Abstract
Background: Multimorbidity presents a key challenge to healthcare systems globally. However, heterogeneity in the definition of multimorbidity and design of epidemiological studies results in difficulty in comparing multimorbidity studies. This scoping review aimed to describe multimorbidity prevalence in studies using large datasets and report the differences in multimorbidity definition and study design. Methods: We conducted a systematic search of MEDLINE, EMBASE, and CINAHL databases to identify large epidemiological studies on multimorbidity. We used the Preferred Reporting Items for Systematic Reviews and Meta-analysis Extension for Scoping Reviews (PRISMA-ScR) protocol for reporting the results. Results: Twenty articles were identified. We found two key definitions of multimorbidity: at least two (MM2+) or at least three (MM3+) chronic conditions. The prevalence of multimorbidity MM2+ ranged from 15.3% to 93.1%, and 11.8% to 89.7% in MM3+. The number of chronic conditions used by the articles ranged from 15 to 147, which were organized into 21 body system categories. There were seventeen cross-sectional studies and three retrospective cohort studies, and four diagnosis coding systems were used. Conclusions: We found a wide range in reported prevalence, definition, and conduct of multimorbidity studies. Obtaining consensus in these areas will facilitate better understanding of the magnitude and epidemiology of multimorbidity.
Keywords: multimorbidity, prevalence, definition, large database
1. Introduction
Multimorbidity, the presence of multiple chronic conditions in an individual [1], challenges the current healthcare system [2]. Individuals with multimorbidity tend to have more complex healthcare needs, and effective management of their multiple chronic conditions is essential [3]. As the proportion of individuals with multimorbidity increases due to the aging population in most developed countries, issues threatening patient safety such as poor coordination of hospital processes, continuity of care, and polypharmacy have also become more prevalent [4,5].
With the exception of relatively uncommon conditions, the majority of visits for the management of chronic conditions and any co-existing conditions are made to primary care physicians, not specialists. Additionally, specialists play a greater role in managing specific conditions of their expertise, but not the other comorbid conditions that the patient may have [6]. Hence, our review chose to focus on the general and primary care populations.
The basic operational definition of multimorbidity includes the following parameters: the minimum number of chronic conditions to determine the presence of multimorbidity and the list of chronic conditions considered [7]. Acute conditions are not included in the definition of multimorbidity as these usually do not result in significant, long-lasting impact on patients’ lives [8]. Factors considered in the conduct of a multimorbidity prevalence study include the choice of study population, data sources, and diagnosis algorithm used. Diagnosis algorithms are used to determine the presence of a chronic condition in an individual, usually involving a combination of diagnosis codes, prescription data, or chronic disease databases [9]. Multimorbidity studies using large datasets require diagnosis algorithms to reliably pick up indicator chronic conditions in their study population. Different diagnosis algorithms used by different studies affect the pick-up rate of chronic diseases and subsequently the reported prevalence rates.
It is difficult to quantify the extent of the burden of multimorbidity, as the design of epidemiological multimorbidity studies varies greatly, and no consensus on the operational definition of multimorbidity exists [10]. A systematic review reported that prevalence was severely underestimated if studies used a list of fewer than 12 chronic conditions, while less variation existed in studies using more than 12 conditions [11]. The lack of consensus in the aforementioned areas resulted in a wide range of multimorbidity prevalence estimates and difficulty in comparing the prevalence among multimorbidity studies [10].
Larger samples are able to provide a more precise estimate of multimorbidity [12]. We defined large database studies as multimorbidity studies that used a study population greater than 500,000. This number was chosen to reflect a large dataset, as datasets of this size would likely have to store their data electronically. They are also more likely to be professionally governed and updated by a specialized data management team [13]. Compared to smaller databases, standards of governance are generally required to ensure diagnoses are reliably coded in large datasets.
Some challenges in using larger databases include the accuracy of the coding system used, and the reliability of coding as hospital records may be coded by non-physicians and may not reflect the actual codes from the physicians’ perspectives [14,15]. This is confounded by the impracticability to go through individual patient notes or obtain a direct account of the patient’s conditions. It has been suggested that patient record review is the best way to collect information about multimorbidity prevalence, as it is not reliant on coding and data entry but rather gathers data from the entire patient record [15,16]. However, this is not feasible in large database studies.
Therefore, a scoping review was conducted on multimorbidity studies using large datasets. The main objective was to describe the range of prevalence of multimorbidity reported by these studies. The secondary objectives of the review were to identify and report the differences in the definitions of multimorbidity and the conduct of these studies.
2. Materials and Methods
This scoping review was reported using Preferred Reporting Items for Systematic Reviews and Meta-analysis Extension for Scoping Reviews (PRISMA-ScR) protocol [17].
Articles were included if they were (a) written in English, (b) involved human participants, (c) had a study population greater than 500,000, (d) included the primary healthcare or general population, and (e) used electronic databases. Articles were excluded if they (a) focused only on comorbidity, (b) used patient-reported data, (c) studied only inpatients, (d) included acute conditions in the list of conditions, (e) used less than 12 conditions to define multimorbidity, or (f) were qualitative, interventional studies, reviews, editorials, systematic reviews, or meta-analyses.
The bibliographic databases of MEDLINE, EMBASE, and CINAHL were searched for all records from the inception date to 8 March 2020 to identify potentially relevant records. The search strategies were drafted and refined through team discussion amongst the authors. The final search strategies for the three databases can be found in Supplementary Table S1. The final search results were exported into EndNote, and duplicates were removed. The electronic database search was supplemented by hand searches of the references listed in the included articles and from Google Scholar.
The articles were screened by two independent reviewers (YPC and ESL) using Covidence. The reviewers sequentially evaluated the titles, abstracts, and then full text of all publications identified by our searches for potentially relevant articles. The reviewers resolved disagreements on article selection and data extraction by consensus and discussion with another reviewer (YX) if needed.
A data-extraction form was jointly developed by two independent reviewers (YPC and ZSC) to determine the type of information to extract. The form captured the relevant information on multimorbidity prevalence, definitions of multimorbidity used (e.g., minimum number of chronic conditions required, number of chronic conditions in the list and the list of chronic conditions), study settings (e.g., country of origin, year of publication and year of data extraction), and conduct of the studies (e.g., study design, population age and type, data sources, and diagnosis algorithms). The form also extracted information on the presence of any data governance or reliability standards of the electronic medical records used by the articles. The prevalence of multimorbidity was manually calculated from data provided in the articles if overall prevalence was not directly reported. In articles reporting prevalence estimates longitudinally over a period of time, we used prevalence estimates of the most recent data.
The two reviewers independently extracted the data, discussed the inputs, and updated the data-extraction form after resolving any disagreements through discussion. Consensus was reached by involving a third reviewer (ESL) for unresolved items.
The list of chronic conditions used by each article to define multimorbidity was compiled and organized into their respective categories based on the body systems. The organization of individual chronic condition into each category was conducted by two independent reviewers (YPC and ESL), and disagreements were resolved until consensus was reached via discussion with a third reviewer (YX). The organized conditions can be found in Supplementary Table S2.
3. Results
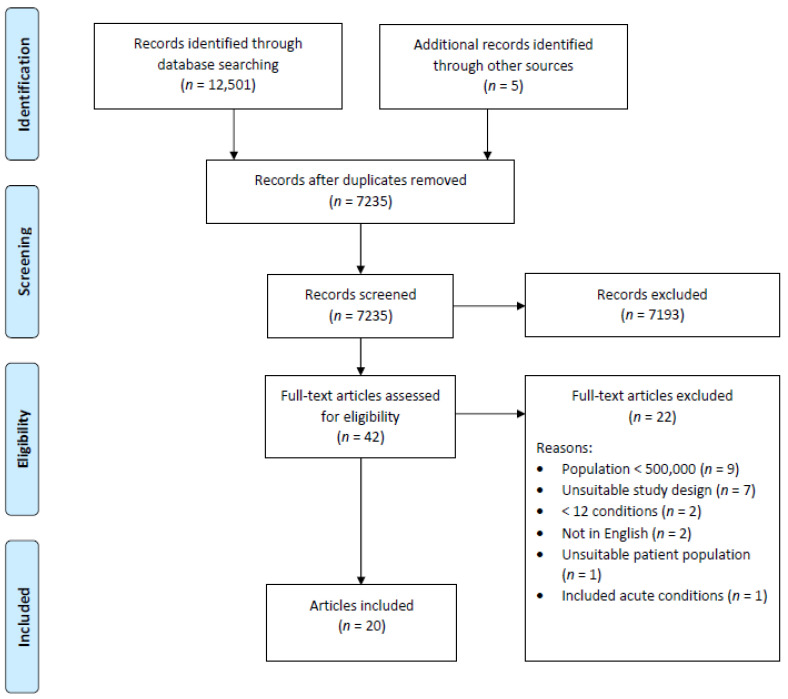
A total of 7235 records were obtained from the searches, and 42 full text articles were assessed for eligibility. Of these, 22 were excluded for various reasons as shown in Figure 1. The remaining 20 articles were selected for this scoping review.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) flow diagram.
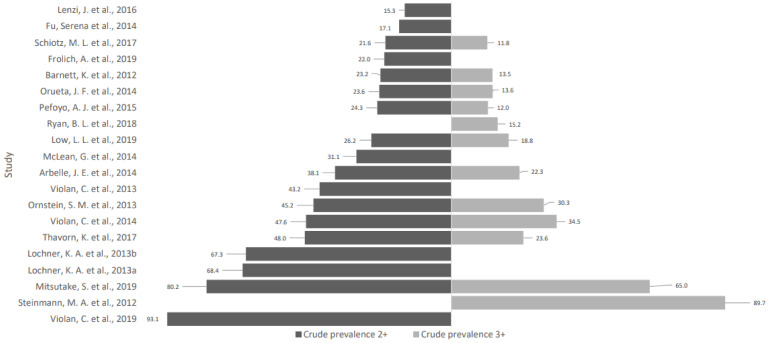
The study characteristics and definitions of multimorbidity used by the 20 articles are presented in Table 1. The chronic conditions used by each article were sorted into 21 body system categories, which are presented in Table 2. The prevalence of multimorbidity with at least two (MM2+) or three (MM3+) chronic conditions is presented in Figure 2. Two articles [18,19] did not provide sufficient data for the calculation of MM2+ prevalence, while eight articles [20,21,22,23,24,25,26,27] did not provide sufficient data for the calculation of MM3+ prevalence.
Table 1.
A comparison of study characteristics and definitions of multimorbidity used in the 20 articles.
| Article Number | Author, Year of Publication | Study Design | Country | Population Age | Population Type | Type of Database | Number of Conditions for MM Definition |
Number of Chronic Conditions in List |
Definition of Chronic Condition | Additional Means to Diagnose Conditions |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Arbelle et al., 2014 [37] | CS | Israel | ≥0 | General population | EMR | 2+ | 40 | No | a. Prescription data b. Hospital discharge codes c. Billing info |
| 2 | Barnett et al., 2012 [28] | CS | Scotland | ≥0 | Primary care | EMR | 2+ | 40 | Yes | Prescription data |
| 3 | Frolich et al., 2019 [20] | CS | Denmark | >16 | a. Primary care b. Secondary care |
EMR | 2+ | 16 | No | a. Prescription data b. Healthcare service utilisation |
| 4 | Fu et al., 2014 [21] | CS | Taiwan | ≥0 | General population | Insurance database |
2+ | 15 | No | No |
| 5 | Lenzi et al., 2016 [22] | CS | Italy | ≥18 | General population | EMR | 2+ | 26 | No | Prescription data |
| 6 | Lochner et al., 2013 [23] | CS | U.S. | ≥0 | Insurance population | Insurance database |
2+ | 15 | No | No |
| 7 | Lochner. et al., 2013 [24] | CS | U.S. | ≥0 | Insurance population | Insurance database |
2+ | 15 | No | No |
| 8 | Low et al., 2019 [35] | CS | Singapore | ≥0 | a. Primary care b. Tertiary care c. Community hospitals |
Government administrative data | 2+ | 48 | No | No |
| 9 | McLean et al., 2014 [25] | CS | Scotland | ≥25 | Primary care | EMR | 2+ | 40 | No | Prescription data |
| 10 | Mitsutake et al., 2019 [36] | CS | Japan | ≥75 | General population | Insurance database |
2+ & 3+ | 22 | No | No |
| 11 | Ornstein et al., 2013 [32] | CS | U.S. | ≥18 | Primary care | EMR | 2+ | 24 | No | No |
| 12 | Orueta et al., 2014 [29] | CS | Spain | ≥0 | General population | EMR | 2+ | 52 | No | Prescription data |
| 13 | Pefoyo et al., 2015 [33] | RC | Canada | 0–105 | General population | Insurance database |
2+ | 16 | No | No |
| 14 | Ryan et al., 2018 [18] | CS | Canada | 0–105 | General population | EMR | 3+ | 17 | No | Prescription data |
| 15 | Schiotz et al., 2017 [30] | CS | Denmark | ≥16 | General population | EMR | 2+ | 16 | No | a. Prescription data b. Healthcare service utilisation |
| 16 | Steinmann et al., 2012 [19] | RC | U.S. | ≥65 | Special group: Veterans | Government administrative data | DNS | 23 | No | No |
| 17 | Thavorn et al., 2017 [34] | RC | Canada | 0–105 | General population | EMR | 2+ | 16 | No | No |
| 18 | Violan et al., 2019 [26] | CS | Spain | ≥65–99 | Primary care | EMR | 2+ | 60 | No | a. Prescription data b. Other clinical parameters |
| 19 | Violan et al., 2013 [27] | CS | Spain | ≥15 | Primary care | EMR | 2+ | 27 | No | No |
| 20 | Violan et al., 2014 [31] | CS | Spain | ≥19 | a. Primary care b. Urban population |
EMR | 2+ | 147 | No | No |
Note: CS—cross-sectional, RC—retrospective cohort, DNS—did not specify, EMR—electronic medical records.
Table 2.
Categories of conditions.
| Category | Number of Articles in the Category | Arbelle, J. E., 2014 | Barnett, K., 2012 | Frolich, A., 2019 | Fu, Serena, 2014 | Lenzi, J., 2016 | Lochner, K. A., 2013 | Lochner, K. A., 2013 | Low, L.L., 2019 | McLean, G., 2014 | Mitsutake, S., 2019 | Ornstein, S. M., 2013 | Orueta, J. F., 2014 | Pefoyo, A. J., 2015 | Ryan, B. L., 2018 | Schiotz, M. L., 2017 | Steinmann, M. A., 2012 | Thavorn, K., 2017 | Violan, C., 2019 | Violan, C., 2013 | Violan, C., 2014 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Cardiovascular | 20 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 2. Endocrine | 20 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 3. Mental health | 20 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 4. Musculoskeletal | 20 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 5. Neurology | 20 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 6. Respiratory | 20 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 7. Neoplasia | 19 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 8. Rheumatology | 15 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| 9. Urology/renal | 15 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| 10. Gastrointestinal | 14 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| 11. Vascular | 11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| 12. Hepatopancreaticobiliary | 10 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| 13. Infectious diseases (communicable) | 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| 14. Ophthalmology | 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| 15. Dermatology | 8 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| 16. Disability | 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||
| 17. Ear Nose and Throat | 6 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||||
| 18. Hematology | 6 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| 19. Immunologic | 5 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||||
| 20. Genetic | 2 | ✓ | ✓ | ||||||||||||||||||
| 21. Others | 2 | ✓ | ✓ |
Note: ✓ represents the presence of conditions from the particular category in the article. Each category consists of conditions involving said body system; “Others” includes “subfertility/infertility”, “weakness/tiredness general” and “transplant status”.
Figure 2.
Crude prevalence of multimorbidity 2+ and 3+.
3.1. Study Characteristics
The majority of the included articles were from Europe (n = 9) [20,22,25,26,27,28,29,30,31], followed by North America (n = 7) [18,19,23,24,32,33,34] and Asia (n = 4) [21,35,36,37]. Seventeen of them were cross-sectional studies, while three articles were retrospective cohort studies [19,33,34].
There was significant variation in the age ranges of the study populations. Seven articles had no limits on age [21,23,24,28,29,35,37], seven articles included adults only [20,22,25,27,30,31,32], three articles included the elderly population only [19,26,36], and three articles included the whole population and only excluded people above the age of 105 [18,33,34]. The target population for majority of the articles was the general population (n = 9) [18,21,22,29,30,33,34,36,37], followed by articles that targeted the primary care populations (n = 8) [20,25,26,27,28,31,32,35] and insurance populations (n = 2) [23,24]. One article used a nationwide sample of community-dwelling elderly veterans receiving care in the United States’ Department of Veterans Affairs healthcare system [19].
3.2. Prevalence of Multimorbidity
The reported prevalence of multimorbidity was varied across the 20 articles. For MM2+ prevalence, 18 articles reported a prevalence ranging from 15.3% to 93.1%. For MM3+ prevalence, 12 articles reported a prevalence ranging from 11.8% to 89.7%. Nineteen articles reported crude prevalence rates, while one article [18] provided a standardized prevalence rate that was standardized against the 1991 Canadian population.
3.3. Definition of Multimorbidity
Most of the articles (85%, n = 17) used two chronic conditions and above to define the presence of multimorbidity. One article used at least three chronic conditions [18], one article used both two and three chronic conditions with a greater emphasis on three chronic conditions [36], while one article did not specify the number of chronic conditions [19].
The number of chronic conditions used in defining multimorbidity varied greatly from 15 to 147. Six out of 21 categories were present in the list of chronic conditions reported by all 20 articles. These six categories were “Cardiovascular”, “Endocrine”, “Mental health”, “Musculoskeletal”, “Neurology”, and “Respiratory”. The presence of the other categories was more diverse, with nine categories present in less than half (n = 10) of the articles. “Genetic conditions” and “Others” were two categories that were only present in two articles [29,31].
3.4. Diagnosis Codes and Algorithms Used
Eleven articles used diagnosis codes solely to denote the presence of chronic conditions. Nine articles used various additional means in their diagnosis algorithms for each condition [18,20,22,25,26,28,29,30,37], where prescription data as an additional means was used by all of them. The coding systems used were varied. A majority of studies used ICD-9-CM [18,19,21,22,23,24,29,32,33,34] or ICD-10-CM codes [18,20,26,27,30,33,34,35,36]; other codes used included Read2 [25] or ICPC-2 [31].
Out of the 20 included articles, only two of them described data governance or reliability standards of the electronic databases used. Arbelle et al. mentioned that the registries in their study were updated daily and automatically using strict algorithms. The algorithms drew data from numerous sources including physicians’ diagnoses, prescription information, data acquired from hospital discharge codes, and billing information from providers. The hospital discharge record database used in the study by Lenzi et al. underwent data quality control by the regional authority before being sent to the Ministry of Health.
4. Discussion
In this scoping review, 20 articles studying multimorbidity using large datasets were identified. There was significant variation in the reported prevalence and definitions of multimorbidity as well as in the conduct of the studies. This finding was aligned with existing literature, which suggested that reported multimorbidity prevalence is still highly varied due to inconsistent definitions of multimorbidity [11] in both large and small studies [38].
4.1. Definitions of Multimorbidity
Most articles used two chronic conditions and above (MM2+) to define the presence of multimorbidity. Only five articles provided the rationale for doing so: two were based on previous systematic reviews [22,28], and three were based on government regulations [23,24,37]. The remaining 15 articles, including those that used three chronic conditions and above (MM3+), did not provide any rationale for their choices. Lenzi et al. mentioned that MM3+ may be more useful in an older study population, but the more general definition of MM2+ was better applied to the general population [22]. The current literature suggested that the majority of the authors supported the use of MM2+ as the minimum number of chronic conditions to determine the presence of multimorbidity.
4.2. List of Conditions Used
The lists of chronic conditions used by the 20 articles ranged between 15 and 147 conditions. Most articles clustered around 15 to 30 conditions, which was partly due to the exclusion of articles with fewer than 12 conditions as recommended by Fortin et al. [11].
The following six categories of conditions were included in all 20 articles: cardiovascular, endocrine, mental health, musculoskeletal, neurology, and respiratory. This suggests greater relevance of these categories in the primary care and general population, which is possibly due to prevalence of conditions, such as acute myocardial infarction (cardiovascular), diabetes mellitus (endocrine), or greater impact on patients’ lives, such as requiring lifelong medications. To derive the lists of conditions, most articles based their lists on previous studies, which was followed by clinical relevance of the conditions. Less commonly, government guidelines, indexes, or systematic reviews were used.
4.3. Inclusion of Mental Health Conditions
All articles included mental health conditions, particularly depression, demonstrating that mental health conditions are significant health problems to consider in multimorbidity. Seven articles [20,22,25,28,30,35,37] grouped chronic conditions into overarching categories of physical and mental health conditions, of which four articles [25,28,30,35] separately analyzed the prevalence of physical–mental, purely physical, or mental health multimorbidity.
While specialists may better provide care for patients with one dominant disease or closely related comorbidities, the management of physical–mental multimorbidity requires holistic care and delicate balance [39]. For example, drugs prescribed for a physical condition may adversely affect mood, while synergistic treatment strategies can improve outcomes in patients with physical–mental multimorbidity [40]. Generalists may integrate the patient’s clinical problems, review medications, and assess the patient holistically [41], and a generalist primary care system is best equipped to manage physical–mental multimorbidity [28].
4.4. Diagnosis Coding Systems and Algorithms Used
Four coding systems were used by the 20 articles: ICD-9-CM, ICD-10-CM, Read2, and ICPC-2. ICD-9-CM and ICD-10-CM were most commonly used by the 20 articles. However, the code accuracy, defined as the extent to which the ICD nosologic code reflects the underlying patient’s disease, is usually low [42]. ICPC-2, designed specifically for the primary care setting, may inaccurately capture conditions less commonly seen in primary care. For all four coding systems, the use of synonyms, acronyms, and abbreviations in medical terminology results in differing codes selected [42], and accurate training is required to reduce coding errors [43]. Hence, coding accuracy and specificity of electronic health records differ amongst the 20 articles.
A lack of consensus on coding systems and diagnosis algorithms results in difficulty comparing among different multimorbidity studies. In studies using different coding systems, imperfect mapping of individual conditions onto a common coding system affects the accuracy of comparison studies. Differences in diagnosis algorithms also affect prevalence estimates of multimorbidity, as conditions may be under-reported if only diagnosis codes are used. Nearly half of the articles (n = 9) in this scoping review used prescription data as an additional means of diagnosing chronic conditions. Future studies may consider including prescription data or other means of confirming diagnoses in their diagnosis algorithms as well as standardizing them for large dataset multimorbidity studies to more accurately estimate multimorbidity prevalence.
Large databases are reliant on accurate clinical coding. Apart from inherent limitations of the coding systems used, ambiguity in patient record documentation and lack of clinical experience of coders affect coding accuracy [44]. While patient record review has been suggested as the best method to derive multimorbidity prevalence as it is not reliant on coding and data entry [15,16], large studies lack access to individual patient notes or direct accounts of patients’ conditions. Errors in coding subsequently implicate the accuracy of research using data from large databases [14].
As such, data governance by a professional body is essential to ensure the reliability of large databases. However, data governance in healthcare is less mature compared to other industries, and no universal standard for healthcare data exists [45]. Most of the articles (n = 18) did not mention any governance standards of the databases used as well. Indicating the presence of data governance is recommended to increase the reliability of multimorbidity studies using large databases.
4.5. Limitations
Our scoping review has some limitations. A categorical approach to analyzing the chronic conditions rather than individual comparison was chosen for feasibility reasons. This made the identification of key chronic conditions difficult.
5. Conclusions
In conclusion, our scoping review found a wide range in prevalence of multimorbidity as reported in studies using a large dataset, from 15.3% to 93.1% in MM2+ and 11.8% to 89.7% in MM3+. This is due to differences in both the definitions of multimorbidity and the conduct of the multimorbidity studies.
Consensus is urgently needed to facilitate comparison across studies as well as ensure reproducibility. Additional research such as a qualitative study using the Delphi method [46] may be important to get consensus where gold standards are absent to create a pre-defined list of key chronic conditions that should be included in multimorbidity studies for large dataset studies.
Methods of diagnosing chronic conditions will also need to be standardized, harmonizing the current established coding systems and diagnosis algorithms. This is especially important as large datasets are reliant on multiple factors to ensure reliability, such as standards of governance of electronic medical records, accuracy of data coding, diagnosis codes, and algorithms used.
Acknowledgments
We would like to thank Zi Siong Chow for his contributions in the process of data extraction.
Supplementary Materials
The searching methodology and list of conditions sorted by category are available online at https://www.mdpi.com/1660-4601/18/4/1673/s1.
Author Contributions
Y.P.C., Y.X., P.S.S.L. and E.S.L. designed the scoping review; Y.P.C. performed the literature search; Y.P.C. and Y.X. extracted, analyzed, and synthesized the data; Y.P.C. wrote the draft; Y.X., P.S.S.L. and E.S.L. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Singapore Ministry of Health’s National Medical Research Council under the Centre Grant Programme (reference number: NMRC/CG/C019/2017).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.van den Akker M., Buntinx F., Metsemakers J.F., Roos S., Knottnerus J.A. Multimorbidity in general practice: Prevalence, incidence, and determinants of co-occurring chronic and recurrent diseases. J. Clin. Epidemiol. 1998;51:367–375. doi: 10.1016/S0895-4356(97)00306-5. [DOI] [PubMed] [Google Scholar]
- 2.Violan C., Foguet-Boreu Q., Flores-Mateo G., Salisbury C., Blom J., Freitag M., Glynn L., Muth C., Valderas J.M. Prevalence, determinants and patterns of multimorbidity in primary care: A systematic review of observational studies. PLoS ONE. 2014;9:e102149. doi: 10.1371/journal.pone.0102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Oostrom S.H., Picavet H.S., de Bruin S.R., Stirbu I., Korevaar J.C., Schellevis F.G., Baan C.A. Multimorbidity of chronic diseases and health care utilization in general practice. BMC Fam. Pract. 2014;15:61. doi: 10.1186/1471-2296-15-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogeli C., Shields A.E., Lee T.A., Gibson T.B., Marder W.D., Weiss K.B., Blumenthal D. Multiple chronic conditions: Prevalence, health consequences, and implications for quality, care management, and costs. J. Gen. Intern. Med. 2007;22(Suppl. 3):391–395. doi: 10.1007/s11606-007-0322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forslund T., Carlsson A.C., Ljunggren G., Ärnlöv J., Wachtler C. Patterns of multimorbidity and pharmacotherapy: A total population cross-sectional study. Fam. Pract. 2020 doi: 10.1093/fampra/cmaa056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starfield B., Lemke K.W., Bernhardt T., Foldes S.S., Forrest C.B., Weiner J.P. Comorbidity: Implications for the importance of primary care in ‘case’ management. Ann. Fam. Med. 2003;1:8–14. doi: 10.1370/afm.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fortin M., Almirall J., Nicholson K. Development of a research tool to document self-reported chronic conditions in primary care. J. Comorb. 2017;7:117–123. doi: 10.15256/joc.2017.7.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foguet-Boreu Q., Violan C., Roso-Llorach A., Rodriguez-Blanco T., Pons-Vigués M., Muñoz-Pérez M.A., Pujol-Ribera E., Valderas J.M. Impact of multimorbidity: Acute morbidity, area of residency and use of health services across the life span in a region of south Europe. BMC Fam. Pract. 2014;15:55. doi: 10.1186/1471-2296-15-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quan H., Sundararajan V., Halfon P., Fong A., Burnand B., Luthi J.C., Saunders L.D., Beck C.A., Feasby T.E., Ghali W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 10.Johnston M.C., Crilly M., Black C., Prescott G.J., Mercer S.W. Defining and measuring multimorbidity: A systematic review of systematic reviews. Eur. J. Public Health. 2019;29:182–189. doi: 10.1093/eurpub/cky098. [DOI] [PubMed] [Google Scholar]
- 11.Fortin M., Stewart M., Poitras M.E., Almirall J., Maddocks H. A systematic review of prevalence studies on multimorbidity: Toward a more uniform methodology. Ann. Fam. Med. 2012;10:142–151. doi: 10.1370/afm.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajian-Tilaki K. Sample size estimation in epidemiologic studies. Caspian. J. Intern. Med. 2011;2:289–298. [PMC free article] [PubMed] [Google Scholar]
- 13.Black N., Barker M., Payne M. Cross sectional survey of multicentre clinical databases in the United Kingdom. BMJ. 2004;328:1478. doi: 10.1136/bmj.328.7454.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng M., Lee S., D’Souza A.G., Doktorchik C.T.A., Quan H. Development and validation of data quality rules in administrative health data using association rule mining. BMC Med. Inform. Decis. Mak. 2020;20:75. doi: 10.1186/s12911-020-1089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei M.Y., Luster J.E., Chan C.-L., Min L. Comprehensive review of ICD-9 code accuracies to measure multimorbidity in administrative data. BMC Health Serv. Res. 2020;20:1–11. doi: 10.1186/s12913-020-05207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fortin M., Bravo G., Hudon C., Vanasse A., Lapointe L. Prevalence of multimorbidity among adults seen in family practice. Ann. Fam. Med. 2005;3:223–228. doi: 10.1370/afm.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tricco A.C., Lillie E., Zarin W., O’Brien K.K., Colquhoun H., Levac D., Moher D., Peters M.D.J., Horsley T., Weeks L., et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 18.Ryan B.L., Bray Jenkyn K., Shariff S.Z., Allen B., Glazier R.H., Zwarenstein M., Fortin M., Stewart M. Beyond the grey tsunami: A cross-sectional population-based study of multimorbidity in Ontario. Can. J. Public Health. 2018;109:845–854. doi: 10.17269/s41997-018-0103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinman M.A., Lee S.J., John Boscardin W., Miao Y., Fung K.Z., Moore K.L., Schwartz J.B. Patterns of multimorbidity in elderly veterans. J. Am. Geriatr. Soc. 2012;60:1872–1880. doi: 10.1111/j.1532-5415.2012.04158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frolich A., Ghith N., Schiotz M., Jacobsen R., Stockmarr A. Multimorbidity, healthcare utilization and socioeconomic status: A register-based study in Denmark. PLoS ONE. 2019;14:e0214183. doi: 10.1371/journal.pone.0214183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu S., Huang N., Chou Y.-J. Trends in the prevalence of multiple chronic conditions in Taiwan from 2000 to 2010: A population-based study. Prev. Chronic Dis. 2014;11:E187. doi: 10.5888/pcd11.140205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lenzi J., Avaldi V.M., Rucci P., Pieri G., Fantini M.P. Burden of multimorbidity in relation to age, gender and immigrant status: A cross-sectional study based on administrative data. BMJ Open. 2016;6:e012812. doi: 10.1136/bmjopen-2016-012812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lochner K.A., Cox C.S. Prevalence of multiple chronic conditions among Medicare beneficiaries, United States, 2010. Prev. Chronic Dis. 2013;10:E61. doi: 10.5888/pcd10.120137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lochner K.A., Goodman R.A., Posner S., Parekh A. Multiple chronic conditions among Medicare beneficiaries: State-level variations in prevalence, utilization, and cost, 2011. Medicare Medicaid Res. Rev. 2013;23:3. doi: 10.5600/mmrr.003.03.b02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLean G., Gunn J., Wyke S., Guthrie B., Watt G.C., Blane D.N., Mercer S.W. The influence of socioeconomic deprivation on multimorbidity at different ages: A cross-sectional study. Br. J. Gen. Pract. 2014;64:e440–e447. doi: 10.3399/bjgp14X680545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Violan C., Foguet-Boreu Q., Fernandez-Bertolin S., Guisado-Clavero M., Cabrera-Bean M., Formiga F., Valderas J.M., Roso-Llorach A. Soft clustering using real-world data for the identification of multimorbidity patterns in an elderly population: Cross-sectional study in a Mediterranean population. BMJ Open. 2019;9:e029594. doi: 10.1136/bmjopen-2019-029594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Violan C., Foguet-Boreu Q., Hermosilla-Perez E., Valderas J.M., Bolibar B., Fabregas-Escurriola M., Brugulat-Guiteras P., Munoz-Perez M.A. Comparison of the information provided by electronic health records data and a population health survey to estimate prevalence of selected health conditions and multimorbidity. BMC Public Health. 2013;13:1–10. doi: 10.1186/1471-2458-13-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnett K., Mercer S.W., Norbury M., Watt G., Wyke S., Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet. 2012;380:37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 29.Orueta J.F., Garcia-Alvarez A., Garcia-Goni M., Paolucci F., Nuno-Solinis R. Prevalence and costs of multimorbidity by deprivation levels in the basque country: A population based study using health administrative databases. PLoS ONE. 2014;9:e89787. doi: 10.1371/journal.pone.0089787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiotz M.L., Stockmarr A., Host D., Glumer C., Frolich A. Social disparities in the prevalence of multimorbidity—A register-based population study. BMC Public Health. 2017;17:422. doi: 10.1186/s12889-017-4314-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Violan C., Foguet-Boreu Q., Roso-Llorach A., Rodriguez-Blanco T., Pons-Vigues M., Pujol-Ribera E., Munoz-Perez M.A., Valderas J.M. Burden of multimorbidity, socioeconomic status and use of health services across stages of life in urban areas: A cross-sectional study. BMC Public Health. 2014;14:530. doi: 10.1186/1471-2458-14-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ornstein S.M., Nietert P.J., Jenkins R.G., Litvin C.B. The prevalence of chronic diseases and multimorbidity in primary care practice: A PPRNet report. J. Am. Board Fam. Med. 2013;26:518–524. doi: 10.3122/jabfm.2013.05.130012. [DOI] [PubMed] [Google Scholar]
- 33.Pefoyo A.J., Bronskill S.E., Gruneir A., Calzavara A., Thavorn K., Petrosyan Y., Maxwell C.J., Bai Y., Wodchis W.P. The increasing burden and complexity of multimorbidity. BMC Public Health. 2015;15:415. doi: 10.1186/s12889-015-1733-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thavorn K., Maxwell C.J., Gruneir A., Bronskill S.E., Bai Y., Kone Pefoyo A.J., Petrosyan Y., Wodchis W.P. Effect of socio-demographic factors on the association between multimorbidity and healthcare costs: A population-based, retrospective cohort study. BMJ Open. 2017;1:e017264. doi: 10.1136/bmjopen-2017-017264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Low L.L., Kwan Y.H., Ko M.S.M., Yeam C.T., Lee V.S.Y., Tan W.B., Thumboo J. Epidemiologic Characteristics of Multimorbidity and Sociodemographic Factors Associated with Multimorbidity in a Rapidly Aging Asian Country. JAMA Netw. Open. 2019;2:e1915245. doi: 10.1001/jamanetworkopen.2019.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitsutake S., Ishizaki T., Teramoto C., Shimizu S., Ito H. Patterns of Co-Occurrence of Chronic Disease among Older Adults in Tokyo, Japan. Prev. Chronic Dis. 2019;16:E11. doi: 10.5888/pcd16.180170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arbelle J.E., Chodick G., Goldstein A., Porath A. Multiple chronic disorders—Health care system’s modern challenge in the Maccabi Health Care System. Isr. J. Health Policy Res. 2014:3. doi: 10.1186/2045-4015-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen H., Manolova G., Daskalopoulou C., Vitoratou S., Prince M., Prina A.M. Prevalence of multimorbidity in community settings: A systematic review and meta-analysis of observational studies. J. Comorbidity. 2019;9 doi: 10.1177/2235042X19870934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mercer S.W., Gunn J., Bower P., Wyke S., Guthrie B. Managing patients with mental and physical multimorbidity. BMJ. 2012;345:e5559. doi: 10.1136/bmj.e5559. [DOI] [PubMed] [Google Scholar]
- 40.Katon W.J., Lin E.H., Von Korff M., Ciechanowski P., Ludman E.J., Young B., Peterson D., Rutter C.M., McGregor M., McCulloch D. Collaborative care for patients with depression and chronic illnesses. N. Engl. J. Med. 2010;363:2611–2620. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrison D., Agur K., Mercer S., Eiras A., Gonzalez-Montalvo J.I., Gruffydd-Jones K. Managing multimorbidity in primary care in patients with chronic respiratory conditions. NPJ Prim. Care Respir. Med. 2016;26:16043. doi: 10.1038/npjpcrm.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Malley K.J., Cook K.F., Price M.D., Wildes K.R., Hurdle J.F., Ashton C.M. Measuring diagnoses: ICD code accuracy. Health Serv. Res. 2005;40:1620–1639. doi: 10.1111/j.1475-6773.2005.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horsky J., Drucker E.A., Ramelson H.Z. Accuracy and Completeness of Clinical Coding Using ICD-10 for Ambulatory Visits. AMIA Annu. Symp. Proc. 2017;2017:912–920. [PMC free article] [PubMed] [Google Scholar]
- 44.Heywood N.A., Gill M.D., Charlwood N., Brindle R., Kirwan C.C., Northwest Research C. Improving accuracy of clinical coding in surgery: Collaboration is key. J. Surg. Res. 2016;204:490–495. doi: 10.1016/j.jss.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 45.Kruse C.S., Goswamy R., Raval Y., Marawi S. Challenges and Opportunities of Big Data in Health Care: A Systematic Review. JMIR Med. Inf. 2016;4:e38. doi: 10.2196/medinform.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thangaratinam S., Redman C.W. The Delphi technique. Obstet. Gynaecol. 2005;7:120–125. doi: 10.1576/toag.7.2.120.27071. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.