Abstract
Carotid atherosclerotic plaque is encountered frequently in patients at high cardiovascular risk, especially in the elderly. When plaque reaches 50% of carotid lumen, it induces haemodynamically significant carotid stenosis, for which management is currently at a turning point. Improved control of blood pressure, smoking ban campaigns, and the widespread use of statins have reduced the risk of cerebral infarction to <1% per year. However, about 15% of strokes are still secondary to a carotid stenosis, which can potentially be detected by effective imaging techniques. For symptomatic carotid stenosis, current ESC guidelines put a threshold of 70% for formal indication for revascularization. A revascularization should be discussed for symptomatic stenosis over 50% and for asymptomatic carotid stenosis over 60%. This evaluation should be performed by ultrasound as a first-line examination. As a complement, computed tomography angiography (CTA) and/or magnetic resonance angiography are recommended for evaluating the extent and severity of extracranial carotid stenosis. In perspective, new high-risk markers are currently being developed using markers of plaque neovascularization, plaque inflammation, or plaque tissue stiffness. Medical management of patient with carotid stenosis is always warranted and applied to any patient with atheromatous lesions. Best medical therapy is based on cardiovascular risk factors correction, including lifestyle intervention and a pharmacological treatment. It is based on the tri-therapy strategy with antiplatelet, statins, and ACE inhibitors. The indications for carotid endarterectomy (CEA) and carotid artery stenting (CAS) are similar: for symptomatic patients (recent stroke or transient ischaemic attack ) if stenosis >50%; for asymptomatic patients: tight stenosis (>60%) and a perceived high long-term risk of stroke (determined mainly by imaging criteria). Choice of procedure may be influenced by anatomy (high stenosis, difficult CAS or CEA access, incomplete circle of Willis), prior illness or treatment (radiotherapy, other neck surgery), or patient risk (unable to lie flat, poor AHA assessment). In conclusion, neither systematic nor abandoned, the place of carotid revascularization must necessarily be limited to the plaques at highest risk, leaving a large place for optimized medical treatment as first line management. An evaluation of the value of performing endarterectomy on plaques considered to be at high risk is currently underway in the ACTRIS and CREST 2 studies. These studies, along with the next result of ACST-2 trial, will provide us a more precise strategy in case of carotid stenosis.
Keywords: Carotid plaque, Carotid endarterectomy, Vulnerability, Carotid artery stenting
Introduction
Carotid atherosclerotic plaque is encountered frequently in patients at high cardiovascular risk, especially in the elderly, with an estimated prevalence of 10% in men and 6% in women after the age of eighty. When plaque reaches 50% of carotid lumen, it induces haemodynamically significant carotid stenosis, for which management is currently at a turning point.
The risk of ipsilateral cerebral infarction with a carotid stenosis was previously estimated to be around 2% per year,1 but it has decreased significantly over the last 20 years. Improved control of blood pressure, smoking ban campaigns, and the widespread use of statins have reduced the risk of cerebral infarction to <1% per year.
However, about 15% of strokes are still secondary to a carotid stenosis, which can potentially be detected by effective imaging techniques. The objective of this work is to offer a practical review, under the authority of the ESC Council of Stroke and the ESC working group on Aorta and Peripheral vessels, for the current desirable management of carotid stenosis.
Imaging of the carotid stenosis
Stenosis evaluation
Stenosis severity is one of the key factors for revascularization indication in case of a carotid plaque. For symptomatic carotid stenosis, current ESC guidelines put a threshold of 70% for formal indication. A revascularization should be discussed for symptomatic stenosis over 50% and for asymptomatic carotid stenosis over 60%. This evaluation should be performed by ultrasound as a first-line examination. The carotid stenosis is estimated through multiple criteria, mostly peak systolic velocity, end-diastolic velocity, ratio of peak velocity of the internal carotid artery over peak velocity of the common carotid artery2 (Table 1).
Table 1.
Spectral Doppler Ultrasound thresholds
| Stenosis (%) | PSV (cm/s) | VICA/VCCA (cm/s) | EDV (cm/s) |
|---|---|---|---|
| ≤49 | <125 | <2.0 | < 40 |
| 50–69 | 125–230 | 2.0–4.0 | 40–100 |
| ≥70 | ≥230 | ≥4.0 | > 100 |
Obtained from Ref.2
PSV, peak systolic velocity in the stenosis; VCCA, peak velocity of common carotid artery; VICA, peak velocity of internal carotid artery; EDV, end diastolic velocity in the stenosis.
The echo B-mode can only be used as an adjunctive method. Several concordant criteria are usually needed. As a complement to ultrasound, computed tomography angiography (CTA) and/or magnetic resonance angiography are recommended for evaluating the extent and severity of extracranial carotid stenoses. In the ACSRS study, the incidence of transient ischaemic attack (TIA) or ipsilateral stroke was 8.2% for 50–69% stenosis, 10.7% for 70–89%, and 19.3% for 90–99%.3 However, trials validating this evaluation are outdated and do not take into account the progress of the medical treatment, especially the use of statins and antithrombotic therapy. Recently, 2017 ESC guidelines integrated plaque characterization by imaging for revascularization indication in case of asymptomatic carotid stenosis above 60% with the assumption that imaging characteristics will identify a subpopulation with higher risk of cerebrovascular accident4,5 (Table 2). Although those parameters are not yet validated in clinical practice, we proposed here to describe the main imaging characteristics of carotid plaques (actual and future ones), which can lead to ‘high-risk lesions’.
Table 2.
Featured associated with increased risk in patient with asymptomatic carotid artery stenosis treated medically
| Clinical |
|
| Cerebral imaging |
|
| Ultrasound imaging |
|
| Magnetic resonance angiography |
|
Obtained from Ref.43
Plaques characteristics
Ultrasound imaging
Stenosis progression
Significant plaque progression, over 20% increased of stenosis, has also been demonstrated as a high-risk plaque feature.6 This tool should be used with great caution, as the inter and intra investigator variability can be considerable. Of note, monitoring the evolution of an asymptomatic carotid plaque is not desirable on a regular basis, and the evaluation of stenosis variation should be performed with available imaging, with the same imaging modality, and if possible, with the same operator.
Plaque surface area
Measurement of plaque surface area is an interesting feature; it is considered as an additional risk factor, when it exceeds 80 mm2. As presented in the ACSRS study, this factor should be integrated into a score, allowing the identification of the plaques at highest risk.7
Plaque echolucency and ulcerations
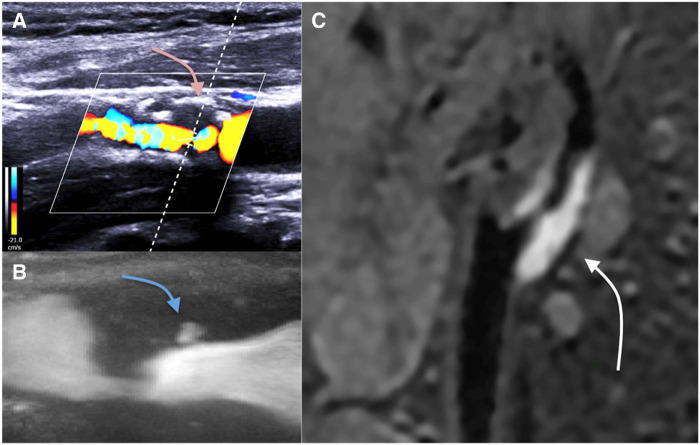
Plaque wall echogenicity could be systematically obtained and its analysis proved to be one of the most used high-risk marker validated by Geroulakos et al.,8 in a qualitative analysis. Hypoechoic structures most often correspond to the lipid core or intra-plaque haemorrhage. This scoring was validated prospectively with an annual risk of 8% for echolucent plaque.9 However, its generalization raises reproducibility issues, which was addressed partially by measure standardization of greyscale median.7 Lastly, within a plaque that may appear iso- or hyper-echoic, the presence of a juxta-luminal anechogenic area should be considered at high risk10 (Figure 1A).
Figure 1.
Examples of vulnerable plaques by imaging. Hypoechogenic juxta-luminal area by B-mode ultrasound and colour Doppler (A); plaque ulceration by ultrafast Doppler imaging (B); 3D T1 hypersignal supporting an intra-plaque haemorrhage by magnetic resonance imaging (C).
Visualization of a micro-flow within the plaque in connection with vascular lumen defines the presence of a plaque ulcer. Initially validated by angiography, this feature is associated with an increased risk of cerebral infarction.11,12 Only the largest ulcerations, however, appear to have the highest risk. To better assess ulcer volume, 3D reconstruction,13 microbubble contrast imaging,14,15 or sensitive Doppler imaging16 might be useful (Figure 1B).
High-intensity transient signals
Presence of high-intensity transient signals (HITS), micro-emboli identified by transcranial Doppler, is also defined as a major factor of high-risk plaque.17 It is necessary, however, to use dedicated devices and not conventional ultrasound examination. In order to raise the sensibility of this technique, HITS detection should indeed be done during a long recording, if possible, at least 1 h.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) has two major advantages: its performance in atherosclerotic tissues analysis (especially for the detection of intra-plaque haemorrhages), and its capacity to perform cerebral structure and parenchyma analysis during the same exam. An MRI dedicated to plaque analysis can thus be used to search for a thin or ruptured fibrous cap, an intraplaque haemorrhage, and a large lipid core18,19 (Figure 1C).
In addition to these recent developments, MRI is a recommended examination modality for silent infarction detection.20 In the ACSRS study, patients with silent embolic infarction had an annual risk of stroke of 3.6 vs. 1.1% without asymptomatic event.21
Computed tomography and positron emission tomography imaging
Computed tomography (CT) accessibility is clearly superior to MRI and CT can also be used for high-risk plaques characterization. It is however less efficient than MRI for tissue analysis, especially for the detection of intraplaque haemorrhages. Calcifications detection can lead to an overestimation of the total plaque surface area, although measurement of plaque volume is easier with this method.22 This technique is still very effective for plaque ulceration detection, thanks to its spatial definition and its capacity of 3D reconstruction.23 Positron emission tomography, although less available than conventional modalities, can detect inflammatory process inside the plaque, with increased glycolytic activity 18F-fluorodeoxyglucose. This technique seems useful by the simple use of the glucose uptake quantification considered as a high-risk marker.24,25 Although very promising, more data are needed to confirm the role of PET scanner in clinical practice.
This is a non-exhaustive review of the proposed markers, according to their validation and use in current practice. In perspective, new high-risk markers are currently being developed, using all of the imaging techniques presented above, but without sufficient evidence. It concerns the evaluation of arterial haemodynamics, including the evaluation of wall shear stress in contact with the plaque,26–28 the evaluation of plaque neovascularization,29,30 or the evaluation of plaque tissue stiffness.31
In addition, although several blood biomarkers are proposed in the literature, our group has chosen not to comment on the use of these biomarkers in clinical practice. While proteomics studies seem to highlight some biomarkers usefulness not detailed here,34,35 scientific evidence is so far insufficient to recommend their use.
Optimal medical treatment
Reducing cardiovascular risk factors
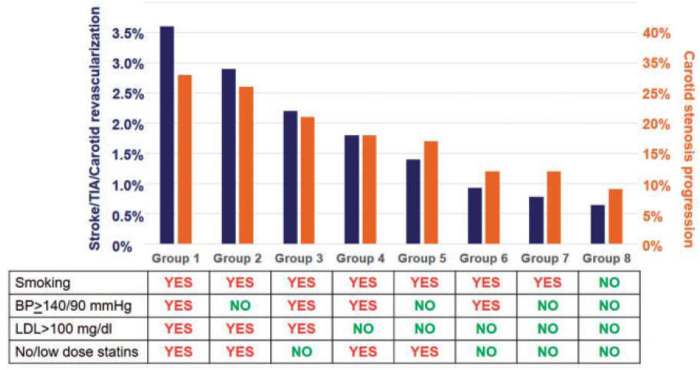
Medical management of patient with carotid stenosis is always warranted and applied to any patient with atheromatous lesions. Best medical therapy is based on cardiovascular risk factors correction, including lifestyle intervention and a pharmacological treatment.36 The goal is to reduce not only the risk of cerebrovascular events but also of global cardiovascular events, especially those involving coronary arteries (Figure 2).
Figure 2.
Annual incidence of neurological ischemic events and stenosis progression in patient with asymptomatic carotid stenosis according to the quality of risk factors management. Extracted from Ref.85 Obtained from Ref.86
Medical treatment begins first by smoking cessation.32 Passive smoking must also be avoided. In addition, regular physical activity and a healthy diet, weight loss, reduced alcohol consumption, and diabetes control are key elements of prevention. In cases of cerebral infarction with evidence of atheromatous arterial lesions, Amarenco et al.33 recently demonstrated the value of an LDLc target of 70 mg/dL (1.8 mmol/L) rather than 100 mg/dL (2.6 mmol/L). An even lower target at 55 mg/dL (1.4 mmol/L) and a reduction in LDLc of >50% of the initial LDLc is now recommended by the European Society of Cardiology since 2019 for patients in secondary cardiovascular prevention and for patients at very high risk.34 This objective is mainly based on the results of Odyssey study with the use of alirocumab, a PCSK9 inhibitor.35 It is of course necessary to start a hypolipemic treatment with a statin. In the event of failure to reach the LDLc target, or intolerance to statins, treatment with ezetimibe should be introduced.36 Blood pressure control is essential in the prevention of cerebral infarction. Pharmacologic treatment should follow the recent ESC guidelines on hypertension,37 and especially angiotensin conversion enzyme inhibitors following the results of HOPE trial.38 It is important to note that targets have evolved with a target systolic pressure <130 mmHg for patients under 65 years of age, and diastolic pressure <80 mmHg in secondary cardiovascular prevention, based primarily on the result of the SPRINT trial.39
Antithrombotic therapy
Strategy of antithrombotic treatment in case of asymptomatic carotid stenosis remains discussed in the literature. The aim of antithrombotic therapy in asymptomatic carotid stenosis is dual: to reduce the risk of stroke directly related to the lesion, as well as modulating the increased risk of other cardiovascular events (e.g. myocardial infarction). Single-antiplatelet therapy (SAPT), especially low-dose aspirin, has shown to reduce the risk of major adverse cardiovascular events (MACE), although the benefit is less certain in case of moderate (i.e. 50–75%) stenosis.40 Dual antiplatelet therapy (DAPT: dual antiplatelet therapy, aspirin, and clopidogrel) has not demonstrated any benefit over SAPT.41 If bleeding risk is low, the ESC guidelines suggest long-term SAPT in patients with asymptomatic >50% stenosis, to reduce not only stroke directly related to carotid lesions but also other cardiovascular events whose risk is increased in the presence of carotid stenosis.42
In the COMPASS trial, 1919 patients with carotid artery disease (either history of carotid revascularization or asymptomatic ≥50% stenosis) have been included. COMPASS compared aspirin 100 mg + rivaroxaban 2.5 mg b.i.d. vs. rivaroxaban 5 mg b.i.d. alone vs. aspirin 100 mg alone.43 In the whole trial, the combination strategy was associated with a significant decrease in MACE, with a similar trend in the carotid artery disease subgroup. However, the report lack granularity to distinguish asymptomatic patients from those revascularized.
Cerebrovascular events secondary to carotid stenosis are at high risk of recurrence,44 and SAPT (aspirin or clopidogrel) is effective in reducing the atherothrombotic risk and is superior over oral anticoagulation.45–47 In the early phase of symptomatic carotid stenosis where the risk of recurrence is particularly high, DAPT reduces the risk of recurrent asymptomatic cerebral embolization and stroke.46–49 It also reduces the risk of stroke recurrence in patients with minor stroke and TIAs, but specific evidence in case of carotid stenosis is lacking.50,51 In a subgroup of patients of the SOCRATES trial with ipsilateral atherosclerotic stenosis, patients under ticagrelor alone had significantly lower MACE rates than those under aspirin.52 In older studies, the combination of dipyridamole and aspirin was superior to aspirin alone to reduce major vascular events in patients with TIA or minor stroke.53,54 This combination appeared as effective as aspirin + clopidogrel, but not superior over clopidogrel alone in the risk reduction of stroke recurrence.55,56 Indeed, data on the efficacy of dipyridamole for cerebrovascular risk reduction are inconsistent.57,58 Of note, COMPASS data presented above cannot be applied to symptomatic carotid stenosis as these patients were excluded from the trial because of intracranial bleeding risk.42
After carotid stenting, DAPT (aspirin + clopidogrel) is generally used,43 but optimal duration is debated. In a meta-analysis of three RCTs,59 the only significant benefit of DAPT over SAPT was a reduction of TIA by 13%. In a nationwide registry, no difference was found in terms of cerebrovascular and cardiovascular events among 2829 patients under DAPT durations of <30 days, 30–41 days, and ≥42 days after carotid stenting.60 Low-dose aspirin reduces periprocedural and long-term events after carotid endarterectomy (CEA; 23,24). Low-dose aspirin was superior to high-dose aspirin on 30-day risk of MACE (3.7% vs. 8.2%; P = 0.002).61
In the meta-analysis collecting data from three RCTs comparing DAPT to SAPT after carotid interventions,60 there was no difference after CEA in death from stroke and TIA between DAPT and single agent antiplatelet therapy, but a significant increase risk of major bleeding and neck haematoma with DAPT. The results of COMPASS trial in patients with carotid artery disease have been presented above. No specific data in patients undergoing CEA is available.
Surgical or endovascular management
Carotid endarterectomy and stenting: description
Principles
Carotid endarterectomy consists of surgical removal of the atherosclerotic material causing stenosis at the carotid bifurcation. It can be undertaken under local or general anaesthetic, although only local anaesthetic offers the opportunity to directly observe and monitor the patient’s neurological status throughout the procedure.
Carotid artery stenting is more frequently carried out under local anaesthetic. It involves passing a wire beyond the stenotic lesion and either employing an umbrella-like filter (to catch any pieces of atheroma dislodged during stent placement) or using balloon inflation in the external/common carotid arteries to encourage reverse flow down the internal carotid (preventing atheromatous emboli being carried up into the intracerebral circulation), whilst the stent is placed across the stenosis and expanded to restore the normal luminal diameter.
More recently, a direct common carotid approach (called ‘TCAR’ for Transcarotid Artery Revascularization) has been shown to be safe and is likely to be employed more frequently in the future; through a mini-incision and using flow-reversal, this avoids traversing the aortic arch and manipulating the catheter into the common carotid origin—this technique may reduce or eliminate the extra ‘minor’ stroke risk currently associated with CAS.62
Prior to intervention, patients should be on lipid-lowering and on antithrombotic medications to stabilize the plaque and discourage thrombosis. Generally, CAS patients have DAPT and heparin during the procedure, with DAPT continuing for about 3 months afterwards, whereas CEA patients have single APT and heparin, with APT continuing thereafter.
Current indications for intervention and procedural risks
The indications for CEA and CAS are similar: for symptomatic patients (non-disabling stroke, TIA within the last 6 months, or multiple episodes of amaurosis fugax) if stenosis >50%. For asymptomatic patients: tight stenosis (>60%) and a perceived high long-term risk of stroke (determined mainly by imaging criteria).
Choice of procedure may be influenced by anatomy (high stenosis, difficult CAS or CEA access, incomplete circle of Willis), prior illness or treatment (radiotherapy, other neck surgery), or patient risk (unable to lie flat, poor AHA assessment). Risks from undertaking CEA include stroke, cranial nerve palsy (or permanent nerve damage), a moderate risk of MI and some risk of infection and bleeding. Risks from CAS include stroke (with more risk of periprocedural minor stroke than CEA), lower bleeding risk than CEA, and a smaller risk of MI. Cranial nerve damage is uncommon after CAS.
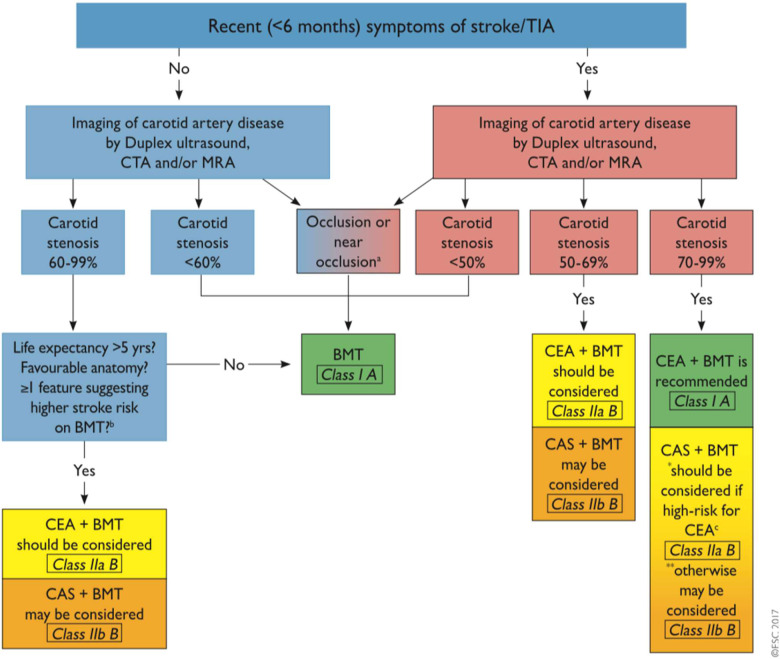
A flowchart for decision-making and current evidence has been published in both the recent European Society for Cardiology Guidelines and the European Society for Vascular Surgery Guidelines43 (Figure 3).
Figure 3.
Management of extracranial carotid artery disease. BMT, best medical therapy; CAS, carotid artery stenting; CEA, carotid endarterectomy; CTA, computed tomography angiography; MRA, magnetic resonance angiography; TIA, transient ischaemic attack. Obtained from Ref.42
Foundations: randomized controlled trials
Carotid endarterectomy and medical treatment vs. medical treatment alone
In symptomatic patients: the two most important randomized trials comparing CEA with BMT (‘Best’ Medical Treatment) were NASCET, ECST published in 1991.63–65
In asymptomatic patients, there are three major trials: VACS randomized males from 1983 to 1987, with follow-up until 1991. ACAS randomized 1662 patients between 1987 and 1993, with follow-up until 1997, and ACST-1 randomized 3120 patients between 1993 and 2003, with follow-up extending to 2008. It can be noticed that medical treatments changed considerably over this 25-year period. In VACS, aspirin was taken by around half of patients, while antihypertensive therapy was also used less commonly than in the other two trials and no patient received statins. During ACAS and ACST-1, use of antihypertensive therapy and antithrombotic treatments increased; these trials also included patients who took fibrates and increasing doses of statins. ACST-1 had longer follow-up and more robust evidence about statins use and found that overall stroke risk was reduced by statins, so was the perioperative stroke risk. This explains the decrease rate of ipsilateral stroke risk, it was 2.3% person-year in trial whom recruited before 2000, compared with 1.0% person-year during the 2000–10 period (P < 0.001).66
Two trials are still recruiting in asymptomatic populations:
CREST-2 is comparing medical treatment alone with medical treatment plus either CAS or CEA—this is not a direct comparison of CEA with CAS, as the patient and clinician choose the CEA or CAS ‘are’ of the trial before randomization between the intervention or medical treatment alone.67 The ACTRIS study is also comparing the effect of endarterectomy in addition to medical treatment vs. medical treatment alone, in patients with asymptomatic carotid plaque >70% NASCET, and with imaging vulnerability criteria.
Outcomes
Symptomatic randomized trials of CEA vs. medical treatment alone:In recently symptomatic patients with <50% stenosis, CEA (plus BMT) did not prevent stroke, but operation did reduce stroke risk in patients with moderate (50–69%) and severe (70–99%) stenosis. Benefit from surgery increased with increasing severity of stenosis, except for ‘near-occlusion’ (defined as a 95–99% stenosis with distal ICA collapse or a narrow calibre lumen with ‘trickle flow’) where there were no obvious benefits from CEA.
In asymptomatic trials, VACS observed no difference in ‘ipsilateral’ or ‘any’ stroke (including the perioperative risk) at 4 years. By contrast, ACAS and ACST observed that CEA conferred significant reductions in ‘any’ stroke (including the perioperative risk), while ACAS reported that CEA significantly reduced 5-year rate of ‘ipsilateral’ stroke. The ACAS and ACST-1 trials were important to the current development of international practice guidelines, which advise that CEA should be performed with a 30-day death/stroke rate <3% and that, before considering surgery, patients should have a predicted survival of at least 5 years.
Important points included:
Gender and CEA—large registries (much larger than these trials) have shown that results of CEA are not influenced by sex and these findings in hundreds of thousands of patients are clear.68
Stenosis severity and stroke risk—these three asymptomatic trials did not show a relationship between stroke risk and severity of stenosis, so patients not undergoing surgery with 60–99% stenosis (few had either 60 or 99%) had very similar stroke risks.
Long-term durability of benefit is particularly important if intervention is undertaken in asymptomatic patients. The ACST-1 trial showed a clear advantage in favour of surgery at 5 years of follow-up and this was maintained until 10 years.
Age and stroke benefit—trials were usually undertaken in patients who were <75 years, although ACST-1 included about 600 older patients—the benefits of CEA were not clear in these older patients although it is unlikely that a very specific cut-off age exists and older patients may benefit from CEA, whilst being at higher risk from stroke and other causes of death within the next 5–10 years.
Carotid endarterectomy vs. carotid artery stenting
Four major trials have recruited patients with recent symptoms (ICSS, CREST, EVA-3S, and SPACE)69–72 and five have completed or are close to completion in patients who are asymptomatic (CREST, SPACE2, Brooks, ACT1, CREST-2, ACST-2).71,73–77 All used contemporary medical treatments in both treatment arms and, as their results have been published in the 2000s and 2010s, their use of lipid-lowering drugs has been more consistent.
Outcomes
Symptomatic randomized trials: endarterectomy vs. stenting.A meta-analysis of over 4000 patients in the ICSS, CREST, EVA-3S, and SPACE trials comparing surgery with stenting in symptomatic patients showed a strong association between increasing age and higher rates of death/stroke after CAS, but not after CEA.In CAS patients, risk increased with age (compared with patients aged < 60 years), CAS patients aged >80 years being four times more likely to suffer a procedural stroke/death [odds ratio (OR) 4.15, 95% confidence interval (CI) 2.20–7.84]. In CEA patients, increasing age was not associated with an increased risk of perioperative stroke/death. When CAS was compared with CEA, the age effect started to become apparent in patients aged 60–65, while CEA was clinically superior to CAS in patients aged >70 years (HR 2.09, 95% CI 1.32–2.32).78 Similar findings (no association between age and procedural risk after CEA) were reported by NASCET in 2001.79
Asymptomatic randomized trials: CEA vs. CASA meta-analysis from four of the five completed asymptomatic RCTs in average risk patients found that CAS patients had a higher chance of periprocedural stroke (OR 1.71, 95% CI 0.99–2.98).75,80–82 These ‘extra’ strokes were mostly minor and, in contrast, the risk of MI was lower after CAS. Long-term review was necessary to determine the durability of CEA vs. CAS and it has shown that both procedures are equally durable, despite their early differences. Trials of CAS vs. CEA are not complete yet, as ACST-2, the largest vascular surgery intervention trial to date has almost finished recruitment and these 5-year results are eagerly awaited in 2021.76 Over 3500 patients have been enrolled in ACST-2 and, with more modern medical treatments and newer stenting techniques, these findings will inform the practice of intervention in asymptomatic patients for the 2020’s.
Recent advances have been made in decision-making for choosing carotid plaque intervention in case of asymptomatic plaque. Early studies had a high risk of stroke due to suboptimal medical treatment. Since these trials, considerable efforts have been made to reduce the risk of stroke, through hygiene-dietary rules and pharmacological treatment (detailed in the paragraph of the medical care).84 Consequently, the best therapeutic option for asymptomatic plaque is now medical except for selected cases. The benefit of CEA and CAS for asymptomatic plaque is now low, and should be limited to patients selected on the basis of long-life expectancy, low surgical risk, and plaque considered vulnerable (or at ‘high risk of stroke’) according to the imaging criteria (detailed in the imaging paragraph). For symptomatic patients with carotid plaques considered to be at high risk of stroke, the benefits of these procedures remain a good option.
Carotid endarterectomy and myocardial revascularization
Myocardial revascularization is accompanied by an increased risk of stroke. The presence of carotid plaque indicates an increased risk of stroke. Nevertheless, the cause of post-operative stroke is multifactorial, including the risk of embolization from the aorta or its branches, atrial fibrillation, etc. No evidence exists for a significant reduction in the risk of stroke with preventive surgery. Therefore, the latest ESC guidelines do not recommend routine carotid surgery and only certain high-risk cases, such as bilateral severe stenosis, should be discussed in a vascular team with a neurologist.83
Conclusion
Neither systematic nor abandoned, the place of carotid revascularization must necessarily be limited to the plaques at highest risk, leaving a large place for optimized medical treatment as first line management. This quite simply delimits the recently symptomatic plaques, for which a cerebral imaging confirms as much as possible the recent character of the cerebral infarction. The situation is much more complex in case of asymptomatic plaques. High-performance imaging for high-risk plaque detection is needed, although no single technique nor morphological marker is universally accepted. An evaluation of the value of performing endarterectomy on plaques considered to be at high risk is currently underway in the ACTRIS and CREST 2 studies. These studies, along with the next result of ACST-2 trial, will provide us a more precise strategy in case of carotid stenosis.
Funding
Professor Halliday’s research was funded by the National Institute for Health Research Oxford Biomedical Research Centre. This paper was published as part of a supplement financially supported by the European Society of Cardiology (ESC), Council on Stroke.
Conflict of interest: none declared.
References
- 1. Walker MD, Marler JR, Goldstein M, Grady PA, Toole JF, Baker WH, Castaldo JE, Chambless LE, Moore WS, Robertson JT, Young B, Howard VJ, Purvis S, Vernon DD, Needham K, Beck P, Celani VJ, Sauerbeck L, Rajcs JA von, Atkins D. Endarterectomy for Asymptomatic Carotid Artery Stenosis. JAMA 1995;273:1421.7723155 [Google Scholar]
- 2. Grant EG, Duerinckx AJ, Saden SE, Melany ML, Hathout GM, Zimmerman PT, Marumoto AK, Cohen SN, Baker JD.. Ability to use duplex US to quantify internal carotid arterial stenoses: fact or fiction? Radiology 2000;214:247–252. [DOI] [PubMed] [Google Scholar]
- 3. Nicolaides AN, Kakkos SK, Griffin M, Sabetai M, Dhanjil S, Tegos T, Thomas DJ, Giannoukas A, Geroulakos G, Georgiou N, Francis S, Ioannidou E, Doré CJ; Asymptomatic Carotid Stenosis and Risk of Stroke (ACSRS) Study Group. Severity of asymptomatic carotid stenosis and risk of ipsilateral hemispheric ischaemic events: results from the ACSRS study. Eur J Vasc Endovasc Surg 2005;30:275–284. [DOI] [PubMed] [Google Scholar]
- 4. Saba L, Francone M, Bassareo PP, Lai L, Sanfilippo R, Montisci R, Suri JS, Cecco CD, Faa G.. CT attenuation analysis of carotid intraplaque hemorrhage. AJNR Am J Neuroradiol 2018;39:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Naim C, Douziech M, Therasse É, Robillard P, Giroux MF, Arsenault F, Cloutier G, Soulez G.. Vulnerable atherosclerotic carotid plaque evaluation by ultrasound, computed tomography angiography, and magnetic resonance imagin: an overview. Can Assoc Radiol J 2014;65:275–286. [DOI] [PubMed] [Google Scholar]
- 6. Hirt LS. Progression rate and ipsilateral neurological events in asymptomatic carotid stenosis. Stroke 2014;45:702–706. [DOI] [PubMed] [Google Scholar]
- 7. Nicolaides AN, Kakkos SK, Kyriacou E, Griffin M, Sabetai M, Thomas DJ, Tegos T, Geroulakos G, Labropoulos N, Dor CJ, Morris TP, Naylor R, Abbott AL, Adovasio R, Ziani B, Alò FP, Cicilioni CG, Ambrosio G, Andreev A, Andreozzi GM, Verlato F, Camporese G, Arosio E, Barkauskas E, Barros DA, Brannigan P, Batchvarova V, Dramov A, Belardi P, Novelli G, Abbott AL.. Asymptomatic internal carotid artery stenosis and cerebrovascular risk stratification. J Vasc Surg 2010;52:1486–1496.e1–5. [DOI] [PubMed] [Google Scholar]
- 8. Geroulakos G, Ramaswami G, Nicolaides A, James K, Labropoulos N, Belcaro G, Holloway M.. Characterization of symptomatic and asymptomatic carotid plaques using high-resolution real-time ultrasonography. Br J Surg 1993;80:1274–1277. [DOI] [PubMed] [Google Scholar]
- 9. Topakian R, King A, Kwon SU, Schaafsma A, Shipley M, Markus HS; for the ACES Investigators. Ultrasonic plaque echolucency and emboli signals predict stroke in asymptomatic carotid stenosis. Neurology 2011;77:751–758. [DOI] [PubMed] [Google Scholar]
- 10. Kakkos SK, Griffin MB, Nicolaides AN, Kyriacou E, Sabetai MM, Tegos T, Makris GC, Thomas DJ, Geroulakos G; Asymptomatic Carotid Stenosis and Risk of Stroke (ACSRS) Study Group. The size of juxtaluminal hypoechoic area in ultrasound images of asymptomatic carotid plaques predicts the occurrence of stroke. J Vasc Surg 2013;57:609–618.e1; discussion 617–8. [DOI] [PubMed] [Google Scholar]
- 11. Eliasziw M, Streifler JY, Fox AJ, Hachinski VC, Ferguson GG, Barnett HJ.. Significance of plaque ulceration in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial. Stroke 1994;25:304–308. [DOI] [PubMed] [Google Scholar]
- 12. Handa N, Matsumoto M, Maeda H, Hougaku H, Kamada T.. Ischemic stroke events and carotid atherosclerosis. Stroke 1995;26:1781–1786. [DOI] [PubMed] [Google Scholar]
- 13. Kuk M, Wannarong T, Beletsky V, Parraga G, Fenster A, Spence JD.. Volume of carotid artery ulceration as a predictor of cardiovascular events. Stroke 2014;45:1437–1441. [DOI] [PubMed] [Google Scholar]
- 14. Saha SA, Gourineni V, Feinstein SB.. The use of contrast-enhanced ultrasonography for imaging of carotid atherosclerotic plaques: current evidence, future directions. Neuroimaging Clin N Am 2016;26:81–96. [DOI] [PubMed] [Google Scholar]
- 15. Steinl D, Kaufmann B.. Ultrasound imaging for risk assessment in atherosclerosis. Int J Mol Sci 2015;16:9749–9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goudot G, Khider L, Pedreira O, Porée J, Alsac JP, Amemiya J-M, Bruneval K, Messas P, Pernot E, Mirault MT.. Innovative multiparametric characterization of carotid plaque vulnerability by ultrasound. Front Physiol Frontiers 2020;11:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Markus HS, King A, Shipley M, Topakian R, Cullinane M, Reihill S, Bornstein NM, Schaafsma A.. Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): a prospective observational study. Lancet Neurol 2010;9:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schindler A, Schinner R, Altaf N, Hosseini AA, Simpson RJ, Esposito-Bauer L, Singh N, Kwee RM, Kurosaki Y, Yamagata S, Yoshida K, Miyamoto S, Maggisano R, Moody AR, Poppert H, Kooi ME, Auer DP, Bonati LH, Saam T.. Prediction of stroke risk by detection of hemorrhage in carotid plaques. JACC Cardiovasc Imaging 2020;13(2 Pt 1):395–406. [DOI] [PubMed] [Google Scholar]
- 19. Takaya N, Yuan C, Chu B, Saam T, Underhill H, Cai J, Tran N, Polissar NL, Isaac C, Ferguson MS, Garden GA, Cramer SC, Maravilla KR, Hashimoto B, Hatsukami TS.. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI–initial results. Stroke 2006;37:818–823. [DOI] [PubMed] [Google Scholar]
- 20. Pascot R, Daoudal A, Cardon A, Godet G, Lucas A, Clochard E, Gauvrit J-Y, Teurnier YL, Kaladji A.. Evaluation by magnetic resonance imaging of silent brain infarcts in preoperative and postoperative asymptomatic carotid surgery. Ann Vasc Surg 2017;43:258–264. [DOI] [PubMed] [Google Scholar]
- 21. Kakkos SK, Sabetai M, Tegos T, Stevens J, Thomas D, Griffin M, Geroulakos G, Nicolaides AN; Asymptomatic Carotid Stenosis and Risk of Stroke (ACSRS) Study Group. Silent embolic infarcts on computed tomography brain scans and risk of ipsilateral hemispheric events in patients with asymptomatic internal carotid artery stenosis. J Vasc Surg 2009;49:902–909. [DOI] [PubMed] [Google Scholar]
- 22. Adraktas DD, Tong E, Furtado AD, Cheng S-C, Wintermark M.. Evolution of CT imaging features of carotid atherosclerotic plaques in a 1-year prospective cohort study. J Neuroimaging 2014;24:1–6. [DOI] [PubMed] [Google Scholar]
- 23. Wintermark M, Jawadi SS, Rapp JH, Tihan T, Tong E, Glidden DV, Abedin S, Schaeffer S, Acevedo-Bolton G, Boudignon B, Orwoll B, Pan X, Saloner D.. High-resolution CT imaging of carotid artery atherosclerotic plaques. AJNR Am J Neuroradiol 2008;29:875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cocker MS, Spence JD, Hammond R, deKemp RA, Lum C, Wells G, Bernick J, Hill A, Nagpal S, Stotts G, Alturkustani M, Adeeko A, Yerofeyeva Y, Rayner K, Peterson J, Khan AR, Naidas AC, Garrard L, Yaffe MJ, Leung E, Prato FS, Tardif J-C, Beanlands RSB; Canadian Atherosclerosis Imaging Network (CAIN) - Project II. [18F]-Fluorodeoxyglucose PET/CT imaging as a marker of carotid plaque inflammation: comparison to immunohistology and relationship to acuity of events. Int J Cardiol 2018;271:378–386. [DOI] [PubMed] [Google Scholar]
- 25. Kelly PJ, Camps-Renom P, Giannotti N, Martí-Fàbregas J, Murphy S, McNulty J, Barry M, Barry P, Calvet D, Coutts SB, Cronin S, Delgado-Mederos R, Dolan E, Fernández-León A, Foley S, Harbison J, Horgan G, Kavanagh E, Marnane M, McDonnell C, O’Donohoe M, Sharma V, Walsh C, Williams D, O’Connell M.. Carotid plaque inflammation imaged by 18F-fluorodeoxyglucose positron emission tomography and risk of early recurrent stroke. Stroke 2019;50:1766–1773. [DOI] [PubMed] [Google Scholar]
- 26. Hariri N, Russell T, Kasper G, Lurie F.. Shear rate is a better marker of symptomatic ischemic cerebrovascular events than velocity or diameter in severe carotid artery stenosis. J Vasc Surg 2019;69:448–452. [DOI] [PubMed] [Google Scholar]
- 27. Goudot G, Poree J, Pedreira O, Khider L, Julia P, Alsac J-M, Laborie E, Mirault T, Tanter M, Messas E, Pernot M.. Wall shear stress measurement by ultrafast vector flow imaging for atherosclerotic carotid stenosis. Ultraschall Med 2019; doi: 10.1055/a-1060-0529. [DOI] [PubMed] [Google Scholar]
- 28. Tuenter A, Selwaness M, Arias Lorza A, Schuurbiers JCH, Speelman L, Cibis M, Lugt AVD, Bruijne M. D, Steen AVD, Franco OH, Vernooij MW, Wentzel JJ.. High shear stress relates to intraplaque haemorrhage in asymptomatic carotid plaques. Atherosclerosis 2016;251:348–354. [DOI] [PubMed] [Google Scholar]
- 29. Schinkel AFL, Oord S. V D, Steen AVD, Laar JV, Sijbrands EJGG.. Utility of contrast-enhanced ultrasound for the assessment of the carotid artery wall in patients with Takayasu or giant cell arteritis. Eur Heart J Cardiovasc Imaging 2014;15:541–546. [DOI] [PubMed] [Google Scholar]
- 30. Camps-Renom P, Prats-Sánchez L, Casoni F, González-de-Echávarri JM, Marrero-González P, Castrillón I, Marín R, Jiménez-Xarrié E, Delgado-Mederos R, Martínez-Domeño A, Guisado-Alonso D, Martí-Fàbregas J.. Plaque neovascularization detected with contrast-enhanced ultrasound predicts ischaemic stroke recurrence in patients with carotid atherosclerosis. Eur J Neurol 2020;27:809–816. [DOI] [PubMed] [Google Scholar]
- 31. Garrard JW, Ummur P, Nduwayo S, Kanber B, Hartshorne TC, West KP, Moore D, Robinson TG, Ramnarine KV.. Shear wave elastography may be superior to greyscale median for the identification of carotid plaque vulnerability: a comparison with histology. Ultraschall der Med 2015;36:386–390. [DOI] [PubMed] [Google Scholar]
- 32. Bullen C. Impact of tobacco smoking and smoking cessation on cardiovascular risk and disease. Expert Rev Cardiovasc Ther 2008;6:883–895. [DOI] [PubMed] [Google Scholar]
- 33. Amarenco P, Kim JS, Labreuche J, Charles H, Abtan J, Béjot Y, Cabrejo L, Cha J-K, Ducrocq G, Giroud M, Guidoux C, Hobeanu C, Kim Y-J, Lapergue B, Lavallée PC, Lee B-C, Lee K-B, Leys D, Mahagne M-H, Meseguer E, Nighoghossian N, Pico F, Samson Y, Sibon I, Steg PG, Sung S-M, Touboul P-J, Touzé E, Varenne O, Vicaut É, Yelles N, Bruckert E.. A comparison of two LDL cholesterol targets after ischemic stroke. N Engl J Med 2020;382:9–19. [DOI] [PubMed] [Google Scholar]
- 34. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen M-R, Tokgozoglu L, Wiklund O; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–118. [DOI] [PubMed] [Google Scholar]
- 35. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby J-F, Tricoci P, White HD, Zeiher AM; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018;379:2097–2107. [DOI] [PubMed] [Google Scholar]
- 36. Murphy SA, Cannon CP, Blazing MA, Giugliano RP, White JA, Lokhnygina Y, Reist C, Im K, Bohula EA, Isaza D, Lopez-Sendon J, Dellborg M, Kher U, Tershakovec AM, Braunwald E.. Reduction in total cardiovascular events with ezetimibe/simvastatin post-acute coronary syndrome: the IMPROVE-IT Trial. J Am Coll Cardiol 2016;67:353–361. [DOI] [PubMed] [Google Scholar]
- 37. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 38.The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000;342:145–153. [DOI] [PubMed] [Google Scholar]
- 39. Sprint Research Group W, Wright JT, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT.. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. King A, Shipley M, Markus H; for the ACES Investigators. The effect of medical treatments on stroke risk in asymptomatic carotid stenosis. Stroke 2013;44:542–546. [DOI] [PubMed] [Google Scholar]
- 41. Bhatt DL, Flather MD, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, Hamm CW, Hankey GJ, Johnston SC, Mak K-H, Mas J-L, Montalescot G, Pearson TA, Steg PG, Steinhubl SR, Weber MA, Fabry-Ribaudo L, Hu T, Topol EJ, Fox KAA; CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007;49:1982–1988. [DOI] [PubMed] [Google Scholar]
- 42. Aboyans V, Ricco J-B, Bartelink M-LEL, Björck M, Brodmann M, Cohnert T, Collet J-P, Czerny M, De Carlo M, Debus S, Espinola-Klein C, Kahan T, Kownator S, Mazzolai L, Naylor AR, Roffi M, Röther J, Sprynger M, Tendera M, Tepe G, Venermo M, Vlachopoulos C, Desormais I; ESC Scientific Document Group. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur Heart J 2018;39:763–816. [DOI] [PubMed] [Google Scholar]
- 43. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, Widimsky P, Hori M, Avezum A, Piegas LS, Branch KRH, Probstfield J, Bhatt DL, Zhu J, Liang Y, Maggioni AP, Lopez-Jaramillo P, O’Donnell M, Kakkar AK, Fox KAA, Parkhomenko AN, Ertl G, Störk S, Keltai M, Ryden L, Pogosova N, Dans AL, Lanas F, Commerford PJ, Torp-Pedersen C, Guzik TJ, Verhamme PB, Vinereanu D, Kim J-H, Tonkin AM, Lewis BS, Felix C, Yusoff K, Steg PG, Metsarinne KP, Cook Bruns N, Misselwitz F, Chen E, Leong D, Yusuf S.. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017;377:1319–1330. [DOI] [PubMed] [Google Scholar]
- 44. Lovett JK, Coull AJ, Rothwell PM.. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology 2004;62:569–573. [DOI] [PubMed] [Google Scholar]
- 45. Fields WS, Lemak NA, Frankowski RF, Hardy RJ.. Controlled trial of aspirin in cerebral ischemia. Stroke 1977;8:301–314. [DOI] [PubMed] [Google Scholar]
- 46. Markus HS, Droste DW, Kaps M, Larrue V, Lees KR, Siebler M, Ringelstein EB.. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using doppler embolic signal detection: the Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial. Circulation 2005;111:2233–2240. [DOI] [PubMed] [Google Scholar]
- 47. Wong KSL, Chen C, Fu J, Chang HM, Suwanwela NC, Huang YN, Han Z, Tan KS, Ratanakorn D, Chollate P, Zhao Y, Koh A, Hao Q, Markus HS; CLAIR study investigators. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol 2010;9:489–497. [DOI] [PubMed] [Google Scholar]
- 48. Palacio S, Hart RG, Pearce LA, Anderson DC, Sharma M, Birnbaum LA, Benavente OR.. Effect of addition of clopidogrel to aspirin on stroke incidence: meta-analysis of randomized trials. Int J Stroke 2015;10:686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Geeganage CM, Diener H-C, Algra A, Chen C, Topol EJ, Dengler R, Markus HS, Bath MW, Bath PMW; for the Acute Antiplatelet Stroke Trialists Collaboration. Dual or mono antiplatelet therapy for patients with acute ischemic stroke or transient ischemic attack: systematic review and meta-analysis of randomized controlled trials. Stroke 2012;43:1058–1066. [DOI] [PubMed] [Google Scholar]
- 50. Wang Y, Johnston SC, Wang Y.. Clopidogrel with aspirin in minor stroke or transient ischemic attack. N Engl J Med 2013;369:1376–1377. [DOI] [PubMed] [Google Scholar]
- 51. Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, Kim AS, Lindblad AS, Palesch YY; Clinical Research Collaboration, Neurological Emergencies Treatment Trials Network, and the POINT Investigators. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med 2018;379:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, Held P, Hill MD, Jonasson J, Kasner SE, Ladenvall P, Minematsu K, Molina CA, Wang Y, Wong KSL, Johnston SC; SOCRATES Steering Committee and Investigators. Efficacy and safety of ticagrelor versus aspirin in acute stroke or transient ischaemic attack of atherosclerotic origin: a subgroup analysis of SOCRATES, a randomised, double-blind, controlled trial. Lancet Neurol 2017;16:301–310. [DOI] [PubMed] [Google Scholar]
- 53. Esprit Study Group H, Halkes PHA, Gijn J van, Kappelle LJ, Koudstaal PJ, Algra A.. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet 2006;367:1665–1673. [DOI] [PubMed] [Google Scholar]
- 54. Dengler R, Diener H-C, Schwartz A, Grond M, Schumacher H, Machnig T, Eschenfelder CC, Leonard J, Weissenborn K, Kastrup A, Haberl R; EARLY Investigators. Early treatment with aspirin plus extended-release dipyridamole for transient ischaemic attack or ischaemic stroke within 24 h of symptom onset (EARLY trial): a randomised, open-label, blinded-endpoint trial. Lancet Neurol 2010;9:159–166. [DOI] [PubMed] [Google Scholar]
- 55. Sacco RL, Diener H-C, Yusuf S, Cotton D, Ôunpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, Bornstein N, Chan BPL, Chen S-T, Cunha L, Dahlöf B, De Keyser J, Donnan GA, Estol C, Gorelick P, Gu V, Hermansson K, Hilbrich L, Kaste M, Lu C, Machnig T, Pais P, Roberts R, Skvortsova V, Teal P, Toni D, VanderMaelen C, Voigt T, Weber M, Yoon B-W.. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med 2008;359:1238–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. King A, Bath PMW, Markus HS.. Clopidogrel versus dipyridamole in addition to aspirin in reducing embolization detected with ambulatory transcranial Doppler: a randomized trial. Stroke 2011;42:650–655. [DOI] [PubMed] [Google Scholar]
- 57.European Stroke Prevention Study. ESPS Group. Stroke;1990;21:1122–1130. [DOI] [PubMed] [Google Scholar]
- 58. Bousser MG, Eschwege E, Haguenau M, Lefaucconnier JM, Thibult N, Touboul D, Touboul PJ.. ‘AICLA’ controlled trial of aspirin and dipyridamole in the secondary prevention of athero-thrombotic cerebral ischemia. Stroke 1983;14:5–14. [DOI] [PubMed] [Google Scholar]
- 59. Barkat M, Hajibandeh S, Hajibandeh S, Torella F, Antoniou GA.. Systematic review and meta-analysis of dual versus single antiplatelet therapy in carotid interventions. Eur J Vasc Endovasc Surg 2017;53:53–67. [DOI] [PubMed] [Google Scholar]
- 60. Jhang K-M, Huang J-Y, Nfor ON, Jian Z-H, Tung Y-C, Ku W-Y, Liaw Y-P.. Is extended duration of dual antiplatelet therapy after carotid stenting beneficial? Medicine (Baltimore) 2015;94:e1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Taylor DW, Barnett HJ, Haynes RB, Ferguson GG, Sackett DL, Thorpe KE, Simard D, Silver FL, Hachinski V, Clagett GP, Barnes R, Spence JD.. Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: a randomised controlled trial. ASA and Carotid Endarterectomy (ACE) Trial Collaborators. Lancet 1999;353:2179–2184. [DOI] [PubMed] [Google Scholar]
- 62. Malas MB, Aridi HD, Kashyap VS, Wang GJ, Motaganahalli RL, Cronenwett J, Eldrup-Jorgensen J, Schermerhorn ML.. Outcomes of transcarotid revascularization with dynamic flow reversal versus carotid endarterectomy in the transcarotid revascularization surveillance project. J Vasc Surg 2019;69:e95–e96. [Google Scholar]
- 63.European Carotid Surgery Trialists’ Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998;351:1379–1387. [PubMed] [Google Scholar]
- 64. Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, Ferguson GG, Fox AJ, Rankin RN, Hachinski VC, Wiebers DO, Eliasziw M; North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445–453. [DOI] [PubMed] [Google Scholar]
- 65. Mayberg MR, Wilson SE, Yatsu F, Weiss DG, Messina L, Hershey LA, Colling C, Eskridge J, Deykin D, Winn HR.. Carotid endarterectomy and prevention of cerebral ischemia in symptomatic carotid stenosis. Veterans Affairs Cooperative Studies Program 309 Trialist Group. JAMA 1991;266:3289–3294. [PubMed] [Google Scholar]
- 66. Hadar N, Raman G, Moorthy D, O'Donnell TF, Thaler DE, Feldmann E, Lau J, Kitsios GD, Dahabreh IJ.. Asymptomatic carotid artery stenosis treated with medical therapy alone: temporal trends and implications for risk assessment and the design of future studies. Cerebrovasc Dis 2014;38:163–173. [DOI] [PubMed] [Google Scholar]
- 67. Howard VJ, Meschia JF, Lal BK, Turan TN, Roubin GS, Brown RD, Voeks JH, Barrett KM, Demaerschalk BM, Huston J, Lazar RM, Moore WS, Wadley VG, Chaturvedi S, Moy CS, Chimowitz M, Howard G, Brott TG; CREST-2 study investigators. Carotid revascularization and medical management for asymptomatic carotid stenosis: protocol of the CREST-2 clinical trials. Int J Stroke 2017;12:770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schmid S, Tsantilas P, Knappich C, Kallmayer M, Breitkreuz T, Zimmermann A, Eckstein H-H, Kuehnl A.. Age but not sex is associated with higher risk of in-hospital stroke or death after carotid artery stenting in symptomatic and asymptomatic carotid stenosis. J Vasc Surg 2019;69:1090–1101.e3. [DOI] [PubMed] [Google Scholar]
- 69. Bonati LH, Dobson J, Featherstone RL, Ederle J, Worp H. V D, Borst G. D, Mali WPTM, Beard JD, Cleveland T, Engelter ST, Lyrer PA, Ford GA, Dorman PJ, Brown MM; International Carotid Stenting Study investigators. Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: the International Carotid Stenting Study (ICSS) randomised trial. Lancet 2015;385:529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Brott TG, Hobson RW, Howard G, Roubin GS, Clark WM, Brooks W, Mackey A, Hill MD, Leimgruber PP, Sheffet AJ, Howard VJ, Moore WS, Voeks JH, Hopkins LN, Cutlip DE, Cohen DJ, Popma JJ, Ferguson RD, Cohen SN, Blackshear JL, Silver FL, Mohr JP, Lal BK, Meschia JF, Crest I.. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010;363:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mas J-L, Arquizan C, Calvet D, Viguier A, Albucher J-F, Piquet P, Garnier P, Viader F, Giroud M, Hosseini H, Hinzelin G, Favrole P, Hénon H, Neau J-P, Ducrocq X, Padovani R, Milandre L, Rouanet F, Wolff V, Saudeau D, Mahagne M-H, Sablot D, Amarenco P, Larrue V, Beyssen B, Leys D, Moulin T, Lièvre M, Chatellier G; EVA-3S Investigators. Long-term follow-up study of endarterectomy versus angioplasty in patients with symptomatic severe carotid stenosis trial. Stroke 2014;45:2750–2756. [DOI] [PubMed] [Google Scholar]
- 72. Eckstein H-H, Ringleb P, Allenberg J-R, Berger J, Fraedrich G, Hacke W, Hennerici M, Stingele R, Fiehler J, Zeumer H, Jansen O.. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol 2008;7:893–902. [DOI] [PubMed] [Google Scholar]
- 73. Eckstein H-H, Reiff T, Ringleb P, Jansen O, Mansmann U, Hacke W, Böckler D, Brückmann H, Debus ES, Fiehler J, Fraedrich G, Mudra H, Schmidli J, Stingele R, Zahn R, Böhm M, Ringelstein EB, Mathias K; SPACE 2 Investigators. SPACE-2: a missed opportunity to compare carotid endarterectomy, carotid stenting, and best medical treatment in patients with asymptomatic carotid stenoses. Eur J Vasc Endovasc Surg 2016;51:761–765. [DOI] [PubMed] [Google Scholar]
- 74. Brooks WH, McClure RR, Jones MR, Coleman TL, Breathitt L.. Carotid angioplasty and stenting versus carotid endarterectomy for treatment of asymptomatic carotid stenosis: a randomized trial in a community hospital. Neurosurgery 2004;54:318–324; discussion 324–5. [DOI] [PubMed] [Google Scholar]
- 75. Rosenfield K, Matsumura JS, Chaturvedi S, Riles T, Ansel GM, Metzger DC, Wechsler L, Jaff MR, Gray W; ACT Investigators. Randomized trial of stent versus surgery for asymptomatic carotid stenosis. N Engl J Med 2016;374:1011–1020. [DOI] [PubMed] [Google Scholar]
- 76. Rudarakanchana N, Dialynas M, Halliday A.. Asymptomatic Carotid Surgery Trial-2 (ACST-2): rationale for a randomised clinical trial comparing carotid endarterectomy with carotid artery stenting in patients with asymptomatic carotid artery stenosis. Eur J Vasc Endovasc Surg 2009;38:239–242. [DOI] [PubMed] [Google Scholar]
- 77. Lal BK, Meschia JF, Brott TG.. Clinical need, design, and goals for the carotid revascularization and medical management for asymptomatic carotid stenosis trial. Semin Vasc Surg 2017;30:2–7. [DOI] [PubMed] [Google Scholar]
- 78. Howard G, Roubin GS, Jansen O, Hendrikse J, Halliday A, Fraedrich G, Eckstein H-H, Calvet D, Bulbulia R, Bonati LH, Becquemin J-P, Algra A, Brown MM, Ringleb PA, Brott TG, Mas J-L; Carotid Stenting Trialists’ Collaboration. Association between age and risk of stroke or death from carotid endarterectomy and carotid stenting: a meta-analysis of pooled patient data from four randomised trials. Lancet 2016;387:1305–1311. [DOI] [PubMed] [Google Scholar]
- 79. Alamowitch S, Eliasziw M, Algra A, Meldrum H, Barnett HJ; North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Risk, causes, and prevention of ischaemic stroke in elderly patients with symptomatic internal-carotid-artery stenosis. Lancet 2001;357:1154–1160. [DOI] [PubMed] [Google Scholar]
- 80. Cui L, Han Y, Zhang S, Liu X, Zhang J.. Safety of stenting and endarterectomy for asymptomatic carotid artery stenosis: a meta-analysis of randomised controlled trials. Eur J Vasc Endovasc Surg 2018;55:614–624. [DOI] [PubMed] [Google Scholar]
- 81. Mannheim D, Karmeli R.. A prospective randomized trial comparing endarterectomy to stenting in severe asymptomatic carotid stenosis. J Cardiovasc Surg 2017. ;58:814–817. [DOI] [PubMed] [Google Scholar]
- 82. Silver FL, Mackey A, Clark WM, Brooks W, Timaran CH, Chiu D, Goldstein LB, Meschia JF, Ferguson RD, Moore WS, Howard G, Brott TG; CREST Investigators. Safety of stenting and endarterectomy by symptomatic status in the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST). Stroke 2011;42:675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke 2009;40:e573–e583. [DOI] [PubMed] [Google Scholar]
- 84. Neumann F-J, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet J-P, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferović PM, Sibbing D, Stefanini GG; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. [DOI] [PubMed] [Google Scholar]
- 85. Shah Z, , Masoomi R, , Thapa R, , Wani M, , Chen J, , Dawn B, , Rymer M, , Gupta K. Optimal medical management reduces risk of disease progression and ischemic events in asymptomatic carotid stenosis patients: a long-term follow-up study. Cerebrovasc Dis 2017;44:150–159. [DOI] [PubMed] [Google Scholar]
- 86. Aboyans V, , Braekkan S, , Mazzolai L, , Sillesen H, , Venermo M, , De Carlo M; ESC Working Group on Aorta and Peripheral Vascular Diseases. The year 2017 in cardiology: aorta and peripheral circulation. Eur Heart J 2018;39:730–738. [DOI] [PubMed] [Google Scholar]