Abstract
The diabetic retinal disease remains a leading cause of vision loss despite currently available screening methods, ocular treatments, and efforts to control metabolic dysfunction. It is now understood that diabetes damages the entire retina and the cellular components of the neurovascular unit. Multiple studies have demonstrated impairment of various aspects of retinal function across the spectrum of retinopathy severity. Here we review these tests, the principles underlying their use, clinical data from multiple publications, the strengths and limitations of the studies, and prospects for their application to understand the pathophysiology of diabetic retinal disease and monitor its response to therapy. We focus on visual acuity, contrast sensitivity, color vision, visual field, and dark adaptation and their use to understand the pathophysiology of diabetic retinopathy and as potential endpoints for clinical trials.
Keywords: diabetic retinopathy, retinal sensory neuropathy, visual function, visual fields, contrast sensitivity
1. Introduction: Beyond microvascular changes
Diabetes mellitus (DM) has become a global pandemic. In 1980, it was estimated that 108 million people had diabetes, but recently the World Health Organization projected that the number of people living with the disease is approaching 422 million, about 8.5% of the world’s adult population.150 Approximately one-third of this population has some form of diabetic retinopathy (DR), and one-10th of these people will later develop sight-threatening DR.152 Thus, DR is now one of the leading causes of vision impairment in the world.
DR develops insidiously and is usually asymptomatic in its early stages. It is divided into two major stages based on clinical examination and fundus photographs: nonproliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR). NPDR is characterized by micro-aneurysms, hemorrhages, lipid exudates, and nerve fiber layer stasis “cotton wool spots”.44 It can then progress into PDR, which is defined by pathological neovascularization in the retina and iris. These fragile new blood vessels can bleed into the vitreous body and then increase the risk of retinal detachment and severe vision loss.30,41,51 In both stages, swelling of the macula can distort foveal integrity and reduce central vision.
Beyond the development of vascular pathology, DR is also a disease of neurodegeneration. Animal and human experiments have revealed cellular injuries in the neural retina evident by apoptosis of inner retinal neurons, thinning of the ganglion cell layer and nerve fiber layer, and defects in the supporting astrocytes.14,15 This neural dysfunction may occur prior to the microvascular maladaptation and detected as subtle defects in vision.9,16 Such defects have been detected by various psychophysical tests, including contrast sensitivity and color vision, despite normal-appearing fundus examination. In short, currently available data highly suggests that diabetes affects the entire neurovascular unit in concert.128
For many years, psychophysical assessments, especially visual acuity, have served as functional outcomes in clinical DR research. Previous studies have reported decreased visual acuity and contrast sensitivity, impaired color vision, loss of visual field, and slowed dark adaptation in people with DR. These visual impairments corresponded with self-reported symptoms, including cloudy or blurred vision, impaired color vision, reduced peripheral vision, and poor night vision.5,45,87 The subjects also reported poor driving ability and significant emotional distress.37,53 These vision tests, however, are not developed specifically to detect visual deficits in people with DR but rather provide a general assessment of the visual pathway. In humans, the visual pathway begins with the perception of light signals by the eyes and terminates with reception of visual details by the brain. Neurological and ophthalmic diseases that affect one or more components of this pathway can lead to abnormal test results. The interpretation of these test results is further limited by the inherent test-retest variability of each instrument. Despite such limitations, psychophysical assessments remain valuable because they can provide information about how the vision is affected by diabetes. Thus, it is important to understand these instruments, their findings, and their limitations in DR research (Table 1).We critically summarize the type of vision loss in people with diabetes, addresses the limitations of psychophysical assessments, and provides recommendations for their future applications. The rationale for this review also lies in the recognition that more comprehensive evaluation of visual function, along with retinal integrity, are needed to enable the development of quantitative severity measures that can reveal new therapeutic targets to address early DR and potentially leads to means to restore vision loss in later stages.9,105,128
Table 1 —
Strengths and drawbacks of psychophysical tests
| Tests | Strengths | Drawbacks | Relationship to retinal physiology |
|---|---|---|---|
| Visual Acuity | Low cost and easily accessible Administer in <1 min High test-retest reliability |
Less sensitive to detect abnormal vision Does not measure retinal function outside of the fovea |
Reflects the integrity of foveal cones. |
| Contrast Sensitivity | Fairly accessible (e.g. Pelli-Robson) Variable testing times, some are ~2—3 min |
Variable costs, depending on the methods Variable test-retest reliability, depending on the methods Does not measure retinal function outside of the macula |
Controls by the wiring of specialized ganglion cells and cone cells to create magnocellular, parvocellular, and koniocellular pathways. |
| Color Vision | Relatively affordable and fairly accessible Administer in <2 min, longer in some methods |
Poor reliability; some methods are heavily affected by the learning effect Does not necessarily measure rod function or retinal function outside of the macula |
Derives from 3 major types of cone cells: short-wavelength, medium-wavelength, and long-wavelength cone types. |
| Visual Field | Can measure both central and peripheral retinal function | Expensive and less accessible Variable testing times can be 2—5 min in some methods but much longer in others (>20 min) Poor reliability, especially when using smaller stimulus or testing defected location |
Indicates the function of photoreceptor pathways to ganglion cells to visual cortex. |
| Dark Adaptation | Measures both rod and cone functions | Less sensitive to detect abnormal vision Expensive and less accessible Long testing times; dark adapting time alone >20—30 min Variable protocols (e.g. different bleaching methods) Limited data on test-retest reliability. |
Determined by the photoreceptor cells and the retinal pigmented epithelium layer. |
2. Visual acuity
Visual acuity (VA) is the common functional measure of foveal photoreceptor function. It has been widely used to define the visual impairment associated with diabetes (Table 2). In most cases, it reveals significant vision loss after the development of PDR, but it is grossly normal in most people with diabetes. The Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) reported that a majority of patients with no retinopathy or NPDR maintained acuity better than 20/40, the minimal VA requirement for driving in most states.86,141 Acuity worse than 20/200 was primarily found in patients with severe PDR, although, in some older adults, cataract, glaucoma, and macular degeneration also contributed to the poor acuity. The National Health and Nutrition Examination Survey (NHANES), from 2005–2006 and 2007–2008, also demonstrated that mean (95% CI) acuity for people with no DR, mild or moderate NPDR, and severe NPDR or PDR were 20/40 (20/40-20/40), 20/50 (20/40-20/50), and 20/250 (20/125-20/400), respectively.147 This pattern of acuity loss aligned with results from prior studies, showing that VA declined with the retinopathy progression and associated with older age, longer duration of diabetes, presence of senile cataract, and presence of macular edema.75,86,100
Table 2 —
Visual acuity in diabetic retinopathy
| Author(s) (year) | No. of eyes (# subjects) | Device | Mean (SD) acuity in letter score equivalence | |||
|---|---|---|---|---|---|---|
| Control | No DR | NPDR | PDR | |||
| Willis et al (2017)147 | No DR = 689 (n = 689) Mild and Moderate DR = 283 (n = 283) Severe NPDR or PDR = 32 (n = 32) |
Nidek ARK-760 (Snellen Equivalent), presenting acuity | ≈ 70 | ≈ 65 | ≈ 30 | |
| Joltikov et al (2017)81 | CN = 18 (n = 18) No DR = 23 (n = 23) Mild NPDR = 19 (n = 19) Moderate NPDR = 15 (n = 15) |
EVA testing (Snellen Equivalent), BCVA | ≈ 90 | ≈ 85 | ≈ 85 | |
| Boynton et al (2015)28 | CN = 15 (n = 15) PDR = 15 (n = 15) PRP = 30 (n = 30) |
EVA (ETDRS Letter Score), BCVA | 90.1 (4.5) | 79.3 (9.4) | ||
| Diabetic Retinopathy Clinical Research Network (2015)151 | Ranibizumab = 191 (n = 191) PRP = 203 (n = 203) |
EVA (ETDRS Letter Score), BCVA | 75.2 (12.5) (baseline) | |||
| Zhu et al (2015)154 | CN = 158 (n = 158) No DR = 141 (n = 141) |
Not Specified (LogMAR), BCVA | ≈ 80 | ≈ 80 | ||
| Jackson et al (2012)77 | CN = 18 (n = 18) No DR = 23 (n = 23) NPDR = 35 (n = 35) |
EVA (ETDRS Letter Score), BCVA | 85.56 (4.83) | 86.48 (4.57) | 81.06 (6.30) | |
| Misra et al (2010)100 | CN = 226 (n = 115) No DR = 281, NPDR = 93 PDR = 8 (n = 205) |
ETDRS chart (LogMAR), BCVA | ≈ 75 | ≈ 70 | ||
| Katz et al (2010)84 | CN = 14 (n = 7) No DR = 17 (n = 9) |
ETDRS chart (LogMAR), BCVA | ≈ 85 | ≈ 85 | ||
EVA, electronic visual acuity; BCVA, best corrected visual acuity; CN, control; DR, diabetic retinopathy; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
Many people, however, with severe DR, can retain relatively normal acuity. For example, in Protocol S of the Diabetic Retinopathy Clinical Research Network’s clinical trial that evaluated the noninferiority of intravitreous ranibizumab compared to panretinal photocoagulation for treating PDR, the baseline mean visual acuity of 394 untreated eyes, of which ~30% had center-involved diabetic macular edema, was around 20/32.151 At the 5-year follow-up, the mean acuity improved to 20/25 in both treatment groups.59
The reported incidence of visual impairment based on VA varies with studies and, sometimes, can be misleading. One reason is the difference between “presenting visual acuity (PVA)” and “best-corrected visual acuity (BCVA).” For example, the NHANES reported the mean PVA of 20/40-20/50 in eyes with mild or moderate retinopathy, whereas other studies showed mean BCVA close to 20/20 in eyes with a similar degree of retinopathy.77,81,147 In this case, the PVA may be confounded by uncorrected refractive errors, leading to worse acuity observed. Another explanation for the difference in the reported acuity is the test design. For instance, the conventional Snellen chart has the drawbacks of having fewer letters at the larger size levels, the inconsistent progression of letter size between lines, and nonuniform spacing between letters and rows. These drawbacks can lead to imprecise quantification of VA. In contrast, the Early Treatment of Diabetic Retinopathy Study (ETDRS) chart standardizes the visual task by using the same number of optotypes at each size level, logarithmic progression between rows, and spacing proportional to letter size in each row; thus, the size of the letters becomes the only significant variable going down the rows. These features explain the small test-retest variability in the ETDRS chart, which, up to 87% of the retest scores, can be within 0.1 logMAR (or 1 line of letters on an eye chart) of the initial test score, and 98% can be within 0.2 logMAR.21 It is important to note that ETDRS testing on an electronic visual acuity tester (EVA) can also produce results slightly different from its standard paper chart and testing procedure. Beck and coworkers reported that 26% of subjects had scores differed by ≥ 0.1 logMAR between the two forms, and up to 6% with scores differed by ≥0.2 logMAR. This variance was thought to be related to the difference in scoring methods and testing strategies: single-letter presentations in electronic ETDRS testing and one-line presentations in the standard ETDRS chart or placing the displays at varying distance from the subjects. Hence, it is essential to clarify which test is used and how it is performed, for example, any encouragement to guess, test room light setting, any aids with target localization, and scoring method, so the results from different studies can be better compared with each other and with fewer unknown variables.
Nevertheless, the testing property of VA makes it a good instrument to study vision loss in diabetes because it is widely available on paper charts, low cost, easy to use, and can be administered in less than a minute. It also has high test-retest reliability. By displaying it on a monitor to control screen lighting, step progression, and score calculation, the VA procedure can be highly efficient and standardized. However, it has several limitations in that it does not evaluate parafoveal and peripheral regions of the retina and can be normal when other components of vision, as well as retinal integrity, are impaired, which has also been observed in people with glaucoma and rod photoreceptor dystrophies.64 In people with retinal degeneration disorder, for example, normal VA can be found in eyes with loss of 60% of cone density near or at the fovea, indicating VA as a crude marker of cone survival and function.55,114 With regard to diabetes, a growing body of evidence supports that neurodegeneration is an early event in the pathogenesis of dr.16,128,132 In the animal models and the retina of diabetic human donors, retinal ganglion cells and amacrine cells were the first neurons to undergo apoptosis.17 The cell death and associated neuronal alterations likely contribute to the thinning of inner retinal layers and the nerve fiber layer detected by optical coherence tomography.47 Despite these structural disruptions, people with diabetes can present with normal visual acuity though revealing diminished contrast sensitivity and impaired color vision.84,154 Thus, when studying the impacts of diabetes on vision loss, one should incorporate other assessments in addition to visual acuity.
3. Contrast sensitivity
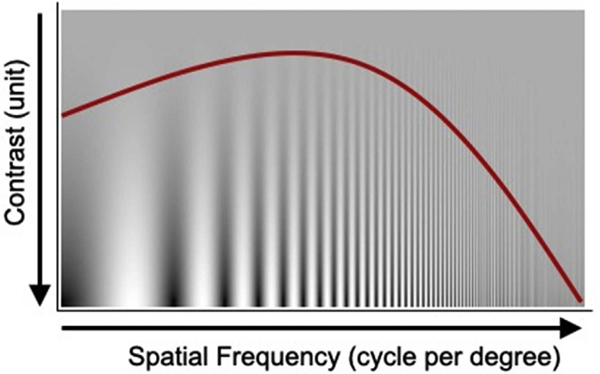
Contrast sensitivity (CS) is a measure of visual ability to distinguish an object from its background, and it is related to the size of the object. In contrast sensitivity tests, the diminishing object size can be depicted by increasing the number of alternating dark and white bars (cycles), known as the spatial frequency. In this case, a contrast sensitivity function (CSF) can be constructed in terms of contrast sensitivity and spatial frequency, as depicted in Fig. 2. This component of vision is largely mediated by retinal ganglion cells and is generally more sensitive to retinal disease than is visual acuity.33,36,117
Fig. 2 –
This contrast sensitivity chart uses sine-wave grating. The red color curve represents the general shape of a contrast sensitivity function of human vision, which peaks in the middle around 5–8 cycle/degree and falls off on either side. (Adapted with permission of Academic Press from Bull. 35)
Similar to visual acuity, CS is also impaired by diabetes and its complications: worsening CS is associated with progressing retinopathy (Table 3). Joltikov and colleagues demonstrated that subjects with diabetes had reduced sensitivity across a range of spatial frequencies compared to those without diabetes, and individuals with moderate NPDR had lower CS than those with mild NPDR or no DR. Andrade et al also reported similar CS impairment and overlaps in the affected range of spatial frequencies from 0.5 to 30.0 cycle per degree (cpd) in patients with DR.8 Other studies, however, found irregular patterns of impaired frequencies due to diabetes. One study reported that patients with no or mild-to-moderate retinopathy had impaired CS at 3.0 and 6.0 cpd while another showed impairment in 6.0 and 12.0 cpd instead.95,139 This variation in impaired spatial frequencies is much more evident in studies that evaluated CS in people with diabetes but no DR. These patients can have impaired sensitivity at low, intermediate, and/or high spatial frequencies despite no clinical evidence of retinopathy.8,46,81,84,139 This observation may suggest that the early neuronal injuries from diabetes can affect multiple spatial frequencies separately, which explains the irregularities of CS alteration in the early stages of DR. Then, as the disease progresses, the whole spectrum becomes affected as more damage accumulates.
Table 3 —
Contrast sensitivity and impaired spatial frequency
| Author(s) (year) | No. of eyes (# subjects) | Device (cpd) | Range of spatial frequency (cpd) with decreased sensitivity (compared to the control group) | ||
|---|---|---|---|---|---|
| No DR | NPDR | PDR | |||
| Joltikov et al (2017)81 | CN = 18 (n = 18) No DR = 23 (n = 23) Mild NPDR = 19 (n = 19) Moderate NPDR = 15 (n = 15) |
qCSF Procedure on AST Platform (1.5, 3, 6, 12, 18 cpd) | 1.5, 3.0, 6.0, 12.0 | 1.5, 3.0, 6.0, 12.0, 18.0 | |
| Stem et al (2016)137 | CN = 23 (n = 23) No DR = 16 (n = 16) Mild to Moderate NPDR = 14 (n = 14) |
Pelli-Robson Chart (~ 1 cpd) | ~1 | ~1 | |
| Boynton et al (2015)27 | CN = 15 (n = 15) PDR = 15 (n = 15) PRP = 30 (n = 30) |
Pelli-Robson Chart (~ 1 cpd) | ~1 | ||
| Zhu et al (2015)154 | CN = 158 (n = 158) No DR = 141 (n = 141) |
Functional Acuity Contrast test (FACT) Optec 6500 Device (1.5, 3, 6, 12, 18 cpd) | 1.5, 3.0, 6.0, 12.0, 18.0 | ||
| Andrade et al (2014)8 | CN = 64 (n = 32) DM = 49 (n = 27): 30 No DR, 13 NPDR, 6 PDR |
Study Designed CS Software (0.2, 0.5, 0.8, 1, 2, 4, 6, 10, 15, 20, 30 cpd) | 15.0, 30.0 | 0.5, 0.8, 1.0, 2.0, 4.0, 6.0, 10.0, 15.0, 20.0, 30.0 | 0.5, 0.8, 1.0, 2.0, 4.0, 6.0, 10.0, 15.0, 20.0, 30.0 |
| Jackson et al (2012)77 | CN = 18 (n = 18) No DR = 23 (n = 23) NPDR = 35 (n = 35) |
Pelli-Robson Chart (~ 1 cpd) | None | ~1 | |
| Georgakopoulos et al (2011)57 | CN = 90 (n = 45) No DR = 120 (n = 60) |
CSV-1000 Device (3, 6, 12, 18 cpd) | 3.0, 6.0, 12.0, 18.0 | ||
| Katz et al (2010)84 | CN = 14 (n = 7) No DR = 17 (n = 9) |
Study Designed Platform Using Gabor Targets (photopic CS: 6, 9,12 cpd; mesopic CS: 3, 6, 9 cpd) | 3.0 (Mesopic setting only) | ||
| Misra et al (2010)100 | CN = 226 (n = 115) DM = 382 (n = 205): 281 No DR, 93 NPDR, 8 PDR |
Pelli-Robson Chart (~ 1 cpd) | ~1 | ~1 | ~1 |
| Di Leo et al (1992)46 | CN = 60 (n = 30) No DR = 48 (n = 24) |
Study Designed CS (static and dynamic test: 0.6, 1.0, 1.4, 2.2, 4.8, 7.1, 9.6, 12.2 cpd) | Dynamic:0.6, 1.0, 1.4, 2.2, 4.8, 7.1, 9.6; Static:1.0,1.4, 2.2, 7.1, 9.6 | ||
| Trick et al (1988)139 | CN 70 (n = 35) No DR = 74 (n = 37) Mild to Moderate NPDR = 40 (n = 20) |
Vistech VCTS 6500 Distance Chart (1.5, 3.0, 6.0, 12.0, 18.0 cpd) | None | 6.0, 12.0 | |
CS, contrast sensitivity; CN, control; DM, diabetes; DR, diabetic retinopathy; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; PRP, pan-retinal photocoagulation; cpd, cycle per degree.
Another possibility for the discrepancy is the different testing methods. For example, even though the CSV-1000 and Vistech VCTS 6500 are both sine-wave grating charts, they cover different spatial frequencies and CS levels and used different step size progression.117 The Vistech chart tests 5 spatial frequencies with CS ranging from 0.5% to 31.62% and step size of ~0.23 log unit per level. In contrast, the CSV-1000 tests 4 spatial frequencies but with a wider range of CS from 0.5% to 67.61% and a smaller step size of ~0.16 log unit per level. The smaller step size progression between levels and wider CS range may make the CSV-1000 more sensitive than the Vistech chart in detecting vision impairment secondary to diabetes.57,139 In addition, the Vistech chart appears to be less reliable than CSV-1000, as previously reviewed.117 Next, while the chart-based tests are more convenient, their limited range of contrast levels restricts the precision and accuracy of the measured contrast sensitivity function (CSF). These limitations led to the development of computer-based programs, such as the quick Contrast Sensitivity Function (qCSF) method that combines with adaptive procedures to minimize these drawbacks. Lesmes et al developed the qCSF method, which based on the Bayesian adaptive strategy, can measure CS across a range of spatial frequencies in a more rapid (~2–3 minutes for a 25-trial session), accurate, and reliable manner compared to conventional CSF tests.48,72,93
In spite of normal acuity and fundus examination, some patients with diabetes can exhibit reduced CS. Di Leo and colleagues evaluated the CS in 24 patients with type 1 diabetes mellitus and without retinopathy and found significant losses on the dynamic and static CS tests.46 In fact, the static CS test could detect 72.9% of the eyes had abnormal CS. Another study investigated the visual functional change in 141 patients with type 2 DM without retinopathy and found reduced CS across a range of spatial frequencies.154 Several other studies also reported similar findings.8,57,81,84 And some even suspected that impaired CS could occur before the diagnosis of diabetes. Dosso and colleagues reported abnormal CS in insulin-resistant obese participants when compared to healthy individuals.49 At the time of testing, the obese subjects had plasma glucose levels within the normal range and below 108 mg/dL. Together, these reports further support the notion that diabetes can induce early nonselective neuronal damage in the visual pathway.
It is clear that CS, taken together, is diminished in many eyes affected by diabetes. Even with normal visual acuity, many eyes with DR can present with reduced sensitivities across a spectrum of spatial frequencies. These findings demonstrate that CS is a more sensitive test than VA in identifying early visual function changes and signs of neurodegeneration in people with diabetes; however, CS is also impaired in many other ophthalmic and neurologic diseases that affect the visual processing pathway, including age- related macular degeneration,88 glaucoma,136 cerebral lesions,26 and multiple sclerosis.116 Thus, it is sensitive but not specific for measuring visual impairment in diabetic eye disease. In addition, in this review, the term “visual” is used rather than “retinal” to refer to functions evaluated with psychophysical assessments because their results indicate the sensitivity of the visual system as a whole instead of the retina alone.
4. Color vision
The human fovea mainly contains three types of cone cells.111 The short-wavelength (S) cones have maximal sensitivity near the spectral blue. The medium wavelength (M) cones have maximal sensitivity near the spectral green. And the long-wavelength (L) cones have maximal sensitivity near the spectral red. Together, they form the basis of trichromatic color vision. Color vision abnormality, thus, can occur after the breakdown of these cells and their supporting units, including horizontal, bipolar, and ganglion cells.42,131
Acquired color vision impairment is common among patients with diabetes (Table 4). A report from the Early Treatment Diabetic Retinopathy Study revealed that 49% (1335 out of 2701) of its patients with DR, ranging from moderate NPDR to early PDR, had color vision abnormalities associated with DR.54 In this cohort, the yellow-blue color defect was most prominent, affecting 32% (862) of these patients, followed by diffuse loss of color discrimination without preferential axis defect (10% or 262) and then red-green color defect (2% or 38). These color defects were associated with factors such as increased age, presence of new vessels, and macular edema severity. Several other studies also reported a significant proportion of patients with DR had a color vision deficit, which mostly occurred in the blue-yellow axis, and this dysfunction became more prevalent with increasing disease severity.8,12,52,62,75,92,103,108,120,139,142 The predominant tritanomaly associated with DR corresponded with the increased cell loss, specifically in the S-cone population, which is responsible for blue-yellow vision.39 Cho and coworkers showed that, in the retinas from donors with diabetic retinopathy, there was an increase in TUNEL-positive S-cones, indicating accelerated apoptosis within this cell population. Another possibility is that S-cones only constituted 7–10% of the cone population while the remaining is made of M-cones and L-cones.4 The nonselective nature of diabetes-induced injuries may affect all three types of cones but has more impacts on blue-yellow color function because of the relatively lower number of S-cones in general. Then, as more M- and L-cones are damaged, the retina develops a wide range of color vision loss.
Table 4 —
Color vision impairment
| Author(s) (year) | No. of eyes (# subjects) | Device | % Subjects with abnormal color vision (if stated, indicates the predominant affected axis) | ||
|---|---|---|---|---|---|
| No DR | NPDR | PDR | |||
| Tan et al (2017)138 | No DR = 849 (n = 849) | Farnsworth Panel D-15 | 22% (Blue-yellow) | ||
| Boynton et al (2015)27 | CN = 15 (n = 15) PDR = 15 (n = 15) PRP = 30 (n = 30) |
Farnsworth Panel D-15 | 26.7% | ||
| Gella et al (2015)56 | No DR = 253 (n = 253) | Farnsworth-Munsell 100-Hue | 39.5% | ||
| Wolff et al (2015)149 | CN = 37 (n = 37) No DR = 22 (n = 22) DR = 25 (n = 25): 9 Mild NPDR, 14 Moderate NPDR, 1 Severe NPDR, 1 PDR with 6 CSME among them |
Adams Desaturated D-15 | 40.9% (Blue-yellow) | 48.0 (Blue-yellow) | |
| Andrade et al (2014)8 | CN = 64 (n = 32) DM = 49 (n = 27): 30 No DR, 13 NPDR, 6 PDR |
Farnsworth-Munsell 100-Hue | 86.7% (Diffused loss) | 90.0% (Diffused loss) | |
| Feitosa-Santana et al (2010)52 | CN for D-15 = 62 (n = 31) CN for CCT = 72 (n = 36) No DR = 61 (n = 31) |
Lanthony Desaturated D-15 Test and Cambridge Color Test (CTT) | D-15: 21.3% (Blue-yellow) CTT: 27.9% (Diffused loss) | ||
| Fong et al (1999)54 | DM = 2701 (n = 2701), ranging from Moderate NPDR to Early PDR and/or with presence of macular edema. | Farnsworth-Munsell 100-Hue | 49.4% (Blue-yellow) | ||
| Hardy et al (1992)62 | CN = 36 (n = 36) No DR = 38 (n = 38) |
Farnsworth-Munsell 100-Hue | 57.0% (Diffused loss) | ||
| Trick et al (1988)139 | CN 70 (n = 35) No DR = 74 (n = 37) Mild to Moderate NPDR = 40 (n = 20) |
Farnsworth-Munsell 100-Hue | 18.9% (Diffused loss) | 25.0% (Diffused loss) | |
CN, control; DM, diabetes; DR, diabetic retinopathy; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; PRP, pan-retinal photocoagulation.
In many people with diabetes, dyschromatopsia can precede clinically evident retinopathy. Tan and colleagues used Farnsworth Panel D-15 to investigate the prevalence of impaired color vision among 849 people with diabetes but without retinopathy.138 They found that 22% had impaired color vision and tritanomaly was the most common defect among the affected eyes. The precise number of color vision abnormalities prior to the development of DR is uncertain and heavily influenced by the type of color vision test used. In a study that used the Farnsworth-Munsell (FM) 100 test, for example, the frequency of color vision impairment in people with no DR was close to 39.5%.56 Another study that used Adams Desaturate D-15 also reported more than 40% of its subjects without DR had significantly elevated color confusion scores than the reference range.149 However, even in patients with severe DR, the Farnsworth Panel D-15 may detect abnormal color vision in less than a third of the subjects.27 These results suggest that the prevalence of color vision impairment associated with diabetes are underestimated with less sensitive tests, such as the Farnsworth Panel D-15. Farnsworth Panel D-15 is a rapid test designed to detect severe discrimination loss, such as loss of blue-yellow color vision. It requires the examinee to arrange 15 discrete colored caps, whereas the FM 100 test relies on 85 colored samples to be arranged in 4 boxes of 21 or 22 colors each. Although it is more time-consuming, the FM 100 can better quantify the degree of color confusion and detect subtle deficits.18 The Adams Desaturated D-15 differs from the Farnsworth Panel D-15 in that the chroma of each cap is reduced by 2 units, making the color less saturated. The desaturate D-15 is also more sensitive than Farnsworth Panel D-15 in detecting acquired color vision defects, for example, in older populations.122
The interpretation of these tests is further complicated by test-retest variability.7,43,73,74,144 It was observed that the variation between tests increased with the magnitude of a test abnormality, so people with worse color vision had more variable outcomes in retesting. In addition, test results are influenced by the learning effect, where the performance improves with familiarity with the test. While evaluating the natural history of diabetes in children, Bronte-Steward and colleagues demonstrated that their performance on the FM 100 improved with subsequent testing over 3-years, which was largely independent of age.32 This confounding factor is not unique to the FM 100 alone. Banford et al also found that scores on the Farnsworth Panel D-15 improved after repeated testing among people with diabetes.13 At baseline, 35% of the patients failed the test; but on the sixth visit, none of them failed. Similarly, when testing with the Lanthony Desaturated D-15, 64% of the same cohort failed at the first visit, and 56% still performed below the normal at the sixth visit. Although Lanthony Desaturated D-15 may experience less learning effect than FM 100 or Farnsworth Panel D-15, it still raises the concern of the learning curve and whether the deficit seen reflects true pathology. These findings suggest that people can learn the correct responses for a color vision test, which may misrepresent them as improvement, despite their underlying color defects being unchanged.
5. Visual fields
Unlike the methods described earlier, visual field tests can examine both the central and peripheral vision. They are usually performed by keeping the subject’s gaze fixed at a fixation point while projecting stimuli of decreasing brightness at predefined locations. Clinically, they play a central role in characterizing and monitoring vision loss in glaucoma, optic neuropathies, and rod-cone dystrophies. It is proposed that the loss of field sensitivity correlates to the extent of ganglion cell and photoreceptor injuries in these diseases.40,61,63,113 Diabetes also induces neuronal, glial, and microglial cell damage in the retina, as mentioned earlier, and these changes are further reflected in perimetric testing as visual field deficits. The application of visual field assessment to DR was first documented in 1943, expanded in 1969119 and successive decades29,31,129,130,148 and then reiterated recently.133 Still, its use in clinical practice or research for DR has been limited despite evidence of visual field changes in people with diabetes.
In the early 1990s, standard automated perimetry was used to demonstrate the visual field loss associated with diabetes (Table 5). In a diabetic cohort with retinopathy level ranging from no retinopathy to severe PDR, Henricsson and Heijl observed that the number of depressed points on the visual field usually increased with the retinopathy level.68 Regions with reduced sensitivity (scotomas) were also correlated with the degree of retinal nonperfusion in the mid-peripheral retina as revealed by fluorescein angiography. However, in eyes with no or mild retinopathy, there was no evidence of field loss. Other studies also reported clear field defects in eyes with more advanced retinopathy as defined by reduced mean deviation (MD) and elevated pattern standard deviation (PSD) as well as decreased foveal threshold or sensitivity.27,81,140,155 In these cases, the reduced MD suggested overall field worsening, elevated PSD indicated more localized patterns of field deficit, and decreased foveal sensitivity implied sole impairment in the fovea. It is important to note that the value of MD and PSD can vary greatly among patients with diabetes, especially in those with worsening retinopathy. In one study, the standard deviations (mean) of MD was 3.76 (−4.95 dB) in patients with moderate NPDR while that of patients with no DR was 2.47 (−1.27 dB), which was still greater than the control group of 0.72 (−0.56 dB).81 This finding implicates that some patients with advanced retinopathy can present with the grossly normal visual field while others can suffer from severe vision loss. Although more severe DR was associated with greater field losses, some longitudinal studies further showed that visual field deterioration could occur without changes in the retinopathy levels. Hellgren et al. monitored the functional and structural changes in people with diabetes over a 5-year period.66,67 After 18 months of followup, 21 of 81 patients with retinopathy ranging from none to moderate retinopathy had a loss of sensitivity in ≥10% of all tested points on the visual field. Two patients showed DR progression but were not among those with visual field declines. At the end of follow-up for at least 3 years, visual field worsening was still observed in patients with and without retinopathy, but the perimetric changes were not correlated with changes in retinopathy severity. These findings suggest that the progression of field deficit and DR can be separate events despite being exposed to the same metabolic disorder.
Table 5 —
Standard automated perimetry (white stimulus on white background) and visual field deficit
| Author(s) (year) | No. of eyes (# subjects) | Device | Impaired visual field parameters (compared to the control group) No DR NPDR PDR | ||
|---|---|---|---|---|---|
| No DR | NPDR | PDR | |||
| Joltikov et al (2017)81 | CN = 18 (n = 18) No DR = 23 (n = 23) Mild NPDR = 19 (n = 19) Moderate NPDR = 15 (n = 15) |
HFA 24–2 SITA-standard strategy | None | ↑ PSD ↓ MD ↓ Foueal Threshold | |
| Boynton et al (2015)27 | CN = 15 (n = 15) PDR = 15 (n = 15) PRP = 30 (n = 30) |
HFA 10–2 SITA-standard and 60–4 | HFA 10–2: ↑ PSD ↓ MD HFA 60–4: ↑ Total Threshold | ||
| Jackson et al (2012)77 | CN = 18 (n = 18) No DR = 23 (n = 23) NPDR = 35 (n = 35) |
HFA 24–2 SITA-standard strategy | None | ↓ Mean Field Sensitiuity | |
| Zico et al (2014)155 | CN = 40 (n = 20) No DR = 40 (n = 20) Mild NPDR = 40 (n = 20) |
Twinfield Analyzer 24–2 SAP | ↓ MD | ↑ PSD ↓ MD | |
| Parravano et al (2008)110 | CN = 30 (n = 30) No DR = 30 (n = 30) |
HFA 30–2 SITA standard strategy | ↑ PSD ↓ MD | ||
| Lobefalo et al (1998)94 | CN = 100 (n = 50) No DR = 100 (n = 50) |
HFA 24–2 SITA-standard strategy | None | ||
| Trick et al (1990)140 | CN 70 (n = 35) No DR = 74 (n = 37) Mild to Moderate NPDR = 40 (n = 20) |
HFA C 30–2 strategy | ↑ PSD ↓ MD | ↑ PSD ↓ MD | |
CN, control; DR, diabetic retinopathy; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; PRP, pan-retinal photocoagulation.
Unlike other psychophysical assessments, visual field testing can provide information on the peripheral regions of the retina as it projects testing points outside of the macula. This property has proven useful in the evaluation of functional changes in response to ocular treatments in studies such as the Diabetic Retinopathy Clinical Research Network Protocol S.59,151 One of the secondary outcome measurements was combined total point scores from the Humphrey visual field 30–2 and 60–4 test patterns to assess peripheral vision changes. In this cohort, the baseline MD was −6.6 dB in spite of mean VA of 20/32.97 As expected, two years after the prescribed treatments, the PRP group had greater loss of peripheral field compared to the ranibizumab group (mean change of −422 dB vs −23 dB, respectively). At the 5-year visit, the mean change in cumulative field sensitivity was −527 dB in the PRP group and −330 dB in the ranibizumab group. Despite both groups having progressive field loss, patients who received ranibizumab unexpectedly showed a rate of decline in the visual field beginning at the 3-year visit that paralleled the PRP-treated group. Even after removing visual field results from those who had subsequent laser treatments, the ranibizumab group still had a 5-year mean change of −201 dB.97 This analysis suggests that factors other than PRP were associated with visual field loss in diabetic eyes treated with ranibizumab. These factors may include age-related vision changes as well as progressive diabetes-induced dysfunction despite treatments.38 Furthermore, it should be noted that the baseline visual field impairment of −6.6 dB in Protocol S subjects is equivalent to that observed in people with advanced glaucoma.1 This fact reveals that people with DR have more advanced retinal damage than fundus photographs reveal, supporting the concept that visual field measurement can be a useful marker in monitoring the progression of DR.
Conventional automated perimetry, however, is less sensitive in detecting abnormal vision in milder disease, such as in the absence of clinically evident retinopathy. While using SAP, several studies reported that eyes with no retinopathy might have slightly reduced field sensitivity compared to the reference group, but the overall difference was not significant.77,81,94,110 This insensitivity had previously been discussed in the context of glaucoma research, where substantial loss of retinal ganglion cells and thinning of the retinal nerve fiber layer associated with optic nerve injury occurred prior to the detection of field abnormality using SAP.85,134 This limitation later led to the development of other types of perimetries, such as the short-wavelength automated perimetry (SWAP) and the frequency doubling perimetry (FDP; also termed FDT or frequency doubling technology) (Tables 6 and 7). Unlike SAP, which presents white stimuli on a white background, SWAP projects blue stimuli on a yellow background. It is thought that the yellow background adapts the rods and two types of cones, middle-wavelength sensitive and long-wavelength sensitive cones, leaving the short-wavelength sensitive cones to detect the targets.121 Lobefalo et al compared SWAP with SAP to determine which test was more sensitive in detecting visual field defects among patients with diabetes but had no signs of DR.94 The blue-on-yellow perimetric program on a Humphrey Field Analyzer revealed reduced field sensitivity among the patients with diabetes, whereas the white-on-white program did not detect such sensitivity loss. Other reports also found that the short-wavelength sensitivity (a narrow band around 440 nm examined by SWAP) may be impaired earlier than the achromatic sensitivity in the course of dr.2,3,107,155 FDP, on the other hand, is based on the principle of so-called frequency doubling illusion. It presents sinusoidal gratings of low spatial frequency that undergo counterphase flickering at a high temporal frequency. It has been proposed that this perimetric method may detect selective ganglion cell loss in the magnocellular pathway as hypothesized in the early optic nerve injury in glaucomatous patients, although the specificity of FDP remains unclear.7,96 While examining the effect of diabetes, Jackson and colleagues found that FDP could detect more field impairment than SAP in patients without retinopathy.77 FDP noted more than 30% of the patients with no DR had abnormal outcomes, whereas SAP only detected at most 17% of them with reduced function. Previous studies also supported that FDP could detect visual dysfunction in people with diabetes prior to the onset of microvascular complications and field defects measurable by achromatic perimetry.81‘110‘112‘115 These field defects also became more common with the onset of visible retinopathy and worsened with the disease evolution.
Table 6 —
Short wavelength automated perimetry (yellow stimulus on white background) and visual field deficit
| Author(s) (year) | No. of eyes (# subjects) | Device | Impaired visual field parameters (compared to the control group) No DR NPDR PDR |
||
|---|---|---|---|---|---|
| No DR | NPDR | PDR | |||
| Joltikov et al (2017)81 | CN = 18 (n = 18) No DR = 23 (n = 23) Mild NPDR = 19 (n = 19) Moderate NPDR = 15 (n =15) |
HFA 24–2 SITA-SWAP strategy | None | ↑ PSD ↓ MD ↓ Foueal Sensitivity | |
| Abrishami et al (2012)2 | CN = 34 (n = 17) No DR = 40 (n = 20) |
HFA 24–2 SITA-SWAP strategy | ↓ MD ↓ Mean Field Sensitivity | ||
| Zico et al (2014)155 | CN = 40 (n = 20) No DR = 40 (n = 20) Mild NPDR = 40 (n = 20) |
Twinfield Analyzer 24–2 SWAP | ↑ PSD ↓ MD | ↑ PSD ↓ MD | |
| Lobefalo et al (1998)94 | CN = 100 (n = 50) No DR = 100 (n = 50) |
HFA 24–2 SITA-SWAP strategy | ↓ Mean Field Sensitivity | ||
CN, control; DR, diabetic retinopathy; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; PRP, pan-retinal photocoagulation.
Table 7 —
Frequency doubling perimetry and visual field deficit
| Author(s) (year) | No. of eyes (# subjects) | Device | Impaired visual field parameters (compared to the control group) | ||
|---|---|---|---|---|---|
| No DR | NPDR | PDR | |||
| Joltikov et al (2017)81 | CN = 18 (n = 18) No DR = 23 (n = 23) Mild NPDR = 19 (n = 19) Moderate NPDR = 15 (n = 15) |
FDP 24–2 and 10–2 strategy | None | FDP 24–2: ↑ PSD ↓ MD FDP 10–2: ↓ MD | |
| Stem et al (2016)137 | CN = 23 (n = 23) No DR = 16 (n = 16) NPDR = 14 (n = 14): 11 Mild and 3 Moderate NPDR. |
FDP 24–2 strategy | None | ↓ MD | |
| Boynton et al (2015)27 | CN = 15 (n = 15) No PRP = 15 (n = 15) PRP = 30 (n = 30) |
FDP 24–2 strategy | ↑ PSD ↓ MD ↓ Foveal Sensitivity | ||
| Pinilla et al (2013)112 | CN = 30 (n = 30) No DR = 55 (n = 55) Mild NPDR = 18 (n = 18) |
FDT C-20 strategy | ↑ PSD | ||
| Jackson et al (2012)77 | CN = 18 (n = 18) No DR = 23 (n = 23) NPDR = 35 (n = 35) |
FDP 24–2 strategy | ↓ Mean Foveal Sensitivity | ↓ Mean Foveal Sensitivity | |
| Parravano et al (2008)110 | CN = 30 (n = 30) No DR = 30 (n = 30) |
FDT 30–2 strategy | ↓ MD | ||
CN, control; DR, diabetic retinopathy; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; PRP, pan-retinal photocoagulation.
In recent years, another form of automated perimetry, known as microperimetry, has gained popularity. This technique can simultaneously measure central retinal sensitivity (up to 20 degrees of the macula) and capture fundus photography, thus correlating functional information with structural data (Table 8). It also minimizes fixation errors through the built-in fundus tracking mechanism and allows a more accurate evaluation of macular function in patients who have unstable and/or parafoveal fixation. Al Shafaee and colleagues compared the macular function of prediabetic and control subjects using microperimetry and found that 40% (20 eyes of 25 patients) with prediabetes and elevated blood sugar level had abnormal fixation pattern and 52% (26 eyes) of them had reduced retinal sensitivity compared to the normal reference level.6 Retinal sensitivity and fixation stability were further impaired with the progression of DR.106 When combined with SD-OCT, microperimetry also demonstrated concurrent loss of retinal sensitivity and thinning of the photoreceptor layer and ganglion cell layer in eyes without overt retinopathy, although the data on this topic is limited.104,143
Table 8 —
Microperimetry and visual field deficit
| Author(s) (year) | No. of eyes (# subjects) | Device | Impaired visual field parameters (compared to the control group) | ||
|---|---|---|---|---|---|
| No DR | NPDR | PDR | |||
| Nittala et al (2012)106 | CN = 40, No DR = 40, Mild NPDR = 40, Moderate NPDR = 30, Severe NPDR = 30, PDR = 30 (n = 160) |
Nidek MP-1 Microperimeter (central 20 degree of macula) | ↓ Mean Foveal Sensitivity, ↓ Mean Retinal Sensitivity | ↓ Mean Foveal Sensitivity, ↓ Mean Retinal Sensitivity | ↓ Mean Foveal Sensitivity, ↓ Mean Retinal Sensitivity |
| Al Shafaee et al (2011)6 | CN = 50 (n = 25) PreDM = 50(n = 25) DM = 50 (n = 25): 20 No DR, 13 Background DR, 17 Macular edemas |
Nidek MP-1 Microperimeter (central 20 degree of macula) | ↓ Mean Retinal Sensitivity ↓ Fixation Pattern | ↓ Mean Retinal Sensitivity ↓ Fixation Pattern | |
| Verma et al (2009)143 | CN = 39 (n = 39) No DR = 39 (n = 39) |
Nidek MP-1 Microperimeter (central 20 degree of macula) | ↓ Mean Retinal Sensitivity | ||
CN, control; DM, diabetes; DR, diabetic retinopathy; NPDR, nonproliferative diabetic retinopathy; PreDM, Pre-diabetic; mean foveal sensitivity, retinal sensitivity in the central 2°; mean retinal sensitivity, retinal sensitivity in the central 20° of macula.
Despite its ability to monitor vision loss associated with diabetes, perimetric testing has its limitations. It is well known that visual field measurement can vary significantly on repeated testing, and the difference between retests is generally larger in patients with more impaired visual sensitivity.65,118 Bengtsson et al also demonstrated that, in patients with DR, both SAP and SWAP exhibited smaller local test-retest variability at test points with normal sensitivity than at test points with reduced sensitivity, although repeated outcomes from the SAP had smaller differences compared to SWAP.23 Such differences were smaller in more central test points as well. These results imply that smaller changes in the peripheral points with reduced sensitivity were more likely due to random chance than actual improvement or deterioration. Some authors have suggested that the high retest variability of SAP started at roughly 25 dB, and detecting any values below 20 dB may have minimal practical use.65,146 The size of the stimulus also contributed to the test-retest variation. It was found that the SAP size III had relatively lower repeatability than a larger stimulus size V testing.145 This finding concurred with the higher retest variability in SAP size III (0.43°) and V (1.72°) compared to Matrix (FDP) perimeter (4°).146 It was hypothesized that the larger stimuli could cover more receptive fields and thus consistently captured the sporadic losses in the entire visual field as compared to the smaller stimuli that only occasionally detected these defects.
6. Dark adaptation
Dark adaptation refers to the slow recovery of visual sensitivity after the cessation of bright light exposure, known as bleaching.76 This index differs from the scotopic sensitivity, the ability to adapt to dark conditions in the absence of a bright flash. Regardless, in the absence of light, rod cells become more active, replacing cone-mediated photopic vision to form scotopic vision. The dark adaptation process reflects the health of the photoreceptor-retinal pigment epithelium interactions and the regeneration of rhodopsin.
Dark adaptometry allows separate recording of rod and cone functions, such as cone recovery rate, cone sensitivity, rod recovery rate, and rod sensitivity (Table 9). Bavinger an colleagues used the AdaptDx Dark Adaptometer to demonstrate that photoreceptor dysfunction progressed with worsening DR, in which the rod function was significantly impaired at the NPDR stage, followed by malfunction of cone cells at the PDR stage.20 These subjects had normal serum retinol and retinol-binding protein (unpublished data) so any impairment in dark adaptation in these individuals did not result from system defects in vitamin A availability. Next, these functional deficits were also correlated with the thinning of the retinal pigmented epithelium layer and photoreceptor inner segment/outer segment layer seen on the OCT. Previous studies also provided evidence of early deterioration of rod function in patients with diabetes prior to the dysfunction of cone cells.58,70,91 Even with mild retinopathy, there were abnormally prolonged rod-mediated, dark-adapted responses despite normal photopic (in the light) function. Arden et al found that, during the first 5 minutes of stimulation after bleaching, people with or without diabetes had similar dark adaptation curves, suggesting grossly unaffected cone function.10 After 5 minutes, the eyes of adults with Type 1 diabetes stopped adapting while the healthy eyes continued to recover their rod function and were able to detect stimuli of lower intensity. At this point, it is not clear why diabetes would affect dark adaptation though Malechka and colleagues reported reduced rhodopsin generation in diabetic rats.98
Table 9 —
Impaired dark adaptation in diabetic retinopathy
| Author(s) (year) | No. of eyes (# subjects) | Device | Impaired dark adaptation parameters (compared to the control group) | ||
|---|---|---|---|---|---|
| No DR | NPDR | PDR | |||
| Stem et al (2016)137 | CN = 23 (n = 23) No DR = 16 (n = 16) NPDR = 14 (n = 14): 11 Mild and 3 Moderate NPDR. |
AdaptDx Dark Adaptometer (flash bleach and stimulating at 5 deg superior to the fovea) | None | None | |
| Boynton et al (2015)27 | CN = 15 (n = 15) PDR = 15 (n = 15) PRP = 30 (n = 30) |
AdaptDx Dark Adaptometer (flash bleach and stimulating at 5 deg superior to the fovea) | None | ||
| Bavinger et al (2016)20 | CN = 23 (n = 23) No DR = 17 (n = 17) Mild NPDR = 7 (n = 7) Moderate NPDR = 4 (n = 4) PDR = 15 (n = 15) PRP = 30 (n = 30) |
AdaptDx Dark Adaptometer (flash bleach and stimulating at 5 deg superior to the fovea) | ↓ Rod Recovery Rate | ↑ Rod Intercept Time ↓ Rod Recovery Rate ↓ Cone Sensitivity | |
| Jackson et al (2012)77 | CN = 18 No DR = 23 NPDR = 35 |
AdaptDx Dark Adaptometer (flash bleach and stimulating at 5 deg superior to the fovea) | None | None | |
| Holfort et al (2010)70 | CN = 17 (n = 17) Mild NPDR = 24 (n = 24) |
AdaptDx Dark Adaptometer (flash bleach and stimulating at 5 deg superior to the fovea) | ↓ Rod Recovery Rate | ||
| Kurtenbach et al (2006)91 | CN = 12 (n = 12) No DR = 10 (n = 10) Mild NPDR = 2 (n = 2) |
Lightproof Goggle Fitted with Dark Adaptation Capacity (3 min bleaching and stimulating within 75 deg of visual field) | ↓ Rod Sensitivity | ||
| Arden et al (1998)10 | CN = 25 (n = 25) DM = 4 (n = 4), ranging from mild background retinopathy to NPDR |
Ganzfeld Electroretinographic Stimulator (120 sec adapting to bright light, subtend 5 deg at 10 deg in the horizontal nasal field) | Impaired Dark-Adapted Response After 5 Minutes | ||
| Greenstein et al (1993)58 | CN = 20 (n = 20) DM = 18 (n = 18), ranging from no DR to NPDR with or without edema. |
Henson-Allen Dark Adaptometer (bleach by 1 min adapting to bright light and stimulating at 20 deg from fovea) | Impaired Dark-Adapted Response After 1400 Seconds | ||
| Henson and North (1979)69 | DM = 29 (n = 29), ranging from no DR to PDR | Henson-Allen Dark Adaptometer (bleach by 1 min adapting to bright light and stimulating at 20 deg from fovea) | Impaired Dark-Adapted Response | ||
CN, control; DR, diabetic retinopathy; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; PRP, pan-retinal photocoagulation.
Other studies, however, revealed less correlation between impaired dark adaptation response and DR. In one study, there was no relationship between the severity of retinopathy and dark adaptation speed.153 Other studies also found no consistent impairment in the rod function among people with diabetes, even in those with PDR.27,77,137 It is important to note that the eyes affected by diabetes tend to have larger variations in their dark adaptation responses, in which some may have severe deficits while others are grossly unaffected. The difference in these results may also be due to test-retest variability, but there is limited evidence on this topic. Another reason that could explain the inconsistency is the different methodologies used by the studies. For example, some of the studies relied on different DR classification systems and did not stratify comparison by the DR stage.10,20,69 Other reasons include using different dark adaptometry devices and testing protocols. For instance, Green-stein and colleagues used a modified Goldmann-Weekers Adaptometer, projecting 1.25° stimuli 15° temporal to the fovea and bleaching of one-second duration. In contrast, Jackson and others used the AdaptRx Dark Adaptometer (MacuLogix, Harrisburg, PA), projecting 2° stimuli 5° superior to the fovea and bleaching with a 2-millisecond flash.20,58,77 Other studies would use light bleaching that lasted several minutes instead of flash bleaching.10,69 Although dark adaptation can provide unique information about rod function and how it is affected by DR, most testing devices only target a single spot of the retina, neglecting other locations. DR affects the retina in a topographically random manner, so many of these tools will miss the visual impairment in the regions not tested.
7. Vision loss in whole
DR is a global disease of the retina, evident by the microvascular, neuronal, glial, and microglial cell pathologies. Its onset and progression are detectable through psychophysical tests, presenting as visual function deficits. These deficits can be measured with respect to visual acuity, contrast sensitivity, color vision, visual field, and dark adaptation. Some visual dysfunction can be observed prior to the development of detectable vasculopathy and then deteriorate with worsening retinopathy. However, most studies have not used sensitive methods such as optical coherence tomography angiography to exclude the presence of subclinical vascular lesions. Further, it is clear that vision becomes severely impaired in more advanced diseases, but the relationship between the severity of DR and the rate of deterioration in visual function is more complicated. For instance, some people with PDR still maintain normal visual acuity, whereas some people with NPDR have worse contrast sensitivity than those with more severe vascular retinopathy (Fig. 1). These observations suggest a nonlinear relationship between microvascular signs of DR and visual dysfunction. In addition to the obvious vascular changes on the fundus examination, subclinical alterations of retinal structure and visual pathways may contribute to the overall decline in visual function. For example, early-onset cataract induced by diabetes can diminish the visual signal reaching the photoreceptors.22,80 In the postretinal segment, diabetes can also impair the visual processing ability of the brain.28,101 All these pathological variables can influence the results of psychophysical tests in relation to diabetes.
Fig. 1 –
Patients with regressed PDR after PRP who had subnormal performance or patient-reported outcomes. The normal reference range was defined as the mean performance of the control group either plus or minus 2 SD, depending on which direction was considered to worsen performance. Red cells indicate abnormal performance. White cells indicate performance within the reference range. Black cells indicate patients not followed-up. (Reprinted with permission of Association of Research in Vision and Ophthalmology from Chen and Gardner. 37)
Most DR studies have conveniently used visual acuity as their primary outcome measurement, and sometimes, they are supplemented with another visual function metric, such as contrast sensitivity, color vision, visual fields, or dark adaptation. However, few studies have simultaneously interrogated multiple components of visual function to create a comprehensive view of the impaired vision. As discussed earlier in this review, each psychophysical test can add new information about the visual function and detect subtle visual deficits that may be missed by other assessments. Visual acuity, for example, only assesses the visual processing ability of the fovea, so it is useful when only interested in the central vision. It does not, however, examine the parafoveal or peripheral retina. In DR, the whole retina is affected, so only measuring visual acuity can miss the functional deficits resulting from peripherally occupying pathologies.50 In some cases, decreased visual acuity may suggest impairments in other psychophysical metrics, but these correlations are weak.37,45 Hence, it is important to examine multiple visual function assessments simultaneously to create a comprehensive understanding of vision loss in diabetes.27,38,59,81,124
DR results from the cumulative damage to the neurovascular unit of the retina. The extent of these damages, however, varies with individual patients and may affect distinct components of vision differently. For example, some patients may present with impaired contrast sensitivity but normal visual acuity. In other patients, their main complaint may be visual field or color vision impairment. These observations prompt the consideration of multi-modal assessments of vision in people with diabetes in future research. First, assessing multiple psychophysical tests in addition to visual acuity can better define the extent of vision impairment as the result of diabetes. This is especially true in people with diabetes but no detectable retinopathy, where metrics such as contrast sensitivity and color vision can detect early loss of vision prior to reduced visual acuity. Then, in more advanced stages of retinopathy, visual field and dark adaptation can identify functional deficits associated with rod dysfunction and other neurovascular degeneration in the peripheral retina. It is also useful to investigate whether early impairments can predict the development of vasculopathy and disease progression. Future diabetes management can monitor these metrics to decide when to initiate a more aggressive treatment plan to prevent worsening retinal pathologies. In addition, a more comprehensive assessment of vision may detect subtle loss or improvement not detected with a single measurement, which is usually done in clinical trials. In most trials related to DR, visual acuity has been the sole primary outcome measurement. This drawback can lead to misleading conclusions when the effect of the treatment is better visualized with other psychophysical metrics.59,124 It is possible that treatment may have varying effects on individuals and different components of vision. Second, multi-modal approaches can clarify the relationship between visual deficits and disruptions in the retinal structure. Although earlier studies demonstrated retinal changes are associated with deterioration in vision, they tend to show a weak correlation and use changes in individual layers of the retina to explain the loss of a single component of the vision.77,81,82 This approach may overlook the cumulative effects of retinal damage in multiple layers or the implication that distortion of a single retinal layer can impair multiple components of vision. In addition, anomalies on retinal imaging should be confirmed by functional tests to best assess clinically significant disease progression. It is still uncertain if structural defects in the retina precede or follow visual dysfunction in diabetes.
8. Novel approaches to Psychophysics
There are multiple methods within each type of visual function test, and their results may not be equivalent. For example, to assess visual acuity, the most common methods include the Snellen chart and the ETDRS chart. The Snellen chart is widely used in the clinical setting, whereas the ETDRS chart was specifically developed for research purposes and has become widely accepted in most clinical studies. Such discrepancy is also true for assessments of contrast sensitivity, color vision, visual field, and dark adaptation. Unfortunately, there is little evidence on which method best captures the deficit in each component of vision, and this should encourage further experimentation and comparison of psychophysical instruments to identify and develop the optimal assessments. The optimal tests should have the following criteria. First, it should be sensitive in detecting vision loss, so that it can reliably measure changes over time. Second, it should be repeatable and have minimal variation between retests. The method of administration should be standardized so that the results can be compared among different studies. Third, it should be accessible and cost-effective. The test should also be quick to administer for application in clinical use.
In recent years, novel approaches to assess visual function have been developed to address the limitations of current methods. One group has created a motion displacement test to assess visual fields using moving line stimuli displayed on a standard laptop computer.109,126 Compared to a standard perimeter, it is portable, easier to administer, and less confounded by the effect of media opacity or corrective error.99 Its test-retest variability is also less influenced by the effect of decreased sensitivity, while SAP results generally show increases in retest variability with decreasing sensitivity. Another approach is to exploit virtual reality technology. Hollander et al developed the Kasha visual field system that uses a virtual reality headset to create a portable automated perimeter.71 This device does not require patients to maintain a particular head position as the headset can adjust comfortably to their needs. It also reduces overall testing time by assessing both eyes during the same field examination. There are also several tablet-based apps developed to measure visual field loss as well as other aspects of visual function, such as visual acuity and contrast sensitivity.11,19,25,48,89,90,123 One team combined tablet computer with an inexpensive “clip-on” eye-tracker, which allows eye movements during testing and no button-pressing or head restraint is required.83 With this platform, the participant is required only to look at the tablet screen and follow a dot with their eye. The authors noted that this method is more suitable for discriminating diseased eyes from healthy eyes, and it is not ready to replace traditional SAP. In addition, a critical limitation of this device is its restricted visual range (within ±15° horizontal and ±9° vertical). Yet, the human peripheral visual field in a monocular vision extends approximately 100° temporally, 60° nasally, 60° upward, and 75° downward.135 Lastly, it is important to note that it is still unclear how robust these tools are when applied to a larger, more diverse cohort, especially in people with diabetes who also faced other disabilities that affect their mobility and function.
9. Electroretinography
We will not discuss electroretinography (ERG) even though many studies have used it to find visual deficits in people with diabetes. It requires specially trained examiners and expensive equipment. The test results can be variable between laboratories because of variations in test setting and instructions, as well as challenges in the analyses that may require subjective manipulations.24,34,60,78,79,102 The procedure is also time consuming as the subject may need to be light or dark-adapted, which can take up to 60 minutes depending on the protocols. These barriers make it less attractive to measure visual deficits in a clinical setting. Its complexity requires a separate review to cover the topic thoroughly.
10. Summary
The progression of diabetes complications in the retina is evident by microvascular changes on fundus examinations and visual deficits on psychophysical assessments. These findings demonstrate the notion that diabetes also leads to neurovascular degeneration. These function-based tools are useful to detect and monitor changes over time, in response to potential treatment and independent from the structural remodeling associated with diabetes. However, the current application of psychophysical tests has been limited. Most studies have relied exclusively on visual acuity to determine vision loss in diabetes. This approach fails to capture the extent of visual deficits during the early course of retinopathy, making it impossible to detect subtle changes in other components of vision overtime. Future DR studies should consider utilizing multiple visual function tests to assess the full extent of vision loss in central and peripheral vision resulting from diabetes. Additional markers of visual function will better define the evolution of the disease and set new goals in preserving vision in people with diabetes. They should also be integrated with retinal imaging to improve the current understanding of the relationship between neural and vascular lesions. This information is essential to develop novel therapeutic approaches to reverse the destructive nature of diabetes in the retina and the visual pathway. Thus, clinical tests of visual function have the potential to reduce the development and progression of DR. Indeed, the Food and Drug Administration recently stated its willingness to consider visual function tests as endpoints for clinical trials.105 This step should accelerate the development of functional endpoints in the evaluation of DR. To date, functional tests have been the primary endpoint in three therapeutic trials, and at least one additional trial is planned that will assess visual function as secondary outcomes (NCT04265261).124,125,127
11. Method of literature search
A literature search was conducted using PubMed and Google scholar for English language studies, and foreign language references when appropriate, on the topics of diabetic retinopathy, diabetes, visual function, psychophysical assessment, visual acuity, color vision, contrast sensitivity, visual field, and dark adaptation. Manuscript references were also used to find articles that evaluated the impacts of diabetes on visual function.
Acknowledgments
Dr. Chen was supported by the NIDDK Summer Research Program (P30DK020572). Dr. Gardner was supported by the National Institutes of Health (R01EY20582 and R24DK082841) and Research to Prevent Blindness.
Footnotes
Disclosures
Dr. Thomas Gardner has received grant support from Zebra Biologics.
REFERENCES
- 1.Abramoff MD, Fort PE, Han IC, Jayasundera KT, Sohn EH, Gardner TW. Approach for a clinically useful comprehensive classification of vascular and neural aspects of diabetic retinal disease. Invest Ophthalmol Vis Sci. 2018;59(1):519–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrishami M, Daneshvar R, Yaghubi Z. Short-wavelength automated perimetry in type I diabetic patients without retinal involvement: a test modification to decrease test duration. Eur J Ophthalmol. 2012;22(2):203–9 [DOI] [PubMed] [Google Scholar]
- 3.Afrashi F, Erakgiin T, Kose S, Ardic K, Mentes J. Blue-on-yellow perimetry versus achromatic perimetry in type 1 diabetes patients without retinopathy. Diabetes Res Clin Pract. 2003;61(1):7–11 [DOI] [PubMed] [Google Scholar]
- 4.Ahnelt PK, Kolb H, Pflug R. Identification of a subtype of cone photoreceptor, likely to be blue sensitive, in the human retina. J Comp Neurol. 1987;255(1):18–34 [DOI] [PubMed] [Google Scholar]
- 5.Akkaya S, Diizova S, Sahin O, Kazokoglu H, Bavbek T. National Eye Institute Visual Function Scale in type 2 diabetes patients. J Ophthalmol. 2016;2016:1549318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Shafaee M, Shenoy R, Bialasiewicz AA, Ganguly SS, Bhargava K. Macular function in prediabetic and diabetic Omani adults: a microperimetric evaluation. Eur J Ophthalmol. 2011;21(6):771–6 [DOI] [PubMed] [Google Scholar]
- 7.Anderson AJ, Johnston AW. Test/retest and inter-test agreement of color aptitude measures. Color Res Appl. 2015;40(3):224–31 [Google Scholar]
- 8.Andrade LCO, Souza GS, Lacerda EMCB, et al. Influence of retinopathy on the achromatic and chromatic vision of patients with type 2 diabetes. BMC Ophthalmol. 2014;14:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366(13):1227–39 [DOI] [PubMed] [Google Scholar]
- 10.Arden GB, Wolf JE, Tsang Y. Does dark adaptation exacerbate diabetic retinopathy? Evidence and a linking hypothesis. Vis Res. 1998;38(11):1723–9 [DOI] [PubMed] [Google Scholar]
- 11.Aslam TM, Parry NRA, Murray IJ, et al. Development and testing of an automated computer tablet-based method for self-testing of high and low contrast near visual acuity in ophthalmic patients. Graefes Arch Clin Exp Ophthalmol. 2016;254(5):891–9 [DOI] [PubMed] [Google Scholar]
- 12.Ayed S, Jeddi A, Kallal Z. Diabetes and color vision disorder detected by the Farnsworth 100 Hue test. Diabetic dyschromatopsia: J Fr Ophtalmol. 1990;13(10):506–10 [PubMed] [Google Scholar]
- 13.Banford D, North RV, Dolben J, Butler G, Owens DR. Longitudinal study of visual functions in young insulin dependent diabetics. Ophthalmic Physiol Opt. 1994;14(4):339–46 [PubMed] [Google Scholar]
- 14.Barber AJ. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(2):283–90 [DOI] [PubMed] [Google Scholar]
- 15.Barber AJ, Antonetti DA, Gardner TW. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. The Penn State Retina Research Group. Invest Ophthalmol Vis Sci. 2000;41(11):3561–8 [PubMed] [Google Scholar]
- 16.Barber AJ, Gardner TW, Abcouwer SF. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52(2):1156–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998;102(4):783–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bassi CJ, Galanis JC, Hoffman J. Comparison of the Farnsworth-Munsell 100-Hue, the Farnsworth D-15, and the L’Anthony D-15 desaturated color tests. Arch Ophthalmol. 1993;111(5):639–41 [DOI] [PubMed] [Google Scholar]
- 19.Bastawrous A, Rono HK, Livingstone IA, et al. Development and validation of a smartphone-based visual acuity test (peek acuity) for clinical practice and community-based fieldwork. JAMA Ophthalmol. 2015;133(8):930–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bavinger JC, Dunbar GE, Stem MS, et al. The effects of diabetic retinopathy and pan-retinal photocoagulation on photoreceptor cell function as assessed by dark adaptometry. Invest Ophthalmol Vis Sci. 2016;57(1):208–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Evid Base Eye Care. 2003;4(3):158–9 [DOI] [PubMed] [Google Scholar]
- 22.Becker C, Schneider C, Aballea S, et al. Cataract in patients with diabetes mellitus–incidence rates in the UK and risk factors. Eye. 2018;32(6):1028–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bengtsson B, Hellgren K-J, Agardh E. Test-retest variability for standard automated perimetry and short-wavelength automated perimetry in diabetic patients. Acta Ophthalmol. 2008;86(2):170–6 [DOI] [PubMed] [Google Scholar]
- 24.Birch DG, Hood DC, Locke KG, Hoffman DR, Tzekov RT. Quantitative electroretinogram measures of phototransduction in cone and rod photoreceptors: normal aging, progression with disease, and test-retest variability. Arch Ophthalmol. 2002;120(8):1045–51 [DOI] [PubMed] [Google Scholar]
- 25.Black J, Jacobs R, Phillips G, et al. An assessment of the iPad as a testing platform for distance visual acuity in adults. BMJ open. 2013;3(6):e002730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bodis-Wollner I Visual acuity and contrast sensitivity in patients with cerebral lesions. Science. 1972;178(4062):769. [DOI] [PubMed] [Google Scholar]
- 27.Boynton GE, Stem MS, Kwark L, Jackson GR, Farsiu S, Gardner TW. Multimodal characterization of proliferative diabetic retinopathy reveals alterations in outer retinal function and structure. Ophthalmology. 2015;122(5):957–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brands AMA, Biessels GJ, de Haan EHF, Kappelle LJ, Kessels RPC. The effects of type 1 diabetes on cognitive performance: A meta-analysis. Diabetes Care. 2005;28(3):726–35 [DOI] [PubMed] [Google Scholar]
- 29.Bresnick GH. Diabetic retinopathy viewed as a neurosensory disorder. Arch Ophthalmol. 1986;104(7):989–90 [DOI] [PubMed] [Google Scholar]
- 30.Bresnick GH, Davis MD, Myers FL, de Venecia G. Clinicopathologic correlations in diabetic retinopathy. II. Clinical and histologic appearances of retinal capillary microaneurysms. Arch Ophthalmol. 1977;95(7):1215–20 [DOI] [PubMed] [Google Scholar]
- 31.Bresnick GH, Korth K, Groo A, Palta M. Electroretinographic oscillatory potentials predict progression of diabetic retinopathy. Preliminary report. Arch Ophthalmol. 1984;102(9):1307–11 [DOI] [PubMed] [Google Scholar]
- 32.Bronte-Stewart JM, Cant JS, Craig JO. The detection of early visual loss in young diabetics. Proc R Soc Med. 1970;63(8):786–8 [PMC free article] [PubMed] [Google Scholar]
- 33.Brown SP, Masland RH. Spatial scale and cellular substrate of contrast adaptation by retinal ganglion cells. Nat Neurosci. 2001;4(1):44–51 [DOI] [PubMed] [Google Scholar]
- 34.Browning DJ, Lee C. Test-retest variability of multifocal electroretinography in normal volunteers and short-term variability in hydroxychloroquine users. Clin Ophthalmol. 2014;8:1467–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bull DR. Chapter 2 - The human visual system. In: Bull DR (ed) Communicating Pictures. Cambridge, MA, Academic Press; 2014, pp 17–61 [Google Scholar]
- 36.Casile A, Victor JD, Rucci M. Contrast sensitivity reveals an oculomotor strategy for temporally encoding space. Elife. 2019;8:e40924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen XD, Gardner TW. Patient-reported outcomes reveal impairments not explained by psychophysical testing in patients with regressed PDR. Transl Vis Sci Technol. 2019;8(4):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen XD, Omari A, Hwang M, et al. Treated PDR reveals age-appropriate vision deterioration but distorted retinal organization. Transl Vis Sci Technol. 2020;9(3):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho NC, Poulsen GL, Ver Hoeve JN, Nork TM. Selective loss of S-cones in diabetic retinopathy. Arch Ophthalmol. 2000;118(10):1393–400 [DOI] [PubMed] [Google Scholar]
- 40.Choi SS, Zawadzki RJ, Keltner JL, Werner JS. Changes in cellular structures revealed by ultra-high resolution retinal imaging in optic neuropathies. Invest Ophthalmol Vis Sci. 2008;49(5):2103–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cogan DG, Toussaint D, Kuwabara T. Retinal vascular patterns. IV. Diabetic retinopathy. Arch Ophthalmol. 1961;66:366–78 [DOI] [PubMed] [Google Scholar]
- 42.Color vision by Peter Gouras – Webvision. https://webvision.med.utah.edu/book/part-vii-color-vision/color-vision/. Accessed April 13, 2020
- 43.Craven BJ. A method for increasing the scoring efficiency of the Farnsworth-Munsell 100-Hue test. Ophthalmic Physiol Opt. 1997;17(2):153–7 [PubMed] [Google Scholar]
- 44.Cunha-Vaz JG. Studies on the pathophysiology of diabetic retinopathy. The blood-retinal barrier in diabetes. Diabetes. 1983;32(Suppl 2):20–7 [DOI] [PubMed] [Google Scholar]
- 45.Cusick M, SanGiovanni JP, Chew EY, et al. Central visual function and the NEI-VFQ-25 near and distance activities subscale scores in people with type 1 and 2 diabetes. Am J Ophthalmol. 2005;139(6):1042–50 [DOI] [PubMed] [Google Scholar]
- 46.Di Leo MA, Caputo S, Falsini B, et al. Nonselective loss of contrast sensitivity in visual system testing in early type I diabetes. Diabetes Care. 1992;15(5):620–5 [DOI] [PubMed] [Google Scholar]
- 47.van Dijk HW, Verbraak FD, Kok PHB, et al. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Invest Ophthalmol Vis Sci. 2010;51(7):3660–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dorr M, Lesmes LA, Lu Z-L, Bex PJ. Rapid and reliable assessment of the contrast sensitivity function on an iPad. Invest Ophthalmol Vis Sci. 2013;54(12):7266–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dosso AA, Yenice-Ustun F, Sommerhalder J, Golay A, Morel Y, Leuenberger PM. Contrast sensitivity in obese dyslipidemic patients with insulin resistance. Arch Ophthalmol. 1998;116(10):1316–20 [DOI] [PubMed] [Google Scholar]
- 50.Duh EJ, Sun JK, Stitt AW. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight. 2017;2(14):e93751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Engerman RL, Bloodworth JM. Experimental diabetic retinopathy in dogs. Arch Ophthalmol. 1965;73:205–10 [DOI] [PubMed] [Google Scholar]
- 52.Feitosa-Santana C, Paramei GV, Nishi M, Gualtieri M, Costa MF, Ventura DF. Color vision impairment in type 2 diabetes assessed by the D-15d test and the Cambridge colour test. Ophthalmic Physiol Opt. 2010;30(5):717–23 [DOI] [PubMed] [Google Scholar]
- 53.Fisher L, Skaff MM, Mullan JT, et al. Clinical depression versus distress among patients with type 2 diabetes: Not just a question of semantics. Diabetes Care. 2007;30(3):542–8 [DOI] [PubMed] [Google Scholar]
- 54.Fong DS, Barton FB, Bresnick GH. Impaired color vision associated with diabetic retinopathy: Early Treatment Diabetic Retinopathy Study Report No. 15. Am J Ophthalmol. 1999;128(5):612–7 [DOI] [PubMed] [Google Scholar]
- 55.Foote KG, Loumou P, Griffin S, et al. Relationship between foveal cone structure and visual acuity measured with adaptive optics scanning laser ophthalmoscopy in retinal degeneration. Invest Ophthalmol Vis Sci. 2018;59(8):3385–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gella L, Raman R, Kulothungan V, Pal SS, Ganesan S, Sharma T. Impairment of colour vision in diabetes with no retinopathy: Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetics Study (SNDREAMS-II, Report 3). PLoS One. 2015;10(6):e0129391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Georgakopoulos CD, Eliopoulou MI, Exarchou AM, Tzimis V, Pharmakakis NM, Spiliotis BE. Decreased contrast sensitivity in children and adolescents with type 1 diabetes mellitus. J Pediatr Ophthalmol Strabismus. 2011;48(2):92–7 [DOI] [PubMed] [Google Scholar]
- 58.Greenstein VC, Thomas SR, Blaustein H, Koenig K, Carr RE. Effects of early diabetic retinopathy on rod system sensitivity. Optom Vis Sci. 1993;70(1):18–23 [DOI] [PubMed] [Google Scholar]
- 59.Gross JG, Glassman AR, Liu D, et al. Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy. JAMA Ophthalmol. 2018;136(10):1138–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grover S, Fishman GA, Birch DG, Locke KG, Rosner B. Variability of full-field electroretinogram responses in subjects without diffuse photoreceptor cell disease. Ophthalmology. 2003;110(6):1159–63 [DOI] [PubMed] [Google Scholar]
- 61.Hamel CP. Cone rod dystrophies. Orphanet J Rare Dis. 2007;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hardy KJ, Lipton J, Scase MO, Foster DH, Scarpello JH. Detection of colour vision abnormalities in uncomplicated type 1 diabetic patients with angiographically normal retinas. Br J Ophthalmol. 1992;76(8):461–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harwerth RS, Carter-Dawson L, Shen F, Smith EL, Crawford M. Ganglion cell losses underlying visual field defects from experimental glaucoma. Invest Ophthalmol Vis Sci. 1999;40(10):2242–50 [PubMed] [Google Scholar]
- 64.Hawkins AS, Szlyk JP, Ardickas Z, Alexander KR, Wilensky JT. Comparison of contrast sensitivity, visual acuity, and humphrey visual field testing in patients with glaucoma. J Glaucoma. 2003;12(2):134–8 [PubMed] [Google Scholar]
- 65.Heijl A, Lindgren A, Lindgren G. Test-retest variability in glaucomatous visual fields. Am J Ophthalmol. 1989;108(2):130–5 [DOI] [PubMed] [Google Scholar]
- 66.Hellgren K-J, Agardh E, Bengtsson B. Progression of early retinal dysfunction in diabetes over time: results of a long-term prospective clinical study. Diabetes. 2014;63(9):3104–11 [DOI] [PubMed] [Google Scholar]
- 67.Hellgren K-J, Bengtsson B, Agardh E. Functional and structural change in diabetic eyes. Interim results from an ongoing longitudinal prospective study. Acta Ophthalmol. 2013;91(7):672–7 [DOI] [PubMed] [Google Scholar]
- 68.Henricsson M, Heijl A. Visual fields at different stages of diabetic retinopathy. Acta Ophthalmol (Copenh). 1994;72(5):560–9 [DOI] [PubMed] [Google Scholar]
- 69.Henson DB, North RV. Dark adaptation in diabetes mellitus. Br J Ophthalmol. 1979;63(8):539–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holfort SK, Jackson GR, Larsen M. Dark adaptation during transient hyperglycemia in type 2 diabetes. Exp Eye Res. 2010;91(5):710–4 [DOI] [PubMed] [Google Scholar]
- 71.Hollander D, Volpe N, Moster M, et al. Use of a portable head mounted perimetry system to assess bedside visual fields. Br J Ophthalmol. 2000;84(10):1185–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hou F, Lesmes LA, Kim W, et al. Evaluating the performance of the quick CSF method in detecting contrast sensitivity function changes. J Vis. 2016;16(6):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hovis JK, Ramaswamy S, Anderson M. Repeatability indices for the adams D-15 test for colour-normal and colour-defective adults. Clin Exp Optom. 2004;87(4–5):326–33 [DOI] [PubMed] [Google Scholar]
- 74.Hovis JK, Ramaswamy S, Anderson M. Repeatability indices for the Farnsworth D-15 test. Vis Neurosci. 2004;21(3):449–53 [DOI] [PubMed] [Google Scholar]
- 75.Ismail GM, Whitaker D. Early detection of changes in visual function in diabetes mellitus. Ophthalmic Physiol Opt. 1998;18(1):3–12 [PubMed] [Google Scholar]
- 76.Jackson GR, Owsley C, McGwin G. Aging and dark adaptation. Vis Res. 1999;39(23):3975–82 [DOI] [PubMed] [Google Scholar]
- 77.Jackson GR, Scott IU, Quillen DA, Walter LE, Gardner TW. Inner retinal visual dysfunction is a sensitive marker of nonproliferative diabetic retinopathy. Br J Ophthalmol. 2012;96(5):699–703 [DOI] [PubMed] [Google Scholar]
- 78.Jacobi PC, Miliczek K-D, Zrenner E. Experiences with the international standard for clinical electroretinography: normative values for clinical practice, interindividual and intraindividual variations and possible extensions. Doc Ophthalmol. 1993;85(2):95–114 [DOI] [PubMed] [Google Scholar]
- 79.Jacobi P, Walter P, Brunner R, Krieglstein G. Reproducibility and intraindividual variability of the pattern electroretinogram. German J Ophthalmol. 1994;3(4–5):216–9 [PubMed] [Google Scholar]
- 80.Javadi M-A, Zarei-Ghanavati S. Cataracts in diabetic patients: A review article. J Ophthalmic Vis Res. 2008;3(1):52–65 [PMC free article] [PubMed] [Google Scholar]
- 81.Joltikov KA, de Castro VM, Davila JR, et al. Multidimensional functional and structural evaluation reveals neuroretinal impairment in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017;58(6):BIO277–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Joltikov KA, Sesi CA, de Castro VM, et al. Disorganization of retinal inner layers (DRIL) and neuroretinal dysfunction in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2018;59(13):5481–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jones PR, Smith ND, Bi W, Crabb DP. Portable perimetry using eye-tracking on a tablet computer—A feasibility assessment. Transl Vis Sci Technol. 2019;8(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Katz G, Levkovitch-Verbin H, Treister G, Belkin M, Ilany J, Polat U. Mesopic foveal contrast sensitivity is impaired in diabetic patients without retinopathy. Graefes Arch Clin Exp Ophthalmol. 2010;248(12):1699–703 [DOI] [PubMed] [Google Scholar]
- 85.Kerrigan-Baumrind LA, Quigley HA, Pease ME, Kerrigan DF, Mitchell RS. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci. 2000;41(3):741–8 [PubMed] [Google Scholar]
- 86.Klein R, Klein BE, Moss SE. Visual impairment in diabetes. Ophthalmology. 1984;91(1):1–9 [PubMed] [Google Scholar]
- 87.Klein R, Moss SE, Klein BE, Gutierrez P, Mangione CM. The NEI-VFQ-25 in people with long-term type 1 diabetes mellitus: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 2001;119(5):733–40 [DOI] [PubMed] [Google Scholar]
- 88.Kleiner RC, Enger C, Alexander MF, Fine SL. Contrast sensitivity in age-related macular degeneration. Arch Ophthalmol. 1988;106(1):55–7 [DOI] [PubMed] [Google Scholar]
- 89.Kollbaum PS, Jansen ME, Kollbaum EJ, Bullimore MA. Validation of an iPad test of letter contrast sensitivity. Optom Vis Sci. 2014;91(3):291–6 [DOI] [PubMed] [Google Scholar]
- 90.Kong YXG, He M, Crowston JG, Vingrys AJ. A comparison of perimetric results from a tablet perimeter and Humphrey field analyzer in glaucoma patients. Transl Vis Sci Technol. 2016;5(6):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kurtenbach A, Mayser HM, Jagle H, Fritsche A, Zrenner E. Hyperoxia, hyperglycemia, and photoreceptor sensitivity in normal and diabetic subjects. Vis Neurosci. 2006;23(3–4):651–61 [DOI] [PubMed] [Google Scholar]
- 92.Lakowski R, Aspinall PA, Kinnear PR. Association between colour vision losses and diabetes mellitus. Ophthalmic Res. 1972;4(3):145–59 [Google Scholar]
- 93.Lesmes LA, Lu Z-L, Baek J, Albright TD. Bayesian adaptive estimation of the contrast sensitivity function: the quick CSF method. J Vis. 2010;10(3):17.1–17.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lobefalo L, Verrotti A, Mastropasqua L, et al. Blue-on-yellow and achromatic perimetry in diabetic children without retinopathy. Diabetes Care. 1998;21(11):2003–6 [DOI] [PubMed] [Google Scholar]
- 95.Loukovaara S, Harju M, Kaaja RJ, Immonen IJR. Topographic change in the central macula coupled with contrast sensitivity loss in diabetic pregnancy. Graefes Arch Clin Exp Ophthalmol. 2003;241(8):607–14 [DOI] [PubMed] [Google Scholar]
- 96.Maddess TL, Henry G. Performance of nonlinear visual units in ocular hypertension and glaucoma. Clin Vis Sci. 1992;7:371–83 [Google Scholar]
- 97.Maguire MG, Liu D, Glassman AR, et al. Visual field changes over 5 years in patients treated with panretinal photocoagulation or ranibizumab for proliferative diabetic retinopathy. JAMA Ophthalmol. 2020;138:285–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Malechka VV, Moiseyev G, Takahashi Y, Shin Y, Ma J-X. Impaired rhodopsin generation in the rat model of diabetic retinopathy. Am J Pathol. 2017;187(10):2222–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Membrev L, Fitzke FW. Effect of lens opacity on white-on-white perimetry, frequency doubling perimetry, and motion detection perimetry. Perimetry Update 2001;259–66 [Google Scholar]
- 100.Misra S, Saxena S, Kishore P, Bhasker SK, Misra A, Meyer CH. Association of contrast sensitivity with LogMAR visual acuity and glycosylated hemoglobin in non-insulin dependent diabetes mellitus. J Ocul Biol Dis Infor. 2010;3(2):60–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moheet A, Mangia S, Seaquist E. Impact of diabetes on cognitive function and brain structure. Ann N Y Acad Sci. 2015;1353:60–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mohidin N, Yap MKH, Jacobs RJ. The repeatability and variability of the multifocal electroretinogram for four different electrodes. Ophthalmic Physiol Opt. 1997;17(6):530–5 [PubMed] [Google Scholar]
- 103.Moloney J, Drury MI. Retinopathy and retinal function in insulin-dependent diabetes mellitus. Br J Ophthalmol. 1982;66(12):759–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Montesano G, Gervasoni A, Ferri P, et al. Structure-function relationship in early diabetic retinopathy: a spatial correlation analysis with OCT and microperimetry. Eye. 2017;31(6):931–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nair P, Aiello LP, Gardner TW, Jampol LM, Ferris FL. Report from the NEI/FDA diabetic retinopathy clinical trial design and endpoints workshop. Invest Ophthalmol Vis Sci. 2016;57(13):5127–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nittala MG, Gella L, Raman R, Sharma T. Measuring retinal sensitivity with the microperimeter in patients with diabetes. Retina. 2012;32(7):1302–9 [DOI] [PubMed] [Google Scholar]
- 107.Nomura R, Terasaki H, Hirose H, Miyake Y. Blue-on-yellow perimetry to evaluate S cone sensitivity in diabetics. Ophthalmic Res. 2000;32(2–3):69–72 [DOI] [PubMed] [Google Scholar]
- 108.Ong GL, Ripley LG, Newsom RSB, Casswell AG. Assessment of colour vision as a screening test for sight threatening diabetic retinopathy before loss of vision. Br J Ophthalmol. 2003;87(6):747–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ong E-L, Zheng Y, Aung T, et al. Performance of the moorfields motion displacement test for identifying eyes with glaucoma. Ophthalmology. 2014;121(1):88–92 [DOI] [PubMed] [Google Scholar]
- 110.Parravano M, Oddone F, Mineo D, et al. The role of Humphrey Matrix testing in the early diagnosis of retinopathy in type 1 diabetes. Br J Ophthalmol. 2008;92(12):1656–60 [DOI] [PubMed] [Google Scholar]
- 111.Pasmanter N, Munakomi S. Physiology, color perception. In: StatPearls. Treasure Island, FL, StatPearls Publishing; 2020 [PubMed] [Google Scholar]
- 112.Pinilla I, Ferreras A, Idoipe M, et al. Changes in frequency-doubling perimetry in patients with type I diabetes prior to retinopathy. Biomed Res Int. 2013;2013:341269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989;107(5):453–64 [DOI] [PubMed] [Google Scholar]
- 114.Ratnam K, Carroll J, Porco TC, Duncan JL, Roorda A. Relationship between foveal cone structure and clinical measures of visual function in patients with inherited retinal degenerations. Invest Ophthalmol Vis Sci. 2013;54(8):5836–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Realini T, Lai MQ, Barber L. Impact of diabetes on glaucoma screening using frequency-doubling perimetry. Ophthalmology. 2004;111(11):2133–6 [DOI] [PubMed] [Google Scholar]
- 116.Regan D, Raymond J, Ginsburg AP, Murray TJ. Contrast sensitivity, visual acuity and the discrimination of Snellen letters in multiple sclerosis. Brain. 1981;104(2):333–50 [DOI] [PubMed] [Google Scholar]
- 117.Richman J, Spaeth GL, Wirostko B. Contrast sensitivity basics and a critique of currently available tests. J Cataract Refract Surg. 2013;39(7):1100–6 [DOI] [PubMed] [Google Scholar]
- 118.Ross DF, Fishman GA, Gilbert LD, Anderson RJ. Variability of visual field measurements in normal subjects and patients with retinitis pigmentosa. Arch Ophthalmol. 1984;102(7):1004–10 [DOI] [PubMed] [Google Scholar]
- 119.Roth JA. Central visual field in diabetes. Br J Ophthalmol. 1969;53(1):16–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roy MS, McCulloch C, Hanna AK, Mortimer C. Colour vision in long-standing diabetes mellitus. Br J Ophthalmol. 1984;68(3):215–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sample PA. Short-wavelength automated perimetry: it’s role in the clinic and for understanding ganglion cell function. Prog Retin Eye Res. 2000;19(4):369–83 [DOI] [PubMed] [Google Scholar]
- 122.Schneck ME, Haegerstrom-Portnoy G, Lott LA, Brabyn JA. Comparison of panel D-15 tests in a large older population. Optom Vis Sci. 2014;91(3):284–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schulz AM, Graham EC, You Y, Klistorner A, Graham SL. Performance of iPad-based threshold perimetry in glaucoma and controls. Clin Exp Ophthalmol. 2018;46(4):346–55 [DOI] [PubMed] [Google Scholar]
- 124.Scott IU, Jackson GR, Quillen DA, et al. Effect of doxycycline vs placebo on retinal function and diabetic retinopathy progression in patients with severe nonproliferative or non-high-risk proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol. 2014;132(5):535–43 [DOI] [PubMed] [Google Scholar]
- 125.Scott IU, Jackson GR, Quillen DA, Klein R, Liao J, Gardner TW. Effect of doxycycline vs placebo on retinal function and diabetic retinopathy progression in mild to moderate nonproliferative diabetic retinopathy: a randomized proof-of-concept clinical trial. JAMA Ophthalmol. 2014;132(9):1137–42 [DOI] [PubMed] [Google Scholar]
- 126.Sharpe GP, Artes PH. Comparison of the moorfields motion displacement test to standard automated perimetry. Invest Ophthalmol Vis Sci. 2010;51(13):5500 [Google Scholar]
- 127.Simo R, Hernandez C, Porta M, et al. Effects of topically administered neuroprotective drugs in early stages of diabetic retinopathy: Results of the EUROCONDOR clinical trial. Diabetes. 2019;68(2):457–63 [DOI] [PubMed] [Google Scholar]
- 128.Simo R, Stitt AW, Gardner TW. Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia. 2018;61(9):1902–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Simonsen SE. Prognostic value of ERG (oscillatory potential) in juvenile diabetics. Acta Ophthalmol Suppl. 1974;123:223–4 [PubMed] [Google Scholar]
- 130.Simonsen SE. The value of the oscillatory potential in selecting juvenile diabetics at risk of developing proliferative retinopathy. Metab Pediatr Ophthalmol. 1981;5(1):55–61 [PubMed] [Google Scholar]
- 131.Simunovic MP. Acquired color vision deficiency. Surv Ophthalmol. 2016;61(2):132–55 [DOI] [PubMed] [Google Scholar]
- 132.Sohn EH, van Dijk HW, Jiao C, et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci U S A. 2016;113(19):E2655–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sohn EH, Han IC, Abramoff MD. Diabetic retinal neurodegeneration-should we redefine retinopathy from diabetes? JAMA Ophthalmol 2019; 10.1001/jamaophthalmol.2019.2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sommer A, Katz J, Quigley HA, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991;109(1):77–83 [DOI] [PubMed] [Google Scholar]
- 135.Spector RH. Visual fields. In: Walker HK, Hall WD, Hurst JW (eds) Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed., Boston, Butterworths; 1990 [PubMed] [Google Scholar]
- 136.Stamper R The effect of glaucoma on central visual function. Trans Am Ophthalmol Soc. 1984;82:792. [PMC free article] [PubMed] [Google Scholar]
- 137.Stem MS, Dunbar GE, Jackson GR, Farsiu S, Pop-Busui R, Gardner TW. Glucose variability and inner retinal sensory neuropathy in persons with type 1 diabetes mellitus. Eye (Lond). 2016;30(6):825–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tan NC, Yip WF, Kallakuri S, Sankari U, Koh YLE. Factors associated with impaired color vision without retinopathy amongst people with type 2 diabetes mellitus: a cross-sectional study. BMC Endocr Disord. 2017;17:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Trick GL, Burde RM, Gordon MO, Santiago JV, Kilo C. The relationship between hue discrimination and contrast sensitivity deficits in patients with diabetes mellitus. Ophthalmology. 1988;95(5):693–8 [DOI] [PubMed] [Google Scholar]
- 140.Trick GL, Trick LR, Kilo C. Visual field defects in patients with insulin-dependent and noninsulin-dependent diabetes. Ophthalmology. 1990;97(4):475–82 [DOI] [PubMed] [Google Scholar]
- 141.U.S. Department of Transportation - National Highway Traffic Safety Administration (NHTSA) - Model Driver Spreening and Evaluation Program: Final Technical Report - Guidelines for Motor Vehicle Administrators - Appendix B. License Renewal Requirements - DOT HS 809 581, May 2003. https://icsw.nhtsa.gov/people/injury/olddrive/modeldriver/3_app_b.htm. Accessed March 10, 2020. [Google Scholar]
- 142.Utku D, Atmaca LS. Farnsworth-Munsell 100-hue test for patients with diabetes mellitus. Ann Ophthalmol. 1992;24(6):205–8 [PubMed] [Google Scholar]
- 143.Verma A, Rani PK, Raman R, et al. Is neuronal dysfunction an early sign of diabetic retinopathy? Microperimetry and spectral domain optical coherence tomography (SD-OCT) study in individuals with diabetes, but no diabetic retinopathy. Eye (Lond). 2009;23(9):1824–30 [DOI] [PubMed] [Google Scholar]
- 144.Victor JD. Evaluation of poor performance and asymmetry in the Farnsworth-Munsell 100-hue test. Invest Ophthalmol Vis Sci. 1988;29(3):476–81 [PubMed] [Google Scholar]
- 145.Wall M, Doyle CK, Zamba KD, Artes P, Johnson CA. The repeatability of mean defect with size III and size V standard automated perimetry. Invest Ophthalmol Vis Sci. 2013;54(2):1345–51 [DOI] [PubMed] [Google Scholar]
- 146.Wall M, Woodward KR, Doyle CK, Artes PH. Repeatability of automated perimetry: a comparison between standard automated perimetry with stimulus size III and V, matrix, and motion perimetry. Invest Ophthalmol Vis Sci. 2009;50(2):974–9 [DOI] [PubMed] [Google Scholar]
- 147.Willis JR, Doan QV, Gleeson M, et al. Vision-related functional burden of diabetic retinopathy across severity levels in the United States. JAMA Ophthalmol. 2017;135(9):926–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wisznia KI, Lieberman TW, Leopold IH. Visual fields in diabetic retinopathy. Br J Ophthalmol. 1971;55(3):183–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wolff BE, Bearse MA, Schneck ME, et al. Color vision and neuroretinal function in diabetes. Doc Ophthalmol. 2015;130(2):131–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.World Health Organization. Global Report on Diabetes. Geneva, World Health Organization; 2016 [Google Scholar]
- 151.Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross JG, Glassman AR, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: A randomized clinical trial. JAMA. 2015;314(20):2137–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yau JWY, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zetterstrom B, Gjotterberg M. Photocoagulation in diabetic retinopathy with special reference to its effect on dark adaptation. Acta Ophthalmol (Copenh). 1973;51(4):512–9 [DOI] [PubMed] [Google Scholar]
- 154.Zhu T, Ma J, Li Y, Zhang Z. Association between retinal neuronal degeneration and visual function impairment in type 2 diabetic patients without diabetic retinopathy. Sci China Life Sci. 2015;58(6):550–5 [DOI] [PubMed] [Google Scholar]
- 155.Zico OA, El-Shazly AA-F, Ahmed EEA-H. Short wavelength automated perimetry can detect visual field changes in diabetic patients without retinopathy. Indian J Ophthalmol. 2014;62(4):383–7 [DOI] [PMC free article] [PubMed] [Google Scholar]