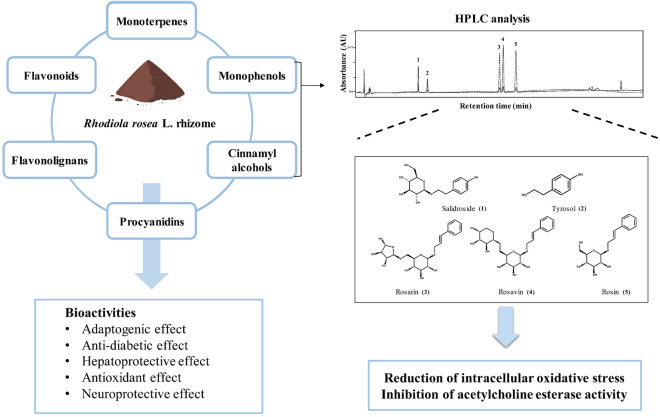
Graphic abstract
Rhodiola rosea L. rhizome has been used as a traditional medicine to treat fatigue, depression, and cognitive dysfunction. We aimed to authenticate R. rosea L. rhizome using the DNA barcoding technique and to quantify its main compounds, total phenolics, total flavonoids, and antioxidant capacity, and then to investigate their neuroprotective effects. The sequences of internal transcribed spacer and trnH-psbA of R. rosea L. rhizomes showed a 99% identity with those of NCBI GenBank database according to BLAST searches. Analysis using reversed-phase HPLC revealed five main compounds in R. rosea L. rhizome. Rhodiola rosea L. rhizome and two bioactive compounds, salidroside and tyrosol, showed free radical scavenging activity. Rhodiola rosea L. rhizome and its identified compounds protected neuronal PC-12 cells against oxidative stress and showed moderate acetylcholinesterase inhibition. Taken together, these results suggest that R. rosea L. rhizomes with bioactives can be used as a functional ingredient with potential for neuroprotection.
Supplementary information
The online version of this article (doi:10.1007/s10068-020-00868-7) contains supplementary material, which is available to authorized users.
Keywords: Acetylcholinesterase, DNA barcoding, Oxidative stress, Salidroside, Tyrosol
Introduction
As a sort of dementia, Alzheimer's disease (AD) is a neurological disorder accompanied by memory impairment and disturbing behaviors, such as cognitive disorder, memory loss, aggressiveness, depression, anxiety, and delusions (Coyle et al., 1983; Dillon et al., 2013). The causes of AD are ascribed to aging, amyloid-beta peptide deposition, hyperphosphorylated tau protein damage, and deficiency of neurotransmitters such as acetylcholine (Coyle et al., 1983; Dillon et al., 2013). Reactive oxygen species (ROS), such as hydroxyl radical, superoxide, and hydrogen peroxide, cause oxidative stress to neurons, inducing apoptosis, which can eventually lead to neurological disorders, such as AD or Parkinson’s disease (Rottkamp et al., 2000). Endogenous antioxidant enzymes like superoxide dismutase, catalase, and glutathione peroxidase and dietary antioxidants including phenolic compounds and vitamins A, C, and E exist in the body, but if their antioxidant activity or production is overwhelmed by oxidative stress or ROS, serious problems such as cell damage can occur (Schreibelt et al., 2007).
Antioxidants can protect neurons against oxidative stress and eventually prevent, delay, and treat neurodegenerative diseases like AD. Also, inhibition of acetylcholinesterase (AChE) can be an effective treatment for AD. Patients with AD have low acetylcholine levels and high AChE activity in neurons, resulting in insufficient neurotransmitters, and ultimately, cognitive dysfunctions (Rottkamp et al., 2000). Synthetic AChE inhibitors such as donepezil and rivastigmine that have been used to treat AD patients cause adverse effects such as digestion disorder, diarrhea, headaches, and dizziness (Mehta et al., 2012). Many phytochemicals such as phenolics were previously reported to act as antioxidants and show cholinesterase-inhibitory effects (Ding et al., 2013; Hillhouse et al., 2004; Kang et al., 2001; Kim et al., 2018). Therefore, finding potent AChE inhibitors and dietary antioxidants from plant-based resources, which have no side effects, could help delay or prevent AD.
Rhodiola rosea L. belongs to the plant family Crassulaceae and is native to arctic regions in Asia, Europe, and North America (Brown et al., 2002). In America and European countries, R. rosea L. is called “golden root,” “arctic root,” or “Siberian golden root,” and in China, “Hong Jing Tian.” Rhodiola rosea L. is a medicinal plant that has been traditionally used to treat various diseases, including fatigue, digestive disorders, and infections (Brown et al., 2002). Also, R. rosea L. has been used to improve brain health declined due to neurological disorders (Brown et al., 2002; Panossian et al., 2010). Rhodiola rosea L. has a wide spectrum of bioactive compounds: monophenols such as tyrosol and salidroside (rhodioloside), cinnamyl alcohol glycosides such as rosarin, rosavin, and rosin, monoterpenes such as rosiridin and rosiridosides, and flavonoids such as rodiolin and rhodiosin (Avula et al., 2009; Panossian et al., 2010). Rosavin and rosin inhibit AChE that is an enzyme terminating signal transmission by hydrolyzing acetylcholine in the synapse (Li et al., 2011). Tyrosol and its glucoside salidroside were previously reported to act as antioxidants and show neuroprotective effects in vitro and in vivo (Di Benedetto et al., 2007; Zhang et al., 2012).
This study investigated total phenolic and flavonoid content, antioxidant capacity, and neuroprotective effects of dry R. rosea L. rhizomes, which have been used in raw material for the preparation of health functional food ingredients in the Republic of Korea. The extraction, amplification, and sequencing of DNA from dry R. rosea L. rhizome were performed to verify its authenticity by analyzing the base sequences of the internal transcribed spacer (ITS) and trnH-psbA based on the NCBI GenBank database. The major bioactive compounds of dry R. rosea L. rhizomes were identified and quantified using the high-performance liquid chromatography (HPLC) system. Neuroprotective effects of dry R. rosea L. rhizome and its identified compounds on intracellular oxidative stress in neuronal PC-12 cell line and AChE activity were evaluated.
Materials and methods
Chemicals
Folin-Ciocalteu’s phenol reagent, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 2,2′-azobis(2-amidino-propane) dihydrochloride (AAPH), 2,2-diphenyl-1-picrylhydrazyl (DPPH), gallic acid, catechin, ascorbic acid, hydrogen peroxide (H2O2), dimethyl sulfoxide (DMSO), 2′,7′-dichlorofluorescin diacetate (DCFH-DA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), AChE, acetylcholine iodide (ATCI), 9-amino-1,2,3,4-tetrahydroacridine hydrochloride hydrate (tacrine), 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), cetyltrimethylammonium bromide (CTAB), and phosphate buffered saline (PBS) were purchased from Sigma Aldrich Co., LLC (St. Louis, MO, USA). Five standard compounds (salidroside, tyrosol, rosarin, rosavin, and rosin; purity > 98%) were purchased from Apexbio Technology LLC (Houston, TX, USA). Roswell Park Memorial Institute (RPMI) 1640 medium, fetal bovine serum (FBS), penicillin/streptomycin, and Dulbecco’s phosphate buffered saline (DPBS) were purchased from Welgene Inc. (Gyeongsan, Republic of Korea). All other reagents used were of analytical or HPLC grade.
Preparation of samples for DNA barcoding
Dry R. rosea L. rhizomes from Canada were obtained from Alberta Rhodiola Rosea Growers Organization (Thorsby, Canada). Rhodiola rosea L. rhizomes were divided into six parts (A − F), and then each part was used for the identification of the sequences of ITS and trnH-psbA using DNA barcoding (Table S1). Rhodiolae Crenulata Radix et Rhizoma (Rhodiola crenulata (Hook. f. et Thomson) H. Ohba) (Voucher no. WKU-2–18-0533) was used as a comparison sample.
DNA extraction, amplification, sequencing, and data analysis
The genomic DNA was extracted according to the manuals of NucleoSpin® Plant II kit (Macherey–Nagel, Düren, Germany). For R. rosea L. rhizome samples, 10% CTAB and 0.7 M NaCl were used to remove the phenolic compounds and polysaccharides. The primers for amplification were ITS1 and ITS4 for ITS (White et al., 1990) and psbAF and trnH2 for trnH-psbA (Sang et al., 1997), as listed in Table S2. For ITS amplification, polymerase chain reaction (PCR) was performed using the TAdvanced thermal cycler (Biometra GmbH, Göttingen, Germany). In brief, 600 nM of target primer set, AccuPower® GoldHotStart Taq PCR PreMix (Bioneer Corp., Daejeon, Republic of Korea), and 50 ng of genomic DNA were used for PCR amplification. PCR cycling conditions for both regions (ITS and trnH-psbA) were as follows: pre-denaturation process (95 °C, 5 min), denaturation process (95 °C, 30 s), annealing process (53 °C, 30 s), extension process (72 °C, 40 s) × 35 cycles, and final extension process (72 °C, 5 min). The amplified PCR product was separated from other gradients using 1.5% agarose gel electrophoresis after staining by the addition of Safe-White™ (Applied Biological Materials Inc., Richmond, BC, Canada). The PCR product separated from the agarose gel was cloned using the TOPcloner™ TA Kit (Enzynomics, Daejeon, Republic of Korea), and the cloned PCR product was sequenced by Bioneer Corperation. DNA sequence was analyzed using ClustalW multiple sequence alignment (BioEdit v7.0.9; available from http://www.mbio.ncsu.edu/BioEdit/page2.html), and the phylogenetic tree based on the neighbor-joining was created using DNADist (BioEdit). For the analysis, DNA sequence data of other Rhodiola species were collected from GenBank (National Center for Biotechnology Information, Bethesda, MD, USA) and previous researches (Zhang et al., 2014; Zhang et al., 2015).
Preparation of R. rosea L. rhizome extract
Dry R. rosea L. rhizomes were ground with a tube mill (Tube Mill 100; IKA, Staufen, Germany) at 11,000 rpm for 2 min. Well ground R. rosea L. rhizomes were extracted with 70% (v/v) aqueous ethanol using an ultrasonic water bath (ESW-2825B; Hwashin Tech. Co., Ltd., Seoul, Republic of Korea) at room temperature for 20 min. The extract was centrifuged at 2,833 × g for 10 min (VS-6000CF1; Vision Scientific Co., Daejeon, Republic of Korea). The supernatant was then filtered through Whatman no. 1 filter paper (Whatman International Ltd., Maidstone, UK) using a chilled Büchner funnel. The residues were re-extracted by repeating the above steps. The filtrate was evaporated using a rotary evaporator (Eyela, Tokyo, Japan) in a 40 °C water bath. The extract of R. rosea L. rhizomes was frozen in a deep freezer and then dried in a freeze-drier for 48 h and kept at −20 °C prior to use.
Quantification of chemical compounds using reversed-phase HPLC
Chemical compounds (salidroside, tyrosol, rosarin, rosavin, and rosin) in the R. rosea L. rhizome extract were quantitatively analyzed using a reversed-phase HPLC system (Alliance e2690; Waters Corp., Milford, MA, USA) equipped with an autosampler, binary pump, and photodiode array detector. A reversed-phase C18 column (InfinityLab Poroshell 120 Å, 2.7 μm, 4.6 × 150 mm; Agilent Technologies, Inc., Santa Clara, CA, USA) was used. Injection volume was 5 μL, and the flow rate was at 0.8 mL/min. The mobile phase A was water with 0.1% (v/v) formic acid, and the mobile phase B was acetonitrile. The gradient elution profile was as follows: 95% A/5% B at 0 min, 78% A/22% B at 15 min, 76% A/24% B at 21 min, 5% A/95% B at 27 min, and 95% A/5% B at 32 min. The wavelengths for detection were set at 280 nm for salidroside and tyrosol and at 245 nm for rosarin, rosavin, and rosin. These compounds were identified by comparing ultraviolet (UV)-visible spectra, retention times, and spiked inputs to commercial standards. The amount of each compound was quantified using calibration curves that relate different concentrations of authentic standards to the areas of their corresponding peaks.
Determination of total phenolic content
The total phenolic content of the R. rosea L. rhizome and its five compounds identified using HPLC was measured using the colorimetric method with Folin-Ciocalteu’s phenol reagent (Singleton and Rossi, 1965). Two hundred microliters of appropriately diluted R. rosea L. rhizomes or each identified compounds were mixed with 2.6 mL of deionized water. Then, 200 μL of Folin-Ciocalteu’s phenol reagent was added to the mixture. At 6 min, 2 mL of 7% (w/v) Na2CO3 solution was added; at 90 min, the absorbance was measured at 750 nm using a spectrophotometer (SPECTRONIC 200; Thermo Fisher Scientific Inc., Waltham, MA, USA). The content of total phenolics was expressed as mg gallic acid equivalents (GAE)/g of R. rosea L. rhizomes or compound.
Determination of total flavonoid content
The total flavonoid content of the R. rosea L. rhizomes and five identified compounds was measured using the method of Liu et al. (2013). Five hundred microliters of appropriately diluted extract of R. rosea L. rhizomes or each identified compound were mixed with 3.2 mL of deionized water. Then, 150 μL of 5% (w/v) NaNO2 solution was added. After 5 min, 150 μL of 10% (w/v) AlCl3 was added. At 6 min, 1 mL of 1 M NaOH was added, and absorbance was measured immediately using a spectrophotometer (SPECTRONIC 200; Thermo Fisher Scientific Inc.). The content of total flavonoids was expressed as mg catechin equivalents (CE)/g of R. rosea L. rhizomes or compound.
Determination of antioxidant capacities
The antioxidant capacities of R. rosea L. rhizomes and their identified compounds were assessed using the ABTS and DPPH radicals (Kim et al., 2002) and expressed in mg vitamin C equivalents (VCE)/g of R. rosea L. rhizomes or compound. The ABTS radical solution was adjusted to an absorbance of 0.650 ± 0.020 at 734 nm using a spectrophotometer (SPECTRONIC 200; Thermo Fisher Scientific Inc.). The reaction between ABTS radicals and the appropriately diluted samples was allowed to proceed at 37 °C for 10 min, and then the decrease in absorbance of the resulting solution was measured at 734 nm using a spectrophotometer (SPECTRONIC 200; Thermo Fisher Scientific Inc.).
For the assay using DPPH radicals, the absorbance of DPPH radicals in 80% (v/v) aqueous methanol was set to 0.650 ± 0.020 at 517 nm using a spectrophotometer (SPECTRONIC 200; Thermo Fisher Scientific Inc.). DPPH radicals and appropriately diluted samples were reacted at 23 °C for 30 min. The absorbance of the resulting solution was monitored at 517 nm using a spectrophotometer (SPECTRONIC 200; Thermo Fisher Scientific Inc.).
Cell culture
Neuronal PC-12 cell line (ATCC, Manassas, VA, USA) was used for measuring the cellular intracellular oxidative stress of R. rosea L. rhizome extract and its five identified compounds. PC-12 cells were cultured in RPMI 1640 medium containing 10% (v/v) heat-inactivated FBS, 100 units/mL penicillin, and 100 μg/mL of streptomycin in a humidified incubator (CO2 incubator BB 15; Thermo Electron LED GmbH, Langenselbold, Germany) with 5% CO2 at 37 °C.
Determination of cytotoxicity of R. rosea L. rhizome extract and its identified compounds
To determine the non-toxic maximal concentration of R. rosea L. rhizome extract and its identified compounds, cytotoxicity was determined using an MTT reduction assay. PC-12 cells were seeded at a density of 2 × 104 cells/well on 96-well plate in RPMI 1640 medium containing FBS for 24 h. After removing the medium, the cells were treated with a serum-free medium containing various concentrations of R. rosea L. rhizome extract or its identified compounds at various concentrations. Following 24 h of incubation, after removing the medium from each well, the serum-free medium was added and incubated for 1 h. Then, the MTT reagent was added and incubated for 3 h. Subsequently, 50 µL of DMSO was added to dissolve purple formazan formed by reduction of MTT. The absorbance was measured using a microplate reader (Infinite M200; Tecan Austria GmbH, Grödig, Austria) at 570 nm (test wavelength) and 630 nm (reference wavelength). Cell viability was expressed in percentage (%) of viable cells relative to control cells cultured without test samples.
Measurement of intracellular oxidative stress
The levels of intracellular oxidative stress were determined by the fluorescent assay using DCFH-DA. PC-12 cells were seeded at a density of 2 × 104 cells/well on 96-well plate in RPMI 1640 medium and incubated for 24 h. Then, cells were treated with non-toxic concentrations of R. rosea L. rhizome extract, each identified compound, and a positive control vitamin C for 24 h. After removing the supernatant, 50 µM DCFH-DA in DPBS was added. At 30 min, intracellular oxidative stress was induced with 100 µM of H2O2 for 1 h. The fluorescence was measured using a microplate reader (Infinite M200; Tecan Austria GmbH) with excitation at 485 nm and emission at 530 nm.
Determination of acetylcholinesterase inhibition
Anticholinesterase activity using AChE, ATCI as a substrate, and DTNB as a color developing reagent was evaluated in a 96-well plate format (Hwang et al., 2017). Briefly, 20 μL of R. rosea L. rhizome extract, each identified compound, tacrine (a positive standard), and distilled water (control) were added to 150 μL DPBS. Subsequently, 20 μL of ATCI substrate (15 mM) and 30 μL of DTNB (10 mM) were added to the mixture. After incubation for 10 min at 37 °C, 20 μL of AChE (0.4 U/mL) was added. After 30 min at 37 °C, we measured the absorbance at 415 nm using a microplate reader (Infinite M200; Tecan Austria GmbH). Measurement of AChE inhibitory activity was done in triplicate. AChE inhibition of R. rosea L. rhizome extract and its five identified compounds was expressed in percentage (%) of control.
Statistical analysis
Data are expressed as mean ± standard deviation of three replicate determinations. Statistical analysis was conducted using the one-way analysis of variance followed by Duncan’s multiple range test (p < 0.05) for multiple comparison using the SAS software (version 9.4; SPSS Inc., Chicago, IL, USA).
Results and discussion
Determination and analysis of ITS and trnH-psbA base sequences
The alignment lengths of the ITS and trnH-psbA barcode data set were found to be 668 and 423 base pairs (bp), respectively (Zhang et al., 2015). The lengths of the ITS of Rhodiolae Crenulata Radix et Rhizoma and the six divided parts of R. rosea L. rhizomes were 662 and 661 bp, respectively, while the length of trnH-psbA was 386 bp (Table S3). The best matches to the sequences of the two candidate barcodes from the seven samples (Rhodiolae Crenulata Radix et Rhizoma and six divided parts of R. rosea L. rhizomes) investigated in this study were identified using BLAST searches for the NCBI database and previous research data which were all from species of Rhodiola (Zhang et al., 2015). The voucher WKU-2-18-0533 showed a 100% identity with the R. crenulata GenBank data (KJ569926.1, KJ569927.1, KP1147718.1, and KM211543.1 in ITS and KJ570055.1 in trnH-psbA). No differences in nucleotide sequences of both ITS and trnH-psbA regions were revealed among the six divided parts (A − F) of R. rosea L. rhizomes (Table S4). Comparing using WKU-2–18-0533, however, 27 and seven variable sites were found in ITS and trnH-psbA regions, respectively. Eighteen transitions, eight transversions, and one indel were found in the ITS region, while four transitions and three transversions were found in the trnH-psbA region. According to the analysis of BLAST results, six divided parts of R. rosea L. rhizomes were 99% identical to R. rosea L. (KJ569949.1 and KJ569947.1 in Fig. S1A). To increase the accuracy of identification, other Rhodiola species data, which were deposited in the NCBI GenBank and reported in the previous research (Zhang et al., 2014), were collected and analyzed (Tables S3 and S4). According to phylogenetic analysis, six parts of R. rosea L. rhizomes were located in the R. rosea group and well distinguished from other species of Rhodiola in both ITS and trnH-psbA regions (Fig. S1). These results confirm that the six parts of R. rosea L. rhizome samples belong to R. rosea.
Rhodiola rosea L. rhizome is a pharmacologically active substance widely used throughout Asia, Europe, and North America for its anti-depressant (Darbinyan et al., 2007), cardioprotective (Maslova et al., 1994), and cognition-enhancing effects (Petkov et al., 1986). There are approximately 24 different species in the genus Rhodiola, such as the roots and rhizomes of R. crenulata (J. D. Hooker & Thomson) H. Ohba, which is included in the Pharmacopoeia of China (Zhang et al., 2015). Rhodiola serrata H. Ohba, R. himalensis (D. Dons) S. H. Fu, R. fastigiata (Hook. f. et Thomson) S. H. Fu, and R. sachalinensis A. Bor have been used as Chinese medicines (Xin et al., 2015; Zhang et al., 2015). Due to such diversity in Rhodiola species, problems of misclassifying some Rhodiola species as R. rosea have increased (Panossian et al., 2010). The extract (containing at least 20 mg/g of rosavin as a marker compound) from only R. rosea L. rhizomes has been used as an ingredient for health functional foods in the Republic of Korea. Therefore, it is important to distinguish R. rosea L. from other Rhodiola species to make a genuine raw material of R. rosea L. when manufacturing health functional foods in the Republic of Korea. Recently, DNA barcoding research has been primarily used for the identification and phylogenetic classification of the Rhodiola species due to their high morphological diversity (Zhang et al., 2014, 2015). With this in mind, before evaluating the neuroprotective effects of R. rosea L. rhizomes, we first verified the authenticity of the raw sample of R. rosea L. rhizomes using both the ITS and trnH-psbA sequence (Tables S3 and S4, Fig. S1).
Quantification of major compounds in R. rosea L. rhizome using reversed-phase HPLC
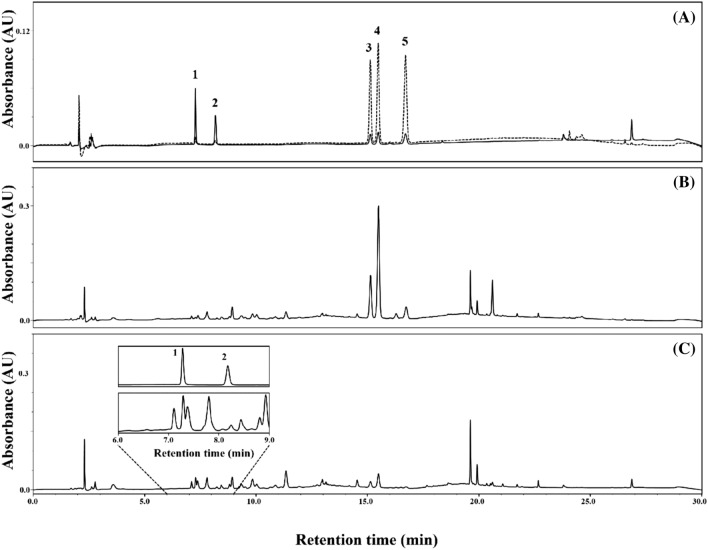
Concentrations of five compounds (salidroside, tyrosol, rosarin, rosavin, and rosin) identified in R. rosea L. rhizome are presented in Table 1. Concentrations of the five major compounds in R. rosea L. rhizome were in the following order from the highest to lowest: salidroside > rosavin > rosarin > tyrosol > rosin. Elution order of the five compounds identified using HPLC was as follows in increasing retention time: salidroside < tyrosol < rosarin < rosavin < rosin (Fig. 2). The sum of the content of three cinnamyl alcohol glycosides (rosarin, rosavin, and rosin) in dry R. rosea L. rhizome was 2.83 mg/g and that of two monophenols (salidroside and tyrosol) was 2.15 mg/g (Table 1).
Table 1.
Concentrations of major cinnamyl alcohol glycosides and monophenols in Rhodiola rosea L. rhizome measured using reversed-phase HPLC
| Compound | Concentration (mg/g dry weight) |
|---|---|
| Salidroside | 1.78 ± 0.03 |
| Tyrosol | 0.37 ± 0.01 |
| Rosarin | 0.79 ± 0.03 |
| Rosavin | 1.74 ± 0.07 |
| Rosin | 0.30 ± 0.01 |
Fig. 2.
HPLC traces of standard mixture (A; dotted line at 248 nm and solid line at 274 nm) and Rhodiola rosea L. rhizome extract at 248 nm (B) and 274 nm (C). Peak 1, salidroside; peak 2, tyrosol; peak 3, rosarin; peak 4, rosavin; and peak 5, rosin
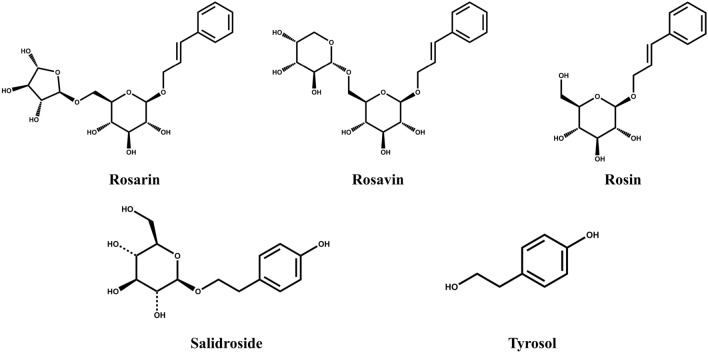
The phytochemical compositions of the genus Rhodiola are different among species, but salidroside are commonly found in various Rhodiola species such as R. sachalinensis, R. crenulata, and R. kirilowii (Panossian et al., 2010). Rhodiola rosea, unlike other Rhodiola species, contains plenty of cinnamyl alcohol glycosides such as rosarin and rosavin, allowing commercial R. rosea product to be standardized according to usually salidroside and rosavin content (Avula et al., 2009; Xin et al., 2015). As shown in Fig. 1, salidroside (p-hydroxyphenethyl-β-D-glucoside) is tyrosol (p-hydroxyphenethyl alcohol) with one glucose, and rosin (cinnamyl-O-β-D-glucoside) is cinnamyl alcohol attached to a glucose. Also, rosin with arabinofuranose is rosarin (cinnamyl-(6′-O-α-L-arabinofuranosyl)-O-β-D-glucopyranoside), and rosin with arabinopyranose is rosavin (cinnamyl-(6′-O-α-L-arabinopyranosyl)-O-β-D-glucopyranoside). Through UV detection, cinnamyl alcohol glycosides were detected at 248 nm, and monophenols at 274 nm (Fig. 2). As shown in Table 1 and Fig. 2, the order of elution was monophenols first, followed by cinnamyl alcohol glycosides in the order of rosarin, rosavin, and rosin, consistent with other studies (Avula et al., 2009; Panossian et al., 2008). Also, the ratio of salidroside to total rosavins (rosarin + rosavin + rosin) was approximately 1:1.6, which is similar to that of various extracts of R. rosea L. root and rhizomes (Peschel et al., 2013).
Fig. 1.
Structures of two monophenols (salidroside and tyrosol) and three cinnamyl alcohol glycosides (rosarin, rosavin, and rosin) in Rhodiola rosea L. rhizome
Total phenolic and flavonoid content of R. rosea L. rhizome
The total phenolic content of R. rosea L. rhizome was 7.94 mg GAE/g (Table 2). The highest total phenolic content among the five bioactive compounds in R. rosea L. rhizome was found in tyrosol, followed by salidroside. Three cinnamyl alcohol glycosides (rosarin, rosavin, and rosin) had zero total phenolic content.
Table 2.
Total phenolic and flavonoid content and antioxidant capacity of Rhodiola rosea L. rhizome and its identified compounds
| Total phenolic content (mg gallic acid equiv./g) | Total flavonoid content (mg catechin equiv./g) | Antioxidant capacity (mg vitamin C equiv./g) | ||
|---|---|---|---|---|
| ABTSa | DPPHb | |||
| Rhodiola rosea L. rhizome | 7.94 ± 0.06c | 0.11 ± 0.01 | 15.55 ± 7.47 | 14.96 ± 0.13 |
| Salidroside | 335.64 ± 11.18 | –d | 394.71 ± 8.99 | – |
| Tyrosol | 814.27 ± 19.42 | – | 914.5 ± 7.37 | – |
| Rosarin | – | – | – | – |
| Rosavin | – | – | – | – |
| Rosin | – | – | – | – |
a2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging assay
b2,2-Diphenyl-1-picrylhydrazyl radical scavenging assay
cData are expressed as means ± standard deviations (n = 3)
dNot detected
Based on our HPLC result, salidroside and tyrosol in a gram of dry weight of R. rosea L. rhizome showed 0.60 and 0.30 mg GAE of total phenolic content, respectively (data not shown). Salidroside and tyrosol can reduce phosphomolybdic–phosphotungstic acid complex due to a phenolic moiety in their structures. Therefore, among the five identified compounds, only salidroside and tyrosol showed their contribution to the total phenolic content when reacted with the complex.
Previously, flavonoids such as flavonols and flavan-3-ols were found in R. rosea L. rhizomes (Avula et al., 2009). Although no flavonoids were identified through reversed-phase HPLC in this study (Table 1), R. rosea L. rhizomes contained 0.11 mg CE/g of total flavonoid content on a dry weight basis (Table 2). However, all of the five identified compounds in R. rosea L. rhizome did not contribute to the total flavonoid content.
Antioxidant capacity
The antioxidant capacities of R. rosea L. rhizome and the five identified compounds measured using the ABTS and DPPH assays are shown in Table 2. In the ABTS assay, R. rosea L. rhizome had an antioxidant capacity of 15.55 mg VCE/g. Among the five compounds tested in this study, the highest antioxidant capacity was shown in tyrosol, followed by salidroside. Like the results of total phenolic content, the other three compounds had no antioxidant capacities according to the ABTS assay. Rhodiola rosea L. rhizome had an antioxidant capacity of 14.96 mg VCE/g in the DPPH assay, whereas all of the five identified compounds had no antioxidant capacities.
Only two phenolic compounds, salidroside and tyrosol, found in R. rosea L. rhizomes showed antioxidant capacities in the ABTS assay due to their free hydroxyl group in the aromatic ring (Table 2). Based on the HPLC results in Table 1, salidroside (1.78 mg/g) contributed approximately 0.70 mg VCE/g to the antioxidant capacity of R. rosea L. rhizome, and tyrosol (0.37 mg/g) contributed approximately 0.34 mg VCE/g to the antioxidant capacity. In the DPPH assay, however, salidroside and tyrosol had no hydrogen-donating ability against DPPH radicals (Table 2). According to a previous report, the lack of antioxidant capacity of tyrosol in the DPPH assay is partly due to its lower lipophilicity that prevents the reaction with DPPH radicals (Damiani et al., 2003). As rosarin, rosavin, and rosin are not phenolic compounds, they are unable to donate hydrogen or electron to free radicals. Thus, rosarin, rosavin, and rosin had no antioxidant capacities in both radical scavenging assays (Table 2).
Effects of R. rosea L. rhizome and its five identified compounds on intracellular oxidative stress in neuronal PC-12 cells
The cytotoxicity of the R. rosea L. rhizomes and the five compounds identified in this study was examined in order to determine the highest concentrations at which they would not be toxic. A cell viability of 90% or above was considered to be non-cytotoxic compared with the control (100%). The R. rosea L. rhizome and its five bioactive compounds had no cytotoxicity against PC-12 cells up to 50 mg/L (data not shown). Therefore, intracellular oxidative stress in PC-12 cells was evaluated at concentrations no greater than 50 mg/L.
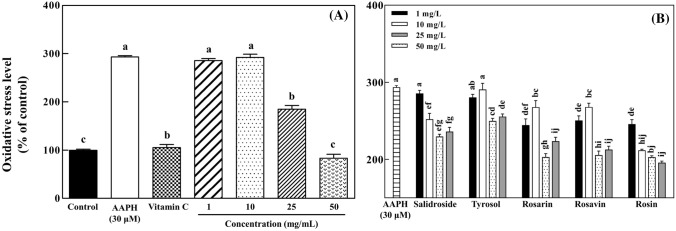
Thirty micromoles of AAPH increased the intracellular oxidative stress in PC-12 cells up to 293.1% compared with the control group (100%; Fig. 3A). Treatment of PC-12 cells with 200 μM vitamin C (positive control) resulted in 105.5% of intracellular oxidative stress. PC-12 cells pretreated with 50 mg/L of R. rosea L. rhizome extract had approximately 83.5% of intracellular oxidative stress of the control cells.
Fig. 3.
Effects of Rhodiola rosea L. rhizome extract (A) and its five identified compounds (B) on intracellular oxidative stress in neuronal PC-12 cells against AAPH-induced oxidative stress measured using the DCFH-DA assay. The data are displayed with mean ± standard deviation (bars) of three replicates. Different letters on the bars indicate significant difference by Duncan’s multiple range test (p < 0.05)
Pretreatments of PC-12 cells with five identified compounds at 50 mg/L showed the intracellular oxidative stress levels as follows in ascending order: rosin (202.3%) > rosarin (202.6%) > rosavin (205.2%) > salidroside (229.2%) > tyrosol (249.3%) (Fig. 3B). The identified compounds at 25 and 50 mg/L had significant (p < 0.05) differences in intracellualr oxidative stress compared with PC-12 cells treated with only AAPH (293.1% of intracellular oxidative stress).
Oxidative stress can cause mitochondrial dysfunction and protein, lipid, and DNA damage, eventually leading to apoptosis. Rhodiola rosea L. rhizomes decreased intracellular oxidative stress in a dose-dependent manner (Fig. 3A). Rosarin, rosavin, and rosin at 25 and 50 mg/L significantly (p < 0.05) decreased AAPH-induced intracellular oxidative stress of PC-12 cells compared with the oxidative stress control. Furthermore, the phenolic compounds (salidroside and tyrosol) significantly (p < 0.05) reduced intracellular oxidative stress of PC-12 cells at all the concentrations used in this study (Fig. 3B). Salidroside may increase the synthesis of antioxidant enzymes such as thioredoxin in neuroblastoma SH-SY5Y cells and protect them from apoptosis induced by oxidative stress via modulation of redox reactions (Zhang et al., 2007). It was also reported that salidroside, as a constituent of R. rosea L., contributes to neuroprotective effects through suppressing the production of nitric oxide and proinflammatory cytokines in the activated microglia (Lee et al., 2013). Tyrosol has been reported to ameliorate oxidative stress and possibly enhance the antioxidant defense system in in vitro and in vivo models (Di Benedetto et al., 2007; Kalaiselvan et al., 2016). Tyrosol was previously reported to protect PC-12 cells from oxidative stress and suppress their apoptotic signaling pathway (Zhang et al., 2012). Intracellular accumulation of phenolic compounds such as tyrosol may reduce cytotoxic oxidative stress in PC-12 cells, leading to increased survival of neurons (Di Benedetto et al., 2007).
Rosarin, rosavin, and rosin had no antioxidant capacity in the in vitro ABTS and DPPH assays in this study, but they protected PC-12 cells against intracellular oxidative stress (Table 2 and Fig. 3B). Rosarin and rosin have been reported to regulate nitric oxide production via modulation of inducible nitric oxide synthase in murine microglial BV2 cells (Lee et al., 2013). Nitric oxide reacts with superoxide to form peroxynitrite, a reactive nitrogen species (RNS), which works as a trigger to deleterious oxidative stress and causes cellular damage such as lipid peroxidation (Weidinger and Kozlov, 2015). Rosarin and rosin may decrease intracellular nitric oxide levels, resulting in decreased production of toxic RNS, thereby protecting neuronal cells (Fig. 3). Therefore, protective effects of R. rosea L. rhizomes against intracellular oxidative stress may be attributed to not only the phenolic antioxidants (salidroside and tyrosol) but also rosarin, rosavin, and rosin.
Inhibitory effects of R. rosea L. rhizome and its five identified compounds on AChE
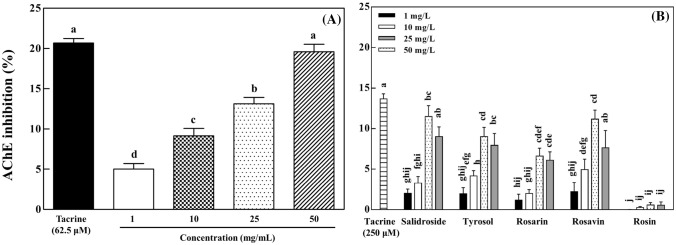
As shown in Fig. 4A, the treatment of R. rosea L. rhizome extract inhibited AChE activity in a dose-dependent manner. The R. rosea L. rhizome extract at 50 mg/L showed a 19.6% of AChE inhibition, similar to that of tacrine (20.7%) at 250 μM. When AChE was treated with five compounds at 50 mg/L, AChE activity was inhibited in the following order from greatest to smallest: salidroside (11.5%) > rosavin (11.2%) > tyrosol (9.0%) > rosarin (6.6%) (Fig. 4B). Rosin did not inhibit AChE activity (Fig. 4B).
Fig. 4.
Inhibitory effects of Rhodiola rosea L. rhizome extract (A) and its five identified compounds (B) on acetylcholinesterase (AChE) activity. The data are displayed with mean ± standard deviation (bars) of three replicates. Different letters on the bars indicate significant difference by Duncan’s multiple range test (p < 0.05)
AChE inhibitors prolong the effect of neurotransmitter acetylcholine in the neuron synapses, thereby enhancing its signal transmission. Bioactive compounds such as phenolics, terpenoids, and alkaloids in plants have been found to show AChE inhibitory activity (Xiao, 2017). It was previously reported that R. rosea L. extract and its flavonols (rhodioflavonoside and rhodiolgin) exhibit AChE inhibition (Hillhouse et al., 2004). In addition, hydroquinone, a type of phenolic compound isolated from R. rosea L. rhizomes, has been reported to inhibit AChE activity (Wang et al., 2007). The extract of R. rosea L. rhizomes used in this study showed moderate AChE inhibition, which is similar to the results of the previous reports (Hillhouse et al., 2004; Wang et al., 2007). Rosarin, rosavin, salidroside, and tyrosol inhibited AChE activity, whereas rosin generally did not (Fig. 4B). Rosavin was found to show higher AChE inhibition (Li et al., 2011), which is consistent with the results of AChE inhibition in this study. In our study, salidroside had more AChE inhibitory activity than its aglycone tyrosol (Fig. 4B). Flavonoid glycosides were previously reported to show higher AChE inhibition than their aglycones (Ding et al., 2013). Similar to the results of AChE inhibition of salidroside and tyrosol, nodakenin showed AChE inhibitory activity similar to its aglycone marmesin (Kang et al., 2001). The results of our study above suggest that the AChE inhibitory activity of the R. rosea L. rhizome extract is due in part to the identified compounds such as salidroside, tyrosol, rosarin, and rosavin.
In conclusion, dry R. rosea L. rhizomes decrease oxidative stress in neural PC-12 cells and inhibit AChE activity. Two monophenols (salidroside and tyrosol) and three cinnamyl alcohol glycosides (rosarin, rosavin, and rosin) are the major bioactive compounds identified in R. rosea L. rhizomes, showing AChE inhibition and neuronal cell protection. The HPLC method used in this study can be applied for quantifying and standardizing bioactive compounds in dry R. rosea L. rhizomes to be used as health functional food ingredients. Further studies of the neuroprotective mechanism of R. rosea L. rhizome and its bioactive compounds in in vivo and ex vivo models are encouraged.
Supplementary information
Acknowledgements
The authors thank Dr. Eun-Sang Hwang (Kyung Hee University) and Ms. Gina Kim (Johns Hopkins University) for editing the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kwan Joong Kim, Email: Joong@khu.ac.kr.
Young Sung Jung, Email: chembio@khu.ac.kr.
Dong Min You, Email: dongmin84@sk.com.
Seung Hyun Lee, Email: shlee1@sk.com.
Guemsan Lee, Email: rasfin@wku.ac.kr.
Kang- Beom Kwon, Email: desson@wku.ac.kr.
Dae-Ok Kim, Email: DOKIM05@khu.ac.kr.
References
- Avula B, Wang YH, Ali Z, Smillie TJ, Filion V, Cuerrier A, Arnason JT, Khan IA. RP-HPLC determination of phenylalkanoids and monoterpenoids in Rhodiola rosea and identification by LC-ESI-TOF. Biomed. Chromatogr. 2009;23:865–872. doi: 10.1002/bmc.1198. [DOI] [PubMed] [Google Scholar]
- Brown RP, Gerbarg PL, Ramazanov Z. Rhodiola rosea: a phytomedicinal overview. HerbalGram. 2002;56:40–52. [Google Scholar]
- Coyle JT, Price DL, DeLong MR. Alzheimer's disease: a disorder of cortical cholinergic innervation. Science. 1983;219:1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- Damiani E, Belaid C, Carloni P, Greci L. Comparison of antioxidant activity between aromatic indolinonic nitroxides and natural and synthetic antioxidants. Free Radic. Res. 2003;37:731–741. doi: 10.1080/1071576031000102169. [DOI] [PubMed] [Google Scholar]
- Darbinyan V, Aslanyan G, Amroyan E, Gabrielyan E, Malmström C, Panossian A. Clinical trial of Rhodiola rosea L extract SHR-5 in the treatment of mild to moderate depression. Nord. J. Psychiatry. 2007;61:343–348. doi: 10.1080/08039480701643290. [DOI] [PubMed] [Google Scholar]
- Di Benedetto R, Vari R, Scazzocchio B, Filesi C, Santangelo C, Giovannini C, Matarrese P, D'Archivio M, Masella R. Tyrosol, the major extra virgin olive oil compound, restored intracellular antioxidant defences in spite of its weak antioxidative effectiveness. Nutr. Metab. Cardiovasc. Dis. 2007;17:535–545. doi: 10.1016/j.numecd.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Dillon C, Serrano CM, Castro D, Leguizamon PP, Heisecke SL, Taragano FE. Behavioral symptoms related to cognitive impairment. Neuropsychiatr. Dis. Treat. 2013;9:1443–1455. doi: 10.2147/NDT.S47133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Ouyang MA, Liu X, Wang RZ. Acetylcholinesterase inhibitory activities of flavonoids from the leaves of Ginkgo biloba against brown planthopper. J. Chem. 2013;2013:645086. doi: 10.1155/2013/645086. [DOI] [Google Scholar]
- Hillhouse BJ, Ming DS, French CJ, Towers GHN. Acetylcholine esterase inhibitors in Rhodiola rosea. Pharm. Biol. 2004;42:68–72. doi: 10.1080/13880200490505636. [DOI] [Google Scholar]
- Hwang JS, Cho CH, Baik MY, Park SK, Heo HJ, Cho YS, Kim D-O. Effects of freeze-drying on antioxidant and anticholinesterase activities in various cultivars of kiwifruit (Actinidia spp.) Food Sci. Biotechnol. 2017;26:221–228. doi: 10.1007/s10068-017-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaiselvan I, Samuthirapandi M, Govindaraju A, Sheeja Malar D, Kasi PD. Olive oil and its phenolic compounds (hydroxytyrosol and tyrosol) ameliorated TCDD-induced heptotoxicity in rats via inhibition of oxidative stress and apoptosis. Pharm. Biol. 2016;54:338–346. doi: 10.3109/13880209.2015.1042980. [DOI] [PubMed] [Google Scholar]
- Kang SY, Lee KY, Sung SH, Park MJ, Kim YC. Coumarins isolated from Angelica gigas inhibit acetylcholinesterase: structure-activity relationships. J. Nat. Prod. 2001;64:683–685. doi: 10.1021/np000441w. [DOI] [PubMed] [Google Scholar]
- Kim D-O, Lee KW, Lee HJ, Lee CY. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food Chem. 2002;50:3713–3717. doi: 10.1021/jf020071c. [DOI] [PubMed] [Google Scholar]
- Kim JW, Im S, Jeong HR, Jung YS, Lee I, Kim KJ, Park SK, Kim DO. Neuroprotective effects of Korean red pine (Pinus densiflora) bark extract and its phenolics. J. Microbiol. Biotechnol. 2018;28:679–687. doi: 10.4014/jmb.1801.01053. [DOI] [PubMed] [Google Scholar]
- Lee Y, Jung JC, Jang S, Kim J, Ali Z, Khan IA, Oh S. Anti-inflammatory and neuroprotective effects of constituents isolated from Rhodiola rosea. Evid. Based Complementary Altern. Med. 2013;2013:514049. doi: 10.1155/2013/514049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-D, Kang S-T, Li G-Y, Li X, Wang J-H. Synthesis of some phenylpropanoid glycosides (PPGs) and their acetylcholinesterase/xanthine oxidase inhibitory activities. Molecules. 2011;16:3580–3596. doi: 10.3390/molecules16053580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C-S, Nam T-G, Han M-W, Ahn S-M, Choi HS, Kim TY, Chun OK, Koo SI, Kim D-O. Protective effect of detoxified Rhus verniciflua Stokes on human keratinocytes and dermal fibroblasts against oxidative stress and identification of the bioactive phenolics. Biosci. Biotechnol. Biochem. 2013;77:1682–1688. doi: 10.1271/bbb.130236. [DOI] [PubMed] [Google Scholar]
- Maslova LV, Kondrat'ev B, Maslov LN, Lishmanov LB. The cardioprotective and antiadrenergic activity of an extract of Rhodiola rosea in stress. Eksp. Klin. Farmakol. 1994;57:61–63. [PubMed] [Google Scholar]
- Mehta M, Adem A, Sabbagh M. New acetylcholinesterase inhibitors for Alzheimer's disease. J. Alzheimer’s Dis. 2012;2012:728983. doi: 10.1155/2012/728983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panossian A, Nikoyan N, Ohanyan N, Hovhannisyan A, Abrahamyan H, Gabrielyan E, Wikman G. Comparative study of Rhodiola preparations on behavioral despair of rats. Phytomedicine. 2008;15:84–91. doi: 10.1016/j.phymed.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Panossian A, Wikman G, Sarris J. Rosenroot (Rhodiola rosea): traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine. 2010;17:481–493. doi: 10.1016/j.phymed.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Peschel W, Prieto JM, Karkour C, Williamson EM. Effect of provenance, plant part and processing on extract profiles from cultivated European Rhodiola rosea L. for medicinal use. Phytochemistry. 2013;86:92–102. doi: 10.1016/j.phytochem.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Petkov VD, Yonkov D, Mosharoff A, Kambourova T, Alova L, Petkov VV. Todorov I. Effects of alcohol aqueous extract from Rhodiola rosea L. roots on learning and memory. Acta Physiol. Pharmacol. Bulg. 1986;12:3–16. [PubMed] [Google Scholar]
- Rottkamp CA, Nunomura A, Raina AK, Sayre LM, Perry G, Smith MA. Oxidative stress, antioxidants, and Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2000;14:S62–S66. doi: 10.1097/00002093-200000001-00010. [DOI] [PubMed] [Google Scholar]
- Sang T, Crawford DJ, Stuessy TF. Chloroplast DNA phylogeny, reticulate evolution, and biogeography of Paeonia (Paeoniaceae) Am. J. Bot. 1997;84:1120–1136. doi: 10.2307/2446155. [DOI] [PubMed] [Google Scholar]
- Schreibelt G, Van Horssen J, Van Rossum S, Dijkstra CD, Drukarch B, de Vries HE. Therapeutic potential and biological role of endogenous antioxidant enzymes in multiple sclerosis pathology. Brain Res. Rev. 2007;56:322–330. doi: 10.1016/j.brainresrev.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA., Jr Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- Wang H, Zhou G, Gao X, Wang Y, Yao W. Acetylcholinesterase inhibitory-active components of Rhodiola rosea L. Food Chem. 2007;105:24–27. doi: 10.1016/j.foodchem.2007.03.031. [DOI] [Google Scholar]
- Weidinger A, Kozlov AV. Biological activities of reactive oxygen and nitrogen species: oxidative stress versus signal transduction. Biomolecules. 2015;5:472–484. doi: 10.3390/biom5020472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. pp. 315–322. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR Protocols: A Guide to Methods and Applications. San Diego, CA, USA: Academic Press Inc.; 1990. [Google Scholar]
- Xiao J. Dietary flavonoid aglycones and their glycosides: which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017;57:1874–1905. doi: 10.1080/10408398.2015.1032400. [DOI] [PubMed] [Google Scholar]
- Xin T, Li X, Yao H, Lin Y, Ma X, Cheng R, Song J, Ni L, Fan C, Chen S. Survey of commercial Rhodiola products revealed species diversity and potential safety issues. Sci. Rep. 2015;5:8337. doi: 10.1038/srep08337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J-Q, Meng S-Y, Wen J, Rao G-Y. Phylogenetic relationships and character evolution of Rhodiola (Crassulaceae) based on nuclear ribosomal ITS and plastid trnL-F and psbA-trnH sequences. Syst. Bot. 2014;39:441–451. doi: 10.1600/036364414X680753. [DOI] [Google Scholar]
- Zhang J-Q, Meng S-Y, Wen J, Rao G-Y. DNA barcoding of Rhodiola (Crassulaceae): a case study on a group of recently diversified medicinal plants from the Qinghai-Tibetan Plateau. PLOS ONE. 2015;10:e0119921. doi: 10.1371/journal.pone.0119921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yu H, Sun Y, Lin X, Chen B, Tan C, Cao G, Wang Z. Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur. J. Pharmacol. 2007;564:18–25. doi: 10.1016/j.ejphar.2007.01.089. [DOI] [PubMed] [Google Scholar]
- Zhang ZX, Song XF, Du XY, Cui Y, Dai JS. Protective effect of tyrosol on apoptosis in PC12 cell induced by paraquat. Afr. J. Pharm. Pharmacol. 2012;6:2224–2228. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.