Abstract
Chronic kidney disease (CKD) is one of the most common chronic non-communicable degenerative diseases and it represents an important risk factor for cardiovascular morbidity and mortality. The Mediterranean diet, in which extra virgin olive oil (EVOO) is the main source of vegetal fats, represents a nutritional-diet regimen that is useful for the treatment of CKD and its comorbidities. We tested two different EVOOs, characterized by a high (Synergy) and medium (Luxolio) content of minor polar compounds (MPCs), detected by HPLC-DAD-MS analysis, in 40 nephropathic patients, at a dose of 40 mL/day for 9 weeks. We evaluated the effects of these two EVOOs on renal function, body composition, oxidative stress, and inflammatory state, after 9 weeks of EVOOs consumption (T1) and after 2 months of wash-out (T2). We observed an improvement of renal function biomarkers (estimated-glomerular filtration rate, albuminuria, azotemia, uric acid), lipid profile, oxidative stress, inflammatory parameters (erythrocyte sedimentation rate, C-reactive protein) and in body composition at T1. These healthy effects were greater and persisted over time after the wash-out period in Synergy patients. The high MPC EVOO content seems to exert an antioxidant and anti-inflammatory effect in nephropathic patients and these protective actions are maintained over time.
Keywords: extra virgin olive oil, polyphenols, antioxidant activity, chronic kidney disease, hydroxytyrosol, oleocanthal
1. Introduction
Chronic kidney disease (CKD) represents one of the leading chronic non-communicable degenerative diseases in the world. CKD is associated with high morbidity and mortality, in addition, the costs of renal replacement therapy (RRT) have increased significantly from 1960 up to today [1]. In fact, the number of people who currently need RRT in the world exceeds 2.5 million and it is assumed that in 2030 this value will double and reach 5.4 million [2]. Furthermore, in many countries, especially in developing countries, there is little possibility of access to RRTs and it is hypothesized that 2.3–7 million adults will experience premature death due to the inability to undergo RRT [2]. Moreover, CKD is now a well-known independent risk factor for cardiovascular diseases (CVDs), specifically, starting from its earliest stages, an increase in cardiovascular morbidity and premature death is observed. This phenomenon can be explained by taking into consideration the cardiovascular (CV) risk factors of the uremic state [3]. In addition, CKD considerably worsens the quality of life of patients who are affected, therefore, it is fundamental to implement new therapeutic strategies aimed at slowing down of its progression and to counteract its comorbidities. Natural bioactive compounds represent a valid therapeutic alternative as they are characterized by a very low presence of side effects [4,5]. From this perspective, nutritional-diet therapy (DNT) plays a key role; in fact, it represents a fundamental part of treatment of CKD at all stages [6,7]. In the early stages of CKD, a “healthy diet” (HD) is recommended, and it is characterized by a high intake of vegetables, fruit, nuts, whole grains, legumes and fish, and low intake of saturated fats and sodium [8]. In fact, a recent meta-analysis revealed that HD was associated with lower mortality in CKD patients than in subjects who did not follow this practice [9]. A prospective population study “Singapore Chinese Health Study” highlighted the possible association between the intake of certain types of food and the incidence of end stage renal disease (ESRD). The authors demonstrated a strong correlation between the consumption of red meat and the risk of developing ESRD [10]. Furthermore, in our previous study we highlighted how the Mediterranean diet (MD) represents a nutritional-diet regimen valid for the treatment of the first stages of CKD, considerably reducing the CV risk associated with the disease [11,12]. This was confirmed by our subsequent study, which divided a population of nephropathic patients based on their genotype for the methylene tetrahydrofolate reductase (MTHFR) enzyme [13].
Among the typical products of an MD, a key food is represented by extra virgin olive oil (EVOO), which is its main source of vegetable fats. A total of 98–99% of the total weight of EVOO is made up by fatty acids, in particular, monounsaturated ones such as oleic acid, and a small percentage (1–2%) by minor polar compounds (MPCs). MPCs, especially of the phenolic variety, seem to be fundamental for the health effects exerted by EVOO. Among these, hydroxytyrosol, tyrosol, oleacin and oleocanthal are of particular importance [14,15]. In this regard, we tested two different EVOOs obtained from synergic-biodynamic agriculture, characterized by a high content of MPCs, evaluated by HPLC-DAD-MS analysis, in nephropathic patients. These EVOOs were administered to a group of CKD patients at a dose of 40 mL/day for 9 weeks in order to evaluate their effects on renal function, body composition, oxidative stress (OS) and inflammatory state. Furthermore, to confirm the potential results obtained, the patients underwent a wash-out period of two months, at the end of which the previously listed parameters were re-tested.
2. Material and Methods
2.1. Samples of EVOOs
EVOOs selected for this study were produced using biphasic pressing technology. The first oil selected was monocultivar Mignolo, produced in Tuscany (Vinci, Florence, Italy) in 2019 (Tuscany Region—European Partnership for Innovation in Agriculture Productivity and Sustainability 2014–2020—BioSynOL, “Oil and Legumes: biodynamic and synergistic crops for naturally fortified foods and innovative products for health and sport”). The second oil chosen was a mix of three cultivars of olives including Moraiolo (33%), Leccino (34%), and Mignolo (33%) produced in Tuscany (Vinci, Florence, Italy) in 2019. The monocultivar Mignolo oil was obtained from a biosynergy and biodynamic crop that was made with leguminous species capable of fixing important nutrients in the soil, that have positively influenced the quality of the olive tree and therefore of the oil produced. The two selected EVOOs were extracted and then analyzed by HPLC-DAD-MS for the evaluation of MPCs content, according to the protocol already described in another study conducted by our research group [16].
2.2. Patients and Methods
In the present clinical study, a group of 40 CKD patients (stages I–IV according to the National Kidney Foundation Kidney—Disease Outcomes Quality Initiative guidelines) [17] under conservative therapy, were enrolled at the UOC of Internal Medicine, Center for Hypertension and Nephrology Unit of the University Hospital Policlinico Tor Vergata (PTV), Rome. Subsequently, these patients were divided into 2 subgroups, matched by age, gender and body mass index (BMI): 20 patients assumed EVOO with medium MPCs (Luxolio, LUX) content and 20 patients assumed EVOO with a high MPCs content (Synergy, SYN).
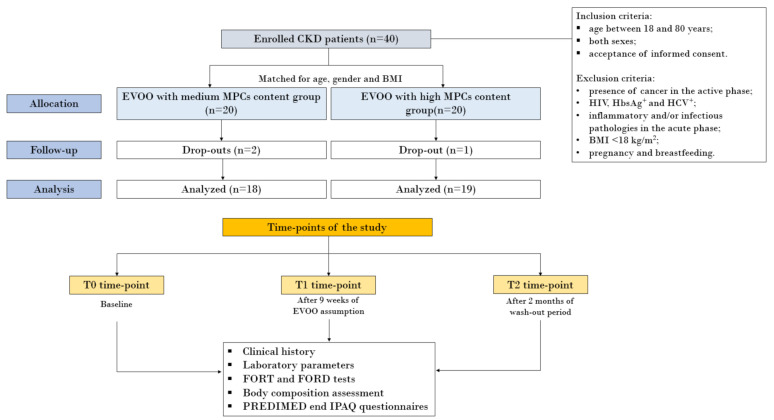
LUX and SYN were administered to nephropathic patients at a dose of 40 mL/day, for 9 weeks. In particular, the EVOOs were consumed raw, as a condiment for dishes. At baseline (T0), we collected the clinical history for each enrolled patient and we evaluated laboratory parameters, such as creatinine, azotemia, uric acid (UA), lipid profile, inflammatory and OS biomarkers, in addition to a body composition assessment. The same parameters were also examined at the two subsequent time-points: at time-point T1 (after 9 weeks of EVOO consumption) and at time-point T2 (after 2 months of wash-out period). The flow-chart of the study design is illustrated in Figure 1.
Figure 1.
Flow-chart of the study. Abbreviations: BMI, body mass index; CKD, chronic kidney disease; EVOO, extra virgin olive oil; FORD, Free Oxygen Radical Defense; FORT, Free Oxygen Radical Test; IPAQ, International Physical Activity Questionnaire; MPCs, minor polar compounds; PREDIMED, Prevención con Dieta Mediterránea.
The study protocol was declared as being compliant with the Helsinki declaration by the PTV Independent Ethics Committee. All enrolled subjects signed an informed consent at the point of enrollment.
The inclusion criteria were individuals aged between 18 and 80 years, both sexes after acceptance of informed consent. The exclusion criteria were presence of cancer in the active phase, HIV, HbsAg+, HCV+, inflammatory and/or infectious pathologies in the acute phase; BMI < 18 kg/m2; pregnancy and breastfeeding. During the period of study, the patients did not undergo therapeutic changes.
All patients followed an Italian standard Mediterranean diet with a controlled protein intake according to CKD stage [18,19]. All patients were instructed to maintain a water intake between 1000–1500 mL/day so as to guarantee a neutral water balance.
2.3. Questionnaires
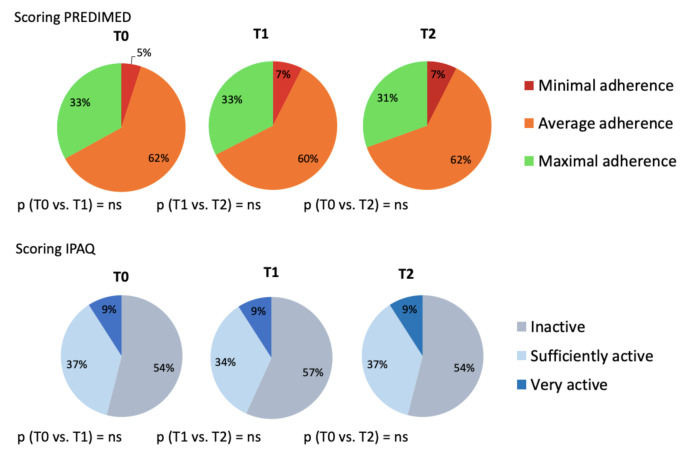
Two questionnaires were administered to all nephropathic patients with the aim to exclude potential biases due to changes in eating habits or physical exercise during the study. PREDIMED (Prevención con Dieta Mediterránea) and IPAQ (International Physical Activity Questionnaire) questionnaires were performed at all time-points of the study (T0, T1, T2). The first assesses the degree of adherence to the Mediterranean diet [20] and the second evaluates the degree of weekly physical activity [21]. The PREDIMED and IPAQ questionnaires did not show any significant difference in the eating habits and lifestyle of the enrolled patients, for the entire study period (Figure 2).
Figure 2.
PREDIMED and IPAQ questionnaires. Abbreviations: IPAQ, International Physical Activity Questionnaire; PREDIMED, Prevención con Dieta Mediterránea.
In addition, to assess the polyphenols intake from other foods, we recorded a 24 h dietary recall at all time-points of the study, highlighting homogeneous polyphenols consumption between the patients, at each time point of the study [22].
2.4. Body Composition Assessment
Anthropometric parameters of all CKD patients were registered according to standard methods [23]. Body weight (kg) was measured to the nearest 0.01 kg, using a balance scale (Seca 711, Hamburg, Germany). Height (m) was measured using a stadiometer to the nearest 0.1 cm (Seca 220, Hamburg, Germany). BMI was calculated as body weight divided by height squared (kg/m2).
For the assessment of body composition, all nephropathic patients underwent bioelectrical impedance analysis (BIA). Resistance, reactance, impedance, and phase angle at 50 KHz frequency were measured using a BIA 101S instruments (Akern/RIL System-Florence). For evaluation of body composition, we considered the following parameters: total body water (TBW), intracellular water (ICW), extracellular water (ECW), body cell mass index (BCMI), fat free mass (FFM), fat mass (FM) and muscle mass (MM) [24]. Not all patients underwent BIA because several participants were pacemaker carriers; specifically, 2 patients from the LUX group and 2 patients from the SYN group.
Moreover, the waist and hip circumferences were measured and the waist to hip ratio was assessed, at all times of the study.
2.5. Laboratory Parameters
At T0, T1 and T2, we performed the following laboratory and urinary exams: azotemia, creatinine, UA, electrolytes, sideremia, glycaemia, lipid profile, chemical–physical urinary examination, albuminuria on morning urine. Moreover, inflammatory parameters such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) and interleukin (IL)-6 were monitored at all time-points of the study. All patients also underwent capillary sampling using CR4000 tool for the oxidative stress evaluation and for the assessment of antioxidant defense mechanisms. In particular, a free oxygen radical test (FORT) and a free oxygen radical defense (FORD) test were performed [25,26]. The first test measures the levels of circulating oxygen free radicals [27] and the second indirectly determines the organisms’ antioxidant capacity by the concentration of ascorbic acid, glutathione, and albumin [28].
Laboratory parameters were monitored by Dimension Vista 1500 (Siemens Healthcare Diagnostics, Milano, Italy), while the lipid profile was detected by standard enzymatic colorimetric techniques (Roche Modular P800, Roche Diagnostics, Indianapolis, IN, USA) and IL-6 was monitored by IMMULITE 2000 XPi Immunoassay System (Siemens Healthcare Diagnostics, Milano, Italy).
All other parameters were analyzed according to standard procedures in the Clinical Chemical Laboratories of the University Hospital, PTV of Rome.
2.6. Statistical Analysis
All data were entered into an Excel spreadsheet (Microsoft, Redmond, WA, USA) and the analysis was performed using the Windows Social Science Statistics Package, version 25.0 (IBM_SPSS, Chicago, IL, USA).
The descriptive statistics consider the mean ± standard deviation for the parameters with normal distribution (after confirmation with histograms and the Kolgomorov–Smirnov test), while for the non-normal variables, they consider the median and the interval (minimum:maximum).
The comparison for the normal variables was carried out with a one-way ANOVA, while the comparisons between treatment over time (T0, T1 and T2) were conducted with a paired t-test for normal variables and a Mann–Whitney test for the non-normal variables. Regarding occurrences (percentages), the chi-square test, possibly corrected by Fisher’s exact test, was performed. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. HPLC-DAD-MS: EVOOs Characterization
The two selected EVOOs were analyzed by HPLC-DAD-MS for evaluation of the MPCs content, the results of the analyses are reported in Table 1. Among the two EVOOs, monocultivar Mignolo (SYN) was the richest in phenolic compounds, with a value of 706.36 mg/kg compared to the blend of Tuscany cultivars (LUX) which presented a content of 485.01 mg/kg of total polyphenols.
Table 1.
High-performance liquid chromatography (HPLC-DAD-MS) analysis of EVOOs samples.
| Compound | SYN | LUX |
|---|---|---|
| Hydroxytyrosol (mg/L *) | 1.78 ± 0.05 | 0.5 ± 0.02 |
| Tyrosol (mg/L *) | 1.71 ± 0.05 | 0.5 ± 0.03 |
| Elenolic acid derivatives (mg/L *) | 198.76 ± 5.96 | 32.26 ± 0.97 |
| Elenolic acid (mg/L *) | 28.15 ± 0.84 | 129.1 ± 3.87 |
| Oleacin (mg/L *) | 121.98 ± 3.66 | 77.54 ± 2.33 |
| Oleocanthal (mg/L *) | 46.02 ± 1.38 | 41.23 ± 1.24 |
| Oleuropein aglycone (mg/L *) | 142.65 ± 4.28 | 23.95 ± 0.72 |
| Secoiridoids derivatives (mg/L *) | 34.29 ± 1.03 | 87.47 ± 2.62 |
| Lignans (mg/L*) | 131.02 ± 3.93 | 92.44 ± 2.77 |
| Total MCP (mg/L*) | 706.36 ± 21.19 | 485.01 ± 14.55 |
* All results are the average of three determinations and the standard error is <2.5%. Abbreviations: LUX, Luxolio; SYN, Synergy.
Through the HPLC-DAD-MS analysis, it was possible to evaluate the content of single molecules. In particular, the values of hydroxytyrosol, tyrosol and derivatives were taken into consideration, as reported by the European Food Safety Authority (EFSA) EU No 432/2012 health claim. In fact, according to EFSA, it is necessary to consume 20 g/day of olive oil with a content of hydroxytyrosol and its derivatives of 5 mg, to have positive effects on health and to yield protection from oxidative stress [29].
The oleocanthal and oleacin content was evaluated in both EVOOs, given the importance of their antioxidant and anti-inflammatory effects, as described in 2005 by Beauchamp [30]. Mignolo (SYN) has an higher content of these molecules compared to LUX. In particular, SYN has a higher content of hydroxytyrosol at 70%, of oleacin and oleocanthal together representing 40%, and of oleuropein aglycone at 80%, in comparison to LUX. Regarding the content of MCPs, it is important to consider their bioavailability. In fact, the biodisponibility of hydroxytyrosol is dose-dependent and exerts physiological and systemic actions after its assumption through EVOO [31]. In particular, hydroxytyrosol is directly absorbed in the small intestine, while other phenolic compounds such as oleuropein aglycone are hydrolyzed, after EVOO consumption, in the small intestine to hydroxytyrosol [32]. In vitro study, investigating hydroxytyrosol stability in aqueous solution at various temperatures and concentrations, revealed that EVOOs with lower hydroxytyrosol concentrations were more susceptible to degradation. On the contrary, EVOOs that are higher in their content of hydroxytyrosol and derivatives were less prone to degradation processes. In addition, the studies available so far show that, in vivo, hydroxytyrosol did not undergo significant degradation processes after one week [33,34]. EVOO with a high content of MPCs stored in bottles, capped with nitrogen and made with special glass (that shielded 99.99% from UV radiation), after 18 months showed stable antioxidant and antiradical activities towards human low-density lipoprotein (LDL) oxidation. These beneficial actions are related to MPC content [35,36,37]. Currently, no human studies have been conducted to evaluate the bioavailability of hydroxytyrosol over time, however, recent studies have highlighted how hydroxytyrosol is more bioavailable in humans than in rats, both as a plasmatic and urinary metabolite [31,38]. Hydroxytyrosol is the molecule immediately available to carry out the antioxidant action of EVOO, while oleuropein aglycone and oleacin exert their antioxidant action by hydrolyzing and releasing hydroxytyrosol. Oleuropein aglycone and oleacin also display anti-inflammatory roles [15]. The synergistic and biodynamic cultivation of the olive trees of Mignolo (SYN) allowed us to obtain EVOO with a higher content of MPCs, compared to EVOO obtained from organic olive trees. Considering the results of the qualitative–quantitative characterization analyzes, the two EVOOs with different MPC contents were selected for application to patients in this study.
3.2. Study in Patients
A total of 40 CKD patients under conservative therapy were recruited for the in vivo study. The patients were divided into two subgroups, homogeneous for age, gender, and BMI. The first group assumed 40 mL/day of EVOO SYN, the second group assumed 40 mL/day of EVOO LUX, for 9 weeks. The epidemiological findings of the two study subgroups and their homogeneity are reported in the Table 2.
Table 2.
Clinical features of the study populations.
| SYN | LUX | p (ANOVA Test) | |
|---|---|---|---|
| N | 20 | 20 | |
| Gender (male/female) | 13/7 | 12/8 | ns |
| Age (years) | 70.6 ± 12.4 | 68.5 ± 11.4 | ns |
| BMI (kg/m2) | 28.9 ± 3.7 | 27.5 ± 5.9 | ns |
All data are expressed as mean ± standard deviation; abbreviations: ns, not significant; BMI, Body mass index; LUX, Luxolio; SYN, Synergy.
During the study, the patients dropped out due to factors unrelated to side effects deriving from EVOO consumption, as reported in Figure 1.
The laboratory and urinary parameters monitored at T0 (baseline), after 9 weeks of EVOO consumption (T1) and after 2 months of wash-out (T2) in the two study groups (SYN and LUX), are reported in Table 3. We observed a significant increase in the estimated glomerular filtration rate (e-GFR), calculated with the chronic kidney disease epidemiology collaboration (CKD-EPI) formula [39], after 9 weeks of SYN consumption (34.5 ± 16.8 mL/min/1.73 m2 vs. 37.7 ± 18.7 mL/min/1.73 m2; p = 0.0235) and this increase was maintained after the wash-out period (34.5 ± 16.8 mL/min/1.73 m2 vs. 38.2 ± 19.5 mL/min/1.73 m2; p = 0.0476), as reported in Table 4. In the SYN group, we also highlighted an albuminuria reduction (31.8 ± 45.2 mg/dL vs. 16.4 ± 24.1 mg/dL; p = 0.0413), an albumin enhancement (4.1 ± 0.3 g/dL vs. 4.3 ± 0.3 g/dL; p = 0.0413), an azotemia decrease (69.6 ± 23.1 mg/dL vs. 59.2 ± 17.3 mg/dL; p = 0.0346), a triglycerides reduction (154.5 ± 82.0 mg/dL vs. 132.7 ± 69.8 mg/dL; p = 0.0053) and a UA decrease (6.7 ± 1.5 mg/dL vs. 5.6 ± 1.2 mg/dL; p = 0.0206) at T1. After 2 months of wash-out, the increase in albumin (p = 0.0041), the reduction in azotemia (p = 0.0389) and the reduction in UA (p = 0.0230) were maintained over time, as reported in Table 4, while the reductions in triglyceride levels were not maintained over time (p = 0.0084). Moreover, we also demonstrated a reduction in inflammatory status, as monitored by CRP (4.2 ± 2.9 mg/L vs. 2.8 ± 2.3 mg/L; p = 0.0011), ERS (41.6 ± 24.7 mm/h vs. 35.1 ± 20.5 mm/h; p = 0.0005) and IL-6 (85.2 ± 110.7 pg/mL vs. 34.3 ± 11.1 pg/mL; p = 0.0321) at T1. Only the reduction in CRP was maintained after 2 months of wash-out (4.2 ± 2.9 mg/L vs. 2.7 ± 2.1 mg/L; p = 0.0007), while the ERS returned to basal levels at T2 (35.1 ± 20.5 mm/h vs. 43.9 ± 27.2 mm/h; p = 0.0218) and the IL-6 reduction was also maintained after the wash-out period (85.2 ± 110.7 vs. 42.8 ± 51.6, p = 0.043) (Table 5 and Table 6).
Table 3.
Laboratory and urinary parameters of two groups of the study.
| SYN | LUX | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 |
p-Value (T0 vs. T1) |
T2 |
p-Value (T1 vs. T2) |
T0 | T1 |
p-Value (T0 vs. T1) |
T2 |
p-Value (T1 vs. T2) |
|
| Creatinine (mg/dL) | 2.1 ± 0.8 | 2.0 ± 0.9 | ns # | 2.1 ± 1.0 | ns # | 2.2 ± 1.2 | 2.1 ± 1.2 | ns # | 2.2 ± 1.2 | ns # |
| e-GFR (mL/min/1.73 m2) | 34.5 ± 16.8 | 37.7 ± 18.7 | 0.0235 # | 38.2 ± 19.5 | ns # | 37.7 ± 20.0 | 40.2 ± 21.0 | ns # | 37.5 ± 20.0 | ns # |
| Albuminuria (mg/dL) | 31.8 ± 45.2 | 16.4 ± 24.1 | 0.0413 # | 28.8 ± 45.1 | ns # | 30.8 ± 59.6 | 18.8 ± 41.13 | ns # | 22.7 ± 55.2 | ns # |
| Albumin (g/dL) | 4.1 ± 0.3 | 4.3 ± 0.3 | 0.0039 # | 4.3 ± 0.3 | ns # | 4.2 ± 0.3 | 4.4 ± 0.4 | 0.0442 # | 4.2 ± 0.3 | 0.0290 # |
| Azotaemia (mg/dL) | 69.6 ± 23.1 | 59.2 ± 17.3 | 0.0346 # | 61.3 ± 17.6 | ns # | 65.4 ± 18.8 | 60.3 ± 22.0 | ns # | 63.7 ± 21.9 | ns # |
| Sodium (mEq/L) | 139.7 ± 3.4 | 139.5 ± 2.5 | ns # | 139.6 ± 2.6 | ns # | 141.6 ± 1.9 | 140.1 ± 2.5 | ns # | 141.2 ± 1.8 | ns # |
| Potassium (mEq/L) | 4.4 ± 0.6 | 4.4 ± 0.6 | ns # | 4.3 ± 0.5 | ns # | 4.5 ± 0.4 | 4.4 ± 0.4 | ns # | 4.5 ± 0.5 | ns # |
| Calcium (mg/dL) | 9.8 ± 0.5 | 9.6 ± 0.4 | ns # | 9.6 ± 0.4 | ns # | 9.8 ± 0.4 | 9.6 ± 0.5 | ns # | 9.7 ± 0.4 | ns # |
| Phosphorus (mg/dL) | 3.4 ± 0.6 | 3.3 ± 0.6 | ns # | 3.3 ± 0.6 | ns # | 3.6 ± 0.6 | 3.5 ± 0.6 | ns # | 3.7 ± 0.7 | ns # |
| TC (mg/dL) | 175.1 ± 44.9 | 169.5 ± 37.5 | ns # | 147.7 ± 47.2 | ns # | 178.9 ± 56.1 | 181.0 ± 47.7 | ns # | 180.9 ± 52.6 | ns # |
| HDL-C (mg/dL) | 41.8 ± 13.3 | 41.3 ± 12.7 | ns # | 42.4 ± 11.7 | ns # | 44.5 ± 12.3 | 47.3 ± 12.3 | ns # | 46.0 ± 11.5 | ns # |
| LDL-C (mg/dL) | 100.6 ± 30.8 | 100.0 ± 29.2 | ns # | 102.1 ± 21.6 | ns # | 112.1 ± 47.1 | 103.6 ± 39.8 | ns # | 112.5 ± 45.7 | ns # |
| Triglycerides (mg/dL) | 154.5 ± 82.0 | 132.7 ± 69.8 | 0.0053 # | 147.0 ± 79.9 | 0.0084 # | 123.4 ± 63.7 | 120.4 ± 55.2 | ns # | 128.2 ± 55.2 | ns # |
| Sideremia (μg/dL) | 86.8 ± 34.2 | 73.6 ± 18.1 | ns # | 73.9 ± 17.6 | ns # | 75.5 ± 22.4 | 68.6 ± 20.4 | ns # | 75.0 ± 21.5 | ns # |
| Glycaemia (mg/dL) | 98.5 ± 25.3 | 96.7 ± 35.4 | ns # | 98.7 ± 37.3 | ns # | 93.4 ± 19.8 | 89.8 ± 13.2 | ns # | 89.2 ± 17.6 | ns # |
| Uric acid (mg/dL) | 6.7 ± 1.5 | 5.6 ± 1.2 | 0.0206 # | 6.1 ± 1.6 | ns # | 6.6 ± 1.4 | 6.0 ± 0.8 | 0.0426 # | 6.3 ± 1.6 | ns # |
| CRP (mg/L) | 4.2 ± 2.9 | 2.8 ± 2.3 | 0.0011 # | 2.7 ± 2.1 | ns # | 5.2 ± 4.5 | 3.0 ± 2.9 | 0.0070 # | 4.89 ± 4.1 | 0.0072 # |
| ESR (mm/h) | 41.6 ± 24.7 | 35.1 ± 20.5 | 0.0005 # | 43.9 ± 27.2 | 0.0218 # | 38.5 ± 29.1 | 30.2 ± 24.4 | 0.0063 # | 36.7 ± 27.7 | 0.0090 # |
All data are expressed as mean ± standard deviation; # for the comparison of each parameter we applied test: t-test for paired data. Values of p ≤ 0.05 are considered statistically significant. Abbreviations: LUX, Luxolio; SYN, Synergy; e-GFR, estimated glomerular filtration rate; TC, total-cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ESR, erythrocyte sedimentation rate; ns, not significant; CRP, C-reactive protein.
Table 4.
p-values of baseline vs. wash-out period of laboratory and urinary parameters.
| SYN | LUX | |
|---|---|---|
|
p-Value (T0 vs. T2) |
p-Value (T0 vs. T2) |
|
| Creatinine (mg/dL) | ns # | ns # |
| e-GFR (mL/min/1.73 m2) | 0.0476 # | ns # |
| Albuminuria (mg/dL) | ns # | ns # |
| Albumin (g/dL) | 0.0041 # | ns # |
| Azotaemia (mg/dL) | 0.0389 # | ns # |
| Sodium (mEq/L) | ns # | ns # |
| Potassium (mEq/L) | ns # | ns # |
| Calcium (mg/dL) | ns # | ns # |
| Phosphorus (mg/dL) | ns # | ns # |
| TC (mg/dL) | ns # | ns # |
| HDL-C (mg/dL) | ns # | ns # |
| LDL-C (mg/dL) | ns # | ns # |
| Triglycerides (mg/dL) | ns # | ns # |
| Sideremia (μg/dL) | ns # | ns # |
| Glycaemia (mg/dL) | ns # | ns # |
| Uric acid (mg/dL) | 0.0230 # | ns # |
| CRP (mg/L) | 0.0007 # | ns # |
| ESR (mm/h) | ns # | ns # |
# Applied test: t-test for paired data. Values of p ≤ 0.05 are considered statistically significant. Abbreviations: LUX, Luxolio; SYN, Synergy; e-GFR, estimated glomerular filtration rate; TC, total-cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; ns, not significant.
Table 5.
Oxidative stress assessment of two groups of the study.
| SYN | LUX | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 |
p-Value (T0 vs. T1) |
T2 |
p-Value (T1 vs. T2) |
T0 | T1 |
p-Value (T0 vs. T1) |
T2 |
p-Value (T1 vs. T2) |
|
| FORT (U) | 289.3 ± 145.8 | 194.0 ± 63.1 | 0.00247 # | 223.3 ± 87.9 | ns # | 352.4 ± 156.5 | 278.6 ± 128.2 | ns # | 337.2 ± 143.7 | ns # |
| FORD (mmol/L Trolox) | 1.3 ± 0.4 | 1.2 ± 0.5 | ns # | 1.1 ± 0.4 | ns # | 1.3 ± 0.5 | 0.9 ± 0.5 | ns # | 1.5 ± 0.6 | ns # |
| IL-6 (pg/mL) | 85.2 ± 110.7 | 34.3 ± 11.1 | 0.0321 # | 42.8 ± 51.6 | ns # | 77.9 ± 98.3 | 59.8 ± 25.7 | ns # | 65.2 ± 34.2 | ns # |
All data are expressed as mean ± standard deviation; # for the comparison of each parameter we applied test: t-test for paired data. Values of p ≤ 0.05 are considered statistically significant. Abbreviations: IL, interleukin; LUX, Luxolio; SYN, Synergy; ns, not significant; FORT, free oxygen radical test; FORD, free oxygen radical defense.
Table 6.
p-value of baseline vs. wash-out period of oxidative stress assessment.
| SYN | LUX | |
|---|---|---|
|
p-Value (T0 vs. T2) |
p-Value (T0 vs. T2) |
|
| FORT (U) | ns # | ns # |
| FORD (mmol/L Trolox) | ns # | ns # |
| IL-6 (pg/mL) | 0.043 # | ns # |
# Applied test: t-test for paired data. Values of p ≤ 0.05 are considered statistically significant. Abbreviations: IL, interleukin; LUX, Luxolio; SYN, Synergy; ns, not significant; FORT, Free Oxygen Radical Test; FORD, Free Oxygen Radical Defense.
In the LUX group, we observed an albumin significant increase (4.2 ± 0.3 g/dL vs. 4.4 ± 0.4 g/dL; p = 0.0442), a significant UA reduction (6.6 ± 1.4 mg/dL vs. 6.0 ± 0.8 mg/dL; p = 0.0426) and an improvement in inflammatory status, highlighted by the reduction in CRP and ESR levels (respectively, 5.2 ± 4.5 mg/L vs. 3.0 ± 2.9 mg/L; p = 0.0070 and 38.5 ± 29.1 mm/h vs. 30.2 ± 24.4 mm/h; p = 0.0063). After 2 months of wash-out, we observed an increase in CRP (3.0 ± 2.9 mg/L vs. 4.89 ± 4.1 mg/L; p = 0.0072) and ESR (30.2 ± 24.4 mm/h vs. 36.7 ± 27.7 mm/h; p = 0.0090) levels and a decrease in serum albumin (4.4 ± 0.4 g/dL vs. 4.2 ± 0.3 g/dL; p = 0.0290) compared to T1.
The OS assessments of the two study subgroups are reported in Table 5. We showed a significant reduction in FORT values (289.3 ± 145.8 U vs. 194.0 ± 63.1 U; p = 0.00247) after 9 weeks of SYN consumption, but this reduction was not maintained after 2 months of wash-out, as reported in Table 6.
The body composition assessments of the two study subgroups are summarized in Table 7. We observed a significant decrease in reactance (39.3 ± 10.8 ohm vs. 42.3 ± 12.3 ohm; p = 0.0391), an TBW increase (52.8 ± 6.1% vs. 55.1 ± 5.9%; p = 0.0465), ICW enhancement (46.8 ± 7.2% vs. 49.9 ± 8.7%; p = 0.0058), a reduction in ECW (53.2 ± 7.2% vs. 50.1 ± 8.7%; p = 0.0058), an MM increase (38.3 ± 6.5% vs. 41.6 ± 7.2%; p = 0.0198) and a BCMI enhancement (9.1 ± 2.7 kg/m2 vs. 10.2 ± 3.6 kg/m2; p = 0.0230), after 9 weeks of SYN consumption. After 2 months of wash-out, we highlighted that the significant variation in reactance and MM, in respect to the baseline, were maintained after wash-out period (respectively, p = 0.0485 and p = 0.0400), as reported in Table 8. Instead, the BCMI values returned to baseline values (10.2 ± 3.6 kg/m2 vs. 9.1 ± 2.9 kg/m kg/m2; p = 0.0296).
Table 7.
Body composition assessment of two groups of the study.
| SYN | LUX | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 |
p-Value (T0 vs. T1) |
T2 |
p-Value (T1 vs. T2) |
T0 | T1 |
p-Value (T0 vs. T1) |
T2 |
p-Value (T1 vs. T2) |
|
| Weight (kg) | 79.1 ± 14.1 | 78.8 ± 13.8 | ns # | 78.4 ± 13.2 | ns # | 75.8 ± 15.8 | 75.6 ± 15.5 | ns # | 76.6 ± 15.0 | ns # |
| BMI (kg/m2) | 28.9 ± 3.7 | 28.8 ± 3.4 | ns # | 28.7 ± 3.7 | ns # | 27.5 ± 5.9 | 27.4 ± 5.8 | ns # | 27.8 ± 5.8 | ns # |
| Waist to hip ratio | 0.93 ± 0.07 | 0.92 ± 0.07 | ns # | 0.92 ± 0.06 | ns # | 0.96 ± 0.09 | 0.95 ± 0.09 | ns # | 0.95 ± 0.8 | ns # |
| Resistance (ohm) | 480.4 ± 87.3 | 450.8 ± 79.1 | ns # | 472.8 ± 96.9 | ns # | 498.7 ± 81.0 | 486.2 ± 98.2 | ns # | 496.0 ± 74.5 | ns # |
| Reactance (ohm) | 39.3 ± 10.8 | 42.3 ± 12.3 | 0.0391 # | 41.9 ± 11.3 | ns # | 41.8 ± 12.1 | 40.2 ± 10.0 | ns # | 41.8 ± 8.7 | ns # |
| Phase angle (°) | 4.6 ± 1.5 | 5.3 ± 2.2 | ns # | 4.8 ± 1.5 | ns # | 4.9 ± 0.5 | 5.4 ± 0.7 | ns # | 4.9 ± 0.5 | ns # |
| TBW (%) | 52.8 ± 6.1 | 55.1 ± 5.9 | 0.0465 # | 53.2 ± 6.3 | ns # | 56.3 ± 7.4 | 60.8 ± 8.3 | 0.0033 # | 54.4 ± 7.4 | 0.0301 # |
| ICW (%) | 46.8 ± 7.2 | 49.9 ± 8.7 | 0.0058 # | 47.0 ± 9.0 | ns # | 48.5 ± 5.0 | 49.5 ± 6.3 | ns # | 45.1 ± 14.4 | ns # |
| ECW (%) | 53.2 ± 7.2 | 50.1 ± 8.7 | 0.0058 # | 53.0 ± 8.8 | ns # | 51.5 ± 3.1 | 50.5 ± 3.5 | ns # | 54.9 ± 3.0 | ns # |
| FM (%) | 34.4 ± 8.2 | 31.5 ± 8.2 | ns # | 32.6 ± 8.0 | ns # | 28.6 ± 9.6 | 22.2 ± 10.1 | ns # | 26.5 ± 8.5 | ns # |
| FFM (%) | 65.6 ± 7.6 | 68.5 ± 8.2 | ns # | 67.4 ± 7.9 | ns # | 71.4 ± 9.6 | 77.8 ± 10.5 | ns # | 73.5 ± 9.9 | ns # |
| MM (%) | 38.3 ± 6.5 | 41.6 ± 7.2 | 0.0198 # | 40.7 ± 7.5 | ns # | 43.9 ± 6.7 | 45.5 ± 5.9 | ns # | 44.3 ± 706 | ns # |
| BCMI (kg/m2) | 9.1 ± 2.7 | 10.2 ± 3.6 | 0.0230 # | 9.1 ± 2.9 | 0.0296 # | 8.9 ± 1.7 | 9.7 ± 2.3 | ns # | 8.3 ± 2.9 | ns # |
All data are expressed as mean ± standard deviation; # for the comparison of each parameter we applied test: t-test for paired data; values of p ≤ 0.05 are considered statistically significant. Abbreviations: LUX, Luxolio; SYN, Synergy; BMI, body mass index; TBW, total body water; ICW, intra cell water; ECW, extra cell water, FM, fat mass; FFM, fat free mass; MM, muscle mass; BCMI, body cellular mass index.
Table 8.
p-value of baseline vs. wash-out period of body composition assessment.
| SYN | LUX | |
|---|---|---|
|
p-Value (T0 vs. T2) |
p-Value (T0 vs. T2) |
|
| Weight (kg) | ns # | ns # |
| BMI (kg/m2) | ns # | ns # |
| Waist/hip ratio | ns # | ns # |
| Resistance (ohm) | ns # | ns # |
| Reactance (ohm) | 0.0485 # | ns # |
| Phase angle (°) | ns # | ns # |
| TBW (%) | ns # | ns # |
| ICW (%) | ns # | ns # |
| ECW (%) | ns # | ns # |
| FM (%) | ns # | ns # |
| FFM (%) | ns # | ns # |
| MM (%) | 0.0400 # | ns # |
| BCMI (kg/m2) | ns # | ns # |
# For the comparison of each parameter we applied test: t-test for paired data; values of p ≤ 0.05 are considered statistically significant. Abbreviations: LUX, Luxolio; SYN, Synergy; BMI, body mass index; TBW, total body water; ICW, intra cell water; ECW, extra cell water, FM, fat mass; FFM, fat free mass; MM, muscle mass; BCMI, body cellular mass index.
In the LUX group, we detected a significant increase in TBW (56.3 ± 7.4% vs. 60.8 ± 8.3%; p = 0.0033) after 9 weeks of EVOO consumption, which decreased after 2 months of wash-out (60.8 ± 8.3% vs. 54.4 ± 7.4%; p = 0.0301).
4. Discussion
Consumption of EVOO MPCs seems to improve renal function biomarkers, in addition to the signs, and the metabolic dysfunctions related to CKD. Currently, in the literature the time scale of nutritional-intervention studies in CKD patients is variable and in most of them, this comprises between one and twenty-four weeks. For this reason, the choice of 9 weeks of treatment with EVOO and of 2 months of wash-out period has been driven by the mean time of treatment present in the literature [11,13,19,40,41,42,43,44].
In our population, in patients treated with SYN, we observed a significant improvement in e-GFR and a reduction in azotemia after 9 weeks of EVOO consumption, which persisted after the wash-out period (Figure 3). We hypothesize that the improvement in renal functional biomarkers was related to three mechanisms. The first, could be due to the reduction in OS, observed in the subgroup treated with SYN. In fact, as presented in the literature, OS can be found starting from the first stages of CKD [45,46] and is characterized by an accumulation of ROS and of advanced oxidation protein products (AOPPS), which cause direct damage to the podocyte and tubulointerstitial levels, at the same time inducing proteinuria [47,48]. The second mechanism could be related to the reduction in low-grade chronic inflammation [49], which is interconnected with the OS and plays a key role in the progression of nephropathy. The factors contributing to the development of the low-grade chronic inflammatory status in nephropathic patients, include increased production of inflammatory cytokines, OS itself, metabolic acidosis, intestinal dysbiosis, and altered metabolism of adipose tissue [50]. The third mechanism is related to the reduction in UA that is observed after SYN and LUX consumption. In fact, previous studies have shown that urate-lowering therapy is associated with slowing the progression of CKD [51,52].
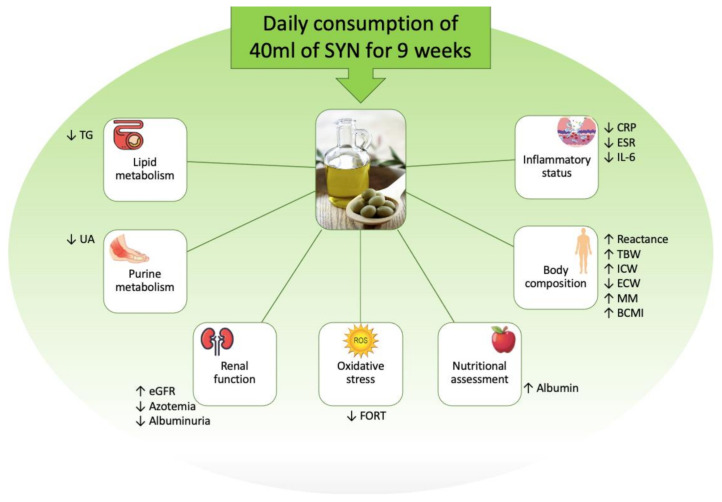
Figure 3.
Outline of the study results. Abbreviations: BCMI, body cell mass index CKD, chronic kidney disease; CRP, C-reactive protein; ECW, extra cellular water; e-GFR, estimated glomerular filtration rate; ESR, erythrocyte sediment rate; FORT, Free Oxygen Radical Test; ICW, intracellular cell water; IL, interleukin; MM, muscle mass; TBW, total body water; TG, triglycerides; UA, uric acid.
Another important result obtained from our study, is the significant albuminuria reduction observed in the SYN subgroup. This amelioration is present not only at T1, but it also persists after the wash-out period (T2). The improvement in albuminuria is important in the clinical management of CKD patients as it is a well-known biomarker of kidney damage and it is one of the main factors associated with the progression of nephropathy. CKD progression seems to also be related to the activation of renal and systemic inflammatory pathways, which induces alterations of the glomerular filtration barrier, with consequent podocyte damage and development of proteinuria [53,54]. The improvement in albuminuria is also attributable to the observed reduction in OS and low-grade inflammatory status.
In our study, we observed a significant reduction in triglycerides in patients treated with SYN, as already highlighted by our preliminary data [55]. This effect seems to be dependent on the consumption of EVOO (SYN). In fact, after the wash-out period, we observed a return to basal values, probably due to CKD itself. Indeed, CKD, starting from the early stages, is characterized by an increase in triglyceride values [56,57]. The decrease in triglycerides levels could be explained by the high content of EVOO polyphenols and oleic acid and by their interaction with the various enzymes involved in energy metabolism [58,59].
Interestingly, in the two subgroups we also observed a decrease in UA values at T1, compared to T0. In detail, patients treated with SYN showed a statistically significant reduction in UA values, after 9 weeks of EVOO consumption, which lasted until the time-point T2. While, in the LUX subgroup, although a statistically significant reduction was observed at T1, this was limited to the period of EVOO intake. In fact, after the wash-out period, the UA concentration increased up to baseline values.
CKD is associated with an increase in UA values due both to its reduced renal excretion and to its altered tubular transport in the nephron [60]. The UA increase observed in CKD patients seems to favor OS through the inhibition of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, altering the vascular response to nitric oxide [61]. Furthermore, it seems to exert a pro-inflammatory action by activating transcription of the factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) [62] and stimulating the synthesis of tumor necrosis factor (TNF)-α and IL-1 [63]. We hypothesized that the UA reduction observed in our patients after 9 weeks of SYN consumption, may be due to two probable mechanisms. The first one involves the intestinal excretion pathway of UA. In fact, the intake of the polyphenols present in the EVOO, may have positively modulated the gut microbiota composition and increased its intestinal excretion, as previously highlighted in the literature [64,65,66,67,68]. The second mechanism could be related to the enhancement of energy metabolism induced by the consumption of SYN, as evidenced by our impedance data, related to basal metabolism and by a previous study [69]. This would lead to a lower availability of adenosine monophosphate (AMP), a UA precursor. The prolonged effect over time is probably due to two mechanisms. The first one is related to the peculiar bioavailability of the antioxidant molecules present in the administered EVOOs. In fact, these molecules, such as oleuropein aglycone and oleacin, represent a reserve of hydroxytyrosol, which is released after hydrolysis reactions. This effect was highlighted by Hu et al. in 2014, in which, the metabolic pathway of hydroxytyrosol—resulting from the hydrolysis of oleuropein aglycone—secondary to consumption of EVOO consumption, was described. The authors showed a slow release and prolonged antioxidant activity of EVOO [70]. Similarly, considering the structure of oleacin—a secoiridoide in presence of hydroxytyrosol—it is hypothesized that the pharmacokinetics described for oleuropein aglycone in EVOO can also be expected for both molecules. Namely, the oleacin shows a prolonged antioxidant activity after consumption of EVOO [70]. This first mechanism may allow for the Mignolo SYN—with a high content of oleacin and oleuropein aglycone—to exert a prolonged antioxidant effect, compared to the EVOO LUX. The second mechanism is related to the impact of EVOO’s polyphenols on the gut microbiota composition. Previous studies have demonstrated that EVOO’s polyphenols are able to modulate gut microbiota and the production of short chain fatty acids (SCFAs). In fact, some studies have demonstrated that EVOO’s polyphenols induce increased production of butyrate or gut bacteria producing butyrate, enhancing the α-divesity of gut micriobiota, which induces beneficial systemic effects that seem to be maintained over the time [68,69].
In our study, we confirmed the anti-inflammatory action of EVOO MCPs [14,15,70]. In fact, our patients after SYN and LUX consumption, showed a significant reduction in both CRP and ESR. Specifically, this anti-inflammatory effect in SYN patients tended to persist over time, as evidenced by the CRP improvement, which was maintained after the wash-out period, while in LUX patients this effect was limited to the period of EVOO consumption. Moreover, in SYN patients, we observed a significant IL-6 reduction after 9 weeks of EVOO consumption, and at T2. The higher presence of oleocanthal in SYN would explain the observed anti-inflammatory effects. In fact, oleocanthal inhibits cyclooxygenase (COX)-1 and COX-2 enzymes in a dose-dependent manner, its anti-inflammatory action seems to be similar to the well-known non-steroidal anti-inflammatory drug, ibuprofen. Beauchamp and collaborators showed that the inhibitory action of oleocanthal on COX-1 and COX-2 enzymes, is greater than that induced by ibuprofen at the same concentration [30]. In our study, the anti-inflammatory action of oleocanthal was preserved by consumption of raw EVOO. In fact, previous studies have shown how cooking reduces the biological activities of oleocanthal [71]. EVOO anti-inflammatory action is due to also the inhibition of TNF-α and IL-4 synthesis, as demonstrated in a cell line derived from human blood (KU812 cells) [72,73].
The observed increase in albumin values, noted in both treated subgroups at T1, is in agreement with previous studies [74,75] and it seems to be associated with reductions in the chronic inflammatory status. Previous data have highlighted how the inflammatory state reduces the synthesis of serum albumin and that its concentration is inversely correlated to that of acute phase proteins [76]. This significant albumin increase in the SYN subgroup persists after wash-out, while the albumin value returns to baseline in the LUX subgroup.
Furthermore, only in patients who consumed SYN, we observed a significant reduction in OS, monitored by FORT. These data do not seem to last over time, as OS tended to rise again 2 months after the wash-out period, yet remaining lower than the baseline values. The antioxidant action is exerted by the EVOO polyphenols, as suggested by previous studies [77,78]. In particular, polyphenols inhibit the enzymes involved in the formation of ROS, exert a scavenger action against ROS, and upregulate the antioxidant defense mechanisms [79,80]. The synergistic antioxidant effect of the MPCs present in high quantities in SYN, is greater in SYN in respect to LUX.
As regards the body composition assessment—monitored by BIA—we, interestingly, observed a BCMI improvement in SYN patients at T1. In the literature, it has been highlighted that BCMI is a very sensitive index for monitoring MM changes, which, in our study population, may be related to uremic sarcopenia [81,82,83]. BCMI seems to be a prognostic index of nutritional, inflammatory, and muscle mass status, as previously observed in geriatric patients [84]. In our study, in addition to the improvement in BCMI, we also observed an increase in MM% in the SYN subgroup at T1 and after the wash-out period (T2). The increase in the two parameters seems to be related to each other and associated with the improvement in the inflammatory state observed.
In addition, we detected an increase in reactance in the SYN subgroup; a parameter related to the cellular integrity and nutritional status of the patients. These data agree with the albumin increase highlighted in the study. In fact, both parameters express an improvement in the patient’s nutritional status [85].
Finally, we demonstrated a TBW% increase in both subgroups (SYN and LUX) at T1 and, at the same time, an improvement in the ICW% to ECW% ratio only in the SYN subgroup. These parameters agree with the observed MM% increase, which is characterized by a high percentage of ICW [86].
5. Conclusions
Our study shows that daily use of an EVOO with high MPC content seems to exert an important anti-inflammatory and antioxidant action in nephropathic patients. Therefore, once again, the pivotal role that nutritional-diet therapy plays in the clinical management of CKD patients and the consequential improvements in their quality of life, exerting positive effects on CKD signs and symptoms, has been highlighted. Thus, this study in nephropathic patients may lay the foundations for further randomized clinical trials that confirm the important role played by EVOO MPCs in the management of CKD.
Acknowledgments
We would like to thank Nadia Consalvo and Tiziana Berardicurti for their nursing assistance. Moreover, we are indebted to Simone Manca di Villahermosa. We thank the Azienda Agricola Fabrizio Tarchi, Vinci, Florence, Italy, for the supply of EVOOs. Tuscany Region—European Partnership for Innovation in Agriculture Productivity and Sustainability 2014–2020—Strategic operational group plan—BioSynOL, “Oil and Legumes: biodynamic and synergistic crops for naturally fortified foods and innovative products for health and sport”. Natural-mente srl, Florence, Italy, for the development of synergistic, organic and biodynamic cultivation.
Author Contributions
Conceptualization, A.N. and A.R.; chemical analysis, S.U., A.R.; investigation, A.N., G.M., S.U., M.D.L., A.P.Z.; data curation, G.M., F.D.D., M.D.L.; writing—original draft preparation, A.N., S.U., M.D.L., A.R.; writing—review and editing, N.D.D.; visualization, S.U., A.P.Z.; supervision, N.D.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Helsinki declaration by the PTV Independent Ethics Committee (Protocol registration number 36/20).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available on request due to privacy restrictions. The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liyanage T., Ninomiya T., Jha V., Neal B., Patrice H.M., Okpechi I., Zhao M.H., Lv J., Garg A.X., Knight J., et al. Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 3.Hill N.R., Fatoba S.T., Oke J.L., Hirst J.A., O’Callaghan C.A., Lasserson D.S., Hobbs F.D. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE. 2016;11:e0158765. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noce A., Marrone G., Di Lauro M., Urciuoli S., Pietroboni Zaitseva A., Wilson Jones G., Di Daniele N., Romani A. Cardiovascular Protection of Nephropathic Male Patients by Oral Food Supplements. Cardiovasc. Ther. 2020;2020:1807941. doi: 10.1155/2020/1807941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noce A., Bocedi A., Campo M., Marrone G., Di Lauro M., Cattani G., Di Daniele N., Romani A. A Pilot Study of a Natural Food Supplement as New Possible Therapeutic Approach in Chronic Kidney Disease Patients. Pharmaceuticals. 2020;13:148. doi: 10.3390/ph13070148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereira R.A., Ramos C.I., Teixeira R.R., Muniz G.A.S., Claudino G., Cuppari L. Diet in Chronic Kidney Disease: An integrated approach to nutritional therapy. Rev. Assoc. Med. Bras. 2020;66:s59–s67. doi: 10.1590/1806-9282.66.s1.59. [DOI] [PubMed] [Google Scholar]
- 7.Di Renzo L., Gualtieri P., Romano L., Marrone G., Noce A., Pujia A., Perrone M.A., Aiello V., Colica C., De Lorenzo A. Role of Personalized Nutrition in Chronic-Degenerative Diseases. Nutrients. 2019;11:1707. doi: 10.3390/nu11081707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rysz J., Franczyk B., Cialkowska-Rysz A., Gluba-Brzozka A. The Effect of Diet on the Survival of Patients with Chronic Kidney Disease. Nutrients. 2017;9:495. doi: 10.3390/nu9050495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly J.T., Palmer S.C., Wai S.N., Ruospo M., Carrero J.J., Campbell K.L., Strippoli G.F. Healthy Dietary Patterns and Risk of Mortality and ESRD in CKD: A Meta-Analysis of Cohort Studies. Clin. J. Am. Soc. Nephrol. 2017;12:272–279. doi: 10.2215/CJN.06190616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lew Q.J., Jafar T.H., Koh H.W., Jin A., Chow K.Y., Yuan J.M., Koh W.P. Red Meat Intake and Risk of ESRD. J. Am. Soc. Nephrol. 2017;28:304–312. doi: 10.1681/ASN.2016030248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Lorenzo A., Noce A., Bigioni M., Calabrese V., Della Rocca D.G., Di Daniele N., Tozzo C., Di Renzo L. The effects of Italian Mediterranean organic diet (IMOD) on health status. Curr. Pharm. Des. 2010;16:814–824. doi: 10.2174/138161210790883561. [DOI] [PubMed] [Google Scholar]
- 12.Andreoli A., Lauro S., Di Daniele N., Sorge R., Celi M., Volpe S.L. Effect of a moderately hypoenergetic Mediterranean diet and exercise program on body cell mass and cardiovascular risk factors in obese women. Eur. J. Clin. Nutr. 2008;62:892–897. doi: 10.1038/sj.ejcn.1602800. [DOI] [PubMed] [Google Scholar]
- 13.Di Daniele N., Di Renzo L., Noce A., Iacopino L., Ferraro P.M., Rizzo M., Sarlo F., Domino E., De Lorenzo A. Effects of Italian Mediterranean organic diet vs. low-protein diet in nephropathic patients according to MTHFR genotypes. J. Nephrol. 2014;27:529–536. doi: 10.1007/s40620-014-0067-y. [DOI] [PubMed] [Google Scholar]
- 14.Romani A., Campo M., Urciuoli S., Marrone G., Noce A., Bernini R. An Industrial and Sustainable Platform for the Production of Bioactive Micronized Powders and Extracts Enriched in Polyphenols From Olea europaea L. and Vitis vinifera L. Wastes. Front. Nutr. 2020;7:120. doi: 10.3389/fnut.2020.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romani A., Ieri F., Urciuoli S., Noce A., Marrone G., Nediani C., Bernini R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients. 2019;11:1776. doi: 10.3390/nu11081776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alessandri S., Ieri F., Romani A. Minor polar compounds in extra virgin olive oil: Correlation between HPLC-DAD-MS and the Folin-Ciocalteu spectrophotometric method. J. Agric. Food Chem. 2014;62:826–835. doi: 10.1021/jf403104a. [DOI] [PubMed] [Google Scholar]
- 17.Kopple J.D. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am. J. Kidney Dis. 2001;37:S66–S70. doi: 10.1053/ajkd.2001.20748. [DOI] [PubMed] [Google Scholar]
- 18.Cupisti A., Brunori G., Di Iorio B.R., D’Alessandro C., Pasticci F., Cosola C., Bellizzi V., Bolasco P., Capitanini A., Fantuzzi A.L., et al. Nutritional treatment of advanced CKD: Twenty consensus statements. J. Nephrol. 2018;31:457–473. doi: 10.1007/s40620-018-0497-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noce A., Vidiri M.F., Marrone G., Moriconi E., Bocedi A., Capria A., Rovella V., Ricci G., De Lorenzo A., Di Daniele N. Is low-protein diet a possible risk factor of malnutrition in chronic kidney disease patients? Cell Death Discov. 2016;2:16026. doi: 10.1038/cddiscovery.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Gonzalez M.A., Garcia-Arellano A., Toledo E., Salas-Salvado J., Buil-Cosiales P., Corella D., Covas M.I., Schroder H., Aros F., Gomez-Gracia E., et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS ONE. 2012;7:e43134. doi: 10.1371/journal.pone.0043134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wanner M., Probst-Hensch N., Kriemler S., Meier F., Autenrieth C., Martin B.W. Validation of the long international physical activity questionnaire: Influence of age and language region. Prev. Med. Rep. 2016;3:250–256. doi: 10.1016/j.pmedr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carter R.L., Sharbaugh C.O., Stapell C.A. Reliability and validity of the 24-hour recall. J. Am. Diet. Assoc. 1981;79:542–547. [PubMed] [Google Scholar]
- 23.Lohman T.G., Roche A.F., Reynaldo Martorell H.M. Anthropometric Standardization Reference Manual. Human Kinetics; Champaign, IL, USA: 1988. [Google Scholar]
- 24.Bellizzi V., Scalfi L., Terracciano V., De Nicola L., Minutolo R., Marra M., Guida B., Cianciaruso B., Conte G., Di Iorio B.R. Early changes in bioelectrical estimates of body composition in chronic kidney disease. J. Am. Soc. Nephrol. 2006;17:1481–1487. doi: 10.1681/ASN.2005070756. [DOI] [PubMed] [Google Scholar]
- 25.Gaman M.A., Epingeac M.E., Diaconu C.C., Gaman A.M. Evaluation of oxidative stress levels in obesity and diabetes by the free oxygen radical test and free oxygen radical defence assays and correlations with anthropometric and laboratory parameters. World J. Diabetes. 2020;11:193–201. doi: 10.4239/wjd.v11.i5.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavlatou M.G., Papastamataki M., Apostolakou F., Papassotiriou I., Tentolouris N. FORT and FORD: Two simple and rapid assays in the evaluation of oxidative stress in patients with type 2 diabetes mellitus. Metabolism. 2009;58:1657–1662. doi: 10.1016/j.metabol.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 27.Cesarone M.R., Belcaro G., Carratelli M., Cornelli U., De Sanctis M.T., Incandela L., Barsotti A., Terranova R., Nicolaides A. A simple test to monitor oxidative stress. Int. Angiol. 1999;18:127–130. [PubMed] [Google Scholar]
- 28.Lewis N.A., Newell J., Burden R., Howatson G., Pedlar C.R. Critical Difference and Biological Variation in Biomarkers of Oxidative Stress and Nutritional Status in Athletes. PLoS ONE. 2016;11:e0149927. doi: 10.1371/journal.pone.0149927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.EFSA Scientific Opinion on the Substantiation of Health Claims Related to Polyphenols in Olive and Protection of LDL Particles from Oxidative Damage (ID 1333, 1638, 1639, 1696, 2865), Maintenance of Normal Blood HDL Cholesterol Concentrations (ID 1639), Maintenance of Normal Blood Pressure (ID 3781), “Anti-Inflammatory Properties” (ID 1882), “Contributes to the Upper respiratory tract health” (ID 3468), “can help to Maintain a Normal Function of Gastrointestinal Tract” (3779), and “Contributes to Body Defences against External Agents” (ID 3467) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. [(accessed on 5 August 2020)]; Available online: https://www.efsa.europa.eu/en/efsajournal/pub/2033.
- 30.Beauchamp G.K., Keast R.S., Morel D., Lin J., Pika J., Han Q., Lee C.H., Smith A.B., Breslin P.A. Phytochemistry: Ibuprofen-like activity in extra-virgin olive oil. Nature. 2005;437:45–46. doi: 10.1038/437045a. [DOI] [PubMed] [Google Scholar]
- 31.Visioli F., Galli C., Grande S., Colonnelli K., Patelli C., Galli G., Caruso D. Hydroxytyrosol excretion differs between rats and humans and depends on the vehicle of administration. J. Nutr. 2003;133:2612–2615. doi: 10.1093/jn/133.8.2612. [DOI] [PubMed] [Google Scholar]
- 32.Pinto J., Paiva-Martins F., Corona G., Debnam E.S., Jose Oruna-Concha M., Vauzour D., Gordon M.H., Spencer J.P. Absorption and metabolism of olive oil secoiridoids in the small intestine. Br. J. Nutr. 2011;105:1607–1618. doi: 10.1017/S000711451000526X. [DOI] [PubMed] [Google Scholar]
- 33.Visioli F., Bernardini E. Extra virgin olive oil’s polyphenols: Biological activities. Curr. Pharm. Des. 2011;17:786–804. doi: 10.2174/138161211795428885. [DOI] [PubMed] [Google Scholar]
- 34.Zafra Gomez A., Luzón-Toro B., Capel-Cuevas S., Morales J. Stability of Hydroxytyrosol in Aqueous Solutions at Different Concentration, Temperature and with Different Ionic Content: A Study Using UPLC-MS. Food Nutr. Sci. 2011;2:1114–1120. doi: 10.4236/fns.2011.210149. [DOI] [Google Scholar]
- 35.Romani A., Lapucci C., Cantini C., Ieri F., Mulinacci N., Visioli F. Evolution of minor polar compounds and antioxidant capacity during storage of bottled extra virgin olive oil. J. Agric. Food Chem. 2007;55:1315–1320. doi: 10.1021/jf062335r. [DOI] [PubMed] [Google Scholar]
- 36.Bernini R., Carastro I., Palmini G., Tanini A., Zonefrati R., Pinelli P., Brandi M.L., Romani A. Lipophilization of Hydroxytyrosol-Enriched Fractions from Olea europaea L. Byproducts and Evaluation of the in Vitro Effects on a Model of Colorectal Cancer Cells. J. Agric. Food Chem. 2017;65:6506–6512. doi: 10.1021/acs.jafc.6b05457. [DOI] [PubMed] [Google Scholar]
- 37.Bernini R., Gilardini Montani M.S., Merendino N., Romani A., Velotti F. Hydroxytyrosol-Derived Compounds: A Basis for the Creation of New Pharmacological Agents for Cancer Prevention and Therapy. J. Med. Chem. 2015;58:9089–9107. doi: 10.1021/acs.jmedchem.5b00669. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-Santiago M., Fonolla J., Lopez-Huertas E. Human absorption of a supplement containing purified hydroxytyrosol, a natural antioxidant from olive oil, and evidence for its transient association with low-density lipoproteins. Pharmacol. Res. 2010;61:364–370. doi: 10.1016/j.phrs.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 39.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y.L., Castro A.F., 3rd, Feldman H.I., Kusek J.W., Eggers P., Van Lente F., Greene T., et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu L.P., Huang X.T., Sun Y., Mao J.X., Wang S., Wang C.D., Bao Z.M. Molecular genetic analysis of heterosis in interspecific hybrids of Argopecten purpuratus x A. irradians irradians. Genet. Mol. Res. 2015;14:10692–10704. doi: 10.4238/2015.September.9.9. [DOI] [PubMed] [Google Scholar]
- 41.McMahon E.J., Campbell K.L., Bauer J.D., Mudge D.W. Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst. Rev. 2015 doi: 10.1002/14651858.CD010070.pub2. [DOI] [PubMed] [Google Scholar]
- 42.Montes-Delgado R., Guerrero Riscos M.A., Garcia-Luna P.P., Martin Herrera C., Pereira Cunill J.L., Garrido Vazquez M., Lopez Munoz I., Suarez Garcia M.J., Martin-Espejo J.L., Soler Junco M.L., et al. [Treatment with low-protein diet and caloric supplements in patients with chronic kidney failure in predialysis. Comparative study] Rev. Clin. Esp. 1998;198:580–586. [PubMed] [Google Scholar]
- 43.Garofalo C., Borrelli S., Provenzano M., De Stefano T., Vita C., Chiodini P., Minutolo R., De Nicola L., Conte G. Dietary Salt Restriction in Chronic Kidney Disease: A Meta-Analysis of Randomized Clinical Trials. Nutrients. 2018;10:732. doi: 10.3390/nu10060732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jing Z., Wei-Jie Y. Effects of soy protein containing isoflavones in patients with chronic kidney disease: A systematic review and meta-analysis. Clin. Nutr. 2016;35:117–124. doi: 10.1016/j.clnu.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 45.Himmelfarb J., Stenvinkel P., Ikizler T.A., Hakim R.M. The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524–1538. doi: 10.1046/j.1523-1755.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- 46.Vaziri N.D. Roles of oxidative stress and antioxidant therapy in chronic kidney disease and hypertension. Curr. Opin. Nephrol. Hypertens. 2004;13:93–99. doi: 10.1097/00041552-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 47.Duni A., Liakopoulos V., Roumeliotis S., Peschos D., Dounousi E. Oxidative Stress in the Pathogenesis and Evolution of Chronic Kidney Disease: Untangling Ariadne’s Thread. Int. J. Mol. Sci. 2019;20:3711. doi: 10.3390/ijms20153711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noce A., Fabrini R., Dessi M., Bocedi A., Santini S., Rovella V., Pastore A., Tesauro M., Bernardini S., Di Daniele N., et al. Erythrocyte glutathione transferase activity: A possible early biomarker for blood toxicity in uremic diabetic patients. Acta Diabetol. 2014;51:219–224. doi: 10.1007/s00592-013-0497-3. [DOI] [PubMed] [Google Scholar]
- 49.Dessi M., Noce A., Agnoli A., De Angelis S., Fuiano L., Tozzo C., Taccone-Gallucci M., Fuiano G., Federici G. The usefulness of the prognostic inflammatory and nutritional index (PINI) in a haemodialysis population. Nutr. Metab. Cardiovasc. Dis. 2009;19:811–815. doi: 10.1016/j.numecd.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 50.Mihai S., Codrici E., Popescu I.D., Enciu A.M., Albulescu L., Necula L.G., Mambet C., Anton G., Tanase C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018;2018:2180373. doi: 10.1155/2018/2180373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsai C.W., Lin S.Y., Kuo C.C., Huang C.C. Serum Uric Acid and Progression of Kidney Disease: A Longitudinal Analysis and Mini-Review. PLoS ONE. 2017;12:e0170393. doi: 10.1371/journal.pone.0170393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goicoechea M., Garcia de Vinuesa S., Verdalles U., Verde E., Macias N., Santos A., Perez de Jose A., Cedeno S., Linares T., Luno J. Allopurinol and progression of CKD and cardiovascular events: Long-term follow-up of a randomized clinical trial. Am. J. Kidney Dis. 2015;65:543–549. doi: 10.1053/j.ajkd.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 53.Burton C., Harris K.P. The role of proteinuria in the progression of chronic renal failure. Am. J. Kidney Dis. 1996;27:765–775. doi: 10.1016/S0272-6386(96)90512-0. [DOI] [PubMed] [Google Scholar]
- 54.Coresh J., Heerspink H.J.L., Sang Y., Matsushita K., Arnlov J., Astor B.C., Black C., Brunskill N.J., Carrero J.J., Feldman H.I., et al. Change in albuminuria and subsequent risk of end-stage kidney disease: An individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol. 2019;7:115–127. doi: 10.1016/S2213-8587(18)30313-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romani A., Bernini R., Noce A., Urciuoli S., Di Lauro M., Pietroboni Zaitseva A., Marrone G., Di Daniele N. Potential Beneficial Effects of Extra Virgin Olive Oils Characterized by High Content in Minor Polar Compounds in Nephropathic Patients: A Pilot Study. Molecules. 2020;25:4757. doi: 10.3390/molecules25204757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zubovic S.V., Kristic S., Prevljak S., Pasic I.S. Chronic Kidney Disease and Lipid Disorders. Med. Arch. 2016;70:191–192. doi: 10.5455/medarh.2016.70.191-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dessi M., Noce A., Bertucci P., Noce G., Rizza S., De Stefano A., Manca di Villahermosa S., Bernardini S., De Lorenzo A., Di Daniele N. Plasma and erythrocyte membrane phospholipids and fatty acids in Italian general population and hemodialysis patients. Lipids Health Dis. 2014;13:54. doi: 10.1186/1476-511X-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Covas M.I., Nyyssonen K., Poulsen H.E., Kaikkonen J., Zunft H.J., Kiesewetter H., Gaddi A., de la Torre R., Mursu J., Baumler H., et al. The effect of polyphenols in olive oil on heart disease risk factors: A randomized trial. Ann. Intern. Med. 2006;145:333–341. doi: 10.7326/0003-4819-145-5-200609050-00006. [DOI] [PubMed] [Google Scholar]
- 59.Tsartsou E., Proutsos N., Castanas E., Kampa M. Network Meta-Analysis of Metabolic Effects of Olive-Oil in Humans Shows the Importance of Olive Oil Consumption With Moderate Polyphenol Levels as Part of the Mediterranean Diet. Front. Nutr. 2019;6:6. doi: 10.3389/fnut.2019.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Obermayr R.P., Temml C., Gutjahr G., Knechtelsdorfer M., Oberbauer R., Klauser-Braun R. Elevated uric acid increases the risk for kidney disease. J. Am. Soc. Nephrol. 2008;19:2407–2413. doi: 10.1681/ASN.2008010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Glantzounis G.K., Tsimoyiannis E.C., Kappas A.M., Galaris D.A. Uric acid and oxidative stress. Curr. Pharm. Des. 2005;11:4145–4151. doi: 10.2174/138161205774913255. [DOI] [PubMed] [Google Scholar]
- 62.Spiga R., Marini M.A., Mancuso E., Di Fatta C., Fuoco A., Perticone F., Andreozzi F., Mannino G.C., Sesti G. Uric Acid Is Associated With Inflammatory Biomarkers and Induces Inflammation Via Activating the NF-kappaB Signaling Pathway in HepG2 Cells. Arterioscler. Thromb. Vasc. Biol. 2017;37:1241–1249. doi: 10.1161/ATVBAHA.117.309128. [DOI] [PubMed] [Google Scholar]
- 63.Martinez-Reyes C.P., Manjarrez-Reyna A.N., Mendez-Garcia L.A., Aguayo-Guerrero J.A., Aguirre-Sierra B., Villalobos-Molina R., Lopez-Vidal Y., Bobadilla K., Escobedo G. Uric Acid Has Direct Proinflammatory Effects on Human Macrophages by Increasing Proinflammatory Mediators and Bacterial Phagocytosis Probably via URAT1. Biomolecules. 2020;10:576. doi: 10.3390/biom10040576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noce A., Marrone G., Di Daniele F., Ottaviani E., Wilson Jones G., Bernini R., Romani A., Rovella V. Impact of Gut Microbiota Composition on Onset and Progression of Chronic Non-Communicable Diseases. Nutrients. 2019;11:1073. doi: 10.3390/nu11051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parkar S.G., Stevenson D.E., Skinner M.A. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int. J. Food Microbiol. 2008;124:295–298. doi: 10.1016/j.ijfoodmicro.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 66.Cory H., Passarelli S., Szeto J., Tamez M., Mattei J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018;5:87. doi: 10.3389/fnut.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duda-Chodak A. The inhibitory effect of polyphenols on human gut microbiota. J. Physiol. Pharmacol. 2012;63:497–503. [PubMed] [Google Scholar]
- 68.Liu X., Lv Q., Ren H., Gao L., Zhao P., Yang X., Yang G., Xu D., Wang G., Yang W., et al. The altered gut microbiota of high-purine-induced hyperuricemia rats and its correlation with hyperuricemia. PeerJ. 2020;8:e8664. doi: 10.7717/peerj.8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Galvao Candido F., Xavier Valente F., da Silva L.E., Goncalves Leao Coelho O., Gouveia Peluzio M.D.C., Goncalves Alfenas R.C. Consumption of extra virgin olive oil improves body composition and blood pressure in women with excess body fat: A randomized, double-blinded, placebo-controlled clinical trial. Eur. J. Nutr. 2018;57:2445–2455. doi: 10.1007/s00394-017-1517-9. [DOI] [PubMed] [Google Scholar]
- 70.Hu T., He X.W., Jiang J.G., Xu X.L. Hydroxytyrosol and its potential therapeutic effects. J. Agric. Food Chem. 2014;62:1449–1455. doi: 10.1021/jf405820v. [DOI] [PubMed] [Google Scholar]
- 71.Cicerale S., Conlan X.A., Barnett N.W., Sinclair A.J., Keast R.S. Influence of heat on biological activity and concentration of oleocanthal--a natural anti-inflammatory agent in virgin olive oil. J. Agric. Food Chem. 2009;57:1326–1330. doi: 10.1021/jf803154w. [DOI] [PubMed] [Google Scholar]
- 72.Perez-Martinez P., Lopez-Miranda J., Blanco-Colio L., Bellido C., Jimenez Y., Moreno J.A., Delgado-Lista J., Egido J., Perez-Jimenez F. The chronic intake of a Mediterranean diet enriched in virgin olive oil, decreases nuclear transcription factor kappaB activation in peripheral blood mononuclear cells from healthy men. Atherosclerosis. 2007;194:e141–e146. doi: 10.1016/j.atherosclerosis.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 73.Lucas L., Russell A., Keast R. Molecular mechanisms of inflammation. Anti-inflammatory benefits of virgin olive oil and the phenolic compound oleocanthal. Curr. Pharm. Des. 2011;17:754–768. doi: 10.2174/138161211795428911. [DOI] [PubMed] [Google Scholar]
- 74.Perez Banasco V., Gil-Cunquero J.M., Borrego F.J., Grasso M., Segura P., Warletta F., Lozano J.L., Costa L.A., Torres J., Gaforio J.J., et al. [Preliminary study on efficacy and tolerance of a “coupage” of olive oil in patients with chronic kidney disease. Nutritonal evaluation] Nefrologia. 2007;27:472–481. [PubMed] [Google Scholar]
- 75.Villarrubia V., Vidal S., Borrego-Utiel F., Gil-Cunquero J., Pérez-Bañasco V. A master formulation of organic extra virgin olive oils increases HDL cholesterol and albumin serum levels in kidney patients, and ameliorates atopic dermatitis in children and adults. Proc. Nutr. Soc. 2010 doi: 10.1017/S0029665110000236. [DOI] [Google Scholar]
- 76.Kaysen G.A., Dubin J.A., Muller H.G., Rosales L., Levin N.W., Mitch W.E., Niddk H.S.G. Inflammation and reduced albumin synthesis associated with stable decline in serum albumin in hemodialysis patients. Kidney Int. 2004;65:1408–1415. doi: 10.1111/j.1523-1755.2004.00520.x. [DOI] [PubMed] [Google Scholar]
- 77.Pitozzi V., Jacomelli M., Zaid M., Luceri C., Bigagli E., Lodovici M., Ghelardini C., Vivoli E., Norcini M., Gianfriddo M., et al. Effects of dietary extra-virgin olive oil on behaviour and brain biochemical parameters in ageing rats. Br. J. Nutr. 2010;103:1674–1683. doi: 10.1017/S0007114509993655. [DOI] [PubMed] [Google Scholar]
- 78.Rossi M., Caruso F., Kwok L., Lee G., Caruso A., Gionfra F., Candelotti E., Belli S.L., Molasky N., Raley-Susman K.M., et al. Protection by extra virgin olive oil against oxidative stress in vitro and in vivo. Chemical and biological studies on the health benefits due to a major component of the Mediterranean diet. PLoS ONE. 2017;12:e0189341. doi: 10.1371/journal.pone.0189341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hussain T., Tan B., Yin Y., Blachier F., Tossou M.C., Rahu N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell Longev. 2016;2016:7432797. doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Possemiers S., Bolca S., Verstraete W., Heyerick A. The intestinal microbiome: A separate organ inside the body with the metabolic potential to influence the bioactivity of botanicals. Fitoterapia. 2011;82:53–66. doi: 10.1016/j.fitote.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 81.Talluri A., Liedtke R., Mohamed E.I., Maiolo C., Martinoli R., De Lorenzo A. The application of body cell mass index for studying muscle mass changes in health and disease conditions. Acta Diabetol. 2003;40:S286–S289. doi: 10.1007/s00592-003-0088-9. [DOI] [PubMed] [Google Scholar]
- 82.Noce A., Marrone G., Rovella V., Cusumano A., Di Daniele N., Casasco M. Beneficial effects of physical activity on uremic sarcopenia. Med. Dello Sport. 2018 doi: 10.23736/S0025-7826.18.03389-6. [DOI] [Google Scholar]
- 83.Noce A., Marrone G., Ottaviani E., Guerriero C., Di Daniele F., Pietroboni Zaitseva A., Di Daniele N. Uremic Sarcopenia and Its Possible Nutritional Approach. Nutrients. 2021;13:147. doi: 10.3390/nu13010147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rondanelli M., Talluri J., Peroni G., Donelli C., Guerriero F., Ferrini K., Riggi E., Sauta E., Perna S., Guido D. Beyond Body Mass Index. Is the Body Cell Mass Index (BCMI) a useful prognostic factor to describe nutritional, inflammation and muscle mass status in hospitalized elderly?: Body Cell Mass Index links in elderly. Clin. Nutr. 2018;37:934–939. doi: 10.1016/j.clnu.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 85.Marra M., Sammarco R., De Lorenzo A., Iellamo F., Siervo M., Pietrobelli A., Donini L.M., Santarpia L., Cataldi M., Pasanisi F., et al. Assessment of Body Composition in Health and Disease Using Bioelectrical Impedance Analysis (BIA) and Dual Energy X-Ray Absorptiometry (DXA): A Critical Overview. Contrast Media Mol. Imaging. 2019;2019:3548284. doi: 10.1155/2019/3548284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martinoli R., Mohamed E.I., Maiolo C., Cianci R., Denoth F., Salvadori S., Iacopino L. Total body water estimation using bioelectrical impedance: A meta-analysis of the data available in the literature. Acta Diabetol. 2003;40:S203–S206. doi: 10.1007/s00592-003-0066-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request due to privacy restrictions. The data presented in this study are available on request from the corresponding author.