Abstract
Amylosucrase (ASase, E.C. 2.4.1.4) is a powerful transglycosylation enzyme that can transfer glucose from sucrose to the hydroxyl (-OH) group of various compounds. In this study, recombinant ASases from Deinococcus geothermalis (DgAS) and Bifidobacterium thermophilum (BtAS) were used to synthesize biosurfactants based on the computational analysis of predicted docking simulations. Successful predictions of the binding affinities, conformations, and three-dimensional structures of three surfactants were computed from receptor-ligand binding modes. DgAS and BtAS were effective in the synthesis of biosurfactants from glyceryl caprylate, glyceryl caprate, and polyglyceryl-2 caprate. The results of the transglycosylation reaction were consistent for both ASases, with glyceryl caprylate acceptor showing the highest concentration, as confirmed by thin layer chromatography. Furthermore, the transglycosylation reactions of DgAS were more effective than those of BtAS. Among the three substrates, glyceryl caprylate glycoside and glyceryl caprate glycoside were successfully purified by liquid chromatography–mass spectrometry (LC–MS) with the corresponding molecular weights.
Keywords: Amylosucrase, Biosurfactant, Docking simulation, Glyceryl glycoside, Transglycosylation
Introduction
In the age of rising global industrialization, as well as its consequences, there has been a ubiquitous desire to explore renewable and sustainable resources, processes, and products. Biosurfactants were developed owing to this need and have become a relatively environmentally safe alternative to the use of chemical surfactants. Surfactants are amphipathic compounds that possess both hydrophilic and hydrophobic components that partition and accumulate between fluid phases of varying polarity. Their properties include reducing surface and interfacial tension, making them suitable for use as detergents, emulsifiers, wetting agents, foaming agents, and dispersants (Sarkar et al. 1989; Sobrinho et al. 2013). The most commonly produced surfactants to date are derived from petroleum. The use of these synthetic surfactants leads to pollution in the form of both byproducts and remnants of the surfactants, which are hazardous to the environment and living organisms, including humans (Fenibo et al. 2019). The growing regard for environmentally safe alternatives began in recent decades, with interest drawn particularly to biosurfactants due to their low toxicity, high biodegradability, wide pH range, and other beneficial properties (Pacwa-Płociniczak et al. 2011). Biosurfactants are categorized based on their microorganism of origin and chemical composition. They are generally classified as glycolipids, polymeric surfactants, lipopeptides, fatty acids, particulate surfactants, or phospholipids (Banat et al. 2010; Rahman and Gakpe 2008). Typically, they are metabolites derived from microorganisms, with a basic amphiphilic form composed of a hydrophobic moiety as a long-chain, hydroxy, or alpha-alkyl-β-hydroxy fatty acid, in addition to a hydrophilic moiety present as a carbohydrate, amino acid, cyclic peptide, phosphate, carboxylic acid, or alcohol (Kosaric and Sukan, 2010). Nonionic surfactants have been suggested to enhance enzymatic activity (Shome et al. 2007) and improve the hydrolytic potential of enzyme-aided production of fermentable sugars from lignocellulosic biomasses (Alhammad et al. 2018). The development of a nontoxic, environmentally friendly food-grade surfactant would increase the limited number of surfactants obtained from natural products that are currently available in the food, pharmaceutical, and household industries. The most common sugar-based surfactants are alkyl polyglycosides, sorbitan esters, and sucrose esters. Moreover, given the global abundance of sucrose, many countries allow the use of sucrose esters as food additives (Ruiz 2008). Glyceride glucosides, as sugar-based surfactants, are inexpensive, have optimal surface activity, and can also be produced from renewable resources, but they are conventionally synthesized by lipases (Soultani et al. 2003).
Amylosucrase (ASase, EC 2.4.1.4) is a multifunctional enzyme known for its role in the processes of transglycosylation, polymerization, and isomerization using sucrose as its sole substrate. Among these ASase activities, the transglycosylation reaction can effectively transfer glucose from sucrose, as a donor molecule, to the hydroxyl (-OH) group of various compounds, as an acceptor molecule (Seo et al. 2020). Among various microorganisms, Bifidobacterium thermophilum and Deinococcus geothermalis are bacteria capable of producing ASase that possesses unique properties, including relatively high thermostability at 50 °C and improved polymerization. B. thermophilum ASase (BtAS) is notable for having the longest half-life at 50 °C, in comparison to other ASases. The bacterium itself is generally regarded as safe and has potential applications in the food industry as a probiotic (Picard et al. 2005). D. geothermalis ASase (DgAS) is not only relatively thermostable but, compared to several other microbial ASases, is also capable of catalyzing transglycosylation reactions for a broad range of acceptor molecules (Seo et al. 2020). The transglycosylation yield of DgAS is approximately 76%, while that of BtAS is approximately 63% (Emond et al. 2008; Kim et al. 2020). High thermostability and high transglycosylation yield of these enzymes confer them advantages as agents of industrial production of biosurfactants. The biosynthesis of nonionic surfactant glycosides by ASase could follow different possibilities in the production of novel biosurfactants, including de novo synthesis of hydrophilic and hydrophobic segments and their subsequent linkage; synthesis of hydrophilic segments and substrate-dependent formation of hydrophobic fragments and subsequent linkage of the two; and synthesis of the hydrophobic segment and substrate-dependent formation of the hydrophilic fragment and its ensuing amalgamation (Kosaric and Sukan 2010).
In this study, the capacity of DgAS and BtAS to synthesize glyceride glycosides from glycerides and sucrose was evaluated. In addition, the structural model of DgAS was generated to demonstrate the manner in which the transglycosylation reaction is facilitated by the enzyme and to ascertain the reason behind the different activities displayed by the enzyme toward substrates with similar structures.
Materials and methods
Chemicals
Crystallized sucrose was purchased from Duchefa Biochemistry (Haarlem, Netherlands). A nickel-nitrilotriacetic acid affinity column (Poly-prep; Bio-Rad Laboratories, Inc., Hercules, CA, USA) with nickel-nitrilotriacetic acid (Ni–NTA) Superflow (Qiagen, Hilden, Germany) was used for the purification of 6 × histidine-tagged recombinant proteins. TLC silica gel 60 F254 25 glass plates (Merck, Darmstadt, Germany) were used for thin layer chromatography (TLC). All other chemicals used were analytical-grade reagents obtained from Sigma-Aldrich Chemical Company (St. Louis, MO, USA).
Expression and purification of recombinant DgAS and BtAS
Recombinant Escherichia coli BL21 harboring pETBtAS (E. coli pETBtAS) and E. coli MC1061 harboring pHCDgAS (E. coli pHCDgAS) was used to express BtAS and DgAS, respectively, according to previous studies (Kim et al. 2020; Seo et al. 2012). The E. coli pHCDgAS cells were cultured in 1 L of Luria–Bertani (LB) medium supplemented with ampicillin (100 μg/mL) at 37 °C with agitation for 18 h. E. coli BL21 pETBtAS was cultured in 1 L of LB medium containing 100 μg/mL ampicillin at 37 °C. At an OD600 value of 0.5–0.6, isopropyl-beta-D-thiogalactoside (IPTG) was added up to a final concentration of 0.5 mM, and the cells were cultured at 18 °C for 20 h to induce protein expression. The cultured cells were harvested by centrifugation (Hanil Combi 514R; Hanil Centrifuge Co., Gimpo, Korea) at 3000 × g for 20 min, following which the supernatant was discarded. Subsequently, the pellet was resuspended in lysis buffer (50 mM NaH2PO4, 300 mM NaCl, and 10 mM imidazole; pH 7.5) and disrupted using an iced ultrasonic bath (Sonifier 450; Branson Ultrasonics Crop., Danbury, CT, USA). Protein extracted from the solution was collected by centrifugation at 10,000 × g for 20 min at 4 °C. Enzymes in the crude cell extract were purified using an Ni–NTA affinity column. The protein obtained from the eluted fraction was dialyzed to remove imidazole, and the enzyme activity was measured before performing further experiments.
Determination of enzymatic properties
Bicinchoninic acid (BCA) assay was used to quantify protein concentration. The BCA protein assay kit (Thermo Fisher Scientific, Agawam, MA, USA) was used with bovine serum albumin as a standard. The sucrose hydrolysis activities of DgAS and BtAS were measured using 3,5-dinitrosalicylic acid (DNS) solution, as described previously (Kim et al. 2019a). A reaction mixture comprising 250 μL 100 mM optimal buffer, 100 μL 25% sucrose, and 100 μL deionized water was used for this purpose, and the enzymatic reaction was initiated by adding 50 μL of enzyme solution to the reaction mixture at 45 °C for 10 min. To stop the reaction, 500 μL of DNS solution was added to the mixture. After boiling for 5 min, the absorbance of the final reaction mixture was measured at 550 nm using a microplate reader (iMark™ Microplate Absorbance Reader; Bio-Rad Laboratories, Inc.). The reducing sugar concentration was calculated using a fructose standard curve. One unit of enzyme was defined as the amount of enzyme that catalyzed 1 μmol of fructose per min under the assay conditions.
Molecular dynamics and docking simulations
The structure of glyceryl caprate (PubChem CID: 92,926), glyceryl caprylate (CID: 3,033,877), and polyglyceryl-2 caprate (CID: 9,905,161) were downloaded from the PubChem Compound database (Kim et al. 2019b). The structure of polyglyceryl-3 stearate was generated from its SMILES notation using the OpenBabel software (version 2.4.1) (Open Babel development team and NextMove Software, Cambridge, UK) (O'Boyle et al. 2011). Subsequently, the structural models of these glycerides were converted into PDBQT (Protein Data Bank, Partial Charge (Q), & Atom Type (T)) format using the AutoDockTools4 script prepare_ligand4.py (Morris et al. 2009). The ensemble structure of DgAS was generated as described in a previous study (Hong et al. 2020). Briefly, the structure of DgAS (Protein Data Bank [PDB] ID: 3UER) was downloaded from the PDB (Guérin et al. 2012). Its structure was further relaxed by a 5 ns molecular dynamics simulation at 300 K using GROMACS suite (Groningen Machine for Chemical Simulations, version 2020) (Abraham et al., 2015), and the conformations were sampled from the trajectory at 50 ps intervals. The simulation was repeated 5 times, and 505 conformations were collected as the structural ensemble of DgAS. Each ligand was docked to each conformation of the DgAS ensemble using AutoDock Vina (version 1.1.1) (Trott and Olson, 2010). The ligand was docked into an isotropic cubic box with a length of 40 Å, the center of which lay between glutamate 326 and aspartate 284. Up to 20 conformations were sampled during each docking simulation.
Analysis of the reactive conformation in docking simulations
A docking pose is considered reactive when the hydroxyl group of a ligand is placed at the active site satisfying the following conditions: First, the distance between the oxygen atom of the hydroxyl group and reactive carbon of the glucose moiety should be less than 4 Å (Distance 1, d1). Second, the distance between the oxygen atom of the hydroxyl group and any of the carboxylic oxygen atoms of glutamate 326 should be less than 4 Å (Distance 2, d2). Finally, the angle between the reactive carbon of the glucose moiety, oxygen atom of the hydroxyl group, and carbon where the hydroxyl group is attached should be more than 100° and less than 140° (Angle 1, a1). The geometric features described above are illustrated in Fig. 1A.
Fig. 1.
Glyceryl caprylate on DgAS. A Illustration of the chamber-like structure surrounding the active site. B Glyceryl caprylate bound to the chambers. C The structure of glyceryl caprylate. D The geometric features of docking poses. The geometry of reactive conformation is demarcated by orange boxes and the number of reactive conformations is noted above each box
Biosynthesis of glyceride glycosides by ASase
Transglycosylation reactions by DgAS and BtAS were carried out under optimal conditions in 50 mM Tris–HCl (pH 8.0) and 50 mM sodium acetate buffers (pH 6.0), respectively. The reactions were performed at 40 °C instead of 45 °C because the transglycosylation activity of DgAS and BtAS was similar to that of other ASases, which are known to have a higher transglycosylation activity at lower temperatures (Seo et al. 2009). Ten types of surfactants (polyglyceryl-10 caprylate/caprate, polyglyceryl-10 dipalmitate, polyglyceryl-10 oleate, polyglyceryl-10 decaoleate, polyglyceryl-3 stearate, polyglyceryl-10 stearate, polyglyceryl-3 polyricinoleate, glyceryl caprate, glyceryl caprylate, and polyglyceryl-2 caprate) were used as acceptor molecules, and 100 µL of 10 U/mL of either DgAS or BtAS was added to 900 µL of substrate solution containing 0.2% sucrose as a glucosyl donor, 0.6% of surfactants as an acceptor, and 50 mM optimal buffer. The mixture was reacted at 40 °C for 24 h and stopped by heating in boiling water for 10 min. The reaction products were subsequently analyzed by TLC and liquid chromatography-mass spectrometry (LC/MS).
Isolation and purification of glyceride glycosides in TLC analysis
The synthesis of glyceride glycosides was confirmed by TLC analysis. TLC strips (12 cm × 10 cm) of F254 25 glass plates were spotted on the bottom with 10 µL samples in a cuboidal TLC developing tank (25 cm × 24 cm × 16 cm; L × H × W) maintained d at 25 °C tank was presaturated with an isopropyl alcohol/ethyl acetate/water (3:1:1; v/v/v) mobile phase. The developed plates were air-dried and sprayed with 0.3% (w/v) 1-naphthol and 5% (v/v) H2SO4 in methanol for detection. To purify the glyceride glycoside, the TLC analysis method described above was slightly modified. Approximately 200 µL of the enzyme reactant was loaded onto each point of a TLC silica gel 60 F254 25 glass plate (10 cm × 10 cm). The glass plate was irrigated with a 3:1:1 (v/v/v) mixture of isopropyl alcohol/ethyl acetate/water for 2 h in a cuboidal TLC tank and following ascending development, the plate was air-dried. The spot on the plate was detected using a reagent containing 1-naphthol and H2SO4 in methanol to verify the separation of the reaction product. The bands corresponding to the glyceride glycosides were marked, and the silica was scraped off of the glass plate to separate them. Deionized water was added to each separated silica gel from the plate and shaken at 37 °C for 24 h to elute the transglycosylation product. Subsequently, the eluted compounds were collected by centrifugation at 10,000 × g for 1 min and used for further experiments.
Molecular weight determination of purified glyceride glycosides
The samples were analyzed by LC/MS on an Agilent Technologies 1200 Series HPLC system using a G1312B binary pump SL (Agilent, Santa Clara, CA, USA). Isolated biosurfactants (5 μL) were loaded on an Agilent column-SL (50 × 2.1 mm; 2 μm particle size) and detected at 265 nm using an Agilent variable wavelength detector SL. The mobile phase used consisted of the following: (A) water acidified with 0.1% formic acid and (B) acetonitrile with 0.1% formic acid, at a flow rate of 0.5 mL/min. The gradient program was started at 5% B over 1 min, increased to 100% B over 9 min, and maintained at 100% B for 1 min. Subsequently, it was decreased to 5% B over 3 min and maintained for 6 min. Mass spectrometry was performed using the following parameters: capillary voltage, -4 kV; desolvation gas flow rate, 11 L/min; and source temperature, 300 °C. Moreover, full-scan data acquisition was performed by scanning from m/z 100 to 1,000 in the profile mode. Furthermore, single ion recording was performed in the positive mode.
Ethics statement
This article does not contain any studies with human or animal subjects performed by any of the authors.
Results and discussion
Molecular dynamics and docking simulations of glyceride compounds from DgAS
Macromolecular docking, which is a type of bioinformatics modeling, encompasses the use of computational simulations to predict the structural models of compounds formed from the interaction of two or more biological macromolecules (Sousa et al. 2006). Through estimates computed in the receptor-ligand binding modes, successful predictions of the binding affinities, conformations, and three-dimensional structure of the complex can be produced. The ASase template used for docking modeling was DgAS (PDB: 3ucq), whose three-dimensional structure was revealed (Guérin et al. 2012). However, BtAS was excluded from the docking modeling as its 3D structure was not revealed. Modeling of the binding structure was performed on the 10 sets of ligands as an acceptor molecule along with DgAS, and the binding structure of four ligands was finalized (Table 1). Compounds containing polyglyceryl-10 were excluded because of the markedly high flexibility of the glyceryl group, making it time-consuming to calculate the docking simulation. Docking simulation results predicted that glyceryl caprylate, glyceryl caprate, polyglyceryl-2 caprate, and polyglyceryl-3 stearate would be reactive to the enzyme. In the case of polyglyceryl-10 caprylate/caprate, the binding positions of neither caprylate nor caprate were specified, and they appeared as a mixture. Some of the molecules within the mixture were similar in form to the terminal section of the glyceryl caprylate structure, which was expected to cause their participation in the simulated response.
Table 1.
List of glyceride substrates run through docking simulation
| Index | Name | PubChem CID | Description |
|---|---|---|---|
| 1 | Polyglyceryl-10 caprylate/caprate | N/A | Exclude a,b |
| 2 | Polyglyceryl-10 dipalmitate | N/A | Exclude a,b |
| 3 | Polyglyceryl-10 oleate | 9,963,243 | Fail b |
| 4 | Polyglyceryl-10 decaoleate | N/A | Exclude a,b |
| 5 | Polyglyceryl-3 stearate | N/A | Success (SMILE, OpenBabel) c |
| 6 | Polyglyceryl-10 stearate | N/A | Exclude b |
| 7 | Polyglyceryl-3 polyricinoleate | N/A | Exclude a |
| 8 | Glyceryl caprate | 92,926 | Success |
| 9 | Glyceryl caprylate | 3,033,877 | Success |
| 10 | Polyglyceryl-2 caprate | 9,905,161 | Success c |
aThe substance was a mixture of several molecules, with a nonspecific location of the fatty acid, forcing the simulation to be put on hold. bThe highly flexible form of polyglyceryl-10 required a longer computational period for calculating docking simulations that were interrupted, possibly due to limited information on the model. c In the absence of stereoscopic structures, 3D computation was created using OpenBabel
For structural insights into glyceride binding on DgAS, glycerides were docked onto the structural ensemble of DgAS as described in a previous study (Hong et al. 2020). DgAS has two chamber-like structures around the enzymatic active site, where the minor one is rather narrow and enclosed by the enzyme, while the larger one is exposed to solvent (Fig. 1A). The docking model showed that glyceryl caprylate can bind to both these chambers (Fig. 1B). Furthermore, the population of docking poses that place the hydroxyl group at the active site in a reactive conformation were evaluated (Fig. 1A), and 203 and 229 docking poses were found for the two hydroxyl groups of glyceryl caprylate (Fig. 1C, 1D). These results suggest that glucose can be transferred to glyceryl caprylate, and its two hydroxyl groups can both be involved in the reaction, although the one closer to the fatty acid moiety is more likely to be modified. The conformation of a docking pose is assumed to be reactive when the geometry of the hydroxyl group satisfies two distance conditions, Distance 1(d1) and Distance 2(d2), and an angle condition, Angle 1(d1), as described in the Materials and Methods (Fig. 1A). In a previous study, (2S)-1-O-α-d-glucosyl-glycerol, (2R)-1-O-α-d-glucosyl-glycerol, and 2-O-α-d-glucosyl-glycerol were produced when the transglycosylation reaction for glycerol as an acceptor molecule was performed by the ASase obtained from Methylobacillus flagellatus KT (MfAS) (Jeong et al. 2014). Therefore, it can be assumed that ASase transfers glucose from sucrose to the hydroxyl group of glycerides as an acceptor molecule.
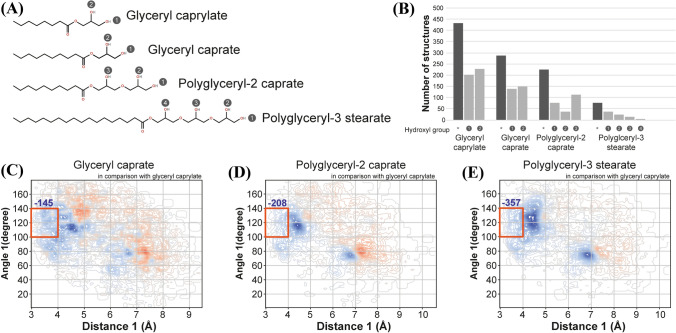
To investigate the substrate selectivity of DgAS, the number of reactive conformations in each glyceride was calculated. The number of reactive conformations was the highest for glyceryl caprylate, followed by glyceryl caprate, polyglyceryl-2 caprate, and polyglyceryl-3 stearate (Fig. 2A, B). Glyceryl caprylate exhibited a highly ‘cramped’ geometry, wherein its hydroxyl oxygen was placed near the reactive carbon of glucose moiety compared to the glycerides (Fig. 2C, D, E). The geometric features of the population of glyceryl caprate showed an increased docking distance of 5 Å, which suggested that its hydroxyl group was moved slightly away from the reactive site (Fig. 2C). As illustrated in Fig. 2B, both chambers would have limited volume, and the lengthened aliphatic chain in glyceryl caprate compared to that of glyceryl caprylate would limit the placement of its hydroxyl groups at the active site. Polyglyceryl-2 caprate has an additional hydroxyl group, where the glucose can be transferred. However, its docking results suggested that the extended glyceryl structure reduced the reactivity of the ligand (Fig. 2D). The hydroxyl group closest to the fatty acid moiety was predicted to be the most reactive (Fig. 2B), suggesting that the binding of the aliphatic chain to the enzyme would place a hydroxyl group at the active site. Polyglyceryl-3 stearate showed the least poses that enabled the reaction (Fig. 2E), which is likely due to its longer aliphatic chain and longer glyceryl group that may limit its access to the substrate binding chamber. The results predicted by simulation are concomitant with the results of the transglycosylation reaction for glycerol by MfAS (Jeong et al. 2014).
Fig. 2.
Reactive binding modes of glycerides. A The structure of glycerides. Each hydroxyl group is marked by a circled number according to its position. B The number of structures with reactive conformation for each hydroxyl group is marked by the circled numbers, while that for entire sites is marked by asterisks. C, D, E The changes in geometric feature distribution of glycerides compared to those of glyceryl caprylate
Biosynthesis of glyceride glycoside using recombinant DgAS and BtAS
DgAS and BtAS were successfully expressed in E. coli strains harboring pHCDgAS (Seo et al. 2012) and pETBtAS (Kim et al. 2020), respectively. The expression of dgas in recombinant E. coli MC1061 was performed with the constitutive expression system, while btas was expressed in recombinant E. coli BL21 following induction with IPTG at a final concentration of 1 mM. DgAS and BtAS fused with 6 × histidine appeared as a single band with a molecular mass of approximately 73 kDa and 66 kDa, respectively, by SDS-PAGE, which was consistent with the estimated molecular mass of the enzymes. The production level of DgAS, reported as enzyme activity, was 33.5 ± 0.8 U/mL, with a protein concentration of 3.5 mg/mL, while the enzyme activity of BtAS was 28.6 ± 0.15 U/mL, with a protein concentration of 18.2 mg/mL. Both enzymes were diluted and calibrated to enzymatic activities of 1 U/mL and subsequently used in transglycosylation reaction experiments.
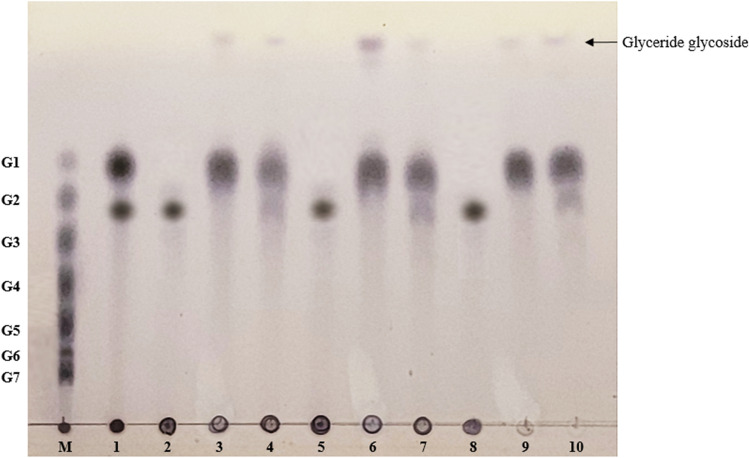
Following the confirmation of ASase functionality and expected formational structures from the docking simulations, glycosylated glycerides were enzymatically synthesized through the intermolecular transglycosylation activity of ASases (Jeong et al. 2014). Transglycosylation activities of DgAS and BtAS were confirmed with sucrose as a donor molecule and glyceryl caprate, glyceryl caprylate, and polyglyceryl-2 caprate as acceptor molecules. The main spots in the TLC analysis with DgAS and BtAS included glucose, fructose, and maltooligosaccharides, as these were the primary products expected from a typical ASase reaction with sucrose and various acceptor molecules (Cho et al. 2011; Jung et al. 2009; Kim et al. 2016; Seo et al. 2009). Unidentified spots appeared at the top, as was expected of transglycosylated surfactants (Fig. 3). In the TLC developing solvent used in this analysis, hydrophobic substances, which were the acceptor molecules, did not appear, but transglycosylated products presented monosaccharides such as glucose and fructose (Cho et al. 2011; Kim et al. 2016; Seo et al. 2012). Therefore, the unidentified spots in TLC analysis generated through the transglycosylation reaction of glyceride with DgAS and BtAS were concluded to be glyceride glycosides to which the glucose from sucrose was transferred. Among the three transglycosylated glycerides, the concentration of glycosylated glyceryl caprylate was the highest, followed by transglycosylated glyceryl caprate. Consistent with the computational simulations—where the number of reactive conformations was the highest for glyceryl caprylate, followed by glyceryl caprate, polyglyceryl-2 caprate, and polyglyceryl-3 stearate—the transglycosylation reaction products determined from the TLC analysis confirmed the synthesis of biosurfactants from these three compounds. These observations were consistent for both DgAS and BtAS, with the highest concentration occurring in the glyceryl caprylate acceptor. A comparison of the results of the reactants with DgAS and BtAS in the TLC analysis indicated that the reactant with DgAS had a lower concentration of sucrose than the reactant with BtAS (Fig. 3). In addition, the concentration of glycosylated surfactants synthesized with DgAS was higher than that of the biosurfactants produced by BtAS. This result indicated that the sucrose hydrolysis and transglycosylation activities of DgAS were better than those of BtAS, as revealed in previous studies (Emond et al. 2008; Kim et al. 2020), such that the concentration of the nonionic surfactants synthesized with DgAS was higher than that of BtAS. Since the substrate binding site of DgAS was structurally wider than that of NpAS (ASase from Neisseria polysaccharea), DgAS might be able to conduct a wider range of transglycosylation reactions using various acceptor molecules (Seo et al. 2016, 2020). However, DgAS produces an amylose-like polymer with a shorter degree of polymerization (DP) than NpAS due to the wider substrate binding site (Seo et al. 2019). Moreover, due to this structural difference, DgAS exhibits higher trehalulose production than NpAS in sucrose isomer production (Guérin et al. 2012). Although the tertiary structure of BtAS has not been revealed, the characteristics of BtAS were similar to those of NpAS: long DP amylose-like polymers were produced, and the production rate of turanose was high among sucrose isomers (Choi et al. 2019; Kim et al. 2020). When a transglycosylation reaction with glycerides as acceptor molecules was attempted using NpAS, the reaction was unsuccessful (data not shown). Therefore, BtAS was considered to have a lower glyceride binding ability than DgAS due to the different substrate binding structure it possesses. As such, it was assumed that the transglycosylation reaction of glyceride by BtAS is lower than that of DgAS.
Fig. 3.
TLC analysis of transglycosylation reaction by DgAS and BtAS. Lane M: standard markers from G1 (glucose) to G7 (maltoheptaose); Lane 1: only sucrose reaction with DgAS; Lane 2: glyceryl caprate transglycosylation reaction without ASases; Lane 3: glyceryl caprate transglycosylation reaction with DgAS; Lane 4: glyceryl caprate transglycosylation reaction with BtAS; Lane 5: glyceryl caprylate transglycosylation reaction without ASases; Lane 6: glyceryl caprylate transglycosylation reaction with DgAS; Lane 7: glyceryl caprylate transglycosylation reaction with BtAS; Lane 8: polyglyceryl-2 caprate transglycosylation without ASases; Lane 9: polyglyceryl-2 caprate transglycosylation reaction with DgAS; and Lane 10: polyglyceryl-2 caprate transglycosylation reaction with BtAS
Purification and molecular weight determination of glyceride glycosides
Three putative glyceride glycosides (glyceryl caprate glycoside, glyceryl caprylate glycoside, and polyglyceryl-2 caprate glycoside) were purified from the TLC plate. Disappointingly, TLC analysis revealed that the polyglyceryl-2 caprate glycoside among the three isolated substances was detected at a very low concentration. Therefore, in the subsequent LC/MS results, the glycoside did not produce a significant result. The molecular weights of the two presumed glyceride glycosides were confirmed using LC/MS. Among the purified glyceride glycosides, the concentration of glyceryl caprylate monoglucoside was the highest, indicating a successful LC/MS analysis. The M + 22.81 molecular ion peak in the mass spectrum was found to have a molecular weight of 241.1, comprising glyceryl caprylate along with sodium adduct ion [M + Na]+ (Fig. 4A). The second highest peak corresponded to the calculated molecular mass of glyceryl caprylate monoglucoside ([M + Na]+: 403.2) (Fig. 4B). Moreover, the molecular weight of the putative glyceryl caprate glycoside was found to be 431.2 m/z [M + Na]+ through LC/MS analysis, which was consistent with the theoretical value of glyceryl caprate monoglucoside (data not shown). However, though LC/MS further showed that one glucose molecule was attached to the two glycerides, it was impossible to confirm whether it was bound to any hydroxyl group. Previous computer docking simulations have indicated that glucose could be similarly bound to the two hydroxyl groups of glyceryl caprylate, and other studies have also shown that MfAS could bind to both hydroxyl groups of glycerol (Jeong et al. 2014). In the future, further studies to reveal the structure of glyceride glycosides should be conducted. It is necessary to investigate the preference of the hydroxyl group in the transglycosylation reaction of ASase using glyceride as an acceptor molecule. Glyceryl caprylate and glyceryl caprate are surfactants that have been widely used in the cosmetic industry. These two surfactants have excellent ability to control the moisture of the skin and have antimicrobial activity than other nonionic surfactants (Herman., 2019). The two biosynthesized biosurfactants were presumed to have been endowed with new properties by the transglycosylation reaction, which may make them easier to use on an industrial scale as they were more soluble in water and that property would allow more enzyme reactions to occur.
Fig. 4.
LC/MS analysis of the glyceryl caprylate transglycosylated by DgAS. The two major peaks are A glyceryl caprylate with sodium ions and B glyceryl caprylate monoglucoside with sodium ions, respectively
A process for the biosynthesis of three glyceride glucosides by DgAS was predicted using molecular dynamics and docking simulations. Glyceride glycosides were successfully synthesized using BtAS and DgAS according to the proposed methodology. The reactive conformations of the binding modes were the highest for glyceryl caprylate, followed by glyceryl caprate and polyglyceryl-2 caprate, and this was found to be consistent with the findings of the TLC analyses, confirming the computational simulation predictions. The use of ASase in nonionic surfactant synthesis could reduce the reliance on petroleum for surfactant production once the process is upscaled and streamlined.
Acknowledgements
This paper was supported by research funds provided to newly appointed professors of Jeonbuk National University in 2019.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ye-Jin Kim, Email: jinatiger@khu.ac.kr.
Inonge Noni Siziya, Email: nonisiziya@gmail.com.
Seungpyo Hong, Email: hsp@kfri.re.kr.
Gil-Yong Lee, Email: enlicoo@kolon.com.
Myung-Ji Seo, Email: mjseo@inu.ac.kr.
Young-Rok Kim, Email: youngkim@khu.ac.kr.
Sang-Ho Yoo, Email: shyoo@sejong.ac.kr.
Cheon-Seok Park, Email: cspark@khu.ac.kr.
Dong-Ho Seo, Email: dhseo@jbnu.ac.kr.
References
- Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1:19–25. doi: 10.1016/j.softx.2015.06.001. [DOI] [Google Scholar]
- Alhammad A, Adewale P, Kuttiraja M, Christopher L. Enhancing enzyme-aided production of fermentable sugars from poplar pulp in the presence of non-ionic surfactants. Bioprocess. Biosyst. Eng. 2018;41:1133–1142. doi: 10.1007/s00449-018-1942-z. [DOI] [PubMed] [Google Scholar]
- Banat IM, Franzetti A, Gandolfi I, Bestetti G, Martinotti MG, Fracchia L, Smyth TJ, Marchant R. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010;87:427–444. doi: 10.1007/s00253-010-2589-0. [DOI] [PubMed] [Google Scholar]
- Cho HK, Kim HH, Seo DH, Jung JH, Park JH, Baek NI, Kim MJ, Yoo SH, Cha J, Kim YR, Park CS. Biosynthesis of (+)-catechin glycosides using recombinant amylosucrase from Deinococcus geothermalis DSM 11300. Enzyme Microb. Technol. 2011;49:246–253. doi: 10.1016/j.enzmictec.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Choi SW, Lee JA, Yoo SH. Sucrose-based biosynthetic process for chain-length-defined α-glucan and functional sweetener by Bifidobacterium amylosucrase. Carbohydr. Polym. 2019;205:581–588. doi: 10.1016/j.carbpol.2018.10.064. [DOI] [PubMed] [Google Scholar]
- Emond S, Mondeil S, Jaziri K, André I, Monsan P, Remaud-Siméon M, Potocki-Véronèse G. Cloning, purification and characterization of a thermostable amylosucrase from Deinococcus geothermalis. FEMS Microbiol. Lett. 2008;285:25–32. doi: 10.1111/j.1574-6968.2008.01204.x. [DOI] [PubMed] [Google Scholar]
- Fenibo EO, Ijoma GN, Selvarajan R, Chikere CB. Microbial surfactants: The next generation multifunctional biomolecules for applications in the petroleum industry and its associated environmental remediation. Microorganisms. 2019;7:581. doi: 10.3390/microorganisms7110581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérin F, Barbe S, Pizzut-Serin S, Potocki-Véronèse G, Guieysse D, Guillet V, Monsan P, Mourey L, Remaud-Siméon M, André I. Structural investigation of the thermostability and product specificity of amylosucrase from the bacterium Deinococcus geothermalis. J. Biol. Chem. 2012;287:6642–6654. doi: 10.1074/jbc.M111.322917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman A. Antimicrobial ingredients as preservative booster and components of self-preserving cosmetic products. Curr. Microbiol. 2019;76:744–754. doi: 10.1007/s00284-018-1492-2. [DOI] [PubMed] [Google Scholar]
- Hong S, Siziya IN, Seo MJ, Park CS, Seo DH. Molecular docking and kinetic studies of the A226N mutant of Deinococcus geothermalis amylosucrase with enhanced transglucosylation activity. J. Microbiol. Biotechn. 2020;30:1436–1442. doi: 10.4014/jmb.2003.03066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JW, Seo DH, Jung JH, Park JH, Baek NI, Kim MJ, Park CS. Biosynthesis of glucosyl glycerol, a compatible solute, using intermolecular transglycosylation activity of amylosucrase from Methylobacillus flagellatus KT. Appl. Biochem. Biotechnol. 2014;173:904–917. doi: 10.1007/s12010-014-0889-z. [DOI] [PubMed] [Google Scholar]
- Jung JH, Seo DH, Ha SJ, Song MC, Cha J, Yoo SH, Kim TJ, Baek NI, Baik MY, Park CS. Enzymatic synthesis of salicin glycosides through transglycosylation catalyzed by amylosucrases from Deinococcus geothermalis and Neisseria polysaccharea. Carbohydr. Res. 2009;344:1612–1619. doi: 10.1016/j.carres.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Kim MD, Jung DH, Seo DH, Jung JH, Seo EJ, Baek NI, Yoo SH, Park CS. Acceptor specificity of amylosucrase from Deinococcus radiopugnans and its application for synthesis of rutin derivatives. J. Microbiol. Biotechn. 2016;26:1845–1854. doi: 10.4014/jmb.1606.06036. [DOI] [PubMed] [Google Scholar]
- Kim ER, Rha CS, Jung YS, Choi JM, Kim GT, Jung DH, Kim TJ, Seo DH, Kim DO, Park CS. Enzymatic modification of daidzin using heterologously expressed amylosucrase in Bacillus subtilis. Food Sci. Biotechnol. 28: 165-174 (2019) [DOI] [PMC free article] [PubMed]
- Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, Li Q, Shoemaker BA, Thiessen PA, Yu B. PubChem 2019 update: improved access to chemical data. Nucleic Acids Res. 47: D1102-D1109 (2019) [DOI] [PMC free article] [PubMed]
- Kim SY, Seo DH, Kim SH, Hong YS, Lee JH, Kim YJ, Jung DH, Yoo SH, Park CS. Comparative study on four amylosucrases from Bifidobacterium species. Int. J. Biol. Macromol. 2020;155:535–542. doi: 10.1016/j.ijbiomac.2020.03.176. [DOI] [PubMed] [Google Scholar]
- Kosaric N, Sukan FV. Biosurfactants: production: properties: applications. CRC Press, Boca Raton, FL, USA. pp. 67 (2010)
- Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open Babel: An open chemical toolbox. J. Cheminformatics. 2011;3:33. doi: 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacwa-Płociniczak M, Płaza GA, Piotrowska-Seget Z, Cameotra SS. Environmental applications of biosurfactants: recent advances. Int. J. Mol. Sci. 2011;12:633–654. doi: 10.3390/ijms12010633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard C, Fioramonti J, Francois A, Robinson T, Neant F, Matuchansky C. Review article: bifidobacteria as probiotic agents–physiological effects and clinical benefits. Aliment. Pharmacol. Ther. 2005;22:495–512. doi: 10.1111/j.1365-2036.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- Rahman P, Gakpe E. Production, characterisation and applications of biosurfactants-Review. Biotechnology. 2008;7:360–370. doi: 10.3923/biotech.2008.360.370. [DOI] [Google Scholar]
- Ruiz CC. Sugar-based surfactants: fundamentals and applications. Boca Raton, FL, USA: CRC Press; 2008. pp. 1–18. [Google Scholar]
- Sarkar A, Goursaud J, Sharma MM, Georgiou G. A critical evaluation of MEOR processes. Situ. 1989;13:207–238. [Google Scholar]
- Seo DH, Jung JH, Ha SJ, Song MC, Cha J, Yoo SH, Kim TJ, Baek NI, Park CS. Highly selective biotransformation of arbutin to arbutin-α-glucoside using amylosucrase from Deinococcus geothermalis DSM 11300. J. Mol. Catal. B:Enzym. 2009;60:113–118. doi: 10.1016/j.molcatb.2009.04.006. [DOI] [Google Scholar]
- Seo DH, Jung JH, Ha SJ, Cho HK, Jung DH, Kim TJ, Baek NI, Yoo SH, Park CS. High-yield enzymatic bioconversion of hydroquinone to α-arbutin, a powerful skin lightening agent, by amylosucrase. Appl. Microbiol. Biotechnol. 2012;94:1189–1197. doi: 10.1007/s00253-012-3905-7. [DOI] [PubMed] [Google Scholar]
- Seo DH, Jung JH, Jung DH, Park S, Yoo SH, Kim YR, Park CS. An unusual chimeric amylosucrase generated by domain-swapping mutagenesis. Enzyme Microb. Technol. 2016;86:7–16. doi: 10.1016/j.enzmictec.2016.01.004. [DOI] [PubMed] [Google Scholar]
- Seo DH, Jung JH, Park CS. Improved polymerization activity of Deinococcus geothermalis amylosucrase by semi-rational design: Effect of loop flexibility on the polymerization reaction. Int. J. Biol. Macromol. 2019;130:177–185. doi: 10.1016/j.ijbiomac.2019.02.139. [DOI] [PubMed] [Google Scholar]
- Seo DH, Yoo SH, Choi SJ, Kim YR, Park CS. Versatile biotechnological applications of amylosucrase, a novel glucosyltransferase. Food Sci. Biotechnol. 2020;29:1–16. doi: 10.1007/s10068-019-00686-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shome A, Roy S, Das PK. Nonionic surfactants: a key to enhance the enzyme activity at cationic reverse micellar interface. Langmuir. 2007;23:4130–4136. doi: 10.1021/la062804j. [DOI] [PubMed] [Google Scholar]
- Sobrinho H, Luna JM, Rufino RD, Porto A, Sarubbo LA. Biosurfactants: classification, properties and environmental applications. pp. 303–330. In: Recent Developments in Biotechnology. Govil JN (ed). Studium Press LLC, USA (2013)
- Soultani S, Ognier S, Engasser J-M, Ghoul M. Comparative study of some surface active properties of fructose esters and commercial sucrose esters. Colloids Surf. A Physicochem. Eng. Asp. 2003;227:35–44. doi: 10.1016/S0927-7757(03)00360-1. [DOI] [Google Scholar]
- Sousa SF, Fernandes PA, Ramos MJ. Protein–ligand docking: current status and future challenges. Proteins. 2006;65:15–26. doi: 10.1002/prot.21082. [DOI] [PubMed] [Google Scholar]
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]