Abstract
The chemical properties that serve as major determinants for the glycemic index (GI) of starchy food and recommended low-GI, carbohydrate-based foods have remained enigmatic. This present work performed a systematic assessment of linkages between chemical properties of foods and GI, and selected low-GI starchy foods. The data were sourced from literature published in various scientific journals. In total, 57 relevant studies and 936 data points were integrated into a database. Both in vitro and in vivo studies on GI values were included. The database was subsequently subjected to a meta-analysis. Meta-analysis from in vitro studies revealed that the two significant factors responsible for the GI of starchy foods were resistant starch and phenolic content (respectively, standardized mean difference (SMD): −2.52, 95% confidence interval (95%CI): −3.29 to −1.75, p (p-value) < 0.001; SMD: −0.72, 95%CI: −1.26 to −0.17, p = 0.005), while the lowest-GI crop type was legumes. Subgroup analysis restricted to the crop species with significant low GI found two crops, i.e., sorghum (SMD: −0.69, 95%CI: −2.33 to 0.96, p < 0.001) and red kidney bean (SMD: −0.39, 95%CI: −2.37 to 1.59, p = 0.001). Meta-analysis from in vivo studies revealed that the two significant factors responsible for the GI of starchy foods were flavonoid and phenolic content (respectively, SMD: −0.67, 95%CI: −0.87 to −0.47, p < 0.001; SMD: −0.63, 95%CI: −1.15 to −0.11, p = 0.009), while the lowest-GI crop type was fruit (banana). In conclusion, resistant starch and phenolic content may have a desirable impact on the GI of starchy food, while sorghum and red kidney bean are found to have low GI.
Keywords: bioactive compounds, carbohydrate foods, diabetes, glycemic index, meta-analysis
1. Introduction
Type 1 diabetes mellitus (T1DM) has become a chronic metabolic disorder world-wide, and the regulation of blood glucose at a near-normal level could best fit the goals of preventing or delaying long-term diabetes complications in T1DM [1]. Insulin treatment alone is inadequate for controlling T1DM; essentially, dietary adjustments are required for the proper regulation of blood glucose level. In addition to type 1 diabetes mellitus, the glycemic index is associated with other non-communicable diseases such as cardiovascular diseases (CVDs), type 2 diabetes, and cancer [2]. Glycemic index (GI) is defined as the blood glucose response measured as the area under the curve (AUC) in response to a test food that an individual consumes under standard conditions and is expressed as a percentage of the AUC following consumption of a reference food that the same individual consumes on a different day [1]. A GI classification system is in common use. In this system, foods are categorized as having low (≤55), medium (55 < GI ≤ 70), or high GI (>70) [2]. Carbohydrates are among the major determinants of postprandial blood glucose and are directly related to the GI. Accordingly, there is a crucial need for information regarding the relationship between various starchy foods and their GI properties. Such information would allow consumers to appropriately adjust their foods based on the GI profile [3,4]. Further, elucidating the chemical properties of starchy foods responsible for GI is essential. These chemical properties can be relevant indicators for selecting foods with low GI [5]. However, the relationship between the chemical properties of starchy foods and GI is not thoroughly known.
Evidence from randomized controlled trials (RCTs) was reported to be inconsistent [4,6,7], while no report was found using systematic review [8] or meta-analysis [9]. Furthermore, five recent trials [10,11,12,13,14] with adequate power have been published and involved new evidence. Therefore, we performed this meta-analysis to explain the chemical property factors affecting the GI of starchy foods and selected low-GI carbohydrate foods.
2. Materials and Methods
2.1. Literature Search
This research referred to the guidelines of a meta-analysis handbook [15]. Relevant studies published in various scientific journals and indexed in electronic databases such as PubMed, Proquest, Science Direct, Cengage Library, and Google Scholar were identified, focusing on the relationship between food chemical properties and GI values.
Keywords used in the search strategy included “chemical properties”, “glycemic index”, “carbohydrate category”, and/or “blood glucose response”. After examining the titles and abstracts, we excluded irrelevant studies. Subsequently, we examined the full texts of all remaining articles to determine eligibility. The discrepancies were verified by discussion and consensus. We also reviewed the identified trials and review articles in reference lists to find any other potential proper reports.
This systematic review and meta-analysis were based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement) checklist. Each section and each subsection should be clearly identified. The search strategy used in the electronic databases employed keywords, inclusion criteria, and exclusion criteria. The keywords that were used in this research were: available carbohydrate, glycemic index, blood glucose, diabetic, weight management, and hypoglycemic. Among those keywords, only glycemic index was detected in the MeSH (Medical Subject Headings) database. The inclusion criteria were the following: peer-reviewed, clinical trial, completely randomized design, in vitro, in vivo, within the last 20 years. Exclusion criteria were the following: letters to the editor, proceedings, abstracts, and book chapters.
2.2. Eligibility Criteria
The eligibility of the trials was determined according to the following criteria: (1) design: completely randomized design that analyzed the relationships between food chemical properties and GI values; (2) population: all research that applied both in vitro and in vivo protocols for determining GI; (3) intervention: comparison between chemical properties, GI value, and selected low-GI carbohydrate-based foods; and (4) data: sufficient information (data) for calculating the standardized mean difference (SMD) and the corresponding 95% confidence interval (CI). Further, all published papers were written in English.
2.3. Data Extraction
Data from each included study were extracted and integrated into the database. The following data were collected: authors, year of publication, country of origin, number of patients or participants, intervention, control, sample/type, method/reference food, outcomes data (GI value), and follow-up.
2.4. Risk of Bias Assessment
To assess the risk of bias, Cochrane risk of bias tool was employed [16]. This was performed by using the following criteria: allocation concealment; random sequence generation; participants and personnel blinding; outcome assessment blinding; selective reporting; incomplete outcome data; and other bias. Each study was evaluated and scored as having “high”, “low”, or “unclear” risk of bias. Patient and clinician blinding was highly difficult and generally not feasible in these tests, and we determined that the main outcome was less vulnerable to being affected by lack of blinding. Consequently, studies with a high risk of bias for any one (or more) of the key domains were considered to have a high risk of bias. Studies for all key domains except blinding were considered to have a low risk of bias; otherwise, studies were considered to have an unclear risk of bias [17].
2.5. Quality of Evidence Assessment
The quality of evidence for primary and secondary outcomes was evaluated according to GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) procedure for risk of bias, inconsistency, indirectness, imprecision, and publication bias, which were categorized as high, moderate, low, or very low [18]. The results were then summarized in tables constructed using the GRADE system [18,19,20] (GRADE version 3.6) (Table 1). Stages of the study, including literature search, data extraction, risk of bias assessment, and evidence grade assessment were performed independently by one author (Frendy Ahmad Afandi/FAA).
Table 1.
GRADE evidence profile for chemical properties and starchy food sources related to GI.
| Quality Assessment | No of Patients/Replicates | Effect | Quality | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of Studies | Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | In Vitro | In Vivo | Total | Relative (95%CI) | Absolute | |
| RS content-GI (follow-up 9 days to 7 months; measured with: blood test and in vitro starch hydrolysis; range of scores: 3–5; better indicated by lower values) | ||||||||||||
| 10 | randomised trials | no serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | strong association | 36 | 100 | 136 | 0.29 | SMD −0.72 lower (−1.00 lower to −0.43 higher) | ⊕⊕⊕⊕ HIGH |
| Dietary Fibre content-GI (follow-up 2 days to 6 weeks; measured with: blood test and in vitro starch hydrolysis; range of scores: 2–3; better indicated by lower values) | ||||||||||||
| 15 | randomised trials | any serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | strong association | 11 | 210 | 221 | 0.22 | SMD −0.32 lower (−0.54 lower to −0.10 higher) | ⊕⊕⊕⊕ HIGH |
| Fat content-GI (follow-up 2 days to 6 weeks; measured with: blood test and in vitro starch hydrolysis; range of scores: 2–3; better indicated by lower values) | ||||||||||||
| 15 | randomised trials | no serious risk of bias | serious | no serious indirectness | no serious imprecision | strong association | 15 | 216 | 231 | 0.21 | SMD 0.05 lower (−0.16 lower to 0.26 higher) | ⊕⊕⊕⊕ HIGH |
| Protein content-GI (follow-up 2 days to 6 weeks; measured with: blood test and in vitro starch hydrolysis; range of scores: 2–3; better indicated by lower values) | ||||||||||||
| 15 | randomised trials | no serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | strong association | 12 | 226 | 238 | 0.20 | SMD −0.47 lower (−0.68 lower to −0.27 higher) | ⊕⊕⊕⊕ HIGH |
| Phenol content-GI (follow-up 15 days; measured with: in vitro starch hydrolysis and blood test; range of scores: 5–7; better indicated by lower values) | ||||||||||||
| 12 | randomised trials | no serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | strong association | 55 | 18 | 73 | 0.38 | SMD −0.67 lower (−1.05 lower to −0.30 higher) | ⊕⊕⊕O MODERATE |
| Flavonoid content-GI (follow-up 2 to 5 weeks; measured with: blood test and in vitro starch hydrolysis; range of scores: 4–6; better indicated by lower values) | ||||||||||||
| 10 | randomised trials | no serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | strong association | 15 | 305 | 320 | 0.20 | SMD −0.65 lower (−0.85 lower to −0.45 higher) | ⊕⊕⊕⊕ HIGH |
| Cereals type (follow-up-; measured with: in vitro starch hydrolysis; range of scores: 4–6; better indicated by lower values) | ||||||||||||
| 5 | randomised trials | no serious risk of bias | no serious inconsistency | no serious indirectness | serious | reporting bias strong association | 18 | 0 | 18 | 1.03 | SMD −2.42 lower (−3.45 lower to −1.39 higher) | ⊕⊕⊕⊕ HIGH |
| Tubers type (follow-up 12 to 20 days; measured with: blood test and in vitro starch hydrolysis; range of scores: 3–4; better indicated by lower values) | ||||||||||||
| 6 | randomised trials | no serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | strong association | 9 | 14 | 23 | 0.68 | SMD −0.25 lower (0.43 lower to −0.93 higher) | ⊕⊕⊕O MODERATE |
| Fruits type (follow-up 15 days; measured with: blood test and in vitro starch hydrolysis; range of scores: 3–4; better indicated by lower values) | ||||||||||||
| 2 | randomised trials | no serious risk of bias | serious | no serious indirectness | serious | reporting bias strong association | 3 | 10 | 13 | 0.85 | SMD 0.25 lower (−0.6 lower to 1.1 higher) | ⊕⊕⊕⊕ HIGH |
| Legumes type (follow-up-; measured with: in vitro starch hydrolysis; range of scores: 3–4; better indicated by lower values) | ||||||||||||
| 4 | randomised trials | no serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | strong association | 10 | 0 | 10 | 1.30 | SMD −2.15 lower (−3.5 lower to −0.9 higher) | ⊕⊕⊕O MODERATE |
2.6. Statistical Analysis
We used weighted analysis using Hedges’ d (Standard Mean Difference/SMD) for statistical methods. The data extracted from selected journals were mean, standard deviation or standard error, and the number of replicate experiments. The SMD with corresponding 95%CI values were pooled using the random-effects model. Exploration of heterogeneity across studies was carried out using the I2 index [20] (the I2 > 50% indicated sufficient heterogeneity), and publication bias was determined using the Begg’s test and Egger’s test (p < 0.05 was considered statistically significant for publication bias). We used Meta-Essentials tools for the meta-analysis process. The criterion of publication bias assessment approach using the GRADE System, consisted of selection, performance, attrition, detection, and reporting bias. The variables used for subgroup analysis were the method of study (in vivo or in vitro), type of reference food, and type of crops.
3. Results
3.1. Trial Selection and Risk of Bias Assessment
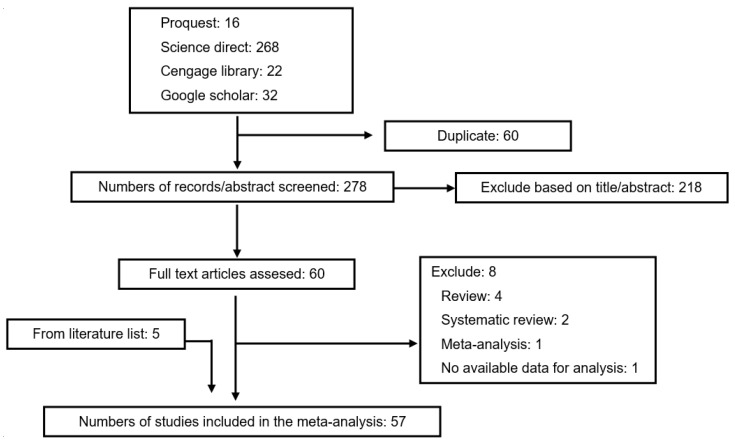
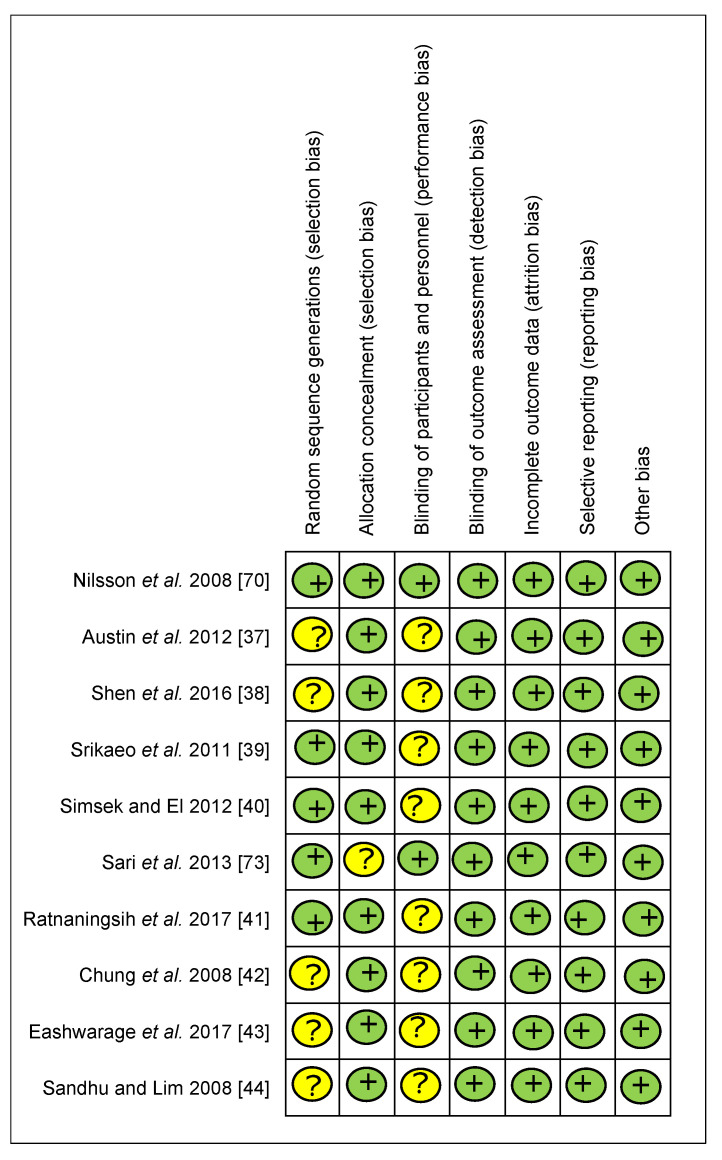
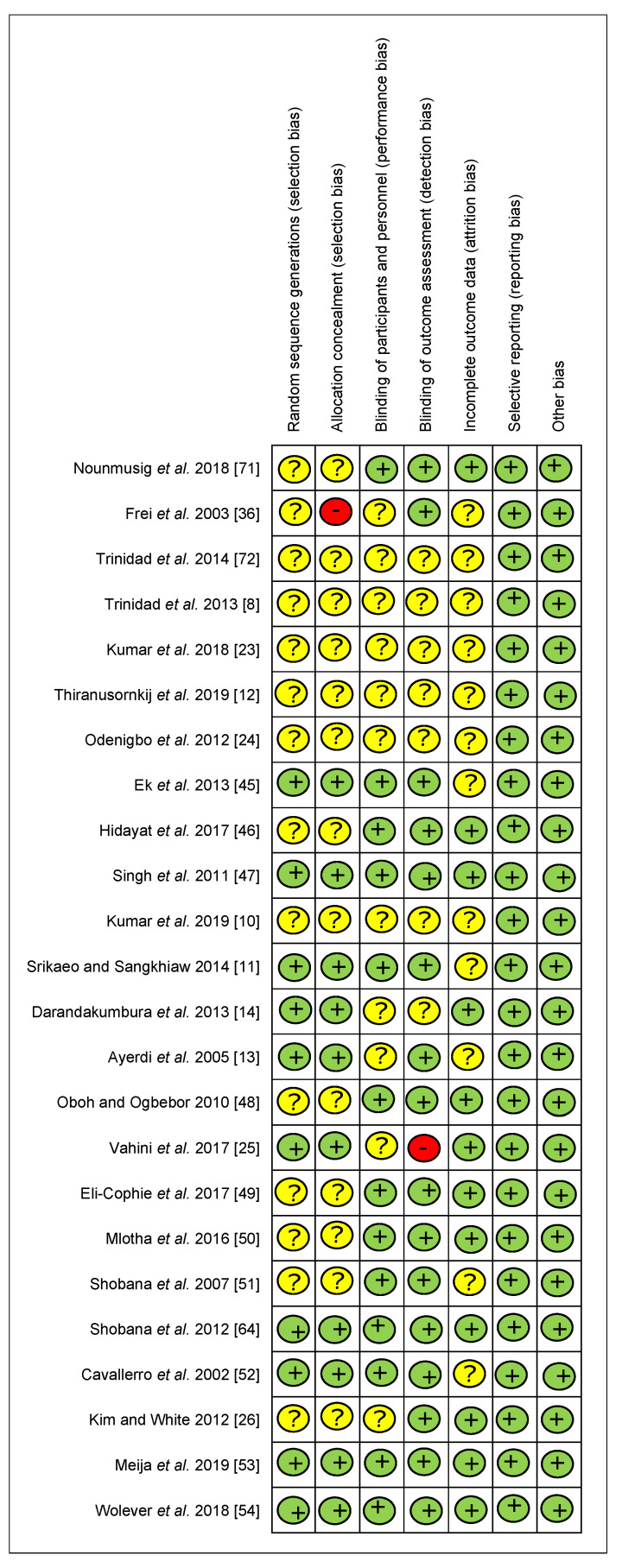
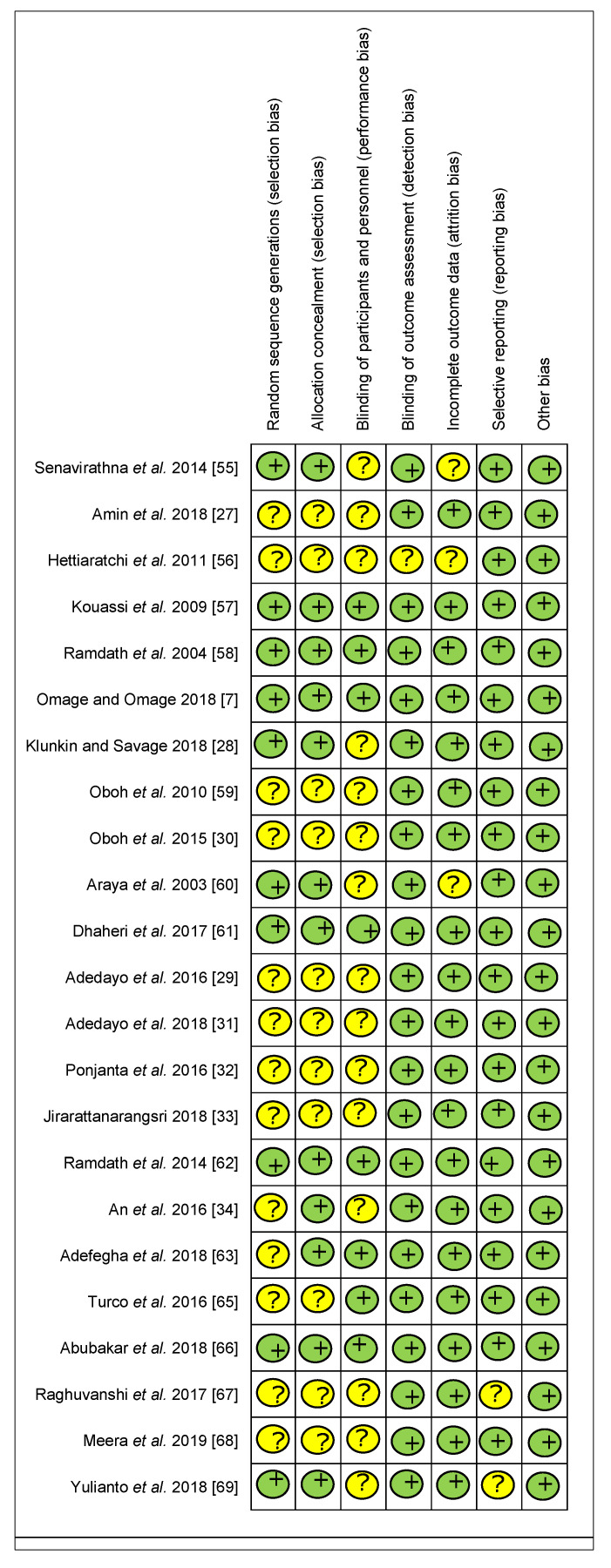
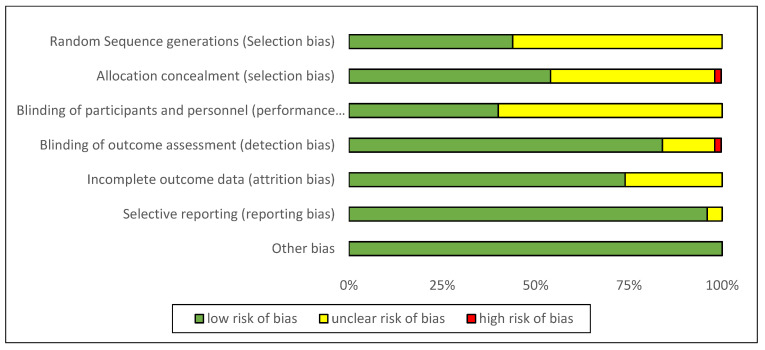
A total of sixty articles (from 338 articles) were selected for full-text review, resulting in fifty-seven articles [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61] that met the inclusion criteria. Eight of them were rejected due to a lack of essential data. Five additional articles [25,26,47,50,51] from reference lists of identified trials were included in the study because they met the inclusion criteria. In total, the meta-analysis involved fifty-seven articles, as exhibited in Figure 1. According to the Cochrane Collaboration tool, eleven trials [6,24,29,32,33,37,38,42,48,51,54] were categorized as being at a low risk of bias, while forty-four were categorized as unclear [17,19,20,21,22,23,25,27,28,30,31,34,35,36,39,40,41,43,44,45,46,47,49,50,52,53,55,56,57,58,59,60,61,62], and two were categorized as being at a high risk of bias [18,26]. Details about the risk of bias are supplied in Figure 2 and Figure 3.
Figure 1.
The preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow chart of the literature review process.
Figure 2.
The result of the risk of bias assessment: each risk of bias item for the included studies (green means low risk of bias, yellow means unclear risk of bias, red means high risk of bias).
Figure 3.
The result of the risk of bias assessment: each risk of bias item is shown as percentages across all included studies.
3.2. Characteristics of Articles
Fifty-seven studies, involving 936 participants, were published from 2002 to 2019. Twenty-six studies [9,10,11,12,17,18,20,21,26,31,35,39,43,44,45,46,47,49,54,55,56,57,59,60,61,62] used an in vitro experiment, while thirty-two studies involved in vivo experiments. Only one study used both in vitro and in vivo data experiments [11]. The in vivo studies involved healthy participants, and some studies used rats [51,58]. The PICOS of this research is defined as Participants, Interventions, Comparisons, Outcomes, and Study Design. Participants in the in vivo experiment were healthy adults. Interventions were lower food chemical properties. Comparisons were higher food chemical properties. Outcomes of this research were resistant starch, dietary fibre, protein, phenolic, and flavonoid content significantly affecting GI starchy foods, while selected low-GI foods were sorghum and red kidney bean. The study design used in this research was the completely randomized design. Among fifty-seven included studies, forty-eight were selected for discussion of the relationship between chemical properties and GI value [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54]. The remaining studies were used for the selection of low-GI carbohydrate-based foods [55,56,57,58,59,60,61,62]. All studies reported changes in glycemic index, four studies [7,17,18,19] reported changes in amylose content to GI, ten studies [9,10,11,12,13,20,21,22,23,24] reported changes in resistant starch (RS) content to GI, fifteen studies reported a change in dietary fibre content to GI, fifteen studies reported a change in fat content to GI, fifteen studies reported a change in protein content to GI, twelve studies reported a change in phenol content to GI, ten studies reported a change in flavonoid content to GI, five studies reported a change in cereal type to GI, six studies reported a change in tuber type to GI, two studies reported a change in fruit type to GI, and four studies reported a change in legume type to GI. Detailed characteristics of eligible studies are presented in Table 2 and Table 3.
Table 2.
Characteristics of in vitro studies included in the systematic review and meta-analysis. RS: resistant starch; GI: glycemic index; M: mean; SD: standard deviation.
| Author/Year | Country | Population | No. of Patient/ Replicates |
Intervention | Control | Sample/Type | Method/Reference Food | GI Value (M ± SD) Intervention/Control | Follow Up |
|---|---|---|---|---|---|---|---|---|---|
| 1. RS Content-GI | |||||||||
| Kumar et al./2018 [23] | India | - | 3 | Lower RS | Higher RS | Flour rice | In vitro/0.2 g maltose | 71.48 ± 0.21 to 69.62 ± 0.45 | - |
| Thiranusornkij et al./2019 [12] | Thailand | - | 3 | Lower RS | Higher RS | Flour rice | In vitro/100 mg white bread | 65.40 ± 4.50 to 87.10 ± 4.85 | - |
| Odenigbo et al./2012 [24] | Canada | - | 3 | Lower RS | Higher RS | French fries potato | In vitro/50 mg white bread | 52.16 ± 2.41 to 53.87 ± 0.89 | - |
| Kumar et al./2019 [10] | India | - | 3 | Lower RS | Higher RS | Flour rice (2 data)-normal light, low light | In vitro/0.2 g glucose | 61.63 ± 1.28 to 72.14 ± 0.86 63.14 ± 0.77 to 74.08 ± 1.06 |
- |
| Srikaeo and Sangkhiaw/2014 [11] | Thailand | - | 3 | Lower RS | Higher RS | Cooked rice noodles | In vitro/100 mg white bread | 66.98 ± 2.68 to 83.66 ± 1.02 | - |
| Ayerdi et al./2005 [13] | Mexico | - | 6 | Lower RS | Higher RS | Cooked bean-corn (3 data)-black beans, tortilla, tortilla-bean | In vitro/1 g white bread | 22.00 ± 3.02 to 27.00 ± 1.81 62.00 ± 3.15 to 75.00 ± 1.52 35.00 ± 1.38 to 51.00 ± 3.81 |
- |
| 2. Dietary Fibre Content-GI | |||||||||
| Vahini et al./2017 [25] | India | - | 2 | Lower dietary fibre | Higher dietary fibre | Corn flour boiled | In vitro/bread | 56.19 ± 1.10 to 82.57 ± 2.25 | - |
| Kim and White/2012 [26] | USA | - | 3 | Lower dietary fibre | Higher dietary fibre | Oat flour (2 data)-raw, heated | In vitro/100 mg white bread | 65.10 ± 1.30 to 61.10 ± 0.90 78.30 ± 1.30 to 85.70 ± 0.70 |
- |
| Amin et al./2018 [27] | India | - | 3 | Lower dietary fibre | Higher dietary fibre | Rice flour by enzymatic hydrolisis | In vitro/50 g white bread | 46.10 ± 0.20 to 88.00 ± 0.20 | - |
| 3. Fat Content-GI | |||||||||
| Klunkin and Savage/2018 [28] | New Zealand | - | 3 | Lower fat | Higher fat | Flour purple rice | In vitro/0.5 g glucose | 48.56 ± 0.03 to 63.11 ± 0.02 | - |
| Kim and White/2012 [26] | USA | - | 3 | Lower fat | Higher fat | Oat flour (2 data)-raw, heated | In vitro/100 mg white bread | 66.70 ± 1.60 to 64.20 ± 0.40 77.20 ± 0.50 to 82.70 ± 0.90 |
- |
| Odenigbo et al./2012 [24] | Canada | - | 3 | Lower fat | Higher fat | French fries sweet potato | In vitro/50 mg white bread | 56.18 ± 0.61 to 54.64 ± 0.71 | - |
| Amin et al./2018 [27] | India | - | 3 | Lower fat | Higher fat | Rice flour by enzymatic hydrolisis | In vitro/50 g white bread | 46.10 ± 0.20 to 88.00 ± 0.20 | - |
| 4. Protein Content-GI | |||||||||
| Kim and White/2012 [26] | USA | - | 3 | Lower protein | Higher protein | Oat flour (2 data)-raw, heated | In vitro/100 mg white bread | 64.20 ± 0.40 to 61.10 ± 0.90 77.20 ± 0.50 to 85.70 ± 0.70 |
- |
| Odenigbo et al./2012 [24] | Canada | - | 3 | Lower protein | Higher protein | French fries sweet potato | In vitro/50 mg white bread | 52.16 ± 2.41 to 56.18 ± 0.61 | - |
| Amin et al./2018 [27] | India | - | 3 | Lower protein | Higher protein | Rice flour by enzymatic hydrolisis | In vitro/50 g white bread | 46.10 ± 0.20 to 88.00 ± 0.20 | - |
| 5. Phenol Content-GI | |||||||||
| Adedayo et al./2016 [29] | Nigeria | - | 3 | Lower phenol | Higher phenol | Banana fruit | In vitro/50 g glucose | 44.95 ± 2.30 to 45.49 ± 2.17 | - |
| Oboh et al./2015 [30] | Nigeria | - | 3 | Lower phenol | Higher phenol | Banana-breadfruit fruit | In vitro/50 mg glucose | 52.78 ± 0.81 to 64.50 ± 1.23 | - |
| Adedayo et al./2018 [31] | Nigeria | - | 3 | Lower phenol | Higher phenol | Foreign and Ofada Rice (raw-cooked)-2 data | In vitro/50 g glucose | 49.00 ± 4.03 to 48.40 ± 3.67 51.80 ± 3.01 to 49.20 ± 2.99 |
- |
| Ponjanta et al./2016 [32] | Thailand | - | 3 | Lower phenol | Higher phenol | Raw rice (white to purple, red, black rice)-3 data | In vitro/50 g white bread | 68.50 ± 0.28 to 83.59 ± 5.38 80.40 ± 4.02 to 81.87 ± 0.68 82.79 ± 2.23 to 78.92 ± 4.12 |
- |
| Jirarattanarangsri/2018 [33] | Thailand | - | 3 | Lower phenol | Higher phenol | Cooked corn (purple waxy corn with ears and without ears)-2 data | In vitro/50 g glucose | 95.80 ± 0.50 to 96.20 ± 0.20 97.20 ± 0.80 to 96.10 ± 0.40 |
- |
| Klunkin and Savage/2018 [28] | New Zealand | - | 3 | Lower phenol | Higher phenol | Cooked cereal (wheat-purple rice) | In vitro/50 g white bread | 48.56 ± 0.03 to 63.11 ± 0.02 | - |
| An et al./2016 [34] | Korea | - | 3 | Lower phenol | Higher phenol | Rice flour (black rice-phenolic enriched extract) | In vitro/5 g white bread | 69.77 ± 1.26 to 79.23 ± 1.63 | - |
| Thiranusornkij et al./2019 [12] | Thailand | - | 3 | Lower phenol | Higher phenol | Rice flour (Hom Mali-Riceberry flour) | In vitro/50 g glucose | 65.40 ± 4.50 to 87.10 ± 4.85 | - |
| Adefegha et al./2018 [63] | Nigeria | - | 3 | Lower phenol | Higher phenol | Wheat (raw flour-paste flour) | In vitro/50 g glucose | 63.15 ± 2.30 to 71.92 ± 2.17 | - |
| Adedayo et al./2018 [35] | Nigeria | - | 3 | Lower phenol | Higher phenol | Yam (white bitter-yellow bitter) | In vitro/25 mg glucose | 56.25 ± 0.81 to 58.95 ± 0.81 | - |
| 6. Flavonoid Content-GI | |||||||||
| Adedayo et al./2018 [31] | Nigeria | - | 3 | Lower flavonoid | Higher flavonoid | Foreign and Ofada Rice (raw-cooked)-2 data | In vitro/50 g glucose | 49.00 ± 4.03 to 48.40 ± 3.67 51.80 ± 3.01 to 49.20 ± 2.99 |
|
| Klunkin and Savage/2018 [28] | New Zealand | - | 3 | Lower flavonoid | Higher flavonoid | Cooked cereal (wheat-purple rice) | In vitro/50 g white bread | 48.56 ± 0.03 to 63.11 ± 0.02 | - |
| An et al./2016 [34] | Korea | - | 3 | Lower flavonoid | Higher flavonoid | Rice flour (black rice-phenolic enriched extract) | In vitro/5 g white bread | 69.77 ± 1.26 to 79.23 ± 1.63 | - |
| Adefegha et al./2018 [63] | Nigeria | - | 3 | Lower flavonoid | Higher flavonoid | Wheat (raw flour-paste flour) | In vitro/50 g glucose | 63.15 ± 2.30 to 71.92 ± 2.17 | - |
| 7. Amylose Content-GI | |||||||||
| Frei et al./2003 [36] | Germany | - | 3 | Low amylose rice | Medium-high amylose rice | Cooked Rice/Brown Rice-milled | In vitro/50 mg white bread | 96.90 ± 4.33 to 68.00 ± 6.41 68.50 ± 5.54 to 87.30 ± 4.68 |
- |
| Lower-Higher Category RS [Selected low GI Carbohydrate] | |||||||||
| 1. Cereals Category | |||||||||
| Thiranusornkij et al./2019 [12] | Thailand | - | 3 | Lower RS | Higher RS | Rice | In vitro/50 g glucose | 65.40 ± 4.50 to 87.10 ± 4.85 | - |
| Frei et al./2003 [36] | Germany | - | 3 | Lower RS | Higher RS | Waxy rice | In vitro/50 mg white bread | 92.30 ± 8.31 to 109.20 ± 1.56 | - |
| Ayerdi et al./2005 [13] | Mexico | - | 6 | Lower RS | Higher RS | Corn | In vitro/1 g white bread | 62.00 ± 3.15 to 75.00 ± 1.52 | - |
| Austin et al./2012 [37] | USA | - | 3 | Lower RS | Higher RS | Sorghum | In vitro/50 mg white bread | 88.00 ± 5.00 to 92.00 ± 4.30 | - |
| Shen et al./2016 [38] | China | - | 3 | Lower RS | Higher RS | Barley | In vitro/50 mg white bread | 51.65 ± 0.02 to 59.67 ± 0.02 | - |
| 2. Tubers Category | |||||||||
| Srikaeo et al./2011 [39] | Thailand | - | 2 | Lower RS | Higher RS | Canna | In vitro/0.5 g rice noodle 100% amylose | 88.00 ± 1.80 to 97.00 ± 2.20 | - |
| Odenigbo et al./2012 [24] | Canada | - | 3 | Lower RS | Higher RS | Sweet potato | In vitro/50 mg white bread | 52.16 ± 2.41 to 53.87 ± 0.89 | - |
| Simsek and El/2012 [40] | Turkey | - | 2 | Lower RS | Higher RS | Taro | In vitro/50 mg white bread | 74.10 ± 1.30 to 86.60 ± 0.80 | - |
| Srikaeo et al./2011 [39] | Thailand | - | 2 | Lower RS | Higher RS | Cassava | In vitro/0.5 g rice noodle 100% amylose | 109.00 ± 1.20 to 105.00 ± 2.40 | - |
| 3. Fruits Category | |||||||||
| Oboh et al./2015 [30] | Nigeria | - | 3 | Lower RS | Higher RS | Papaya-breadfruit | In vitro/50 mg glucose | 64.50 ± 1.23 to 55.29 ± 1.33 | - |
| 4. Legumes Category | |||||||||
| Ratnaningsih et al./2017 [41] | Indonesia | - | 2 | Lower RS | Higher RS | Cowpea | In vitro/100 mg white bread | 45.46 ± 0.23 to 47.74 ± 0.19 | - |
| Chung et al./2008 [42] | Canada | - | 2 | Lower RS | Higher RS | Red kidney bean | In vitro/50 mg white bread | 12.00 ± 0.10 to 12.20 ± 0.40 | - |
| Eashwarage et al./2017 [43] | Sri Lanka | - | 3 | Lower RS | Higher RS | Mung bean | In vitro/1 g white bread | 41.54 ± 0.25 to 42.05 ± 0.09 | - |
| Sandhu and Lim/2008 [44] | South Korea | - | 3 | Lower RS | Higher RS | Pea | In vitro/50 g white bread | 44.20 ± 0.60 to 48.90 ± 0.40 | - |
Table 3.
Characteristics of in vivo studies included in the systematic review and meta-analysis. RS: resistant starch; GI: glycemic index; M: mean; SD: standard deviation.
| Author/Year | Country | Population | No. of Patient/Replicates | Intervention | Control | Sample/Type | Method/Reference Food | GI Value (M ± SD) Intervention/Control | Follow Up |
|---|---|---|---|---|---|---|---|---|---|
| 1. RS Content-GI | |||||||||
| Ek et al./2013 [45] | Australia | Adults (healthy) | 10, 27/10 (12 males, 17 females) | Lower RS | Higher RS | Boiled potato (4′) | In vivo/50 mg glucose | 82.00 ± 9.49 to 93.00 ± 31.62 | 12–20 days |
| Hidayat et al./2017 [46] | Indonesia | Adults (healthy) | 10, 10/10 | Lower RS | Higher RS | Corn based-rice analogues | In vivo/50 g glucose | 34.79 ± 2.11 to 40.77 ± 2.12 | 12 days |
| Singh et al./2011 [47] | Jamaica | Adults (healthy) | 10/10–5 males, 5 females | Lower RS | Higher RS | Cooked sweet potato (4 data)-boiled, fried, baked, roasted | In vivo/50 g glucose | 49.00 ± 12.65 to 46.00 ± 12.65 68.00 ± 9.49 to 75.00 ± 9.49 91.00 ± 9.49 to 87.00 ± 12.65 89.00 ± 9.49 to 90.00 ± 9.49 |
7 months |
| Srikaeo and Sangkhiaw/2014 [11] | Thailand | Adults (healthy) | 10/10 -5 males, 5 females | Lower RS | Higher RS | Cooked rice noodles | In vivo/50 g bread | 51.84 ± 1.95 to 63.62 ± 4.69 | 9 days |
| Darandakumbura et al./2013 [14] | Srilanka | Adults (healthy) | 10/10–5 males, 5 females | Lower RS | Higher RS | Cooked rice (3 data)-cultivar, cooking method, parboiled | In vivo/50 g glucose | 68.00 ± 2.00 to 70.00 ± 1.00 71.00 ± 2.00 to 72.00 ± 2.00 71.00 ± 1.00 to 68.00 ± 1.00 |
1.5 months (42 days) |
| 2. Dietary Fibre Content-GI | |||||||||
| Oboh and Ogbebor/2010 [48] | Nigeria | Adults (healthy) | 10–5 males, 5 females | Lower dietary fibre | Higher dietary fibre | Maize cooking method | In vivo/50 g glucose | 38.89 ± 3.13 to 21.32 ± 4.40 | 15 days |
| Eli-Cophie et al./2017 [49] | Ghana | Adults (healthy) | 10/10–8 males, 2 females | Lower dietary fibre | Higher dietary fibre | Cassava Corn-Fermented Corn (local food in Ghana) | In vivo/50 g glucose | 41.00 ± 21.50 to 73.00 ± 15.81 | 21 days |
| Mlotha et al./2016 [50] | Malawi | Adults (healthy) | 11/11–6 males, 5 females | Lower dietary fibre | Higher dietary fibre | Cooked dehulled-whole maize (porridges) | In vivo/50 g glucose | 106.72 ± 47.83 to 74.90 ± 46.22 | 21 days |
| Shobana et al./2007 [51] | India | Adults (healthy) | 8/8–5 males, 3 females | Lower dietary fibre | Higher dietary fibre | Cooked expanded rice-wheat (food formulation) | In vivo/50 g white bread | 55.40 ± 9.00 to 105.00 ± 6.00 | 25 days |
| Cavallerro et al./2002 [52] | Italy | Adults (healthy) | 8/8–3 males, 5 females | Lower dietary fibre | Higher dietary fibre | 100% Bread Wheat-20% Water Extracted Fraction Barley | In vivo/50 g glucose | 69.67 ± 20.34 to 89.49 ± 35.10 | 15 days |
| Meija et al./2019 [53] | Latvia-Norway | Adults (healthy) | 21/18 9–5 male, 4 female |
Lower dietary fibre | Higher dietary fibre | Cooked wheat-triticale | In vivo/50 g glucose | 53.85 ± 4.86 to 75.76 ± 3.26 | 5 days |
| Wolever et al./2018 [54] | Canada | Adults (healthy) | 51/40–24 male, 16 female | Lower dietary fibre | Higher dietary fibre | Cooked rice cereal-oat | In vivo/50 g rice cereal | 53.85 ± 4.86 to 75.76 ± 3.26 | 2–6 weeks |
| Senavirathna et al./2014 [55] | Sri Lanka | Adults (healthy) | 10/10 | Lower dietary fibre | Higher dietary fibre | Cooked underutilized tuber | In vivo/50 g white bread | 82.00 ± 25.30 to 64.00 ± 28.46 | ≈15 days |
| Hettiaratchi et al./2011 [56] | Sri Lanka | Adults (healthy) | 10 | Lower dietary fibre | Higher dietary fibre | Banana fruit | In vivo/50 g white bread | 61.00 ± 15.81 to 67.00 ± 22.14 | ≈15 days |
| Kouassi et al./2009 [57] | Cote d’Ivoire | Adults (healthy) | 10–10 males | Lower dietary fibre | Higher dietary fibre | Cooked yam (2 data)-oven, boiled | In vivo/50 g glucose | 66.00 ± 34.64 to 53.00 ± 22.52 54.00 ± 32.91 to 60.00 ± 31.18 |
≈27 days |
| Ramdath et al./2004 [58] | Carribean | Adults (healthy) | 8–10-4 males, 4–6 females | Lower dietary fibre | Higher dietary fibre | Cooked staple in Carribean (Tannia-green banana) | In vivo/50 g white bread | 61.00 ± 15.81 to 67.00 ± 22.14 | ≈1 months |
| Omage and Omage/2018 [7] | Nigeria | Adults (healthy) | 240/80–40 males, 40 females | Lower dietary fibre | Higher dietary fibre | Cooked mixed rice bean-rice plantain | In vivo/50 g glucose | 89.74 ± 9.84 to 86.60 ± 49.82 | 2 days |
| 3. Fat Content-GI | |||||||||
| Oboh and Ogbebor/2010 [48] | Nigeria | Adults (healthy) | 10–5 males, 5 females | Lower fat | Higher fat | Cooked maize | In vivo/50 g glucose | 38.89 ± 3.13 to 21.32 ± 4.40 | ≈9 days |
| Mlotha et al./2016 [50] | Malawi | Adults (healthy) | 11–6 males, 5 females | Lower fat | Higher fat | Cooked maize | In vivo/50 g glucose | 106.72 ± 47.83 to 74.90 ± 46.22 | 21 days |
| Shobana et al./2007 [51] | India | Adults (healthy) | 8–5 males, 3 females | Lower fat | Higher fat | Cooked rice (popped rice) | In vivo/50 g white bread | 109.00 ± 8.00 to 55.40 ± 9.00 | 25 days |
| Cavallerro et al./2002 [52] | Italy | Adults (healthy) | 8–3 males, 5 females | Lower fat | Higher fat | Bread (20% Water-Extracted Fraction Barley-50% Sieved Fraction Barley) | In vivo/50 g glucose | 74.46 ± 43.78 to 69.67 ± 20.34 | 15 days |
| Wolever et al./2018 [54] | Canada | Adults (healthy) | 40–24 male, 16 female | Lower fat | Higher fat | Cooked rice cereal-oat | In vivo/50 g rice cereal | 62.98 ± 9.07 to 100.00 ± 0.00 | 2–6 weeks |
| Senavirathna et al./2014 [55] | Sri Lanka | Adults (healthy) | 10 | Lower fat | Higher fat | Cooked underutilized tuber | In vivo/50 g white bread | 110.00 ± 25.30 to 69.00 ± 12.65 | ≈15 days |
| Shobana et al./2012 [64] | India | Adults (healthy) | 23/23–12 males, 11 females | Lower fat | Higher fat | Cooked rice | In vivo/50 g glucose | 77.00 ± 19.18 to 72.00 ± 21.58 | 8 days |
| Hettiaratchi et al./2011 [56] | Sri Lanka | Adults (healthy) | 10 | Lower fat | Higher fat | Banana fruit | In vivo/50 g white bread | 69.00 ± 28.46 to 61.00 ± 18.97 | ≈15 days |
| Kouassi et al./2009 [57] | Cote d’Ivoire | Adults (healthy) | 10–10 males | Lower fat | Higher fat | Cooked yam (2 data)-oven, boiled | In vivo/50 g glucose | 70.00 ± 31.18 to 53.00 ± 22.52 67.00 ± 27.71 to 51.00 ± 22.52 |
≈27 days |
| Ramdath et al./2004 [58] | Carribean | Adults (healthy) | 8–10 | Lower fat | Higher fat | Cooked staple in Carribean (white yam-dasheen) | In vivo/50 g white bread | 88.00 ± 28.46 to 109.00 ± 44.27 | ≈1 months |
| Omage and Omage/2018 [7] | Nigeria | Adults (healthy) | 80–40 males, 40 females | Lower fat | Higher fat | Cooked mixed rice bean-rice plantain | In vivo/50 g glucose | 86.93 ± 56.71 to 86.60 ± 49.82 | 2 days |
| 4. Protein Content-GI | |||||||||
| Mlotha et al./2016 [50] | Malawi | Adults (healthy) | 11–6 males, 5 females | Lower protein | Higher protein | Cooked dehulled-whole maize (porridges) | In vivo/50 g glucose | 106.72 ± 47.83 to 121.97 ± 38.99 | 21 days |
| Shobana et al./2007 [51] | India | Adults (healthy) | 8–5 males, 3 females | Lower protein | Higher protein | Cooked millet-wheat (food formulation) | In vivo/50 g white bread | 55.40 ± 9.00 to 93.40 ± 7.00 | 25 days |
| Cavallerro et al./2002 [52] | Italy | Adults (healthy)) | 8–3 males, 5 females | Lower protein | Higher protein | Bread (50% Barley Flour-100% Bread Wheat) | In vivo/50 g glucose | 89.49 ± 35.10 to 85.42 ± 38.81 | 15 days |
| Wolever et al./2018 [54] | Canada | Adults (healthy) | 40–24 male, 16 female | Lower protein | Higher protein | Cooked rice cereal-oat | In vivo/50 g rice cereal | 62.98 ± 9.07 to 100.00 ± 0.00 | 2–6 weeks |
| Senavirathna et al./2014 [55] | Sri Lanka | Adults (healthy) | 10 | Lower protein | Higher protein | Cooked underutilized tuber | In vivo/50 g white bread | 64.00 ± 28.46 to 69.00 ± 12.65 | ≈15 days |
| Shobana et al./2012 [64] | India | Adults (healthy) | 23–12 males, 11 females | Lower protein | Higher protein | Cooked rice | In vivo/50 g glucose | 70.20 ± 17.26 to 77.00 ± 19.18 | 8 days |
| Kouassi et al./2009 [57] | Cote d’Ivoire | Adults (healthy) | 10–10 males | Lower protein | Higher protein | Cooked yam (2 data)-oven, boiled | In vivo/50 g glucose | 53.00 ± 22.52 to 56.00 ± 20.78 60.00 ± 31.18 to 54.00 ± 32.91 |
≈27 days |
| Ramdath et al./2004 [58] | Carribean | Adults (healthy) | 8–10 | Lower protein | Higher protein | Cooked staple in Carribean (tannia-green banana) | In vivo/50 g white bread | 88.00 ± 28.46 to 109.00 ± 44.27 | ≈1 months |
| Omage and Omage/2018 [7] | Nigeria | Adults (healthy) | 80–40 males, 40 females | Lower protein | Higher protein | Cooked mixed rice bean-rice plantain | In vivo/50 g glucose | 86.93 ± 56.71 to 89.74 ± 9.84 | 2 days |
| Oboh et al./2010 [59] | Nigeria | Adults (healthy) | 5 | Lower protein | Higher protein | Cooked legumes (Cowpea black white-Pigeonpea brown) | In vivo/50 g glucose | 24.00 ± 22.36 to 30.00 ± 24.60 | ≈24 days |
| Araya et al./2003 [60] | Chile | Adults (healthy) | 10 males | Lower protein | Higher protein | Cooked legumes (bean-lentil) | In vivo/50 g white bread | 49.30 ± 29.50 to 76.80 ± 43.40 | ≈3–6 weeks |
| Dhaheri et al./2017 [61] | United Arab Emirates | Adults (healthy) | 37/15- 6 males, 9 females | Lower protein | Higher protein | Emirate cuisine (balalet-chami) | In vivo/50 g glucose | 60.00 ± 36.00 to 63.00 ± 19.36 | ≈2 months |
| 5. Phenol Content-GI | |||||||||
| Ramdath et al./2014 [62] | Canada | Caucasian Adults (healthy) | 9/9–3 males, 6 females | Lower phenol | Higher phenol | Pigmented potatoes (white-purple; red-yellow)-2 data | In vivo/50 g glucose | 77.00 ± 27.00 to 93.00 ± 51.00 78.00 ± 42.00 to 81.00 ± 48.00 |
≈15 days |
| Turco et al./2016 [65] | Italy | Adults (healthy) | 13- 2 males, 11 females | Lower phenol | Higher phenol | Cooked pasta wheat-bean | In vivo/50 g glucose | 40.00 ± 16.22 to 72.00 ± 28.84 | ≈15 days |
| 6. Flavonoid Content-GI | |||||||||
| Turco et al./2016 [65] | Italy | Adults (healthy) | 13/13- 2 males, 11 females | Lower flavonoid | Higher flavonoid | Cooked pasta wheat-bean | In vivo/50 g glucose | 40.00 ± 16.22 to 72.00 ± 28.84 | ≈15 days |
| Abubakar et al./2018 [66] | Malaysia | Rats weighing 160–200 g | 60 males Sprague-Dawley rats | Lower flavonoid | Higher flavonoid | Rice flour (germinated brown rice, brown rice, white rice)-3 data | In vivo/500 mg glucose | 67.60 ± 2.27 to 64.30 ± 3.51 67.70 ± 2.04 to 81.80 ± 2.70 65.60 ± 3.51 to 84.70 ± 2.81 |
≈5 weeks |
| Raghuvanshi et al./2017 [67] | India | Adults (healthy) | 10 | Lower flavonoid | Higher flavonoid | Cooked rice (red-white rice) | In vivo/50 g glucose | 71.70 ± 2.63 to 63.15 ± 0.91 | ≈15 days |
| Meera et al./2019 [68] | India | Adults (healthy) | 12 | Lower flavonoid | Higher flavonoid | Cooked rice (red-white rice) | In vivo/50 g glucose | 47.19 ± 11.09 to 69.74 ± 15.59 61.69 ± 13.86 to 56.27 ± 14.90 |
≈15 days |
| Yulianto et al./2018 [69] | Indonesia | Adults (healthy) | 12 | Lower flavonoid | Higher flavonoid | Cooked rice (cinnamon bark, pandan leaf, bay leaf extract)-3 data | In vivo/50 g glucose | 29.00 ± 8.65 to 32.00 ± 8.70 33.00 ± 13.70 to 40.00 ± 13.92 31.00 ± 8.06 to 37.00 ± 10.69 |
≈1 month |
| Nilsson et al./2008 [70] | Swedish | Adults (healthy) | 12/12–7 males, 5 females | Lower flavonoid | Higher flavonoid | Cooked cereal (rye-wheat; oat-barley)-2 data | In vivo/50 g glucose | 79.00 ± 45.03 to 73.00 ± 65.82 49.00 ± 24.25 to 85.00 ± 45.03 |
≈28 days |
| 7. Amylose Content-GI | |||||||||
| Nounmusig et al./2018 [71] | Thailand | Adults (healthy) | 22 22/9 |
Low amylose rice | Medium-high amylose rice | Cooked rice/White rice | In vivo/50 g glucose | 90.70 ± 36.00 to 66.10 ± 33.00 90.70 ± 36.00 to 63.80 ± 37.50 90.70 ± 36.00 to 66.20 ± 24.00 90.70 ± 36.00 to 54.60 ± 19.50 90.70 ± 36.00 to 48.10 ± 18.60 |
3 weeks |
| Trinidad et al./2014 [72] | Philippines | Adults (healthy) | 12 12/12 |
Low amylose rice | Medium-high amylose rice | Cooked rice/White rice-milled (3 data) and brown rice (3 data) | In vivo/50 g glucose | 69.00 ± 13.86 to 59.00 ± 10.39 85.00 ± 10.39 to 59.00 ± 10.39 94.00 ± 17.32 to 59.00 ± 10.39 61.00 ± 10.39 to 57.00 ± 10.39 69.00 ± 13.86 to 57.00 ± 10.39 77.00 ± 17.32 to 57.00 ± 10.39 |
≈27 days |
| Trinidad et al./2013 [8] | Philippines | Adults (healthy) | 10 10/10 |
Low amylose rice | Medium-high amylose rice | Cooked Rice/White Rice-milled (3 data) and brown rice (1 data) | In vivo/50 g glucose | 59.00 ± 12.65 to 50.00 ± 9.49 75.00 ± 12.65 to 63.00 ± 9.49 70.00 ± 12.65 to 57.00 ± 9.49 55.00 ± 6.32 to 51.00 ± 3.16 |
≈1 month |
| Lower-Higher Category RS [Selected low GI Carbohydrate] | |||||||||
| 1. Tubers Category | |||||||||
| Ek et al./2013 [45] | Australia | Adults (healthy) |
10 | Lower RS | Higher RS | Potato | In vivo/50 mg glucose | 82.00 ± 9.49 to 93.00 ± 31.62 | 12–20 days |
| Sari et al./2013 [73] | Indonesia | Rats Wistar 200–220 g age 2–3 months | 4 | Lower RS | Higher RS | Underutilized tubers | In vivo/50 g glucose | 22.40 ± 0.00 to 20.60 ± 0.49 | ≈12 days |
| 2. Fruits Category | |||||||||
| Hettiaratchi et al./2011 [56] | Sri Lanka | Adults (healthy) |
10 | Lower dietary fibre | Higher dietary fibre | Banana | In vivo/50 g white bread | 67.00 ± 22.14 to 69.00 ± 28.46 | ≈15 days |
Note: Explanation in intervention and control column stated that lower fat and higher fat or lower protein and higher protein; for example, Wolever et al. (2018) [54] and Ramdath et al. (2004) [58]. This means that the data used in the control are the data that have a proximate or bioactive compound analysis result (like fat, protein, phenolic, flavonoid, and others) that is higher than the data used in the intervention, such as in, e.g., Ramdath et al. (2004), who compared tannia and green banana. Green banana had higher protein content (2.7 g per serving food for glycemic index (GI) test) than tannia (2.6 g per serving food for GI test). The GI value of green banana is 109.00 ± 44.27, while that of tannia is 88.00 ± 28.46. Therefore, we input the GI value of green banana as the control data and the GI value of tannia as intervention data.
3.3. Primary Outcome
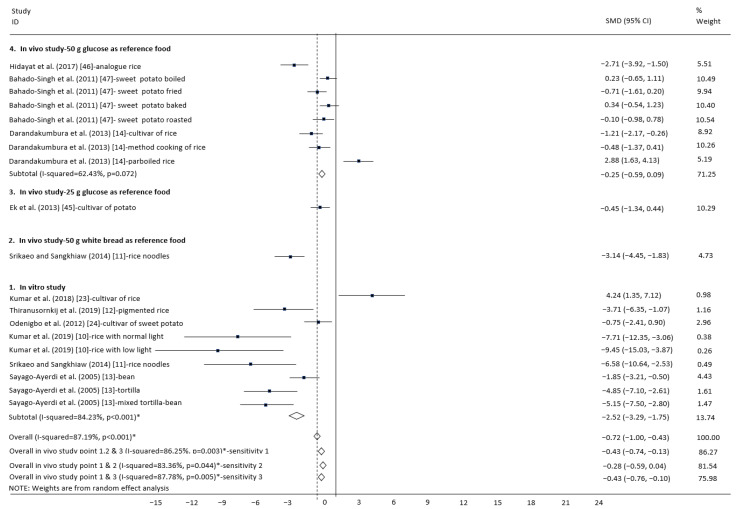
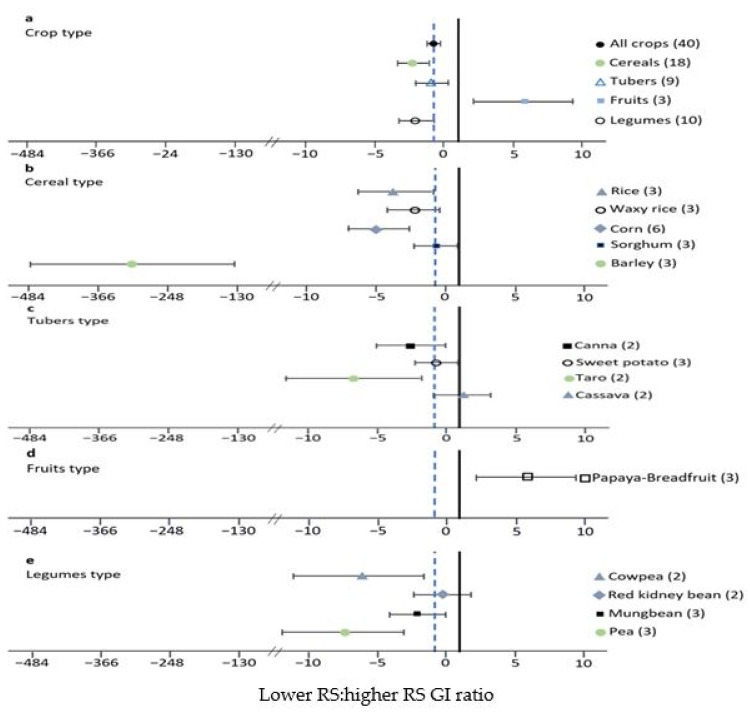
The primary outcome was the chemical properties determinant responsible for the GI of starchy foods and selected low-GI carbohydrate-based foods in the forest plots and funnel plots graphic (Figure 4, Figure 5a, Figure 6, and Figure 7a). All studies (a total of 936 participants) provided data on the determinant of chemical properties (912 participants) and selected low-GI carbohydrate-based foods (twenty-four participants). Compared to other chemical properties, resistant starch and phenolic content significantly reduced the GI value of starchy foods (respectively, SMD: −2.52, 95%CI: −3.29 to −1.75, p < 0.001; SMD: −0.72, 95%CI: −1.26 to −0.17, p = 0.005) with heterogeneity (respectively, I2 = 84.23%, p < 0.001; I2 = 73.64%, p = 0.005) (Table 2, Table 4, and Table 5). The lowest GI crop type is legumes (SMD: −2.15, 95%CI: −3.45 to −0.85, p < 0.001) with heterogeneity (I2 = 72.97%, p < 0.001) (Figure 7). The heterogeneity among these studies may result from discrepancies in the population and control group.
Figure 4.
Subgroup analysis of the glycemic index according to the differing resistant starch content in carbohydrate foods. Standardized mean difference (SMD), confidence interval (CI), and point represent the estimated overall effect size (with 95%CIs) for each study. Positive values indicate relatively higher resistant starch content in carbohydrate foods. Negative values indicate relatively lower resistant starch content in carbohydrate foods.
Figure 5.
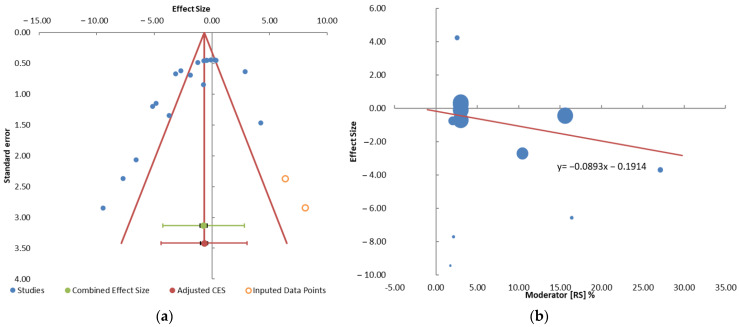
(a) The funnel plot of the effect of differing resistant starch content in carbohydrate foods-to-GI; (b) the meta-regression between resistant starch content (%) and effect size.
Figure 6.
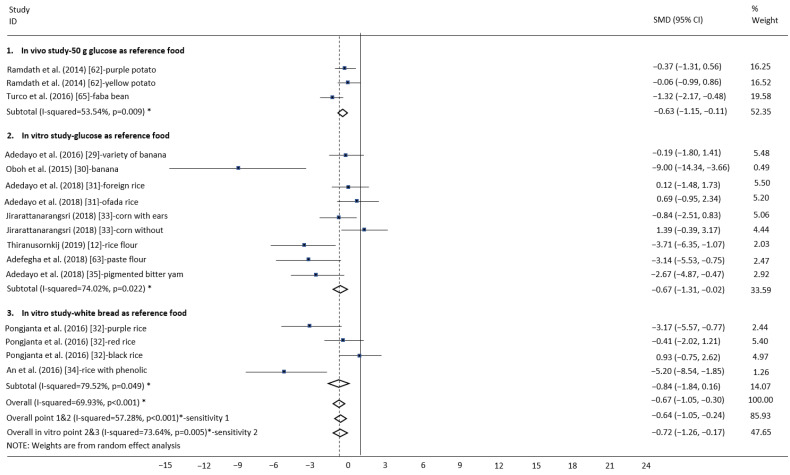
Subgroup analysis of the glycemic index according to the differing phenolic content in carbohydrate foods. Standardized mean difference (SMD), confidence interval (CI), and point represent the estimated overall effect size (with 95%CIs) for each study. Positive values indicate relatively higher phenolic content in carbohydrate foods. Negative values indicate relatively lower phenolic content in carbohydrate foods.
Figure 7.
Influence of different crop type (a), cereal type (b), tuber type (c), fruit type (d), and legume type (e) on lower-RS to higher-RS glycemic index ratio in in vitro studies. Forest plot of cumulative effect size and 95% confidence interval (CI) of different crop types comparing lower RS and higher RS on GI. Bold lines indicate the robust model. Only crop type was represented by at least seventeen observations. Values are mean effect sizes with 95% confidence intervals. The number of studies in each class is written in parentheses. The dotted line shows the cumulative effect size across all classes.
Table 4.
Chemical properties of starchy foods determined to affect the GI in in vitro studies.
| No. | Chemical Properties | n | I2 | p-Value | Significancy One Tailed α 5% | d+ | % (Δ to 1 Value) | Determinant Order |
|---|---|---|---|---|---|---|---|---|
| 1 | RS content | 9 | 84.23 | <0.001 | significant | −2.52 | 352 | 1 |
| 2 | Dietary fibre content | 3 | 91.20 | 0.348 | not significant | −0.37 | 137 | 4 |
| 3 | Fat content | 3 | 86.39 | 0.078 | not significant | 0.91 | 9 | 6 |
| 4 | Protein content | 3 | 90.80 | 0.251 | not significant | −0.51 | 151 | 3 |
| 5 | Phenolic content | 13 | 73.64 | 0.005 | significant | −0.72 | 172 | 2 |
| 6 | Flavonoid content | 3 | 71.74 | 0.309 | not significant | −0.26 | 126 | 5 |
Table 5.
Selected low-GI carbohydrate foods using meta-analysis in in vitro studies.
| No. | Item | n | I2 (%) | p-Value | Significancy One Tailed α 5% | % Weight | CI | GI Value from Study [4,21,22] | Selected Low GI Carbohydrate Foods |
|---|---|---|---|---|---|---|---|---|---|
| Crop Type | |||||||||
| 1 | All crops | 40 | 86.35 | <0.001 | significant | 100.00 | 0.44 | - | - |
| Cereals | 11.67 | 1.03 | 56.44 | - | |||||
| Tubers | 18.58 | 0.68 | 55.97 | - | |||||
| Fruits | 42.79 | 0.85 | 50.76 | - | |||||
| Legumes | 26.96 | 1.30 | 41.94 | √ | |||||
| Cereal Type | |||||||||
| 2 | Rice | 18 | 81.41 | <0.001 | significant | 15.13 | 2.64 | 67.9 | - |
| Waxy rice | 25.12 | 2.05 | 79 | - | |||||
| Corn | 20.88 | 2.25 | 48 | - | |||||
| Sorghum | 38.87 | 1.65 | 32 | √ | |||||
| Barley | 0.00 | 175 | 40 | - | |||||
| Tuber Type | |||||||||
| 3 | Canna | 9 | 51.59 | 0.076 | not significant | 18.63 | 2.64 | 19.87 | - |
| Sweet potato | 47.45 | 1.66 | 70 | ||||||
| Taro | 5.24 | 4.99 | 63.1 | ||||||
| Cassava | 28.68 | 2.13 | 78.7 | ||||||
| Fruit Type | |||||||||
| 4 | Papaya-Breadfruit | 3 | - | <0.001 | significant | 100.00 | 3.63 | 64.5 | - |
| Legume Type | |||||||||
| 5 | Cowpea | 10 | 72.97 | <0.001 | significant | 7.57 | 4.71 | 41 | - |
| Red bean | 42.84 | 1.98 | 26 | √ | |||||
| Mungbean | 41.20 | 2.02 | 76 | - | |||||
| Pea | 8.39 | 4.47 | 35 | - | |||||
3.4. Subgroup Analysis
Crop species, i.e., sorghum and red kidney bean, had low GI (respectively, SMD: −0.69, 95%CI: −2.33 to 0.96, p < 0.001; SMD: −0.39, 95%CI: −2.37 to 1.59, p = 0.001), with heterogeneity (respectively, I2 = 81.4%, p < 0.001; I2 = 73.0%, p = 0.001). All results of subgroup analyses are presented in Table 5 and Figure 7.
3.5. Secondary Outcomes
The contribution of six chemical properties to GI and five source types of carbohydrates to GI can be seen in Table 4 and Table 5. Forty-eight studies [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54] reported on the relationship between chemical properties and GI, while nine studies reported on the source types of carbohydrates to GI, respectively. Compared to other chemical properties of starchy foods or the source of carbohydrate-based foods, fat content did not reduce GI (SMD: 0.05, 95%CI: −0.16 to 0.27, p = 0.312; I2 = 93.64%), as was also found in tuber type (SMD: −0.25, 95%CI: −0.93 to 0.43, p = 0.233; I2 = 79.4%) or fruit type (SMD: 0.25, 95%CI: −0.60 to 1.10, p = 0.284; I2 = 89.3%).
3.6. Strength of Evidence and Publication Bias
The quality of evidence was evaluated by the GRADE system. The level of evidence of RS content was at level A and highly recommended. Phenol content and legume type were at level B and moderately recommended. All evidence profiles for the primary and secondary outcomes are provided in Table 5. For the meta-analysis of RS content to GI food, any publication bias was observed by Begg’s test and Egger’s test (Begg’s, p = 0.020; Egger’s, p = 0.004) (Figure 5a). For phenol content on GI food, any publication bias was observed by Begg’s test and Egger’s test (Begg’s, p = 0.001; Egger’s, p = 0.008). For the meta-analysis of legume type on GI food, any publication bias was observed by Begg’s test and Egger’s test (Begg’s, p = 0.087; Egger’s, p = 0.077).
3.7. In Vitro Laboratory Simulation Experiments
Compared to other chemical properties, resistant starch and phenolic content significantly reduced the GI value of starchy foods (respectively, SMD: −2.52, 95%CI: −3.29 to −1.75, p < 0.001; SMD: −0.72, 95%CI: −1.26 to−0.17, p = 0.005), with heterogeneity (respectively, I2 = 84.23%, p < 0.001; I2 = 73.64%, p = 0.005) (Table 2, Table 4, and Table 5). The lowest GI crop type is legumes (SMD: −2.15, 95%CI: −3.45 to −0.85, p < 0.001), with heterogeneity (I2 = 72.97%, p < 0.001) (Figure 7). The heterogeneity among these studies may result from discrepancies in the population and control group. Crop species, i.e., sorghum and red kidney bean had low GI (respectively, SMD: −0.69, 95%CI: −2.33 to 0.96, p < 0.001; SMD: −0.39, 95%CI: −2.37 to 1.59, p = 0.001), with heterogeneity (respectively, I2 = 81.4%, p < 0.001; I2 = 73.0%, p = 0.001) (Table 5 and Figure 7).
3.8. In Vivo Experiments
In all in vivo studies, compared to other chemical properties, flavonoid and phenolic content significantly reduced the GI value of starchy foods (respectively, SMD: −0.67, 95%CI: −0.87 to −0.47, p < 0.001; SMD: − 0.63, 95%CI: −1.15 to−0.11, p = 0.009), with heterogeneity (respectively, I2 = 97.34%; I2 = 53.54%) (Table 3, Table 6, and Table 7). In in vivo studies with 50 g glucose as the reference food, compared to other chemical properties, phenolic and flavonoid content significantly reduced the GI value of starchy foods (respectively, SMD: −0.63, 95%CI: −1.15 to −0.11, p = 0.009; SMD: −0.42, 95%CI: −0.72 to −0.12, p = 0.003), with heterogeneity (respectively, I2 = 53.54%; I2 = 87.50%) (Table 6). The lowest GI crop type is fruit (banana) (SMD: −0.07, 95%CI: −0.95 to 0.80, p = 0.433) (Table 3 and Table 7).
Table 6.
Chemical properties of starchy foods determined to affect the GI in in vivo studies with 50 g glucose as the reference food.
| No. | Chemical properties | n | I2 | p Value | Significancy One Tailed α 5% | d+ | % (Δ to 1 Value) | Determinant Order |
|---|---|---|---|---|---|---|---|---|
| 1 | RS content | 8 | 62.43 | 0.072 | not significant | −0.25 | 125 | 3 |
| 2 | Dietary fibre content | 8 | 89.55 | 0.041 | significant | −0.23 | 123 | 4 |
| 3 | Fat content | 7 | 79.06 | 0.033 | significant | 0.23 | 77 | 6 |
| 4 | Protein content | 8 | 0.00 | <0.001 | significant | −0.13 | 113 | 5 |
| 5 | Phenolic content | 3 | 53.54 | 0.009 | significant | −0.63 | 163 | 1 |
| 6 | Flavonoid content | 7 | 87.50 | 0.003 | significant | −0.42 | 142 | 2 |
Table 7.
Selected low-GI carbohydrate foods using meta-analysis in in vivo studies.
| No | Item | n | I2 (%) |
p-Value | Significancy One Tailed α 5% | % Weight | CI | GI Value from Study [4,21,22] | Selected Low GI Carbohydrate Foods |
|---|---|---|---|---|---|---|---|---|---|
| Crop Type | |||||||||
| 1 | All crops | 24 | 86.35 | 0.413 | not significant | 100.00 | 0.53 | - | - |
| Tubers | 61.35 | 0.68 | 56.44 | - | |||||
| Fruits | 38.65 | 0.85 | 50.76 | - | |||||
| Tuber Type | |||||||||
| 2 | Potato | 14 | 59.76 | 0.440 | not significant | 89.56 | 0.89 | 82 | - |
| Underutilized tuber | 10.44 | 2.60 | - | - | |||||
| Fruit Type | |||||||||
| 3 | Banana | 10 | - | 0.433 | not significant | 100.00 | 0.88 | 52.78 | - |
4. Discussion
4.1. Relationship between Food Chemical Properties and GI
This work systematically reviewed the current accessible literature and found that, in general, among the various chemical properties, the presence of resistant starch and phenolic compounds exerted significant impacts on the GI of starchy foods. It is noteworthy that evidence of this finding was consistent with the previous study. Moreover, some chemical properties show an essential impact on the GI beyond those mentioned, i.e., flavonoid, protein, dietary fibre, and amylose, which were negatively correlated with GI. Further, we found that the crop type with the lowest GI value was legumes. Subgroup analysis revealed that significant low-GI crop species were sorghum and red kidney bean, while the subgroup analysis was restricted to trials that compared some crop species. This may relate to a larger quantity of RS and phenolic compounds in the crop species.
No meta-analysis has been conducted on the effect of resistant starch levels on starchy food’s GI. Previous researchers stated that the relationship between resistant starch levels and GI is a negative correlation [19]. Resistant starch is starch that cannot be digested by the small intestine within 120 min after consumption but that the large intestine can ferment. Resistant starch is a linear molecule of α-1,4-d-glucan, which is obtained mainly from the retrogradation of the amylose fraction and has a relatively low molecular weight (1.2 × 105 Da) [74]. The results of a previous meta-analysis showed that resistant starch can reduce blood sugar levels and fasting insulin [9]. The same thing can be seen from the results of the meta-regression between resistant starch levels and effect size (Figure 5b), which has a negative slope (−0.09). The mechanism for decreasing GI is that resistant starch cannot be digested by digestive enzymes due to its compact molecular structure, such that there is no increase in blood sugar levels [74].
Furthermore, meta-analysis has not been conducted on the effect of dietary fibre levels on starchy foods’ GI. Previous researchers stated that the relationship between dietary fibre levels and GI is negatively correlated [6,35]. The same thing can be seen from the result of the meta-regression between dietary fibre content and effect size, which has a negative slope equal to −0.11. The mechanism of action of dietary fibre to reduce GI is by slowing down the rate of digestion of starch and increasing the duration of intestinal transit so that dietary fibre serves as a physical barrier in digestion in the intestine, thus slowing down the interaction between enzymes and substrates. In addition, the degree of viscosity of the dietary fibre is positively related to the extent of the flattening of the postprandial glucose response [75].
Meta-analysis of the effect of fat content on starchy foods’ GI has not been carried out. Previous researchers stated that the relationship of fat content to GI is negatively correlated [76]. The same thing can be seen from the results of the meta-regression between fat content and effect size, which has a negative slope as much as −0.07. The mechanism of GI reduction is that fat slows the rate at which the stomach empties, creates a steric hindrance for the enzyme [77], and interacts with amylose to form a very strong matrix, named amylo-lipid, which digestive enzymes have trouble digesting [75].
In addition, meta-analysis of the effect of protein levels on starchy foods’ GI has not been done. Previous researchers stated that the relationship between protein levels and GI is negatively correlated [55]. The mechanism for decreasing GI is that a protein allegedly stimulates insulin secretion so that blood glucose is not excessive and is under control [78]. However, the meta-regression outcome between protein levels and effect size shows a different result, which has a positive slope of 0.02.
No meta-analysis has been conducted on the effect of phenol levels on starchy foods’ GI. Previous researchers stated that the relationship between phenol levels and GI is negatively correlated [79]. The same can be seen from the result of the meta-regression between phenol levels and effect size, which has a negative slope, as much as −0.0001. The mechanism of GI reduction is that phenol inhibits the α-amylase enzyme and the α-glucosidase enzyme [80].
No meta-analysis has been conducted on the effect of flavonoid levels on starchy foods’ GI. Previous researchers stated that the relationship between flavonoid levels and GI is negatively correlated [66]. The same can be seen from the result of the meta-regression between fat content and effect size, which has a negative slope, as much as −0.0002. The mechanism of GI reduction is that flavonoids inhibit the α-amylase and α-glucosidase enzymes [80].
4.2. Comparability of In Vitro Results to Forecast In Vivo Correlations to Chemical Properties and GI
This study used both the in vivo and in vitro methods. This consideration was based on previous studies in which in vitro test results had the same trend as in vivo tests, though in vitro tests tended to have absolute values (overestimation) about 5–25 higher than those of in vivo tests [11,81,82]. That gap was strengthened by the results of the absolute value of SMD chemical properties towards the GI of food, which, in vitro, has a higher value compared to in vivo (Table 4 and Table 6). Other considerations are several theories stating that at least 10 studies must be carried out in a meta-analysis. In the analysis, subgroup analysis and sensitivity analysis are performed. Subgroup analysis was performed based on the variable type of study and reference food. This can provide an overview of two aspects if both methods are used and if only the in vivo method with 50 g glucose reference food os used (Table 6). For in vivo tests, we used and compared only those using 50 g glucose reference food. This is done because the results show that if the reference food is 50 g of white bread or white rice, it is necessary to first convert the GI value with multiplier factors, respectively, 0.77 and 0.69 [83]. If the reference food used is 25 g glucose, different results would be obtained, which would need to be multiplied by 0.67 as a conversion factor [84].
For in vivo food model simulation, we suggest some recommendations. First, our study found that RS and phenolic content had positive effects on the reduction of the GI value of starchy foods. Such a correlation is stable and reliable. Thus, RS and phenolic content should be recommended for the chemical properties of starchy foods determined to affect the GI value. Second, to date, little attention has been paid to the study of the chemical properties determinant that affects the glycemic index of starchy foods and selected low-GI carbohydrates using meta-analysis. The determinant factor discussed in this study can be a meaningful direction for further research. Finally, comprehensive in vivo trials are warranted to validate [17] the positive impact of these findings.
4.3. Determinant of Chemical Properties Affecting the GI and Low-GI Carbohydrate Foods
In our study, the determinant effect of RS or phenolic content on GI and legume, as the selected low-GI carbohydrate food, was in accordance with the previous meta-analysis [9]. Nevertheless, differences between our study and the previous analysis should be noted. First, the previous meta-analysis included thirteen trials with the involvement of 428 participants for the RS effect, while for phenolic content to GI, no meta-analysis research was found until now. However, Ramdath et al. (2014) [62] asserted that there was a significant inverse correlation between polyphenol content and the GI of potatoes (r = −0.825; p < 0.05; n = 4). In the case of the relation between legume and GI, the previous study using meta-analysis included forty trials totaling 253 participants. We included ninety-eight trials, and added subgroup analysis based on chemical properties and the source of starchy foods according to the control group, enabling us to reach a more robust conclusion by eliminating interference factors. Our meta-analysis found that heterogeneity among trials was due mainly to the design of different control group, rather than population. In addition, we assessed the quality of the evidence and the strength of the recommendations. Thus, our work was the latest and most comprehensive one.
Low-GI foods, such as legumes, had the lowest GI compared to other carbohydrate foods because legumes have relatively higher levels of resistant starch and bioactive compounds. Beans’ resistant starch levels were 24.7% [74]. In the cereal group, red sorghum had the lowest GI (Table 5), apparently due to the higher content of bioactive compounds and resistant starch compared to other cereal groups. Red sorghum’s resistant starch levels ranged from 3.34 to 65.36 g/100 g [85]. The phenol compound contained in sorghum is 445–2850 μg/g [86]. In the legumes, red beans had the lowest GI (Table 5), presumably due to the higher content of bioactive compounds and resistant starch compared to other legumes. The resistant starch content of red bean starch is 21.27% [87], while the total phenol content can reach 4871 mg gallic acid equivalents (GAE)/100 g dry weight [88]. Previous studies [89] only determined the glycemic index of various staple carbohydrate-rich foods in the UK diet in five groups, such as breakfast cereals, breads, pastas, and potatoes using ten subjects.
Our study also has limitations. Though this meta-analysis includes high-quality studies, the sample sizes are small. Additionally, there is enough heterogeneity among studies, which could alter the reliability of the results. Trials with higher-quality samples and larger sample sizes are required to confirm the current results.
4.4. Critical Review GI as Indicator for Classifying Healthy Foods and the Alternative Concept
Although the concept of GI is widely used in explaining the causes of diabetes, some scientists consider that GI is not accurate enough to explain this. The concept of GI is considered inappropriate to classify a food as healthy or not or to describe its impact on human health. Several aspects of criticism of the GI concept include reproducibility, its impact on physiological effects, and levels and standards of the reference food used [90]. Therefore, it is necessary to use other indicators related to the character of carbohydrates besides digestibility, such as the types of food fibre found in foodstuffs and the levels of bioactive compounds contained therein [91]. Substitutes for the concept of GI proposed by health nutrition researchers include a new method for classifying starch digestion by modeling amylolysis of plant foods using first-order enzyme kinetic principles. This research opens new horizons and supports the relationship between levels of resistant starch, dietary fibre, phenolic, flavonoids, and the value of food GI.
The results evidenced that resistant starch and phenolic content reduce the GI value of starchy foods. As regards in vitro studies, it is well known that resistant starch is negatively correlated to GI, but the results obtained for the phenolic content in this systematic review were not obvious, even though part of phenolics were bound to fibre compounds known to reduce the digestion in the flattening of postprandial glucose response. Moreover, phenols inhibit the α-amylase and α-glucosidase enzymes. Among cereals, sorghum—a gluten free cereal—is the only one that reveals high-resistant starch and low GI. This is a very interesting result due to the fact that gluten-free foods generally are low in fibre and high in GI. This finding could be useful to investigate the potential of sorghum as gluten-free products.
5. Conclusions
The present work successfully elucidated that resistant starch, phenolic, flavonoid, protein, and dietary fibre content exerted crucial roles in reducing the glycemic index of starchy foods. Among the starchy foods, sorghum and red kidney bean were identified to have low-GI properties. Sorghum and kidney beans have a low GI because both contain relatively high resistant starch and phenolic compounds. The relationship between levels of resistant starch, phenolic, flavonoids, protein, and fibre to GI was a negatively correlation. Resistant starch causes steric hindrance in the molecular structure of the starch, while phenolic compounds (including flavonoids) are capable of inhibiting the α-amylase and α-glucosidase enzymes. The mode of action of resistant starch in reducing GI is making the enzyme unable to hydrolyze and disrupting the hydrolysis on non-resistant starch (which creates steric hindrance). Nevertheless, microbes ferment the resistant starch in the colon so that the body will not absorb it as glucose. Protein is supposed to stimulate the secretion of insulin so that blood glucose is not excessive and can be controlled. The fibre functions as an inhibitor of physical digestion in the intestine, thereby slowing down the interactions between enzymes with the substrates.
Acknowledgments
The authors are grateful to DPIS (Direktorat Publikasi Ilmiah dan Informasi Strategis), IPB University for providing technical support during the writing of this manuscript.
Author Contributions
Conceptualization—F.A.A., C.H.W., D.N.F., N.E.S., and A.J.; Formal analysis—F.A.A., C.H.W., D.N.F., N.E.S., and A.J.; Data curation—F.A.A., C.H.W., D.N.F., N.E.S., and A.J.; Investigation—F.A.A., C.H.W., D.N.F., N.E.S., and A.J.; Methodology— F.A.A., D.N.F., and A.J.; Supervision—C.H.W., D.N.F., N.E.S., and A.J.; Validation— D.N.F., C.H.W., D.N.F., N.E.S., and A.J.; Visualization—F.A.A., C.H.W., D.N.F., N.E.S. and A.J.; Writing—original draft, F.A.A., C.H.W., D.N.F., N.E.S., and A.J.; Project administration— F.A.A. and D.N.F.; Resources— F.A.A., C.H.W., D.N.F., N.E.S. and A.J.; Writing—review & editing, F.A.A., C.H.W., D.N.F., N.E.S. and A.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Venn B.J., Green T.J. Glycemic index and glycemic load: Measurement issues and their effect on diet–disease relationships. Eur. J. Clin. Nutr. 2007;61:S122–S131. doi: 10.1038/sj.ejcn.1602942. [DOI] [PubMed] [Google Scholar]
- 2.ISO . International Standard International Standard. Volume 26642:2010. The International Organization for Standardization; Geneva, Switzerland: 2010. [Google Scholar]
- 3.Diederichs T., Herder C., Roßbach S., Roden M., Wudy S.A., Nöthlings U., Alexy U., Buyken A.E. Carbohydrates from sources with a higher glycemic index during adolescence: Is evening rather than morning intake relevant for risk markers of type 2 diabetes in young adulthood? Nutrients. 2017;9:591. doi: 10.3390/nu9060591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson S.V., Winham D.M., Hutchins A.M. Bean and rice meals reduce postprandial glycemic response in adults with type 2 diabetes: A cross-over study. Nutr. J. 2012;11:1. doi: 10.1186/1475-2891-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giri S., Banerji A., Lele S.S., Ananthanarayan L. Starch digestibility and glycaemic index of selected Indian traditional foods: Effects of added ingredients. Int. J. Food Prop. 2017;20:S290–S305. doi: 10.1080/10942912.2017.1295387. [DOI] [Google Scholar]
- 6.Jenkins D.J.A., Kendall C.W.C., Augustin L.S.A., Mitchell S., Sahye-Pudaruth S., Blanco Mejia S., Chiavaroli L., Mirrahimi A., Ireland C., Bashyam B., et al. Effect of legumes as part of a low glycemic index diet on glycemic control and cardiovascular risk factors in type 2 diabetes mellitus: A randomized controlled trial. Arch. Intern. Med. 2012;172:1653–1660. doi: 10.1001/2013.jamainternmed.70. [DOI] [PubMed] [Google Scholar]
- 7.Omage K., Omage S.O. Evaluation of the glycemic indices of three commonly eaten mixed meals in Okada, Edo State. Food Sci. Nutr. 2018;6:220–228. doi: 10.1002/fsn3.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trinidad T.P., Mallillin A.C., Encabo R.R., Sagum R.S., Felix A.D., Juliano B.O. The effect of apparent amylose content and dietary fibre on the glycemic response of different varieties of cooked milled and brown rice. Int. J. Food Sci. Nutr. 2013;64:89–93. doi: 10.3109/09637486.2012.700922. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y., Chen J., Song Y.H., Zhao R., Xia L., Chen Y., Cui Y.P., Rao Z.Y., Zhou Y., Zhuang W., et al. Effects of the resistant starch on glucose, insulin, insulin resistance, and lipid parameters in overweight or obese adults: A systematic review and meta-analysis. Nutr. Diabetes. 2019;9:1–11. doi: 10.1038/s41387-019-0086-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar A., Panda D., Biswal M., Dey P., Behera L., Baig M.J., Nayak L., Ngangkham U., Sharma S. Low Light Stress Influences Resistant Starch Content and Glycemic Index of Rice (O. sativa L) Starch Staerke. 2019;71 doi: 10.1002/star.201800216. [DOI] [Google Scholar]
- 11.Srikaeo K., Sangkhiaw J. Effects of amylose and resistant starch on glycaemic index of rice noodles. LWT Food Sci. Technol. 2014;59:1129–1135. doi: 10.1016/j.lwt.2014.06.012. [DOI] [Google Scholar]
- 12.Thiranusornkij L., Thamnarathip P., Chandrachai A., Kuakpetoon D., Adisakwattana S. Comparative studies on physicochemical properties, starch hydrolysis, predicted glycemic index of Hom Mali rice and Riceberry rice flour and their applications in bread. Food Chem. 2019;283:224–231. doi: 10.1016/j.foodchem.2019.01.048. [DOI] [PubMed] [Google Scholar]
- 13.Sáyago-Ayerdi S.G., Tovar J., Osorio-Díaz P., Paredes-López O., Bello-Pérez L.A. In vitro starch digestibility and predicted glycemic index of corn tortilla, black beans, and tortilla-bean mixture: Effect of cold storage. J. Agric. Food Chem. 2005;53:1281–1285. doi: 10.1021/jf048652k. [DOI] [PubMed] [Google Scholar]
- 14.Darandakumbura H.D.K., Wijesinghe D.G.N.G., Rohitha Prasantha B.D. Sri Lanka Effect of Processing Conditions and Cooking Methods on Resistant Starch, Dietary Fibre and Glycemic Index of Rice. Trop. Agric. Res. 2013;24:163–174. [Google Scholar]
- 15.Borenstein M., Hedges L.V., Higgins J.P.T., Rothstein H.R. Introduction to Meta-Analysis. John Wiley & Sons Ltd.; Hoboken, NJ, USA: 2009. Fixed-Effect Versus Random-Effects Models; pp. 77–86. [Google Scholar]
- 16.Viswanathan M., Ansari M.T., Berkman N.D., Chang S., Hartling L., McPheeters M., Santaguida P.L., Shamliyan T., Singh K., Tsertsvadze A., et al. Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions. Agency for Healthcare Research and Quality; Rockville, MD, USA: 2012. [PubMed] [Google Scholar]
- 17.Fu S., Li L., Deng S., Zan L., Liu Z. Effectiveness of advanced carbohydrate counting in type 1 diabetes mellitus: A systematic review and meta-analysis. Sci. Rep. 2016;6:1–8. doi: 10.1038/srep37067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guyatt G.H. Guyatt 2008—What is “quality of evidence” and why is it important to clinicians. BMJ. 2008;336:995–998. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldet G., Howick J. Understanding GRADE: An introduction. J. Evid. Based Med. 2013;6:50–54. doi: 10.1111/jebm.12018. [DOI] [PubMed] [Google Scholar]
- 20.Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Alonso-Coello P., Schünemann H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. Chin. J. Evid. Based Med. 2009;9:8–11. doi: 10.1136/bmj.39489.470347.AD. [DOI] [Google Scholar]
- 21.Prasad M.P.R., Rao B.D., Kalpana K., Rao M.V., Patil J.V. Glycaemic index and glycaemic load of sorghum products. J. Sci. Food Agric. 2015;95:1626–1630. doi: 10.1002/jsfa.6861. [DOI] [PubMed] [Google Scholar]
- 22.Chandrasekara A., Josheph Kumar T. Roots and tuber crops as functional foods: A review on phytochemical constituents and their potential health benefits. Int. J. Food Sci. 2016;2016 doi: 10.1155/2016/3631647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar A., Sahoo U., Baisakha B., Okpani O.A., Ngangkham U., Parameswaran C., Basak N., Kumar G., Sharma S.G. Resistant starch could be decisive in determining the glycemic index of rice cultivars. J. Cereal Sci. 2018;79:348–353. doi: 10.1016/j.jcs.2017.11.013. [DOI] [Google Scholar]
- 24.Odenigbo A., Rahimi J., Ngadi M., Amer S., Mustafa A. Starch digestibility and predicted glycemic index of fried sweet potato cultivars. Funct. Foods Health Dis. 2012;2:280. doi: 10.31989/ffhd.v2i7.83. [DOI] [Google Scholar]
- 25.Vahini J., Bhaskarachary K., Rao M.V.V. Effect of cooking on glycemic index of commonly consumed corn in inda. Int. J. Food Nutr. Sci. 2017;6:25–30. [Google Scholar]
- 26.Kim H.J., White P.J. In vitro digestion rate and estimated glycemic index of oat flours from typical and high β-glucan oat lines. J. Agric. Food Chem. 2012;60:5237–5242. doi: 10.1021/jf300429u. [DOI] [PubMed] [Google Scholar]
- 27.Amin T., Naik H.R., Hussain S.Z., Mir M.A., Jabeen A. In vitro digestion, physicochemical and morphological properties of low glycemic index rice flour prepared through enzymatic hydrolysis. Int. J. Food Prop. 2018;21:2632–2645. doi: 10.1080/10942912.2018.1545789. [DOI] [Google Scholar]
- 28.Klunklin W., Savage G. Physicochemical, antioxidant properties and in vitro digestibility of wheat–purple rice flour mixtures. Int. J. Food Sci. Technol. 2018;53:1962–1971. doi: 10.1111/ijfs.13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adedayo B.C., Oboh G., Oyeleye S.I., Olasehinde T.A. Antioxidant and Antihyperglycemic Properties of Three Banana Cultivars (Musa spp.) Scientifica. 2016;2016:1–5. doi: 10.1155/2016/8391398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oboh G., Ademosun A.O., Akinleye M., Omojokun O.S., Boligon A.A., Athayde M.L. Starch composition, glycemic indices, phenolic constituents, and antioxidative and antidiabetic properties of some common tropical fruits. J. Ethn. Foods. 2015;2:64–73. doi: 10.1016/j.jef.2015.05.003. [DOI] [Google Scholar]
- 31.Adedayo B.C., Adebayo A.A., Nwanna E.E., Oboh G. Effect of cooking on glycemic index, antioxidant activities, α-amylase, and α-glucosidase inhibitory properties of two rice varieties. Food Sci. Nutr. 2018;6:2301–2307. doi: 10.1002/fsn3.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pongjanta J., Chomsri N.-O., Meechoui S. Correlation of pasting behaviors with total phenolic compounds and starch digestibility of indigenous pigmented rice grown in upper Northern Thailand. Funct. Foods Health Dis. 2016;6:133–143. doi: 10.31989/ffhd.v6i3.231. [DOI] [Google Scholar]
- 33.Jirarattanarangsri W. The Effect of Traditional Thermal Cooking Processes on Anthocyanins, Total Phenolic Content, Antioxidant Activities and Glycemic Index in Purple Waxy Corn. Food Appl. Biosci. J. 2018;6:154–166. [Google Scholar]
- 34.An J.S., Bae I.Y., Han S.I., Lee S.J., Lee H.G. In vitro potential of phenolic phytochemicals from black rice on starch digestibility and rheological behaviors. J. Cereal Sci. 2016;70:214–220. doi: 10.1016/j.jcs.2016.06.010. [DOI] [Google Scholar]
- 35.Adedayo B., Okeke B.M., Oyeleye S.I., Oboh G. Effects of boiling on the estimated Glycemic Index (eGI) and α- amylase/α-glucosidase inhibitory properties of two Bitter Yam (Dioscorea dumetorum) spp. Biokemistri. 2018;30:21–31. [Google Scholar]
- 36.Frei M., Siddhuraju P., Becker K. Studies on the in vitro starch digestibility and the glycemic index of six different indigenous rice cultivars from the Philippines. Food Chem. 2003;83:395–402. doi: 10.1016/S0308-8146(03)00101-8. [DOI] [Google Scholar]
- 37.Lemlioglu-austin D., Turner N.D., Mcdonough C.M. Effects of Sorghum [Sorghum bicolor (L.) Moench] Crude Extracts on Starch Digestibility, Estimated Glycemic Index (EGI), and Resistant Starch (RS) Contents of Porridges. Molecules. 2012;17:11124–11138. doi: 10.3390/molecules170911124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen R., Zang W., Dong J. Preparation, structural characteristics and digestibility of resistant starches from highland barley, oats and buckwheat starches. J. Food Nutr. Res. 2016;55:303–312. [Google Scholar]
- 39.Srikaeo K., Mingyai S., Sopade P.A. Physicochemical properties, resistant starch content and enzymatic digestibility of unripe banana, edible canna, taro flours and their rice noodle products. Int. J. Food Sci. Technol. 2011;46:2111–2117. doi: 10.1111/j.1365-2621.2011.02724.x. [DOI] [Google Scholar]
- 40.Simsek S., El S.N. Production of resistant starch from taro (Colocasia esculenta L. Schott) corm and determination of its effects on health by in vitro methods. Carbohydr. Polym. 2012;90:1204–1209. doi: 10.1016/j.carbpol.2012.06.039. [DOI] [PubMed] [Google Scholar]
- 41.Ratnaningsih N., Harmayani E., Marsono Y. In vitro Starch Digestibility and Estimated Glycemic Index of Indonesian Cowpea Starch (Vigna unguiculata) Pak. J. Nutr. 2017;16:1–8. doi: 10.3923/pjn.2017.1.8. [DOI] [PubMed] [Google Scholar]
- 42.Chung H., Liu Q., Pauls K.P., Fan M.Z., Yada R. In vitro starch digestibility, expected glycemic index and some physicochemical properties of starch and flour from common bean (Phaseolus vulgaris L.) varieties grown in Canada. Food Res. Int. 2008;41:869–875. doi: 10.1016/j.foodres.2008.03.013. [DOI] [Google Scholar]
- 43.Eashwarage I.S. Dietary fibre, resistant starch and in-vitro starch digestibility of selected elevencommonly consumed legumes (Mung bean, Cowpea, Soybean and Horse Gram) in Sri Lanka. Res. J. Chem. Sci. 2017;7:27–33. [Google Scholar]
- 44.Sandhu K.S., Lim S. Digestibility of legume starches as influenced by their physical and structural properties. Carbohydr. Polym. 2008;71:245–252. doi: 10.1016/j.carbpol.2007.05.036. [DOI] [Google Scholar]
- 45.Ek K.L., Wang S., Copeland L., Brand-Miller J.C. Discovery of a low-glycaemic index potato and relationship with starch digestion in vitro. Br. J. Nutr. 2014;111:699–705. doi: 10.1017/S0007114513003048. [DOI] [PubMed] [Google Scholar]
- 46.Hidayat B., Akmal S., Muslihudin M., Suhada B. Assessment of Corn-Based Rice Analogues Made from Modified Corn Flour and Cassava Starch Which Processed by Granulation Method as Functional Food. Food Sci. Qual. Manag. 2017;61:19–24. [Google Scholar]
- 47.Bahado-Singh P.S., Riley C.K., Wheatley A.O., Lowe H.I.C. Relationship between processing method and the glycemic indices of ten sweet potato (Ipomoea batatas) cultivars commonly consumed in Jamaica. J. Nutr. Metab. 2011;2011 doi: 10.1155/2011/584832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oboh H.A., Ogbebor V.O. The Effect of Processing on the Glycemic Index and Glycemic Load of Maize (Zea Mays) Biology. 2010;25:46–51. [Google Scholar]
- 49.Eli-Cophie D., Agbenorhevi J.K., Annan R.A. Glycemic index of some local staples in Ghana. Food Sci. Nutr. 2017;5:131–138. doi: 10.1002/fsn3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mlotha V., Mwangwela A.M., Kasapila W., Siyame E.W.P., Masamba K. Glycemic responses to maize flour stiff porridges prepared using local recipes in Malawi. Food Sci. Nutr. 2016;4:322–328. doi: 10.1002/fsn3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shobana S., Kumari S.R.U., Malleshi N.G., Ali S.Z. Glycemic response of rice, wheat and finger millet based diabetic food formulations in normoglycemic subjects. Int. J. Food Sci. Nutr. 2007;58:363–372. doi: 10.1080/09637480701252229. [DOI] [PubMed] [Google Scholar]
- 52.Cavallero A., Empilli S., Brighenti F., Stanca A.M. High (1 → 3, 1 → 4)-β-glucan barley fractions in bread making and their effects on human glycemic response. J. Cereal Sci. 2002;36:59–66. doi: 10.1006/jcrs.2002.0454. [DOI] [Google Scholar]
- 53.Meija L., Havensone G., Lejnieks A. Postprandial Glycaemic and Insulinaemic Responses after Consumption of Activated Wheat and Triticale Grain Flakes. J. Nutr. Metab. 2019;2019 doi: 10.1155/2019/6594896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolever T.M.S., Jones P.J.H., Jenkins A.L., Mollard R.C., Wang H., Johnston A., Johnson J., Chu Y.F. Glycaemic and insulinaemic impact of oats soaked overnight in milk vs. cream of rice with and without sugar, nuts, and seeds: A randomized, controlled trial. Eur. J. Clin. Nutr. 2019;73:86–93. doi: 10.1038/s41430-018-0329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Senavirathna R.M.I.S.K., Ekanayake S., Jansz E.R., Welihinda J. Proximate composition, glycemic indices, and some factors affecting glycemic indices of underutilized tubers. Starch Staerke. 2014;66:1041–1048. doi: 10.1002/star.201400059. [DOI] [Google Scholar]
- 56.Hettiaratchi U.P.K., Ekanayake S., Welihinda J. Chemical compositions and glycemic responses to banana varieties. Int. J. Food Sci. Nutr. 2011;62:307–309. doi: 10.3109/09637486.2010.537254. [DOI] [PubMed] [Google Scholar]
- 57.Kouassi N.K., Tiahou G.G., Abodo J.R.F., Camara-Cisse M., Amani G.N.G. Influence of the variety and cooking method on glycemic index of yam. Pak. J. Nutr. 2009;8:993–999. doi: 10.3923/pjn.2009.993.999. [DOI] [Google Scholar]
- 58.Ramdath D.D., Isaacs R.L.C., Teelucksingh S., Wolever T.M.S. Glycaemic index of selected staples commonly eaten in the Caribbean and the effects of boiling v. crushing. Br. J. Nutr. 2004;91:971–977. doi: 10.1079/BJN20041125. [DOI] [PubMed] [Google Scholar]
- 59.Oboh H., Osagie A., Omotosho A. Glycemic response of some boiled legumes commonly eaten in nigeria. Diabetol. Croat. 2010;39:125–131. [Google Scholar]
- 60.Araya H., Pak N., Vera G., Alviña M. Digestion rate of legume carbohydrates and glycemic index of legume-based meals. Int. J. Food Sci. Nutr. 2003;54:119–126. doi: 10.1080/0963748031000084061. [DOI] [PubMed] [Google Scholar]
- 61.Al Dhaheri A.S., Henry C.J.K., Mohamad M.N., Ohuma E.O., Ismail L.C., Al Meqbaali F.T., Jarrar A.H. Glycaemic index and glycaemic load values of commonly consumed foods in the United Arab Emirates. Br. J. Nutr. 2017;117:1110–1117. doi: 10.1017/S0007114517001027. [DOI] [PubMed] [Google Scholar]
- 62.Ramdath D.D., Padhi E., Hawke A., Sivaramalingam T., Tsao R. The glycemic index of pigmented potatoes is related to their polyphenol content. Food Funct. 2014;5:909–915. doi: 10.1039/c3fo60395d. [DOI] [PubMed] [Google Scholar]
- 63.Adefegha S.A., Olasehinde T.A., Oboh G. Pasting Alters Glycemic Index, Antioxidant Activities, and Starch-Hydrolyzing Enzyme Inhibitory Properties of Whole Wheat Flour. Food Sci. Nutr. 2018;6:1591–1600. doi: 10.1002/fsn3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shobana S., Kokila A., Lakshmipriya N., Subhashini S., Ramya Bai M., Mohan V., Malleshi N.G., Anjana R.M., Henry C.J.K., Sudha V. Glycaemic Index of Three Indian Rice Varieties. Int. J. Food Sci. Nutr. 2012;63:178–183. doi: 10.3109/09637486.2011.615300. [DOI] [PubMed] [Google Scholar]
- 65.Turco I., Bacchetti T., Bender C., Zimmermann B., Oboh G., Ferretti G. Polyphenol Content and Glycemic Load of Pasta Enriched with Faba Bean Flour. Funct. Foods Health Dis. 2016;6:291. doi: 10.31989/ffhd.v6i5.254. [DOI] [Google Scholar]
- 66.Abubakar B., Yakasai H.M., Zawawi N., Ismail M. Compositional analyses of white, brown and germinated forms of popular Malaysian rice to offer insight into the growing diet-related diseases. J. Food Drug Anal. 2018;26:706–715. doi: 10.1016/j.jfda.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raghuvanshi R., Dutta A., Tewari G., Suri S. Qualitative Characteristics of Red Rice and White Rice Procured from Local Market of Uttarakhand: A Comparative Study. J. Rice Res. 2017;10:49–53. [Google Scholar]
- 68.Meera K., Smita M., Haripriya S., Sen S. Varietal influence on antioxidant properties and glycemic index of pigmented and non-pigmented rice. J. Cereal Sci. 2019;87:202–208. doi: 10.1016/j.jcs.2019.03.005. [DOI] [Google Scholar]
- 69.Yulianto W.A., Suryani C.L., Susiati A.M., Luwihana S. Evaluation of Chromium Fortified- Parboiled Rice Coated with Herbal Extracts: Resistant Starch and Glycemic Index. Int. Food Res. J. 2018;25:2608–2613. [Google Scholar]
- 70.Nilsson A.C., Östman E.M., Granfeldt Y., Björck I.M.E. Effect of cereal test breakfasts differing in glycemic index and content of indigestible carbohydrates on daylong glucose tolerance in healthy subjects. Am. J. Clin. Nutr. 2008;87:645–654. doi: 10.1093/ajcn/87.3.645. [DOI] [PubMed] [Google Scholar]
- 71.Nounmusig J., Kongkachuichai R., Sirichakwal P.P., Yamborisut U., Charoensiri R., Vanavichit A. The effect of low and high glycemic index based rice varieties in test meals on postprandial blood glucose, insulin and incretin hormones response in prediabetic subjects. Int. Food Res. J. 2018;25:835–841. [Google Scholar]
- 72.Trinidad T.P., Mallillin A.C., Sagum R.S., Felix A.D.R., Tuaño A.P.P., Juliano B.O. Relative effect of apparent amylose content on the glycemic index of milled and brown rice. Philipp. Agric. Sci. 2014;97:405–408. [Google Scholar]
- 73.Sari I.P., Lukitaningsih E., Rumiyati R., Setiawan I. Glycaemic Index of Uwi, Gadung, and Talas Which Were Given on Rat. Tradit. Med. J. 2013;18:127–131. [Google Scholar]
- 74.Fuentes-Zaragoza E., Riquelme-Navarrete M.J., Sánchez-Zapata E., Pérez-Álvarez J.A. Resistant starch as functional ingredient: A review. Food Res. Int. 2010;43:931–942. doi: 10.1016/j.foodres.2010.02.004. [DOI] [Google Scholar]
- 75.Jenkins D.J.A., Kendall C.W.C., Augustin L.S.A., Franceschi S., Hamidi M., Marchie A., Jenkins A.L., Axelsen M. Glycemic index: Overview of implications in health and disease. Am. J. Clin. Nutr. 2002;76:266–273. doi: 10.1093/ajcn/76.1.266S. [DOI] [PubMed] [Google Scholar]
- 76.Osman N.M.H., Mohd-Yusof B.N., Ismail A. Estimating Glycemic Index of Rice-Based Mixed Meals by Using Predicted and Adjusted Formulae. Rice Sci. 2017;24:274–282. doi: 10.1016/j.rsci.2017.06.001. [DOI] [Google Scholar]
- 77.Dupuis J.H., Liu Q., Yada R.Y. Methodologies for Increasing the Resistant Starch Content of Food Starches: A Review. Compr. Rev. Food Sci. Food Saf. 2014;13:1219–1234. doi: 10.1111/1541-4337.12104. [DOI] [Google Scholar]
- 78.Dworatzek P.D., Arcudi K., Gougeon R., Husein N., Sievenpiper J.L., Williams S.L. Nutrition Therapy. Can. J. Diabetes. 2013;37:S45–S55. doi: 10.1016/j.jcjd.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 79.Bule M., Abdurahman A., Nikfar S., Abdollahi M., Amini M. Antidiabetic effect of quercetin: A systematic review and meta-analysis of animal studies. Food Chem. Toxicol. 2019;125:494–502. doi: 10.1016/j.fct.2019.01.037. [DOI] [PubMed] [Google Scholar]
- 80.Afandi F., Wijaya C., Faridah D., Suyatma N. Relationship between Carbohydrate Content and the Glycemic Index in High-Carbohydrate Foods. Pangan. 2019;28:145–159. doi: 10.33964/jp.v28i2.422. [DOI] [Google Scholar]
- 81.Ferrer-Mairal A., Peñalva-Lapuente C., Iglesia I., Urtasun L., De Miguel-Etayo P., Remón S., Cortés E., Moreno L.A. In vitro and in vivo assessment of the glycemic index of bakery products: Influence of the reformulation of ingredients. Eur. J. Nutr. 2012;51:947–954. doi: 10.1007/s00394-011-0272-6. [DOI] [PubMed] [Google Scholar]
- 82.Fujiwara N., Hall C., Jenkins A. BBEДEHИE Page 1 of 23. Cereal Chem. J. 2017;94:110–116. doi: 10.1094/CCHEM-04-16-0119-FI. [DOI] [Google Scholar]
- 83.Foster-Powell K., Holt S.H., Brand-Miller J.C. International Table of Glycemic Index and Glycemic Load values. Am. J. Clin. Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- 84.Lee B.M., Wolever T.M.S. Effect of glucose, sucrose and fructose on plasma glucose and insulin responses in normal humans: Comparison with white bread. Eur. J. Clin. Nutr. 1998;52:924–928. doi: 10.1038/sj.ejcn.1600666. [DOI] [PubMed] [Google Scholar]
- 85.Teixeira N.D.C., Queiroz V.A.V., Rocha M.C., Amorim A.C.P., Soares T.O., Monteiro M.A.M., De Menezes C.B., Schaffert R.E., Garcia M.A.V.T., Junqueira R.G. Resistant starch content among several sorghum (Sorghum bicolor) genotypes and the effect of heat treatment on resistant starch retention in two genotypes. Food Chem. 2016;197:291–296. doi: 10.1016/j.foodchem.2015.10.099. [DOI] [PubMed] [Google Scholar]
- 86.Xiong Y., Zhang P., Warner R.D., Fang Z. Sorghum Grain: From Genotype, Nutrition, and Phenolic Profile to Its Health Benefits and Food Applications. Compr. Rev. Food Sci. Food Saf. 2019;18:2025–2046. doi: 10.1111/1541-4337.12506. [DOI] [PubMed] [Google Scholar]
- 87.Reddy C.K., Suriya M., Haripriya S. Physico-chemical and functional properties of Resistant starch prepared from red kidney beans (Phaseolus vulgaris.L) starch by enzymatic method. Carbohydr. Polym. 2013;95:220–226. doi: 10.1016/j.carbpol.2013.02.060. [DOI] [PubMed] [Google Scholar]
- 88.Yang Q.Q., Gan R.Y., Ge Y.Y., Zhang D., Corke H. Polyphenols in Common Beans (Phaseolus vulgaris L.): Chemistry, Analysis, and Factors Affecting Composition. Compr. Rev. Food Sci. Food Saf. 2018;17:1518–1539. doi: 10.1111/1541-4337.12391. [DOI] [PubMed] [Google Scholar]
- 89.Anton L.M., Gambell J.M., Lee D.M., Bryant S.P., Jebb S.A. Determination of Glycemic Index pf Various Stable Carbohydrate-rich Foods in the UK diet. Eur. J. Clin. Nutr. 2009;62:279–285. doi: 10.1038/sj.ejcn.1602723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Devries J.W. Glycemic Index: The Analytical Perspective. Cereal Foods World. 2007;52:45–49. doi: 10.1094/CFW-52-2-0045. [DOI] [Google Scholar]
- 91.Edwards C.H., Warren F.J., Milligan P.J., Butterworth P.J., Ellis P.R. A Novel Method for Classifying Starch Digestion by Modelling the Amylolysis of Plant Foods Using First-Order Enzyme Kinetic Principles. Food Funct. 2014;5:2751–2758. doi: 10.1039/C4FO00115J. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.