Abstract
Objective
To identify risk factors associated with nonmelanoma skin cancer (NMSC) occurrence and survival in children.
Study design
This was a multicenter, retrospective, case-control study of patients <20 years of age diagnosed with NMSC between 1995 and 2015 from 11 academic medical centers. The primary outcome measure was frequency of cases and controls with predisposing genetic conditions and/or iatrogenic exposures, including chemotherapy, radiation, systemic immunosuppression, and voriconazole.
Results
Of the 124 children with NMSC (40 with basal cell carcinoma, 90 with squamous cell carcinoma), 70% had at least 1 identifiable risk factor. Forty-four percent of the cases had a predisposing genetic condition or skin lesion, and 29% had 1 or more iatrogenic exposures of prolonged immunosuppression, radiation therapy, chemotherapy, and/or voriconazole use. Prolonged immunosuppression and voriconazole use were associated with squamous cell carcinoma occurrence (cases vs controls; 30% vs 0%, P = .0002, and 15% vs 0%, P = .03, respectively), and radiation therapy and chemotherapy were associated with basal cell carcinoma occurrence (both 20% vs 1%, P < .0001). Forty-eight percent of initial skin cancers had been present for >12 months prior to diagnosis and 49% of patients were diagnosed with ≥2 skin cancers. At last follow-up, 5% (6 of 124) of patients with NMSC died. Voriconazole exposure was noted in 7 cases and associated with worse 3-year overall survival ( P = .001).
Conclusions
NMSC in children and young adults is often associated with a predisposing condition or iatrogenic exposure. High-risk patients should be identified early to provide appropriate counseling and management.
Failure to recognize risk factors for nonmelanoma skin cancer (NMSC) in children can result in delays in diagnosis and increased tumor burden. In contrast to adults, where chronic sun exposure is a primary risk factor, NMSC has been reported in children with heritable and congenital conditions, such as tumor syndromes, photosensitivity disorders, and various birthmarks.1–3 Moreover, pediatric NMSC is considered rare and is not otherwise well described. A recent, retrospective study of a small cohort of pediatric patients identified iatrogenic exposures of prolonged immunosuppression, radiation therapy, chemotherapy, and voriconazole in 46% of patients with NMSC.4 Iatrogenic risk factors associated with occurrence of NMSC are established in the adult literature and include exposure to prolonged immunosuppression, radiation therapy, and voriconazole therapy.5–9 Given the increasing number of long-term survivors from childhood cancer and other chronic medical conditions, pediatric cases of NMSC because of iatrogenic factors may be more common than previously reported.10–12 In this multicenter, case-control study, we investigated characteristics of NMSC in children and young adults and identified risk factors associated with NMSC occurrence and overall survival. We also explored access to dermatologic care in these patients.
Methods
The Dana-Farber Cancer Institute institutional review board (protocol #15–156) approved this study (primary site), as well as the institutional review boards did at 10 other institutions. Reliance and data use agreements were established between Dana-Farber Cancer Institute and each additional site.
Study Design
This was a multicenter, retrospective, case-control study. At each site, all patients less than 20 years of age upon initial histopathologic diagnosis of squamous cell carcinoma (SCC) or basal cell carcinoma (BCC) between January 1, 1995, and June 30, 2015, were identified. Patients with no clinical information in the electronic medical record were excluded from the study.
Controls were selected from patients seen in the Dermatology Program at Boston Children’s Hospital from 2000 to 2015 without a history of skin cancer. Controls were matched to cases at a 1:1 by sex and age group (1–5 years, 6–10 years, 11–15 years, and 16–19 years old).
Medical records were reviewed and nonidentifiable data were entered into a REDCap database (Vanderbilt University, Tennessee). Demographic data were obtained including age at diagnosis, sex, and race. Additional data obtained from records included past medical history (such as history of malignancy, stem cell or organ transplantation, genetic syndrome, or birthmark), medication history (focusing on chemotherapeutic and immunosuppressive agents, and voriconazole), and radiation exposure (total body irradiation and localized radiation therapy in cGy). Histopathology records and outcomes, including skin cancer directed therapies, response to therapy, and death, were recorded.
Study Definitions
Unless otherwise specified in this paper, the term SCC includes SCC in situ, SCC invasive, and keratoacanthomas. Chemotherapy was defined as a medication used for treatment of cancer and excluded agents that were employed as part of a transplant conditioning regimen, as exposure to conditioning agents is very short term, and not comparable with treatment regimens of chemotherapy. Prolonged immunosuppression was defined as systemic immunosuppressive therapy for >6 months.
Statistical Analyses
Descriptive analyses were performed to summarize patient demographics and disease status. Proportions of patients with predisposing genetic conditions, predisposing skin lesions, and iatrogenic factors were calculated for each type of skin cancer. χ2 or Fisher exact tests were used to identify the association between a risk factor and the occurrence of skin cancer; recurrence of skin cancer; or a subsequent new primary skin cancer (defined as a diagnosis different from the initial skin cancer diagnosis). Overall survival time was calculated from initial skin diagnosis until death, or last contact if the patient was alive. Survival curves were generated using the methods of Kaplan and Meier, with standard errors according to Greenwood. Survival curves were compared using a log-rank test. P values of < .05 were considered statistically significant.
Results
Patients with an initial histopathologic diagnosis of NMSC (n = 124) were identified as cases. Forty cases had at least 1 SCC, 95 patients had at least 1 BCC, 11 patients had both BCC and SCC, and 9 patients had a diagnosis of melanoma in addition to NMSC. There were a total of 856 skin cancers, including 716 BCC, 131 SCC (36 in situ, 87 invasive lesions, and 8 keratoacanthomas), and 9 melanomas among patients with NMSC; 49% of cases were diagnosed with ≥2 skin cancers (median: 1, range: 1–126).
Demographics
The median age at initial NMSC diagnosis was 14.1 years (range 2.2–19.8) in patients with SCC and 12.2 years (range 0.9–19.8) in patients with BCC (Table I); 28% (11 of 40) of patients with SCC and 44% (42 of 95) of patients with BCC were 10 years or younger at time of initial diagnosis. Of those with data available, 33% (11 of 33) of patients with SCC and 24% (18 of 74) of patients with BCC were non-Hispanic white.
Table I.
Demographic and clinical characteristics of NMSC cases (n = 124)
| n (%) |
|||
|---|---|---|---|
| Characteristics | Patients with SCC (n = 40)* | Patients with BCC (n = 95)* | Overall (n = 124) |
| Age at diagnosis in y (median, range) | 14.1 (2.2–19.8) | 12.2 (0.9–19.8) | 13.2 (0.9–19.8) |
| Follow-up time after initial skin cancer in years† [n, median (range)] | n = 36, 4 (0–20.7) | n = 92, 3.3 (0.0–22.1) | n = 118, 3.4 (0.0–22.1) |
| Age at diagnosis | |||
| 0–5 y | 6 (15) | 12 (13) | 15 (12) |
| 6–10 y | 5 (13) | 30 (32) | 33 (27) |
| 11–15 y | 17 (43) | 29 (31) | 44 (35) |
| >15 y | 12 (30) | 24 (25) | 32 (26) |
| Sex | |||
| Male | 18 (45) | 44 (46) | 58 (47) |
| Female | 22 (55) | 51 (54) | 66 (53) |
| Race | |||
| White | 22/33 (67) | 56/74 (76) | 75/99 (76) |
| Black | 2/33 (6) | 5/74 (7) | 5/99 (5) |
| Hispanic | 6/33 (18) | 7/74 (9) | 11/99 (11) |
| Asian | 1/33 (3) | 4/74 (5) | 4/99 (4) |
| Middle-Eastern | 2/33 (6) | 1/74 (1) | 3/99 (3) |
| American Indian | 0/33 (0) | 1/74 (1) | 1/99 (1) |
| Unknown | 7 | 21 | 25 |
| Life status at last follow-up | |||
| Dead | 4 (10) | 3 (3) | 6 (5) |
| Alive | 35 (88) | 87 (92) | 112 (90) |
| Unknown | 1 (2) | 5 (5) | 6 (5) |
| Predisposing genetic conditions | 18 (45) | 47 (49) | 55 (44) |
| Basal cell nevus syndrome | 1 (3) | 34 (36) | 34 (27) |
| Bloom syndrome | 0 | 1 (1) | 1 (1) |
| Dyskeratosis congenita | 2 (5) | 0 (0) | 2 (2) |
| Epidermolysis bullosa | 1 (3) | 0 (0) | 1 (1) |
| Incontinentia pigmenti | 1 (3) | 0 (0) | 1 (1) |
| Primary immunodeficiency | 2 (5) | 1 (1) | 2 (2) |
| Xeroderma pigmentosum | 11 (28) | 11 (12) | 14 (11) |
| Predisposing skin lesions | 3 (8) | 10 (11) | 12 (10) |
| Chronic ulcer | 1 (3) | 0 (0) | 1 (1) |
| Epidermolysis bullosa | 1 (3) | 0 (0) | 1 (1) |
| Morphea | 1 (3) | 0 (0) | 1 (1) |
| Nevus sebaceus | 0 (0) | 9 (9) | 9 (7) |
| Wart | 1 (3) | 1 (1) | 1 (1) |
| Iatrogenic exposures | 14 (35) | 25 (26) | 36 (29) |
| Prolonged immunosuppression | 12 (30) | 5 (5) | 16 (13) |
| Radiation therapy | 5 (13) | 19 (20) | 21 (17) |
| Chemotherapy | 3 (8) | 19 (20) | 21 (17) |
| Voriconazole use | 6 (15) | 2 (2) | 7 (6) |
| Other pertinent medical history | |||
| Prior oncologic diagnosis | 7/38 (18) | 24/92 (26) | 28/120 (23) |
| HSCT | 6 (15) | 8 (8) | 13/124 (10) |
| Organ transplant | 6 (15) | 2 (2) | 7/124 (6) |
| No identifiable risk factors‡ | 12 (30) | 26 (27) | 37/124 (30) |
Groups are not mutually exclusive. Denominators are 40 for the SCC group, 95 for the BCC group, 11 for SCC and BCC group, 9 for the melanoma group unless otherwise specified.
Censored patients are those who have not died at the time of last contact.
No identifiable risk factors: Defined as having no predisposing genetic conditions, no predisposing skin lesions, and no iatrogenic exposures.
Clinical Characteristics and Access to Dermatologic Care
Of all NMSC cases, 70% (87 of 124) had a genetic condition, skin lesion, and/or iatrogenic exposure known to increase risk for NMSC; 30% had no identifiable risk factors.
Forty-four percent of patients had a genetic condition, and 10% had a primary skin lesion associated with increased risk for NMSC. The most common genetic conditions were basal cell nevus syndrome in 36% (34 of 95) of patients with BCC and xeroderma pigmentosum in 28% (11 of 40) of patients with SCC. In addition, 29% of all patients with NMSC had exposure to 1 or more medical treatments (iatrogenic exposures) increasing their risk for skin cancer, including prolonged systemic immunosuppression, radiation therapy, chemotherapy, and voriconazole. Our case sample included 23% (28 of 120) of patients with history of oncologic conditions, 10% (13 of 124) with hematopoietic stem cell transplantation (HSCT), and 6% (7 of 124) with solid organ transplants (Table I).
There were 14 patients with both iatrogenic exposures and predisposing genetic conditions. Genetic conditions of these 14 patients were basal cell nevus syndrome (8), Bloom syndrome (1), dyskeratosis congenita (1), xeroderma pigmentosum (2), and primary immunodeficiency (2).
Excluding patients with both predisposing genetic conditions and iatrogenic exposures, the median age of initial NMSC diagnosis in patients with predisposing genetic conditions was significantly lower than that of patients with iatrogenic exposures (9.2 years [0.9–19.4, n = 41] vs 15.4 years [5.6–19.7, n = 22], P = .001).
Median time from known onset of skin lesion to diagnosis of NMSC was 9 months (0–127, n = 48 patients with known data). Fifty-eight percent (28 of 48) of initial skin cancers had been present for >6 months prior to diagnosis, and 48% (23 of 48) of initial skin cancers had been present for >12 months prior to diagnosis. Ninety-three percent (100 of 108) were diagnosed with their initial skin cancer by a dermatologist. Forty-four percent (24 of 54) had never been seen by a dermatologist prior to skin cancer diagnosis, including 32% (6 of 19) of those with iatrogenic exposures. Twenty-six percent (32 of 124) of patients had pre-existing skin lesions suggestive of photodamage or cancer risk, including 21 with actinic keratoses, 10 with atypical nevi, 10 with lentigos, and 2 with porokeratoses (these conditions were not mutually exclusive).
Iatrogenic Risk Factors
Tests of association of risk factors for the occurrence of skin cancer are detailed in Table II. Patients with SCC were significantly more likely to have received (1) prolonged immunosuppression; or (2) voriconazole, than age- and sex-matched controls (30% vs 0%, P = .0002 and 15% vs 0%, P = .026, respectively). Patients with BCC were significantly more likely to have received (1) radiation therapy; or (2) chemotherapy, than age- and sex-matched controls (20% vs 1%, P ≤ .0001, and 20% vs 1%, P < .0001, respectively). Of the 19 patients with BCC who received chemotherapy, 78.9% (15 of 19) also received radiation therapy. All (9 of 9) patients with SCC with iatrogenic exposures had only a history of prolonged immunosuppression, and 85% (11 of 13) of patients with BCC with iatrogenic exposures had only a history of radiation therapy.
Table II.
Tests of association of risk factors for the occurrence of skin cancer, within patients with SCC (n = 40) and within patients with BCC (n = 95)
| Risk factors | n (%) |
P value† | n (%) |
P value† | ||
|---|---|---|---|---|---|---|
| SCC (n = 40)* | Control (n = 40)* | BCC (n = 95)* | Control (n = 95) * | |||
| Prolonged immunosuppression | 12 (30) | 0 (0) | .0002 | 5 (5) | 1 (1) | .2 |
| Voriconazole use | 6 (15) | 0 (0) | .03 | 2 (2) | 0 (0) | .5 |
| Radiation therapy | 5 (13) | 0 (0) | .055 | 19 (20) | 1 (1) | <.0001 |
| Chemotherapy | 3 (8) | 0 (0) | .2 | 19 (20) | 1 (1) | <.0001 |
| Prior oncologic diagnosis | 7/38 (18) | 0/39 (0) | .005 | 90/93 (97) | 68/92 (74) | <.0001 |
| HSCT | 6 (15) | 0 (0) | .03 | 8 (8) | 0 (0) | .007 |
| Organ transplant | 6 (15) | 0 (0) | .03 | 2 (2) | 0 (0) | .5 |
Bold values are P < 0.05 that are statistically significant.
Denominator is 40 for patients with SCC and matched controls, and 95 for patients with BCCs and matched controls unless otherwise specified.
Using Fisher exact test.
Of 16 patients who received prolonged immunosuppressive therapy, median duration of immunosuppression was 6.3 years (1.3–15.3, n = 10). Of 7 patients known to have received voriconazole therapy, median duration was 2.8 years (0–5, n = 6). Of 21 patients who received radiation therapy, 17 received localized radiation therapy with a median total cumulative dose of 2400 cGy (54–12640, n = 13). Eighty-two percent (14 of 17) of patients with BCC and known exposure to localized radiation therapy developed BCC at the site of radiation therapy.
Of 13 patients who received HSCT, median time from transplant to skin cancer diagnosis was 7.8 years (2.3–13.5, n = 13). Of 7 patients known to have received organ transplant, median time from transplant to skin cancer diagnosis was 6.1 years (1–15.3, n = 6).
Skin Cancer Characteristics
The most common locations for all forms of SCC (n = 53) were the face (43%), leg (19%), and scalp (15%). The most common locations for BCC (n = 95) were the face (42%), scalp (31%), and back (22%).
Of those with invasive SCC (n = 29), 10% of patients (3 of 29) had lymph node involvement, and 7% (2 of 29) had metastases. All 3 patients with lymph node involvement had histopathologic evidence of perineural invasion, and one of them had invasion beyond fat. One patient with metastatic disease had perineural invasion and invasion beyond fat; this patient also had lymph node involvement.
Outcomes
For patients who were alive at last contact, overall median follow-up time was 3.4 years (0–22.1, n = 118) (4 years [0–20.7, n = 36] in SCC; 3.3 years [0–22.1, n = 92] in BCC). There were no significant associations between risk factors and subsequent skin cancer (data not shown).
Death was reported in 6 patients; 3 patients with SCC, 2 patients with BCC, and 1 with both BCC and SCC. One death was related to skin cancer or associated therapies, 4 deaths were related to other causes, and the cause of death in 1 case was not recorded. Patients treated with voriconazole had lower overall survival compared with patients who were not treated with voriconazole (P = .001) (Table III).
Table III.
Identification of risk factors prognostic of overall survival in NMSC cases (n = 123)*
| Risk factors | n/N (%)† | 5-y Overall survival ± SE (%) | P value‡ |
|---|---|---|---|
| Prolonged immunosuppression | |||
| Yes | 16 (13) | 84 ± 10.8 | .2 |
| No | 107 (87) | 96 ± 2.5 | |
| Radiation therapy | |||
| Yes | 20 (16) | 95 ± 4.9 | .97 |
| No | 103 (84) | 93 ± 3.5 | |
| Chemotherapy | |||
| Yes | 20 (16) | 100 ± 0 | .3 |
| No | 103 (84) | 92 ± 3.5 | |
| Voriconazole use | |||
| Yes | 7 (6) | 53.3 ± 24.8§ | .001 |
| No | 116 (94) | 96.2 ± 2.2§ | |
| Prior oncologic diagnosis | |||
| Yes | 29/121 (24) | 96 ± 3.8 | .7 |
| No | 92/121 (76) | 92 ± 3.8 | |
| HSCT | |||
| Yes | 13 (11) | 77.9 ± 14.1 | .053 |
| No | 110 (89) | 96 ± 2.4 | |
| Organ transplant | |||
| Yes | 7 (6) | 100 ± 0 | .5 |
| No | 116 (94) | 93 ± 3.1 | |
Bold values are P < 0.05 that are statistically significant.
One patient is missing survival data.
Denominator is 123 unless otherwise specified.
P value of log-rank test.
Overall survival estimates are for 3 years.
Discussion
Our study suggests that the majority of children at risk for NMSC can be identified prior to diagnosis. In our cohort of 124 children with NMSC from 11 major tertiary centers, we found that 70% of patients had at least 1 identifiable risk factor, including a predisposing genetic condition, predisposing skin lesion, and/or iatrogenic exposure.
Forty-four percent of our cohort had a predisposing genetic condition, most commonly basal cell nevus syndrome and xeroderma pigmentosum, and presented at a significantly younger age than those with iatrogenic exposures. Early diagnosis of these patients can significantly reduce tumor burden and quality of life through vigilant sun protection practices and appropriate treatment of skin cancers.13,14 Pediatricians and pediatric subspecialists are on the front line of providers who can make an early diagnosis and should be aware of cutaneous and systemic findings associated with these conditions. Dysmorphic facial features including frontal bossing and macrocephaly, palmar pits, odontogenic cysts, bifid ribs, and early childhood diagnosis of medulloblastoma are some findings that can point providers toward the diagnosis of basal cell nevus syndrome.15 Evidence of photosensitivity, including easy sunburning, lentigos, and telangiectasias in photodistributed locations, can suggest genodermatoses associated with DNA fragility or disrepair such as xeroderma pigmentosum.16 Interestingly, 25% of patients with a predisposing genetic condition also had 1 or more iatrogenic exposures associated with increased risk for NMSC. Thus, presence of a predisposing condition should not preclude providers from considering other contributing factors for an individual patient’s skin cancer burden.
Twenty-nine percent of our cohort received at least 1 medical treatment known to increase risk for skin cancer, including prolonged systemic immunosuppression, radiation therapy, chemotherapy, and voriconazole. We identified prolonged immunosuppression and voriconazole exposure as risk factors for development of SCC, and radiation therapy and chemotherapy as risk factors for development of BCC in children. Prolonged immunosuppression has been shown to increase risk for NMSC in adults with history of organ transplantation and HSCT, and 1 recent study suggested an increased risk of NMSC in a single center cohort of pediatric HSCT recipients.5,8,9,17 Prolonged immunosuppression has been shown to impair immune surveillance of dysplastic cells, and systemic calcineurin inhibitors may heighten risk of NMSC by interfering with P53 signaling and nucleotide excision repair.18–20 The use of systemic mammalian target of rapamycin (mTOR) inhibitors, such as sirolimus or everolimus, as alternative immunosuppressive agents may decrease the risk of subsequent skin cancers in organ transplant recipients.21 Of note, none of the cases in our sample received these agents as part of their immunosuppressive regimen. These agents are currently being used in children with tuberous sclerosis, and neural tumors and may be considered in children with SCC who require systemic immunosuppression.
Voriconazole, a triazole used for antifungal prophylaxis and treatment, has been associated with increased risk for SCC in adult lung transplant patients and more recently in adult HSCT recipients.7,22–24 One study identified high cumulative dose exposure as a stronger risk factor than duration of therapy.22 Voriconazole metabolites may induce both phototoxicity and photocarcinogenesis by sensitizing keratinocytes to ultraviolet A, inducing DNA fragility and disrupting DNA repair.25,26 Although data on the photocarcinogenic effects of voriconazole on children are lacking, 1 study found that 47% of children on voriconazole for more than 6 months experienced phototoxic reactions, suggesting that children are vulnerable to acute and perhaps long-term photo-induced effects of voriconazole.27 We also identified voriconazole as a risk factor for decreased overall survival. However, only 1 of 6 deceased patients died of skin cancer related causes, suggesting that voriconazole exposure may be an indication of poor overall health status rather than a predictor of skin cancer associated mortality.
Radiation therapy is a known risk factor for development of BCC, with childhood exposure to radiation therapy shown to increase exponentially the risk for BCC as compared with radiation therapy exposure in adulthood.28 Data through the Childhood Cancer Survivor Study shows that although 1 Gy of radiation increases one’s risk for BCC, a dose-dependent relationship exists, with 35 Gy or more to the skin increasing the likelihood of developing BCC by 40 times that of cancer survivors who were not treated with radiation.6 Our data confirm that children who have received radiation therapy are at risk for BCC and require routine dermatologic surveillance, particularly at the site of radiation exposure. To our knowledge, chemotherapy has not been previously identified as an independent risk factor for BCC, and given the significant overlap between patients in our cohort who received radiation therapy and chemotherapy, further studies are needed to confirm this finding.
In contrast to adults, most children have not had sufficient sun exposure to develop NMSC as a consequence of photodamage. Thus, the occurrence of NMSC in children can inform us of mechanisms in cutaneous tumorigenesis caused by iatrogenic exposures. In our cohort, all patients with SCC with iatrogenic exposures received prolonged immunosuppression, and 85% of patients with BCC with iatrogenic exposures received radiation therapy. Our data suggest strong associations and distinct pathogenic mechanisms between prolonged immunosuppression and SCC development, and radiation therapy and BCC development. As mentioned above, systemic immunosuppressive agents alone or in combination with voriconazole may interfere with P53 signaling and nucleotide excision repair mechanisms implicated in SCC development. Just as chronic ultraviolet light exposure has been shown to inactivate tumor suppressor genes, such as PTCH and P53, via a double hit phenomenon or loss of heterozygosity, similar mutations could be found in children with exposure to radiation therapy and chemotherapy, explaining their propensity for BCC development.29,30 Future tissue analyses including exome sequencing, immune cell characterization, and tumor suppressor and oncogene expression profiling in this cohort may help refine our understanding of these proposed mechanisms.
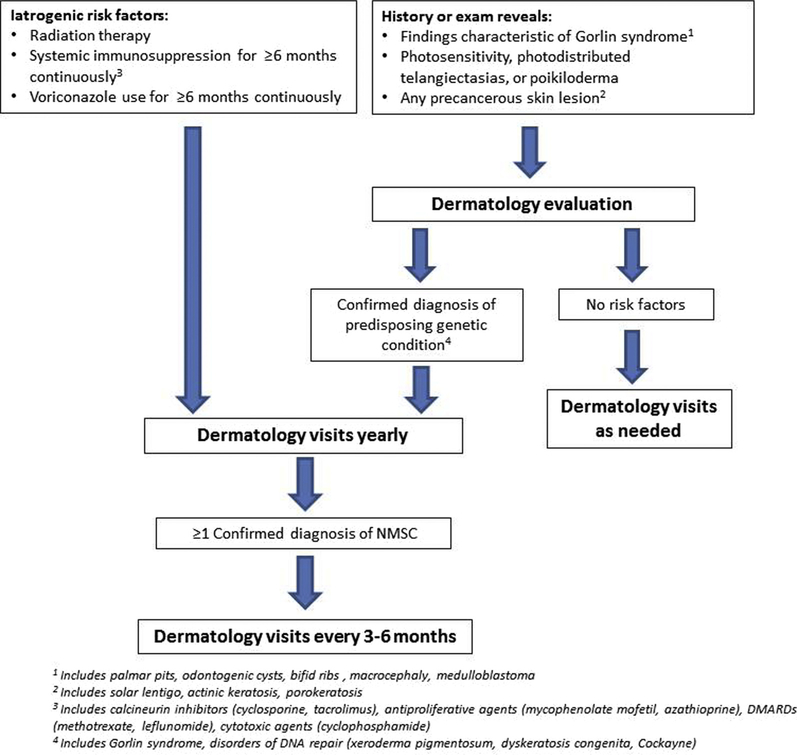
Our data also suggest gaps in dermatologic care in our cohort, including lack of routine skin cancer surveillance and delays in skin cancer diagnosis. Forty-four percent of patients were never seen by a dermatologist prior to initial skin cancer diagnosis despite identifiable risk factors in 70% of patients. Of those with iatrogenic risk factors, 32% had never been seen by a dermatologist prior to their skin cancer diagnosis. In addition, 48% of initial skin cancers had been present for 12 months or longer prior to diagnosis. These data corresponds to prior studies showing that delays in skin cancer diagnosis are common in children.4,31,32 We also found a high tumor burden in our cohort, with an overall count of 856 skin cancers in our cohort of 124 patients and 2 or more skin cancers diagnosed in 49% of patients. Although the median age of skin cancer diagnosis was 13.2 years, 39% of patients were 10 years of age or younger. Thus, routine skin cancer surveillance and guidance regarding preventative measures for at-risk children of any age could decrease time to diagnosis and tumor burden. Based on our findings, we have summarized our recommendations for skin cancer screening in the Figure.
Figure.
Suggested pediatric nonmelanoma skin cancer screening recommendations.
Our study was limited by its retrospective design, which resulted in missing data and potential inaccuracies in recording. Follow-up time was relatively short, and, thus, complete data on long-term outcomes including tumor burden and mortality were not available. Tissue analysis was not performed which could help us to understand the mechanisms of NMSC development in these patients.
The majority of pediatric NMSC cases are associated with predisposing conditions and iatrogenic exposures. We identified prolonged immunosuppression and voriconazole as risk factors for SCC, and radiation therapy and chemotherapy as risk factors for BCC. Providers should be aware of early presentations of genetic skin cancer predisposition syndromes, and recommend routine skin cancer surveillance to children exposed to prolonged immunosuppression, voriconazole use, and/or radiation therapy. Early recognition of children at risk for NMSC can minimize delays in diagnosis and tumor burden. ■
Acknowledgments
Supported by the Pediatric Dermatology Research Alliance and the American Skin Association.
Glossary
- BCC
Basal cell carcinoma
- HSCT
Hematopoietic stem cell transplantation
- NMSC
Nonmelanoma skin cancer
- SCC
Squamous cell carcinoma
Footnotes
The authors declare no conflicts of interest.
References
- 1.Holman JD, Dyer JA. Genodermatoses with malignant potential. Curr Opin Pediatr 2007;19:446–54. [DOI] [PubMed] [Google Scholar]
- 2.Xiang F, Lucas R, Hales S, Neale R. Incidence of nonmelanoma skin cancer in relation to ambient UV radiation in white populations, 1978–2012: empirical relationships. JAMA Dermatol 2014;150:1063–71. [DOI] [PubMed] [Google Scholar]
- 3.Leiter U, Eigentler T, Garbe C. Epidemiology of skin cancer. Adv Exp Med Biol 2014;810:120–40. [DOI] [PubMed] [Google Scholar]
- 4.Khosravi H, Schmidt B, Huang JT. Characteristics and outcomes of nonmelanoma skin cancer (NMSC) in children and young adults. J Am Acad Dermatol 2015;73:785–90. [DOI] [PubMed] [Google Scholar]
- 5.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med 2003;348:1681–91. [DOI] [PubMed] [Google Scholar]
- 6.Watt TC, Inskip PD, Stratton K, Smith SA, Kry SF, Sigurdson AJ, et al. Radiation-related risk of basal cell carcinoma: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst 2012;104:1240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer JP, Boker A, Metchnikoff C, Binstock M, Boettger R, Golden JA, et al. High cumulative dose exposure to voriconazole is associated with cutaneous squamous cell carcinoma in lung transplant recipients. J Heart Lung Transplant 2013;31:694–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curtis RE, Metayer C, Rizzo JD, Socié G, Sobocinski KA, Flowers ME, et al. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood 2005;105:3802–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leisenring W, Friedman DL, Flowers ME, Schwatrz JL, Deeg HJ. Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J Clin Oncol 2006;24:1119–26. [DOI] [PubMed] [Google Scholar]
- 10.Rork JF, Margossian SP, Nambudiri V, Huang JT. Nonmelanoma skin cancer in childhood after hematopoietic stem cell transplant: a report of 4 cases. J Pediatr Hematol Oncol 2014;36:224–7. [DOI] [PubMed] [Google Scholar]
- 11.Unal S, Cetin M, Gumruk F. Basal cell carcinoma after treatment of childhood acute lymphoblastic leukemia and concise review of the literature. Pediatr Dermatol 2015;32:e82–5. [DOI] [PubMed] [Google Scholar]
- 12.Cowen EW, Nguyen JC, Miller DD, McShane D, Arron ST, Prose NS, et al. Chronic phototoxicity and aggressive squamous cell carcinoma of the skin in children and adults during treatment with voriconazole. J Am Acad Dermatol 2010;62:31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solis DC, Kwon GP, Ransohoff KJ, Li S, Chahal HS, Ally MS, et al. Risk factors for basal cell carcinoma among patients with basal cell nevus syndrome: development of a basal cell nevus syndrome patient registry. JAMA Dermatol 2017;153:189–92. [DOI] [PubMed] [Google Scholar]
- 14.Moriwaki S, Kanda F, Hayashi M, Yamashita D, Sakai Y, Nishigori C, et al. Xeroderma pigmentosum clinical practice guidelines. J Dermatol 2017;44:1087–96. [DOI] [PubMed] [Google Scholar]
- 15.Gorlin RJ. Nevoid basal cell carcinoma (Gorlin) syndrome. Genet Med 2004;6:530–9. [DOI] [PubMed] [Google Scholar]
- 16.Giordano CN, Yew YW, Spivak G, Lim HW. Understanding photodermatoses associated with defective DNA repair: syndromes with cancer predisposition. J Am Acad Dermatol 2016;75:855–70. [DOI] [PubMed] [Google Scholar]
- 17.Song JS, London WB, Hawryluk EB, Guo D, Sridharan M, Fisher DE, et al. Risk of melanocytic nevi and nonmelanoma skin cancer in children after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2017;52:989–97. [DOI] [PubMed] [Google Scholar]
- 18.Muehleisen B, Jiang SB, Gladsjo JA Gerber M, Hata T, Gallo RL. Distinct innate immune gene expression profiles in non-melanoma skin cancer of immunocompetent and immunosuppressed patients. PLoS One 2012;7: e40754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuschal C, Thoms KM, Schubert S, Schäfer A, Boeckmann L, Schön MP, et al. Skin cancer in organ transplant recipients: effects of immunosuppressive medications on DNA repair. Exp Dermatol 2012;21:2–6. [DOI] [PubMed] [Google Scholar]
- 20.Wheless L, Jacks S, Mooneyham Potter KA, Leach BC, Cook J. Skin cancer in organ transplant recipients: more than the immune system. J Am Acad Dermatol 2014;71:359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okut G, Alp A, Tatar E, Simsek C, Tugmen C, Uslu A. Nonmelanoma skin cancers after kidney transplant: our 15 years of experience with mammalian target of rapamycin inhibitors. Exp Clin Transplant 2017;15:236–9. [DOI] [PubMed] [Google Scholar]
- 22.Feist A, Lee R, Osborne S, Lane J, Yung G. Increased incidence of cutaneous squamous cell carcinoma in lung transplant recipients taking long-term voriconazole. J Heart Lung Transplant 2012;31:1177–81. [DOI] [PubMed] [Google Scholar]
- 23.Wojenski DJ, Bartoo GT, Merten JA, Dierkhising RA, Barajas MR, El-Azhary RA, et al. Voriconazole exposure and the risk of cutaneous squamous cell carcinoma in allogeneic hematopoietic stem cell transplant patients. Transpl Infect Dis 2015;17:250–8. [DOI] [PubMed] [Google Scholar]
- 24.Kuklinski LF, Li S, Karagas MR, Weng WK, Kwong BY. Effect of voriconazole on risk of nonmelanoma skin cancer after hematopoietic cell transplantation. J Am Acad Dermatol 2017;77:706–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haylett AK, Felton S, Denning D, Denning DW, Rhodes LE. Voriconazole-induced photosensitivity: photobiological assessment of a case series of 12 patients. Br J Dermatol 2013;168:179–85. [DOI] [PubMed] [Google Scholar]
- 26.Ona K, Oh DH. Voriconazole N-oxide and its ultraviolet B photoproduct sensitize keratinocytes to ultraviolet A. Br J Dermatol 2015;173:751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheu J, Hawryluk EB, Guo, London WB, Huang JT. Voriconazole phototoxicity in children: a retrospective review. J Am Acad Dermatol 2015;72:314–20. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz JL, Kopecky KJ, Mathes R, Leisenring WM, Friedman DL, Deeg HJ. Basal cell skin cancer after total-body irradiation and hematopoietic cell transplantation. Radiat Res 2009;171:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan H, Oro AE, Scott M, Khavari PA. Induction of basal cell carcinoma features in transgenic human skin expressing Sonic Hedgehog. Nat Med 1997;3:788–92. [DOI] [PubMed] [Google Scholar]
- 30.Brown VL, Harwood CA, Crook, Cronin JG, Kelsell DP, Proby CM. p16INK4a and p14ARF tumor suppressor genes are commonly inactivated in cutaneous squamous cell carcinoma. J Invest Dermatol 2004;122:1284–92. [DOI] [PubMed] [Google Scholar]
- 31.Murray JE, Cannon B. Basal-cell cancer in children and young adults. N Engl J Med 1960;262:440–3. [DOI] [PubMed] [Google Scholar]
- 32.Milstone EB, Helwig EB. Basal cell carcinoma in children. Arch Dermatol 1973;108:523–7. [PubMed] [Google Scholar]