Abstract
Piperine is a bio-active compound found in pepper, including Piper nigrum L. and P. longum L. It has a strong, pungent flavour and several pharmacologic benefits. However, the risks of piperine have not yet been characterized. In this study, piperine in black pepper and some selected foods was determined to characterise the risk of exposure to piperine. Piperine in black pepper, curry and noodle was analysed by high-performance liquid chromatography–ultraviolet detection, which was validated through the measurement of performance parameters. The mean concentrations of piperine in black pepper, powdered curry, retorted curry, instant noodle and cup noodle were 4,418, 28, 3.4, 4.3 and 4.2 mg/100 g, respectively. The estimated dietary exposure to piperine was 123.66 μg/kg body weight/day, and the margin of exposure calculated by the no-observed-adverse-effect level of piperine, was 162. The piperine from food does not cause an adverse health effect to the public in Korea.
Keywords: Curry, Noodle, Margin of exposure, No-observed-adverse-effect level, Piper nigrum L
Introduction
Piperine is an amide alkaloid. It is a very weak base, with a yellow crystalline appearance (Pruthi, 1999). It is the main secondary metabolite in pepper (Piper nigrum L.) and long pepper (Piper longum L.) fruit, and it also occurs in Balinese long pepper (Piper retrofractum Vahl) fruit (Ferreira et al., 2011; Gaikar and Raman, 2002). Thus, consumers of black pepper could have a high intake of piperine.
Piperine (trans–trans) is one of four geometric isomers, including iso-piperine (cis–trans), chavicine (cis-cis) and iso-chavicine (trans–cis), all containing two double bonds (Hertwig et al., 2015; Ternes and Krause, 2002). Although its analogues (piperanine, piperettine, piperylin A, piperolein B and pipericine) have also been isolated from black pepper (Gorgani et al., 2017a), piperine imparts the pungency to black pepper (Gorgani et al., 2017b).
Black pepper has been used as a herbal medicine in Asia, including China and India, for treating and alleviating pain, muscular pains, chills, rheumatism, influenza and fever (Parthasarathy et al., 2008). Black pepper tea can relieve migraine headaches, strep throat, and poor digestion (Parthasarathy et al., 2008). Piperine exerts various health effects. It is a strong antioxidant and a scavenger of free radicals, which can damage biological tissues. It relieves oxidative stress in mice induced by a high-fat diet and acetaminophen-triggered liver damage (Sabina et al., 2010; Vijayakumar et al., 2004). Piperine has immunomodulatory and anti-tumour activity, inhibiting solid tumour development and decreasing lung metastasis in mice (Pradeep and Kuttan, 2002; Sunila and Kuttan, 2004). Anti-inflammatory activity is another bio-activity of piperine, leading to reduced inflammation or swelling (Mujumdar et al., 1990). Some studies have deciphered its anti-inflammatory mechanism in animal models (Bang et al., 2009; Mujumdar et al., 1990; Tasleem et al., 2014). Piperine is also a bio-enhancer. It increases the bio-availability and the effect of drugs by regulating membrane dynamics. Piperine can interact with lipids and hydrophobic parts of proteins and enhance membrane permeability (Khajuria et al., 1998, 2002).
Several analytical methods have been developed to analyze piperine. Piperine was determined in oleoresin and crystallised piperine by ultraviolet (UV) spectrophotometry at 343 nm (ASTA, 1997). Anagha et al. (2011) developed a high-performance–thin-layer chromatography method for convenient and rapid screening of piperine. Another established approach to analysing piperine is high-performance liquid chromatography (HPLC) with UV detection (Anagha et al., 2011; Ko, 1995 ; Santosh et al. 2005; Shingate et al. 2013). More recently,Chandra et al. (2015) described an ultra-performance liquid chromatography–electrospray ionisation-tandem mass spectrometry to determine piperine and other chemical constituents in black pepper.
Piperine concentrations vary among different pepper types. It is found at 3,000–6,650 mg/100 g in P. nigrum L. fruit and at 790 mg/100 g in root, whereas P. longum has comparatively lower levels of 600–1,600 mg/100 g in fruit and 310 mg/100 g in root (Ko, 1995; Hu et al., 1996; Mukherjee, 2002; Santosh et al., 2005). Despite the known health advantages of piperine derived from black pepper, there would be the risks of exposure to piperine. Every chemical substance has an associated risk of exposure, no matter how high its hazard. Some studies addressed that piperine has hepatic dysfuction, significant increase of aspartate aminotransferase and alanine aminotransferase in serum, liver histopathological changes, increase in hepatocyte degeneration, steatosis, and reduction in total leucocytes in animal experiments (Da Silva Cardoso et al., 2009; Dogra et al., 2004; Rao et al., 2015). Therefore, it is important to not only characterize the risk but also the benefit by exposure to piperine from consuming black pepper and other piperine-containing foods in human populations. In this study, the concentrations of piperine in black pepper and some other foods incorporated with black pepper were determined, and the risks of exposure to it through dietary intake in Korea were characterised.
Materials and methods
Chemicals and materials
Methanol and acetonitrile (HPLC grade) were supplied by Fisher Scientific (Pittsburgh, PA, USA). Deionised water (18.2 MΩ), used to prepare the HPLC eluent, was produced using an ultra-pure water system (OmniaTap6, Stakpure, Niederahr, Germany). The liquid chromatography solvents were filtered through 0.45 µm HVLP filters (Millipore, Burlington, MA, USA). The piperine reference standard and citric acid were purchased from Sigma–Aldrich (St. Louis, MO, USA).
Samples
Samples (n = 208) of black pepper (n = 71), powdered curry (n = 30), retorted curry (n = 18), instant noodle (n = 36) and instant cup noodle (n = 53) were collected from retail markets and supermarkets in Seoul, Korea, in 2019. Curry and noodle are the main food types in Korea that use black pepper as an ingredient. All samples were locally manufactured and are highly consumed among the Korean population. Before piperine analysis, all samples were stored under refrigeration at − 20 °C.
Sample preparation
Piperine was extracted, as described by Santosh et al. (2005) with a minor modification. First, all samples were homogenised (HR 2860, Philips, Shanghai, China). For black pepper, approximately 0.1 g of sample was placed in a 50 mL conical tube, and the volume completed to 50 mL with methanol. The mixture was ultrasonically extracted at 50 °C for 20 min, then cooled at room temperature, and filtered through a 0.45-μm Minisart RC (regenerated cellulose) syringe filter (Sartorius, Göttingen, Germany). The extract was transferred to vials for HPLC analysis. The same extraction procedure was used for liquid samples, such as retorted curry and some instant noodles/cup noodles, but with 5.0 g of sample, and similarly for solid samples, such as powdered curry and some instant noodles/cup noodles, but with 0.5 g of sample.
Determination of piperine by HPLC–UV
Piperine was determined as addressed by Santosh et al. (2005) with modifications. The HPLC analysis was carried out in an Agilent 1100 HPLC (Agilent, Santa Clara, CA, USA) equipped with a diode array detector (DAD) and an Eclipse C18 Plus analytical column (4.5 × 150 mm, 5 μm particle size; Agilent) at 25 °C. The mobile phase of 1% citric acid and acetonitrile at 55:45 (v/v) ratio was maintained at a constant flow rate of 1.0 mL/min. The injection volume was 10 μL, Detection was by DAD at 340 nm, and the run time was 20 min. For the calibration curves, three different points were obtained with standard solutions, and the results were expressed as milligrams per 100 g of sample.
Method validation
Method validation was conducted by obtaining several performance parameters, including specificity, the limit of detection (LOD), the limit of quantification (LOQ), range of linearity, accuracy and precision through the whole procedure. Some samples were analysed to find the procedural blank samples with a low concentration of piperine to validate the method. Specificity was established by identifying a piperine peak from noise peaks in the chromatograph of the samples and identified with UV spectrum. LOD and LOQ were statistically calculated from the results obtained by analysing the lowest concentration (0.08 mg/100 g) on the calibration curve. The calibration curve for piperine was obtained by three regression equations of six calibration standards in the range 0.08–20 mg/100 g. The linearity of the standard curve was confirmed by calculating the correlation coefficient (R2). Accuracy was estimated by analysing total 18 samples (6 samples for each) fortified with 0.5, 2 and 5% of the standard and calculating the recoveries. Precision was confirmed by calculating repeatability (RSDr) and reproducibility (RSDR), which were the relative standard deviations (RSDs) acquired by analysing the test samples three times per day for three consecutive days.
Exposure estimation and risk characterization
The piperine concentration in black pepper and some other foods containing black pepper as an ingredient, and their consumption data were used to estimate exposure to piperine in the Korean population. Data on the consumption of black pepper and foods containing black pepper were obtained from the first (2016) and second (2017) rounds of the seventh Korea National Health and Nutrition Examination Survey (KNHANES) conducted from 2016 to 2018 (KCDC, 2016, 2017). The seventh KNHANES involved 15,176 participants from seven metropolitan cities (Seoul, Busan, Daegu, Incheon, Gwangju, Daejeon, and Ulsan) and nine provinces (Gyeonggi, Gangwon, Chungbuk, Chungnam, Jeonbuk, Jeonnam, Gyeongbuk, Gyeongnam, and Jeju) in Korea. The food intake amounts were collected by inspectors visiting each randomly selected home and asking the occupants about their food consumption based on 24-h recall.
The daily dietary exposure to piperine was calculated by multiplying the concentration of piperine by the consumption amount and dividing by the average body weight (bw) (Eq.1), considering each food. The dietary intake was estimated with respect to the whole population.
Daily dietary exposure (mg/kg bw/day).
| 1 |
The risk of exposure to piperine was characterised based on the margin of exposure (MOE) approach. Piperine did not show mutagenicity in the standard Ames assay with various strains of Salmonella typhimurium and no genotoxicity in CHO cells (JECFA, 2006; Thiel et al., 2014). The MOE was calculated by diving the no-observed-adverse-effect level (NOAEL), as the health guidance value, by the estimated exposure (Eq.2):
| 2 |
An acceptable MOE value of 100 for piperine was used based on a factor of 10 for the difference between animals and humans and a factor of 10 for the inter-individual human variation (EPA, 2012).
Results and discussion
Method validation
Performance parameters, including LOD, LOQ, the linearity of the calibration curve, accuracy and precision were obtained (Table 1). Specificity was confirmed by distinguishing the peak of piperine at the retention time of 9.89 min from noise. LOD and LOQ were 0.015 and 0.049 mg/100 g, respectively. The calibration curve of piperine showed good linearity (> 0.9999). The concentration of piperine in the procedural blank sample was 3.5 g/100 g, and recoveries of piperine in black pepper at 0.5, 2 and 5 g/100 g were 100.82, 100.27 and 99.31%, respectively. Repeatability (RSDr) ranged from 0.05 to 0.08%, and reproducibility (RSDR) ranged from 1.35 to 2.14%.
Table 1.
Linear equation, limit of detection (LOD), limit of quantification (LOQ), accuracy and precision obtained for piperine at 0.5, 2, 5 g/100 g
| Linear equation | Detection limit (mg/100 g) |
Accuracy (%) | Precision | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RSDr1(%) | RSDR2(%) | ||||||||||
| LOD | LOQ | 0.5 | 2 | 5 | 0.5 | 2 | 5 | 0.5 | 2 | 5 | |
| (R2 = 0.9999) | 0.015 | 0.049 | 100.8 | 100.3 | 99.3 | 0.05 | 0.08 | 0.06 | 1.75 | 2.14 | 1.35 |
1 RSDr:Relative standard deviation of repeatability (intra-day tests)
2 RSDR: Relative standard deviation of reproducibility (inter-day tests)
Occurrence of piperine in food
Table 2 shows the concentrations of piperine in black pepper and selected foods containing black pepper. Black pepper (P. nigrum L.) contained a piperine concentration range of 2,531–8,073 mg/100 g, with a mean concentration of 4,418 ± 946 mg/100 g. In previous studies, piperine was detected in the fruit of P. nigrum at 4,500 mg/100 g and 3,000–6,000 mg/100 g (Mukherjee, 2002; Santosh et al., 2005). Ko (1995) determined a piperine content of 4.970 mg/100 g in black pepper. These concentrations are similar to those in this study and higher relative to the root of P. nigrum of 790 mg/100 g. Piper fruits have a higher level of piperine relative to roots.
Table 2.
Concentrations of piperine in black pepper and its containing products
| Food | n | Piperine (mg/100 g) | Ref | |
|---|---|---|---|---|
| Mean a | Range | |||
| Black pepper (Piper nigrum L.) | 71 | 4418 ± 946 | 2531–8073 | This study |
| Curry, powdered | 30 | 28.0 ± 22.3 | 2.3–73.6 | |
| Curry, retorted | 18 | 3.4 ± 1.9 | 0.4–6.0 | |
| Noodle, instant | 36 | 4.3 ± 3.8 | 0.1–14.9 | |
| Noodle, cup | 53 | 4.2 ± 3.6 | 0.2–17.4 | |
| Fruit (P. longum) | – | 879 | – | (Santosh et al., 2005) |
| Root (P. longum) | – | 310 | – | |
| Fruit (P. nigrum) | – | 4500 | – | |
| Fruit (P. nigrum) | – | – | 3000–6000 | (Mukherjee, 2002) |
| Fruit (P. longum) | – | – | 600–1600 | |
| Black pepper power (P. nigrum) | 15 | 4970 ± 860 | 3760–6650 | (Ko, 1995) |
| Root (P. nigrum) | – | 790 | – | (Hu et al. 1996) |
aMean: average ± standard deviation
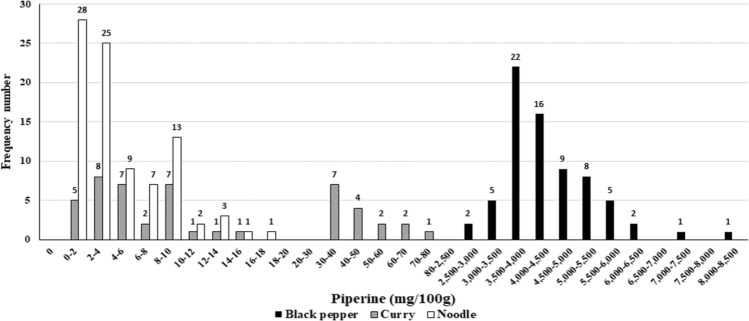
Powdered curry contained more piperine (28.0 mg/100 g) compared with retorted curry (3.4 mg/100 g), and the amount in instant noodle was similar (4.3 mg/100 g) to that in cup noodle (4.2 mg/100 g), There are no previous studies to compare the concentration of piperine in curry and noodle. Figure 1 shows the frequency distribution histogram of the piperine in black pepper and its products consumed in Korea.
Fig. 2.
Frequency distribution histogram of piperine for black pepper and its containing products consumed in Korea
Fig. 1.
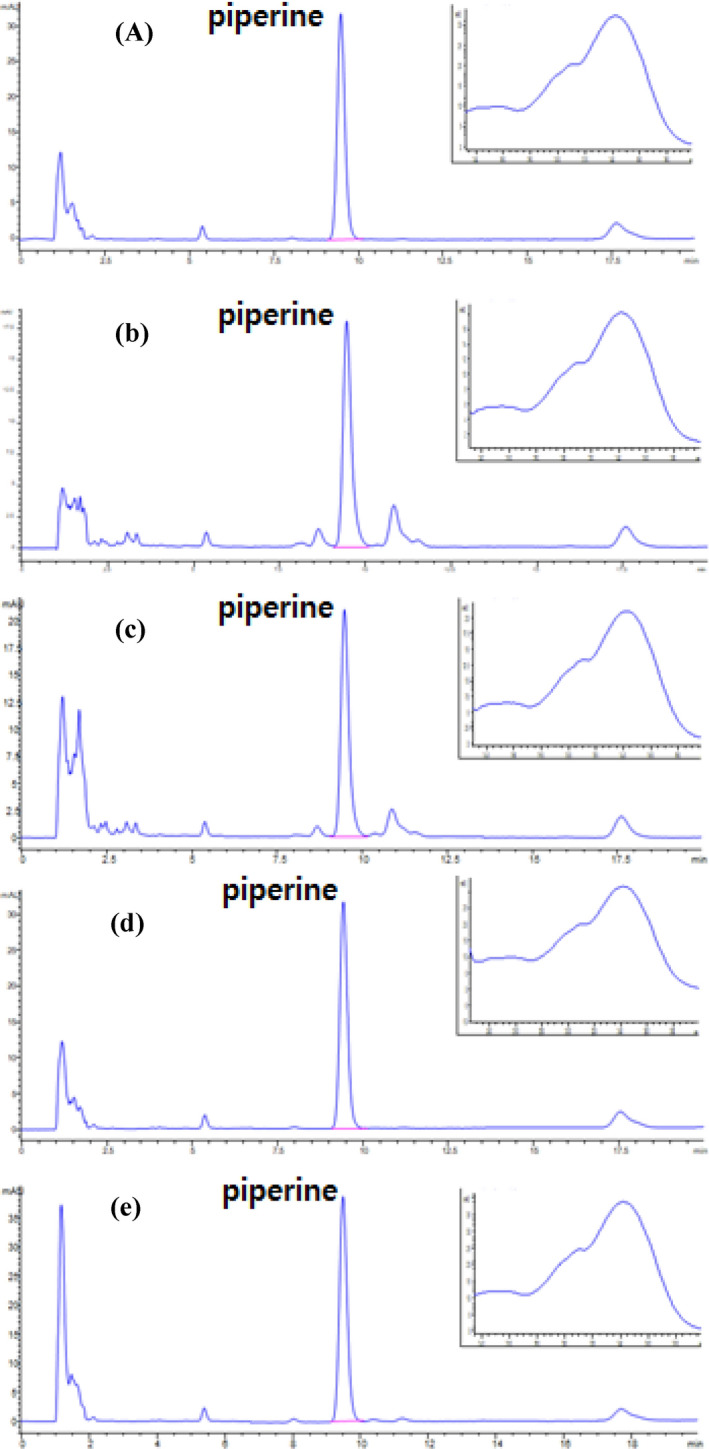
HPLC chromatogram and spectrum of piperine in a black pepper; b curry, powdered; c curry, retorted; d noodle, instant; e noodle, cup
Exposure estimation and risk characterisation
Table 3 shows the daily consumption data for black pepper, powdered curry, retorted curry, instant noodle and cup noodle by the whole population in Korea, and Table 4 shows the estimated exposure to piperine in food. In Korea, the average consumption of instant noodle (dry product) is 9.198 g/day, and cup noodle is 2.816 g/day, retorted curry is 0.444 g/day, powdered curry is 0.419 g/day and black pepper is 0.155 g/day. Literature data indicate an average daily consumption of black pepper in the USA of 0.359 g/day, and Koreans consume two-fold more black pepper than Americans (Jin and Han, 2010).
Table 3.
Daily consumptions of black pepper and its containing products by Korean in 2016 to 2017
| Food | Black pepper | Curry, powdered | Curry, retorted | Noodle, instant | Noodle, cup |
|---|---|---|---|---|---|
| Daily consumption (g/day) | 0.155 | 0.419 | 0.444 | 9.198 | 2.815 |
Table 4.
Estimated exposure to piperine by consuming black pepper and its containing products, and its risk characterization for Korean population
| Food | Estimated exposure (μg/kg bw/day) a |
Margin of Exposure |
|---|---|---|
| Black pepper | 113.00 | 177 |
| Curry, powdered | 1.94 | 10,309 |
| Curry, retorted | 0.25 | 80,000 |
| Noodle, instant | 6.53 | 3,063 |
| Noodle, cup | 1.95 | 10,256 |
| Total | 123.66 | 162 |
abw body weight
The estimated average exposure to piperine by consuming food was 123.66 μg/kg bw/day, with the highest contribution from black pepper, followed by instant noodle, cup noodle, powdered curry and retorted curry, at 113.00, 6.53, 1.95, 1.94 and 0.25 μg/kg bw/day, respectively. Among the tested foods, black pepper was the primary food contributing to exposure to piperine, at 91.4%, whereas the exposure from curry and noodle consumption was low.
A NOAEL of 20 mg/kgbw/day for piperine was suggested by the Joint Food Agricultural Organisation (FAO)/World Health Organisation (WHO) Expert Committee on Food Additives (JECFA) from a toxicology study feeding different doses of black pepper or oleoresin to 6 rats (Thiel et al., 2014). In this study, the NOAEL of 20 mg/kg bw/day was used for calculating the MOE for piperine from dietary intake of food. The MOE value was above 100, indicating that piperine from food does not cause adverse health effects in consumers (Table 4).
Factors influencing the risk characterisation could be uncertainties of the food consumption data obtained from the 24-h recall survey. The health guidance value characterised by animal toxicological experiments could also cause uncertainty. Other food we did not consider for exposure estimation but which could be incorporated with black pepper might also increase the risk of piperine via dietary intake. In the future, it is necessary to analyse more types of food that would contain piperine.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1D1A1B03036312).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Joon-Goo Lee, Email: capbox@korea.kr.
A.-Young Kim, Email: ayoung0814@seoultech.ac.kr.
Dae-Won Kim, Email: kdw3566@seoultech.ac.kr.
Young-Jun Kim, Email: kimyj@seoultech.ac.kr.
References
- American Spice Trade Association (ASTA). Method 12(1): 52–53 (1997).
- Anagha R, Anuradha U, Arvind M. HPTLC method for analysis of piperine in fruits of Piper species. J. planar. chroma. 2011;24:57–59. doi: 10.1556/JPC.24.2011.1.11. [DOI] [Google Scholar]
- Bang JS, Choi HM, Sur B-J, Lim S-J, Kim JY, Yang H-I, Yoo MC, Hahm D-H, Kim kS. Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1β-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis. Res. Ther. 11: 1–9 (2009). [DOI] [PMC free article] [PubMed]
- Chandra P, Pandey R, Srivastva M, Rameshlumar KB, Kumar B. Quantitative determination of chemical constituents of Piper spp. using UPLC-ESI-MS/MS. Ind. Crop. Prod. 76: 967–976 (2015)
- Da Silva Cardoso V, Ribeiro de Lima CA, Freire de Lima ME, Dorneles LEG, Teixeira Filho WL, Lisboa RS, Da silva Guedes JrD, Direito GM, Danelli MDGM. Oral piperine administration to broiler checkens. Ciencia. Rural. 39:1521–1526 (2009)
- Dogra RK, Khanna S, Shanker R. Immunotoxicological effects of piperine in mice. Toxicol. 2004;196:229–236. doi: 10.1016/j.tox.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Enviornmental Protection Agency (EPA), Section 13: Quantitative risk assessment calculations, sustainable futures/p2 framework manual 2012 EPA-748-B12–001, Environmental Protection Agency (EPA) Washington, DC, USA. pp.11 (2012)
- Ferreira C, Soares DC, Barreto-Junior CB, Nascimento MT, Lima LF, Delorenzi JC, Lima ME, Atella GC, Folly E, Carvalho TM, Saraiva EM, Silva LHP. Leishmanicidal effects of piperine, its derivatives, and analogues on Leishmania amazonensis. Phytochemistry. 2011;72:2155–2164. doi: 10.1016/j.phytochem.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Gaikar VG, Raman G. Process for extraction of piperine from piper species. Google Patents (2002)
- Gorgani L, Mohammadi M, Najafpour GD, Nikzad M. Sequential microwave-ultrasound-assisted extraction for isolation of piperine from black pepper (Piper nigrum L.) Food Bioproc. Tech. 2017;10:2199–2207. doi: 10.1007/s11947-017-1994-0. [DOI] [Google Scholar]
- Gorgani L, Mohammadi M, Najafpour GD, Nikzad M. Piperine-the bioactive compound of black pepper: from isolation to medicinal formulations. Compr. Rev. Food Sci. F. 2017;16:124–140. doi: 10.1111/1541-4337.12246. [DOI] [PubMed] [Google Scholar]
- Hertwig C, Reineke K, Ehlbeck J, Knorr D, Schlter O. Decontamination of whole black pepper using different cold atmospheric pressure plasma applications. Food Control. 2015;55:221–229. doi: 10.1016/j.foodcont.2015.03.003. [DOI] [Google Scholar]
- Hu S, Ao P, Liu D. Pharmacognostical studies on the roots of Piper nigrum L. III: Determination of essential oil and piperine. Acta. Hortic. 426: 179-182 (1996)
- The Joint FAO/WHO Expert Committee on Food Additvies (JECFA). Safety evaluation of certain food additives and contaminants. Sixty-fifth meeting of the Joint FAO/WHO Expert Committee on Food Additvies, WHO Food Additives: 56. IPCS, WHO, Geneva (2006)
- Jin MJ, Han HK. Effect of piperine, a major component of black pepper, on the intestinal absorption of fexofenadine and its implication on food-drug interaction. J. Food Sci. 2010;75:H93–H96. doi: 10.1111/j.1750-3841.2010.01542.x. [DOI] [PubMed] [Google Scholar]
- Khajuria A, Thusu N, Zutshi U. Piperine modulates permeability characteristics of intestine by inducing alterations in membrane dynamics: influence on brush border membrane fluidity, ultrastructure, and enzyme kinetics. Phytomedicine. 2002;9:224–231. doi: 10.1078/0944-7113-00114. [DOI] [PubMed] [Google Scholar]
- Khajuria A, Zutshi U, Bedi K. Permeability characteristics of piperine on oral absorption: an active alkaloid from peppers and a biovailability enhancer. Indian J. Exp. boil. 1998;36:46–50. [PubMed] [Google Scholar]
- Ko JM. A study on quantitative method of piperine in pure ground black pepper. J. Food Hyg. 1995;10:169–174. [Google Scholar]
- Korea Centers for Disease Control and Prevention (KCDC). The seventh Korea national health & nutrition examination survey VII-2. Osong (Korea), Korea Centers for Disease Control and Prevention (2016) https://www.khidi.or.kr/kps/dhraStat/result1?menuId=MENU01652&year=2016
- Korea Centers for Disease Control and Prevention (KCDC). The seventh Korea national health & nutrition examination survey VII-2. Osong (Korea), Korea Centers for Disease Control and Prevention (2017) https://www.khidi.or.kr/kps/dhraStat/result1?menuId=MENU01652&year=2017
- Mujumdar AM, Dhuley JN, Deshmukh VK, Raman PH, Naik SR. Anti-inflammatory activity of piperine. Jpn. J. Med. Sci. Biol. 1990;43:95–100. doi: 10.7883/yoken1952.43.95. [DOI] [PubMed] [Google Scholar]
- Mukherjee PK (2002). Quality control of herbal drugs, Business Horizons: New Delhi.pp.755–760
- Parthasarathy VA, Chempakam B, Zachariah TJ. Chemistry of spices. London: CABI; 2008. [Google Scholar]
- Pradeep C, Kuttan G. Effect of piperine on the inhibition of lung metastasis industry of induced B16F–10 melanoma cells in mice. Clin. Exp. Metastasis. 2002;19:703–708. doi: 10.1023/A:1021398601388. [DOI] [PubMed] [Google Scholar]
- Pruthi J. Quality assurance in spices and spice products, modern methods of analysis. New Delhi, India: Allied Publishers Ltd; 1999. [Google Scholar]
- Rao PJ, Kolla SD, Elshaari F, Elshaari F, Awamy HE, Elfrady M, Singh R, Belkhier A, Srikumar S, Said AR, Dhopoide SJ, Ramanujam R, Elbarassi I, Peela LT, Argi A. Effect of piperine on liver fuction of CF-1 albino mice. Infect. Disord. Drug Targets. 2015;15:131–134. doi: 10.2174/1871526515666150724114616. [DOI] [PubMed] [Google Scholar]
- Sabina EP, Souriyan ADH, Jackline D, Rasool MK. Piperine, an active ingredient of black pepper attenuates acetaminophen-induced hepatotoxicity in mice. Asian Pac. J. Trop. Med. 2010;3:971–976. doi: 10.1016/S1995-7645(11)60011-4. [DOI] [Google Scholar]
- Santosh MK, Shaila D, Rajyalakshmi I, Rao IS. RP-HPLC Method for determination of piperine from Piper longum Linn, and Piper nigrum Linn. E. J. Chem. 2005;2:131–135. doi: 10.1155/2005/627029. [DOI] [Google Scholar]
- Shingate PN, Dongre PP, Kannur DM. New method development for extraction and isolation of piperine from black pepper. Int. J. Pharm. Sci. 2013;4:3165–3170. [Google Scholar]
- Sunila E, Kuttan G. Immunomodulatory and antitumor activity of piper longum Linn. and piperine. J. Ethnopharmacol. 90: 339–346 (2004) [DOI] [PubMed]
- Tasleem F, Azhar I, Ali Sn, Perveen S, Mahmood ZA. Analgesic and anti-inflammatory activities of Piper nigum L. Asian Pac. J. Trop. Med. 7: 461–468 (2014) [DOI] [PubMed]
- Ternes W, Krause EL. Characterization and determination of piperine and piperine isomers in eggs. Anal. Bioanal. Chem. 2002;374:155–160. doi: 10.1007/s00216-002-1416-6. [DOI] [PubMed] [Google Scholar]
- Thiel A, Buskens C, Woehrle T, Etheve S, Schoenmakers A, Fehr M, Beilstein P. Black pepper constituent piperine: genotoxicity studies in vitro and in vivo. Food Chem. Toxicol. 2014;66:350–357. doi: 10.1016/j.fct.2014.01.056. [DOI] [PubMed] [Google Scholar]
- Vijayakumar R, Surya D, Nalini N. Antioxidant efficacy of black pepper (Piper nigrum L.) and piperine in rats with high-fat-diet-induced oxidative stress. Redox Report. 2004;9:105–110. doi: 10.1179/135100004225004742. [DOI] [PubMed] [Google Scholar]