Abstract
Relapse of neonatal meningitis is most commonly caused by Escherichia coli. Management to prevent relapse varies and evidence is limited. We present four cases of relapsing neonatal E. coli meningitis in Denmark in 2016–2017 and review the current literature on this subject. During the primary episodes, our patients received cephalosporin for 3 weeks and gentamicin for the first 3 days. The only identified risk factor was delayed CSF sterilization in three of four cases and no repeated lumbar puncture. Relapse occurred after 2–28 days; one case with ventriculitis and one with empyema. Relapses were treated for 6–14 weeks with monotherapy. No children had an underlying disease predisposing to E. coli meningitis. There is generally a trend towards reducing invasive procedures, e.g., lumbar puncture and the length of intravenous antibiotics in pediatric infectious diseases, but our cases highlight a condition where the opposite might be needed.
Keywords: neonate, meningitis, E. coli, lumbar puncture
1. Introduction
Relapse of neonatal meningitis is most commonly caused by Escherichia coli, occurring in 2–21% of infants despite potent antibiotics [1,2], but risk factors are scarcely described. Management of neonatal E. coli meningitis to prevent relapse varies and the evidence is limited. We present four cases of neonatal E. coli meningitis with relapse and discuss strategies for prevention.
2. Cases
The four infants with relapse E. coli meningitis were diagnosed in Denmark in 2016–2017; a country with approx. 60,000 live births per year. The cases are summarized in Table 1 and detailed in Appendix A. The E. coli isolates were susceptible to the initial therapy, including third generation cephalosporins and gentamicin. During the primary episodes, the first lumbar punctures were performed 20–58 h after initiation of targeted therapy, and three were still culture positive despite ongoing treatment. None of the three culture-positive cases had repeated CSF examination to secure sterilization. Cerebral magnetic resonance imaging (MRI) during or shortly after cessation of therapy was without signs of persistent infection, and abdominal ultrasound with no urinary tract malformations. All cases were treated for 3 weeks in total. The relapse E. coli episodes occurred 2–28 days after completion of the initial 3 weeks therapy, all with isolates with an identical susceptibility pattern, and case 3 with identical strain shown by whole genome sequencing. There was no evidence of ESBL (extended spectrum beta-lactamase) production. At relapse, MRI was abnormal in two cases showing signs of ventriculitis (case 3) and empyema (case 4), while case 1 and 2 had normal MRI. The relapses were treated for 6–14 weeks. No children had an underlying disease predisposing to E. coli meningitis.
Table 1.
Cases of relapse of neonatal Echerichia coli meningitis.
| Case | First E. coli Meningitis | Relapse E. coli Meningitis | Follow-Up | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | GA at birth (weeks) |
BW (gram) |
Age (days) |
Treatment | Cerebral MRI | Urinary ultra-sound | Time from completion (days) | Treatment | Age (years) |
Under-lying disease | Sequelae * | |
| 1 | F | 36 + 3 | 2130 | 3 | Cefotaxime 21 days + gentamicin day 1–5 |
Minor hemorrhagic parenchymal infarctions. No signs of infection. (without contrast) |
Normal | 4 | Meropenem 1 week followed by ceftriaxone 9 weeks |
4.0 | No | No |
| 2 | M | 34 + 0 | 1980 | 5 | Cefotaxime 21 days + gentamicin day 1–3 |
Normal (with contrast) |
Normal | 11 | Cefotaxime 1 week, meropenem 1 week followed by ceftriaxone 12 weeks |
3.5 | No | No |
| 3 | M | 34 + 5 | 2280 | 45 | Cefotaxime 20 days + gentamicin day 1–7 |
Ischemic changes of cortical parenchyma. No signs of infection. (without contrast) |
Normal | 28 | Ceftriaxone 8 weeks | 3.5 | No | Moderate sensory disabilities, visual impairment, delayed development. No signs of hydrocephalus or cerebral palsy. Persistent changes on MRI. |
| 4 | F | 37 + 1 | 2940 | 13 | Cefotaxime 22 days + gentamicin day 1–3 |
Minor hemorrhagic parenchymal infarctions. No signs of infection. (without contrast) |
Normal | 2 | Meropenem + ciprofloxacin 6 weeks followed by ceftriaxone 3 weeks |
4.0 | No | No |
F = Female; M = Male; GA = Gestational Age; BW = Birth Weight; MRI = Magnetic Resonance Imaging. * Developmental milestones and audiology were investigated, in addition to signs of cerebral palsy and hydrocephalus.
At follow-up, all children were healthy, although case 3 had moderately delayed developmental milestones without other signs of neurological disease.
3. Discussion
We present four cases of relapsing neonatal E. coli meningitis despite 3 weeks of targeted treatment. There were no apparent risk factors during the primary meningitis episode, except for persistent bacterial growth in the CSF 1–2½ days after initiation of antibiotics in three of four cases and lack of repeated lumbar puncture to secure sterilization.
The need to perform a repeat lumbar puncture is a frequent consideration for the neonatologist, and recommendations vary due to limited evidence. Standard textbooks recommend a repeated lumbar puncture 48–72 h into treatment of E. coli meningitis to document CSF sterilization and provide reassurance of effective therapy in infants with this particularly virulent organism and guide treatment duration [3,4]. The UK guidelines do not recommend repeat lumbar puncture in neonates with good clinical recovery [5]. In fact, many clinicians do not routinely perform this invasive procedure in a stabilized infant [6,7]. In this study, all four children were treated with adequate antibiotics and showed good clinical recovery, but relapse still occurred. None of the three culture-positive cases had repeated lumbar puncture performed, and their relapses may have been caused by delayed sterilization of CSF and an unfulfilled need for longer duration treatment.
Our findings encourage the use of repeat lumbar puncture, although we acknowledge that (1) other factors, not identified, could have been involved in the relapses, and (2) the association between persistent CSF growth and subsequent relapse has not been addressed systematically. However, studies have shown an increased risk of complications and death in those with positive repeat lumbar puncture, found in 10–15% of cases [7,8,9,10]. Delayed CSF sterilization should lead to prolonged antibiotic therapy [1,2], but may also warrant consideration of increasing the dose to achieve higher CNS concentrations, changing the antibiotic to a drug with lower minimal inhibitory concentration (MIC), or even adding an additional antibiotic. Furthermore, cerebral imaging should be considered, since the persistence of bacterial growth can indicate a purulent focus (e.g., ventriculitis) that may require surgical intervention or increased duration of antimicrobial therapy [3].
Combination antibiotic therapy in primary E. coli meningitis has been suggested for improving treatment, e.g., adding ciprofloxacin, which has good penetration to CSF and cerebral tissue, as well as bactericidal activity and low MIC against E. coli. However, no randomized trials have explored this, but a retrospective study showed that first-line adjunct ciprofloxacin to neonates with primary E. coli meningitis did not decrease the proportion of infants with CSF sterilization failure after a median of 49 h of initiation of therapy (11% vs. 14%), and ciprofloxacin did not improve neurological outcome or mortality [10]. Gentamicin was added as initial empiric therapy in all our cases, as often recommended in septic neonates, but this did not prevent relapse.
Lumbar puncture at the end of therapy is generally not recommended in uncomplicated cases since abnormal CSF findings often persist irrespective of successful treatment [3].
Neuroimaging is also used to identify children at risk of complications, which may require prolonged treatment or surgery, i.e., ventriculitis, cerebral abscess, empyema, or persistent parameningeal foci. It is recommended shortly before cessation of antibiotics, even in uncomplicated courses. In all our cases, cerebral MRI during or shortly after the primary episode was without signs of persistent infection and not indicative of relapse. This suggests that the patients harbored residual infectious foci within the brain substances or paraventricular regions not evident on MRI, illustrating that a normal MRI does not exclude subsequent relapse. However, three of the MRI’s were performed without contrast, which may have limited visualization of persistent infection.
All children had initial high levels of C-reactive protein (mean 201 mg/L, range 165–260) with fast decline within a few days after treatment initiation. All children had normal C-reactive protein at termination of antibiotic treatment. Procalcitonin was not measured systematically. We therefore do not have data to support that inflammatory markers followed an unusual pattern or that normal patterns can exclude relapse. Hopefully, future research will identify biomarkers that can monitor persistent silent infection.
Some underlying conditions predispose to neonatal E. coli infection. Urinary tract malformation is a risk factor for E. coli meningitis [2] and thus a potential reservoir for relapse, and ultrasound of the urinary tract is indicated in primary neonatal E. coli meningitis or sepsis. All our cases had normal urinary tract anatomy.
Primary immunodeficiency has not been associated with neonatal E. coli meningitis, relapse or recurrence. The susceptibility to E. coli infection in neonates is well explained by their immature immune system including a poor antibody response to the K1 capsular antigen carried by the majority of E. coli strains. Transitional neutropenia is common in Gram-negative bacterial meningitis, but neonatal E. coli meningitis has not been reported to occur particularly in children with congenital neutropenia or other phagocyte disorders. Thus, routine testing for primary immunodeficiency is not indicated in case of neonatal E. coli meningitis or relapse.
Dermal sinus tracts malformation is a well-described risk factor to E. coli meningitis, and a careful physical examination of the spine is essential, particularly in episodes which re-occur after a longer interval (e.g., 3 weeks). None of our cases had spinal malformations.
Galactosemia is rare hereditary disorder of carbohydrate metabolism predisposing to neonatal E. coli infection and should therefore be considered in the case of invasive E. coli infection [11]. Screening for galactosemia is included in some newborn screening programs or can be specifically tested. Also, children with galactosemia are unable to tolerate mother’s milk and will normally present early in life with failure to thrive and elevated liver enzymes. None of our children had galactosemia.
None of our cases were of full-term gestation. Three were premature, and one was born ‘early term’ (GA 37 + 1). This is consistent with the existing literature showing E. coli meningitis to be 7-fold more frequent in preterm neonates [9]. To our knowledge, it has not been explored if prematurity is related to an increased risk of relapse per se, but it is likely that children who are not born at full-term gestation are also at higher risk of relapse of their meningitis. Thus, a higher vigilance could be considered for these patients.
All cases presented were interpreted as relapse of the primary infection in contrary to recurrent meningitis, which is traditionally defined as a secondary meningitis occurring more than 21 days after cessation of therapy, often with a different organism [12]. In such cases, cranial anatomical defects and primary immunodeficiency should be ruled out [13]. The secondary episode in case 3 did in fact occur 28 days after cessation of therapy, but the E. coli strain was shown to be identical by whole genome sequencing.
Treatment
Treatment of relapsing E. coli meningitis in children is very sparsely reported, and guidance for the clinician is minimal, including choice of antibiotics and duration of treatment. Our cases, including one with ventriculitis and one with empyema, received prolonged treatment (8–14 weeks) decided by the treating physician, without additional relapse. In case 4, it was chosen to treat with additional ciprofloxacin for the first 3 weeks.
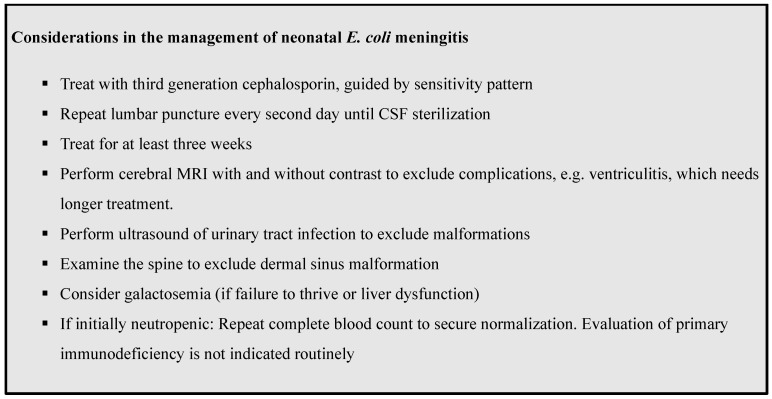
Our cases demonstrate that neonatal E. coli meningitis is a complex disease with risk of relapse. If possible, early involvement of a specialist in Pediatric Infectious Diseases is advisable. Figure 1 summarizes important clinical key points to consider when treating an infant with E. coli meningitis.
Figure 1.
Key Clinical Points.
4. Conclusions
These cases illustrate the risk of relapsing infection in neonates with E. coli meningitis. There were no clinical clues except delayed CSF sterilization in three of four cases. Our findings support the use of repeat lumbar puncture even in stable children to ensure CSF sterilization and guide treatment. As there is generally a trend towards reducing invasive procedures and the length of intravenous antibiotics in pediatric infectious diseases, our cases highlight a situation where the opposite might be needed.
Acknowledgments
We thank the parents of the infants described for allowing us to review and share the medical details of their child.
Appendix A
Table A1.
Description of Cases.
| Case 1 |
| A female born prematurely at gestational age (GA) 36 weeks + 3 days, birth weight (BW) 2130 g, admitted to the neonatal department. Presented at age 3 days with fever and drowsiness with elevated inflammatory parameters (CRP 123 mg/L) and was treated with cefuroxime and gentamicin in suspicion of sepsis. After 24 h, she was irritable and shivering and therapy was changed to meningitis treatment (cefotaxime, ampicillin, and gentamicin). Lumbar puncture was performed after 58 h of treatment and cerebrospinal fluid (CSF) grew E. coli sensitive to cefotaxime. Cefotaxime was continued as monotherapy for 21 days. Cerebral magnetic resonance imaging (MRI) at completion of therapy was normal except for two very minor bleedings. The girl was thriving. Four days after completion of antibiotic treatment, the girl was readmitted with fever, respiratory distress, and cardiac failure, and referred to intensive care unit for mechanical ventilation, inotropes, and empirically treated for meningitis with cefotaxime and gentamicin. Blood culture was positive for E. coli sensitive to cefotaxime. CSF examination was postponed to day 3 of relapse due to clinical instability. It was culture negative, but with elevated CSF white blood cells (722 mill/L) and marked hypoglycorrhachia (0.0), a high percentage of neutrophiles (375 mill/L= 49%), and elevated CSF protein 2.29 g/L, all suggestive of acute infection in the CNS. Antibiotic therapy was changed to ceftriaxone monotherapy for 9 weeks. At follow-up, the girl had normal neurological development and no apparent sequelae to the infection. |
| Case 2 |
| A male born prematurely at GA 34 + 0, BW 1980 g, admitted to the neonatal department. Presented at age 5 days with drowsiness and elevated inflammatory parameters (CRP 145 mg/L) and was treated with ampicillin and gentamicin in suspicion of sepsis. After 24 h, therapy was changed to cefotaxime and ampicillin due to clinical worsening, and lumbar puncture was performed. CSF culture was positive for E. coli, sensitive to third generation cephalosporins. Transfontanellar ultrasound showed thickened meninges. Cefotaxime was continued for 3 weeks. Urinary tract ultrasound was normal. MRI of cerebrum and repeat TFU was normal. A lumbar puncture was performed at the end of treatment showed elevated WBC 111 mill/L and protein 1,67 g/L with negative culture. At discharge, the boy was thriving. Eleven days after completion of antibiotic therapy, the boy was readmitted due to severe vomiting and irritability. He was treated with cefotaxime and gentamicin in suspicion of relapsing meningitis. Blood and CSF cultures were positive E. coli, sensitive to third generation cephalosporins. The E. coli strain was typed as K1. Repeated lumbar puncture after 36 h was without bacterial growth. MRI of cerebrum and spine and ultrasound of urinary tract and abdomen were normal. Ceftriaxone was continued for 12 weeks. At follow-up, the boy had normal neurological development and no sequelae to the infection. |
| Case 3 |
| A male born prematurely at GA 34 + 5, BW 2280 g without neonatal complications. Admitted from home at age 6 weeks (corrected GA 41 + 4) with fever and septic appearance and was treated with ampicillin, gentamicin, and cefotaxime in suspicion of meningitis and transferred to intensive care unit. Blood culture was drawn at admission and was positive for E. coli. Lumbar puncture was performed 21 h after initiation of antibiotics and was also positive for E. coli, sensitive to third generation cephalosporins. Cerebral MRI showed ischemic changes of cortical parenchyma, but no signs of abscess or ventriculitis. Cefotaxime was continued for 21 days. A lumbar puncture was performed at the end of treatment, showing elevated WBC (110 mill/L) and protein (1.72 g/L) with negative culture and PCR. Four weeks after completion of antibiotic therapy, the boy was readmitted with fever and irritability. He was treated with ampicillin and ceftriaxone in suspicion of relapsing meningitis. Blood culture was positive for E. coli, sensitive to third generation cephalosporins. CSF culture was negative, but PCR positive for E. coli. The whole genome sequenced of the strain was found to be identical to the previous episode of E. coli meningitis. Treatment was changed to ceftriaxone monotherapy for a total of 8 weeks as out-patient therapy after stabilization. Repeated CSF cultures at day 38 and 47 were negative. MRI of cerebrum and spine showed signs of ventriculitis and cortical changes from the former episode of meningitis. MRI of abdomen was normal. At follow-up, the boy had moderately delayed neurological development, but no signs of hydrocephalus or cerebral palsy. |
| Case 4 |
| A female born at term at GA 37 + 1, BW 2940 g without neonatal complications. Admitted from home at age 13 days with drowsiness, irritability, poor feeding, and elevated inflammatory parameters (CRP 57 g/L), and treated with gentamicin, ampicillin, and cefotaxime in suspicion for meningitis. Blood culture was positive for E. coli, sensitive to third generation cephalosporins. CSF obtained 48 h after initiated therapy was positive for E coli. Cefotaxime was continued as monotherapy for 21 days. An MRI at completion of therapy was normal except for signs of very minor bleedings. Two days after completion of antibiotic treatment, the patient was readmitted with irritability and rising CRP (53 mg/L). She was treated with gentamicin, ampicillin, and cefotaxime in suspicion of relapsing meningitis. CSF culture was positive for E. coli, sensitive to third generation cephalosporins. The treatment was changed to meropenem and ciprofloxacin. Cerebral MRI showed bilateral minor empyema and early stage of communicating hydrocephalus. MRI was repeated 4 weeks later with regression of both empyema and hydrocephalus. Urinary tracts ultrasound was normal. Repeated CSF culture at day 12 was negative. Ciprofloxacin and meropenem were discontinued after 6 weeks of treatment and changed to ceftriaxone for an additional 3 weeks as out-patient therapy. CSF culture 3 days after the end of treatment was negative. At follow-up, the girl had normal neurological development and no sequelae to the infection. |
Author Contributions
Conceptualization, N.H.V. and U.N.; methodology, N.H.V. and U.N.; software, N.H.V. and M.B.M.; validation, N.H.V. and M.B.M.; formal analysis, N.H.V., M.B.M. and U.N.; investigation, N.H.V., M.B.M. and U.N.; resources, all authors; data curation, all authors.; writing—original draft preparation, N.H.V.; writing—review and editing, All authors; visualization, N.H.V.; supervision, U.N.; project administration, U.N.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. Formal ethical review and approval was not required for this retrospective case study.
Informed Consent Statement
Written informed consent to publish this paper has been obtained from both parents/legal guardians.
Data Availability Statement
Further details on the patients can be accessed by contacting the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Anderson S.G., Gilbert G.L. Neonatal Gram Negative Meningitis: A 10-Year Review, with Reference to Outcome and Relapse of Infection. J. Paediatr. Child Health. 1990;26:212–216. doi: 10.1111/j.1440-1754.1990.tb02432.x. [DOI] [PubMed] [Google Scholar]
- 2.Unhanand M., Mustafa M.M., McCracken G.H., Nelson J.D. Gram-Negative Enteric Bacillary Meningitis: A Twenty-One-Year Experience. J. Pediatr. 1993;122:15–21. doi: 10.1016/S0022-3476(05)83480-8. [DOI] [PubMed] [Google Scholar]
- 3.Heath P.T., Yusoff N.K.N., Baker C.J. Neonatal Meningitis. Arch. Dis. Child.-Fetal Neonatal Ed. 2003;88:F173–F178. doi: 10.1136/fn.88.3.F173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarah S. Long Principles and Practice of Pediatric Infectious Diseases. 3rd ed. Churchill Livingstone; London, UK: 2012. [Google Scholar]
- 5.National Collaborating Centre for Women’s and Children’s Health (UK) Bacterial Meningitis and Meningococcal Septicaemia: Management of Bacterial Meningitis and Meningococcal Septicaemia in Children and Young People Younger than 16 Years in Primary and Secondary Care. RCOG Press; London, UK: 2010. National Institute for Health and Clinical Excellence: Guidance. [PubMed] [Google Scholar]
- 6.Agarwal R., Emmerson A.J. Should Repeat Lumbar Punctures Be Routinely Done in Neonates with Bacterial Meningitis? Results of a Survey into Clinical Practice. Arch. Dis. Child. 2001;84:451–452. doi: 10.1136/adc.84.5.450d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenberg R.G., Benjamin D.K., Jr., Cohen-Wolkowiez M., Clark R.H., Cotten C.M., Laughon M., Smith P.B. Repeat Lumbar Punctures in Infants with Meningitis in the Neonatal Intensive Care Unit. J. Perinatol. 2011;31:425–429. doi: 10.1038/jp.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basmaci R., Bonacorsi S., Bidet P., Biran V., Aujard Y., Bingen E., Béchet S., Cohen R., Levy C. Escherichia Coli Meningitis Features in 325 Children From 2001 to 2013 in France. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2015;61:779–786. doi: 10.1093/cid/civ367. [DOI] [PubMed] [Google Scholar]
- 9.Ouchenir L., Renaud C., Khan S., Bitnun A., Boisvert A.-A., McDonald J., Bowes J., Brophy J., Barton M., Ting J., et al. The Epidemiology, Management, and Outcomes of Bacterial Meningitis in Infants. Pediatrics. 2017;140 doi: 10.1542/peds.2017-0476. [DOI] [PubMed] [Google Scholar]
- 10.Tauzin M., Ouldali N., Lévy C., Béchet S., Cohen R., Caeymaex L. Combination Therapy with Ciprofloxacin and Third-Generation Cephalosporin versus Third-Generation Cephalosporin Monotherapy in Escherichia Coli Meningitis in Infants: A Multicentre Propensity Score-Matched Observational Study. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2018 doi: 10.1016/j.cmi.2018.12.026. [DOI] [PubMed] [Google Scholar]
- 11.Lak R., Yazdizadeh B., Davari M., Nouhi M., Kelishadi R. Newborn Screening for Galactosaemia. Cochrane Database Syst. Rev. 2020;6:CD012272. doi: 10.1002/14651858.CD012272.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaad U.B., Nelson J.D., McCracken G.H. Recrudescence and Relapse in Bacterial Meningitis of Childhood. Pediatrics. 1981;67:188–195. [PubMed] [Google Scholar]
- 13.Tebruegge M., Curtis N. Epidemiology, Etiology, Pathogenesis, and Diagnosis of Recurrent Bacterial Meningitis. Clin. Microbiol. Rev. 2008;21:519–537. doi: 10.1128/CMR.00009-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Further details on the patients can be accessed by contacting the corresponding author.