Abstract
An increasing number of studies have shown that Faecalibacterium prausnitzii (F. prausnitzii) is a promising anti-inflammatory bacterium that colonizes in the gut and that gut microbiota dysbiosis plays an important role in the pathogenesis of inflammatory bowel disease (IBD). In this study, we report the gut microbiota profile of 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis mice treated with F. prausnitzii and its supernatant on the basis of high-throughput sequencing. We interestingly found that both F. prausnitzii and its metabolites exerted protective effects against colitis in mice, which ameliorated gut dysbiosis, with an increase in bacterial diversity and the abundance of short-chain fatty acid (SCFA)-producing bacteria and a decrease in serum TNF-α and the abundance of Proteinbacteria, Acidobacteria, and Bacteroidetes. These findings will provide further evidence of the anti-inflammatory effect of F. prausnitzii, which presents therapeutic potential for IBD treatment.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13568-021-01197-6.
Keywords: Inflammatory bowel disease, Gut microbiota, F. prausnitzii, Supernatant, Short chain fatty acid
Introduction
Inflammatory bowel disease (IBD) is a group of refractory digestive diseases including Crohn's disease (CD) and ulcerative colitis (UC). At present, there is a lack of satisfactory targeted drugs and therapies for the IBD. A large proportion of patients show complications or cancer development or require surgical treatment after using 5-aminosalicylates (5-ASA), steroids, immunomodulators, biological agents and other drugs. Compared with other gastrointestinal diseases, widespread concern about IBD has been aroused because of the high cost of its treatment and high relapse rate. To date, however, the pathogenesis of IBD has not been fully elucidated. It is well known that the gut microbiota plays a substantial role in the pathogenesis of IBD. There is increasing interest in the development of microbiota-modulating therapies for IBD, such as the use of fecal microbiota transplantation (FMT), probiotics and prebiotics.
Faecalibacterium prausnitzii (F. prausnitzii), is an anaerobic bacterium that mainly exists in the ileocecal and terminal ileum. Accumulating studies have shown that the abundance of F. prausnitzii is significantly reduced in the stool of patients with acute IBD, especially in those with acute UC, but there is no difference in the proportion of F. prausnitzii between patients in IBD remission and healthy controls (Sokol et al. 2009). Decreases in the abundance of F. prausnitzii in the intestine are significantly related to the activity of CD (Friswell et al. 2010; Sokol et al. 2008). A reduced abundance of F. prausnitzii in the intestinal mucosa of CD patients is positively correlated with the degree of intestinal lesions during surgery (Sokol et al. 2008). Sokol et al. (2009) compared the fecal microbiota of patients with acute CD, those in CD remission and patients with UC and found that the abundance of Firmicutes [Clostridium leptum and Clostridium coccoides] and the ratio of the Firmicutes/Bacteroides phyla were reduced in the stool of patients with acute IBD and infectious enteritis. After recurrence in patients with UC, if the abundance of F. prausnitzii in the intestine is restored, it will be beneficial to disease remission (Varela et al. 2013). In animal models and in vitro, F. prausnitzii may regulate the metabolites of the gastrointestinal tract and peripheral blood to reduce the occurrence of colitis (Miquel et al. 2015). In addition to its direct anti-inflammatory effects (Friswell et al. 2010), F. prausnitzii improves the permeability of the intestinal epithelium in an IBD rat model induced by dextran sodium sulfate (DSS) (Carlsson et al. 2013), which further confirms the positive protective effect of F. prausnitzii on IBD. While the investigation of F. prausnitzii has gradually become a research focus and hotspot, its mechanism in IBD is still unclear. Therefore, it is of great scientific significance and clinical value to explore the molecular mechanism of F. prausnitzii in IBD. In this study, we aimed to investigate the effect of F. prausnitzii on the gut microbiota and to evaluate its protective ability when therapeutically used in acute TNBS-induced murine colitis.
Materials and methods
Bacteria culture
Faecalibacterium prausnitzii (F. prausnitzii, ATCC27766), kindly provided by Professor Chenggong Yu from Nanjing Medical University, was grown in LYHBHI medium [(brain heart infusion (37 g/L), yeast extract (5 g/L), blood crystal (5 mg/L)], plus maltose (1 g/L), cellobiose (1 g/L), cysteine (0.5 g/L) at 37 °C in an anaerobic incubator (97% carbon dioxide and 3% hydrogen). According to the optical density (OD) at 600 nm, the growth curve and live bacteria count were calculated based on the absorbance value in combination with the bacterial plate count (Qiu et al. 2013; Sokol et al. 2008). The F. prausnitzii supernatant was collected from bacterial cultures at 1 × 109 CFU/mL.
Animal model
Sixty 8- to 10-week-old male mice were purchased from Guangdong Medical Laboratory Animal Center (GMLAC, Foshan, Guangdong, China). These mice were kept under SPF conditions according to relevant laws and regulations of the laboratory animal ethics committee of GMLAC.
The mice were divided into 5 groups (N = 12 per group): EtOH control (EtOH), TNBS (TNBS), TNBS + 5-ASA (TNBS + ASA), TNBS + F. prausnitzii (TNBS + Fp), and TNBS + F. prausnitzii supernatant (TNBS + SN). Four groups were induced to develop colitis via the intrarectal administration of TNBS (Sigma Aldrich), which was dissolved in 50% ethanol at a dose of 25 mg/L for administration at 0.1 mL/per mouse. Before 24 h of colitis induction, 5-ASA (Sigma, 7.5 mg/mL, 0.1 mL/10 g body weight), F. prausnitzii (2–4 × 1010 CFU/kg), the F. prausnitzii supernatant (0.1 mL/10 g body weight) and PBS (0.1 mL/10 g body weight) were administered intragastrically to the mice once, which was repeated daily for the next 7 days. The EtOH control mice received PBS orally once in the first 24 h and on each of the following 7 days and were injected with 0.1 mL of a 50% ethanol solution in the rectum once in the second day. The experiment ended 24 h after the last intervention.
Colonic pathology
At the end of the experiment, 6 mice were randomly selected from each group. Their fresh colon tissues were collected, washed, fixed in a 4% neutral formaldehyde solution for 24 h, and routinely dehydrated, embedded, cut into 4 μm sections, which were then stained with hematoxylin–eosin (H–E). The colonic histopathological score was assessed blindly using a previously published grading system (Fuss et al. 2002).
TNF-α detection in mouse serum
TNF-α concentrations in mouse serum were quantified with an ELISA kit (Abcam, ab100747) according to the manufacturer’s recommendations.
Stool sampling, short-chain fatty acids (SCFAs) detection and microbiota analysis
Stool samples from mice were collected by stimulating the anus and immediately frozen in a − 80 °C refrigerator. Fecal SCFAs were detected by gas chromatography–mass spectrometry (GC–MS) as previously described (He et al. 2020; Wang et al. 2019). Stool DNA extraction was performed with the Qiagen DNA Stool MiniKit (Qiagen, Cat No. 51504). PCR and 16S rRNA sequencing were conducted using a MiSeq System (Illumina, Inc.). Bioinformatic analysis was performed as previously described (Zhou et al. 2018b, c). For the statistical analysis, we used the unpaired Student’s t-test, Wilcox test and Mann–Whitney test to compare two groups. One-way ANOVA, the Kruskal–Wallis test and Tukey’s posttest were used for comparisons of three groups. The results were considered statistically significant when P < 0.05 (*P < 0.05, **P < 0.01, ***P < 0.005). All analyses were performed with Prism GraphPad 6.0 and SPSS 19.0 software.
Results
F. prausnitzii and its supernatant relieve symptoms and reduce body weight and colonic inflammation in mice
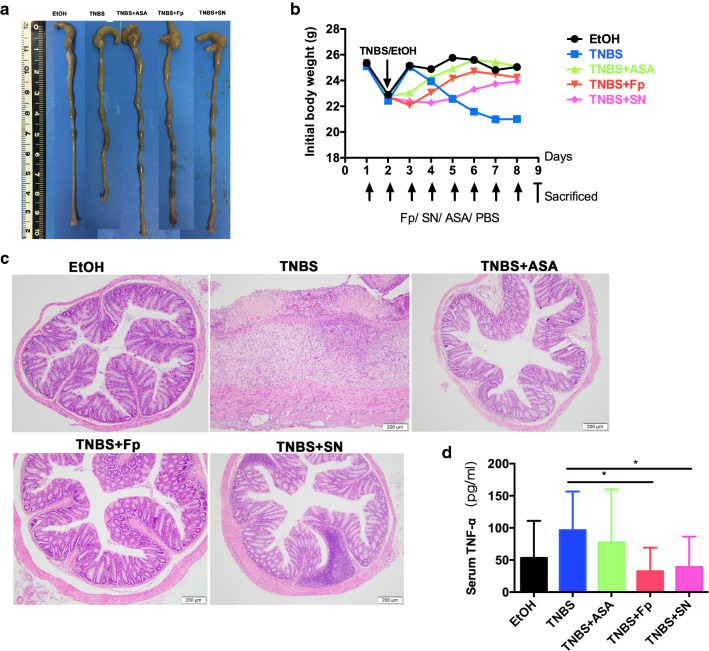
Mice became symptomatic, showing symptoms such as weight loss, loose stool, and bloody diarrhea by day 2–3 of intrarectal TNBS administration. The symptoms were significantly improved by F. prausnitzii and its supernatant. Compared with the control group, the mice in the TNBS model group exhibited a markedly shorter colon length. The gross observation of the bowel revealed that the intestines of the mice in the EtOH group were pink, smooth and intact, while the gut lumen of the mice from the TNBS group showed swelling, congestion, a thinner colon wall, disappearance of mucosal folds, and a shortened colon. In some segments, large amounts of loose, unshaped or bloody feces were deposited in the gut lumen. After longitudinal incision, obvious ulcers were observed, and a few of the specimens exhibited intestinal adhesions and intestinal obstruction. Most of the changes observed in the gross observation of the gut were mitigated by F. prausnitzii and its supernatant to some extent. Histological examination also showed that F. prausnitzii and its supernatant could also ameliorate mucosal inflammation and histopathologic damage compared with the TNBS model group. Compared with the TNBS model group, the serum TNF-α concentrations in F. prausnitzii (32.51 ± 16.41 vs. 96.55 ± 16.06) and its supernatant (39.27 ± 16.70 vs. 96.55 ± 16.06) were also significantly decreased (Fig. 1).
Fig. 1.
Effects of F. prausnitzii and its supernatant on intestinal inflammation in an enteritis mouse model. a Colon length; b body weight; c histological HE; d serum TNF-α concentration. Data are the mean ± SD. *P < 0.05
F. prausnitzii and its supernatant increase gut microbiota diversity
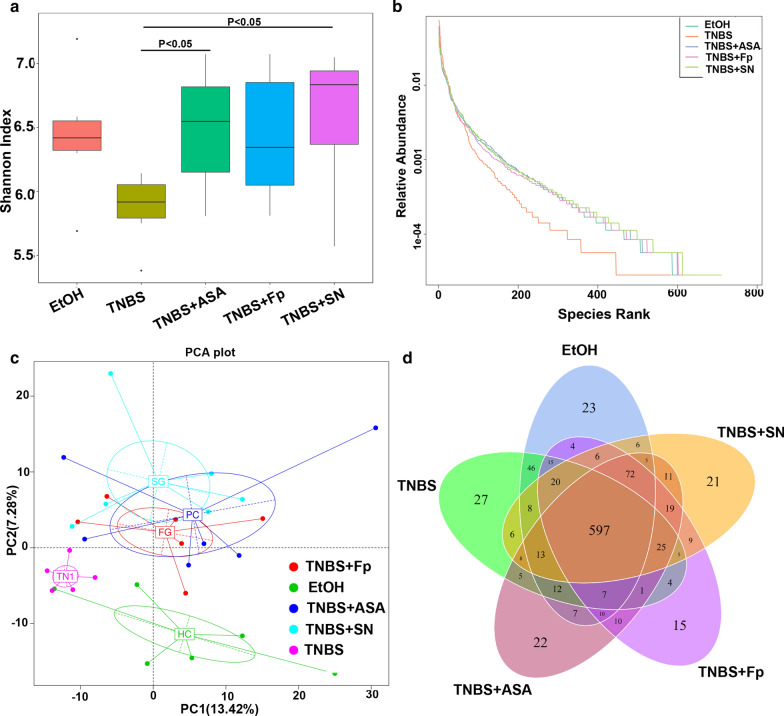
Mounting evidence shows that gut microbiota dysbiosis plays a significant role in the occurrence and development of IBD. To further study the effects of F. prausnitzii and its supernatant on the gut microbiota in IBD, we used 16S sequencing to further clarify the differences in the gut microbiome. Our findings indicated that the microbial diversity in the TNBS model group was significantly different from that in the EtOH control group, which was generally accompanied by a decrease in the diversity (Shannon index and species rank abundance curve) of gut microbiota. Treatment with both F. prausnitzii and its supernatant increased the diversity of the gut microbiota, especially in the supernatant group (P < 0.05). Based on the analysis of the number of OTUs, the 5 groups shared 597 OTUs, 23 of which were unique to the EtOH control group, while 27 were unique to the TNBS model group, and 22 were unique to the TNBS + ASA group. There were 15 and 21 unique OTUs in F. prausnitzii and its supernatant, respectively (Fig. 2).
Fig. 2.
F. prausnitzii and its supernatant significantly improved the diversity of the gut microbiota in colitis mice. a Shannon index; b species rank abundance curve; c PCA; d Venn diagram
F. prausnitzii and its supernatant restore gut microbiota dysbiosis and increase the abundance of SCFA- producing bacteria
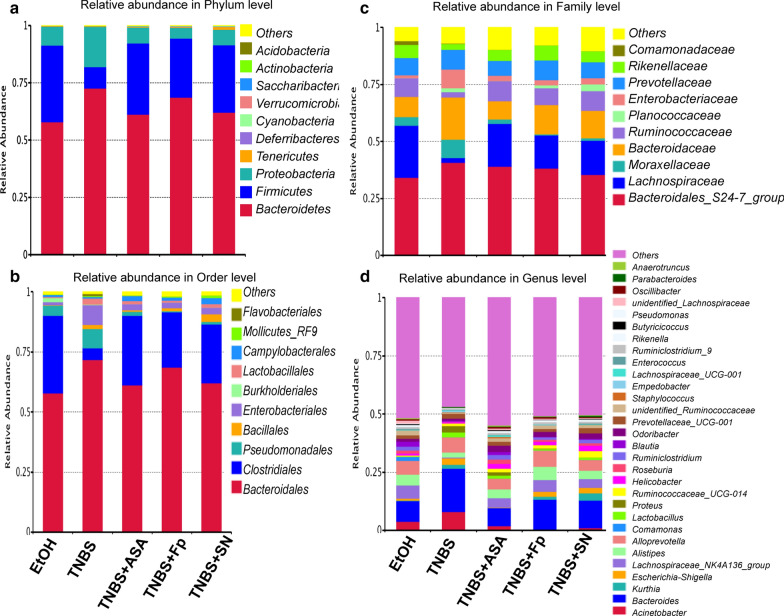
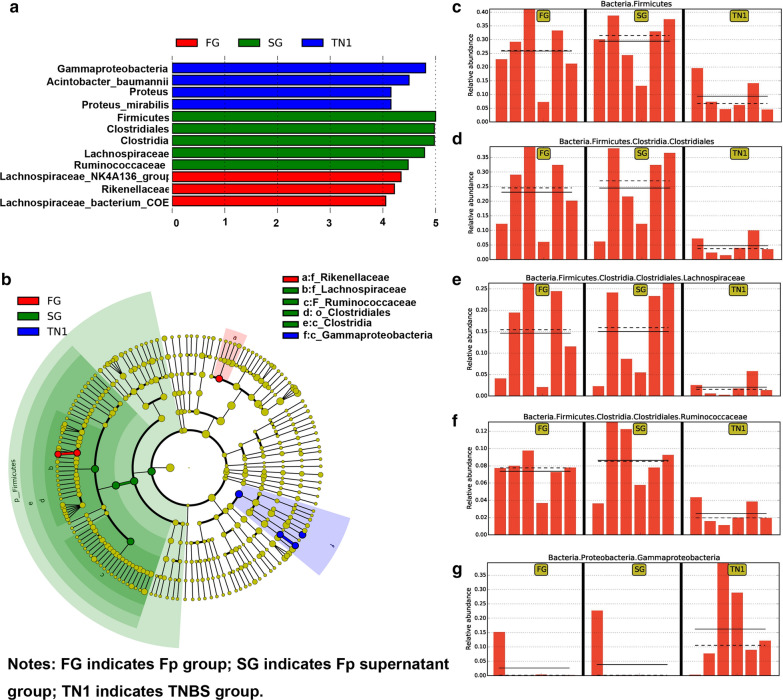
We further explored the changes in the gut microbiota composition, as shown by the relative abundance (Fig. 3), OTU level (Fig. 4) and LefSc biomarkers (Fig. 5). Compared with the TNBS model group, treatment with both F. prausnitzii and its supernatant increased the abundance of Firmicutes, Cyanobacteria and Clostridiales, and decreased the abundance of Proteinbacteria, Acidobacteria, and Bacteroidetes. At the genus level, F. prausnitzii increased the abundance of Butyricicoccus, Roseburia, Ruminiclostridium, Lachnospiraceae, Oscillibacter, Anaerotruncus, Ruminococcaceae, Alistipes, Rikenella, Allspirophore, Deisulfoprecotella, and Mucispirllum, which was more obvious in the F. prausnitzii supernatant group than in the TNBS model group. Notably, the Butyricicoccus, Roseburia, Lachnospiraceae, Ruminococcaceae, Rikenella, and Eubacterium_xylanophilum are short-chain fatty acid (SCFA)-producing bacteria.
Fig. 3.
Histogram of taxon levels. a Phylum level; b order level; c family level, and d genus level
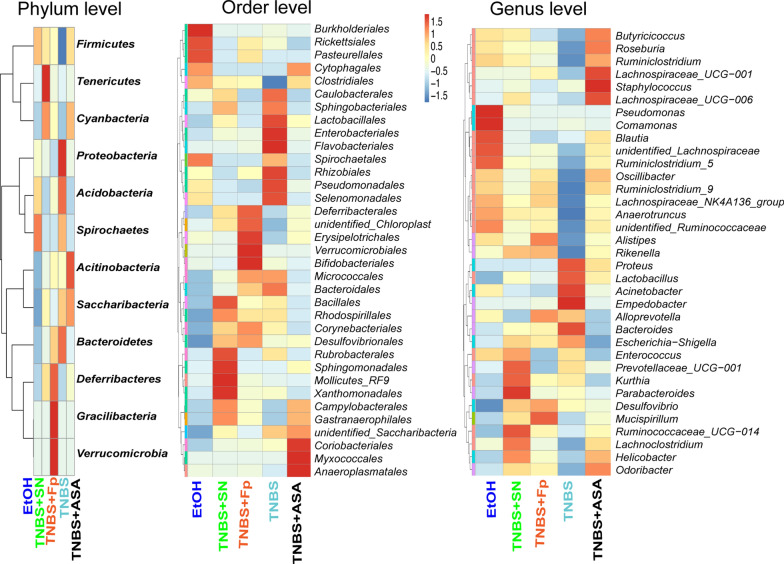
Fig. 4.
Microbial heatmap of the top OTUs at the phylum, order, and genus levels
Fig. 5.
Microbial biomarkers in the TNBS model, F. prausnitzii and its supernatant groups. a Linear discriminant analysis (LDA) score; b LDA effect size (LEfSe), and relative abundance of c Firmicutes, d Clostridiales, e Lachnospiraceae, f Ruminococcaceae, and g Gammaproteobacteria
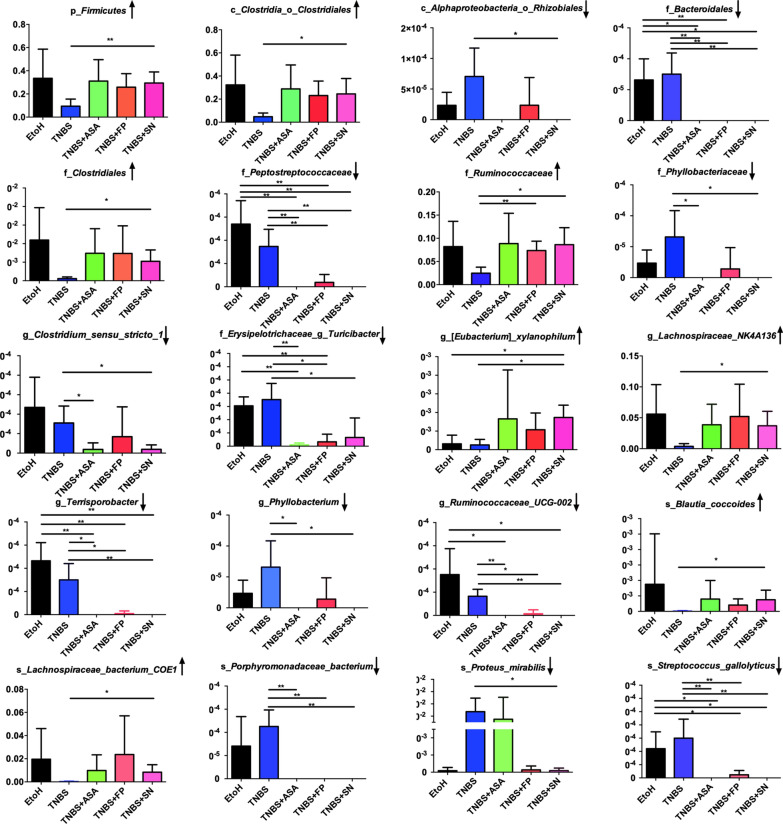
Next, we investigated significant taxonomic shifts in the microbial community through MetaStats analysis. Compared with the TNBS model group, F. prausnitzii treatment significantly increased the abundance of Ruminococcaceae (P < 0.01) and decreased the abundance of Peptostreptococcaceae (P < 0.01), Ruminococcaceae_UCG (P < 0.05), Terrisporobacter (P < 0.05), and Turicibacter (P < 0.05). These changes were the most obvious in the F. prausnitzii supernatant group (TNBS + SN) and were characterized by increased the abundance of Firmicutes (P < 0.01), Clostridiales (P < 0.05), Ruminococcaceae (P < 0.05), Clostridiales_vadinBB60 (P < 0.05), Lachnospiraceae_NK4A136 (P < 0.05), Eubacterium_xylanophilum (P < 0.05), Lachnospiraceae_bacterium_COE1 (P < 0.05), Blautia_coccoides (P < 0.05), and reducing the abundance of Rhizobiales (P < 0.05), Peptostreptococcaceae (P < 0.01), Phyllobacteriaceae (P < 0.05), Ruminococcaceae_UCG-002 (P < 0.01), Terrisporobacter (P < 0.01), Turicibacter (P < 0.05), Clostridium_sensu_stricto (P < 0.05), Phyllobacterium (P < 0.05), Proteus_mirabilis (P < 0.05), Streptococcus_gallolyticus (P < 0.01), and Porphyromonadaceae_bacterium (P < 0.01) (Fig. 6). Interestingly, Lachnospiraceae, Ruminococcaceae, Blautia and Eubacterium_xylanophilum are also SCFAs-producing bacteria. In addition, to determine the alterations of SCFAs after treatment of F. prausnitzii and its supernatant, fecal SCFAs including acetic acid, propionic acid, butyric acid and amyl acid were detected. As shown in Additional file 1: Figure S1, both F. prausnitzii and its supernatant could increase the content of SCFAs in the feces compared with the TNBS model group, further indicating that F. prausnitzii and its supernatant increase the abundance of SCFA-producing bacteria.
Fig. 6.
MetaStat analysis at different taxon classification levels. The X axis represents the sample groups, and the Y axis represents species abundance
Discussion
Inflammatory bowel diseases (IBD) are multifactorial disorders characterized by a partially unclear etiology and pathogenic mechanisms including gut microbiota dysbiosis. Our previous study (Zhou et al. 2018c) showed that the fecal microbiota of humans provides promising universal and promising biomarkers for the evaluation of IBD severity and IFX treatment efficacy. The composition of the intestinal microbiome and microbial-derived metabolites seem to influence the healthy state of the host both by both regulating metabolism-related pathways and affecting the expression of different genes via epigenetic effects (De Musis et al. 2020). Based on this, it has been suggested that the gut microbiota might be a promising therapeutic target in IBD. Accumulating studies have suggested that probiotic administration is useful for IBD.
Faecalibacterium prausnitzii, a representative commensal bacterium of the Firmicutes phylum, can decompose undigested cellulose in the intestinal lumen to produce SCFAs such as butyric acid, which not only provide energy for intestinal mucosal epithelial cells, but also benefit the intestinal environment and the stability of the intestinal mucosal barrier. Sokol et al. (2009) confirmed that the administration of F. prausnitzii A2-165 or its supernatant significantly decreased colitis severity induced by TNBS and restored gut microbiota dysbiosis, as demonstrated by real-time quantitative PCR (qPCR) analysis. F. prausnitzii A2-165 also exerted anti-inflammatory effects on both cellular and colitis animal models by blocking NF-KB activation and IL-8 production (Sokol et al. 2009), facilitating the induction of IL-10 in human and murine dendritic cells and modulating T cell responses (Rossi et al. 2016). Furthermore, F. prausnitzii (strain A2-165) or its supernatant has been confirmed to induce a significant reduction in colitis severity by downregulating MPO, pro-inflammatory cytokines, and T-cell levels (Martin et al. 2014), and protecting the intestinal epithelial barrier(Carlsson et al. 2013; Martin et al. 2015) in colitis mouse models. F. prausnitzii (ATCC 27766) or its supernatant can also exert protective effects by inhibiting Th17 differentiation and IL-17A secretion in both DSS-induced mouse colitis (Huang et al. 2016) and TNBS-induced colorectal colitis in rats (Zhang et al. 2014, 2019), and upregulating regulatory T cells (Qiu et al. 2013) and butyrate production to maintain Th17/Treg balance (Zhou et al. 2018a) in a colitis rat model and in vitro. In addition, the F. prausnitzii HTF-F strain and the extracellular polymeric matrix (EPM) exerted anti-inflammatory effects on the clinical parameters measured in the DSS model (Rossi et al. 2015).
In this study, we report the gut microbiota profile of TNBS-induced colitis mice treated with F. prausnitzii (ATCC 27766) and its supernatant on the basis of high-throughput sequencing, and we found that treatment with F. prausnitzii and its supernatant significantly improved the colitis-related symptoms, increased the gut microbial diversity and mitigated gut dysbiosis. Our previous study (Zhou et al. 2018c) confirmed a relative decrease in SCFA-producing bacteria, including F. prausnitzii, Lachnospiraceae, Ruminococcaceae and Roseburia, which all belong to Clostridiales within the Firmicutes phylum, in patients with IBD. In this study, we interestingly found that the protective effects of F. prausnitzii and its supernatant may result from an increase in the abundance of SCFA-producing bacteria such as Butyricicoccus, Roseburia, Lachnospiraceae, Ruminococcaceae, Rikenella, Eubacterium_xylanophilum and Blautia, indicating the restoration of gut microbiota dysbiosis after F. prausnitzii or supernatant treatment. The mechanism behind the potential protective role of F. prausnitzii on gut microbiota may associate with competition for nutrients, metabolites and occupying effects to regulate commensal organisms and restrain pathogenic bacteria. It was confirmed that butyrate-producing bacteria belong to the Firmicutes phylum, mainly including Clostridium leptum and Clostridium coccoides (Kumari et al. 2013), which are mainly represented by Faecalibacterium prausnitzii and Roseburia, respectively (Louis and Flint 2009).
SCFAs are organic fatty acids with aliphatic tails of five or fewer carbons that are mainly produced by anaerobic microorganisms fermenting indigestible carbohydrates. In vitro studies of human intestinal cells have shown that butyrate can promote intestinal mucosal repair and inhibit the formation of inflammatory cytokines, thereby exerting an anti-inflammatory effect (Augenlicht et al. 2002; Segain et al. 2000). Through intestinal perfusion, sodium butyrate can also promote colonic mucosal healing in TNBS-induced colitis. In vitro studies have shown that butyrate also exhibits antitumor effects, including the reduction of the secretion of tumor necrosis factor (TNF) in intestinal epithelial cells (Segain et al. 2000), and induction of the differentiation and apoptosis of tumor cells, thereby inhibiting tumor growth (Pryde et al. 2002). In addition, SCFAs especially butyrate could regulate gut microbiota by modulating intestinal lumen pH values that are beneficial for SCFAs-producing bacteria (Canani et al. 2011; Guilloteau et al. 2010). Moreover, butyrate could preserve epithelial hypoxia and limit the overgrowth of nitrate respiration-dependent bacteria to maintain intestinal homeostasis (Byndloss et al. 2017; Winter et al. 2013).
In summary, this work showed that both F. prausnitzii and its metabolites exerted protective effects against colitis in mice, which ameliorated gut dysbiosis, with an increase in bacterial diversity and the abundance of SCFA-producing bacteria and a decrease in serum TNF- α and the abundance of Proteinbacteria, Acidobacteria, and Bacteroidetes. These findings will provide further evidence of the anti-inflammatory effect of F. prausnitzii, which presents therapeutic potential for IBD treatment.
Supplementary Information
Additional file 1: Figure S1. The fecal SCFAs concentrations including acetic acid (A), propionic acid (B), butyric acid (C) and amyl acid (D).
Acknowledgements
We thank Professor Chenggong Yu and Dr. Mingming Zhang from Nanjing Medical University for kindly providing Faecalibacterium prausnitzii (F. prausnitzii, ATCC27766).
Abbreviations
- IBD
Inflammatory bowel disease
- TNBS
2,4,6-Trinitrobenzenesulfonic acid
- SCFAs
Short-chain fatty acids
- CD
Crohn’s disease
- UC
Ulcerative colitis
- 5-ASA
Five-aminosalicylates
- FMT
Fecal microbiota transplantation
- DSS
Dextran sodium sulfate
- OD
Optical density
- CFU
Colony forming unit
- SN
Supernatant
- SPF
Specific pathogen free
- GMLAC
Guangdong Medical Lab Animal Center
- PBS
Phosphate-buffered saline
- ELISA
Enzyme linked immunosorbent assay
- HE
Hematoxylin–eosin
- ANOVA
Analysis of variance
- OTU
Operational taxonomic unit
- LDA
Linear discriminant analysis
- LEfse
Linear discriminant analysis (LDA) effect size
- EPM
Extracellular polymeric matrix
- TNF
Tumor necrosis factor
Authors’ contributions
YLZ conducted the study, collected the data, and wrote the manuscript; HMX conducted the mouse model and collected the data; JX, XG, HLZ, collected the data; YC interpret the data and guide the strain culture; YQN and YJZ directed the project. All authors read and approved the final manuscript.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (81700487, 81871905), Guangdong Medical Science and Technology Research Fund (A2019243), Natural Science Foundation of Guangdong Province (2020A1515011000), Guangzhou Planned Project of Science and Technology (202002030293, 202002020012), and the Fundamental Research Funds for the Central Universities, SCUT (2018MS82).
Availability of data and materials
The data used to support the findings of this study are available in NCBI-SRA under accession number PRJNA669888 (https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA669888).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Youlian Zhou and Haoming Xu contributed equally to this work
Contributor Information
Youlian Zhou, Email: eyyoulianzhou@scut.edu.cn.
Haoming Xu, Email: 771805286@qq.com.
Jing Xu, Email: 201820144495@mail.scut.edu.cn.
Hailan Zhao, Email: 201910108691@mail.scut.edu.cn.
Ye Chen, Email: yechen_fimmu@163.com.
Yongjian Zhou, Email: eyzhouyongjian@scut.edu.cn.
Yuqiang Nie, Email: eynieyuqiang@scut.edu.cn.
References
- Augenlicht LH, Mariadason JM, Wilson A, Arango D, Yang W, Heerdt BG, Velcich A. Short chain fatty acids and colon cancer. J Nutr. 2002;132(12):3804S–3808S. doi: 10.1093/jn/132.12.3804S. [DOI] [PubMed] [Google Scholar]
- Byndloss MX, Olsan EE, Rivera-Chavez F, Tiffany CR, Cevallos SA, Lokken KL, Torres TP, Byndloss AJ, Faber F, Gao Y, Litvak Y, Lopez CA, Xu G, Napoli E, Giulivi C, Tsolis RM, Revzin A, Lebrilla CB, Baumler AJ. Microbiota-activated PPAR-gamma signaling inhibits dysbiotic Enterobacteriaceae expansion. Science. 2017;357(6351):570–575. doi: 10.1126/science.aam9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. 2011;17(12):1519–1528. doi: 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson AH, Yakymenko O, Olivier I, Hakansson F, Postma E, Keita AV, Soderholm JD. Faecalibacterium prausnitzii supernatant improves intestinal barrier function in mice DSS colitis. Scand J Gastroenterol. 2013;48(10):1136–1144. doi: 10.3109/00365521.2013.828773. [DOI] [PubMed] [Google Scholar]
- De Musis C, Granata L, Dallio M, Miranda A, Gravina AG, Romano M. Inflammatory bowel diseases: the role of gut microbiota. Curr Pharm Des. 2020;26(25):2951–2961. doi: 10.2174/1381612826666200420144128. [DOI] [PubMed] [Google Scholar]
- Friswell M, Campbell B, Rhodes J. The role of bacteria in the pathogenesis of inflammatory bowel disease. Gut Liver. 2010;4(3):295–306. doi: 10.5009/gnl.2010.4.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss IJ, Boirivant M, Lacy B, Strober W. The interrelated roles of TGF-beta and IL-10 in the regulation of experimental colitis. J Immunol. 2002;168(2):900–908. doi: 10.4049/jimmunol.168.2.900. [DOI] [PubMed] [Google Scholar]
- Guilloteau P, Martin L, Eeckhaut V, Ducatelle R, Zabielski R, Van Immerseel F. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr Res Rev. 2010;23(2):366–384. doi: 10.1017/S0954422410000247. [DOI] [PubMed] [Google Scholar]
- He H, Xu H, Xu J, Zhao H, Lin Q, Zhou Y, Nie Y. Sodium butyrate ameliorates gut microbiota dysbiosis in lupus-like mice. Front Nutr. 2020;7:604283. doi: 10.3389/fnut.2020.604283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XL, Zhang X, Fei XY, Chen ZG, Hao YP, Zhang S, Zhang MM, Yu YQ, Yu CG. Faecalibacterium prausnitzii supernatant ameliorates dextran sulfate sodium induced colitis by regulating Th17 cell differentiation. World J Gastroenterol. 2016;22(22):5201–5210. doi: 10.3748/wjg.v22.i22.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari R, Ahuja V, Paul J. Fluctuations in butyrate-producing bacteria in ulcerative colitis patients of North India. World J Gastroenterol. 2013;19(22):3404–3414. doi: 10.3748/wjg.v19.i22.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294(1):1–8. doi: 10.1111/j.1574-6968.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- Martin R, Chain F, Miquel S, Lu J, Gratadoux JJ, Sokol H, Verdu EF, Bercik P, Bermudez-Humaran LG, Langella P. The commensal bacterium Faecalibacterium prausnitzii is protective in DNBS-induced chronic moderate and severe colitis models. Inflamm Bowel Dis. 2014;20(3):417–430. doi: 10.1097/01.MIB.0000440815.76627.64. [DOI] [PubMed] [Google Scholar]
- Martin R, Miquel S, Chain F, Natividad JM, Jury J, Lu J, Sokol H, Theodorou V, Bercik P, Verdu EF, Langella P, Bermudez-Humaran LG. Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model. BMC Microbiol. 2015;15:67. doi: 10.1186/s12866-015-0400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel S, Leclerc M, Martin R, Chain F, Lenoir M, Raguideau S, Hudault S, Bridonneau C, Northen T, Bowen B, Bermudez-Humaran LG, Sokol H, Thomas M, Langella P. Identification of metabolic signatures linked to anti-inflammatory effects of Faecalibacterium prausnitzii. mBio. 2015 doi: 10.1128/mBio.00300-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;217(2):133–139. doi: 10.1111/j.1574-6968.2002.tb11467.x. [DOI] [PubMed] [Google Scholar]
- Qiu X, Zhang M, Yang X, Hong N, Yu C. Faecalibacterium prausnitzii upregulates regulatory T cells and anti-inflammatory cytokines in treating TNBS-induced colitis. J Crohn's Colitis. 2013;7(11):e558–e568. doi: 10.1016/j.crohns.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Rossi O, Khan MT, Schwarzer M, Hudcovic T, Srutkova D, Duncan SH, Stolte EH, Kozakova H, Flint HJ, Samsom JN, Harmsen HJ, Wells JM. Faecalibacterium prausnitzii strain HTF-F and its extracellular polymeric matrix attenuate clinical parameters in DSS-induced colitis. PLoS ONE. 2015;10(4):e0123013. doi: 10.1371/journal.pone.0123013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi O, van Berkel LA, Chain F, Tanweer Khan M, Taverne N, Sokol H, Duncan SH, Flint HJ, Harmsen HJ, Langella P, Samsom JN, Wells JM. Faecalibacterium prausnitzii A2-165 has a high capacity to induce IL-10 in human and murine dendritic cells and modulates T cell responses. Sci Rep. 2016;6:18507. doi: 10.1038/srep18507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segain JP, Raingeard de la Bletiere D, Bourreille A, Leray V, Gervois N, Rosales C, Ferrier L, Bonnet C, Blottiere HM, Galmiche JP. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut. 2000;47(3):397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottiere HM, Dore J, Marteau P, Seksik P, Langella P. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105(43):16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier I, Beaugerie L, Cosnes J, Corthier G, Marteau P, Dore J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15(8):1183–1189. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- Varela E, Manichanh C, Gallart M, Torrejon A, Borruel N, Casellas F, Guarner F, Antolin M. Colonisation by Faecalibacterium prausnitzii and maintenance of clinical remission in patients with ulcerative colitis. Aliment Pharmacol Ther. 2013;38(2):151–161. doi: 10.1111/apt.12365. [DOI] [PubMed] [Google Scholar]
- Wang S, Lv D, Jiang S, Jiang J, Liang M, Hou F, Chen Y. Quantitative reduction in short-chain fatty acids, especially butyrate, contributes to the progression of chronic kidney disease. Clin Sci. 2019;133(17):1857–1870. doi: 10.1042/CS20190171. [DOI] [PubMed] [Google Scholar]
- Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J, Lawhon SD, Popova IE, Parikh SJ, Adams LG, Tsolis RM, Stewart VJ, Baumler AJ. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science. 2013;339(6120):708–711. doi: 10.1126/science.1232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Qiu X, Zhang H, Yang X, Hong N, Yang Y, Chen H, Yu C. Faecalibacterium prausnitzii inhibits interleukin-17 to ameliorate colorectal colitis in rats. PLoS ONE. 2014;9(10):e109146. doi: 10.1371/journal.pone.0109146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zhou L, Wang Y, Dorfman RG, Tang D, Xu L, Pan Y, Zhou Q, Li Y, Yin Y, Zhao S, Wu J, Yu C. Faecalibacterium prausnitzii produces butyrate to decrease c-Myc-related metabolism and Th17 differentiation by inhibiting histone deacetylase 3. Int Immunol. 2019;31(8):499–514. doi: 10.1093/intimm/dxz022. [DOI] [PubMed] [Google Scholar]
- Zhou L, Zhang M, Wang Y, Dorfman RG, Liu H, Yu T, Chen X, Tang D, Xu L, Yin Y, Pan Y, Zhou Q, Zhou Y, Yu C. Faecalibacterium prausnitzii produces butyrate to maintain Th17/Treg balance and to ameliorate colorectal colitis by inhibiting histone deacetylase 1. Inflamm Bowel Dis. 2018;24(9):1926–1940. doi: 10.1093/ibd/izy182. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Ou Z, Tang X, Zhou Y, Xu H, Wang X, Li K, He J, Du Y, Wang H, Chen Y, Nie Y. Alterations in the gut microbiota of patients with acquired immune deficiency syndrome. J Cell Mol Med. 2018;22(4):2263–2271. doi: 10.1111/jcmm.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Xu ZZ, He Y, Yang Y, Liu L, Lin Q, Nie Y, Li M, Zhi F, Liu S, Amir A, Gonzalez A, Tripathi A, Chen M, Wu GD, Knight R, Zhou H, Chen Y. Gut microbiota offers universal biomarkers across ethnicity in inflammatory bowel disease diagnosis and infliximab response prediction. mSystems. 2018 doi: 10.1128/mSystems.00188-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. The fecal SCFAs concentrations including acetic acid (A), propionic acid (B), butyric acid (C) and amyl acid (D).
Data Availability Statement
The data used to support the findings of this study are available in NCBI-SRA under accession number PRJNA669888 (https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA669888).