Abstract
With limited high-level evidence, we carried out a comparative effectiveness study for the effect of proton beam therapy (PBT) on overall survival compared to external-beam radiotherapy (EBRT) and brachytherapy (BT) among patients with localized prostate cancer using a national database. PBT was associated with a significant overall survival benefit compared to EBRT and had a similar performance as BT.
Background:
There are few comparative outcomes data regarding the therapeutic delivery of proton beam therapy (PBT) versus the more widely used photon-based external-beam radiation (EBRT) and brachytherapy (BT). We evaluated the impact of PBT on overall survival (OS) compared to EBRT or BT on patients with localized prostate cancer.
Patients and Methods:
The National Cancer Data Base (NCDB) was queried for 2004–2015. Men with clinical stage T1–3, N0, M0 prostate cancer treated with radiation, without surgery or chemotherapy, were included. OS, the primary clinical outcome, was fit by Cox proportional hazard model. Propensity score matching was implemented for covariate balance.
Results:
There were 276,880 eligible patients with a median follow-up of 80.9 months. A total of 4900 (1.8%) received PBT, while 158,111 (57.1%) received EBRT and 113,869 (41.1%) BT. Compared to EBRT and BT, PBT patients were younger and were less likely to be in the high-risk group. On multivariable analysis, compared to PBT, men had worse OS after EBRT (adjusted hazard ratio [HR] = 1.72; 95% confidence interval [CI], 1.51–1.96) or BT (adjusted HR = 1.38; 95% CI, 1.21–1.58). After propensity score matching, the OS benefit of PBT remained significant compared to EBRT (HR = 1.64; 95% CI, 1.32–2.04) but not BT (adjusted HR = 1.18; 95% CI, 0.93–1.48). The improvement in OS with PBT was most prominent in men ≤ 65 years old with low-risk disease compared to other subgroups (interaction P < .001).
Conclusion:
In this national data set, PBT was associated with a significant OS benefit compared to EBRT, and with outcomes similar to BT. These results remain to be validated by ongoing prospective trials.
Keywords: Brachytherapy, Comparative effectiveness, External-beam radiation, Localized prostate cancer, Proton beam radiation
Introduction
Men diagnosed with localized prostate cancer have a variety of treatment options, including active surveillance, radical prostatectomy, and radiotherapy, with radiotherapy options including brachytherapy (BT), photon-based external-beam radiotherapy (EBRT), or particle-based external radiotherapy, such as proton beam therapy (PBT). EBRT may be delivered in conventional fractionation over 8 to 9 weeks, with moderate hypofractionation over 4 to 6 weeks, or with ultrahypofractionation including stereotactic body radiotherapy over 2 to 3 weeks. Over the past decades, radiation treatment delivery has continued to become more technologically sophisticated, as clinicians have sought to improve the conformality of the radiation dose to the target while limiting radiation to nontarget normal tissues and to improve the accuracy and precision of treatment through daily localization or image guidance, which are seen as a key prerequisites to higher doses and shorter treatment courses. Many of these technological advances have increased the costs of radiation treatment before high-quality data emerged to demonstrate an improvement in outcomes.1–6
Proton therapy delivers radiotherapy with proton particles rather than with photons (X-rays). A PBT treatment plan may provide an improved therapeutic ratio as a result of its unique physical characteristics compared to photon-based EBRT. Specifically, protons demonstrate a Bragg peak, allowing the maximum radiation dose deposition from each proton beam to be matched to the location and depth of the tumor. Each proton beam has a finite range, meaning that after the beam reaches the depth of the target, there is essentially no radiation dose deposition beyond the target. For men with prostate cancer, proton therapy treatment plans typically reduce the radiation dose to the rectum, bladder, and other nontarget normal pelvic tissues relative to photon-based EBRT using intensity-modulated radiotherapy (IMRT),7 although single-institution retrospective studies have not identified a measurable difference in physician-assessed acute or late toxicities.8,9
While proton therapy represents a small percentage of radiation therapy treatments for prostate cancer and remains a relatively scarce resource, the growth in proton therapy from approximately 2% to 5% of EBRT cases between 2004 to 2012,10 the increased cost of treatment,11 the increasing number of proton centers, the sometimes questionable marketing claims,12 and the lack of randomized data demonstrating its superiority to alternative treatments for prostate cancer have underscored the scrutiny this treatment modality continues to receive. Previous population-based analyses from the Surveillance, Epidemiology, and End Results (SEER) database, a linked SEER-Medicare analysis, and the MarketScan Commercial Claims and Encounters database have reached conflicting conclusions about the acute and late toxicity profile of proton therapy relative to IMRT.13–15
We therefore queried the National Cancer Data Base (NCDB) in order to carry out a retrospective comparative effectiveness study to address whether PBT results in better overall survival (OS) than conventional radiation modalities (eg, EBRT, BT) among patients with localized prostate cancer.
Patients and Methods
Patient Population
Cases of localized prostate cancer treated with definitive radiotherapy between 2004 and 2015 were identified in the NCDB. The National Cancer Data Base is a joint project between the American College of Surgeons and the American Cancer Society that provides deidentified data from over 1500 hospitals affiliated with the Commission on Cancer program, which represents approximately 70% of new cancer diagnoses in the United States.16,17 The inclusion criteria were patients with clinical T1–3, N0, M0 disease who received radiation as the first-course treatment directed to the prostate and/or pelvis. For EBRT and PBT cohorts, only men receiving ≥ 60 Gy were included. Patients who had metastatic disease, who had received a prior cancer diagnosis, who underwent surgery, who received cytotoxic chemotherapy, or who were designated as receiving palliative care were excluded (Supplemental Table 1 in the online version). Clinical information, including tumor grade, clinical T stage, prostate-specific antigen (PSA), Gleason score, percentage of positive biopsy cores (available for 2010–2015), addition of concomitant androgen deprivation therapy (ADT), and Charlson-Deyo comorbidity index, were acquired. Facility and demographic data were assessed. We considered type of treating center (academic vs. nonacademic); age at diagnosis, race/ethnicity, primary insurance payer (government, private insurance, uninsured, unknown), and median income quartiles by residential zip code (<$38,000, $38,000-$47,999, $48,000-$62,999, $63,000+); and patient’s distance from the treatment center (calculated in miles by great circle distance).
Three treatment cohorts were identified: EBRT, including 3-dimension conformal radiotherapy or intensity-modulated radiotherapy; PBT; and BT, including monotherapy or as a boost with EBRT. OS was defined as months from the start date of radiation to the last follow-up date or death from any cause.
Statistical Analysis
Data analyses were performed by in SAS 9.4 software (SAS Institute, Cary, NC) by SAS macros developed by the Biostatistics and Bioinformatics Shared Resource at the Winship Cancer Institute.18 The significance level was set at .05. Descriptive statistics and univariate associations (eg, chi-square test, ANOVA) were applied to characterize the study population and to examine background differences among the 3 cohorts. A multivariable logistic regression model was used to identify the factors associated with the utilization of PBT. OS was evaluated by the Kaplan-Meier method and modeled by the Cox proportional hazard model. The subgroup analyses were performed by considering the interaction term into the multivariable model. A backward elimination procedure built all multivariable models, with removal criteria of P > .05.
To further balance covariates across the 3 cohorts, we implemented 1:1:1 generalized propensity score matching (GPSM).19,20 GPSM was estimated by multinomial logistic regression using the 3-level cohort as an outcome and all mentioned covariates as the predictors. The covariate balance was calibrated by the absolute standardized difference (ASD), where ASD > 0.2 was considered a substantial imbalance.21,22 A multivariable Cox proportional hazard model was applied to the final matched data set with a robust variance estimator to account for the paired nature of the data.23 GPSM can only balance cohorts across observed features, and unobserved confounders may influence the observed findings.24,25 A sensitivity analysis for unobserved confounders was performed through a simulation based on the matched sample, in which a confounder variable was simulated with a varying magnitude of association with either treatment assignment or OS. We examined the sensitivity parameters required for an unmeasured confounder to drive the estimate to nonsignificance (P > .05).24 The R package ‘survSens’ was used in the sensitivity analysis.
Results
Patient Characteristics
A total of 276,880 eligible patients were identified, with a median follow-up of 80.9 months. The median age was 68 years. Patients were majority (76.1%) non-Hispanic white, 81.1% resided in a metro area, and 35.3% were covered by private insurance. The National Comprehensive Cancer Network (NCCN) disease risk stratifications were high (24.9%), intermediate (43%), and low (32.1%), and 41.4% received ADT. A total of 4900 (1.8%) received PBT, 158,111 (57.1%) EBRT, and 113,869 (41.1%) BT (Table 1). The median follow-up per cohort was 62.5 (PBT), 76.5 (EBRT), and 88.7 (BT) months (P < .001 by reverse Kaplan-Meier method).
Table 1.
Baseline Characteristics for Study Population by Study Cohorts and Multivariable Logistic Regression for Factors That Predict Utilization of Proton Therapy
| Characteristic | Total | Radiation Modality | ASDa | Probability of Proton Delivery, Odds Ratio (95% CI) | ||
|---|---|---|---|---|---|---|
| PBT | EBRT | BT | ||||
| Patients | 276,880 | 4900 (1.8) | 158,111 (57.1) | 113,869 (41.1) | ||
| Age at diagnosis | ||||||
| ≤65 y | 103,504 (37.4) | 2288 (46.7) | 48,607 (30.7) | 52,609 (46.2) | 0.33 | 1.31 (1.17–1.46)d |
| >65 y | 173,376 (62.6) | 2612 (53.3) | 109,504 (69.3) | 61,260 (53.8) | 0.33 | Ref |
| Median (IQR) | 68 (62–74) | 66 (61–71) | 70 (64–75) | 66 (61–72) | 0.44 | |
| Race/ethnicity | ||||||
| Non-Hispanic white | 210,820 (76.1) | 4226 (86.2) | 117,212 (74.1) | 89,382 (78.5) | 0.31 | 4.41 (3.58–5.42)d |
| Non-Hispanic black | 45,173 (16.3) | 283 (5.8) | 27,611 (17.5) | 17,279 (15.2) | 0.37 | 2.41 (1.86–3.12)d |
| Hispanic | 10,968 (4.0) | 216 (4.4) | 7451 (4.7) | 3301 (2.9) | 0.09 | 2.23 (1.71–2.91)d |
| Other/unknown | 3902 (1.4) | 35 (0.7) | 2189 (1.4) | 1678 (1.5) | 0.07 | 1.35 (0.88–2.09)d |
| Asian | 6017 (2.2) | 140 (2.9) | 3648 (2.3) | 2229 (2) | 0.06 | Ref |
| Median income quartiles, 2008–2012 | ||||||
| <$38,000 | 49,771 (18.0) | 439 (9) | 29,987 (19) | 19,345 (17) | 0.29 | NSb |
| $38,000–$47,999 | 63,412 (22.9) | 916 (18.7) | 36,428 (23) | 26,068 (22.9) | 0.11 | |
| $48,000–$62,999 | 72,585 (26.2) | 1359 (27.7) | 41,947 (26.5) | 29,279 (25.7) | 0.05 | |
| $63,000+ | 91,112 (32.9) | 2186 (44.6) | 49,749 (31.5) | 39,177 (34.4) | 0.27 | |
| No high school diploma, 2008–2012 | ||||||
| ≥21.0% | 45,225 (16.3) | 701 (14.3) | 27,374 (17.3) | 17,150 (15.1) | 0.08 | NSb |
| 13.0%–20.9% | 71,105 (25.7) | 955 (19.5) | 41,634 (26.3) | 28,516 (25) | 0.16 | |
| 7.0%–12.9% | 91,944 (33.2) | 1531 (31.2) | 51,717 (32.7) | 38,696 (34) | 0.06 | |
| <7.0% | 68,606 (24.8) | 1713 (35) | 37,386 (23.6) | 29,507 (25.9) | 0.25 | |
| Urban/rural 2013 | ||||||
| Metro | 224,640 (81.1) | 4136 (84.4) | 129,246 (81.7) | 91,258 (80.1) | 0.11 | 1.28 (1.08–1.51) |
| Rural | 5676 (2.0) | 41 (0.8) | 2994 (1.9) | 2641 (2.3) | 0.12 | 0.83 (0.45–1.54) |
| Unknown | 5539 (2.0) | 174 (3.6) | 3171 (2) | 2194 (1.9) | 0.10 | 1.96 (1.46–2.62) |
| Urban | 41,025 (14.8) | 549 (11.2) | 22,700 (14.4) | 17,776 (15.6) | 0.13 | Ref |
| Primary insurance | ||||||
| Other/unknown | 22,000 (7.9) | 229 (4.7) | 15,073 (9.5) | 6698 (5.9) | 0.19 | 0.61 (0.51–0.74)d |
| Medicare | 157,183 (56.8) | 2542 (51.9) | 96,732 (61.2) | 57,909 (50.9) | 0.21 | 1.65 (1.48–1.84)d |
| Private | 97,697 (35.3) | 2129 (43.4) | 46,306 (29.3) | 49,262 (43.3) | 0.30 | Ref |
| Year of diagnosis (quartiles) | ||||||
| 2004–2006 | 82,740 (29.9) | 1108 (1.3) | 40,518 (49.0) | 41,114 (49.7) | 0.30 | Ref |
| 2007–2009 | 75,624 (27.3) | 1178 (1.6) | 41,452 (54.8) | 32,994 (43.6) | 0.11 | 1.26 (1.11–1.43)d |
| 2010–2012 | 65,641 (23.7) | 1492 (2.3) | 40,491 (61.7) | 23,658 (36.0) | 0.22 | 1.95 (1.71–2.20)d |
| 2013–2015 | 52,875 (19.1) | 1122 (2.1) | 35,650 (67.4) | 16,103 (30.5) | 0.23 | 2.25 (1.98–2.56)d |
| Facility type | ||||||
| Nonacademic/research Program | 195,950 (70.8) | 64 (1.3) | 112,562 (71.2) | 83,324 (73.2) | 2.22 | Ref |
| Academic/research Program | 80,930 (29.2) | 4836 (98.7) | 45,549 (28.8) | 30,545 (26.8) | 2.22 | 216.85 (165.3–284.5)d |
| Facility location | ||||||
| East | 134,306 (48.5) | 342 (7) | 75,736 (47.9) | 58,228 (51.1) | 1.11 | 2.94 (2.35–3.66)d |
| West | 32,247 (11.6) | 4445 (90.7) | 17,746 (11.2) | 10,056 (8.8) | 2.85 | 152.43 (125–185.9)d |
| Central/Mountain | 110,327 (39.8) | 113 (2.3) | 64,629 (40.9) | 45,585 (40) | 1.06 | Ref |
| Great circle distance (miles), median (IQR) | 9.1 (4.3–20.4) | 317 (42.2–78.7) | 8 (3.8–16.6) | 10.7 (4.9–24.8) | 1.58 | 1.14 (1.14–1.15)d |
| Charlson-Deyo score | ||||||
| 0 | 237,529 (85.8) | 4353 (88.8) | 135,826 (85.9) | 97,350 (85.5) | 0.1 | 1.70 (1.22–2.37)d |
| 1 | 31,947 (11.5) | 483 (9.9) | 17,651 (11.2) | 13,813 (12.1) | 0.07 | 1.64 (1.15–2.34)d |
| 2+ | 7404 (2.7) | 64 (1.3) | 4634 (2.9) | 2706 (2.4) | 0.11 | Ref |
| Sequence number | ||||||
| 00 | 257,769 (93.1) | 4844 (98.9) | 146,335 (92.6) | 106,590 (93.6) | 0.32 | 2.89 (2.12–3.93)d |
| 01 | 19,111 (6.9) | 56 (1.1) | 11,776 (7.4) | 7279 (6.4) | 0.32 | Ref |
| Grade | ||||||
| Well/moderately differentiated | 143,911 (52.0) | 3042 (62.1) | 69,235 (43.8) | 71,634 (62.9) | 0.39 | 1.21 (1.10–1.33)d |
| Unknown | 6366 (2.3) | 60 (1.2) | 4035 (2.6) | 2271 (2) | 0.10 | 0.70 (0.51–0.96) |
| Poorly/undifferentiated | 126,603 (45.7) | 1798 (36.7) | 84,841 (53.7) | 39,964 (35.1) | 0.38 | Ref |
| AJCC clinical T stage | ||||||
| T1 | 187,636 (67.8) | 3081 (62.9) | 100,753 (63.7) | 83,802 (73.6) | 0.23 | 1.69 (1.25–2.28)d |
| T2 | 79,964 (28.9) | 1735 (35.4) | 49,839 (31.5) | 28,390 (24.9) | 0.23 | 2.02 (1.49–2.73)d |
| T3 | 9280 (3.4) | 84 (1.7) | 7519 (4.8) | 1677 (1.5) | 0.19 | Ref |
| PSA (ng/mL) | ||||||
| <10 | 204,047 (73.7) | 4090 (83.5) | 106,050 (67.1) | 93,907 (82.5) | 0.39 | 2.02 (1.63–2.50)d |
| 10–20 | 44,470 (16.1) | 652 (13.3) | 31,105 (19.7) | 12,713 (11.2) | 0.24 | 1.67 (1.32–2.10)d |
| >20 | 28,363 (10.2) | 158 (3.2) | 20,956 (13.3) | 7249 (6.4) | 0.37 | — |
| Gleason score | ||||||
| 2–6 | 118,508 (42.8) | 2457 (50.1) | 51,294 (32.4) | 64,757 (56.9) | 0.51 | NSb |
| 7 | 110,811 (40.0) | 2041 (41.7) | 69,625 (44) | 39,145 (34.4) | 0.20 | |
| 8–10 | 47,561 (17.2) | 402 (8.2) | 37,192 (23.5) | 9967 (8.8) | 0.37 | |
| NCCN risk group | ||||||
| Low | 88,965 (32.1) | 1872 (38.2) | 34,956 (22.1) | 52,137 (45.8) | 0.52 | NICc |
| Intermediate | 119,047 (43.0) | 2486 (50.7) | 71,205 (45) | 45,356 (39.8) | 0.22 | |
| High | 68,868 (24.9) | 542 (11.1) | 51,950 (32.9) | 16,376 (14.4) | 0.55 | |
| ADT | ||||||
| No | 162,147 (58.6) | 4229 (86.3) | 78,381 (49.6) | 79,537 (69.8) | 0.86 | 3.36 (2.98–3.7)d |
| Yes | 114,733 (41.4) | 671 (13.7) | 79,730 (50.4) | 34,332 (30.2) | 0.86 | Ref |
| Regional + boost radiation dose (Gy) | ||||||
| Mean (SD) | 79.3 (37.4) | 80.8 (24.7) | 79.2 (37.7) | NA | 0.04 | NICc |
| Biopsy positive (2010–2015) | ||||||
| <50% | 53,789 (59.5) | 1080 (70.8) | 32,603 (54.9) | 20,106 (68) | 0.33 | NICc |
| ≥50% | 36,683 (40.5) | 445 (29.2) | 26,759 (45.1) | 9479 (32) | 0.33 | |
| Months of radiation start from diagnosis (quartiles) | ||||||
| ≤2.2 | 68,523 (24.7) | 737 (15) | 42,918 (27.1) | 24,868 (21.8) | 0.30 | |
| >2.2–3.2 | 69,118 (25.0) | 1153 (23.5) | 39,186 (24.8) | 28,779 (25.3) | 0.04 | |
| >3.2–4.6 | 69,480 (25.1) | 1371 (28) | 39,790 (25.2) | 28,319 (24.9) | 0.07 | |
| >4.6 | 69,759 (25.2) | 1639 (33.4) | 36,217 (22.9) | 31,903 (28) | 0.24 | |
| Mean (SD) | 3.8 (2.6) | 4.5 (3.2) | 3.7 (2.6) | 3.9 (2.5) | 0.32 | 1.07 (1.05–1.08)d |
Data are presented as n (%) unless otherwise indicated. For univariate association with radiation modalities, all P < .001.
Abbreviations: ADT = androgen deprivation therapy; AJCC = American Joint Committee on Cancer; ASD = absolute standardized difference; BT = brachytherapy; CI = confidence interval; EBRT = external-beam radiotherapy; IQR = interquartile range; NCCN = National Comprehensive Cancer Network; NIC = not initially considered in univariate model; NS = not selected; PBT = proton beam therapy; PSA = prostate-specific antigen; SD = standard deviation.
Value of > 0.2 is considered substantial difference in distribution.
NCCN risk group was derived from clinical T stage, PSA, and Gleason score. Radiotherapy dose was not available for BT patients. Biopsy core positivity information was only available after 2010; these data were not considered in multivariable model to avoid collinearity issue or sample size reduction.
NS by variable backward elimination at a significance level of .05.
Statistically significant (P < .05).
As shown in Table 1, there were significant differences in the baseline covariates between patients in the PBT cohort compared to the other two radiation modalities. With the large sample size, the P value was < .001 for all univariate associations. Evaluating the ASD, the 3 greatest differences in cohorts were in facility location, facility type, and the great circle distance. A total of 98.7% of PBT patients were treated in academic/research facilities and 90.7% at facilities located on the West Coast (ASD = 2.85). On average, patients traveled a longer distance to receive PBT (median, 317 miles) than for EBRT (8 miles) or BT (10.7 miles). Examining the ASD and the logistic regression model, PBT patients were more likely to be younger than 65 and non-Hispanic white, to reside in a metro area, to be covered by Medicare, to be diagnosed in more recent years, to have a lower comorbidity score, and to be more likely to have well/moderately differentiated tumor grade, earlier T stage, lower PSA, or favorable NCCN risk group. The percentage of biopsy-positive cores ≥ 50% was also lower in PBT patients (29.2%) than in EBRT (45.1%) and BT (32%) patients (ASD = 0.33). PBT patients were less often prescribed ADT (13.7% vs. 50.4% or 30.2%, ASD = 0.86) and experienced a longer time from diagnosis to initiation of radiotherapy (average time from diagnosis to start of radiation was 4.5, 3.7, and 3.9 months for PBT, EBRT, and BT, respectively). In addition, fewer PBT patients were found to have a sequence number of 1 (1.1% vs. 7.4% or 6.4% for EBRT or BT), which indicates the existence of a secondary tumor diagnosis after the current prostate cancer diagnosis.
Overall, on the basis of the distribution of observed baseline covariates across the 3 study cohorts, PBT patients in general had more favorable clinical prognostic features (eg, age, comorbidity score, grade, NCCN risk group) than EBRT or BT patients, while the EBRT and BT cohorts were more similar to each other. There was a clear increase in the utilization trend for PBT (1.3%, 1.6%, 2.3%, 2.1%) and EBRT (49%, 55%, 62%, 67%) with decreasing use of BT (50%, 44% 36%, 31%) over the aggregated diagnostic years of 2004–2006, 2007–2009, 2010–2012, and 2013–2015.
Survival Analysis
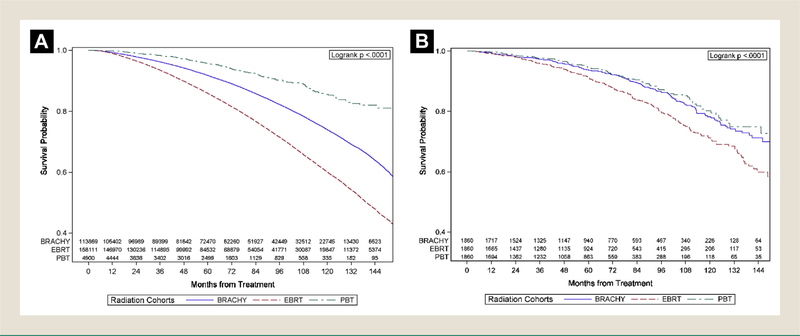
By the Kaplan-Meier method, the 10-year survival rates were 85.6%, 60.1%, and 74% for the PBT, EBRT, and BT groups, respectively (Figure 1A). With PBT set as the reference group, the hazard ratio (HR) was 3.43 (95% confidence interval [CI], 3.04–3.87) for EBRT and 2.03 (95% CI, 1.8–2.29) for BT (all P < .001) in the univariate analysis (Table 2). After controlling for the other covariates in multivariable analysis (MVA), the HR for death was 1.72 (95% CI, 1.51–1.96) for EBRT and 1.38 (95% CI, 1.21–1.58) for BT compared to PBT (all P < .001).
Figure 1.
Kaplan-Meier Curves With Number at Risk of Overall Survival by 3 Comparison Cohorts. (A) Original Study Population. (B) Propensity Score–matched Population
Table 2.
Univariate and Multivariable Cox Proportional Hazard Model for Association With Overall Survival in Study Population and Generalized Propensity Score—Matched Samples
| Characteristic | UVA | MVAa | MVA Matched Sampleb | |||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P | Hazard Ratio (95% CI) | P | Hazard Ratio (95% CI) | P | |
| Radiation cohort | ||||||
| EBRT vs. PBT | 3.43 (3.04–3.87) | <.001 | 1.72 (1.51–1.96) | <.001 | 1.64 (1.32–2.04) | <.001 |
| BT vs. PBT | 2.03 (1.80–2.29) | <.001 | 1.38 (1.21–1.58) | <.001 | 1.18 (0.93–1.48) | .168 |
| EBRT vs. BT | 1.69 (1.66–1.72) | <.001 | 1.24 (1.22–1.27) | <.001 | 1.39 (1.15–1.69) | <.001 |
| Age at diagnosis | 1.07 (1.07–1.07) | <.001 | 1.05 (1.05–1.05) | <.001 | 1.07 (1.06–1.08) | <.001 |
| Race/ethnicity | ||||||
| Non-Hispanic white vs. Asian | 1.39 (1.30–1.48) | <.001 | 1.33 (1.25–1.42) | <.001 | NS | |
| Non-Hispanic black vs. Asian | 1.38 (1.29–1.47) | <.001 | 1.43 (1.33–1.53) | <.001 | ||
| Hispanic vs. Asian | 1.01 (0.93–1.09) | .843 | 0.95 (0.87–1.03) | .201 | ||
| Other/unknown vs. Asian | 1.01 (0.91–1.12) | .848 | 1.14 (1.03–1.26) | .014 | ||
| Median income quartiles, 2008–2012 | ||||||
| <$38,000 | 1.34 (1.31–1.37) | <.001 | 1.20 (1.16–1.24) | <.001 | NS | |
| $38,000–$47,999 | 1.44 (1.40–1.47) | <.001 | 1.14 (1.11–1.17) | <.001 | ||
| $48,000–$62,999 | 1.21 (1.19–1.24) | <.001 | 1.09 (1.06–1.12) | <.001 | ||
| Versus $63,000+ | — | — | ||||
| No high school diploma, 2008–2012 | ||||||
| ≥21.0% vs. < 7.0% | 1.26 (1.23–1.29) | <.001 | 1.22 (1.18–1.26) | <.001 | 1.75 (1.39–2.21) | <.001 |
| 13.0%–20.9% vs. < 7.0% | 1.16 (1.13–1.18) | <.001 | 1.15 (1.12–1.19) | <.001 | 1.48 (1.17–1.87) | .001 |
| 7.0%–12.9% vs. < 7.0% | 1.34 (1.30–1.37) | <.001 | 1.09 (1.07–1.12) | <.001 | 1.14 (0.91–1.44) | .250 |
| Urban/rural, 2013 | NS | NS | ||||
| Urban vs. metro | 1.16 (1.14–1.19) | <.001 | ||||
| Rural vs. metro | 1.24 (1.17–1.31) | <.001 | ||||
| Unknown vs. metro | 0.93 (0.87–0.99) | .019 | ||||
| Primary insurance | ||||||
| Other/unknown vs. private | 1.69 (1.63–1.75) | <.001 | 1.34 (1.29–1.39) | <.001 | NS | |
| Medicare vs. Private | 2.08 (2.04–2.12) | <.001 | 1.17 (1.15–1.20) | <.001 | ||
| Year of diagnosis | ||||||
| 2004–2006 vs. 2013–2015 | 0.93 (0.86–0.94) | <.001 | 0.97 (0.94–1.01) | .237 | NS | |
| 2007–2009 vs. 2013–2015 | 0.94 (0.90–0.98) | .035 | 1.00 (0.97–1.04) | .757 | ||
| 2010–2012 vs. 2013–2015 | 0.98 (0.94–1.03) | .416 | 1.05 (1.01–1.09) | .017 | ||
| Facility type | ||||||
| Academic/research program, no vs. yes | 1.16 (1.14–1.18) | <.001 | 1.05 (1.03–1.07) | <.001 | NS | |
| Facility Location | ||||||
| East vs. West | 1.18 (1.15–1.22) | <.001 | 1.10 (1.07–1.14) | <.001 | 1.31 (1.05–1.63) | .018 |
| Central/Mountain vs. West | 1.36 (1.32–1.40) | <.001 | 1.14 (1.11–1.18) | <.001 | 1.24 (0.85–1.80) | .257 |
| Great circle distance (unit = 50 miles) | 0.96 (0.96–0.97) | <.001 | 0.99 (0.98–0.99) | <.001 | 0.96 (0.94–0.98) | <.001 |
| Charlson-Deyo score | ||||||
| 2+ vs. 0 | 2.28 (2.19–2.38) | <.001 | 2.11 (2.02–2.20) | <.001 | NS | |
| 1 vs. 0 | 1.42 (1.38–1.45) | <.001 | 1.39 (1.36–1.43) | <.001 | ||
| Sequence number | ||||||
| 01 vs. 00 | 2.54 (2.49–2.60) | <.001 | 2.27 (2.22–2.31) | <.001 | 3.09 (2.25–4.23) | <.001 |
| Grade | ||||||
| Poorly/undifferentiated | 1.62 (1.60–1.65) | <.001 | NS | NS | ||
| Unknown | 1.37 (1.26–1.48) | <.001 | ||||
| Vs. well/moderately differentiated | Ref | |||||
| AJCC clinical T stage | ||||||
| T3 vs. T1 | 1.77 (1.70–1.84) | <.001 | 1.28 (1.23–1.34) | <.001 | NS | |
| T2 vs. T1 | 1.31 (1.29–1.33) | <.001 | 1.10 (1.08–1.12) | <.001 | ||
| PSA (ng/mL) | ||||||
| >20 vs. < 10 | 1.56 (1.53–1.60) | <.001 | 1.27 (1.24–1.30) | <.001 | 1.47 (1.07–2.02) | .017 |
| 10–20 vs. < 10 | 1.56 (1.53–1.60) | <.001 | 1.26 (1.23–1.28) | <.001 | 1.11 (0.90–1.38) | .334 |
| Gleason score | ||||||
| 8–10 vs. 2–6 | 2.28 (2.23–2.33) | <.001 | 1.58 (1.54–1.62) | <.001 | 1.44 (1.12–1.85) | .004 |
| 7 vs. 2–6 | 1.47 (1.44–1.49) | <.001 | 1.20 (1.17–1.22) | <.001 | 1.14 (0.95–1.37) | .156 |
| Risk group | ||||||
| High vs. low | 1.55 (1.52–1.58) | <.001 | NICc | NICc | ||
| Intermediate vs. low | 2.22 (2.17–2.27) | <.001 | ||||
| ADT, yes vs. no | 1.41 (1.39–1.43) | <.001 | 0.96 (0.94–0.98) | <.001 | NS | |
| Positive biopsy cores (2010–2015), ≥ 50% vs. < 50% | 1.60 (1.53–1.67) | <.001 | NICc | NICc | ||
| Months of radiation start from diagnosis (quartiles) | ||||||
| ≤2.2 vs. > 4.6 | 1.12 (1.10–1.15) | <.001 | 1.03 (1.01–1.06) | .013 | NS | |
| >2.2–3.2 vs. > 4.6 | 1.08 (1.05–1.10) | <.001 | 1.03 (1.01–1.06) | .013 | ||
| >3.2–4.6 vs. > 4.6 | 1.04 (1.01–1.06) | .002 | 0.99 (0.97–1.02) | .565 | ||
Abbreviations: ADT = androgen deprivation therapy; AJCC = American Joint Committee on Cancer; ASD = absolute standardized difference; BT = brachytherapy; CI = confidence interval; EBRT = external-beam radiotherapy; IQR = interquartile range; MVA = multivariate analysis; NCCN = National Comprehensive Cancer Network; NIC = not initially considered in multivariable model; NS = not selected by variable backward elimination; PBT = proton beam therapy; PSA = prostate-specific antigen; UVA = univariate analysis.
Cox proportional hazard model was fitted followed by backward variable elimination with significance level of P < .05.
There were 1860 patients in each radiation cohort in final matched set. Maximum ASD is 0.163. Cox proportional hazard model was fitted followed by backward variable elimination with significance level of P < .05.
NCCN risk group was derived from clinical T stage, PSA, and Gleason score. Percentage of positive biopsy cores (2010–2015) was only available after 2010. NCCN risk groups were not considered in multivariable model to avoid collinearity or sample size reduction.
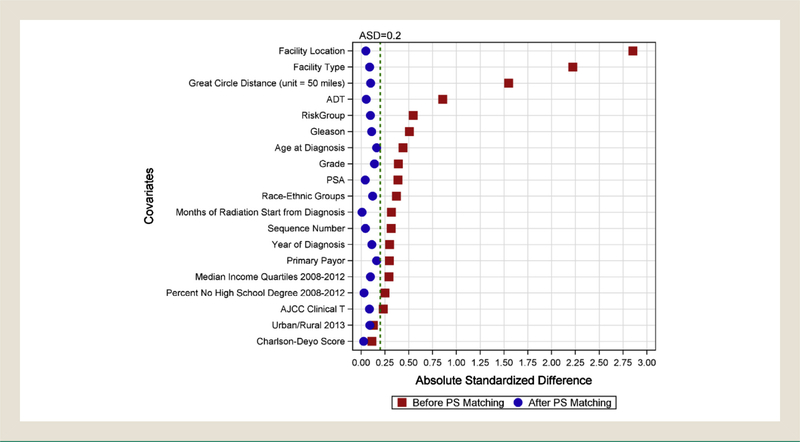
The GPSM approach identified 1860 matched cases from each cohort with all covariates balanced within ASD < 0.2. Figure 2 illustrates the covariate balance improvement from the original study population to the matched samples. The covariate distribution in the matched sample is presented in Supplemental Table 2 in the online version. In the multivariable Cox regression model of the matched samples, we found no statistically significant difference in OS between PBT and BT (HR = 1.18; 95% CI, 0.93–1.48; P = .168). However, relative to PBT, EBRT was associated with inferior OS (HR = 1.64; 95% CI, 1.32–2.04; P < .001). In the matched samples, the 10-year survival rates were 80.2%, 71.3%, and 78.3% for PBT, EBRT, and BT, respectively (Figure 1B). Comparing EBRT and BT, EBRT was associated with inferior OS compared to BT in both the original sample (HR = 1.24; 95% CI, 1.22–1.27) and in the matched sample following MVA (HR = 1.39; 95% CI, 1.15–1.69).
Figure 2.
Summary of Covariate Balance Improvement Measured by Absolute Standardized Difference before and after Generalized Propensity Score Matching
Subgroup Analysis
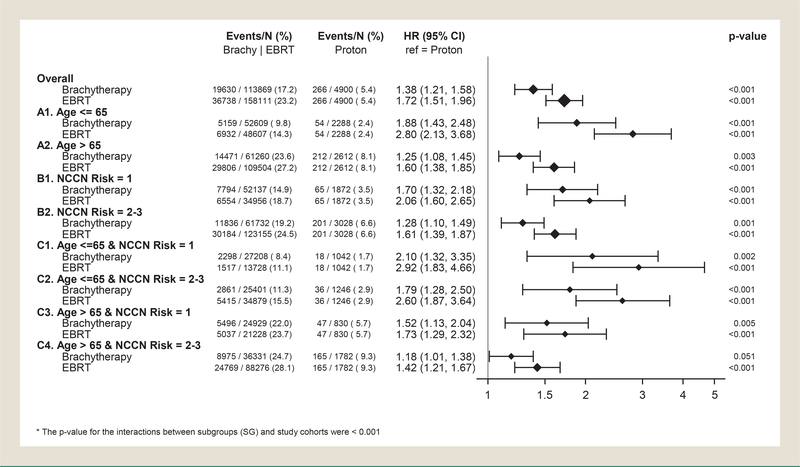
By exploring the difference in OS by treatment modality across a variety of patient subgroups defined by the baseline covariates, we identified that patients who were ≤ 65 years old and who were categorized as having NCCN low-risk disease had the greatest potential difference in OS between PBT and BT or EBRT. With PBT was the reference group, the overall HR was 1.38 for BT, increasing to 1.88 for patients with age at diagnosis ≤ 65 years (subgroup A1), to 1.70 for those who had NCCN low-risk disease (subgroup B1), and to 2.1 for those who were both ≤ 65 with low-risk disease (subgroup C1) (Figure 3). For EBRT, the overall HR was 1.72, increasing to 2.8 for the patients with age at diagnosis ≤ 65 (subgroup A1), to 2.06 for those who with NCCN low-risk disease (subgroup B1), and to 2.92 for those who were both ≤ 65 with low-risk disease (subgroup C1). For the combination subgroups by age and NCCN risk (subgroups C1-C4), there was a clear decreasing trend of HR for both BT (2.1, 1.79, 1.52, 1.18) and EBRT (2.92, 2.6, 1.73, 1.42) compared to PBT (interaction P < .001), suggesting that the magnitude of improvement in OS associated with PBT was less in older patients and those with higher-risk disease. Because of the low numbers of events for PBT patients, this subgroup analysis was not performed in the matched samples.
Figure 3.
Summary of Subgroup Analysis by Multivariable Cox Proportional Hazard Model With Forest Plots. (A) Stratified by Age Group. (B) Stratified by National Comprehensive Cancer Network (NCCN) Risk Group. (C) Stratified by Combinations of (A) and (B) Strata
Sensitivity Analysis for Percentage of Positive Biopsy Cores
A sensitivity analysis was performed for the more recently diagnosed patients (2010–2015) in which we further controlled for the possible confounding effect from the percentage of positive biopsy cores (≥50% vs. < 50%) in MVA and propensity score–matching analysis (Supplemental Table 3 in the online version). During 2010–2015, there were 90,472 eligible patients, 1525 (1.7%) of whom received proton therapy. Most patient characteristics were similar to those in the original study population. In MVA, patients with percentage of positive biopsy cores ≥ 50% had a greater hazard ratio for death (HR = 1.22; 95% CI, 1.16–1.28) compared to those with percentage of positive biopsy cores < 50%. In MVA, with PBT as the reference, the HR was 2.31 (95% CI, 1.54–3.48) for EBRT and 1.84 (95% CI, 1.22–2.76) for BT. After GPSM matched analysis, the HR was 2.08 (95% CI, 1.19–3.65) for EBRT and 1.96 (95% CI, 1.10–3.47) for BT. The sensitivity analysis confirmed the previous findings, with a greater magnitude of improved OS observed with PBT compared to the findings in Table 2. Regarding the comparisons between EBRT and BT, EBRT was associated with inferior OS compared to BT (HR = 1.26; 95% CI, 1.19–1.33) in MVA, while there was no statistical difference between EBRT and BT in the GPSM analysis (HR = 1.07; 95% CI, 0.7–1.62; P = .77).
Sensitivity Analysis for Unmeasured Confounder
A sensitivity analysis was performed to simulate the effect of unmeasured confounders.24 As Supplemental Figure 1 in the online version shows, when comparing EBRT to PBT, the sensitivity parameters required for an unmeasured confounder to drive the estimate to nonsignificance are larger than the coefficients for the observed confounders by at least 100%. To drive the estimate to 0, the sensitivity parameter of the unmeasured confounder would have to be > 400% larger than the largest coefficient estimate for the observed covariates. This suggests that the effect size of an unmeasured confounder would need to be very large relative to known prognostic variables to alter the findings, and that the estimated PBT versus EBRT difference appears robust to unobserved confounders. In the sensitivity analysis for BT versus EBRT, a few of the observed confounders are in proximity to the boundary of nonsignificance. This suggests that an unmeasured confounder with an effect size similar to one of several known variables could reduce the observed difference in OS between BT and EBRT.
Discussion
Our analysis confirmed earlier analyses of the NCDB showing significant differences in patient characteristics between those receiving proton therapy and other radiation modalities.10 Among other factors, the relatively limited geographic accessibility of PBT as well as the varied and more restrictive insurance coverage for PBT are expected to underpin the considerable selection biases, including potential unmeasured socioeconomic differences between patients who could access PBT. We used a generalized propensity score matching technique to balance the treatment cohorts by all available covariates and achieved well-matched cohorts by established prognostic variables. Nevertheless, an inherent limitation of retrospective analysis is the inability to account for unmeasured and unknown confounding variables. Therefore, it is possible that some, or all, of the residual difference observed in outcome among matched cohorts still reflects the powerful role of provider and patient self-selection. The limited utilization of nonproton treatment modalities at proton centers—and conversely the lack of proton therapy offered at nonproton centers—limited the ability to account for a potential facility cluster effect in our analysis.
The matched sample focuses on a subpopulation of patients who shared the same baseline characteristics across the 3 cohorts (Supplemental Table 2 in the online version). Hence, conclusions based on the matched sample may not be generalizable to the entire original study population. Treatment with ultra hypofractionated radiation regimens including stereotactic body radiotherapy were not included in this analysis. Finally, metastasis-free survival or cause-specific survival may be more relevant endpoints for early-stage prostate cancer, given its long natural history and the competing risk of death from other causes, but data for these alternate endpoints are not available in the NCDB. Despite these limitations, our sensitivity analysis found that the observed difference in OS between PBT and EBRT was robust to an unmeasured confounder, whose effect size would need to be significantly larger than any prognostic variable included in the model to alter the findings.
Is a difference in OS by radiation modality plausible? Biochemical disease control results from prospective single-institution studies of PBT are excellent.26–29 There is a growing interest in understanding and potentially exploiting differences in relative biologic effectiveness between proton therapy and photon therapy for an enhanced therapeutic effect in the tumor30 and in the potential for reduced immunosuppressive impact of proton therapy through reduced nontarget radiation exposure.31 Some preclinical work suggests that proton therapy may be more damaging to treatmentresistant cancer stem cells or better prime them for immune response.32,33
While in other disease sites a potential reduction in treatment toxicities with PBT may be hypothesized to improve survival, grade 5 acute or late toxicities after prostate cancer radiotherapy are exceedingly rare. One possible exception is the risk of a secondary malignancy. A systematic review of published clinical data confirms that secondary malignancies are observed after prostate radiotherapy, and that the risk may increase in patients followed for more than 10 years.34 Prior radiobiologic modeling has predicted a reduced rate of secondary malignancy after proton therapy compared to IMRT in prostate cancer.35 In this study, we observed a significantly lower rate of secondary cancers among men treated with PBT (1.1%) compared to EBRT (7.4%) and BT (6.4%) (Table 1). The rates were 1% (PBT), 10.9% (EBRT), and 8.6% (BT) among patients who were followed for 10+ years and diagnosed in 2004–2006 in this study population. The definition of sequence number is the order of current cancer diagnosis among all possible cancer diagnoses in a patient’s lifetime. A value of 1 for the sequence number means that the patient had at least one other cancer diagnosis after current cancer diagnosis, but it is not possible to say that those subsequent cancers were radiation induced or consequential in OS. Further investigation in this direction is needed.
In our GPSM analysis, patients treated with BT had similar 10-year OS (78.3%) as those treated with PBT (80.2%), which was greater than that observed in EBRT patients (71.3%). BT can provide excellent radiation dose localization within the prostate and a favorable toxicity profile. A large retrospective analysis found improved biochemical control with BT compared to EBRT for low-risk disease.36,37 Despite the effectiveness of BT, there is a well-documented decline in utilization over time,38–40 which was also observed in our study, at least part of which may be related to less favorable reimbursement relative to the true cost of BT care.1
This analysis supports the continued efforts to acquire high-quality data to assess the comparative effectiveness of proton therapy relative to other treatment modalities for prostate cancer, preferably through patient enrollment onto multi-institutional patient registries or prospective clinical trials, consistent with the model policy on proton therapy from the American Society for Radiation Oncology. Two prospective trials, PARTiQOL (NCT01617161) and COMPPARE (NCT03561220), will provide additional valuable comparative data on treatment toxicity, patient-reported quality of life, and PSA control between PBT and IMRT. In the interim, this large-scale retrospective study provides comparative outcomes data that support the rationale for ongoing and future trials, and to our knowledge, it is the first study demonstrating a possible long-term survival benefit with PBT in a contemporary prostate cancer cohort treated in the United States.
Conclusion
To our knowledge, this is the first analysis to evaluate survival rates between PBT, photon EBRT, and BT in men with localized prostate cancer. After adjustment for multiple confounding variables by GPSM, we found that PBT was associated with more favorable OS than EBRT and similar outcomes as BT. Patient enrollment onto ongoing and future prospective comparative clinical trials and patient registries is encouraged to further define the optimal role of PBT in the treatment of localized prostate cancer.
Supplementary Material
Clinical Practice Points.
Is proton beam therapy (PBT) for localized prostate cancer associated with improved overall survival compared to photon-based external-beam radiotherapy (EBRT) or brachytherapy (BT)? Clinical evidence is needed to guide informed decisions and resource allocation.
In this national population-based data set, a propensity score–matched analysis balanced for differences in baseline covariates found that proton therapy was associated with a significant improvement in overall survival compared to EBRT (hazard ratio = 1.64; P < .001), with similar outcomes as BT (hazard ratio = 1.18; P = .168). The magnitude of benefit was greatest in younger men with lower-risk disease. PBT was also associated with a lower risk of secondary malignancy.
These findings support continued efforts to acquire high-quality data to assess the comparative utilization and cost-effectiveness of proton therapy relative to other treatment modalities for prostate cancer.
To our knowledge, this is the first analysis to evaluate survival differences among PBT, EBRT, and BT in men with localized prostate cancer.
Patient enrollment onto ongoing and future prospective comparative clinical trials and patient registries is encouraged to further define the optimal role of PBT in the treatment of localized prostate cancer.
Acknowledgments
Supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and National Institutes of Health/National Cancer Institute (award P30CA138292).
Footnotes
Disclosure
The authors have stated that they have no conflict of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The data used in the study are derived from a deidentified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology used, or the conclusions drawn from these data by the investigators.
Supplemental Data
Supplemental figure and tables accompanying this article can be found in the online version at https://doi.org/10.1016/j.clgc.2020.08.009.
References
- 1.Dutta SW, Bauer-Nilsen K, Sanders JC, et al. Time-driven activity-based cost comparison of prostate cancer brachytherapy and intensity-modulated radiation therapy. Brachytherapy 2018; 17:556–63. [DOI] [PubMed] [Google Scholar]
- 2.Gray C, Campbell K. High Dose Rate Brachytherapy Versus Low Dose Rate Brachytherapy for the Treatment of Prostate Cancer: A Review of Clinical Effectiveness and Cost-Effectiveness. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; February 14, 2019. [PubMed] [Google Scholar]
- 3.Goldin GH, Sheets NC, Meyer AM, et al. Comparative effectiveness of intensity-modulated radiotherapy and conventional conformal radiotherapy in the treatment of prostate cancer after radical prostatectomy. JAMA Intern Med 2013; 173:1136–43. [DOI] [PubMed] [Google Scholar]
- 4.Das S, Liu T, Jani AB, et al. Comparison of image-guided radiotherapy technologies for prostate cancer. Am J Clin Oncol 2014; 37:616–23. [DOI] [PubMed] [Google Scholar]
- 5.Kamran SC, Light JO, Efstathiou JA. Proton versus photon-based radiation therapy for prostate cancer: emerging evidence and considerations in the era of value-based cancer care. Prostate Cancer Prostatic Dis 2019; 22:509–21. [DOI] [PubMed] [Google Scholar]
- 6.Halpern JA, Sedrakyan A, Hsu WC, et al. Use, complications, and costs of stereotactic body radiotherapy for localized prostate cancer. Cancer 2016; 122:2496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryant C, Henderson RH, Hoppe BS, et al. Controversies in proton therapy for prostate cancer. Chin Clin Oncol 2016; 5:55. [DOI] [PubMed] [Google Scholar]
- 8.Santos PMG, Barsky AR, Vapiwala N. Proton beam therapy after radical prostatectomy. Cancer 2020; 126:1135–6. [DOI] [PubMed] [Google Scholar]
- 9.Fang P, Mick R, Deville C, et al. A case-matched study of toxicity outcomes after proton therapy and intensity-modulated radiation therapy for prostate cancer. Cancer 2015; 121:1118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahal BA, Chen YW, Efstathiou JA, et al. National trends and determinants of proton therapy use for prostate cancer: a National Cancer Data Base study. Cancer 2016; 122:1505–12. [DOI] [PubMed] [Google Scholar]
- 11.Schroeck FR, Jacobs BL, Bhayani SB, Nguyen PL, Penson D, Hu J. Cost of new technologies in prostate cancer treatment: systematic review of costs and cost effectiveness of robotic-assisted laparoscopic prostatectomy, intensity-modulated radiotherapy, and proton beam therapy. Eur Urol 2017; 72:712–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corkum MT, Liu W, Palma DA, et al. Online advertising and marketing claims by providers of proton beam therapy: are they guideline-based? Radiat Oncol 2018; 13:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheets NC, Goldin GH, Meyer AM, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA 2012; 307:1611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu JB, Soulos PR, Herrin J, et al. Proton versus intensity-modulated radiotherapy for prostate cancer: patterns of care and early toxicity. J Natl Cancer Inst 2013; 105:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan HY, Jiang J, Hoffman KE, et al. Comparative toxicities and cost of intensity-modulated radiotherapy, proton radiation, and stereotactic body radiotherapy among younger men with prostate cancer. J Clin Oncol 2018; 36:1823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008; 15:683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for outcomes research: a review. JAMA Oncol 2017; 3:1722–8. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Nickleach DC, Zhang C, Switchenko JM, Kowalski J. Carrying out streamlined routine data analyses with reports for observational studies: introduction to a series of generic SAS® macros. F1000Res 2018; 7:1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Nickleach D, Lipscomb J. Propensity score matching for multiple treatment comparisons in observational studies. Paper presented at: 59th World Statistics Congress; August 25–30, 2013; Hong Kong. Available at: https://www.isi-web.org/index.php/publications/proceedings. Accessed: September 2, 2020. [Google Scholar]
- 20.Liu Y, Kowalski J, Gillespie TW. Propensity score approach for multiple treatment options and its application in cancer outcome research. Paper presented at: 2016 Joint Statistical Meeting; July 30-August 4 2016, Chicago, IL. Abstract 319875. [Google Scholar]
- 21.Kim HY. Statistical notes for clinical researchers: effect size. Restor Dent Endod 2015; 40:328–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simulat Comput 2009; 38:1228–34. [Google Scholar]
- 23.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc 1989; 84:1074–8. [Google Scholar]
- 24.Huang R, Xu RAO, Dulai PS. Sensitivity analysis of treatment effect to unmeasured confounding in observational studies with survival and competing risks outcomes. Stat Med 2020. [DOI] [PubMed] [Google Scholar]
- 25.Liu W, Kuramoto SJ, Stuart EA. An introduction to sensitivity analysis for unobserved confounding in nonexperimental prevention research. Prev Sci 2013; 14:570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pugh TJ, Choi S, Nogueras-Gonzalaez GM, et al. Proton beam therapy for localized prostate cancer: results from a prospective quality-of-life trial. Int J Part Ther 2016; 3:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bryant C, Smith TL, Henderson RH, et al. Five-year biochemical results, toxicity, and patient-reported quality of life after delivery of dose-escalated image guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2016; 95:422–34. [DOI] [PubMed] [Google Scholar]
- 28.Henderson RH, Bryant C, Hoppe BS, et al. Five-year outcomes from a prospective trial of image-guided accelerated hypofractionated proton therapy for prostate cancer. Acta Oncol 2017; 56:963–70. [DOI] [PubMed] [Google Scholar]
- 29.Mendenhall NP, Hoppe BS, Nichols RC, et al. Five-year outcomes from 3 prospective trials of image-guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2014; 88:596–602. [DOI] [PubMed] [Google Scholar]
- 30.Guan F, Bronk L, Titt U, et al. Spatial mapping of the biologic effectiveness of scanned particle beams: towards biologically optimized particle therapy. Sci Rep 2015; 5:9850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ebner DK, Tinganelli W, Helm A, et al. The immunoregulatory potential of particle radiation in cancer therapy. Front Immunol 2017; 8:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gameiro SR, Malamas AS, Bernstein MB, et al. Tumor cells surviving exposure to proton or photon radiation share a common immunogenic modulation signature, rendering them more sensitive to T cell–mediated killing. Int J Radiat Oncol Biol Phys 2016; 95:120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, Lin SH, Fang B, Gillin M, Mohan R, Chang JY. Therapy-resistant cancer stem cells have differing sensitivity to photon versus proton beam radiation. J Thorac Oncol 2013; 8:1484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murray L, Henry A, Hoskin P, Siebert FA, Venselaar J, PROBATE group of GEC ESTRO. Second primary cancers after radiation for prostate cancer: a systematic review of the clinical data and impact of treatment technique. Radiother Oncol 2014; 110:213–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fontenot JD, Lee AK, Newhauser WD. Risk of secondary malignant neoplasms from proton therapy and intensity-modulated X-ray therapy for early-stage prostate cancer. Int J Radiat Oncol Biol Phys 2009; 74:616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grimm P, Billiet I, Bostwick D, et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int 2012; 109(suppl 1):22–9. [DOI] [PubMed] [Google Scholar]
- 37.Morris WJ, Tyldesley S, Rodda S, et al. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE-RT trial): an analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys 2017; 98:275–85. [DOI] [PubMed] [Google Scholar]
- 38.Mahmood U, Pugh T, Frank S, et al. Declining use of brachytherapy for the treatment of prostate cancer. Brachytherapy 2014; 13:157–62. [DOI] [PubMed] [Google Scholar]
- 39.Martin JM, Handorf EA, Kutikov A, et al. The rise and fall of prostate brachytherapy: use of brachytherapy for the treatment of localized prostate cancer in the National Cancer Data Base. Cancer 2014; 120:2114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel S, Ragab O, Demanes DJ, Chen AM, Kamrava M. Brachytherapy: where has it gone.again? J Clin Oncol 2016; 34:1155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.