Abstract
Epidemiological and experimental studies have associated oral and systemic exposures to the herbicide paraquat (PQ) with Parkinson’s disease. Despite recognition that airborne particles and solutes can be directly translocated to the brain via olfactory neurons, the potential for inhaled PQ to cause olfactory impairment has not been investigated. This study sought to determine if prolonged low-dose inhalation exposure to PQ would lead to disposition to the brain and olfactory impairment, a prodromal feature of Parkinson’s disease. Adult male and female C57BL/6J mice were exposed to PQ aerosols in a whole-body inhalation chamber for 4 h/day, 5 days/week for 4 weeks. Subsets of mice were sacrificed during and after exposure and PQ concentrations in various brain regions (olfactory bulb, striatum, midbrain, and cerebellum) lung, and kidney were quantified via mass spectrometry. Alterations in olfaction were examined using an olfactory discrimination paradigm. PQ inhalation resulted in an appreciable burden in all examined brain regions, with the highest burden observed in the olfactory bulb, consistent with nasal olfactory uptake. PQ was also detected in the lung and kidney, yet PQ levels in all tissues returned to control values within 4 weeks post exposure. PQ inhalation caused persistent male-specific deficits in olfactory discrimination. No effects were observed in females. These data support the importance of route of exposure in determination of safety estimates for neurotoxic pesticides, such as PQ. Accurate estimation of the relationship between exposure and internal dose is critical for risk assessment and public health protection.
Keywords: paraquat, inhalation, Parkinson’s disease, olfactory impairment
Many pesticides, including the commonly used herbicide paraquat (1,1’-dimethyl-4,4’-bipyridinium dichloride, PQ), can produce neurotoxic effects (Costa, 2008; Richardson et al., 2019). Exposure to pesticides can occur through several routes: ingestion of residues in food and water, dermal penetration following deposition on skin or contact with contaminated surfaces, and inhalation of airborne aerosols (Gangemi et al., 2016; Mamane et al., 2015). Occupational exposure to pesticides in agricultural workers is a well-documented occurrence (Baldi et al., 2012; Quandt et al., 2006) and is associated with adverse health outcomes (Damalas and Eleftherohorinos, 2011; Lee et al., 2011), including neurodegenerative diseases (Brouwer et al., 2017; Gorell et al., 1998; Moisan et al., 2015; Wang et al., 2011). Research indicates that pesticide applicators may be continuously exposed to low levels of respirable pesticides, including PQ (Chester and Ward, 1984; Chester and Woollen, 1982; Lee et al., 2009; Baharuddin et al., 2011); however, inhalation is virtually overlooked in basic animal research and risk assessment (Lee et al., 2002; Dowling and Seiber, 2002). Typically, acute inhalation LC50 values for pesticides are considered when setting exposure limits, but such measures may overlook critical toxicological outcomes that occur at lower doses and repeated exposures. Additionally, inhalation presents the opportunity for translocation into the brain via olfactory neurons. Indeed, constituents of air pollution and other inhaled toxicants can enter the central nervous system (CNS) through this route (Maher et al., 2016; Oberdorster et al., 2004). Despite the reality of inhalation exposure to pesticides, data on the toxicokinetic profiles of inhaled pesticides, such as PQ, at relevant exposure levels is sorely lacking.
Introduced in 1961, PQ is a broad spectrum herbicide that destroys plant tissue through the production of oxygen radicals and disruption of photosynthesis (Bus et al., 1976a). The toxicity of this compound in animals likely occurs through a similar oxidative mechanism (Bus et al., 1976b; Di Monte et al., 1986; Smith, 1987). Following systemic absorption, PQ accumulates primarily in lung and secondarily in kidney (Li et al., 2011; Rose et al., 1974), where its toxic effects have been well-recognized in humans (Dinis-Oliveira et al., 2008; Matthew et al., 1968; Smith, 1987) and rodents (Dinis-Oliveira et al., 2006; Seidenfeld et al., 1978; Wyatt et al., 1981); however, subtle or delayed consequences at low, relevant doses have not been extensively investigated. Although PQ is charged and hydrophilic, it is able to cross the blood-brain-barrier following systemic absorption via a widely expressed family of neutral amino acid transporters (Shimizu et al., 2001). Toxicokinetic studies have revealed that, following intraperitoneal (i.p.) injection, systemically absorbed PQ distributes throughout the brain, where it can be retained for weeks (Prasad et al., 2007). The retention half-life of PQ (10 mg/kg, i.p.) in the midbrain was estimated to be 28 days (Prasad et al., 2009). Although these and other studies (Litteljohn et al., 2011; McCormack et al., 2005) demonstrate that PQ has the potential to exert negative effects on the CNS, they are difficult to extrapolate to humans due to the use of high doses and routes of exposure not encountered by agricultural workers (eg, injection). Despite its prohibition in many countries (Donley, 2019), PQ application continues to be widespread; the use of PQ in the United States has increased in recent years, with over 15 million pounds of active ingredient used in 2017, a 10-year high (USGS NAWQA). Spray drift of PQ can affect nearby communities (Ames et al., 1993; Chester and Ward, 1984), but agricultural workers remain most likely to experience inhalation exposure to PQ (Chester and Woollen, 1982; Lee et al., 2009).
In addition to providing a portal of entry into the CNS, the olfactory system itself is especially vulnerable to toxicants because its primary sensory neurons, located in the nasal mucosa, are in direct contact with the environment (Elder et al., 2006; Oberdorster et al., 2004). A recent study found that farmers who experienced acute pesticide exposure were 49% more likely to develop olfactory impairment later in life (Shrestha et al., 2019). In addition to negatively impacting quality of life (eg, decreased appetite, diminished hazard detection), olfactory impairment is associated with increased risk of Parkinson’s disease (PD; Doty, 2007, 2012; Ross et al., 2008) and Alzheimer’s disease (Ruan et al., 2012). Olfactory impairment independently predicts all-cause mortality (Gopinath et al., 2012; Schubert et al., 2017) and is increasingly recognized as a public health concern, particularly in older adults (Murphy, 2002). In rodents, PQ injection (10 mg/kg, i.p.) reduces time spent sniffing novel scents (Czerniczyniec et al., 2011), suggesting that PQ may lead to olfactory dysfunction regardless of route of administration. Thus, olfactory impairment may serve as a useful marker of neurotoxicity following pesticide exposure.
This study sought to determine if prolonged low-dose PQ inhalation, a translationally relevant exposure paradigm, would produce different concentrations in the brain, lung and kidney compared with concentrations reported using the common injection model of administration. PQ was selected due to its continued widespread use, substantial available data regarding its effects in rodent models via injected or oral routes (Dinis-Oliveira et al., 2008; Tsai, 2013) and its epidemiological association with PD in agricultural settings (Tanner et al., 2011). We also sought to assess functional changes in olfaction, which may serve as a sensitive indicator of pesticide exposure in humans (Shrestha et al., 2019) and as a prodromal feature of PD (Schapira et al., 2017). As such, outcome measures included quantitation of PQ in various tissues and brain regions across time, coupled with an olfactory discrimination paradigm.
MATERIALS AND METHODS
Animals
Male and female C57BL/6J mice were obtained from Jackson Laboratories. Mice were pair-housed in standard mouse caging with approximately 3 mm high performance bedding (BioFresh), provided standard rodent chow (LabDiet Autoclavable Diet 5010) and water ad libitum, and maintained at 22°C ± 2°C under a 12-h light-dark cycle (lights on at 06:00) at the University of Rochester Medical Center. At postnatal day 120, male and female animals were randomly assigned to either PQ or filtered-air control groups. Within these 2 treatment groups, animals were further randomly divided into either behavior or nonbehavior control groups; behavioral enrichment has been shown to mitigate the effects of toxicants like PQ (Wu et al., 2011). This allocation yielded sample sizes of n = 8–9/treatment/behavior group for males and n = 5/treatment/behavior group for females. Treatment/group allocations are summarized in Supplementary Table 1.
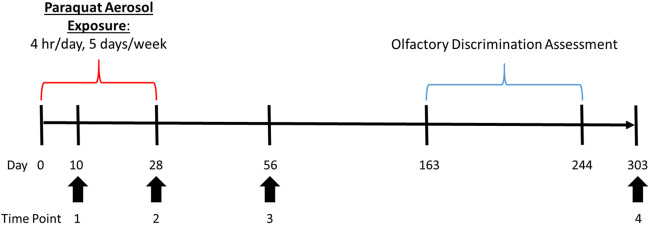
To assess whether PQ inhalation leads to disposition of PQ to the brain, a subset of male mice was sacrificed at 3 time points during and after exposure (Figure 1). These time points were chosen to capture the burden of PQ throughout the exposure, based on the previously published retention half-life of PQ in the brain (28 days; Prasad et al., 2007, 2009). Only male mice were used for kinetic time points, as previous studies have established that there is no difference in toxicokinetic profile of PQ between males and females (Litchfield et al., 1973). All mice were weighed weekly (every Friday) from the beginning of exposure to the completion of the study to monitor for signs of systemic toxicity. All mice used in this study were treated humanely and with regard for the alleviation of suffering. All procedures were approved by the Institutional Animal Care and Use Committees at the University of Rochester.
Figure 1.
Experimental timeline. Male and female C57BL/6J mice were exposed to either HEPA-filtered air (99.9% effective) or PQ aerosols (130 μg/m3) beginning on experimental day 0. Subsets of male mice were sacrificed on experimental days 10, 28, 56, and 303 (Time points Nos 1–4) and brain, lung, and kidney were collected for PQ quantitation. Bronchoalveolar lavage fluid was collected from a subset of males immediately following the end of exposure (Time point No. 2) to assess the acute effects of PQ inhalation on the lung. In order to assess the long-term effects of PQ inhalation on the lung, perfused lungs were collected from additional subset of males at Time point No. 4 for histological analysis. Olfactory discrimination training/testing began on experimental day 163, 135 days after the end of exposure. Abbreviations: PQ, paraquat; HEPA, high-efficiency particulate air.
Inhalation exposure paradigm
Whole-body inhalation exposures were conducted in the University of Rochester Inhalation Core Facility. Exposures were conducted 5 days/week for 4 weeks. Animals were exposed to either high-efficiency particulate air-filtered (99.9% effective) air or PQ aerosols for 4 h/day beginning at 08:30, during which time the mice were singly housed in small mesh chambers inside the 30-l stainless steel-reinforced Lexan exposure chamber. The exposure chamber was maintained at 40–45% relative humidity and 22–24°C. PQ aerosols were generated by adding a 0.2 mg/ml solution of PQ in ddH2O (made fresh on each day of exposure) into an ultrasonic nebulizer (Ultrasonic2000, Nouvag Dental and Medical Equipment). Clean, dry air (approximately 500 ml/min) was passed through the nebulizer at a frequency of 2.4 MHz to produce a PQ mist, which then passed through a heated drying tube and cold trap to remove moisture. The resulting dry aerosol was mixed with diluting air and entrained into the exposure chamber at a rate of 25–30 l/min. A peristaltic pump (Masterflex, Cole Palmer Inc.) was used to maintain the PQ solution within the nebulizer for the duration of the exposure (approximately 18 ml/h). Real-time exposure measurements were performed using a condensation particle counter (CPC Model 3022 A, TSI Inc.) to generate a continuous particle count concentration. Aerosol particle size distribution was measured once per exposure using an electrostatic classifier (SMPS Model 3071, TSI Inc.). Filters (Pallflex Membrane Filters, Pall Life Science) were collected hourly to determine gravimetric exposure concentration by mass; PQ on filters was also measured chemically at an absorbance wavelength of 259 nm using a UV-Vis spectrophotometer (HP-Agilent Technologies). PQ (PQ dichloride hydrate, >98.9% purity) was purchased from Sigma Aldrich.
In order to further evaluate PQ aerosol size and shape, particles were collected during exposure using an electrostatic precipitator (Model: 02-1400 Point-to-Plane; In-Tox Products). Aerosols were drawn from the exposure chamber (250 ml/min) and passed through the electron microscopy grid assembly (400 mesh Cu-coated holey carbon grids, 3/8 inch EM planchettes; Electron microscopy Sciences) for 10 min, sampling a total of 2.5 l. The particles were then visualized via transmission electron microscopy in the University of Rochester Microscopy Core.
Tissue collection
At each of the specified time points shown in Figure 1, and at the conclusion of behavioral assessment, various tissues were collected from both behavior and nonbehavior groups. Mice were weighed and then sacrificed by rapid decapitation without the use of sedative and fresh brains were removed and hemisected. The order of sacrifice was counterbalanced by sex, treatment, and behavior (behavior/nonbehavior) to preclude circadian variation within a single group. The olfactory bulb, striatum, ventral midbrain, cerebellum, and brain stem were extracted from each brain, after which they were flash-frozen. Additionally, the left lung and left kidney were collected from every mouse for PQ quantitation, with the exception of the last time point, where only lung tissue and brain were collected.
PQ quantification
To determine whether PQ enters the brain following inhalation, PQ concentrations in various tissues were quantified by the University of Rochester Mass Spectrometry core. Frozen tissues were thawed, weighed, and diluted in 12% acetic acid spiked with 500 pg/ml of the internal standard, PQD8 (>98% purity, Sigma Aldrich), and homogenized for 10 s (30 s in the case of kidney tissue only) via ultra-sonication (SLPe digital sonifier, Branson Ultrasonics). The resulting homogenized solution was boiled for 5 minutes, then spun at 10 000 × g at 4°C for 90 min and supernatant was collected. Proteins were then precipitated by adding 4 volumes of acetonitrile and centrifuging for 5 min at 15 000 × g; supernatants were collected and placed in clean autosampler vials for analysis via LC-MS/MS.
PQ analysis was conducted on a Dionex Ultimate 3000 UHPLC coupled to a Q Exactive Plus mass spectrometer (Thermo Fisher). PQ and PQD8 were separated on an InfinityLab Poroshell 120 HILIC-Z 2.1 × 100 mm column (Agilent). The mobile phases were: (A) 20 mM ammonium formate in 0.1% formic acid and (B) 20 mM ammonium formate in 90% acetonitrile with 0.1% formic acid. The flow rate was set to 800 µl/min, and the column oven was set to 30°C. 10 µl of each sample was injected, and the analytes were eluted with a gradient beginning at 80% A and holding for 0.5 min, before ramping to 60% B in 0.5 min. After holding at 60% B for 0.5 min, the gradient returned to 80% B in 0.25 min and column was re-equilibrated for 2.25 min; the total runtime was 4 min.
The Q Exactive Plus was operated in positive mode with a heated electrospray ionization source. The spray voltage was set to 4.0 kV, the sheath gas flow rate was set to 50, and the auxiliary gas flow rate was set to 10, while the capillary temperature was set to 350°C. A parallel reaction monitoring method was used to detect PQ and PQD8. The [M + H] precursor ions of 186.1153 m/z (PQ) and 194.1645 m/z (PQD8) were isolated with 1.0 m/z isolation width and then fragmented in the collision cell with collision energy of 25. The maximum injection time was set to 200 ms, while the automatic gain control (AGC) target was set to 2e5. The resulting fragment ions were detected in the Orbitrap with a resolution of 17,500 at m/z 200. Fragment ions 171.0916 (PQ) and 179.1408 (PQD8) were used to quantify the analytes. Chromatograms were extracted with a 10 ppm mass error and peaks were integrated using the LC Quan node of the XCalibur software (Thermo Fisher). The PQ: PQD8 peak area ratios of the samples were compared with those of the standard curve to determine analyte concentrations.
Lung lavage and histology
To assess whether PQ aerosol inhalation led to acute lung damage, a subset of male mice (n = 3/treatment) at Time point No. 2 (Figure 1) were sacrificed by CO2 inhalation and lungs were lavaged with phosphate-buffered saline (PBS, pH 7.2) and bronchoalveolar lavage fluid was collected. Lavage fluid was then assessed for gross markers of lung damage by measuring lactate dehydrogenase (LDH) enzyme activity (CyQUANT Cytotoxicity Assay, ThermoFisher) and protein content (Pierce BCA Protein Assay, ThermoFisher).
To determine whether inhaled PQ exposure produced pulmonary fibrosis, as has been observed with higher doses of PQ (Borges et al., 2014; Chen et al., 2013; Isha et al., 2018; Tomita et al., 2007), lungs were collected from another subset of nonbehavior mice at the last time point (Figure 1) for histological observation. These mice were sacrificed by Euthasol (pentobarbital and phenytoin; Virbac AH, Inc.) injection (10 µl/g). Lungs were perfused via cardiac puncture using PBS (pH 7.4) and then instilled with 1 ml 3% paraformaldehyde in PBS and transferred to histology cassettes and submerged in fixative overnight at 4°C, after which they were transferred to 70% ethanol until sectioning. After being paraffin embedding, lungs were sectioned at 5 µm, mounted and stained using Gomori’s trichrome. Representative images (3–5/lung section) were captured with a 20× objective on a brightfield microscope (Olympus BX53 microscope mounted with a Qimaging Retiga 2000 R camera) and collagen (marker of fibrosis) was assessed following a previously described method (Borges et al., 2014). In brief, fibrous connective tissue within the lung parenchyma was estimated by isolating the green shade pixels in the real image. The resulting binary image was analyzed using established thresholding methods (Montgomery et al., 2013) in ImageJ (National Institutes of Health) by an investigator blinded to treatment conditions. Blue-green labeling of collagen bands surrounding terminal bronchioles provided a positive control for the stain within each animal.
Olfactory discrimination paradigm
To assess whether PQ inhalation altered olfactory behavior, an olfactory discrimination assay was carried out in which male and female mice were trained to drink water containing 1 scent (positive) and to avoid water containing a different scent (negative). To enhance motivation to drink, all mice were placed on a water-restriction schedule for 48 h prior to all training and testing sessions. Mice were given free access to water for no < 24 h after each day of training or testing. All mice were weighed daily throughout olfactory assessment and monitored for signs of dehydration. All sessions were conducted in an open Plexiglass arena (dimensions: 30.5 × 30.5 × 30.5 cm); the 4 walls of the arena were covered with construction paper to eliminate visual cues. Water-deprived mice were habituated to the arena for 10 min prior to the first training session. Mice were then presented with a circular glass dish (9 cm in diameter, 1-cm tall) containing water for 4 min; if a mouse did not drink from the dish within the 4-min session, it was encouraged to drink by having its front paws and nose placed inside the dish. Once all of the mice were readily drinking from the dish, the volume of water was gradually reduced down to a single 20 µl drop in the center of the dish over the course of 3 sessions.
Next, mice were trained to associate 1 scent with pure water (positive scent) and the other scent with water containing a noxious stimulus (negative scent). The 2 scents used were almond and vanilla extract (McCormick and Company, Inc., Hunt Valley, Maryland), which were counterbalanced across treatment and sex. If a mouse was assigned vanilla as its positive scent, almond would be its negative scent, and vice versa. These scents were chosen for their lack of ecological relevance. During positive association training sessions, mice were placed in the arena with a single dish containing 8 µl of positive scent in 12 µl of water for 4 min. Once again, if a mouse did not drink by the end of the session, they were encouraged to do so. To preclude side bias, the location of the dish within the arena was randomized across sessions. Negative association training was conducted in the same manner using each mouse’s negative scent in water with 1% (m/v) of the bittering agent denatonium benzoate (Sigma Aldridge). The training criterion for both the positive and negative scent training required a given mouse to freely consume the water drop (positive) or abstain from drinking (negative) for 5 consecutive sessions.
After scent association training was completed in all mice, their ability to discriminate between the 2 scents was measured. Water-deprived mice were placed in the same arena with 2 dishes, 1 containing water with positive scent, the other containing water with negative scent. The odorless bittering agent was always included in the water with negative scent to avoid reinforcement of an incorrect choice. The placement of the positive scent dish relative to the negative scent dish was counterbalanced across treatments and subjects. Discrimination testing was initially conducted using a 100:0 ratio of the 2 scents (ie, 8 µl of positive scent in 12 µl water vs 8 µl of negative scent in 12 µl water). This was followed by a 90:10 ratio (ie, 7.2 µl of positive scent with 0.8 µl of the negative scent in 12 µl water vs 7.2 µl negative scent with 0.8 µl of the positive scent in 12 µl water). In total, 4 ratios were tested: 100:0, 90:10, 80:20, and 70:30 (Supplementary Figure 1). Mice were retrained to the same mastery criterion on each new ratio before discrimination testing. If a mouse drank first from the dish containing the negative scent, this was considered an incorrect choice. Because all mice successfully completed positive and negative association training, failure to drink from either dish before the end of the test was considered an incorrect choice during the discrimination phase. This task yielded discrete data (ie, correct vs incorrect choices). Prior to olfactory discrimination training/testing, locomotor behavior and motor coordination was assessed by spontaneous open-field locomotor activity, inverted screen test, and rota-rod apparatus performance; these data are described elsewhere(Anderson et al., in preparation). All sessions were video recorded and scored by an investigator blinded to treatment group using Noldus video software (The Observer XT 13).
Statistical analyses
Overall analyses were initially conducted with sex as a factor. On nearly every endpoint, data confirmed either main effects of sex or sex × time interactions in repeated-measures analyses. For that reason, and given that previous studies consistently demonstrate sex differences in the behavioral and neuropathological effects of PQ exposure (Litteljohn et al., 2011), all analyses were subsequently conducted stratified by sex. Endpoints with repeated data collection across weeks (body weight) were analyzed using a repeated-measures analysis of variance. For endpoints that did not include a repeated-measures component (lung endpoints), treatment differences were analyzed using t tests. The discrete olfactory discrimination data were analyzed using a Chi square test. Statistical analyses were conducted using JMP Pro 14.0 (SAS Institute Inc.). p Values ≤ .05 were considered statistically significant.
RESULTS
Exposure Conditions
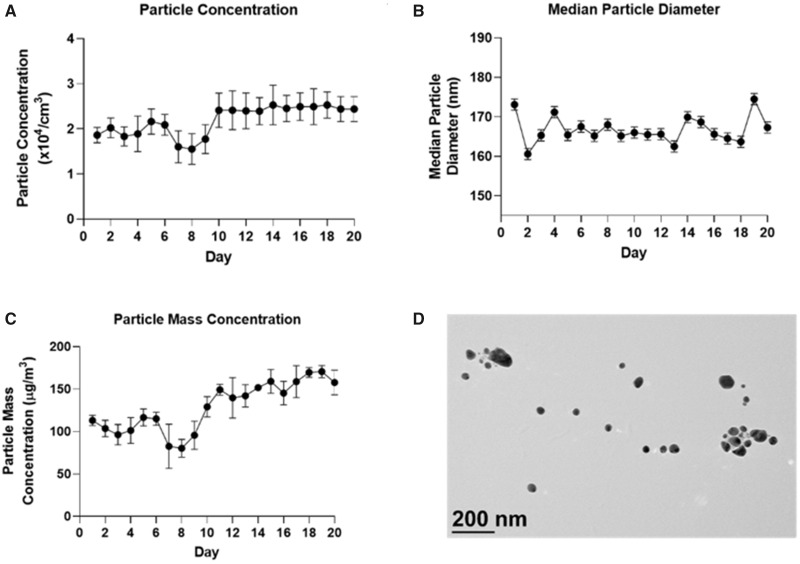
As shown in Figure 2, the average particle count across 20 days of exposure was 21 900 ± 3320 particles/cm3. The average particle mass concentration was 130 ± 10 µg/m3. The median particle diameter was 166 ± 3.1 nm. The geometric SD was 1.43.
Figure 2.
Exposure parameters. Daily (A) mean ± SD particle concentration, (B) median ± SD particle diameter, (C) mean ± SD particle mass concentration and, (D) TEM image of paraquat aerosols. Abbreviations: SD, standard deviation; TEM, transmission electron microscopy.
Body Weight and Pulmonary Endpoints
Prior to exposure, the average body weight of male (nonbehavior groups only) mice was 32.4 ± 0.95 g and 31.0 ± 0.84 g in air and PQ-treated mice, respectively. The average body weight of female mice was 24.0 ± 1.0 and 24.5 ± 0.44 g in air and PQ-treated groups, respectively. At the end of exposure, the average body weight of male (nonbehavior groups only) mice was 31.2 ± 0.86 and 31.0 ± 0.86 g in filter-air and PQ-treated animals, respectively. The average body weight of female mice was 23.5 ± 1.22 and 23.6 ± 0.19 g in filtered-air and PQ-exposed mice, respectively. Over the course of behavioral testing (275 days), all animals steadily gained weight (Supplementary Figure 2). No significant treatment-related changes in body weight were noted in males (F[1,15] = 2.6, p = .13) or females (F[1,8] = 0.17, p = .69).
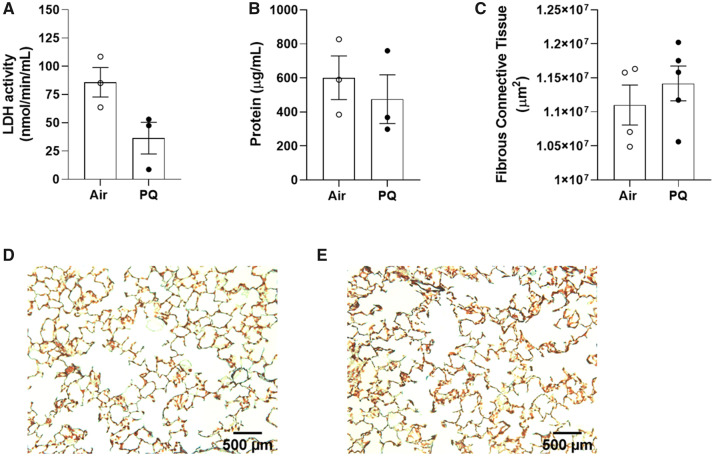
There was no statistically significant difference in LDH activity of the bronchoalveolar lavage fluid between air control (85.8 ± 13.0 nmol/min/ml) and PQ-exposed males (36.4 ± 14.0 nmol/min/ml) immediately following the end of exposure (t[4] = 2.6, p = .061; Figure 3A), nor was there any difference in the lavage fluid protein content (t[4] = 0.65, p = .55; Figure 3B). Histological analysis of the lungs at the end of the experiment (Time point No. 4, 275 days after the end of exposure) yielded no signs of fibrosis, as there was no difference in the area of fibrous connective tissue between the treatment groups (t[7] = 0.82, p = .44; Figs. 3C–E).
Figure 3.
Lung endpoints. Group means ± SE for LDH activity (A) and protein content (B) of bronchoalveolar lavage fluid collected from a subset of males (n = 3/group) immediately following the end of exposure (Time point No. 2). Group means ± SE for fibrous connective tissue area in lungs collected from a separate subset of males (n = 4–5/group) at the conclusion of the experiment (Time point No. 4), more than 300 days after the end of exposure (C). Representative images of lungs sections stained with Gomorri’s trichrome from air-control (D) and PQ-exposed (E) mice. Abbreviations: SE, standard error; LDH, lactate dehydrogenase; PQ, Paraquat.
PQ Quantitation
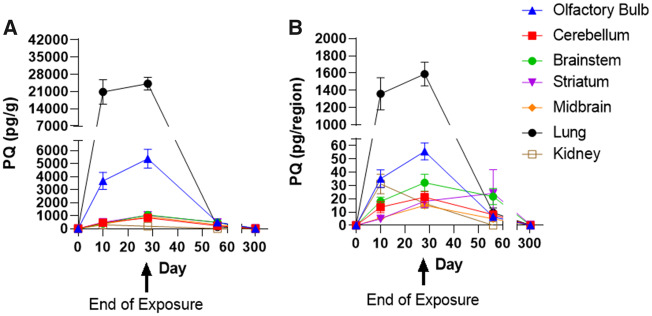
As shown in Figure 4, PQ was detected in all regions of the brain that were analyzed. The concentrations of PQ were highest in all brain regions immediately following the end of exposure (Time point No. 2), at 5,374 ± 718.7 pg/g in olfactory bulb, 497 ± 101 pg/g in the striatum, 346 ± 52.1 pg/g in the midbrain, 832.4 ± 212.2 pg/g in cerebellum, and 1,056 ± 229.7 pg/g in the brainstem. The highest tissue concentration of PQ was detected in the lungs during the exposure (20 848 ± 5002.0 pg/g). Notably, within the brain, levels in olfactory bulb were substantially higher than in any other brain region. Although PQ was detected in the kidney (303.3 ± 65.74 pg/g), the concentration present was lower than any other tissue examined. By 28 days postexposure (Time point No. 3), the concentration of PQ had decreased substantially in all regions that were examined (Figure 4A). By the end of the experiment (Time point No. 4, 278 days after the end of exposure), PQ levels were below the limit of detection in all tissues examined. In order to assess the relative burden of PQ across brain regions, the amount of PQ per region/organ was also plotted (Figure 4B). This analysis revealed that both the olfactory bulb and the brainstem had higher PQ burdens compared with the other regions analyzed. As there was not a clear, linear relationship between the concentration of PQ at Time points Nos 3 and 4, we were not able to estimate the retention half-life or the clearance rate for PQ in individual tissues or the body as a whole.
Figure 4.
PQ quantitation in tissues. A, Group mean ± SE PQ concentration (pg/g); B, Group mean ± SE tissue burden (pg/organ) in PQ-exposed male brain regions, lung, and kidney. Samples size of n = 5/time point/region. Abbreviations: PQ, paraquat; SE, standard error.
Olfactory Discrimination
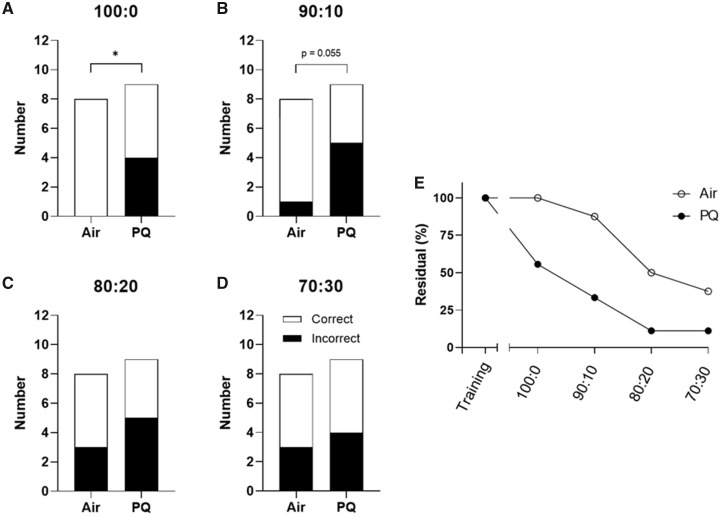
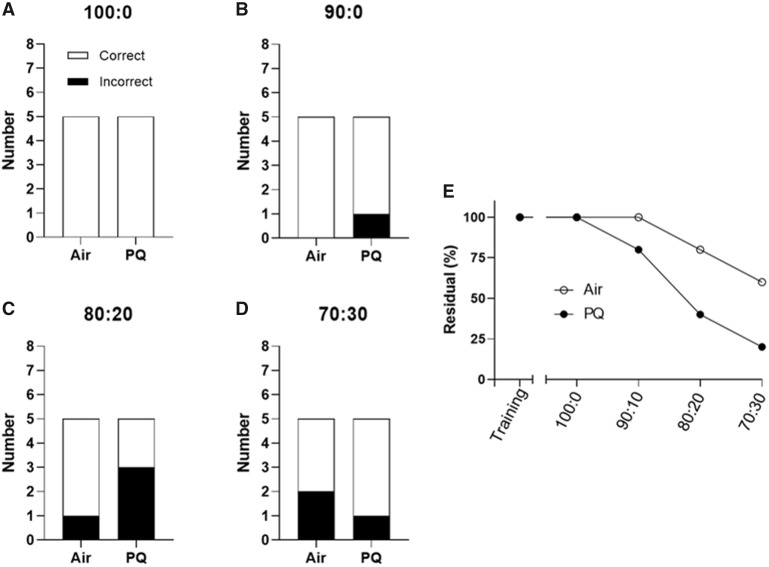
Male-specific deficits were observed in olfactory discrimination testing. All mice met the mastery criterion during the initial training as well as during the training sessions occurring prior to testing at each new scent ratio. However, PQ-exposed males made significantly more incorrect choices than the filtered-air controls in the 100:0 test (χ2 [1, n = 17] = 6.19, p = .013; Figure 5A). There was a trend toward significance in the 90:10 test (χ2 [1, n = 17) = 3.68, p = .055; Figure 5B). The difference in performance between the 2 groups was not present at either the 80:20 (χ2 [1, n = 17] = 0.56, p = 0.45; Figure 5C) or the 70:30 ratios (χ2 [1, n = 17] = 0.084, p = .77; Figure 5D). We did not observe any statistically significant effects of PQ on olfactory discrimination in females: in the 100:0 test, all filtered-air control and PQ-exposed females made the correct choice (Figure 6B). There was no difference between the groups in the 90:10 test (χ2 [1, n = 10] = 1.50, p = .22; Figure 6B), the 80:20 test (χ2 (1, n = 10] = 1.73, p = .19; Figure 6C), or the 70:30 test (χ2 [1, n = 10] = 0.483, p = .49; Figure 6D). As both groups in each sex had accuracy values approximating 50% at the 80:20 and 70:30 ratios, indicative of chance performance, lower and thus more difficult ratios (ie, 60:40) were not examined.
Figure 5.
Male olfactory discrimination performance. Total number of correct and incorrect responses made by air control and PQ-exposed males (n = 8–9/group) in a 2-choice olfactory discrimination test at (A) 100:0 ratio of positive: negative scents in the correct choice (least difficult), (B) 90:10, (C) 80:20, and (D) 70:30 (most difficult). As there was a possibility that a mouse with impaired olfaction could guess correctly in a 2-option test, the residual (%) of mice within each group that never made an error was also visualized to further highlight the differences between treatment groups (E). All mice were able to correctly identify single scents during training. Olfactory discrimination training/testing began 135 days after the end of exposure. *, significant main effect of PQ at p ≤ .05 following Chi square analysis. Abbreviation: PQ, paraquat.
Figure 6.
Female olfactory discrimination performance. Total number of correct and incorrect responses made by air control and PQ-exposed females (n = 5/group) in a 2-choice olfactory discrimination test at (A) 100:0 ratio of positive: negative scents in the correct choice (least difficult), (B) 90:10, (C) 80:20, and (D)70:30 (most difficult). As there was a possibility that a mouse with impaired olfaction could guess correctly in a 2-option test, the residual (%) of mice within each group that never made an error was also visualized to further highlight the differences between treatment groups (E). All mice were able to correctly identify single scents during training. Olfactory discrimination training/testing began 135 days after the end of exposure. No significant treatment effects were noted following Chi square analysis. Abbreviation: PQ, paraquat.
As there was a possibility that mice could guess correctly in a 2-option test, the residual (%) of mice within each group that never made an error was also examined and plotted across ratios (Figs. 5E and 6E). This visualization demonstrated that while 3 out of 8 (38%) air control male mice made the correct choice in all 4 tests, only 1 out of 9 (11%) PQ-exposed males did the same. In females, 3 out of 5 (60%) filtered-air controls made the correct choice in every test, while only 1 out of 5 (20%) of PQ-exposed females did the same.
DISCUSSION
This study sought to determine whether whole-body inhalation of PQ aerosols (4 h/day, 5 days/week for 4 weeks), a realistic route of exposure, would lead to disposition to the brain and functional deficits in behavior in mice. This study demonstrated that under such conditions, PQ entered all examined regions of the brain, with the highest concentration seen in the olfactory bulb. PQ was also detected within the lungs and kidney, but PQ levels in all tissues returned to control values 28 days postexposure. Additionally, PQ exposure produced a persistent olfactory discrimination impairment that was only seen in males.
Toxicokinetic studies of brain PQ accumulation suggest a retention half-life of approximately 28 days (Prasad et al., 2007, 2009). This estimation is based on PQ accumulation in the ventral midbrain of 150–500 pg/g tissue following a single PQ administration (10 mg/kg, i.p.; Prasad et al., 2007) and accumulation in the striatum of 1000 pg/g tissue following repeated administration (10 mg/kg, i.p., 6 injections across 2 weeks (Prasad et al., 2009). Time points for this study were chosen in accordance with aforementioned toxicokinetic studies of PQ that had used systemic injection routes of administration. Although it is difficult to estimate the brain half-life of PQ, the data here suggest the brain retention half-life of inhaled PQ is <28 days, given the observed temporal distribution of PQ concentrations. PQ was undetectable in the brain at the final time point (275 days postexposure). PQ was also cleared from the lung and undetectable at the last time point. Accumulation of PQ into the lung is accepted as a mechanism for its pulmonary toxicity (Rose et al., 1974; Smith, 1987), but this was not observed in this study. The redistribution of PQ from the lung to other organs following inhalation may preclude the reported lung-seeking behavior of PQ following systemic administration. Previous kinetic studies of PQ demonstrate near-complete (>90%) excretion of PQ in urine with no modification or degradation, suggesting that rodents are incapable of metabolizing PQ (Litchfield et al., 1973). Such findings are consistent with clinical reports of PQ excretion following human ingestion, which indicate PQ is excreted intact in urine (Beebeejaun et al., 1971; Kim et al., 2016). As PQ is not metabolized by humans or rodents, the observed clearance in this study may be due to excretion alone. Future studies should investigate the manner in which PQ is excreted from the brain and how that may alter its neurotoxicity.
These findings—consistent with evidence reporting translocation of other particulate materials (Oberdorster et al., 2004)—strongly suggest that inhaled PQ has the potential to enter the brain via the olfactory nerve following deposition on the nasal olfactory mucosa (Elder et al., 2006; Oberdorster et al., 2004). The relatively high burden of PQ in the brain stem suggests that translocation along the trigeminal nerve following deposition in the nasopharyngeal cavity may have also occurred (Lochhead and Thorne, 2012). There are a few key differences in inhalation between rodents and humans: rodents are obligate nose breathers, which may increase the likelihood of xenobiotic entry to the brain along the olfactory nerve relative to humans, who breathe through both the nose and mouth (Maher et al., 2016). Despite these differences, it is accepted that inhaled substances have a privileged transportation to the brain through these pathways (Kozlovskaya et al., 2014; Lochhead and Thorne, 2012; Prediger et al., 2012).
In addition to providing a portal of entry into the CNS that bypasses the blood-brain-barrier, the olfactory system itself is vulnerable to xenobiotic-mediated damage and functional deficits therein may be indicative of underlying pathologies (Dintica et al., 2019; Doty, 2007; Doty et al., 1992). Male PQ-exposed mice made significantly more errors in an olfactory discrimination test despite being able to correctly identify scents presented in isolation during training. This suggests PQ has a limited impact on odor detection threshold, but can adversely alter odor discrimination processing, which is consistent with other reports on the toxicity of PQ. It appears that, rather than damaging the odorant receptor-expressing olfactory neurons directly, PQ generates lesions in downstream processing, possibly in the olfactory bulb or tubercle. PQ-mediated damage to the olfactory bulb is consistent with the high burden of PQ we observed in this region relative to other brain regions. Correspondingly, acute systemic administration (i.p. injection) of PQ has also been reported to cause olfactory deficits in mice at doses much higher than that used here (Czerniczyniec et al., 2011). Thus, the olfactory deficits observed in this study are likely not an artifact of the route of exposure, but an inherent manifestation of PQ-mediated neurotoxicity. Future studies should investigate whether inhalation of PQ disrupts olfactory neurons or associated olfactory-processing circuits.
Regardless of the pathophysiological mechanisms, these findings suggest olfactory function may be useful in screening individuals who have been exposed to airborne pesticides. Although it has been demonstrated that acute exposure to pesticides impairs olfaction in farmers (Shrestha et al., 2019), these data indicate that even low-dose PQ inhalation exposures can have adverse effects on olfaction. The persistent olfactory impairment observed following inhaled PQ requires expanded investigation, given the well-established association between PQ and PD (Brouwer et al., 2017; Costello et al., 2009; Tanner et al., 2011). Olfactory impairment, or hyposmia, is observed in almost 90% of PD patients and is recognized as a common prodromal symptom of neurodegeneration (Doty, 2007, 2012; Ross et al., 2008; Ruan et al., 2012; Schapira et al., 2017). Future studies should investigate whether low-level inhalation exposure to PQ disrupts dopaminergic neurons in a manner consistent with the trajectory of PD.
It is difficult to compare exposure parameters from this study directly to what workers may be exposed to in the field. Few studies have examined the concentration of airborne PQ in the field during spraying. Seiber and Woodrow (1981) reported that PQ was present at a range of 4.31–10.7 µg/m3 1 m downwind of the field during spraying. Although the highest values detected on the perimeter of the field are about 10-fold lower than the concentration used in this study, workers within the field may experience higher levels. Chester and Woollen (1982) reported that workers manually spraying PQ in the field were exposed to average daily PQ concentrations of 4.89 ± 20.77 µg/m3. The highest concentration that a worker was exposed to on a single day (93.12 µg/m3) is comparable with our exposure. Meteorological conditions (eg, wind speed and direction, temperature, humidity) can influence the movement of airborne pesticide aerosols, which are difficult to account for in field studies and may explain some of the variation (Costanzini et al., 2018; Woodrow et al., 2018). Meteorological factors can also influence the size and respirability of the droplets after spraying. There are several routes for a nonvolatile pesticide, such as PQ, to become stabilized in the atmospheric phase, such as erosion following soil deposition or primary spray drift (Bedos et al., 2002; Costanzini et al., 2018; Sarigiannis et al., 2013; Woodrow et al., 2018; Yao et al., 2008). Pesticides with similar vapor pressures also have the potential to be transported far from the application site (Hageman et al., 2006), likely through mechanisms other than secondary volatilization. At least 1 documented case exists of community exposure to aerosolized PQ through primary spray drift following applicator error (Ames et al., 1993). In view of our findings, namely the lung deposition of inhaled PQ followed by disposition to the brain and persistent olfactory impairment, even low-level inhalation exposure to PQ may be cause for concern.
Despite the strengths of this study, there are limitations that merit discussion. This study was not designed to investigate the temporal dynamics of PQ-mediated functional alterations. Although these data suggest that PQ inhalation leads to male-specific olfactory deficits that persisted long after exposure, it cannot be determined when (and at what PQ concentration in the olfactory bulb) these deficits first presented, which would be useful in assessing olfaction as functional readout for pesticide exposure. Behavioral testing closer to exposure could have assisted in answering this question. As there was not a linear relationship between the concentration of PQ at Time points Nos 3 and 4, it was not possible to estimate the CNS half-life or clearance rate for PQ. Due to the study design and the tissues selected for analysis, this study is also unable to address the route by which PQ is excreted from the brain. Tissue collection closer to the end of exposure again would have allowed for better analysis of PQ kinetics. Future studies should examine how PQ is removed from the brain following disposition. Although previous studies using systemic administration suggested no sex differences in the tissue distribution of PQ (Litchfield et al., 1973), these data suggest that low-level inhalation of PQ leads to different distribution than previously reported with injection studies (Prasad et al., 2007, 2009). Thus, it remains unclear whether there are sex differences in the disposition of inhaled PQ; the addition of a female time course would have allowed for analysis of sex differences in kinetics. Finally, while histopathological analysis of the lung suggested that fibrosis was not present at the end of the study, the temporal dynamics of lung inflammation cannot be determined. As it is well known that PQ can lead to an acute inflammatory response in the lung (Borges et al., 2014; Chen et al., 2013; Isha et al., 2018; Tomita et al., 2007), future studies should examine whether low-dose inhalation of PQ leads to pulmonary inflammation. A better understanding of how inhaled pesticides may distribute throughout various organ systems may further an understanding of their potential impacts on the nervous system.
Taken together, these data suggest that prolonged low-level inhalation exposure to PQ aerosols leads to translocation of PQ to the brain, as well as persistent male-specific olfactory deficits consistent with the prodromal trajectory of PD. Thus, neurotoxicity should be considered when evaluating inhalation exposure to pesticides. Specifically, functional endpoints such as olfactory discrimination are sensitive outcome measures for PQ-mediated neurotoxicity. More research is needed to identify mechanisms of sex-dependent olfactory effects. Finally, these data support the need for the establishing protective regulations for applying neurotoxic pesticides, such as PQ, in order to protect public health.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
FUNDING
National Institutes of Health (ES025541, P30 ES00247, and 5T32HL066988-19).
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary Material
REFERENCES
- Ames R. G., Howd R. A., Doherty L. (1993). Community exposure to a paraquat drift. Arch. Environ. Health 48, 47–52. [DOI] [PubMed] [Google Scholar]
- Baharuddin M. R. B., Sahid I. B., Noor M. A. B. M., Sulaiman N., Othman F. (2011). Pesticide risk assessment: A study on inhalation and dermal exposure to 2,4-D and paraquat among Malaysian paddy farmers. J. Environ. Sci. Health B 46, 600–607. [DOI] [PubMed] [Google Scholar]
- Baldi I., Lebailly P., Rondeau V., Bouchart V., Blanc-Lapierre A., Bouvier G., Canal-Raffin M., Garrigou A. (2012). Levels and determinants of pesticide exposure in operators involved in treatment of vineyards: Results of the PESTEXPO Study. J. Expo. Sci. Environ. Epidemiol. 22, 593–600. [DOI] [PubMed] [Google Scholar]
- Bedos C., Cellier P., Calvet R., Barriuso E., Gabrielle B. (2002). Mass transfer of pesticides into the atmosphere by volatilization from soils and plants: Overview. Agronomie 22, 21–33. [Google Scholar]
- Beebeejaun A. A., Beevers G., Rogers W. N. (1971). Paraquat poisoning - Prolonged excretion. Clin. Toxicol. 4, 397–407. [DOI] [PubMed] [Google Scholar]
- Borges E. L., de Barros Pinheiro M., Prata L. O., Sales W. A., Silva Y. A. J. B., Caliari M. V., da Glória Rodrigues-Machado M. (2014). Effect of lung fibrosis on glycogen content in different extrapulmonary tissues. Lung 192, 125–131. [DOI] [PubMed] [Google Scholar]
- Brouwer M., Huss A., van der Mark M., Nijssen P. C. G., Mulleners W. M., Sas A. M. G., van Laar T., de Snoo G. R., Kromhout H., Vermeulen R. C. H., et al. (2017). Environmental exposure to pesticides and the risk of Parkinson’s disease in the Netherlands. Environ. Int. 107, 100–110., [DOI] [PubMed] [Google Scholar]
- Bus J. S., Cagen S. Z., Olgaard M., Gibson J. E. (1976a). A mechanism of paraquat toxicity in mice and rats. Toxicol. Appl. Pharmacol. 35, 501–513. [DOI] [PubMed] [Google Scholar]
- Bus J. S., Aust S. D., Gibson J. E. (1976. b). Paraquat toxicity: Proposed mechanism of action involving lipid peroxidation. Environ. Health Perspect. 16, 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Nie Y-C., Luo Y-L., Lin F., Zheng Y-F., Cheng G-H., Wu H., Zhang K-J., Su W-W., Shen J-G., et al. (2013). Protective effects of naringin against paraquat-induced acute lung injury and pulmonary fibrosis in mice. Food Chem. Toxicol. 58, 133–140., [DOI] [PubMed] [Google Scholar]
- Chester G., Ward R. J. (1984). Occupational exposure and drift hazard during aerial application of paraquat to cotton. Arch. Environ. Contamin. Toxicol. 13, 551–563. [DOI] [PubMed] [Google Scholar]
- Chester G., Woollen B. H. (1982). Studies of the occupational exposure of Malaysian plantation workers to paraquat. Br. J. Ind. Med. 39, 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa L. G. (2008). Neurotoxicity of pesticides: A brief review. Front. Biosci. 13, 1240–1249. [DOI] [PubMed] [Google Scholar]
- Costanzini S., Teggi S., Bigi A., Ghermandi G., Filippini T., Malagoli C., Nannini R., Vinceti M. (2018). Atmospheric dispersion modelling and spatial analysis to evaluate population exposure to pesticides from farming processes. Atmosphere 9, 38. [Google Scholar]
- Costello S., Cockburn M., Bronstein J., Zhang X., Ritz B. (2009). Parkinson’s disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am. J. Epidemiol. 169, 919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniczyniec A., Karadayian A. G., Bustamante J., Cutrera R. A., Lores-Arnaiz S. (2011). Paraquat induces behavioral changes and cortical and striatal mitochondrial dysfunction. Free Radic. Biol. Med. 51, 1428–1436. [DOI] [PubMed] [Google Scholar]
- Damalas C. A., Eleftherohorinos I. G. (2011). Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 8, 1402–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Monte D., Sandy M. S., Ekström G., Smith M. T. (1986). Comparative studies on the mechanisms of paraquat and 1-methyl-4-phenylpyridine (MPP+) cytotoxicity. Biochem. Biophys. Res. Commun. 137, 303–309. [DOI] [PubMed] [Google Scholar]
- Dinis-Oliveira R. J., Duarte J. A., Sánchez-Navarro A., Remião F., Bastos M. L., Carvalho F. (2008). Paraquat poisonings: Mechanisms of lung toxicity, clinical features, and treatment. Crit. Rev. Toxicol. 38, 13–71. [DOI] [PubMed] [Google Scholar]
- Dinis-Oliveira R. J., Valle M. J. D. J., Bastos M. L., Carvalho F., Sánchez Navarro A. (2006). Kinetics of paraquat in the isolated rat lung: Influence of sodium depletion. Xenobiotica 36, 724–737. [DOI] [PubMed] [Google Scholar]
- Dintica C. S., Marseglia A., Rizzuto D., Wang R., Seubert J., Arfanakis K., Bennett D. A., Xu W. (2019). Impaired olfaction is associated with cognitive decline and neurodegeneration in the brain. Neurology 92, e700–e709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donley N. (2019). The USA lags behind other agricultural nations in banning harmful pesticides. Environ. Health 18, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty R. L. (2007). Olfaction in Parkinson’s disease. olfaction in Parkinson’s disease. Parkinsonism Relat. Dis. 13, S225–S228. [DOI] [PubMed] [Google Scholar]
- Doty R. L. (2012). Olfaction in Parkinson’s disease and related disorders. Neurobiol. Dis. 46, 527–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty R. L., Stern M. B., Pfeiffer C., Gollomp S. M., Hurtig H. I. (1992). Bilateral olfactory dysfunction in early stage treated and untreated idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 55, 138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling K. C., Seiber J. N. (2002). Importance of respiratory exposure to pesticides among agricultural populations. Int. J. Toxicol. 21, 371–381. [DOI] [PubMed] [Google Scholar]
- Elder A., Gelein R., Silva V., Feikert T., Opanashuk L., Carter J., Potter R., Maynard A., Ito Y., Finkelstein J., et al. (2006). Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ. Health Perspect. 114, 1172–1178., [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangemi S., Gofita E., Costa C., Teodoro M., Briguglio G., Nikitovic D., Tzanakakis G., Tsatsakis A. M., Wilks M. F., Spandidos D. A., et al. (2016). Occupational and environmental exposure to pesticides and cytokine pathways in chronic diseases (Review). Int. J. Mol. Med. 38, 1012–1020., [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath B., Sue C. M., Kifley A., Mitchell P. (2012). The association between olfactory impairment and total mortality in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 67A, 204–209. [DOI] [PubMed] [Google Scholar]
- Gorell J. M., Johnson C. C., Rybicki B. A., Peterson E. L., Richardson R. J. (1998). The risk of Parkinson’s disease with exposure to pesticides, farming, well water, and rural living. Neurology 50, 1346–1350. [DOI] [PubMed] [Google Scholar]
- Hageman K. J., Simonich S. L., Campbell D. H., Wilson G. R., Landers D. H. (2006). Atmospheric deposition of current-use and historic-use pesticides in snow at national parks in the Western United States. Environ. Sci. Technol. 40, 3174–3180. [DOI] [PubMed] [Google Scholar]
- Isha I. T., Alam Z. H. M. N., Shaha B. K., Bari M. S., Bari M. Z. J., Chowdhury F. R. (2018). Paraquat induced acute kidney injury and lung fibrosis: A case report from Bangladesh. BMC Res. Notes 11, 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.-J., Kim H.-K., Lee H., Bae J.-S., Kown J.-T., Gil H.-W., Hong S.-Y. (2016). Toxicokinetics of paraquat in Korean patients with acute poisoning. Korean J. Physiol. Pharmacol. 20, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlovskaya L., Abou-Kaoud M., Stepensky D. (2014). Quantitative analysis of drug delivery to the brain via nasal route. J. Control Release 189, 133–140. [DOI] [PubMed] [Google Scholar]
- Lee K., Park E.-K., Stoecklin-Marois M., Koivunen M. E., Gee S. J., Hammock B. D., Beckett L. A., Schenker M. B. (2009). Occupational paraquat exposure of agricultural workers in large Costa Rican farms. Int. Arch. Occup. Environ. Health 82, 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., McLaughlin R., Harnly M., Gunier R., Kreutzer R. (2002). Community exposures to airborne agricultural pesticides in California: Ranking of inhalation risks. Environ. Health Perspect. 110, 1175–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-J., Mehler L., Beckman J., Diebolt-Brown B., Prado J., Lackovic M., Waltz J., Mulay P., Schwartz A., Mitchell Y., et al. (2011). Acute pesticide illnesses associated with off-target pesticide drift from agricultural applications: 11 States, 1998-2006. Environ. Health Perspect. 119, 1162–1169., [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Peng X., Yang H., Wang H., Shu Y. (2011). Deficiency of multidrug and toxin extrusion 1 enhances renal accumulation of paraquat and deteriorates kidney injury in mice. Mol. Pharm. 8, 2476–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litchfield M. H., Daniel J. W., Longshaw S. (1973). The tissue distribution of the bipyridylium herbicides diquat and paraquat in rats and mice. Toxicology 1, 155–165. [DOI] [PubMed] [Google Scholar]
- Litteljohn D., Nelson E., Bethune C., Hayley S. (2011). The effects of paraquat on regional brain neurotransmitter activity, hippocampal BDNF and behavioural function in female mice. Neurosci. Lett. 502, 186–191. [DOI] [PubMed] [Google Scholar]
- Lochhead J. J., Thorne R. G. (2012). Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 64, 614–628. [DOI] [PubMed] [Google Scholar]
- Maher B. A., Ahmed I. A. M., Karloukovski V., MacLaren D. A., Foulds P. G., Allsop D., Mann D. M. A., Torres-Jardón R., Calderon-Garciduenas L. (2016). Magnetite pollution nanoparticles in the human brain. Proc. Natl. Acad. Sci. U.S.A. 113, 10797–10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamane A., Baldi I., Tessier J.-F., Raherison C., Bouvier G. (2015). Occupational exposure to pesticides and respiratory health. Eur. Respir. Rev. 24, 306–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew H., Logan A., Woodruff M. F. A., Heard B. (1968). Paraquat poisoning - Lung transplantation. Br. Med. J. 3, 759–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack A. L., Atienza J. G., Johnston L. C., Andersen J. K., Vu S., Di Monte D. A. (2005). Role of oxidative stress in paraquat-induced dopaminergic cell degeneration. J. Neurochem. 93, 1030–1037. [DOI] [PubMed] [Google Scholar]
- Moisan F., Spinosi J., Delabre L., Gourlet V., Mazurie J.-L., Bénatru I., Goldberg M., Weisskopf M. G., Imbernon E., Tzourio C., et al. (2015). Association of Parkinson’s disease and its subtypes with agricultural pesticide exposures in men: A case-control study in France. Environ. Health Perspect. 123, 1123–1129., [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S. L., Narrow W. C., Mastrangelo M. A., Olschowka J. A., O’Banion M. K., Bowers W. J. (2013). Chronic neuron- and age-selective down-regulation of TNF receptor expression in triple-transgenic Alzheimer disease mice leads to significant modulation of amyloid- and tau-related pathologies. Am. J. Pathol. 182, 2285–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C. (2002). Prevelance of olfactory impairment in older adults. JAMA 288, 2307–2312. [DOI] [PubMed] [Google Scholar]
- Oberdorster G., et al. (2004). Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 16, 437–445. [DOI] [PubMed] [Google Scholar]
- Pesticide National Synthesis Project. Estimated Annual Agricultural Pesticide Use. Paraquat: 1992–2017. (2020). Available at: http://water.usgs.gov/nawqa/pnsp/usage/maps/show_map.php?year=2017&map=PARAQUAT&hilo=H. Accessed September 29, 2020. [Google Scholar]
- Prasad K., Tarasewicz E., Mathew J., Strickland P. A. O., Buckley B., Richardson J. R., Richfield E. K. (2009). Toxicokinetics and toxicodynamics of paraquat accumulation in mouse brain. Exp. Neurol. 215, 358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K., Winnik B., Thiruchelvam M. J., Buckley B., Mirochnitchenko O., Richfield E. K. (2007). Prolonged toxicokinetics and toxicodynamics of paraquat in mouse brain. Environ. Health Perspect. 115, 1448–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prediger R. D. S., Aguiar A. S., Matheus F. C., Walz R., Antoury L., Raisman-Vozari R., Doty R. L. (2012). Intranasal administration of neurotoxicants in animals: Support for the olfactory vector hypothesis of Parkinson’s disease. Neurotox. Res. 21, 90–116. [DOI] [PubMed] [Google Scholar]
- Quandt S. A., Hernández-Valero M. A., Grzywacz J. G., Hovey J. D., Gonzales M., Arcury T. A. (2006). Workplace, household, and personal predictors of pesticide exposure for farmworkers. Environ. Health Perspect. 114, 943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. R., Fitsanakis V., Westerink R. H. S., Kanthasamy A. G. (2019). Neurotoxicity of pesticides. Acta Neuropathol. 138, 343–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. S., Smith L. L., Wyatt I. (1974). Evidence of energy-dependent accumulation of paraquat into rat lung. Nature 252, 314–315. [DOI] [PubMed] [Google Scholar]
- Ross G. W., Petrovitch H., Abbott R. D., Tanner C. M., Popper J., Masaki K., Launer L., White L. R. (2008). Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann. Neurol. 63, 167–173. [DOI] [PubMed] [Google Scholar]
- Ruan Y., Zheng X.-Y., Zhang H.-L., Zhu W., Zhu J. (2012). Olfactory dysfunctions in neurodegenerative disorders. J. Neurosci. Res. 90, 1693–1700. [DOI] [PubMed] [Google Scholar]
- Sarigiannis D. A., Kontoroupis P., Solomou E. S., Nikolaki S., Karabelas A. J. (2013). Inventory of pesticide emissions into the air in Europe. Atmos. Environ. 75, 6–14. [Google Scholar]
- Schapira A. H. V., Chaudhuri K. R., Jenner P. (2017). Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 18, 435–450. [DOI] [PubMed] [Google Scholar]
- Schubert C. R., Fischer M. E., Pinto A. A., Klein B. E. K., Klein R., Tweed T. S., Cruickshanks K. J. (2017). Sensory impairments and risk of mortality in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 72, 710–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiber J. N., Woodrow J. E. (1981). Sampling and analysis of airborne residues of paraquat in treated cotton field environments. Arch. Environ. Contam. Toxicol. 10, 133–149. [DOI] [PubMed] [Google Scholar]
- Seidenfeld J. J., Wycoff D., Zavala D. C., Richerson H. B. (1978). Paraquat lung injury in rabbits. Br. J. Ind. Med. 35, 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K., Ohtaki K., Matsubara K., Aoyama K., Uezono T., Saito O., Suno M., Ogawa K., Hayase N., Kimura K., et al. (2001). Carrier-mediated processes in blood-brain barrier penetration and neural uptake of paraquat. Brain Res. 906, 135–142. [DOI] [PubMed] [Google Scholar]
- Shrestha S., Kamel F., Umbach D. M., Freeman L. E. B., Koutros S., Alavanja M., Blair A., Sandler D. P., Chen H. (2019). High pesticide exposure events and olfactory impairment among U.S. farmers. Environ. Health Perspect. 127, 017005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. L. (1987). Mechanism of paraquat toxicity in lung and its relevance to treatment. Hum. Toxicol. 6, 31–36. [DOI] [PubMed] [Google Scholar]
- Tanner C. M., Kamel F., Ross G. W., Hoppin J. A., Goldman S. M., Korell M., Marras C., Bhudhikanok G. S., Kasten M., Chade A. R., et al. (2011). Rotenone, paraquat, and Parkinson’s disease. Environ. Health Perspect. 119, 866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita M., Okuyama T., Katsuyama H., Miura Y., Nishimura Y., Hidaka K., Otsuki T., Ishikawa T. (2007). Mouse model of paraquat-poisoned lungs and its gene expression profile. Toxicology 231, 200–209. [DOI] [PubMed] [Google Scholar]
- Tsai W.-T. (2013). A review on environmental exposure and health risks of herbicide paraquat. Toxicol. Environ. Chem. 95, 197–206. [Google Scholar]
- Wang A., Costello S., Cockburn M., Zhang X., Bronstein J., Ritz B. (2011). Parkinson’s disease risk from ambient exposure to pesticides. Eur. J. Epidemiol. 26, 547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodrow J. E., Gibson K. A., Seiber J. N. (2018) Pesticides and related toxicants in the atmosphere. Rev. Environ. Contam. Toxicol. 247, 147–196. [DOI] [PubMed] [Google Scholar]
- Wu S.-Y., Wang T.-F., Yu L., Jen C. J., Chuang J.-I., Wu F.-S., Wu C.-W., Kuo Y.-M. (2011). Running exercise protects the substantia nigra dopaminergic neurons against inflammation-induced degeneration via the activation of BDNF signaling pathway. Brain Behav. Immun. 25, 135–146. [DOI] [PubMed] [Google Scholar]
- Wyatt I., Doss A. W., Zavala D. C., Smith L. L. (1981). Intrabronchial instillation of paraquat in rats: Lung morphology and retention study. Br. J. Ind. Med. 38, 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Harner T., Blanchard P., Tuduri L., Waite D., Poissant L., Murphy C., Belzer W., Aulagnier F., Sverko E., et al. (2008). Pesticides in the atmosphere across Canadian agricultural regions. Environ. Sci. Technol. 42, 5931–5937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.