Watch a video presentation of this article
Watch an interview with the author
Answer an questions and earn CME
Abbreviations
- HCA
hepatocellular adenoma
- H&E
hematoxylin and eosin
- HNF‐1α
hepatocyte nuclear factor 1α
- MRI
magnetic resonance imaging
Case
A 27‐year‐old female patient currently taking oral contraceptives is referred to be seen after being found to have three adenomas, the largest being 4 cm. She is contemplating pregnancy in the future.
Hepatocellular adenomas (HCA) are rare (~0.01% of the population) benign tumors that typically occur in the absence of chronic liver disease. HCAs are believed to be monoclonal tumors that develop as an interaction between gene defect and environmental exposure. The strongest risk factor for HCA is prolonged exposure to oral contraceptives. Although incidence in the general population is close to 1 case per 1 million people, women taking oral contraceptives have a significantly higher incidence, closer to 30 cases per 1 million people. 1 Other risk factors include glycogen storage disease type I and III, anabolic androgens, and the presence of obesity and the metabolic syndrome. There appears to be a rising incidence of HCA in the absence of oral contraceptives, although it is unclear if this relates to rising prevalence of risk factors such as metabolic syndrome or increased incidental detection in light of increased use of abdominal imaging. 2
Although an increasingly common presentation of HCA is an incidental finding on imaging for other reasons, 25% to 50% of cases can present with right upper quadrant or epigastric discomfort. Contrast‐enhanced magnetic resonance imaging (MRI) is likely the best imaging modality for diagnosis (Fig. 1); however, imaging can often be nondiagnostic given variation in HCA appearance, reflecting the spectrum of molecular subtypes, as well as a dearth of distinguishing features. Specifically, it can be difficult to distinguish HCA from focal nodular hyperplasia in some cases. A cost‐effectiveness analysis of diagnostic strategies in this case found that gadoxetic acid–enhanced MRI was likely the most cost‐effective approach. 3 Although most adenomas have a benign natural history, potential serious complications include rupture, hemorrhage, and malignant transformation into hepatocellular carcinoma. Hemorrhage and malignant transformation have been reported in 27.2% and 4.2% of patients with HCA, respectively, although both are significantly less likely in lesions smaller than 5 cm in maximum diameter. 4 , 5 Notably, HCAs in men have a significantly higher risk for malignant transformation, reflecting differences in molecular subclasses between men and women.
FIG 1.
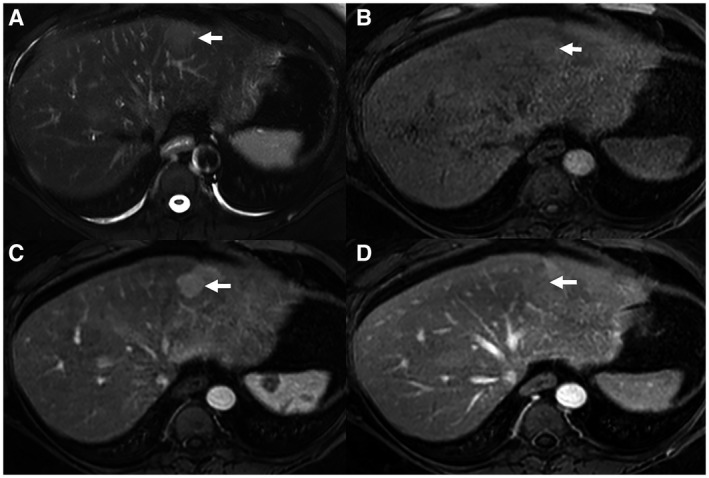
MRI images of HCA. (A) Axial T2‐weighted image demonstrates a left hepatic lobe lesion that is mildly high in signal intensity relative to the normal surrounding hepatic parenchyma. (B) Axial T1‐weighted precontrast image shows that the left hepatic lobe lesion is isointense to the background normal liver parenchyma. (C) Axial T1‐weighted arterial‐phase postcontrast image shows the left hepatic lobe lesion with arterial‐phase hyperenhancement relative to hepatic parenchyma. (D) Axial T1‐weighted delayed venous‐phase postcontrast image shows that the left hepatic lobe lesion becomes isointense to the hepatic parenchyma. Imaging findings favor a lesion of benign hepatocellular origin, such as adenoma or focal nodular hyperplasia.
HCA is no longer considered a homogeneous disease, with recognition of four distinct molecular subtypes: HNF‐1α inactivated (30% to 35% of cases), inflammatory (40% to 50%), β‐catenin activated (10% to 15%), and unclassified (<10%). 6 In addition to these subgroups being linked to molecular pathways, they are associated with distinct histological features (Fig. 2), imaging appearances, and clinical presentations, including risk for complications. Notably, β‐catenin–activated HCAs have a significantly higher risk for malignant transformation. Although these data could enable a precision approach to HCA management, routine biopsy of HCA for subtyping is not currently recommended given the risk for biopsy‐related complications. In cases where biopsy is otherwise done for diagnostic reasons, subtyping should be performed to better understand the risk for downstream complications. There are increasing data supporting the ability of MRI to identify hepatocyte nuclear factor 1α (HNF‐1α)–inactivated or inflammatory HCA, although it has insufficient accuracy to differentiate β‐catenin–activated and unclassified HCA. 7 Continued refinement and validation of MRI‐based HCA subtyping may enable tailoring of surveillance intervals and management in the future.
FIG 2.
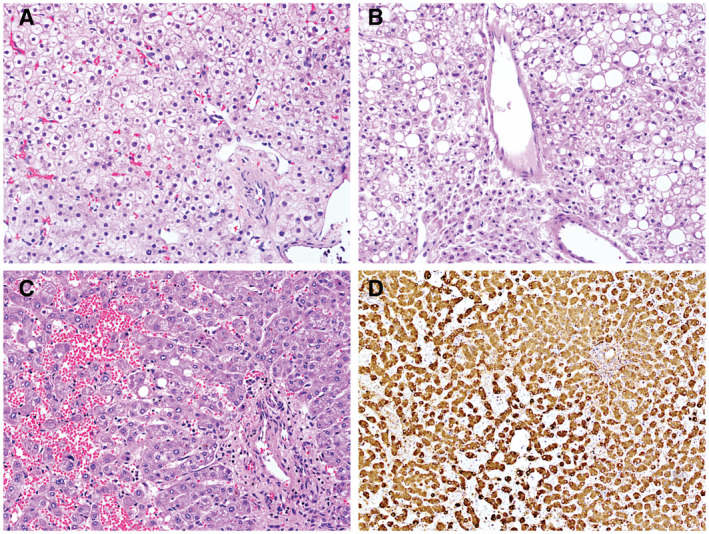
Pathology features of HCA. (A) H&E stain from a HCA (original magnification ×20). (B) HCA with prominent steatosis (H&E, original magnification ×20). (C) Inflammatory HCA with dilated and congested sinusoids (H&E, original magnification ×20). (D) Positive C‐reactive protein immunohistochemical staining in an inflammatory HCA (original magnification ×20).
Treatment decisions for HCA are largely based on patient gender, as well as HCA size and location (Fig. 3). 8 , 9 HCAs in men or those with known β‐catenin activation are high risk for malignant transformation, independent of size, and should be referred for consideration of early surgical resection. In women, the first step in the management of HCA is discontinuation of oral contraceptives, which can lead to regression of HCA in many cases. There are limited data demonstrating that weight loss can also induce regression of HCAs, although the threshold of required weight loss remains to be defined. 10 Repeat contrast‐enhanced MRI is recommended after 6 months to assess for interval change in size. Lesions that remain larger than 5 cm, exhibit interval growth, or are exophytic are at increased risk for rupture and malignant transformation and should be considered for surgical resection. In poor surgical candidates, alternatives, such as embolization for larger lesions and ablation for smaller lesions, can be considered. For women with lesions less than 5 cm, continued observation is recommended, particularly if there is evidence of stability or regression after stopping oral contraceptives, given a lower risk for complications. Albeit in the absence of robust data, it is recommended to repeat imaging every 6 months for 12 to 24 months to define stable disease, at which point annual imaging can be performed. Surveillance intervals may be extended to biennial imaging in cases where the lesion remains stable or regresses over longer periods. The point at which observation can be discontinued is undefined, although it can be considered in patients with small, stable lesions given a low risk for malignant transformation or rupture. The risk for complications for patients with multiple HCAs is primarily driven by the size of the largest nodule, so management of these patients is driven by the size of the largest tumor. HCA is a rare indication for liver transplantation, but this can be considered in cases of HCA who are at increased risk for malignant transformation (e.g., β‐catenin activation or glycogen storage disease) who are not amenable to surgical resection (e.g., multiple HCA lesions). 11
FIG 3.
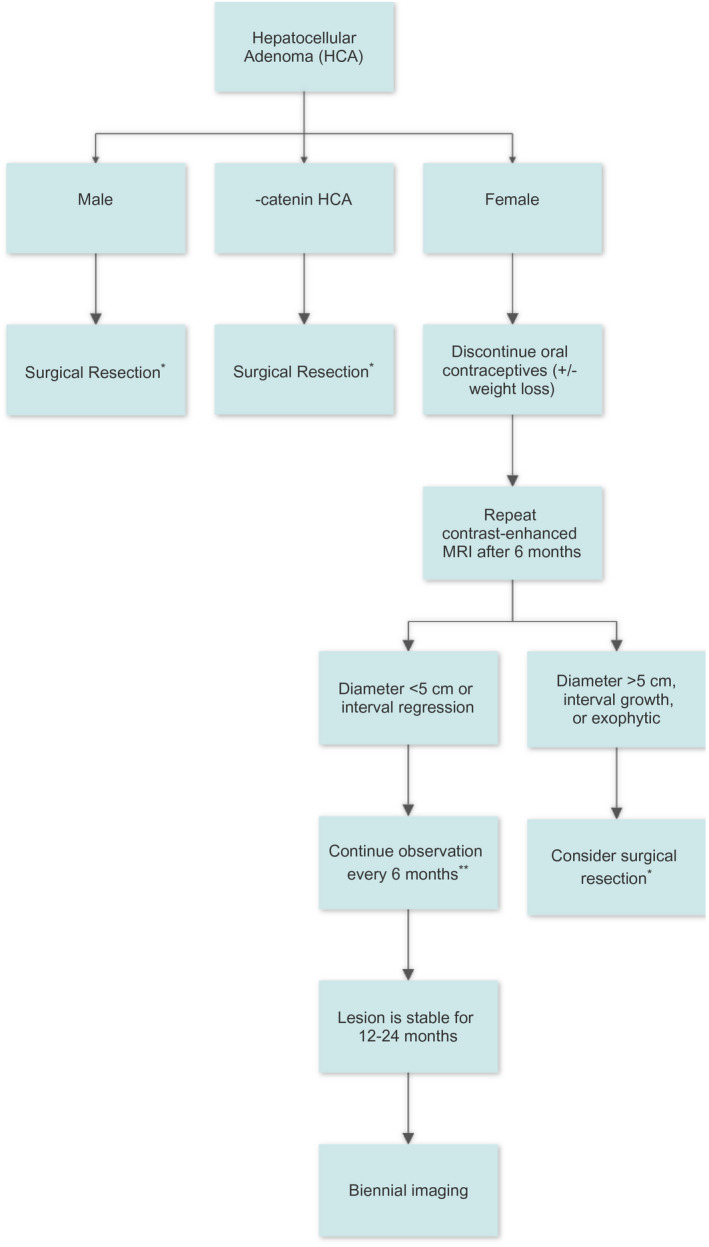
Management algorithm for patients with HCA. *If patient is a poor surgical candidate, can consider local ablation or transarterial embolization. **Shorter intervals can be considered in some cases, such as pregnancy or developing metabolic syndrome.
Given the hormone‐responsive nature of HCA, pregnancy can induce significant growth of HCA. However, pregnancy does not need to be discouraged, particularly for women with HCAs smaller than 5 cm, and an individualized approach using shared decision making is recommended. 12 Elective resection of known HCAs, particularly larger or subcapsular lesions, may be considered before pregnancy. For other HCAs, including those that are newly diagnosed during pregnancy, close observation every 2 to 3 months is recommended to monitor for interval growth. If there is significant growth, treatment with surgical resection or embolization can be considered depending on the trimester. In the absence of growth, no treatment is necessary and vaginal delivery can be pursued.
For the young female patient with three adenomas who is contemplating pregnancy, we would first recommend stopping oral contraceptives and encourage weight loss if she is overweight. Although it is important to discuss the risk for HCA growth during pregnancy, we would reinforce that pregnancy is not contraindicated. After she has discontinued oral contraceptives, we would repeat a contrast‐enhanced MRI 6 months later. If there is interval growth, we would recommend surgical consultation to discuss resection given increased risk for complications. If the lesion is stable or regresses, imaging can be repeated every 6 months for another 6 to 12 months, at which time surveillance can be extended to annual imaging if there is continued stability or regression. Continued communication about pregnancy is important, because surveillance intervals should be shortened to every 3 months at that time.
Potential conflict of interest: A.G.S. consults for Bayer, Eisai, Genetech, BMS, Exelixis, Roche, Exact Sciences, Glycotest, Wako, and TARGET Pharma.
References
- 1. Rooks JB, Ory HW, Ishak KG, et al. Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. JAMA 1979;242:644‐648. [PubMed] [Google Scholar]
- 2. Chang CY, Hernandez‐Prera JC, Roayaie S, et al. Changing epidemiology of hepatocellular adenoma in the United States: review of the literature. Int J Hepatol 2013;2013:604860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Suh CH, Kim KW, Park SH, et al. A cost‐effectiveness analysis of the diagnostic strategies for differentiating focal nodular hyperplasia from hepatocellular adenomas. Eur Radiol 2018;28:214‐225. [DOI] [PubMed] [Google Scholar]
- 4. Stoot J, Coelen R, de Jong MC, et al. Malignant transformation of hepatocellular adenomas into hepatocellular carcinoma: a systematic review including more than 1600 adenoma cases. HPB (Oxford) 2010;12:509‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Aalten SM, de Man RA, Uzermans JN, et al. Systematic review of haemorrahge and rupture of hepatocellular adenomas. Br J Surg 2012;99:911‐916.22619025 [Google Scholar]
- 6. Zucman‐Rossi J, Jeannot E, Nhieu JT, et al. Genotype‐phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology 2006;43:515‐524. [DOI] [PubMed] [Google Scholar]
- 7. Ronot M, Bahrami S, Calderaro J, et al. Hepatocellular adenomas: accuracy of magnetic resonance imaging and liver biopsy in subtype classification. Hepatology 2011;53:1182‐1191. [DOI] [PubMed] [Google Scholar]
- 8. Marrero JA, Anh J, Reddy R; American College of Gastroenterology . ACG clinical guideline: the diagnosis and management of focal liver lesions. Am J Gastroenterol 2014;109:1328‐1347. [DOI] [PubMed] [Google Scholar]
- 9. European Association for the Study of the Liver (EASL) . EASL Clinical Practice Guidelines on the management of benign liver tumours. J Hepatol 2016;65:386‐398. [DOI] [PubMed] [Google Scholar]
- 10. Gevers T, Spanier BWM, Veendrick PB, et al. Regression of hepatocellular adenoma after bariatric surgery in severe obese patients. Liver Int 2018;38:2134‐2136. [DOI] [PubMed] [Google Scholar]
- 11. Alagusundaramoothy S, Vilchez V, Zanni A, et al. Role of transplantation in the treatment of benign solid tumors of the liver. JAMA Surg 2015;150:337‐342. [DOI] [PubMed] [Google Scholar]
- 12. Broker ME, Ijzermans JN, van Aalten SM, et al. The management of pregnancy in women with hepatocellular adenoma: a plea for an individualized approach. Int J Hepatol 2012;2012:725735. [DOI] [PMC free article] [PubMed] [Google Scholar]


