Abstract
Steroid 5-α reductase (5AR) is responsible for the reduction of steroids to 5-α reduced metabolites, such as the reduction of testosterone to 5-α dihydrotestosterone (DHT). A new adverse outcome pathway (AOP) for 5AR inhibition to reduce female reproduction in fish (AOP 289) is under development to clarify the antiestrogenic effects of 5AR inhibitors in female fish. A sensitive method for the DHT analysis using chemical derivatization and liquid chromatography–tandem mass spectrometry was developed. A cell-based 5AR inhibition assay that utilizes human cell lines, a transient overexpression system, and fish cell lines was developed. The measured IC50 values of two well-known 5AR inhibitors, finasteride and dutasteride, were comparable in the different systems. However, the IC50 of dutasteride in the fish cell lines was lower than that in the human cell lines. Finasteride showed a higher IC50 against the RTG-2 cell line. These results demonstrated that 5ARs inhibition could differ in terms of structural characteristics among species. The assay has high sensitivity and reproducibility and is suitable for the application in 5AR inhibition screening for various endocrine disruption chemicals (EDCs). Future studies will continue to evaluate the quantitative inhibition of 5AR by EDCs to compare the endocrine-disrupting pathway in different species.
Keywords: 5α-reductase inhibitors, dihydrotestosterone, in vitro, dutasteride, finasteride, adverse outcome pathway
1. Introduction
Steroid 5-α reductase (5AR, EC. 1.3.99.5) is a membrane-bound protein that is responsible for reducing steroids such as testosterone, progesterone, and androstenedione to 5-α reduced metabolites such as 5-α dihydrotestosterone (DHT), 5-α dihydroprogesterone and androstanedione, respectively. There are three isoforms of 5AR in humans: SRD5A1, SRD5A2, and SRD5A3. SRD5A1 and SRD5A2 have functionality for 5-α reduction of steroids in humans. DHT is a more potent androgen than testosterone and has a function in androgen receptor activation [1,2,3]. The regulation of 5AR is important for the treatment of benign prostate hyperplasia (BPH) and prostate cancer (PC), and 5AR inhibitors have also been used for the treatment of baldness [4,5,6].
5AR inhibition was suggested as a new molecular initiating event (MIE) in the adverse outcome pathway (AOP) 289 [7]. AOP 289, which is entitled ‘Inhibition of 5α-reductase leading to impaired fecundity in female fish’, describes the effects of 5AR on reducing estradiol and further decreasing egg production via vitellogenin reduction. 5AR is expressed in both sexes, and DHT is involved in estradiol (E2) level regulation [8]. Even though a lower expression of 5AR was detected in females, its inhibition reduced the fecundity of fish and affected several aspects of reproductive endocrine functions in both sexes of fathead minnows [9]. For the development of a quantitative AOP for 5AR inhibition, a quantitative structure–activity relationship is required for endocrine disruption chemical (EDC) evaluation. Several methods have been described for screening the pharmacological aspects of 5AR inhibitors, but experimental data are limited in fishes for screening for endocrine disruption.
In practice, 5AR inhibition studies are traditionally conducted using radioactive substrates with thin layer chromatography or high-performance liquid chromatography (HPLC) detection [10,11]. A native substrate method without radiolabeled isotopes that utilizes a spectrophotometric method [12] and a HPLC-UV detection method was also developed [13]. However, these methods have not been extensively applied due to their limitations, which include safety issues with radiometric assays and low sensitivity. The liquid chromatography-tandem mass spectrometry (LC-MS/MS) method can be used for high-throughput screening (HTS) techniques, and combinational chemistry during drug discovery and development has led to a tremendous increase in the number of compounds to be evaluated for potential 5AR inhibition [14,15]. Recently, sensitive chemical derivatization methods for DHT detection in LC-MS/MS were developed [16].
In the present study, using this chemical derivatization technique, a cell incubation method was developed, and the metabolites of the substrates were determined in a single assay using LC-MS/MS for HTS of 5AR inhibition. LNCaP clone FGC (LNCaP) and DU-145 cells that express the SRD5A1 gene, SW-13 cells that express the SRD5A1 and SRD5A2 genes, and HEK-293 cells with transient overexpression of the SRD5A1 and SRD5A2 genes were compared to establish the enzyme inhibition method. In addition, to understand species differences in 5AR between fish and humans, the inhibition of 5AR was compared in the 5AR-expressing zebrafish liver cells (ZFL) and rainbow trout gonad cell lines (RTG-2).
2. Results
2.1. Method Validation
2.1.1. Linearity of the Calibration Curve and LLOQ
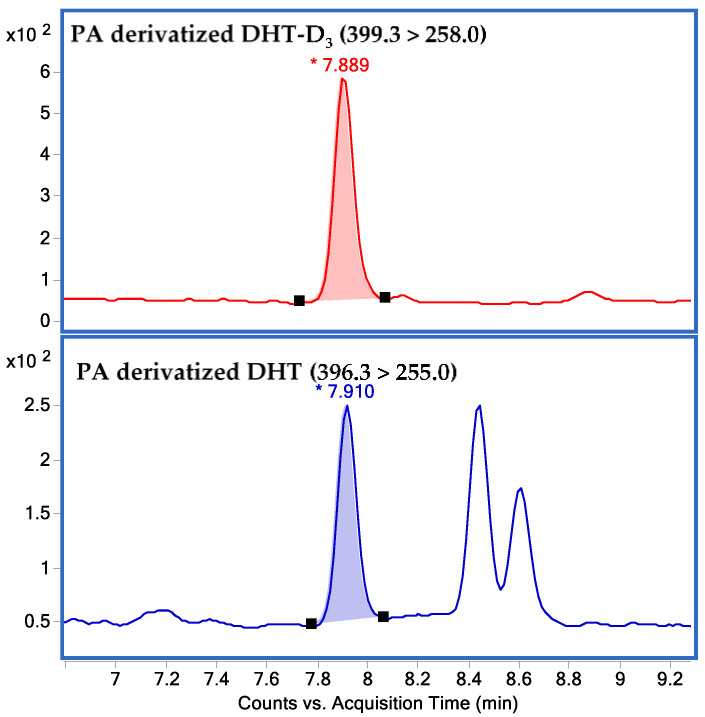
The 1/x weighted linear regression calibration curve for DHT was obtained by plotting the MRM peak area ratio (analyte/IS) versus the concentration over the working range 0.01–1000 nM for the assay media. The 1/x weighted linear correlation coefficient (R2) for DHT exceeded 0.995. The LLOQ of this method for DHT was 0.05 nM. Chromatograms of 2-picolinic acid (PA)-derivatized DHT and DHT-d3 are presented in Figure 1.
Figure 1.
Chromatograms of 2-picolinic acid (PA)-derivatized 5-α dihydrotestosterone (DHT) and DHT-D3.
2.1.2. Accuracy and Precision
The method accuracy and precision that were determined using the low QC, medium QC and high QC samples are presented in Table 1. The inter day accuracies for the low, medium, and high QC samples were 102.3, 104.0, and 95.0%, respectively, and the intraday accuracies for the low, medium, and high QC samples were 101, 98.9, and 95.5%, respectively. The interday precisions were 1.3% for low QC, 0.7% for medium QC, and 1.6% for high QC, and the intraday precisions were 0.9% for low QC, 2.5% for medium QC, and 1.3% for high QC. Acceptable method accuracies and precisions on the QC samples were obtained.
Table 1.
Method accuracy and precision (n = 5).
| Low QC | Medium QC | High QC | |
|---|---|---|---|
| CV%—inter day a | 1.3 | 0.7 | 1.6 |
| CV%—intra day b | 0.9 | 2.5 | 1.3 |
| Accuracy%—inter day | 102.3 | 104.0 | 95.0 |
| Accuracy%—intra day | 101.0 | 98.9 | 95.5 |
a Coefficient of variation within days; b Coefficient of variation between 3 consecutive days.
2.2. Assay Application in Human Cell Lines
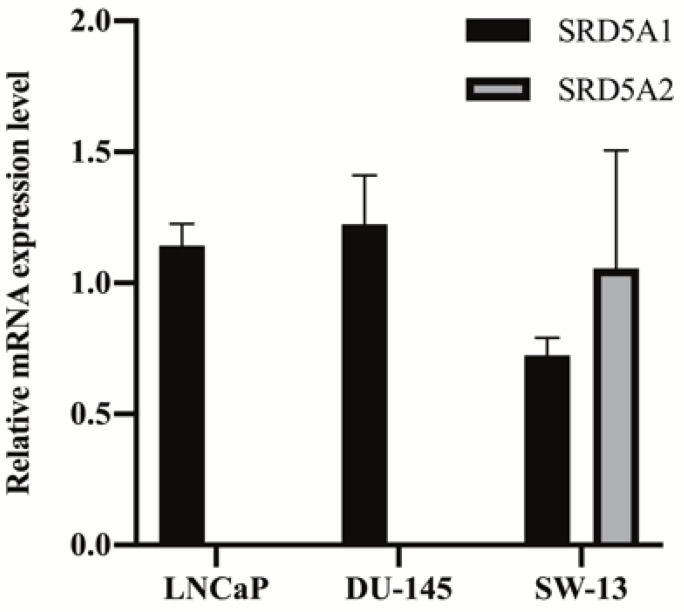
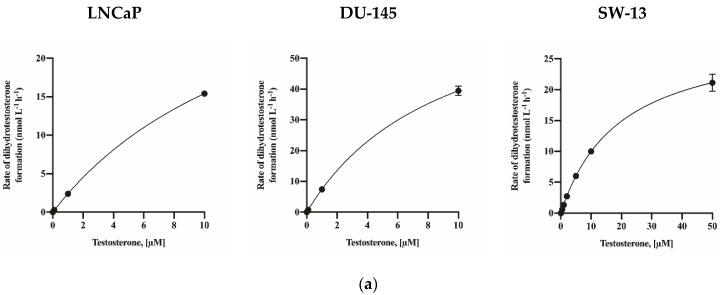
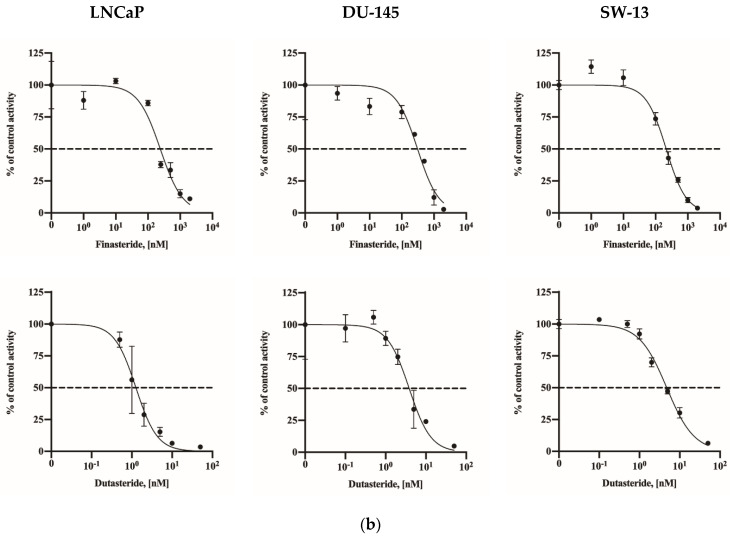
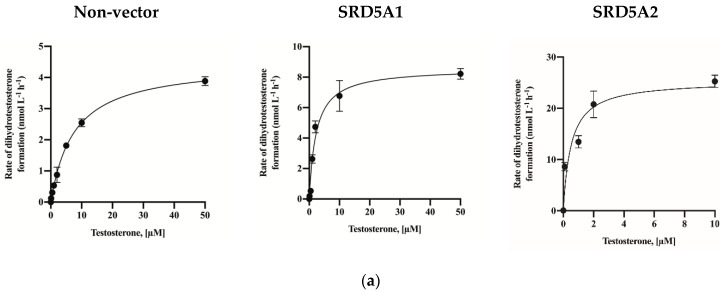
The gene expression levels of SRD5A1 and SRD5A2 in LNCaP, DU-145, and SW-13 cells are presented in Figure 2. All cell lines showed SRD5A1 expression, but SRD5A2 expression was identified only for SW-13 cells. For the calculation of KM, testosterone treatment was applied in increments of 0 to 10 µM in the LNCaP and DU-145 cells and in increments of 0 to 50 µM in the SW-13 cells for 3 h. The de novo synthesized DHT levels were measured (Figure 3a). The calculated values of KM and Vmax are presented in Table 2. The Vmax value of the DU-145 cells was 75.55 nmol/L/h, which exceeded those of the other two cell lines. Based on the calculated KM value as the substrate concentration, inhibition assays were conducted by treating the cells with a selective SRD5A2 inhibitor, namely, finasteride, and a dual SRD5A1 and SRD5A2 inhibitor, namely, dutasteride (Figure 3b). The IC50 value of each inhibitor was calculated and is presented in Table 3.
Figure 2.
Quantitative PCR analysis for measuring the mRNA expression levels of SRD5A1 and SRD5A2 in the LNCaP, DU-145, and SW-13 cell lines. The data are expressed as the mean ± standard deviation (SD) of three repeated experiments.
Figure 3.
Activity of 5α-reductase (a) and inhibitory effects of finasteride and dutasteride (b) on LNCaP, DU-145, and SW-13 cells. The data are expressed as the mean ± standard deviation (SD) of three repeated experiments.
Table 2.
Vmax and KM value for testosterone in each cell line.
| Vmax (nmol L−1 h−1) | KM (nM) | ||
|---|---|---|---|
| Human cell lines | LNCaP | 38.67 (34.36–45.16) * | 15.10 (12.35–19.25) |
| DU-145 | 75.55 (66.61–90.45) | 9.15 (7.01–12.81) | |
| SW-13 | 29.35 (27.94–30.90) | 19.42 (17.31–21.88) | |
| Overexpression lines | Non vector | 4.473 (4.29–4.66) | 7.56 (6.74–8.46) |
| SRD5A1 | 8.584 (7.86–9.35) | 2.29 (1.70–3.09) | |
| SRD5A2 | 22.52 (20.01–25.42) | 0.36 (0.18–0.65) | |
| Fish cell lines | ZFL | 116.60 (106.9–126.9) | 0.46 (0.30–0.68) |
| RTG-2 | 13.09 (11.88–14.37) | 1.12 (0.78–1.61) |
* The values in parentheses are 95% confidence intervals.
Table 3.
IC50 values of finasteride and dutasteride in each cell line.
| IC50 Value | |||
|---|---|---|---|
| Finasteride (nM; 95% CI *) | Dutasteride (nM; 95% CI) | ||
| Human cell lines | LNCaP | 241.0 (185.8–303.9) | 1.26 (1.02–1.57) |
| DU-145 | 308.5 (217.0–415.5) | 3.83 (3.10–4.78) | |
| SW-13 | 213.5 (180.2–250.7) | 4.75 (4.26–5.32) | |
| Overexpression lines | SRD5A1 | 332.8 (260.9–424.8) | 1.27 (0.76–2.06) |
| SRD5A2 | 69.83 (33.65–133.3) | 1.19 (0.96–1.47) | |
| Fish cell lines | ZFL | 142.4 (121.5–165.7) | 7.33 (6.12–8.77) |
| RTG-2 | 2667 (2394–2952) | 13.19 (10.73–16.54) | |
* The values in parentheses are 95% confidence intervals.
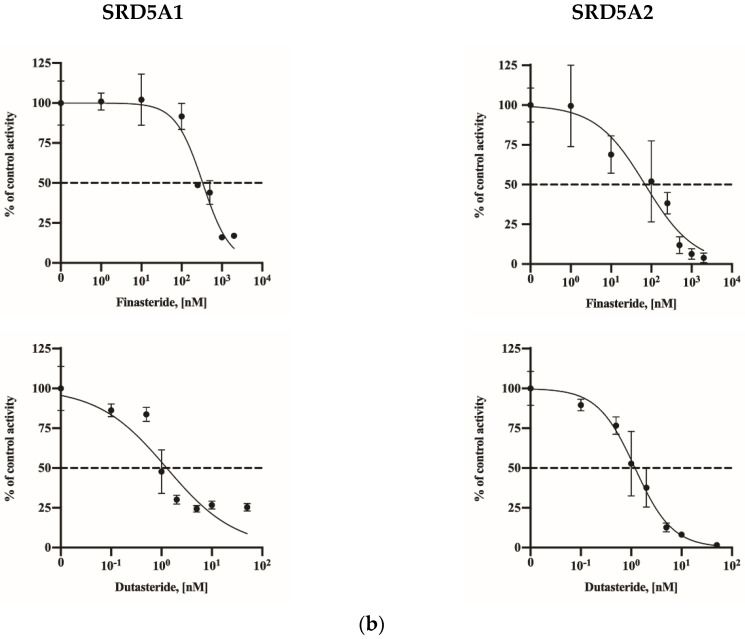
2.3. Assay Application in SRD5A2-Overexpressing HEK-293 Cells
For the calculation of KM, testosterone was added in increments of 0 to 50 µM to non-vector- and SRD5A1-HEK293 cells and in increments of 0 to 10 µM to SRD5A2-HEK293 cells 24 h after transfection. The DHT levels were measured (Figure 4a). The calculated KM and Vmax values are presented in Table 2. SRD5A2 showed a higher production rate (Vmax and KM were 22.52 and 0.36 nM, respectively) due to the higher affinity of the enzyme for testosterone. Based on the calculated KM values, inhibition assays were conducted by treating the cells with selective SRD5A2 inhibitor finasteride and the dual SRD5A1 and SRD5A2 inhibitor dutasteride (Figure 4b). The IC50 values were calculated (Table 3).
Figure 4.
Activity of 5α-reductase (a) and inhibitory effects of finasteride and dutasteride (b) on transfected HEK-293 cells. The data are expressed as the mean ± standard deviation (SD) of three repeated experiments.
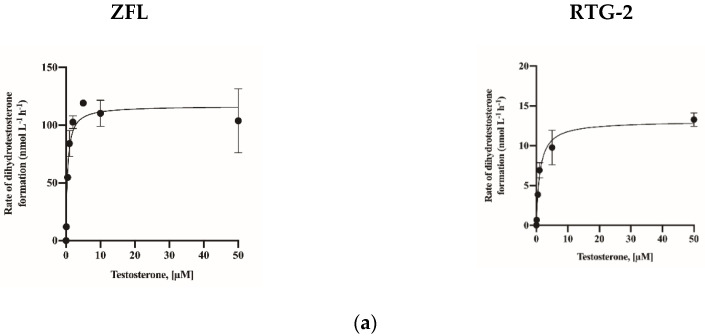
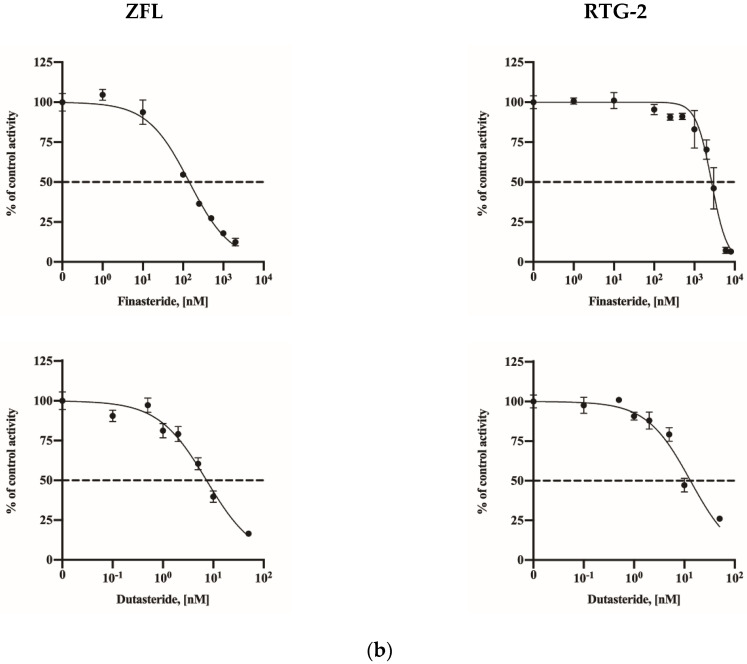
2.4. Assay Application in a Fish Cell Line
For the calculation of KM for optimized assay conditions, ZFL and RTG-2 cells were treated with testosterone in increments of 0 to 50 µM. The DHT levels were measured (Figure 5a). The calculated KM and Vmax values are presented in Table 2. Based on the calculated KM values, inhibition assays were conducted (Figure 5b). Both the RTG-2 and ZFL cells showed lower KM values than human cell lines. The Vmax value of the ZFL cells (116.6 nmol/L/h) substantially exceeded those of the 5AR-overexpressing cell line and the human cell lines (29.35 in LNCaP, 75.55 in DU-145, and 29.35 in SW-13). The IC50 value of finasteride in the RTG-2 cells was 2459 nM, which exceeded those of the 5AR-overexpressing cell line and the human cell lines (1.27 in SRD5A1 and 1.16 in SRDA2-overexpressing HEK cells). Furthermore, the IC50 values of dutasteride in both fish cell lines exceeded those of the 5AR-overexpressing cell line and the human cell lines (Table 3).
Figure 5.
Activity of 5α-reductase (a) and inhibitory effects of finasteride and dutasteride (b) on and zebrafish liver cells (ZFL) and rainbow trout gonad (RTG-2) cells. The data are expressed as the mean ± standard deviation (SD) of three repeated experiments.
3. Discussion
Fluorinated anhydride acylation methods are widely used for gas chromatography mass spectrometry (GC-MS) for steroid quantification. Similar to the acylation reaction of fluorinated anhydrides and the hydroxyl group of the seventeenth carbon position in the steroid reaction, derivatization using PA showed a higher sensitivity in the detection of 17-OH steroids, such as corticosteroids, in ESI-LC/MS [17]. Recently, LC-MS based quantification methods for androgens such as DHT that utilize various sample sources were developed, and 5AR inhibition studies were conducted [16,18,19,20,21,22]. The method in the present study requires an additional derivatization step compared to the direct measurement. However, compared to these reports, the LLOQ of DHT (14.5 pg/mL) in the present study showed higher sensitivity than hydroxylamine hydrochloride derivatization [18] or direct measurement [19,23] by using LLE after PA derivatization. Also, methods using solid-phase extraction have been developed for the detection of steroids, but these methods are not efficient in time and cost -effective compared with liquid-liquid extraction [16,24,25]. The present study also used 2 times the liquid-liquid extraction step using MTBE after and before derivatization, this process increased the recovery of target compounds from 69 to 74% to 89–108% [16]. The lower limit of quantification of other studies using spectrophotometric method for DHT were from 0.2–10 nM [12,22,26], and other studies using radioactive substrates were range of 25 to 250 ng. The comparison study between immunoassay and LC/MS detection of DHT showed that the variation of detection was relatively more significant in immunoassay than in MS systems [27]. Thus, the method in the present study has an advantage for the detection of DHT than other methods.
A cell-based assay has additional factors that need to optimizing assay condition, but it has more reliability to in vivo system than purified enzyme or centrifuged fraction. Inhibition of 5AR reduced the DHT levels in tissues and can affect the androgen receptor (AR) expression [28,29,30]. Steroids such as androgens, estrogens and corticosteroids and inhibitors of 5AR are widely utilized in pharmacological applications, and these chemicals may act as EDCs and substantially impact fish and other species that are exposed to the environment [31,32]. We compared the 5AR activities and inhibition rates of 5AR by finasteride and dutasteride between human cell lines and fish cell lines. The Vmax and KM values in human cell lines were the largest in the DU-145 cells (Table 2). This result may be related to AR signaling. The LNCaP cell line was AR-positive, whereas the DU-145 cell line was AR-negative. DHT can be metabolized to DHT-glucuronide by the uridine diphosphate-glucuronosyltransferase (UGT) 2B15 and 2B17 enzymes in prostate cells, and these enzymes are modulated by AR [33]. It is possible that the rate of DHT production in AR-negative DU-145 cells exceeds those in other cell lines. The optimal pH of SRD5A1 activity is a broad range from 6.0 to 8.5, and the range for SRD5A2 is from 5.0 to 5.5 [12,33,34]. The steroid affinity of SRD5A2 is 10–20 times higher than that of SRD5A1 under optimal conditions [35].
Under transient transfection conditions, the Vmax values in HEK-293 cells that were transfected with SRD5A1 and SRD5A2 were approximately 2 times and 5 times larger, respectively, than those of the nontransfected HEK-293 cells. The KM values in HEK-293 cells that were transfected with SRD5A1 and SRD5A2 were approximately 3.3 times and 21 times smaller, respectively. The transfected cell lines did not show a higher Vmax compared to human cell lines, but the KM values decreased; hence, we assume that transient conditions can be used for the comparison of specific enzyme inhibition.
Both fish cell lines were more sensitive to testosterone treatment than human cell lines, and the ZFL cells were more sensitive than the RTG-2 cells. Other studies showed that the activity of 5AR in goldfish (Carassius auratus) was high in nonreproductive tissues such as the liver, brain and pituitary tissues, and it was reported that the expression pattern of SRD5A2 in toadfish (Opsanus tau) was significantly higher in the liver than in the gonad, in contrast to that in humans [36,37]. In the case of rainbow trout (Oncorhynchus mykiss), SRD5A activity was confirmed in the skin of males and females [38,39]. Although we did not measure the 5AR activity in whole tissue cells, our results demonstrated that fish cell lines are more sensitive to testosterone than human cell lines. The results showed a clear difference in steroid metabolism between the human and fish cell lines. In addition, the activity of 5AR in fish liver cells exceeded that in gonad cells.
The results of the 5AR inhibition assay demonstrated that dutasteride was more potent than finasteride in all cell lines. This is because dutasteride, which is a 5AR dual inhibitor, had a higher 5AR inhibition efficiency, and this tendency was similar to that observed in previous studies [39,40]. However, all the fish cell lines except ZFL on finasteride showed relatively lower sensitivity than human cell lines, and the IC50 value of RTG-2 on finasteride was 14 times larger than those on other cell lines. The IC50 values of dutasteride in fish cell lines exceeded those in human cell lines.
Similar to our results, other studies also reported that the activity of inhibitors differs among species. The inhibitory effects of finasteride, which mainly inhibits SRD5A2, were similar among dogs, monkeys, and humans, whereas finasteride inhibited both SRD5A1 and SRD5A2 in rats [41,42]. In addition, in a comparison of rat and human IC50 values comparisons of finasteride using rat 5α-reductase in prostate microsome were 11 nM, 13 nM, and 237 nM, and IC50 values of dutasteride to rat and human 5α-reductase were in the range of 0.2–7 nM [14,43,44,45,46,47]. It was suggested that the difference in amino acid sequences may present a differential response to inhibitors [42]. The amino acid sequence identity of SRD5A1 in humans and fish was approximately 50.2–51.7%, and for SRD5A2 the amino acid identity was detected as 42.4–52.3% (Table 4). Due to the difference in amino acid sequences, the enzymes may differ structurally, and accordingly, the interactions between the substrate or inhibitor and the enzymes can also differ. This suggests that known EDCs may exert various adverse effects on several species through other interactions; thus, future studies are necessary for identifying differences in the impact of EDCs among species.
Table 4.
Percentage of amino acid identity of human, zebrafish, and rainbow trout 5ARs.
| Zebrafish | Rainbow Trout | ||
| srd5a1 | srd5a1 | ||
| Human srd5a1 | 51.7 | 50.2 | |
| Zebrafish | Rainbow Trout | ||
| srd5a2a | srd5a2b | srd5a2a | |
| Human srd5a2 | 52.3 | 42.4 | 50.2 |
Data were compared with human 5ARs amino acid sequence. The percentage of amino acid identity was compared using NCBI’s BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 4 February 2021) and UniProt (http://www.uniprot.org, accessed on 4 February 2021). The sequences used for analysis are as follows (species, gene_GenBank GI ID): (Human, srd5a1_4507201, srd5a2_39812447); (Zebrafish, srd5a1_11549628, srd5a2a_62955375, srd5a2b_62202806); (Rainbow trout, srd5a1_1211289547, srd5a2_1211257249).
4. Materials and Methods
4.1. Chemicals and Reagents
Fetal bovine serum (FBS), Leibowitz’s L-15 medium, the Roswell Park Memorial Institute (RPMI) 1640 medium, Ham’s F12 medium, Eagle’s minimal essential medium (EMEM), Dulbecco’s modified Eagle’s medium (DMEM), the Opti-MEM medium, a penicillin/streptomycin solution and trypsin were obtained from GIBCO (Grand Island, NY, USA). Trout serum was purchased from Caisson Laboratories (Smithfield, VA, USA). Mouse epidermal growth factor (EGF) and HEPES were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Sodium bicarbonate, bovine insulin, DHT, DHT-D3 solution, methyl-tertiary-butyl ether (MTBE), trimethylamine (TEA), tetrahydrofuran (THF), 2- PA, 4-(dimethylamino) pyridine (DMAP), 2-methyl-6-nitrobenzoic anhydride (MNBA), and acetic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA), and HPLC-grade formic acid was purchased from Fisher Scientific (Pittsburgh, PA, USA). MS-grade methanol and water were obtained from VWR (Westchester, NY, USA). The stock solution and internal standard were prepared in methanol. The derivatization reagent was prepared by dissolving 25.0 mg of PA, 10.0 mg of DMAP, and 20.0 mg of MNBA in 1 mL of THF (Yamashita et al., 2009) and vortexing. Then, the mixture was left at room temperature for at least 5 min before the sample pretreatment.
4.2. Cell Culture
HEK-293, LNCaP, DU-145, SW-13, and ZFL cell lines were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured according to their instructions. The HEK-293 cells were cultured in a high-glucose DMEM that contained 10% FBS, 100 units/mL penicillin, and 100 µg/mL streptomycin. The DU-145 cells were cultured in EMEM that contained 10% FBS, 100 units/mL penicillin, and 100 µg/mL streptomycin. The LNCaP cells were cultured in RPMI 1640 that contained 10% FBS, 100 units/mL penicillin, and 100 µg/mL streptomycin at 37 °C in 5% CO2. SW-13 cells were cultured in Leibovitz’s L-15 medium with 10% FBS at 37 °C without CO2. ZFL cells were cultured in a complete medium that was composed of 50% L-15, 35% DMEM medium, and 15% F12 medium that contained 0.15 g/mL sodium bicarbonate, 15 mM HEPES, 0.01 mg/mL bovine insulin, 50 ng/mL mouse EGF, 5% FBS, and 0.5% trout serum at 28 °C without CO2. RTG-2 cells were obtained from Prof. Kristin Schirmer (EAWAG, Switzerland) and cultured in the L-15 medium with 5% FBS, 100 units/mL penicillin, and 100 µg/mL streptomycin at 20 °C without CO2.
4.3. Transient Overexpression
SRD5A1 and SRD5A2 expression vectors were purchased from GenScript (pcDNA3.1+/C-(K)-DYK-SRD5A1, OHu02727D, and pcDNA3.1+/C-(K)-DYK-SRD5A2, OHu18065D, respectively). Transient overexpression was induced using transfection of cDNA with lipofectamine (Thermo Fisher Scientific, Waltham, MA, USA). HEK-293 cells were seeded in 24-well plates at a density of 105 cells per well and incubated at 37 °C in an atmosphere of 5% CO2. After overnight culture, 500 ng of cDNA and 0.75 µL of the Lipofectamine 3000 reagent were diluted in the Opti-MEM medium and incubated for 15 min for DNA-lipid complex formation. The DNA-lipid complex was added to the wells and incubated for 6 h. After incubation, the sample-treated medium was changed to the complete culture medium and incubated for 18 h.
4.4. Cell Culture Assay Application
All cells were seeded on a 24-well plate. The seeding densities of the DU-145, LNCaP, and SW-13 cells were 0.5 × 105 cells per well. The ZFL and RTG-2 cells were seeded at densities of 1.0 × 105 cells and 2.0 × 105 cells, respectively. After overnight culture, the culture media was aspirated from each well and treated with testosterone that was diluted in the complete medium for 3 h and 6 h. In the case of transiently transfected HEK-293 cells, the testosterone treatment was applied after transient overexpression under the same conditions as other cell lines. The treated media were collected from each well and centrifuged at 3000× g for 5 min at 4 °C. The supernatants were stored at −80 °C until needed. A selective SRD5A2 inhibitor, namely, finasteride, and a dual inhibitor of SRD5A1 and SRD5A2, namely, dutasteride, were used as inhibitors of 5-α reductase. The seeding conditions of all cells were the same as those previously described. After overnight culture, the culture medium was aspirated, and the cells were cotreated with a medium that contained testosterone and inhibitors for 3 h. The medium was collected from each well and centrifuged at 3000× g for 5 min at 4 °C. The supernatants were stored at −80 °C until analysis.
4.5. qRT PCR
The total RNA was isolated using a column-based kit (Qiagen, Valencia, CA, USA). cDNA was synthesized from 500 ng of the total RNA using a high-capacity RNA-to-cDNA kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. qRT-PCR assays were conducted using a TaqMan gene expression assay on a 7500 FAST real-time PCR system (Applied Biosystems). The TaqMan assay ID is as follows (Gene, assay ID): RPLO0, Hs00420895_gH; SRD5A1, Hs00165843_m1; SRD5A2, Hs00165843_m1.
4.6. Sample Preparation
A method that was modified by [15] was used for DHT extraction from the samples. Each sample, which included the calibration, QC, and assay medium, was placed in 1.5 mL PP tubes and spiked with a 0.5 ng/mL DHT-D3 internal standard prior to extraction. All sample tubes were vortexed for 5 s, and the samples were extracted using a liquid-liquid extraction (LLE) method via the addition of 600 µL of MTBE. The samples were vortexed and centrifuged at 4500× g rpm for 5 min, and the organic phase was transferred into glass tubes. The extraction step was repeated once, and the organic phase extracts were dried under a stream of nitrogen. After the samples were dried, 100 µL of the derivatization reagent and 100 µL of TEA were added for DHT derivatization. The samples were vortexed and incubated at room temperature, and 1 mL of 10% acetic acid was added to stop the reaction after 30 min of incubation. The LLE step, which was conducted before the derivatization step, was repeated twice. The organic phase extracts were collected, dried under a stream of nitrogen, and reconstituted in 50 µL of 80% methanol that contained 0.1% formic acid for LC-MS/MS analysis.
4.7. Instrumental Conditions
The extracts were analyzed for DHT via ultra-performance LC-MS/MS (Agilent 1200/6460C QQQMSD coupled Jet Stream technology electrospray ion (ESI) source; Agilent Technologies, Santa Clara, CA, USA). To separate the analytes, a Kinetex XB-C18 column (2.1 mm × 150 mm, 2.6 μm) that was fitted with a ZORBAX Eclipse Plus C18 guard column (2.1 mm × 5 mm, 1.8 μm) was used. The mobile phase solvents were 0.1% formic acid and methanol, with a flow rate of 300 µL/min for 14 min and a sample injection volume of 10 µL. The gradient started at 5% methanol, was increased to 90% with a 3 min ramp, and was maintained until 5 min. Then, the ramp was increased to 95% methanol until 13 min. At 13.1 min, the ramp was decreased to 5% methanol, which was maintained until 14 min. Mass spectrometry was conducted in the positive ion electrospray mode and multiple reaction mode (MRM) to identify and quantify DHT. The MRM transitions are 396.3 > 255.0 and 273.0 for PA-derivatized DHT and 399.3 > 258.0 and 276.0 for DHT-D3, respectively. The optimized MS conditions are as follows: gas temperature of 350 °C, gas flow of 10 L/min, nebulizer gas pressure of 45 psi, sheath gas temperature of 350 °C, sheath gas flow of 11 L/min, capillary voltage of 3500 V, nozzle voltage of 500 V, and collision energies of 16 V for DHT and 14 V for DHT-D3.
4.8. Calibration Curve and LLOQ
A linear calibration curve was established using a standard solution that consisted of a concentration series of 0.1, 0.5, 1, 5, 10, 50, 100, 500, and 1000 nM DHT with 5 ng/mL DHT-D3. The calibrators for DHT were prepared in an assay medium with a blank (which contained only 5 ng/mL DHT-D3). To evaluate the linearity of the calibration curve, a 1/x weighting linear regression was used. The LLOQ was defined as the lowest concentration of the calibrators at which the signal sensitivity was 3-fold higher than those of the corresponding blank samples.
4.9. Accuracy and Precision
The accuracy and precision of the method were evaluated using intra- and interday quality control (QC) samples. Five replicates each of low QC, medium QC, and high QC samples were prepared by spiking into standard solutions of DHT and DHT-D3 in an assay medium. Their concentrations are 5, 50, and 500 nM, respectively, which represent 100% DHT accuracy of each QC set. The method accuracy was evaluated based on the recoveries (%) that were calculated for each QC spiking level. The precision of the method was expressed as the coefficient of variation (CV, %). CV was determined by dividing the relative standard deviations of the QC samples by the average DHT concentration of the QC samples. The interday accuracy and precision were determined via three parallel analyses of three sets of QC samples (low, medium, and high). The intraday accuracy and precision were determined via analysis of five replicate samples of each QC set for 3 consecutive days.
4.10. Data Analysis
The LC-MS/MS data were analyzed with the MassHunter quantitative analysis software (Agilent). The DHT inhibition in the presence of inhibitors was expressed as a percentage of the corresponding control value. Each point was expressed as the mean ± S.D. A sigmoid-shaped curve was fitted to the data, and the enzyme kinetic module and inhibition parameter IC50 were calculated by fitting the Hill equation to the data using nonlinear regression (least-squares best fit modeling) of the plot of the percent control activity vs. concentration of the test inhibitor using GraphPad Prism 8 (GraphPad Software Inc., San Diego, CA, USA). Control samples (without the inhibitor) were assayed in each analytical run. The amount of metabolite in each sample (relative to the control samples) was plotted vs. the inhibitor concentration.
5. Conclusions
The present study established cell-based 5AR inhibition assay models using quantitative LC-MS/MS analysis. Using this method, all the fish cell lines except the ZFL cell line for finasteride showed significantly higher IC50 values for dutasteride and finasteride. This method can be used as a tool for 5AR inhibitor screening in the early stages of drug discovery. In future studies, the inhibitory potency of chemicals will be evaluated for predicting endocrine disruption via a 5AR inhibition assay to develop quantitative AOPs for 5AR inhibition in fishes.
Author Contributions
C.S.R., S.K.K. and Y.J.K. conceived and designed the experiments; D.K., H.C. and R.E. performed the experiments, and analyzed the data; D.K., H.C. and C.S.R. wrote the paper; S.K.K., C.S.R. and Y.J.K. reviewed and edited the entire manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a National Research Council of Science and Technology (NST) grant by the Korean government (MSIP) (No. CAP-17-01-KIST Europe) and the Korea Institute of Science and Technology Europe basic research program (Project no. 12101).
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bruchovsky N., Wilson J.D. The conversion of testosterone to 5α-androstan-17-beta-ol-3-one by rat prostate in vivo and in vitro. J. Biol. Chem. 1968;243:2012–2021. doi: 10.1016/S0021-9258(18)93542-8. [DOI] [PubMed] [Google Scholar]
- 2.Russell D.W., Wilson J.D. Steroid 5α-reductase: Two genes/two enzymes. Annu. Rev. Biochem. 1994;63:25–61. doi: 10.1146/annurev.bi.63.070194.000325. [DOI] [PubMed] [Google Scholar]
- 3.Langlois V.S., Zhang D., Cooke G.M., Trudeau V.L. Evolution of steroid-5α-reductases and comparison of their function with 5beta-reductase. Gen. Comp. Endocrinol. 2010;166:489–497. doi: 10.1016/j.ygcen.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 4.McConnell J.D., Wilson J.D., George F.W., Geller J., Pappas F., Stoner E. Finasteride, an inhibitor of 5α-reductase, suppresses prostatic dihydrotestosterone in men with benign prostatic hyperplasia. J. Clin. Endocrinol. Metab. 1992;74:505–508. doi: 10.1210/jcem.74.3.1371291. [DOI] [PubMed] [Google Scholar]
- 5.Diani A.R., Mulholland M.J., Shull K.L., Kubicek M.F., Johnson G.A., Schostarez H.J., Brunden M.N., Buhl A.E. Hair growth effects of oral administration of finasteride, a steroid 5α-reductase inhibitor, alone and in combination with topical minoxidil in the balding stumptail macaque. J. Clin. Endocrinol. Metab. 1992;74:345–350. doi: 10.1210/jcem.74.2.1309834. [DOI] [PubMed] [Google Scholar]
- 6.McConnell J.D., Roehrborn C.G., Bautista O.M., Andriole G.L., Dixon C.M., Kusek J.W., Lepor H., McVary K.T., Nyberg L.M., Clarke H.S., et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N. Engl. J. Med. 2003;349:2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 7.Ryu C.S., Sung B., Baik S., Kim Y.J., Lee Y. Inhibition of 5α-Reductase Leading to Impaired Fecundity in Female Fish. [(accessed on 11 December 2020)]; Available online: https://aopwiki.org/aops/289.
- 8.García-García M., Sánchez-Hernández M., García-Hernández M.P., García-Ayala A., Chaves-Pozo E. Role of 5α-dihydrotestosterone in testicular development of gilthead seabream following finasteride administration. J. Steroid Biochem. Mol. Biol. 2017;174:48–55. doi: 10.1016/j.jsbmb.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Margiotta-Casaluci L., Courant F., Antignac J.P., Le Bizec B., Sumpter J.P. Identification and quantification of 5α-dihydrotestosterone in the teleost fathead minnow (Pimephales promelas) by gas chromatography-tandem mass spectrometry. Gen. Comp. Endocrinol. 2013;191:202–209. doi: 10.1016/j.ygcen.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Andersson S., Bishop R.W., Russell D.W. Expression cloning and regulation of steroid 5α-reductase, an enzyme essential for male sexual differentiation. J. Biol. Chem. 1989;264:16249–16255. doi: 10.1016/S0021-9258(18)71614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raynaud J.P., Cousse H., Martin P.M. Inhibition of type 1 and type 2 5α-reductase activity by free fatty acids, active ingredients of permixon. J. Steroid Biochem. Mol. Biol. 2002;82:233–239. doi: 10.1016/S0960-0760(02)00187-5. [DOI] [PubMed] [Google Scholar]
- 12.Iwai A., Yoshimura T., Wada K., Watabe S., Sakamoto Y., Ito E., Miura T. Spectrophotometric method for the assay of steroid 5α-reductase activity of rat liver and prostate microsomes. Anal. Sci. 2013;29:455–459. doi: 10.2116/analsci.29.455. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda H., Sato N., Yamazaki M., Naruto S., Kubo M. Testosterone 5α-reductase inhibitory active constituents from Anemarrhenae Rhizoma. Biol. Pharm. Bull. 2001;24:586–587. doi: 10.1248/bpb.24.586. [DOI] [PubMed] [Google Scholar]
- 14.Mitamura K., Narukawa H., Mizuguchi T., Shimada K. Degradation of estrogen conjugates using titanium dioxide as a photocatalyst. Anal. Sci. 2004;20:3–4. doi: 10.2116/analsci.20.3. [DOI] [PubMed] [Google Scholar]
- 15.Abe M., Ito Y., Oyunzul L., Oki-Fujino T., Yamada S. Pharmacologically relevant receptor binding characteristics and 5α-reductase inhibitory activity of free fatty acids contained in saw palmetto extract. Biol. Pharm. Bull. 2009;32:646–650. doi: 10.1248/bpb.32.646. [DOI] [PubMed] [Google Scholar]
- 16.Gorityala S., Yang S., Montano M.M., Xu Y. Simultaneous determination of dihydrotestosterone and its metabolites in mouse sera by LC-MS/MS with chemical derivatization. J. Chromatogr. B. 2018;1090:22–35. doi: 10.1016/j.jchromb.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashita K., Takahashi M., Tsukamoto S., Numazawa M., Okuyama M., Honma S. Use of novel picolinoyl derivatization for simultaneous quantification of six corticosteroids by liquid chromatography-electrospray ionization tandem mass spectrometry. J. Chromatogr. A. 2007;1173:120–128. doi: 10.1016/j.chroma.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 18.Srivilai J., Rabgay K., Khorana N., Waranuch N., Nuengchamnong N., Ingkaninan K. A new label-free screen for steroid 5α-reductase inhibitors using LC-MS. Steroids. 2016;116:67–75. doi: 10.1016/j.steroids.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Cao Z., Lu Y., Cong Y., Liu Y., Li Y., Wang H., Zhang Q., Huang W., Liu J., Dong Y., et al. Simultaneous quantitation of four androgens and 17-hydroxyprogesterone in polycystic ovarian syndrome patients by LC-MS/MS. J. Clin. Lab. Anal. 2020;34:23539. doi: 10.1002/jcla.23539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan J.J.Y., Pan J., Sun L., Zhang J., Wu C., Kang L. Bioactives in Chinese proprietary medicine modulates 5α-reductase activity and gene expression associated with androgenetic alopecia. Front. Pharmacol. 2017;8:194. doi: 10.3389/fphar.2017.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D., Zhang M. Rapid quantitation of testosterone hydroxyl metabolites by ultra-performance liquid chromatography and mass spectrometry. J. Chromatogr. B. 2007;855:290–294. doi: 10.1016/j.jchromb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 22.Jain R., Monthakantirat O., Tengamnuay P., De-Eknamkul W. Identification of a new plant extract for androgenic alopecia treatment using a non-radioactive human hair dermal papilla cell-based assay. BMC Complementary Altern. Med. 2015;16:18. doi: 10.1186/s12906-016-1004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nouri M.-Z., Kroll K.J., Webb M., Denslow N.D. Quantification of steroid hormones in low volume plasma and tissue homogenates of fish using LC-MS/MS. Gen. Comp. Endocrinol. 2020;296:113543. doi: 10.1016/j.ygcen.2020.113543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Licea-Perez H., Wang S., Szapacs M.E., Yang E. Development of a highly sensitive and selective UPLC/MS/MS method for the simultaneous determination of testosterone and 5α-dihydrotestosterone in human serum to support testosterone re-placement therapy for hypogonadism. Steroids. 2008;73:601–610. doi: 10.1016/j.steroids.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita K., Miyashiro Y., Maekubo H., Okuyama M., Honma S., Takahashi M., Numazawa M. Development of highly sensitive quantification method for testosterone and dihydrotestosterone in human serum and prostate tissue by liquid chro-matography-electrospray ionization tandem mass spectrometry. Steroids. 2009;74:920–926. doi: 10.1016/j.steroids.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Koseki J., Matsumoto T., Matsubara Y., Tsuchiya K., Mizuhara Y., Sekiguchi K., Nishimura H., Watanabe J., Kaneko A., Hattori T., et al. Inhibition of Rat 5α-Reductase activity and testosterone-induced sebum synthesis in hamster sebocytes by an extract of Quercus acutissima Cortex. Evid. Based Complementary Altern. Med. 2015;2015:853846. doi: 10.1155/2015/853846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dorgan J.F., Fears T.R., McMahon R.P., Friedman L.A., Patterson B.H., Greenhut S.F. Measurement of steroid sex hormones in serum: A comparison of radioimmunoassay and mass spectrometry. Steroids. 2002;67:151–158. doi: 10.1016/S0039-128X(01)00147-7. [DOI] [PubMed] [Google Scholar]
- 28.Bauman T.M., Sehgal P.D., Johnson K.A., Pier T., Bruskewitz R.C., Ricke W.A., Huang W. Finasteride treatment alters tissue specific androgen receptor expression in prostate tissues. Prostate. 2014;74:923–932. doi: 10.1002/pros.22810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L.G., Liu X.M., Kreis W., Budman D.R. Down-regulation of prostate-specific antigen expression by finasteride through inhibition of complex formation between androgen receptor and steroid receptor-binding consensus in the promoter of the PSA gene in LNCaP cells. Cancer Res. 1997;57:714–719. [PubMed] [Google Scholar]
- 30.Audet-Walsh É., Yee T., Tam I.S., Giguère V. Inverse regulation of DHT synthesis enzymes 5α-reductase Types 1 and 2 by the androgen receptor in prostate cancer. Endocrinology. 2017;158:1015–1021. doi: 10.1210/en.2016-1926. [DOI] [PubMed] [Google Scholar]
- 31.Chang H., Wan Y., Hu J. Determination and source apportionment of five classes of steroid hormones in urban rivers. Environ. Sci. Technol. 2009;43:7691–7698. doi: 10.1021/es803653j. [DOI] [PubMed] [Google Scholar]
- 32.Schmid S., Willi R.A., Fent K. Effects of environmental steroid mixtures are regulated by individual steroid receptor signaling. Aquat. Toxicol. 2020;226:105562. doi: 10.1016/j.aquatox.2020.105562. [DOI] [PubMed] [Google Scholar]
- 33.Bao B.-Y., Chuang B.-F., Wang Q., Sartor O., Balk S.P., Brown M., Kantoff P.W., Lee G.-S.M. Androgen receptor mediates the expression of UDP-glucuronosyltransferase 2 B15 and B17 genes. Prostate. 2008;68:839–848. doi: 10.1002/pros.20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Span P.N., Smals A.G., Sweep C.G., Benraad T.J. Rat steroid 5α-reductase kinetic characteristics: Extreme pH-dependency of the type II isozyme in prostate and epididymis homogenates. J. Steroid. Biochem. Mol. Biol. 1995;54:185–192. doi: 10.1016/0960-0760(95)00125-J. [DOI] [PubMed] [Google Scholar]
- 35.Normington K., Russell D.W. Tissue distribution and kinetic characteristics of rat steroid 5α-reductase isozymes. Evidence for distinct physiological functions. J. Biol. Chem. 1992;267:19548–19554. doi: 10.1016/S0021-9258(18)41809-1. [DOI] [PubMed] [Google Scholar]
- 36.Martyniuk C.J., Bissegger S., Langlois V.S. Current perspectives on the androgen 5α-dihydrotestosterone (DHT) and 5 α-reductases in teleost fishes and amphibians. Gen. Comp. Endocrinol. 2013;194:264–274. doi: 10.1016/j.ygcen.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 37.Pasmanik M., Schlinger B.A., Callard G.V. In Vivo steroid regulation of aromatase and 5α-reductase in goldfish brain and pituitary. Gen. Comp. Endocrinol. 1988;71:175–182. doi: 10.1016/0016-6480(88)90308-5. [DOI] [PubMed] [Google Scholar]
- 38.Latz M., Reinboth R. Androgen metabolism in the skin of the rainbow trout (Oncorhynchus mykiss) Fish Physiol. Biochem. 1993;11:281–286. doi: 10.1007/BF00004576. [DOI] [PubMed] [Google Scholar]
- 39.Bramson H.N., Hermann D., Batchelor K.W., Lee F.W., James M.K., Frye S.V. Unique preclinical characteristics of GG745, a potent dual inhibitor of 5AR. J. Pharmacol. Exp. Ther. 1997;282:1496–1502. [PubMed] [Google Scholar]
- 40.Tian G., Mook R., Moss M.L., Frye S.V. Mechanism of time-dependent inhibition of 5α-reductases by. DELTA.1-4-Azasteroids: Toward perfection of rates of time-dependent inhibition by using ligand-binding energies. Biochemistry. 1995;34:13453–13459. doi: 10.1021/bi00041a024. [DOI] [PubMed] [Google Scholar]
- 41.Azzolina B., Ellsworth K., Andersson S., Geissler W., Bull H.G., Harris G.S. Inhibition of rat alpha-reductases by finasteride: Evidence for isozyme differences in the mechanism of inhibition. J. Steroid. Biochem. Mol. Biol. 1997;61:55–64. doi: 10.1016/S0960-0760(97)00002-2. [DOI] [PubMed] [Google Scholar]
- 42.Levy M.A., Brandt M., Sheedy K.M., Holt D.A., Heaslip J.I., Trill J.J., Ryan P.J., Morris R.A., Garrison L.M., Bergsma D.J. Cloning, expression and functional characterization of type 1 and type 2 steroid 5α-reductases from cynomolgus monkey: Comparisons with human and rat isoenzymes. J. Steroid Biochem. Mol. Biol. 1995;52:307–319. doi: 10.1016/0960-0760(94)00183-M. [DOI] [PubMed] [Google Scholar]
- 43.Häußler A., Allegrini P., Biollaz M., Batzl C., Scheidegger E., Bhatnagar A. CGP 53153: A new potent inhibitor of 5α-reductase. J. Steroid Biochem. Mol. Biol. 1996;57:187–195. doi: 10.1016/0960-0760(95)00260-X. [DOI] [PubMed] [Google Scholar]
- 44.Hirosumi J., Nakayama O., Fagan T., Sawada K., Chida N., Inami M., Takahashi S., Kojo H., Notsu Y., Okuhara M. FK143, a novel nonsteroidal inhibitor of steroid 5α-reductase: (1) In vitro effects on human and animal prostatic enzymes. J. Steroid Biochem. Mol. Biol. 1995;52:357–363. doi: 10.1016/0960-0760(94)00187-Q. [DOI] [PubMed] [Google Scholar]
- 45.Di Salle E., Giudici D., Radice A., Zaccheo T., Ornati G., Nesi M., Panzeri A., Delos S., Martin P. PNU 157706, a novel dual type I and II5α-reductase inhibitor. J. Steroid Biochem. Mol. Biol. 1998;64:179–186. doi: 10.1016/S0960-0760(97)00158-1. [DOI] [PubMed] [Google Scholar]
- 46.Xu Y., Dalrymple S.L., Becker R.E., Denmeade S.R., Isaacs J.T. Pharmacologic basis for the enhanced efficacy of dutasteride against prostatic cancers. Clin. Cancer Res. 2006;12:4072–4079. doi: 10.1158/1078-0432.CCR-06-0184. [DOI] [PubMed] [Google Scholar]
- 47.Mitamura K., Ogasawara C., Shiozawa A., Terayama E., Shimada K. Determination method for steroid 5α-reductase activity using liquid chromatography/atmospheric pressure chemical ionization-mass spectrometry. Anal. Sci. 2005;21:1241–1244. doi: 10.2116/analsci.21.1241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.