Abstract
For mass production of liposomes, we designed a plastic micro-channel device on the basis of 5 μm of micro-nozzle array forming T-junction with 100 μm depth of micro-channel. A micro-channel unit for synthesizing liposomes consisted of two micro-nozzle arrays for mixing two solutions as well as delivery and recovery channels for supplying solutions and collecting liposome suspension. The number of micro-nozzles was approximately 2400 for a micro-channel unit, and seven units were applied independently on a micro-channel plate. The plastic micro-channel plate was injection-molded for mass production using a micro-channel stamper previously fabricated by UV lithography and nickel electroforming process. A plastic cover plate with seven pairs of inlet and outlet ports was machined by mechanical milling and drilling and was assembled with a micro-channel plate using a holder to form a liposome synthesizing device. Flow and mixing of solutions in the micro-channels were tested using colored water to check the micro-fluidic characteristics of the device. Finally, a L-α-phosphatidylcholine (SOY PC) liposome was synthesized using EtOH solution of SOY PC (95%) and saline (0.85% NaOH solution) to find that the liposomes were around 230 and 260 nm in diameter, depending on the flow rate of the lipid solution.
Keywords: liposome, micro-nozzle array, micro-channel, injection molding, plastic
1. Introduction
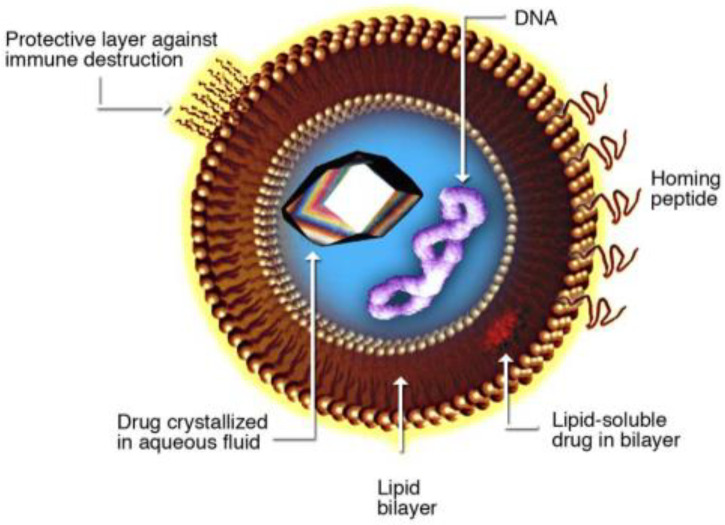
Liposome is a spherical lipid bilayer (Figure 1) that can encapsulate liquid containing various functional substances such as drugs, proteins, and nutrients [1]. Size of the liposome ranges from several tens of nanometers to several micrometers depending on the lipid, buffer solution, and process conditions such as flow rate of solution. Due to the good protective and controlling ability of the functional substance contained within, liposome is more widely used for applications in drug delivery [2,3,4,5,6,7], health supplements [8,9], cosmetics [10,11], oral vaccine [12,13], and biosensors [14].
Figure 1.
Schematic of liposome.
To synthesize liposome, several bulk synthesis methods are used, such as ultrasonic emulsification [15], extrusion [16,17,18], and microfluidizer [19]. However, liposome synthesized by these bulk processes is generally larger than several hundreds of nanometers and shows a relatively broad size distribution. Smaller and more uniformly sized liposome has been synthesized successfully using various types of micro-fluidic channels such as T-junction channels, mostly in small amounts [20,21]. Although liposome synthesis using such channels shows outstanding performance regarding uniformity and control of size, the low throughput makes it difficult for industrial use. To solve this issue, researchers conducted studies on liposome synthesis using micro-pore membrane as a micro-fluidic platform, showing good productivity due to the large number of micro-pores on the membrane and parallel synthesizing through each pore [22,23].
In this study, a micro-channel device was designed on the basis of T-junction type of micro-channel array for scalable production of liposome. The micro-channel plate was injection-molded using thermoplastic for mass production, mechanical strength, and dimensional stability. The injection-molded micro-channel plate was sealed through mechanical assembly using a plastic cover plate with inlet and outlet ports. Using the micro-channel device, we synthesized L-α-phosphatidylcholine (SOY PC) liposomes; afterwards, their size and distribution were measured.
2. Design and Fabrication of a Micro-Channel Device for Synthesizing Liposome
2.1. Design for Micro-Channel Device
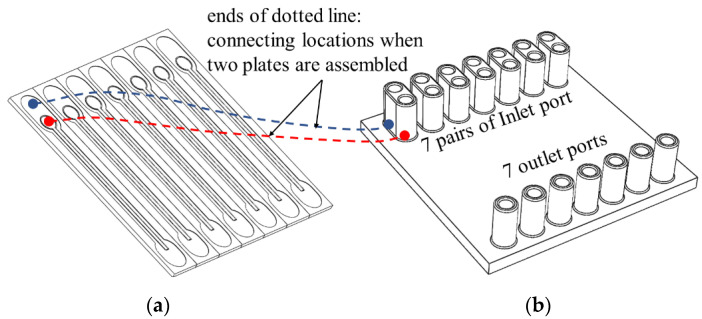
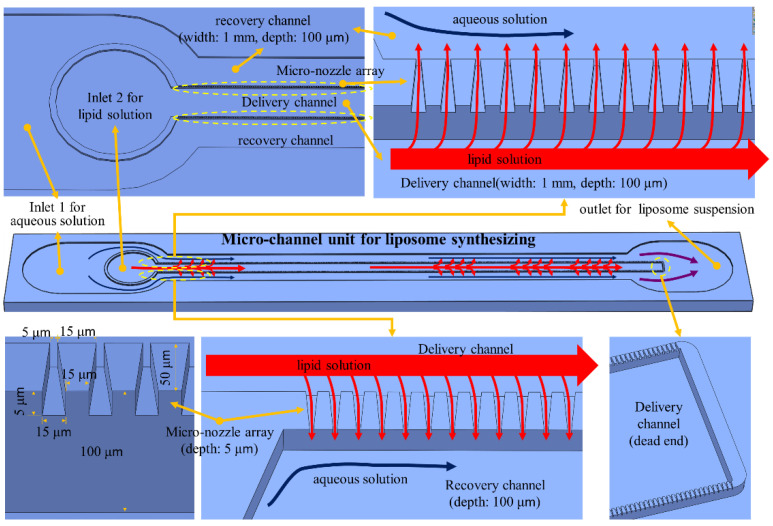
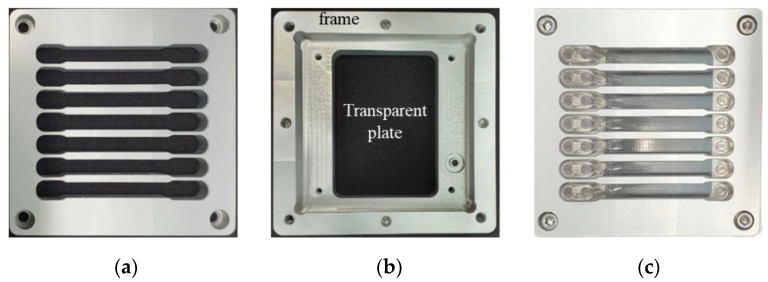
The micro-channel device for synthesizing liposome consists of a micro-channel plate and a cover plate with inlet and outlet ports (Figure 2). A micro-channel unit for liposome synthesis was designed to include a micro-nozzle array, a delivery channel, and two recovery channels (Figure 3). The delivery channel to deliver lipid solution is placed at the center and connected to two micro-nozzle arrays on both sides. To form multiple micro-T junctions, each micro-nozzle array connects outside with a recovery channel to supply aqueous solution for mixing with lipid solution passing through micro-nozzles from the delivery channel and to collect liposome suspension of the mixture at the same time. Two recovery channels merge into a single port to collect liposome suspension into a bottle through a tube. In this design, the lipid solution can only be mixed with the aqueous solution by passing through micro-nozzles after the delivery channel delivers it. A 37 mm long linear micro-nozzle array contains approximately 1220 micro-nozzles that gradually taper towards the exit. The dimension of a micro-nozzle is 15 μm and 5 μm in width at entrance and exit, respectively, and 50 μm in length and 5 μm in height. The delivery channel and recovery channel were designed to be 1 mm in width and 100 μm in depth to reduce pressure loss along the channel.
Figure 2.
Parts for liposome synthesizing micro-channel device: (a) micro-channel plate and (b) cover plate.
Figure 3.
Schematics and design for a unit liposome-synthesizing micro-channel.
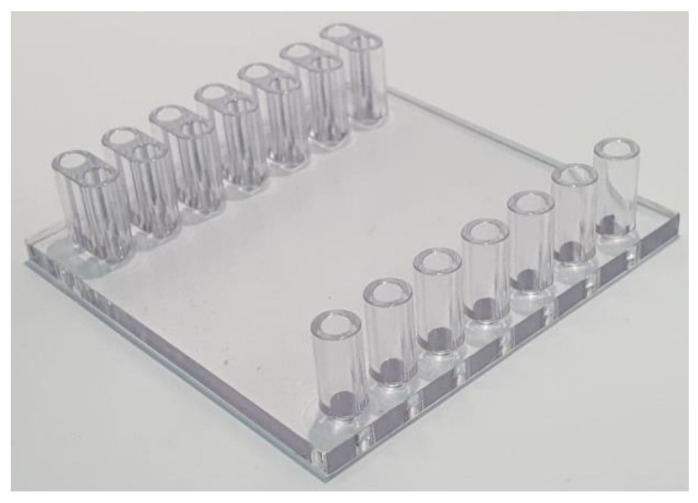
As shown in Figure 2, seven liposome synthesizing micro-channel units were applied to a micro-channel plate, which is 50 mm × 50 mm in size and 2 mm in thickness. The cover plate has seven pairs of ports connecting to delivery channel and recovery channel. Each pair of ports consists of two inlet ports for supplying lipid solution and aqueous solution each, and one outlet port for collecting liposome suspension.
2.2. Fabrication of the Plastic Device
First, two parts of the liposome-synthesizing device, a micro-channel plate, and a cover plate, as shown in Figure 2, were injection-molded using thermoplastic resin or machined mechanically on plastic plate; details on the stamper fabrication process, injection molding conditions, mechanical machining by milling and drilling, and plastic materials used for the parts are explained in Section 2.3 and Section 2.4. Two plates were assembled tightly using a holder to seal the micro-channels on the micro-channel plate with the cover plate (Figure 4). The micro-channel plate was injection-molded using a nickel stamper, fabricated as explained in the following section because the micro-nozzles cannot be machined mechanically due to their size. Moreover, injection molding is suitable for mass production of micro-channel plate.
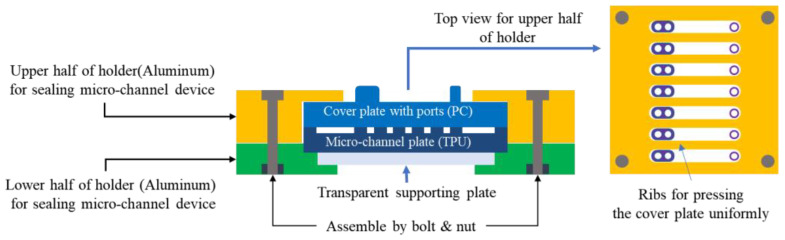
Figure 4.
Schematic of a holder for assembling a micro-channel plate and a cover plate.
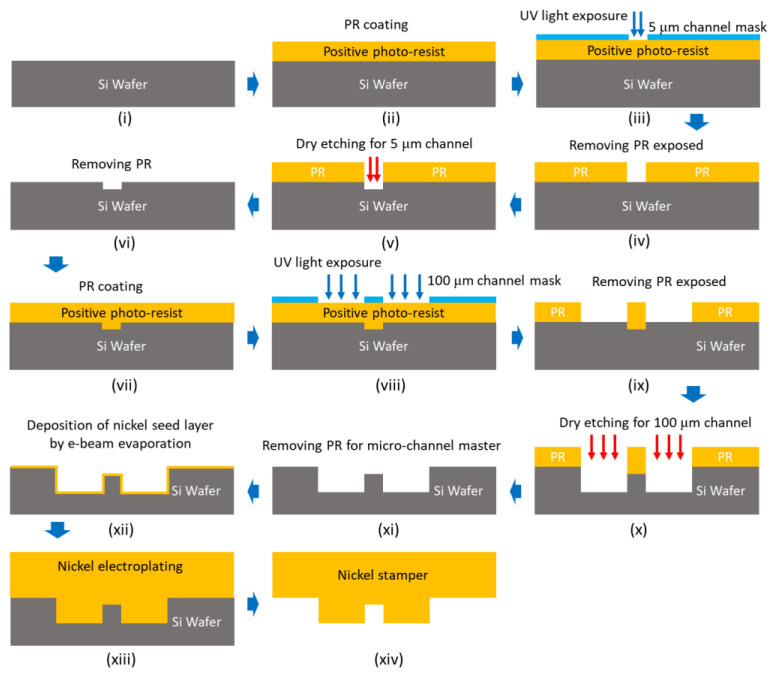
2.3. Fabrication of Micro-Channel Stamper
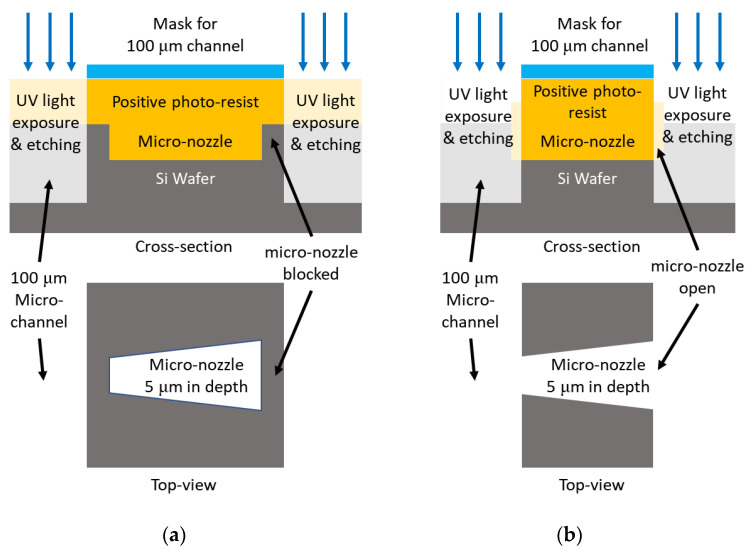
For injection molding of micro-channel plate, we fabricated a durable nickel stamper to be 0.5 mm in thickness and to have negative structure for micro-channel based on UV lithography process on silicon wafer and nickel electroforming process. The overall fabrication process for nickel stamper is shown in Figure 5. To fabricate stamper with channels of different depths, 5 μm-deep micro-nozzles, and 100 μm-deep delivery and recovery channel, we first fabricated silicon pattern master by repeating photo-resisting patterning and etching process twice, each using a different pattern mask, shown in steps (iii) and (viii) in Figure 5. Misalignment of these two masks can cause disconnection between the micro-nozzles and delivery or recovery channel, as shown in Figure 6a. To prevent this, we designed the masking region of the second mask aligned to the micro-nozzle array that was previously machined during steps (iii)–(vi) to be shorter by 5 μm than the length of the micro-nozzle to ensure the ends of the micro-nozzle were open, as shown in Figure 6b.
Figure 5.
Fabrication procedure for nickel stamper including photo lithography and electroforming.
Figure 6.
Schematic for (a) micro-nozzle blocked due to misalignment of mask and (b) design for second mask.
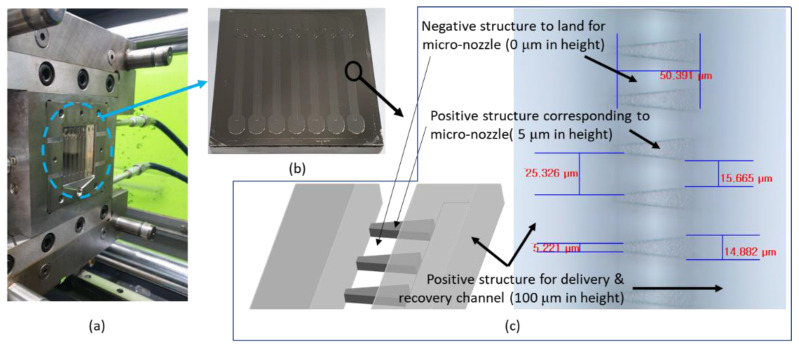
As shown in Figure 7, a nickel stamper was welded on a metal block, one of the parts for injection-molding mold. The microscopic image shows relief structures on the nickel stamper, which correspond to micro-nozzles on injection-molded micro-channel plate, and the regions out of focus correspond to delivery and recovery channel or lands in micro-nozzle array.
Figure 7.
Images for (a) half of mold with an insert for injection molding, (b) micro-channel stamper insert, and (c) micro-channels and micro-nozzles on the stamper.
2.4. Injection Molding of Plastic Micro-Channel Plate and Mechanical Machining of Cover Plate
After mounting the nickel stamper block on the wall of the mold cavity, as shown in Figure 7, we repeatedly injection-molded the micro-channel plate using thermoplastic urethane (TPU, Lubrizol TPU-195A, Wickliffe, OH, USA), one of the thermoplastic elastomers. For injection-molding of TPU micro-channel plate, plasticizing temperature and mold temperature were 210 °C and 30 °C, respectively. The injection rate was 60 mm/s, and packing pressure of 900 kgf/cm2 was applied first for 1 s and then 800 kgf/cm2 for 2.5 s.
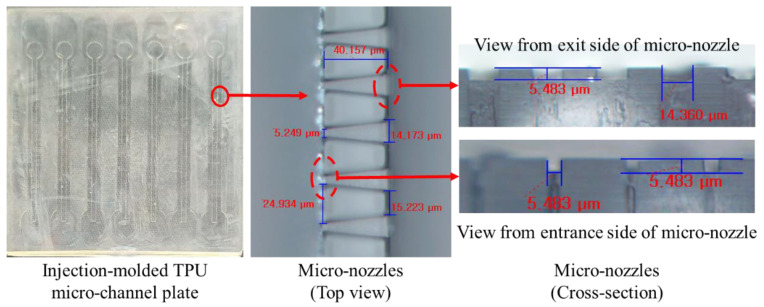
Figure 8 shows the top and cross-sections of injection-molded micro-channel plate and microscopic image micro-nozzles. From the microscopic image, we can see that the micro-nozzle was injection-molded well to the corner of the land of micro-nozzle. The cycle time for micro-channel plate was less than 30 s, which is fast enough for mass production.
Figure 8.
Photo for an injection-molded thermoplastic urethane (TPU) micro-channel plate and microscopic images for micro-nozzles.
As shown in Figure 9, the cover plate with inlet and outlet ports was machined by mechanical milling and drilling using polycarbonate workpiece, since the structures for cover plate are good enough to be machined directly. The cover plate can also be injection-molded easily for mass production.
Figure 9.
A cover plate with inlet and outlet ports machined by mechanical milling and drilling using polycarbonate block.
2.5. Sealing of Micro-Channel Device
For sealing micro-channels and making the micro-channel device work, we stacked the micro-channel plate and cover plate and inserted them between two halves of an aluminum holder assembled by bolting to press the plates tightly (Figure 4). As seen in Figure 10a, the upper half of the holder has ribs to press the cover plate with ports more evenly. The lower half of the holder presses the micro-channel plate injection molded with TPU, which is deformed easily and therefore is good for sealing of micro-channels when covered with a rigid plate. The lower half of the holder consists of a rigid outer frame and a transparent plate for monitoring the flow in the channel (Figure 10b). The transparent plate also supports the channel plate to prevent deformation or bending when the channel plate and cover plate are assembled tightly, which may result in leakage from the channels.
Figure 10.
Aluminum holder machined mechanically for micro-channel device: (a) upper half of holder, (b) lower half of holder, and (c) assembly of holder and micro-channel device.
3. Mixing Fluids and Synthesizing Liposome Using Micro-Channel Device
3.1. Test for Mixing of Two Fluids in Micro-Channel Device
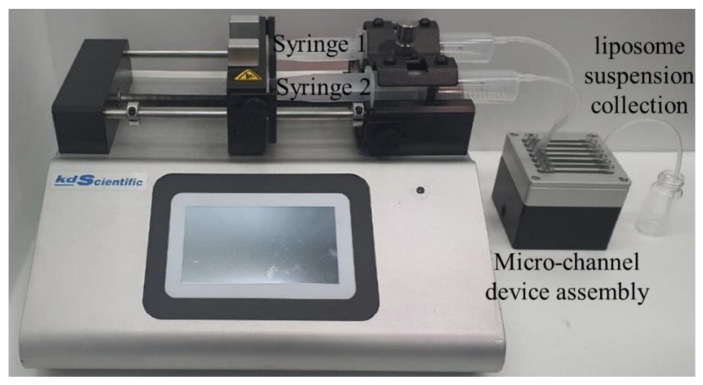
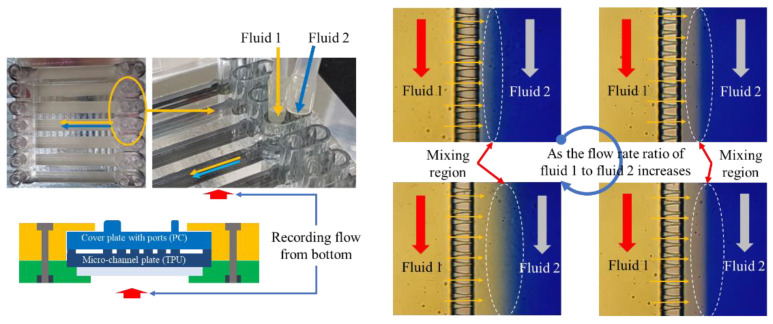
For testing flow and mixing of fluids through micro-nozzle and channels, we used two types of water, one yellow and the other blue, for visualization of flow and mixing. The yellow water was supplied to the delivery channel through the inlet port for lipid solution (inlet port 2 in Figure 3) and flowed into recovery channel through the micro-nozzle to mix with the blue water supplied to the recovery channel through inlet port for buffer solution (inlet port 1 in Figure 3). The flow rate for each fluid was controlled independently using a syringe pump, as shown in Figure 11.
Figure 11.
Experiment set-up for flow and mixing test or synthesizing liposome using micro-channel device.
When we supplied yellow and blue water to the inlet ports, some microscopic images were taken on interfacial regions of yellow and blue water along the recovery channel where two fluids mixed, with the neighboring region including micro-nozzle array and delivery channel (Figure 12). The interface between two fluids was formed parallel to the recovery channel due to the flow injected orthogonally from micro-nozzles arrayed closely in a row. Depending on the flow rate of fluid through inlet 1 for lipid solution, the location of the interface moved close to the micro-nozzle array or away from the micro-nozzle array.
Figure 12.
Flow and mixing visualization in micro-channel device.
3.2. Synthesizing Liposome Using Micro-Channel Device
SOY PC (95%) (L-α-phosphatidylcholine, Avanti Polar Lipids, Inc., alabaster, AL, USA) and saline (0.85% NaOH solution) were used to synthesize liposome using micro-channel device. A total of 0.775 g of granular SOY PC was mixed with 10 mL of EtOH for 3 h using a magnetic stirrer to produce 10 mM of SOY PC EtOH solution. This SOY PC solution was supplied to the micro-channel device through inlet port 1 at two different flow rates, 3 mL/h and 4.5 mL/h. Saline, 0.85% of NaOH solution, was supplied through inlet port 2 at a 30 mL/h flow rate (Figure 3). Liposome synthesis experiments were repeated three times, each for a set of flow rate conditions using the same SOY PC EtOH solution and saline.
The liposome suspension was heated up to 80 °C for 1 h so that it could be volatilized after being collected through the outlet port from recovery channel where the two solutions were mixed. The boiled liposome suspension was cooled down to room temperature and stored in a chamber at 5 °C for 20 h. NANOPHOX (Sympatec GmbH, Clausthal-Zellerfeld, Germany), a photon cross-correlation spectroscopy (PCCS), was used to analyze the size of the liposome in the suspension.
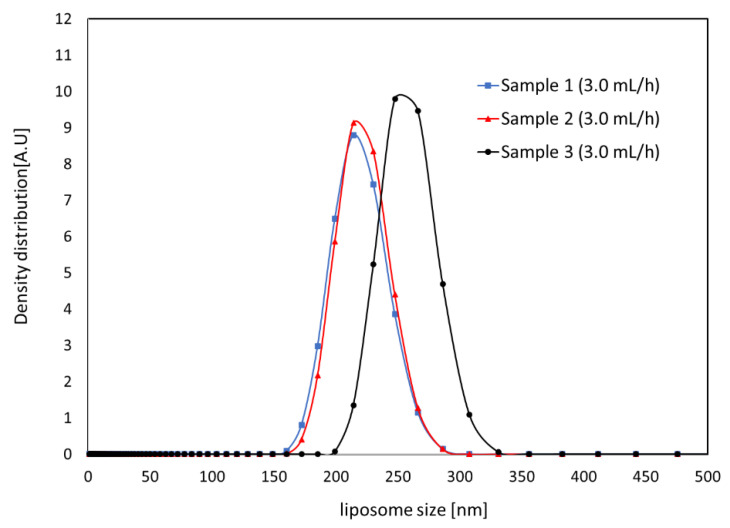
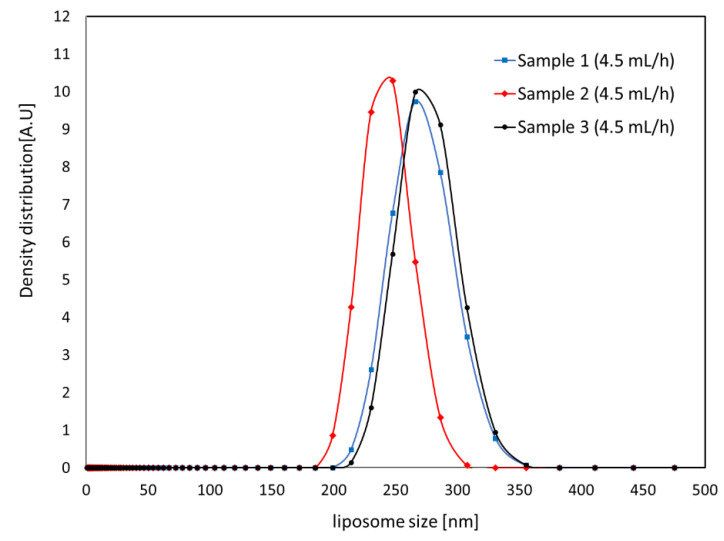
The results for the measured size of the SOY PC liposomes synthesized by micro-channel device are shown in Figure 13 and Figure 14. The graph shows the intensity of the signal depending on the size of the liposome in the solution. The average diameter, , and standard deviation, , of the liposomes in a sample were estimated by Equation (1) and Equation (2), respectively, to observe size variation in the sample, and the results are shown in Table 1. For both flow rate conditions faster than 30 mL/h of total flow rate, liposome was smaller than 300 nm with approximately 10% of standard deviation of average size.
| (1) |
| (2) |
where and are diameter of liposome and signal density from spectroscopy for liposome of diameter , respectively.
Figure 13.
Liposome size distribution measured for three samples synthesized at 3.0 mL/h of lipid solution and 30 mL/h of saline.
Figure 14.
Liposome size distribution measured for three samples synthesized at 4.5 mL/h of lipid solution and 30 mL/h of saline.
Table 1.
Average and standard deviation for liposomes.
| Sample | Lipid Solution 3 mL/h, Saline 30 mL/h | Lipid Solution 4.5 mL/h, Saline 30 mL/h | ||
|---|---|---|---|---|
| Average Size (nm) | Standard Deviation | Average Size (nm) | Standard Deviation | |
| I | 217 | 22 | 270 | 25 |
| II | 220 | 21 | 242 | 20 |
| III | 257 | 22 | 274 | 24 |
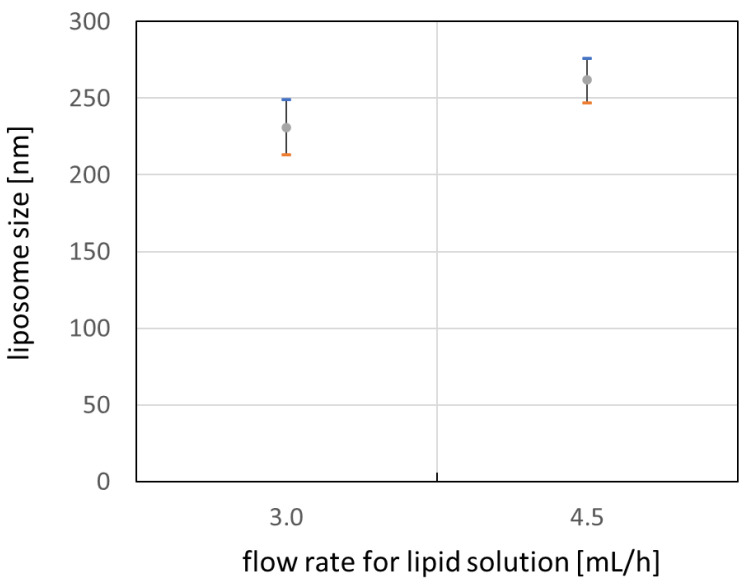
Figure 15 shows average size of liposomes for each flow rate conditions with error bar of one standard deviation. It is noticed that the size of the liposome can be controlled by flow rate of the lipid solution or buffer. As the ratio of lipid solution flow rate to the buffer flow rate increases, the characteristic time for mixing two solution increases also. This increase in mixing time may enhance the growth of the lipid bilayer and result in larger liposomes when the lipid bilayer forms a vesicle after linear growth [24,25,26]. However, extended experimental investigation may be required to figure out the effect on the size of the liposome for a wider range of flow rate conditions.
Figure 15.
Liposome size depending on flow rate for lipid solution.
From the viewpoint of throughput, 30 mL/h of flow rate in this work is much higher than a flow rate less than 1 mL/h for typical single T-junction micro-channel [21]. This indicates that the throughput for synthesizing liposome can be enhanced greatly by T-junction array formed by micro-nozzles and recovery channel. In addition, synthesizing liposome is scalable since the throughput can be enhanced by magnitude of the number of T-junction arrays in a micro-channel device when the solutions are supplied simultaneously to all arrays at the same time.
4. Conclusions
A high-throughput plastic micro-channel device for liposome synthesis was designed and fabricated by injection molding or mechanical machining depending on part and assembling the parts. The micro-channel plate, a core part of the liposome synthesizing device, was injection-molded with a cycle time of less than 30 s, which is fast enough for mass production. The micro-channel device showed that fluids flowed and mixed well through micro-nozzles and micro-channels. Using the micro-channel device, we synthesized SOY PC liposome by mixing EtOH solution of SOY PC and saline (0.85% NaOH solution). The size of the liposome varied depending on the flow rate conditions of solutions. In the case of 30 mL/h of flow rate for saline, the liposome diameter was 230 nm or 260 nm for 3.0 mL/h or 4.5 mL/h SOY PC solution, respectively. The size of each liposome sample had a standard deviation of less than 20 nm, which indicates good uniformity in size.
Overall, this study implies that the throughput for synthesizing liposome can be enhanced linearly by using multiple micro-channel units. Additional extensive studies are required to figure out the precise correlation between size of the liposome and flow rate.
Author Contributions
Conceptualization, Y.-E.Y. and S.K.K.; methodology, S.-W.W. and Y.K.J.; software, Y.K.J.; formal analysis, S.-W.W., Y.K.J., Y.-E.Y., and S.K.K.; investigation, S.-W.W. and Y.K.J.; data curation, S.-W.W. and Y.K.J.; writing—original draft preparation, S.-W.W. and Y.K.J.; writing—review and editing, Y.-E.Y. and S.K.K.; visualization, S.-W.W.; supervision, Y.-E.Y. and S.K.K.; project administration, Y.-E.Y.; funding acquisition, Y.-E.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Technology Innovation Program (20003670) funded by the Ministry of Trade, Industry and Energy (MOTIE, Korea) and the Institute Project of Korea Institute of Machinery and Materials (NK232E).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Torchilin V.P. Multifunctional nanocarriers. Adv. Drug Deliv. Rev. 2006;58:1532. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Cevc G., Schätzlein A., Blume G. Transdermal drug carriers: Basic properties, optimization and transfer efficiency in the case of epicutaneously applied peptides. J. Control. Release. 1995;36:3–16. doi: 10.1016/0168-3659(95)00056-E. [DOI] [Google Scholar]
- 3.Kono K. Thermosensitive polymer-modified liposomes. Adv. Drug Deliv. Rev. 2001;53:307–319. doi: 10.1016/S0169-409X(01)00204-6. [DOI] [PubMed] [Google Scholar]
- 4.Needham D., Dewhirst M.W. The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors. Adv. Drug Deliv. Rev. 2001;53:285–305. doi: 10.1016/S0169-409X(01)00233-2. [DOI] [PubMed] [Google Scholar]
- 5.Drummond D.C., Zignani M., Leroux J. Current status of pH-sensitive liposomes in drug delivery. Prog. Lipid Res. 2000;39:409–460. doi: 10.1016/S0163-7827(00)00011-4. [DOI] [PubMed] [Google Scholar]
- 6.Ran R., Middelberg A.P., Zhao C.X. Microfluidic synthesis of multifunctional liposomes for tumour targeting. Colloids Surf. B Biointerfaces. 2016;148:402–410. doi: 10.1016/j.colsurfb.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Pattni B.S., Chupin V.V., Torchilin V.P. New Developments in Liposomal in Drug Delivery. Chem. Rev. 2015;115:10938–10966. doi: 10.1021/acs.chemrev.5b00046. [DOI] [PubMed] [Google Scholar]
- 8.Reza Mozafari M., Johnson C., Hatziantoniou S., Demetzos C. Nanoliposomes and their applications in food nanotechnology. J. Liposome Res. 2008;18:309. doi: 10.1080/08982100802465941. [DOI] [PubMed] [Google Scholar]
- 9.Thompson A.K., Singh H. Preparation of Liposomes from Milk Fat Globule Membrane Phospholipids Using a Microfluidizer. J. Dairy Sci. 2006;89:410–419. doi: 10.3168/jds.S0022-0302(06)72105-1. [DOI] [PubMed] [Google Scholar]
- 10.Betz G., Aeppli A., Menshutina N., Leuenberger H. In vivo comparison of various liposome formulations for cosmetic application. Int. J. Pharm. 2005;296:44–54. doi: 10.1016/j.ijpharm.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 11.Rahimpour Y., Hamishehkar H. Liposomes in cosmeceutics. Expert Opin. Drug Deliv. 2012;9:443–455. doi: 10.1517/17425247.2012.666968. [DOI] [PubMed] [Google Scholar]
- 12.Minato S., Iwanaga K., Kakemi M., Yamashita S., Oku N. Application of polyethyleneglycol (PEG)-modified liposomes for oral vaccine: Effect of lipid dose on systemic and mucosal immunity. J. Control. Release. 2003;89:189–197. doi: 10.1016/S0168-3659(03)00093-2. [DOI] [PubMed] [Google Scholar]
- 13.Gerdts V., Mutwiri G.K., Tikoo S.K., Babiuk L.A. Mucosal delivery of vaccines in domestic animals. Vet. Res. 2006;37:487–510. doi: 10.1051/vetres:2006012. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Hens A., Fernandez-Romero J.M. The Role of Liposomes in Analytical Processes. Trends Anal. Chem. 2005;24:9–19. doi: 10.1016/j.trac.2004.07.017. [DOI] [Google Scholar]
- 15.Maulucci G., De Spirito M., Arcovito G., Boffi F., Castellano A.C., Briganti G. Particle Size Distribution in DMPC Vesicles Solutions Undergoing Different Sonication Times. Biophys. J. 2005;88:3545–3550. doi: 10.1529/biophysj.104.048876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson F., Hunt C.A., Szoka F.C., Vail W.J., Papahadjopoulos D. Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes. Biochim. Biophys. Acta. 1979;557:9–23. doi: 10.1016/0005-2736(79)90085-3. [DOI] [PubMed] [Google Scholar]
- 17.Mayer L.D., Hope M.J., Cullis P.R. Vesicles of Variable Sizes Produced by a Rapid Extrusion Procedure. Biochim. Biophys. Acta. 1986;858:161–168. doi: 10.1016/0005-2736(86)90302-0. [DOI] [PubMed] [Google Scholar]
- 18.Macdonald R.C., Macdonald R.I., Menco B.P.M., Takeshita K., Subbarao N.K., Hu L.R. Small-Volume Extrusion Apparatus for Preparation of Large, Unilamellar Vesicles. Biochim. Biophys. Acta. 1991;1061:297–303. doi: 10.1016/0005-2736(91)90295-J. [DOI] [PubMed] [Google Scholar]
- 19.Vemuri S., Yu C.D., Wangsatorntanakun V., Roosdorp N. Large-scale production of liposomes by a microfludizer. Drug Dev. Ind. Pharm. 1990;16:2243–2256. doi: 10.3109/03639049009043797. [DOI] [Google Scholar]
- 20.Carugo D., Bottaro E., Owen J., Stride E., Nastruzzi C. Liposome production by microfluidics: Potential and limiting factors. Sci. Rep. 2016;6:25876. doi: 10.1038/srep25876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jahn A., Stavis S.M., Hong J.S., Vreeland W.N., DeVoe D.L., Gaitan M. Microfluidic Mixing and the Formation of Nanoscale Lipid Vesicles. ACS Nano. 2010;4:2077–2087. doi: 10.1021/nn901676x. [DOI] [PubMed] [Google Scholar]
- 22.Cho H., Kim J., Suga K., Ishigami T., Park H., Bang J.W., Seo S., Choi M., Chang P.S., Umakoshi H., et al. Microfluidic platforms with monolithically integrated hierarchical apertures for the facile and rapid formation of cargo-carrying vesicles. Lab Chip. 2015;15:373. doi: 10.1039/C4LC01096E. [DOI] [PubMed] [Google Scholar]
- 23.Jo M., Park K.M., Park J.Y., Yu H., Choi S.J., Chang P.S. Microfluidic assembly of mono-dispersed liposome and its surface modification for enhancing the colloidal stability. Colloids Surf. A. 2020;586:124202. doi: 10.1016/j.colsurfa.2019.124202. [DOI] [Google Scholar]
- 24.Balbino T.A., Aoki N.T., Gasperini A.A., Oliveira C.L., Azzoni A.R., Cavalcanti L.P., Lucimara G. Continuous flow production of cationic liposomes at high lipid concentration in microfluidic devices for gene delivery applications. Chem. Eng. J. 2013;226:423–433. doi: 10.1016/j.cej.2013.04.053. [DOI] [Google Scholar]
- 25.Zook J.M., Vreeland W.N. Vreeland Effects of temperature, acyl chain length, and flow-rate ratio on liposome formation and size in a microfluidic hydrodynamic focusing device. Soft Matter. 2010;6:1352–1360. doi: 10.1039/b923299k. [DOI] [Google Scholar]
- 26.Hertzog D.E., Michalet X., Jäger M., Kong X., Santiago J.G., Weiss S., Bakajin O. Femtomole mixer for microsecond kinetic studies of protein folding. Anal. Chem. 2004;76:7169–7178. doi: 10.1021/ac048661s. [DOI] [PMC free article] [PubMed] [Google Scholar]