Abstract
Several pre-clinical and clinical trials show that exogenous pulmonary surfactant has clinical efficacy in inflammatory lung diseases, especially ARDS. By infecting type II alveolar cells, COVID-19 interferes with the production and secretion of the pulmonary surfactant and therefore causes an increase in surface tension, which in turn can lead to alveolar collapse. The use of the pulmonary surfactant seems to be promising as an additional therapy for the treatment of ARDS. COVID-19 causes lung damage and ARDS, so beneficial effects of surfactant therapy in COVID-19-associated ARDS patients are conceivable, especially when applied early in the treatment strategy against pulmonary failure. Because of the robust anti-inflammatory and lung protective efficacy and the current urgent need for lung-supportive therapy, the exogenous pulmonary surfactant could be a valid supportive treatment of COVID-19 pneumonia patients in intensive care units in addition to the current standard of ARDS treatment
Keywords: COVID-19, Lung infections, SARS-Cov-2, ARDS
1. Introduction
Acute Respiratory Distress Syndrome (ARDS) is a syndrome characterized by cardiogenic or non-cardiogenic respiratory failure, mild, moderate, or severe oxygenation impairment (Vassiliou et al., 2020;). Pathophysiologically, there is observed damage to the capillary endothelium and alveolar epithelium. It can also occur fluid accumulation in the alveolar space, reduced lung compliance, imbalanced lung ventilation flow ratio, decreased lung volume, and refractory dyspnea (Vassiliou et al., 2020; Bracco, 2020).
Dysfunction of the alveolar epithelium determines an alteration of the surface tension with consequent harmful gas exchange and lung lesions. During the mechanism of dysfunction of the alveolar epithelium, an important role is related to alveolar cells called type I and II. Type II contain secretory granular organelles fused with the cell membranes. These organelles, called lamellar-bodies, produce pulmonary surfactant andt is excreted into the alveolar space (Mirastschijski et al., 2020). If type II is damaged, production and secretion of pulmonary surfactant to the alveolar space will be considerably reduced and, consequently, lung ventilation flow (Zebialowicz Ahlström et al., 2019).
One of the most significant issues related to SARS-CoV-2 infection is its ability to affect the respiratory system: because of the high viral tropism for lung epithelial cells, it can colonize and replicate within type II cell (Mirastschijski et al., 2020). By infecting type II, COVID-19 interposes with the production and secretion of the pulmonary surfactant and therefore causes an increase in surface tension, leading to alveolar collapse. The tendency towards lung collapse is countered by inspiratory movement; however, inspiration simultaneously causes pressure in the interstitial space.
This phenomenon causes inflammatory liquids and molecules from the bloodstream to be called into the interstitial space, causing the onset of interstitial pneumonia and possibly acute respiratory distress syndrome (ARDS) (Zebialowicz Ahlström et al., 2019).
During the pandemic, (Gattinoni et al. (2020) have proposed two different phenotypes of COVID-19 patients, with different pathophysiology and progression. Type 1, “nonARDS”, patients had radiological features of SARS-CoV-2 pneumonia with almost regular respiratory system compliance, and severe hypoxemia is primarily due to high intrapulmonary shunting Gattinoni et al. (2020). On the other hand, Type 2 subjects are affected by severe ARDS with low compliance value Gattinoni et al. (2020). Theoretically, type 2 phenotype is due to the disease’s natural evolution and initial respiratory management, according to Gattinoni et al. and non-ARDS type may transit to ARDS-type by self-inflicted or ventilator-induced lung injury (Gattinoni et al. (2020)). Interestingly, Schousboe et al., starting from Gattinoni’s hypothesis, compared ARDS in SARS-CoV-2 to neonatal respiratory distress syndrome, caused by surfactant deficiency, suggesting an assessment of surfactant levels to the evaluation of COVID-19 patients (Bollag and Gonzales, 2020).
Because there are no specific antiviral treatments against SARS-CoV-2, barring the recent approval of remdesivir (Beigel et al., 2020), it is necessary to find alternative supportive symptomatic treatments to prevent ARDS and pulmonary failure, among the most common causes of COVID-19 mortality (Matera et al., 2020).
This work aims to summarize the current evidence on the use of exogenous pulmonary surfactant in ARDS with the purpose of highlighting the efficacy and safety of exogenous surfactant in reducing or preventing endotracheal intubation and the duration of mechanical ventilation in patients with COVID-19.
2. Methods
A literature search was performed using the PubMed database and the Cochrane library. Search terms included “pulmonary surfactant”, “COVID-19” and “Acute Respiratory Distress Syndrome”. The MeSH terms were “pulmonary surfactant” [All Fields] AND {“Acute Respiratory Distress Syndrome” [All Fields] OR (“COVID-19” [All Fields] AND “pulmonary surfactant” [All Fields]. A search period of 1 January 2010 to 15 January 2021 was selected to review narratively.
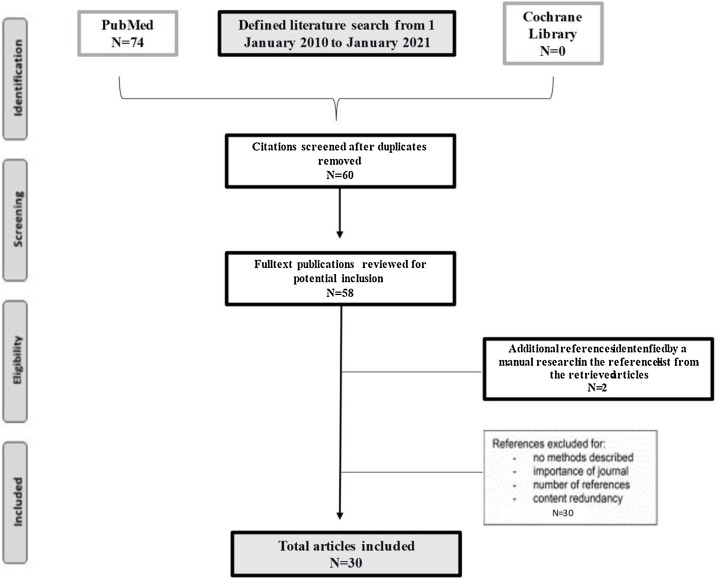
Seventy-four articles met the search criteria applied ( Fig. 1 ). Of these, 60 titles appeared to be relevant to pulmonary surfactant use in ARDS and 14 titles were excluded because they were not focused on the medical application of exogenous pulmonary surfactant. An additional thirty articles were then excluded after abstract review. Thirty articles were found to support the clinical efficacy of the exogenous surfactant in inflammatory lung diseases (Fig. 2 ).
Fig. 1.
Narrative literature review flow chart.
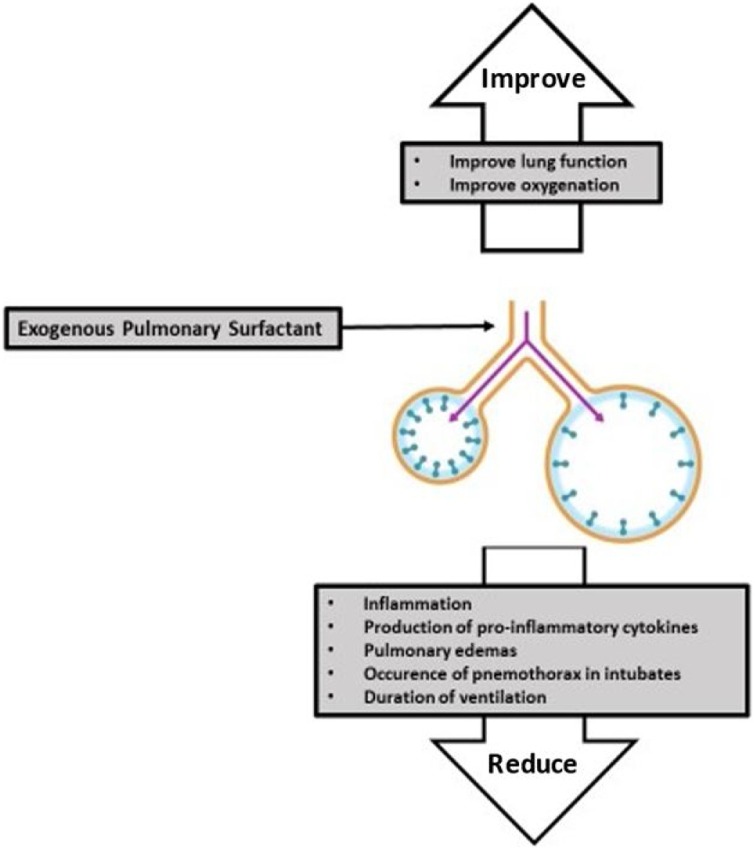
Fig. 2.
Valuable properties of Exogenous Pulmonary Surfactant.
3. Results
3.1. Use of pulmonary surfactant in ARDS
The use of exogenous surfactant in the treatment of ARDS has been investigated in several trials. Some of these studies support its use however, otherhave shown that there is no significant improvement. Nine articles were found to support the clinical efficacy of the exogenous surfactant in inflammatory lung diseases: i) Two of them are pre-clinical trials on animals (rabbits and lambs) (Zebialowicz Ahlström et al., 2019; Van Zyl and Smith, 2013); ii) three describe clinical trials on infants and children, two of which are meta-analyses (Chakraborty and Kotecha, 2013; More et al., 2014) and a comparative study (Liu et al., 2017); iii) three are meta-analyses (Meng et al., 2012, 2019; Zhang et al., 2013) describing the results obtained from several randomized clinical trials on adults; iv) one article is a retrospective case-control pilot study (Piva et al., 2021)
Ttwo articles were found to not support the exogenous surfactant's clinical efficacy in inflammatory lung diseases to improve mortality and oxygenation for adult ARDS patients (Bracco, 2020; Dushianthan et al., 2012).
Pre-clinical trials on animals showed how exogenous surfactant use resulted in improved lung function and decreased pulmonary oedema.
The first trial is a comparative study (Zebialowicz Ahlström et al., 2019) of two pulmonary surfactants, rSP-C33Leu (surfactant protein C analogue) and Curosurf® (poractant alfa), used to reduce pulmonary inflammation as a model of ARDS in rabbits ( Table 1 ). After induction of ARDS, 23 animals were allocated randomly to three groups: a) control group, n = 7: no surfactant treatment; b) treatment with Curosurf®, n = 8; or c) treatment with rSP-C33Leu surfactant, n = 8 (Zebialowicz Ahlström et al., 2019). Both preparations were administered with two intratracheal boluses, and the rabbits were subsequently ventilated for the next three hours (Zebialowicz Ahlström et al., 2019). In the control group, an air bolus was given instead of surfactant (Zebialowicz Ahlström et al., 2019). After treatment, the animals were set in a prone position, and physiological data were recorded every 30 min, considering blood gases and respiratory parameters (Zebialowicz Ahlström et al., 2019). Both surfactant preparations improved lung function and decreased inflammation, level of pro-inflammatory cytokines and formation of pulmonary oedema. Curosurf® showed better efficacy in improving lung parameters than rSP-C33Leu (Zebialowicz Ahlström et al., 2019).
Table 1.
Main features of Curosurf and Solnatide.
| Curosurf | Solnatide | |
|---|---|---|
| Generic name | Poractant alfa | AP301 |
| Origin and composition | Non-pyrogenic pulmonary surfactant Extract of natural porcine lung surfactant 99 % polar lipids (mainly phospholipids) and 1% hydrophobic low molecular weight proteins (surfactant associated proteins SP-B and SP-C) |
Synthetic peptide < 20 aminoacids Intended to activate the pulmonary sodium ion channels (ENaC) |
| Preservatives | No | No |
| Administration | Intratracheal use only | Orally inhaled |
| Indications | Rescue treatment of RDS in premature infants | Acute Respiratory Distress Syndrome (ARDS) and various forms of Pulmonary Oedema |
| Controindications | No specific controindications | No specific controindications |
| Off-label | Adult with ARDS | No |
| Compassionate Use | No | Yes |
| Population target | Premature infants | > 18 years old |
| PROs | Reduces mortality Reduces pneumothoraces associated with RDS |
Activate alveolar fluid clearance Reduce the leakage of blood and fluids from the capillaries in the air space Accelerating the dissolution of alveolar oedema Reduce barrier damage in the lungs Inhibits production of ROS and PKC-alpha activation Restores ENaC activity Protects and thus restores the barrier integrity of endothelial and epithelial cells |
| CONs | Risk fo adverse reactions such as bradycardia, hypotension, endotracheal tube blockage, and oxygen desaturation during administration | RCT trials ongoing |
| Drug ‘n’ drug interactions | Not available | Not available |
ARDS: acute respiratory distress syndrome; RDS: respiratory distress syndrome.
The second study (Van Zyl and Smith, 2013), performed on preterm lambs, compares Curosurf® with Synsurf®, a synthetic surfactant. In this randomized controlled trial (six lambs/group), the pharmacological substances have been dispensed at 100 mg/kg; normal saline was administered to the control group (Van Zyl and Smith, 2013). The respiratory and the lung function parameters were measured before and after the treatment for the next five hours (Van Zyl and Smith, 2013). Both surfactants resulted in better oxygenation (significant increase in PaO2 / FiO2 ratio) in the first 30 min after treatment, and two pharmacological substances have not demonstrated significant differences (Van Zyl and Smith, 2013).
Moreover, several clinical trials of surfactant replacement in infants and children have been conducted. Two meta-analyses (Chakraborty and Kotecha, 2013; ; More et al., 2014) describe the pulmonary surfactant benefits in neonatology. Preterm infants have immature lungs that produce an inadequate amount of surfactant to decrease the surface tension between air and alveoli and are more likely to develop ARDS, so they need respiratory support (Chakraborty and Kotecha, 2013; ;). The use of an exogenous pulmonary surfactant in intubated infants immediately after birth or after developing ARDS reduced the occurrence of pneumothorax, pulmonary interstitial emphysema and neonatal mortality (Chakraborty and Kotecha, 2013; ; More et al., 2014).
In a study of 60 infants (Liu et al., 2017), the use of intubation, mechanical ventilation and administration of pulmonary surfactant (experimental group, n = 30) is compared with the use of intubation and mechanical ventilation only (control group, n = 30). PaO2, PaCO2 and PaO2/FiO2 values were measured before and after treatment (Liu et al., 2017). The PaO2 level in both groups continually increased with the duration of time, starting from before treatment, then six hours after treatment and 12 h after treatment, and was highest at 24 h after treatment (Liu et al., 2017). The PaCO2 level in both groups continually decreased with time, starting from before treatment, then six hours after treatment and12 h after treatment, and was lowest at 24 h after treatment (Liu et al., 2017). The ratio of PaO2/FiO2 in the observational group and the control group continually increased with the duration of time, starting from before treatment, six hours after treatment and 12 h after treatment, and was highest at 24 h after treatment (Liu et al., 2017). The results show that levels of all parameters improved in the two groups, and the improvement effect in the experimental group was better than in the control group (Liu et al., 2017).
In addition to animal and infants studies, several studies are supporting the use of exogenous surfactant in adults. Three meta-analyses (Meng et al., 2012, 2019; Zhang et al., 2013) describe the results obtained from several randomized clinical studies. These studies included the administration of the exogenous pulmonary surfactant in adults with acute lung injury and ARDS.
Mortality 28 or 30 days after randomization was the first outcome, the secondary was the change in the ratio of PaO2/FiO2 in the first 24 h or after 120 h, the number of ventilation-free days and any adverse effects (Meng et al., 2012, 2019; Zhang et al., 2013). The use of pulmonary surfactant improved oxygenation during the first 24 h after treatment and appeared to reduce the duration of ventilation (Meng et al., 2012, 2019; Zhang et al., 2013).
A recent retrospective study investigated the feasibility, efficacy and safety of off-label use of Curosurf on COVID-19 positive ARDS adult patients as well as duration of mechanical ventilation and mortality (Piva et al., 2021).
Although the study showed how there is no evidence of acute decompensation after the installation of surfactants and mortality has shown 28 days; there were no acute effects decompensation on patients treated (Piva et al., 2021). The data are still preliminary and limited. Further randomized clinical trials are needed (Piva et al., 2021).
The different results between the treatment of preterm infants and adults could be due to the different aetiology of lung damage, the method and time of administration of the surfactant, or the dose and kind of surfactant used. Additional studies are needed in adults to obtain conclusive results.
3.2. Use of exogenous pulmonary surfactant and other drug in clinical practice for ARDS
In the clinical practice of several hospitals, the treatment of respiratory distress syndrome (RDS) in preterm infants and the prophylaxis in preterm infants with the risk of developing RDS is based on the use of Curosurf® (2021).
Currently, no specific contraindications to the use of Curosurf® or interactions with other drugs are known. Most of the adverse effects reported in the data sheet occur with uncommon or rare frequency (Curosurf®, 2021).
From the technical data sheet, Curosusf® is indicated for the treatment of preterm infants with RDS but an off-label use could be indicated for adults with ARDS (Curosurf®, 2021).
Kumar describe a novel and innovative physical therapeutic intervention for COVID-19 related ARDS (Kumar, 2020): co-somministration of exogenous pulmonary surfactant and ambroxol may provide synergistic benefits. The prolonged presence of ambroxol in lungs can stimulate phospholipid synthesis and plays a role in generation and secretion of pulmonary surfactant (Kumar, 2020). Moreover, ambroxol can increases the expression levels of SP-B and SP-C and hence may contribute to the interfacial turnover of the surfactant (Kumar, 2020).
Another but new drug is Solnatide, a synthetic peptide composed of 16 amino acids normally found in nature, understudy for the treatment of ARDS ( Table 1) (Anon, 2021).
Solnatide deactivates the pulmonary sodium ion channels (ENaC), thus accelerating the dissolution of alveolar edema. Besides, Solnatide inhibits the production of reactive species of oxygen (ROS).
The drug reduces the level of myosin light chain (MLC) phosphorylation, thus protecting and restoring the integrity of the barrier of endothelial and epithelial cells. It has no pro-inflammatory activity and does not lead to increased chemokine production or increased infiltration of neutrophils (Anon, 2021). A clinical study evaluated the local and systemic safety of multiple sequential ascending doses of Solnatide inhaled for seven days orally (Anon, 2021). This is a phase IIb, randomized, placebo-controlled, double-blind, dose-escalation study. Patients in this trial had moderate to severe pulmonary permeability edema and ARDS. This trial reviewed the potential efficacy endpoints for a future phase III study (NCT03567577) (Anon, 2021). Solnatide deactivates the pulmonary sodium ion channels (ENaC), thus accelerating the dissolution of alveolar edema (Anon, 2021). Besides, Solnatide inhibits the production of reactive species of oxygen (ROS) (Anon, 2021). The drug reduces the level of myosin light chain (MLC) phosphorylation, thus protecting and restoring the integrity of the barrier of endothelial and epithelial cells (Anon, 2021). It has no pro-inflammatory activity and does not lead to increased chemokine production or increased infiltration of neutrophils (Anon, 2021).
Agenzia Italiana del Farmaco (AIFA) has authorized the compassionate use program with Solnatide for the treatment of pulmonary permeability edema in COVID-19 patients with acute pulmonary failure. Solnatide is a drug currently unauthorized, but in clinical development for the treatment of acute respiratory distress syndrome (ARDS) and pulmonary edema as reported above (Veldhuizen et al., 2020).
4. Discussion
Pulmonary surfactant is composed of 90 % lipids and 10 % proteins (Bracco, 2020; ; ; Mirastschijski et al., 2020): i) Lipids: 80 % phosphatidylcholine (of which about 40 % is dipalmitoylphosphatidylcholine), 10 % phosphatidylglycerol and small quantities of phosphatidic acid, phosphatidylinositol, phosphatidylethanolamine and cholesterol, ii) Proteins: four specific surfactant proteins (SPs); SP-A, SP-B, SP-C and SP-D (Bracco, 2020; ; ; Mirastschijski et al., 2020). Surfactant works by lowering superficial-tension between air and liquid interface within the alveoli of the lungs (Bracco, 2020; ; ; Mirastschijski et al., 2020). Lowering the surface tension decreases breathing and prevents alveolar collapse at the end of expiration (Bracco, 2020; ; ; Mirastschijski et al., 2020). Surfactant’s components are bio-synthesised and processed in particular cellular structures of the type II cells like Golgi bodies and endoplasmic reticulum (Chakraborty and Kotecha, 2013; More et al., 2014). The transport and the storage of surfactant lipids and proteins, except SP-A, occur in lamellar bodies, after going through maturation cell process (Chakraborty and Kotecha, 2013; More et al., 2014).
The release of the surfactant into the alveolar space is the result of a mechanism of exocytosis that involves the membranes of the lamellar bodies which fuse with the membranes of the epithelial cells (Chakraborty and Kotecha, 2013; More et al., 2014). In lungs, the surfactant reservoir is represented by a structure called tubular myelin inside which phospholipids and surfactant proteins, particularly SP-A, both assemble; tubular myelin plays an essential role the alveolar breathing process and improves the insertion of lipids into the air-liquid interface (Chakraborty and Kotecha, 2013; More et al., 2014). During the breathing process, high pressures in low lung volumes favour the desorption of surfactant lipids. A portion of desorbed lipid follows the following steps: it is recycled by type II cells, endocytosed through multivesicular bodies and finally stored in lamellar bodies and secreted. Other elements of the surfactant can be recovered in tubular myelin through an extracellular process; macrophages absorb the rest for degradation (Chakraborty and Kotecha, 2013; More et al., 2014).
Several pre-clinical and clinical trials show that exogenous pulmonary surfactant has clinical efficacy in inflammatory lung diseases, especially ARDS (Zebialowicz Ahlström et al., 2019; Van Zyl and Smith, 2013; Chakraborty and Kotecha, 2013; ; More et al., 2014; Liu et al., 2017; Meng et al., 2012, 2019; Zhang et al., 2013). Pre-clinical trials on rabbits and lambs show that pulmonary surfactants improve lung function and reduce inflammation, production of pro-inflammatory cytokines, and pulmonary oedema, leading to significant improvement in oxygenation (Zebialowicz Ahlström et al., 2019; Van Zyl and Smith, 2013). Clinical trials on infants show that using an exogenous pulmonary surfactant in intubated infants immediately after birth or after developing ARDS significantly reduces the occurrence of pneumothorax, pulmonary interstitial emphysema and neonatal mortality and improves the levels of oxygen parameters (Chakraborty and Kotecha, 2013; More et al., 2014). Randomised clinical trials on adults show that the administration of pulmonary surfactant improves oxygenation during the first 24 h after treatment and appears to reduce the duration of ventilation. The use of this preparation in adults is not associated with reduced mortality or reduced ventilation duration. However, several studies have reported how surfactant administration has not been shown to improve mortality (Bracco, 2020) and oxygenation for adult ARDS patients (Bracco, 2020; Dushianthan et al., 2012).
There is no convincing proof that surfactant in COVID-19 patients is dysfunctional (Veldhuizen et al., 2020). However, indirect evidence indicates that surfactant disorders play a significant part theoretically in COVID-19 lung dysfunction (Zuo et al., 2020; Mason, 2020a, b; Islam and Khan, 2020). We know that SARS-CoV-2 infects the type II alveolar surfactant cells and surfactant proteins have been downregulated because of SARS-CoV-2 infections (Zuo et al., 2020; Mason, 2020a, b; Islam and Khan, 2020). Furthermore, current evidence stated benefits on surfactant use in ARDS if administered a highly functional exogenous surfactant preparation in early respiratory failure, near to the beginning of mechanical ventilation if not combined with it (Lewis and Veldhuizen, 2003). Centred on the above considerations, several groups have undertaken studies to hypothesise exogenous surfactant value in COVID-19 patients (Grocott, 2020; Lewis, 2020; Lenclud, 2020; Keller and Huang, 2020; Howard, 2020).
The use of the pulmonary surfactant seems to be promising as an additional therapy for the treatment of ARDS. COVID-19 causes lung damage and ARDS, so the use of pulmonary surfactant as Curosurf® could be hypothesized for the treatment of this viral infection, therefore be beneficial if applied in the early stages of the treatment strategy against pulmonary insufficiency. Furthermore the therapeutic combination of surfactant and ambroxol could be a potential approach an urgent and immediate COVID-19 clinical trial (Bollag and Gonzales, 2020).
5. Conclusions
ARDS is a syndrome characterized by non-cardiogenic respiratory failure, to mild, moderate, or severe oxygenation impairment. Despite the treatments used as lung ventilation, morbidity and mortality remains high. Surfactant dysfunction is a characteristic of patients with ARDS. Exogenous surfactant replacement in animal models of ARDS and neonatal RDS shows consistent improvements in gas exchange and survival. However, several adult studies have shown improved oxygenation but no survival benefit. The different results between the treatment of preterm infants and adults could be due to the different etiology of lung damage. Dose and delivery method, type of surfactant used are some of the limitations of published surfactant replacement clinical trials. (Meng et al., 2012) Further studies are needed in adults to obtain conclusive results. However, since ARDS are among the complications of COVID-19, the use of exogenous surfactant or Solnatide can be hypothesized for the treatment of this viral infection.
Declaration of Competing Interest
There are no conflicts of interest to report for any authors.
References
- https://www.aifa.gov.it/programmi-di-uso-compassionevole-covid-19.
- Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of Covid-19 - final report. N. Engl. J. Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag W.B., Gonzales J.N. Phosphatidylglycerol and surfactant: a potential treatment for COVID-19? Med. Hypotheses. 2020;144(November) doi: 10.1016/j.mehy.2020.110277. Epub 2020 Sep 16. PMID: 33254581; PMCID: PMC7493731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracco L. Covid-19, type II alveolar cells and surfactant . J Med - Clin Res & Rev. 2020;4(4):1–3. [Google Scholar]
- Chakraborty M., Kotecha S. Pulmonary surfactant in newborn infants and children. Breathe. 2013;9(December 6) doi: 10.1183/20734735.006513. [DOI] [Google Scholar]
- Curosurf® 2 vials 1,5 ml 80 mg/ml (120 mg/vial) italian data sheet - https://www.codifa.it/cont/codica-ricerca/27/ricerca.asp?id_scheda=3663.
- Dushianthan A., Cusack R., Goss V., Postle A.D., Grocott M.P. Clinical review: Exogenous surfactant therapy for acute lung injury/acute respiratory distress syndrome--where do we go from here? Crit. Care. 2012;16(November 6):238. doi: 10.1186/cc11512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L., Chiumello D., Rossi S. COVID-19 pneumonia: ARDS or not? Crit. Care. 2020;24(April 1):154. doi: 10.1186/s13054-020-02880-z. PMID: 32299472; PMCID: PMC7160817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grocott M.P. 2020. A Clinical Trial of Nebulized Surfactant for the Treatment of Moderate to Severe COVID-19 (COVSurf). ClinicalTrials.gov identifier NCT04362059. [Google Scholar]
- Howard C. 2020. Poractant Alfa - Curosurf and SARS-COV-19 ARDS (Covid- 19) ClinicalTrials.gov identifierNCT04502433. [Google Scholar]
- Islam Abmmk, Khan M.-A.-A.-K. Lung transcriptome of a COVID-19 patient and systems biology predictions suggest impaired surfactant production which may be druggable by surfactant therapy. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-76404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S., Huang Y.C.T. 2020. The Safety and Preliminary Efficacy of Lucinactant in Adults With COVID-19. ClinicalTrials.gov identifierNCT04389671. [Google Scholar]
- Kumar P. Co-aerosolized Pulmonary Surfactant and Ambroxol for COVID-19 ARDS Intervention: What Are We Waiting for? Front. Bioeng. Biotechnol. 2020;25(September 8) doi: 10.3389/fbioe.2020.577172. PMID: 33102461; PMCID: PMC7546362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenclud C. 2020. Curosurf® in Adult Acute Respiratory Distress Syndrome Due to COVID-19 (Caards-1) ClinicalTrials.gov identifierNCT04384731. [Google Scholar]
- Lewis J. 2020. London’s Exogenous Surfactant Study for COVID19 (LESSCOVID). ClinicalTrials.gov identifier NCT04375735. [Google Scholar]
- Lewis J.F., Veldhuizen R. The role of exogenous surfactant in the treatment of acute lung injury. Annu. Rev. Physiol. 2003;65(1):613–642. doi: 10.1146/annurev.physiol.65.092101.142434. [DOI] [PubMed] [Google Scholar]
- Liu J., Liu G., Wu H., et al. Efficacy study of pulmonary surfactant combined with assisted ventilation for acute respiratory distress syndrome management of term neonates. Exp. Ther. Med. 2017;14(September 3):2608–2612. doi: 10.3892/etm.2017.4839. Epub 2017 Jul 25. PubMed PMID: 28947918; PubMed Central PMCID: PMC5609315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R.J. Thoughts on the alveolar phase of COVID-19. Am. J. Physiol. Lung Cell Mol. Physiol. 2020;319(1):L115–L120. doi: 10.1152/ajplung.00126.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. J. 2020;55(4):4. doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matera M.G., Rogliani P., Calzetta L., Cazzola M. Pharmacological management of COVID-19 patients with ARDS (CARDS): a narrative review. Respir. Med. 2020;171(September) doi: 10.1016/j.rmed.2020.106114. Epub 2020 Aug 4. PMID: 32795902; PMCID: PMC7402220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H., Sun Y., Lu J., et al. Exogenous surfactant may improve oxygenation but not mortality in adult patients with acute lung injury/acute respiratory distress syndrome: a meta-analysis of 9 clinical trials. JCardiothoracVascAnesth. 2012;26(October 5):849–856. doi: 10.1053/j.jvca.2011.11.006. Epub 2012 Jan 20. PubMed PMID: 22265270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng S.S., Chang W., Lu Z.H., Xie J.F., Qiu H.B., Yang Y., Guo F.M. Effect of surfactant administration on outcomes of adult patients in acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. BMC Pulm. Med. 2019;19(January 1):9. doi: 10.1186/s12890-018-0761-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirastschijski U., Dembinski R., Maedler K. Lung surfactant for pulmonary barrier restoration in patients with COVID-19 pneumonia. Front Med (Lausanne). 2020;22(May 7):254. doi: 10.3389/fmed.2020.00254. PMID: 32574339; PMCID: PMC7256165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- More K., Sakhuja P., Shah P.S. Minimally invasive surfactant administration in preterm infants: a meta-narrative review. JAMA Pediatr. 2014;168(October 10):901–908. doi: 10.1001/jamapediatrics.2014.1148. Review. PubMed PMID: 25089718. [DOI] [PubMed] [Google Scholar]
- Piva S., DiBlasi R.M., Slee A.E., et al. Surfactant therapy for COVID-19 related ARDS: a retrospective case-control pilot study. Respir. Res. 2021;22(January 1):20. doi: 10.1186/s12931-020-01603-w. PMID: 33461535; PMCID: PMC7812332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zyl J.M., Smith J. Surfactant treatment before first breath for respiratory distress syndrome in preterm lambs: comparison of a peptide-containing synthetic lung surfactant with porcine-derived surfactant. Drug Des. Devel. Ther. 2013;29(August 7):905–916. doi: 10.2147/DDDT.S47270. eCollection 2013. PubMed PMID: 24039400; PubMed Central PMCID: PMC3769412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassiliou A.G., Kotanidou A., Dimopoulou I., Orfanos S.E. Endothelial damage in acute respiratory distress syndrome. Int. J. Mol. Sci. 2020;21(November 22):8793. doi: 10.3390/ijms21228793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen R.A.W., Zuo Y.Y., Petersen N.O., Lewis J.F., Possmayer F. The COVID-19 pandemic: a target for surfactant therapy? Expert Rev. Respir. Med. 2020;28(December):1–12. doi: 10.1080/17476348.2021.1865809. Epub ahead of print. PMID: 33331197. [DOI] [PubMed] [Google Scholar]
- Zebialowicz Ahlström J., Massaro F., Mikolka P., et al. Synthetic surfactant with a recombinant surfactant protein C analogue improves lung function and attenuates inflammation in a model of acute respiratory distress syndrome in adult rabbits. Respir. Res. 2019;20(November 1):245. doi: 10.1186/s12931-019-1220-x. PubMed PMID: 31694668; PubMed Central PMCID: PMC6836435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.N., Sun J.P., Xue X.Y., et al. Exogenous pulmonary surfactant for acute respiratory distress syndrome in adults: a systematic review and meta-analysis. ExpTher Med. 2013;5(January 1):237–242. doi: 10.3892/etm.2012.746. Epub 2012 Oct 15. PubMed PMID: 23251275;PubMed Central PMCID: PMC3524286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y.Y., Uspal W.E., Wei T. Airborne transmission of COVID-19: aerosol dispersion, lung deposition, and virus-receptor interactions. ACS Nano. 2020 doi: 10.1021/acsnano.0c08484. [DOI] [PubMed] [Google Scholar]