Abstract
Thyroid carcinoma is the most frequent endocrine neoplasia. Different types of thyroid carcinoma are described: well-differentiated papillary thyroid carcinoma (PTC), poorly differentiated thyroid carcinoma (PDTC), follicular thyroid carcinoma (FTC), anaplastic thyroid carcinoma (ATC), and medullary thyroid carcinoma (MTC). MTC is inherited as an autosomal dominant trait in 25% of cases. The genetic landscape of thyroid carcinoma has been largely deciphered. In PTC, genetic alterations have been found in about 95% of tumors: BRAF mutations and RET rearrangements are the main genetic alterations. BRAF and RAS mutations have been confirmed to play an important role also in PDTC and ATC, together with TP53 mutations that are fundamental in tumor progression. It has also been clearly demonstrated that telomerase reverse transcriptase (TERT) promoter mutations and TP53 mutations are present with a high-frequency in more advanced tumors, frequently associated with other mutations, and their presence, especially if simultaneous, is a signature of aggressiveness. In MTC, next-generation sequencing confirmed that mutations in the RET gene are the most common molecular events followed by H-RAS and K-RAS mutations. The comprehensive knowledge of the genetic events responsible for thyroid tumorigenesis is important to better predict the biological behavior and better plan the therapeutic strategy for specific treatment of the malignancy based on its molecular profile.
Keywords: thyroid cancer, oncogenes, molecular signature, RET, BRAF, TERT, p53
1. Introduction
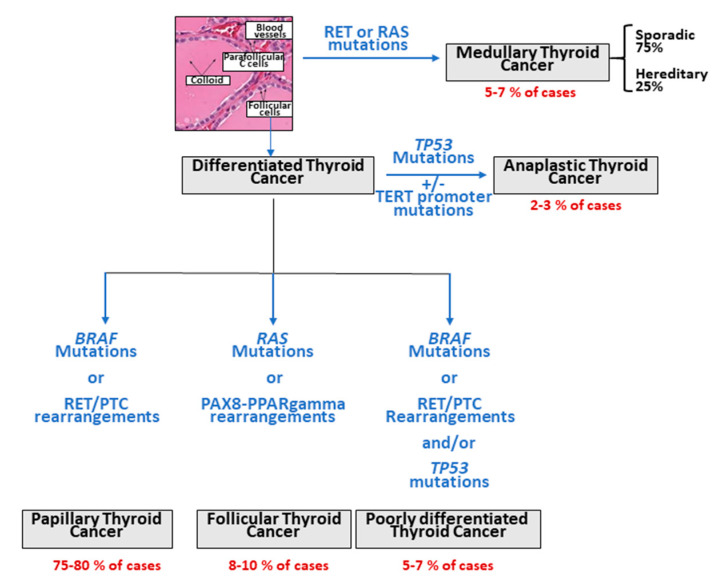
Thyroid carcinoma is the most frequently reported endocrine neoplasia and represents 3–4% of all human tumors. Accordingly to their histological features, different types of thyroid carcinoma are described: well-differentiated papillary thyroid carcinoma (PTC, 75–80%), poorly differentiated thyroid carcinoma (PDTC, 5–7%), follicular thyroid carcinoma (FTC, 8–10%), anaplastic thyroid carcinoma (ATC, 2–3%), which originate from follicular cells, and medullary thyroid carcinoma (MTC, 5–7%), which derives from parafollicular C-cells (Figure 1) [1]. PTC, FTC and ATC are very rarely familial (only about 5% of PTC patients), while MTC is inherited as an autosomal dominant trait in 25% of cases.
Figure 1.
Different histological types of thyroid carcinomas and most relevant/driver molecular alterations.
Hereditary MTC can occur as an isolated form, familial MTC (FMTC) in which only tumors of the thyroid gland are present, or in association with neoplasia of other endocrine organs (i.e., parathyroid and adrenal glands), thus giving rise to the multiple endocrine neoplasia type 2 (MEN 2) syndromes. MEN 2 are then distinguished into two different subtypes (i.e., MEN 2A, MEN 2B) according to the different clinical manifestations [2].
In the last decades, many studies have been performed to find the genetic alterations involved in the pathogenesis of thyroid cancer. The first studies were mainly based on the analysis of one or a few genes, and only a limited number of gene alterations were investigated. Finally, after the improvement of sequencing techniques (next-generation sequencing, NGS) that are nowadays able to investigate large portions of the genome and even the whole-genome, exome or transcriptome or any other “home” with a quite easy approach, the genomic landscape of the different histotypes of thyroid cancer has been deciphered [3,4,5,6]. The aim of this review is to describe genetic markers relevant to thyroid cancer.
2. Oncogenic Alterations in PTC, FTC, PDTC and ATC
The great majority of the alterations involved in the pathogenesis of these tumors are represented by somatic mutations that likely occur in the early steps of the tumoral transformation process. The most frequent driver events can be either point mutations or gene rearrangements, mainly affecting the MAPK pathway and phosphatidylinositol-3 kinase (PI3K)/AKT pathway. The mutated genes that affect these pathways encode cell-membrane receptors with tyrosine kinases activity such as RET and NTRK1 and intracellular signal transducers, among which BRAF and RAS. On the other hand, PI3K/AKT pathway, which is mainly involved in FTC initiation, is driven by activating mutations in RAS, PIK3CA, and AKT1 as well as by inactivation of PTEN. Mutations of TP53 and Wnt/βcatenin are indeed involved in the progression from PTC to PDTC and ATC. Other altered genes, such as TERT, a ribonucleoprotein polymerase that maintains telomere ends, have been described in all the histological thyroid cancer types, with a significantly higher prevalence in aggressive and undifferentiated tumors, indicating their role in thyroid cancer progression.
Here following we will discuss the major players in the process able to transform a normal follicular cell into a malignant cell and to determine tumoral proliferation.
2.1. RET Rearrangements
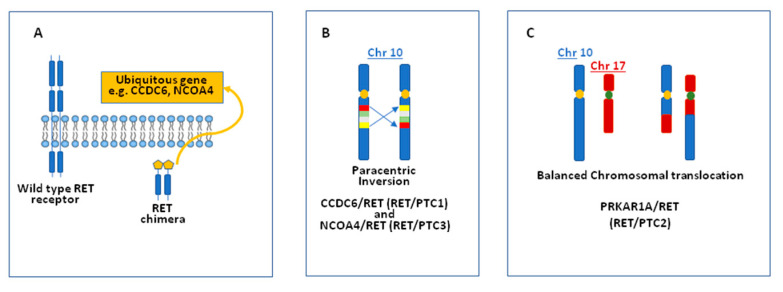
The first rearrangement of the RET gene (named RET/PTC1) was described several years ago using DNA transfection analysis on NIH3T3 cells [7]. As a result of this rearrangement, the tyrosine kinase domain of the RET gene is fused to the promoter region of the CCDC6 gene (formerly called H4) that drives the ligand-independent activation of the RET/PTC protein. Soon after the first description of the RET/PTC1 other RET fusions have been discovered. In particular, the RET/PTC3 rearrangement, in which RET is fused to the promoter region of the NCOA4 gene (also known as ELE1), was described in a post-Chernobyl thyroid tumor [8]. Over the years, several RET/PTC rearrangements have been reported in thyroid carcinoma, all characterized by the fusion of the tyrosine kinase domain of the receptor with a ubiquitous driver gene that allows the illegitimate kinase expression in cells that commonly do not express it (i.e., follicular cells) (Figure 2A). The gene rearrangements can be due to either a paracentric intrachromosomal 10 inversion or a translocation between chromosome 10 and another one (Figure 2B,C).
Figure 2.
Mechanisms of RET/papillary thyroid carcinoma (PTC) activation. (A): the tyrosine kinase domain of the RET gene is constitutively activated by the fusion with a ubiquitous gene; (B): a paracentric inversion on chromosome 10 leads to the formation of a RET chimera as RET/PTC1 and RET/PTC3; (C): a chromosomal translocation leads to the formation of a RET chimera as RET/PTC2.
In most cases, these rearrangements differ for the involvement of different partner genes, but in few cases, different RET/PTC rearrangements are characterized by the occurrence of different breakpoints giving origin to longer or shorter chimeras [9]. The high prevalence of RET/PTC in post-Chernobyl thyroid tumors (87%) highlighted a strong relation between RET rearrangements and radiation exposure [10,11]. Years later, it has been proposed by in vitro experiments that the spatial proximity of the loci involved in RET/PTC rearrangements predisposes their mis-joining as a consequence of double-stranded breaks produced by ionizing radiation [12,13,14,15].
RET/PTC rearrangements have been found at a higher frequency in radiation-exposed children than in adults (probably due to the high proliferation rate of thyroid follicular cells in childhood) [10,11,16]. It is a matter of fact that with the increase of the latency period from the nuclear accident, the prevalence of RET/PTC rearrangements declined [17]. Interestingly a statistically significant decrease in the RET/PTC prevalence also has been reported by some authors in sporadic PTC [18]. A relatively low prevalence of RET/PTC rearrangements (6.3%) has been reported when using a next-generation sequencing approach [3]. Although to a much lower extent, rearrangements of the RET gene have also been reported in benign nodules as well as in Hashimoto thyroiditis [10,19,20].
The prognostic value of RET/PTC rearrangement in thyroid cancer has not been fully clarified yet. In a consecutive series of 1510 patients with thyroid cancer, RET/PTC-positive cases tended to be more aggressive with respect to RAS-positive cases [21].
In addition, among PTC tumors with a RET rearrangement, RET/PTC1 was found to be associated with a small, classic PTC variant [22]. At variance, RET/PTC3 rearrangement is prevalent in the solid variant of PTC and with a more aggressive clinical presentation both in post-Chernobyl childhood thyroid cancer [23] and in sporadic cases [24]. A low prevalence of RET rearrangements, mainly in carcinoma associated with a differentiated component, have been found in PDTC and ATC [25].
2.2. Other Rearrangements
The possibility to use sequencing techniques characterized by a very high sensitivity has allowed the identification of additional rearrangements, other than of RET, in thyroid cancer, mainly in radiation-induced tumors. Although gene rearrangements involving BRAF oncogene were previously described by Ciampi et al. [26] in a series of radiation-induced post-Chernobyl thyroid cancer, they have also been reported in the TGCA study in sporadic cases [3]. In particular, a total of 13/484 (2.7%) fusion of the BRAF gene with different gene partners were identified (Table 1). Very few cases (4/484 (0.8%)) of PAX8/PPARgamma fusions were also found in the TCGA series, in particular in the follicular variant of PTC. Conversely, PAX8/PPARgamma fusions are much more frequent in FTC, being present in about one-third of them and with a prevalence ranging from 12 to 56% [27,28].
Table 1.
BRAF rearrangements in thyroid cancers.
| Gene Fusion | Prevalence | Type of Cancer | Reference |
|---|---|---|---|
| AKAP9/BRAF | Radiation-induced | [26] | |
| SND1/BRAF | 3/33 | Sporadic | [3] |
| AGK/BRAF | 1/33 | Sporadic | [3] |
| AP3B1/BRAF | 1/33 | Sporadic | [3] |
| BLC2L11/BRAF | 1/33 | Sporadic | [3] |
| CCNY/BRAF | 1/33 | Sporadic | [3] |
| ERC1/BRAF | 1/33 | Sporadic | [3] |
| FAM114A2/BRAF | 1/33 | Sporadic | [3] |
| MACF1/BRAF | 1/33 | Sporadic | [3] |
| MKRN1/BRAF | 1/33 | Sporadic | [3] |
| SVOPL/BRAF | 1/33 | Sporadic | [3] |
| ZC3HAB1/BRAF | 1/33 | Sporadic | [3] |
Although to a less extent, translocations were also found in other genes such as NTRK (1.2%), THADA (1.2%), ALK (0.8%) and FGFR2 (0.4%) [3]. In addition to PTC, ALK fusions were found with a high prevalence in PDTC and ATC [29]. A high prevalence (55.5%) of less common gene fusions (STRN/ALK, TPR/NTRK1, SQSTM1/NTRK3, AFAP1L2/RET, and PPFIBP2/RET) (n = 5) were also reported in Fukushima PTC previously found to be negative (n = 9) for the classical oncogenes, thus confirming the strong correlation between radiation exposure and gene translocations [30].
2.3. BRAF Point Mutations
The BRAF gene encodes for a protein belonging to the serine/threonine-protein kinase family. This protein plays a role in regulating the MAP kinase/ERKs signaling pathway, which affects cell division, differentiation, and secretion. Mutations in this gene are associated with various human cancers, including non-Hodgkin lymphoma, colorectal cancer, malignant melanoma, thyroid carcinoma, non-small cell lung carcinoma, and adenocarcinoma of the lung. The most common mechanism of BRAF activation is the c.1799T > A, p.V600E (COSM476) point mutation that, according to the Cosmic database (https://cancer.sanger.ac.uk/cosmic), accounts for 51% of PTCs. A similar rate of BRAF mutations in PTC (59.7%) also has been reported by the Cancer Genome Atlas Research (TGCA) study [3] that was performed by NGS on a series of about 500 PTC cases. All point mutations and the rearrangements lead to the activation of BRAF kinase and to the chronic stimulation of the MAPK pathway.
The BRAF-V600E mutation constitutes 98–99% of all BRAF mutations found in thyroid cancer, but other alterations, including other point mutations, in-frame insertion/deletion and rearrangements, have been reported (Table 2). However, BRAF “rare” mutations are mainly present in the follicular variant of PTC and correlate with a good outcome [29]. Interestingly BRAF amplifications have been frequently found in BRAF wild-type tumors [30]
Table 2.
Rare BRAF mutation in thyroid cancers according to the COSMIC database.
| Nucleotide Change | Amino Acid Substitution | Mutation Type | Prevalence |
|---|---|---|---|
| c.1834C > T | p.Q612 | Substitution nonsense | 2/40,072 |
| c.1778G > A | p.G593D | Substitution missense | 1/21,576 |
| c.1793C > T | p.A598V | Substitution missense | 2/21,576 |
| c.1796C > G | p.T599R | Substitution missense | 1/21,576 |
| c.? | p.T599I | Substitution missense | 3/21,576 |
| c.1801A > G | p.K601E | Substitution missense | 54/21,576 |
| c.1794_1795insGTT | p.A598_T599insV | Insertion inframe | 8/13 |
| c.1795_1796insTAA | p.A598_T599insI | Insertion inframe | 1/13 |
| c.1795_1796ins27 | p.A598_T599insKKIGDFGLA | Insertion inframe | 1/13 |
| c.1796_1797insTAC | p.T599_V600insT | Insertion inframe | 1/13 |
| c.1797_1798ins9 | p.T599_V600insETT | Insertion inframe | 1/13 |
| c.1798_1799ins18 | p.T599_V600insDFGLAT | Insertion inframe | 1/13 |
| c.? | p.K601del | Deletion inframe | 3/6 |
| p.V600_W604del | p.V600_W604del | Deletion inframe | 1/6 |
| c.1801_1803delAAA | p.K601del | Deletion inframe | 1/6 |
| c.1801_1812del12 | p.K601_W604del | Deletion inframe | 1/6 |
| c.1799_1814 > ATGT | p.V600_S605 > DV | Complex | 1/33 |
| c.1796_1809 > TC | p.T599_R603 > I | Complex | 4/33 |
| c.1799_1801delTGA | p.V600_K601 > E | Complex | 15/33 |
| c.1798_1798G > TACA | p.V600 > YM | Complex | 4/33 |
| c.1796_1798CAG > TAGCTT | p.T599_V600 > IAL | Complex | 2/33 |
| c.? | p.T599_V600 > IYI | Complex | 1/33 |
| c.? | p.T599_R603 > I | Complex | 1/33 |
| c.? | p.V600_K601 > E | Complex | 1/33 |
| c.? | p.V600 > YM | Complex | 1/33 |
| c.1799_1801delTGA | p.V600_K601 > E | Complex | 3/33 |
A different prevalence of BRAF mutations has been observed according to different morphological variants of PTC, and the highest prevalence was found in the tall cell PTC than in the classic variant, while a rather low prevalence has been reported in the follicular variant [21], where also some rare BRAF mutations are found [29].
Although not confirmed in an American series [31], a statistically significant increase in the BRAF mutation prevalence in sporadic PTC cases has been reported in parallel with the abovementioned decrease in RET rearrangement prevalence [18,32,33]. Since it has been demonstrated that high iodine levels could be a risk factor for BRAF mutations [34], the increased occurrence of BRAF mutations in PTC has been hypothesized, although never demonstrated, to be related to the intake of prophylactic iodine that has become increasingly recommended. Another hypothesis is that exposure to pollutants can be responsible for the induction of BRAF-V600E mutation, and in particular, a correlation has been found between a higher prevalence of BRAF-V600E mutation and the living close to the Etna volcano [35].
BRAF mutations have been shown not to be a major event in post-Chernobyl thyroid carcinomas as well in a not irradiated pediatric population, while these alterations have been found to be highly prevalent in thyroid carcinomas in the young population of Fukushima, suggesting a different mechanism of the tumoral transformation in the 2 groups [36]. The BRAF-V600E mutation has also been demonstrated to be subclonal or even oligoclonal with a different technical approach [36,37,38,39]. These findings led to hypothesize that this mutation might not always be the first transforming genetic event but rather a secondary event in PTC tumorigenesis [40,41]. Nevertheless, a high percentage of BRAF-V600E mutated alleles, that indicates a clonal origin and development of tumoral cells, correlated with a specific PTC molecular subtype and predicts a poorer disease outcome [36].
Several studies have investigated the role of BRAF-V600E mutations as prognostic markers and have shown a strong correlation with poor clinicopathological outcomes of PTC. In particular, a close association of BRAF mutation with extrathyroidal extension, lymph node metastasis, and advanced TNM stages III/IV of PTC, which are well-documented risk factors associated with increased rates of recurrence and mortality of thyroid cancer, have been reported [42,43,44,45,46]. The role of the BRAF-V600E mutation as a poor prognostic factor has also been reported in low-risk intrathyroidal tumors [44]. Despite the above-reported studies, the association between the BRAF-V600E mutations with increased tumor aggressiveness and poor prognosis in PTC is still under debate [47,48]. At variance, PTC cases with rare BRAF mutations, and in particular the BRAF-K601E, show an indolent behavior similar to the cases with BRAF wild-type [29,49].
The BRAF-V600E mutation also occurs in about 30% of PDTC and 40% of ATC [4,50,51]. It is interesting to note that many of these carcinomas are characterized by the presence of well-differentiated areas, and BRAF-V600E is present in both tumor components assuming the hypothesis that this mutation is an early event in tumor development and dedifferentiation [52].
2.4. TERT Mutations
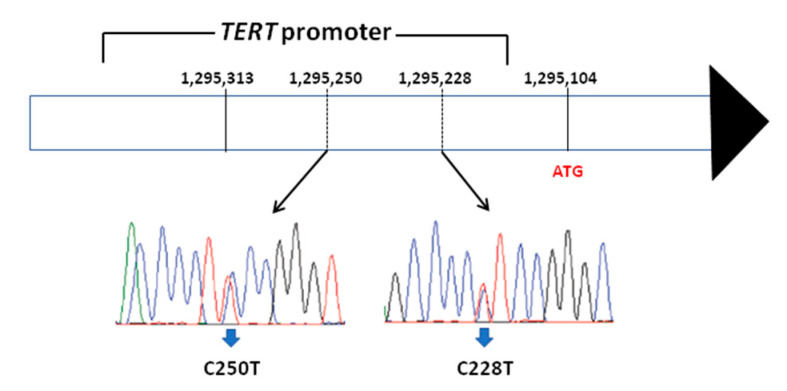
Telomerase reverse transcriptase (TERT) is the catalytic domain of telomerase whose role is to add telomeres, to preserve chromosomal integrity and genome stability [53,54]. In addition, TERT has been shown to play a major role in the activation of telomerase during malignant transformation of cells [55,56]. In 2013, using whole-genome sequencing, mutations in the promoter of the TERT gene have been described in melanoma [57,58] and in other human cancers, among which thyroid cancer [59]. The two most common TERT promoter mutations occurring in thyroid cancer are located in the promoter region (chr: 5, 1,295,228 C > T (C228T) and 1,295,250 C > T (C250T)) (Figure 3).
Figure 3.
Schematic representation of TERT gene with the indication of the 2 most frequent mutations localized in the gene promoter, responsible for the transcription of the gene starting from the ATG codon.
These two mutations are mutually exclusive, suggesting that both the two mutations are highly transforming. The most frequent TERT mutation in thyroid carcinoma is the C228T mutation, and its prevalence is increasing with the increase of the level of aggressiveness being lower in the less aggressive PTC and very high in the more aggressive ATC. A similar association of TERT mutations and aggressiveness of the disease is also observed when comparing different histological variants of PTC. Pediatric thyroid tumors, which are considered not so aggressive, have been found to be negative for the presence of TERT promoter mutations [58]. To date, no TERT promoter mutations have been found in benign thyroid diseases and in MTC [59,60].
TERT promoter mutations have been found to correlate strictly with poor clinicopathological features and bad outcomes of the tumor [60]. A significant association between TERT promoter mutations with older age at diagnosis, tumor size, extrathyroidal invasion, vascular invasion, lymph node and distant metastasis, advanced stage and mortality has been reported [61]. TERT promoter mutations have been found to be associated with the presence of the BRAF-V600E mutations: the coexistence of BRAF-V600E and TERT mutation has been demonstrated to define a particularly aggressive group of thyroid tumors and in particular with tumor recurrence and mortality [60].
2.5. RAS Mutations
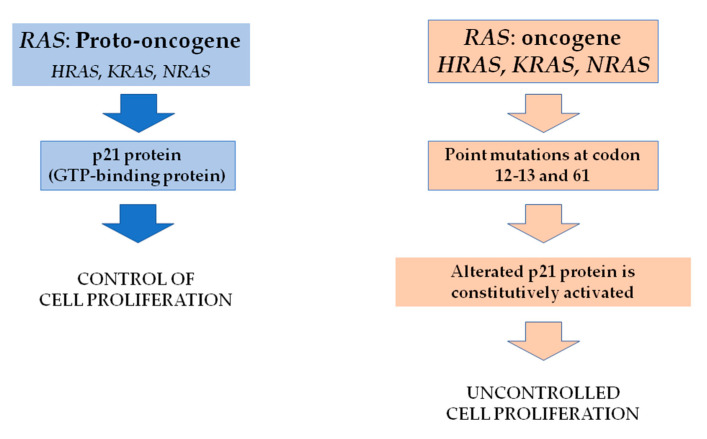
Proteins of the RAS family are G-proteins able to activate the MAPK and other signaling pathways. In their active state, RAS proteins bind GTP; when GTP is hydrolyzed to GDP, ras proteins assume their inactive state (Figure 4).
Figure 4.
RAS gene activation in cancer: The RAS oncogene encodes for the p21 protein. In its native state, p21 controls cell growth and differentiation. When a point mutation occurs at codons 12, 13 and 61, p21 is constitutively activated, leading to uncontrolled cell growth.
Three isoforms of the RAS gene exist: H-RAS, K-RAS and N-RAS being N-RAS the most mutated in differentiated thyroid tumors, mainly at codon 12, 13 and 61 and H-RAS, K-RAS in MTC. According to the data of the TGCA study that published the results of a comprehensive next-generation study on PTC [3], the overall prevalence of N-RAS, H-RAS and K-RAS was 8.5%, 3.5% and 1%, respectively, very similar to that derived from the COSMIC database. Interestingly all RAS mutations reported by the TGCA study are in the follicular variant of PTC; thus, the relative frequency of the mutations in this subgroup is really higher in keeping with the evidence that RAS mutations are a major leading event in FTC [62]. Based on the presence of BRAF or RAS mutations, two main classes of PTC have been identified and have been named “BRAF-like and RAS-like” [3,62]. In detail, the first group of tumors was mainly constituted by classical or tall cell variants and with a significant reduction of the expression of the thyroid differentiation genes. At variance, the RAS-like PTCs were mainly follicular variants characterized by a high degree of differentiation. Nevertheless, RAS mutations have been found to be particularly relevant also in poorly differentiated and anaplastic thyroid cancer, where are frequently associated with other mutations such as TERT promoter mutation [63]. Somatic mutations in the RAS family have been reported in FTC. N-RAS mutations at codon 61 have been found to be mutated at a prevalence varying from 15% up to 40% of FTC [64,65]. New insights from a single-center and a large patient cohort of RAS mutated FTC have been shown to increase the metastatic potential and disease-specific mortality.
2.6. EIF1AX Mutations
The TGCA study [3] identified EIF1AX as a novel cancer gene in PTC. Mutations of EIF1AX were found in 1.5% of cases. The EIF1AX gene encodes for a eukaryotic translation initiator factor involved in the control of the initiation of protein synthesis. Exons 2, 5 and 6 of the EIF1AX gene have been further analyzed in other studies [66] and 3/86 (2.3%) PTC, 1/4 (25%) ATC and 2/27 (7.4%) follicular adenomas were found to carry one of these somatic mutations. The important role of the EIF1AX gene in thyroid tumorigenesis came up also by the NGS studies on PDTC, ATC and FTC in which an EIF1AX gene mutation was found in 11%, 9%–13% and 5.1% of cases, respectively [4,51,65]. According to the data of the TGCA study on PTC, the EIF1AX mutations result to be mutually exclusive with any other mutation. At variance, EIF1AX mutations seem to co-occur with RAS mutations in more advanced tumors.
2.7. TP53 Mutations
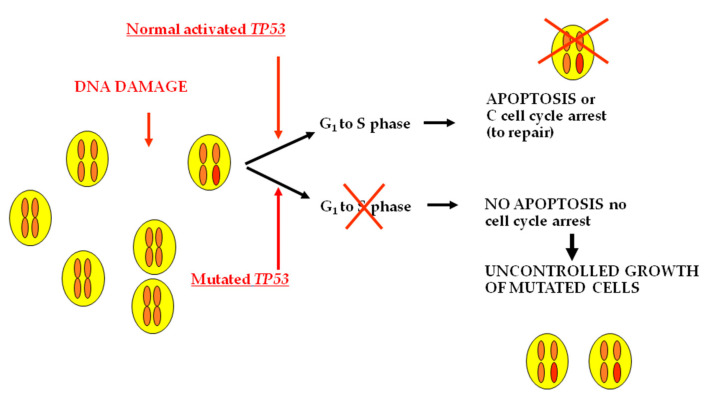
The TP53 gene-encoded protein is involved in several cellular processes. In response to cellular stress, it can induce cell cycle arrest, apoptosis, senescence, DNA repair, or changes in metabolism (Figure 5).
Figure 5.
TP53 gene encodes for a protein involved in the control of the cell cycle. When DNA damage occurs, TP53 is able to induce the arrest of the cell cycle, and mutated cells cannot give origin to altered clones. Mutated TP53 loses the ability to stop the growth of mutated cells. Thus, tumoral clones take over.
In thyroid cancer, mainly in ATC, TP53 mutations are prevalently located in exons 5–9, and codon 273 is the most frequently involved [67,68]. The profile of TP53 mutations has been changing over the years mainly because the recent development of the highly sensitive NGS has allowed the identification of genetic alterations that are present at very low prevalence. By Sanger sequencing inactivating TP53 mutations were reported in about 26% of PDTC [69] and in about 80% of ATC. No TP53 expression or mutations were found in normal thyroid or in benign lesions [70]. According to the data reported by the TGCA study, TP53 mutations are present in a negligible percentage (0.7%) of PTC. At variance, using a last-generation sequencing approach [4,50,51], the prevalence of TP53 mutations is rather elevated in PDTC and ATC. The overall prevalence of inactivating TP53 mutations in ATC, which varied slightly in the aforementioned studies, is approximately 58% [63]. TP53 mutations can be found in ATC either associated with other genetic alterations typical of PTC or FTC, thus suggesting that ATC can derive from the dedifferentiation of well-differentiated longstanding thyroid cancer or as a unique genetic event, thus suggesting a direct role of these mutations in transforming the normal follicular cell into an undifferentiated tumoral cell. In this regard, it is worth noting that Landa et al. showed that none of the nine patients with TP53-positive ATC had any mutation in other components of the MAPK pathway, supporting the hypothesis that ATC can directly develop from follicular cells. This hypothesis is also supported by the observation that when a tumor is composed of a mixture of well-differentiated and undifferentiated areas, TP53 mutations are restricted to the undifferentiated areas of the tumor. TP53 mutations can also be found in PDTC, but their prevalence is significantly lower with respect to that found in ATC (73% vs. 8%) [4]. Moreover, in series in which the PDTC identification was done according to the Turin classification, TP53 mutations were demonstrated to be highly prevalent in ATC but completely absent in PDTC, suggesting a different genetic origin of the two malignancies [71]. A few prevalences of TP53 mutations have also been reported in FTC [65]
3. Oncogenic Alterations in MTC
The genetic landscape of medullary thyroid cancer (MTC) is not yet fully discovered, and about 40% of sporadic MTC and 2% of hereditary cases are still orphans of driver mutations. At present, mutations in the RET gene, both somatic and germline, appear to be the most important genetic events in MTC [9,72]. With the exception of the RAS gene, very few alternative gene alterations have been described in MTC [5,6,73]. As an alternative to point mutations, ALK and RET rearrangements have also been reported in few cases [73,74].
Here following we will discuss the major players in the process able to transform a normal parafollicular C-cell into a malignant cell and give origin to MTC.
3.1. RET Mutations
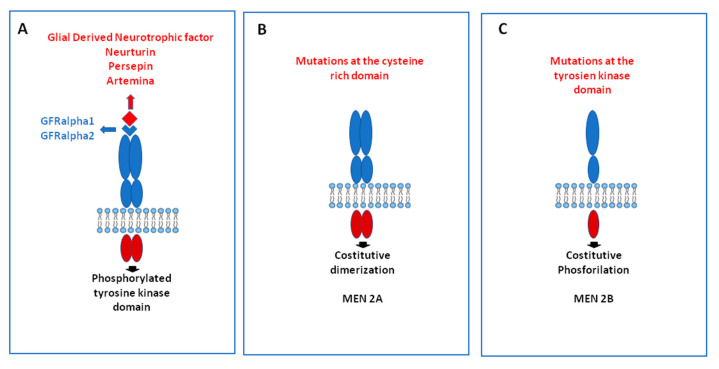
The RET proto-oncogene encodes for a tyrosine kinase transmembrane receptor whose ligands are members of the glial-derived neurotrophic factor family. Following the binding with the ligands, two RET receptor molecules make a dimer and initiate the activation of the receptor. When point mutations occur, ligand-independent activation of the receptor takes place (Figure 6).
Figure 6.
Mechanisms of activation of the RET gene: in physiological conditions, the binding of a RET ligand, which is mediated by a co-receptor, induces the dimerization of two to RET molecules, causing the phosphorylation of the tyrosine kinase domain (A). When a point mutation in the cysteine domain is present, as it happens in multiple endocrine neoplasia (MEN) type 2A syndrome, the constitutive dimerization of 2 RET molecules occurs, and the receptor is activated independently by ligand-binding (B). Alternatively, if the mutation occurs in the tyrosine kinase domain, as it happens in MEN 2B syndrome, constitutive phosphorylation activates the RET receptor independently by ligand-binding (C).
Activation of RET stimulates multiple downstream pathways such as the mitogen-activated protein kinase (MAPK), the phosphoinositide 3-kinase (PI3K). Activating RET point mutations in MTC were first described in 1993 [75]. Since that time, many studies have been performed in hereditary and sporadic cases demonstrating the oncogenic driver role of RET mutations in MTC.
Hereditary cases: RET germline mutations have been found in more than 98% of MEN 2 kindreds, and only a few families affected by hereditary MTC are “orphans” of germline mutations [9]. In addition to RET mutations, recently, a germline ESR2 mutation has been identified in a family as a novel cause of familial MTC/CCH and provides important insights into a novel mechanism causing increased RET expression in tumorigenesis [76]. However, so far, it appears that this germline ESR2 mutation is a “private” mutation of that specific family since no other kindreds carrying the same germline mutation have been described [77].
The causative role of germline RET mutations in MEN 2 syndromes and the strict correlation between genotype and phenotype was clearly demonstrated by the study of the International RET Consortium that collected and published very important data about the RET mutations and the clinical and pathological features of 477 kindred affected by MEN 2A, MEN 2B and FMTC [78]. Following this study, several MTC series have been reported and summarized in reviews and or guidelines. One of the most important observation is that the classical MEN 2A phenotype is mainly associated with mutations in the RET cysteine codons 609, 611, 618, and 620 in exon 10 and, mostly, with the C634R mutation in exon 11. Secondly, MEN 2B was almost exclusively associated with the M918T mutation in exon 16. Few MEN 2B families have been found to have the A883F mutations: in these families, MTC is less aggressive than the M918T MTC tumors. By contrast, in FMTC cases, mutations were distributed among different codons/exons of the RET gene [78], but they are mainly concentrated in non-cysteine codons, such as codon 804 in exon 14 and codons 883 and 891 in exon 15 [78,79]. Almost all mutations reported to date are listed in public databases (www.hgmd.cf.ac.uk; www.arup.utah.edu/database/MEN2; www.ensembl.org, accessed date: 30 November 2020) (Table 3).
Table 3.
Distribution of germline RET mutation in hereditary medullary thyroid carcinoma (MTC).
| Location | Protein Change | Classification | MEN2 Phenotype |
|---|---|---|---|
| Exon 5 | p.V292M | Pathogenic | MEN2A and FMTC |
| p.T338I | Pathogenic | ||
| Exon 7 | p.505_506del | Pathogenic | MEN2A |
| Exon 8 | p.C515S/W | Pathogenic | |
| p.G533C | Pathogenic | ||
| Exon 10 | p.C609R/G/Y/S/F | Pathogenic | MEN2A and FMTC |
| p.C611S/R/G/YF/W | |||
| p.C618S/R/G/Y/F/W | |||
| p.C620S/R/G/L/F/W/Y | |||
| Exon 11 | p.D631Y/A/G/V/E | Pathogenic/uncertain | MEN2A |
| p.C634S/R/G/Y/L/W | Pathogenic | ||
| p.K666E/R | Pathogenic | ||
| Exon 13 | p.E768D | Pathogenic | MEN2A and FMTC |
| p.L790F | Pathogenic | ||
| Exon 14 | p.V804M | Pathogenic | MEN2A and FMTC |
| p.V804L | Pathogenic | ||
| p.Y806C | Benign | ||
| Exon 15 | p.A883T | Pathogenic | FMTC, MEN2B and MEN 2A |
| p.A883F | Pathogenic | ||
| p.S891A | Pathogenic | ||
| p.S904F | Pathogenic | ||
| Exon 16 | p.M918T | Pathogenic | MEN2B and FMTC |
| p.M918V | Pathogenic |
As clearly reported in the ATA guidelines, not all mutations confer the same aggressiveness to MTC [80,81], and this aggressiveness is correlated with the transforming ability of the RET mutation. We and others demonstrated that M918T mutation and mutations ad codon 634 are more transforming than non-cysteine mutations [82]. Because of the pathogenic role of germline RET mutations and their correlation with phenotype, all cases of MTC, both those with a clear familial-positive history and those apparently sporadic, must be submitted to the RET genetic screening. This will allow the correct identification of the hereditary cases and of the gene carriers among their first-degree relatives. These latter will be studied for the presence of an undiagnosed but already present MTC or for their potentiality to develop the tumor if not yet present. The planning of a prophylactic surgical treatment or the follow-up strategy will be done according to the age of the patients, the type of RET mutation and the levels of serum calcitonin [83,84].
Sporadic cases: According to data collected from several studies and published in a public database (COSMIC, https://cancer.sanger.ac.uk/cosmic), RET somatic mutations have also been found in 932/2107 (44%) sporadic MTC tissues. The most frequent RET somatic alterations in sporadic MTC are point mutations, but deletions and insertions have also been reported. Although RET somatic mutations have been found at different codons, the M918T mutation in exon 16 is the most frequently reported, especially in more advanced cases [83,85]. The prevalence of RET somatic mutations in MTC was around 50% of cases in series in which direct sequencing was adopted for the analysis. With the introduction of advanced sequencing methodologies (next-generation sequencing, NGS) that allowed the deep sequencing of the larger portion of the genome, the role of somatic RET mutations as main drivers in MTC had been confirmed. As reported in a large series [86], RET mutations are almost always mutually exclusive, and only in few cases, multiple RET somatic mutations are present, suggesting that MTC is a rather stable tumor. This evidence was also reported in additional studies [87,88] that demonstrated that only in about 20% of cases a different RET mutation profile could be found when comparing primary tumor and its corresponding metastases. NGS studies have also allowed the definition of the frequency of the mutated allele (AF) and have demonstrated that larger tumors not only are characterized by a higher prevalence of RET somatic mutations [89] but also have a higher AF corresponding to a higher number of mutated cells. This evidence suggests the hypothesis that the presence of a RET mutation, particularly M918T, is able to induce a growth advantage resulting in the formation of larger and clonal tumors.
The presence of somatic RET mutations has been found to be correlated with a worse prognosis of the tumor and shorter survival [90,91]. The correlation between RET somatic mutation and a worse outcome is strengthened by the evidence that the prevalence of RET somatic mutations is higher in patients with a more advanced tumor and lower in patients with smaller tumors [85]. At variance with the RET genetic screening in the familial form, the search for somatic RET mutation in the tumoral tissue is not yet part of the routine clinical practice, although it is highly desirable to be new anti-RET-specific drugs under development.
3.2. RAS Mutations
Interestingly, in the last years, RAS mutations have also been found in MTC [86,92,93]. In 2011, a Portuguese group [92] found somatic HRAS and KRAS mutations in 15/26 (57.7%) and in 3/26 (11.5%) RET wild-type MTC cases. At variance, only 1/40 (2.5%) RET-positive case had a somatic RAS mutation, indicating that RAS and RET mutations are mutually exclusive in MTC. In the Ciampi et al. series [86], RAS mutations were present in 69.2% (18/26) of RET-negative cases and in only 2.5% of RET-positive sporadic MTC, confirming that activation of the RAS and RET proto-oncogenes represents alternative genetic events in sporadic MTC tumorigenesis. Although with different prevalence, likely due to technical and/or ethical reasons, these results have been confirmed by several groups. It is also worth noting that RAS mutation identifies a subgroup of MTC with less aggressive behavior when compared with cases with RET mutations [86].
4. Conclusions
In the last years, much progress has been made in deciphering the genetic landscape of thyroid carcinoma. In PTC, the results of the study of the TGCA study definitively demonstrated that genetic alterations are present in about 95% of tumors and that two main classes of tumors (BRAF- and RAS-like), each with their own clinical and biological behavior, can be distinguished. In addition to PTC, important achievements have also been reached for PDTC and ATC. BRAF and RAS mutations have been confirmed to play an important role in the pathogenesis of these tumors, and TP53 mutations have been found to be fundamental in tumor progression. It also has been clearly demonstrated that TERT promoter mutations and TP53 mutations are present with a high-frequency in more advanced tumors, frequently associated with other mutations, and their presence is a sign of aggressiveness. Similarly, the presence of several genetic alterations in the same tumoral tissue is correlated with a higher degree of dedifferentiation and probability of a bad outcome. As MTC are concerned, the whole-exome sequencing and target sequencing studies confirmed that mutations in the RET gene are the most common molecular events followed by H-RAS and K-RAS mutations. The comprehensive knowledge of the genetic events responsible for thyroid tumorigenesis, as well as for any other human tumor, is important to better predict the biological behavior and to better plan the therapeutic strategy for specific treatment of the malignancy on its molecular profile.
Author Contributions
C.R. and R.E. equally contributed to the preparation of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants to R.E. from Associazione Italiana per la Ricerca sul Cancro (AIRC, Investigator grant 2018, project code 21790) and by the PRIN project 2017YTWKWH.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fagin J.A., Wells S.A. Biologic and Clinical Perspectives on Thyroid Cancer. N. Engl. J. Med. 2016;375:1054–1067. doi: 10.1056/NEJMra1501993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romei C., Pardi E., Cetani F., Elisei R. Genetic and Clinical Features of Multiple Endocrine Neoplasia Types 1 and 2. J. Oncol. 2012:1–15. doi: 10.1155/2012/705036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal N., Akbani R.B., Aksoy A., Ally A., Arachchi H., Sylvia L.A., Auman J.T., Balasundaram M., Balu S., Baylin S.B., et al. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676–690. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landa I., Ibrahimpasic T., Boucai L., Sinha R., Knauf J.A., Shah R.H., Dogan S., Ricarte-Filho J.C., Krishnamoorthy G.P., Xu B., et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J. Clin. Investig. 2016;126:1052–1066. doi: 10.1172/JCI85271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agrawal N., Jiao Y., Sausen M., Leary R., Bettegowda C., Roberts N.J., Bhan S., Ho A.S., Khan Z., Bishop J., et al. Exomic Sequencing of Medullary Thyroid Cancer Reveals Dominant and Mutually Exclusive Oncogenic Mutations in RET and RAS. J. Clin. Endocrinol. Metab. 2013;98:E364–E369. doi: 10.1210/jc.2012-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simbolo M., Mian C., Barollo S., Fassan M., Mafficini A., Neves D., Scardoni M., Pennelli G., Rugge M., Pelizzo M.R., et al. High-throughput mutation profiling improves diagnostic stratification of sporadic medullary thyroid carcinomas. Virchows Arch. 2014;465:73–78. doi: 10.1007/s00428-014-1589-3. [DOI] [PubMed] [Google Scholar]
- 7.Fusco A., Grieco M., Santoro M., Berlingieri M.T., Pilotti S., Pierotti M.A., Della Porta G., Vecchio G. A new oncogene in human thyroid papillary carcinomas and their lymph-nodal metastases. Nat. Cell Biol. 1987;328:170–172. doi: 10.1038/328170a0. [DOI] [PubMed] [Google Scholar]
- 8.Santoro M., Dathan N.A., Berlingieri M.T., Bongarzone I., Paulin C., Grieco M., Pierotti M.A., Vecchio G., Fusco A. Molecular characterization of RET/PTC3; A novel rearranged version of the RETproto-oncogene in a human thyroid papillary carcinoma. Oncogene. 1994;9:509–516. [PubMed] [Google Scholar]
- 9.Romei C., Ciampi R., Elisei R. A comprehensive overview of the role of the RET proto-oncogene in thyroid carcinoma. Nat. Rev. Endocrinol. 2016;12:192–202. doi: 10.1038/nrendo.2016.11. [DOI] [PubMed] [Google Scholar]
- 10.Elisei R., Romei C., Vorontsova T., Cosci B., Veremeychik V., Kuchinskaya E., Basolo F., Demidchik E.P., Miccoli P., Pinchera A., et al. RET/PTC Rearrangements in Thyroid Nodules: Studies in Irradiated and Not Irradiated, Malignant and Benign Thyroid Lesions in Children and Adults1. J. Clin. Endocrinol. Metab. 2001;86:3211–3216. doi: 10.1210/jcem.86.7.7678. [DOI] [PubMed] [Google Scholar]
- 11.Nikiforov Y.E., Rowland J.M., Bove K.E., Monforte-Munoz H., Fagin J.A. Distinct pattern of ret oncogene rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res. 1997;57:1690–1694. [PubMed] [Google Scholar]
- 12.Nikiforova M.N., Stringer J.R., Blough R., Medvedovic M., Fagin J.A., Nikiforov Y.E. Proximity of Chromosomal Loci That Participate in Radiation-Induced Rearrangements in Human Cells. Science. 2000;290:138–141. doi: 10.1126/science.290.5489.138. [DOI] [PubMed] [Google Scholar]
- 13.Gandhi M., Evdokimova V., Nikiforov Y.E. Mechanisms of chromosomal rearrangements in solid tumors: The model of papillary thyroid carcinoma. Mol. Cell. Endocrinol. 2010;321:36–43. doi: 10.1016/j.mce.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandhi M., Medvedovic M., Stringer J.R., Nikiforov Y.E. Interphase chromosome folding determines spatial proximity of genes participating in carcinogenic RET/PTC rearrangements. Oncogene. 2005;25:2360–2366. doi: 10.1038/sj.onc.1209268. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi M., Evdokimova V., Nikiforov Y.E. Frequency of close positioning of chromosomal loci detected by FRET correlates with their participation in carcinogenic rearrangements in human cells. Genes. Chromosom. Cancer. 2012;51:1037–1044. doi: 10.1002/gcc.21988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarzab B., Handkiewicz-Junak D. Differentiated thyroid cancer in children and adults: Same or distinct disease? Hormones. 2007;6:200–209. [PubMed] [Google Scholar]
- 17.Rabes H.M., Demidchik E.P., Sidorow J.D., Lengfelder E., Beimfohr C., Hoelzel D., Klugbauer S. Pattern of radiation-induced RET and NTRK1 rearrangements in 191 post-chernobyl papillary thyroid carcinomas: Biological, phenotypic, and clinical implications. Clin. Cancer Res. 2000;6:1093–1103. [PubMed] [Google Scholar]
- 18.Romei C., Fugazzola L., Puxeddu E., Frasca F., Viola D., Muzza M., Moretti S., Nicolosi M.L., Giani C., Cirello V., et al. Modifications in the Papillary Thyroid Cancer Gene Profile Over the Last 15 Years. J. Clin. Endocrinol. Metab. 2012;97:E1758–E1765. doi: 10.1210/jc.2012-1269. [DOI] [PubMed] [Google Scholar]
- 19.Guerra A., Sapio M.R., Marotta V., Campanile E., Moretti M.I., Deandrea M., Motta M., Limone P.P., Fenzi G., Rossi G., et al. Prevalence of RET/PTC rearrangement in benign and malignant thyroid nodules and its clinical application. Endocr. J. 2011;58:31–38. doi: 10.1507/endocrj.K10E-260. [DOI] [PubMed] [Google Scholar]
- 20.Sheils O.M., O’Leary J., Uhlmann V., Lüttich K., Sweeney E.C. Ret/PTC-1 Activation in Hashimoto Thyroiditis. Int. J. Surg. Pathol. 2000;8:185–189. doi: 10.1177/106689690000800305. [DOI] [PubMed] [Google Scholar]
- 21.Yip L., Nikiforova M.N., Yoo J.Y., McCoy K.L., Stang M.T., Armstrong M.J., Nicholson K.J., Ohori N.P., Coyne C., Hodak S.P., et al. Tumor genotype determines phenotype and disease-related outcomes in thyroid cancer: A study of 1510 patients. Ann. Surg. 2015;262:515–519. doi: 10.1097/SLA.0000000000001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soares P., Fonseca E., Wynford-Thomas D., Sobrinho-Simões M. Sporadic ret-rearranged papillary carcinoma of the thyroid: A subset of slow growing, less aggressive thyroid neoplasms? J. Pathol. 1998;185:71–78. doi: 10.1002/(SICI)1096-9896(199805)185:1<71::AID-PATH42>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 23.Thomas G.A., Bunnell H., Cook H.A., Williams E.D., Nerovnya A., Cherstvoy E.D., Tronko N.D., Bogdanova T.I., Chiappetta G., Viglietto G., et al. High Prevalence of RET/PTC Rearrangements in Ukrainian and Belarussian Post-Chernobyl Thyroid Papillary Carcinomas: A Strong Correlation between RET/PTC3 and the Solid-Follicular Variant1. J. Clin. Endocrinol. Metab. 1999;84:4232–4238. doi: 10.1210/jc.84.11.4232. [DOI] [PubMed] [Google Scholar]
- 24.Romei C., Ciampi R., Faviana P., Agate L., Molinaro E., Bottici V., Basolo F., Miccoli P., Pacini F., Pinchera A., et al. BRAFV600E mutation, but not RET/PTC rearrangements, is correlated with a lower expression of both thyroperoxidase and sodium iodide symporter genes in papillary thyroid cancer. Endocr. Relat. Cancer. 2008;15:511–520. doi: 10.1677/ERC-07-0130. [DOI] [PubMed] [Google Scholar]
- 25.Mochizuki K., Kondo T., Nakazawa T., Iwashina M., Kawasaki T., Nakamura N., Yamane T., Murata S.-I., Ito K., Kameyama K., et al. RET rearrangements and BRAF mutation in undifferentiated thyroid carcinomas having papillary carcinoma components. Histopathology. 2010;57:444–450. doi: 10.1111/j.1365-2559.2010.03646.x. [DOI] [PubMed] [Google Scholar]
- 26.Ciampi R., Knauf J.A., Kerler R., Gandhi M., Zhu Z., Nikiforova M.N., Rabes H.M., Fagin J.A., Nikiforov Y.E. Oncogenic AKAP9-BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer. J. Clin. Investig. 2005;115:94–101. doi: 10.1172/JCI23237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikiforova M.N., Biddinger P.W., Caudill C.M., Kroll T.G., Nikiforov Y.E. PAX8-PPARgamma rearrangement in thyroid tumors: RT-PCR and immunohistochemical analyses. Am. J. Surg. Pathol. 2002;26:1016–1023. doi: 10.1097/00000478-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Boos L.A., Dettmer M., Schmitt A., Rudolph T., Steinert H., Moch H., Sobrinho-Simões M., Komminoth P., Perren A. Diagnostic and prognostic implications of the PAX8-PPARγ translocation in thyroid carcinomas-a TMA-based study of 226 cases. Histopathology. 2013;63:234–241. doi: 10.1111/his.12150. [DOI] [PubMed] [Google Scholar]
- 29.Torregrossa L., Viola D., Sensi E., Giordano M., Piaggi P., Romei C., Materazzi G., Miccoli P., Elisei R., Basolo F. Papillary Thyroid Carcinoma with Rare Exon 15 BRAF Mutation Has Indolent Behavior: A Single-Institution Experience. J. Clin. Endocrinol. Metab. 2016;101:4413–4420. doi: 10.1210/jc.2016-1775. [DOI] [PubMed] [Google Scholar]
- 30.McKelvey B.A., Zeiger M.A., Umbricht C.B. Characterization of TERT and BRAF copy number variation in papillary thyroid carcinoma: An analysis of the cancer genome atlas study. Genes Chromosom. Cancer. 2020;122 doi: 10.1002/gcc.22928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung C.K., Little M.P., Lubin J.H., Brenner A.V., Wells S.A.J., Sigurdson A.J., Nikiforov Y.E. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J. Clin. Endocrinol. Metab. 2014;99:E276–E285. doi: 10.1210/jc.2013-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smyth P., Finn S., Cahill S., O’Regan E., Flavin R., O’Leary J., Sheils O. Ret/PTC and BRAF Act as Distinct Molecular, Time-Dependant Triggers in a Sporadic Irish Cohort of Papillary Thyroid Carcinoma. Int. J. Surg. Pathol. 2005;13:1–8. doi: 10.1177/106689690501300101. [DOI] [PubMed] [Google Scholar]
- 33.Vuong H.G., Altibi A.M., Abdelhamid A.H., Ngoc P.U.D., Quan V.D., Tantawi M.Y., Elfil M., Vu T.L.H., Elgebaly A., Oishi N., et al. The changing characteristics and molecular profiles of papillary thyroid carcinoma over time: A systematic review. Oncotarget. 2016;8:10637–10649. doi: 10.18632/oncotarget.12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guan H., Ji M., Bao R., Yu H., Wang Y., Hou P., Zhang Y., Shan Z., Teng W., Xing M. Association of High Iodine Intake with the T1799A BRAF Mutation in Papillary Thyroid Cancer. J. Clin. Endocrinol. Metab. 2009;94:1612–1617. doi: 10.1210/jc.2008-2390. [DOI] [PubMed] [Google Scholar]
- 35.Pellegriti G., De Vathaire F., Scollo C., Attard M., Giordano C., Arena S., Dardanoni G., Frasca F., Malandrino P., Vermiglio F., et al. Papillary Thyroid Cancer Incidence in the Volcanic Area of Sicily. J. Natl. Cancer Inst. 2009;101:1575–1583. doi: 10.1093/jnci/djp354. [DOI] [PubMed] [Google Scholar]
- 36.Guerra A., Fugazzola L., Marotta V., Cirillo M., Rossi S., Cirello V., Forno I., Moccia T., Budillon A., Vitale M. A High Percentage of BRAFV600E Alleles in Papillary Thyroid Carcinoma Predicts a Poorer Outcome. J. Clin. Endocrinol. Metab. 2012;97:2333–2340. doi: 10.1210/jc.2011-3106. [DOI] [PubMed] [Google Scholar]
- 37.Guerra A., Sapio M.R., Marotta V., Campanile E., Rossi S., Forno I., Fugazzola L., Budillon A., Moccia T., Fenzi G., et al. The primary occurrence of BRAF(V600E) is a rare clonal event in papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2012;97:517–524. doi: 10.1210/jc.2011-0618. [DOI] [PubMed] [Google Scholar]
- 38.Kim M.-H., Bae J.S., Lim D.-J., Lee H., Jeon S.R., Park G., Jung C.K. Quantification of BRAF V600E alleles predicts papillary thyroid cancer progression. Endocr. Relat. Cancer. 2014;21:891–902. doi: 10.1530/ERC-14-0147. [DOI] [PubMed] [Google Scholar]
- 39.Finkel A., Liba L., Simon E., Bick T., Prinz E., Sabo E., Ben-Izhak O., Hershkovitz D. Subclonality for BRAF Mutation in Papillary Thyroid Carcinoma Is Associated with Earlier Disease Stage. J. Clin. Endocrinol. Metab. 2016;101:1407–1413. doi: 10.1210/jc.2015-4031. [DOI] [PubMed] [Google Scholar]
- 40.Xing M. BRAFV600E mutation and papillary thyroid cancer: Chicken or egg? J. Clin. Endocrinol. Metab. 2012;97:2295–2298. doi: 10.1210/jc.2012-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vasko V., Hu S., Wu G., Xing J.C., Larin A., Savchenko V., Trink B., Xing M., Larin A. High Prevalence and Possible de Novo Formation of BRAF Mutation in Metastasized Papillary Thyroid Cancer in Lymph Nodes. J. Clin. Endocrinol. Metab. 2005;90:5265–5269. doi: 10.1210/jc.2004-2353. [DOI] [PubMed] [Google Scholar]
- 42.Ha L.N., Iravani A., Nhung N.T., Hanh N.T.M., Hutomo F., Son M.H. Relationship between clinicopathologic factors and FDG avidity in radioiodine-negative recurrent or metastatic differentiated thyroid carcinoma. Cancer Imaging. 2021;21:1–8. doi: 10.1186/s40644-020-00378-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elisei R., Ugolini C., Viola D., Lupi C., Biagini A., Giannini R., Romei C., Miccoli P., Pinchera A., Basolo F. BRAFV600Emutation and outcome of patients with papillary thyroid carcinoma: A 15-year median follow-up study. J. Clin. Endocrinol. Metab. 2008;93:3943–3949. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]
- 44.Elisei R., Viola D., Torregrossa L., Giannini R., Romei C., Ugolini C., Molinaro E., Agate L., Biagini A., Lupi C., et al. TheBRAFV600E Mutation Is an Independent, Poor Prognostic Factor for the Outcome of Patients with Low-Risk Intrathyroid Papillary Thyroid Carcinoma: Single-Institution Results from a Large Cohort Study. J. Clin. Endocrinol. Metab. 2012;97:4390–4398. doi: 10.1210/jc.2012-1775. [DOI] [PubMed] [Google Scholar]
- 45.Tufano R.P., Teixeira G.V., Bishop J., Carson K.A., Xing M. BRAF mutation in papillary thyroid cancer and its value in tailoring initial treatment: A systematic review and meta-analysis. Medicine. 2012;91:274–286. doi: 10.1097/MD.0b013e31826a9c71. [DOI] [PubMed] [Google Scholar]
- 46.Xing M., Alzahrani A.S., Carson K.A., Viola D., Elisei R., Bendlova B., Yip L., Mian C., Vianello F., Tuttle R.M., et al. Association Between BRAF V600E Mutation and Mortality in Patients with Papillary Thyroid Cancer. JAMA. 2013;309:1493–1501. doi: 10.1001/jama.2013.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim T.Y., Kim W.B., Song J.Y., Rhee Y.S., Gong G., Cho Y.M., Kim S.Y., Kim S.C., Hong S.J., Shong Y.K. The BRAF mutation is not associated with poor prognostic factors in Korean patients with conventional papillary thyroid microcarcinoma. Clin. Endocrinol. 2005;63:588–593. doi: 10.1111/j.1365-2265.2005.02389.x. [DOI] [PubMed] [Google Scholar]
- 48.Liu R.-T., Chen Y.-J., Chou F.-F., Li C.-L., Wu W.-L., Tsai P.-C., Huang C.-C., Cheng J.-T. No correlation between BRAFV600E mutation and clinicopathological features of papillary thyroid carcinomas in Taiwan. Clin. Endocrinol. 2005;63:461–466. doi: 10.1111/j.1365-2265.2005.02367.x. [DOI] [PubMed] [Google Scholar]
- 49.Afkhami M., Karunamurthy A., Chiosea S., Nikiforova M.N., Seethala R., Nikiforov Y.E., Coyne C. Histopathologic and Clinical Characterization of Thyroid Tumors Carrying the BRAF(K601E) Mutation. Thyroid. 2016;26:242–247. doi: 10.1089/thy.2015.0227. [DOI] [PubMed] [Google Scholar]
- 50.Jeon M.J., Chun S.-M., Kim D., Kwon H., Jang E.K., Kim T.Y., Kim W.B., Shong Y.K., Jang S.J., Song D.E. Genomic Alterations of Anaplastic Thyroid Carcinoma Detected by Targeted Massive Parallel Sequencing in a BRAFV600E Mutation-Prevalent Area. Thyroid. 2016;26:683–690. doi: 10.1089/thy.2015.0506. [DOI] [PubMed] [Google Scholar]
- 51.Kunstman J.W., Juhlin C.C., Goh G., Brown T.C., Stenman A., Healy J.M., Rubinstein J.C., Choi M., Kiss N., Nelson-Williams C., et al. Characterization of the mutational landscape of anaplastic thyroid cancer via whole-exome sequencing. Hum. Mol. Genet. 2015;24:2318–2329. doi: 10.1093/hmg/ddu749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nikiforova M.N., Kimura E.T., Gandhi M., Biddinger P.W., Knauf J.A., Basolo F., Zhu Z., Giannini R., Salvatore G., Fusco A., et al. BRAF Mutations in Thyroid Tumors Are Restricted to Papillary Carcinomas and Anaplastic or Poorly Differentiated Carcinomas Arising from Papillary Carcinomas. J. Clin. Endocrinol. Metab. 2003;88:5399–5404. doi: 10.1210/jc.2003-030838. [DOI] [PubMed] [Google Scholar]
- 53.Greider C.W., Blackburn E.H. Tracking telomerase. Cell. 2004;116:S83–S87. doi: 10.1016/S0092-8674(04)00053-4. [DOI] [PubMed] [Google Scholar]
- 54.Blasco M.A. Telomeres and human disease: Ageing, cancer and beyond. Nat. Rev. Genet. 2005;6:611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 55.Feng J., Funk W.D., Wang S.S., Weinrich S.L., Avilion A., Chiu C.P., Adams R.R., Chang E., Allsopp R.C., Yu J., et al. The RNA component of human telomerase. Science. 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 56.Janknecht R. On the road to immortality: hTERT upregulation in cancer cells. FEBS Lett. 2004;564:9–13. doi: 10.1016/S0014-5793(04)00356-4. [DOI] [PubMed] [Google Scholar]
- 57.Horn S., Figl A., Rachakonda P.S., Fischer C., Sucker A., Gast A., Kadel S., Moll I., Nagore E., Hemminki K., et al. TERT Promoter Mutations in Familial and Sporadic Melanoma. Science. 2013;339:959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 58.Huang F.W., Hodis E., Xu M.J., Kryukov G.V., Chin L., Garraway L.A. Highly Recurrent TERT Promoter Mutations in Human Melanoma. Science. 2013;339:957–959. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu X., Bishop J., Shan Y., Pai S., Liu D., Murugan A.K., Sun H., El-Naggar A.K., Xing M. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr. Relat. Cancer. 2013;20:603–610. doi: 10.1530/ERC-13-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu X., Qu S., Liu R., Sheng C., Shi X., Zhu G., Murugan A.K., Guan H., Yu H., Wang Y., et al. TERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathological characteristics of thyroid cancer. J. Clin. Endocrinol. Metab. 2014;99:E1130–E1136. doi: 10.1210/jc.2013-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Melo M., Da Rocha A.G., Vinagre J., Batista R., Peixoto J., Tavares C., Celestino R., Almeida A., Salgado C., Eloy C., et al. TERT Promoter Mutations Are a Major Indicator of Poor Outcome in Differentiated Thyroid Carcinomas. J. Clin. Endocrinol. Metab. 2014;99:E754–E765. doi: 10.1210/jc.2013-3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoo S.-K., Lee S., Kim S.-J., Jee H.-G., Kim B.-A., Cho H., Song Y.S., Cho S.W., Won J.-K., Shin J.-Y., et al. Comprehensive Analysis of the Transcriptional and Mutational Landscape of Follicular and Papillary Thyroid Cancers. PLoS Genet. 2016;12:e1006239. doi: 10.1371/journal.pgen.1006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Molinaro E., Romei C., Biagini A., Sabini E., Agate L., Mazzeo S., Materazzi G., Sellari-Franceschini S., Ribechini A., Torregrossa L., et al. Anaplastic thyroid carcinoma: From clinicopathology to genetics and advanced therapies. Nat. Rev. Endocrinol. 2017;13:644–660. doi: 10.1038/nrendo.2017.76. [DOI] [PubMed] [Google Scholar]
- 64.Fukahori M., Yoshida A., Hayashi H., Yoshihara M., Matsukuma S., Sakuma Y., Koizume S., Okamoto N., Kondo T., Masuda M., et al. The association between RAS gene mutations and clinical characteristics in follicular thyroid tumors: New insights from a single center and a large patient cohort. Thyroid. 2012;22:683–689. doi: 10.1089/thy.2011.0261. [DOI] [PubMed] [Google Scholar]
- 65.Nicolson N.G., Murtha T.D., Dong W., Paulsson J.O., Choi J., Barbieri A.L., Brown T.C., Kunstman J.W., Larsson C., Prasad M.L., et al. Comprehensive Genetic Analysis of Follicular Thyroid Carcinoma Predicts Prognosis Independent of Histology. J. Clin. Endocrinol. Metab. 2018;103:2640–2650. doi: 10.1210/jc.2018-00277. [DOI] [PubMed] [Google Scholar]
- 66.Karunamurthy A., Panebianco F., Hsiao S.J., Vorhauer J., Nikiforova M.N., Chiosea S., Nikiforov Y.E. Prevalence and phenotypic correlations of EIF1AX mutations in thyroid nodules. Endocr. Relat. Cancer. 2016;23:295–301. doi: 10.1530/ERC-16-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soares P., Lima J., Preto A., Castro P., Vinagre J., Celestino R., Couto J.P., Prazeres H., Eloy C., Maximo V., et al. Genetic Alterations in Poorly Differentiated and Undifferentiated Thyroid Carcinomas. Curr. Genom. 2011;12:609–617. doi: 10.2174/138920211798120853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tavares C., Melo M., Teijeiro J.M.C., Soares P., Sobrinho-Simões M. Endocrine tumours: Genetic predictors of thyroid cancer outcome. Eur. J. Endocrinol. 2016;174:R117–R126. doi: 10.1530/EJE-15-0605. [DOI] [PubMed] [Google Scholar]
- 69.Donghi R., Longoni A., Pilotti S., Michieli P., Della Porta G., Pierotti M.A. Gene p53 mutations are restricted to poorly differentiated and undifferentiated carcinomas of the thyroid gland. J. Clin. Investig. 1993;91:1753–1760. doi: 10.1172/JCI116385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fagin J.A., Matsuo K., Karmakar A., Chen D.L., Tang S.H., Koeffler H.P. High prevalence of mutations of the p53 gene in poorly differentiated human thyroid carcinomas. J. Clin. Investig. 1993;91:179–184. doi: 10.1172/JCI116168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Romei C., Tacito A., Molinaro E., Piaggi P., Cappagli V., Pieruzzi L., Matrone A., Viola D., Agate L., Torregrossa L., et al. Clinical, pathological and genetic features of anaplastic and poorly differentiated thyroid cancer: A single institute experience. Oncol. Lett. 2018;15:9174–9182. doi: 10.3892/ol.2018.8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takahashi M., Kawai K., Asai N. Roles of the RET Proto-oncogene in Cancer and Development. JMA J. 2020;3:175–181. doi: 10.31662/jmaj.2020-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ji J.H., Oh Y.L., Hong M., Yun J.W., Lee H.-W., Kim D., Ji Y., Kim D.-H., Park W.-Y., Shin H.-T., et al. Identification of Driving ALK Fusion Genes and Genomic Landscape of Medullary Thyroid Cancer. PLoS Genet. 2015;11:e1005467. doi: 10.1371/journal.pgen.1005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grubbs E.G., Ng P.K.-S., Bui J., Busaidy N.L., Chen K., Lee J.E., Lu X., Lu H., Meric-Bernstam F., Mills G.B., et al. RET fusion as a novel driver of medullary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2015;100:788–793. doi: 10.1210/jc.2014-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mulligan L.M., Kwok J.B.J., Healey C.S., Elsdon M.J., Eng C., Gardner E., Love D.R., Mole S.E., Moore J.K., Papi L., et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nat. Cell Biol. 1993;363:458–460. doi: 10.1038/363458a0. [DOI] [PubMed] [Google Scholar]
- 76.Smith J., Read M.L., Hoffman J., Brown R., Bradshaw B., Campbell C., Cole T., Navas J.D., Eatock F., Gundara J.S., et al. Germline ESR2 mutation predisposes to medullary thyroid carcinoma and causes up-regulation of RET expression. Hum. Mol. Genet. 2016;25:1836–1845. doi: 10.1093/hmg/ddw057. [DOI] [PubMed] [Google Scholar]
- 77.Ruiz-Ferrer M., Fernández R.M., Navarro E., Antinolo G., Borrego S. ESR2 Gene and Medullary Thyroid Carcinoma. Thyroid. 2017;27:1456–1457. doi: 10.1089/thy.2017.0171. [DOI] [PubMed] [Google Scholar]
- 78.Eng C., Clayton D., Schuffenecker I., Lenoir G., Cote G., Gagel R.F., Van Amstel H.K., Lips C.J., Nishisho I., Takai S., et al. The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. JAMA. 1996;276:1575–1579. doi: 10.1001/jama.1996.03540190047028. [DOI] [PubMed] [Google Scholar]
- 79.Wells S.A., Pacini F., Robinson B.G., Santoro M. Multiple Endocrine Neoplasia Type 2 and Familial Medullary Thyroid Carcinoma: An Update. J. Clin. Endocrinol. Metab. 2013;98:3149–3164. doi: 10.1210/jc.2013-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wells S.A., Asa S.L., Dralle H., Elisei R., Evans D.B., Gagel R.F., Lee N.Y., Machens A., Moley J.F., Pacini F., et al. Revised American Thyroid Association Guidelines for the Management of Medullary Thyroid Carcinoma. Thyroid. 2015;25:567–610. doi: 10.1089/thy.2014.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chair R.T.K., Eng C., Evans D.B., Francis G.L., Gagel R.F., Gharib H., Moley J.F., Pacini F., Ringel M.D., Schlumberger M., et al. Medullary Thyroid Cancer: Management Guidelines of the American Thyroid Association. Thyroid. 2009;19:565–612. doi: 10.1089/thy.2008.0403. [DOI] [PubMed] [Google Scholar]
- 82.Cosci B., Vivaldi A., Romei C., Gemignani F., Landi S., Ciampi R., Tacito A., Molinaro E., Agate L., Bottici V., et al. In silico and in vitro analysis of rare germline allelic variants of RET oncogene associated with medullary thyroid cancer. Endocr. Relat. Cancer. 2011;18:603–612. doi: 10.1530/ERC-11-0117. [DOI] [PubMed] [Google Scholar]
- 83.Elisei R., Romei C., Renzini G., Bottici V., Cosci B., Molinaro E., Agate L., Cappagli V., Miccoli P., Berti P., et al. The timing of total thyroidectomy in RET gene mutation carriers could be personalized and safely planned on the basis of serum calcitonin: 18 years experience at one single center. J. Clin. Endocrinol. Metab. 2012;97:426–435. doi: 10.1210/jc.2011-2046. [DOI] [PubMed] [Google Scholar]
- 84.Sherman S.I., Clary D.O., Elisei R., Schlumberger M.J., Cohen E.E.W., Schoffski P., Wirth L.J., Mangeshkar M., Aftab D.T., Brose M.S. Correlative analyses of RET and RAS mutations in a phase 3 trial of cabozantinib in patients with progressive, metastatic medullary thyroid cancer. Cancer. 2016;122:3856–3864. doi: 10.1002/cncr.30252. [DOI] [PubMed] [Google Scholar]
- 85.Romei C., Casella F., Tacito A., Bottici V., Valerio L., Viola D., Cappagli V., Matrone A., Ciampi R., Piaggi P., et al. New insights in the molecular signature of advanced medullary thyroid cancer: Evidence of a bad outcome of cases with double RET mutations. J. Med. Genet. 2016;53:729–734. doi: 10.1136/jmedgenet-2016-103833. [DOI] [PubMed] [Google Scholar]
- 86.Ciampi R., Mian C., Fugazzola L., Cosci B., Romei C., Barollo S., Cirello V., Bottici V., Marconcini G., Pelizzo M.R., et al. Evidence of a low prevalence of ras mutations in a large medullary thyroid cancer series. Thyroid. 2012;23 doi: 10.1089/thy.2012-0207. [DOI] [PubMed] [Google Scholar]
- 87.Eng C., Mulligan L.M., Healey C.S., Houghton C., Frilling A., Raue F., Thomas G.A., Ponder B.A. Heterogeneous mutation of the RET proto-oncogene in subpopulations of medullary thyroid carcinoma. Cancer Res. 1996;56:2167–2170. [PubMed] [Google Scholar]
- 88.Romei C., Ciampi R., Casella F., Tacito A., Torregrossa L., Ugolini C., Basolo F., Materazzi G., Vitti P., Elisei R. RET mutation heterogeneity in primary advanced medullary thyroid cancers and their metastases. Oncotarget. 2018;9:9875–9884. doi: 10.18632/oncotarget.23986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Romei C., Ugolini C., Cosci B., Torregrossa L., Vivaldi A., Ciampi R., Tacito A., Basolo F., Materazzi G., Miccoli P., et al. Low prevalence of the somatic M918T RET mutation in micro-medullary thyroid cancer. Thyroid. 2012;22:476–481. doi: 10.1089/thy.2011.0358. [DOI] [PubMed] [Google Scholar]
- 90.Elisei R., Cosci B., Romei C., Bottici V., Renzini G., Molinaro E., Agate L., Vivaldi A., Faviana P., Basolo F., et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: A 10-year follow-up study. J. Clin. Endocrinol. Metab. 2008;93:682–687. doi: 10.1210/jc.2007-1714. [DOI] [PubMed] [Google Scholar]
- 91.Mian C., Pennelli G., Barollo S., Cavedon E., Nacamulli D., Vianello F., Negro I., Pozza G., Boschin I.M., Pelizzo M.R., et al. Combined RET and Ki-67 assessment in sporadic medullary thyroid carcinoma: A useful tool for patient risk stratification. Eur. J. Endocrinol. 2011;164:971–976. doi: 10.1530/EJE-11-0079. [DOI] [PubMed] [Google Scholar]
- 92.Moura M.M., Cavaco B.M., Pinto A.E., Leite V. High prevalence of RAS mutations in RET-negative sporadic medullary thyroid carcinomas. J. Clin. Endocrinol. Metab. 2011;96:E863–E868. doi: 10.1210/jc.2010-1921. [DOI] [PubMed] [Google Scholar]
- 93.Boichard A., Croux L., Al Ghuzlan A., Broutin S., Dupuy C., Leboulleux S., Schlumberger M., Bidart J.M., Lacroix L. Somatic RAS mutations occur in a large proportion of sporadic RET-negative medullary thyroid carcinomas and extend to a previously unidentified exon. J. Clin. Endocrinol. Metab. 2012;97:E2031–E2035. doi: 10.1210/jc.2012-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]