Abstract
To determine the compositions of Forsythia suspensa leaves tea (FSLT) and its safety, the chemical compounds were analysed with some methods, and the toxicity was evaluated in Kunming mice and Wistar rats. The results showed that FSLT contained rich flavonoid, lignans, triperpene acids, amino acids, and mineral elements. In the acute toxicity study, none of the mice died, and no obvious poisoning symptoms were observed after 14 days in mice at the dose of 15 mg/g·body weight (bw) FSLT; in the sub-chronic toxicity, no abnormal or dead rat was found at the dose of 1, 3, and 10 mg/g·bw during 90 days feeding administration; there was no significant difference in bw and food consumption; no significant differences were found in each hematology and serum biochemistry parameter and organ/body weight ratio comparing with the control experimental group. The results revealed that the FSLT has low or no toxicity via oral administration. Therefore, FSLT is very suitable and safe to be used as a new resource food.
Keywords: Forsythia suspensa leaves tea (FSLT), Chemical compound, Acute toxicity, Sub-chronic toxicity
Introduction
Forsythia suspensa is a plant widely grown in China, Korea, Japan and many European countries (Jiao et al., 2013). Its fruit, named “Lianqiao”, is a famous traditional Chinese medicine in China. The herb has been used to prepare over 40 traditional Chinese medicines, such as Shuanghuanglian Oral Liquid, Yinqiao Jiedu Tablet, and Qinlian Tablet. Some studies found that the leaves of F. suspensa contain many bioactive compounds, such as forsythin, forsythoside A, rutin, hyperin, quercetin, triterpene acids, ursolic acid, oleanolic acid (Wang et al., 2018; Zhao et al., 2020). Qu et al. (2008) reported that the content of forsythin in F. suspense leaves was much higher than that in the fruits. Moreover, it was confirmed that the forsythin from F. suspensa leaves had low or no toxicity by acute and sub-chronic oral administration in rodent models (Han et al., 2017). In addition, F. suspensa leaves showed the function of eliminating constipation, modulating blood lipids, anti-fatigue, anti-senile, anti-influenza activity, antioxidant activity, and lowering blood pressure (Xu et al., 2019; Yuan et al., 2014). However, most of F. suspensa leaves were discarded with no further utilization. Therefore, the comprehensive development and utilization of the leaves of F. suspensa is needed.
Owing to the pursuit to green and healthy food, more and more people liked to use natural plants for their health care needs. Indeed, many plants had been used to provide regular foods and health remedies in the past. Drinking tea is a healthy and favorable life style in China. It has been shown to have many beneficial effects in health, such as reducing cardiovascular diseases and cancers (Sun et al., 2019). With the coming of population ageing and the improvement of living standard, the demand for food and medicine homology products is increasing. Therefore, the deep processing of plant leaves can not only improve its economic benefits, but also produce new healthy products, for example, mulberry leaf tea, olive leaf tea, mango leaf tea, and honeysuckle leaf tea (Feng et al., 2018; Ferdousi et al., 2019; Medina et al., 2018; Oh et al., 2013).
The green tea prepared from F. suspensa leaves is commonly used as a health-tea in China. However, plants can contain some ingredients that might cause side effects in humans. It is known that the production of ingredients of plants is affected by some factors, such as the climate, locality, and seasonality (Aires et al., 2011). To promote its use in food and other fields, we should determine the main ingredients and evaluate the safety of the leaf tea. Although FSLT was used widely in some areas of China, little researches had been performed in the field of basic toxicological pharmacology analyses. Therefore, it is necessary to evaluate its safety using standardized experimental methods.
In this study, F. suspensa leaves were processed into tea according to preparation method of green tea. Firstly, its chemical compounds were analyzed. Then, its safety was evaluated by acute toxicity and sub-chronic toxicity experiments. The results will be an important reference to better understand the safety of FSLT for further development and application.
Materials and methods
FSLT processing procedure and preparation of extracts
The leaves of F. suspensa were collected in May (2018) from West of Henan Province, central China. The leaves were processed according to processing technology of green tea (Carloni et al., 2013). First, the fresh F. suspensa leaves were roasted in a pan at 200 °C for 5–10 min. Then, the leaves were rubbed into tea shape. Finally, the leaves were put into an oven at 90 °C for 20–30 min. The water content was about 5%. About 400 g fresh leaves can make 100 g FSLT. The FSLT was smashed and passed with a 40 mesh sieve. The FSLT powder was refluxed twice with 55% ethanol at 70 °C, and the ratio of powder to liquid was 1:30. The extract was concentrated under reduced pressure and controlled temperature (50 °C) using rotary evaporator. The FSLT extract dry power I was obtained with vacuum freeze drying technology at –40 °C and stored at –20 °C for chemical, compounds analysis. The FSLT powder was soaked twice for 20 min in 90 °C water, and the ratio of material to liquid was 1:30. The water extract was concentrated with a rotary evaporator at 60 °C. The FSLT extract dry power II was obtained with vacuum freeze drying technology at –40 °C, and stored at –20 °C for toxicity analysis.
Experimental animals
Healthy Kunming mice and Wistar rats of clean grade were provided by Animal Experiment Center in Henan University of Science and Technology. These animals were housed in plastic cages with the conditions of 22–26 °C, 50–70% relative humidity and 12 h light/dark cycle. These animals were given a standard feed and water during the experiment period. All procedures were performed according to the published guidelines of the China Council on Animal Care and approved by Institutional Animal Care and Use Committees of Henan University of Science and Technology (IRB numbers: 4103050043305).
Chemical compounds analysis
The contents of protein, soluble polysaccharide and fat were measured by using Lowry’s method, sulfuric acid-anthrone colorimetric method, and Soxhlet extraction method, respectively. The contents of ash and crude fiber were measured according to China National Standard GB/T 8306-2013, and SN/T 0913-2000, respectively. Total lignans and triterpene acid were determined by UV-spectrophotometry (UV2600, Shimadzu, Japan) method (Zhao et al., 2013). The content of total flavonoids was determined according to the AlCl3 colorimetry method (Jiang et al., 2018). The contents of rutin, hyperin, quercetin, oleanolic acid, ursolic acid, forsythoside A, and phillyrin were determined by HPLC (1260, Agilent, USA) method (Yuan et al., 2015a; 2015b). The contents of calcium, sodium, potassium, iron, zinc, and manganeses were determined by Atomic Absorption Photometer (AA700, PerkinElmer, USA). The content of Vitamin C (VC) was determined by 2,6-dichloro phenol indophenol method. Amino acid contents were determined using an Automatic Amino Acid Analyzer (A300, MembraPure GmbH, Germany) (Kabelova et al., 2008).
Acute toxicity
The method of maximum tolerated dose (MTD) was used to evaluate the acute toxicity of FSLT. In the study, 10 male and 10 female healthy Kunming mice (18–22 g) were used. The FSLT extract dry power I (15 g) was added in 60 mL distilled water, then the test sample was shaken well before gavage. All mice were fed with the sample (the experimental group) or the negative control group (NC: distilled water) after 14 h fasting period. The animals were given by oral gavage for two times, with a dose of 0.03 mL/g·body weight (bw) and an interval of 6 h. The total dose was 15 mg/g·bw. The mice were observed for 14 days. All signs of toxicity and deaths were recorded.
Sub-chronic toxicity
Eighty Wistar rats (40 males and 40 females, 60–84 g) were used in the study. These rats were divided into two sex-groups with three dose-groups and one negative control group (total 8 groups and 10 rats in each group). The three-dose groups were treated with FSLT extract dry power II of 1.0 (T1), 3.0 (T2), and 10.0 mg/g·bw (T3), respectively. The FSLT extract dry power was dissolved in distilled water and administered to the rats by oral gavage. The negative control group (NC) were orally administered with distilled water. During the 90 days exposure time, body weight and food consumption were measured every two weeks. The behavior, poisoning symptoms, and death of the animals were observed and recorded every day. After feeding for 90 days, the rats were sacrificed, then the blood was obtained from the neck vein for the analysis of biochemical and hematological parameters. The following hematological parameters were determined, including white blood cell count (WBC), red blood cell count (RBC), lymphocyte (Lym), neutrophil (Neut), and hemoglobin levels (Hb). Serum was obtained by centrifugation at 3775×g for 10 min at 4 °C. The following biochemical parameters were determined, including glutamic-pyruvic transaminase (GPT), glutamic-oxalacetic transaminase (GOT), blood urea nitrogen (BUN), creatinine (CRE), triglyceride (TG), total cholesterol (TC), blood glucose (GLU), total protein (TP), and albumin (ALB). In addition, some organs, including the liver, spleen, testis, and kidney, were removed and weighed. The ratios of organ to body weight were calculated (Xu et al., 2015).
Micronucleus test of polychromatic erythrocyte (PCE)
The micronucleus test of PCE was determined according to the standard methods from National Standards of PR China. Fifty Kunming mice (twenty-five males and twenty-five females, 18–22 g) were randomly divided into five groups (five males and five females in each group) including three dose-groups, one negative control group (NC: distilled water), and one positive control group (PC: 2.5 mg/mL cyclophosphamide, 50 mg/g·bw). The three-dose groups were treated with FSLT extract dry power II of 1.25 (T1), 2.5 (T2), and 5.0 mg/g·bw (T3), respectively. All tested samples were given by oral gavage for two times. The interval of two times was 24 h. After 6 h of the second feeding, all mice were sacrificed. The femurs from each mouse were taken to prepare the smear of bone marrow. Each smear was stained in Giemsa solution (4%), and PCE was counted using a microscopy according to the previous report (Sumiya et al., 2008). One thousand PCEs in each mouse were examined (total 5000 PCEs every group).
Sperm abnormality assay
Twenty-five male mice were randomly divided into five groups including three dose-groups, one negative control group (NC: distilled water) and one positive control group (PC: 2.5 mg/mL cyclophosphamide, 0.05 mg/g·bw). The three-dose groups were treated with FSLT extract dry power II of 1.25 (T1), 2.5 (T2), and 5.0 mg/g·bw (T3), respectively. All tested samples were daily given by oral gavage for five days. The animals were killed at the thirty-fifth day after the first gavage. The bilateral epididymides of mice were taken out and placed in 0.9% saline. Smears were prepared on clean slides and stained with eosin (Zhang et al., 2016). Morphology of sperms was observed using a microscope. One thousand sperm cells each mouse were counted, and the percentage (%) of abnormal sperm was calculated.
Statistical analysis
Statistical analysis was performed by one-way analysis of variance using the SPSS statistics software version 25.0 (SPSS Inc., Chicago, IL, USA) and Kruskal–Wallis test. Minimum significant difference test was performed on the data to determine the difference with statistical significance. All the experiments were performed at three times, and results were expressed as means ± standard deviations.
Results and discussion
Nutrient compounds of FSLT
Forsythia suspensa leaves have been used as a health tea to nourish the body for a long time. Some studies found that the leaves contained many kinds of compounds (Jiao et al., 2013; Ge et al., 2016). When F. suspensa leaves are processed into green tea, some compounds may change. To obtain the comprehensive composition information of FSLT, the content of measured compounds are shown in Table 1. The FSLT contained total flavonoid, total lignans, triperpene acids, and many kinds of bioactive compounds, including rutin, hyperin, quercetin, ursolic acid, oleanolic acid, phillyrin, and forsythoside A. Among of them, total lignans, forsythoside A, and forsythin were main biologically active components in FSLT, and their contents in FSLT were 59.31 ± 2.14 mg/g, 18.20 ± 0.83 mg/g, and 26.54 ± 1.18 mg/g, respectively. The FSLT was formed from fresh leaves with the process of high temperature roasting and dehydration, the contents of total lignans, forsythoside A, and forsythin in FSLT were slightly lower that of F. suspensa leaves according to our report (Yuan et al., 2015a; 2015b). The content of triperpene acids, ursolic acid, oleanolic acid, rutin, and hyperin were also slightly lower that of F. suspensa leaves (Yuan et al., 2015a; 2015b). The reason for the decrease of the compound content might be that the high temperature processing destroyed the structures of the compounds by polymerization and degradation (Yuan et al., 2020). Amino acids and Vc were the important nutrient compounds in FSLT, and their contents were 127.24 ± 6.37 mg/g and 3.80 ± 0.14 mg/g, respectively. Amino acid analysis showed that 17 kinds of amino acids were found in FSLT. The number of amino acids was similar to other green teas, and the content of amino acids was higher than the Xinyang Maojian green tea (41.50 mg/g) (Guo and Zhu, 1998). Among of them, Glu, Asp, and Leu were the top three amino acids, and their contents were 18.14 ± 0.63, 14.67 ± 0.81, and 11.84 ± 0.79 mg/g, respectively. The content of seven essential amino acids was 48.7 mg/g, accounting for 38.3% of the total amino acids. The results also showed that FSLT contained rich mineral elements. The content of soluble polysaccharide (36.4 mg/g) was slightly higher than the green tea (30.70 mg/g). The contents of carbohydrate, protein, fat, crude fiber, and ash were 532.38 ± 20.22, 193.52 ± 6.41, 4.39 ± 0.52, 72.73 ± 5.54, and 56.36 ± 3.22 mg/g, respectively. Their contents were similar to the Wanyuan green tea (Li et al., 2009a; 2009b). So, these ingredients are the basis of its beneficial effect on health in FSLT.
Table 1.
Compounds of F. suspensa leaves tea
| Ingredients | Content(mg/g) | Ingredients | Content(mg/g) |
|---|---|---|---|
| Protein | 193.52 ± 6.41 | Manganese | 0.12 ± 0.001 |
| Fat | 4.39 ± 0.52 | Calcium | 4.22 ± 0.24 |
| Ash | 56.36 ± 3.22 | Total amino acids | 127.24 ± 6.37 |
| Crude fiber | 72.73 ± 5.54 | L-Asp | 14.67 ± 0.81 |
| Carbohydrate | 532.38 ± 20.22 | L-Thr | 6.42 ± 0.32 |
| Soluble polysaccharide | 36.44 ± 2.23 | L-Ser | 6.73 ± 0.18 |
| Total flavonoids | 37.96 ± 2.38 | L-Glu | 18.14 ± 0.63 |
| Rutin | 2.76 ± 0.12 | L-Pro | 5.82 ± 0.34 |
| Hyperin | 6.77 ± 0.41 | L-Gly | 7.16 ± 0.41 |
| Quercetin | 0.22 ± 0.01 | L-Ala | 9.08 ± 0.45 |
| Triterpene acids | 25.37 ± 1.12 | L-Cys | 1.78 ± 0.12 |
| Ursolic acid | 14.26 ± 0.53 | L-Val | 8.72 ± 0.37 |
| Oleanolic acid | 7.46 ± 0.30 | L-Met | 1.50 ± 0.13 |
| Total lignans | 59.31 ± 2.14 | L-Ile | 5.91 ± 0.22 |
| Forsythin | 26.54 ± 1.18 | L-Leu | 11.84 ± 0.79 |
| Forsythoside A | 18.20 ± 0.83 | L-Tyr | 5.67 ± 0.20 |
| Vitamin C | 3.80 ± 0.14 | L-Phe | 7.20 ± 0.41 |
| Iron | 0.21 ± 0.03 | L-Lys | 7.21 ± 0.47 |
| Potassium | 10.21 ± 0.83 | L-His | 2.12 ± 0.18 |
| Sodium | 0.03 ± 0.002 | L-Arg | 7.27 ± 0.34 |
| Zinc | 0.04 ± 0.002 |
Acute toxicity
For safety evaluating, acute toxicity is firstly an important index to identify the harmful effects of the new resource food. In the study, MTD method was used. The results showed that none of the mice died at the experimental dose (15 mg/g·bw) during 14 days. The body weight of female mice increased from 18.42 ± 0.97 g to 28.90 ± 0.70 g, increasing by 56.89%, and that of male mice increased from 18.92 ± 1.37 g to 32.24 ± 1.03 g, increasing by 70.40%. The results also showed that the body growth rate in the male mice was higher than that in female mice. During the experiment period, no obvious poisoning symptoms were observed, and no secretion or swelling was found in eyes, nose or mouth in the acute toxicity mice. Therefore, the results confirmed that the acute oral toxicity of the FSLT was no toxicity in mice. Ai et al. (2011) studied the acute toxicity and mutagenicity of F. suspensa leaves in rats, and confirmed that F. suspensa leaves were no obvious poisoning symptoms at the dose of 60 g/kg, which was higher than that in this study. Li et al. (2013) studied the acute toxicity of the water extract and the ethanol extract of F. suspensa leaves, and the results showed that the two extracts had no toxicity at the maximum dose. Due to the yield of 3.20%, the dose of 15 mg/g·bw was the adequate intake, the mice were feed approximately 10 g FSLT every day. So, the results showed that the FSLT was safety.
Body weight and feed intake
Generally, body weight changes and food consumption are important indicators of toxicity after giving to drugs and chemicals (Han et al., 2017). In the study, the body weights of the Wistar rats were determined every two weeks. During 90 days experiment, the behavior of all rats in each group was normal. None of the Wistar rats died, and their body weight increased normally. The secretions and swelling was not found in their eyes, nose, and mouth. Their coats were clean and smooth, without hyperemia and edema in the mucosa.
The change of body weight was shown in Table 2, and the feed intake and feed utilization rate were shown in Fig. 1A and B, respectively. Compared to initial weight, the body weights of female mice and male mice in T2 group were increased by 337.68% (233 g) and 630.56% (454 g), respectively. In NC group, the weight gain of female mice and male mice were increased by 352.17% (243 g) and 652.78% (470 g) compared to the initial weight, respectively. Owing to the treatment, the increase of body weight in the experimental groups was slower than that in the control group, but there was no significant difference (p > 0.05). The results indicated that the FSLT had the function of delaying weight gain and suggested that the FSLT could be used as a diet tea. Also, feed intake and feed utilization rate for all dose groups were no significant differences in both sexes throughout the administration period (p > 0.05), and feed intake and feed utilization rate in male mice were higher than those in female mice.
Table 2.
Change of the body weight of rats, main organ weights and organ/body weights of rats in sub-chronic toxicity
| Groups | NC | T1 | T2 | T3 | NC | T1 | T2 | T3 |
|---|---|---|---|---|---|---|---|---|
| Female | Male | |||||||
| Initial weight (g) | 69.31 ± 5.22 | 69.46 ± 5.76 | 69.43 ± 4.45 | 69.72 ± 4.43 | 72.56 ± 8.12 | 72.78 ± 7.85 | 72.87 ± 7.48 | 72.56 ± 7.37 |
| 1 week weight (g) | 101.40 ± 7.71 | 101.14 ± 7.22 | 100.25 ± 10.03 | 101.23 ± 7.58 | 109.02 ± 12.32 | 107.13 ± 13.33 | 106.78 ± 16.64 | 102.87 ± 13.08 |
| 3 week weight (g) | 171.02 ± 12.24 | 171.12 ± 7.43 | 171.81 ± 10.24 | 163.45 ± 10.89 | 235.67 ± 13.72 | 218.24 ± 22.86 | 223.78 ± 19.02 | 214.45 ± 17.32 |
| 5 week weight (g) | 216.67 ± 16.12 | 211.90 ± 11.12 | 210.36 ± 13.14 | 205.22 ± 10.93 | 342.90 ± 13.92 | 326.78 ± 21.45 | 335.13 ± 21.83 | 328.67 ± 12.16 |
| 7 week weight (g) | 255.04 ± 16.21 | 247.48 ± 13.92 | 248.22 ± 17.06 | 239.93 ± 15.94 | 423.67 ± 15.03 | 403.67 ± 26.68 | 410.14 ± 25.08 | 398.67 ± 14.87 |
| 9 week weight (g) | 278.38 ± 15.42 | 268.32 ± 12.14 | 270.92 ± 16.34 | 260.67 ± 17.44 | 453.83 ± 14.58 | 451.78 ± 29.82 | 457.78 ± 22.42 | 446.58 ± 15.28 |
| 11 week weight (g) | 298.23 ± 23.04 | 283.52 ± 12.33 | 286.58 ± 15.12 | 276.72 ± 17.53 | 515.33 ± 23.48 | 496.54 ± 34.30 | 506.88 ± 25.03 | 495.58 ± 6.78 |
| 13 week weight (g) | 312.45 ± 24.82 | 308.91 ± 9.30 | 302.46 ± 13.78 | 292.34 ± 17.25 | 542.80 ± 26.14 | 518.91 ± 35.42 | 527.08 ± 28.58 | 520.32 ± 5.71 |
| Weight gain (g) | 243.14 ± 19.60 | 239.55 ± 3.54 | 233.38 ± 9.33 | 222.62 ± 12.82 | 470.24 ± 18.02 | 446.13 ± 27.57 | 454.21 ± 21.10 | 446.76 ± 8.34 |
| Liver weight (g) | 7.94 ± 0.66 | 7.42 ± 0.45 | 7.65 ± 0.42 | 7.47 ± 0.70 | 14.05 ± 1.44 | 13.87 ± 1.54 | 14.01 ± 1.33 | 13.10 ± 1.26 |
| Spleen weight (g) | 0.56 ± 0.09 | 0.59 ± 0.06 | 0.62 ± 0.09 | 0.57 ± 0.10 | 1.03 ± 0.12 | 1.01 ± 0.22 | 0.97 ± 0.15 | 0.89 ± 0.15 |
| Kidney weight (g) | 1.90 ± 0.13 | 1.87 ± 0.09 | 1.87 ± 0.14 | 1.81 ± 0.15 | 3.40 ± 0.41 | 3.25 ± 0.43 | 3.19 ± 0.12 | 2.99 ± 0.32 |
| Testis weight (g) | – | – | – | – | 3.76 ± 0.34 | 3.63 ± 0.29 | 3.86 ± 0.23 | 3.60 ± 0.36 |
| Liver/bw (%) | 2.55 ± 0.18 | 2.40 ± 0.17 | 2.54 ± 0.11 | 2.56 ± 0.17 | 2.59 ± 0.31 | 2.67 ± 0.25 | 2.66 ± 0.18 | 2.52 ± 0.24 |
| Spleen/bw (%) | 0.18 ± 0.03 | 0.19 ± 0.02 | 0.20 ± 0.04 | 0.20 ± 0.03 | 0.19 ± 0.03 | 0.19 ± 0.04 | 0.18 ± 0.02 | 0.17 ± 0.03 |
| Kidey/bw (%) | 0.61 ± 0.05 | 0.61 ± 0.04 | 0.62 ± 0.03 | 0.62 ± 0.04 | 0.63 ± 0.08 | 0.63 ± 0.08 | 0.61 ± 0.04 | 0.58 ± 0.06 |
| Testis/bw (%) | – | – | – | – | 0.69 ± 0.06 | 0.70 ± 0.04 | 0.73 ± 0.05 | 0.69 ± 0.07 |
NC mice without FSLT, T1 mice treated with 1.25 mg/g·bw, T2 mice treated with 2.5 mg/g·bw, T3 mice treated with 5.0 mg/g·bw. Compared with NC, *P < 0.05,**P < 0.01
Fig. 1.
Total feed intake and feed utilization rate for Wistar rats feed the diet containing F. suspensa leaf tea for 90 days. Control: rats without the leaf tea, T1: rats treated with 1.0 mg/g·bw, T2: rats treated with 3.0 mg/g·bw, T3: rats treated with 10.0 mg/g·bw. Grey bars represents female rats, while black bars represents male rats
Change of organ/body weight ratio
The ratio of relative organ weight with body weight is also an important index for evaluating the toxicity. Table 2 showed the results of the organ (liver, spleen, kidney, and testis) weights and the ratio of organ to body weight after FSLT administration period. There were no significant differences in organ weights and the ratios of organs to body weights between each dose group and control group (p > 0.05). According to the previous report (Li et al., 2009a; 2009b), all of the values were in the normal range. The results confirmed that FSLT had no significant effect on the main organs of the Wistar rats.
Micronucleus test of PCE
Micronucleus is a rapid method for the detection of chemical poisons that damage chromosomes and interfere with cell mitosis. Micronucleus is thought to be the genetic material that remains outside the nucleus during anaphase of cell mitosis due to the disruption of intracellular chromosomes or the influence of spindles. Therefore, micronucleus test can check the two genetic endpoints of chromosomal integrity alteration and chromosomal segregation alteration which were induced by chemical poisons or physical factors (Gong et al., 2012).
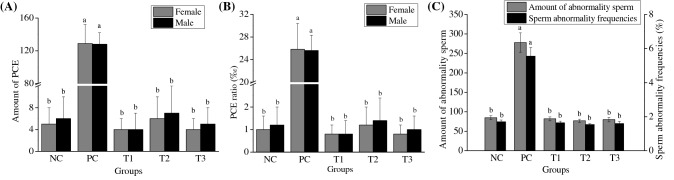
In the study, the micronucleus of mouse bone marrow cells were measured, and the data were examined with the Poisson distribution in Fig. 2. The results were shown in Fig. 2A and Fig. 2B. The amount of micronucleus was no differences (p > 0.05) comparing the NC with the each dose group. But in the PC, there were 129 PCE in female and 128 PCE in male mice, which showed significantly difference (p < 0.01, Fig. 2A). The micronucleus rates of PCE in NC, T1, T2, T3, and PC group in the female mice were 1.02 ± 0.71‰, 0.81 ± 0.84‰, 1.24 ± 0.84‰, 0.82 ± 0.45‰, and 25.80 ± 4.60‰, respectively. There were no significant differences between the NC group and the each dose group (p > 0.05), while PC group showed highly significant difference comparing to the NC group (p < 0.01). In male mice, the micronucleus rates of PCE in the NC, T1, T2, T3, and PC group were 1.23 ± 0.84‰, 0.83 ± 0.84‰, 1.44 ± 1.14‰, 1.02 ± 0.71‰, and 25.56 ± 2.72‰, respectively. Also, PC group showed highly significant differences compared to the NC group (p < 0.01). The results illustrated that there was no increase in the incidence of hyponuclear formation of PEC in anaphase of mitosis under the action of FSLT. The study confirmed that FLST did not showed mutagenic effects at the tested dose.
Fig. 2.
Results of PCE examination and in sperm abnormality examination in Kunming mice treated with different FSLT concentration. NC: mice without FSLT, PC: mice treated with 50.0 mg/g·bw cyclophosphamide, T1: mice treated with 1.25 mg/g·bw, T2: mice treated with 2.5 mg/g·bw, T3: mice treated with 5.0 mg/g·bw. (A) amount of PCE, (B) PCE rate. Different lower case letters for amount of PCE or PCE rate indicate significant differences at p < 0.01
Sperm abnormality assay
The result of mouse sperm abnormality test after the treatment with FSLT was shown in Fig. 2C. In the 5000 examined sperms, there were 85, 82, 77, 80, and 278 sperms in NC, T1, T2, T3, and PC groups, respectively. The sperm abnormality frequencies were 1.64%, 1.54%, and 1.60% in three dose groups (T1, T2, and T3), respectively. The abnormality frequencies at the test dose were in the normal range, and they showed no significant differences compared with that of negative group (p > 0.05). Moreover, the abnormality frequencies were lower than that of positive group (5.62%, p < 0.01). The results verified that FSLT did not cause the increase in sperm abnormality rate in mice within the range of experimental dose.
Hematology and serum biochemistry
Hematological parameters are important index to determine drug toxicity (Wang et al., 2010). WBC, lymphocyte and nertrohpil play important roles in the response of immunity. Hb and RBC are relative with oxygen-carrier and the excretion of CO2. However, the decline of many various physiological functions was induced by low and high volatility Hb in contrast (Weber and Vinogradov, 2001). After poisoning, these hematology parameters will significantly change.
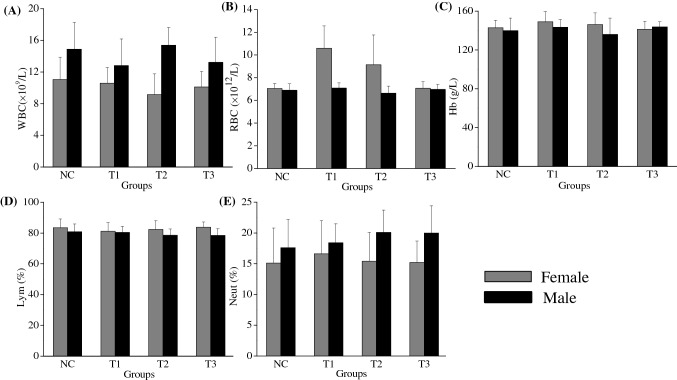
In the study, the results about hematology test were shown in Fig. 3. In female mice, compared to negative control group, FSLT at the maximum dose of 10 mg/g·bw unobviously increased WBC levels from 11.05(± 2.82) × 109/L to 10.10(± 1.79) × 109/L in Fig. 3A, RBC levels from 7.02(± 0.34) × 1012/L to 7.07(± 0.55) × 1012/L in Fig. 3B, Hb levels from 143.72 ± 7.73 g/L to 141.19 ± 8.41 g/L in Fig. 3C, lymphocyte rate from 83.31 ± 5.20% to 83.62 ± 3.50% in Fig. 3D, neutrophil rate from 15.22 ± 5.50% to 15.10 ± 3.71% in Fig. 3E, respectively. In male mice, compared to the control group, FSLT at the dose of 10 g/kg·bw also showed unobvious increase in WBC levels from 14.88(± 3.39) × 109/L to 13.21(± 3.18) × 109/L in Fig. 3A, RBC levels from 6.89(± 0.57) × 1012/L to 6.97(± 0.44) × 1012/L in Fig. 3B, Hb levels from 138.81 ± 14.12 g/L to 142.73 ± 5.24 g/L in Fig. 3C, lymphocyte rate from 80.82 ± 5.11% to 78.42 ± 4.42% in Fig. 3D, neutrophil rate from 17.63 ± 4.60% to 20.02 ± 4.43% in Fig. 3E, respectively. Among of the hematology parameters, the account of RBC in female rats slightly decreased following the increase of the concentration of FSLT. The reason and the mechanism will need further study. According to the previous report (Gao et al., 2008), all of the parameters were in the normal range. FSLT at the maximum dose of 10 mg/g·bw did not show significant influence on these parameters in the male and female rats (p > 0.05). The results indicated that these concentrations of FSLT were no toxicity to the both sex rats.
Fig. 3.
The serum hematological results in both sex Wistar rats treated with leaf tea for 90 days. (A) WBC, (B) RBC, (C) Hb, (D) lymphocyte, and (E) neutrophil. Control: rats without the FSLT, T1: rats treated with 1.0 mg/g·bw, T2: rats treated with 3.0 mg/g·bw, T3: rats treated with 10.0 mg/g·bw. Grey bars represents female rats, while black bars represents male rats
Serum biochemistry is an indication of drug toxicity in the liver and kidney (Mukinda and Eagles, 2010). For serum biochemistry, the biochemical examinations were listed in Fig. 4. In male and female rats, all the biochemical parameters in the dose groups, including GPT levels in Fig. 4A, GOT levels in Fig. 4B, BUN levels in Fig. 4C, CRE levels in Fig. 4D, TG levels in Fig. 4E, GLU levels in Fig. 4F, TC levels in Fig. 4G, TP levels in Fig. 4H, and ALB levels in Fig. 4I, showed no significant decrease/increase compared to the negative control group (p > 0.05). TC and BUN levels reached the minimum value in T1 groups, and GLU levels reached the minimum value in T2 group. But the decrease showed no significant difference comparing to the negative control. It was agreed with previous report that F. suspensa leaves could significantly reduce the concentrations of GLU and TC (Liu et al., 2006). The reason for the decrease will need further study in vivo. According to the previous report (Gao et al., 2008), all of the parameters were in the normal range in this study. The results showed that the FSLT had no adverse effects on liver and kidney function, lipid metabolism, protein metabolism and glucose metabolism, and the results indicated that FSLT was nontoxic.
Fig. 4.
The serum biochemical examination in both sex Wistar rats treated with different FSLT concentration for 90 days. (A) GPT, (B) GOT, (C) BUN, (D) CRE, (E) TG, (F) GLU, (G) TC, (H) TP, and (I) ALB. Grey bars represents female rats, while black bars represents male rats
In conclusion, FSLT was formed with the processing of high temperature roasting and dehydration. The study showed that FSLT contained many kinds of ingredients, including flavonoid, lignans, triperpene acids, amino acids, and Vc. These compounds were the basis of FSLT which showed its function. The results of acute toxicity experiments revealed that the maximum tolerated dose of FSLT to mice was greater than 15 mg/g·bw, and none of the mice died at the experimental dose. There was no significant difference in body weight and feed intake between the experimental group and the control group. Organ/body weight ratio, micronucleus test of PCE, sperm abnormality assay, the hematological parameters and serum biochemistry showed that all of the values were in the normal range, and there were no significant differences between each dose group and negative control group. However, there were significant differences between the PC and other groups in the amount of PCE, PCE rate, the amount of abnormality sperm, and sperm abnormality frequencies. So, the current investigation verified the non-toxicity of FSLT. But, the comparison of FSLT with other leave teas has not yet been studied. In the future, the chemical compositions are still worth to further study, and the toxicity evaluation of in vitro and in vivo of FSLT also needs to research in order to better employ it as a new resource food.
Acknowledgment
This study was funded by the National Natural Science Foundation of Henan Province (No. 172102310543).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Human and animal rights
All institutional and national guidelines for the care and use of laboratory animals were followed.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Da-Hong Wang, Email: wangdahong2003@163.com.
Meng-Yang Wang, Email: mengyangwang121@163.com.
Wen-Hao Shen, Email: shenwenhao1995@163.com.
Jiang-Feng Yuan, Email: jfyuan@haust.edu.cn.
References
- Ai X, Dong XT, Wang LJ, Han X, Luo CR. Acute toxicity test and micronucleus test of Forsythia Suspensa leaves in mice. J. Anhui Agr. Sci. 2011;39:6397–6398. [Google Scholar]
- Aires A, Fernandes C, Carvalho R, Bennett RN, Saavedra MJ, Rosa EAS. Seasonal effects on bioactive compounds and antioxidant capacity of six economically important brassica vegetables. Molecules. 2011;16:6816–6832. doi: 10.3390/molecules16086816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carloni P, Tiano L, Padella L, Bacchetti T, Customu C, Kay A, Damiani E. Antioxidant activity of white, green and black tea obtained from the same tea cultivar. Food Res. Int. 2013;53:900–908. doi: 10.1016/j.foodres.2012.07.057. [DOI] [Google Scholar]
- Feng J, Liu Y, Shi X, Wang Q. Potential of hyperspectral imaging for rapid identification of true and false honeysuckle tea leaves. J. Food Meas. Charact. 2018;12:2184–2192. doi: 10.1007/s11694-018-9834-0. [DOI] [Google Scholar]
- Ferdousi F, Araki R, Hashimoto K, Isoda H. Olive leaf tea may have hematological health benefit over green tea. Clin. Nutr. 2019;38:2952–2955. doi: 10.1016/j.clnu.2018.11.009. [DOI] [PubMed] [Google Scholar]
- Gao MZ, Chai WJ, Wang YY, Wang RC, Zhang HY. Investigation on the range of baseline hematological parameters and serum biochemical values in clean Wistar rat and immunolotgical parameters in clean ICR mice. J. Med. Pest Control. 2008;24:675–677. [Google Scholar]
- Ge Y, Wang Y, Chen P, Wang Y, Hou C, Wu Y, Zhang M, Li L, Huo C, Shi Q, Gao H. Polyhydroxytriterpenoids and phenolic constituents from Forsythia suspensa (Thunb.) Vahl leaves. J. Agric. Food Chem. 2016;64:125–131. doi: 10.1021/acs.jafc.5b04509. [DOI] [PubMed] [Google Scholar]
- Gong J, Huang H, Mi Q, Ni H, Ji X, Yao J. Research progress on micronucleus technology. Biotechnol. Bull. 2012;3:49–56. [Google Scholar]
- Guo G, Zhu X. Probe into relation between chemical components and quality of Xinyang Maojian tea. J. Xinyang Agri. Coll. 1998;4:17–20. [Google Scholar]
- Han Z, Lei X, Zhang H, Liu L, Chen Z, Yang W, Lun Z. Evaluating the safety of forsythin from Forsythia suspensa leaves by acute and sub-chronic oral administration in rodent models. Asian Pacific J. Trop. Med. 2017;10:47–51. doi: 10.1016/j.apjtm.2016.10.011. [DOI] [PubMed] [Google Scholar]
- Jiang C, Zeng Z, Huang Y, Zhang X. Chemical compositions of Pu’er tea fermented by Eurotium cristatum and their lipid-lowering activity. LWT-Food Sci. Technol. 2018;98:204–211. doi: 10.1016/j.lwt.2018.08.007. [DOI] [Google Scholar]
- Jiao J, Gai QY, Luo M, Wang W, Gu CB, Zhao CJ, Zu YG, Wei FY, Fu YJ. Comparison of main bioactive compounds in tea infusions with different seasonal Forsythia suspensa leaves by liquid chromatography–tandem mass spectrometry and evaluation of antioxidant activity. Food Res. Int. 2013;53:857–863. doi: 10.1016/j.foodres.2012.12.018. [DOI] [Google Scholar]
- Kabelova I, Dvorakova M, Cizkova H, Dostalek P, Melzoch K. Determination of free amino acids in beers: A comparison of Czech and foreign brands. J. Food Composit. Anal. 2008;21:736–741. doi: 10.1016/j.jfca.2008.06.007. [DOI] [Google Scholar]
- Li J, Zhang C, Xu Z, Ni S. The organic component and its value of tea in Wanyuan County in Sichuan Province. J. Anhui Agri. Sci. 2009;37:8457–8460. [Google Scholar]
- Li X, Guo W, Chen F, Li Q, Wei Y, Fan Y. Experimental study on acute toxicity of different extracts from Forsythia Suspense leaves. Feed Res. 2013;1:11–12. [Google Scholar]
- Li Y, Gao H, Sun J, He J. Research on body weight and reference value of main organ of Wistar rats. Pratic. Prevent Med. 2009;16:1708–1711. [Google Scholar]
- Liu J, Yang JX, Li XD. The experimental study on the oxidation resistance of the extracts of Forsythia suspense leaves to grease. J. Shanxi In. Edu. 2006;22:93–95. [Google Scholar]
- Medina Ramírez N, de Queiróz JH, Machado Rocha, Ribeiro S, Lopes Toledo RC, Castro Moreira ME, Mafra CL, dos Anjos Benjimin L, de Morais Clelho C, Paranho Veloso M, Stampimi Duarte Martimo H. Mango leaf tea promotes hepatoprotective effects in obese rats. J. Funct. Foods 49: 437-446 (2018)
- Mukinda JT, Eagles PFK. Acute and sub-chronic oral toxicity profiles of the aqueous extract of Polygala fruticosa in female mice and rats. J. Ethnopharmacol. 2010;128:236–240. doi: 10.1016/j.jep.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Oh J, Jo H, Cho AR, Kim SJ, Han J. Antioxidant and antimicrobial activities of various leaf herbal teas. Food Control. 2013;31:403–409. doi: 10.1016/j.foodcont.2012.10.021. [DOI] [Google Scholar]
- Qu H, Li B, Li X, Tu G, Lü J, Sun W. Qualitative and quantitative analyses of three bioactive compounds in different parts of Forsythia suspensa by high-performance liquid chromatography-electrospray ionization-mass spectrometry. Microchem. J. 2008;89:159–164. doi: 10.1016/j.microc.2008.02.002. [DOI] [Google Scholar]
- Sumiya T, Ikeda Y, Broadmeadowb A, May K, Pritchard L, Horne C, Burlinson B. Himematsutake (Iwade Strain 101) extract (ABM-FD): Genetic toxicology and a 3-month dietary toxicity study in rats. Food Chem. Toxicol. 2008;46:1949–1959. doi: 10.1016/j.fct.2008.01.035. [DOI] [PubMed] [Google Scholar]
- Sun M, Shen Z, Zhou Q, Wang M. Identification of the antiglycative components of Hong Dou Shan (Taxus chinensis) leaf tea. Food Chem. 2019;297:124942. doi: 10.1016/j.foodchem.2019.06.009. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang W, Wang Y, Peng D, Ihsan A, Huang X, Huang L, Liu Z, Dai M, Zhou W, Yuan ZH. Acute and sub-chronic oral toxicological evaluations of quinocetone in Wistar rats. Regul. Toxicol. Pharm. 2010;58:421–427. doi: 10.1016/j.yrtph.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhou Q, Shi N, Huo C, Jin S. A new bisepoxyligan gulcoside from the leaves of Forsythia suspense. Chem. Nat. Compounds. 2018;54:1038–1040. doi: 10.1007/s10600-018-2549-y. [DOI] [Google Scholar]
- Weber RE, Vinogradov SN. Nonvertebrate hemoglobins: functions and molecular adaptations. Physiol. Rev. 2001;81:569–628. doi: 10.1152/physrev.2001.81.2.569. [DOI] [PubMed] [Google Scholar]
- Xu K, Lin Y, Zhang R, Lan M, Chen C, Li S, Zou C, Chen C, Zhang T, Yuan Z. Evaluation of safety of iridoids rich fraction from Valeriana jatamansi Jones: acute and sub-chronic toxicity study in mice and rats. J. Ethnopharmacol. 2015;172:386–394. doi: 10.1016/j.jep.2015.06.046. [DOI] [PubMed] [Google Scholar]
- Xu X, Saadeldeen FSA, Xu L, Zhao Y, Wei J, Wang HMD, Liu Z, Kang W. The mechanism of phillyrin from the leaves of Forsythia suspensa for improving insulin resistance. Biomed. Res. Int. 2019;7:1–7. doi: 10.1155/2019/3176483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JF, Liu XQ, Yang JX, Cui XQ. Forsythia suspense leaves, a plant: extraction, purification and antioxidant activity of main active compounds. European Food Res. Technol. 2014;238:527–533. doi: 10.1007/s00217-014-2179-y. [DOI] [Google Scholar]
- Yuan JF, Qiu ZJ, Liu JL, Zhang ZQ. Determination of total lignans, forsythiaside A, forsythiaside B and phillyrin in Forsythia suspensa leaves from Henan and Shanxi. Nat. Prod. Res. Dev. 27: 845-848, 864 (2015)
- Yuan JF, Wang TT, Wang DH, Zhou GH, Zou GX, Wang Y, Gong MG, Zhang B. Effect of microwave on changes of gallic acid and resveratrol in a model extraction solution. Food Bioprocess Tech. 2020;13:1246–1254. doi: 10.1007/s11947-020-02452-7. [DOI] [Google Scholar]
- Yuan JF, Zhao JF, Sun JJ, He LM, Zhang ZQ, Liu H. Determination of flavonoids and triterpene acids in Forsythia suspensa leaves from Henan and Shanxi Provinces. Food Sci. 2015;36:164–167. [Google Scholar]
- Zhang J, Gao X, Pan Y, Xu N, Jia L. Toxicology and immunology of Ganoderma lucidum polysaccharides in Kunming mice and Wistar rats. Int. J. Biol. Macromol. 2016;85:302–310. doi: 10.1016/j.ijbiomac.2015.12.090. [DOI] [PubMed] [Google Scholar]
- Zhao JL, Liu LX, Wei L, Tong LJ, Sun LX. Study on the determination of total lignans in Wikstroemia indica. Northwest Pharm. J. 2013;28:226–227. [Google Scholar]
- Zhao L, Xiang KL, Liu RL, Xie ZP, Zhang SM, Dai SJ. Anti-inflammatory and anti-viral labdane diterpenoids from the fruits of Forsythia suspense. Bioorg. Chem. 2020;96:103651. doi: 10.1016/j.bioorg.2020.103651. [DOI] [PubMed] [Google Scholar]