Abstract
Skinned cardiac muscle preparations are widely used to study contractile function of myofilament proteins and pathophysiological changes. The current methods applied in these biomechanical studies include detergent permeabilization of freshly isolated papillary muscle, ventricular trabeculae, surgically dissected ventricular muscle strips, mechanically blended cardiac muscle bundles or myocytes, and enzymatically isolated single cardiomyocytes. To facilitate and expand the skinned cardiac muscle approach, we have developed an efficient and readily practical method for mechanical studies of skinned mouse cardiac papillary muscle strips prepared from cryosections. Longitudinal papillary muscle strips of 120–150 micron wide cut from 35–70 micron thick cryosections are mounted to force transducer and chemically skinned for force-pCa, sarcomere length-tension relationship and rate of tension redevelopment studies. In addition to more effective skinning and perfusion than that with whole papillary muscle and much higher yield of useful preparations than that from trabeculae, this new methodology has two more major advantages. One is to allow for the use of frozen cardiac muscle in storage to maximize the value of muscle samples, facilitating resource sharing among research institutions without the need of transferring live animals or fresh biopsies. The other is that the integrity of the muscle strips is well preserved during the preparation and mechanical studies, allowing coupled characterization of myofilament proteins. The combined power of biomechanics and protein biochemistry can provide novel insights into integrative physiological and pathophysiological mechanisms of cardiac muscle contraction while the high yield of high-quality muscle strips also provides an efficient platform for development of therapeutic reagents.
Keywords: Cardiac muscle contractility, cryosections, force-pCa relationship, myofilament proteins, sarcomere length, skinned papillary muscle
Introduction
The rapid development of transgenesis and gene editing technology has made the use of mouse models of human disease a readily accessible resource to investigate molecular mechanisms in integrative physiological and pathophysiological systems, including the studies of cardiac function and heart failure. While in vivo and organ level assays, such as echocardiography, magnetic resonance imaging, hemodynamic measurement via catheterization and ex vivo working hearts can comprehensively evaluate the overall cardiac performance (Feng et al., 2008a; Feng et al., 2008b; Feng & Jin, 2010, 2018; Lindsey et al., 2018; Feng & Jin, 2019), quantitative biomechanical studies on skinned cardiac muscle preparations has a critical value in the understanding of cardiac muscle contractility at the myofilament level for mechanistic insights into heart function and diseases (Vahl et al., 1997). Measurements of the activation and force development of skinned cardiac muscle in the absence of calcium handling system, metabolic factors and neurohormonal influence provide assessment of the function and dysfunction of myofilament motor and regulatory proteins during contraction and relaxation (Vahl et al., 1997).
Ventricular trabeculae and papillary muscles with longitudinally organized cardiomyocytes have been used for cardiac muscle contractility studies. Skinned trabeculae have relatively small size to allow perfusion of oxygen and chemicals into the core region, minimizing ischemia-like effect. Sarcomere length (SL) in a skinned trabecula can be directly measured using laser diffraction (van Heuningen et al., 1982; Stuyvers et al., 2002) or microscopic imaging (Bardswell et al., 2010) of the striations. A major limitation with mouse hearts, however, is that only very few suitably formed trabeculae with optimal size and shape are present in the right ventricle.
Mouse left ventricular papillary muscles are easy to collect for contractility studies. However, their larger size imposes a limitation to the diffusion of oxygen and nutrients, causing ischemia-like injuries in the core region (Widen & Barclay, 2005) and slow or partial response to chemical reagents during functional measurements. Manually splitting fresh papillary muscle into smaller diameters is time consuming with significant and inevitable tissue damages. The measurement of SL in skinned papillary muscle with laser diffraction or microscopic imaging also depends on the making of sufficiently thin strips (Lee et al., 2010).
Enzymatically isolated single cardiomyocytes from adult mouse heart provide another approach for contractility measurement in permeabilized preparations (Liang et al., 2008). An advantage of using isolated single cardiomyocytes is the exclusion of mechanical complications from the extracellular matrix (Chung & Granzier, 2011; King et al., 2011). However, the force produced by a single cell is small, the isolation procedure unavoidably introduces ischemia-reperfusion injury accompany with a large proportion of dead cardiac cells, and the individual cells from endocardial and epicardial regions cannot be easily tracked and their different mechanical properties can generate variations (Cazorla et al., 2005; Ait Mou et al., 2008).
Experimentally calibrated mechanical blending of cardiac muscle tissue has been used to obtain small bundles of cardiomyocytes for permeabilized preparations that permit effective perfusion and measurement of SL using microscopic imaging (McDonald et al., 1998; Patel et al., 2001). However, the process of mechanical blending could cause uncontrolled and extensive structural damage especially stretching injury of the myofibrils.
The intrinsic limitations of the current methodologies call for a more efficient method to prepare high quality skinned cardiac muscle for comprehensive mechanical studies. A previous study of mechanically disrupted multicellular tissue strips from frozen cardiac muscle was able to measure the contractility of skinned preparations (Patel et al., 2001). Based on this observation, we also demonstrated that myofibrils isolated from frozen mouse hearts have preserved Ca2+-regulated myosin ATPase activity (Gunther et al., 2016). Extending the premise of using frozen muscle for contractility studies, here we report the development of a method using frozen sections of mouse papillary muscle to obtain skinned cardiac muscle strips with physiologically regulated contractility, clear striations under bright field light microscope, and the integrity of myofilament proteins with preserved in vivo posttranslational modifications. This new methodology produces a high yield of high quality and uniform skinned cardiac muscle strips per mouse heart, allowing comprehensive studies of force-pCa relationship and the rate of tension redevelopment (Ktr) at various SL followed by biochemical verification of myofilament proteins. This time- and cost-effective methodology has a combined power of mechanical and biochemical analyses for mechanistic and translational studies and the logistically significant advantage of using frozen muscle tissues.
Material and Methods
Animals and ethical approval
Male and female C57BL/6 mice 3–8 months old were used in the present study. All animal protocols are approved by the Institutional Animal Care and Use Committee of Wayne State University (#17–12-0438) and were conducted in accordance with the Guiding Principles in the Care and Use of Animals, under the guidelines of the Council of the American Physiological Society.
Flash freezing of mouse left ventricular papillary muscles
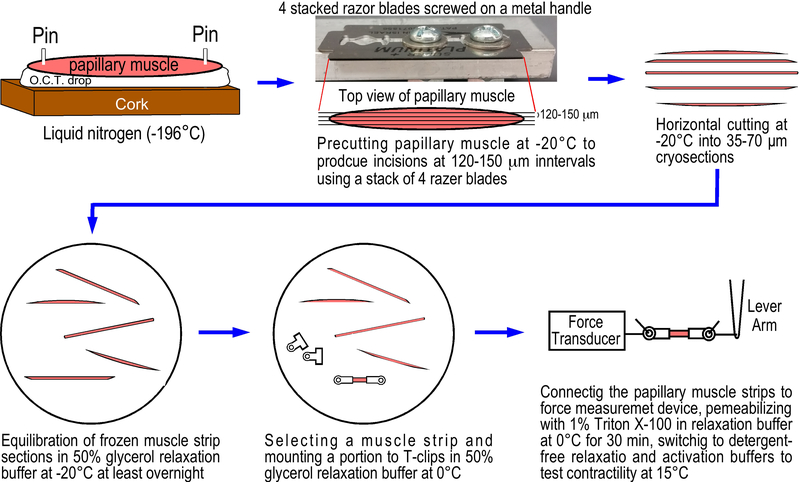
Mice were anesthetized using isoflurane. After opening the thorax, the heart was rapidly isolated with aortic arch attached. After cut-open the free wall of right ventricle, left ventricular papillary muscles were exposed by cutting the septum from the root of aorta. Papillary muscles with tendon at one end and a piece of ventricular wall at the other end were carefully isolated avoiding stretching. The muscle was pinned horizontally with two 30 gauge needles on a piece of cork that were pre-wrapped with aluminum foil to facilitate later removal of the frozen tissue block. After filling a small drop of optimal cutting temperature tissue freezing media (O.C.T., 10.24% polyvinyl alcohol, 4.26% polyethylene glycol, 85.5% non-reactive) in between the papillary muscle and the cork (Fig. 1), the block was quickly submerged in liquid nitrogen for rapid freezing. After equilibration in a −20°C freezer for at least 30 min, the specimen was placed on dry ice to quickly remove the needles before the tissue temperature dropping again. The O.C.T. tissue block was then easily separated from the foil-wrapped cork and placed in a 1.7 mL Eppendorf tube for long term storage in a −80°C freezer.
Figure.1. Preparation of skinned mouse left ventricular papillary muscle strips from cryosections.
A freshly isolated mouse papillary muscle was pinned on a piece of cork on a drop of O.C.T. compound. After flash freezing in liquid nitrogen, the frozen muscle tissue block was transferred to a cryostat set at −20°C, precut from the top manually using a stack of 4 razor blades screwed tightly on a metal handle at 120–150 μm intervals (based on the original thickness of the blades) to make partial incisions along the long axis of the papillary muscle in a depth about one-third (~150μm) of the muscle diameter. The precut portion of the papillary muscle was cryosectioned horizontally to produce 35 μm or 70 μm thick and 120–150 μm wide frozen muscle strips. The frozen muscle strips were immediately transferred into −20°C cold 50% glycerol relaxation buffer in a 35 mm cell culture dish to equilibrate at −20°C for at least 24 hours. The muscle strips were then mounted with aluminum T-clips at 0°C in 50% glycerol relaxation buffer. After attaching to force transducer and length lever arm, the muscle strip was skinned for 30 min in 1% Triton X-100 in relaxation buffer at 4–6°C. After switch to detergent-free solutions, the preparation is ready for contractility studies at 15°C.
Cryosectioning of mouse ventricular papillary muscles
In a cryostat set at −20°C, the frozen mouse papillary muscle was precut along the long axis using an aligned stack of 4 thin razor blades (Fig. 1), of which the spaces in between allow the creation of 4 parallel incisions at 120–150 μm intervals in the depth of approximately 150 μm. The muscle tissue was then aligned parallel with the knife of cryostat to cut the precut portion into 35 μm or 70 μm thick frozen strips. The frozen muscle strips were gently picked up using the tips of a brush and transferred into a 35 mm cell culture dish containing cold (−20°C) liquid form of a relaxation solution (50% glycerol [v/v], BES 40 mM, EGTA 10 mM, MgCl2 6.86 mM, ATP 5.96 mM, DTT 1 mM, K-propionate 3.28 mM, pH 7.0, creatine phosphate 33 mM, creatine kinase 200 U/mL and protease inhibitor cocktail). The muscle strips initially spread on the surface of the relaxation solution and transformed from solid frozen to soft tissue during an incubation of 24 hours in a −20°C freezer. All steps were carried out at low temperature avoiding rising above −20°C to preserve myofibril structure and function.
Mounting mouse papillary muscle strips for force measurement
The 35 mm cell culture dish containing the mouse papillary muscle strips in glycerol relaxation buffer was placed on a thermo-controlled stage at 0°C, which was customer made using two pairs of thermoelectric Peltier (TEC1–12709 Thermoelectric Peltier Cooler, 12V, 90W) with a working temperature range from −17°C to 50°C when coupled to a room temperature liquid cooling system (Fig. 2A). Under a dissecting microscope set at 25x or 50x magnification, papillary muscle strips with cardiomyocytes organized parallelly along the long axis were selected (Fig. 2B).
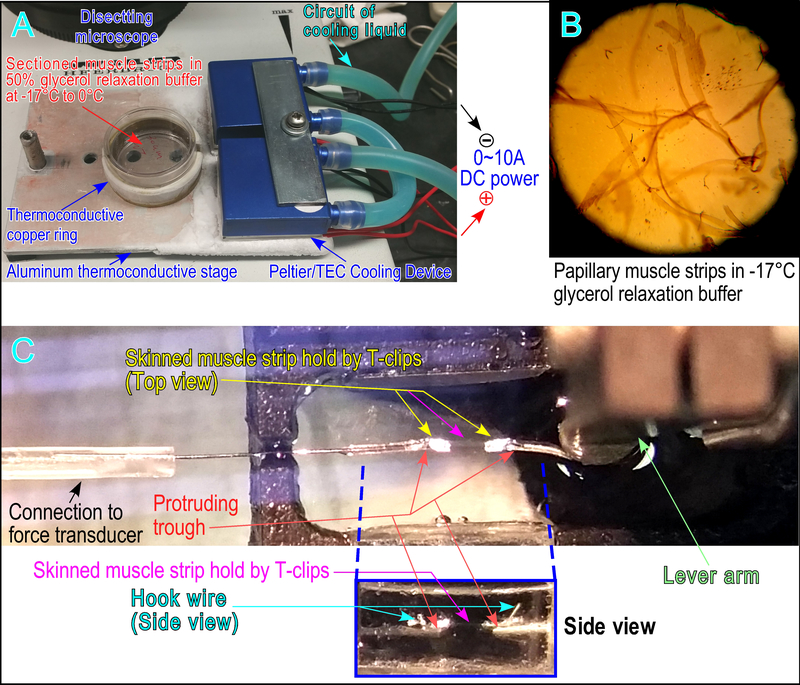
Figure 2. System set up for muscle strip selection, mounting and contractility measurements.
(A) A thermo-controlled aluminum stage was customer-made using two pairs of thermal electric coupler/Peltier with adjustable electric DC current to control the desired temperature on the surface of the aluminum plate. Heat produced by the Peltiers is removed by heat sinks connected to a water circulatory cooling system. The stage was placed under a dissection microscope with an underneath light source to luminate through the drilled holes. The muscle strips were placed in 50% glycerol relaxation buffer in a 35 mm cell culture dish with temperature controlled at 0°C. A thermo-conductive coper ring was attached to the culture dish to stabilize the temperature inside the dish. (B) Bright field view with underneath lightening visualizes the muscle strips under a dissecting microscope for selecting strip with properly organized myofibrils to mount to a pair of T-clips in 50% glycerol relaxation buffer at −17°C. (C) A skinned papillary muscle strip with T-clips was attached to metal wire hooks connected to force transducer and length-controlled lever arm. A pair of metal troughs (seen in the side view box) made from half-removed 30-gauge needles were glued underneath the hook as a support to prevent over-the-range movement of the T-clips when the muscle strip was lifted out of solution during the switch of buffer chambers.
A pair of aluminum T-clips were attached to the two ends of the strip while care was taken to wrap them by pressing with proper force to avoid crashing the tissue or loose holding. The papillary muscle strip was then transferred to a skinning buffer (relaxation buffer plus 1%-Triton X-100) in a chamber of an ASI 8-chamber thermal controlled stage (802D) using a customer-made coiled platinum wire as a spatula that effectively avoids stretching damage of the muscle strip during transfer.
During 30 min of skinning at 4–6°C, the papillary muscle strip with T-clips was connected to a stainless steel wire hook glued to an ASI lever arm length controller (322C) and another stainless steel wire hook connected to the glass tube of an ASI force transducer (403A) using melted wax. A horizontally protruding trough made from a 30-gauge grinded-open needle was glued underneath of each hook to prevent dragging down during chamber switches (Fig. 2C).
Contractility measurement of skinned papillary muscle strips
After 30 min, the skinning solution was removed and the muscle strip was rinsed twice to remove remaining Triton X-100 using detergent-free pCa 9.0 relaxation buffer made from mixing pCa 10.0 and pCa 4.0 buffers. The force transducer was calibrated to zero when the muscle strip was set slack at 15°C. Sarcomere striations were photographed through a 20x objective lens with 10x eyepiece to obtain 200x magnification using a digital camera attached to the inverted microscope (Fig. 3). The average static state SL was measured at pCa 9.0 in at least three different fields. The initial SL was adjusted to 2.3 μm for pre-activation at pCa 4.5 to tighten the mounting to the T-clips.
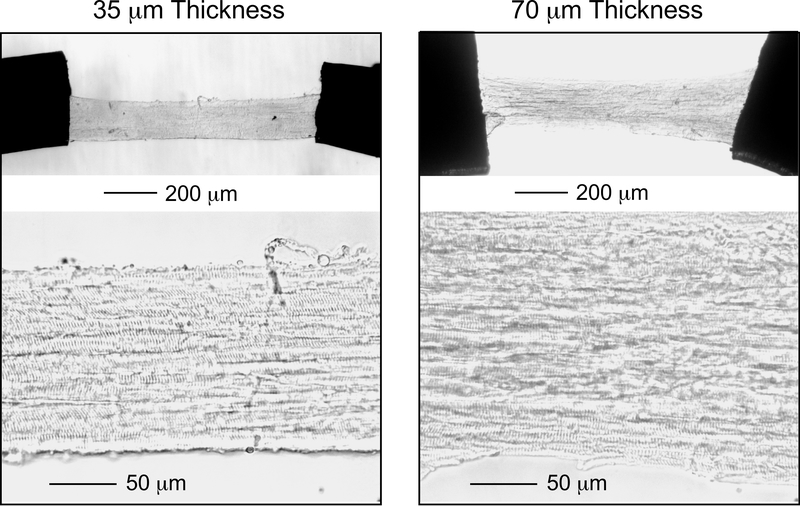
Figure. 3. Visual validation of the structure of 35 μm and 70 μm thick cryosection-generated skinned mouse left ventricular papillary muscle strips.
Papillary muscle strips with longitudinally and parallelly organized cardiomyocytes were selected and mounted via T-clips for contractility measurement (upper low magnification image). The lower left high magnification image of a 35 μm thick strip shows clear striations of skinned cardiomyocytes across the entire strip adjusted at a sarcomere length of 2.3 μm. The lower right image of a 70 μm thicker strip shows visible although less clear striations.
Muscle strips remained intact with secure mounting after the initial contraction would represent those with acceptable longitudinal precutting and sectioning parallel to cardiomyocytes and myofibrils since strips with angled cuts would split in the middle during the maximum level pre-activation. The qualified strip was re-adjusted for SL to test isometric force production at SL of 2.3 μm, 2.15 μm and 2.0 μm for activation at a series of Ca2+ concentrations by incubating in pCa 9.0, 6.5, 6.3, 6, 5.8, 5.5, 5, and 4.5 buffers (BES 40 mM, EGTA 10 mM, MgCl2 6.64 mM, ATP 6.23 mM, DTT 1 mM, K-propionate 2.09 mM, Ca-EGTA 10 mM, creatine phosphate 33 mM, Creatine kinase 200U/mL with protease cocktail, pH 7.0) made from mixing pCa 10.0 and pCa 4.0 buffers at various ratios to provide the given free [Ca2+] calculated using the program of Fabiato and Fabiato (Fabiato & Fabiato, 1979; Lee et al., 2010).
The muscle strip was then returned to SL of 2.3 μm to re-measure Ca2+ activated force production. Strips with no more than 20% run-down of force were continued to test the rate of tension redevelopment (Ktr) at pCa 5.8 as described previously (Hanft & McDonald, 2010). The measurements were performed at SL of 2.3, 2.15 and 2 μm with 15% step-shortening of total muscle length for 20 ms, 105% step-lengthening for 2 ms, and then step-returning to the initial length. The force redevelopment after muscle length return to the initial length for 16 seconds was selected for data fitting analysis.
The force production and muscle length changes were recorded simultaneously via an A/D interface (ASI, 604C) using computer software (ASI, ASI-600). At the end of the experiment, the muscle tissue was recovered with the T-clips into a 1.7 mL tube and rapidly frozen in liquid nitrogen for storage at −80°C for later analysis of myofilament protein contents.
Data fitting and analysis
Forces activated at different Ca2+ concentrations were fitted with Hill equation (Lee et al., 2010):
where n is the Hill coefficient. The pCa for half-maximal activation was calculated as pCa50 = (−logK)/n for use as an indicator of myofibril calcium sensitivity.
Tension redevelopment following a slack–re-stretch maneuver was fitted by a single exponential equation (Hanft & McDonald, 2010):
where F is tension at time t, Fmax is maximal tension, ktr is the rate constant of force development, and Fres represents any residual tension immediately after the slack–re-stretch maneuver.
In vitro PKA phosphorylation of skinned papillary muscle strips
In vitro phosphorylation of myofilament proteins was tested in cryosection-generated skinned papillary muscle strips. Since mouse hearts normally have near maximum phosphorylation of myofilament proteins such as cardiac troponin I (TnI) and myosin binding protein C in vivo, we applied 30 minutes ex vivo perfusion to diminish the effect of systemic sympathetic and adrenergic stimulations and reduce the baseline phosphorylation of myofilament proteins. Treatment of the skinned muscle strips with the catalytic subunit of bovine PKA and control was done in relaxation solution at 37°C for 30 min. The muscle trips were then recovered and stored at −80°C for later analysis.
SDS-PAGE, Pro-Q staining and Western blotting
The skinned cardiac papillary muscle strips recovered after contractility studies and stored at −80°C were directly lyzed in 10 μL SDS-PAGE sample buffer containing 50 mM Tris–HCl, pH 8.8, 10% glycerol, 0.1% bromophenol blue, 2% SDS, and 3% β-mercaptoethanol by sonication on ice (two 1 second pulses at 10% power using a microprobe). The samples were heated at 80°C for 5 min, vortexed, centrifuged in a Beckman Coulter Microfuge 18 at 14,000 rpm for 5 min, and resolved on 14% SDS-PAGE gel with an acrylamide-to-bisacrylamide ratio of 180:1. The protein bands were stained with Coomassie Blue R250. Duplicate gel was electrically blotted on nitrocellulose membranes at 5 mA/cm2 for 15 min using a semi-dry transfer apparatus (Bio-Rad, Hercules, CA, USA). The membranes were blocked with Tris-buffered saline containing 1% bovine serum albumin and individually incubated with mouse anti-troponin T (TnT) monoclonal antibody (mAb) CT3 (Jin et al., 2000) or mouse anti-TnI mAb TnI-1 (Jin et al., 2001) at 4°C overnight. The membranes were subsequently washed, incubated with alkaline phosphatase-labeled anti-mouse IgG secondary antibody (ThermoFisher Sci.), and developed in 5-bromo-4-chloro-3-indolylphosphate/nitrobluetetrazolium substrate solution to visualize the target protein bands.
Pro-Q diamond phosphoprotein staining of SDS-gels was performed according to the manufacture’s instruction (Invitrogen, ThermoFisher Sci.) with minor modifications. Briefly, the gels were fixed in 50% methanol and 10% acetic acid overnight. After washing 3 times in deionized water for 10 min each, the gels were stained with Pro-Q Diamond reagent in a dark box at room temperature for 90 min. After de-staining four times with 20% acetonitrile in 50 mM sodium acetate, pH 4.0, for 30 min each, the gels were washed twice with deionized water for 5 min each and then scanned on a Typhoon 9410 fluorescence imager (Typhoon Trio, GE Healthcare) using fluorescence mode (450V, high sensitivity, green laser excitation at 532nm and recording emission at 560nm long pass).
Statistical analysis
Contractility data analyzed for statistical significance. Values are presented as mean ± SD and examined using one-way ANOVA repeated measures.
Results
Cryosection-generated skinned mouse papillary muscle strips have preserved myofibril structure
The new methodology we developed to prepare skinned mouse papillary muscle strips from frozen sections reproducibly yields an average of 6–10 useful strips per heart for contractility studies, significantly higher than that of mouse trabeculae preparations. The bright field images in Fig. 3 demonstrate examples of 35 μm and 70 μm thick, 120–150 μm wide mouse papillary muscle strips containing longitudinally aligned cardiomyocytes with clear and uniform striations, allowing an easy determination of SL with light microscope using 20x objective lens.
Cryosection-generated skinned papillary muscle strips have preserved physiological contractility and length-tension response
The functionality of skinned papillary muscle strips prepared from cryosections was first tested for resting tension at pCa 9 and Ca2+-activated maximum tension at pCa 4.5. The results showed that the cryosection-generated skinned papillary muscle strips have preserved Ca2+-activated force production. Similar to that previously shown in skinned ventricular papillary muscle strips prepared using traditional dissection methods (Lee et al., 2010), resting tension and Ca2+-activated maximum tension of our preparations both exhibited SL dependence, where increasing SL from 2.0 μm to 2.15 μm and 2.3 μm produced increased tension development (Fig. 4A, inset table). The data demonstrate that the cryosection-generated skinned mouse papillary muscle strips retain native myofibril structure and function, validating their use in contractility studies.
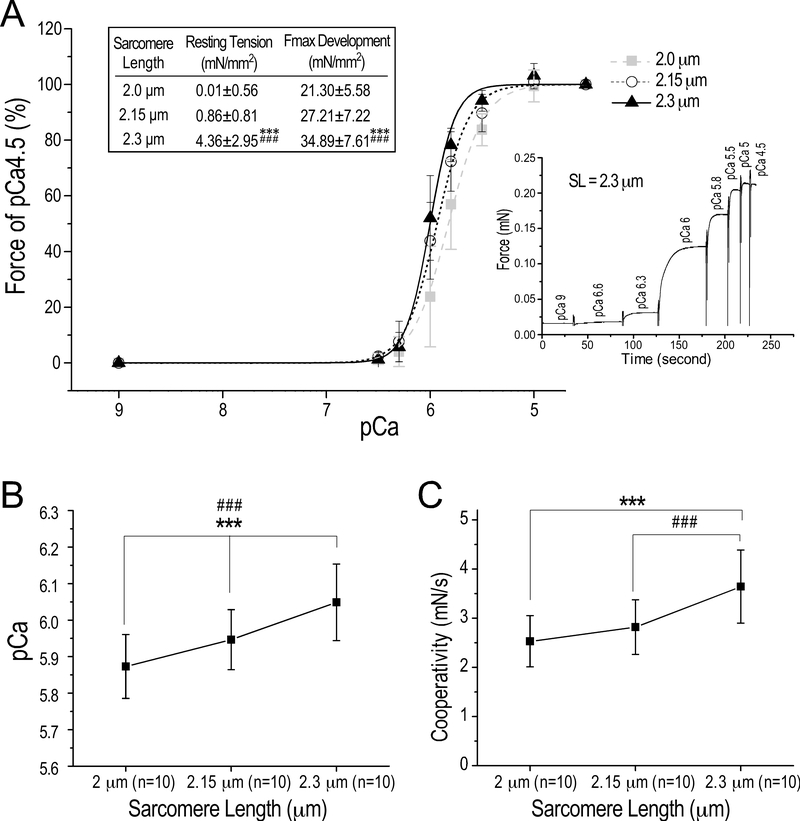
Figure 4. Preserved Ca2+-activated contractility of cryosection-generated skinned papillary muscle strips with positive responses to increase in sarcomere length.
(A) At SL of 2.0 μm, 2.15 μm and 2.3 μm, Ca2+ activated isometric contraction of skinned mouse papillary muscle strips was tested at pCa 9, 6.5, 6.3, 6, 5.8, 5.5, 5 and 4.5. A representative trace of the step increases of isometric force at increasing Ca2+ concentrations is shown. When SL was increased from 2.0 μm, to 2.15 μm and 2.3 μm, the cross-sectional area-normalized maximal tension development at pCa 4.5 of the muscle strips increased as expected for skinned fibers prepared from fresh cardiac muscle, indicating preserved functionality. The Hill fitted plots demonstrate corporative force development with left shifts of the force-pCa curve when SL was increased from 2.0 μm to 2.15 μm and to 2.3 μm, reflecting increases in Ca2+ sensitivity at longer SL (inset table). (B) pCa50, i.e., the Ca2+ concentration for 50% maximum force production in the Hill fitted force-pCa curve, increased significantly when SL was increased. (C) The cooperativity of Ca2+ activation of contraction also increased at longer SL, indicating increased myofilament contractility. The values are presented as mean± SD. N = 10 strips from 6 mouse hearts. ***P<0.001 vs SL 2.0 μm and ###P<0.001 vs SL 2.15 μm in one-way ANOVA repeated measures with pos hoc test.
Cryosection-generated skinned papillary muscle strips have preserved SL dependence of Ca2+ sensitivity and cooperativity
Force-pCa relationship was examined to validate the functionality of skinned papillary muscle strips prepared from cryosections in standard biomechanical analyses. A representative trace of the step increases of isometric force development at pCa 9, 6.5, 6.3, 6, 5.8, 5.5, 5 and 4.5 is shown in Fig. 4A. Normalized to cross-sectional area, the Hill fit force-pCa curves in Fig. 4A show force development, Ca2+ sensitivity and cooperativity of cryosection-generated skinned papillary muscle strips as expected for preparations from fresh muscles.
The cryosection-generated skinned papillary muscle strips preserved the positive relationship between resting SL and active tension development. When SL increased from 2.0 μm to 2.15 μm and 2.3 μm, the muscle strips responded with anticipated length dependence of Ca2+ sensitivity indicated by a left shift of pCa50 (Fig. 4B). As an indicator of contractility (Gordon et al., 2000), the cooperativity of Ca2+-activation of cryosection-generated skinned papillary muscle strips was similar to that expected for a high quality skinned cardiac muscle prepared using classic methods (Konhilas et al., 2003; Bohlooli Ghashghaee et al., 2017) with preserved SL dependence (Fig. 4C).
Capacity of cryosection-generated skinned papillary muscle strips for measuring tension redevelopment and the effect of stretch activation
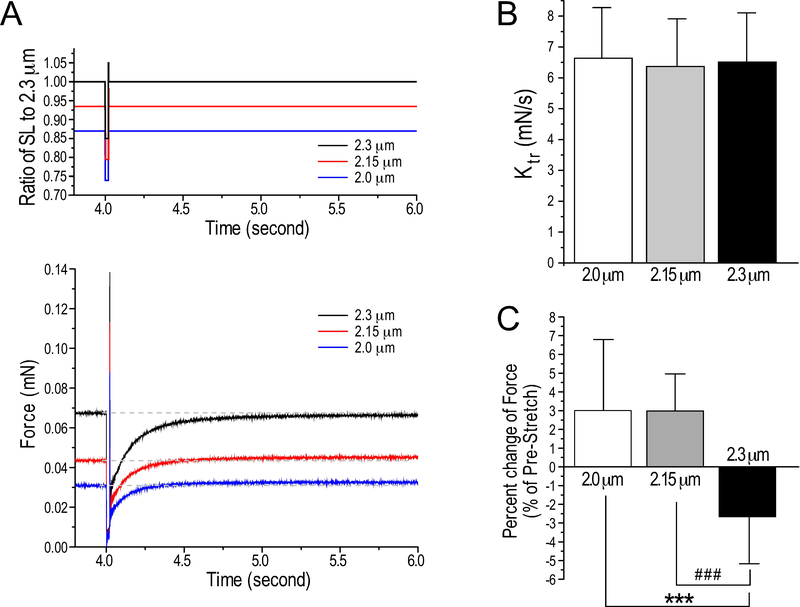
To further evaluate the structural and functional preservation of cryosection-generated skinned papillary muscle strips for comprehensive contractility studies, force redevelopment assay that requires an integrity of the strips for undergoing rapid mechanical manipulations of muscle length involving stretch was tested in the same strips after force-pCa curve measurements (Fig. 5A). The results showed rate of tension redevelopment (Ktr) values similar to that expected for skinned preparations of fresh cardiac muscle (Hanft & McDonald, 2010). There was no difference of Ktr at the different SL examined (Fig. 5B), consistent with the notion that Ktr is not related to SL (Hanft & McDonald, 2010).
Figure 5. Measurement of the rate of force redevelopment in cryosection-generated skinned papillary muscle strips.
(A) Force redevelopment was studied in skinned papillary muscle strips activated at pCa 5.8. After recording developed force at SL of 2.0 μm, 2.15 μm and 2.3 μm, the muscle was rapidly shortened to 85% of the original length for 20 ms and then step stretched to 105% of the original SL for 2 ms before returning to the original SL (the upper panel). The data were fitted exponentially to determine the rate of force redevelopment (Ktr) after the muscle returned to the original length (the lower panel). (B) The results showed no difference of Ktr at the different SL tested. (C) The step stretch of the skinned muscle strips produced a redeveloped force higher than the original level at SL 2 μm and 2.15 μm whereas the redeveloped force was lower than the original level at SL 2.3 μm. The values are presented as mean± SD. N = 10 strips from 6 mouse hearts. ***P<0.001 vs SL at 2.0 μm and ###P<0.001 vs SL at 2.15 μm in one-way ANOVA repeated measures with pos hoc test.
Using the same muscle strips, we also investigated whether the effect of stretch activation of force was preserved. Sub-maximumly activated at pCa 5.8, a 2 ms 5% stretch increased the redeveloped force to a level higher than the original force at SL 2.0 μm and 2.15 μm, implicating stretch-induced additional activation of cross bridges (Fig. 5A and 5C). In contrast, the redeveloped force was lower than the original level at SL 2.3 μm, possibly due to maximized activation at long SL (Fig. 5A and 5C).
Preservation of myofilament proteins in cryosection-generated papillary muscle strips
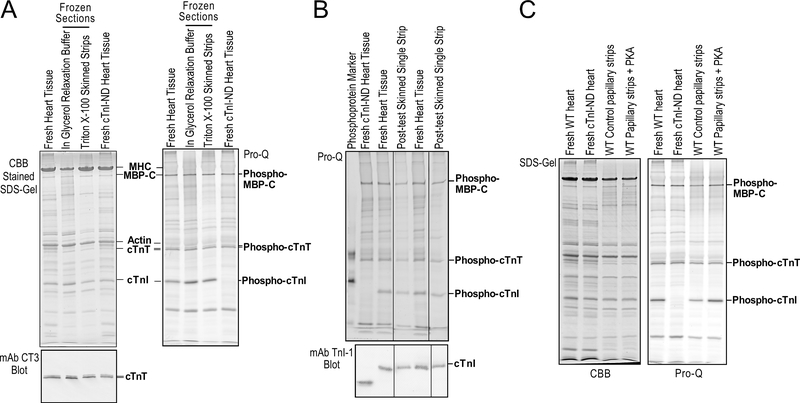
An advantage of the larger and more uniform size of the cryosection-generated skinned papillary muscle strips than that of mouse trabeculae or single cardiomyocytes is to recover sufficient amount of protein from a strip after a series of mechanical studies for biochemical characterizations. To demonstrate the preservation of myofilament protein integrity and their native phosphorylation status, the SDS-gel and Pro-Q phosphoprotein stain in Fig. 6A show no detectable degradation of myofilament proteins with preserved phosphorylation of myosin binding protein C (MBP-C), cardiac TnT and cardiac TnI after the skinning procedure as compared to that in freshly isolated mouse cardiac muscle. The Pro-Q phosphoprotein stain in Fig. 6B shows that the representative phosphorylated myofilament proteins, MBP-C, cardiac TnT and cardiac TnI, remained their phosphorylation levels in the strips after a series of contractility measurements. The results in Fig. 6 C further demonstrate that PKA treatment of the cryosection-generated skinned papillary muscle strips in vitro produced specific phosphorylation of cardiac TnI and MBP-C, indicating the preserved integrity and native structure of myofilament proteins that sustained physiological posttranslational regulations. The excellent capacity for allowing biochemical analysis of the same muscle strips studied for contractility adds a significant value to this new methodology of skinned fiber preparation.
Figure 6. Preserved integrity and phosphorylation state of myofilament proteins in cryosection-generated skinned cardiac muscle strips.
(A) Coomassie brilliant blue (CBB) and ProQ phosphoprotein stained SDS-PAGE gels and mAb CT3 Western blot showed the protein profiles of cryosection-generated skinned papillary muscle strips prior to and post skinning. In comparison with fresh papillary muscle homogenate control, the results demonstrate preserved integrity of myofilament proteins, myosin heavy chain (MHC), myosin binding protein C (MBP-C), actin, cardiac TnT (cTnT) and cardiac TnI (cTnI) and in vivo phosphorylation state of MBP-C, cTnT and cTnI. Transgenic mouse heart contains solely N-terminal phosphorylation sites deleted cTnI (cTnI-ND) was used as a control of non-phosphorylated cTnI. (B) The protein samples recovered from skinned papillary muscle strips after contractility experiments were sufficient for SDS-PAGE, phosphoprotein staining, and Western blotting analysis. The Pro-Q stained SDS-gel and mAb TnI-1 Western blots demonstrate that the cryosection-generated skinned cardiac muscle strips preserved the native state of myofilament protein phosphorylation as shown by that of MBP-C, cTnT and cTnI together with cTnI-ND control. (C) PKA treatment of cryosection-generated skinned cardiac muscle strips from a mouse heart after ex vivo perfusion to diminish the effective of systemic adrenergic tune and lower baseline level of myofilament phosphorylation produced significant and specific increases in the phosphorylation of MBP-C and cTnI, indicating preserved myofilament structure for posttranslational physiologic regulations. Fresh cardiac muscles of wild type and cTnI-ND mice were used as controls.
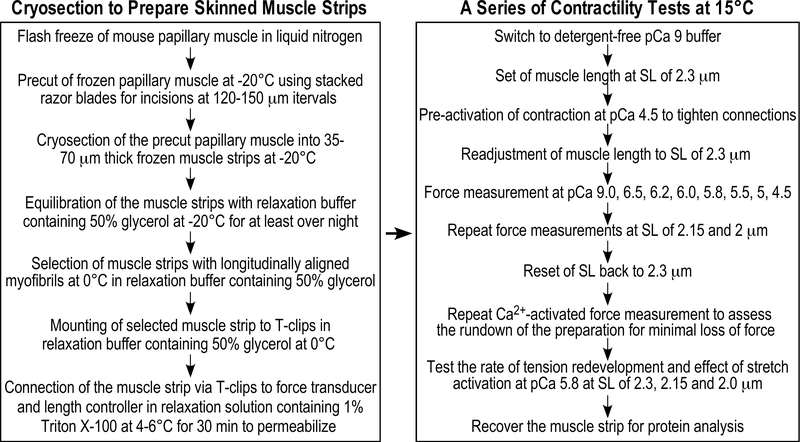
The established protocol to prepare cryosection-generated skinned muscle strips
The new methodology using frozen sections of mouse papillary muscle to prepare skinned cardiac muscle strips for biomechanical studies is summarized in Fig. 7 with the following key steps: a) flash freezing of papillary muscle to preserve structure and function, b) cryosectioning to generate longitudinal muscle strips of 35 μm or 70 μm by 120–150 μm in cross-sectional area and store in glycerol relaxation buffer at −20°C, c) mounting the strip to force transducer and skinning in relaxation solution with detergent, d) performing a series of mechanical measurements at various SL, and e) recovering the strip for biochemical characterizations.
Figure 7. Summary of the preparation of cryosection-generated skinned papillary muscle strips for contractility study.
The flowchart to the left outlines the protocol to prepare cryosection-generated skinned papillary muscle strips. The flowchart to the right summarizes a series of contractility measurements that can be obtained from a single skinned mouse papillary muscle strips using this new method that reliably produces high yield and high quality skinned cardiac muscle strips.
Discussion
The new methodology developed in the present study proves the concept that skinned muscle strips from frozen heart tissue can retain physiological functionality. The thin preparations of muscle strips provide clear image of striations for determining SL as well as effective perfusion for functional studies. The near uniformed size of the cryosection-generated muscle strips adds reproducibility with reduces data variations. Validation of contractility and preservation of myofilament protein integrity demonstrate that this new method produces high yield of high quality muscle strips per mouse heart with preserved physiological function. In comparison with that of traditional skinned fiber methods, its advantages and limitations are discussed as follows.
High yield of skinned muscle strips per mouse heart for use in mechanical studies
From the two papillary muscles of a mouse left ventricle with diameters of 200–500 μm, longitudinal cryosectioning can produce 100–150 pieces of 35 μm thick and 120–150 μm wide strips. From these strips, an average of 6–10 strips with properly aligned myofibrils can be readily obtained. This reproducible yield of useful skinned muscle strips from one mouse heart is much higher than the average of less than one useful trabecula from a mouse heart, allowing more effective studies with multiple or repeated experiments using each and every individual animal, especially valuable when working on genetically modified mice with limited supply.
A unique advantage of the cryosection-generated muscle strips is that they have uniform thickness and very small variation in width, which produces similar cross-sectional areas of the preparations to reduce experimental variations and increase reproducibility. The 35–70 μm thick papillary muscle strips are close to the diameter of a single cardiomyocyte, making chemical perfusion during buffer changes effective to produce cleaner functional responses (Fig. 4A). We recently demonstrated the effectiveness of this method on testing a cardiac TnI-originated peptide, HcTnI-C27, for pharmacological effect on reducing the Ca2+-sensitivity of skinned cardiac muscle without decreasing maximum force production (Wong et al., 2019). While human iPSCs-derived cardiomyocytes are useful for drug and toxicity screening (Kussauer et al., 2019), the new method expands the use of in vivo developed cardiac muscles with an increased scale than that of the traditional methods for validation and optimization of cardiac drugs, especially in disease models to provide physiologically more relevant and quantitative force and kinetic evaluations beyond the high-throughput contractility assay of human stem cell-derived cardiomyocytes (Miklas et al., 2019).
Preserved contractility of skinned mouse cardiac muscle strips
The cryosection-generated skinned cardiac muscle strips have plausibly preserved myofibril structures (Fig. 3), providing the foundation for preserved functionality as shown by the contractility data (Figs. 4 and 5). It is known that cryoprotectant such as hydroxyethyl starch, dimethylsulfoxide and glycerol can help to preserve cellular structure and viability during cryopreservation and thaw of living cells (Luyet & Rapatz, 1970; Knorpp et al., 1971). While our rapid freezing of mouse papillary muscle with O.C.T. compound in liquid nitrogen at −196°C avoids the generation of visible ice crystals as it has been broadly used in pathology labs and muscle histology studies, the thawing protocol we developed using 50% glycerol relaxation buffer containing protease inhibitors (Fig. 1) can restore the cardiac muscle strips to physiological condition with preserved contractility. Protein profiles and phosphorylation states of strips and post experimental samples confirmed the preservation of myofilament proteins and physiological posttranslational modifications (Fig. 6).
Contractility of the cryosection-generated cardiac muscle strips was excellently preserved with normalized maximum force development higher than previous reported in skinned cardiomyocytes from enzymatic isolation (20.7±2.3 mN/mm2 at SL 2.2 μm, Stelzer et al., 2006), or in skinned multicellular cardiac muscle strips from mechanical disruption of frozen tissue (18.3±1.5 mN/mm2 at SL 2.35 μm (Patel et al., 2001). The pCa50 and cooperativity of the cryosection-generated cardiac muscle strips are also comparable with numerous data published on skinned mouse cardiac muscle studies in the literature.
Real time imaging of SL for length-tension relationship studies
Sarcomere length is a crucial factor in muscle contractility and in investigations on myofilament regulatory mechanisms. The precise and uniform thickness of 35 μm of cryosection-generated skinned papillary muscle strips provides clear image of striations under a simple light microscope for the measurement of SL (Fig. 3). Such clear striation image cannot be obtained from skinned whole papillary muscle (van Heuningen et al., 1982; Stuyvers et al., 2002), or skinned trabeculae with diameter larger than 50–100 μm (Bardswell et al., 2010). The striation of muscle strips starts to become unclear in cryosections of 40 μm thick but still visible at 70 μm (Fig. 3).
Being able to readily measure SL in cryosection-generated skinned papillary muscle strips allows real time monitoring of length-tension relationship in studies of Frank-Starling mechanism. Frank-Starling response of the heart is the essential inotropic regulation for beat-by-beat adjustment of ventricular function, which is based on resting SL and tension to alter myofilament Ca2+-sensitivity (Sequeira & van der Velden, 2015). Therefore, precise and real time measurements of SL during contractility studies of skinned muscle strips is an essential requirement in studies on Frank-Starling mechanism.
Frank-Starling response of cardiac muscle has been implicated to involve stretch activation (Sequeira & van der Velden, 2015). Consistent with studies by other investigators (Hanft & McDonald, 2010), the cryosection-generated skinned cardiac muscle strips showed Ktr independent of SL (Fig. 5). While the maximum rate of myosin ATPase is not altered by SL, the higher-than-baseline level of post-stretch redeveloped force at SL 2.0 μm and 2.15 μm suggests a role of stretch activation in Frank-Starling response of the heart before plateaued activation is reached at SL 2.3 μm (Fig. 5).
Skinned muscle strips for long term storage and easy transportation
This new method makes it possible to use frozen papillary muscles to prepare skinned muscle strips for contractility study. Flash frozen papillary muscle tissues can be kept in 1.7 mL centrifuge tubes for long term storage in −80°C freezer or liquid nitrogen tank. The ultra-low temperature provides a reliable cryopreservation condition to preserve myofibril structure and myofilament protein integrity. The cryosection-generated skinned cardiac muscle strips can be stored in 50% glycerol relaxation buffer at −20°C in 3–4 weeks for used in multiple experiments without notable loss of contractility (data not shown).
Both frozen papillary muscles and cryosection-generated skinned cardiac muscle strips can be easily shipped to collaborators at other institutions without time-consuming procedures and high cost required for the transfer of live animals. This advantage would also facilitate the use of banked animal and human cardiac muscle samples for contractility studies, especially in collaborative research requiring complementary expertise and experimental systems available at multiple institutions.
Direct coupling of biochemical analysis of myofilament proteins with fiber contractility
The relatively large size of the cryosection-generated skinned cardiac muscle strips permits sufficient amount of tissue to be recovered after mechanical studies for biochemical characterization of myofilament proteins. In addition to the preserved sarcomere striations indicating structural integrity functionality, our data demonstrate the preservation of integrity and in vivo phosphorylation of myofilament proteins as well as the effects of in vitro biochemical treatment (Fig. 6). This capacity adds an advantage to couple biochemical analysis and treatment with contractility studies using the same muscle strips, which is highly valuable in providing molecular level mechanistic insights.
Limitations and directions for improvement
Summarized in Table 1, the new method of preparing skinned cardiac muscle strips from mouse left ventricular papillary muscles meet all of the best possible expectations in the yield of useful strips, minimum tissue damage, easy SL measurement, uniform cross-sectional area, use of both fresh and frozen samples, and recovering material for protein analysis. However, this approach does have some specific limitations.
Table 1.
Comparison of cryosection preparation of skinned papillary muscle strips with other methods
| Preparations from mouse heart | Source | Yield | Dissection injury | Sarcomere Length measurement | Uniform cross-sectional area | Sample requirement | Analysis of myofibril proteins |
|---|---|---|---|---|---|---|---|
| Trabeculae | Right ventricle | Low | Minimum | Laser diffraction or microscopic images | No | Fresh isolation in limited time | Yes |
| Dissecting papillary muscles | Left ventricle | Skill dependent | Yes | Depending on size | Skill dependent | Fresh isolation in limited time | Yes |
| Blended myofibril | Left ventricle | High | Yes, especially mechanical stretch | Brightfield microscopic images | No | Frozen or fresh muscle | Limited |
| Enzymatic isolated cardiomyocyte | Left ventricle | High | Proteolytic protein damage | Brightfield microscopic images | No | Fresh isolation in limited time | Very limited |
| Strips of frozen papillary muscle sections | Left ventricle | High | Minimum | Brightfield microscopic images | Yes | Frozen or fresh muscle | Yes |
The rapid freezing protocol does not include preconditioning with cryopreservant which is known to protect live cells during freeze-thaw for cell cultures (Taylor et al., 2019) since we emphasize on the preservation of muscle functions. Further development to add a preconditioning step may allow to preserve intracellular membrane structures such as the SR Ca2+ handling system to produce plasma membrane penetration-only preparations.
An important step is the manual selection of strips with properly aligned myofibrils. Trained eyes with proper focusing of the dissecting microscope at both low and high magnifications, adjusting the view angle and transmission light intensity, and using an additional light souse in a different angle can make this critical and potentially time-consuming step efficient in screening a large number of strips to select high quality fibers. The constantly controlled low temperature thermostage effectively helps to preserve the tissue during strip selections.
As that accompanying with other skinned fiber studies, tissue injury at the T-clip mounting sites needs to be minimized by very gentle closing of the T-clips. The initial precontraction is a critical step to preclude preparations that were loosely mounted or damaged during clipping since those would break near the holding ends. Use of a water-resistant glue may help to improve mounting while avoiding tight clipping-caused tissue damage.
We have observed minor heterogeneity of SL across the cryosection-generated skinned muscle strips, which may introduce variations in SL measurement. Therefore, resting SL of a strip should be measured in at least three different areas to obtain the representative average. Possibly related to the heterogeneity of resting SL that could produce heterogeneity in force production, we also observed SL variation in some part of the skinned fiber during the activation of isometric contractions. Adding isotonic contraction to the experimental protocol may be considered to further investigate the contractility of the preparations (McDonald et al., 1998).
In summary, the new methodology reported here presents a time- and cost-effective high efficiency approach for physiological and pathophysiological studies of muscle function. It is readily applicable by research labs in the field and can significantly extend the utilization of genetically modified mouse hearts and patient samples in multi-institutional investigations, especially valuable by eliminating the need of transferring live animals or fresh muscle tissues.
New Findings.
Skinned cardiac muscle preparations are widely used in physiological and pathophysiological research. The current methods using freshly isolated papillary muscle, ventricular trabeculae, surgically dissected ventricular muscle strips, mechanically blended cardiac muscle bundles, or enzymatically isolated single cardiomyocytes are of low efficiency with restricted applications. We have developed a new method using frozen cardiac papillary muscles and cryosectioning to reliably obtain uniform cardiac muscle strips with high yields. Experimental results demonstrate that this new methodology significantly increases the efficiency and application of quantitative biomechanical studies using skinned muscle fibers with an additional advantage of no need for transferring alive animals.
Acknowledgements
This work was supported in part by grants from the National Institutes of Health HL127691 and HL138007 (to JPJ).
Abbreviations
- Ktr
rate of tension redevelopment
- LV
left ventricle
- mAb
monoclonal antibody
- PKA
protein kinase A
- SL
sarcomere length
- TnI
troponin I
- TnT
troponin T
Footnotes
Competing interests
None.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Ait Mou Y, le Guennec JY, Mosca E, de Tombe PP & Cazorla O (2008). Differential contribution of cardiac sarcomeric proteins in the myofibrillar force response to stretch. Pflugers Arch 457, 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardswell SC, Cuello F, Rowland AJ, Sadayappan S, Robbins J, Gautel M, Walker JW, Kentish JC & Avkiran M (2010). Distinct sarcomeric substrates are responsible for protein kinase D-mediated regulation of cardiac myofilament Ca2+ sensitivity and cross-bridge cycling. J Biol Chem 285, 5674–5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlooli Ghashghaee N, Tanner BCW & Dong WJ (2017). Functional significance of C-terminal mobile domain of cardiac troponin I. Arch Biochem Biophys 634, 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazorla O, Szilagyi S, Le Guennec JY, Vassort G & Lacampagne A (2005). Transmural stretch-dependent regulation of contractile properties in rat heart and its alteration after myocardial infarction. FASEB J 19, 88–90. [DOI] [PubMed] [Google Scholar]
- Chung CS & Granzier HL (2011). Contribution of titin and extracellular matrix to passive pressure and measurement of sarcomere length in the mouse left ventricle. J Mol Cell Cardiol 50, 731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A & Fabiato F (1979). Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 75, 463–505. [PubMed] [Google Scholar]
- Feng HZ, Biesiadecki BJ, Yu ZB, Hossain MM & Jin JP (2008a). Restricted N-terminal truncation of cardiac troponin T: a novel mechanism for functional adaptation to energetic crisis. J Physiol 586, 3537–3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng HZ, Chen M, Weinstein LS & Jin JP (2008b). Removal of the N-terminal extension of cardiac troponin I as a functional compensation for impaired myocardial beta-adrenergic signaling. J Biol Chem 283, 33384–33393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng HZ & Jin JP (2010). Coexistence of cardiac troponin T variants reduces heart efficiency. Am J Physiol Heart Circ Physiol 299, H97–H105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng HZ & Jin JP (2018). A protocol to study ex vivo mouse working heart at human-like heart rate. J Mol Cell Cardiol 114, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng HZ & Jin JP (2019). Transgenic expression of carbonic anhydrase III in cardiac muscle demonstrates a mechanism to tolerate acidosis. Am J Physiol Cell Physiol 317, C922–C931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AM, Homsher E & Regnier M (2000). Regulation of contraction in striated muscle. Physiol Rev 80, 853–924. [DOI] [PubMed] [Google Scholar]
- Gunther LK, Feng HZ, Wei H, Raupp J, Jin JP & Sakamoto T (2016). Effect of N-Terminal Extension of Cardiac Troponin I on the Ca(2+) Regulation of ATP Binding and ADP Dissociation of Myosin II in Native Cardiac Myofibrils. Biochemistry 55, 1887–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanft LM & McDonald KS (2010). Length dependence of force generation exhibit similarities between rat cardiac myocytes and skeletal muscle fibres. J Physiol 588, 2891–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JP, Chen A, Ogut O & Huang QQ (2000). Conformational modulation of slow skeletal muscle troponin T by an NH(2)-terminal metal-binding extension. Am J Physiol Cell Physiol 279, C1067–1077. [DOI] [PubMed] [Google Scholar]
- Jin JP, Yang FW, Yu ZB, Ruse CI, Bond M & Chen A (2001). The highly conserved COOH terminus of troponin I forms a Ca2+-modulated allosteric domain in the troponin complex. Biochemistry 40, 2623–2631. [DOI] [PubMed] [Google Scholar]
- King NM, Methawasin M, Nedrud J, Harrell N, Chung CS, Helmes M & Granzier H (2011). Mouse intact cardiac myocyte mechanics: cross-bridge and titin-based stress in unactivated cells. J Gen Physiol 137, 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knorpp CT, Starkweather WH, Spencer HH & Weatherbee L (1971). The preservation of erythrocytes at liquid nitrogen temperatures with hydroxyethyl starch: the removal of hydroxyethyl starch from erythrocytes after thawing. Cryobiology 8, 511–516. [DOI] [PubMed] [Google Scholar]
- Konhilas JP, Irving TC, Wolska BM, Jweied EE, Martin AF, Solaro RJ & de Tombe PP (2003). Troponin I in the murine myocardium: influence on length-dependent activation and interfilament spacing. J Physiol 547, 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kussauer S, David R & Lemcke H (2019). hiPSCs Derived Cardiac Cells for Drug and Toxicity Screening and Disease Modeling: What Micro- Electrode-Array Analyses Can Tell Us. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Peng J, Radke M, Gotthardt M & Granzier HL (2010). Calcium sensitivity and the Frank-Starling mechanism of the heart are increased in titin N2B region-deficient mice. J Mol Cell Cardiol 49, 449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B, Chung F, Qu Y, Pavlov D, Gillis TE, Tikunova SB, Davis JP & Tibbits GF (2008). Familial hypertrophic cardiomyopathy-related cardiac troponin C mutation L29Q affects Ca2+ binding and myofilament contractility. Physiol Genomics 33, 257–266. [DOI] [PubMed] [Google Scholar]
- Lindsey ML, Kassiri Z, Virag JAI, de Castro Bras LE & Scherrer-Crosbie M (2018). Guidelines for measuring cardiac physiology in mice. Am J Physiol Heart Circ Physiol 314, H733–H752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyet B & Rapatz G (1970). A review of basic researches on the cryopreservation of red blood cells. Cryobiology 6, 425–482. [DOI] [PubMed] [Google Scholar]
- McDonald KS, Wolff MR & Moss RL (1998). Force-velocity and power-load curves in rat skinned cardiac myocytes. J Physiol 511 ( Pt 2), 519–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklas JW, Salick MR & Kim DH (2019). High-Throughput Contractility Assay for Human Stem Cell-Derived Cardiomyocytes. Circ Res 124, 1146–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JR, Fitzsimons DP, Buck SH, Muthuchamy M, Wieczorek DF & Moss RL (2001). PKA accelerates rate of force development in murine skinned myocardium expressing alpha- or beta-tropomyosin. Am J Physiol Heart Circ Physiol 280, H2732–2739. [DOI] [PubMed] [Google Scholar]
- Sequeira V & van der Velden J (2015). Historical perspective on heart function: the Frank-Starling Law. Biophys Rev 7, 421–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer JE, Fitzsimons DP & Moss RL (2006). Ablation of myosin-binding protein-C accelerates force development in mouse myocardium. Biophys J 90, 4119–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuyvers BD, McCulloch AD, Guo J, Duff HJ & ter Keurs HE (2002). Effect of stimulation rate, sarcomere length and Ca(2+) on force generation by mouse cardiac muscle. J Physiol 544, 817–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, Weegman BP, Baicu SC & Giwa SE (2019). New Approaches to Cryopreservation of Cells, Tissues, and Organs. Transfus Med Hemother 46, 197–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahl CF, Timek T, Bonz A, Kochsiek N, Fuchs H, Schaffer L, Rosenberg M, Dillmann R & Hagl S (1997). Myocardial length-force relationship in end stage dilated cardiomyopathy and normal human myocardium: analysis of intact and skinned left ventricular trabeculae obtained during 11 heart transplantations. Basic Res Cardiol 92, 261–270. [DOI] [PubMed] [Google Scholar]
- van Heuningen R, Rijnsburger WH & ter Keurs HE (1982). Sarcomere length control in striated muscle. Am J Physiol 242, H411–420. [DOI] [PubMed] [Google Scholar]
- Widen C & Barclay CJ (2005). Resting metabolism of mouse papillary muscle. Pflugers Arch 450, 209–216. [DOI] [PubMed] [Google Scholar]
- Wong S, Feng HZ & Jin JP (2019). The evolutionarily conserved C-terminal peptide of troponin I is an independently configured regulatory structure to function as a myofilament Ca(2+)-desensitizer. J Mol Cell Cardiol 136, 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]