Abstract
Simple Summary
Ovarian cancer survival rates are poor, with most deaths occurring from cancer recurrence following initial remission. Accordingly, there is a significant need for treatments that prevent relapse. Here, using a therapeutic vaccine against a mouse model of ovarian cancer, we evaluate a personalized vaccine that could be delivered to patients during their remission period. We show that mice that receive a combination of cowpea mosaic virus nanoparticles (CPMV) and irradiated tumor cells overwhelmingly reject tumor challenges in a T cell-dependent manner. Accordingly, we extend the demonstrated potential of CPMV as a vaccine adjuvant. We provide initial evidence that vaccines delivered during periods of clinical remission, using previously resected tumor tissue and an immune adjuvant, may comprise a feasible strategy of ovarian cancer treatment.
Abstract
Ovarian cancer is the deadliest gynecological malignancy. Though most patients enter remission following initial interventions, relapse is common and often fatal. Accordingly, there is a substantial need for ovarian cancer therapies that prevent relapse. Following remission generated by surgical debulking and chemotherapy, but prior to relapse, resected and inactivated tumor tissue could be used as a personalized vaccine antigen source. The patient’s own tumor contains relevant antigens and, when combined with the appropriate adjuvant, could generate systemic antitumor immunity to prevent relapse. Here, we model this process in mice to investigate the optimal tumor preparation and vaccine adjuvant. Cowpea mosaic virus (CPMV) has shown remarkable efficacy as an immunostimulatory cancer therapy in ovarian cancer mouse models, so we use CPMV as an adjuvant in a prophylactic vaccine against a murine ovarian cancer model. Compared to its codelivery with tumor antigens prepared in three other ways, we show that CPMV co-delivered with irradiated ovarian cancer cells constitutes an effective prophylactic vaccine against a syngeneic model of ovarian cancer in C57BL/6J mice. Following two vaccinations, 72% of vaccinated mice reject tumor challenges, and all those mice survived subsequent rechallenges, demonstrating immunologic memory formation. This study supports remission-stage vaccines using irradiated patient tumor tissue as a promising option for treating ovarian cancer, and validates CPMV as an antitumor vaccine adjuvant for that purpose.
Keywords: ovarian cancer, immunotherapy, vaccine, CPMV, T cell-dependent, adjuvant, nanoparticle, cowpea mosaic virus
1. Introduction
A serous ovarian carcinoma diagnosis carries a dismal prognosis. Due to the cancer’s nonspecific clinical symptoms, the majority of patients are diagnosed with stage III or stage IV disease, among which the five-year survival rates are 42% and 26%, respectively [1]. The current standard of care includes surgical debulking, during which large quantities of tumor are removed from the peritoneal cavity. Surgery is generally followed by carboplatin and paclitaxel chemotherapy and, while most patients enter remission, many later relapse with chemo-resistant tumors [2]. Following relapse, most patients succumb to their disease. Accordingly, there is a significant need for therapies that could be applied during remission to prevent relapse when the tumor burden is very low.
Cancer immunotherapies have revolutionized clinical oncology, particularly in the treatment of certain cancers, such as melanoma. However, traditional immunotherapies, such as immune checkpoint blockades, have proven largely unsuccessful in treating ovarian cancer [3,4,5,6]. This is likely due to the fact that the ovarian tumor microenvironment is strongly immunosuppressive [7]. While immune checkpoint blockade therapies rely upon revitalizing an existing T cell response, the intense immunosuppression provided by the abundance of M2-type tumor-associated macrophages (TAMs), regulatory T cells (Tregs), and myeloid-derived suppressor cells (MDSCs) in the ovarian cancer microenvironment generally prevents a robust and effective T cell response [8,9,10,11,12,13].
Immune checkpoint blockade therapies have not successfully treated ovarian cancer, and the field is actively investigating other ovarian cancer immunotherapies, including vaccines [14]. An autologous dendritic cell vaccine against mucin 1 (MUC-1), a tumor-associated antigen (TAA), had promising results in phase II clinical trials [15]. Another promising approach relevant to this study used hypochlorous acid-oxidized ovarian whole tumor lysates to treat dendritic cells, ultimately inducing an anti-tumor CD8+ T cell response and extending survival outcomes [16]. Peptide vaccines, such as those directed against another TAA, NY-ESO-1, have shown promise [17,18,19]. Peptide vaccines, or antibodies targeting another TAA relevant to ovarian cancer, sperm surface protein 17 (Sp17), have been studied in mice and humans [20,21,22]. Further, PANVAC is a therapeutic poxviral vaccine containing the genes for the tumor-associated antigens MUC-1 and carcinoembryonic antigen (CEA), as well as immunostimulatory genes CD80, intracellular adhesion molecule-1 (ICAM1), and leukocyte function-associated antigen-3 (LFA3) [23]. It was recently shown that the prophylactic injection of freeze–thawed lysates of a murine ovarian cancer stem-like cell expressing high levels of ROR-1 increased mouse survival [24]. Another study indicated that the prophylactic injection of a TAA, Sp17, and CpG oligodeoxynucleotide, a toll-like receptor (TLR) 9 agonist, dramatically extended survival in mice [25]. Although moderately efficacious, all of these vaccines are therapeutic and designed to treat active disease. This study focused on vaccines designed to be delivered during remission, when disease is clinically undetectable, an approach that we call “remission-stage vaccines”, and we model with prophylactic vaccines.
Cowpea mosaic virus (CPMV) is a 30 nm icosahedral nanoparticle, which contains a bipartite ssRNA genome within a protein capsid and does not have an envelope [26]. Studies by our labs and others have shown that CPMV nanoparticles are immunostimulatory and are recognized by TLRs 7/8 [27,28]. Compared to tobacco mosaic virus, the in-situ delivery of CPMV in a mouse model of melanoma significantly increased survival [29]. The in-situ delivery of CPMV in a murine model of lung melanoma conferred a strong survival benefit that depended upon neutrophils, adaptive immune cells, IL-12, and IFNγ [28]. CPMV has also proven efficacious when delivered as an in-situ vaccine in mouse models of intracranial glioma and breast cancer [30,31]. Furthermore, either standard or slow-release versions of CPMV delivered intraperitoneally significantly delayed the growth of established murine ovarian tumors in the highly aggressive ID8/VEGFA/defb29 model [28,32]. In this same model, the presence of pre-existing anti-CPMV antibodies in mice pre-exposed to CPMV significantly increased survival [33]. CPMV increased the levels of IL-6, TNFα, IFNγ, and GM-CSF produced by non-adherent cells from the peritoneal cavities of treated mice ex vivo, while decreasing levels of TGFβ and IL-10. It also increased the levels of tumor-infiltrating neutrophils and activated dendritic cells [34]. Furthermore, CPMV delivered in combination with radiation therapy induced dramatic tumor regression in the same murine ovarian cancer model [35].
Based on CPMV’s efficacy as a therapy in early-stage established murine ovarian cancer, and its immunostimulatory properties, we use CPMV as an adjuvant to model treatment during patients’ periods of remission. We propose to use the tumor as a remission-stage vaccine antigen source. By using the tumor as the antigen source, the vaccine is fully personalized. Our approach is particularly attractive in the ovarian cancer context because the vast majority of serous ovarian cancer patients undergo surgical debulking, which generates hundreds of grams or multiple kilograms of patient tumor, which is currently discarded. We suggest that this tumor could be retained, disaggregated, inactivated to ensure cells cannot divide, and frozen for future use as the antigen source in a remission-stage vaccine. The inactivated tumor tissue will contain many of the tumor-associated antigens or neoantigens that would be carried by tumors during disease relapse. When combined with adjuvant, the treated tumor tissue could be administered to patients with the goal of preventing fatal relapse. In the murine context, we modeled this as a prophylactic vaccine. With that in mind, we performed these studies that address two central issues: (1) how best to inactivate the tumor so it cannot grow in the patient but retains optimal immunogenicity, and (2) how to pair that inactivated tumor tissue with the ideal vaccine adjuvant.
In this investigation, murine tumor cells were prepared in four different ways (irradiation, freeze–thawed lysates, heat-shocked lysates, and HOCl-oxidized lysates) to enhance the immunogenicity of the vaccine’s antigen. [16,36,37,38,39,40,41,42,43]. We used CPMV as an adjuvant and compared it to monophosphoryl lipid A (MPLA), a bacterial cell wall component and potent TLR 4 agonist that is an FDA-approved vaccine adjuvant [44,45]. We also compared CPMV to DMXAA, a murine STING agonist and antivascular agent [46]. Following the administration of two vaccines, the mice were challenged with live murine ovarian cancer cells, with survival used as a measure of vaccine efficacy.
Reported here are the results of investigating multiple tumor cell treatments and adjuvants to identify the optimal tumor vaccine. We observed that mice treated with the combination of CPMV and irradiated cells generated a robust T cell-dependent response, which induced superior survival against live tumor cell challenge and subsequent rechallenge, indicating the formation of immune memory. Here, we show that the codelivery of CPMV nanoparticles and lethally irradiated ovarian cancer cells could form the basis of an effective remission-stage ovarian cancer vaccine.
2. Results
2.1. CPMV Is an Effective Adjuvant When Combined with Irradiated Syngeneic Ovarian Cancer Cells
MPLA is an FDA-approved vaccine adjuvant that stimulates TLR 4 on antigen-presenting cells (APCs) [44,45]. Furthermore, because MPLA is widely used and easily obtained, it serves as a useful point of reference against which CPMV, the novel adjuvant, can be compared [47,48]. We compared the efficacies of these adjuvants in the ID8/VEGFA/defb29 ovarian cancer cell line, which is aggressive and closely mirrors human serous ovarian carcinoma; it is poorly immunogenic, metastasizes throughout the peritoneal cavity, and causes syngeneic C57BL/6J mice to rapidly develop acute ascites, making it an ideal model of serous ovarian cancer in humans [49].
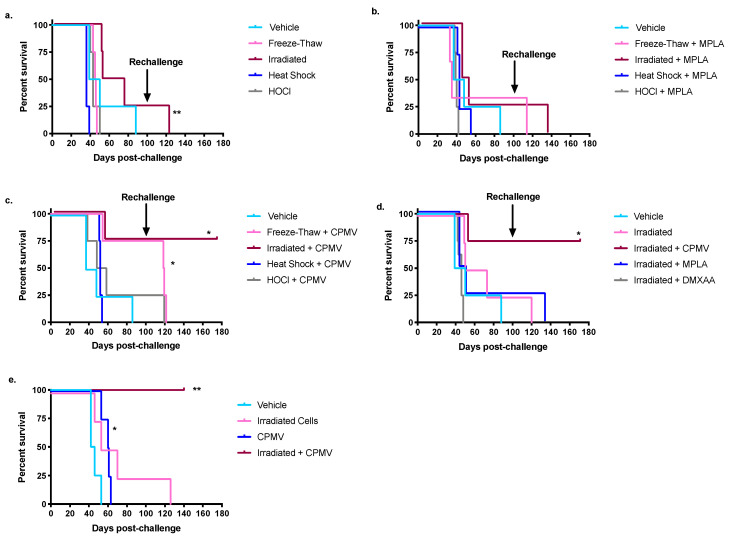
To compare the efficacy of CPMV and MPLA as adjuvants against the ID8/VEGFA/defb29 cell line, they were co-administered with a variety of different antigen preparations. Each immune adjuvant was co-delivered intraperitoneally (IP) with irradiated tumor cells, freeze–thawed tumor lysates, heat-shocked tumor cell lysates, or HOCl-oxidized tumor cell lysates. All antigen preparations were selected because they dependably kill the tumor cells and have previously been found to increase tumor cell immunogenicity [16,36,37,38,39,40,41,42,43]. Mice were given two identical vaccines one week apart, followed by a live tumor cell challenge one week after the second vaccine. We followed the survival of mice given different antigen and adjuvant combinations after their live tumor cell challenge (Figure 1).
Figure 1.
The combination of cowpea mosaic virus nanoparticles (CPMV) and irradiated cells significantly extends survival in a mouse model of ovarian cancer. ID8/VEGFA/defb29 cells were inactivated for intraperitoneal (IP) vaccine injection in one of four different ways: irradiation, freeze–thaw, heat shock, or HOCl oxidation (see methods for cell preparation). Following two vaccinations seven days apart, mice were challenged with live tumor cells and survival tracked. (a) Irradiated p = 0.007 compared to freeze–thaw, n = 4 in all groups; (b) cells co-delivered IP with 100 µg MPLA. Irradiated p = 0.35 compared to freeze–thaw and p = 0.59 compared to vehicle, n = 4 in all groups except freeze–thaw + MPLA where n = 3; (c) inactivated cells were co-delivered IP with 100 µg CPMV, n = 4 in all groups, freeze–thaw p = 0.03, irradiated p = 0.03 compared to vehicle; (d) mice received irradiated ID8/VEGFA/defb29 cells co-delivered IP with PBS, 100 µg CPMV, 100 µg MPLA, or 250 µg DMXAA. n = 4 in all groups except irradiated + DMXAA where n = 8. Irradiated + CPMV p = 0.03 or less when compared to any other group; (e) n = 4 in all groups. Irradiated + CPMV p = 0.007 or less compared to any other group. (a–e) When twice the average length of the survival of vehicle-treated mice had passed, surviving mice were rechallenged with 5 × 106 cells, as denoted by the arrows. p values compare survival curves with a log-rank (Mantel–Cox) test. All p values are compared to vehicle-treated controls unless otherwise noted ** 0.001 < p < 0.01; * 0.01 < p < 0.05.
Without adjuvant, there was a modest survival advantage provided by the irradiated tumor cells, but none of the other cell preparations yielded a statistically significant survival benefit (Figure 1a). This suggested that, of the preparations tested, radiation was the best option, and combination with adjuvant would strengthen its efficacy. MPLA is a weakly effective adjuvant against the ID8/VEGFA/defb29 murine ovarian cancer cell line when combined with irradiated cells (Figure 1b). Indeed, none of the tumor antigen preparations in combination with MPLA conferred a significant survival advantage beyond the survival of mice given the same antigen preparations without adjuvant. MPLA was not an effective adjuvant in combination with irradiated tumor cells or freeze–thawed lysates, as it did not provide a survival benefit when compared to vehicle-treated mice (Figure 1b) (p = 0.59 and p = 0.57, respectively). Mice treated with HOCl-oxidized cells and MPLA lived roughly as long as mice treated with HOCl-oxidized cells alone, showing that MPLA is not an effective adjuvant when combined with HOCl-oxidized cells (Figure 1a,b) (p = 0.82). Groups treated with heat-shocked lysates in combination with MPLA showed no significant difference between their survival and that of the vehicle-treated mice (p = 0.81) (Figure 1b). Because the vaccines that included MPLA as an adjuvant were ineffective, we performed experiments changing the MPLA dose, the amount of antigen included in the vaccine, and the route of injection, but all formulations remained ineffective (Figure S1). We also investigated the combination of irradiated cells and DMXAA, a murine STING agonist, but it, too, did not extend mouse survival (p = 0.28) (Figure 1d).
Like MPLA, CPMV lacked consistent efficacy when combined with heat-shocked tumor lysates or HOCl-oxidized tumor lysates (Figure 1c) (p = 0.80 and p = 0.53, respectively). The combination of CPMV and freeze–thawed lysates initially appeared effective, since 75% of the mice survived to rechallenge (Figure 1c) (p = 0.02). However, despite the significant survival benefit, the mice succumbed to their rechallenge within the expected forty-day timeline, suggesting that they did not mount a protective memory response (Figure 1c).
When combined with irradiated cells, CPMV significantly extended mouse survival and outperformed both MPLA and DMXAA as an adjuvant (Figure 1c,d) (p = 0.03 compared to MPLA and p = 0.003 compared to DMXAA). Overall, then, compared to MPLA and DMXAA, CPMV provided a far superior survival benefit to mice in the ID8/VEGFA/defb29 model, particularly in combination with irradiated cells.
In the ID8/VEGFA/defb29 ovarian cancer model the best vaccine combined CPMV with irradiated tumor cells. Compared to the other vaccines tested, the co-administration of CPMV and irradiated cells extended survival the longest, was the only vaccine that enabled total tumor rejection in any mice, and provided mice with the ability to withstand rechallenge, justifying further characterization.
To confirm that both antigen and adjuvant, or in this case irradiated cells and CPMV, were necessary for vaccine efficacy, the survival of mice vaccinated with both irradiated cells and CPMV was compared to mice vaccinated with irradiated cells alone or CPMV alone. Both CPMV and irradiated cells were required to confer survival benefit against the ID8/VEGFA/defb29 cell line (Figure 1e). At 140 days post-challenge, which is over three times as long as it generally takes for the vehicle-treated mice to reach the endpoint, all mice vaccinated with both irradiated cells and CPMV remained alive and tumor-free (p = 0.006). In contrast, by day 65, all mice treated with CPMV alone had reached the endpoint criteria. While we did observe that CPMV treatment alone provided a statistically significant survival benefit, the benefit was not comparable to that provided by the complete vaccine (Figure 1e) (p = 0.02). Similarly, all mice treated with irradiated cells alone succumbed to their cancer. Compared to CPMV alone and to irradiated cells alone, the combination vaccine significantly increased survival (p = 0.007 for each), indicating that both irradiated cells and CPMV are necessary for full vaccine efficacy.
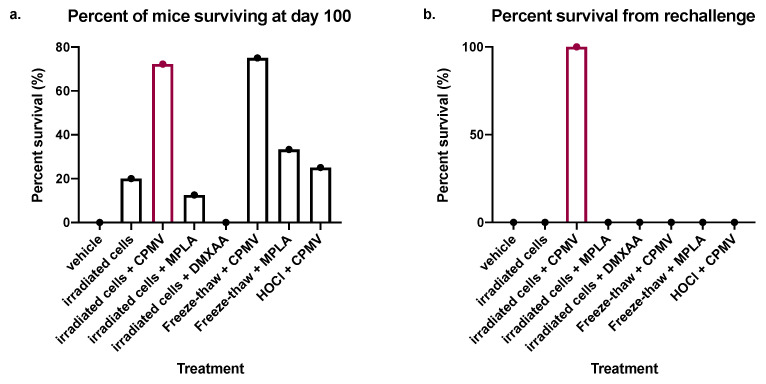
We selected survival for 100 days after a challenge with ID8/VEGFA/defb29 tumor cells as the indication of rejection of the challenge. While vehicle-treated control mice reached endpoint criteria around 40 days post-challenge, over 40% of vaccine-treated mice in this study survived for 100 days. CPMV provided extremely dependable protection against rechallenge when combined with either irradiated cells or freeze–thawed cells, with 75–100% of mice given those vaccines surviving for 100 days or more with no signs of ascites development (Figure 1c–e). However, other treatments also sporadically enabled the survival of challenged mice for 100 days at frequencies between 25 and 33% in some experiments, namely, irradiated cells only (Figure 1a), freeze–thaw or irradiated + MPLA (Figure 1b,d), HOCl + CPMV (Figure 1c), and irradiated cells only (Figure 1a,d,e). In Figure 2a, we show the total percent survival of all mice at 100 days. These composite data clearly established the combination of CPMV and irradiated cells, or CPMV and freeze–thawed cells, as the best vaccine to mediate resistance to the primary challenge, and these treatments were roughly equal in protecting mice from primary tumor challenge.
Figure 2.
The prophylactic codelivery of irradiated cells and CPMV provides robust, long-term protection from ID8/VEGFA/defb29 tumor challenge and rechallenge. (a) Combination data from all survival experiments. Mice received treated ID8/VEGFA/defb29 cells, co-delivered IP with or without adjuvant. The panel shows the percent of mice (out of the total number that received that treatment in all experiments) that survived to day 100 to be rechallenged. (b) The panel shows the percent of mice that survived 60 days after being rechallenged. All groups with any mice surviving to rechallenge are included, as are all groups that included irradiated cells and all vehicle-treated mice. (a,b) Vehicle-treated n = 24, irradiated cells n = 20, irradiated cells + CPMV n = 20, irradiated cells + MPLA n = 8, irradiated cells + DMXAA n = 8, freeze–thaw + CPMV n = 4, freeze–thaw + MPLA n = 3, HOCl + CPMV n = 4.
While protection from the primary tumor challenge is an important assessment of vaccine efficacy, the establishment of protective immune memory is also very important. Accordingly, mice that survived the primary challenge for 100 days with no sign of ascites development were rechallenged to assess their ability to reject tumors months after vaccination, providing an indication of immune memory. The rechallenge data for each experiment and subsequent survival are shown in Figure 1a–d. We selected survival for at least 60 days following rechallenge as an indication of established, protective immune memory. Only mice treated with the combination of CPMV and irradiated cells survived 60 days following rechallenge (Figure 1 and Figure 2b). This study of longer-term protection clearly established the combination of CPMV and irradiated cells as superior to any other vaccine, including the combination of freeze–thawed cells and CPMV. Additionally, two mice vaccinated with CPMV and irradiated cells that survived 100 days after rechallenge (200 days after primary challenge) were rechallenged a second time at that 200 day mark, after which they survived to 300 days, when they were rechallenged for the third time. Both animals survived until 450 days after the primary challenge and had no ascites when the experiment was concluded (data not shown). Overall, these data show the unique ability of the vaccine combining CPMV and irradiated cells to establish long-term, protective memory against a mouse model of ovarian cancer.
2.2. The Survival Benefit Provided by the Combination of CPMV and Irradiated Cells Is T Cell-Dependent
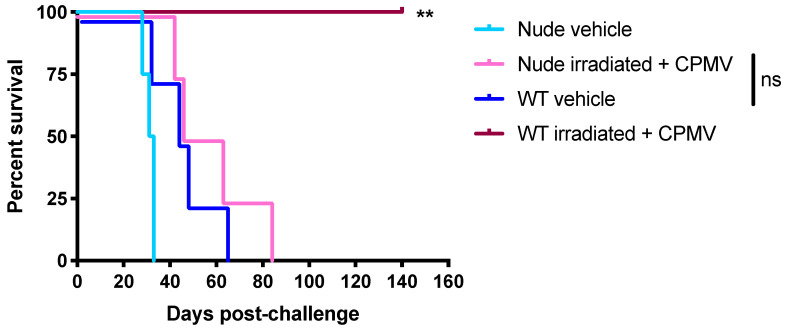
To begin to understand the immunological mechanisms of the vaccine combining irradiated cells and CPMV, the survival of vaccinated wild-type mice was compared to the survival of vaccinated nude mice that lack T cells. Comparing the vaccine’s efficacy in nude mice to its efficacy in wild-type mice elucidates the importance of T cells to the anti-tumor immune response (Figure 3).
Figure 3.
The survival benefit provided by the co-delivery of CPMV and irradiated cells is T cell-dependent. Irradiated ID8/VEGFA/defb29 cells were co-delivered IP with 100 µg CPMV to C57BL/6J (WT) or NU/J (nude) mice. Following two vaccinations seven days apart, mice were challenged with 5 × 106 live tumor cells. n = 4 in all groups. Compared to WT vehicle: WT irradiated + CPMV p = 0.007, nude irradiated + CPMV p = 0.29, and nude vehicle p = 0.10. Compared to nude vehicle, nude irradiated + CPMV p = 0.009. p values compare survival curves with a log-rank (Mantel–Cox) test. All p values are compared to vehicle-treated controls unless otherwise noted. ** 0.001 < p < 0.01; ns p > 0.05.
The survival benefit conferred by the vaccine combining irradiated cells is T cell-dependent. Vehicle-treated nude mice succumbed to their tumors very quickly, likely because they lacked even the immune pressure of the anti-tumor T cells in unvaccinated mice (Figure 3). However, the difference in survival between nude unvaccinated mice and wild-type unvaccinated mice was not significant (p = 0.10). All vaccinated wild-type mice remained tumor-free at day 140, which was over three times the average survival of vehicle-treated mice, and they experienced a significant survival advantage compared to unvaccinated wild-type mice (p = 0.007). The survival curves of the wild-type unvaccinated mice and the vaccinated nude mice closely mirrored one another, and there was no significant difference in the survival between these two groups (p = 0.29). While all of the vaccinated wild-type mice rejected their tumors, none of the vaccinated nude mice rejected their tumors, providing clear evidence that the vaccine’s immunological mechanism requires T cells. Perhaps the innate immune activation provided by CPMV allows a protective T cell response to be primed, ultimately causing tumor rejection.
3. Discussion
Despite enormous advances in clinical cancer immunotherapies over the last two decades, none have shown clinical efficacy in treating ovarian carcinomas. Though a combination of surgical, chemotherapeutic, and radiological interventions often induces clinical remission, serous ovarian cancer generally returns. Accordingly, there is a significant need for patient-centered immunotherapies that could be delivered during patients’ remission to prevent disease relapse [50]. Immunotherapies, including but not limited to vaccines, have the best opportunity to completely eliminate disease during remission, when low tumor burden leads to relatively weak tumor-mediated immunosuppression. As such, we believe that a vaccine that actually cures disease, rather than one that extends survival, is best delivered during clinical remission before disease relapse. In order for that vaccine to induce a strong immune reaction, it must include an adjuvant. Furthermore, to specifically direct the vaccine against tumor cells, the vaccine must include an antigen source. If immunogenic tumor antigens overlap between the primary tumor removed during surgical debulking and the tumor present at relapse, then the primary tumor serves as a useful antigen source. Because a relatively large amount of tumor tissue is discarded from each patient’s surgery, the tumor tissue removed during surgery serves as a patient-specific and readily available antigen source for a personalized cancer vaccine.
In this investigation, we show that the combination of CPMV and irradiated murine ovarian cancer cells constitutes an effective, T cell-dependent prophylactic vaccination against an aggressive syngeneic mouse model of ovarian cancer. CPMV, an immunostimulatory plant viral nanoparticle that has previously shown promise as a therapeutic agent, was compared to MPLA, a TLR 4 agonist, and DMXAA, a murine STING agonist. CPMV was a consistently more effective adjuvant than either MPLA or DMXAA (Figure 1). To determine the best antigen source for the vaccine, four different tumor preparations were compared—ionizing irradiation, freeze–thawed lysates, heat-shocked lysates, and hypochlorous acid-oxidized lysates—and we observed that irradiated tumor cells were the most effective vaccine antigen. Together, the combination of CPMV and irradiated tumor cells enabled the majority of treated mice to reject the primary tumor challenge, as well as subsequent tumor challenges over a prolonged period.
Unsurprisingly, both CPMV (the adjuvant) and irradiated cells (the antigen) were necessary for vaccine efficacy, supporting the expected vaccine function (Figure 1e). Most mice vaccinated with irradiated tumor cells and CPMV survived both the original tumor challenge and at least one rechallenge, with 70–75% of vaccinated mice surviving the initial challenge and all mice surviving rechallenge (Figure 1 and Figure 2). The remarkable efficacy of CPMV in extending survival in the highly aggressive ID8/VEGFA/defb29 model is consistent with other studies, which showed that CPMV moderately extended the survival of tumor-bearing mice when delivered therapeutically [32,35,51,52]. Accordingly, our present data suggests a novel application for CPMV as an effective adjuvant in remission-stage vaccines that block ovarian cancer relapse.
This sort of robust response is most often accomplished by CD8+ T cells, and our vaccine was rendered minimally effective in nude mice lacking T cells (Figure 3). Furthermore, since vaccinated mice survived tumor rechallenges delivered 80–100 days after their original tumor challenge, they appear to have robust immunological memory against the ID8/VEGFA/defb29 tumor cell line (Figure 2). While most tumor immunotherapies involve CD8+ T cells, the data we present here are also consistent with a reliance upon CD4+ T cells, either for their own cytokine production or for their ability to help mount a protective B cell response; these possibilities cannot be ruled out [53]. Though more studies are necessary to thoroughly explain the vaccine’s mechanisms, it is clear that the vaccine functions via a T cell-dependent immunological mechanism. These data corroborate earlier findings indicating that a vaccine consisting of CPMV conjugated to NY-ESO-1 induces an antigen-specific CD8+ T cell response [54].
The activation of TLR signaling appears to be involved in CPMV’s efficacy as an adjuvant. CPMV’s mechanism of immune cell activation is not yet fully understood, but CPMV does signal through TLR 7/8 and requires Syk signaling, while MPLA signals through TLR 4; perhaps differences in TLR activation account for the contrasting survival benefits [27]. A number of studies support CPMV’s ability to act as an immune adjuvant for in-situ vaccines [27,28,34,51,52]. Furthermore, CD11b+ monocytes from mouse ascites activated ex vivo with a combination of TLR 4 and TLR 9 agonists moderately extended survival when provided prophylactically, and led to tumor rejection and long-term memory in the therapeutic setting, further underscoring the important role of TLR stimulation in effective ovarian cancer immunotherapies [55]. Accordingly, though more studies are needed to fully elucidate the mechanism of the vaccine we describe here, it seems likely that CPMV activates innate immune cells, allowing the priming of an anti-tumor T cell response.
Vaccines with whole tumor cell antigens have long been an area of interest in ovarian cancer immunotherapies [56,57,58,59]. Of the various antigen preparations that we tested, the ionizing irradiation of tumor cells provided the best survival benefit (particularly in combination with CPMV) (Figure 1a,c). Ionizing radiation has not been used extensively as an antigen preparation technique in the past. However, perhaps most famously, irradiated tumor cells expressing GM-CSF comprise GVAX, an early approach which helped galvanize the field of cancer immunotherapy [60]. Furthermore, irradiation has been found to increase dendritic cell activation and mouse survival compared to freeze–thawed lysates in a dendritic cell vaccine against mouse models of glioma and melanoma [36,37]. Most probably, this is because gamma irradiation increases the expression of tumor antigens or oxidation-associated molecular patterns (specific types of danger-associated molecular patterns), which vigorously activate APCs [60,61]. Furthermore, irradiation increases the expression of the T cell costimulatory molecule CD80 on a variety of tumor cells, which increases tumor cell immunogenicity [62]. These past findings are consistent with our observation that irradiated tumor cells alone (without adjuvant) increased mouse survival (Figure 1a).
Freeze–thawing, heat-shocking, and HOCl-oxidizing tumor cells were generally less effective antigen preparation techniques when combined with CPMV or MPLA. To our knowledge, no studies have examined these techniques in the prophylactic context in ovarian cancer, but the therapeutic literature has suggested that preparing tumor cells in these ways can be advantageous. Freeze–thawed lysates delivered in combination with CPMV initially conferred a survival benefit, but did not establish immunological memory, as mice succumbed to rechallenge (Figure 1c). Freeze–thawed lysates are used regularly and are often used to prepare tumor cell lysates in successful dendritic cell vaccines [43,59,63]. For this reason, we compared various different freeze–thawed lysates to irradiated cells. However, it is possible that freeze–thawed lysates can decrease the ability of dendritic cells to respond to TLR stimulation, which may explain why their combination with CPMV was not as effective as the combination of CPMV and irradiated cells was [64]. Other groups have found that treating ovarian cancer cells with HOCl has improved the ability of dendritic cells to prime anti-tumor T cell responses and extend survival [16,41,42,65,66]. Our results did not corroborate the efficacy of HOCl oxidation as an antigen preparation technique. Because the heat treatment of cells causes an increased expression of immunogenic heat shock proteins, heat-shocked lysates have also been used to induce anti-tumor immune responses. Others have found that heat-shocking tumor cells can intensify the anti-tumor immune response, and they have made effective dendritic cell vaccines against colon cancer with heat-shocked tumor cells [38,39]. Studies have even examined the optimal methods of heat-shocking tumor cells for vaccine preparation, which were used to inform our method of heat-shocking tumor cells [40]. The results from our study do not corroborate previous studies that have found heat-shocked lysates to be effective.
The results we describe here align with previous work regarding the role of T cells in ovarian cancer. Human patients with ovarian cancer are capable of mounting modest anti-tumor CD8+ T cell responses, though clinically these responses do not appear sufficient to protect patients; while it is very difficult to understand the level of response that exists early in disease development, the fact that clinical disease develops suggests that the T cell response is not sufficiently protective [67,68,69,70,71]. Perhaps the strategy modeled here, a combination of robust T cell priming and strategic delivery of the vaccine during times of low tumor burden, would invigorate T cell responses enough to prevent relapse in humans. Furthermore, there is reason to believe that genetically modifying the patient’s own resected tumor tissue as a component of a personalized cancer vaccine would be useful in the clinical setting. When patients’ own autologous whole tumor cells are engineered to co-express GM-CSF and a shRNA against furin, subsequently irradiated, and delivered to ovarian cancer patients during periods of remission, blood samples from treated patients have increased levels of IFNγ, and treated patients experience a significant survival benefit [72].
Mice that received two prophylactic vaccines consisting of irradiated cells and CPMV had a significant survival benefit. We suggest here that, due to the low (clinically undetectable) tumor burden present during remission, prophylactic vaccine delivery provides a model for remission-stage vaccines. However, we acknowledge that prophylactic vaccines do not perfectly model remission-stage vaccines; during clinical remission, the immune system is no longer naïve to cancer antigens, and tumor immune-editing can occur.
Mice treated with the best vaccine combination in this study, irradiated cells and CPMV, experienced a remarkable survival rate compared to other therapies reported in the literature with this model. In many of our trials, 70–75% of vaccinated mice survived the initial challenge and rechallenge, and our total combined cohort indicates that 100% of rechallenged mice survived their rechallenge (Figure 1, Figure 2 and Figure 3). In contrast, other studies examining CPMV in this model cite a more modest survival benefit, with roughly 25% surviving challenge and rechallenge or, more frequently, no mice remaining tumor-free [33,34,51].
Immune checkpoint blockade therapies, which are widely used cancer immunotherapies, have at best a 15% overall response rate in ovarian cancer patients [3]. Some of the most promising murine ovarian cancer therapies to date combined various immunotherapies, such as a STING agonist with anti-PD-1 immune checkpoint blockade or GVAX, or FVAX, anti-41BB and anti-PD-1 or PD-L1 [73,74]. Compared to other murine vaccine studies, including dendritic cell vaccines, the vaccine developed in this study confers a much greater survival advantage [16,24,75]. In a study wherein mice were treated with a triple checkpoint blockade therapy, 20% of the mice remained tumor-free [76]. However, another group that engineered CAR T cells with the NKG2D receptor observed excellent mouse survival rates in ovarian cancer, and is moving toward clinical trials [77,78]. One of the most promising prospective ovarian cancer vaccines targets Sp17 and utilizes CpG, a TLR 9 agonist, as an adjuvant [25]. While our vaccine provides comparable survival, the predominance of tumor escape in single antigen vaccines has become apparent. Accordingly, the present investigation provides an important, effective direction for the development of multiple antigen-targeted ovarian cancer immunotherapies.
4. Materials and Methods
4.1. Animals
Six-week-old female C57BL/6J and athymic nude (NU/J) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). All mice were housed in the Norris Cotton Cancer Center vivarium in accordance with Institutional Animal Care and Use Committee guidelines.
4.2. Tumor Models
The ID8/VEGFA/defb29 murine ovarian serous carcinoma cell line was generated as previously described [49]. Cells were cultured at 37 °C in RPMI complete media (RPMI 1640 (HyClone, Marlborough, MA, USA) supplemented with 10% (v/v) fetal bovine serum (Gibco, Waltham, MA, USA), 1 mmol/L sodium pyruvate (Life Technologies, Waltham, MA, USA), 1% (v/v) penicillin/streptomycin mixture (Gibco, Waltham, MA, USA), and 2 mmol/L L-glutamine (Gibco, Waltham, MA, USA)). Cells were harvested and washed with RPMI 1640. Eight-week-old mice were challenged with 5 × 106 tumor cells in 400 µL sterile PBS intraperitoneally on day 0 after receiving vaccines on days −14 and −7. After challenge, the mice were weighed regularly to monitor ascites formation. Mice were euthanized with carbon dioxide when they reached the humane endpoint of 33 g of weight, indicating significant ascites formation. Many surviving mice were rechallenged around 100 days after their initial tumor challenge. The mice were never given vaccines following tumor challenge or rechallenge.
4.3. Vaccine Antigen Preparation
To prepare freeze–thawed lysates, the cells were washed with PBS and harvested. Cells were transferred to 15 mL conical tubes and resuspended in RPMI complete media. Tubes were submerged in a dry ice–ethanol slurry for 10 min. Cells were then allowed to thaw to room temperature in a room-temperature water bath. The freeze–thaw cycle was repeated five times. The freeze–thaw procedure was adapted from Chiang et al. (2011) and Herr et al. (2000) [43,63]. Cell lysis was confirmed via trypan blue exclusion. Cells were resuspended in sterile PBS at a concentration of 25 × 106 cells per mL. Lysates were injected intraperitoneally on days −14 and −7, with 5 × 106 cells delivered to each mouse simultaneously with adjuvant injection.
To prepare irradiated cells, cells were washed with PBS and harvested. Cells were transferred to conical tubes in RPMI complete media and irradiated with 70 Gray (10 Gy per min for 7 min) ionizing gamma radiation from a cesium source. The preliminary experiments confirmed complete cell death following this radiation procedure. Cells were resuspended in sterile PBS at a concentration of 25 × 106 cells per mL. Cells were placed on ice until just prior to vaccination. Irradiated cells were injected on days −14 and −7, with 5 × 106 cells delivered to each mouse simultaneously with adjuvant injection.
To prepare heat-shocked lysates, cells were washed with PBS and harvested. Cells were resuspended in RPMI complete media and heat-shocked in a water bath at 43 °C for 30 min. Cells were removed from the water bath and incubated at 37 °C for 1 h. Cells were then subjected to five freeze–thaw cycles, as described above. The heat-shocked lysate procedure was adapted from Ito et al. (2005) [40]. Cells were resuspended in sterile PBS at a concentration of 25 × 106 cells per mL. Lysates were injected intraperitoneally on days −14 and −7, with 5 × 106 cells delivered to each mouse simultaneously with adjuvant injection.
To prepare hypochlorous acid-oxidized lysates, cells were washed with PBS and harvested. Cells were resuspended in 0.06 M HOCl in HBSS and incubated at 37 °C for 30 min. Cells were gently agitated to encourage oxidation, and then returned to the incubator for another 30 min. The HOCl-oxidation procedure was adapted from Chiang et al. (2006) [41]. Cells were centrifuged at 5000 rpm for 5 min and washed twice with PBS. Cells were subjected to five freeze–thaw cycles, as described above, before being resuspended in sterile PBS at a concentration of 25 × 106 cells per mL. Lysates were injected intraperitoneally on days −14 and −7, with 5 × 106 cells delivered to each mouse simultaneously with adjuvant injection.
Vaccines were entirely prophylactic; mice were never given vaccines after tumor challenge or rechallenge. Two doses were provided due to the preponderance of the literature suggesting that both primary and secondary immune responses are important for prophylactic vaccine efficacy [79,80].
4.4. Vaccine Adjuvant Preparation
MPLA (Sigma Aldrich, (St. Louis, MO, USA) was dissolved in 2.5% (v/v) sterile DMSO in ET-free PBS to a concentration of 0.50 µg MPLA/µL. Each mouse received 200 µL MPLA solution (100 µg MPLA) on day 14 and day 7, simultaneously with antigen injection. DMXAA was dissolved in 2.5% (v/v) sterile DMSO in ET-free PBS to a concentration of 1.25 µg MPLA/µL. Each mouse received 200 µL DMXAA solution (250 µg DMXAA) on days −14 and −7, simultaneously with antigen injection [81].
CPMV nanoparticles were prepared as previously described and were verified to have < 50 endotoxin units per mg protein [82]. CPMV was diluted in PBS to a concentration of 100 µg per 200 µL PBS. Each mouse received 200 µL CPMV solution (100 µg CPMV) injected intraperitoneally on days −14 and −7, simultaneously with antigen injection.
4.5. Statistical Analysis
All statistical analyses were performed with GraphPad Prism 8 (San Diego, CA, USA). All p values reported compare survival curves using the log-rank (Mantel–Cox) test. All experimental curves were compared to the relevant vehicle-treated controls unless otherwise stated.
5. Conclusions
This study shows that remission-stage ovarian cancer vaccines using irradiated tumor cells can effectively and significantly increase survival in a mouse model, suggesting the possibility of a similar potential in human serous ovarian cancer patients. These results suggest that, in other cancers in which patients frequently experience long periods of remission before their cancers recur, the development of inactivated tumor cell vaccines to be delivered during that period of remission could be useful. After testing a variety of tumor cell treatments and established as well as experimental adjuvants, we found that only the combination of CPMV and irradiated cells enabled the vast majority of mice to respond to both the original tumor challenge and the rechallenge. These studies support our proposition of CPMV as a novel tumor vaccine adjuvant. Overall, the combination of irradiated cells and CPMV together provides a protective and T cell-dependent ovarian cancer vaccine against a mouse model of ovarian cancer, and opens doors for future studies in cancer immunotherapy.
Acknowledgments
This study was supported by Dartmouth Mouse Modeling Shared Resource, Dartmouth Irradiation, Preclinical Imaging and Microscopy Shared Resource and Dartlab Immune Monitoring Shared Resource, which receive support from the Dartmouth Norris Cotton Cancer Center, through NCI funded grant 5P30CA023108.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/13/4/627/s1, Figure S1: MPLA is an ineffective adjuvant against the ID8/VEGFA/defb29 murine ovarian cancer model.
Author Contributions
Conceptualization, C.T.S. and S.F.; data curation, C.T.S.; formal analysis, C.T.S.; funding acquisition, N.F.S. and S.F.; investigation, C.T.S., G.H., C.M. and J.F.; methodology, C.T.S., G.H. and S.F.; project administration, S.F.; resources, F.A.V., V.B., J.F., N.F.S. and S.F.; supervision, S.F.; validation, C.T.S. and G.H.; visualization, C.T.S.; writing—original draft, C.T.S.; writing—review and editing, C.T.S., G.H., N.F.S. and S.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Cancer Institute of the US National Institutes of Health, grant numbers U01CA218292, R01CA224605, 1R01CA253615 awarded to Steinmetz and Fiering. Steinmetz acknowledges support through the Shaughnessy Family Fund for Nano-ImmunoEngineering (nanoIE) at UCSD.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Dartmouth Institutional Animal Care and Use Committee (IACUC) (protocol code 2137, approved 5 February 2018).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Fiering and Steinmetz are co-founders of and have a financial interest in Mosaic Immunoengineering Inc. The other authors declare no potential conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Torre L.A., Trabert B., DeSantis C.E., Miller K.D., Samimi G., Runowicz C.D., Gaudet M.M., Jemal A., Siegel R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018;68:284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Della Pepa C., Tonini G., Pisano C., Di Napoli M., Cecere S.C., Tambaro R., Facchini G., Pignata S. Ovarian cancer standard of care: Are there real alternatives? Chin. J. Cancer. 2015;34:17–27. doi: 10.5732/cjc.014.10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamanishi J., Mandai M., Ikeda T., Minami M., Kawaguchi A., Murayama T., Kanai M., Mori Y., Matsumoto S., Chikuma S., et al. Safety and Antitumor Activity of Anti-PD-1 Antibody, Nivolumab, in Patients With Platinum-Resistant Ovarian Cancer. J. Clin. Oncol. 2015;33:4015–4022. doi: 10.1200/JCO.2015.62.3397. [DOI] [PubMed] [Google Scholar]
- 4.Mahoney K.M., Rennert P.D., Freeman G.J. Combination cancer immunotherapy and new immunomodulatory targets. Nat. Rev. Drug Discov. 2015;14:561–584. doi: 10.1038/nrd4591. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y.F., Zou J.P., Mu J., Wijesuriya R., Ono S., Walunas T., Bluestone J., Fujiwara H., Hamaoka T. Enhanced induction of antitumor T-cell responses by cytotoxic T lymphocyte-associated molecule-4 blockade: The effect is manifested only at the restricted tumor-bearing stages. Cancer Res. 1997;57:4036–4041. [PubMed] [Google Scholar]
- 6.Kudrin A. Overview of cancer vaccines: Considerations for development. Hum. Vaccines Immunother. 2012;8:1335–1353. doi: 10.4161/hv.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Worzfeld T., Pogge von Strandmann E., Huber M., Adhikary T., Wagner U., Reinartz S., Muller R. The Unique Molecular and Cellular Microenvironment of Ovarian Cancer. Front. Oncol. 2017;7:24. doi: 10.3389/fonc.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagemann T., Wilson J., Burke F., Kulbe H., Li N.F., Pluddemann A., Charles K., Gordon S., Balkwill F.R. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J. Immunol. 2006;176:5023–5032. doi: 10.4049/jimmunol.176.8.5023. [DOI] [PubMed] [Google Scholar]
- 9.Landskron J., Helland O., Torgersen K.M., Aandahl E.M., Gjertsen B.T., Bjorge L., Tasken K. Activated regulatory and memory T-cells accumulate in malignant ascites from ovarian carcinoma patients. Cancer Immunol. Immunother. 2015;64:337–347. doi: 10.1007/s00262-014-1636-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang D.K., Peterson E., Sun J., Goudie C., Drapkin R.I., Liu J.F., Matulonis U., Zhu Q., Marasco W.A. Anti-CCR4 monoclonal antibody enhances antitumor immunity by modulating tumor-infiltrating Tregs in an ovarian cancer xenograft humanized mouse model. Oncoimmunology. 2016;5:e1090075. doi: 10.1080/2162402X.2015.1090075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Condamine T., Ramachandran I., Youn J.I., Gabrilovich D.I. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu. Rev. Med. 2015;66:97–110. doi: 10.1146/annurev-med-051013-052304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang B., Chen F., Xu Q., Han L., Xu J., Gao L., Sun X., Li Y., Li Y., Qian M., et al. Revisiting ovarian cancer microenvironment: A friend or a foe? Protein Cell. 2018;9:674–692. doi: 10.1007/s13238-017-0466-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniel D., Urs S., Guley K., Krueger S., Draper D., Wong A., Evens H., Higginbottom C., Saims D., Wise S., et al. Abstract 5691: Evaluation of immunomodulatory agents in classically immunologically ’cold’ cancers using syngeneic mouse models of breast and ovarian cancer. Cancer Res. 2018;78:5691. doi: 10.1158/1538-7445.Am2018-5691. [DOI] [Google Scholar]
- 14.Hu Z., Ott P.A., Wu C.J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol. 2018;18:168–182. doi: 10.1038/nri.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray H.J., Benigno B., Berek J., Chang J., Mason J., Mileshkin L., Mitchell P., Moradi M., Recio F.O., Michener C.M., et al. Progression-free and overall survival in ovarian cancer patients treated with CVac, a mucin 1 dendritic cell therapy in a randomized phase 2 trial. J. Immunother. Cancer. 2016;4:34. doi: 10.1186/s40425-016-0137-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiang C.L., Kandalaft L.E., Tanyi J., Hagemann A.R., Motz G.T., Svoronos N., Montone K., Mantia-Smaldone G.M., Smith L., Nisenbaum H.L., et al. A dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: From bench to bedside. Clin. Cancer Res. 2013;19:4801–4815. doi: 10.1158/1078-0432.CCR-13-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raza A., Merhi M., Inchakalody V.P., Krishnankutty R., Relecom A., Uddin S., Dermime S. Unleashing the immune response to NY-ESO-1 cancer testis antigen as a potential target for cancer immunotherapy. J. Transl. Med. 2020;18:140. doi: 10.1186/s12967-020-02306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabbatini P., Tsuji T., Ferran L., Ritter E., Sedrak C., Tuballes K., Jungbluth A.A., Ritter G., Aghajanian C., Bell-McGuinn K., et al. Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clin. Cancer Res. 2012;18:6497–6508. doi: 10.1158/1078-0432.CCR-12-2189. [DOI] [PubMed] [Google Scholar]
- 19.Ishihara M., Tono Y., Miyahara Y., Muraoka D., Harada N., Kageyama S., Sasaki T., Hori Y., Soga N., Uchida K., et al. First-in-human phase I clinical trial of the NY-ESO-1 protein cancer vaccine with NOD2 and TLR9 stimulants in patients with NY-ESO-1-expressing refractory solid tumors. Cancer Immunol. Immunother. 2020;69:663–675. doi: 10.1007/s00262-020-02483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiang S.D., Gao Q., Wilson K.L., Heyerick A., Plebanski M. A Nanoparticle Based Sp17 Peptide Vaccine Exposes New Immuno-Dominant and Species Cross-reactive B Cell Epitopes. Vaccines. 2015;3:875–893. doi: 10.3390/vaccines3040875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song J.X., Cao W.L., Li F.Q., Shi L.N., Jia X. Anti-Sp17 monoclonal antibody with antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity activities against human ovarian cancer cells. Med. Oncol. 2012;29:2923–2931. doi: 10.1007/s12032-011-0137-0. [DOI] [PubMed] [Google Scholar]
- 22.Brunette L.L., Mhawech-Fauceglia P.Y., Ji L., Skeate J.G., Brand H.E., Lawrenson K., Walia S., Chiriva-Internati M., Groshen S., Roman L.D., et al. Validity and prognostic significance of sperm protein 17 as a tumor biomarker for epithelial ovarian cancer: A retrospective study. BMC Cancer. 2018;18:970. doi: 10.1186/s12885-018-4880-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gulley J.L., Arlen P.M., Tsang K.Y., Yokokawa J., Palena C., Poole D.J., Remondo C., Cereda V., Jones J.L., Pazdur M.P., et al. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin. Cancer Res. 2008;14:3060–3069. doi: 10.1158/1078-0432.CCR-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu D., Yu X., Wang J., Hui X., Zhang Y., Cai Y., Ren M., Guo M., Zhao F., Dou J. Ovarian Cancer Stem Cells with High ROR1 Expression Serve as a New Prophylactic Vaccine for Ovarian Cancer. J. Immunol. Res. 2019;2019:9394615. doi: 10.1155/2019/9394615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiriva-Internati M., Yu Y., Mirandola L., Jenkins M.R., Chapman C., Cannon M., Cobos E., Kast W.M. Cancer testis antigen vaccination affords long-term protection in a murine model of ovarian cancer. PLoS ONE. 2010;5:e10471. doi: 10.1371/journal.pone.0010471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yildiz I., Lee K.L., Chen K., Shukla S., Steinmetz N.F. Infusion of imaging and therapeutic molecules into the plant virus-based carrier cowpea mosaic virus: Cargo-loading and delivery. J. Control. Release. 2013;172:568–578. doi: 10.1016/j.jconrel.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albakri M.M., Veliz F.A., Fiering S.N., Steinmetz N.F., Sieg S.F. Endosomal toll-like receptors play a key role in activation of primary human monocytes by cowpea mosaic virus. Immunology. 2020;159:183–192. doi: 10.1111/imm.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lizotte P.H., Wen A.M., Sheen M.R., Fields J., Rojanasopondist P., Steinmetz N.F., Fiering S. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat. Nanotechnol. 2016;11:295–303. doi: 10.1038/nnano.2015.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray A.A., Wang C., Fiering S., Steinmetz N.F. In Situ Vaccination with Cowpea vs Tobacco Mosaic Virus against Melanoma. Mol. Pharm. 2018;15:3700–3716. doi: 10.1021/acs.molpharmaceut.8b00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerstetter-Fogle A., Shukla S., Wang C., Beiss V., Harris P.L.R., Sloan A.E., Steinmetz N.F. Plant Virus-Like Particle In Situ Vaccine for Intracranial Glioma Immunotherapy. Cancers. 2019;11:515. doi: 10.3390/cancers11040515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai H., Wang C., Shukla S., Steinmetz N.F. Cowpea Mosaic Virus Immunotherapy Combined with Cyclophosphamide Reduces Breast Cancer Tumor Burden and Inhibits Lung Metastasis. Adv. Sci. (Weinh.) 2019;6:1802281. doi: 10.1002/advs.201802281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Czapar A.E., Tiu B.D.B., Veliz F.A., Pokorski J.K., Steinmetz N.F. Slow-Release Formulation of Cowpea Mosaic Virus for In Situ Vaccine Delivery to Treat Ovarian Cancer. Adv. Sci. (Weinh.) 2018;5:1700991. doi: 10.1002/advs.201700991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shukla S., Wang C., Beiss V., Steinmetz N.F. Antibody Response against Cowpea Mosaic Viral Nanoparticles Improves In Situ Vaccine Efficacy in Ovarian Cancer. ACS Nano. 2020;14:2994–3003. doi: 10.1021/acsnano.9b07865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C., Fiering S.N., Steinmetz N.F. Cowpea Mosaic Virus Promotes Anti-Tumor Activity and Immune Memory in a Mouse Ovarian Tumor Model. Adv. Ther. 2019;2 doi: 10.1002/adtp.201900003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel R., Czapar A.E., Fiering S., Oleinick N.L., Steinmetz N.F. Radiation Therapy Combined with Cowpea Mosaic Virus Nanoparticle in Situ Vaccination Initiates Immune-Mediated Tumor Regression. ACS Omega. 2018;3:3702–3707. doi: 10.1021/acsomega.8b00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vandenberk L., Garg A.D., Verschuere T., Koks C., Belmans J., Beullens M., Agostinis P., De Vleeschouwer S., Van Gool S.W. Irradiation of necrotic cancer cells, employed for pulsing dendritic cells (DCs), potentiates DC vaccine-induced antitumor immunity against high-grade glioma. Oncoimmunology. 2016;5:e1083669. doi: 10.1080/2162402X.2015.1083669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prasad S.J., Farrand K.J., Matthews S.A., Chang J.H., McHugh R.S., Ronchese F. Dendritic cells loaded with stressed tumor cells elicit long-lasting protective tumor immunity in mice depleted of CD4+CD25+ regulatory T cells. J. Immunol. 2005;174:90–98. doi: 10.4049/jimmunol.174.1.90. [DOI] [PubMed] [Google Scholar]
- 38.Koido S., Hara E., Homma S., Mitsunaga M., Takahara A., Nagasaki E., Kawahara H., Watanabe M., Toyama Y., Yanagisawa S., et al. Synergistic induction of antigen-specific CTL by fusions of TLR-stimulated dendritic cells and heat-stressed tumor cells. J. Immunol. 2007;179:4874–4883. doi: 10.4049/jimmunol.179.7.4874. [DOI] [PubMed] [Google Scholar]
- 39.Ying M., Zhen Q., Liu S., Gong F., Xie Y. Treatment of established colon carcinoma-bearing mice by dendritic cells pulsed with lysates of heat-treated tumor cells. Sci. China C Life Sci. 2009;52:831–835. doi: 10.1007/s11427-009-0112-6. [DOI] [PubMed] [Google Scholar]
- 40.Ito A., Fujioka M., Tanaka K., Kobayashi T., Honda H. Screening of cytokines to enhance vaccine effects of heat shock protein 70-rich tumor cell lysate. J. Biosci. Bioeng. 2005;100:36–42. doi: 10.1263/jbb.100.36. [DOI] [PubMed] [Google Scholar]
- 41.Chiang C.L., Ledermann J.A., Rad A.N., Katz D.R., Chain B.M. Hypochlorous acid enhances immunogenicity and uptake of allogeneic ovarian tumor cells by dendritic cells to cross-prime tumor-specific T cells. Cancer Immunol. Immunother. 2006;55:1384–1395. doi: 10.1007/s00262-006-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiang C.L., Ledermann J.A., Aitkens E., Benjamin E., Katz D.R., Chain B.M. Oxidation of ovarian epithelial cancer cells by hypochlorous acid enhances immunogenicity and stimulates T cells that recognize autologous primary tumor. Clin. Cancer Res. 2008;14:4898–4907. doi: 10.1158/1078-0432.CCR-07-4899. [DOI] [PubMed] [Google Scholar]
- 43.Chiang C.L., Hagemann A.R., Leskowitz R., Mick R., Garrabrant T., Czerniecki B.J., Kandalaft L.E., Powell D.J., Jr., Coukos G. Day-4 myeloid dendritic cells pulsed with whole tumor lysate are highly immunogenic and elicit potent anti-tumor responses. PLoS ONE. 2011;6:e28732. doi: 10.1371/journal.pone.0028732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cluff C.W. Monophosphoryl lipid A (MPL) as an adjuvant for anti-cancer vaccines: Clinical results. Lipid A Cancer Ther. 2009;667:111–123. doi: 10.1007/978-1-4419-1603-7_10. [DOI] [PubMed] [Google Scholar]
- 45.Mata-Haro V., Cekic C., Martin M., Chilton P.M., Casella C.R., Mitchell T.C. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 46.Weiss J.M., Guerin M.V., Regnier F., Renault G., Galy-Fauroux I., Vimeux L., Feuillet V., Peranzoni E., Thoreau M., Trautmann A., et al. The STING agonist DMXAA triggers a cooperation between T lymphocytes and myeloid cells that leads to tumor regression. Oncoimmunology. 2017;6:e1346765. doi: 10.1080/2162402X.2017.1346765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi M., Chen X., Ye K., Yao Y., Li Y. Application potential of toll-like receptors in cancer immunotherapy: Systematic review. Medicine (Baltim.) 2016;95:e3951. doi: 10.1097/MD.0000000000003951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Srivastava A.K., Dinc G., Sharma R.K., Yolcu E.S., Zhao H., Shirwan H. SA-4-1BBL and monophosphoryl lipid A constitute an efficacious combination adjuvant for cancer vaccines. Cancer Res. 2014;74:6441–6451. doi: 10.1158/0008-5472.CAN-14-1768-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Conejo-Garcia J.R., Benencia F., Courreges M.C., Kang E., Mohamed-Hadley A., Buckanovich R.J., Holtz D.O., Jenkins A., Na H., Zhang L., et al. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat. Med. 2004;10:950–958. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- 50.Chu C.S., Boyer J., Schullery D.S., Gimotty P.A., Gamerman V., Bender J., Levine B.L., Coukos G., Rubin S.C., Morgan M.A., et al. Phase I/II randomized trial of dendritic cell vaccination with or without cyclophosphamide for consolidation therapy of advanced ovarian cancer in first or second remission. Cancer Immunol. Immunother. 2012;61:629–641. doi: 10.1007/s00262-011-1081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang C., Beiss V., Steinmetz N.F. Cowpea Mosaic Virus Nanoparticles and Empty Virus-Like Particles Show Distinct but Overlapping Immunostimulatory Properties. J. Virol. 2019;93 doi: 10.1128/JVI.00129-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shukla S., Wang C., Beiss V., Cai H., Washington T., 2nd, Murray A.A., Gong X., Zhao Z., Masarapu H., Zlotnick A., et al. The unique potency of Cowpea mosaic virus (CPMV) in situ cancer vaccine. Biomater. Sci. 2020;8:5489–5503. doi: 10.1039/D0BM01219J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nielsen J.S., Sahota R.A., Milne K., Kost S.E., Nesslinger N.J., Watson P.H., Nelson B.H. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin. Cancer Res. 2012;18:3281–3292. doi: 10.1158/1078-0432.CCR-12-0234. [DOI] [PubMed] [Google Scholar]
- 54.Patel B.K., Wang C., Lorens B., Levine A.D., Steinmetz N.F., Shukla S. Cowpea Mosaic Virus (CPMV)-Based Cancer Testis Antigen NY-ESO-1 Vaccine Elicits an Antigen-Specific Cytotoxic T Cell Response. ACS Appl. Bio Mater. 2020;3:4179–4187. doi: 10.1021/acsabm.0c00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adams S.F., Grimm A.J., Chiang C.L., Mookerjee A., Flies D., Jean S., McCann G.A., Michaux J., Pak H., Huber F., et al. Rapid tumor vaccine using Toll-like receptor-activated ovarian cancer ascites monocytes. J. Immunother. Cancer. 2020;8 doi: 10.1136/jitc-2020-000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chiang C.L., Benencia F., Coukos G. Whole tumor antigen vaccines. Semin. Immunol. 2010;22:132–143. doi: 10.1016/j.smim.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiang C.L., Coukos G., Kandalaft L.E. Whole Tumor Antigen Vaccines: Where Are We? Vaccines. 2015;3:344–372. doi: 10.3390/vaccines3020344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ophir E., Bobisse S., Coukos G., Harari A., Kandalaft L.E. Personalized approaches to active immunotherapy in cancer. Biochim. Biophys. Acta. 2016;1865:72–82. doi: 10.1016/j.bbcan.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 59.Chiang C.L., Kandalaft L.E., Coukos G. Adjuvants for enhancing the immunogenicity of whole tumor cell vaccines. Int. Rev. Immunol. 2011;30:150–182. doi: 10.3109/08830185.2011.572210. [DOI] [PubMed] [Google Scholar]
- 60.Dranoff G., Jaffee E., Lazenby A., Golumbek P., Levitsky H., Brose K., Jackson V., Hamada H., Pardoll D., Mulligan R.C. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl. Acad. Sci. USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vandenberk L., Garg A.D., Agostinis P., Verschuere T., Koks C., De Vleeschouwer S., Van Gool S. Irradiation of necrotic tumor cells used to pulse dendritic cells (DCs) potentiates DC vaccine-induced anti-tumor immunity in a mouse model of high-grade glioma. J. Immunother. Cancer. 2014;2 doi: 10.1186/2051-1426-2-S3-P163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morel A., Fernandez N., De La Coste A., Haddada H., Viguier M., Polla B.S., Antoine B., Kahn A. Gamma-ray irradiation induces B7.1 costimulatory molecule neoexpression in various murine tumor cells. Cancer Immunol. Immunother. 1998;46:277–282. doi: 10.1007/s002620050488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herr W., Ranieri E., Olson W., Zarour H., Gesualdo L., Storkus W.J. Mature dendritic cells pulsed with freeze-thaw cell lysates define an effective in vitro vaccine designed to elicit EBV-specific CD4(+) and CD8(+) T lymphocyte responses. Blood. 2000;96:1857–1864. doi: 10.1182/blood.V96.5.1857.h8001857_1857_1864. [DOI] [PubMed] [Google Scholar]
- 64.Tirapu I., Lewis A., Kreutz M., McLinden H., Diebold S.S. Freeze-and-thaw-disrupted tumour cells impair the responsiveness of DC to TLR stimulation. Eur. J. Immunol. 2008;38:2740–2750. doi: 10.1002/eji.200838284. [DOI] [PubMed] [Google Scholar]
- 65.Kandalaft L.E., Powell D.J., Jr., Chiang C.L., Tanyi J., Kim S., Bosch M., Montone K., Mick R., Levine B.L., Torigian D.A., et al. Autologous lysate-pulsed dendritic cell vaccination followed by adoptive transfer of vaccine-primed ex vivo co-stimulated T cells in recurrent ovarian cancer. Oncoimmunology. 2013;2:e22664. doi: 10.4161/onci.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tanyi J.L., Bobisse S., Ophir E., Tuyaerts S., Roberti A., Genolet R., Baumgartner P., Stevenson B.J., Iseli C., Dangaj D., et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aao5931. [DOI] [PubMed] [Google Scholar]
- 67.Webb J.R., Milne K., Watson P., Deleeuw R.J., Nelson B.H. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin. Cancer Res. 2014;20:434–444. doi: 10.1158/1078-0432.CCR-13-1877. [DOI] [PubMed] [Google Scholar]
- 68.Consortium O.T.T.A., Goode E.L., Block M.S., Kalli K.R., Vierkant R.A., Chen W., Fogarty Z.C., Gentry-Maharaj A., Toloczko A., Hein A., et al. Dose-Response Association of CD8+ Tumor-Infiltrating Lymphocytes and Survival Time in High-Grade Serous Ovarian Cancer. JAMA Oncol. 2017;3:e173290. doi: 10.1001/jamaoncol.2017.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stanske M., Wienert S., Castillo-Tong D.C., Kreuzinger C., Vergote I., Lambrechts S., Gabra H., Gourley C., Ganapathi R.N., Kolaschinski I., et al. Dynamics of the Intratumoral Immune Response during Progression of High-Grade Serous Ovarian Cancer. Neoplasia. 2018;20:280–288. doi: 10.1016/j.neo.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wick D.A., Webb J.R., Nielsen J.S., Martin S.D., Kroeger D.R., Milne K., Castellarin M., Twumasi-Boateng K., Watson P.H., Holt R.A., et al. Surveillance of the tumor mutanome by T cells during progression from primary to recurrent ovarian cancer. Clin. Cancer Res. 2014;20:1125–1134. doi: 10.1158/1078-0432.CCR-13-2147. [DOI] [PubMed] [Google Scholar]
- 71.Deniger D.C., Pasetto A., Prickett T.D., Gartner J.J., Bharathan M., Tran E., Robbins P.F., Rosenberg S.A. Mutated Tumor Neoantigens Are Recognized by Tumor Infiltrating Lymphocytes from Metastatic Ovarian Cancer. Cancer Immunother. Cancer Vaccines II. 2016;24:S155. doi: 10.1016/S1525-0016(16)33200-2. [DOI] [Google Scholar]
- 72.Oh J., Barve M., Matthews C.M., Koon E.C., Heffernan T.P., Fine B., Grosen E., Bergman M.K., Fleming E.L., DeMars L.R., et al. Phase II study of Vigil(R) DNA engineered immunotherapy as maintenance in advanced stage ovarian cancer. Gynecol. Oncol. 2016;143:504–510. doi: 10.1016/j.ygyno.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 73.Ghaffari A., Peterson N., Khalaj K., Vitkin N., Robinson A., Francis J.A., Koti M. STING agonist therapy in combination with PD-1 immune checkpoint blockade enhances response to carboplatin chemotherapy in high-grade serous ovarian cancer. Br. J. Cancer. 2018;119:440–449. doi: 10.1038/s41416-018-0188-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duraiswamy J., Freeman G.J., Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 2013;73:6900–6912. doi: 10.1158/0008-5472.CAN-13-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yuan J., Kashiwagi S., Reeves P., Nezivar J., Yang Y., Arrifin N.H., Nguyen M., Jean-Mary G., Tong X., Uppal P., et al. A novel mycobacterial Hsp70-containing fusion protein targeting mesothelin augments antitumor immunity and prolongs survival in murine models of ovarian cancer and mesothelioma. J. Hematol. Oncol. 2014;7:15. doi: 10.1186/1756-8722-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang R.Y., Francois A., McGray A.R., Miliotto A., Odunsi K. Compensatory upregulation of PD-1, LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in metastatic ovarian cancer. Oncoimmunology. 2017;6:e1249561. doi: 10.1080/2162402X.2016.1249561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barber A., Zhang T., Sentman C.L. Immunotherapy with chimeric NKG2D receptors leads to long-term tumor-free survival and development of host antitumor immunity in murine ovarian cancer. J. Immunol. 2008;180:72–78. doi: 10.4049/jimmunol.180.1.72. [DOI] [PubMed] [Google Scholar]
- 78.Spear P., Barber A., Sentman C.L. Collaboration of chimeric antigen receptor (CAR)-expressing T cells and host T cells for optimal elimination of established ovarian tumors. Oncoimmunology. 2013;2:e23564. doi: 10.4161/onci.23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clem A.S. Fundamentals of vaccine immunology. J. Glob. Infect. Dis. 2011;3:73–78. doi: 10.4103/0974-777X.77299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Watson B., Boardman C., Laufer D., Piercy S., Tustin N., Olaleye D., Cnaan A., Starr S.E. Humoral and cell-mediated immune responses in healthy children after one or two doses of varicella vaccine. Clin. Infect. Dis. 1995;20:316–319. doi: 10.1093/clinids/20.2.316. [DOI] [PubMed] [Google Scholar]
- 81.Jing W., McAllister D., Vonderhaar E.P., Palen K., Riese M.J., Gershan J., Johnson B.D., Dwinell M.B. STING agonist inflames the pancreatic cancer immune microenvironment and reduces tumor burden in mouse models. J. Immunother. Cancer. 2019;7:115. doi: 10.1186/s40425-019-0573-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Murray A.A., Sheen M.R., Veliz F.A., Fiering S.N., Steinmetz N.F. In Situ Vaccination of Tumors Using Plant Viral Nanoparticles. Methods Mol. Biol. 2019;2000:111–124. doi: 10.1007/978-1-4939-9516-5_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.