On the 30th of January 2020, the WHO declared a public health emergency of international concern, the outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV 2), causing coronavirus disease (COVID-19), a new infectious disease that emerged in Wuhan, China at the end of 2019 and expeditiously spread to several other countries worldwide [1].
While COVID-19 may present with a multitude of manifestations, commonly including cough, sore throat and anosmia, it mainly presents as a viral pneumonia. Although the vast majority of patients do not experience severe respiratory distress requiring hospitalization, more critically ill patients (commonly elderly patients and those with underlying medical conditions such as cardiovascular disease, diabetes and chronic respiratory disease) often require invasive mechanical ventilation.
Due to the uncontrolled spread of this pandemic, and as such, an exponential rise in the number of symptomatic patients, there has been a dramatic escalation in the numbers of severe cases of COVID-19 patients requiring invasive respiratory support. As such, rapid adaptation of clinical practice is essential. Unfortunately, due to the paucity of evidence-based studies on COVID-19 infection (in part due to its novelty), intensivists and acute care surgeons have struggled in dealing with critically ill COVID-19 patients in regards to critical care decision making.
A recent meta-analysis study revealed that 26% of patients with COVID-19 infection were admitted to ICU globally and the prevalence of mortality among COVID-19 patients in the ICU reached up to 31% [2].
Early reports out of Wuhan, China mentioned that around 71% of critically ill patients with SARS-CoV-2 pneumonia required mechanical ventilation [3].
Another study from Lombardy Region, Italy suggests that many patients needed intubation for at least 1–2 weeks and the intensive care unit (ICU) stay seems to be relatively long [4]. This actively led to an increase in the number of candidates for tracheostomy globally.
The estimated percentage of tracheostomies performed among patients with severe COVID-19 infection varies from 29.3% [5] to 36.1% [6], 40.9% [7] and 53% [8] as reported in the literature.
Because of the nature of COVID-19 infection and its ability to affect multi-organs systems, a multidisciplinary team should handle the decision of tracheostomy on a case-by-case basis. Weighing the risks and benefits for both patient and health care workers (HCW).
Many studies have been conducted to prove the safety of this procedure. Thus far, the available data on the timing [early vs. late], location [bed side vs. OR] and techniques [percutaneous vs surgical opening vs hybrid] of tracheostomy during the current pandemic are conflicting. Few studies focused on how tracheostomy would affect the clinical course of this disease and described the outcomes of tracheostomy after a considerable period of follow up.
The aim of this review is to reach a forthright statement on whether tracheostomy gives superior outcomes for COVID-19 patients who are ventilator-dependent or with a predicted prolonged intubation and if it improves their prognosis. We will also highlight evidence based guidelines and protocols for performing tracheostomy safely and effectively during the COVID-19 pandemic.
1. Methods and material
We reviewed the available literature with interest in assessing the harms and benefits of carrying out tracheostomies in patients who are candidates for this procedure.
1.1. Inclusion criteria
-
✓
Sample size ≥ 10
-
✓
Diagnosis of COVID-19
-
✓
Describe the outcomes in at least one of the following areas:
-
➢
Rate of complications
-
➢
Percentage of weaning from mechanical ventilation (MV)
-
➢
Percentage of successful decannulation
1.2. Exclusion criteria
Studies that didn't report the complications, rate of successful weaning from MV and/or decannulation among COVID-19 patients who underwent tracheostomy were excluded. Studies with an inadequate follow up period after the procedure, and small case series and case reports were excluded as well.
1.3. Search strategy
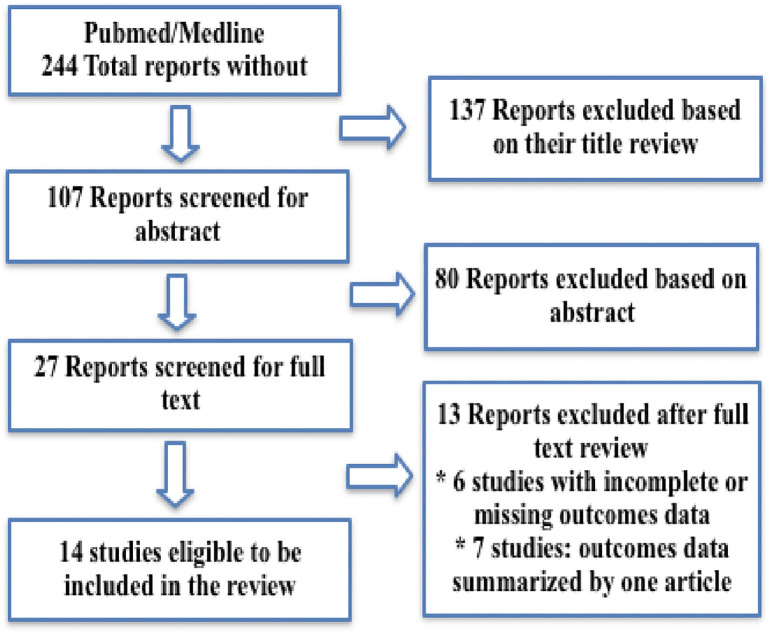
A comprehensive literature search in PubMed database looking for all available English reports and articles published during the year 2020 among COVID-19 patients who were tracheostomized as apart of their critical care management using the following terms: tracheostomy/tracheotomy and COVID-19/SARS CoV2. Followed by an analysis of the titles and abstracts [Fig. 1 ].
Fig. 1.
Shows exclusion and exclusion criteria of search strategy.
2. Results
In total, 244 reports and articles were identified after the initial search, of which 137 unrelated reports were excluded based on their titles, and the rest included for abstract review. Reports that met the inclusion criteria were gathered for formal review and full-text assessed by two independent authors. After full screening, selected articles were utilised in this review to compare their data.
The extracted data included: author name, country, sample size, type of tracheostomy, timing, the complications up to and including death, rate of successful weaning from MV and decannulation [Table 1, Table 2 ].
Table 1.
Studies selected for the review.
| Study | Country | Sample size | PDT vs OST | Timing from ETI | Complication rate | |
|---|---|---|---|---|---|---|
| Martin-Villares C. et al. [9] | Spain | 1890a | 429 | 1461 | 12 (median) | 60/1890 |
| Breik O. et al. [10] | UK | 100 | 75 | 25 | 13.9 (mean) | 13/100 |
| Angel L. et al. [11] | USA | 98 | 98 | 0 | 10.6 (mean) | 5/98 |
| Yeung et al. [7] | UK | 72 | 28 | 44 | 17 (median) | 7/72 intraOP 10/72 late |
| Tornari C. et al. [5] | UK | 78b | 73 | 2 | 16 (median) | 13/69 |
| Picetti E. et al. [8] | Italy | 66 | 0 | 66 | 6.1(mean) | 9/66 |
| Chao T.N. et al. [12] | USA | 53 | 29 | 24 | 19.7 (mean) | 2/53 |
| Botti C. et al. [13] | Italy | 44 | 15 | 29 | 7 (median) | 25/44 |
| Floyd E. et al. [14] | USA | 38 | 0 | 38 | 24 (median) | 4/38 |
| Zuazua-Gonzalez A. et al. [6] | Spain | 30 | 0 | 30 | 10.1 (mean) | 18/30 |
| Glibbery N. et al. [15] | UK | 28 | 3 | 25 | 17 (mean) | Minimal |
| Volo et al. [16] | Italy | 23 | 1 | 22 | 13 (mean) | 1/23 |
| Zhang X. et al. [17] | China | 11 | 6 | 5 | 16.8 (mean) | 3/11 |
| Broderick D. et al. [18] | UK | 10 | 0 | 10 | 11–27 days | 2/10 |
| Total | – | 2541 | 760 | 1781 | – | 172/2504 (6.7%) |
PDT: percutaneous dilatational tracheostomy, OST: open surgical treacheostomy, ETI: endotracheal intubation.
274 participant missing data.
78 of which 69 were included in the review, 3 underwent hybrid tracheostomies.
Table 2.
Percentage of MV weaning, decannulation and death among tracheostomized COVID-19 patients.
| Study | Sample size | Follow up period/day | MV weaning %/days | Decannulation % | Deaths % |
|---|---|---|---|---|---|
| Martin-Villares C. et al. [9] | 1890a | 30 days | 842/1616 (52.1%) | 683/1616 | 383/1616 (23.7%) |
| Breik O. et al. [10] | 100 | 30 days | 85/100 (85%) | 84/100 mean 14 days | 15/100 (15%) |
| Angel L. et al. [11] | 98 | Mean 11 (SD 6) | 32/98 (33%) | 8/98 (8%) | 7/98 (7%) |
| Yeung. et al. [7] | 72 | Median 26 (IQR18.8–3) | 44/72 61.1% median 10 days | 25/72 | 7/72 (9.7%) |
| Tornari C. et al. [5] | 78b | Median 21 (IQR 15–28) | 46/69 (66.6%) | 35/69 | 4/78 (5.1%) |
| Picetti E. et al. [8] | 66 | Retrospective study | Mean 21.4 days (SD 7.2) | N/A | 15/66 (22.7%) |
| Chao T.N. et al. [12] | 53 | Minimum 14 days PT | 30/53 (56.6%) 11.8 days (SD 6.9) | 7/53 (13.2%) 16.6 days (SD 5.0) | 6/53 (11.3%) |
| Botti C. et al. [13] | 44 | Median 22 days PI | Median 35 days PI (18–79) | Mean 36 (10–77) day PT | 15/44 (34.1%) |
| Floyd E. et al. [14] | 38 | Minimum 14 days PT | 21/38 (55.2%) median 10 days | 7/38 (18.4%) median 14 days | 2/38 (5.3%) |
| Zuazua-Gonzalez A. et al. [6] | 30 | Minimum 14 days PT | 7/30 mean 28.14 days (SD 10.1) | N/A | 17/30 (56.7%) |
| Glibbery N. et al. [15] | 28 | Mean 57.7 PT (SD11.7) | 25/28 mean 13.4 days PT | N/A mean 15.8 day PT | 2/28 (7.1%) |
| Volo et al. [16] | 23 | Median 50 days | N/A | 6/23 (26%) | 9/23 (39%) |
| Zhang X et al. [17] | 11 | Retrospective study | 9/11 (81.8%) mean 6.9 days | N/A | N/A |
| Broderick D. et al. [18] | 10 | 2–22 day | 7/10 (70%) | 6/10 (60%) | 0 |
| Total | 2541 | – | 1148/2125 (54%) | 861/2079 (41.4%) | 482/2256 (21.4%) |
MV: mechanical ventilation, IQR: interquartile range, SD: standard deviation, PT: post tracheostomy, PI: post intubation. N/A: not available.
274 participant missing data.
78 of which 69 were included in the review.
A total of 14 studies with 2541 participants were included in this review. Of these, 5 studies were conducted in the UK, 3 studies from Italy, 3 studies from the USA, one from China and 2 studies from Spain (of which one study [9] is a multicentre prospective observational study collecting data from the UK, Italy, China, Japan and Spain).
1781 patients underwent open surgical tracheostomy while 757 had their tracheostomies with percutaneous dilatational technique, and only 3 patients underwent hybrid tracheostomy.
283 participants were excluded due to incomplete or missing follow up data. All studies but one reported the number of patients who had immediate or short term complications related to the procedure which was estimated to be 6.7%. This figure is similar to the percentage of short-term tracheostomy related complications seen in non COVID-19 patients [38].
The percentage of fruitful weaning from MV was 54%, which was calculated from the studies that reported the number of tracheostomized patients who were successfully liberated from invasive mechanical ventilators (1148/2125). Similarly, 861/2079 (41.4%) underwent successful decannulation until the date of studies publication so these numbers are for approximate estimation.
Including all studies but one, the total number of deaths (thus the mortality rate) calculated among tracheostomised COVID-19 patients in the ICU was 21.4% (482/2256) which is possibly an underestimation because many of the patients were still undergoing management in the ICU at the time of the studies release.
However, this preliminary outcome seems to be promising, as the ICU mortality among COVID-19 patients on IMV is 41.6% [39].
3. Discussion
As of December 1st, 2020, the total number of COVID-19 cases had reached over 61.8 million reported cases with nearly 1.4 million deaths globally since the start of the pandemic [19].
In medium and low income countries, shortly after the first case of SARS-CoV2 infection was reported, this outbreak rapidly evolved into a national health Crisis. As this devastating pandemic raged on, the world was leaning on experts to help deal with this outbreak. It was anticipated that healthcare demand would exceed supply, and by now most countries have exhausted their medical resources and ICU capacities have been overwhelmed. This placed frontline staff under immense pressure to adopt measures that would help take the edge off and free up desperately needed ICU beds in order to maximize their capacity. On that ground, experts started to think up new strategies. For instance, a modified weaning approach from mechanical ventilation was adopted by the Brompton Hospital, during the first wave of COVID-19 pandemic in London [20], by using 32 tracheostomy-portable ventilators weaning approach, they freed up 32 ICU ventilators and saved 230 ICU ventilator days. This result gives some hope, but needs evaluation for feasibility and cost-effectiveness.
3.1. Argument for and against tracheostomy in COVID-19 era
Indeed, even before COVID-19 outbreak the benefit of tracheostomy to patients who need prolonged invasive mechanical ventilation is well established. It allows weaning of relaxation and sedation drugs thus reducing delirium in ICU patients [21], it facilitates pulmonary hygiene and suctioning to get rid of excessive secretions, helps in decreasing dead space, lessening respiratory effort, giving more time for the lungs to recover, and enhances weaning from mechanical ventilation, therefore shortening ICU length of stay [22].
Moreover, the endotracheal tube itself may cause a variety of complications. It may damage the vocal cords if left in for more than 10 days, and it may cause subglottic stenosis and laryngeal granulomas as well as perioral pressure sores if intubation is prolonged. These issues may be avoided if tracheostomy is done [[23], [24]].
Not to mention the fact that prone-postioning used in the management of COVID-19 in the ICU can lead to upper airway edema (along with edema from COVID-19) which increases the propensity to laryngeal edema, hence precluding extubation, so airway protection by tracheostomy may be beneficial [25].
On the other hand, there are many general procedural and post procedural complications of tracheostomy that are well known, in COVID-19 patients and otherwise. These include bleeding, pneumothorax, false passage, tube dislodgment, stoma infection, air leak and vocal cord palsy [23].
Furthermore, it is widely known that tracheostomies are aerosol generating procedures [26], and as such, they pose a significant risk of SARS-CoV2 transmission to the surgeon and the team members within the operating theatre. Previous studies show that the risk of viral transmission from tracheostomy is second only to intubation [40]. Therefore, it is crucial to follow strict evidence based general and procedural guidelines when performing tracheostomy to reduce the risk of viral transmission to medical personnel.
Experts in the field suggest that applying the essential infection-control measures would lessen the viral transmissibility. As evidenced by this review, in all the tracheostomies carried out in multiple centres worldwide, regardless of the timing or the technique, none of the health care workers or hospital staff involved in the procedure, be it surgeons, anaesthesiologists or nurses, reported any symptoms and/or demonstrated seroconversion within 2 weeks of this procedure, provided that they all followed the guidelines and committed to the use of appropriate PPE and adhered to strict donning and doffing procedures [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18]].
Based on the above-mentioned information, benefits must be balanced against the fear of the risks.
The earliest recommendations from the British Laryngological Association (BLA) and ENT-UK suggest that tracheostomy should be considered after at least 14 days of intubation in COVID-19 patients [27].
Similarly, the New York Head and Neck Society recommends waiting until 21 days after the onset of symptoms to perform tracheostomy in COVID-19 patients except in situations in which it would significantly improve the prognosis of the patient [28].
Consistently, The American Academy of Otolaryngology–Head and Neck Surgery advised to wait at least 2–3 weeks with negative COVID-19 testing preferred before considering the procedure [29].
As highlighted above, these recommendations were due in a part to the possibility of viral transmission particularly to healthcare workers involved in performing this procedure or providing care for the patients afterwards. Therefore, deferring the procedure when feasible until the viral load potentially dropped off would decrease the risk of transmission.
In addition, the prognosis in critically ill COVID-19 patients seems to be bad, with some regions publishing alarmingly high ICU mortality rates that reached up to 78%, as in Wuhan, China [30], despite the fact that other reports from Wuhan described lower ICU mortality rates of 16% [31] and 49% [32] along with studies from Italy, USA and UK reporting ICU mortality rates of 26% [33], 39% [34] and 51.6% [35] respectively. As of yet the prognosis for severe COVID-19 patients requiring critical care is poorly understood, and some publications were released while a significant portion of patients remained in the ICU which may lead to an underestimation of the actual mortality rate.
With scarcity of data on the positive influence of early tracheostomy on the prognosis and the clinical course of the disease, many would prefer to delay the decision of tracheostomy until having better idea about the prognosis in each selected case, and to identify the patients who have a sufficient chance of survival. In this context, many adopted the worldwide recommendation for tracheostomy timing [12,14,36]. In fact, a recent study conducted by Volo et al. [16] found that the ICU mortality rate of non-tracheostomized patients was 35% compared to 18% in patients with tracheostomy, with two thirds of these tracheostomies being performed early.
However, others focused their efforts to establish an evidence-based statement on the safety and effectiveness of early tracheostomy and to prove its positive influence on the prognosis along with liberation of ventilators and sorely needed ICU beds [6,8,11,13].
One significant study conducted by Queen Elizabeth Hospital Birmingham COVID-19 airway team indicates that tracheostomized patients exhibited better survival rate than the non-tracheostomized after one month of follow up, and showed that early tracheostomies done within 14 days of intubation were associated with shorter duration on ventilators and reduced ICU stay. Consequently they were able to gain approximately 448 bed days by performing 64 earlier tracheostomies [10].
Another article recently published in the Respiratory Care Journal [37] reviewed several guidelines and protocols from different institutions, which concluded that tracheostomy can be carried out safely as early as 7 days after intubation in COVID-19 positive patients without jeopardizing the safety of patients and healthcare workers.
So far, there was insufficient data to decide whether the location and technique of this procedure would affect the outcomes, although one study suggests that earlier MV weaning could be achieved in patients who underwent surgical open tracheostomies compared to percutaneous technique [7]. However, as we have highlighted previously, a multidisciplinary team should make the decision based on patient prognostic factors and institutional resources.
3.2. Procedural guidelines
As evidenced above, it is widely known that tracheostomies are aerosol generating procedures, and as such, pose a significant risk of SARS-CoV2 transmission to the surgeon and the team members within the operating theatre. Therefore, regardless of the timing and setting of the procedure, it is crucial to follow strict evidence based general and procedural guidelines when performing tracheostomy to reduce the risk of viral transmission to medical personnel. Below we will highlight some of these guidelines and protocols.
The procedure must ideally be done in a negative pressure operating theatre [[40], [41], [42],[45], [46], [47], [48], [49]], with only the most experienced healthcare staff available present during the operation to reduce staff exposure [40,44,[46], [47], [48], [49]]. All personnel present in the operating theatre must follow appropriate donning and doffing protocols, and use appropriate PPE (N95 mask, surgical gown, coverall, face shield, double gloving, head cover and shoe cover) [[40], [41], [42], [43], [44], [45], [46], [47], [48], [49]].
Before starting the procedure, when switching from the ventilator to the anesthesia machine, the endotracheal tube must be clamped to avoid aerosolisation of the virus, followed by deep closed circuit suctioning of the tube [48]. The anesthesiologist must achieve deep neuromuscular blockade with complete paralysis to avoid the patients cough reflex [40,[48], [49]].
In order to reduce airflow through the surgical incision site, and by extension, aerosolization of viral particles, the endotracheal tube must be advanced until the cuff is distal to the site of tracheal window creation, followed by hyperinflation of the cuff [[44], [45], [46], [47], [48], [49]]. Pre‑oxygenation must be done (with PEEP), followed by cessation of ventilation after an exhalation before surgical incision is performed [40,[47], [48], [49]].
When performing the surgical incision to create the tracheal window, care must be taken to avoid damaging the endotracheal tubes cuff, and electrocautery should be avoided to reduce vapours [40,41,45,48]. After creating the tracheal window, the endotracheal tube must be clamped with cessation of ventilation, deflation of the cuff, and removing of the tube until the lower portion is seen just proximal to the tracheal window site [[40], [41], [42],[47], [48], [49]].
A cuffed, non-fenestrated tracheostomy tube is then inserted, the introducer replaced with a non-fenestrated inner tube, a heat and moisture exchanger connected and the cuff immediately inflated. Once this is done, the circuit can be reattached and ventilation resumed [40,41,[46], [47], [48], [49]]. After confirming the tracheostomy tube is in place, the clamped endotracheal tube may be removed and discarded, and the tracheostomy tube may be secured with stay sutures [40,41,44,46,48,49].
Post operatively, any medical personnel dealing with the tracheostomy (suctioning/dressing) must wear full PPE. Furthermore, changing the dressing of the tracheostomy must be done only if necessary, and the first tracheostomy change should be delayed until the tests negative for SARS-CoV2 to reduce unnecessary risk to healthcare staff [41,44,46].
4. Conclusion
In our review, we aimed to elaborate the possible gain from performing tracheostomy for candidate COVID-19 patients and weighing the benefits with the complications and the risk of this procedure. We believe the evidence shown above shows that, given proper safety protocols and guidelines are followed, the benefits of tracheostomy (and specifically early tracheostomy) in appropriate candidates outweighs the risks to the patient and involved healthcare personnel. In actuality, more data is needed with a large scale randomized controlled trial (RCT) comparing the outcomes between patients who underwent tracheostomy with ICU patients who did not and evaluating the correlation of the timing or the technique with such outcomes.
References
- 1.https://www.who.int/news/item/27-04-2020-who-timeline---covid-19
- 2.Abate S.M., Ahmed Ali S., Mantfardo B., Basu B. Rate of intensive care unit admission and outcomes among patients with coronavirus: a systematic review and meta-analysis. PLoS One. 2020 Jul 10;15(7) doi: 10.1371/journal.pone.0235653. PMID: 32649661; PMCID: PMC7351172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Xiaobo, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G., Zangrillo A., Zanella A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tornari C., Surda P., Takhar A., et al. Tracheostomy, ventilatory wean, and decannulation in COVID-19 patients. Eur Arch Otorhinolaryngol. 2020 doi: 10.1007/s00405-020-06187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuazua-Gonzalez A., Collazo-Lorduy T., Coello-Casariego G., et al. Surgical tracheostomies in COVID-19 patients: indications, technique, and results in a second-level Spanish hospital. OTO Open. 2020 doi: 10.1177/2473974X20957636. July. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeung E., Hopkins P., Auzinger G., Fan K. Challenges of tracheostomy in COVID-19 patients in a tertiary centre in inner city London. Int J Oral Maxillofac Surg. 2020;49(11):1385–1391. doi: 10.1016/j.ijom.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Picetti E., Fornaciari A., Taccone F.S., et al. Safety of bedside surgical tracheostomy during COVID-19 pandemic: a retrospective observational study. PLoS One. 2020;15(9) doi: 10.1371/journal.pone.0240014. Published 2020 Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin-Villares C., Perez Molina-Ramirez C., Bartolome-Benito M., Bernal-Sprekelsen M. COVID ORL ESP Collaborative Group (*). Outcome of 1890 tracheostomies for critical COVID-19 patients: a national cohort study in Spain [published online ahead of print, 2020 Aug 4] Eur Arch Otorhinolaryngol. 2020:1–8. doi: 10.1007/s00405-020-06220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Queen Elizabeth Hospital Birmingham COVID-19 Airway Team Safety and 30-day outcomes of tracheostomy for COVID-19: a prospective observational cohort study. Br J Anaesth. 2020;125(6):872–879. doi: 10.1016/j.bja.2020.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angel L., Kon Z.N., Chang S.H., et al. Novel percutaneous tracheostomy for critically ill patients with COVID-19. Ann Thorac Surg. 2020;110(3):1006–1011. doi: 10.1016/j.athoracsur.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao, Tiffany N et al. “Outcomes after tracheostomy in COVID-19 patients.” Ann Surg, vol. 272,3 e181–e186. 11 Jun. 2020, doi: 10.1097/SLA.0000000000004166. [DOI] [PMC free article] [PubMed]
- 13.Botti Cecilia, et al. The role of tracheotomy and timing of weaning and decannulation in patients affected by severe COVID-19. Ear Nose Throat J. 2020;145561320965196:9. doi: 10.1177/0145561320965196. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Floyd E., Harris S.S., Lim J.W., Edelstein D.R., Filangeri B., Bruni M. Early data from case series of tracheostomy in patients with SARS-CoV-2. Otolaryngology–Head and Neck Surgery. 2020;163(6):1150–1152. doi: 10.1177/0194599820940655. [DOI] [PubMed] [Google Scholar]
- 15.Glibbery N., et al. Tracheostomy in the coronavirus disease 2019 patient: evaluating feasibility, challenges and early outcomes of the 14-day guidance. J Laryngol Otol. 2020;134(8):688–695. doi: 10.1017/S0022215120001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volo T., Stritoni P., Battel I., et al. Elective tracheostomy during COVID-19 outbreak: to whom, when, how? Early experience from Venice, Italy [published online ahead of print, 2020 Jul 12] Eur Arch Otorhinolaryngol. 2020:1–9. doi: 10.1007/s00405-020-06190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X., Huang Q., Niu X., et al. Safe and effective management of tracheostomy in COVID-19 patients. Head Neck. 2020;42(7):1374–1381. doi: 10.1002/hed.26261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broderick Damian, et al. Surgical tracheostomies in COVID-19 patients: a multidisciplinary approach and lessons learned. Oral Oncol. 2020;106 doi: 10.1016/j.oraloncology.2020.104767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.https://www.who.int/publications/m/item/weekly-epidemiological-update---1-december-2020
- 20.Singh S., Hind M., Jordan S., et al. Weaning by surgical tracheostomy and portable ventilators released ICU ventilators during coronavirus disease 2019 surge in London. Crit Care Explor. 2020;2(8) doi: 10.1097/CCE.0000000000000193. Published 2020 Aug 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.https://www.entnet.org/content/tracheotomy-recommendations-during-covid-19-pandemic
- 22.Cheung NH, Napolitano LM. Tracheostomy: epidemiology, indications, timing, technique, and outcomes. Respir Care 2014 Jun;59(6):895–915; discussion 916-9. doi: 10.4187/respcare.02971. PMID: 24891198. [DOI] [PubMed]
- 23.Andriolo B.N., Andriolo R.B., Saconato H., Atallah Á.N., Valente O. Early versus late tracheostomy for critically ill patients. Cochrane Database Syst Rev. 2015;1(1) doi: 10.1002/14651858.CD007271.pub3. Published 2015 Jan 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu C.C., Livingstone D., Dixon E., Dort J.C. Early versus late tracheostomy: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2015 Feb;152(2):219–227. doi: 10.1177/0194599814561606. Epub 2014 Dec 12. PMID: 25505259. [DOI] [PubMed] [Google Scholar]
- 25.Naunheim M.R., Zhou A.S., Puka E., et al. Laryngeal complications of COVID-19. Laryngoscope Investigative Otolaryngology. 2020:1–8. doi: 10.1002/lio2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson T, Deibert D, Wyatt G, et al. Classification of aerosol-generating procedures: a rapid systematic review. BMJ Open Resp Res 2020;7:e000730. doi:10.1136/bmjresp-2020-000730. [DOI] [PMC free article] [PubMed]
- 27.https://www.britishlaryngological.org/news/tracheostomy-guideline-covid-19
- 28.Miles B.A., Schiff B., Ganly I., et al. Tracheostomy during SARS-CoV-2 pandemic: recommendations from the New York Head and Neck Society. Head Neck. 2020;42(6):1282–1290. doi: 10.1002/hed.26166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.https://www.entnet.org/content/tracheotomy-recommendations-during-covid-19-pandemic
- 30.Zhou Fei, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Zunyou, McGoogan Jennifer M. Characteristics of and important lessons from the coronavirus disease, (COVID-19) outbreak in China: summary of a report of 72,314 cases from the chinese center for disease control and prevention. JAMA. 2019 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 33.Giacomo G., Alberto Z., Alberto Z., Massimo A., Luca C., Antonio C., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matthew J.C., Matthew R.B., Darryl A., Samuel D.J., Benjamin J.M., Elizabeth M.B., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020 doi: 10.1016/s0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takhar A., Walker A., Tricklebank S., Wyncoll D., Hart N., Jacob T., et al. Recommendation of a practical guideline for safe tracheostomy during the COVID-19 pandemic. Eur Arch Otorhinolaryngol. 2020;21:1–12. doi: 10.1007/s00405-020-05993-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishio Naoki, et al. Surgical strategy and optimal timing of tracheostomy in patients with COVID-19: early experiences in Japan. Auris Nasus Larynx. 19 Nov. 2020 doi: 10.1016/j.anl.2020.11.004. S0385-8146(20)30306-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiang S.S., Aboutanos M.B., Jawa R.S., Kaul S.K., Houng A.P., Dicker R.A., et al. Controversies in tracheostomy for patients with COVID-19: the when, where, and how. Respir Care. 2020 Nov;65(11):1767–1772. doi: 10.4187/respcare.08100. Epub 2020 Sep 1. PMID: 32873749. [DOI] [PubMed] [Google Scholar]
- 38.Young D., Harrison D.A., Cuthbertson B.H., Rowan K., TracMan Collaborators FT Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. JAMA. 2013;309(20):2121–2129. doi: 10.1001/jama.2013.5154. [DOI] [PubMed] [Google Scholar]
- 39.Armstrong R.A., Kane A.D., Cook T.M. Outcomes from intensive care in patients with COVID-19: a systematic review and meta-analysis of observational studies. Anaesthesia. 2020 Oct;75(10):1340–1349. doi: 10.1111/anae.15201. Epub 2020 Jul 15. PMID: 32602561. [DOI] [PubMed] [Google Scholar]
- 40.Michetti C.P., Burlew C.C., Bulger E.M., et al. Trauma Surg Acute Care Open. 2020;5 doi: 10.1136/tsaco-2020-000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacob Tony. Framework for open tracheostomy in COVID-19 patients, ENTUK website. https://www.entuk.org/sites/default/files/files/COVID%20tracheostomy%20guidance_compressed.pdf [DOI] [PMC free article] [PubMed]
- 42.Tay J.K., Khoo M.L., Loh W.S. Surgical considerations for tracheostomy during the COVID-19 pandemic: lessons learned from the severe acute respiratory syndrome outbreak. JAMA Otolaryngol Head Neck Surg. 2020 doi: 10.1001/jamaoto.2020.0764. Published online March 31. [DOI] [PubMed] [Google Scholar]
- 43.Chan J.Y.K., Wong E.W.Y., Lam W. Practical aspects of otolaryngologic clinical services during the 2019 novel coronavirus epidemic: an experience in Hong Kong. JAMA Otolaryngol Head Neck Surg. 2020 doi: 10.1001/jamaoto.2020.0488. Published online March 20. [DOI] [PubMed] [Google Scholar]
- 44.Harrison Laura, Ramsden James, et al. Tracheostomy guidance during the COVID-19 pandemic, ENTUK website. March 19, 2020. https://www.entuk.org/tracheostomy-guidance-during-covid-19-pandemic
- 45.Mattioli F., Fermi M., Ghirelli M., et al. Tracheostomy in the COVID-19 pandemic. Eur Arch Otorhinolaryngol. 2020 doi: 10.1007/s00405-020-05982-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chow Tiffany, Braslow Benjamin, et al. Tracheotomy in ventilated patients with COVID-19, Annals of Surgery website. https://journals.lww.com/annalsofsurgery/Documents/Tracheotomy%20in%20ventilated%20patients%20with%20COVID19.pdf [DOI] [PMC free article] [PubMed]
- 47.NTSP considerations for tracheostomy in the Covid-19 outbreak, National tracheostomy safety project website. March 20, 2020. http://www.tracheostomy.org.uk/storage/files/NTSP%20COVID_19%20tracheostomy%20guidance%2031_3_20.pdf
- 48.Broderick D., et al. Surgical tracheostomies in Covid-19 patients: important considerations and the “5Ts” of safety. Br J Oral Maxillofac Surg. 2020 doi: 10.1016/j.bjoms.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pichi B., Mazzola F., Bonsembiante A., et al. CORONA-steps for tracheotomy in COVID-19 patients: a staff-safe method for airway management [published online ahead of print, 2020 Apr 6] Oral Oncol. 2020;105 doi: 10.1016/j.oraloncology.2020.104682. [DOI] [PMC free article] [PubMed] [Google Scholar]