Abstract
Background & Aims:
Magnetic resonance imaging proton density fat fraction (MRI-PDFF) offers promise as a non-invasive biomarker of treatment response in early-phase nonalcoholic steatohepatitis (NASH) trials. We performed a systematic review to quantify the association between a ≥ 30% reduction in MRI-PDFF and histologic response in NASH.
Methods:
We searched the Cochrane Library, Embase, Medline and trial registries through May 2020 for early-phase clinical trials that incorporated MRI-PDFF and examined histologic response following intervention in adults with NASH. Subjects were classified as MRI-PDFF responders (relative decline in liver fat ≥30%) or non-responders (relative decline in liver fat <30%). MRI-PDFF responders versus non-responders were compared. Primary outcome was histologic response defined as a 2-point improvement in NAFLD Activity Score with at least 1-point improvement in lobular inflammation or ballooning. Secondary outcome was NASH resolution. Proportions and random effects odds ratios (OR) with corresponding 95% confidence intervals (CI) were calculated.
Results:
Seven studies met inclusion criteria, comprising 346 subjects (median age 51 years; 59% female; 46% with diabetes). MRI-PDFF responders were significantly more likely to have a histologic response (51% vs 14%, p <0.001; OR 6.98, 95% CI 2.38–20.43, p<0.001) and NASH resolution (41% vs 7%, p<0.001; OR 5.45, 95% CI 1.53–19.46, p=0.009) compared to non-responders.
Conclusions:
This meta-analysis demonstrates that a ≥30% relative decline in MRI-PDFF is associated with higher odds of histologic response and NASH resolution. These results support the use of MRI-PDFF in non-invasive monitoring of treatment response in early-phase NASH clinical trials and provide helpful data for sample-size estimation for histology-based assessment.
Keywords: steatohepatitis, clinical trial, steatosis, liver biopsy, histology
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the leading cause of chronic liver disease worldwide affecting 30% of the global population.1 NAFLD is an umbrella term that includes a spectrum of histologic features ranging from nonalcoholic fatty liver (NAFL), the non-progressive form of NAFLD, to steatohepatitis (NASH), the progressive form of NAFLD.2 NASH is typically characterized by presence of hepatic steatosis in zone-3, lobular inflammation, and ballooning, with or without peri-sinusoidal fibrosis. Patients with NASH can have a progressive course leading to cirrhosis and the consequences of end-stage liver disease including hepatocellular carcinoma.3–5 Several therapies are in various stages of clinical development for the treatment of NASH but none are U.S. Food and Drug Administration (FDA) approved.
Liver biopsy with histology assessment is the gold standard for diagnosis and assessment of treatment response in NASH for clinical care and in clinical trials. Biopsy is required by the FDA for all NASH clinical trials seeking conditional approval of pharmacologic therapy. The NASH Clinical Research Network (NASH CRN) Histologic Scoring System provides criteria for scoring key features of NAFLD including steatosis, inflammation, ballooning, and fibrosis. The NAFLD Activity Score (NAS) is a summary score including steatosis (0–3), lobular inflammation (0–3) and ballooning (0–2).6 Liver biopsy is currently a required endpoint for clinical trials to document histologic improvement.7 Two accepted histologic endpoints in NASH clinical trials are a 2-point improvement in NAS (with at least one point derived from improvement in lobular inflammation or ballooning) and NASH resolution, defined as absence of ballooning with 0–1 lobular inflammation, without worsening of fibrosis.8, 9
Although it is still considered the gold standard and is mandated in trials seeking conditional approval, liver biopsy has well described limitations, namely sampling error, inter- and intra-observer differences in histologic assessment and procedural risk inherent to its invasive nature including hospitalization for pain, bleeding and rarely death.10–12 In addition, it was recently recognized by the FDA that a requirement of liver biopsy may limit clinical trial participation due its logistical burdens and risks.9
Non-invasive modalities are needed to replace liver biopsy for the diagnosis and monitoring of NASH in clinical trials.13 Magnetic resonance imaging (MRI) technologies have emerged as safe alternatives to liver biopsy for some contexts of use.14 In particular, MRI-estimated proton density fat fraction (MRI-PDFF) is a reliable and accurate non-invasive biomarker of liver fat content in patients with NASH.15 MRI-PDFF has been validated as accurate when compared to reference standards of MR spectroscopy16, 17 and histology.18, 19 MRI-PDFF is highly reproducible20 and in a single study was superior to liver histology in assessing longitudinal changes in liver fat in patients with NASH.21 Importantly, MRI-PDFF is sensitive to even small longitudinal changes (<5%) in liver fat content.17 MRI-PDFF also has excellent linearity, bias, and precision across different field strengths, imager manufacturers, and reconstruction methods.22
Although MRI-PDFF is a biomarker of liver fat content, and not of NASH per se, emerging data from both single-center as well as multicenter randomized controlled clinical trials with paired MRI-PDFF and liver histology have shown that longitudinal reductions in MRI-PDFF are associated with histologic response in NASH patients, especially those treated with anti-steatogenic drugs.19, 23, 24 Moreover, two studies independently have identified the same optimal cutoff (≥30%) for MRI-PDFF reduction to predict histologic response.14, 18 Based on a post hoc analysis of the 72-week FLINT trial, this cutoff has both 81% specificity and 81% positive predictive value for discerning histologic improvement and resolution of NASH.18 However, these studies are limited by relatively small sample sizes. Larger studies would be needed to verify the association of this cutoff as a threshold for assessing histological response and to establish its role as a clinical trial endpoint.
The aim of our systematic review and meta-analysis was to assess the evidence for MRI-PDFF reduction as a predictor of histologic response in patients with NASH. The primary outcome was to examine the association between a ≥30% reduction in MRI-PDFF and histologic response, defined as a ≥ 2-point improvement in NAS (with at least one point derived from improvement in lobular inflammation or ballooning) without worsening of fibrosis stage. The secondary outcome of this study was to examine the association between a ≥30% reduction in MRI-PDFF and histologic resolution of NASH.
Materials and Methods
We performed a systematic review of the medical literature according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.25 This systematic review was registered with The International Prospective Register of Systematic Reviews (PROSPERO), an open access online database of systematic review protocols on health-related topics (CRD42020184023). Institutional review board approval was not required for this systematic review.
Identification of Studies and Searches
A detailed search was conducted by a Medical Information Consultant (KK) using indexing languages including Medical Subject Headings and free text terms for NAFLD, NASH, MRI and liver biopsy. The search strategy is available in Supplementary Table 1. The following databases were searched through May 2020: Cochrane Library (Ovid), Embase (Ovid) and Medline (Ovid). Several clinical trial registries were also searched including ClinicalTrials.gov. Search results were combined into an Endnote database (version X8.2, Clarivate Analytics, Philadelphia, USA) for reference management and imported into the Rayyan web and mobile app for systematic reviews (Qatar Computing Research Institute, Doha, Qatar).26 Reference lists for all eligible studies were screened for additional studies as were identified systematic reviews and meta-analyses.
Study Selection
Studies were selected if they met the following inclusion criteria: (1) Study design: randomized controlled trials, non-randomized controlled trials or comparative studies in human subjects. (2) Population: adults (age ≥18 years) with biopsy-proven NASH. (3) Exposure: MRI-PDFF measurement before and after an intervention aimed to improve NASH or NAFLD. (4) Outcome measures: data on the primary or secondary outcome measures (below) provided. (5) English publication language. To fully extract the desired data, we excluded abstracts where study investigators were unable to provide additional information required.
Primary outcome measure was histologic response defined as ≥ 2-point improvement in NAS, with at least one point derived from improvement in lobular inflammation or ballooning, without worsening of fibrosis stage. Secondary outcome measure was NASH resolution as defined as ballooning of 0 and lobular inflammation of 0–1 on follow-up biopsy.
All authors had access to the study data and reviewed and approved the final manuscript.
Data Extraction and Risk of Bias Assessment
The following study-level data was extracted from included studies: author, study year, country, study design, enrollment period and definition of NASH. Subject-level data that was extracted included age, sex, metabolic comorbidities (e.g., diabetes, hypertension, hyperlipidemia), body mass index (BMI), and baseline and end-of-treatment histologic scores (NAS elements and fibrosis stage) so that histologic change for both the primary and secondary outcomes could be determined. Where necessary, authors were contacted for unpublished data to complete subject-level data extraction. MRI characteristics were also extracted including field strength, MRI vendor, reconstruction method and data analysis method.
Study risk of bias was adjudicated using Cochrane Risk of Bias Tool Version 2 (ROB2).27 The ROB2 tool has five domains: randomization process (where available), derivations from intended interventions, missing outcome data, measurement of the outcome and selection of the reported result. For each domain, the study reporting is graded as yes, partly yes, partly no, no or no information. Based on this assessment, each domain is then assigned an overall risk of bias: (1) Low (2) Some concerns or (3) High.
Statistical Analysis
The Cochrane Handbook for Systematic Reviews of Interventions was used as a guide for data analysis.28 The number of subjects who underwent paired liver biopsy and MRI-PDFF before and after intervention were extracted for each study. The number of events were then extracted into two groups, those subjects who had a ≥30% reduction in MRI-PDFF, termed MRI-PDFF responders, versus those who did not, or MRI-PDFF non-responders. Descriptive analysis of the studies identified, excluded, and included, and meta-analysis of the reported study effect measures was performed using Review manager software (Rev-Man version 5.3; Copenhagen; The Nordic Cochrane Centre; The Cochrane Collaboration; 2014). Pooled odds ratios (ORs) between the two groups of subjects for each outcome were estimated by weighting the study-specific risk ratios by the inverse of their individual variance. DerSimonian and Laird random-effects models were used to calculate corresponding 95% confidence intervals (CIs) to account for between- and within-study variability.29, 30 Between-study variability was then separately assessed using the Cochran’s Q statistic (p<0.05). 29,30 The proportion of heterogeneity accounted for by between-study variability was estimated using the I2 index and adjudicated to be significant if I2 was >75%.29, 30 Publication bias was assessed with post-hoc funnel plot asymmetry and the Egger test (Supplementary Figure 1).31
Results
Study Selection
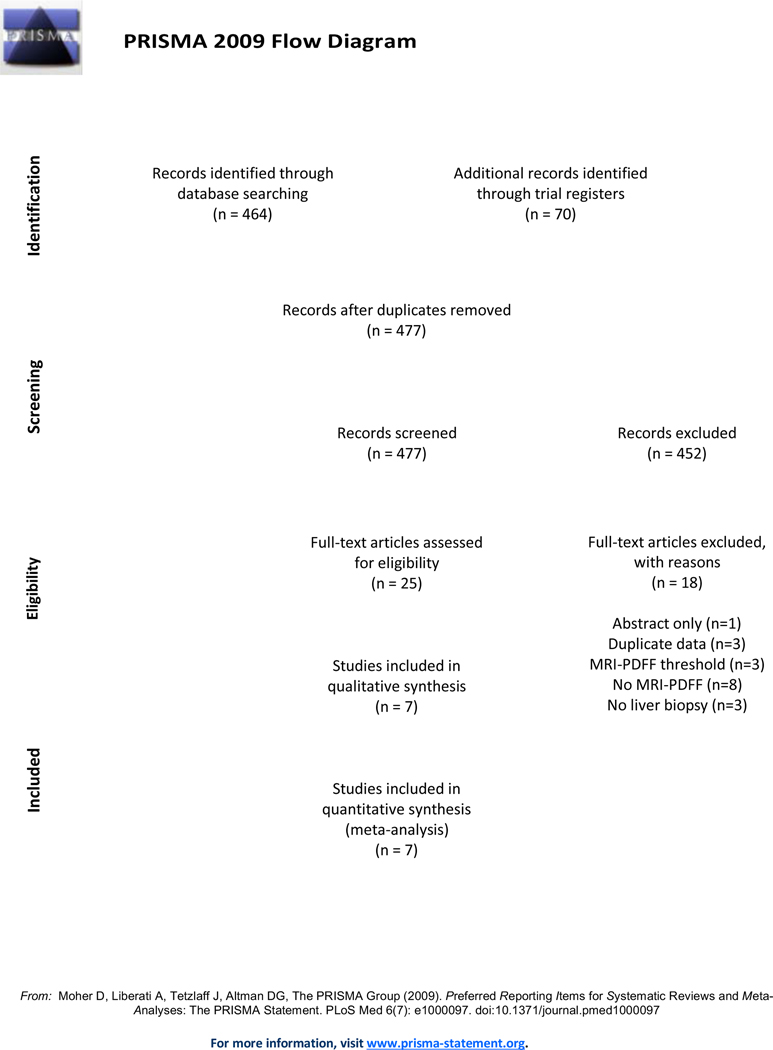
After removing duplicates, the database search identified a total of 407 abstracts and titles. An additional 70 study titles were found from trial registers. After review of all titles and full study texts, a total of seven studies met inclusion criteria for this systematic review (Figure 1). Citations and reasons for study exclusion following review of publication full text are found in Supplementary Table 2.
Figure 1.
PRISMA diagram
Study and Patient Characteristics
346 subjects were included from the seven studies meeting criteria for this review. Study and patient characteristics are shown in Table 1. All seven studies were controlled clinical trials, six of which were randomized. Study intervention duration ranged from 16–72 weeks and included five anti-steatogenic medications, one supplement and one exercise-based trial. The median average subject age was 51 years (range 47–61 years) and 59% of participants were female. Metabolic comorbidities were common as 46% were diabetic (range 32–67%). Median average BMI was 34kg/m2 (range 31–35kg/m2). Fibrosis stage evaluated by liver biopsy and distribution across studies by fibrosis stage or by mean fibrosis stage is shown in Table 1. Mean fibrosis stage was 1.6 (range 0.5–2.3). Median NAS was 5.0 (range 4.6–5.5). Mean MRI-PDFF was 18.2% (range 13.4–20.2%). MRI characteristics are described in Supplementary Table 3.
Table 1.
Characteristics of Included Studies
| First author (year) and country | Study design, time period | Population diagnosis | Population demographics | NASH severity |
|---|---|---|---|---|
| Argo et al. (2015), USA |
RCT, 2007 to 2010 |
34 subjects (biopsy proven NASH, NAS > or = 4) 28 subjects with biopsy and MRI-PDFF |
Median Age: 47 years Male: 38% (n=13) DM:32% (n=11) HTN:50% (n=17) HLD:15% (n=5) BMI, median 33kg/m2 |
Mean liver fat (MRI-PDFF): 18.4 % (fish oil and 11.3 % (placebo) Mean NAS: 5.4 (fish oil) and 4.9 (placebo) Mean Fibrosis: 2.1 (fish oil) and 1.6 (placebo) |
| Jayakumar et al.
(2018), USA and Canada |
RCT, June 2015 to March 2016 |
72 subjects (liver biopsy within 3 months of screening consistent with a diagnosis of NASH and stage 2/3 fibrosis according to the NASH CRN histologic scoring. All patients with NAS ≥5 and at least 3 of the metabolic syndromes 65 subjects with biopsy and MRI-PDFF |
Median Age: 56 years Male: 29% (n=19) DM: 63% (n=45) HTN: n/a HLD: n/a BMI, median 33kg/m2 |
NAS:
7–8: 12 (19%) Fibrosis: F2: 21 (32%) F3: 44 (68%) |
| Le et al. (2012), USA | RCT, September 2009 to January 2011 |
50 subjects (Hepatic steatosis of >5% by MRI-PDFF and liver biopsy with evidence of NASH, defined by the presence of all of the following features including steatosis, ballooning degeneration and lobular inflammation with or without fibrosis or suspicious for NASH defined as NAS ≥4) |
Mean Age: 48 years Male: 46% (n=23) DM: 36% (n=18) HTN: n/a HLD: n/a BMI, mean: 31kg/m2 |
Mean liver fat (MRI-PDFF): 14.2% (colesevelam) and 17.9% (placebo) Mean NAS: 4.7 (colesevelam) and 4.6 (placebo) Mean Fibrosis: 1.2 (colesevelam) and 1.1 (placebo) |
| Loomba et al. (2020), USA |
Secondary analysis of RCT, March 2011 to Dec 2012 |
78 subjects (Biopsy-proven NASH and NAS ≥4) | Mean Age: 53 years Male: 37% (n=29) DM: 51% (n=40) HTN: 53% (n=41) HLD: 53% (n=41) BMI, mean: 34kg/m2 |
Mean liver fat (MRI-PDFF): 18.0% (obeticholic acid) and 20.0% (placebo) Mean NAS: 5.1 (obeticholic acid) and 5.4 (placebo) Mean fibrosis stage: 1.9 (obeticholic acid) and 1.7 (placebo) |
| Loomba R et al. (2020), USA | Secondary analysis of RCT, October 2016 to July 2017 |
125 subjects (Biopsy confirmed NASH defined as F1–3 and NAS ≥4 and hepatic fat fraction of ≥10% by MRI-PDFF) 107 subjects with biopsy and MRI-PDFF |
Mean Age: 50 years Male: 50% (n=62) DM: 39% (n=49) HTN: 50% (n=63) HLD: n/a BMI, mean: 35kg/m2 |
Mean liver fat (MRI-PDFF): 20.2 (resmetirom) and 19.6 (placebo) NAS: Mean 4.9 (resmetirom) and 4.8 (placebo) NAS ≥5: 47 (resmetirom) vs 19 (placebo) Fibrosis: Fibrosis 0: 1 vs 2 Fibrosis 1: 47 vs 19 Fibrosis 2: 18 vs 13 Fibrosis 3: 18 vs 7 |
| Patel et al. (2016), USA |
Secondary analysis of RCT, Jan 2013 to Dec 2013 |
35 subjects (Liver biopsy-confirmed NASH as defined by the NASH CRN histologic scoring system and MRI-PDFF ≥5% hepatic steatosis) |
Median Age: 61 years for histological responders and 49 years for non-responders Male: 34% (n=12) DM: mean A1c 6.1% HTN: n/a HLD: n/a BMI, median: 31kg/m2 in responders and 35 kg/m2 in non-responders |
Mean liver fat (MRI-PDFF): 13.4% (responders) vs 18.2% (non-responders) Mean NAS: 5.5 (histologic responders) vs 5 (non-responders) Mean fibrosis: 0.5 (histologic responders) vs 1 (non-responders) |
| Stine et al. (2020), USA |
Controlled trial, May 2018 to May 2019 |
3 subjects (Biopsy proven NASH defined by NASH-CRN and NAS ≥5) |
Mean age: 55 years Male: 67% (n=2) DM: 67% (n=2) HTN: 100% (n=3) HLD: 67% (n=2) BMI, mean: 34kg/m2 |
Mean liver fat (MRI-PDFF): 19.4% Mean NAS: 5.3 Mean fibrosis: 2.3 |
BMI=body mass index; CRN=Clinical Research Network; DM=diabetes; F=fibrosis; HLD=hyperlipidemia; HTN=hypertension; MRI-PDFF=magnetic resonance imaging proton density fat fraction; NAS=NASH Activity Score; NASH=nonalcoholic steatohepatitis; RCT=randomized controlled trial; USA=United States of America
All studies had the same definition of histologic response which was an improvement in NAS by ≥2 points without worsening of fibrosis
NASH resolution defined either by 0 ballooning and 0–1 lobular inflammation as assed by NAS
Risk of Bias Assessment
Most included studies were adjudicated to have a low risk of bias (Table 2). Three studies were judged to overall have some concerns for bias.14, 32, 33 The study by Jayakumar et al.14 analyzing data from the phase II selonsertib trial was open label and thus there were minor concerns due to lack of placebo when derivations from intended interventions were assessed. The study by Loomba et al.33, which was also a post-hoc analysis of phase II clinical trial data from the resmetirom trial, had more women and diabetics in the treatment group so despite a well-described randomization process, the study was judged to have some concern for bias. The study by Stine et al.32 was a single arm open label study with a controlled exercise intervention. The lack of two study arms and no randomization process were determined to be of minor concern.
Table 2.
Risk of Bias Assessment of Included Studies
| First author (year) | Randomization process | Derivations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported result | Overall bias |
|---|---|---|---|---|---|---|
| Argo et al. (2015) | Low | Low | Low | Low | Low | Low |
| Le et al. (2012) | Low | Low | Low | Low | Low | Low |
| Patel et al. (2016) | Low | Low | Low | Low | Low | Low |
| Jayakumar et al. (2018) | Low | Some concerns# | Low | Low | Low | Some concerns |
| Loomba et al. (2020) | Low | Low | Low | Low | Low | Low |
| Loomba R et al. (2020) | Some concerns## | Low | Low | Low | Low | Some concerns |
| Stine et al. (2020) | N/A### | Low | Low | Low | Low | Some concerns |
- open label study design (participants and study staff aware of treatment group)
-more women and diabetics in the treatment group
-single arm open label study
Primary Outcome: ≥ 2 point improvement in NAS
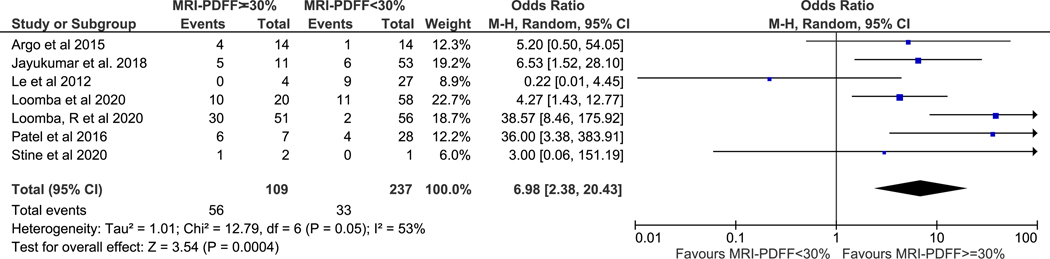
All seven studies contributed data to this analysis. In aggregate, there were 109 MRI-PDFF responders, 237 MRI-PDFF non-responders, 89 histologic responders, and 257 histologic non-responders. The pooled histologic response rate among MRI-PDFF responders versus MRI-PDFF non-responders was 51% and 14%, (p-value <0.001), respectively. Meta-analysis demonstrated that MRI-PDFF responders had higher odds of histologic response (pooled OR 6.98, 95% CI 2.38–20.43, p<0.001) when compared to MRI-PDFF non-responders (Figure 2). Moderate heterogeneity was observed (I2=53%).
Figure 2. MRI-PDFF response as defined as ≥ 30% decline predicts histologic response.
Subjects with MRI-PDFF response were nearly seven-fold more likely to have histologic response
Secondary Outcome: NASH resolution
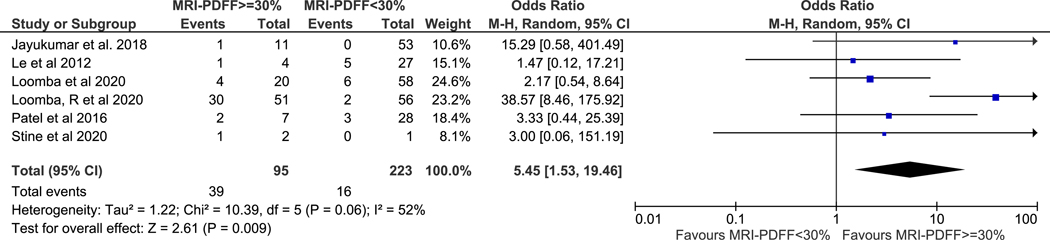
The study by Argo et al.34 had no events of interest (0 of 28 subjects had NASH resolution) and an OR was unable to be calculated for this study. Therefore, only six studies with 318 subjects were included in the secondary outcome analysis. In aggregate, there were 95 MRI-PDFF responders, 223 MRI-PDFF non-responders, 55 NASH resolution, and 263 without NASH resolution. The pooled NASH resolution rate among MRI-PDFF responders versus MRI-PDFF non-responders was 41% versus 7%, (p-value <0.001), respectively. Meta-analysis demonstrated MRI-PDFF responders had higher odds of histologic response (pooled OR 5.45, 95% CI 1.53–19.46, p=0.009) when compared to MRI-PDFF non-responders (Figure 3). Moderate heterogeneity was again observed (I2=52%).
Figure 3. MRI-PDFF response as defined as ≥ 30% decline predicts NASH resolution.
Subjects with MRI-PDFF response were nearly five and a half-fold more likely to have NASH resolution
Discussion:
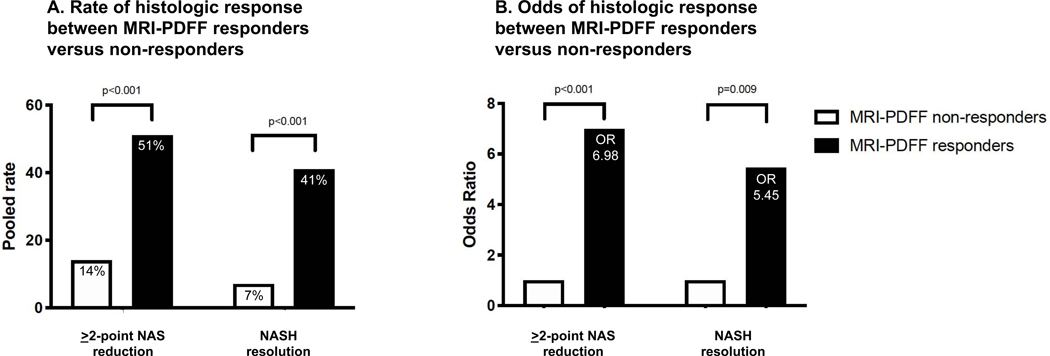
This systematic review and meta-analysis found a consistent body of evidence from international controlled clinical trials demonstrating that a ≥ 30% reduction in MRI-PDFF may be a useful non-invasive biomarker for both a ≥ 2-point improvement in NAS and for NASH resolution. MRI-PDFF responders following a clinical trial intervention are substantially more likely to have histologic improvement than non-responders – by a factor of seven when histologic response is determined by NAS reduction and by a factor of over five when determined by NASH resolution (Figure 4). The methodologic rigor of this study as evidenced by high-quality evidence with low overall bias provides new confidence to support the use of MRI-PDFF as an accurate and reliable alternative to invasive liver biopsy, especially in early phase clinical trials in NASH. These data provide validation for the use of ≥ 30% decline in MRI-PDFF as a clinically meaningful reduction in liver fat associated with histologic response in NASH.
Figure 4.
The rate of 2-point improvement in NAS in MRI-PDFF responders versus non-responders was 51% and 15%, p-value < 0.001, and the rate of NASH resolution in MRI-PDFF responders versus non-responders was 41% and 7%, p-value < 0.001 (figure 4-A), respectively.
The odds of 2-point improvement in NAS in MRI-PDFF responders versus non-responders was 6.98 (95% CI, 2.38–20.43, p-value < 0.001), and the odds of NASH resolution in MRI-PDFF responders versus non-responders was 5.45 (95% CI, 1.53–19.46, p-value 0.009), (figure 4-B), respectively.
In the Context of Current Recommendations and Evidence
A recent statement from the FDA in December 20189 provides guidance for clinical trial design for patients with noncirrhotic NASH with liver fibrosis in order to slow the progress of, halt, or reverse disease progression. It is the goal of all NASH trial design to improve clinical outcomes including reducing the need for liver transplantation. As fibrosis is slow to progress in NASH, the FDA recognizes that surrogate endpoints for both disease progression to cirrhosis and/or survival are necessary. Current best practice suggestions from the FDA are based exclusively on histologic improvement as an endpoint to predict future clinical benefit and to allow accelerated drug approval. Yet, the FDA recognizes the inherent limitations of invasive liver biopsy and encourages identification of biochemical or noninvasive imaging biomarkers that could eventually replace liver biopsies. Given that the controlled clinical trials included in this meta-analysis all used FDA suggested definitions for histologic response and the strength of our findings, we would submit that longitudinal MRI-PDFF could be used as an endpoint in early phase clinical trials in NASH. This is important for trial feasibility and for dissemination to clinical practice following effective drug development and approval, a context where monitoring for treatment response with non-invasive imaging biomarkers is even more important, especially when cost to payers must be considered.
Previous studies have demonstrated that reduction in liver fat measured by MRI-PDFF is correlated with other non-invasive NASH biomarkers including those measuring hepatic apoptosis and necroinflammation such as cytokeratin-18 or fibroblast growth factor (FGF)-21 and those measuring fibrosis, such as Enhance Liver Fibrosis (ELF) score, pro-C3, TIMP-1 and hepatic collagen.14, 32 While we were not able to perform a qualitative pooled analysis of these non-invasive biomarkers and their relationship to MRI-PDFF response, this nonetheless offers an intriguing avenue for future study. If a similar cutoff of response can be established for histologic response, presumably the combination of non-invasive imaging with MRI-PDFF and serum biomarkers would provide additional support in choosing non-invasive methods over liver biopsy in future trial design.
Strengths and Limitations
To date, this is the only systematic review and meta-analysis to calculate a pooled measure of effect for a liver MRI-PDFF cutoff to be used as a non-invasive biomarker for histologic improvement in NASH. In addition, the methodologically robust assessment provided by this study indicates that further investigation into this topic is unlikely to change our conclusions and that full-confidence in putting forth these findings into clinical trial design is warranted. We were also able to extend the medical literature on this topic by contacting and receiving additional quantitative data not reported in the original manuscripts for six of the seven included studies. This allows us to ensure our meta-analysis is inclusive of comprehensive information necessary to make these strong conclusions, a fact that is supported by our findings of little or no publication bias.
We do however recognize that there are limitations to our work, some of which are intrinsic to the study design in that missing data is a problem for all comparative effectiveness studies and several important patient level covariates were unable to be obtained, limiting our ability to calculate pooled performance metrics including sensitivity, specificity and positive predictive value for the 30% MRI-PDFF cutoff and to perform a sensitivity analysis. A post hoc analyses of the FLINT trial validated the cutoff of ≥30% reduction in MRI-PDFF with excellent performance metrics of 81% specificity and 81% positive predictive value.18 A post hoc analysis of the phase II selonsertib trial found similar accuracy for >30% reduction in MRI-PDFF with 87% specificity and 87% negative predictive value.19 Our study was also unable to determine a dose-dependent relationship between different relative MRI-PDFF cutoffs and likelihood of histologic response or NASH resolution, however, we recognize this presents an interesting avenue for further study. Knowing with certainly how much histologic benefit could be expected for each 1, 5 or 10% reduction in MRI-PDFF respectively, would have great clinical implications. We were also unable to assess improvement in one-stage of hepatic fibrosis, an important long-term NASH trial endpoint due to both limited data and the fact that four of the seven trials included patients with NASH without requiring fibrosis at baseline. As additional studies that utilize MRI-PDFF paired with histologic assessment in patients with NASH and stage 1–3 fibrosis become available this association can then be assessed.
Other limitations include that the majority of these trials were conducted in North America. We also encountered the inability to include multiple large randomized controlled trials that did not utilize both paired liver biopsy and MRI-PDFF.35–42 If a different study design had been elected, these large sample sizes would have increased the power of this study. Additionally, moderate heterogeneity was encountered for each endpoint analyzed, which may be attributed to differences in study intervention (e.g., pharmacologic, supplement or exercise training), study duration and study population. We also recognize that a subgroup analysis based on pharmacotherapy class, supplement type or exercise intervention would also add additional value to our work, however, given the relatively few number of included studies, the degree of effect for each treatment type was unable to be determined.
Despite these limitations, our pooled measure of effect for both outcomes was highly statistically significant. Further studies are needed to examine the association between MRI-PDFF and its association with long-term outcomes and risk of fibrosis progression.23
Conclusion:
MRI-PDFF offers an accurate and reliable biomarker for assessment of treatment response in early phase NASH trials. Future NASH Phase 2A clinical trials may consider ≥ 30% reduction in liver fat as measured by MRI-PDFF as the primary endpoint to measure treatment response, especially in early-phase clinical trials for anti-steatotic drugs. Quantitative decline in MRI-PDFF can be used to determine the expected histologic response and NASH resolution rate in clinical drug development and may be useful in determining sample-size estimates for Phase 2b/Phase 3 trials in NASH. Further research is needed to define the role of MRI-PDFF in clinical trials of drugs with different mechanisms of action, including antifibrotics, and to clarify the role of MRI-PDFF in late-phase clinical trials and clinical care.
Supplementary Material
Publication bias. No significant publication bias was observed for (a) histologic response or (b) NASH resolution
Acknowledgments
Grants and Financial Support: This grant was funded in part by NIH grant L30 DK118601 (Stine). This grant was funded in part by NIH/NIDDK grant R01DK12378, U01DK61734, R01DK106419, DoD PRCRP CA170674P2 and NIH/NIEHS 5P42ES010337 (Loomba).
JS- Research Funding from Target Pharma, Inc, AM- none, AB- none, JW-none, CKA-none, SS-none, KF receives grant support from Bayer, GE, and Siemens and serves as a consultant for Innovis, CS reports grants from GE, Siemens, Philips, Bayer, Gilead; personal fees from Wolters Kluwer for educational royalties outside the submitted work; personal fees for consultation from Blade, Boehringer, and Epigenomics; consultation under the auspices of the University to AMRA, BMS, Exact Sciences, GE Digital, and IBM-Watson; lab service agreements from Enanta, Gilead, ICON, Intercept, Nusirt, Shire, Synageva, Takeda; RL serves as a consultant or advisory board member for Arrowhead Pharmaceuticals, AstraZeneca, Bird Rock Bio, Boehringer Ingelheim, Bristol-Myer Squibb, Celgene, Cirius, CohBar, Conatus, Eli Lilly, Galmed, Gemphire, Gilead, Glympse bio, GNI, GRI Bio, Intercept, Ionis, Janssen Inc., Merck, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Pfizer, Prometheus, Sanofi, Siemens, and Viking Therapeutics. In addition, his institution has received grant support from Allergan, Boehringer-Ingelheim, Bristol-Myers Squibb, Cirius, Eli Lilly and Company, Galectin Therapeutics, Galmed Pharmaceuticals, GE, Genfit, Gilead, Intercept, Grail, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, NuSirt, Pfizer, pH Pharma, Prometheus, and Siemens. He is also co-founder of Liponexus, Inc.
Abbreviations:
- (NASH)
nonalcoholic steatohepatitis
- (MRI-PDFF)
Magnetic resonance imaging proton density fat fraction
- (NAFLD)
Nonalcoholic fatty liver disease
- (NAS)
NAFLD Activity Score
- (OR)
odds ratios
- (FDA)
Food and Drug Administration
- (MRE)
Magnetic resonance elastography
- (PRISMA)
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- (PROSPERO)
The International Prospective Register of Systematic Reviews
- (BMI)
body mass index
- (ROB2)
Cochrane Risk of Bias Tool Version 2
- (GRADE)
Grading of Recommendations, Assessment, Development and Evaluations
- (FGF)
fibroblast growth factor
- (ELF)
Enhance Liver Fibrosis
- (CRN)
Clinical research network
- (DM)
Diabetes
- (F)
Fibrosis
- (HLD)
Hyperlipidemia
- (HTN)
Hypertension
Footnotes
Disclosures:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol 2019;70:531–544. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, et al. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2017. [DOI] [PubMed] [Google Scholar]
- 3.Parikh ND, Marrero WJ, Wang J, et al. Projected increase in obesity and non-alcoholic-steatohepatitis-related liver transplantation waitlist additions in the United States. Hepatology 2019;70:487–495. [DOI] [PubMed] [Google Scholar]
- 4.Loomba R, Lim JK, Patton H, et al. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stine JG, Wentworth BJ, Zimmet A, et al. Systematic review with meta-analysis: risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment Pharmacol Ther 2018;48:696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- 7.Filozof C, Chow S-C, Dimick-Santos L, et al. Clinical endpoints and adaptive clinical trials in precirrhotic nonalcoholic steatohepatitis: Facilitating development approaches for an emerging epidemic. Hepatology communications 2017;1:577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noncirrhotic Nonalcoholic Steatohepatitis With Liver Fibrosis: Developing Drugs for Treatment Guidance for Industry. https://www.fda.gov/media/119044/download 2018.
- 9.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/noncirrhotic-nonalcoholic-steatohepatitis-liver-fibrosis-developing-drugs-treatment. Accessed Apr 30 2020.
- 10.Seeff LB, Everson GT, Morgan TR, et al. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol 2010;8:877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002;97:2614–8. [DOI] [PubMed] [Google Scholar]
- 12.Poynard T, Lenaour G, Vaillant JC, et al. Liver biopsy analysis has a low level of performance for diagnosis of intermediate stages of fibrosis. Clin Gastroenterol Hepatol 2012;10:657–63.e7. [DOI] [PubMed] [Google Scholar]
- 13.Rinella ME, Tacke F, Sanyal AJ, et al. Report on the AASLD/EASL Joint Workshop on Clinical Trial Endpoints in NAFLD. Hepatology 2019;70:1424–1436. [DOI] [PubMed] [Google Scholar]
- 14.Jayakumar S, Middleton MS, Lawitz EJ, et al. Longitudinal correlations between MRE, MRI-PDFF, and liver histology in patients with non-alcoholic steatohepatitis: Analysis of data from a phase II trial of selonsertib. J Hepatol 2019;70:133–141. [DOI] [PubMed] [Google Scholar]
- 15.Di Martino M, Pacifico L, Bezzi M, et al. Comparison of magnetic resonance spectroscopy, proton density fat fraction and histological analysis in the quantification of liver steatosis in children and adolescents. World J Gastroenterol 2016;22:8812–8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Middleton MS, Heba ER, Hooker CA, et al. Agreement Between Magnetic Resonance Imaging Proton Density Fat Fraction Measurements and Pathologist-Assigned Steatosis Grades of Liver Biopsies From Adults With Nonalcoholic Steatohepatitis. Gastroenterology 2017;153:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Middleton MS, Van Natta ML, Heba ER, et al. Diagnostic accuracy of magnetic resonance imaging hepatic proton density fat fraction in pediatric nonalcoholic fatty liver disease. Hepatology 2018;67:858–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loomba R, Neuschwander-Tetri BA, Sanyal A, et al. Multicenter validation of association between decline in MRI-PDFF and histologic response in nonalcoholic steatohepatitis. Hepatology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayamkumar S, Loomba R, Lawitz E, et al. Reductions in hepatic proton density fat fraction (PDFF) predict histologic improvement in a multi-center clinical trial of subjects with nonalcoholic steatohepatitis (NASH). Canadian Liver Journal 2018;1:96–97. [Google Scholar]
- 20.Kang GH, Cruite I, Shiehmorteza M, et al. Reproducibility of MRI-determined proton density fat fraction across two different MR scanner platforms. J Magn Reson Imaging 2011;34:928–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noureddin M, Lam J, Peterson M, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology (Baltimore, Md.) 2013;58:1930–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yokoo T, Serai SD, Pirasteh A, et al. Linearity, Bias, and Precision of Hepatic Proton Density Fat Fraction Measurements by Using MR Imaging: A Meta-Analysis. Radiology 2018;286:486–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ajmera V, Park CC, Caussy C, et al. Magnetic Resonance Imaging Proton Density Fat Fraction Associates With Progression of Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2018;155:307–310.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel J, Bettencourt R, Cui J, et al. Association of noninvasive quantitative decline in liver fat content on MRI with histologic response in nonalcoholic steatohepatitis. Therapeutic Advances in Gastroenterology 2016;9:692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions: Checklist and Explanations. Ann Intern Med. 2015;162:777–84. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 26.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. [Google Scholar]
- 29.Haidich AB. Meta-analysis in medical research. Hippokratia. 2010;14:29–37. [PMC free article] [PubMed] [Google Scholar]
- 30.Murad MH, Montori VM, Ioannidis JP, et al. How to read a systematic review and meta-analysis and apply the results to patient care: users’ guides to the medical literature. JAMA. 2014;312:171–9. doi: 10.1001/jama.2014.5559. [DOI] [PubMed] [Google Scholar]
- 31.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Bmj 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stine JG, Xu D, Schmitz K, et al. Exercise Attenuates Ribosomal Protein Six Phosphorylation in Fatty Liver Disease. Dig Dis Sci 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loomba R BP, Guy C, Bashir M, Harrison S. Magnetic resonance imaging-proton density fat fraction (MRI-PDFF) to predict treatment response on NASH liver biopsy: a secondary analysis of the resmetirom randomized placebo controlled Phase 2 clinical trial. EASL International Liver Congress 2020. [Google Scholar]
- 34.Argo CK, Patrie JT, Lackner C, et al. Effects of n-3 fish oil on metabolic and histological parameters in NASH: a double-blind, randomized, placebo-controlled trial. Journal of Hepatology 2015;62:190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362:1675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Younossi ZM, Ratziu V, Loomba R, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019;394:2184–2196. [DOI] [PubMed] [Google Scholar]
- 37.Ratziu V, Sanyal A, Harrison SA, et al. Cenicriviroc Treatment for Adults with Nonalcoholic Steatohepatitis and Fibrosis: Final Analysis of the Phase 2b CENTAUR Study. Hepatology 2020. [DOI] [PubMed] [Google Scholar]
- 38.Harrison SA, Abdelmalek MF, Caldwell S, et al. Simtuzumab Is Ineffective for Patients With Bridging Fibrosis or Compensated Cirrhosis Caused by Nonalcoholic Steatohepatitis. Gastroenterology 2018;155:1140–1153. [DOI] [PubMed] [Google Scholar]
- 39.Harrison SA, Alkhouri N, Davison BA, et al. Insulin sensitizer MSDC-0602K in non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled phase IIb study. J Hepatol 2020;72:613–626. [DOI] [PubMed] [Google Scholar]
- 40.Loomba R, Kayali Z, Noureddin M, et al. GS-0976 Reduces Hepatic Steatosis and Fibrosis Markers in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2018;155:1463–1473.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harrison SA, Rossi SJ, Paredes AH, et al. NGM282 Improves Liver Fibrosis and Histology in 12 Weeks in Patients With Nonalcoholic Steatohepatitis. Hepatology 2020;71:1198–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study.Lancet 2016;387:679–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Publication bias. No significant publication bias was observed for (a) histologic response or (b) NASH resolution