Abstract
The ability of cells to promote plasminogen activation on their surfaces is now well recognized, and several distinct cell surface proteins have been demonstrated to function as plasminogen receptors. Here, we review studies demonstrating that plasminogen bound to cells, in addition to plasminogen directly bound to fibrin, plays a major role in regulating fibrin surveillance. We focus on the ability of specific plasminogen receptors on eukaryotic cells to promote fibrinolysis in the in vivo setting by reviewing data obtained predominantly in murine models. Roles for distinct plasminogen receptors in fibrin surveillance in intravascular fibrinolysis, immune cell recruitment in the inflammatory response, wound healing, and lactational development are discussed.
Keywords: plasminogen, fibrinolysis, plasminogen receptors
1. Introduction
The ability of cells to promote plasminogen activation on their surfaces is now well recognized, and several distinct cell surface proteins have been demonstrated to function as plasminogen receptors. Here we review studies demonstrating that plasminogen bound to cells, in addition to plasminogen directly bound to fibrin, has a major role in regulating fibrinolysis. We focus on the ability of specific plasminogen receptors on eukaryotic cells to promote fibrinolysis in the in vivo setting by reviewing data obtained predominantly in murine models.
2. Similarities in Plasminogen Activation on Fibrin and on Cell Surfaces
Over 25 years ago, the ability of plasminogen to interact with platelets was first demonstrated [1]. Subsequently, the presence of plasminogen binding sites on virtually every cell type examined (with the exception of red cells) has been established (reviewed in [2]). The interaction of plasminogen with cell surfaces mimics the interaction of plasminogen with fibrin. That is, when plasminogen is bound to cells, its activation is markedly enhanced compared with the reaction in solution reviewed in [3]) and Figure 1. Similarly to plasminogen activation on fibrin [4], this enhancement takes place upon the concomitant localization of plasminogen activators, tissue type plasminogen activator (t-PA) and/or urokinase (u-PA) on the cell surface.
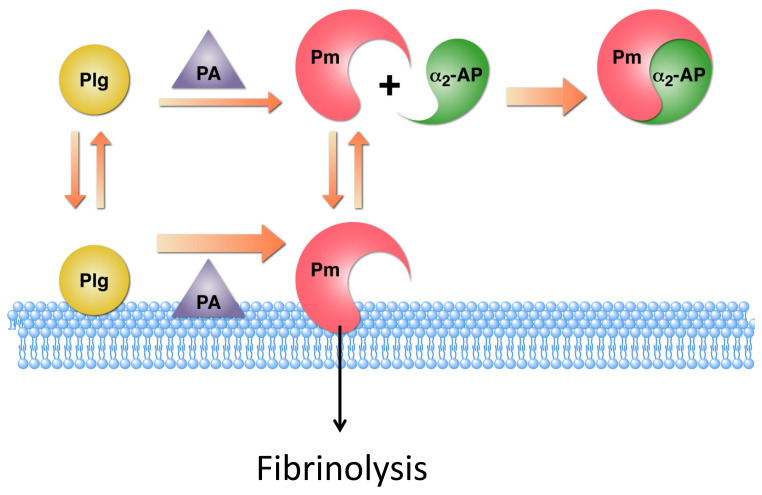
Figure 1.
Enhancement of plasminogen activation on the cell surface. Activation of cell-associated plasminogen (Plg) to plasmin (Pm) by cell-associated plasminogen activators (PA) is markedly enhanced compared to the reaction in solution. The Pm formed remains on the cell surface, where it is relatively protected from its inhibitor, α2-antiplasmin (α2-AP). This results in the association of the proteolytic activity of plasmin with the cell surface to function in fibrinolysis. This figure is modified from a figure originally published in © 2012 Miles, L.A.; Andronicos, N.M.; Chen, E.I.; Baik, N.; Bai, H.; Parmer, C.M.; Lighvani, S.; Nangia, S.; Kiosses, W.B.; Kamps, M.P.; Yates, J.R., III; Parmer, R.J. Published in under CC BY 3.0 license. Available online: http://dx.doi.org/10.5772/31113 [7].
Furthermore, as with the interaction of plasminogen with fibrin, its interaction with eukaryotic cell surfaces is mediated by the lysine binding sites within plasminogen kringles [5] that interact with the carboxyl-terminal lysyl residues exposed on cell surfaces. In additional concordance with plasminogen activation on fibrin, the removal of carboxyl-terminal basic residues with carboxypeptidase B (CpB) results in the complete loss of the ability of cell surfaces to promote plasminogen activation [6].
Conformational changes induced by the interaction of plasminogen with cellular receptors also contribute to enhanced plasminogen activation on cell surfaces. It has been suggested that a major effect of the interaction of Glu-plasminogen with cell surfaces is to induce a conformational change in Glu-plasminogen that mimics the conformation of the more readily activated Lys-plasminogen, without the necessary N-terminal plasmic cleavage. However, recent studies indicate that the interaction of Glu-plasminogen with cell surfaces induces a conformational change that then promotes plasmic cleavage to the more readily activated Lys-plasminogen form [8,9,10,11].
On both the cell surface and on the fibrin surface, the formed plasmin is relatively protected from its major inhibitor, α2-antiplasmin [12,13] (Figure 1). As the initial interaction with plasmin is mediated via the C-terminal lysine of α2-antiplasmin [14], the protection from inhibition on cell surfaces is most likely due to the competition between the C-terminus of α2-antiplasmin and the C-terminal lysines exposed on cell surfaces.
The result of the foregoing interactions is the localization of the proteolytic activity of plasmin on cell surfaces, suggesting that when cells are located in the proximity of fibrin, cell surface plasminogen receptors have the potential to promote fibrinolysis in vivo.
3. Cellular Plasminogen Receptors
Numerous studies have addressed the molecular identity of plasminogen binding sites on eukaryotic cells. Consistent with the high capacity of cells for plasminogen and the diversity of cell types that interact with plasminogen, several cell surface molecules with plasminogen-binding function have been identified. CpB-sensitive cellular plasminogen receptors now comprise three categories: (1) a transmembrane protein synthesized with a C-terminal lysine and exposing a C-terminal lysine on the cell surfaces, plasminogen receptor KT (Plg-RKT) [15]; (2) proteins synthesized with C-terminal basic residues and lacking transmembrane domains, and having well-established intracellular functions, including α-enolase [16,17], cytokeratin 8 [18,19], S100A10 (in complex with annexin A2 within the annexin A2 heterotetramer (AIIt)) [20], histone H2B [21] and TIP49a [22], and (3) proteins that require proteolytic processing in order to expose a C-terminal basic residue to permit plasminogen binding, including actin [23,24,25]. A CpB-insensitive component of plasminogen binding is present on eukaryotic cells that does not promote plasminogen activation [6], which includes tissue factor [26] and non-protein gangliosides [27]. Additionally, transmembrane proteins (αIIbβ3 [28], αMβ2 [29,30] and α5β1 [30], as well as amphoterin [31] and GP330 [32]) bind plasminogen, but are not synthesized with C-terminal basic residue, and whether these proteins undergo proteolysis to reveal C-terminal basic residues is not known. Specific review articles regarding the identification, functions and mechanisms of action of plasminogen receptors can be found in the volume introduced by reference [33].
The role of cell surface-associated plasmin in fibrinolysis in vivo is most likely carried out by plasminogen receptors exposing C-terminal lysines on the cell surfaces. Of this category, genetic deletion resulting in viable mice has been performed only for annexin A2 [34], S100A10 [35] and Plg-RKT [36]. The assessment of the functions of other plasminogen receptors, H2B and α-enolase, has been carried out using antibody inhibition approaches [37,38].
4. Intravascular Fibrinolysis
Plasminogen-deficient mice exhibit severe spontaneous thrombosis in multiple organs [39,40]. The annexin A2/S100A10 heterotetrameric complex (AIIt) plays a clear role in regulating intravascular fibrinolysis, in both spontaneously arising thrombosis and challenge models [41]. The separate components of AIIt regulate each other’s expression [42,43,44,45]. Consequently, the genetic deletion of either annexin A2 or S100A10 in mice results in the deletion of the other component [44]. Annexin A2 deficiency results in spontaneous microvascular fibrin deposition in kidney, lung, heart, spleen liver and small intestine [34], while spontaneous fibrin deposition has been examined and demonstrated in the lung, liver and kidney of S100A10-deficient mice [35]. In addition, S100A10-deficient mice exhibit a four-fold reduction in bleeding time following tail clipping, consistent with decreased fibrinolysis of blood clots [35]. In contrast, Plg-RKT deficiency does not have a significant effect on spontaneous fibrin deposition based on the examination of hematoxylin and eosin-stained tissues, including kidney, lung, liver, brain, heart muscle, skeletal muscle, lymheph nodes, thymus, spleen, stomach, small intestine, large intestine, colon, cecum, rectum, pancreas, adrenal, uterus, ovaries, vas deferens, testes, and epididymis [36], and genetic deletion of Plg-RKT has no effect on bleeding time in response to tail clipping [46]. In contrast, Plg-RKT deletion results in impaired fibrinolysis during wound healing and lactational development, as described below in Section 6 and Section 7. These differential effects on intravascular and tissue fibrinolysis may relate to differences in specific cell types present adjacent to and within fibrin clots in the local environment, as well as to the age of thrombi.
A role for AIIt in intravascular fibrinolysis is also demonstrated in challenge models. In response to FeCl3-induced carotid artery thrombosis, annexin A2-deficient mice exhibit impaired clearance of acute arterial thrombi [47]. In a batroxobin-induced clot lysis model, S100A10-deficient mice show a dramatic decrease in the ability to clear fibrin clots, compared with wild type controls [35]. These challenge models have not been tested in Plg-RKT-deficient mice to date.
5. Immune Cell Recruitment in the Inflammatory Response
Studies in plasminogen-deficient mice have revealed an essential role for plasminogen in macrophage and lymphocyte recruitment in the pro-inflammatory phase of the immune response [48,49,50,51]. Recent studies employing the thioglycollate-induced model of sterile peritonitis have established that impaired fibrinolysis is a major contributor to impaired macrophage recruitment in plasminogen-deficient mice [52]. Genetic deficiency of fibrinogen or the removal of the αMβ2-binding domain of fibrinogen corrects the defect in macrophage recruitment in plasminogen-deficient mice [52]. These results were interpreted to suggest a requirement for fibrinolysis to disrupt the interaction of leukocytes with fibrino(ogen) via integrin αMβ2 [52]. In addition, the removal of fibrin from the extravascular space also involves a plasmin-dependent endocytotic pathway on CCR2-positive macrophages [53].
The participation of plasminogen receptors that expose C-terminal basic amino acids on the cell surface is essential for optimal macrophage recruitment in sterile peritonitis [54]. Antibody inhibition approaches have revealed roles for H2B [37], α-enolase [37], and Plg-RKT [55] in macrophage recruitment in this model, as well as a role for α-enolase in lung inflammation [38]. Genetic deletion has also revealed roles for S100A10 [56] and Plg-RKT [36,57] in macrophage recruitment in sterile peritonitis. The direct effects of specific plasminogen receptors on fibrinolysis have not been investigated, but are likely to take place, based on the requirement for fibrinolysis in this model [52].
The participation of multiple plasminogen receptors in inflammatory macrophage recruitment is consistent with a muti-step process that may utilize distinct plasminogen receptors at different steps. Thus, traversing different barriers with distinct extracellular matrix compositions may require the involvement of distinct plasminogen receptors at distinct sites, and also requires fibrin(ogen)-independent steps. In addition to fibrinolysis and fibrin endocytosis, MMP9 activation is required for optimal plasminogen-dependent macrophage recruitment [58]. Notably, impaired activation of MMP9 is exhibited by S100A10-deficient macrophages in culture [56], as well as with the treatment of mice with an anti-Plg-RKT monoclonal antibody in the sterile peritonitis model [55]. In addition, synthesis of the chemotactic cytokine, CCL2, that promotes macrophage recruitment, is impaired in models of immune cell recruitment in plasminogen-deficient mice [50,51], and Plg-RKT deficiency results in decreased CCL2 synthesis [51]. Furthermore, the expression of distinct plasminogen receptors is likely to be distinct on distinct macrophage subtypes, as exemplified by the increased expression of Plg-RKT on proinflammatory monocytes and macrophages [57].
6. Wound Healing
Wound healing is a complex and central pathophysiologic process that proceeds in overlapping, sequential steps [59]. In response to wounding, vascular permeability increases, leading to extravascular fibrin deposition in the wounded area as a key hemostatic event [60]. Subsequently, the proliferation phase of wound healing is characterized by fibrin clearance via fibrinolysis, and keratinocyte proliferation and migration through the fibrin-rich extracellular matrix [61].
Studies in plasminogen-deficient patients have documented an essential requirement for plasminogen in normal wound healing [62,63,64,65,66]. Impaired fibrinolysis in affected individuals leads to the formation of ligneous fibrin-rich pseudomembranes at mucosal sites during wound healing [67], most commonly seen in the conjunctivae and less frequently in the periodontal and gingival mucosae. In addition, the involvement of the mucosae of the respiratory tract and upper and lower gastrointestinal tracts can result in life threatening pseudomembranous obstruction [64].
Impaired wound healing in plasminogen-deficient mice was first demonstrated in an incisional skin wounding model [68]. Wounds from plasminogen-deficient mice were characterized by fibrin(ogen)-rich deposits in front of migrating keratinocytes, while fibrin(ogen) was uniformly present in wounds of wild type mice, leading to the conclusion that a major factor in the impaired wound healing in plasminogen-deficient mice is the decreased ability of keratinocytes to proteolytically dissect the fibrin-rich extracellular matrix [68]. Impairment in healing following myocardial infarction was also demonstrated in plasminogen-deficient mice, although fibrin deposition was not evaluated [69]. Impaired fibrinolysis is observed in several other models of wound healing in plasminogen-deficient mice. Plasminogen-deficient mice spontaneously develop otitis media with extensive fibrin deposition and abnormal keratin formation in the tympanic membrane, middle ear cavity and external ear canal [70]. Furthermore, several steps in the healing of perforated tympanic membranes, including the removal of fibrin, are impaired in these mice [71]. In concordance with the sites of impaired wound healing in plasminogen-deficient humans, plasminogen-deficient mice develop periodontitis with detachment of gingival tissue that is characterized by fibrin deposition [72]. Plasminogen is required for several steps of the wound healing process. Long after re-epithelialization, plasminogen-deficient mice exhibit extensive fibrin deposition in the wounded area, indicating inefficient debridement, thus emphasizing an additional role for plasminogen in the termination of the wound healing response [73]. In a model of radiation injury, fibrin deposition is present in the wound, and treatment with plasminogen accelerates healing and removes fibrin deposits [74]. In view of the many examples of impaired fibrin clearance in plasminogen-deficient mice, it is most noteworthy that the healing defect in plasminogen-deficient mice is corrected by the genetic deletion of fibrinogen [75], leading to the suggestion that the primary function of plasminogen in wound healing is fibrinolysis [76].
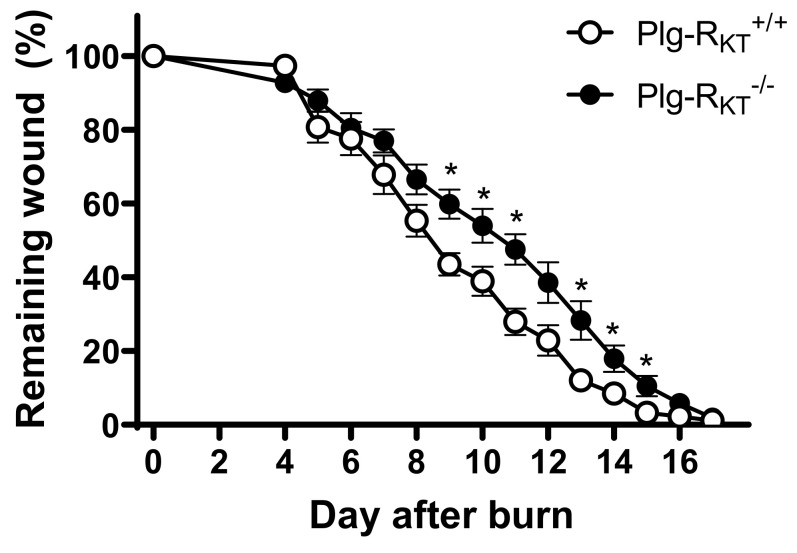
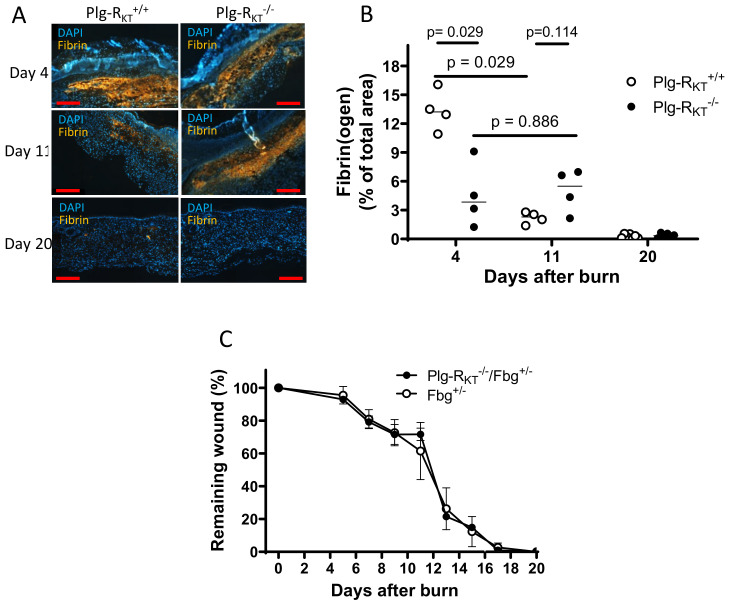
Recent studies have demonstrated a novel key role for plasminogen in wound healing, in addition to orchestrating fibrinolysis. Plasminogen binds to macrophages and neutrophils and is transported to the wound area, where the level of plasminogen is locally increased. This induces intracellular signaling and cytokine release [76]. Notably, the recruitment of immune cells to cutaneous wounds is not affected by plasminogen deficiency [68,73,76] and thus, the role of plasminogen in the initial stages of inflammation is predominantly the induction of intracellular signaling. Earlier studies in vitro documented the plasmin-dependent stimulation of intracellular signaling pathways, and cytokine release by monocytes and macrophages that depends on the interaction of plasmin with cell surfaces [77,78,79]. Notably, the deletion of Plg-RKT in mice (Plg-RKT−/− mice) leads to a significant delay in cutaneous wound healing (Figure 2) [80]. Consistent with studies in plasminogen-deficient mice, the rate of fibrin clearance is markedly impaired in wounds of Plg-RKT−/− mice (Figure 3A,B) [80]. Moreover, the genetic reduction of fibrinogen levels by 50% in Plg-RKT−/− mice corrects the wound healing impairment in these mice (Figure 3C) [80].
Figure 2.
Effect of Plg-RKT deficiency on burn wound healing. (A) Quantification of the remaining wound area at different time points after wounding of male Plg-RKT−/− mice (●) and Plg-RKT+/+ litter mates (○) (n = 6). The mixed effects model (REML) showed a significant effect for time (p < 0.0001, f = 396.6), and for genotype (p = 0.040, f = 6.00), and a significant time X genotype interaction (p < 0.0001, f = 4.289). Post hoc testing was done with 2-tailed t tests at each time point * p < 0.05. This figure is modified from a figure originally published in [80]. Creative common license available at: http://creativecommons.org/licenses/by/4.0/.
Figure 3.
Enhanced fibrin(ogen) content in Plg-RKT−/− wound tissue and effect of fibrinogen heterozygosity on burn wound healing. (A) Immunohistochemical staining for fibrin in the tissue of Plg-RKT−/− and Plg-RKT+/+ mice collected at different time points after induction of burn wounds (n = 4). Scale bar = 200 µm. (B) Quantification of fibrinogen area in Panel A (%) based on immunostaining using Image J software https://imagej.nih.gov/ij/download.html. (C) Quantification of the remaining wound area at different time points after burn wounding of male Plg-RKT−/−/Fgn+/− (n = 4) mice and Plg-RKT+/+/Fgn+/− mice (n = 5). Post hoc testing was done by Mann–Whitney test. This figure is modified from a figure originally published in [80]. Creative common license available at: http://creativecommons.org/licenses/by/4.0/.
Plg-RKT deletion also impairs immune cell function in wound healing, although there is no significant effect on immune cell recruitment to the wound site [80] (similar to results with plasminogen-deficient mice [68,73,76]). The injection of mice with an anti-Plg-RKT blocking monoclonal antibody impairs plasminogen transport to the wound area [80]. Furthermore, the specific deletion of Plg-RKT in myeloid cells results in a prominent and highly significant delay in wound healing [80].
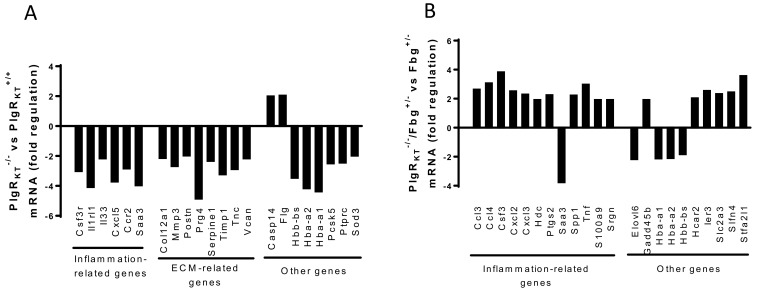
A comparison of gene expression profiles in wound tissue of Plg-RKT−/− mice and their wild type littermates showed the downregulation of six genes participating in the inflammatory response, including cytokines, and eight genes related to the extracellular matrix (Figure 4A) [80]. Remarkably, in Plg-RKT−/− mice with genetic heterozygosity for fibrinogen, the expression of eleven inflammation-related genes was upregulated, while these genes were not upregulated in Plg-RKT−/− wound tissue on a fibrinogen wild type background (Figure 4B) [80].
Figure 4.
Effect of Plg-RKT deletion on gene expression in wound tissue. Gene expression was studied using mRNA sequencing in samples taken at day 11 (n = 4). All the genes had corrected p-value ≤ 0.05. (A) Genes whose expression changed ≥ 1.5-fold in Plg-RKT−/− compared with Plg-RKT+/+ tissue. (B) Genes whose expression changed ≥ 1.5-fold in Plg-RKT−/−/Fibrinogen+/− compared with Plg-RKT+/+/Fibrinogen+/− tissue. This figure is modified from a figure originally published in [80]. Creative common license available at: http://creativecommons.org/licenses/by/4.0/.
Thus, Plg-RKT regulates wound healing via promoting fibrinolysis as well as regulating plasminogen-dependent cytokine release and intracellular signaling. Moreover, these latter functions are related by the effects of fibrin(ogen) on intracellular signaling mediated by Plg-RKT.
7. Lactation
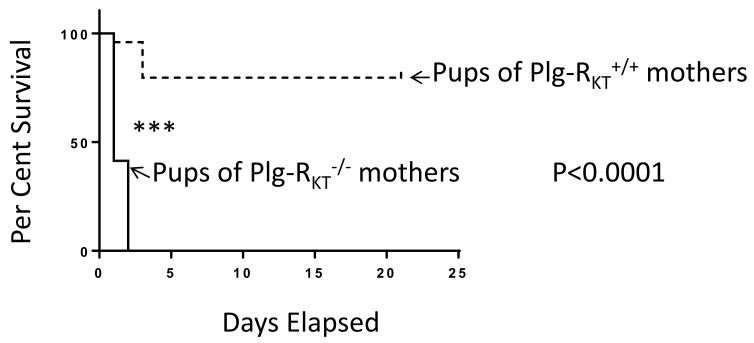
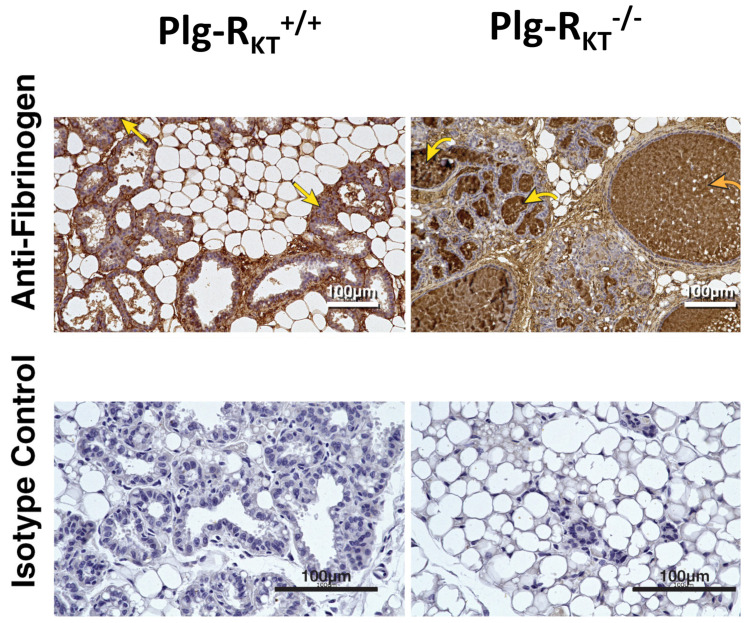
Plasminogen plays an essential role in lactational development in the mouse mammary gland [81,82]. Whereas 60% of offspring of plasminogen-deficient mice are alive at postpartum day 2, 75% of pups die by postpartum day 17 [82]. Although milk synthesis is not affected, fibrin deposition is noted in the ducts of plasminogen-deficient mice, consistent with the blockade of ducts, preventing the release of milk. Consequently, the lactation defect is rescued when plasminogen-deficient mice are bred into a heterozygous fibrinogen background [82]. Plg-RKT−/− mice exhibit a more severe lactation defect in which all offspring of Plg-RKT−/− mice die by postpartum day 2 (Figure 5) [83]. A massive accumulation of fibrin is present in the alveoli and ducts of these mice (Figure 6) [83].
Figure 5.
Plg-RKT null mice cannot successfully lactate. Survival data are shown for a cohort of offspring of Plg-RKT+/+ (dashed lines) and Plg-RKT−/− (solid lines) primiparous female littermates: 48 offspring of Plg-RKT+/+ mice and 30 offspring of Plg-RKT−/− mice. **** p < 0.0001 Log-rank (Mantel Cox test). This research was originally published in Miles, L.A.; Baik, N.; Bai, H.; Makarenkova, H.P.; Kiosses, W.B.; Krajewski, S.; Castellino, F.J.; Valenzuela, A.; Varki, N.M.; Mueller, B.M.; Parmer, R.J. The plasminogen receptor, Plg-RKT, is essential for mammary lobuloalveolar development and lactation. J. Thromb. Haemost. 2018, 16, 919–932 [83], and is reprinted with permission from John Wiley and Sons.
Figure 6.
Plg-RKT deletion alters the ECM in lactating mammary glands. Abdominal mammary glands were harvested from Plg-RKT+/+ and Plg-RKT−/− female mice two days postpartum and stained with anti-fibrin(ogen) antibody (the curved yellow arrow labels represent fibrin deposition in alveoli; the curved orange arrow labels represent fibrin deposition in dilated ducts; the straight yellow arrows labels represent fibrin deposition in adipose tissue) or non-immune rabbit IgG control. This research was originally published in Miles, L.A.; Baik, N.; Bai, H.; Makarenkova, H.P.; Kiosses, W.B.; Krajewski, S.; Castellino, F.J.; Valenzuela, A.; Varki, N.M.; Mueller, B.M.; Parmer, R.J. The plasminogen receptor, Plg-RKT, is essential for mammary lobuloalveolar development and lactation. J. Thromb. Haemost. 2018, 16, 919–932. [83] Reprinted with permission from John Wiley and Sons.
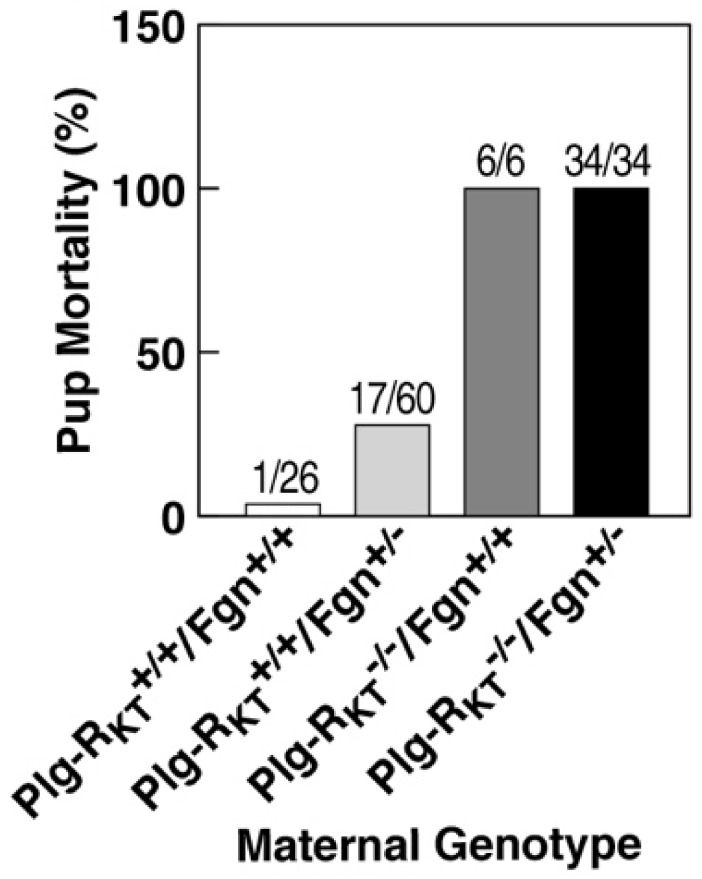
However, pup survival is not rescued by fibrinogen heterozygosity (Figure 7) [83]. Thus, in addition to an effect on fibrinolysis, the deletion of Plg-RKT is likely to exert other plasminogen- and fibrin(ogen)-independent effects on lactational development. These include the downregulation of EGF synthesis and, consequently, the downregulation of the pro-survival protein, Mcl-1, leading to increased apoptosis and decreased alveolar development [83]. The phenotypes of plasminogen and Plg-RKT−/− mammary glands are also distinct, in that plasminogen deficiency results in delays in ductal development during puberty [82] that are not observed in Plg-RKT−/− glands [83]. Recently, the loss of S100A10 has been shown to impair ductal development during the early stages of mammary development [84]. Thus, mammary development provides an example of the utilization of multiple plasminogen receptors at distinct steps in a physiologic process.
Figure 7.
Effect of fibrinogen heterozygosity on alveolar development and lactational competence in Plg-RKT−/− mice. Pup mortality at day 2 postpartum as a function of maternal genotype. This research was originally published in Miles, L.A.; Baik, N.; Bai, H.; Makarenkova, H.P.; Kiosses, W.B.; Krajewski, S.; Castellino, F.J.; Valenzuela, A.; Varki, N.M.; Mueller, B.M.; Parmer, R.J. The plasminogen receptor, Plg-RKT, is essential for mammary lobuloalveolar development and lactation. J. Thromb. Haemost. 2018, 16, 919–932. [83] Reprinted with permission from John Wiley and Sons.
8. Conclusions
Emerging evidence demonstrates a role for specific plasminogen receptors in fibrinolysis in both physiological and pathological conditions in vivo. In addition, fibrin(ogen) appears to reciprocally modulate the function of plasminogen receptors. Results have been obtained by the genetic deletion of specific receptors and through the use of function-blocking antibodies. Results with antibody blockade are more limited, most likely due to the expense and the requirement for repeated injections in these in vivo experiments. Plasminogen receptors exposing C-terminal lysines on cell surfaces that have been genetically deleted to date are components of the AIIt complex (annexin A2 and S100A10) and Plg-RKT. The deletion of plasminogen receptors that are synthesized with C-terminal basic residues, lack transmembrane domains and have additional essential intracellular functions is likely to result in non-viable mice. The creation of transgenic mice bearing a plasminogen receptor with a C-terminal K to A substitution (that is not predicted to interact with plasminogen) should yield informative results in the future. In addition, the investigation of the functions of AIIt and Plg-RKT in other fibrinolysis-dependent functions that are impaired in plasminogen-deficient mice should yield further information regarding the in vivo roles of these plasminogen receptors in fibrin surveillance.
Author Contributions
L.A.M., L.N., M.W., Y.S., T.N. and R.J.P. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by NIH Grants HL081046 and HD101133 (to L.A.M.), and HL149511 (to R.J.P. and L.A.M.), The Erling-Persson Family Foundation (to T.N.), The Cancer Research Foundation in Northern Sweden (to T.N.), the Medical Faculty of Umeå University (to T.N.), and by Merit Review Award I01BX003933 from the U.S. Department of Veterans Affairs (to R.J.P.). L.N. received a travel grant from the International Society of Fibrinolysis and Proteolysis.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Miles L.A., Plow E.F. Binding and activation of plasminogen on the platelet surface. J. Biol. Chem. 1985;260:4303–4311. doi: 10.1016/S0021-9258(18)89264-X. [DOI] [PubMed] [Google Scholar]
- 2.Miles L.A., Parmer R.J. Plasminogen receptors: The first quarter century. Semin. Thromb. Hemost. 2013;39:329–337. doi: 10.1055/s-0033-1334483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miles L.A., Lighvani S., Baik N., Parmer C.M., Khaldoyanidi S., Mueller B.M., Parmer R.J. New insights into the role of Plg-RKT in macrophage recruitment. Int. Rev. Cell Mol. Biol. 2014;309:259–302. doi: 10.1016/B978-0-12-800255-1.00005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoylaerts M., Rijken D.C., Lijnen H.R., Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. J. Biol. Chem. 1982;257:2912–2919. doi: 10.1016/S0021-9258(19)81051-7. [DOI] [PubMed] [Google Scholar]
- 5.Miles L.A., Dahlberg C.M., Plow E.F. The cell-binding domains of plasminogen and their function in plasma. J. Biol. Chem. 1988;263:11928–11934. doi: 10.1016/S0021-9258(18)37875-X. [DOI] [PubMed] [Google Scholar]
- 6.Félez J., Miles L.A., Fábregas P., Jardi M., Plow E.F., Lijnen R.H. Characterization of cellular binding sites and interactive regions within reactants required for enhancement of plasminogen activation by tPA on the surface of leukocytic cells. Thromb. Haemost. 1996;76:577–584. doi: 10.1055/s-0038-1650625. [DOI] [PubMed] [Google Scholar]
- 7.Miles L.A., Andronicos N.M., Chen E.I., Baik N., Bai H., Parmer C.M., Lighvani S., Nangia S., Kiosses W.B., Kamps M.P., et al. Identification of the Novel Plasminogen Receptor, Plg-RKT in Proteomics—Human Diseases and Protein Functions. IntechOpen Limited; London, UK: 2011. [DOI] [Google Scholar]
- 8.Gong Y., Kim S.O., Felez J., Grella D.K., Castellino F.J., Miles L.A. Conversion of glu-plasminogen to lys-plasminogen is necessary for optimal stimulation of plasminogen activation on the endothelial cell surface. J. Biol. Chem. 2001;276:19078–19083. doi: 10.1074/jbc.M101387200. [DOI] [PubMed] [Google Scholar]
- 9.Miles L.A., Castellino F.J., Gong Y. Critical role for conversion of glu-plasminogen to Lys-plasminogen for optimal stimulation of plasminogen activation on cell surfaces. Trends Cardiovasc. Med. 2003;13:21–30. doi: 10.1016/S1050-1738(02)00190-1. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L., Gong Y., Grella D.K., Castellino F.J., Miles L.A. Endogenous plasmin converts Glu-plasminogen to Lys-plasminogen on the monocytoid cell surface. J. Thromb. Haemost. 2003;1:1264–1270. doi: 10.1046/j.1538-7836.2003.00155.x. [DOI] [PubMed] [Google Scholar]
- 11.Han J., Baik N., Kim K.H., Yang J.M., Han G.W., Gong Y., Jardi M., Castellino F.J., Felez J., Parmer R.J., et al. Monoclonal antibodies detect receptor-induced binding sites in Glu-plasminogen. Blood. 2011;118:1653–1662. doi: 10.1182/blood-2010-11-316943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plow E.F., Freaney D.E., Plescia J., Miles L.A. The plasminogen system and cell surfaces: Evidence for plasminogen and urokinase receptors on the same cell type. J. Cell Biol. 1986;103:2411–2420. doi: 10.1083/jcb.103.6.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall S.W., Humphries J.E., Gonias S.L. Inhibition of cell surface receptor-bound plasmin by a2-antiplasmin and a2-macroglobulin. J. Biol. Chem. 1991;266:12329–12336. doi: 10.1016/S0021-9258(18)98900-3. [DOI] [PubMed] [Google Scholar]
- 14.Lu B.G., Sofian T., Law R.H., Coughlin P.B., Horvath A.J. Contribution of conserved lysine residues in the alpha2-antiplasmin C terminus to plasmin binding and inhibition. J. Biol. Chem. 2011;286:24544–24552. doi: 10.1074/jbc.M111.229013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andronicos N.M., Chen E.I., Baik N., Bai H., Parmer C.M., Kiosses W.B., Kamps M.P., Yates J.R., III, Parmer R.J., Miles L.A. Proteomics-based discovery of a novel, structurally unique, and developmentally regulated plasminogen receptor, Plg-RKT, a major regulator of cell surface plasminogen activation. Blood. 2010;115:1319–1330. doi: 10.1182/blood-2008-11-188938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miles L.A., Dahlberg C.M., Plescia J., Felez J., Kato K., Plowz E.F. Role of cell-surface lysines in plasminogen binding to cells: Identification of alpha-Enolase as a candidate plasminogen receptor. Biochemistry. 1991;30:1682–1691. doi: 10.1021/bi00220a034. [DOI] [PubMed] [Google Scholar]
- 17.Redlitz A., Fowler B.J., Plow E.F., Miles L.A. The role of an enolase-related molecule in plasminogen binding to cells. Eur. J. Biochem. 1995;227:407–415. doi: 10.1111/j.1432-1033.1995.tb20403.x. [DOI] [PubMed] [Google Scholar]
- 18.Hembrough T.A., Vasudevan J., Allietta M.M., Glass W.F., Gonias S.L. A cytokeratin 8-like protein with plasminogen-binding activity is present on the external surfaces of hepatocytes, HepG2 cells and breast carcinoma cell lines. J. Cell Sci. 1995;108:1071–1082. doi: 10.1242/jcs.108.3.1071. [DOI] [PubMed] [Google Scholar]
- 19.Hembrough T.A., Kralovich K.R., Li L., Gonias S.L. Cytokeratin 8 released by breast carcinoma cells in vitro binds plasminogen and tissue-type plasminogen activator and promotes plasminogen activation. Biochem. J. 1996;317:763–769. doi: 10.1042/bj3170763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kassam G., Le B.H., Choi K.S., Kang H.M., Fitzpatrick S.L., Louie P., Waisman D.M. The p11 subunit of the annexin II tetramer plays a key role in the stimulation of t-PA-dependent plasminogen activation. Biochemistry. 1998;37:16958–16966. doi: 10.1021/bi981713l. [DOI] [PubMed] [Google Scholar]
- 21.Herren T., Burke T.A., Das R., Plow E.F. Identification of histone H2B as a regulated plasminogen receptor. Biochemistry. 2006;45:9463–9474. doi: 10.1021/bi060756w. [DOI] [PubMed] [Google Scholar]
- 22.Hawley S.B., Tamura T., Miles L.A. Purification, cloning, and characterization of a profibrinolytic plasminogen-binding protein, TIP49a. J. Biol. Chem. 2001;276:179–186. doi: 10.1074/jbc.M004919200. [DOI] [PubMed] [Google Scholar]
- 23.Dudani A.K., Ganz P.R. Endothelial cell surface actin serves as a binding site for plasminogen, tissue plasminogen activator and lipoprotein(a) Br. J. Haematol. 1996;95:168–178. doi: 10.1046/j.1365-2141.1996.7482367.x. [DOI] [PubMed] [Google Scholar]
- 24.Miles L.A., Andronicos N.M., Baik N., Parmer R.J. Cell-surface actin binds plasminogen and modulates neurotransmitter release from catecholaminergic cells. J. Neurosci. 2006;26:13017–13024. doi: 10.1523/JNEUROSCI.2070-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briens A., Bardou I., Lebas H., Miles L.A., Parmer R.J., Vivien D., Docagne F. Astrocytes regulate the balance between plasminogen activation and plasmin clearance via cell-surface actin. Cell Discov. 2017;3 doi: 10.1038/celldisc.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan Z., Larson P.J., Bognacki J., Raghunath P.N., Tomaszewski J.E., Kuo A., Canziani G., Chaiken I., Cines D.B., Higazi A.A. Tissue factor regulates plasminogen binding and activation. Blood. 1998;91:1987–1998. doi: 10.1182/blood.V91.6.1987. [DOI] [PubMed] [Google Scholar]
- 27.Miles L.A., Dahlberg C.M., Levin E.G., Plow E.F. Gangliosides interact directly with plasminogen and urokinase and may mediate binding of these components to cells. Biochemistry. 1989;280:9337–9343. doi: 10.1021/bi00450a014. [DOI] [PubMed] [Google Scholar]
- 28.Miles L.A., Ginsberg M.H., White J.G., Plow E.F. Plasminogen interacts with human platelets through two distinct mechanisms. J. Clin. Invest. 1986;77:2001–2009. doi: 10.1172/JCI112529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pluskota E., Soloviev D.A., Bdeir K., Cines D.B., Plow E.F. Integrin alphaMbeta2 orchestrates and accelerates plasminogen activation and fibrinolysis by neutrophils. J. Biol. Chem. 2004;279:18063–18072. doi: 10.1074/jbc.M310462200. [DOI] [PubMed] [Google Scholar]
- 30.Lishko V.K., Novokhatny V.V., Yakubenko V.P., Prokvolit S.H.V., Ugarova T.P. Characterization of plasminogen as an adhesive ligand for integrins {alpha}M{beta}2 (Mac-1) and {alpha}5{beta}1(VLA-5) Blood. 2004;104:719–726. doi: 10.1182/blood-2003-09-3016. [DOI] [PubMed] [Google Scholar]
- 31.Parkkinen J., Raulo E., Merenmies J., Nolo R., Kajander E.O., Baumann M., Rauvala H. Amphoterin, the 30-kDa protein in a family of HMG1-type polypeptides. Enhanced expression in transformed cells, leading edge localization, and interactions with plasminogen activation. J. Biol. Chem. 1993;268:19726–19738. doi: 10.1016/S0021-9258(19)36575-5. [DOI] [PubMed] [Google Scholar]
- 32.Kanalas J.J., Makker S.P. Identification of the rat Heymann nephritis autoantigen (GP330) as a receptor site for plasminogen. J. Biol. Chem. 1991;266:10825–10829. doi: 10.1016/S0021-9258(18)99093-9. [DOI] [PubMed] [Google Scholar]
- 33.Miles L.A., Plow E.F., Waisman D.M., Parmer R.J. Plasminogen receptors. J. Biomed. Biotechnol. 2012;2012 doi: 10.1155/2012/130735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ling Q., Jacovina A.T., Deora A., Febbraio M., Simantov R., Silverstein R.L., Hempstead B., Mark W.H., Hajjar K.A. Annexin II regulates fibrin homeostasis and neoangiogenesis in vivo. J. Clin. Invest. 2004;113:38–48. doi: 10.1172/JCI19684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Surette A.P., Madureira P.A., Phipps K.D., Miller V.A., Svenningsson P., Waisman D.M. Regulation of fibrinolysis by S100A10 in vivo. Blood. 2011;118:3172–3181. doi: 10.1182/blood-2011-05-353482. [DOI] [PubMed] [Google Scholar]
- 36.Miles L.A., Baik N., Lighvani S., Khaldoyanidi S., Varki N.M., Bai H., Mueller B.M., Parmer R.J. Deficiency of plasminogen receptor, Plg-RKT, causes defects in plasminogen binding and inflammatory macrophage recruitment in vivo. J. Thromb. Haemost. 2017;15:155–612. doi: 10.1111/jth.13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das R., Burke T., Plow E.F. Histone H2B as a functionally important plasminogen receptor on macrophages. Blood. 2007;110:3763–3772. doi: 10.1182/blood-2007-03-079392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wygrecka M., Marsh L.M., Morty R.E., Henneke I., Guenther A., Lohmeyer J., Markart P., Preissner K.T. Enolase-1 promotes plasminogen-mediated recruitment of monocytes to the acutely inflamed lung. Blood. 2009;113:5588–5598. doi: 10.1182/blood-2008-08-170837. [DOI] [PubMed] [Google Scholar]
- 39.Bugge T.H., Flick M.J., Daugherty C.C., Degen J.L. Plasminogen deficiency causes severe thrombosis but is compatible with development and reproducton. Genes. Dev. 1995;9:794–807. doi: 10.1101/gad.9.7.794. [DOI] [PubMed] [Google Scholar]
- 40.Ploplis V.A., Carmeliet P., Vazirzadeh S., Van V.I., Moons L., Plow E.F., Collen D. Effects of disruption of the plasminogen gene on thrombosis, growth, and health in mice. Circulation. 1995;92:2585–2593. doi: 10.1161/01.CIR.92.9.2585. [DOI] [PubMed] [Google Scholar]
- 41.Madureira P.A., Surette A.P., Phipps K.D., Taboski M.A., Miller V.A., Waisman D.M. The role of the annexin A2 heterotetramer in vascular fibrinolysis. Blood. 2011;118:4789–4797. doi: 10.1182/blood-2011-06-334672. [DOI] [PubMed] [Google Scholar]
- 42.Puisieux A., Ji J., Ozturk M. Annexin II up-regulates cellular levels of p11 protein by a post-translational mechanisms. Biochem. J. 1996;313:51–55. doi: 10.1042/bj3130051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zobiack N., Rescher U., Ludwig C., Zeuschner D., Gerke V. The annexin 2/S100A10 complex controls the distribution of transferrin receptor-containing recycling endosomes. Mol. Biol. Cell. 2003;14:4896–4908. doi: 10.1091/mbc.e03-06-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He K.L., Deora A.B., Xiong H., Ling Q., Weksler B.B., Niesvizky R., Hajjar K.A. Endothelial cell annexin A2 regulates polyubiquitination and degradation of its binding partner S100A10/p. J. Biol. Chem. 2008;283:19192–19200. doi: 10.1074/jbc.M800100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hou Y., Yang L., Mou M., Hou Y., Zhang A., Pan N., Qiang R., Wei L., Zhang N. Annexin A2 regulates the levels of plasmin, S100A10 and Fascin in L5178Y cells. Cancer Invest. 2008;26:809–815. doi: 10.1080/07357900801898664. [DOI] [PubMed] [Google Scholar]
- 46.Whyte C.S., Morrow G.B., Baik N., Booth N.A., Jalal M.M., Parmer R.J., Miles L.A., Mutch N.J. Exposure of plasminogen and the novel plasminogen receptor, Plg-RKT, on activated human and murine platelets. Blood. 2020 doi: 10.1182/blood.2020007263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bavil A.A., Hayes S., Goretzki L., Kroger M., Anders J., Hendriks R. Convenient and versatile subcellular extraction procedure, that facilitates classical protein expression profiling and functional protein analysis. Proteomics. 2004;4:1397–1405. doi: 10.1002/pmic.200300710. [DOI] [PubMed] [Google Scholar]
- 48.Ploplis V.A., French E.L., Carmeliet P., Collen D., Plow E.F. Plasminogen deficiency differentially affects recruitment of inflammatory cell populations in mice. Blood. 1998;91:2005–2009. doi: 10.1182/blood.V91.6.2005. [DOI] [PubMed] [Google Scholar]
- 49.Plow E.F., Ploplis V.A., Busuttil S., Carmeliet P., Collen D. A role of plasminogen in atherosclerosis and restenosis models in mice. Thromb. Haemost. 1999;82:4–7. [PubMed] [Google Scholar]
- 50.Busuttil S.J., Ploplis V.A., Castellino F.J., Tang L., Eaton J.W., Plow E.F. A central role for plasminogen in the inflammatory response to biomaterials. J. Thromb. Haemost. 2004;2:1798–1805. doi: 10.1111/j.1538-7836.2004.00916.x. [DOI] [PubMed] [Google Scholar]
- 51.Vago J.P., Sugimoto M.A., Lima K.M., Lima N.G.L., Baik N., Teixeira M.M., Perretti M., Parmer R.J., Miles L.A., Sousa L.P. Plasminogen and the Plasminogen Receptor, Plg-RKT, Regulate Macrophage Phenotypic, and Functional Changes. Front. Immunol. 2019;10:1458. doi: 10.3389/fimmu.2019.01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silva L.M., Lum A.G., Tran C., Shaw M.W., Gao Z., Flick M.J., Moutsopoulos N.M., Bugge T.H., Mullins E.S. Plasmin-mediated fibrinolysis enables macrophage migration in a murine model of inflammation. Blood. 2019;134:291–303. doi: 10.1182/blood.2018874859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Motley M.P., Madsen D.H., Jurgensen H.J., Spencer D.E., Szabo R., Holmbeck K., Flick M., Lawrence D.A., Castellino F.J., Weigert R., et al. A CCR2 macrophage endocytic pathway mediates extravascular fibrin clearance in vivo. Blood. 2016;127:1085–1096. doi: 10.1182/blood-2015-05-644260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swaisgood C.M., Schmitt D., Eaton D., Plow E.F. In vivo regulation of plasminogen function by plasma carboxypeptidase B. J. Clin. Invest. 2002;110:1275–1282. doi: 10.1172/JCI0215082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lighvani S., Baik N., Diggs J.E., Khaldoyanidi S., Parmer R.J., Miles L.A. Regulation of macrophage migration by a novel plasminogen receptor Plg-RKT. Blood. 2011;118:5622–5630. doi: 10.1182/blood-2011-03-344242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Connell P.A., Surette A.P., Liwski R.S., Svenningsson P., Waisman D.M. S100A10 regulates plasminogen-dependent macrophage invasion. Blood. 2010;116:1136–1146. doi: 10.1182/blood-2010-01-264754. [DOI] [PubMed] [Google Scholar]
- 57.Thaler B., Baik N., Hohensinner P.J., Baumgartner J., Panzenbock A., Stojkovic S., Demyanets S., Huk I., Kaun R.G., Kaun C., et al. Differential expression of Plg-RKT and its effects on migration of proinflammatory monocyte and macrophage subsets. Blood. 2019;134:561–567. doi: 10.1182/blood.2018850420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gong Y., Hart E., Shchurin A., Plow H.J. Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. J. Clin. Invest. 2008;118:3012–3024. doi: 10.1172/JCI32750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rose L.F., Chan R.K. The Burn Wound Microenvironment. Adv. Wound Care. 2016;5:106–118. doi: 10.1089/wound.2014.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hettiaratchy S., Dziewulski P. ABC of burns: Pathophysiology and types of burns. BMJ. 2004;328:1427–1429. doi: 10.1136/bmj.328.7453.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Donaldson D.J., Mahan J.T. Keratinocyte migration and the extracellular matrix. J. Invest. Dermatol. 1988;90:623–628. doi: 10.1111/1523-1747.ep12560762. [DOI] [PubMed] [Google Scholar]
- 62.Mingers A.M., Heimburger N., Zeitler P., Kreth H.W., Schuster V. Homozygous type I plasminogen deficiency. Semin. Thromb. Hemost. 1997;23:259–269. doi: 10.1055/s-2007-996099. [DOI] [PubMed] [Google Scholar]
- 63.Schuster V., Mingers A.M., Seidenspinner S., Nüssgens Z., Pukrop T., Kreth H.W. Homozygous mutations in the plasminogen gene of two unrelated girls with ligneous conjunctivitis. Blood. 1997;90:958–966. doi: 10.1182/blood.V90.3.958. [DOI] [PubMed] [Google Scholar]
- 64.Klammt J., Kobelt L., Aktas D., Durak I., Gokbuget A., Hughes Q., Irkec M., Kurtulus I., Lapi E., Mechoulam H., et al. Identification of three novel plasminogen (PLG) gene mutations in a series of 23 patients with low PLG activity. Thromb. Haemost. 2011;105:454–460. doi: 10.1160/TH10-04-0216. [DOI] [PubMed] [Google Scholar]
- 65.Shapiro A.D., Nakar C., Parker J.M., Albert G.R., Moran J.E., Thibaudeau K., Thukral N., Hardesty B.M., Laurin P., Sandset P.M. Plasminogen replacement therapy for the treatment of children and adults with congenital plasminogen deficiency. Blood. 2018;131:1301–1310. doi: 10.1182/blood-2017-09-806729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rouatbi A., Chebbi A., Bouguila H. Ligneous conjunctivitis due to plasminogen deficit: Diagnostic and therapeutic approach. With literature review. J. Fr. Ophtalmol. 2018;41:916–919. doi: 10.1016/j.jfo.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 67.Schuster V., Hugle B., Tefs K. Plasminogen deficiency. J. Thromb. Haemost. 2007;5:2315–2322. doi: 10.1111/j.1538-7836.2007.02776.x. [DOI] [PubMed] [Google Scholar]
- 68.Romer J., Bugge T.H., Pyke C., Lund L.R., Flick M.J., Degen J.L., Dano K. Impaired wound healing in mice with a disrupted plasminogen gene. Nat. Med. 1996;2:287–292. doi: 10.1038/nm0396-287. [DOI] [PubMed] [Google Scholar]
- 69.Creemers E., Cleutjens J., Smits J., Heymans S., Moons L., Collen D., Daemen M., Carmeliet P. Disruption of the plasminogen gene in mice abolishes wound healing after myocardial infarction. Am. J. Pathol. 2000;156:1865–1873. doi: 10.1016/S0002-9440(10)65060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eriksson P.O., Li J., Ny T., Hellstrom S. Spontaneous development of otitis media in plasminogen-deficient mice. Int. J. Med. Microbiol. 2006;296 doi: 10.1016/j.ijmm.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 71.Li J., Eriksson P.O., Hansson A., Hellstrom S., Ny T. Plasmin/plasminogen is essential for the healing of tympanic membrane perforations. Thromb. Haemost. 2006;96:512–519. doi: 10.1160/TH06-03-0168. [DOI] [PubMed] [Google Scholar]
- 72.Sulniute R., Lindh T., Wilczynska M., Li J., Ny T. Plasmin is essential in preventing periodontitis in mice. Am. J. Pathol. 2011;179 doi: 10.1016/j.ajpath.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sulniute R., Shen Y., Guo Y.Z., Fallah M., Ahlskog N., Ny L., Rakhimova O., Broden J., Boija H., Moghaddam A., et al. Plasminogen is a critical regulator of cutaneous wound healing. Thromb. Haemost. 2016;115:1001–1009. doi: 10.1160/TH15-08-0653. [DOI] [PubMed] [Google Scholar]
- 74.Fallah M., Viklund E., Bäckman A., Brodén J., Lundskog B., Johansson M., Blomquist M., Wilczynska M., Ny T. Plasminogen is a master regulator and a potential drug candidate for the healing of radiation wounds. Cell Death Dis. 2020;11:201. doi: 10.1038/s41419-020-2397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bugge T.H., Kombrinck K.W., Flick M.J., Daugherty C.C., Danton M.J.S., Degen J.L. Loss of fibrinogen rescues mice from the pleiotropic effects of plasminogen deficiency. Cell. 1996;87:709–719. doi: 10.1016/S0092-8674(00)81390-2. [DOI] [PubMed] [Google Scholar]
- 76.Shen Y., Guo Y., Mikus P., Sulniute R., Wilczynska M., Ny T., Li J. Plasminogen is a key proinflammatory regulator that accelerates the healing of acute and diabetic wounds. Blood. 2012;119:5879–5887. doi: 10.1182/blood-2012-01-407825. [DOI] [PubMed] [Google Scholar]
- 77.Syrovets T., Jendrach M., Rohwedder A., Schule A., Simmet T. Plasmin-induced expression of cytokines and tissue factor in human monocytes involves AP-1 and IKKbeta-mediated NF-kappaB activation. Blood. 2001;97:3941–3950. doi: 10.1182/blood.V97.12.3941. [DOI] [PubMed] [Google Scholar]
- 78.Li Q., Laumonnier Y., Syrovets T., Simmet T. Plasmin triggers cytokine induction in human monocyte-derived macrophages. Arter. Thromb. Vasc. Biol. 2007;27:1383–1389. doi: 10.1161/ATVBAHA.107.142901. [DOI] [PubMed] [Google Scholar]
- 79.Syrovets T., Lunov O., Simmet T. Plasmin as a proinflammatory cell activator. J. Leukoc. Biol. 2012;92:509–519. doi: 10.1189/jlb.0212056. [DOI] [PubMed] [Google Scholar]
- 80.Ny L., Parmer R.J., Shen Y., Holmberg S., Baik N., Bäckman A., Broden J., Wilczynska M., Ny T., Miles L.A. The plasminogen receptor, Plg-R(KT), plays a role in inflammation and fibrinolysis during cutaneous wound healing in mice. Cell Death Dis. 2020;11:1054. doi: 10.1038/s41419-020-03230-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lund L.R., Bjorn S.F., Sternlicht M.D., Nielsen B.S., Solberg H., Usher P.A., Osterby R., Christensen I.J., Stephens R.W., Bugge T.H., et al. Lactational competence and involution of the mouse mammary gland require plasminogen. Development. 2000;127:4481–4492. doi: 10.1242/dev.127.20.4481. [DOI] [PubMed] [Google Scholar]
- 82.Green K.A., Nielsen B.S., Castellino F.J., Romer J., Lund L.R. Lack of plasminogen leads to milk stasis and premature mammary gland involution during lactation. Dev. Biol. 2006;299:164–175. doi: 10.1016/j.ydbio.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 83.Miles L.A., Baik N., Bai H., Makarenkova H.P., Kiosses W.B., Krajewski S., Castellino F.J., Valenzuela A., Varki N.M., Mueller B.M., et al. The Plasminogen Receptor Plg-RKT, is Essential for Mammary Lobuloalveolar Development, Lactation. J. Thromb. Haemost. 2018;16:919–932. doi: 10.1111/jth.13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bharadwaj A.G., Dahn M.L., Liu R.Z., Colp P., Thomas L.N., Holloway R.W., Marignani P.A., Too C.K., Barnes P.J., Godbout R., et al. S100A10 Has a Critical Regulatory Function in Mammary Tumor Growth and Metastasis: Insights Using MMTV-PyMT Oncomice and Clinical Patient Sample Analysis. Cancers (Basel) 2020;12 doi: 10.3390/cancers12123673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.