elderly and Medicaid waivers restrict possibilities for addressing caregiver health as a national priority issue of public health urgency. More funding and appropriate coverage for interventions shown to be effective in promoting caregiver emotional health, such as respite care, family daycare, optimism training and psychosocial support programs (De Oliveira, Sousa, & Orrell, 2019; Diaz, Ponsoda, & Belena, 2020; Tretteteig, Vatne, & Rokstad, 2016; Zhong, Wang, & Nicholas, 2020), are needed.
Conclusion
This nationally representative study observed depressive symptoms of older individuals increasing by 30% or more after the diagnosis of ADRD in a partner, with larger impacts following longer exposures following a partner’s ADRD diagnosis. The findings demonstrate the cumulative and clinically meaningful effects of partner ADRD on depressive symptoms among older partner caregivers during the development of a progressive disease. Clinicians and policymakers should prioritize family-centered health care practices and policies that can improve just-in-time and ongoing supportive services and mental health care for the vulnerable population of individuals diagnosed with ADRD, as well as their partner caregivers.
Acknowledgments
Funding: Predoctoral Fellowship Training Grant (2018-Current). T32 NR016914 Complexity: Innovations in Promoting Health and Safety. (Program Director: Titler)
Appendix A
Table S1.
Associations Between Partner Dementia Status and Respondent Depressive Symptoms, 2006-2014 (n=28,989)
| Partner ADRD Status |
Respondent Depressive Symptoms (controlling for anxiety/depression medication use) |
|||
|---|---|---|---|---|
| Incident Rate Ratio [95% CI] |
Marginal Difference [95% CI] |
p |
Percent Change (%) |
|
| Less Recent | 1.27 | 0.34 | ||
| Diagnosis | [1.19, 1.36] | [0.23, 0.45] | <0.001 | 28 |
| 1.20 | 0.25 | |||
| Recent Diagnosis | [1.10, 1.32] | [0.12, 0.39] | <0.001 | 20 |
| Respondent Depressive Symptoms (not controlling for anxiety/depression medication use) |
||||
| Less Recent | 1.29 | 0.35 | ||
| Diagnosis | [1.20, 1.38] | [0.24, 0.46] | <0.001 | 29 |
| 1.22 | 0.27 | |||
| Recent Diagnosis | [1.11, 1.34] | [0.13, 0.41] | <0.001 | 22 |
Note: A less recent diagnosis indicates a respondent who had a partner that received any ADRD diagnosis 2 or more years earlier. A recent diagnosis indicates a respondent who had a partner that received any ADRD diagnosis in the last 2 years. Two separate zero-inflated negative binomial models were estimated comparing recent and less recent partner ADRD diagnosis with no partner ADRD diagnosis. The results presented in the first 2 rows are when controlling for anxiety/depression prescription medication use. Results in the second 2 rows do not control for use of anxiety/depression prescription medications. Percentage changes are based on marginal effects given a mean of 1.22 depressive symptoms among respondents of partners without ADRD (e.g. [0.34/1.22]=30%).
Table S2.
Associations Between Partner Dementia Status and Respondent Depressive Symptoms, 2004-2014 (n=44,965)
| Partner ADRD Status |
Respondent Depressive Symptoms (controlling frequency of light physical activity) |
|||
|---|---|---|---|---|
| Incident Rate Ratio [95% CI] |
Marginal Difference [95% CI] |
p |
Percent Change (%) |
|
| Less Recent | 1.33 | 0.38 | ||
| Diagnosis | [1.26, 1.40] | [0.30, 0.46] | <0.001 | 33 |
| Recent Diagnosis | 1.23 | 0.27 | ||
| [1.14, 1.33] | [0.17, 0.38] | <0.001 | 23 | |
| Respondent Depressive Symptoms (not controlling for frequency of light physical activity) |
||||
| Less Recent | 1.32 | 0.38 | ||
| Diagnosis | [1.25,1.40] | [0.30, 0.46] | <0.001 | 33 |
| Recent Diagnosis | 1.23 | 0.27 | ||
| [1.14, 1.33] | [0.17, 0.38] | <0.001 | 23 | |
Note: A less recent diagnosis indicates a respondent who had a partner that received any ADRD diagnosis 2 or more years earlier. A recent diagnosis indicates a respondent who had a partner that received any ADRD diagnosis in the last 2 years. Two separate zero-inflated negative binomial models were estimated comparing recent and less recent partner ADRD diagnosis with no partner ADRD diagnosis. The results presented in the first 2 rows are when controlling for frequency of light physical activity. Results in the second 2 rows do not control for frequency of light physical activity. Percentage changes are based on marginal effects given a mean of 1.15 depressive symptoms among respondents of partners without ADRD (e.g. [0.38/1.15]=30%).
Table S3.
Caregiver Depressive Symptoms according to Partner ADRD Status Estimated Using Fixed Effects Specifications, 2000-2014 (n=46,931)
| Partner ADRD Status |
Incident Rate Ratio [95% CI] |
Marginal Difference [95% CI] |
p |
Percent Change (%) |
|---|---|---|---|---|
| 1.26 | 0.23 | |||
| Any Diagnosis | [1.18, 1.34] | [0.17, 0.29] | <0.001 | 16 |
| 1.32 | 0.28 | |||
| Less Recent Diagnosis | [1.22, 1.42] | [0.20, 0.35] | <0.001 | 19 |
| 1.20 | 0.19 | |||
| Recent Diagnosis | [1.12, 1.30] | [0.11, 0.23] | <0.001 | 13 |
Note: Partner with any diagnosis includes those with a recent or less recent diagnosis. A less recent diagnosis indicates a respondent who had a partner that received any ADRD diagnosis 2 or more years earlier. A recent diagnosis indicates a respondent who had a partner that received any ADRD diagnosis in the last 2 years. Two separate zero-inflated negative binomial models were estimated. The first model’s results are presented in the row titled “Any Diagnosis”, which compared respondents with partners with a partner diagnosed with any ADRD, and those with partners never reporting a diagnosis of ADRD. Results from the second model, which compared respondents with less recently diagnosed partners and those with partners recently diagnosed with respondents with no partner ADRD diagnosis, are presented in the rows titled “Less Recent Diagnosis” and “Recent Diagnosis”, respectively. Percentage changes are based on marginal effects given a mean of 1.44 depressive symptoms among caregivers of partners without ADRD (e.g. [0.23/1.44]=16%).
Footnotes
Conflict of Interest: All authors report no potential or perceived COIs.
This study used publicly available, already de-identified data and was determined to be not regulated by the IRB at the University of Michigan.
Contributor Information
Melissa L. Harris, University of Michigan School of Nursing, 400 N. Ingalls St. Ann Arbor, MI 48109.
Marita G. Titler, University of Michigan School of Nursing, 400 N. Ingalls St. Ann Arbor, MI 48109.
Geoffrey J. Hoffman, University of Michigan School of Nursing, 400 N. Ingalls St. Ann Arbor, MI 48109.
References
- Adams KB (2008). Specific effects of caring for a spouse with dementia: differences in depressive symptoms between caregiver and non-caregiver spouses. International Psychogeriatrics, 20(3), 508–520. doi: 10.1017/S1041610207006278 [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association. (2019). Alzheimer’s Disease Facts and Figures. Retrieved from Chicago, IL: https://www.alz.org/media/Documents/alzheimers-facts-and-figures.pdf [Google Scholar]
- Atchley RC (1989). A Continuity Theory of Normal Aging. The Gerontologist, 29(2), 183–190. doi: 10.1093/geront/29.2.183 [DOI] [PubMed] [Google Scholar]
- Ballard CG, Eastwood C, Gahir M, & Wilcock G (1996). A follow up study of depression in the carers of dementia sufferers. BMJ, 312(7036), 947. doi: 10.1136/bmj.312.7036.947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer K, Schwarzkopf L, Graessel E, & Holle R (2014). A claims data-based comparison of comorbidity in individuals with and Campbell P, Wright J, Oyebode J, Job D, Crome P, Bentham P, … Lendon C, (2008). Determinants of burden in those who care for someone with dementia. International Journal of Geriatric Psychiatry, 23(10), 1078–1085. doi:doi: 10.1002/gps.2071 [DOI] [PubMed] [Google Scholar]
- Bevans M, & Sternberg EM (2012). Caregiving burden, stress, and health effects among family caregivers of adult cancer patients. JAMA, 307(4), 398–403. doi: 10.1001/jama.2012.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors MH, Seeher K, Teixeira-Pinto A, Woodward M, Ames D, & Brodaty H (2019). Mild Cognitive Impairment and Caregiver Burden: A 3-Year-Longitudinal Study. The American Journal of Geriatric Psychiatry, 27(11), 1206–1215. doi: 10.1016/j.jagp.2019.05.012 [DOI] [PubMed] [Google Scholar]
- De Oliveira D, Sousa L, & Orrell M (2019). Improving health-promoting self-care in family carers of people with dementia: A review of interventions. Clinical Interventions in Aging Volume 14. doi: 10.2147/CIA.S190610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz A, Ponsoda JM, & Belena A (2020). Optimism as a key to improving mental health in family caregivers of people living with Alzheimer’s disease. Aging and Mental Health, 1–9. doi: 10.1080/13607863.2020.1715342 [DOI] [PubMed] [Google Scholar]
- Elder JGH (1995). The life course paradigm: Social change and individual development. In Examining lives in context: Perspectives on the ecology of human development. (pp. 101–139). Washington, DC, US: American Psychological Association. [Google Scholar]
- Fauth E, Hess K, Piercy K, Norton M, Corcoran C, Rabins P, … Tschanz J (2012). Caregivers’ relationship closeness with the person with dementia predicts both positive and negative outcomes for caregivers’ physical health and psychological well-being. Aging & Mental Health, 16(6), 699–711. doi: 10.1080/13607863.2012.678482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiest KM, Currie SR, Williams JVA, & Wang J (2011). Chronic conditions and major depression in community-dwelling older adults. Journal of Affective Disorders, 131(1), 172–178. doi: 10.1016/j.jad.2010.11.028 [DOI] [PubMed] [Google Scholar]
- Gaugler JE, Jutkowitz E, Peterson CM, & Zmora R (2018). Caregivers dying before care recipients with dementia. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 4, 688–693. doi: 10.1016/j.trci.2018.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianattasio KZ, Wu Q, Glymour MM, & Power MC (2019). Comparison of Methods for Algorithmic Classification of Dementia Status in the Health and Retirement Study. Epidemiology (Cambridge, Mass.), 30(2), 291–302. doi: 10.1097/EDE.0000000000000945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill DP, Hubbard RA, Koepsell TD, Borrie MJ, Petrella RJ, Knopman DS, & Kukull WA (2013). Differences in rate of functional decline across three dementia types. Alzheimer’s & Dementia, 9(5, Supplement), S63–S71. doi: 10.1016/j.jalz.2012.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givens JL, Mezzacappa C, Heeren T, Yaffe K, & Fredman L (2014). Depressive Symptoms Among Dementia Caregivers: Role of Mediating Factors. The American Journal of Geriatric Psychiatry, 22(5), 481–488. doi: 10.1016/j.jagp.2012.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JM, Riffin C, Havyer RD, Biggar VS, Comer M, Frangiosa TL, & Bangerter LR (2019). Integrating Family Caregivers of People With Alzheimer’s Disease and Dementias into Clinical Appointments: Identifying Potential Best Practices. Journal of Applied Gerontology, 0733464819880449. doi: 10.1177/0733464819880449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek A, Brettschneider C, Ernst A, Posselt T, Wiese B, Prokein J, … König H-H (2016). Longitudinal predictors of informal and formal caregiving time in community-dwelling dementia patients. Social Psychiatry and Psychiatric Epidemiology, 51(4), 607–616. doi: 10.1007/s00127-015-1138-7 [DOI] [PubMed] [Google Scholar]
- Han B (2002). Depressive Symptoms and Self-Rated Health in Community-Dwelling Older Adults: A Longitudinal Study. Journal of the American Geriatrics Society, 50(9), 1549–1556. doi: 10.1046/j.1532-5415.2002.50411.x [DOI] [PubMed] [Google Scholar]
- Health and Retirement Study, (RAND HRS) public use dataset. Produced and distributed by the University of Michigan with funding from the National Institute on Aging (grant number NIA U01AG009740). Ann Arbor, MI, (2018). [Google Scholar]
- Hidayat B, & Pokhrel S (2010). The selection of an appropriate count data model for modelling health insurance and health care demand: case of Indonesia. International journal of environmental research and public health, 7(1), 9–27. doi: 10.3390/ijerph7010009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill H, Yeates S, & Donovan J (2018). You, me, us – Creating connection: Report on a program to support and empower couples to navigate the challenges of dementia (innovative practice). Dementia, 1471301218786592. doi: 10.1177/1471301218786592 [DOI] [PubMed] [Google Scholar]
- Hoffman GJ, Burgard S, Mendez-Luck CA, & Gaugler JE (2019). Interdependence in Health and Functioning Among Older Spousal Caregivers and Care Recipients. Western Journal of Nursing Research, 41(5), 685–703. doi: 10.1177/0193945918781057 [DOI] [PubMed] [Google Scholar]
- Hoffman GJ, Hays RD, Wallace SP, Shapiro MF, & Ettner SL (2017). Depressive symptomatology and fall risk among community-dwelling older adults. Social Science & Medicine, 178, 206–213. doi: 10.1016/j.socscimed.2017.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd MD, Martorell P, Delavande A, Mullen KJ, & Langa KM (2013). Monetary costs of dementia in the United States. The New England Journal of Medicine, 368(14), 1326–1334. doi: 10.1056/NEJMsa1204629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hybels CF, Blazer DG, Pieper CF, Burchett BM, Hays JC, Fillenbaum GG, … Berkman LF (2006). Sociodemographic Characteristics of the Neighborhood and Depressive Symptoms in Older Adults: Using Multilevel Modeling in Geriatric Psychiatry. The American Journal of Geriatric Psychiatry, 14(6), 498–506. doi: 10.1097/01.JGP.0000194649.49784.29 [DOI] [PubMed] [Google Scholar]
- Jensen RE, Moinpour CM, Potosky AL, Lobo T, Hahn EA, Hays RD, … Eton DT (2017). Responsiveness of 8 Patient-Reported Outcomes Measurement Information System (PROMIS) measures in a large, community-based cancer study cohort. Cancer, 123(2), 327–335. doi: 10.1002/cncr.30354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joling KJ, van Marwijk HWJ, Veldhuijzen AE, van der Horst HE, Scheltens P, Smit F, & van Hout HPJ (2015). The Two-Year Incidence of Depression and Anxiety Disorders in Spousal Caregivers of Persons with Dementia: Who is at the Greatest Risk? The American Journal of Geriatric Psychiatry, 23(3), 293–303. doi: 10.1016/j.jagp.2014.05.005 [DOI] [PubMed] [Google Scholar]
- Karim J, Weisz R, Bibi Z, & ur Rehman S (2015). Validation of the Eight-Item Center for Epidemiologic Studies Depression Scale (CES-D) Among Older Adults. Current Psychology, 34(4), 681–692. doi: 10.1007/s12144-014-9281-y [DOI] [Google Scholar]
- Kershaw T, Ellis KR, Yoon H, Schafenacker A, Katapodi M, & Northouse L (2015). The Interdependence of Advanced Cancer Patients’ and Their Family Caregivers’ Mental Health, Physical Health, and Self-Efficacy over Time. Annals of Behavioral Medicine, 49(6), 901–911. doi: 10.1007/s12160-015-9743-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, & Moen P (2002). Retirement Transitions, Gender, and Psychological Well-Being: A Life-Course, Ecological Model. The Journals of Gerontology: Series B, 57(3), P212–P222. doi: 10.1093/geronb/57.3.P212 [DOI] [PubMed] [Google Scholar]
- Köhler S, van Boxtel MPJ, van Os J, Thomas AJ, O’Brien JT, Jolles J, …Allardyce J (2010). Depressive Symptoms and Cognitive Decline in Community-Dwelling Older Adults. Journal of the American Geriatrics Society, 58(5), 873–879. doi: 10.1111/j.1532-5415.2010.02807.x [DOI] [PubMed] [Google Scholar]
- Langa KM, Llewellyn DJ, Lang IA, Weir DR, Wallace RB, Kabeto MU, & Huppert FA (2009). Cognitive health among older adults in the United States and in England. BMC Geriatrics, 9(1), 23. doi: 10.1186/1471-2318-9-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lethin C, Renom-Guiteras A, Zwakhalen S, Soto-Martin M, Saks K, Zabalegui A, … Karlsson S (2017). Psychological well-being over time among informal caregivers caring for persons with dementia living at home. Aging & Mental Health, 21(11), 1138–1146. doi: 10.1080/13607863.2016.1211621 [DOI] [PubMed] [Google Scholar]
- Lyons KS, & Lee CS (2018). The Theory of Dyadic Illness Management. Journal of Family Nursing 24(1), 8–28. doi: 10.1177/1074840717745669 [DOI] [PubMed] [Google Scholar]
- Matthews KA, Xu W, Gaglioti AH, Holt JB, Croft JB, Mack D, & McGuire LC (2019). Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimer’s & Dementia, 75(1), 17–24. doi: 10.1016/j.jalz.2018.06.3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod LD, Cappelleri JC, & Hays RD (2016). Best (but oft-forgotten) practices: expressing and interpreting associations and effect sizes in clinical outcome assessments. The American Journal of Clinical Nutrition, 103(3), 685–693. doi: 10.3945/ajcn.115.120378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta KM, & Yeo GW (2017). Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 73(1), 72–83. doi: 10.1016/j.jalz.2016.06.2360 [DOI] [PubMed] [Google Scholar]
- Millenaar JK, de Vugt ΜE, Bakker C, van Vliet D, Pijnenburg YAL, Koopmans RTCM, & Verhey FRJ (2016). The Impact of Young Onset Dementia on Informal Caregivers Compared with Late Onset Dementia: Results from the NeedYD Study. The American Journal of Geriatric Psychiatry, 24(6), 467–474. doi: 10.1016/j.jagp.2015.07.005 [DOI] [PubMed] [Google Scholar]
- Monin J, Doyle M, Levy B, Schulz R, Fried T, & Kershaw T (2016). Spousal Associations Between Frailty and Depressive Symptoms: Longitudinal Findings from the Cardiovascular Health Study. Journal of the American Geriatrics Society, 64(4), 824–830. doi: 10.1111/jgs.14023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel MA, Kaluzynski TS, & Templeton VH (2015). Quality Dementia Care: Integrating Caregivers Into a Chronic Disease Management Model. Journal of Applied Gerontology, 36(2), 195–212. doi: 10.1177/0733464815589986 [DOI] [PubMed] [Google Scholar]
- Norton MC, Piercy KW, Rabins PV, Green RC, Breitner JCS, Østbye T, … Tschanz JT (2009). Caregiver-Recipient Closeness and Symptom Progression in Alzheimer Disease. The Cache County Dementia Progression Study. The Journals of Gerontology: Series B, 64B(5), 560–568. doi: 10.1093/geronb/gbp052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinquart M, & Sörensen S (2006). Helping caregivers of persons with dementia: which interventions work and how large are their effects? International Psychogeriatrics, 18(4), 577–595. doi: 10.1017/S1041610206003462 [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement, 1(3), 385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- Rusbult CE, & Buunk BP (1993). Commitment Processes in Close Relationships: An Interdependence Analysis. Journal of Social and Personal Relationships, 10(2), 175–204. doi: 10.1177/026540759301000202 [DOI] [Google Scholar]
- Schulz R, Beach SR, Czaja SJ, Martire LM, & Monin JK (2020). Family Caregiving for Older Adults. Annual Review of Psychology, 71(1), 635–659. doi: 10.1146/annurev-psych-010419-050754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Beach SR, Hebert RS, Martire LM, Monin JK, Tompkins CA, & Albert SM (2009). Spousal Suffering and Partner’s Depression and Cardiovascular Disease: The Cardiovascular Health Study. The American Journal of Geriatric Psychiatry, 17(3), 246–254. doi: 10.1097/JGP.0b013e318198775b [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC [Google Scholar]
- Steinberg M, Corcoran C, Tschanz JT, Huber C, Welsh-Bohmer K, Norton MC, … Lyketsos CG (2006). Risk factors for neuropsychiatric symptoms in dementia: the Cache County Study. International Journal of Geriatric Psychiatry, 21(9), 824–830. doi: 10.1002/gps.1567ataCorp. 2017. [DOI] [PubMed] [Google Scholar]
- Tretteteig S, Vatne S, & Rokstad AMM (2016). The influence of day care centres for people with dementia on family caregivers: an integrative review of the literature. Aging & Mental Health, 20(5), 450–462. doi: 10.1080/13607863.2015.1023765 [DOI] [PubMed] [Google Scholar]
- van der Lee J, Bakker TJEM, Duivenvoorden HJ, & Droes R-M (2017). Do determinants of burden and emotional distress in dementia caregivers change over time? Aging & Mental Health, 21(3), 232–240. doi: 10.1080/13607863.2015.1102196 [DOI] [PubMed] [Google Scholar]
- Whitlatch CJ, Judge K, Zarit SH, & Femia E (2006). Dyadic Intervention for Family Caregivers and Care Receivers in Early-Stage Dementia. The Gerontologist, 46(5), 688–694. doi: 10.1093/geront/46.5.688 [DOI] [PubMed] [Google Scholar]
- Zhong Y, Wang J, & Nicholas S (2020). Social support and depressive symptoms among family caregivers of older people with disabilities in four provinces of urban China: the mediating role of caregiver burden. BMC Geriatrics, 20(1), 3. doi: 10.1186/s12877-019-1403-9 [DOI] [PMC free article] [PubMed] [Google Scholar]


