Abstract
Simple Summary
The tumor microenvironment plays a major role in the progression and drug resistance of pancreatic cancer. Cancer-associated fibroblasts are the major stromal cells and source of extracellular matrix proteins forming the dense stromal tumor microenvironment. Targeting cancer-associated fibroblasts has been deemed a promising therapeutic strategy. However, depleting cancer-associated fibroblasts may also have tumor-promoting effects due to their functional heterogeneity. It is therefore important to target selectively the tumor-promoting subtype of cancer-associated fibroblasts. Furthermore, deactivating fibroblasts, or reprograming tumor-promoting cancer-associated fibroblasts to tumor-restraining cancer-associated fibroblasts are considered as therapy for pancreatic cancer.
Abstract
Pancreatic cancer is the fourth leading cause of cancer deaths in the United States both in female and male, and is projected to become the second deadliest cancer by 2030. The overall five-year survival rate remains at around 10%. Pancreatic cancer exhibits a remarkable resistance to established therapeutic options such as chemotherapy and radiotherapy, due to dense stromal tumor microenvironment. Cancer-associated fibroblasts are the major stromal cell type and source of extracellular matrix proteins shaping a physical and metabolic barrier thereby reducing therapeutic efficacy. Targeting cancer-associated fibroblasts has been considered a promising therapeutic strategy. However, depleting cancer-associated fibroblasts may also have tumor-promoting effects due to their functional heterogeneity. Several subtypes of cancer-associated fibroblasts have been suggested to exhibit tumor-restraining function. This review article summarizes recent preclinical and clinical investigations addressing pancreatic cancer therapy through targeting specific subtypes of cancer-associated fibroblasts, deprogramming activated fibroblasts, administration of mesenchymal stem cells, as well as reprogramming tumor-promoting cancer-associated fibroblasts to tumor-restraining cancer-associated fibroblasts. Further, inter-cellular mediators between cancer-associated fibroblasts and the surrounding tissue microenvironment are discussed. It is important to increase our understanding of cancer-associated fibroblast heterogeneity and the tumor microenvironment for more specific and personalized therapies for pancreatic cancer patients in the future.
Keywords: pancreatic cancer, tumor microenvironment, cancer immunotherapy, reprogramming cancer-associated fibroblasts, mesenchymal stem cells, tumor-promoting cancer-associated fibroblasts, tumor-restraining cancer-associated fibroblasts
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a devastating disease with an unfavorable outcome. Currently, pancreatic cancer is the fourth leading cause of cancer deaths in the United States both in female and male [1], and is projected to become the second deadliest cancer by 2030 [2]. Although a number of studies have shown significant progress in survival of pancreatic cancer patients by combination chemotherapies [3,4,5,6,7], the overall five-year survival rate stands still at 10% [1]. Pancreatic cancer exhibits a significant resistance to established therapeutic options such as chemotherapy and radiotherapy, in part due to the dense stromal tumor microenvironment [8]. Cancer-associated fibroblasts (CAF) are the major stromal cell type and source of extracellular matrix proteins shaping a physical and metabolic barrier thereby reducing therapeutic efficacy [9,10]. Targeting CAFs and disrupting ECM have been considered as promising therapeutic strategies. The cellular component of the desmoplastic stroma in pancreatic cancer is composed primarily of myofibroblasts with alpha-smooth muscle actin (α-SMA) expression [11]. Several studies have shown that the number of α-SMA-positive CAFs correlates with shorter overall survival in esophageal and pancreatic cancer patients [12,13], suggesting that depletion of α-SMA-positive cells can be a promising therapeutic strategy in cancer patients. However, deletion of α-SMA-positive cells in a pancreatic cancer preclinical mouse model (Ptf1-Cre; lox-stop-lox-KrasG12D/+; Tgfbr2lox/lox) leads to invasive, undifferentiated tumors that are more hypoxic and reduces animal survival [11]. In another pancreatic cancer mouse model called KPC (Pdx1-Cre; lox-stop-lox-KrasG12D/+; lox-stop-lox-Trp53R172H/+) [14], deletion of α-SMA-positive cells diminishes animal survival [11]. Focal adhesion kinase (FAK) is a key intracellular effector of ECM signaling, which is activated upon ECM-induced integrin receptor activation [15], suggesting that targeting FAK can be a therapeutic option in cancer. Deletion of FAK in the fibroblast-specific protein 1 (FSP-1)-positive cells in mice (Fsp1-Cre; Faklox/lox) however leads to increased breast and pancreatic cancer growth [16]. Furthermore, low FAK expression is associated with shorter overall survival of breast and pancreatic cancer patients [16]. These studies imply that removal of cells based solely on positivity of α-SMA or FAK is not effective in limiting tumor aggressiveness. It has been demonstrated that static normal fibroblasts suppress polyoma virus-transformed tumor cells [17], suggesting that an innate function of fibroblasts is to protect against tumorigenesis [18]. Several subtypes of cancer-associated fibroblasts exhibit a tumor-restraining function [10,19]. Therefore, identification and better characterization of tumor-promoting CAF subtypes is important. Targeting inter-cellular mediators between cancer-associated fibroblasts and the surrounding microenvironment, deprogramming activated fibroblasts, administration of mesenchymal stem cells, as well as reprogramming tumor-promoting cancer-associated fibroblasts to tumor-restraining cancer-associated fibroblasts, have been considered as potential cancer therapeutic strategies.
2. Targeting Cancer-Associated Fibroblast Subtypes
Fibroblast Activation Protein (FAP) is a type II transmembrane cell surface serine protease and shares high sequence homology with the Dipeptidyl Peptidase (DPP) 4 [20]. FAP is frequently (90%) expressed, predominantly in CAFs, in patients with pancreatic cancer [21]. High expression of FAP is associated with shorter overall survival and disease-free survival in pancreatic cancer patients. Global knockout of Fap delays pancreatic tumor progression and prolongs the survival in KPC mice [22]. In line with this, conditional ablation of FAP-positive cells (using Diphtheria Toxin Receptor (DTR) expressing selectively in FAP-positive cells) introduced into the KPC mouse line leads to inhibition of pancreatic tumor development [23]. A FAP monoclonal antibody conjugated to DM1, a cytotoxic drug tubulin-binding maytansinoid with antimitotic activity, called FAP5-DM1, inhibits tumor growth leading to complete regressions in lung, pancreas, head and neck in xenograft cancer models [24]. FAP-specific chimeric antigen receptor (CAR) T cells have been generated that present a specific targeting effect on FAP-positive cells [25,26]. Adoptive transfer of FAP-specific CAR T cells reduces FAP-positive stromal cells and concomitantly decreases tumor xenograft growth [25,26]. Adoptive transfer of anti-FAP CAR T cells inhibits the growth of pancreatic cancers in KPC mice [27]. However, in another study, infusion of FAP CAR T cells led to bone toxicity and cachexia in non-tumor bearing wild-type mice as well as in global Rag1-deficient mice bearing a stroma-rich pancreatic cancer xenograft with strong FAP-positivity [28]. Bone marrow stromal cells express FAP and therefore FAP-reactive T cells can recognize and kill the cells [28,29,30]. A phase 2 trial of FAP inhibition using a humanized monoclonal antibody sibrotuzumab failed due to ongoing tumor progression in colorectal cancer patients (NCT02198274) (Table 1) [31,32]. Targeting FAP-positive stromal cells by CAR T cells remains a promising therapeutic option. However, additional markers are further required to increase specificity for FAP-positive CAFs in pancreatic cancer. A phase 1/2 study with talabostat mesylate (BXCL701) (Figure 1) in combination with the anti-programmed cell death 1 (PD-1) monoclonal antibody pembrolizumab recruits prostate cancer patients to assess safety and efficacy of the combined treatment (NCT03910660) (Table 1). Talabostat is a specific inhibitor of dipeptidyl peptidases such as FAP [33]. Several clinical trials use RO6874281, FAP-targeted IL-2 variant, as a FAP inhibitor (Figure 1). RO6874281 carries an engineered IL-2 variant lacking binding ability to IL-2Rα. Affinity to IL-2Rβγ is retained, resulting in activation of CD8+ T cells and natural killer (NK) cells, but reduced activity on immunosuppressive regulatory T (Treg) cells. The antibody part of RO6874281 binds with high affinity to bind FAP, and mediate retention and accumulation in malignant lesions [34]. A phase 1 clinical study is currently ongoing for evaluating safety and therapeutic activity of RO6874281 in combination with pembrolizumab in patients with metastatic melanoma (NCT03875079) (Table 1). Another phase 1 study is ongoing to evaluate safety, pharmacokinetics, and therapeutic activity of RO6874281 as a single agent or in combination with trastuzumab or cetuximab for patients with breast cancer, head and neck cancer (NCT02627274) (Table 1). For patients with advanced/metastatic head and neck, esophageal, and cervical cancers, a phase 2 study evaluates the therapeutic activity of RO6874281 together with atezolizumab (also known as MPDL3280A, an engineered anti-PD-L1 antibody [35]), Gemcitabine and a mitotic spindle poison Vinorelbine (NCT03386721) (Table 1) [36]. For patients with metastatic pancreatic cancer, a phase 1/2 study of multiple immunotherapy-based treatment combinations is underway (NCT03193190) (Table 1).
Table 1.
Overview of several clinical trials targeting fibroblast activation protein, CXCL12 and CXCR4 axis, and LRRC15.
| Intervention/Treatment | Condition or Disease | NCT Number | Stage of Clinical Trial | Recruitment Status (Recruiting, Completed, not yet Recruiting. Last Update) | Last Update |
|---|---|---|---|---|---|
| BIBH1 (Sibrotuzumab) | Metastatic colorectal cancer | NCT02198274 | Phase 2 | Completed | 23 July 2014 |
| Talabostat mesylate (BXCL701) Pembrolizumab |
Prostate cancer | NCT03910660 | Phase 1/2 | Recruiting | 5 November 2020 |
| RO6874281 Atezolizumab (MPDL3280A), an engineered anti-PD-L1 antibody Gemcitabine Vinorelbine |
Advanced/Metastatic head and neck, esophageal and cervical cancers | NCT03386721 | Phase 2 | Recruiting | 27 April 2020 |
| RO6874281 with Trastuzumab or Cetuximab |
Solid tumor Breast cancer Cancer of head and neck |
NCT02627274 | Phase 1 | Recruiting | 14 October 2020 |
| RO6874281 Pembrolizumab |
Metastatic melanoma | NCT03875079 | Phase 1 | Recruiting | 9 October 2020 |
| Nab-Paclitaxel Gemcitabine Oxaliplatin Leucovorin Fluorouracil Atezolizumab Cobimetinib PEGGH20 BL-8040 Selicrelumab Bevacizumab RO6874281 AB928 Tiragolumab Tocilizumab |
Metastatic pancreatic adenocarcinoma | NCT03193190 | Phase 1,2 | Recruiting | 27 October 2020 |
| Cemiplimab (REGN-2810, Libtayo) Plerixafor (AMD3100, Mozobil) |
Metastatic pancreatic cancer | NCT04177810 | Phase 2 | Recruiting | 9 November 2020 |
| Plerixafor (Mozobil) | Metastatic pancreatic cancer Metastatic colorectal cancer Ovarian Serous Adenocarcinoma |
NCT02179970 (CAM-PLEX) |
Phase 1 | Completed | 23 July 2019 |
| BL-8040 Pembrolizumab Onivyde/5-FU/leucovorin |
Metastatic pancreatic cancer | NCT02826486 (COMBAT) | Phase 2 | Active, not recruiting | 9 June 2020 |
| ABBV-085 | Advanced solid tumors | NCT02565758 | Phase 1 | Completed | 5 April 2019 |
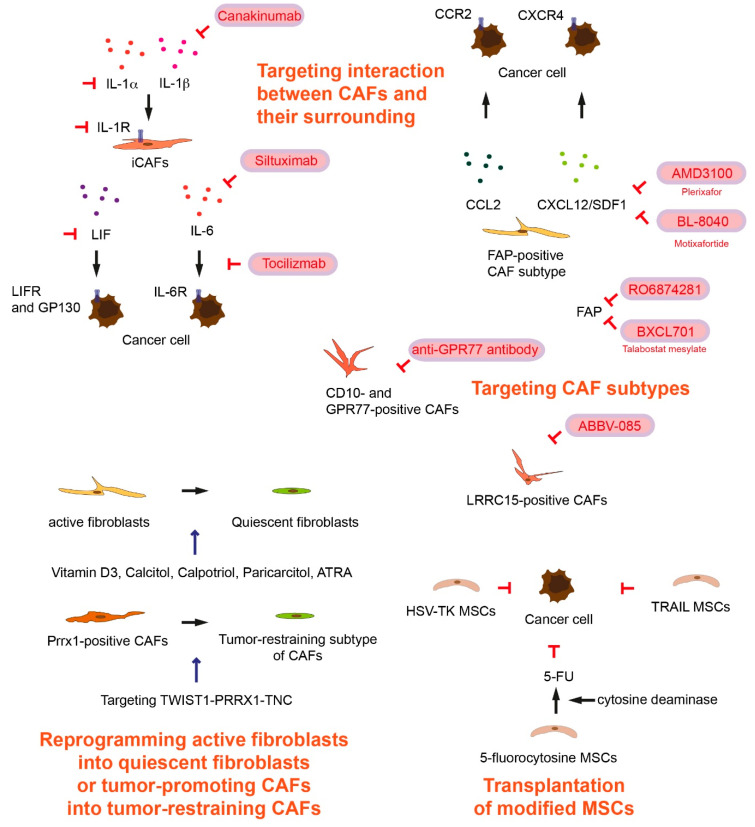
Figure 1.
Therapeutic potential of targeting cancer-associated fibroblasts (CAFs), targeting interaction between CAFs and their surroundings, reprogramming active fibroblasts into quiescent fibroblasts or tumor promoting CAFs into tumor-restraining CAFs, and transplantation of modified mesenchymal stem cells (MSCs). The inhibition symbols are colored in red.
FAP-positive CAFs attract Gr-1+/CD11b+ myeloid cells, and co-injection of FAP-positive CAFs and tumor cells into immunocompetent mice increases the frequency of infiltration of macrophages [37]. FAP-positive CAFs are a major source of chemokine CC-chemokine ligand 2 (CCL2) also known as monocyte chemoattractant protein-1 (MCP-1) (Figure 1). Administration of neutralizing anti-CCL2 antibody or CCL2 knockdown by shRNA blocks migration of Gr-1+/CD11b+ myeloid cells toward cell culture supernatants from FAP-positive CAFs [37]. The CCL2 and CC chemokine receptor type 2 (CCR2, MCP-1 receptor) axis is important for monocyte recruitment [38]. In global CCR2 knockout mice, FAP-positive CAFs fail to promote tumor growth in a liver tumor xenograft model [37]. In a doxycycline-inducible pancreatic cancer mouse model (Ptf1-Cre; TetO-KrasG12D/+; Rosa26rtTa-IRES-EGFP; lox-stop-lox-Trp53R172H/+), depletion of CD11b+ myeloid cells (Itgam-DTR) increased intratumoral CD8+ T cells [39,40]. It has been shown that CAFs expressing FAP suppress antitumor immunity [29]. FAP-positive CAFs express CXCL12 where T cell are excluded [23]. CXCL12 plays a role in tumoral immunosuppression. CXCL12, also known as stromal cell-derived factor 1 (SDF1) is the primary ligand for the C-X-C motif chemokine receptor 4 (CXCR4) (Figure 1). The CXCL12 and CXCR4 axis promotes pancreatic cancer development, invasion, and metastasis [41,42]. Targeting the CXCL12-CXCR4 axis by treatment with a specific CXCR4 inhibitor AMD3100 (Figure 1), also known as Plerixafor, reverses FAP-positive CAF-mediated immunosuppression, infiltration of CD8+ T cells into the tumor. Further AMD3100 treatment attenuates pancreatic cancer development in KPC mice [23]. The CXCL12-CXCR4 signaling pathway can recruit Treg cells [42]. However, Treg cells are not critically involved once immunosuppressive role of CXCL12 is neutralized. The precise roles of CXCL12 in immunosuppression still need to be clarified. A phase 1 study (CAM-PLEX study) for assessing the safety of continuous administration of Plerixafor (AMD3100) in patients with advanced pancreatic, ovarian and colon cancer has been completed (NCT 02179970) (Table 1). The results of the phase 1 study demonstrate that continuous administration of Plerixafor induces intratumoral CD8+ and natural killer (NK) cell accumulation in patients with pancreatic and colorectal cancer [43]. BL-8040 (motixafortide) is a CXCR4 antagonist, and a phase 2 study shows that BL-8040 increases CD8+ T cell tumor infiltration, decreases myeloid-derived suppressor cells and circulating regulatory T cells (COMBAT trial) (NCT02826486) (Table 1) [44]. These data suggest that alteration of tumor microenvironment by stromal and immune modulation is an appropriate therapeutic approach [32].
It has been shown that chemoresistant and chemosensitive tumors contain functionally distinct CAF subtypes. A study compared CAFs from chemoresistant breast cancer biopsies and CAFs from chemosensitive before neoadjuvant chemotherapy, and identified that cell-surface markers CD10 and G protein-coupled receptor 77 (GPR77) are upregulated in the CAFs from chemoresistant tumors [45]. CD10 is a zinc-dependent metalloproteinase, and GPR77, also known as C5L2, coded by the C5AR2 gene, is an alternative receptor for complement C5a [46]. High expression of CD10- and GPR77-positive CAFs (Figure 1) are associated with docetaxel or cisplatin chemoresistance and shorter disease-free survival in breast and lung cancer patients [45]. CD10 and GPR77-positive CAFs sustain cancer stemness and promote tumor chemoresistance [45]. Treatment with a neutralizing anti-GPR77 antibody suppresses the activity of NF-κB, IL-6, and IL-8 secretion, abolishes establishment of breast cancer patient-derived xenograft in the immunocompromised mice, and restores docetaxel chemosensitivity [45].
Leucine-rich repeat containing 15 (LRRC15) is expressed on CAFs (Figure 1) in many solid cancers including pancreatic cancer as well as on a subset of cancer cells of mesenchymal origin [47,48]. A LRRC15-positive CAF subpopulation was identified in a pancreatic cancer mouse model (Pdx1-Cre; lox-stop-lox-KrasG12D/+; p16/p19lox/lox) [48]. Mouse tumor spheroids cultured with LRRC-positive CAFs grow larger than those in media alone, suggesting that LRRC-positive CAFs can directly enhance tumor growth [48]. ABBV-085 is a monomethyl auristatin E (MMAE)-containing antibody-drug conjugate directed against LRRC15, and ABBV-085 inhibits cancer cells tumor xenograft growth [47]. A phase 1 study with ABBV-085 to evaluate the safety and pharmacokinetics in patients with advanced solid tumors has been completed (NCT02565758) (Table 1). ABBV-085 was well-tolerated in the phase 1 study in patients with advanced sarcomas [49].
3. Targeting Interactions between Cancer-Associated Fibroblasts and Their Surrounding in Tumor Microenvironment
Another strategy is targeting signaling molecules involved in the interaction between CAFs and their surrounding environment. IL-6 from CAFs inhibits NK cell activity, and activates signal transducer and activator of transcription 3 (STAT3) in pancreatic cancer cells [50]. IL-6 and PD-L1 antibody (BioXCell) blockade combination therapy reduces tumor progression and extends overall survival in a murine pancreatic cancer model (Pdx1-Cre; lox-stop-lox-KrasG12D/+; lox-stop-lox-Trp53R270H/+; Brca2lox/lox) [51]. Siltuximab is an anti-IL-6 chimeric monoclonal antibody (Figure 1) [52]. A phase 1/2 study is currently recruiting metastatic pancreatic cancer patients for treatment with siltuximab and spartalizumab (a monoclonal antibody directed against human PD-1) (NCT04191421) (Table 2). Tocilizumab is a monoclonal antibody that competitively inhibits the binding of IL-6 to IL-6R (Figure 1) [53]. A phase 2 study (PACTO) investigates administration of tocilizumab in combination with gemcitabine and nab-paclitaxel for patients with unresectable pancreatic cancer (NCT02767557) (Table 2). Another phase 2 study with ipilimumab, nivolumab, tocilizumab and stereotactic body radiotherapy (SBRT) (TRIPPLE-R) has been launched for advanced pancreatic cancer patients (NCT04258150) (Table 2).
Table 2.
Overview of clinical trials with targeting interaction between tumor-promoting cancer-associated fibroblasts and surrounding microenvironment.
| Intervention/Treatment | Condition or Disease | NCT Number | Stage of Clinical Trial | Recruitment Status (Recruiting, Completed, not yet Recruiting. Last Update) | Last Update |
|---|---|---|---|---|---|
| Siltuximab Spartalizumab |
Metastatic pancreatic cancer | NCT04191421 | Phase 1,2 | Recruiting | 20 August 2020 |
| Tocilizumab Gemcitabine Nab-Paclitaxel |
Unresectable pancreatic cancer |
NCT02767557 (PACTO) |
Phase 2 | Recruiting | 21 April 2020 |
| Ipilimumab Nivolumab Tocilizumab SBRT |
Pancreatic cancer |
NCT04258150 (TRIPPLE-R) |
Phase 2 | Recruiting | 21 April 2020 |
| Canakinumab Spartalizumab Nab-paclitaxel Gemcitabine |
Metastatic pancreatic cancer |
NCT04581343 (PanCAN-SR1) |
Phase 1 | Recruiting | 20 October 2020 |
It has been demonstrated that IL-1 and transforming growth factor β (TGF-β) are key regulators of CAF heterogeneity [54]. The majority of fibroblasts in human pancreatic tumors as well as in tumors from KPC mice express FAP and low levels of α-SMA, whereas a subpopulation of FAP-positive cells exhibit elevated α-SMA expression called myofibroblastic CAFs (myCAFs) [55]. TGF-β downregulates IL-1R1 and promotes differentiation into myCAFs [54]. IL-1 activates NF-κB signaling and leukemia inhibitory factor (LIF) expression in a CAF subtype named inflammatory CAFs (iCAFs) [54]. Canakinumab is a human anti-IL-1β monoclonal antibody (Figure 1) [56]. A phase 1 study assesses safety and tolerability of canakinumab, spartalizumab, with the chemotherapy combination of gemcitabine and nab-paclitaxel in metastatic pancreatic cancer patients (NCT04581343) (Table 2). IL-1β is one of the IL-1 cytokines, canakinumab targets only one arm of the regulatory cytokines. Targeting IL-1α or IL-1R1 could also be therapeutic options. LIF further activates Janus kinase (JAK)/STAT signaling for IL-6 expression [54]. LIF can be a key paracrine factor produced by activated pancreatic stellate cells (PSCs) acting on cancer cells via activating STAT3 signaling [57]. In PSC-conditioned medium-stimulated human pancreatic cancer cells, LIF receptor (LIFR) and its co-receptor IL-6 signal transducer (IL6ST, also known as gp130) are only receptors identified as interacting partners with STAT3 (immunoprecipitation) [57]. Conditional deletion of LIFR leads to reduced pancreatic tumor progression and prolonged survival in a pancreatic cancer mouse model (Pdx1-Cre; lox-stop-lox-KrasG12D/+; Trp53lox/lox; Lifrlox/lox; lox-stop-lox-Rosa26Luc/Luc). Similar to genetic depletion of Lifr, administration of LIF neutralizing antibody increases overall survival and gemcitabine chemosensitivity in Pdx1-Cre; lox-stop-lox-KrasG12D/+; Trp53lox/lox; lox-stop-lox-Rosa26Luc/Luc mice [57].
4. Transplantation of Modified Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) exist in many tissues that have an important role in tissue regeneration [58]. However, MSCs (such as bone marrow-derived and adipose-derived MSCs) can migrate to tumors and differentiate into cancer-associated MSCs and tumor-promoting CAFs [10,58]. The complex cellular and molecular interaction, paracrine and reciprocal factors between MSCs and the surrounding microenvironment can lead to different function of MSCs and outcomes [10,58]. However, MSCs can be used for anti-cancer treatment because of their characteristic tendency to home to tumor sites. This property of MSCs makes these cells attractive candidates as drug delivery vehicles [58]. For treatment of gastrointestinal cancer, bone marrow-derived MSC-delivery of Herpes simplex virus type 1 thymidine kinase (HSV-TK) under the control of the CCL5-promotor has been used. After cell delivery, a cytotoxic drug ganciclovir is administered, which is phosphorylated and activated by HSV-TK for cancer cell death (Figure 1). This TREAT-ME1 study in phase 1/2 (NCT02008539) has been terminated and concluded as safe and tolerable (Table 3) [59,60,61]. Adipose-derived MSCs modified to express yeast cytosine deaminase co-administered with of the non-toxic prodrug 5-flurocytosine inhibit colon cancer cells tumor xenograft growth in immunocompromised mice [62]. Cytosine deaminase converts 5-flurocytosine to 5-fluorouracil (5-FU) (Figure 1) [62]. Co-administration of 5-flurocytosine and cytosine deaminase-expressing MSCs also inhibits melanoma cells tumor xenograft growth in nude mice [63].
Table 3.
Overview of clinical trials with transplantation of mesenchymal stem cells.
| Intervention/Treatment | Condition or Disease | NCT Number | Stage of Clinical Trial | Recruitment Status (Recruiting, Completed, not yet Recruiting. Last Update) | Last Update |
|---|---|---|---|---|---|
| MSC_apceth_101 | Advanced gastrointestinal cancer | NCT02008539 | Phase 1, 2 | Terminated | 27 March 2017 |
| Oncolytic measles virus encoding thyroidal sodium iodide symporter infected MSCs | Recurrent ovarian, peritoneal or fallopian tube cancer | NCT02068794 | Phase 1, 2 | Recruiting | 3 December 2020 |
| MSCTRAIL | Lung cancer |
NCT03298763 (TACTICAL) |
Phase 1, 2 | Recruiting | 31 March 2020 |
MSCs can also be transfected with apoptosis-inducing factors (Figure 1). A phase 1/2 study with oncolytic measles virus encoding thyroidal sodium iodide symporter (MV-NIS)-infected MSCs recruits patients with recurrent ovarian, peritoneal, or fallopian tube cancer (NCT02068794) (Table 3). Oncolytic measles virus preferentially infects tumor cells and induces syncytia formation and apoptosis [64]. TNF-related apoptosis-inducing ligand (TRAIL)-transduced MSCs are resistant to TRAIL-induced apoptosis, due to low expression of the death receptors TRAIL-R1 and TRAIL-R2 in comparison with tumor cells [58]. A phase 1/2 study (NCT03298763, TACTICAL trial) is underway to recruit lung cancer patients using MSCs to deliver the TRAIL (Table 3). Taken together, several preclinical and clinical studies have demonstrated a great potential of MSCs in cancer therapy, yet it needs to be clarified whether MSCs keep their cellular status or transdifferentiate into tumor-promoting CAFs during therapies.
5. Reprogramming Active Fibroblasts into Quiescent Fibroblasts or Tumor-Promoting Cancer-Associated Fibroblasts into Cancer-Restraining Cancer-Associated Fibroblasts
Rather than complete depletion of CAF subtypes, reprogramming active fibroblasts into quiescent fibroblasts or the tumor-promoting subtype of CAFs into the tumor-restraining subtype of CAFs can be deemed to block pancreatic cancer progression and to render cancer cells more responsive to treatment (Figure 1) [10,19]. Vitamin D is a pluripotent fat-soluble prohormone and plays a critical role in calcium homeostasis, cell differentiation, proliferation as well as apoptosis. Furthermore, vitamin D deficiency is associated with increased risk of developing several types of cancer [65]. Epidemiological cohort studies have shown that higher intake of Vitamin D reduces pancreatic cancer risk [66]. Calcitriol (1α,25-dihydroxyvitamin D3, 1,25(OH)2D3) enhances gemcitabine efficiency to inhibit pancreatic cancer xenografts [67]. Vitamin D3 binds with Vitamin D receptor (VDR) and retinoid X receptor (RXR) to form a heterodimer complex regulating downstream signaling pathways [65]. Activation of VDR in PSCs by calcipotriol (vitamin D3 analog) attenuates inflammation and fibrosis. Furthermore, administration of calcipotriol in combination with gemcitabine enhances survival of KPC mice [68]. High VDR expression in CAFs is associated with longer overall survival and progression-free survival in colorectal cancer patients [69]. Currently several clinical studies with high-dose of vitamin D3 or paricalcitol (analog of the active form of vitamin D2) [70] are underway. A phase 3 study with high-dose vitamin D3 for treating pancreatic cancer patients has been launched (NCT03472833) (Table 4). Further an early phase 1 study with paricalcitol/gemcitabine/Nab-paclitaxel/Nivolumab for resectable pancreatic cancer patients (NCT03519308), and a phase 1 study with paricalcitol/5-FU/leucovorin/liposomal irinotecan for advanced pancreatic cancer (NCT03883919) have been initiated (Table 4). A phase 2 study with paricalcitol in combination with pembrolizumab has been completed (NCT03331562). Other phase 2 studies with paricalcitol/gemcitabine/Nab-paclitaxel (NCT04617067) or with paricalcitol/gemcitabine/hydroxychloroquine/Nab-paclitaxel (NCT04524702) are currently recruiting pancreatic cancer patients (Table 4).
Table 4.
Overview of clinical trials for reprogramming active fibroblasts into quiescent fibroblasts or tumor-promoting cancer-associated fibroblasts into tumor-restraining cancer-associated fibroblasts.
| Intervention/Treatment | Condition or Disease | NCT Number | Stage of Clinical Trial | Recruitment Status (Recruiting, Completed, not yet Recruiting. Last Update) | Last Update |
|---|---|---|---|---|---|
| High-dose Vitamin D3 | Pancreatic cancer | NCT03472833 | Phase 3 | Recruiting | 14 September 2020 |
| Pembrolizumab paricalcitol |
Pancreatic cancer, advanced pancreatic cancer, metastatic pancreatic cancer | NCT03331562 | Phase 2 | Completed | 8 October 2020 |
| Nivolumab Nab-Paclitaxel Gemcitabine Paricalcitol |
Resectable pancreatic cancer | NCT03519308 | Early Phase 1 | Recruiting | July 30, 2020 |
| Paricalcitol Gemcitabine and Nab-paclitaxel |
Advanced pancreatic cancer | NCT04617067 | Phase 2 | Recruiting | 9 November 2020 |
| 5-FU Leucovorin Liposomal irinotecan Paricalcitol |
Advanced pancreatic cancer | NCT03883919 | Phase 1 | Recruiting | 27 August 2020 |
| Gemcitabine Hydroxychloroquine Nab-paclitaxel Paricalcitol |
Advanced or metastatic pancreatic cancer | NCT04524702 | Phase 2 | Recruiting | 8 October 2020 |
| ATRA Gemcitabine Nab-paclitaxel |
Pancreatic cancer |
NCT03307148 (STAR_PAC) |
Phase 1 | Completed | 22 January 2020 |
| ATRA Gemcitabine Nab-paclitaxel |
Pancreatic cancer |
NCT04241276 (STARPAC2) |
Phase 2 | Not yet recruiting | 27 January 2020 |
Retinoids are natural and synthetic vitamin A derivatives such as all-trans retinoic acid (ATRA), 9-cis retinoic acid, and 13-cis retinoid acid, control cellular differentiation, growth, and apoptosis. ATRA activates retinoic acid receptors and is the principal activator of retinoic acid signaling [71]. ATRA induces quiescence of PSCs [72]. Quiescent PSCs produce Secreted Frizzled Related Protein 4 (SFRP4), which sequesters Wnt molecules and inhibits Wnt-meditated signal transduction. ATRA administration leads to a reduction of activated stroma as well as reduction of cancer cell proliferation in KPC mice. Further ATRA treatment increases apoptosis of cancer cells associated with a decrease in nuclear β-catenin and increase in stromal SFRP4 in KPC mice [72]. The combination of gemcitabine and ATRA results in inhibition of tumor growth in gemcitabine-resistant pancreatic cancer xenograft in mice. In pancreatic tumor xenograft with high fatty acid binding protein 5 (FABP5) expression and no cellular retinoic acid binding protein 2 (CRABP2) expression is resistant to the treatment with ATRA [73]. FABP5 delivers retinoic acid to peroxisome proliferator activated receptor β/δ (PPARβ/δ), while its homolog CRABP2 delivers retinoic acid to retinoic acid receptor (RAR). Both FABP5 and CRABP2 localize in the cytoplasm in the absence of ligand. Upon binding retinoic acid, they translocate into the nucleus to form a complex with either PPARβ/δ or RAR. PPARβ/δ or RAR forms heterodimer with RXR to regulate gene expression [74]. Heat shock protein 47 (HSP47) is a collagen-specific molecular chaperon that is required for the proper folding and secretion of collagen [75]. HSP47 enhances deposition of ECM proteins and promotes cancer progression in a breast cancer xenograft [76]. Co-delivery of ATRA and HSP47 siRNA, based on PEGylated polyethylenimine-coated gold nanoparticles system, induces PSC quiescence, thereby promoting drug delivery and enhancing the anti-tumor efficacy of gemcitabine in pancreatic cancer xenografts [75]. Juxtatumoral compartments of pancreatic cancer patient samples contain increased numbers of myeloperoxidase- and CD68-positive cells but less CD8-positve cells than in pan-stromal compartments. Pancreatic cancer patients with high density of CD8-positive T cells in the juxtatumoral compartment exhibit longer survival. ATRA administration increases CD8-positive T cells in juxtatumoral compartment in KPC mice [77]. A phase 1 clinical trial with ATRA in combination with gemcitabine and Nab-Paclitaxel (STARPAC study) has been completed (NCT03307148) (Table 4) [78]. A subsequent phase 2 study (ATRA/gemcitabine/Nab-paclitaxel) (STARPAC2) is currently underway (NCT04241276) (Table 4).
The homeodomain transcription factor paired-related homeobox 1 (PRRX1) regulates epithelial to mesenchymal transition (EMT) [79], and considered a driver of cellular plasticity during pancreatic ductal development, acinar-to-ductal metaplasia (ADM) formation and carcinogenesis [80]. One of two major isoforms of PRRX1, PRRX1b promotes EMT (de-differentiation), invasion, and tumor differentiation while PRRX1a stimulates mesenchymal to epithelial transition (MET) (re-differentiation), metastatic outgrowth as well as tumor differentiation [81]. These two isoforms of PRRX1 have therefore a reciprocal relationship in regulating cellular plasticity and regulate tumor progression at different stages [81]. In Pdx1-Cre; lox-stop-lox-KrasG12D/+; Ink4alox/+ murine pancreatic cancer and in pancreatic cancer patient specimens, PRRX1 is expressed in CAFs. High expression levels of PRRX1 in CAFs are associated with squamous subtypes of pancreatic cancer [82]. It has been shown that patients with the squamous subtype have shorter median survival [83]. PRRX1-deficient CAFs display attenuated cellular plasticity and differentiate into myCAFs [82]. Although it cannot be concluded that α-SMA-positive cells are simply tumor-restraining CAFs (see the Section 1), targeting PRRX1 can be a promising strategy for reprograming tumor-promoting subtype of CAFs to tumor-restraining subtype of CAFs. Twist family basic helix-loop-helix transcription factor (TWIST1) induces trans-differentiation of quiescent fibroblasts to CAFs [84]. TWIST1 is highly expressed in CAFs and a positive regulator of PRRX1, which subsequently increases the expression of Tenascin C (TNC). By forming a positive feedback loop, TNC increases TWIST1 expression [85]. Targeting TWIST1-PRRX1-TNC is hence a promising therapeutic target to deprogram activated fibroblasts or reprogram tumor-promoting CAFs to tumor-restraining CAFs.
6. Conclusions
Currently, various preclinical and clinical strategies have been developed to target subpopulations of tumor-promoting CAFs. Some of the most commonly used CAF markers are expressed on other cell types leading to side effects of CAF-targeted therapy. Further identification of tumor-promoting CAF subpopulations and specific markers are important to develop precise targeting strategies against the tumor-promoting CAF subtypes. Another strategy of pancreatic cancer therapy is targeting signaling molecules involved in the interaction between CAFs and their surrounding environment. Underlying mechanisms for CAF accumulation in cancers and identification of CAF subsets populating the tumor microenvironment would help in establishing therapeutic strategies to reprogram the tumor-promoting microenvironment into a tumor-suppressive microenvironment. Reprogramming active fibroblasts into quiescent fibroblasts or tumor-promoting subtype of CAFs into tumor-restraining subtype of CAFs can be used to block pancreatic cancer progression, to reprogram the tumor microenvironment, and to render cancer cells more responsive to treatments.
Author Contributions
Writing—original draft preparation, Y.S., resources, V.B., supervision and revisions, J.K. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge the financial support within the funding programme Open Access Publishing by the German Research Foundation (DFG).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L., Smith B.D., Aizenberg R., Rosenzweig A.B., Fleshman J.M., Matrisian L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D., Chau I., Stocken D.D., Valle J.W., Smith D., Steward W., Harper P.G., Dunn J., Tudur-Smith C., West J., et al. Phase III Randomized Comparison of Gemcitabine Versus Gemcitabine Plus Capecitabine in Patients with Advanced Pancreatic Cancer. J. Clin. Oncol. 2009;27:5513–5518. doi: 10.1200/JCO.2009.24.2446. [DOI] [PubMed] [Google Scholar]
- 4.Conroy T., Desseigne F., Ychou M., Bouché O., Guimbaud R., Bécouarn Y., Adenis A., Raoul J.-L., Gourgou-Bourgade S., De La Fouchardière C., et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff D.D., Ervin T., Arena F.P., Chiorean E.G., Infante J., Moore M., Seay T., Tjulandin S.A., Ma W.W., Saleh M.N., et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neoptolemos J.P., Palmer D.H., Ghaneh P., Psarelli E.E., Valle J.W., Halloran C.M., Faluyi O., O’Reilly D.A., Cunningham D., Wadsley J., et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–1024. doi: 10.1016/S0140-6736(16)32409-6. [DOI] [PubMed] [Google Scholar]
- 7.Conroy T., Hammel P., Hebbar M., Ben Abdelghani M., Wei A.C., Raoul J.-L., Choné L., Francois E., Artru P., Biagi J.J., et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018;379:2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 8.Kleeff J., Korc M., Apte M., La Vecchia C., Johnson C.D., Biankin A.V., Neale R.E., Tempero M., Tuveson D.A., Hruban R.H., et al. Pancreatic cancer. Nat. Rev. Dis. Prim. 2016;2:16022. doi: 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- 9.Sunami Y., Rebelo A., Kleeff J. Lipid Metabolism and Lipid Droplets in Pancreatic Cancer and Stellate Cells. Cancers. 2017;10:3. doi: 10.3390/cancers10010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sunami Y., Häußler J., Kleeff J. Cellular Heterogeneity of Pancreatic Stellate Cells, Mesenchymal Stem Cells, and Can-cer-Associated Fibroblasts in Pancreatic Cancer. Cancers. 2020;12:3770. doi: 10.3390/cancers12123770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Özdemir B.C., Pentcheva-Hoang T., Carstens J.L., Zheng X., Wu C.-C., Simpson T.R., Laklai H., Sugimoto H., Kahlert C., Novitskiy S.V., et al. Depletion of Carcinoma-Associated Fibroblasts and Fibrosis Induces Immunosuppression and Accelerates Pancreas Cancer with Reduced Survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujita H., Ohuchida K., Mizumoto K., Nakata K., Yu J., Kayashima T., Cui L., Manabe T., Ohtsuka T., Tanaka M. α-Smooth Muscle Actin Expressing Stroma Promotes an Aggressive Tumor Biology in Pancreatic Ductal Adenocarcinoma. Pancreas. 2010;39:1254–1262. doi: 10.1097/MPA.0b013e3181dbf647. [DOI] [PubMed] [Google Scholar]
- 13.Underwood T.J., Hayden A.L., Derouet M., Garcia E., Noble F., White M.J., Thirdborough S., Mead A., Clemons N., Mellone M., et al. Cancer-associated fibroblasts predict poor outcome and promote periostin-dependent invasion in oesophageal adenocarcinoma. J. Pathol. 2015;235:466–477. doi: 10.1002/path.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hingorani S.R., Wang L., Multani A.S., Combs C., Deramaudt T.B., Hruban R.H., Rustgi A.K., Chang S., Tuveson D.A. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 15.Sulzmaier F.J., Jean C., Schlaepfer D.D. FAK in cancer: Mechanistic findings and clinical applications. Nat. Rev. Cancer. 2014;14:598–610. doi: 10.1038/nrc3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demircioglu F., Wang J., Candido J., Costa A.S.H., Casado P., Delgado B.D.L., Reynolds L.E., Gomez-Escudero J., Newport E., Rajeeve V., et al. Cancer associated fibroblast FAK regulates malignant cell metabolism. Nat. Commun. 2020;11:1290. doi: 10.1038/s41467-020-15104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stoker M.G., Shearer M., O’Neill C. Growth inhibition of polyoma-transformed cells by contact with static normal fibroblasts. J. Cell Sci. 1966;1:297–310. doi: 10.1242/jcs.1.3.297. [DOI] [PubMed] [Google Scholar]
- 18.Klein G. Evolutionary aspects of cancer resistance. Semin. Cancer Biol. 2014;25:10–14. doi: 10.1016/j.semcancer.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Miyai Y., Esaki N., Takahashi M., Enomoto A. Cancer-associated fibroblasts that restrain cancer progression: Hypotheses and perspectives. Cancer Sci. 2020;111:1047–1057. doi: 10.1111/cas.14346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitzgerald A.A., Weiner L.M. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev. 2020;39:783–803. doi: 10.1007/s10555-020-09909-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen S.J., Alpaugh R.K., Palazzo I., Meropol N.J., Rogatko A., Xu Z., Hoffman J.P., Weiner L.M., Cheng J.D. Fibroblast Activation protein and its relationship to clinical outcome in pancreatic adenocarcinoma. Pancreas. 2008;37:154–158. doi: 10.1097/MPA.0b013e31816618ce. [DOI] [PubMed] [Google Scholar]
- 22.Lo A., Li C.-P., Buza E.L., Blomberg R., Govindaraju P., Avery D., Monslow J., Hsiao M., Puré E. Fibroblast activation protein augments progression and metastasis of pancreatic ductal adenocarcinoma. JCI Insight. 2017;2 doi: 10.1172/jci.insight.92232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feig C., Jones J.O., Kraman M., Wells R.J.B., Deonarine A., Chan D.S., Connell C.M., Roberts E.W., Zhao Q., Caballero O.L., et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. USA. 2013;110:20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostermann E., Garin-Chesa P., Heider K.H., Kalat M., Lamche H., Puri C., Kerjaschki D., Rettig W.J., Adolf G.R. Effective immunoconjugate therapy in cancer models targeting a serine protease of tumor fibroblasts. Clin. Cancer Res. 2008;14:4584–4592. doi: 10.1158/1078-0432.CCR-07-5211. [DOI] [PubMed] [Google Scholar]
- 25.Kakarla S., Chow K.K.H., Mata M., Shaffer D.R., Song X.-T., Wu M.-F., Liu H., Wang L.L., Rowley D.R., Pfizenmaier K., et al. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol. Ther. 2013;21:1611–1620. doi: 10.1038/mt.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L.-C.S., Lo A., Scholler J., Sun J., Majumdar R.S., Kapoor V., Antzis M., Cotner C.E., Johnson L.A., Durham A.C., et al. Targeting Fibroblast Activation Protein in Tumor Stroma with Chimeric Antigen Receptor T Cells Can Inhibit Tumor Growth and Augment Host Immunity without Severe Toxicity. Cancer Immunol. Res. 2014;2:154–166. doi: 10.1158/2326-6066.CIR-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lo A., Wang L.-C.S., Scholler J., Monslow J., Avery D., Newick K., O’Brien S., Evans R.A., Bajor D.J., Clendenin C., et al. Tumor-Promoting Desmoplasia Is Disrupted by Depleting FAP-Expressing Stromal Cells. Cancer Res. 2015;75:2800–2810. doi: 10.1158/0008-5472.CAN-14-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tran E., Chinnasamy D., Yu Z., Morgan R.A., Lee C.-C.R., Restifo N.P., Rosenberg S.A. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J. Exp. Med. 2013;210:1125–1135. doi: 10.1084/jem.20130110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraman M., Bambrough P.J., Arnold J.N., Roberts E.W., Magiera L., Jones J.O., Gopinathan A., Tuveson D.A., Fearon D.T. Suppression of Antitumor Immunity by Stromal Cells Expressing Fibroblast Activation Protein-alpha. Science. 2010;330:827–830. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 30.Roberts E.W., Deonarine A., Jones J.O., Denton A.E., Feig C., Lyons S.K., Espeli M., Kraman M., McKenna B., Wells R.J., et al. Depletion of stromal cells expressing fibroblast activation protein-α from skeletal muscle and bone marrow results in cachexia and anemia. J. Exp. Med. 2013;210:1137–1151. doi: 10.1084/jem.20122344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofheinz R.-D., Al-Batran S.-E., Hartmann F., Hartung G., Jäger D., Renner C., Tanswell P., Kunz U., Amelsberg A., Kuthan H., et al. Stromal Antigen Targeting by a Humanised Monoclonal Antibody: An Early Phase II Trial of Sibrotuzumab in Patients with Metastatic Colorectal Cancer. Oncol. Res. Treat. 2003;26:44–48. doi: 10.1159/000069863. [DOI] [PubMed] [Google Scholar]
- 32.Ho W.J., Jaffee E.M., Zheng L. The tumour microenvironment in pancreatic cancer—clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 2020;17:527–540. doi: 10.1038/s41571-020-0363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cunningham C.C. Talabostat. Expert Opin. Investig. Drugs. 2007;16:1459–1465. doi: 10.1517/13543784.16.9.1459. [DOI] [PubMed] [Google Scholar]
- 34.Melero I., Alvarez E.C., Mau-Sorensen M., Lassen U., Lolkema M., Robbrecht D., Gomez-Roca C., Martin-Liberal J., Tabernero J., Ros W., et al. 412PD—Clinical activity, safety, and PK/PD from a phase I study of RO6874281, a fibroblast activation protein (FAP) targeted interleukin-2 variant (IL-2v) Ann. Oncol. 2018;29:viii134–viii135. doi: 10.1093/annonc/mdy279.400. [DOI] [Google Scholar]
- 35.Sunshine J.C., Taube J.M. PD-1/PD-L1 inhibitors. Curr. Opin. Pharmacol. 2015;23:32–38. doi: 10.1016/j.coph.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregory R.K., Smith I.E. Vinorelbine—A clinical review. Br. J. Cancer. 2000;82:1907–1913. doi: 10.1054/bjoc.2000.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang X., Lin Y., Shi Y., Li B., Liu W., Ying-Hong S., Dang Y., Chu Y., Fan J., He R. FAP Promotes Immunosuppression by Cancer-Associated Fibroblasts in the Tumor Microenvironment via STAT3–CCL2 Signaling. Cancer Res. 2016;76:4124–4135. doi: 10.1158/0008-5472.CAN-15-2973. [DOI] [PubMed] [Google Scholar]
- 38.Tacke F. Targeting hepatic macrophages to treat liver diseases. J. Hepatol. 2017;66:1300–1312. doi: 10.1016/j.jhep.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 39.Collins M.A., Brisset J.-C., Zhang Y., Bednar F., Pierre J., Heist K.A., Galbán C.J., Galbán S., Di Magliano M.P. Metastatic Pancreatic Cancer Is Dependent on Oncogenic Kras in Mice. PLoS ONE. 2012;7:e49707. doi: 10.1371/journal.pone.0049707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y., Velez-Delgado A., Mathew E., Li D., Mendez F.M., Flannagan K., Rhim A.D., Simeone D.M., Beatty G.L., Di Magliano M.P. Myeloid cells are required for PD-1/PD-L1 checkpoint activation and the establishment of an immunosuppressive environment in pancreatic cancer. Gut. 2017;66:124–136. doi: 10.1136/gutjnl-2016-312078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sleightholm R.L., Neilsen B.K., Li J., Steele M.M., Singh R.K., Hollingsworth M.A., Oupický D. Emerging roles of the CXCL12/CXCR4 axis in pancreatic cancer progression and therapy. Pharmacol. Ther. 2017;179:158–170. doi: 10.1016/j.pharmthera.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 42.Nagarsheth N., Wicha M.S., Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017;17:559–572. doi: 10.1038/nri.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biasci D., Smoragiewicz M., Connell C.M., Wang Z., Gao Y., Thaventhiran J.E.D., Basu B., Magiera L., Johnson T.I., Bax L., et al. CXCR4 inhibition in human pancreatic and colorectal cancers induces an integrated immune response. Proc. Natl. Acad. Sci. USA. 2020;117:28960–28970. doi: 10.1073/pnas.2013644117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bockorny B., Semenisty V., Macarulla T., Borazanci E., Wolpin B.M., Stemmer S.M., Golan T., Geva R., Borad M.J., Pedersen K.S., et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: The COMBAT trial. Nat. Med. 2020;26:878–885. doi: 10.1038/s41591-020-0880-x. [DOI] [PubMed] [Google Scholar]
- 45.Su S., Chen J., Yao H., Liu J., Yu S., Lao L., Wang M., Luo M., Xing Y., Chen F., et al. CD10+GPR77+ Cancer-Associated Fibroblasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness. Cell. 2018;172:841–856.e16. doi: 10.1016/j.cell.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 46.Gerard N.P., Lu B., Liu P., Craig S., Fujiwara Y., Okinaga S., Gerard C. An Anti-inflammatory Function for the Complement Anaphylatoxin C5a-binding Protein, C5L2. J. Biol. Chem. 2005;280:39677–39680. doi: 10.1074/jbc.C500287200. [DOI] [PubMed] [Google Scholar]
- 47.Purcell J., Tanlimco S.G., Hickson J.A., Fox M., Sho M., Durkin L., Uziel T., Powers R., Foster K., McGonigal T., et al. LRRC15 Is a Novel Mesenchymal Protein and Stromal Target for Antibody–Drug Conjugates. Cancer Res. 2018;78:4059–4072. doi: 10.1158/0008-5472.CAN-18-0327. [DOI] [PubMed] [Google Scholar]
- 48.Dominguez C.X., Müller S., Keerthivasan S., Koeppen H., Hung J., Gierke S., Breart B., Foreman O., Bainbridge T.W., Castiglioni A., et al. Single-Cell RNA Sequencing Reveals Stromal Evolution into LRRC15+ Myofibroblasts as a Determinant of Patient Response to Cancer Immunotherapy. Cancer Discov. 2019;10:232–253. doi: 10.1158/2159-8290.CD-19-0644. [DOI] [PubMed] [Google Scholar]
- 49.Demetri G.D., Luke J.J., Hollebecque A., Powderly J.D., Spira A.I., Subbiah V., Lai D.W., Yue H., Kasichayanula S., Gulbranson S., et al. First-in-human phase 1 study of ABBV-085, an antibody-drug conjugate (ADC) targeting LRRC15, in sarcomas and other advanced solid tumors. J. Clin. Oncol. 2019;37:3004. doi: 10.1200/JCO.2019.37.15_suppl.3004. [DOI] [PubMed] [Google Scholar]
- 50.Huang H., Zhang Y., Gallegos V., Sorrelle N., Zaid M.M., Toombs J., Du W., Wright S., Hagopian M., Wang Z., et al. Targeting TGF βR2-mutant tumors exposes vulnerabilities to stromal TGF β blockade in pancreatic cancer. EMBO Mol. Med. 2019;11:e10515. doi: 10.15252/emmm.201910515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mace T.A., Shakya R., Pitarresi J.R., Swanson B., McQuinn C.W., Loftus S., Nordquist E., Cruz-Monserrate Z., Yu L., Young G., et al. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut. 2018;67:320–332. doi: 10.1136/gutjnl-2016-311585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rossi J.-F., Lu Z.-Y., Jourdan M., Klein B. Interleukin-6 as a Therapeutic Target. Clin. Cancer Res. 2015;21:1248–1257. doi: 10.1158/1078-0432.CCR-14-2291. [DOI] [PubMed] [Google Scholar]
- 53.Sebba A. Tocilizumab: The first interleukin-6-receptor inhibitor. Am. J. Health Pharm. 2008;65:1413–1418. doi: 10.2146/ajhp070449. [DOI] [PubMed] [Google Scholar]
- 54.Biffi G., Oni T.E., Spielman B., Hao Y., Elyada E., Park Y., Preall J., Tuveson D.A. IL1-Induced JAK/STAT Signaling Is Antagonized by TGFβ to Shape CAF Heterogeneity in Pancreatic Ductal Adenocarcinoma. Cancer Discov. 2019;9:282–301. doi: 10.1158/2159-8290.CD-18-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Öhlund D., Handly-Santana A., Biffi G., Elyada E., Almeida A.S., Ponz-Sarvise M., Corbo V., Oni T.E., Hearn S.A., Lee E.J., et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 2017;214:579–596. doi: 10.1084/jem.20162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schenk K.M., Reuss J.E., Choquette K., Spira A.I. A review of canakinumab and its therapeutic potential for non-small cell lung cancer. Anti-Cancer Drugs. 2019;30:879–885. doi: 10.1097/CAD.0000000000000832. [DOI] [PubMed] [Google Scholar]
- 57.Shi Y., Gao W., Lytle N.K., Huang P., Yuan X., Dann A.M., Ridinger-Saison M., DelGiorno K.E., Antal C.E., Liang G., et al. Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nat. Cell Biol. 2019;569:131–135. doi: 10.1038/s41586-019-1130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi Y., Du L., Lin L., Wang Y. Tumour-associated mesenchymal stem/stromal cells: Emerging therapeutic targets. Nat. Rev. Drug Discov. 2017;16:35–52. doi: 10.1038/nrd.2016.193. [DOI] [PubMed] [Google Scholar]
- 59.Niess H., Von Einem J.C., Thomas M.N., Michl M., Angele M.K., Huss R., Günther C., Nelson P.J., Bruns C.J., Heinemann V. Treatment of advanced gastrointestinal tumors with genetically modified autologous mesenchymal stromal cells (TREAT-ME1): Study protocol of a phase I/II clinical trial. BMC Cancer. 2015;15:237. doi: 10.1186/s12885-015-1241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Von Einem J.C., Peter S., Günther C., Volk H.-D., Grütz G., Salat C., Stoetzer O., Nelson P.J., Michl M., Modest D.P., et al. Treatment of advanced gastrointestinal cancer with genetically modified autologous mesenchymal stem cells—TREAT-ME-1—A phase I, first in human, first in class trial. Oncotarget. 2017;8:80156–80166. doi: 10.18632/oncotarget.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Einem J.C., Guenther C., Volk H., Grütz G., Hirsch D., Salat C., Stoetzer O., Nelson P.J., Michl M., Modest D.P., et al. Treatment of advanced gastrointestinal cancer with genetically modified autologous mesenchymal stem cells: Results from the phase 1/2 TREAT-ME-1 trial. Int. J. Cancer. 2019;145:1538–1546. doi: 10.1002/ijc.32230. [DOI] [PubMed] [Google Scholar]
- 62.Kucerova L., Altanerova V., Matuskova M., Tyciakova S., Altaner C. Adipose Tissue–Derived Human Mesenchymal Stem Cells Mediated Prodrug Cancer Gene Therapy. Cancer Res. 2007;67:6304–6313. doi: 10.1158/0008-5472.CAN-06-4024. [DOI] [PubMed] [Google Scholar]
- 63.Kucerova L., Matuskova M., Pastorakova A., Tyciakova S., Jakubikova J., Bohovic R., Altanerova V., Altaner C. Cytosine deaminase expressing human mesenchymal stem cells mediated tumour regression in melanoma bearing mice. J. Gene Med. 2008;10:1071–1082. doi: 10.1002/jgm.1239. [DOI] [PubMed] [Google Scholar]
- 64.Hutzen B., Pierson C.R., Russell S.J., Galanis E., Raffel C., Studebaker A.W. Treatment of medulloblastoma using an oncolytic measles virus encoding the thyroidal sodium iodide symporter shows enhanced efficacy with radioiodine. BMC Cancer. 2012;12:508. doi: 10.1186/1471-2407-12-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goyal H., Perisetti A., Rahman M.R., Levin A., Giuseppe L. Vitamin D and Gastrointestinal Cancers: A Narrative Review. Dig. Dis. Sci. 2018;64:1098–1109. doi: 10.1007/s10620-018-5400-1. [DOI] [PubMed] [Google Scholar]
- 66.Skinner H.G., Michaud D.S., Giovannucci E., Willett W.C., Colditz G.A., Fuchs C.S. Vitamin D Intake and the Risk for Pancreatic Cancer in Two Cohort Studies. Cancer Epidemiol. Biomark. Prev. 2006;15:1688–1695. doi: 10.1158/1055-9965.EPI-06-0206. [DOI] [PubMed] [Google Scholar]
- 67.Yu W.-D., Ma Y., Flynn G., Muindi J.R., Kong R.-X., Trump D.L., Johnson C.S. Calcitriol enhances gemcitabine antitumor activity in vitro and in vivo by promoting apoptosis in a human pancreatic carcinoma model system. Cell Cycle. 2010;9:3094–3101. doi: 10.4161/cc.9.15.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sherman M.H., Yu R.T., Engle D.D., Ding N., Atkins A.R., Tiriac H., Collisson E.A., Connor F., Van Dyke T., Kozlov S., et al. Vitamin D Receptor-Mediated Stromal Reprogramming Suppresses Pancreatitis and Enhances Pancreatic Cancer Therapy. Cell. 2014;159:80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferrer-Mayorga G., Gómez-López G., Barbáchano A., Fernández-Barral A., Peña C., Pisano D.G., Cantero R., Rojo F., Muñoz A., Larriba M.J. Vitamin D receptor expression and associated gene signature in tumour stromal fibroblasts predict clinical outcome in colorectal cancer. Gut. 2016;66:1449–1462. doi: 10.1136/gutjnl-2015-310977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Merrigan S., Kennedy B. Vitamin D receptor agonists regulate ocular developmental angiogenesis and modulate expression of dre-miR-21 and VEGF. Br. J. Pharmacol. 2017;174:2636–2651. doi: 10.1111/bph.13875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Slavikova V., Chlapek P., Mazanek P., Sterba J., Veselska R. Traffic lights for retinoids in oncology: Molecular markers of retinoid resistance and sensitivity and their use in the management of cancer differentiation therapy. BMC Cancer. 2018;18:1–13. doi: 10.1186/s12885-018-4966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Froeling F.E., Feig C., Chelala C., Dobson R., Mein C.E., Tuveson D., Clevers H., Hart I.R., Kocher H.M. Retinoic Acid–Induced Pancreatic Stellate Cell Quiescence Reduces Paracrine Wnt–β-Catenin Signaling to Slow Tumor Progression. Gastroenterology. 2011;141:1486–1497.e14. doi: 10.1053/j.gastro.2011.06.047. [DOI] [PubMed] [Google Scholar]
- 73.Gupta S., Pramanik D., Mukherjee R., Campbell N.R., Elumalai S., De Wilde R.F., Hong S.-M., Goggins M.G., De Jesus-Acosta A., Laheru D., et al. Molecular Determinants of Retinoic Acid Sensitivity in Pancreatic Cancer. Clin. Cancer Res. 2012;18:280–289. doi: 10.1158/1078-0432.CCR-11-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Noy N. Non-classical Transcriptional Activity of Retinoic Acid. Macromol. Protein Complexes III Struct. Funct. 2016;81:179–199. doi: 10.1007/978-94-024-0945-1_7. [DOI] [PubMed] [Google Scholar]
- 75.Han X., Li Y., Xu Y., Zhao X., Zhang Y., Yang X., Wang Y., Zhao R., Anderson G.J., Zhao Y., et al. Reversal of pancreatic desmoplasia by re-educating stellate cells with a tumour microenvironment-activated nanosystem. Nat. Commun. 2018;9:1–18. doi: 10.1038/s41467-018-05906-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu J., Xiong G., Fu H., Evers B.M., Zhou B.P., Xu R. Chaperone Hsp47 Drives Malignant Growth and Invasion by Modulating an ECM Gene Network. Cancer Res. 2015;75:1580–1591. doi: 10.1158/0008-5472.CAN-14-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ene–Obong A., Clear A.J., Watt J., Wang J., Fatah R., Riches J.C., Marshall J.F., Chin-Aleong J., Chelala C., Gribben J.G., et al. Activated Pancreatic Stellate Cells Sequester CD8+ T Cells to Reduce Their Infiltration of the Juxtatumoral Compartment of Pancreatic Ductal Adenocarcinoma. Gastroenterology. 2013;145:1121–1132. doi: 10.1053/j.gastro.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kocher H.M., Basu B., Froeling F.E.M., Sarker D., Slater S., Carlin D., DeSouza N.M., De Paepe K.N., Goulart M.R., Hughes C., et al. Phase I clinical trial repurposing all-trans retinoic acid as a stromal targeting agent for pancreatic cancer. Nat. Commun. 2020;11:1–9. doi: 10.1038/s41467-020-18636-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ocaña O.H., Córcoles R., Fabra Á., Moreno-Bueno G., Acloque H., Vega S., Barrallo-Gimeno A., Cano A., Nieto M.A. Metastatic Colonization Requires the Repression of the Epithelial-Mesenchymal Transition Inducer Prrx1. Cancer Cell. 2012;22:709–724. doi: 10.1016/j.ccr.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 80.Reichert M., Takano S., Von Burstin J., Kim S.-B., Lee J.-S., Ihida-Stansbury K., Hahn C., Heeg S., Schneider G., Rhim A.D., et al. The Prrx1 homeodomain transcription factor plays a central role in pancreatic regeneration and carcinogenesis. Genes Dev. 2013;27:288–300. doi: 10.1101/gad.204453.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takano S., Reichert M., Bakir B., Das K.K., Nishida T., Miyazaki M., Heeg S., Collins M.A., Marchand B., Hicks P.D., et al. Prrx1 isoform switching regulates pancreatic cancer invasion and metastatic colonization. Genes Dev. 2016;30:233–247. doi: 10.1101/gad.263327.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feldmann K., Maurer C., Peschke K., Teller S., Schuck K., Steiger K., Engleitner T., Öllinger R., Nomura A., Wirges N., et al. Mesenchymal Plasticity Regulated by Prrx1 Drives Aggressive Pancreatic Cancer Biology. Gastroenterology. 2021;160:346–361. doi: 10.1053/j.gastro.2020.09.010. [DOI] [PubMed] [Google Scholar]
- 83.Bailey P., Initiative A.P.C.G., Chang D.K., Nones K., Johns A.L., Patch A.-M., Gingras M.-C., Miller D.K., Christ A.N., Bruxner T.J.C., et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 84.Lee K.-W., Yeo S.-Y., Sung C.O., Kim S.-H. Twist1 Is a Key Regulator of Cancer-Associated Fibroblasts. Cancer Res. 2014;75:73–85. doi: 10.1158/0008-5472.CAN-14-0350. [DOI] [PubMed] [Google Scholar]
- 85.Yeo S.-Y., Lee K.-W., Shin D., An S., Cho K.-H., Kim S.-H. A positive feedback loop bi-stably activates fibroblasts. Nat. Commun. 2018;9:1–16. doi: 10.1038/s41467-018-05274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]