Abstract
Carotenoids are an essential group of compounds that may be obtained by microbiological synthesis. They are instrumental in various areas of industry, medicine, agriculture, and ecology. The increase of carotenoids’ demand at the global market is now essential. At the moment, the production of natural carotenoids is more expensive than obtaining their synthetic forms, but several new approaches/directions on how to decrease this difference were developed during the last decades. This review briefly describes the information accumulated until now about the beneficial effects of carotenoids on human health protection, their possible application in the treatments of various diseases, and their use in the food and feed industry. This review also describes some issues that are linked with biotechnological production of fungal and yeasts carotenoids, as well as new approaches/directions to make their biotechnological production more efficient.
Keywords: yeast, carotenoids, pigments
1. Introduction
Carotenoids are an essential group of compounds that can be synthesized by some bacteria, yeasts, and molds. They are largely produced by plants, especially green leafy plants, for which some of them play a crucial role in photosynthesis [1,2,3,4]. In this process, they help absorb light but also play an important role in removing excess solar energy [5]. In the case of microorganisms, the main role of carotenoids is to protect cells against the negative influence of reactive forms of oxygen and radiation [6]. Carotenoids have applications in various areas of industry, medicine, agriculture, and ecology. A lot of information has been accumulated during the last decades about their possible health-protecting effects [7,8,9,10,11]. It is known that carotenoids cannot be synthesized in humans and animals. Therefore, people and animals need to obtain them from their diet [4,12]. Carotenoids may provide cosmetic benefits [7,10,13]. Moreover, their great importance in food production, as natural colorants, is well-known (Figure 1). The global market of carotenoids grew very promptly: In 2017, it reached the value of 1.5 billion USD. Based on the expectations of experts, it should reach $2.0 billion by 2022, at a compound annual growth rate of 5.7% for the period of 2017–2022 [14].
Figure 1.
Current and potential use of carotenoids.
Carotenoids are lipid-soluble, mainly terpenoid pigments of 40 carbon atoms. It is thought that the most important structural characteristic of carotenoids is their conjugated double bonds (CDBs) that are largely responsible for their physicochemical properties. For instance, CDBs are responsible for the color of most carotenoids. At least seven CDBs are necessary for obtaining a colored carotenoid [10]. Carotenoids can be divided into two groups. One of them is “oxygen-free carotenes”, e.g., α-carotene, β-carotene, ¥-carotene, lycopene, and torulene. The second group is “oxygen-containing xanthophylls”, e.g., astaxanthin, lutein, zeaxanthin, β-cryptoxanthin, fucoxanthin, and canthaxanthin [4,15]. Carotenoids can also be divided into provitamin A and non-provitamin A compounds [16]. The major provitamin A carotenoids are β-carotene, α-carotene, and β-cryptoxanthin. The carotenoids that are mainly studied so far are β-carotene, lycopene, astaxanthin, lutein, and zeaxanthin [17].
It is well-known that filamentous fungi and yeasts may produce, besides carotenoids, a lot of other various pigments, including melanins, flavins, phenazines, quinones, and others. One rather new pigment being researched is the red pigment accumulated by Saccharomyces cerevisiae mutants for ADE1 and ADE2, the product of the polymerization of 1-(5′-phosphoribosyl)-5-aminoimidazole containing several amino acid residues. This red pigment is a mixture of polymers containing a different number of monomers (4–10) and is characterized by a molecular weight from 2 to 10 kDa [17,18,19,20].
2. Characteristics of Some Fungal Carotenoids
The group of yeast that can synthesize carotenoids includes Phaffia rhodozyma (and its teleomorph Xanthophyllomyces dendrorhous) and species of the genera Rhodosporidium, Rhodotorula, Sporobolomyces, and Sporidiobolus [21,22,23]. Among the molds, the Blakeslea trispora species is of the greatest importance [24]. The biosynthesis of carotenoids in fungal cells begins with the conversion of acetyl-CoA, which is formed in the process of β-oxidation of fatty acids in the mitochondria. According to the pathway of mevalonic acid, several biochemical reactions catalyzed by specific reductases, kinases, and decarboxylases produce a five-carbon carotenoid precursor, isopentenyl pyrophosphate (IPP). The addition reactions of three IPPs lead to the formation of geranyl–geranyl pyrophosphate (GGPP), with 20 carbon atoms per molecule. The condensation of the two GGPP particles, catalyzed by phytoene synthase, produces phytoene (C40). It is a precursor to lycopene biosynthesis. Depending on the type of microorganisms, lycopene can be next transformed into β-carotene, γ-carotene, torulene, lutein, torularhodin, zeaxanthin, and astaxanthin [25].
2.1. β-Carotene
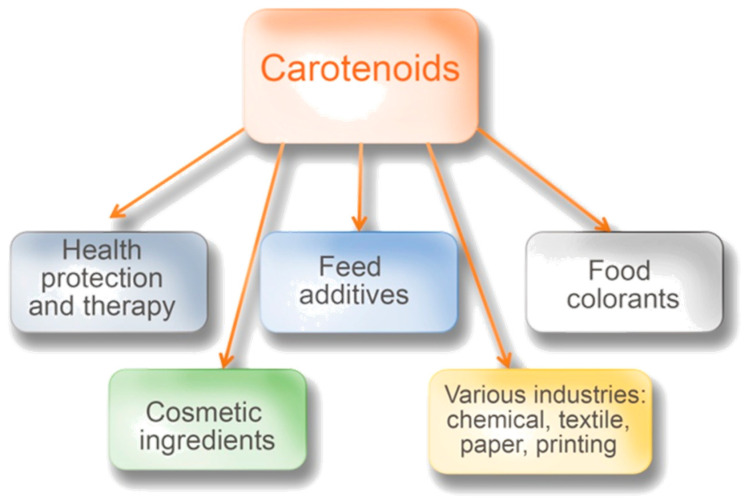
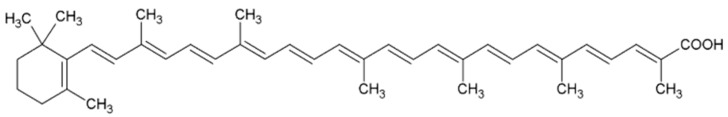
β-carotene is an isoprenoid compound with the chemical formula C40H56 and a molecular weight of 536.88 g/mol (Figure 2). The molecule of this compound consists of two β-ionone rings connected by a polyene chain containing nine conjugated double bonds. Due to the structure of β-carotene and the system of double bonds, this compound shows a maximum absorbance at 450 nm and is characterized by a color from yellow to orange [26,27]. One molecule of this compound can be converted by specific intestinal enzymes into two molecules of vitamin A, and therefore β-carotene is the main source of this vitamin in the diet [26]. On an industrial scale, it is obtained by chemical and biotechnological methods, using the microalgae Dunaliella salina or Bl. trispora mold [28]. Efficient producers of microbial β-carotene also include the yeast Rhodotorula glutinis [6,29], Rhodotorula mucilaginosa [30], and Sporidiobolus pararoseus [31,32].
Figure 2.
The structural formula of β-carotene.
2.2. Astaxanthin
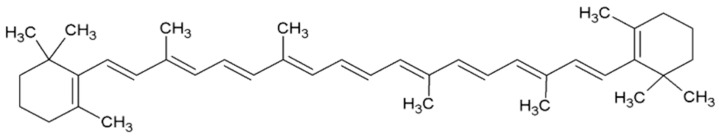
Astaxanthin (C40H52O4, 596.85 g/mol) (Figure 3) belongs to the group of xanthophylls. Two polar β-ionone rings are connected by a non-polar chain. Each ring contains one hydroxyl group and one ketone group. In total, there are 13 double bonds in the astaxanthin molecule, which determines the strong antioxidant properties of this compound. The presence of ketone and hydroxyl groups gives astaxanthin the ability to esterify and determines its polar character. Due to the presence of hydroxyl groups in the β-ionone rings, astaxanthin is an optically active compound. Chiral centers occur at positions C-3 and C-3′, and, therefore, there are three isomers of astaxanthin: enantiomers (3S, 3′S and 3R, 3′R) and a meso form (3R, 3′S) [33,34,35]. The main fungal producer of this compound is the Xanthophyllomyces dendrorhous yeast, which synthesizes mainly the (3R, 3′R) isomer [36].
Figure 3.
The structural formula of astaxanthin.
2.3. Torulene
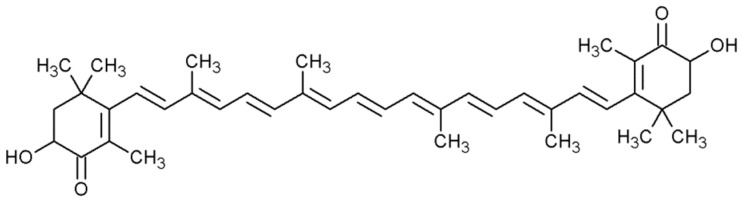
Torulene (C40H54, 534.9 g/mol) belongs to the carotenes group. The torulene molecule is composed of one β-ionone ring with a polyene chain containing 12 conjugated double bonds (Figure 4). It is orange or orange-red in color, which depends on the concentration. The main microbial producers of torulene are yeasts belonging to the genus Rhodotorula [6], the yeast species Sporidiobolus pararoseus [37,38], and molds of the genus Neurospora [39]. Torulene has antioxidant [40] and anticancer properties [41].
Figure 4.
The structural formula of torulene.
2.4. Torularhodin
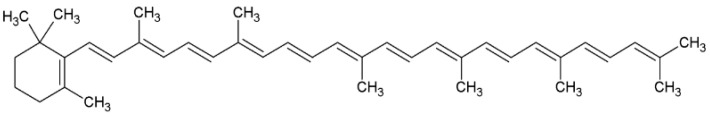
Torularhodin (C40H52O2, 564.84 g/mol) has a structure similar to torulene. The only difference is the presence of a carboxyl group at the end of the polyene chain (Figure 5). For this reason, this compound belongs to the group of xanthophylls. Torularhodin shows a polar character and dark pink color [6]. The main microbial producers of this compound are Rh. mucilaginosa [42,43] and Sporobolomyces ruberrimus yeast [44,45].
Figure 5.
The structural formula of torularhodin.
3. Carotenoids and Human Health
It is well-known that carotenoids are compounds that are very important for human health. They can prevent a deficiency in vitamin A, which is known as the essential compound for the promotion of growth, embryonal development, and visual function. The lipophilicity of carotenoids determines their subcellular distribution; they are enriched in membranes and other lipophilic compartments, i.e., lipid droplets [16]. It is supposed that carotenoids in membranes can protect them as antioxidants. Besides that, polar carotenoids can regulate membrane fluidity [21,22]. One of their functions is linked to the protection of our vision. The deficiency of carotenoids can lead to blindness, and as it was reviewed in the literature it is a serious problem for children, especially in developing countries [23,46]. Carotenoids are vital for the protection of the retina by preventing cataracts and age-related macular degeneration [46,47,48]. There is definite evidence that shows the efficiency for eye health of lutein and zeaxanthin. They may reduce the risk for age-related macular eye diseases and lead to the improvement of visual performance that also includes positive effects, such as contrast sensitivity, glare tolerance, and photo-stress recovery [49].
Torularhodin is a carotenoid produced mainly by the yeast genera Rhodotorula and Sporobolomyces; it has strong antimicrobial properties and may become a new natural antibiotic [50,51,52,53]. The antimicrobial properties of torularhodin can also be used in the production of films for coating medical implants [54,55].
The efficiency of carotenoids’ use is known for the protection and therapy of various chronic diseases. chronic diseases. They exhibit an anti-inflammatory property and may activate the immune response of an organism [56]. It was shown that the use of lycopene-enriched foods might decrease the risk of developing atherosclerosis and other cardiovascular diseases [57,58,59]. Such beneficial results are most likely linked to the ability of lycopene to reduce systemic and high-density lipoprotein-associated inflammation and to modulate high-density lipoprotein functionality [60]. It was shown that supplementation with lycopene significantly decreased systolic blood pressures [59,61]. Astaxanthin has also been reported to exert a preventive action against atherosclerotic cardiovascular diseases by the reduction of oxidative stress and inflammation and the enhancement of lipid metabolism and glucose metabolism [62]. Supplying the body with astaxanthin allows us to effectively reduce the negative effects resulting from the oxidation and degradation of cellular elements. Another study revealed that lycopene might limit the release of proinflammatory cytokines and chemokines [63]. One study also speculated that lycopene might affect the immune functions modulating the cellular redox environment and cell-to-cell interactions and influence anti-inflammatory transcription factors, such as peroxisome-proliferator-activated receptor [64]. Several results, summarized by Rao and Rao [59], reported the involvement of lycopene and β-carotene in bone health and in preventing or decreasing the risk of osteoporosis. Such a positive effect of lycopene in decreasing osteoporosis risk was also shown in postmenopausal women [65]. Similar effects were also described for β-cryptoxanthin [49]. Lycopene consumption was demonstrated to improve bone strength, by reducing bone resorption, and to protect from type 2 diabetes, by enhancing glucose homeostasis [66,67,68].
The role of various carotenoids in the prevention of other chronic diseases was also studied [59]. Moreover, the use of lycopene in the cases of male infertility led to the improvements of sperm motility, sperm motility index, sperm morphology, and functional sperm concentration, and finally resulted in a 36% increase of successful pregnancies [59]. The possible use of lycopene in recovering the cases of alcohol-induced liver injury was also suggested [21]. Carotenoids might have beneficial effects on weight management and obesity [49,69]. It is expected that future studies could reveal a positive role of carotenoids in the treatments of other diseases, i.e., skin disorders, rheumatoid arthritis, periodontal diseases, and others [49,70].
β-Carotene and lutein have positive effects on cognitive performance [49]. The putative positive role of lycopene in the treatments of neurodegenerative diseases, including Alzheimer’s disease, was also studied [60,71]. It was thought that lutein is linked to the possible control of inflammation-related neurodegenerative disorders [72], while torularhodin can be used as a neuroprotective agent against H2O2-induced oxidative stress, due to its strong antioxidant activity [73]. Lycopene exhibited protection against amyotrophic lateral sclerosis disorder in humans [59,74].
Interesting information was received, in recent years, regarding the possible medical application of a red pigment that accumulated in S. cerevisiae mutants. It is known that “conformational diseases” in humans and animals are linked to abnormal aggregation of proteins and the formation of amyloid fibrils. The red pigment accumulated in S. cerevisiae mutants for ADE1 and ADE2 can bind amyloid fibrils and disturb their interactions with chaperones that, in turn, lead to the inhibition of prion “multiplication” and amyloid fibril formation [75,76,77]. It was also shown that yeast mutants, which accumulate this pigment, had lower amyloid content than wild-type parental strains. It was shown that this red pigment accumulation reduced cloned human amyloid-β aggregation. The conclusion was made that red yeast pigment has potential importance in therapy for Alzheimer’s and Parkinson’s diseases [19,20].
Carotenoids have characteristics of antioxidants [78,79,80,81]. They quench 1O2 and increase the levels of glutathione and glutathione peroxidase [4,82,83]. β-Carotene can be used for sun protection and sunburn prevention [16,84]. Carotenoids are efficient blue-light filters; they protect against photo-oxidative damages lipids, proteins, and DNA, thus preventing premature ageing of the skin and skin cancer [16,84,85,86]. It was also suggested that astaxanthin might be used as a potential anti-ageing agent [87]. β-Carotene reduces the risk of developing neoplastic diseases, and also inhibits the promotion and progression of neoplasms.
Very promising findings were also obtained on the putative efficiency of using carotenoids against some types of cancer [46,88]. The anticancer activity of some carotenoids, i.e., α-carotene, β-carotene, lycopene, torulene, torularhodin, and some others, was studied regarding prostate, breast, colon, lung, oral, gastric, and skin cancers, in addition to hepatoma, leukemia, uveal melanoma, etc. [4,52,53,66,88,89,90,91,92,93,94,95,96]. Synergistic inhibition of prostate and breast cancer cell growth was evident under the influence of combinations of low concentrations of various carotenoids [97]. The use of reporter gene assays of the transcriptional activity of the androgen receptor in hormone-dependent prostate cancer cells and the electrophile/antioxidant response element (EpRE/ARE) transcription system enabled the observation of combinations of several carotenoids (e.g., lycopene, phytoene, and phytofluene) to synergistically inhibit the androgen receptor activity and activate the EpRE/ARE system and suggested their use in the therapy and prevention of this type of cancer [97]. In the experiments performed by Prakash et al. [98], estrogen-receptor (ER) positive MCF-7 and ER-negative Hs578T and MDA-MB-231 human breast cancer cells were treated with carotenoids. Among them, β-carotene significantly reduced the growth of MCF-7 and Hs578T cells, and lycopene inhibited the growth of MCF-7 and MDA-MB-231 cells. Similar effects were also shown for astaxanthin [62]. Authors concluded that carotenoids inhibit the growth of both studied breast cancer cell lines, indicating that estrogen receptor status is an important factor for the responsiveness of breast cancer cells to carotenoid treatments [98]. The use of food rich in various carotenoids was found to decrease the risk of lung and stomach cancers [81,99,100]; although, in the case of lung cancer, negative results were obtained for β-carotene for smokers and asbestos workers. In these cases, β-carotene supplementation was associated with an increased risk of lung and gastric cancers [49,101]. It is supposed that the cancer-preventive effects exhibited by various carotenoids might also be linked to their induction and stimulation of intercellular communications via gap junctions, which are important for the regulation of cell growth, differentiation, and apoptosis [21]. More recently, lycopene was found to inhibit tumor metastasis by slowing down cell-cycle progression and inhibiting the proliferation of diverse cancer cell lines [66]. A detailed description of the different effects and mechanisms of anticancer activity of carotenoids (cell-cycle arrest, apoptosis-inducing effect, and anti-metastasis effect) is reported in some recent reviews [4,102,103].
Carotenoids may act as chemoprotective agents against cellular mutagenesis and malignant transformation [79,81,104,105,106]. Protective effects expressed by β-carotene and other carotenoids were demonstrated against the mutagenic potential of 8-methoxypsoralen, cyclophosphamide, 1-methyl-3-nitro-1-nitrosoguanidine, benzo(α)pyrene, quinolones, and ultraviolet light, using Salmonella typhimurium as a cell model system [81,107,108,109,110]. β-Carotene and other carotenoids (canthaxanthin, α-carotene, and lycopene) can inhibit malignant transformation induced by 3-methylcholanthrene, or X-ray treatment in the fibroblast cell line [81,111,112].
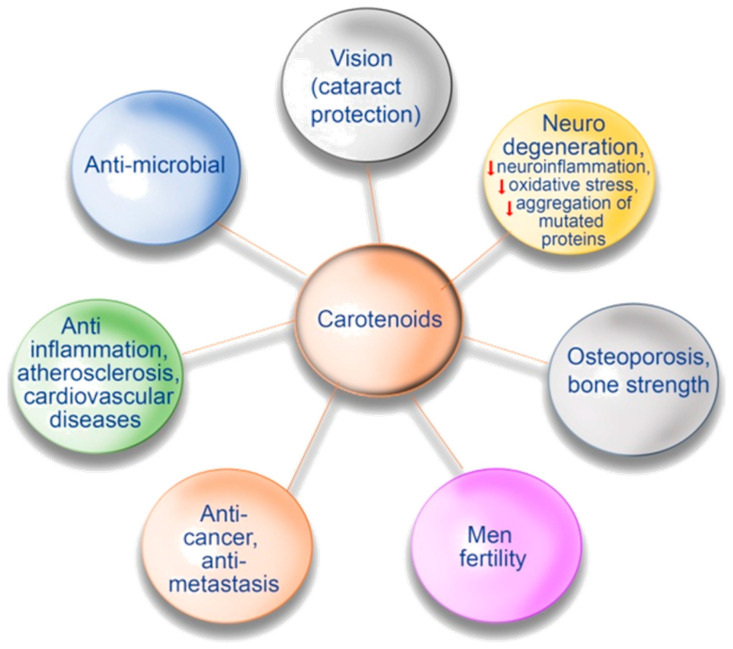
Antioxidant and anti-ageing effects of astaxanthin led to its wide use in cosmetics [113]. Besides all of these examples of the positive effects of carotenoids on human health, there are also data that β-carotene and astaxanthin may have immunoprotective effects, whereas lutein can prevent oxidative stress in eye tissues, as well as has antiviral activity against hepatitis B virus [113]. Figure 6 briefly presents the possible positive effects of carotenoids for human health described above.
Figure 6.
Possible positive effects of carotenoids on human health.
4. Carotenoids and Other Microbial Pigments as Feed Additives and Colorants
Carotenoids are widely used in salmon and trout farming and in the poultry and food industry as feed additives and natural food colorants, which can give from yellow to red colors [7,114,115,116]. From an economic viewpoint, astaxanthin is the third most important carotenoid after β-carotene and lutein, due to its importance in aquaculture, and the chemical, pharmaceutical, and food industries [3]. In salmon and trout farming, it is widely used as a pigment for fish meat. Feed supplementation of carotenoids essentially improves the health of poultry birds and enhances the quality of eggs and meat. Carotenoids are very important for the pigmentation of egg yolk, skin, legs, beak, comb, feather, and fat. The use of carotenoids as alternative feed ingredients gives the possibility to replace synthetic medicine and nutrients in poultry industry [117].
Many yeasts belonging to different genera have been extensively studied during the last decades as potential efficient producers of various pigments (especially of mixtures of carotenoids). Among them, the most forthcoming is Ph. rhodozyma [118,119]. The production of astaxanthin has been scaled-up to the industrial level in the last decades [116,120,121]. On the other hand, the yellow carotenoid pigment zeaxanthin can be used as an additive in poultry, as well as in the cosmetics and food industries. Canthaxanthin is another carotenoid pigment that is already used in aquafeed for farmed salmonids [122]. Besides these food-related applications of carotenoids, they may serve as alternative coloring agents that are in demand in different industries, such as the textile, plastic, paint, paper, and printing industries [122,123,124,125].
The analysis of food consumer requirements revealed a growing rejection of synthetic food dyes during the last decade. The use of some synthetic colorants in food and cosmetic processing has recently been banned due to their hyperallerginicity, carcinogenicity, and other toxicological problems [122,123]. For example, the astaxanthin produced chemically is not approved for human consumption, due to the presence of by-products [126]. Correspondingly, a growing demand for dyes of natural origin is becoming increasingly more popular. It is well-known that natural coloring agents can be extracted from various plants, algae, and microorganisms (i.e., bacteria, yeasts, and fungi), which can produce various pigments [127]. Some food-grade microbial pigments are already produced biotechnologically. Among them is the hydroxyanthraquinoid pigment Arpink red, which is produced by a strain of Penicillium oxalicum var. armeniaca isolated from soil by the Czech company Ascolor Biotech s.r.o. The patent covering Arpink Red also claims its anticancer effects for applications in the food and pharmaceutical fields [127]. Another example is the yellow vitamin riboflavin (vitamin B2). It can be produced by the yeast species Meyerozyma (formerly Candida) guilliermondii or Debaryomyces subglobosus and by the dimorphic fungus Eremothecium ashbyi (and its heterotypic synonym Ashbya gossypii); the latter is used for industrial-scale production [126,128].
Recent studies explored the possibility of replacing the use of yellow pigments from the fungus Monascus sp. (which are not approved for the use in EU and USA, because of the risk of possible contamination by the nephrotoxic and hepatotoxic metabolite citrinin), with similar pigments produced by non-mycotoxigenic strains of the fungal genus Talaromyces [124,128,129,130,131,132]. Marine fungi are also studied as promising sources of novel pigments [128]. New findings in this area that give new possibilities for modern biotechnology are described in detail in the review by Dufosse et al. [128].
5. Biotechnology
Some carotenoids are now produced at the industrial level, using microbial strains. Although the fungal species Mucor circinelloides, Phycomyces blakesleeanus, and Bl. trispora are well-known producers of β-carotene, Bl. trispora is the main one used for industrial production. This species is very interesting in biotechnology; however, the need to co-cultivate (+) and (–) sexual mating types of this fungus makes this technology quite complex. This species is nonpathogenic and nontoxigenic [127,133]. The first biotechnological company that started the production of β-carotene in Western Europe, at the industrial level, between 1995 and 2001, was the Dutch company Gist-brocades (now DSM); meanwhile, in the Soviet Union, production of β-carotene in Eastern Europe started a decade earlier [131]. The Spanish Company Vitatene (now DSM) started the production of lycopene from Bl. trispora for the European market in 2003 [131].
One of the approaches for further improvement of these technologies may be connected with the use of new selected or mutant strains, as well as the improvement of fermentation conditions [126,134,135]. Carotenoids can be synthesized by different groups of microorganisms. However, given the efficiency of the biosynthesis process and economic factors, yeasts of the genus Rhodotorula deserve special attention. Another possible approach to increase the production of carotenoids may include applying different stress factors. It was shown that, in the case of oxidative, osmotic, and salt stress, Rh. glutinis, Rh. mucilaginosa, and Sporidiobolus (formerly Sporobolomyces) salmonicolor produce significantly higher amounts of carotenoids [116,136,137]. In a recent study, it was revealed that low temperature caused an increase in the biosynthesis of carotenoids by Rhodotorula toruloides (formerly Rhodotorula gracilis) in media containing agro-industrial waste-potato wastewater and glycerol. The induction of osmotic stress and low temperature intensified the biosynthesis of β-carotene (up to 73.9% of the total carotenoid content). In the conditions of oxidative stress, the yeast synthesized torulene (up to 82.2%) more efficiently than under other conditions, whereas white-light irradiation increased the production of torularhodin (up to 20.0%) [138].
One more efficient approach to this problem was the engineering of the carotenoid pathway [46,139]. New achievements reached in genetic and metabolic engineering of microorganisms made it possible to optimize host microorganisms to use as advanced microbial cell factories. It was demonstrated that the best combinations of mutations identified for β-carotene production were also beneficial for the production of lycopene [140]. It was shown that recombinant microbial cell factories can be engineered on the basis of an oleaginous yeast, Yarrowia lipolytica, to produce astaxanthin by submerged fermentation [126]. Recently, a study directed to metabolic engineering of S. cerevisiae demonstrated the potential of a yeast-based process for β-carotene production [141]. Recent reviews give insights into microbial engineering principles for the overproduction of carotenoids and describe key strategies and current advances in engineering of the metabolism of carotenoid-producing microorganisms for maximizing carotenoid production [140,142,143]. It was reported that chemical mutagenesis led to the obtaining of Bl. trispora strains, which produced 100-fold higher amounts of β-carotene, compared with the wild-type strain [134]. The same organism is also proposed for the industrial production of lycopene [134,144]. It is expected that further identification of genes important in the carotenogenic pathway will be reached in the next few years and will lead to obtaining higher quantities of carotenoids at the industrial level [116].
Because of the economic efficiency, biotechnological production of carotenoids can be significantly increased when the costs are diminished by the use of waste or by-products from other biotechnologies as the main substrate for microorganisms. Various ideas are proposed accordingly [126,134,135,145]. In this context, corn syrup, sugarcane bagasse, wheat bran, rice bran, silage, whey, and crude glycerin are alternatives for producing carotenoids [146,147]. These products are found in abundance due to the production of biodiesel, sugar, and corn processing [148,149,150,151]. Interesting research showed that, besides the raw glycerin from biodiesel production, spent brewer’s yeast from the breweries may also be used to substitute carbon and nutrient sources, to produce carotenoids (β-carotene, torularhodin, torulene, and γ-carotene) by Rhodotorula strains [152]. An interesting proposal for carotenoids production using spent coffee grounds was published. Considering that, at the moment, coffee is the second largest product in the world (after petroleum) and that the industrial production of instant coffee in 2012 yielded about 330.000 tons of spent coffee grounds, this idea seems rather promising. The best results on carotenoids’ production using spent coffee grounds was obtained by using the yeast species Sporobolomyces roseus (other strains they have studied were Rh. glutinis, Rh. mucilaginosa, and Cystofilobasidium capitatum) [153,154]. Three yeast strains isolated in the Brazilian forests, belonging to the species Sporidiobolus pararoseus, Rh. mucilaginosa, and Pichia fermentans, were found to produce cryptoxanthin and β-carotene when they were cultivated in the media containing parboiled rice water and crude glycerol or parboiled rice water and sugar cane molasses [155,156].
There are also some other possibilities of using industrial wastes. For example, researchers have explored the possibility of obtaining simultaneously high-value carotenoids and lipids for biodiesel production [157]. The yield of synthesized carotenoids (mainly, β-carotene) using Rh. glutinis cultivated on brewery wastewater as a carbon source was rather low, but the idea is interesting and needs further investigations and development [158]. Utilization of agro-industrial waste in fermentation is an important source that may provide nutrient sources for the fast growth of microorganisms and enhances their pigment production. Utilization of these substrates also reduces the accumulation of biomass in large quantities, which may cause deterioration to the environment [122,159]. Fruit wastes derived from orange, pomegranate, and pineapple can be used as a culture medium for β-carotene production from Rh. mucilaginosa (formerly Rh. rubra) [160]. Working in the same direction, a set of cold-adapted pigmented yeast strains were isolated from plants and food samples. Some yeast strains that may synthesize both carotenoids and extracellular enzymes—lipases and cellulases—were identified (S. roseus and S. pararoseus). Because of this, it is expected that these strains might be interesting for the development of biotechnological production of carotenoids using cheap substrates, such as agro-industrial waste, including also lignocellulose [160]. Some studies showed the possibility to develop a new bioprocess that gives possibility to produce β-carotene from the xylose fraction of lignocellulosic biomass, using engineered S. cerevisiae strain [161,162].
Thinking of the large-scale biotechnological production of various carotenoids, it is clear that various strategies must be used. Besides the already mentioned above selection of over-producing strains, obtaining genetically modified hyper-producing strains and metabolic engineering of strain-producers, and the use of cheap carbon and nitrogen sources from waste and by-products from other existing technologies, it is necessary to remember that carotenoid synthesis in microorganisms depends on the number of factors, which are summarized in the review by Mata-Gomez et al. [3]. These factors are carbon and nitrogen sources, light, temperature, aeration, metal ions, and especially some trace elements, and the addition of some chemicals (such as ethanol and acetic acid) into the growth medium. It was shown that the use of carbon sources, such as ethanol, could provoke an increase in the synthesis of pigments [3,120,163,164]. Their production is positively affected by white light [3,165]. Temperature is another important factor which influences carotenoids synthesis [166]. It was revealed for Rh. glutinis that the temperature of 25 °C favored the synthesis of β-carotene and torulene, while a temperature range of 30–35 °C favored the torularhodin biosynthesis [167,168].
Correct aeration is one more important factor affecting carotenoid biosynthesis [149]. An increased rate of stirring led to an essential increase in carotenoid production by Rh. mucilaginosa [169]. The addition of 0.5 M NaCl to the fresh water used in the preparation of nutrient media for engineered carotenoids producing S. cerevisiae strain increased the production of β-carotene by almost two times. An increase in the C:N ratio further improved carotenoid production by this strain [170]. The nitrogen sources were revealed as the main factors that most efficiently influenced the intracellular accumulation of carotenoids in yeast Rh. mucilaginosa [169,171]. Metal ions (such as Ba, Fe, Mg, Ca, Zn, and Co) and especially some trace elements (such as Al, Zn, and Mn) are very important for carotenoids synthesis in various species of Rhodotorula [170]. A recent study has described the potential of the ascomycetous yeast species Y. lipolytica as a β-carotene-producing cell factory, reporting the highest titer of recombinant β-carotene produced to date [172]. The medium optimization (C:N ratio, possible addition of glycerol) led to an improvement of up to 50% in the yield of β-carotene production in the best of the conditions [173,174]. Some other approaches were also proposed to increase pigment production. One of them is the immobilization of culture-producers. Alipour et al. found that the addition of the natural loofa sponge immobilized Rh. mucilaginosa (formerly Rh. rubra) in a cell-immobilized airlift photobioreactor considerably increased the production of carotenoids [175].
At the same time, in summarizing the existent literature, it was concluded that, despite the large amount of the information linked with various issues of carotenoids’ effects, their importance, and the biotechnology of their production published during last years, some of these substances that have health-promoting activities still require further study. These are lutein, zeaxanthin, α-carotene, β-cryptoxanthin, phytoene, torulene, and torularhodin [10].
6. Conclusions
Carotenoids represent a very large group of various compounds. These pigments have been studied for more than 120 years [176]. During this time, a substantial amount of information regarding their chemistry, structure, and mechanisms of functional activities has been accumulated. Due to the health-promoting properties of carotenoids, there is a growing interest in the methods of obtaining and enriching them, primarily in food products. For many years, they have been widely used in salmon and trout farming and the poultry and food industry, as feed additives and natural food colorants. The increase in the demand for carotenoids in the global market was essential during the last years, and it is expected that there will be an annual growth rate of their production at the level of 5.7%. The main quantity of these compounds produced by industry has synthetic origin. At the same time, during the last decades, there has been a marked increase in negative associations of these pigment consumers with the use of synthetic carotenoids, some of which may be hyperallergenic, carcinogenic, and may have some other toxicological problems. The alternative is to use natural carotenoids from plants or those produced by microorganisms. Currently, the production of natural carotenoids is more expensive than obtaining their synthetic forms, but a lot of new approaches/directions on how to decrease this difference was recently developed. The first strategy is based on the use of industrial waste and by-products; the further search for new efficient strain-producers, as well as the selection of existing industrial strains; and the application of physiological, metabolic, and genetic-engineering methods. The second strategy is to obtain carotenoids from invasive plant species that cause enormous economic and infrastructural damage and loss of biodiversity. Examples of such plants are Japanese knotweed (Fallopia japonica Houtt.) and Bohemian knotweed (Fallopia x bohemica) [24]. It is well-known that, besides carotenoids, microorganisms synthesize many other pigments that are also of essential importance for various areas of human activities and are actively studied or already produced in large amounts by modern biotechnology. Some of them were briefly mentioned in this review, and plenty of special reviews devoted to them were recently published [125,177,178]. However, the information about the potential application of carotenoids that were obtained during the last decades testifies that they are still underestimated. We expect that the unique characteristics of carotenoids will lead to further demand for natural carotenoids in the coming years and much more efficient use of these compounds in various areas, including those associated with the prevention and therapy of various human diseases. Simultaneously, this should give an additional incentive to further study this unique group of natural pigments.
Acknowledgments
L.B. is grateful to Erasmus Traineeship EU Program for his scholarship that allowed him to spend a few months at the University of Latvia.
Author Contributions
Conceptualization, A.R.; writing—original draft preparation, A.R., I.G., L.B., and P.B.; writing—review and editing, A.R., P.B., A.M.K., and M.K.; supervision, A.R.; project administration, A.R. and M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dufosse L., Galaup P., Yaron A., Arad S.M., Blanc P., Chidambara Murthy K.N., Ravishankar G.A. Microorganisms and microalgae as sources of pigments for food use: A scientific oddity or an industrial reality? Trends Food Sci. Technol. 2005;16:389–406. doi: 10.1016/j.tifs.2005.02.006. [DOI] [Google Scholar]
- 2.Das A., Yoon S.H., Lee S.H., Kim J.Y., Oh D.K., Kim S.W. An update on microbial carotenoid production. Application of recent metabolic engineering tools. Appl. Microbiol. Biotechnol. 2007;77:505–512. doi: 10.1007/s00253-007-1206-3. [DOI] [PubMed] [Google Scholar]
- 3.Mata-Gomez L.C., Montanez J.C., Mendez-Zavala A., Aguilar C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Factor. 2014;13:12. doi: 10.1186/1475-2859-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niranjana R., Gayathri R., Mol S.N., Sugawara T., Hirata T., Miyashita K., Ganesan P. Carotenoids modulate the hallmarks of cancer cells. J. Funct. Foods. 2015;18:968–985. doi: 10.1016/j.jff.2014.10.017. [DOI] [Google Scholar]
- 5.Riso P., Brusamolino A., Scalfi L., Porrini M. Bioavailability of carotenoids from spinach and tomatoes. Nutr. Metab. Cardiovasc. Dis. 2004;14:150–156. doi: 10.1016/S0939-4753(04)80035-8. [DOI] [PubMed] [Google Scholar]
- 6.Di Mascio P., Murphy M.E., Sies H. Antioxidant defense systems: The role of carotenoids, tocopherols, and thiols. Am. J. Clin. Nutr. 1991;53:194S–200S. doi: 10.1093/ajcn/53.1.194S. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Concepcion M., Avalos J., Bonet M.L., Boronat A., Gomez-Gomez L., Hornero-Mendez D., Limon M.C., Meléndez-Martínez A.J., Olmedilla-Alonso B., Palou A., et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018;70:62–93. doi: 10.1016/j.plipres.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Meléndez-Martínez A.J. An overview of carotenoids, apocarotenoids and vitamin A in agro-food, nutrition, health and disease. Mol. Nutrit. Food Res. 2019;63:1801045. doi: 10.1002/mnfr.201801045. [DOI] [PubMed] [Google Scholar]
- 9.Meléndez-Martínez A.J., Stinco C.M., Mapelli-Brahm P. Skin carotenoids in public health and nutricosmetics. The emerging roles and applications of the UV light-absorbing colourless carotenoids phytoene and phytofluene. Nutrients. 2019;11:1093. doi: 10.3390/nu11051093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mapelli-Brahm P., Barba F.J., Remize F., Garcia C., Fessard A., Khaneghah A.M., Sant’Ana A.S., Lorenzoe J.M., Montesano D., Meléndez-Martínez A.J. The impact of fermentation processes on the production, retention and bioavailability of carotenoids: An overview. Trends Food Sci. Technol. 2020;99:389–401. doi: 10.1016/j.tifs.2020.03.013. [DOI] [Google Scholar]
- 11.Britton G. Carotenoid research: History and new perspectives for chemistry in biological systems. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2020;1865:158699. doi: 10.1016/j.bbalip.2020.158699. [DOI] [PubMed] [Google Scholar]
- 12.Woodside J.V., McGrath A.J., Lyner N., McKinley M.C. Carotenoids and health in older people. Maturitas. 2015;80:63–68. doi: 10.1016/j.maturitas.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Meléndez-Martínez A.J., Mapelli-Brahm P., Stinco C.M. The colourless carotenoids phytoene and phytofluene: From dietary sources to their usefulness for the functional foods and nutricosmetics industries. J. Food Compos. Anal. 2018;67:91–103. doi: 10.1016/j.jfca.2018.01.002. [DOI] [Google Scholar]
- 14.BCC Research The Global Market for Carotenoids. [(accessed on 20 December 2020)];2018 Available online: https://www.bccresearch.com/market-research/food-and-beverage/the-global-market-for-carotenoids.html.
- 15.Aziz E., Batool R., Akhtar W., Rehman S., Shahzad T., Malik A., Shariati M.A., Laishevtcev A., Plygun S., Heydari M., et al. Xanthophyll: Health benefits and therapeutic insights. Life Sci. 2020;240:117104. doi: 10.1016/j.lfs.2019.117104. [DOI] [PubMed] [Google Scholar]
- 16.Stahl W., Sies H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta. 2005;1740:101–107. doi: 10.1016/j.bbadis.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Fernandes A.S., do Nascimento T.C., Jacob-Lopes E., De Rosso V.V., Zepka L.Q. Introductory Chapter: Carotenoids—A brief overview on its structure, biosynthesis, synthesis, and applications. Prog. Carotenoid Res. 2018:1–16. doi: 10.5772/intechopen.79542. [DOI] [Google Scholar]
- 18.Amen T.R., Mikhailova E.V., Alenin V.V., Artyomov A.V., Dementyev P.A., Khodorkovskii M.A., Artamonova T.O., Kuznetsova I.M., Soidla T.R., Nevzglyadova O.V. Structural and functional characteristics of various forms of red pigment of yeast Saccharomyces cerevisiae and its synthetic analog. Cell Tissue Biol. 2013;7:86–94. doi: 10.1134/S1990519X13010021. [DOI] [Google Scholar]
- 19.Nevzglyadova O.V., Mikhailova E.V., Amen T.R., Zenin V.V., Artemov A.V., Kostyleva E.I., Mezhenskaya D.A., Rodin D.I., Saifitdinova A.F., Khodorkovskii M.A., et al. Yeast red pigment modifies amyloid beta growth in Alzheimer disease models in both Saccharomyces cerevisiae and Drosophila melanogaster. Amyloid. 2015;22:100–111. doi: 10.3109/13506129.2015.1010038. [DOI] [PubMed] [Google Scholar]
- 20.Nevzglyadova O.V., Mikhailova E.V., Artemov A.V., Ozerova Y.E., Ivanova P.A., Golomidov I.M., Bolshakova O.I., Zenin V.V., Kostyleva E.I., Soidla T.R., et al. Yeast red pigment modifies cloned human α-synuclein pathogenesis in Parkinson disease models in Saccharomyces cerevisiae and Drosophila melanogaster. Neurochem. Intern. 2018;120:172–181. doi: 10.1016/j.neuint.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Tapiero H., Townsend D.M., Tew K.D. The role of carotenoids in the prevention of human pathologies. Biomed. Pharmacother. 2004;58:100–110. doi: 10.1016/j.biopha.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seel W., Baust D., Sons D., Albers M., Etzbach L., Fuss J., Lipski A. Carotenoids are used as regulators for membrane fluidity by Staphylococcus xylosus. Sci. Rep. 2020;10:330. doi: 10.1038/s41598-019-57006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Black R.E., Allen L.H., Bhutta Z.A., Caulfield L.E., De Onis M., Ezzati M., Mathers C., Rivera J. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet. 2008;371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 24.Metličar V., Vovk I., Albreht A. Japanese and Bohemian Knotweeds as Sustainable Sources of Carotenoids. Plants. 2019;8:384. doi: 10.3390/plants8100384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mussagy C.U., Winterburn J., Santos-Ebinuma V.C., Pereira J.F.B. Production and extraction of carotenoids produced by microorganisms. Appl. Microbiol. Biotechnol. 2019;103:1095–1114. doi: 10.1007/s00253-018-9557-5. [DOI] [PubMed] [Google Scholar]
- 26.Gul K., Tak A., Singh A.K., Singh P., Yousuf B., Wani A.A., Yildiz F. Chemistry, encapsulation, and health benefits of β-carotene—A review. Cogent Food Agric. 2015;1:1018696. doi: 10.1080/23311932.2015.1018696. [DOI] [Google Scholar]
- 27.Grune T., Lietz G., Palou A., Ross A.C., Stahl W., Tang G., Thurnham D., Yin S.A., Biesalski H.K. Beta-carotene is an important vitamin A source for humans. J. Nutr. 2010;140:2268S–2285S. doi: 10.3945/jn.109.119024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raja R., Hemaiswarya S., Rengasamy R. Exploitation of Dunaliella for β-carotene production. Appl. Microbiol. Biotechnol. 2007;74:517–523. doi: 10.1007/s00253-006-0777-8. [DOI] [PubMed] [Google Scholar]
- 29.Bhosale P., Gadre R.V. Manipulation of temperature and illumination conditions for enhanced beta-carotene production by mutant 32 of Rhodotorula glutinis. Lett. Appl. Microbiol. 2002;34:349–353. doi: 10.1046/j.1472-765X.2002.01095.x. [DOI] [PubMed] [Google Scholar]
- 30.Sharma R., Ghoshal G. Optimization of carotenoids production by Rhodotorula mucilaginosa (MTCC-1403) using agro-industrial waste in bioreactor: A statistical approach. Biotechnol. Rep. 2020;25:e00407. doi: 10.1016/j.btre.2019.e00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han M., He Q., Zhang W.G. Carotenoids production in different culture conditions by Sporidiobolus pararoseus. Prep. Biochem. Biotechnol. 2012;42:293–303. doi: 10.1080/10826068.2011.583974. [DOI] [PubMed] [Google Scholar]
- 32.Manowattana A., Techapun C., Laokuldilok T., Phimolsiripol Y., Chaiyaso T. Enhancement of β-carotene-rich carotenoid production by a mutant Sporidiobolus pararoseus and stabilization of its antioxidant activity by microencapsulation. J. Food Process. Preserv. 2020;44:e14596. doi: 10.1111/jfpp.14596. [DOI] [Google Scholar]
- 33.Schmidt I., Schewe H., Gassel S., Jin C., Buckingham J., Hümbelin M., Sandmann G., Schrader J. Biotechnological production of astaxanthin with Phaffia rhodozyma/Xanthophyllomyces dendrorhous. Appl. Microbiol. Biotechnol. 2011;89:555–571. doi: 10.1007/s00253-010-2976-6. [DOI] [PubMed] [Google Scholar]
- 34.Ambati R.R., Phang S.M., Ravi S., Aswathanarayana R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications: A review. Mar. Drugs. 2014;12:128–152. doi: 10.3390/md12010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jannel S., Caro Y., Bermudes M., Petit T. Novel insights into the biotechnological production of Haematococcus pluvialis-derived astaxanthin: Advances and key challenges to allow its industrial use as novel food ingredient. J. Mar. Sci. Eng. 2020;8:789. doi: 10.3390/jmse8100789. [DOI] [Google Scholar]
- 36.Rodríguez-Sáiz M., Fuente J., Barredo J. Xanthophyllomyces dendrorhous for the industrial production of astaxanthin. Appl. Microbiol. Biotechnol. 2010;88:645–658. doi: 10.1007/s00253-010-2814-x. [DOI] [PubMed] [Google Scholar]
- 37.Li C., Li B., Zhang N., Wei N., Wang Q., Wang W., Xie Y., Zou H. Salt stress increases carotenoid production of Sporidiobolus pararoseus NGR via torulene biosynthetic pathway. J. Gen. Appl. Microbiol. 2019;65:111–120. doi: 10.2323/jgam.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Wei C., Wu T., Ao H., Qian X., Wang Z., Sun J. Increased torulene production by the red yeast, Sporidiobolus pararoseus, using citrus juice. Prep. Biochem. Biotechnol. 2019;10:1–8. doi: 10.1080/10826068.2019.1663533. [DOI] [PubMed] [Google Scholar]
- 39.Hausmann A., Sandmann G. A single five-step desaturase is involved in the carotenoid biosynthesis pathway to β-carotene and torulene in Neurospora crassa. Fung. Gen. Biol. 2000;30:147–153. doi: 10.1006/fgbi.2000.1212. [DOI] [PubMed] [Google Scholar]
- 40.Dimitrova S., Pavlova K., Lukanov L., Korotkova E., Petrova E., Zagorchev P., Kuncheva M. Production of metabolites with antioxidant and emulsifying properties by Antarctic strain Sporobolomyces salmonicolor AL1. Appl. Biochem. Biotechnol. 2013;169:301–311. doi: 10.1007/s12010-012-9983-2. [DOI] [PubMed] [Google Scholar]
- 41.Du C., Guo Y., Cheng Y., Han M., Zhang W., Qian H. Anti-cancer effects of torulene, isolated from Sporidiobolus pararoseus, on human prostate cancer LNCaP and PC-3 cells via a mitochondrial signal pathway and the down-regulation of AR expression. RSC Adv. 2017;7:2466–2474. doi: 10.1039/C6RA24721K. [DOI] [Google Scholar]
- 42.Ungureanu C., Ferdes M., Chirvase A.A., Mocanu E. Method for torularhodin separation and analysis in the yeast Rhodotorula rubra aerobically cultivated in lab bioreactor; Proceedings of the Icheap-10: 10th International Conference on Chemical and Process Engineering, Pts 1–3; Florence, Italy. 8–11 May 2011; pp. 943–948. [Google Scholar]
- 43.Ungureanu C., Ferdes M., Chirvase A.A. Torularhodin biosynthesis andn extraction by yeast cells of Rhodotorula rubra. Rev. Chim. 2012;63:316–318. [Google Scholar]
- 44.Cardoso L.A., Jäckel S., Karp S.G., Framboisier X., Chevalot I., Marc I. Improvement of Sporobolomyces ruberrimus carotenoids production by the use of raw glycerol. Bioresour. Technol. 2016;200:374–379. doi: 10.1016/j.biortech.2015.09.108. [DOI] [PubMed] [Google Scholar]
- 45.Kanno K.Y.F., Karp S.G., Rodrigues C., Tanobe V.O.A., Soccol C.R., da Costa Cardoso L.A. Influence of organic solvents in the extraction and purification of torularhodin from Sporobolomyces ruberrimus. Biotechnol. Lett. 2020 doi: 10.1007/s10529-020-03023-8. [DOI] [PubMed] [Google Scholar]
- 46.Sandmann G. Carotenoids of biotechnological importance. Adv. Biochem. Eng. Biotechnol. 2015;148:449–467. doi: 10.1007/10_2014_277. [DOI] [PubMed] [Google Scholar]
- 47.Gupta S.K., Trivedi D., Srivastava S., Joshi S., Halder N., Verma S.D. Lycopene attenuates oxidative stress induced experimental cataract development: An in vitro and in vivo study. Nutrition. 2003;19:794–799. doi: 10.1016/S0899-9007(03)00140-0. [DOI] [PubMed] [Google Scholar]
- 48.SanGiovanni J.P., Neuringer M. The putative role of lutein and zeaxanthin as protective agents against age-related macular degeneration: Promise of molecular genetics for guiding mechanistic and translational research in the field. Am. J. Clin. Nutr. 2012;96:1223S–1233S. doi: 10.3945/ajcn.112.038240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eggersdorfer M., Wyss A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018;652:18–26. doi: 10.1016/j.abb.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 50.Ungureanu C., Ferdes M. Evaluation of antioxidant and antimicrobial activities of torularhodin. Adv. Sci. Lett. 2012;5:1–4. doi: 10.1166/asl.2012.4403. [DOI] [Google Scholar]
- 51.Keceli T.M., Erginkaya Z., Turkkan E., Kaya U. Antioxidant and antibacterial effects of carotenoids extracted from Rhodotorula glutinis strains. Asian J. Chem. 2013;25:42–46. doi: 10.14233/ajchem.2013.12377. [DOI] [Google Scholar]
- 52.Kot A.M., Błażejak S., Kurcz A., Gientka I., Kieliszek M. Rhodotorula glutinis—Potential source of lipids, carotenoids, and enzymes for use in industries. Appl. Microbiol. Biotechnol. 2016;100:6103–6117. doi: 10.1007/s00253-016-7611-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kot A.M., Błażejak S., Gientka I., Kieliszek M., Brys J. Torulene and torularhodin: “new” fungal carotenoids for industry? Microb. Cell Fact. 2018;17:49. doi: 10.1186/s12934-018-0893-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ungureanu C., Popescu S., Purcel G., Tofan V., Popescu M., Sălăgeanu A., Pîrvu C. Improved antibacterial behavior of titanium surface with torularhodin-polypyrrole film. Mater. Sci. Eng. C. 2014;42:726–733. doi: 10.1016/j.msec.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 55.Ungureanu C., Dumitriu C., Popescu S., Enculescu M., Tofan V., Popescu M., Pirvu C. Enhancing antimicrobial activity of TiO2/Ti by torularhodin bioinspired surface modification. Bioelectrochemistry. 2016;107:14–24. doi: 10.1016/j.bioelechem.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Haddad N., Levy R. The synergistic anti-inflammatory effects of lycopene, lutein, β-carotene, and carnosic acid combinations via redox-based inhibition of NF-kB signaling. Free Radic. Biol. Medic. 2012;53:1381–1391. doi: 10.1016/j.freeradbiomed.2012.07.078. [DOI] [PubMed] [Google Scholar]
- 57.Katan M.B., Grundy S.M., Jones P., Law M., Miettinen T., Paoletti R. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin. Proc. 2003;78:965–968. doi: 10.1016/S0025-6196(11)63144-3. [DOI] [PubMed] [Google Scholar]
- 58.Rao A.V. Lycopene, tomatoes, and the prevention of coronary heart disease. Exp. Biol. Med. 2002;227:908–913. doi: 10.1177/153537020222701011. [DOI] [PubMed] [Google Scholar]
- 59.Rao A.V., Rao L.G. Carotenoids and human health. Pharmacol. Res. 2007;55:207–216. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 60.McEneny J., Wade L., Young I.S., Masson L., Duthie G., McGinty A., McMaster C., Thies F. Lycopene intervention reduces inflammation and improves HDL functionality in moderately overweight middle-aged individuals. J. Nutr. Biochem. 2013;24:163–168. doi: 10.1016/j.jnutbio.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 61.Kim J.Y., Paik J.K., Kim O.Y., Park H.V., Lee J.H., Jang Y., Lee J.H. Effects of lycopene supplementation on oxidative stress and markers of endothelial function in healthy men. Atherosclerosis. 2011;215:189–195. doi: 10.1016/j.atherosclerosis.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 62.Ramesh C., Vinithkumar N.V., Kirubagaran R., Venil C.K., Dufossé L. Multifaceted applications of microbial pigments: Current knowledge, challenges and future directions for public health implications. Microorganisms. 2019;7:186. doi: 10.3390/microorganisms7070186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gouranton E., Thabuis C., Riollet C., Malezet-Desmoulins C., El Yazidi C., Amiot M.J., Borel P., Landrier J.F. Lycopene inhibits proinflammatory cytokine and chemokine expression in adipose tissue. J. Nutr. Biochem. 2011;22:642–648. doi: 10.1016/j.jnutbio.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 64.Palozza P., Simone R., Catalano A., Monego G., Barini A., Mele M.C., Parrone N., Trombino S., Picci N., Ranelletti F.O. Lycopene prevention of oxysterol-induced proinflammatory cytokine cascade in humn macrophages: Inhibition of NF-κB nuclear binding and increase in PPARγ expression. J. Nutr. Biochem. 2011;22:259–268. doi: 10.1016/j.jnutbio.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 65.Rao L.G., Mackinnon E.S., Josse R.G., Murray T.M., Strauss A., Rao A.V. Lycopene consumption decreases oxidative stress and bone resorption markers in postmenopausal women. Osteoporosis Int. 2007;18:109–115. doi: 10.1007/s00198-006-0205-z. [DOI] [PubMed] [Google Scholar]
- 66.Viuda-Martos M., Sanchez-Zapata E., Sayas-Barberá E., Sendra J., Perez-Alvares A., Fernandes-Lopez J. Tomato and tomato byproducts. Human health benefits of lycopene and its application to meat products: A review. Crit. Rev. Food Sci. Nutr. 2014;54:1032–1049. doi: 10.1080/10408398.2011.623799. [DOI] [PubMed] [Google Scholar]
- 67.Thies F., Mills L.M., Moir S., Masson L.F. Cardiovascular benefits of lycopene: Fantasy or reality? Proc. Nutr. Soc. 2017;76:122–129. doi: 10.1017/S0029665116000744. [DOI] [PubMed] [Google Scholar]
- 68.Walallawita U.S., Wolber F.M., Ziv-Gal A., Kruger M.C., Heyes J.A. Potential role of lycopene in the prevention of postmenopausal bone loss: Evidence from molecular to clinical studies. Int. J. Mol. Sci. 2020;21:7119. doi: 10.3390/ijms21197119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rao A.V., Ray M.R., Rao L.G. Lycopene. Adv. Food Nutr. Res. 2006;51:99–164. doi: 10.1016/S1043-4526(06)51002-2. [DOI] [PubMed] [Google Scholar]
- 70.Bonet M.L., Ribot J., Galmés S., Francisca Serra F., Palou A. Carotenoids and carotenoid conversion products in adipose tissue biology and obesity: Pre-clinical and human studies. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2020;1865:158676. doi: 10.1016/j.bbalip.2020.158676. [DOI] [PubMed] [Google Scholar]
- 71.Rao A.V., Balachandran B. Role of oxidative stress and antioxidants in neurodegenerative diseases. Nutr. Neurosci. 2003;5:291–309. doi: 10.1080/1028415021000033767. [DOI] [PubMed] [Google Scholar]
- 72.Wu W., Li Y., Wu Y., Zhang Y., Wang Z., Liu X. Lutein suppresses inflammatory responses through Nrf2 activation and NF-κB inactivation in lipopolysaccharide-stimulated BV-2 microglia. Mol. Nutr. Food Res. 2015;59:1663–1673. doi: 10.1002/mnfr.201500109. [DOI] [PubMed] [Google Scholar]
- 73.Wu J.L., Wang W.Y., Cheng Y.L., Du C., Qian H. Neuroprotective effects of torularhodin against H2O2-induced oxidative injury and apoptosis in PC12 cells. Pharmazie. 2015;70:17–23. [PubMed] [Google Scholar]
- 74.Longnecker M.P., Kamel F., Umbach D.M., Munsat T.L., Shefner J.M., Lansdell L.W., Sandler D.P. Dietary intake of calcium, magnesium and antioxidants in relation to risk of amyotrophic lateral sclerosis. Neuroepidemiology. 2000;19:210–216. doi: 10.1159/000026258. [DOI] [PubMed] [Google Scholar]
- 75.Mikhailova E.V., Artemov A.V., Snigirevskaya E.S., Artamonova T.O., Khodorkovskii M.A., Soidla T.R., Nevzglyadova O.V. Effect of red pigment on insulin fibril formation in vitro. Cell Tiss. Biol. 2011;5:580–585. doi: 10.1134/S1990519X11060095. [DOI] [PubMed] [Google Scholar]
- 76.Nevzglyadova O.V., Artemov A.V., Mittenberg A.G., Mikhailova E.V., Kuznetsova I.M., Turoverov K.K., Soidla T.R. Effect of red pigment on amyloidization of yeast. Cell Tiss. Biol. 2010;4:152–166. doi: 10.1134/S1990519X10020057. [DOI] [PubMed] [Google Scholar]
- 77.Nevzglyadova O.V., Kuznetsova I.M., Mikhailova E.V., Artamonova T.O., Artemov A.V., Mittenberg A.G., Kostyleva E.I., Turoverov K.K., Khodorkovskii M.A., Soidla T.R. The effect of red pigment on amyloidization of yeast proteins. Yeast. 2011;28:505–526. doi: 10.1002/yea.1854. [DOI] [PubMed] [Google Scholar]
- 78.Burton G.W., Ingold K.U. Beta-carotene: An unusual type of lipid antioxidant. Science. 1984;224:569–573. doi: 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]
- 79.Krinsky N.I. Antioxidant functions of carotenoids. Free Radic. Biol. Med. 1989;7:617–635. doi: 10.1016/0891-5849(89)90143-3. [DOI] [PubMed] [Google Scholar]
- 80.Kiokias S., Gordon M.H. Dietary supplementation with a natural carotenoid mixture decreases oxidative stress. Eur. J. Clin. Nutr. 2003;57:1135–1140. doi: 10.1038/sj.ejcn.1601655. [DOI] [PubMed] [Google Scholar]
- 81.Krinsky N.I., Johnson E.J. Carotenoid actions and their relation to health and disease. Mol. Asp. Med. 2005;26:459–516. doi: 10.1016/j.mam.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 82.Sarada S., Dipti P., Anju B., Pauline T., Kain A., Sairam M., Sharma S., Ilavazhagan G., Kumar D., Selvamurthy W. Antioxidant effect of β-carotene on hypoxia induced oxidative stress in male albino rats. J. Ethnopharmacol. 2002;79:149–153. doi: 10.1016/S0378-8741(01)00360-9. [DOI] [PubMed] [Google Scholar]
- 83.Amarowicz R. Lycopene as a natural antioxidant. Eur. J. Lipid Sci. Technol. 2011;113:675–677. doi: 10.1002/ejlt.201100157. [DOI] [Google Scholar]
- 84.Sies H., Stahl W. Nutritional protection against skin damage from sunlight. Annu. Rev. Nutr. 2004;24:173–200. doi: 10.1146/annurev.nutr.24.012003.132320. [DOI] [PubMed] [Google Scholar]
- 85.Berneburg M., Krutmann J. Photoimmunology, DNA repair and photocarcinogenesis. J. Photochem. Photobiol. B. 2000;54:87–93. doi: 10.1016/S1011-1344(00)00024-5. [DOI] [PubMed] [Google Scholar]
- 86.Krutmann J. Ultraviolet A radiation-induced biological effects in human skin: Relevance for photoaging and photodermatosis. J. Dermatol. Sci. 2000;23:S22–S26. doi: 10.1016/S0923-1811(99)00077-8. [DOI] [PubMed] [Google Scholar]
- 87.Nishino H., Tokuda H., Murakoshi M., Satomi Y., Masuda M., Onozuka M., Yamaguchi S., Takayasu J., Tsuruta J., Okuda M. Cancer prevention by natural carotenoids. Biofactors. 2000;13:89–94. doi: 10.1002/biof.5520130115. [DOI] [PubMed] [Google Scholar]
- 88.Ajila C., Brar S. Role of dietary antioxidants in cancer. In: Shankar S., Srivastava R.K., editors. Nutrition, Diet and Cancer. Springer; Dordrecht, Germany: 2012. pp. 377–412. [DOI] [Google Scholar]
- 89.Tanaka T., Shnimizu M., Moriwaki H. Cancer chemoprevention by carotenoids. Molecules. 2012;17:3202–3242. doi: 10.3390/molecules17033202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haddad N.F., Teodoro A.J., Oliveira F.L., Soares N., de Mattos R.M., Hecht F., Dezonne R.S., Vairo L., Goldenberg R.C., Gomes F.C.A., et al. Lycopene and β-carotene induce growth inhibition and proapoptotic effects on ACTH-secreting pituitary adenoma cells. PLoS ONE. 2013;8:e62773. doi: 10.1371/journal.pone.0062773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Holzapfel N.P., Holzapfel B.M., Champ S., Feldthusen J., Clements J., Hutmacher D.W. The potential role of lycopene for the prevention and therapy of prostate cancer: From molecular mechanisms to clinical evidence. Int. J. Mol. Sci. 2013;14:14620–14646. doi: 10.3390/ijms140714620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rokkaku T., Kimura R., Ishikawa C., Yasumoto T., Senba M., Kanaya F., Mori N. Anticancer effects of marine carotenoids, fucoxanthin and its deacetylated product, fucoxanthinol, on osteosarcoma. Int. J. Oncol. 2013;43:1176–1186. doi: 10.3892/ijo.2013.2019. [DOI] [PubMed] [Google Scholar]
- 93.Gloria N.F., Soares N., Brand C., Oliveira F.L., Borojevic R., Teodoro A.J. Lycopene and beta-carotene induce cell cycle arrest and apoptosis in human breast cancer cell lines. Anticancer Res. 2014;34:1377–1386. [PubMed] [Google Scholar]
- 94.Sugawara T., Ganesan P., Li Z., Manabe Y., Hirata T. Siphonaxanthin, a green algal carotenoid, as a novel functional compound. Marine Drugs. 2014;12:3660–3668. doi: 10.3390/md12063660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zu K., Mucci L., Rosner B.A., Clinton S.K., Loda M., Stampfer M.J., Giovannucci E. Dietary lycopene, angiogenesis, and prostate cancer: A prospective study in the prostate-specific antigen era. J. Natl. Cancer Inst. 2014;106:djt430. doi: 10.1093/jnci/djt430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Du C., Li Y., Guo Y., Han M., Zhang W., Qian H. The suppression of torulene and torularhodin treatment on the growth of PC-3 xenograft prostate tumors. Biochem. Biophys. Res. Commun. 2016;469:1146–1152. doi: 10.1016/j.bbrc.2015.12.112. [DOI] [PubMed] [Google Scholar]
- 97.Linnewiel-Hermoni K., Khanin M., Danilenko M., Zango G., Amosi Y., Levy J., Sharoni Y. The anti-cancer effects of carotenoids and other phytonutrients resides in their combined activity. Arch. Biochem. Biophys. 2015;572:28–35. doi: 10.1016/j.abb.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 98.Prakash P., Russell R.M., Krinsky N.I. In vitro inhibition of proliferation of estrogen-dependent and estrogen-independent human breast cancer cells treated with carotenoids or retinoids. J. Nutr. 2001;131:1574–1580. doi: 10.1093/jn/131.5.1574. [DOI] [PubMed] [Google Scholar]
- 99.Block G., Patterson B., Subar A. Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutr. Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 100.Ziegler R.G., Mayne S.T., Swanson C.A. Nutrition and lung cancer. Cancer Causes Control. 1996;7:157–177. doi: 10.1007/BF00115646. [DOI] [PubMed] [Google Scholar]
- 101.Omen G.S., Goodman G.E., Thornquist M.D., Balmes J., Cullen M.R., Glass A., Keogh J.P., Meyskens F.L., Valanis B., Williams J.H., et al. Effects of a combination of beta-carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 102.Rowles J.L., III, Erdman J.W., Jr. Carotenoids and their role in cancer prevention. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2020;1865:158613. doi: 10.1016/j.bbalip.2020.158613. [DOI] [PubMed] [Google Scholar]
- 103.Lim J.Y., Wang X.-D. Mechanistic understanding of β-cryptoxanthin and lycopene in cancer prevention in animal models. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2020;1865:158652. doi: 10.1016/j.bbalip.2020.158652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bendich A. Carotenoids and immune response. J. Nutr. 1989;119:112–115. doi: 10.1093/jn/119.1.112. [DOI] [PubMed] [Google Scholar]
- 105.Krinsky N.I. Actions of carotenoids in biological systems. Annu. Rev. Nutr. 1993;13:561–587. doi: 10.1146/annurev.nu.13.070193.003021. [DOI] [PubMed] [Google Scholar]
- 106.Shultz T.D., Chew B.P., Seaman W.R., Luedecke L.O. Inhibitory effects of conjugated dienoic derivatives of linoleic acid and beta-carotene on /the in vitro growth of human cancer cells. Cancer Lett. 1992;63:125–133. doi: 10.1016/0304-3835(92)90062-Z. [DOI] [PubMed] [Google Scholar]
- 107.Santamaria L., Bianchi A., Andreoni L., Santagati G., Arnaboldi A., Bermond P. 8-Methoxypsoralen photocarcinogenesis and its prevention by dietary carotenoids. Preliminary results. Med. Biol. Environ. 1984;12:533–537. [Google Scholar]
- 108.Belisario M.A., Pecce R., Battista C., Panza N., Pacilio G. Inhibition of cyclophosphamide mutagenicity by β-carotene. Biomed. Pharmacother. 1985;39:445–448. [PubMed] [Google Scholar]
- 109.Azuine M.A., Goswami U.C., Kayal J.J., Bhide S.V. Antimutagenic and anticarcinogenic effects of carotenoids and dietary palm oil. Nutr. Cancer. 1992;17:287–295. doi: 10.1080/01635589209514198. [DOI] [PubMed] [Google Scholar]
- 110.Arriaga-Alba M., Rivera-Sanchez R., Parra-Cervantes G., Barro-Moreno F., Flores-Paz R., Garcıa-Jimenez E. Antimutagenesis of β-carotene to mutations induced by quinolone on Salmonella typhimurium. Arch. Med. Res. 2000;31:156–161. doi: 10.1016/S0188-4409(00)00046-1. [DOI] [PubMed] [Google Scholar]
- 111.Pung A.O., Rundhaug J.E., Yoshizawa C.N., Bertram J.S. β-Carotene and canthaxanthin inhibit chemically- and physically-induced neoplastic transformation in 10T1/2 cells. Carcinogenesis. 1988;9:1533–1539. doi: 10.1093/carcin/9.9.1533. [DOI] [PubMed] [Google Scholar]
- 112.Bertram J.S., Pung A., Churley M., Kappock T.J., IV, Wilkins L.R., Cooney R.V. Diverse carotenoids protect against chemically induced neoplastic transformation. Carcinogenesis. 1991;12:671–678. doi: 10.1093/carcin/12.4.671. [DOI] [PubMed] [Google Scholar]
- 113.Saini R.K., Keum Y.-S. Microbial platforms to produce commercially vital carotenoids at industrial scale: An updated review of critical issues. J. Ind. Microbiol. Biotechnol. 2019;46:657–674. doi: 10.1007/s10295-018-2104-7. [DOI] [PubMed] [Google Scholar]
- 114.Tyczkowski J.K., Hamilton P.B. Absorption, transport, and deposition in chickens of lutein diester, a carotenoid extracted from Marigold (Tagetes erecta) petals. Poult. Sci. 1986;65:1526–1531. doi: 10.3382/ps.0651526. [DOI] [PubMed] [Google Scholar]
- 115.Mortensen A. Carotenoids and other pigments as natural colorants. Pure Appl. Chem. 2006;78:1477–1491. doi: 10.1351/pac200678081477. [DOI] [Google Scholar]
- 116.Mannazzu I., Landolfo S., Lopes da Silva T., Buzzini P. Red yeasts and carotenoid production: Outlining a future for non-conventional yeasts of biotechnological interest. World J. Microbiol. Biotechnol. 2015;31:1665–1673. doi: 10.1007/s11274-015-1927-x. [DOI] [PubMed] [Google Scholar]
- 117.Nabi F., Arain M.A., Rajput N., Alagawany M., Soomro J., Umer M., Soomro F., Wang Z., Ye R., Liu J. Health benefits of carotenoids and potential application in poultry industry: A review. J. Anim. Physiol. Anim. Nutrit. 2020;104:104–1809. doi: 10.1111/jpn.13375. [DOI] [PubMed] [Google Scholar]
- 118.Johnson E.A., An G.H. Astaxanthin from microbial sources. Crit. Rev. Biotechnol. 1991;11:297–326. doi: 10.3109/07388559109040622. [DOI] [Google Scholar]
- 119.Johnson E.A. Phaffia rhodozyma: Colorfull odyssey. Int. Microbiol. 2003;6:169–174. doi: 10.1007/s10123-003-0130-3. [DOI] [PubMed] [Google Scholar]
- 120.Marcoleta A., Niklitschek M., Wozniak A., Lozano C., Alcaıno J., Baeza M., Cifuentes V. Glucose and ethanol-dependent transcriptional regulation of the astaxanthin biosynthesis pathway in Xanthophyllomyces dendrorhous. BMC Microbiol. 2011;11:190. doi: 10.1186/1471-2180-11-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martinez-Moya P., Niehaus K., Alcaıno J., Baeza M., Cifuentes V. Proteomic and metabolomic analysis of the carotenogenic yeast Xanthophyllomyces dendrorhous using different carbon sources. BMC Genomics. 2015;16:289. doi: 10.1186/s12864-015-1484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aruldass C.A., Dufosse L., Ahmad W.A. Current perspective of yellowish-orange pigments from microorganisms—A review. J. Clean. Prod. 2018:168–182. doi: 10.1016/j.jclepro.2018.01.093. [DOI] [Google Scholar]
- 123.Tuli H.S., Chaudhary P., Beniwal V., Sharma A.K. Microbial pigments as natural color sources: Current trends and future perspectives. J. Food Sci. Technol. 2014;52:4669–4678. doi: 10.1007/s13197-014-1601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lagashetti A.C., Dufossé L., Singh S.K., Singh P.N. Fungal pigments and their prospects in different industries. Microorganisms. 2019;7:604. doi: 10.3390/microorganisms7120604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Venil C.K., Velmurugan P., Dufossé L., Devi P.R., Ravi A.V. Fungal pigments: Potential coloring compounds for wide ranging applications in textile dyeing. J. Fungi. 2020;6:68. doi: 10.3390/jof6020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tramontin L.R.R., Kildegaard K.R., Sudarsan S., Borodina I. Enhancement of astaxanthin biosynthesis in oleaginous yeast Yarrowia lipolytica via microalgal pathway. Microorganisms. 2019;7:472. doi: 10.3390/microorganisms7100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dufosse L. Microbial production of food grade pigments. Food Technol. Biotechnol. 2006;44:313–321. [Google Scholar]
- 128.Dufosse L., Fouillaud M., Caro Y., Mapari S.A.S., Sutthiwong N. Filamentous fungi are large-scale producers of pigments and colorants for the food industry. Curr. Opin. Biotechnol. 2014;26:56–61. doi: 10.1016/j.copbio.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 129.Mapari S.A.S., Hansen M.E., Meyer A.S., Thrane U. Computerized screening for novel producers of Monascus-like food pigments in Penicillium species. J. Agric. Food Chem. 2008;56:9981–9989. doi: 10.1021/jf801817q. [DOI] [PubMed] [Google Scholar]
- 130.Mapari S.A.S., Meyer A.S., Thrane U., Frisvad J.C. Identification of potentially safe promising fungal cell factories for the production of polyketide natural food colorants using chemotaxonomic rationale. Microb. Cell Fact. 2009;8:24. doi: 10.1186/1475-2859-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dufossé L. Red colourants from filamentous fungi: Are they ready for the food industry? J. Food Compos. Anal. 2018;69:156–161. doi: 10.1016/j.jfca.2017.11.002. [DOI] [Google Scholar]
- 132.Morales-Oyervides L., Ruiz-Sánchez J.P., Oliveira J.C., Sousa-Gallagher M.J., Mendez-Zavala A., Giuffrida D., Dufossé L., Montañez J. Biotechnological approaches for the production of natural colorants by Talaromyces/Penicillium: A review. Biotechnol. Adv. 2020;43:107601. doi: 10.1016/j.biotechadv.2020.107601. [DOI] [PubMed] [Google Scholar]
- 133.Böhme K., Richter C., Pätz R. New insights into mechanisms of growth and β-carotene production in Blakeslea trispora. Biotechnol. J. 2006;1:1080–1084. doi: 10.1002/biot.200600096. [DOI] [PubMed] [Google Scholar]
- 134.Mehta B.J., Obraztsova I.N., Cerda-Olmedo E. Mutants and intersexual heterokaryons of Blakeslea trispora for production of β-carotene and lycopene. Appl. Environ. Microbiol. 2003;69:4043–4048. doi: 10.1128/AEM.69.7.4043-4048.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Reyes L.H., Gomez J.M., Kao K.C. Improving carotenoids production in yeast via adaptive laboratory evolution. Metab. Eng. 2014;21:26–33. doi: 10.1016/j.ymben.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 136.Marova I., Breierova E., Koci R., Friedl Z., Slovak B., Pokorna J. Influence of exogenous stress factors on production of carotenoids by some strains of carotenogenic yeasts. Ann. Microbiol. 2004;54:73–85. [Google Scholar]
- 137.Marova I., Carnecka M., Halienova A., Koci R., Breierova E. Production of carotenoid/ergosterol supplemeted biomass by red yeast Rhodotorula glutinis grown under external stress. Food Technol. Biotech. 2010;48:56–61. [Google Scholar]
- 138.Kot A.M., Błażejak S., Gientka I., Kieliszek M., Brys J., Reczek L., Pobiega K. Effect of exogenous stress factors on the biosynthesis of carotenoids and lipids by Rhodotorula yeast strains in media containing agro-industrial waste. World J. Microbiol. Biotechnol. 2019;35:157. doi: 10.1007/s11274-019-2732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sandmann G. Genetic manipulation of carotenoid biosynthesis: Strategies, problems and achievements. Trend Plant. Sci. 2001;6:14–17. doi: 10.1016/S1360-1385(00)01817-3. [DOI] [PubMed] [Google Scholar]
- 140.Godara A., Rodriguez M.A.G., Weatherston J., Peabody G.L., Wu H.-J., Kao K.C. Beneficial mutations for carotenoid production identified from laboratory-evolved Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2019;46:1793–1804. doi: 10.1007/s10295-019-02241-y. [DOI] [PubMed] [Google Scholar]
- 141.Fathi Z., Tramontin L.R.R., Ebrahimipour G., Borodina I., Darvishi F. Metabolic engineering of Saccharomyces cerevisiae for production of β-carotene from hydrophobic substrates. FEMS Yeast Res. 2021:foaa068. doi: 10.1093/femsyr/foaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang C., Zhao S., Shao X., Park J.-B., Jeong S.-H., Park H.-J., Kwak W.-J., Wei G., Kim S.-W. Challenges and tackles in metabolic engineering for microbial production of carotenoids. Microb. Cell Fact. 2019;18:55. doi: 10.1186/s12934-019-1105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li C., Swofford C.A., Sinskey A.J. Modular engineering for microbial production of carotenoids. Metabol. Eng. Commun. 2020;10:e00118. doi: 10.1016/j.mec.2019.e00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li L., Liu Z., Jiang H., Mao X. Biotechnological production of lycopene by microorganisms. Appl. Microbiol. Biotechnol. 2020;104:10307–10324. doi: 10.1007/s00253-020-10967-4. [DOI] [PubMed] [Google Scholar]
- 145.Da Silva Rios D.A., de Matos Borba T., Kalil S.J., de Medeiros Burkert J.F. Rice parboiling wastewater in the maximization of carotenoids bioproduction by Phaffia rhodozyma. Ciência e Agrotecnologia. 2015;39:401–410. doi: 10.1590/S1413-70542015000400011. [DOI] [Google Scholar]
- 146.Machado W.R.C., Burkert J.F.D.M. Optimization of agroindustrial medium for the production of carotenoids by wild yeast Sporidiobolus pararoseus. Afr. J. Agric. Res. 2015;9:209–219. doi: 10.5897/AJMR2014.7096. [DOI] [Google Scholar]
- 147.Da Silva S.R.S., Stamford T.C.M., Albuquerque W.W.C., Vidal E.E., Stamford T.L.M. Reutilization of residual glycerin for the produce β-carotene by Rhodotorula minuta. Biotechnol. Lett. 2020;42:437–443. doi: 10.1007/s10529-020-02790-8. [DOI] [PubMed] [Google Scholar]
- 148.Imandi S.B., Bandaru V.R., Somalanka S.R., Garapati H.R. Optimization of medium constituents for the production of citric acid from byproduct glycerol using Doehlert experimental design. Enzyme Microb. Technol. 2007;40:1367–1372. doi: 10.1016/j.enzmictec.2006.10.012. [DOI] [Google Scholar]
- 149.Saenge C., Cheirsilp B., Suksaroge T.T., Bourtoom T. Potential use of oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process. Biochem. 2011;46:210–218. doi: 10.1016/j.procbio.2010.08.009. [DOI] [Google Scholar]
- 150.Valduga E., Rausch Ribeiro A.H., Cence K., Colet R., Tiggemann L., Zeni J., Toniazzo G. Carotenoids production from a newly isolated Sporidiobolus pararoseus strain using agroindustrial substrates. Biocatal. Agric. Biotechnol. 2014;3:207–213. doi: 10.1016/j.bcab.2013.10.001. [DOI] [Google Scholar]
- 151.Cipolatti E.P., Remedi R.D., dos Santos Sá C., Rodrigues A.B., Ramos J.M.G., Burkert C.A.V., Furlong E.B., de Medeiros Burkert J.F. Use of agroindustrial byproducts as substrate for production of carotenoids with antioxidant potential by wild yeasts. Biocatal. Agric. Biotechnol. 2019;20:101208. doi: 10.1016/j.bcab.2019.101208. [DOI] [Google Scholar]
- 152.Rodrigues T.A., Schueler T.A., da Silva A.J.R., Sérvulo E.F.C., Oliveira F.J.S. Valorization of solid wastes from the brewery and biodiesel industries for the bioproduction of natural dyes. Braz. J. Chem. Eng. 2019;36:99–107. doi: 10.1590/0104-6632.20190361s20170608. [DOI] [Google Scholar]
- 153.Petrik S., Obruca S., Benesova P., Marova I. Bioconversion of spent coffee grounds into carotenoids and other valuable metabolites by selected red yeast strains. Biochem. Eng. J. 2014;90:307–315. doi: 10.1016/j.bej.2014.06.025. [DOI] [Google Scholar]
- 154.Obruca S., Benesova P., Kucera D., Petrik S., Marova I. Biotechnological conversion of spent coffee grounds into polyhydroxyalkanoates and carotenoids. New Biotechnol. 2015;32:569–574. doi: 10.1016/j.nbt.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 155.Otero D.M., Bulsing B.A., Huerta K.d.M., Rosa C.A., Zambiazi R.C., Burkert C.A.V., Burkert J.F.d.M. Carotenoid-producing yeasts in the Brazilian biodiversity: Isolation, identification and cultivation in agroindustrial waste. Braz. J. Chem. Engineer. 2019;36:117–129. doi: 10.1590/0104-6632.20190361s20170433. [DOI] [Google Scholar]
- 156.Pereira R.N., da Silveira J.M., de Medeiros Burkert J.F., Ores J.d.C., Burkert C.A.V. Simultaneous lipid and carotenoid production by stepwise fedpbatch cultivation of Rhodotorula mucilaginosa with crude glycerol. Braz. J. Chem. Eng. 2019;36:1099–1108. doi: 10.1590/0104-6632.20190363s20190199. [DOI] [Google Scholar]
- 157.Schneider T., Graeff-Hönninger S., French W.T., Hernandez R., Merkt N., Claupein W., Hetrick M., Pham P. Lipid and carotenoid production by oleaginous red yeast Rhodotorula glutinis cultivated on brewery effluents. Energy. 2013;61:34–43. doi: 10.1016/j.energy.2012.12.026. [DOI] [Google Scholar]
- 158.Dineshkumar R., Sen R. A sustainable perspective of microalgal biorefinery for co-production and recovery of high-value carotenoid and biofuel with CO2 valorization. Biofuels Bioprod. Bioref. 2020;14:879–897. doi: 10.1002/bbb.2107. [DOI] [Google Scholar]
- 159.Panesar R., Kaur S., Panesar P.S. Production of microbial pigments utilizing agro-industrial waste: A review. Curr. Opin. Food Sci. 2015;1:70–76. doi: 10.1016/j.cofs.2014.12.002. [DOI] [Google Scholar]
- 160.Korumilli T., Mishra S. Carotenoid production by Rhodotorula sp. on fruit waste extract as a sole carbon source and optimization of key parameters. Iran J. Chem. Chem. Eng. 2014;33:89–99. [Google Scholar]
- 161.Cheng M.-H., Sun L., Jin Y.-S., Dien B., Singh V. Production of xylose enriched hydrolysate from bioenergy sorghum and its conversion to β-carotene using an engineered Saccharomyces cerevisiae. Bioresour. Technol. 2020;308:123275. doi: 10.1016/j.biortech.2020.123275. [DOI] [PubMed] [Google Scholar]
- 162.Sun L., Atkinson C.A., Lee Y.-G., Jin Y.-S. High-level β-carotene production from xylose by engineered Saccharomyces cerevisiae without overexpression of a truncated HMG1 (tHMG1) Biotechnol. Bioeng. 2020;117:3522–3532. doi: 10.1002/bit.27508. [DOI] [PubMed] [Google Scholar]
- 163.Chreptowicz K., Mierzejewska J., Tkácová J., Młynek M., Certik M. Carotenoid-producing yeasts: Identification and characteristics of environmental isolates with a valuable extracellular enzymatic activity. Microorganisms. 2019;7:653. doi: 10.3390/microorganisms7120653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gu W.L., An G.H., Johnson E.A. Ethanol increases carotenoid production in Phaffia rhodozyma. J. Ind. Microbiol. Biotechnol. 1997;19:114–117. doi: 10.1038/sj.jim.2900425. [DOI] [PubMed] [Google Scholar]
- 165.Zhang Z., Zhang X., Tan T. Lipid and carotenoid production by Rhodotorula glutinis under irradiation/high-temperature and dark/low-temperature cultivation. Bioresour. Technol. 2014;157:149–153. doi: 10.1016/j.biortech.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 166.Frengova G.I., Simova E.D., Beshkova D.M. Effect of temperature changes on the production of yeast pigments co-cultivated with lacto-acid bacteria in whey ultrafiltrate. Biotechnol. Lett. 1995;17:1001–1006. doi: 10.1007/BF00127443. [DOI] [Google Scholar]
- 167.Buzzini P., Martini A. Production of carotenoids by strains of Rhodotorula glutinis cultured in raw materials of agro-industrial origin. Bioresour. Technol. 2000;71:41–44. doi: 10.1016/S0960-8524(99)00056-5. [DOI] [Google Scholar]
- 168.Malisorn C., Suntornsuk W. Optimization of β-carotene production by Rhodotorula glutinis DM28 in fermented radish brine. Bioresour. Technol. 2008;99:2281–2287. doi: 10.1016/j.biortech.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 169.Machado W.R.C., da Silva L.G., Vanzela E.S.L., Del Bianchi V.L. Evaluation of the process conditions for the production of microbial carotenoids by the recently isolated Rhodotorula mucilaginosa URM 7409. Braz. J. Food Technol. 2019;22:e2018267. doi: 10.1590/1981-6723.26718. [DOI] [Google Scholar]
- 170.Guo Y., Xie S., Yuan J.S., Kao K.C. Effects of seawater on carotenoid production and lipid content of engineered Saccharomyces cerevisiae. Fermentation. 2019;5:6. doi: 10.3390/fermentation5010006. [DOI] [Google Scholar]
- 171.Buzzini P., Martini A., Gaetani M., Turchetti B., Pagnoni U.M., Davoli P. Optimization of carotenoid production by Rhodotorula graminis DBVPG 7021 as a function of trace element concentration by means of response surface analysis. Enzyme Microb. Technol. 2005;36:687–692. doi: 10.1016/j.enzmictec.2004.12.028. [DOI] [Google Scholar]
- 172.Larroude M., Celinska E., Back A., Thomas S., Nicaud J.M., Ledesma-Amaro R. A synthetic biology approach to transform Yarrowia lipolytica into a competitive biotechnological producer of b-carotene. Biotechnol. Bioeng. 2017;115:464–472. doi: 10.1002/bit.26473. [DOI] [PubMed] [Google Scholar]
- 173.Miller K.K., Alper H.S. Yarrowia lipolytica: More than an oleaginous workhorse. Appl. Microbiol. Biotechnol. 2019;103:9251–9262. doi: 10.1007/s00253-019-10200-x. [DOI] [PubMed] [Google Scholar]
- 174.Jacobsen I.H., Ledesma-Amaro R., Martinez J.L. Recombinant β-carotene production by Yarrowia lipolytica—Assessing the potential of micro-scale fermentation analysis in cell factory design and bioreaction optimization. Front. Bioengineer. Biotechnol. 2020;8:29. doi: 10.3389/fbioe.2020.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Alipour S., Habibi A., Taavoni S., Varmira K. β-Carotene production from soap stock by loofa-immobilized Rhodotorula rubra in an airlift photobioreactor. Process. Biochem. 2016;54:9–19. doi: 10.1016/j.procbio.2016.12.013. [DOI] [Google Scholar]
- 176.Britton G., Liaaen-Jensen S., Pfander H. Carotenoids: A Colourful History. Fagtrykk; Trondheim, Norway: 2017. [Google Scholar]
- 177.Pailliè-Jiménez M.E., Stincone P., Brandelli A. Natural pigments of microbial origin. Front. Sustain. Food Syst. 2020;4:590439. doi: 10.3389/fsufs.2020.590439. [DOI] [Google Scholar]
- 178.Singh T., Heer K. A review: Microbial anticancer pigments. Plant Cell Biotechnol. Mol. Biol. 2020;21:1–7. [Google Scholar]