Abstract
Simple Summary
Low hemoglobin (Hb) values—indicating a condition of anemia—are related to impaired nutrition and immune system status, suggesting reduced tolerance to therapies in oncologic patients. In fact, it has been shown that pre-treatment anemia predicts poor outcomes in many neoplastic diseases. Similarly, red cell distribution width—which is a measure of the size of variation of circulating erythrocytes—has been shown to be closely related to poor prognosis both in cardiovascular and in oncologic diseases. The use of the Hb-to-red cell distribution width (RDW) ratio (HRR)—which merges data coming from the two blood parameters—is a prognostic marker in esophageal squamous cell carcinoma, small cell lung cancer, and several other types of solid tumors, emerging as an independent prognostic factor for overall survival and disease-free survival. The aim of the present study was to investigate the prognostic role of pre-operative HRR in resected-pulmonary adenocarcinoma patients undergoing a multidisciplinary treatment.
Abstract
Background: The ratio of hemoglobin to red cell distribution width (HRR) has been described as an effective prognostic factor in several types of cancer. The aim of this study was to investigate the prognostic role of preoperative HRR in resected-lung-adenocarcinoma patients. Methods: We enrolled 342 consecutive patients. Age, sex, surgical resection, adjuvant treatments, pathological stage, preoperative hemoglobin, red cell distribution width, and their ratio were recorded for each patient. Results: Mean age was 66 years (SD: 9.0). There were 163 females (47.1%); 169 patients (49.4%) had tumors at stage I, 71 (20.8%) at stage II, and 102 (29.8%) at stage III. In total, 318 patients (93.0%) underwent lobectomy, and 24 (7.0%) pneumonectomy. Disease-free survival multivariable analysis disclosed an increased hazard ratio (HR) of relapse for preoperative HRR lower than 1.01 (HR = 2.20, 95%CI: (1.30–3.72), p = 0.004), as well as for N1 single-node (HR = 2.55, 95%CI: (1.33–4.90), p = 0.005) and multiple-level lymph node involvement compared to N0 for both N1 (HR = 9.16, 95%CI:(3.65–23.0), p < 0.001) and N2 (HR = 10.5, 95%CI:(3.44–32.2, p < 0.001). Conclusion: Pre-operative HRR is an effective prognostic factor of disease-free survival in resected-lung-adenocarcinoma patients, together with the level of pathologic node involvement.
Keywords: hemoglobin/red blood cell distribution width ratio (HRR), lung adenocarcinoma, disease-free survival
1. Introduction
Low hemoglobin (Hb) values—indicating a condition of anemia—are related to impaired nutrition and immune system status, suggesting reduced tolerance to therapies in oncologic patients [1]. In fact, it has been shown that pre-treatment anemia predicts poor outcomes in many neoplastic diseases [2,3,4]. Similarly, red cell distribution width (RDW)—which is a measure of the size of variation of circulating erythrocytes—has been shown to be closely related to poor prognosis both in cardiovascular and in oncologic diseases [5,6,7,8]. RDW, in fact, has recently emerged as one of the markers potentially implicated in the inflammatory process and in oxidative stress, as well as in endothelial dysfunction in vessel diseases, a group of alterations implicated both in neoplastic and in non-neoplastic pathophysiology [9]. High baseline values of RDW—defined as anisocytosis—correlate with poor outcomes at least for lung cancer [10,11], breast cancer [12], and renal cancer [13] patients. The use of the Hb-to-RDW ratio (HRR)—which merges data coming from the two blood parameters—was first proposed as a prognostic marker in esophageal squamous cell carcinoma patients [4] and later in small cell lung cancer (SCLC) patients [14], emerging as an independent prognostic factor for overall survival (OS) and disease-free survival (DFS). Although clinical guidelines clearly suggest when to offer adjuvant treatments to patients and when to limit post-operative care to a dedicated follow-up program, they also recommend further evaluation of each patient in a multidisciplinary meeting in order to better customize every treatment to each patient’s need [15]. Further prognostic tools may therefore be taken into consideration to optimize the post-operative strategy and to formulate a more accurate prognostic forecast. The aim of the present study was to investigate the prognostic role of preoperative HRR in resected-pulmonary adenocarcinoma patients undergoing multidisciplinary treatment, in terms of both oncologic outcomes and post-operative complications. The HRR, in fact, reflecting nutritional status, immune system efficiency, and inflammatory condition, may affect not only long-term outcomes but even post-operative complications.
2. Materials and Methods
This was an observational retrospective study. Data were collected prospectively, entered into our institutional general thoracic database at the point of care, reviewed, and double-checked retrospectively. Three hundred and forty-two consecutive lung adenocarcinoma patients operated in the last two years (from 2018 to 2019) were analyzed. Patients operated before 2018 were not enrolled due to the only recent introduction of RDW in the standard preoperative assessment of our patients. Written informed consent to undergo the procedure and for the use of clinical and imaging data for scientific or educational purposes, or both, was obtained from all patients before the operation; a blank copy of the written informed consent was provided. Operability was assessed by whole-body computed tomography (CT), whole-body fluorodeoxynucleotide positron emission tomography (PET), and invasive staging procedures, including endobronchial ultrasonographic bronchoscopy (EBUS) and transbronchial needle aspiration (TBNA), as appropriate. The functional status was routinely examined by blood gas analysis and spirometry and by lung perfusion scanning and cardiopulmonary exercise testing (CPET) in the case of planned pneumonectomy. Comorbidities were stratified according to an adapted Charlson comorbidity index [16]. Post-operative death was defined as 30-day mortality or longer if mortality occurred during hospitalization. Complications were classified according to the Thoracic Morbidity and Mortality classification system as minor (grade I and II) and major (grade IIIa, grade IIIb, grade IVa, grade IVb, grade V) [17]. Age, sex, smoking status, type of surgical resection, neo-adjuvant and adjuvant treatments, pathological stage, T and N status, (N0, N1a or N1b, N2 single station or N2 multiple stations) tumor size, pre-operative Hb and RDW and their ratio (HRR), neutrophils, lymphocytes, neutrophil- to-lymphocytes ratio, and lactate dehydrogenase (LDH) levels were recorded for each patient. Outpatient follow-up was performed in order to record the date of relapse (if any); early-stage patients received out-patient follow-up twice a year (every six months); locally advanced-stage patients received out-patient follow-up three times per year (every four months).
Statistical Methods
Patients’ characteristics, treatments, and procedures were summarized either by count and percent or mean and standard deviation (SD) for categorical and continuous variables, respectively. Disease-free survival was defined as the time from the date of surgery to the last follow-up date without any sign of disease; patients alive without disease at the last follow-up date entered the analysis as time-censored observations. Death from any cause before evidence of recurrence entered the univariate and multivariable disease-free survival analysis as a competing event for relapse. Results were tabulated as hazard ratios (HR) with 95% confidence intervals (CI). Only the variables that were significant in the univariate analysis were included in the multivariable analysis, except Hb and RDW because of their collinearity with HRR. Other collinear variables (pT, stage, and pN) were analyzed together with the significant variables in the univariate analysis, using three stratified-by-treatment multivariable models; of the three models, it was decided to keep the one with the lowest Akaike Information Criterion (AIC) as the best explanatory model. A plot of the cumulative incidence functions for relapse by the median of HRR was produced. Cumulative incidence functions were compared by the Gray’s test. Overall survival was defined as the time from the date of surgery to the date of death from any cause. Complication (any, major, and minor) risks according to the median HRR cut-off were estimated using a logistic regression analysis; results were tabulated as odds ratios (OR) with 95% CIs. The median of the follow-up time was calculated by the inverted Kaplan–Meier method. All tests were two-tailed and considered significant at the 5% level. All analyses were done using SAS 9.4 (SAS Institute Inc., N.C., Cary, USA).
3. Results
Baseline patients’ characteristics and treatments are summarized in Table 1.
Table 1.
Patients’ characteristics and treatments.
| Characteristic | Level | All Patients a n = 342 |
|---|---|---|
| Age at Surgery, years | 66.0 (9.0) | |
| ≤70 | 228 (66.7) | |
| >70 | 114 (33.3) | |
| Tumor Size, mm | 30.4 (19.7) | |
| Hemoglobin (g/dL) | 13.5 (1.6) | |
| Low (<11.8) | 47 (13.7) | |
| Normal (11.8–15.8) | 272 (79.5) | |
| High (>15.8) | 23 (6.7) | |
| RDW (%) | 14.1 (2.0) | |
| HRR b | 0.98 (0.19) | |
| Female Gender | 163 (47.1) | |
| Smoker | Yes | 264 (77.2) |
| No | 75 (21.9) | |
| missing | 3 (0.9) | |
| pN | N0 | 204 (59.7) |
| N1 | 67 (19.6) | |
| Single station | 55 (16.1) | |
| Multiple station | 12 (3.5) | |
| N2 | 71 (20.8) | |
| Single station | 54 (15.8) | |
| Multiple station | 17 (5.0) | |
| N Status (n = 138) c | Single station | 109 (79.0) |
| Multiple stations | 29 (21.0) | |
| Pathological Stage | pT1 | 136 (39.8) |
| pT2 | 141 (41.2) | |
| pT3 | 46 (13.5) | |
| pT4 | 19 (5.6) | |
| Stage | 1A,1B | 169 (49.4) |
| 2A,2B | 71 (20.8) | |
| 3A,3B | 102 (29.8) | |
| Grading | 0 | 22 (6.4) |
| 1 | 20 (5.9) | |
| 2 | 159 (46.5) | |
| 3 | 112 (32.8) | |
| missing | 29 (8.5) | |
| Surgery | Open | 188 (54.8) |
| Minimal Invasive Surgery | 154 (45.2) | |
| Adjuvant Treatments | Yes | 93 (27.2) |
| unknown | 6 (1.8) | |
| Neoadjuvant Treatments | Yes | 54 (15.8) |
| unknown | 4 (1.2) | |
| Any treatment | Yes | 121 (35.4) |
| unknown | 4 (1.2) | |
| Procedures d | Lobectomy | 318 (93.0) |
| Pneumonectomy | 24 (7.0) |
a Statistics are means (SD) for: age, tumor size, hemoglobin, red cell distribution width (RDW), HRR; N (column%) otherwise; b HRR = hemoglobin/RDW ratio; c N1 and N2 patients subgroup only; d See text for details.
Forty patients (11.4%) had comorbidities. Among these, 20 patients (5.7%) had a previous history of myocardial infarction, 5 patients (1.4%) suffered from diabetes mellitus without end-organ damage, 4 patients (1.1%) suffered from peripheral vascular disease, 3 patients (0.86%) suffered from cerebrovascular disease, 3 patients (0.86%) suffered from connective tissue disease, 2 patients (0.5%) suffered from chronic obstructive pulmonary disease, 2 patients (0.5%) suffered from mild liver disease, and 1 patient (0.2%) suffered from moderate kidney disease. Seventy-five patients (21.6%) had a previous history of neoplastic disease.
Significant risk factors for relapse in univariate analysis were tumor size (HR = 1.21, 95% CI: (1.04–1.40), p = 0.01), Hb level (HR = 0.84, 95% CI: (0.72–0.97), p = 0.02), RDW (HR = 1.16, 95% CI: (1.05–1.28), p = 0.003), and HRR as both a continuous variable (HR = 0.15, 95% CI: (0.05–0.50), p = 0.002) and categorized according to its median cut-off (HR = 0.41, 95% CI: (0.25–0.68), p < 0.001); pT2 and pT3 stages but not pT4 also showed an increased hazard for relapse compared to pT1, as well as Stages 2A, 2B and 3A, 3B vs. Stage 1A,1B (Table 2).
Table 2.
Disease-free survival univariate analysis.
| Risk Factor at Surgery | Events/At Risk | HR (95% CI) | p-Value | |
|---|---|---|---|---|
| Age | 65/342 | 1.17 a (0.99–1.38) | 0.07 | |
| Tumor Size | 65/342 | 1.21 b (1.04–1.40) | 0.01 | |
| Hemoglobin | 65/342 | 0.84 c (0.72–0.97) | 0.02 | |
| RDW% | 65/342 | 1.16 c (1.05–1.28) | 0.003 | |
| HRR | ||||
| Continuous | 65/342 | 0.15 c (0.05–0.50) | 0.002 | |
| Median cut-off | <1.01 | 44/170 | 1 | |
| ≥1.01 | 21/172 | 0.41 (0.25–0.68) | <0.001 | |
| pN | N0 | 23/204 | 1 | |
| N1 Single Station | 15/55 | 3.30 (1.78–6.11) | <0.001 | |
| N1 Multiple Stations | 5/12 | 6.47 (2.37–17.7) | <0.001 | |
| N2 Single Station | 12/54 | 1.79 (0.87–3.67) | 0.12 | |
| N2 Multiple Stations | 19/17 | 9.25 (3.57–24.0) | <0.001 | |
| N Status d | Single station | 27/109 | 1 | |
| Multiple stations | 15/29 | 2.84 (1.43–5.63) | 0.003 | |
| Gender | Female | 27/161 | 1 | |
| Male | 38/181 | 1.23 (0.76–1.98) | 0.41 | |
| Smoker | No | 12/75 | 1 | |
| Yes | 53/264 | 0.85 (0.49–1.49) | 0.58 | |
| Surgery | Open | 42/188 | 1 | |
| Minimal Invasive Surgery | 23/154 | 0.66 (0.41–1.06) | 0.08 | |
| pN | N0 | 23/204 | 1 | |
| N1 | 20/67 | 3.69 (2.10–6.48) | <0.001 | |
| N2 | 22/71 | 2.85 (1.55–5.27) | <0.001 | |
| pT | pT1 | 12/136 | 1 | |
| pT2 | 35/141 | 2.22 (1.18–4.18) | 0.01 | |
| pT3 | 13/46 | 3.01 (1.35–6.71) | 0.007 | |
| pT4 | 5/19 | 3.33 (0.99–11.2) | 0.05 | |
| Grading | 0 | 4/22 | 1 | |
| 1 | 0/20 | Not estimable | - | |
| 2 | 22/159 | 0.74 (0.26–2.16) | 0.58 | |
| 3 | 30/112 | 1.80 (0.62–5.25) | 0.28 | |
| Stage | 1A,1B | 16/169 | 1 | |
| 2A,2B | 17/71 | 2.00 (1.08–3.69) | 0.03 | |
| 3A,3B | 32/102 | 3.72 (2.06–6.69) | <0.001 | |
| Treatments | No | 32/217 | 1 | |
| Yes | 33/121 | 1.67 (1.02–2.73) | 0.04 | |
| Procedure | Lobectomy | 60/318 | 1 | |
| Pneumonectomy | 5/24 | 1.99 (0.74–5.37) | 0.17 |
a by five-year increase; b by 10 mm increase; c by one unit increase; d N1 and N2 patients subgroup only; median follow-up = 13 months.
Lymph node hilar involvement (N1) and ipsilateral lymph node mediastinal involvement (N2) showed a significantly increased hazard for relapse compared to patients without lymph node involvement (N0): HR = 3.69 95% CI: (2.10–6.48), p < 0.001 and HR = 2.85 95% CI: (1.55–5.27), p < 0.001, respectively. Multiple- vs. single-station involvement was also a significantly risk factor for relapse HR = 2.84, 95% CI: (1.43–5.63), p < 0.001 (Table 2). Treatments were also significantly associated with an increased hazard for relapse, though borderline significant (p = 0.04) (Table 2). Risk factors that remained significant in the multivariable analysis were HRR for values ≥1.01 vs. values <1.01 (HR = 0.46 95% CI: (0.27–0.77), p = 0.004), N1 single station vs. N0 (HR = 2.55, 95% CI: (1.33–4.90), p = 0.005), N1 multiple station vs. N0 (HR = 9.16, 95% CI: (3.65–23.0), p < 0.001), and N2 multiple stations vs. N0 (HR = 10.5, 95% CI: (3.44–32.2), p < 0.001); N2 single station was not a significant risk factor for relapse compared to N0 (HR = 1.82, 95% CI: (0.70–4.74), p = 0.11) (Table 3).
Table 3.
Disease-free survival multivariable analysis treatment-adjusted.
| Risk Factor at Surgery | HR (95% CI) | p-Value | |
|---|---|---|---|
| Tumor Size | 1.18 a (0.99–1.40) | 0.06 | |
| HRR Median cut-off | ≥1.01 | 1 | |
| <1.01 | 2.20 (1.30–3.72) | 0.004 | |
| pN | N0 | 1 | |
| N1 Single Station | 2.55 (1.33–4.90) | 0.005 | |
| N1 Multiple Stations | 9.16 (3.65–23.0) | <0.001 | |
| N2 Single Station | 2.29 (0.83–6.33) | 0.11 | |
| N2 Multiple Stations | 10.5 (3.44–32.2) | <0.001 |
a by 10 mm-unit increase; median follow-up = 13 months.
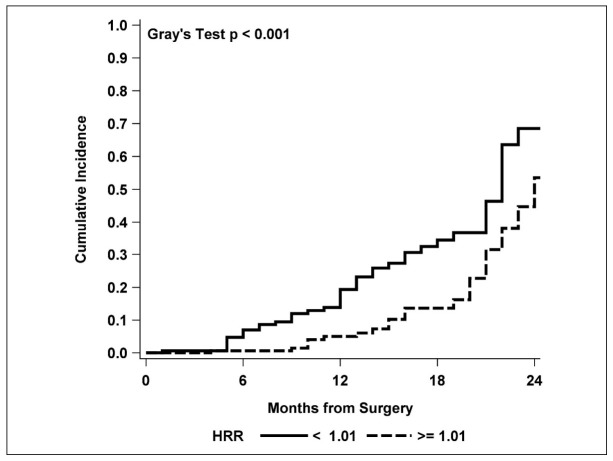
Comparison of the cumulative incidence functions for relapse also showed a higher and significant increase with time for HRR values lower than 1.01 vs. HRR ≥1.01 (p < 0.001) (Figure 1).
Figure 1.
Cumulative incidence functions for relapse by the HRR median cut-off value.
Fifty-six patients (16.1%) had post-operative complications: 18 patients (5.1%) had grade I complications, 23 patients (6.6%) had grade II complications, 7 patients (2.0%) had grade III complications, 2 patients (0.5%) had grade IV complications, six patients (1.7%) had grade V complications—5 of them receiving right pneumonectomy, and 1 left pneumonectomy—and then died within 30 days of the operation or during hospitalization. Pre-operative HRR did not disclose any prognostic value either for any complications (p = 0.26) or for minor (p = 0.53) and major complications (p = 0.29). (Table 4).
Table 4.
Estimates of the risk for major, minor, and any grade complications (odd ratios) according to the HRR median cut-off.
| Complication | HRR | OR (95% CI) | p-Value |
|---|---|---|---|
| Major | <1.01 | 1 | |
| ≥1.01 | 1.82 (0.60–5.55) | 0.29 | |
| Minor | <1.01 | 1 | |
| ≥1.01 | 1.24 (0.64–2.40) | 0.53 | |
| Any complications | <1.01 | 1 | |
| ≥1.01 | 1.41 (0.78–2.53) | 0.26 |
4. Discussion
Complete blood count (CBC) is routinely performed in neoplastic patients before starting almost any kind of treatment. Several parameters tested in a standard CBC have recently been shown to have predictive value in many neoplastic diseases. These include Hb, RDW, neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) [18,19].
There is now clear evidence that the host response to systemic inflammation is closely related to tumor development and its progression; similarly, higher values of RDW—defined as anisocytosis—have been correlated to systemic inflammation and thus to an aggressive tumor behavior [20].
Nevertheless, although many studies disclosed interesting findings about the prognostic value of RDW in neoplastic disease, it has been postulated that RDW alone—without further indicators—might not completely represent the systematic inflammatory condition of the patient, in particular when chemotherapy has already been administered. For this reason, Sun et coll.—considering that Hb is a well-established marker of nutritional status—combined RDW and Hb values to build a new prognostic index for esophageal squamous cell carcinoma, disclosing a significant association between the HB/RDW ratio and clinical characteristics and survival outcomes in this cohort of patients [4].
Both RDW and Hb are influenced by many non-neoplastic conditions, and the Hb/RDW ratio is therefore a means of reducing the potential bias due to interfering factors, reflecting a more global health status of the patient. Our findings suggest that the HRR is an effective prognostic tool in terms of disease-free survival; in fact, considering the HRR as a categorical variable, patients with pre-operative HRR lower than 1.01 presented a higher risk for shorter disease-free survival, suggesting a stricter follow-up for patients with early-stage diseases or encouraging adjuvant treatments for those with tumors in more advanced stages. Similarly, considering HRR as a continuous variable, we observed recurrence risk reductions up to 85% for each unit of increase of the pre-operative HRR.
The HRR is a simple, inexpensive laboratory test included in standard CBC routinely performed before surgery; it does not require any additional procedure, cost, or dedicated equipment; its cost-effectiveness, considering its valuable prognostic efficacy, represents an added value of the test. Although we demonstrated a clear association only between preoperative HRR and DFS, we may assume that the HRR could be useful during the follow-up too. Further studies are required to explore this hypothesis.
As expected, our study disclosed a higher incidence of recurrence for patients with advanced-stage tumors and mediastinal involvement; interestingly, single-station-N2 disease did not show a significant risk increase (p = 0.12) that, instead, we observed in diseases with N1 multiple- (p < 0.001) and N2 multiple- (p < 0.001) station involvement. Although this may simply be due to a sampling effect, we could argue that single-station-N2 disease may represent a more favorable subgroup of patients, as reported in recent literature [21,22,23,24,25].
Finally, no difference was observed between open and minimally invasive approaches, thus confirming the now well-known concept that the minimally invasive approach is at least not inferior to open surgery in terms of oncological results; in any case, we adopted a minimally invasive approach frequently for patients with an early-stage disease and only in a few cases for carefully selected N2 patients [26,27].
Several limitations of the present study need to be considered: this is a single-center, non-randomized retrospective study, with a limited follow-up due to the only recent introduction of RDW in the standard pre-operative assessment of our patients. This conditioned only a small number of deaths, which were mostly due to post-operative major complications rather than to oncologic causes. For these reasons, we focused our analysis on disease-free survival rather than on overall survival (Table S1). Similarly, we did not explore the impact of pre-operative comorbidities on overall survival.
5. Conclusions
Pre-operative HRR is an effective prognostic factor of disease-free survival for resected-lung-adenocarcinoma patients; together with the number of pathologic node stations involved, it could therefore be considered as a further tool for planning adjuvant treatments and setting up a patient-tailored follow-up program.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/13/4/710/s1, Table S1: All multivariable subdistribution Hazard Ratios (sHR) for treatment adjusted Disease Free Survival.
Author Contributions
F.P. conceived the study; F.P., M.C., D.R., S.R., and L.S. contributed to the design and implementation of the research, to the analysis of the results, and to the writing of the manuscript. A.C., G.M., E.P. contributed to the design and implementation of the research, to the analysis of the results, and to the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the Italian Ministry of Health with “Ricerca Corrente”, “5 × 1000” funds.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki; ethical review and approval were waived for this study, due to the retrospective nature of the study and the previous consents obtained.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Available on request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ye X., Liu J., Chen Y., Wang N., Lu R. The impact of hemoglobin level and transfusion on the outcomes of chemotherapy in gastric cancer patients. Int. J. Clin. Exp. Med. 2015;8:4228–4235. [PMC free article] [PubMed] [Google Scholar]
- 2.Shin N.R., Lee Y.Y., Kim S.H., Choi C.H., Kim T.J., Lee J.W., Bae D.S., Kim B.G. Prognostic value of pretreatment haemoglobin level in patients with early cervical cancer. Obstet. Gynecol. Sci. 2014;57:28–36. doi: 10.5468/ogs.2014.57.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cordella C., Luebbers H.T., Rivelli V., Grätz K.W., Kruse A.L. An evaluation of the preoperative hemoglobin level as a prognostic factor for oral squamous cell carcinoma. Head Neck Oncol. 2011;3:35. doi: 10.1186/1758-3284-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun P., Zhang F., Chen C. The ratio of hemoglobin to red cell distribution width as a novel prognostic parameter in esophageal squamous cell carcinoma: A retrospective study from southern China. Oncotarget. 2016;7:42650–42660. doi: 10.18632/oncotarget.9516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riedl J., Posch F., Königsbrügge O., Lötsch F., Reitter E.M., Eigenbauer E., Marosi C., Schwarzinger I., Zielinski C., Pabinger I., et al. Red Cell Distribution Width and Other Red Blood Cell Parameters in Patients with Cancer: Association with Risk of Venous Thromboembolism and Mortality. PLoS ONE. 2014;9:e111440. doi: 10.1371/journal.pone.0111440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W., Li X., Wang M., Ge X., Li F., Huang B., Peng J., Li G., Lu L., Yu Z., et al. Association between red cell distribution width and the risk of heart events in patients with coronary artery disease. Exp. Ther. Med. 2015;9:1508–1514. doi: 10.3892/etm.2015.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrella F., Radice D., Guarize J., Piperno G., Rampinelli C., De Marinis F., Spaggiari L. The Impact of Multidisciplinary Team Meetings on Patient Management in Oncologic Thoracic Surgery: A Single-Center Experience. Cancers. 2021;13:228. doi: 10.3390/cancers13020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellingsen T.S., Lappegård J., Skjelbakken T., Brækkan S.K., Hansen J.B. Impact of red cell distribution width on future risk of cancer and all-cause mortality among cancer patients—The Tromsø Study. Haematologica. 2015;100:e387–e389. doi: 10.3324/haematol.2015.129601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L., Wang C., Wu S., Li Y., Guo W., Liu M. Red blood cell distribution width is associated with mortality after acute ischemic stroke: A cohort study and systematic review. Ann. Transl. Med. 2020;8:81. doi: 10.21037/atm.2019.12.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warwick R., Mediratta N., Shackcloth M., Shaw M., McShane J., Poullis M. Preoperative red cell distribution width in patients undergoing pulmonary resections for non-small-cell lung cancer. Eur. J. Cardiothorac Surg. 2014;45:108–113. doi: 10.1093/ejcts/ezt275. [DOI] [PubMed] [Google Scholar]
- 11.Fanti S., Farsad M., Battista G., Monetti F., Montini G., Chiti A., Savelli G., Petrella F., Bini A., Nanni C. Somatostatin Receptor Scintigraphy for Bronchial Carcinoid Follow-Up. Clin. Nucl. Med. 2003;28:548–552. doi: 10.1097/00003072-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Seretis C., Seretis F., Lagoudianakis E., Gemenetzis G., Salemis N.S. Is Red Cell Distribution Width a Novel Biomarker of Breast Cancer Activity? Data From a Pilot Study. J. Clin. Med. Res. 2013;5:121–126. doi: 10.4021/jocmr1214w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang F.M., Xu G., Zhang Y., Ma L.L. Red Cell Distribution Width Is Associated with Presence, Stage, and Grade in Patients with Renal Cell Carcinoma. Dis. Markers. 2014;2014:860419. doi: 10.1155/2014/860419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu F., Yang S., Tang X., Liu W., Chen H., Gao H. Prognostic value of baseline hemoglobin-to-red blood cell distribution width ratio in small cell lung cancer: A retrospective analysis. Thorac. Cancer. 2020;11:888–897. doi: 10.1111/1759-7714.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Postmus P.E., Kerr K.M., Oudkerk M., Senan S., Waller D.A., Vansteenkiste J., Escriu C., Peters S. ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017;28(Suppl. 4):iv1–iv21. doi: 10.1093/annonc/mdx222. [DOI] [PubMed] [Google Scholar]
- 16.Charlson M.E., Pompei P., Ales K.L., Mackenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Ivanovic J., Al-Hussaini A., Al-Shehab D., Threader J., Villeneuve P.J., Ramsay T., Maziak D.E., Gilbert S., Shamji F.M., Sundaresan R.S., et al. Evaluating the reliability and reproducibility of the Ottawa Thoracic Morbidity and Mortality classification system. Ann. Thorac Surg. 2011;91:387–393. doi: 10.1016/j.athoracsur.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 18.Arigami T., Okumura H., Matsumoto M., Uchikado Y., Uenosono Y., Kita Y., Owaki T., Mori S., Kurahara H., Kijima Y., et al. Analysis of the Fibrinogen and Neutrophil–Lymphocyte Ratio in Esophageal Squamous Cell Carcinoma: A Promising Blood Marker of Tumor Progression and Prognosis. Medicine (Baltimore) 2015;94:e1702. doi: 10.1097/MD.0000000000001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrella F., Diotti C., Rimessi A., Spaggiari L. Pulmonary metastasectomy: An overview. J. Thorac Dis. 2017;9:S1291–S1298. doi: 10.21037/jtd.2017.03.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwal S. Red cell distribution width, inflammatory markers and cardiorespiratory fitness: Results from the National Health and Nutrition Examination Survey. Indian Heart J. 2012;64:380–387. doi: 10.1016/j.ihj.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hishida T., Yoshida J., Ohe Y., Aokage K., Ishii G., Nagai K. Surgical Outcomes After Initial Surgery for Clinical Single-Station N2 Non-Small-Cell Lung Cancer. Jpn. J. Clin. Oncol. 2014;44:85–92. doi: 10.1093/jjco/hyt164. [DOI] [PubMed] [Google Scholar]
- 22.Kowalewski J., Szczęsny T.J. Is Single-Station N2 Disease on PET-CT an Indication for Primary Surgery in Lung Cancer Patients? J. Thorac Dis. 2017;9:4828–4831. doi: 10.21037/jtd.2017.10.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casiraghi M., Maisonneuve P., Piperno G., Bellini R., Brambilla D., Petrella F., De Marinis F., Spaggiari L. Salvage Surgery After Definitive Chemoradiotherapy for Non–small Cell Lung Cancer. Semin. Thorac. Cardiovasc. Surg. 2017;29:233–241. doi: 10.1053/j.semtcvs.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Petrella F., Leo F., Veronesi G., Solli P., Borri A., Galetta D., Gasparri R., Lembo R., Radice D., Scanagatta P., et al. “Salvage” surgery for primary mediastinal malignancies: Is it worthwhile? J. Thorac. Oncol. 2008;3:53–58. doi: 10.1097/JTO.0b013e31815e6d54. [DOI] [PubMed] [Google Scholar]
- 25.Yazgan S., Ucvet A., Gursoy S., Samancilar O., Yagci T. Single-station skip-N2 Disease: Good Prognosis in Resected Non-Small-Cell Lung Cancer (Long-Term Results in skip-N2 Disease) Interact. Cardiovasc. Thorac. Surg. 2019;28:247–252. doi: 10.1093/icvts/ivy244. [DOI] [PubMed] [Google Scholar]
- 26.Petrella F., Chieco P., Solli P., Veronesi G., Borri A., Galetta D., Gasparri R., Spaggiari L. Which factors affect pulmonary function after lung metastasectomy? Eur. J. Cardiothorac. Surg. 2009;35:792–796. doi: 10.1016/j.ejcts.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Spaggiari L., Sedda G., Maisonneuve P., Tessitore A., Casiraghi M., Petrella F., Galetta D. A Brief Report on Survival After Robotic Lobectomy for Early-Stage Lung Cancer. J. Thorac. Oncol. 2019;14:2176–2180. doi: 10.1016/j.jtho.2019.07.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available on request.