Abstract
FK866 possesses various functional properties, such as anti-angiogenic, anti-cancer, and anti-inflammatory activities. We previously demonstrated that premature senescence of human dental pulp cells (hDPCs) was induced by hydrogen peroxide (H2O2). The present study aimed to investigate whether H2O2-induced premature senescence of hDPCs is affected by treatment with FK866. We found that FK866 markedly inhibited the senescent characteristics of hDPCs after exposure to H2O2, as revealed by an increase in the number of senescence-associated β-galactosidase (SA-β-gal)-positive hDPCs and the upregulation of the p21 and p53 proteins, which acts as molecular indicators of cellular senescence. Moreover, the stimulatory effects of H2O2 on cellular senescence are associated with oxidative stress induction, such as excessive ROS production and NADPH consumption, telomere DNA damage induction, and upregulation of senescence-associated secretory phenotype factors (IL-1β, IL-6, IL-8, COX-2, and TNF-α) as well as NF-κB activation, which were all blocked by FK866. Thus, FK866 might antagonize H2O2-induced premature senescence of hDPCs, acting as a potential therapeutic antioxidant by attenuating oxidative stress-induced pathologies in dental pulp, including inflammation and cellular senescence.
Keywords: hydrogen peroxide, senescence, human dental pulp cells, reactive oxygen species, telomere damage, inflammation, SASP factors, NF-κB
1. Introduction
Dental pulp is a soft tissue comprising nerves, blood vessels, lymphatic vessels, connective tissue, and various types of cells within a tooth [1]. Moreover, it includes several antibacterial substances, hormones, and immune cells that protect the teeth from external invasive bacteria or irritants and prevent the accumulation of extrinsic bacteria or substances inside the dental pulp [2,3]. Aging and stress-related conditions such as inflammation cause dental pulp cell senescence, leading to the aging of the pulp tissue and impaired tooth protection [4,5,6]. Therefore, it is important to understand the molecular mechanisms that underlie the senescence process of dental pulp cells, in order to maintain healthy teeth.
Cellular senescence is a phenomenon of irreversible cell cycle arrest that limits the replicative life span of cells, which might contribute to age-associated organ diseases [7,8]. Most cells undergo normal cellular senescence with increasing age, while premature cellular senescence can be acutely triggered by various stressors, including ionizing radiation, hyperglycemia, and oxidative stress [9,10,11]. Oxidative stress is a damaging response and refers to excessive intracellular levels of reactive oxygen species (ROS) [12]. Previous studies reported that excess ROS can cause damage to vital biomolecules, such as proteins, lipids, and DNA; moreover, excessive molecular damage in cells might lead to cellular senescence [13]. Since hydrogen peroxide (H2O2) induces oxidative stress and causes premature senescence in various cell types [14], the in vitro model of premature cellular senescence induced by application of H2O2 has been used as an efficient tool for studying human aging.
One of the hallmarks of cellular senescence is an acquisition of senescence-associated secretory phenotype (SASP) [15,16]. The SASP consists of a range of secreted factors, such as inflammatory cytokines, growth factors, chemokines, and enzymes that act in auto/paracrine manners [17]. Short-term acute induction of the SASP can prove to be beneficial for tissue regeneration and tumor suppression through the clearance of dysfunctional cells, whereas long-term chronic exposure to SASP factors in aging or lesions facilitates inflammatory, tumorigenic, and age-associated diseases [18]. The expression of key SASP genes is transcriptionally regulated by the NF-κB transcription factor in response to senescence inducers [19,20]. Upon exposure to the inducers, the degradation of the inhibitors of NF-κB (IkBs), following phosphorylation, leads to the NF-κB activation and nuclear translocation, affecting the transcription of the NF-κB-dependent SASP genes [19,20,21].
We recently reported that visfatin induced senescence in human dental pulp cells (hDPCs), which was inhibited by FK866, a noncompetitive small-molecule inhibitor of visfatin [22]; however, the role of FK866 in H2O2-induced cellular senescence remains unknown. Therefore, the present study was conducted to evaluate whether FK866 protects hDPCs from premature senescence induced by H2O2. To achieve this, we assessed the in vitro effects of H2O2 on hDPCs with regard to the expression of a series of senescence markers, including the senescence-associated β-galactosidase, and the expression of the p21 and p53 proteins. We further aimed to reveal the mechanism through which FK866 affects H2O2-induced senescence in hDPCs via molecular analyses, such as ROS production, NADPH consumption, telomere dysfunction, and SASP genes expression.
2. Materials and Methods
2.1. Reagents
Hydrogen peroxide (H2O2) was obtained from Junsei (Tokyo, Japan). FK866 was purchased from Adipogen (Switzerland). The antibodies used in the present study were as follows—rabbit anti-p21 (Santa Cruz Biotech, Dallas, TX, USA), mouse monoclonal anti-p53 (Calbiochem, San Diego, CA, USA), mouse monoclonal anti-β-Actin (Abcam, Cambridge, MA, USA), mouse monoclonal anti-TRF1 (Santa Cruz Biotechnology, Dallas, TX, USA), rabbit anti-phospho (Ser139)-histone H2AX (γH2AX) (Cell Signaling Technology, Danvers, MA, USA), mouse monoclonal anti-NF-κB p65 (Santa Cruz Biotech, Dallas, TX, USA), horseradish peroxidase-conjugated goat anti-rabbit, and anti-mouse IgG (Thermo Fisher Scientific, Waltham, MA, USA), Alexa Fluor® 488-conjugated goat anti-mouse IgG, and Alexa Fluor® 594-conjugated goat anti-rabbit IgG (Invitrogen, Camarillo, CA, USA).
2.2. Cell Culture
A cell line of immortalized human dental pulp cells (hDPCs) [23] were cultured in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% heat-inactivated fetal bovine serum (FBS, GibcoTM, Gaithersburg, MD, USA) and 1% antibiotics–antimycotics (GibcoTM, USA). Primary human umbilical vein endothelial cells (HUVECs) were purchased from CLONETICS (Lonza, Bazel, Switzerland). HUVECs were plated on 0.2% gelatin-coated plate and cultured in sterile endothelial growth medium with trace element and growth factors. Murine bone marrow-derived macrophages (mBMDM) out of the femur and tibia of hind limbs of 6- to 8-week old mice were generated by isolating the bone marrow. Cells were differentiated directly in 100 mm culture dish in the presence of 20 ng/mL macrophage colony-stimulating factor (M-CSF) (Peprotech, Hamburg, Germany) for up to 7 days. At day 3, fresh M-CSF was added. Human dental pulp stem cells (hDPSCs) were purchased from Lonza Inc. (PT-5025, Walkersville, MD, USA). The hDPSCs were cultured in StemMACSTM MSC Expansion media Kit XF (130-104-182, Miltenyi Biotec., Inc., Somerville, MA, USA) with 20% FBS and 1% antibiotics–antimycotics. The cells were incubated at 37 °C in a humidified 5% CO2 atmosphere.
2.3. Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from hDPCs using a TRIzol reagent kit (Invitrogen, Carlsbad, CA, USA). cDNA synthesis was performed using 1 μg of total RNA with MaximeRT premix (iNtRON Biotechnology, Sungnam, South Korea). The oligonucleotide primer sequences used for PCR were as Table 1.
Table 1.
The oligonucleotide primer sequences used for PCR.
| Gene | Accession Number | Primer Sequence |
|---|---|---|
| GAPDH | NM_001256799.3 | Forward: 5′-ATCTTCCAGGAGCGAGATCC-3′ |
| Reverse: 5′-AGGAGGCATTGCTGATGATC-3′ | ||
| IL-1β | NM_000576.3 | Forward: 5′-GGATATGGAGCAACAAGTGG-3′ |
| Reverse: 5′-ATGTACCAGTTGGGGAACTG-3′ | ||
| IL-6 | NM_000600.5 | Forward: 5′-AGATTCCAAAGATGTAGCCG-3′ |
| Reverse: 5′-TCTTTGCTGCTTTCACACAT-3′ | ||
| IL-8 | NM_000584.4 | Forward: 5′-ATGACTTCCAAGCTGGCCGTGGCT-3′ |
| Reverse: 5′-CTCAGCCCTCTTCAAAAACTTCTC-3′ | ||
| COX-2 | NM_000963.4 | Forward: 5′-TTCTTTGCCCAGCACTTCAC-3′ |
| Reverse: 5′-CTGCTCATCACCCCATTCAC-3′ | ||
| TNF-α | NM_000594.4 | Forward: 5′-GAGTGACAAGCCTGTAGCCCA-3′ |
| Reverse: 5′-GCAATGATCCCAAAGTAGACC-3′ |
2.4. Western Blot Analysis
The harvested cells were lysed in radioimmunoprecipitation assay buffer (RIPA) (iNtRON Biotechnology, Sungnam, Korea) containing a protease inhibitor cocktail (Roche, Mannheim, Germany). Protein extracts (30 μg/lane) were separated by SDS-PAGE and transferred to a nitrocellulose membrane (Amersham Pharmacia Biotech, Little Chalfont, UK). The membrane was blocked with 5% skim milk in PBS containing 0.1% Tween 20 for 1 h at room temperature and probed with the appropriate antibodies. The signal was developed using an ECL detection system (Amersham Pharmacia Biotech, Little Chalfont, UK).
2.5. SA-β-galactosidase Staining Assay
The degree of SA-β-galactosidase activity was measured using a senescence assay kit (Senescence Cells Histochemical Staining; Sigma-Aldrich, St. Louis, MO, USA), according to the manufacturer’s protocol. The cells were washed twice in 1× PBS and fixed in 1× fixation buffer for 6–7 min at 25 °C, washed in 1× PBS, and incubated overnight with SA-β-galactosidase staining solution at 37 °C without CO2. Thereafter, the cells were imaged under a microscope (Olympus, IX71; Toyko, Japan).
2.6. ROS Measurement
The ROS-ID® Total ROS detection kit (Enzo, Farmingdale, NY, USA) was used to measure the total intracellular ROS generation. For measurement of intracellular ROS levels, the cells (1 × 104 cells) were seeded in a 96-well dark plate overnight and incubated with oxidative stress detection reagent in cell culture media at 37 °C in a dark room for 30 min, as recommended by the manufacturer. Fluorescence was measured at a wavelength of 490 nm (excitation) / 525 nm (emission), using a fluorescent microplate reader (BIOTEK, Cytation3, USA). For ROS detection in cells, the cells (5 × 103 cells) were seeded in a 24-well plate with poly L-lysine-coated coverslips overnight, and incubated with oxidative stress detection reagent in cell culture media at 37 °C in a dark room for 30 min, following the manufacturer’s instructions. After washing with buffer salts thrice, the cells were analyzed using a confocal microscope LSM510 (Carl Zeiss, Oberkochen, Germany).
2.7. NADP/NADPH Assay
The NADP/NADPH levels were assessed using a colorimetric NADP/NADPH assay kit (Abcam, Cambridge, MA, USA), following the manufacturer’s instructions. The cells were lysed in the assay buffer provided in the kit. The lysates were deproteinized by passing through a 10-kD spin column (Biovision, Milpitas, CA, USA). Thereafter, the assay was performed in a 24-well plate, and the absorbance of the samples was measured using a Multimode Plate Reader Victor X3, P (Perkin Elmer, Hopkinton, MA, USA) at 450 nm.
2.8. Immunocytochemistry
Cells cultured on poly L-lysine-coated coverslips were washed thrice with phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde/PBS for 10 min at room temperature, permeabilized with 0.01% Triton X-100 in PBS for 15 min at room temperature, and then washed thrice with PBS for 5 min. Thereafter, the cells were blocked with 1% BSA/0.3% Triton X-100/PBS for 1 h and labeled with the appropriate primary antibodies. After overnight incubation at 4 °C, the cells were incubated with Alexa Fluor 488-conjugated secondary antibodies for 1 h at 25 °C. Coverslips were mounted on slides with a fluorescent mounting medium containing DAPI (Vector Laboratories, Burlingame, CA, USA). Images were acquired using a confocal microscope (LSM 510; Carl Zeiss; Oberkochen, Germany).
2.9. Statistical Analysis
Data are presented as the mean ± standard deviation (SD) obtained from at least three independent experiments. Statistical analysis was performed using Student’s t-test for data points and ANOVA for curves.
3. Results
3.1. FK866 Alleviates H2O2-Induced Premature Senescence in hDPCs
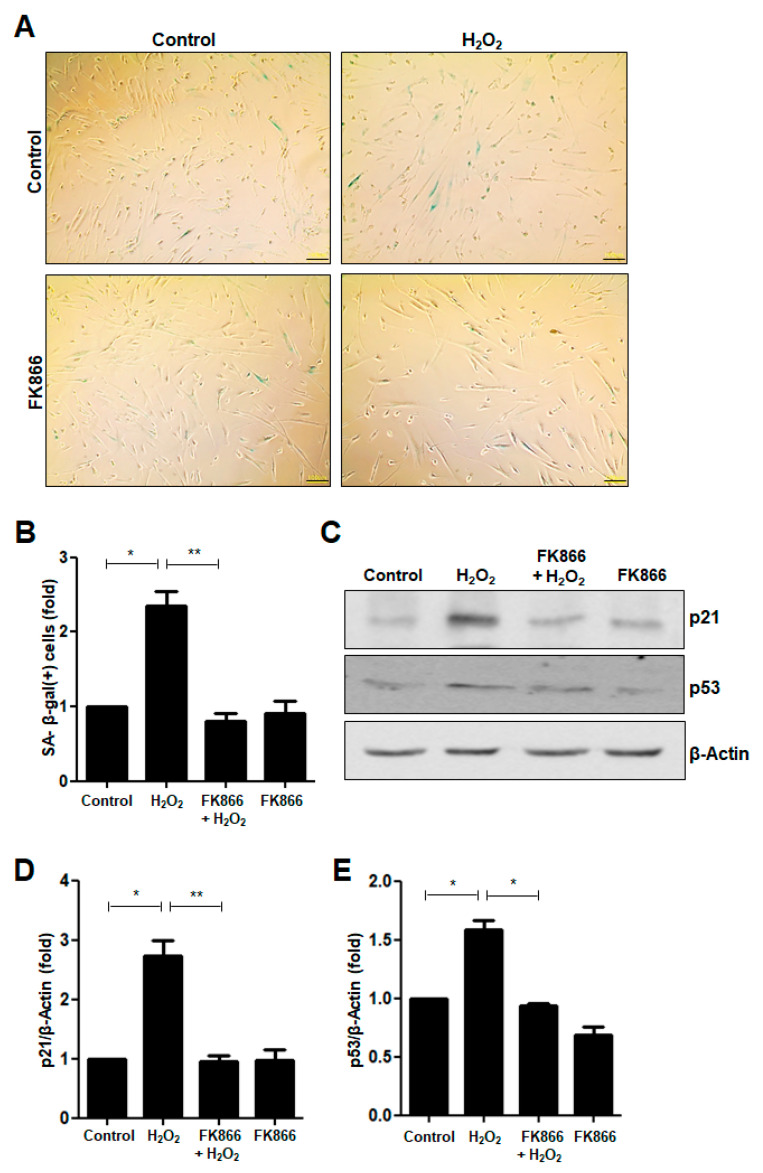
We recently reported that 400 nM H2O2 did not affect the survival of hDPCs and induced premature senescence of immortalized hDPCs [24]. In this study, we confirmed that H2O2 increased the fraction of cells that was stained positive for SA-β-galactosidase (SA-β-gal), a marker of cellular senescence (Figure 1A,B). To address the effect of FK866 on H2O2-induced premature senescence, the hDPCs were pretreated with FK866 before H2O2 treatment. As shown in Figure 1A,B, the increase in the number of SA-β-gal(+) cells elicited by H2O2 was reversed by the FK866 pretreatment. We examined whether FK866 affects H2O2-induced premature senescence in other different cells including vascular endothelial cells and macrophages, and obtained similar results using HUVEC (Figure S1A,B) and mBMDM (Figure S1C,D). In the case of human dental pulp stem cells (hDPSCs), treatment with 400 nM H2O2 had no significant effect on the induction of cellular senescence (Figure S1E).
Figure 1.
Effect of FK866 on H2O2-induced premature senescence in hDPCs. (A) The hDPCs were stimulated with H2O2 (400 nM) for 24 h and stained for detecting the activity of senescence-associated (SA)-β-galactosidase. Representative image of SA-β-galactosidase staining. Scale bar: 100 μm. (B) Quantitative results for the percentage of cells stained positively with SA-β-galactosidase. (C) The hDPCs were pretreated with FK866 (10 µM) for 2 h and then stimulated with H2O2 (400 nM) for 24 h. Cell extracts were subjected to Western Blot analysis for detecting the levels of the p21 and p53 proteins. β-Actin was used as the loading control. (D,E) Densitometric analysis for assessing the relative protein levels were normalized to the levels of β-Actin: D, p21; E, p53. * p < 0.1, ** p < 0.01.
The change in the fraction of SA-β-gal(+) cells among the H2O2-treated hDPCs was evaluated by detecting the expression of the p21 and p53 proteins, which are well-known senescence markers. H2O2 increased the protein levels of p21 (Figure 1C,D) and p53 (Figure 1C,E) in hDPCs. When pretreated with FK866 before H2O2 treatment, the H2O2-induced increase in the levels of p21 and p53 proteins was significantly attenuated (Figure 1C–E).
3.2. FK866 Attenuates H2O2-Induced Telomere Damage in hDPCs
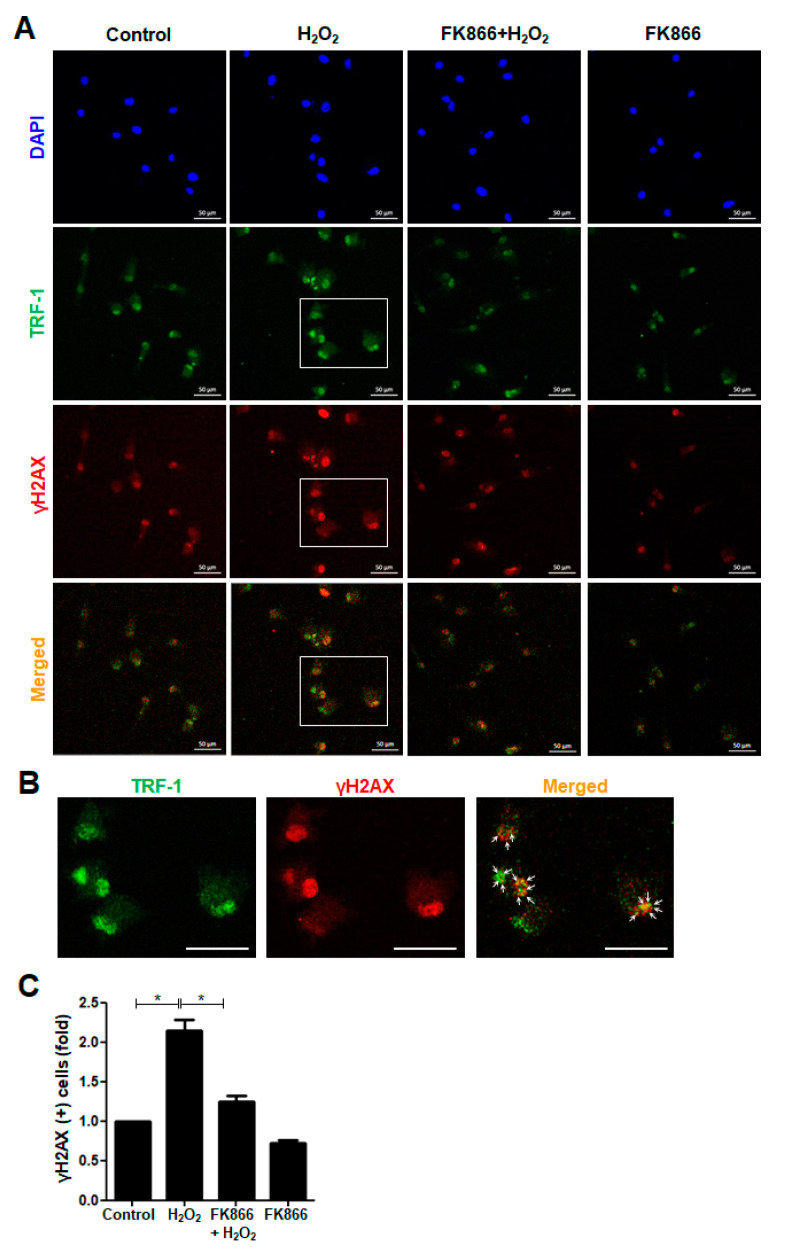
H2O2, the most abundant ROS, causes DNA damage via the promotion of intracellular oxidative stress [14,25]. To examine whether H2O2 treatment influences the DNA damage response in hDPCs, we performed immunocytochemistry and detected γH2AX foci formed at the sites where the DNA was damaged and at sites with uncapped dysfunctional telomeres. The intensity of γH2AX-positive immunofluorescence staining increased in response to H2O2 treatment (Figure 2A,B). Moreover, H2O2 increased the number of γH2AX foci that were scattered over the nucleus and colocalized with telomeric TRF-1 signals (Figure 2A,B). Furthermore, the H2O2-induced increase in both the intensity and number of γH2AX-positive cells was significantly attenuated by pretreatment with FK866 (Figure 2A,C).
Figure 2.
Effect of FK866 on H2O2-induced telomere damage formation in hDPCs. The hDPCs were pretreated with FK866 (10 µM) for 2 h and then stimulated with H2O2 (400 nM) for 24 h. (A) Immunofluorescence analysis of TRF-1 (Alexa fluoro (AF)-488, green) and γH2AX (AF-594, red) in hDPCs. The cells were analyzed using a confocal microscope. The nuclei were counterstained with DAPI (blue). (B) Higher magnification images of the boxed areas (A) showing double immunoreactivity (yellow) for TRF-1(green) and γH2AX(red). Arrows in the merged images point to sites of colocalization. Scale bar: 50 μm. (C) Quantitative result for the percentage of γH2AX-positive cells. * p < 0.1.
3.3. FK866 Reverses H2O2-Induced Upregulation of NADPH Consumption and ROS Production
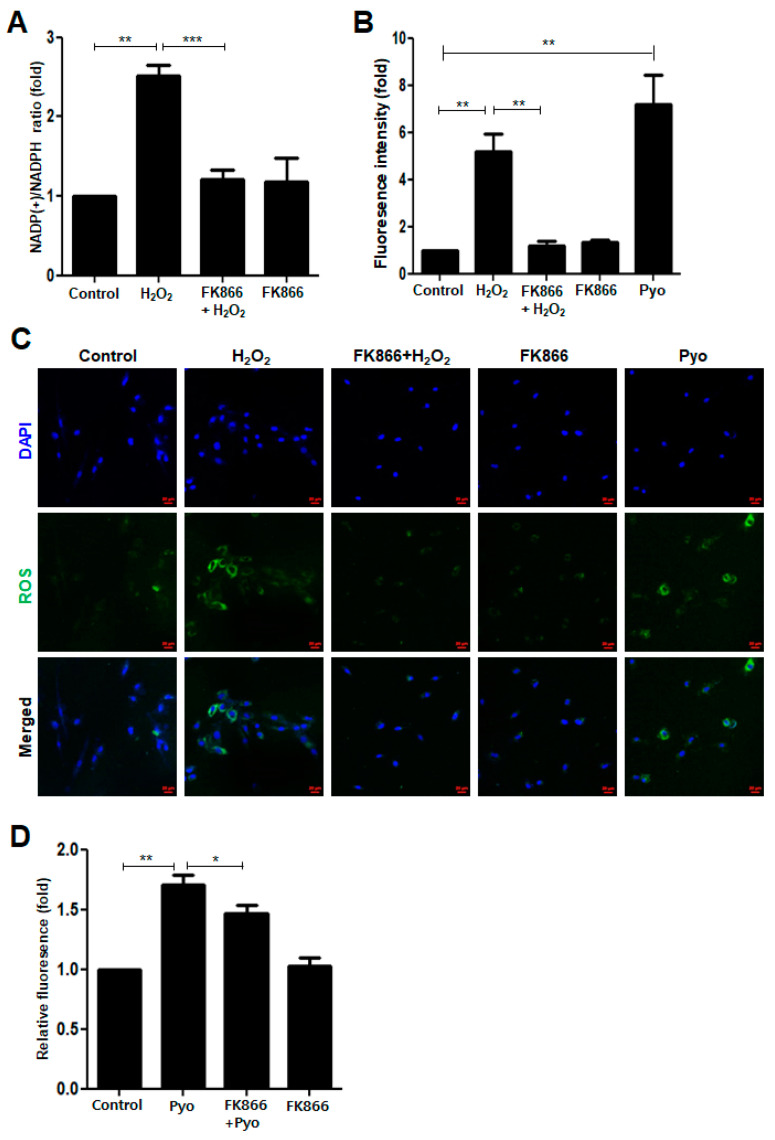
DNA damage is caused by various stresses including oxidative stress [26]. Unless such DNA damage is repaired, cells undergo premature senescence [15,27]. To examine whether oxidative stress is involved in H2O2-induced senescence in hDPCs, we measured the NADP+/NADPH ratio and ROS production, two major indicators of oxidative stress [26,28]. As shown in Figure 3A, H2O2 increased the NADP+/NADPH ratio, which was reversed by the FK866 treatment. In accordance with the changes in the NADP+/NADPH ratio, intracellular ROS levels were remarkably elevated after H2O2 treatment in hDPCs, whereas FK866 pretreatment reversed the H2O2-induced increase in ROS levels (Figure 3B). Similar results were obtained with HUVEC (Figure S2A), mBMDM (Figure S2B), and hDPSCs (Figure S2C). Moreover, in the immunofluorescence analysis, the green intensity per cell was higher in the H2O2-treated hDPCs than in the control cells (Figure 3C). The stimulatory effect of H2O2 on intracellular ROS production was verified using pyocyanin (Pyo) as a positive “ROS generator” control (Figure 3B,C). Furthermore, FK866 decreased the extent of Pyo- induced ROS production (Figure 3D).
Figure 3.
Effect of FK866 on H2O2-induced NADPH consumption and ROS production in hDPCs. (A–C) The hDPCs were pretreated with or without FK866 (10 µM) for 2 h and then stimulated with H2O2 (400 nM) for 24 h or pyocyanin (250 µM) for 30 min. Measurement of the NADP(+)/NADPH ratio in H2O2-treated hDPCs with or without FK866 pretreatment (A). Measurement of the total intracellular ROS levels in H2O2-treated hDPCs with or without FK866 pretreatment using a fluorescent microplate reader (B) and a confocal microscope (C). Pyocyanin, a redox-active compound that generates ROS, was used as a positive control for ROS production. The nuclei were counterstained with DAPI (blue). Scale bar: 20 μm. (D) The hDPCs were pretreated with FK866 (10 µM) for 2 h and then stimulated with pyocyanin (Pyo) for 30 min. * p < 0.1, ** p < 0.01, *** p < 0.001.
3.4. FK866 Reverses H2O2-Induced Upregulation of SASP Factors
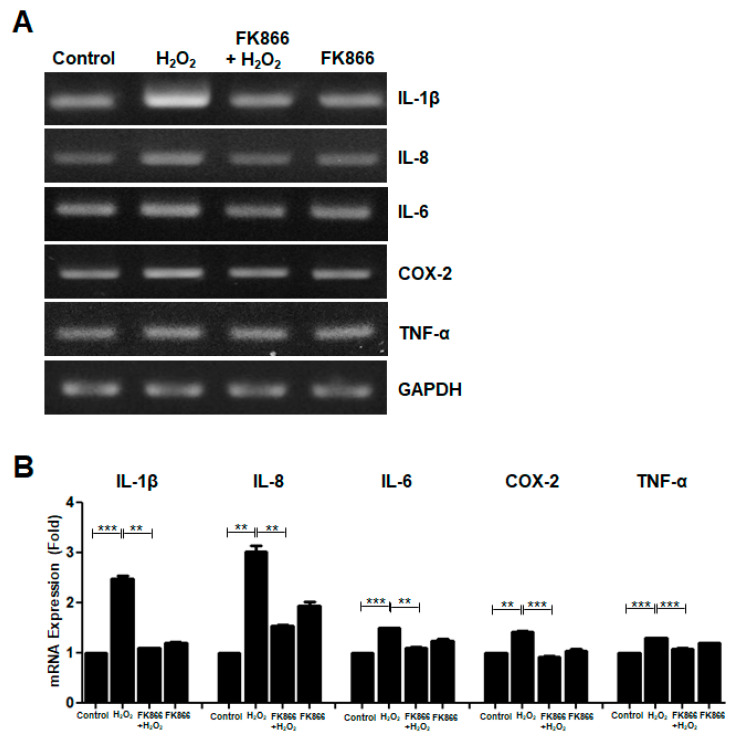
The SASP is a hallmark of cellular senescence [16]. To investigate whether H2O2 affects the induction of SASP, the expression levels of several SASP factors were examined by RT-PCR in H2O2-treated hDPCs. H2O2 increased the mRNA levels of SASP factors such as IL-1β, IL-8, IL-6, COX-2, and TNF-α, which were all reduced by pretreatment with FK866 in hDPCs (Figure 4A,B).
Figure 4.
Effect of FK866 on the H2O2-induced upregulation of SASP factors in hDPCs. The hDPCs were pretreated with FK866 (10 µM) for 2 h and then stimulated with H2O2 (400 nM) for 24 h. (A) The cell lysates were subjected to RT-PCR for determining the mRNA levels of the indicated SASP markers. GAPDH was used as an internal control. (B) Densitometric analysis for assessing the relative mRNA expression level of each SASP factor normalized to the mRNA level of GAPDH. ** p < 0.01, *** p < 0.001.
3.5. FK866 Reverses H2O2-Induced NF-κB Activation
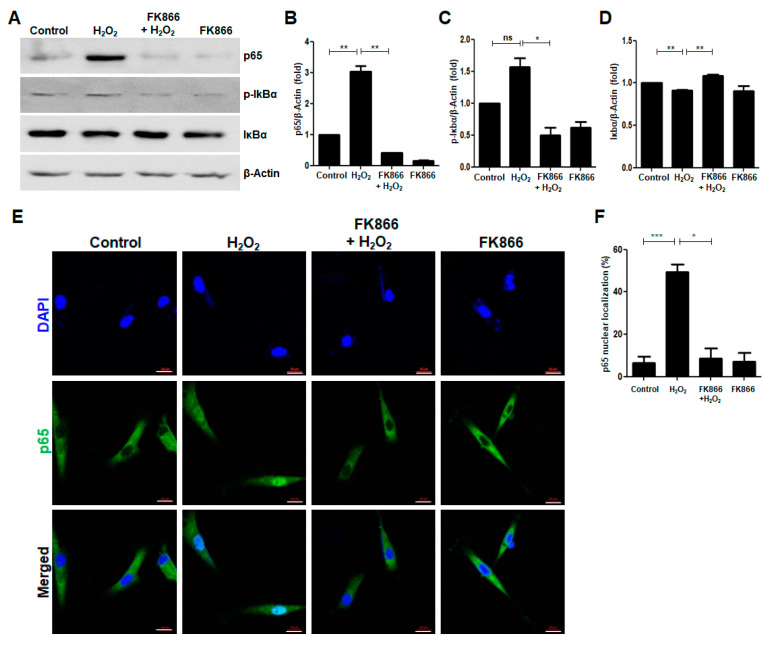
Since NF-κB is one of main transcription factors involved in the activation of SASP in response to senescence inducers [17,19,20], Western Blotting and immunocytochemistry were performed to examine whether H2O2 affects NF-κB activation in hDPCs. It was observed that H2O2 increased the protein levels of the NF-κB p65 and the phosphorylation of Ser-32 in IκBα (p-IκBα), whereas the levels of IκBα protein decreased in H2O2-treated hDPCs (Figure 5A–C). Furthermore, H2O2-induced nuclear translocation of the p65 subunit of NF-κB was abrogated by the FK866 pretreatment (Figure 5E,F).
Figure 5.
Effect of FK866 on H2O2-induced upregulation of NF-κB p65 activation in hDPCs. The hDPCs were pretreated with FK866 (10 µM) for 2 h and then stimulated with H2O2 (400 nM) for 24 h. (A) The cell extracts were subjected to Western Blot analysis for detecting the expression of the NF-κB p65, p-IκBα, and IκBα proteins. β-Actin was used as the loading control. (B–D) Densitometric analysis for assessing relative protein levels normalized to the levels of β-Actin: B, p65; C, p-IκBα; D, IκBα. (E) Immunocytochemical analysis of NF-κB p65 protein localization in the H2O2-treated cells. The cells were visualized using a fluorescence microscope. The nuclei were counterstained with DAPI (blue). (F) Quantitative results for the percentage of nuclear localization of NF-κB p65. Scale bar: 20 μm. * p < 0.1, ** p < 0.01, *** p < 0.001.
4. Discussion
In the present study, we demonstrated the effect of FK866 on the senescence of human dental pulp cells in an in vitro model of H2O2-induced premature cellular senescence. This was the first study to report that FK866 exerts protective effects against senescence in H2O2-treated hDPCs. FK866 inhibited H2O2-induced ROS generation, NADPH consumption, and upregulation of SASP in hDPCs. Furthermore, FK866 prevented H2O2-induced NF-κB activation in hDPCs.
Oxidative stress is implicated in normal aging and age-associated diseases, including neurodegenerative diseases, cardiovascular diseases, diabetes, and cancers [29,30,31,32,33]. Overproduction of intracellular ROS causes oxidative stress, which majorly contributes to the induction and progression of cellular senescence. Moreover, H2O2 activates the signaling pathways to promote ROS production and thus, induces premature cellular premature senescence via oxidative stress in various cells, such as human retinal epithelial cells, hippocampal neurons, vascular endothelial cells, and human periodontal ligament cells [25,34,35,36,37]. In previous studies, we reported that H2O2 caused premature senescence in hDPCs; however, the involvement of oxidative stress in this process was not elucidated [24]. In the present study, we demonstrated that H2O2 induces senescence in hDPCs by stimulating NADPH consumption and increasing ROS generation, whereas pretreatment with FK866 significantly abrogated this effect, suggesting that FK866 acts as an antioxidant against oxidative stress-induced senescence in hDPCs during H2O2 treatment. Furthermore, we found that the ROS generator pyocyanin remarkably increased ROS production in hDPCs, whereas FK866 decreased pyocyanin-induced ROS production. Therefore, based on these results, it is conceivable that FK866 prevents H2O2-induced oxidative stress in hDPCs and further protects the cells against oxidative stress-induced senescence. Thus, it would be interesting to study whether FK866 exerts antioxidant effects on other oxidative stress-related human diseases, such as viral infection, cardiovascular diseases, and oral mucosal diseases [34,35,36,37,38,39,40].
FK866 exhibits anti-inflammatory and antitumor effects [41]; however, its role in cellular senescence is debatable. The results of the present study revealed that FK866 attenuated H2O2-induced senescence in hDPCs, which was accompanied by a decrease in ROS production. Similar effects of FK866 were observed in vascular endothelial cells (HUVEC) and macrophages (mBMDM). On the other hand, in the case of human dental pulp stem cells (hDPSCs), FK866 exerted inhibitory effects on H2O2-induced ROS production, but the effect of FK866 on cellular senescence could not be further analyzed due to negative SA-β-gal staining in hDPSCs that were treated with 400 nM H2O2 for 1 day. It was recently reported that cellular senescence of primary human DPSCs isolated from dental pulp tissues was induced after incubation with 100 µM H2O2 for 7 days [42]; we therefore consider future research to examine whether culture conditions with much higher concentrations and longer time of H2O2 treatment could lead to the senescence of hDPSCs and to address if the senescence could be reversed by FK866. Based on these results, it is likely that the protective role of FK866 as an antioxidant against oxidative stress-induced senescence is probably conserved in several cell types, which is in accordance with the results on human endothelial cells [43], but is inconsistent with the stimulatory effects of FK866 on the senescence of other cell types [44,45]. The reason for this discrepancy is unknown; however, this might result from the differences in the cell type- and context-dependent effects of FK866.
In this study, we found that FK866 reversed H2O2-induced NF-κB p65 activation as well as cellular senescence. Thus, considering growing evidence that the NF-κB activation plays an important role in senescence and aging processes [7,17,20,46,47], it is likely that FK866 inactivated-NF-κB p65 might have an impact on FK866-protection in H2O2-induced senescence. At present, we do not know whether the protection of FK866 against H2O2-induced senescence is directly or indirectly attributable to the inactivation of NF-κB p65 in hDPCs. Thus, additional studies are needed to investigate the effects of NF-κB p65 inactivation on FK866-protection in H2O2-induced senescence as well as on H2O2-induced senescence, through which the relationship between FK866-protection in H2O2-induced senescence and NF-κB p65 pathway would be clarified.
Hydrogen peroxide was used in dentistry since 1913 to control periodontal disease and reduce tooth plaque [48]. H2O2 exhibits antimicrobial activity in the oral cavity by releasing oxygen, destroying the cell wall of bacteria, and subsequently exerting a pathogenic effect against both gram-positive and gram-negative bacteria; thus, it could be used for commercial dentifrices in periodontal therapy [48,49]. A recent study reported that H2O2 produced by Streptococcus sanguinis reduced the biomass of Porphyromonas gingivalis [50]. Aggregatibacter actinomycetemcomitans protects P. gingivalis from H2O2 attack via catalase-mediated H2O2 degradation, which consequently aided the survival of anaerobic P. gingivalis in S. sanguinis-P. gingivalis-A. actinomycetemcomitans tri-species biofilms under micro-aerobic conditions, indicating the therapeutic role of H2O2 in treating P. gingivalis-mediated periodontal diseases [50]. In addition, H2O2 is frequently used in dentistry as a tooth bleaching agent due to its oxidative reactivity [48,49]. In addition to these advantages, H2O2 is widely known as a cytotoxic substance [51]. H2O2 is produced in vivo by the dismutation of superoxide radicals, and presumably, most human cells are exposed to different levels of H2O2. Exposure to high concentrations of H2O2 corrodes tissues, organs, and mucous membranes [49,51]. Sublethal H2O2 exposure leads to cellular senescence in tissues and organs [52]. Our results demonstrated that sublethal H2O2 induced premature senescence in hDPCs. Thus, considering that numerous environmental stressors trigger H2O2 production, the increased use of agents containing or generating H2O2 in oral hygiene requires continuous monitoring, and safety concerns regarding the senescent effects of H2O2 on oral tissues and cells exist.
5. Conclusions
In conclusion, the present study demonstrated that FK866 attenuates oxidative stress and reverses oxidative stress-induced senescence in hDPCs. Therefore, FK866 might serve as a potential pharmacological agent used to maintain and promote the health of dental pulp tissues.
Acknowledgments
We would like to thank Editage.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3921/10/2/271/s1. Figure S1. Effect of FK866 on H2O2-induced premature senescence in HUVEC, mBMDM, and hDPSCs; Figure S2. Effect of FK866 on H2O2-induced ROS production in HUVEC, mBMDM, and hDPSCs.
Author Contributions
Conceptualization, S.-K.B.; Data curation, C.Y.O., S.P. and S.-K.B.; Formal analysis, C.Y.O., S.P. and S.-K.B.; Funding acquisition, S.-K.B.; Investigation, C.Y.O. and S.P.; Methodology, C.Y.O. and S.P.; Project administration, S.-K.B.; Resources, H.-O.J., T.T., O.-H.L., M.-K.B. and S.-K.B.; Supervision, S.-K.B.; Visualization, C.Y.O. and S.P.; Writing—original draft, C.Y.O. and S.-K.B.; Writing—review & editing, O.-H.L., M.-K.B. and S.-K.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. NRF-2018R1A5A2023879) (to Bae S-K).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All datasets generated for this study are included in the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yu C., Abbott P.V. An Overview of the Dental Pulp: Its Functions and Responses to Injury. Aust. Dent. J. 2007;52:S4–S16. doi: 10.1111/j.1834-7819.2007.tb00525.x. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg M., Farges J.C., Lacerda-Pinheiro S., Six N., Jegat N., Decup F., Septier D., Carrouel F., Durand S., Chaussain-Miller C., et al. Inflammatory and Immunological Aspects of Dental Pulp Repair. Pharmacol. Res. 2008;58:137–147. doi: 10.1016/j.phrs.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farges J.C. Understanding Dental Pulp Innate Immunity—A Basis for Identifying New Targets for Therapeutic Agents that Dampen Inflammation. J. Appl. Oral Sci. 2009;17 doi: 10.1590/S1678-77572009000300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung H.Y., Lee E.K., Choi Y.J., Kim J.M., Kim D.H., Zou Y., Kim C.H., Lee J., Kim H.S., Kim N.D., et al. Molecular Inflammation as an Underlying Mechanism of the Aging Process and Age-Related Diseases. J. Dent. Res. 2011;90:830–840. doi: 10.1177/0022034510387794. [DOI] [PubMed] [Google Scholar]
- 5.Lee Y.H., Kim G.E., Cho H.J., Yu M.K., Bhattarai G., Lee N.H., Yi H.K. Aging of in Vitro Pulp Illustrates Change of Inflammation and Dentinogenesis. J. Endod. 2013;39:340–345. doi: 10.1016/j.joen.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 6.Murray P.E., Stanley H.R., Matthews J.B., Sloan A.J., Smith A.J. Age-Related Odontometric Changes of Human Teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002;93:474–482. doi: 10.1067/moe.2002.120974. [DOI] [PubMed] [Google Scholar]
- 7.Childs B.G., Durik M., Baker D.J., van Deursen J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015;21:1424–1435. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blagosklonny M.V. Prospective treatment of age-related diseases by slowing down aging. Am. J. Pathol. 2012;181:1142–1146. doi: 10.1016/j.ajpath.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 9.Ji J., Tian Y., Zhu Y.Q., Zhang L.Y., Ji S.J., Huan J., Zhou X.Z., Cao J.P. Ionizing irradiation inhibits keloid fibroblast cell proliferation and induces premature cellular senescence. J. Dermatol. 2015;42:56–63. doi: 10.1111/1346-8138.12702. [DOI] [PubMed] [Google Scholar]
- 10.Toussaint O., Medrano E.E., von Zglinicki T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp. Gerontol. 2000;35:927–945. doi: 10.1016/S0531-5565(00)00180-7. [DOI] [PubMed] [Google Scholar]
- 11.Ksiazek K., Breborowicz A., Jörres A., Witowski J. Oxidative stress contributes to accelerated development of the senescent phenotype in human peritoneal mesothelial cells exposed to high glucose. Free Radic. Biol. Med. 2007;42:636–641. doi: 10.1016/j.freeradbiomed.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Egea G., Jiménez-Altayó F., Campuzano V. Reactive Oxygen Species and Oxidative Stress in the Pathogenesis and Progression of Genetic Diseases of the Connective Tissue. Antioxidants. 2020;9:1013. doi: 10.3390/antiox9101013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cirilli I., Orlando P., Marcheggiani F., Dludla P.V., Silvestri S., Damiani E., Tiano L. The Protective Role of Bioactive Quinones in Stress-induced Senescence Phenotype of Endothelial Cells Exposed to Cigarette Smoke Extract. Antioxidants. 2020;9:1008. doi: 10.3390/antiox9101008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z., Wei D., Xiao H. Methods of cellular senescence induction using oxidative stress. Humana Press, Totowa, NJ Biological Aging. Methods Mol. Biol. 2013;1048:135–144. doi: 10.1007/978-1-62703-556-9_11. [DOI] [PubMed] [Google Scholar]
- 15.Dodig S., Cepelak I., Pavic I. Hallmarks of Senescence and Aging. Biochem. Med. 2019;29:030501. doi: 10.11613/BM.2019.030501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borodkina A.V., Deryabin P.I., Giukova A.A., Nikolsky N.N. “Social Life” of Senescent Cells: What is SASP and Why Study it? Acta. Nat. 2018;10:4–14. doi: 10.32607/20758251-2018-10-1-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopes-Paciencia S., Saint-Germain E., Rowell M.C., Ruiz A.F., Kalegari P., Ferbeyre G. The senescence-associated secretory phenotype and its regulation. Cytokine. 2019;117:15–22. doi: 10.1016/j.cyto.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Ohtani N. Deciphering the mechanism for induction of senescence-associated secretory phenotype (SASP) and its role in aging and cancer development. J. Biochem. 2019;166:289–295. doi: 10.1093/jb/mvz055. [DOI] [PubMed] [Google Scholar]
- 19.Faget D.V., Ren Q., Stewart S.A. Unmasking senescence: Context-dependent effects of SASP in cancer. Nat. Rev. Cancer. 2019;19:439–453. doi: 10.1038/s41568-019-0156-2. [DOI] [PubMed] [Google Scholar]
- 20.Osorio F.G., López-Otín C., Freije J.M. NF-κB in premature aging. Aging. 2012;4:726–727. doi: 10.18632/aging.100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolcet X., Llobet D., Pallares J., Matias-Guiu X. NF-κB in development and progression of human cancer. Virchows Arch. 2005;446:475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 22.Ok C.Y., Park S., Jang H.O., Takata T., Bae M.K., Kim Y.D., Ryu M.H., Bae S.K. Visfatin Induces Senescence of Human Dental Pulp Cells. Cells. 2020;9:193. doi: 10.3390/cells9010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitagawa M., Ueda H., Iizuka S., Sakamoto K., Oka H., Kudo Y., Ogawa I., Miyauchi M., Tahara H., Takata T. Immortalization and characterization of human dental pulp cells with odontoblastic differentiation. Arch. Oral Biol. 2007;52:727–731. doi: 10.1016/j.archoralbio.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Park S., Bak K.J., Ok C.Y., Park H.J., Jang H.O., Bae M.K., Bae S.K. Melatonin Rescues Human Dental Pulp Cells from Premature Senescence Induced by H2O2. Int. J. Oral. Biol. 2017;42:91–97. doi: 10.11620/IJOB.2017.42.3.091. [DOI] [Google Scholar]
- 25.Giorgio M., Trinei M., Migliaccio E., Pelicci P.G. Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007;8:722–728. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- 26.Kay J., Thadhani E., Samson L., Engelward B. Inflammation-Induced DNA Damage, Mutations and Cancer. DNA Repair. 2019;83:102673. doi: 10.1016/j.dnarep.2019.102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marazita M.C., Dugour A., Marquioni-Ramella M.D., Figueroa J.M., Suburo A.M. Oxidative Stress-Induced Premature Senescence Dysregulates VEGF and CFH Expression in Retinal Pigment Epithelial Cells: Implications for Age-Related Macular Degeneration. Redox Biol. 2016;7:78–87. doi: 10.1016/j.redox.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y., Ji L., Jiang R., Zheng L., Liu D. Oxidized High-Density Lipoprotein Induces the Proliferation and Migration of Vascular Smooth Muscle Cells by Promoting the Production of ROS. J. Atheroscler. Thromb. 2014;21:204–216. doi: 10.5551/jat.19448. [DOI] [PubMed] [Google Scholar]
- 29.Luo J., Mills K., le Cessie S., Noordam R., van Heemst D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing. Res. Rev. 2020;57:100982. doi: 10.1016/j.arr.2019.100982. [DOI] [PubMed] [Google Scholar]
- 30.Singh A., Kukreti R., Saso L., Kukreti S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules. 2019;24:1583. doi: 10.3390/molecules24081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papaconstantinou J. The Role of Signaling Pathways of Inflammation and Oxidative Stress in Development of Senescence and Aging Phenotypes in Cardiovascular Disease. Cells. 2019;8:1383. doi: 10.3390/cells8111383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nobakht-Haghighi N., Rahimifard M., Baeeri M., Rezvanfar M.A., Moini Nodeh S., Haghi-Aminjan H., Hamurtekin E., Abdollahi M. Regulation of aging and oxidative stress pathways in aged pancreatic islets using alpha-lipoic acid. Mol. Cell. Biochem. 2018;449:267–276. doi: 10.1007/s11010-018-3363-3. [DOI] [PubMed] [Google Scholar]
- 33.Gorrini C., Harris I.S., Mak T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 34.Chae S.Y., Park S.Y., Park G. Lutein protects human retinal pigment epithelial cells from oxidative stress-induced cellular senescence. Mol. Med. Rep. 2018;18:5182–5190. doi: 10.3892/mmr.2018.9538. [DOI] [PubMed] [Google Scholar]
- 35.Xu T.Z., Shen X.Y., Sun L.L., Chen Y.L., Zhang B.Q., Huang D.K., Li W.Z. Ginsenoside Rg1 protects against H2O2-induced neuronal damage due to inhibition of the NLRP1 inflammasome signalling pathway in hippocampal neurons in vitro. Int. J. Mol. Med. 2019;43:717–726. doi: 10.3892/ijmm.2018.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruan Y., Wu S., Zhang L., Chen G., Lai W. Retarding the senescence of human vascular endothelial cells induced by hydrogen peroxide: Effects of 17beta-estradiol (E2) mediated mitochondria protection. Biogerontology. 2014;15:367–375. doi: 10.1007/s10522-014-9507-2. [DOI] [PubMed] [Google Scholar]
- 37.Kuang Y., Hu B., Feng G., Xiang M., Deng Y., Tan M., Li J., Song J. Metformin prevents against oxidative stress-induced senescence in human periodontal ligament cells. Biogerontology. 2020;21:13–27. doi: 10.1007/s10522-019-09838-x. [DOI] [PubMed] [Google Scholar]
- 38.Camini F.C., da Silva Caetano C.C., Almeida L.T., de Brito Magalhães C.L. Implications of oxidative stress on viral pathogenesis. Arch. Virol. 2017;162:907–917. doi: 10.1007/s00705-016-3187-y. [DOI] [PubMed] [Google Scholar]
- 39.Poprac P., Jomova K., Simunkova M., Kollar V., Rhodes C.J., Valko M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017;38:592–607. doi: 10.1016/j.tips.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Sardaro N., Della Vella F., Incalza M.A., DI Stasio D., Lucchese A., Contaldo M., Laudadio C., Petruzzi M. Oxidative Stress and Oral Mucosal Diseases: An Overview. In Vivo. 2019;33:289–296. doi: 10.21873/invivo.11474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu T.Y., Zhang S.L., Dong G.Q., Liu X.Z., Wang X., Lv X.Q., Qian Q.J., Zhang R.Y., Sheng C.Q., Miao C.Y. Discovery and Characterization of Novel Small-Molecule Inhibitors Targeting Nicotinamide Phosphoribosyltransferase. Sci. Rep. 2015;5:10043. doi: 10.1038/srep10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yaghoobi M.M., Sheikoleslami M., Ebrahimi M. Effects of hydrogen peroxide, doxorubicin and ultraviolet irradiation on senescence of human dental pulp stem cells. Arch. Oral Biol. 2020;117:104819. doi: 10.1016/j.archoralbio.2020.104819. [DOI] [PubMed] [Google Scholar]
- 43.Villalobos L.A., Uryga A., Romacho T., Leivas A., Sanchez-Ferrer C.F., Erusalimsky J.D., Peiro C. Visfatin/Nampt Induces Telomere Damage and Senescence in Human Endothelial Cells. Int. J. Cardiol. 2014;175:573–575. doi: 10.1016/j.ijcard.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 44.Yang N.C., Song T.Y., Chang Y.Z., Chen M.Y., Hu M.L. Up-Regulation of Nicotinamide Phosphoribosyltransferase and Increase of NAD+ Levels by Glucose Restriction Extend Replicative Lifespan of Human Fibroblast Hs68 Cells. Biogerontology. 2015;16:31–42. doi: 10.1007/s10522-014-9528-x. [DOI] [PubMed] [Google Scholar]
- 45.Pi C., Yang Y., Sun Y., Wang H., Sun H., Ma M., Lin L., Shi Y., Li Y., Li Y., et al. Nicotinamide Phosphoribosyltransferase Postpones Rat Bone Marrow Mesenchymal Stem Cell Senescence by Mediating NAD(+)-Sirt1 Signaling. Aging. 2019;11:3505–3522. doi: 10.18632/aging.101993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Venkatachalam G., Surana U., Clément M.V. Replication stress-induced endogenous DNA damage drives cellular senescence induced by a sub-lethal oxidative stress. Nucleic Acids Res. 2017;45:10564–10582. doi: 10.1093/nar/gkx684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tilstra J.S., Robinson A.R., Wang J., Gregg S.Q., Clauson C.L., Reay D.P., Nasto L.A., St Croix C.M., Usas A., Vo N., et al. NF-kappaB inhibition delays DNA damage-induced senescence and aging in mice. J. Clin. Investig. 2012;122:2601–2612. doi: 10.1172/JCI45785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marshall M.V., Cancro L.P., Fischman S.L. Hydrogen peroxide: A review of its use in dentistry. J. Periodontol. 1995;66:786–796. doi: 10.1902/jop.1995.66.9.786. [DOI] [PubMed] [Google Scholar]
- 49.Walsh L.J. Safety issues relating to the use of hydrogen peroxide in dentistry. Aust. Dent. J. 2000;45:257–269. doi: 10.1111/j.1834-7819.2000.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 50.Zhu B., Macleod L.C., Newsome E., Liu J., Xu P. Aggregatibacter actinomycetemcomitans mediates protection of Porphyromonas gingivalis from Streptococcus sanguinis hydrogen peroxide production in multi-species biofilms. Sci. Rep. 2019;20:4944. doi: 10.1038/s41598-019-41467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watt B.E., Proudfoot A.T., Vale J.A. Hydrogen peroxide poisoning. Toxicol. Rev. 2004;23:51–57. doi: 10.2165/00139709-200423010-00006. [DOI] [PubMed] [Google Scholar]
- 52.Osorio F.G., Soria-Valles C., Santiago-Fernández O., Freije J.M., López-Otín C. NF-κB signaling as a driver of ageing. Int. Rev. Cell Mol. Biol. 2016;326:133–174. doi: 10.1016/bs.ircmb.2016.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated for this study are included in the article.