Abstract
Recent animal and small clinical studies have suggested depression is related to altered lipid and amino acid profiles. However, this has not been examined in a population-based sample, particularly in women. We identified multiple metabolites associated with depression as potential candidates from prior studies. Cross-sectional data from three independent samples of postmenopausal women were analyzed, including women from the Women's Health Initiative-Observational Study (WHI-OS, n=926), the WHI-Hormone Trials (WHI-HT; n=1,325), and the Nurses' Health Study II Mind-Body Study (NHSII-MBS; n=218). Positive depression status was defined as having any of the following: elevated depressive symptoms, antidepressant use, or depression history. Plasma metabolites were measured using liquid chromatography-tandem mass spectrometry (21 phosphatidylcholines [PCs], 7 lysophosphatidylethanolamines, 5 ceramides, 3 branched chain amino acids and 9 neurotransmitters). Associations between depression status and metabolites were evaluated using multivariable linear regression; results were pooled by random-effects meta-analysis with multiple testing adjustment using the Benjamini-Hochberg false discovery rate (FDR). Prevalence rates of positive depression status were 24.4% (WHI-OS), 25.7% (WHI-HT) and 44.7% (NHSII-MBS). After multivariable adjustment, positive depression status was associated with higher levels of glutamate and PC 36:1/38:3, and lower levels of tryptophan and GABA-to-glutamate and GABA-to-glutamine ratio (FDR-p<0.05). Positive associations with LPE 18:0/18:1 and inverse associations with valine and serotonin were also observed, although these associations did not survive FDR adjustment. Associations of positive depression status with several candidate metabolites including PC 36:1/38:3 and amino acids involved in neurotransmission suggest potential depression-related metabolic alterations in postmenopausal women, with possible implications for later chronic disease.
Keywords: depression, metabolomics, women, cardiometabolic disease, lipids, amino acids
Introduction
A substantial body of research demonstrates that depression is associated with increased risk of a range of chronic diseases. The evidence has been particularly strong with regard to cardiometabolic diseases, such as metabolic syndrome, diabetes, heart disease and stroke [1-5], which are among the leading causes of morbidity and mortality in developed countries. For example, in the Women’s Health Initiative (WHI), the incidence rate for coronary heart disease (CHD) per 1,000 person-years was 5.18 for women using tricyclic antidepressants (TCA), 4.73 for women using selective serotonin reuptake inhibitors (SSRI), and 5.38 for women using other antidepressants, compared to 3.81 for women who did not use any antidepressants [1]. Similar differences in cardiovascular disease (CVD) incidence were also observed comparing women with versus without depressive symptoms [2]. As a result, investigators have speculated about mechanisms by which depression might systematically affect cardiometabolic function. Prior work has suggested that depression may lead to chronic inflammation and altered glucose regulation, along with greater likelihood of engaging in unhealthy behaviors, albeit also noting that the relationships of depression with these factors may be bidirectional [6-9]. However, identification of molecular mechanisms linking depression with adverse cardiometabolic outcomes remains limited. Advances in metabolomic profiling technologies provide an opportunity to understand the metabolic signature associated with depression, which may shed additional light on depression-related pathogenesis of cardiometabolic disease.
Several experimental and small-scale clinical studies have examined depression in relation to plasma metabolites. In some of this work, depression has been associated with circulating levels of several lipid metabolites and amino acids. For example, compared with healthy controls, patients with major depressive disorder had elevated plasma phosphatidylcholines (PC), lysophosphatidylethanolamines (LPE) and ceramides, and lower tryptophan levels [10]. In animal models, rats who were versus were not exposed to chronic stress (a manipulation that induces depression) exhibited an increase in glutamate and the leucine-to-isoleucine ratio and a decrease in glutamine and valine [11]. However, existing studies on depression and metabolomic profiles generally included small sample sizes (e.g., <200 for cases and controls combined), did not control adequately for potential confounding factors (e.g., body mass index [BMI]), and did not examine associations specifically in women, who appear to be more susceptible to both depression and the cardiometabolic consequences of depression than men [12-14]. Particularly, no study has evaluated the associations between depression and plasma metabolites in postmenopausal women, a large segment of the population with heightened vulnerability to depression and substantially increased risk of cardiovascular disease [15, 16].
We identified several groups of metabolites that prior work has linked to depression (in human or experimental animal models) as candidate metabolites, including (1) PCs, (2) LPEs, (3) ceramides, (4) branched-chain amino acids (BCAA) and (5) amino acid neurotransmitters. We evaluated associations of depression status with these candidate metabolites in samples of postmenopausal women participating in the WHI or the Nurses’ Health Study II (NHSII). We hypothesized that alterations in these metabolites may reflect important metabolic changes due to depression that ultimately contribute to development of cardiometabolic disease.
Methods
Study population.
We used existing cross-sectional data from the WHI and the NHSII. The WHI is a large multicenter study of women comprised of a clinical trial of hormone therapy (WHI-HT) and a long-running prospective observational study (WHI-OS) [17]. Between 1994 and 1998, postmenopausal women aged 50–79 years were recruited through mass mailing campaigns and media awareness programs across 40 US clinical centers. Eligibility criteria and recruitment methods have been described previously [17]. WHI-HT randomized 27,347 postmenopausal women to a combined estrogen and progestin versus placebo arm, or estrogen only versus placebo arm, according to hysterectomy status. WHI-OS included 93,676 postmenopausal women who were either ineligible or unwilling to participate in the randomized trial, with prospective follow-up for occurrence of multiple health outcomes, including CVD. At baseline, all WHI participants completed the initial screening, a questionnaire assessment (including depression assessment) and a physical examination with fasting blood collection. A nested case-control study was conducted in WHI-OS and WHI-HT to examine the association of plasma metabolomics (obtained from assays of blood collected at baseline) with subsequent CHD risk [18]; this included 1,153 women who were free of CHD at baseline and developed CHD (i.e., cases) and 1,153 women who did not develop CHD (i.e., controls) during follow-up. CHD cases and controls were frequency-matched on age, race/ethnicity, hysterectomy status, and 2-year enrollment windows. The WHI metabolomics study was approved by the institutional review boards of the Brigham and Women’s Hospital.
NHSII is an ongoing prospective cohort study of 116,429 US female registered nurses. At enrollment in 1989, all participants completed a questionnaire reporting on their demographics, lifestyle factors, and medical history. Biennial follow-up questionnaires have been mailed to all participants to update exposure information and disease diagnoses. Return of the completed questionnaires implied consent to use the data in ongoing research. The Mind-Body Study (NHSII-MBS) was a NHSII substudy of 233 postmenopausal women, which oversampled women with childhood abuse to facilitate identification of biologic markers of psychosocial stress [19]. In 2013, MBS participants (age: 49–67 years) provided a fasting blood sample for metabolomics assay and completed a comprehensive online psychosocial assessment including depression assessment. The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health.
Cross-sectional data for the current study are provided by three independent samples from WHI and NHSII as described above: (1) 472 CHD case-control pairs from WHI-OS, (2) 681 CHD case-control pairs from WHI-HT, and (3) 226 women from NHSII-MBS. After excluding women with missing data on depression measures, 926 WHI-OS participants (n=18 excluded), 1,325 WHI-HT participants (n=37 excluded), and 218 MBS participants (n=8 excluded) were included in our analytic sample. While NHSII-MBS women are predominantly White, women participating in WHI are more diverse including 15% Black and 6% Hispanic women.
Assessment of depression status.
Depression status was assessed considering current symptoms, history of depression, and use of antidepressants. In WHI, current symptoms were assessed on the baseline questionnaire using 6 items from the validated Center for Epidemiological Studies Depression Scale (CES-D-6) that queried depressive symptoms in the past week [2]. Response options ranged from 0 to 3, with higher scores indicating higher depressive symptoms. Consistent with prior studies, we derived a sum score across all items and considered a CES-D-6 score≥5 as representing elevated current depressive symptoms. The 6-item CES-D has been previously found to correlate strongly with the full 20-item scale (r=0.88); compared with the established threshold of ≥16 using the full CES-D scale, the threshold of ≥5 in the 6-item scale had 95% agreement to classify current depression at levels considered to be clinically relevant [2]. Two questions from the Diagnostic Interview Schedule [20] included on the baseline questionnaire were used to assess history of depression. Participants self-reported whether they had been (1) depressed for two weeks or more in the past year, or (2) depressed for much of the time for two years or more in their lifetimes. Participants who answered yes to both questions were considered to have history of depressed mood. Use of antidepressants was also assessed at the baseline visit. Participants provided drug labels to permit an inventory of their current medications at that time, and information was recorded regarding use of any of the following antidepressant medications including TCAs, SSRIs, monoamine oxidase inhibitors, modified cyclics, or any other antidepressants. Participants demonstrating use of any of these medications were considered to be using antidepressants. The prevalence of current depressive symptoms, history of depressed mood and antidepressant use was 16.7%, 11.6% and 6.5%, respectively in the WHI-HT sample and 15.6%, 11.2% and 6.3%, respectively in the WHI-OS sample. In comparison, the rates of current depressive symptoms, history of depressed mood and antidepressant use in the entire WHI-OS cohort were 15.8%, 12.3%, and 7.7%, respectively [2].
In the NHSII-MBS, current depression status was assessed by the validated 10-item CES-D scale, and a CES-D score≥10 was used to indicate elevated depressive symptoms. Prior work has shown 97% agreement in classification of clinically relevant depression with the threshold of ≥10 on the 10-item scale versus the threshold of ≥16 on the full 20-item scale [21]. Physician-diagnosed depression was self-reported on every biennial NHSII main questionnaire since 2003. Participants who reported the diagnosis on 2013 or any prior questionnaires were considered to have history of physician-diagnosed depression. Regular antidepressant use in the past two years, including TCAs (e.g., Elavil, Tofranil, Pamelor), SSRIs (e.g., Prozac, Zoloft, Paxil, Celexa, Lexapro, Luvox) and other antidepressants (e.g., Wellbutrin, Effexor, Remeron, Cymbalta), was self-reported on the MBS questionnaire. In the MBS, the rates of current depressive symptoms, history of physician-diagnosed depression and antidepressant use were 18.8%, 33.0% and 24.8%, respectively. These rates were somewhat higher than the corresponding estimates in the full NHSII cohort in 2013 (18.9%, 28.2% and 21.6%, respectively), likely due to overrepresentation of women who experienced early life stress in the MBS.
In all three WHI and MBS datasets, the primary analysis was based on a composite depression status variable defined as positive according to the presence of at least one of the following conditions: presence of elevated current depressive symptoms, history of depression, or antidepressant use. In secondary analyses, associations with current versus past depression were evaluated by defining current depression as the presence of elevated current depressive symptoms or current antidepressant use regardless of history of depression, past depression as the presence of history of depression without current depressive symptoms or antidepressant use, considering participants with neither current nor past depression as the reference group. We also evaluated depression status according to antidepressant use by categorizing participants into four mutually exclusive groups according to: (1) presence of current depressive symptoms or history of depression with antidepressant use; (2) presence of current depressive symptoms or history of depression without antidepressant use; (3) antidepressant use with neither current depressive symptoms nor history of depression; and (4) no current depressive symptoms, history of depression or antidepressant use (reference).
Blood collection and metabolomics assay.
In WHI, baseline fasting blood samples were collected and processed at local centers into separate aliquots containing serum, plasma and buffy coat, which were frozen and shipped to a central repository for long-term storage at −70°C [17]. In NHSII-MBS, participants had their blood drawn in sodium heparin tubes by a colleague or at a local clinic and shipped with an ice pack via overnight courier to our laboratory, where it was processed into plasma, red blood cells and white blood cells and stored in the vapor phase of liquid nitrogen freezers [19]. In both cohorts, blood samples were divided into small aliquots before storage to minimize the number of freeze-thaw cycles.
Profiling of plasma metabolomics from all study samples was conducted at the Broad Institute of the Massachusetts Institute of Technology and Harvard University using the same liquid chromatography-tandem mass spectrometry (LC-MS/ MS) method. A C8-positive platform, which connected a Shimadzu Nexera X2 U-HPLC (Shimadzu Corp) to a Exactive Plus orbitrap mass spectrometer (Thermo Fisher Scientific), was used to measure polar and non-polar plasma lipids, including 21 phosphatidylcholines (PCs), 7 lysophosphatidylethanolamines (LPEs) and 4 ceramides that were consistently measured in all three datasets. A HILIC-positive platform composed of a Shimadzu Nexera X2 U-HPLC (Shimadzu Corp) coupled to a Q Exactive hybrid quadrupole orbitrap mass spectrometer (Thermo Fisher Scientific) was used to measure water soluble metabolites, including 3 branched chain amino acids (BCAAs, namely leucine [Leu], isoleucine [Ile] and valine [Val]) and 9 amino acid neurotransmitters (γ-aminobutyric acid [GABA], glutamate [Glu], glutamine [Gln], glycine [Gly], histidine [His], phenylalanine [Phe], tryptophan [Trp], serotonin [5-HT], tyrosine [Tyr]) that were also consistently measured across all three datasets. In light of the well-established Gln-Glu/GABA cycle in neurofunction, we further considered two ratio measures: GABA/Glu ratio and Gln/Glu ratio. Internal standard peak areas were monitored for quality control and to ensure system performance throughout analyses. Pooled plasma reference samples were also inserted every twenty samples as an additional quality control. Targeted data were processed using MultiQuant software (AB SCIEX; Framingham, MA) or TraceFinder (Thermo Fisher Scientific; Waltham, MA) for automated LC-MS peak integration. In our pilot testing of the metabolomics platform, 92% of metabolites had excellent assay reproducibility, nearly 90% of metabolites were stable over 1–2 years within women, and about 75% of metabolites were stable with delayed processing [22]. The metabolite categories with the best assay reproducibility and within-person stability were lipids and lipid metabolites, bile acids, amino acids and their derivatives, organic acids, and vitamins. In WHI, the median coefficient of variation (range) was 7.8% (4.6, 21.7) for candidate lipids and 11.6% (8.4, 16.7) for candidate amino acids; the corresponding statistics were 8.3% (4.1, 20.1) and 9.6% (6.2, 23.3), respectively, in MBS.
Covariate assessment.
At baseline, WHI participants self-reported age, race/ethnicity and smoking status. Medical history (hypertension and diabetes) and current medication use (aspirin, statin, other lipid-lowering medications and hormone therapy) were ascertained from interviewer-administered questionnaires. Weight was measured on a balance beam scale and height using a wall-mounted stadiometer by trained staff. Diet and alcohol consumption were assessed using a validated food frequency questionnaire (FFQ), and diet quality was evaluated by the Alternative Healthy Eating Index (AHEI) [23]. Recreational physical activity was evaluated by metabolic equivalent task (MET)-hours per week based on responses to the WHI brief physical activity inventory [24]. Sleep quality was assessed by the WHI insomnia rating scale [25].
In NHSII, birth date, race/ethnicity and height were self-reported at baseline. At blood collection, MBS participants reported their current weight, smoking status and medication use. On every NHSII biennial questionnaire, participants reported whether they had been diagnosed with diabetes or hypertension in the past two years. Diet quality measured by AHEI was derived from dietary information collected in 2011 using a validated semi-quantitative FFQ [26]. MET-hours per week was calculated from a validated questionnaire to quantify recreational physical activity [27]. The Pittsburgh Sleep Quality Index was used to assess habitual sleep quality [28].
Statistical analysis.
Due to right-skewed metabolite distributions, all metabolite values were natural log-transformed. Values below the limit of detection (<1% of the sample except for tryptophan) were imputed using half of the observed minimum metabolite level. Log-transformed metabolite values were then converted to z-scores with a mean of 0 and a SD of 1 in each sample separately to facilitate comparison of different metabolites on the same scale. Pairwise correlations between metabolite z-scores were calculated using Spearman’s correlation coefficients.
In the primary analysis within each dataset, multivariable linear regression models were fit to examine the associations between the composite depression status (see definition above) and individual candidate metabolites, with the metabolite z-score as the dependent variable and binary depression status as a predictor. The analysis adjusted for age (continuous), race/ethnicity (white, non-white), BMI (continuous), current medication use (aspirin, statin, other lipid-lowering medications and hormone therapy; yes, no) and co-morbidities (hypertension and diabetes; yes, no). Missing covariates (<3% of the sample) were assigned to the sample-specific median. Effect estimates from each dataset were then pooled by random-effects meta-analysis [29, 30]; potential heterogeneity across datasets was tested using the Q statistic for consistency of the association across studies. The Q statistic is calculated as a weighted sum of squares of the effects around its mean, where the weights are proportional to the inverse of the sum of the study-specific variance plus the common between-study variance estimated in a non-iterative method-of-moments procedure. To account for multiple testing and control for the false discovery rate (FDR), p-values from the meta-analysis within each metabolite category (i.e., 22 PCs, 7 LPEs, 4 ceramides and 14 amino acids measures) were used to derive an FDR-adjusted p value using the positive FDR approach [31]. An FDR cutoff of 5% (implying that no more than 5% of significant findings are truly null) was used to identify metabolites that were statistically significantly associated with depression status. For lipid metabolites, the strength of the associations (measured by -log10P or β coefficient) with the carbon chain length and double bond content of the fatty acid chains were examined in linear models, with carbon chain length and double bond content modeled as predictors. In secondary analyses, for metabolites that were nominally significant in the primary meta-analysis, we further examined their associations with alternative depression definitions as described above to assess whether the findings differed by current versus past depression or by antidepressant use.
Several sensitivity analyses were performed. First, we adjusted for additional behavioral factors that may be associated with both depression status and metabolite levels, including current smoking (yes, no), dietary quality, physical activity, sleep quality, caffeine intake and alcohol drinking. Second, we evaluated the associations of the continuous CESD symptom score with metabolites among women who did not use antidepressant medications. Third, we evaluated the relationships of the three depression indicators (namely, presence of elevated depressive symptoms, history of depression and antidepressant use) with metabolites by including them simultaneously in each multivariable model, with adjustment for potential confounders as noted above. Finally, given racial/ethnic differences in rates of depression and metabolomic profiles [32, 33], we repeated the analyses restricted to white women. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and R statistical packages version 3.2.5.
Results
Based on the composite depression status definition, the prevalence of positive depression status was 25.7% in the WHI-HT sample, 24.4% in the WHI-OS sample, and 44.7% in the MBS, which oversampled women with early abuse (Table 1). Women with versus without positive depression status were less likely to be white and more likely to have diabetes, hypertension, use statins or other lipid-lowering medications; they were also more likely to have higher BMI, lower physical activity and alcohol consumption, and poorer sleep quality. Among participants with positive depression status across the three samples, only 15–20% of participants reported a history of depression without current depressive symptoms or antidepressant use (Supplemental Table 1). Further, in the two older WHI samples, only 12–13% of participants with current depressive symptoms or history of depression reported antidepressant use, whereas in the more recent NHSII-MBS, 52% of these participants reported antidepressant use.
Table 1.
Age-standardized characteristics of the study sample by depression status
| WHI-OS (1994) |
WHI-HT (1994) |
NHSII-MBS (2013) |
||||
|---|---|---|---|---|---|---|
| Depression status | ||||||
| No | Yes | No | Yes | No | Yes | |
| N | 700 | 226 | 984 | 341 | 122 | 96 |
| Age, years | 67.7 (6.8) | 66.5 (6.8) | 67.1 (6.8) | 65.6 (7.1) | 60.6 (4.2) | 60.5 (3.8) |
| Non-white, % | 25 | 27 | 15 | 19 | 2 | 7 |
| Medical factors | ||||||
| Type 2 diabetes, % | 9 | 15 | 13 | 19 | 3 | 9 |
| Hypertension, % | 40 | 45 | 41 | 47 | 30 | 38 |
| Aspirin use, % | 23 | 20 | 29 | 30 | 31 | 39 |
| Statin use, % | 8 | 10 | 13 | 14 | 23 | 31 |
| Other lipid-lowering drug, % | 1 | 2 | 2 | 4 | 3 | 6 |
| Hormone therapy, % | 41 | 41 | 7 | 4 | 23 | 25 |
| Lifestyle factors | ||||||
| Body mass index, kg/m2 | 27.6 (6.2) | 28.6 (6.2) | 29.2 (5.8) | 29.7 (6.0) | 26.0 (6.3) | 27.8 (5.8) |
| Current smoker, % | 7 | 12 | 12 | 19 | 3 | 3 |
| Physical activity, MET-hrs/week1 | 13.5 (13.9) | 9.8 (10.6) | 9.9 (12.0) | 8.3 (10.4) | 31.0 (27.7) | 24.3 (24.0) |
| Diet quality score2 | 69.0 (11.2) | 66.6 (11.3) | 66.6 (10.7) | 65.4 (11.0) | 67.9 (13.7) | 68.8 (12.3) |
| Sleep quality score3 | 6.1 (4.2) | 8.9 (5.0) | 6.5 (4.3) | 9.4 (4.9) | 5.6 (2.7) | 7.8 (3.9) |
| Caffeine intake, mg/day | 151.1 (131.5) | 148.2 (128.2) | 177.6 (148.3) | 178.2 (150.6) | 167.8 (138.0) | 186.9 (151.1) |
| Alcohol intake, g/day | 5.5 (11.7) | 4.4 (10.6) | 4.3 (9.4) | 3.5 (12.3) | 9.4 (12.6) | 5.9 (9.9) |
Assessed by the WHI brief physical activity inventory in WHI and a validated questionnaire including individual types of moderate and vigorous activities in NHSII
Measured by HEI-2005 in WHI and AHEI-2010 in NHSII
Measured by the WHI Insomnia Rating Scale in WHI (range: 0-20) and the Pittsburgh Sleep Quality Index in NHSII (range: 0-21), with higher scores indicating poor sleep quality
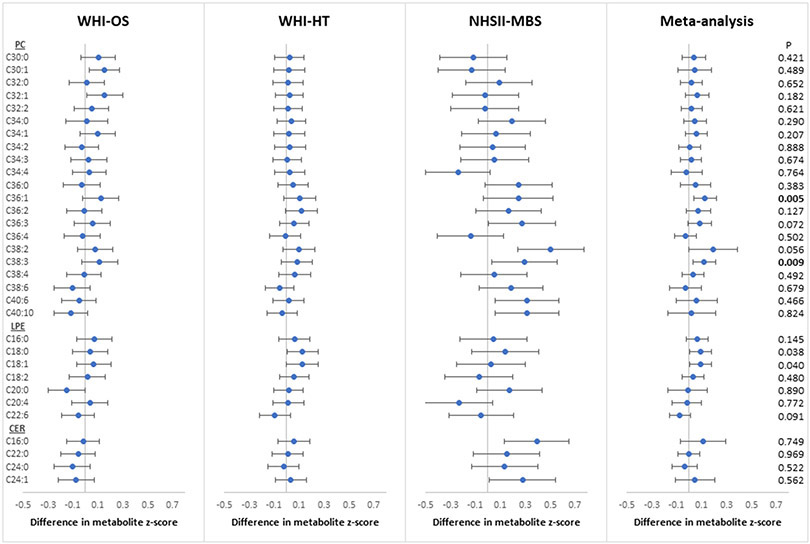
In the meta-analysis of candidate lipid metabolites, positive depression status was associated with increased relative abundance of 2 PCs (PC 36:1, 38:3) at the FDR-adjusted threshold of 0.05 after adjustment for age, race/ethnicity, BMI, medication use and co-morbidities (Figure 1). The mean differences in metabolite z-score in SD units from meta-analysis were 0.13 (95% CI: 0.04, 0.22) for PC 36:1 and 0.12 (95% CI: 0.03, 0.21) for PC 38:3 (FDR-p<0.05). At a less stringent FDR threshold of 0.1, positive depression status was associated with increased relative abundance of PC 36:3 with a mean difference of 0.09 SD (95% CI: 0.00, 0.18) and PC 38:2 with a mean difference of 0.19 SD (95% CI: 0.00, 0.39). Of note, the statistical significance for PC 38:2 in meta-analysis was primarily driven by the NHSII-MBS estimate (mean difference=0.50 SD; 95% CI: 0.24, 0.77), with significant between-sample heterogeneity (p-heterogeneity=0.02); no significant between-sample differences were observed for the other PCs (p-heterogeneity>0.25). Further, these four PCs were moderately to strongly inter-correlated within each sample (Spearman correlation>0.6 in general; Supplemental Figure 1). Interestingly, positive depression status was most strongly associated with PCs with longer carbon chains and lower double bond content. The mean change in -log(p-value) was 0.39 (95% CI: 0.14, 0.64; p-trend=0.007) per unit increase in carbon chain length and −0.46 (95% CI: −0.76, −0.17; p-trend=0.007) per unit increase in double bond content (Supplemental Table 2 and Supplemental Figure 2). In the meta-analysis of candidate LPEs and ceramides with depression, none of the associations were robust to FDR correction, although increases in relative abundance of LPE 18:0 and 18:1 among those with positive depression status were evident at the FDR threshold of 0.10.
Figure 1.
Associations of depression status (defined as the presence of any of elevated current depressive symptoms, history of depression or antidepressant use) with plasma lipids, including phosphatidylcholines (PC), lysophosphatidylethanolamines (LPE) and ceramides (CER), adjusted for age, race/ethnicity, BMI, hypertension, diabetes, aspirin use, statin use, other lipid-lowering medications and hormone therapy. P: nominal p-value; Bold: FDR-p<0.05
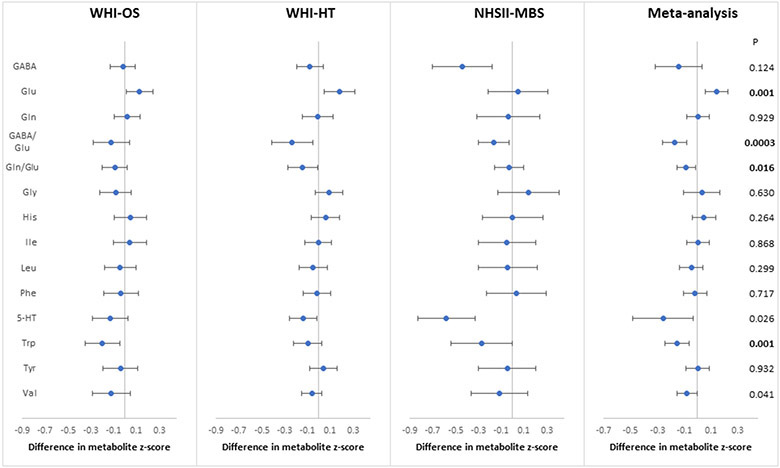
In the meta-analysis of 12 candidate amino acids with depression status, the strongest associations were observed for Glu and Trp (FDR-p< 0.05), with consistent trends across datasets (p-heterogeneity>0.38; Figure 2). These two amino acids were generally uncorrelated with the lipid metabolites examined above or with each other (Spearman r<0.3 across the three samples; Supplemental Figure 1). Comparing women with versus without positive depression status, the average difference in multivariable-adjusted metabolite z-score from meta-analysis was 0.14 SD (95% CI: 0.06, 0.23; p=0.001) for Glu and −0.15 (95% CI: −0.24, −0.06; p=0.001) for Trp. Although depression status was not associated with either Gln or GABA, their ratios to Glu were inversely associated with positive depression status in the meta-analysis at FDR-p<0.05. The average z-score difference was −0.17 SD (95% CI: −0.26, −0.08; p=0.0003) for GABA/Glu and −0.09 SD (95% CI: −0.15, −0.02; p=0.016) for Gln/Glu. Positive depression status was less strongly associated with valine (β=−0.08; 95% CI: −0.15, −0.01; p=0.04) and 5-HT (β=−0.26; 95% CI: −0.48, −0.03; p=0.03), each satisfying a less stringent FDR threshold of 0.10. No significant associations were observed for other candidate amino acids in relation to depression status.
Figure 2.
Associations of depression status (defined as the presence of any of elevated current depressive symptoms, history of depression or antidepressant use) with branched-chain amino acids and amino acid neurotransmitters in plasma, adjusted for age, race/ethnicity, BMI, hypertension, diabetes, aspirin use, statin use, other lipid-lowering medications and hormone therapy. P: nominal p-value; Bold: FDR-p<0.05
Abbreviations: GABA: γ-aminobutyric acid; Glu: glutamate; Gln: glutamine; Gly: glycine; His: histidine; Ile: isoleucine; Leu: leucine; Phe: phenylalanine; 5-HT: serotonin; Trp: tryptophan; Tyr: tyrosine; Val: valine.
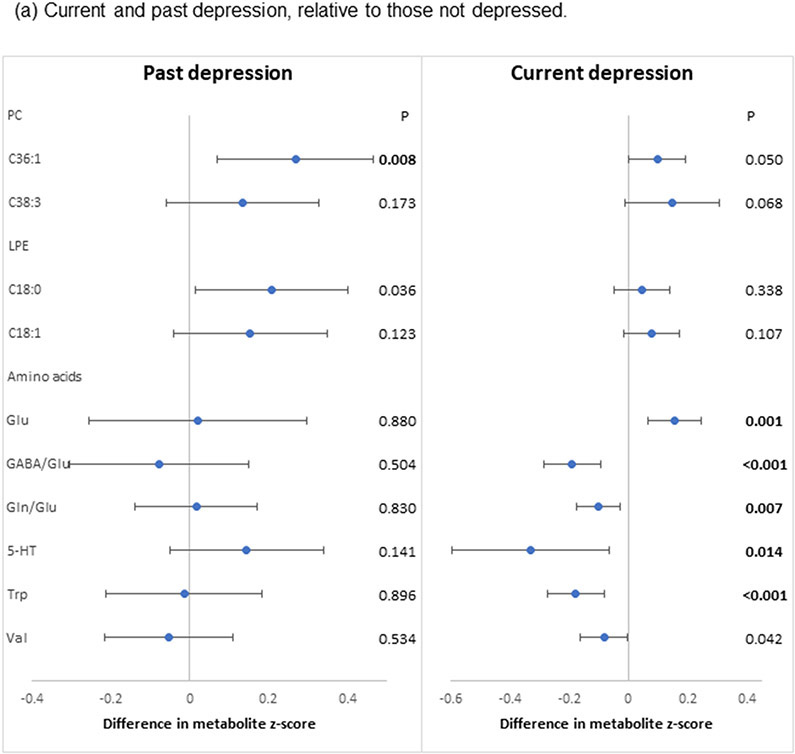
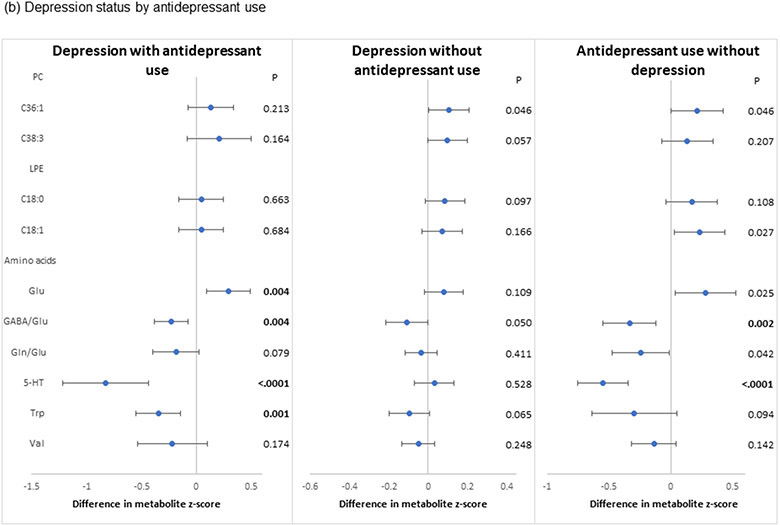
In the secondary analyses, the two PCs (PC 36:1, 38:3) that were significantly associated with the primary composite depression definition exhibited largely similar associations when considering positive depression status as defined by current versus past depression (Figure 3a). Associations of the two LPEs (LPE 18:0, 18:1) that showed nominal statistical significance with the primary composite definition were also similar between current versus past depression. However, the inverse associations with Trp, 5-HT, valine and the two ratio measures and the positive association with Glu were observed only for current and not past depression. In analyses of depression status by antidepressant use (Figure 3b), associations were similar for the two PCs and the two LPEs relative to findings from the primary analyses, although none met the threshold for statistical significance after FDR adjustment. By contrast, the two antidepressant use groups (with or without depression) showed strong associations with amino acids.
Figure 3.
Pooled associations of depression characteristics with plasma lipid and amino acid metabolites identified in the primary analyses, adjusted for age, race/ethnicity, BMI, hypertension, diabetes, aspirin use, statin use, other lipid-lowering medications and hormone therapy. (a) Associations with current and past depression. Participants were categorized into three groups - past depression (defined as history of depression without current depressive symptoms or current antidepressant use), current depression (defined as current elevated depressive symptoms or current antidepressant use regardless of depression history) and those with no evidence of depression (no current depressive symptoms, no antidepressant use, and no depression history). The reference group included women with no evidence of depression. (b) Associations with depression status by antidepressant use. Participants were categorized into four groups – (1) presence of current depressive symptoms or history of depression with antidepressant use; (2) presence of current depressive symptoms or history of depression without antidepressant use; (3) antidepressant use with neither current depressive symptoms nor history of depression; and (4) no current depressive symptoms, history of depression or antidepressant use (reference). P: nominal p-value; Bold: FDR-p<0.05
Abbreviations: GABA: γ-aminobutyric acid; Glu: glutamate; Gln: glutamine; 5-HT: serotonin; Trp: tryptophan; Val: valine.
In sensitivity analyses, additional adjustment for behavioral factors somewhat attenuated the associations described above, but findings were similar overall (Supplemental Figures 3 and 4). Relative to findings in the full sample, restricting the analysis to white women resulted in similar associations of positive depression status with amino acid metabolites but weaker associations with lipid metabolites (Supplemental Figures 5 and 6). Similar to the primary results, analysis of the continuous CESD score among women with higher symptoms levels but who did not use antidepressants revealed positive associations with PC 36:1 and PC 38:3 and a strong inverse association with Trp (Supplemental Figures 7 and 8). However, there were no associations of symptoms levels with Glu (p=0.75), GABA/Glu ratio (p=0.56) or Gln/Glu ratio (p=0.64). Similarly, after simultaneous adjustment of the three depression indicators (presence of elevated depressive symptoms, history of depression and antidepressant use), the associations with amino acids were strongest with antidepressant use (Supplemental Tables 3) whereas the associations with PCs were more strongly associated with depression history (Supplemental Tables 4).
Discussion
Among three independent samples of postmenopausal women, we observed consistent associations between positive depression status and altered levels of several lipid and amino acid candidate metabolites, including PC 36:1, 38:3, glutamate, the GABA/glutamate ratio, the glutamine/glutamate ratio and tryptophan. Associations were robust after accounting for multiple factors that may be associated with either metabolomic profiles or depression status. Analyses focusing on a depressive symptom score among women who did not use antidepressants resulted in similar associations for lipid metabolites and tryptophan, although associations with glutamate, GABA/glutamate ratio and glutamine/glutamate ratio diminished. These findings suggest that altered lipid metabolism in relation to depression is not solely attributable to use of antidepressants and potential pharmacologic effects. By contrast, amino acid profiles may be more strongly linked to antidepressant use, a finding consistent with other work showing that antidepressants may have normalizing roles in glutamate and GABA metabolism by directly acting on glutamatergic and GABAergic receptors [34, 35]. Collectively, these data suggest that depression status is associated with multiple metabolites that in turn could be linked to the pathogenesis of major cardiometabolic diseases.
Our findings were similar to some prior evidence. For example, one recent study found that levels of several lipid classes were increased among patients with major depressive disorder compared to healthy controls, including LPE 18:2 and PC 32:1 [10]. Another study reported worse progression of depressive symptoms over 12 weeks among depressed patients with higher versus lower plasma levels of PC 18:1 at baseline [36]. Our findings suggest that positive depression status may be associated with PCs and LPEs with similar carbon numbers and double-bond content, namely PC 36:1, 38:3 and LPE 18:0, 18:1. Further, these associations did not differ appreciably by antidepressant use or by past versus current depression status, suggesting that depression may have a long-term relationship with altered lipid profiles that is not strongly modified by antidepressant use.
However, previous studies on depression and lipids have been very mixed, with a wide range of metabolites identified. For example, a qualitative systematic review of 14 studies focusing on the relationship between ceramides and depression reported that depression status was most strongly linked to ceramides C18:0 and C20:0 [37], but we did not measure either of these metabolites on our semi-targeted metabolomics platform. In another larger Dutch study of 742 individuals, depression and anxiety symptoms were inversely correlated with PC O 36:4 (an ether phospholipid) and its ratio to ceramide 20:0 [38]. Several other small human studies have also yielded mixed results regarding lipid dysregulation in patients with depression [39-41], in part due to different methods for evaluating lipidomics (i.e., different types of lipids were quantified) as well as different age/sex distributions. For example, one study found elevations in LDL, VLDL and unsaturated lipids in depressed patients, but none of the PC, LPE or ceramides that we evaluated were measured in that study [39]. Future studies using consistent methods for more comprehensive coverage of lipid metabolites would be instrumental for clarifying and confirming specific lipid species that may be altered in depression.
There is longstanding interest in the relationship of depression with different types of neurotransmitters, although prior studies have generally been small and focused on individual types of neurotransmitters. Recently, one study examined a panel of 19 plasma neurotransmitters involved in GABAergic, catecholaminergic and serotonergic pathways, and found that 9 metabolites were significantly altered between 50 patients with major depressive disorder versus 50 healthy controls [42]. We evaluated 4 of these 9 metabolites (GABA, Gln, Tyr and Trp) in our study; however, positive depression status was inversely associated only with Trp, which prior work has suggested may act to reduce depression. For example, repeated sleep deprivation, which has been proposed as a possible treatment for depression [43, 44], induces acute elevations in plasma Trp levels [45]. However, in the Netherlands Study of Depression and Anxiety, Trp was not significantly associated with depressive symptoms in the overall sample or among individuals with current major depressive disorder [46]. Of note, Trp is a precursor to 5-HT, which also showed a trend toward an inverse association with depression status in our primary analysis. Antidepressant medications, such as SSRIs, act on 5-HT receptors to increase the extracellular level of 5-HT and thereby reduce symptoms of major depressive disorder and anxiety disorder. This is consistent with our observation of the striking difference in whether depression status was associated with 5-HT depending on antidepressant use, and more studies are needed to understand the potential impact of antidepressant use on other amino acids.
We observed a positive association of depression status with Glu, while a previous study found that depression was inversely associated with Gln, positively associated with GABA and unassociated with Glu [42]. However, it is important to note that this previous study included male and female Chinese adults and considered few covariates (e.g., co-morbidities), making comparisons with the current study difficult. Interestingly, in our study, positive depression status was more strongly associated with the GABA/Glu ratio and the Gln/Glu ratio than with the individual metabolites. Glutamate is the most abundant excitatory neurotransmitter whereas GABA is a major inhibitory neurotransmitter in the human central nervous system. Taken together, these findings suggest that depression may be associated with imbalances between excitatory and inhibitory neurotransmitters [47].
Prior work has suggested that higher depression is associated with increased risk of cardiometabolic diseases [2-5]. A large number of metabolites have been identified to predict risk of cardiometabolic disease [48, 49], and it is notable that several candidate metabolites associated with depression status in the current study are the same as, or share common features with, metabolites that other work has linked to cardiometabolic diseases. For example, in the Framingham Heart Study, lipids characterized by lower carbon number and double bond content, including PC 34:2 and LPE 18:2, were positively associated with increased diabetes risk [50]. In several longitudinal human studies of coronary heart disease (including the WHI), glutamate, glutamine and their ratio were consistently associated with risk of metabolic disorders and cardiovascular disease [18, 51-53]. The Prevención con Dieta Mediterránea (PREDIMED) Study also found that higher baseline tryptophan levels were associated with lower risk of incident cardiovascular disease [54]. In contrast, although several prospective studies have linked BCAAs and aromatic amino acids (tyrosine and phenylalanine) with higher risk of diabetes and cardiovascular disease [55-57], we did not find strong associations of these metabolites with depression in the current study . An interesting and important avenue for future research may be to examine more broadly whether other metabolites or metabolic pathways may mediate the association of depression with cardiometabolic disease risk.
Strengths of our study include the large sample size, utilization of the same platform across studies for metabolomic assays with strict quality control, and availability of a large number of relevant covariates for consideration. However, several limitations should be noted. First, our study was cross-sectional, thus temporal relationships could not be established. For example, while we initially hypothesized that alterations in metabolite levels may represent the metabolic consequences of depression, due to the cross-sectional design we cannot exclude the possibility that certain metabolites (e.g., amino acids involved in neurotransmission) may also play a role in the pathogenesis of depression. Numerous studies have documented that individuals with depression and co-morbid cardiometabolic conditions may be more likely to initiate and adhere to antidepressant treatment compared with individuals with depression only [58, 59], thus the observed associations between antidepressant use and several amino acids cannot be simply interpreted as an effect of antidepressants. Prospective longitudinal studies are needed to gain greater insight into the direction of the associations and whether relationships may be bidirectional. Second, our primary depression assessment combined information regarding current depressive symptoms, antidepressant use and history of depression rather than considering clinical diagnostic criteria. While this composite indicator maximized our ability to identify women who experienced some form of depression, such as those with subclinically elevated symptom levels, it is possible that the individual components we considered have heterogeneous effects, which could influence observed associations with plasma metabolites. However, it is worth noting that our composite definition of depression has been associated with risk of chronic disease in prior work [60-62], suggesting that this definition may capture important biological signals connecting depression with cardiometabolic health outcomes. Third, between-study differences in depression status measures (e.g., 6-item versus 10-item CESD, history of depressed mood versus history of physician-diagnosed depression), as well as imbalance in sample size and differences in population characteristics (e.g., age distribution, racial/ethnic composition, temporal variation in recognition/diagnosis of depression, length of blood sample storage, etc.), may have limited our ability to detect consistent signals for certain metabolites. Notably, while many pre-analytical processes, such as processing and storage of blood samples, were similar between WHI and MBS [63, 64], the WHI blood samples have been stored for a substantially longer period compared to MBS. However, the potential impact of long-term blood storage on metabolome stability remains unclear [63, 64], and would likely lead to non-differential measurement errors and attenuate the associations. As a result, we primarily relied on the meta-analysis results, in conjunction with the association patterns across samples, to evaluate associations of depression status with metabolites. Finally, the three independent samples were comprised exclusively of postmenopausal women. Whether our findings can be generalized to premenopausal women or men requires additional investigation.
In conclusion, using a candidate metabolite approach based on a priori hypotheses in three independent samples of postmenopausal women, we found that depression status was associated with altered plasma levels of certain lipid and amino acid metabolites. Replication of our results in other populations is warranted, and if confirmed, may have implications for understanding pathways potentially linking depression, metabolites, and cardiometabolic disease. These findings may also provide empirical evidence that would suggest the value of conducting more active screening and surveillance for depression to prevent subsequent development of cardiometabolic disease.
Supplementary Material
Acknowledgements
Metabolomic analysis in the Women’s Health Initiative (WHI) was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contract HHSN268201300008C. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C.
We thank the participants and the staff of the Nurses’ Health Study II for their valuable contributions. The Nurses’ Health Study II is supported by the National Institutes of Health (UM1 CA176726, R01 CA67262). This work was supported by the National Institutes of Health (R01 AG051600, R01 CA163451). T.H. is supported by K01 HL143034. The authors do not have any potential conflicts of interest to report. The authors assume full responsibility for analyses and interpretation of these data.
Footnotes
Disclosures
The authors have nothing to disclose.
References
- 1.Smoller JW, Allison M, Cochrane BB, Curb JD, Perlis RH, Robinson JG et al. Antidepressant use and risk of incident cardiovascular morbidity and mortality among postmenopausal women in the Women’s Health Initiative study. Arch Intern Med 2009; 169(22): 2128–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wassertheil-Smoller S, Shumaker S, Ockene J, Talavera GA, Greenland P, Cochrane B et al. Depression and cardiovascular sequelae in postmenopausal women. The Women’s Health Initiative (WHI). Arch Intern Med 2004; 164(3): 289–298. [DOI] [PubMed] [Google Scholar]
- 3.Yu M, Zhang X, Lu F, Fang L. Depression and Risk for Diabetes: A Meta-Analysis. Can J Diabetes 2015; 39(4): 266–272. [DOI] [PubMed] [Google Scholar]
- 4.Pan A, Keum N, Okereke OI, Sun Q, Kivimaki M, Rubin RR et al. Bidirectional association between depression and metabolic syndrome: a systematic review and meta-analysis of epidemiological studies. Diabetes Care 2012; 35(5): 1171–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB. Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review. Jama 2011; 306(11): 1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimaki M. Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav Immun 2015; 49: 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wesolowska K, Elovainio M, Hintsa T, Jokela M, Pulkki-Raback L, Lipsanen J et al. Is the association between depressive symptoms and glucose bidirectional? A population-based study. Health Psychol 2018; 37(7): 603–612. [DOI] [PubMed] [Google Scholar]
- 8.Whooley MA, de Jonge P, Vittinghoff E, Otte C, Moos R, Carney RM et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. Jama 2008; 300(20): 2379–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whooley MA, Wong JM. Depression and cardiovascular disorders. Annu Rev Clin Psychol 2013; 9: 327–354. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Zheng P, Zhao X, Zhang Y, Hu C, Li J et al. Discovery and validation of plasma biomarkers for major depressive disorder classification based on liquid chromatography-mass spectrometry. J Proteome Res 2015; 14(5): 2322–2330. [DOI] [PubMed] [Google Scholar]
- 11.Shi B, Tian J, Xiang H, Guo X, Zhang L, Du G et al. A (1)H-NMR plasma metabonomic study of acute and chronic stress models of depression in rats. Behav Brain Res 2013; 241: 86–91. [DOI] [PubMed] [Google Scholar]
- 12.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62(6): 593–602. [DOI] [PubMed] [Google Scholar]
- 13.Demmer RT, Gelb S, Suglia SF, Keyes KM, Aiello AE, Colombo PC et al. Sex differences in the association between depression, anxiety, and type 2 diabetes mellitus. Psychosom Med 2015; 77(4): 467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Möller-Leimkühler AM. Gender differences in cardiovascular disease and comorbid depression. Dialogues Clin Neurosci 2007; 9(1): 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karel MJ. Aging and depression: vulnerability and stress across adulthood. Clin Psychol Rev 1997; 17(8): 847–879. [DOI] [PubMed] [Google Scholar]
- 16.Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease. The Framingham Study. Ann Intern Med 1978; 89(2): 157–161. [DOI] [PubMed] [Google Scholar]
- 17.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol 2003; 13(9 Suppl): S5–17. [DOI] [PubMed] [Google Scholar]
- 18.Paynter NP, Balasubramanian R, Giulianini F, Wang DD, Tinker LF, Gopal S et al. Metabolic Predictors of Incident Coronary Heart Disease in Women. Circulation 2018; 137(8): 841–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang T, Trudel-Fitzgerald C, Poole EM, Sawyer S, Kubzansky LD, Hankinson SE et al. The Mind-Body Study: study design and reproducibility and interrelationships of psychosocial factors in the Nurses’ Health Study II. Cancer Causes Control 2019; 30(7): 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Arch Gen Psychiatry 1981; 38(4): 381–389. [DOI] [PubMed] [Google Scholar]
- 21.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med 1994; 10(2): 77–84. [PubMed] [Google Scholar]
- 22.Townsend MK, Clish CB, Kraft P, Wu C, Souza AL, Deik AA et al. Reproducibility of metabolomic profiles among men and women in 2 large cohort studies. Clin Chem 2013; 59(11): 1657–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.George SM, Ballard-Barbash R, Manson JE, Reedy J, Shikany JM, Subar AF et al. Comparing indices of diet quality with chronic disease mortality risk in postmenopausal women in the Women’s Health Initiative Observational Study: evidence to inform national dietary guidance. Am J Epidemiol 2014; 180(6): 616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol 2003; 13(9 Suppl): S107–121. [DOI] [PubMed] [Google Scholar]
- 25.Huang T, Zeleznik OA, Poole EM, Clish CB, Deik AA, Scott JM et al. Habitual sleep quality, plasma metabolites and risk of coronary heart disease in post-menopausal women. Int J Epidemiol 2019; 48(4): 1262–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985; 122(1): 51–65. [DOI] [PubMed] [Google Scholar]
- 27.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 1994; 23(5): 991–999. [DOI] [PubMed] [Google Scholar]
- 28.Huang T, Poole EM, Vetter C, Rexrode KM, Kubzansky LD, Schernhammer E et al. Habitual sleep quality and diurnal rhythms of salivary cortisol and dehydroepiandrosterone in postmenopausal women. Psychoneuroendocrinology 2017; 84: 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hertzmark E, Spiegelman D. The SAS METAANAL Macro. 2012.
- 30.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7(3): 177–188. [DOI] [PubMed] [Google Scholar]
- 31.Storey JDJJotRSSSB. A direct approach to false discovery rates. 2002; 64(3): 479–498. [Google Scholar]
- 32.Dunlop DD, Song J, Lyons JS, Manheim LM, Chang RW. Racial/ethnic differences in rates of depression among preretirement adults. Am J Public Health 2003; 93(11): 1945–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel MJ, Batch BC, Svetkey LP, Bain JR, Turer CB, Haynes C et al. Race and sex differences in small-molecule metabolites and metabolic hormones in overweight and obese adults. Omics 2013; 17(12): 627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G et al. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry 2002; 7 Suppl 1: S71–80. [DOI] [PubMed] [Google Scholar]
- 35.Musazzi L, Treccani G, Mallei A, Popoli M. The action of antidepressants on the glutamate system: regulation of glutamate release and glutamate receptors. Biol Psychiatry 2013; 73(12): 1180–1188. [DOI] [PubMed] [Google Scholar]
- 36.Czysz AH, South C, Gadad BS, Arning E, Soyombo A, Bottiglieri T et al. Can targeted metabolomics predict depression recovery? Results from the CO-MED trial. Transl Psychiatry 2019; 9(1): 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dinoff A, Herrmann N, Lanctot KL. Ceramides and depression: A systematic review. J Affect Disord 2017; 213: 35–43. [DOI] [PubMed] [Google Scholar]
- 38.Demirkan A, Isaacs A, Ugocsai P, Liebisch G, Struchalin M, Rudan I et al. Plasma phosphatidylcholine and sphingomyelin concentrations are associated with depression and anxiety symptoms in a Dutch family-based lipidomics study. J Psychiatr Res 2013; 47(3): 357–362. [DOI] [PubMed] [Google Scholar]
- 39.Zheng P, Gao HC, Li Q, Shao WH, Zhang ML, Cheng K et al. Plasma metabonomics as a novel diagnostic approach for major depressive disorder. J Proteome Res 2012; 11(3): 1741–1748. [DOI] [PubMed] [Google Scholar]
- 40.Paige LA, Mitchell MW, Krishnan KR, Kaddurah-Daouk R, Steffens DC. A preliminary metabolomic analysis of older adults with and without depression. Int J Geriatr Psychiatry 2007; 22(5): 418–423. [DOI] [PubMed] [Google Scholar]
- 41.Assies J, Pouwer F, Lok A, Mocking RJ, Bockting CL, Visser I et al. Plasma and erythrocyte fatty acid patterns in patients with recurrent depression: a matched case-control study. PLoS One 2010; 5(5): e10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan JX, Xia JJ, Deng FL, Liang WW, Wu J, Yin BM et al. Diagnosis of major depressive disorder based on changes in multiple plasma neurotransmitters: a targeted metabolomics study. Transl Psychiatry 2018; 8(1): 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boland EM, Rao H, Dinges DF, Smith RV, Goel N, Detre JA et al. Meta-Analysis of the Antidepressant Effects of Acute Sleep Deprivation. J Clin Psychiatry 2017; 78(8): e1020–e1034. [DOI] [PubMed] [Google Scholar]
- 44.Dallaspezia S, Benedetti F. Sleep deprivation therapy for depression. Curr Top Behav Neurosci 2015; 25: 483–502. [DOI] [PubMed] [Google Scholar]
- 45.Davies SK, Ang JE, Revell VL, Holmes B, Mann A, Robertson FP et al. Effect of sleep deprivation on the human metabolome. Proc Natl Acad Sci U S A 2014; 111(29): 10761–10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quak J, Doornbos B, Roest AM, Duivis HE, Vogelzangs N, Nolen WA et al. Does tryptophan degradation along the kynurenine pathway mediate the association between pro-inflammatory immune activity and depressive symptoms? Psychoneuroendocrinology 2014; 45: 202–210. [DOI] [PubMed] [Google Scholar]
- 47.Shabel SJ, Proulx CD, Piriz J, Malinow R. Mood regulation. GABA/glutamate co-release controls habenula output and is modified by antidepressant treatment. Science 2014; 345(6203): 1494–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ussher JR, Elmariah S, Gerszten RE, Dyck JR. The Emerging Role of Metabolomics in the Diagnosis and Prognosis of Cardiovascular Disease. J Am Coll Cardiol 2016; 68(25): 2850–2870. [DOI] [PubMed] [Google Scholar]
- 49.Guasch-Ferre M, Hruby A, Toledo E, Clish CB, Martinez-Gonzalez MA, Salas-Salvado J et al. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis. Diabetes Care 2016; 39(5): 833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rhee EP, Cheng S, Larson MG, Walford GA, Lewis GD, McCabe E et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest 2011; 121(4): 1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng S, Rhee EP, Larson MG, Lewis GD, McCabe EL, Shen D et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 2012; 125(18): 2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wurtz P, Havulinna AS, Soininen P, Tynkkynen T, Prieto-Merino D, Tillin T et al. Metabolite profiling and cardiovascular event risk: a prospective study of 3 population-based cohorts. Circulation 2015; 131(9): 774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shah SH, Bain JR, Muehlbauer MJ, Stevens RD, Crosslin DR, Haynes C et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet 2010; 3(2): 207–214. [DOI] [PubMed] [Google Scholar]
- 54.Yu E, Ruiz-Canela M, Guasch-Ferre M, Zheng Y, Toledo E, Clish CB et al. Increases in Plasma Tryptophan Are Inversely Associated with Incident Cardiovascular Disease in the Prevencion con Dieta Mediterranea (PREDIMED) Study. J Nutr 2017; 147(3): 314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011; 17(4): 448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruiz-Canela M, Toledo E, Clish CB, Hruby A, Liang L, Salas-Salvado J et al. Plasma Branched-Chain Amino Acids and Incident Cardiovascular Disease in the PREDIMED Trial. Clin Chem 2016; 62(4): 582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Magnusson M, Lewis GD, Ericson U, Orho-Melander M, Hedblad B, Engstrom G et al. A diabetes-predictive amino acid score and future cardiovascular disease. Eur Heart J 2013; 34(26): 1982–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Puyat JH, Kazanjian A, Wong H, Goldner E. Comorbid Chronic General Health Conditions and Depression Care: A Population-Based Analysis. Psychiatr Serv 2017; 68(9): 907–915. [DOI] [PubMed] [Google Scholar]
- 59.Menear M, Dore I, Cloutier AM, Perrier L, Roberge P, Duhoux A et al. Chronic physical comorbidity burden and the quality of depression treatment in primary care: a systematic review. J Psychosom Res 2015; 78(4): 314–323. [DOI] [PubMed] [Google Scholar]
- 60.Pan A, Lucas M, Sun Q, van Dam RM, Franco OH, Manson JE et al. Bidirectional association between depression and type 2 diabetes mellitus in women. Arch Intern Med 2010; 170(21): 1884–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whang W, Kubzansky LD, Kawachi I, Rexrode KM, Kroenke CH, Glynn RJ et al. Depression and risk of sudden cardiac death and coronary heart disease in women: results from the Nurses’ Health Study. J Am Coll Cardiol 2009; 53(11): 950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang T, Poole EM, Okereke OI, Kubzansky LD, Eliassen AH, Sood AK et al. Depression and risk of epithelial ovarian cancer: Results from two large prospective cohort studies. Gynecol Oncol 2015; 139(3): 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yin P, Lehmann R, Xu G. Effects of pre-analytical processes on blood samples used in metabolomics studies. Anal Bioanal Chem 2015; 407(17): 4879–4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Townsend MK, Bao Y, Poole EM, Bertrand KA, Kraft P, Wolpin BM et al. Impact of Pre-analytic Blood Sample Collection Factors on Metabolomics. Cancer Epidemiol Biomarkers Prev 2016; 25(5): 823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.